- 1Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 2College of First Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
Osteoporosis is a systemic metabolic disorder characterized by compromised bone strength and increased fracture risk. Exosomes, extracellular vesicles measuring 40–160 nm in diameter, are critical mediators of intercellular communication. Among their bioactive components, microRNAs (miRNAs) have garnered attention for their role in the pathogenesis of Osteoporosis. Through complementary binding to the 3′ untranslated regions of target genes, miRNAs regulate key processes such as bone formation, bone resorption, angiogenesis, and bone immunity. This review provides a comprehensive summary of the regulatory roles and underlying mechanisms of miRNAs in osteoporosis, offering insights into potential therapeutic strategies.
1 Introduction
Osteoporosis (OP) is a metabolic bone disease characterized by reduced bone mass and deterioration of bone microarchitecture, resulting in increased bone fragility and susceptibility to fractures (1, 2). In recent years, OP has become increasingly prevalent among the elderly, contributing to greater fracture risk, diminished quality of life, and increased mortality in severe cases (3). According to the International Osteoporosis Foundation, osteoporosis imposes a substantial global burden, with approximately 1 in 3 women and 1 in 5 men aged over 50 experiencing osteoporotic fractures (4). Using the WHO definition, OP affects approximately 6.3% of men and 21.2% of women over 50 globally, suggesting approximately 500 million people may be affected (5). These trends underscore the urgent need to address OP as a significant global public health challenge, especially in the context of an ageing population.
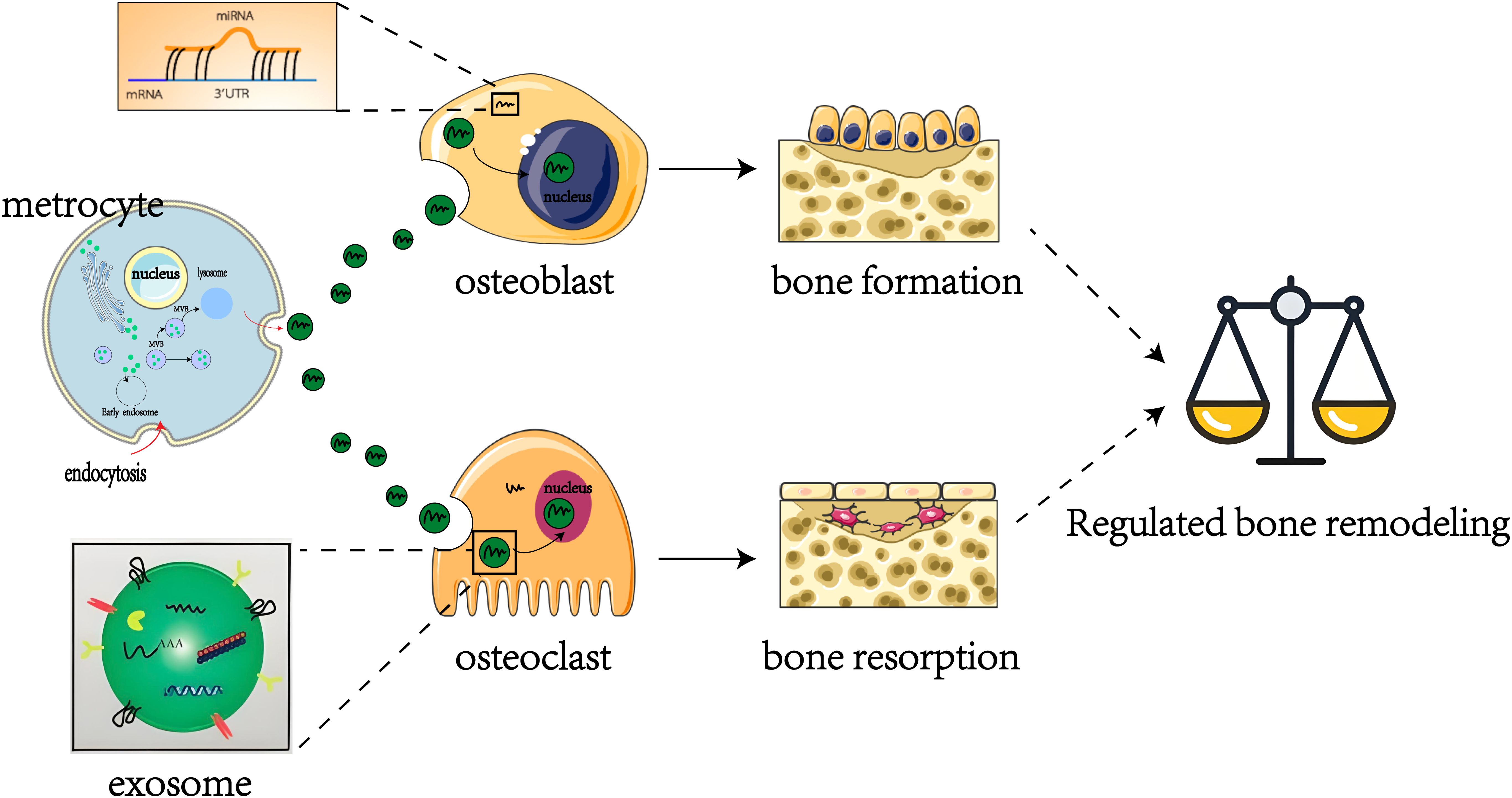
Exosomes are double-layered lipid membrane vesicles, 40–160 nm in diameter, produced within the endosomal compartments of most eukaryotic cells, and primarily contain biomolecules such as proteins, RNAs, and lipids that play significant roles in intercellular communication (6, 7). MicroRNAs (miRNAs), critical epigenetic regulators, participate in bone development, homeostasis, and repair processes by modulating the differentiation and activity of osteoblasts and osteoclasts, and are strongly linked to osteoporosis pathogenesis (8, 9). miRNA expression is regulated at multiple levels, including epigenetic mechanisms such as DNA methylation and histone modifications, as well as proteins regulating their maturation. Furthermore, miRNA expression is significantly influenced by environmental factors such as diet (e.g., vitamin D), exercise, pharmaceuticals, hormones, smoking, and even circadian rhythms, which alter their circulating levels (8). It has been found that miRNAs, as important active components of exosomes, can be transported to recipient cells via exosomes, thereby affecting the post-transcriptional expression of target genes and regulating the life activities of recipient cells (10, 11). Increasing evidence indicates that exosomal miRNAs may exert influence over the skeletal microenvironment by regulating gene expression through post-transcriptional gene silencing, which is either directly or indirectly implicated in the bone remodeling process (12–14). Several studies have reviewed the importance of miRNAs in the pathobiology of human disease (15, 16). miRNAs, acting upstream in the gene expression pathway, exhibit changes in circulating levels that can earlier reflect biological effects in the skeletal system, thus making them more sensitive potential biomarkers than classical protein biomarkers (8). The aim of this review is to provide an overview of the role of exosomal miRNAs in bone remodeling and their regulatory mechanisms in OP.
2 The biogenesis of miRNAs
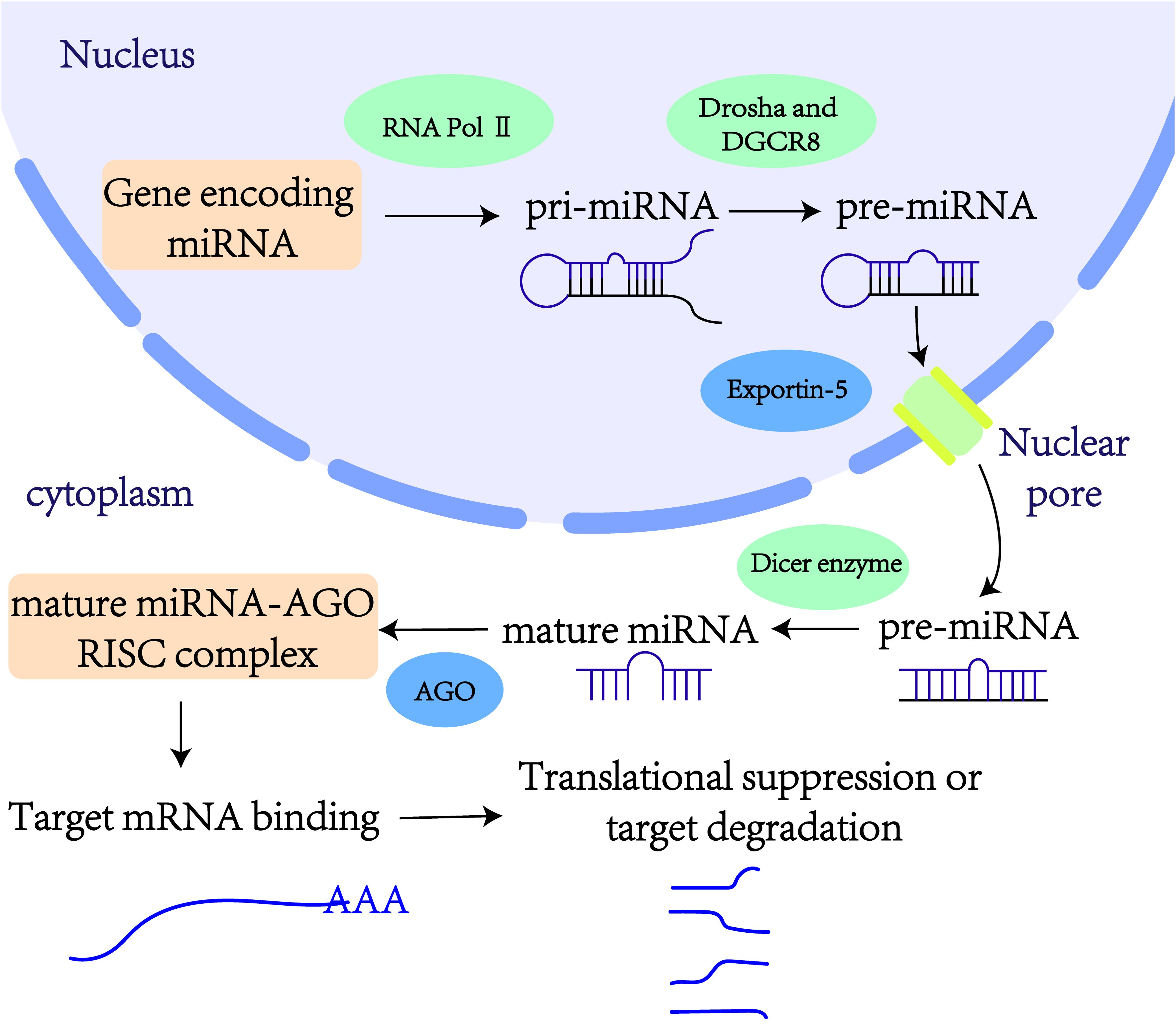
miRNAs are small endogenous non-coding RNA molecules, typically 19–25 nucleotides in length (17). In 1993, Victor Ambros and colleagues first identified the lin-4 gene as being involved in the developmental regulation of the nematode Caenorhabditis elegans (18). This discovery marked lin-4 as the first member of the miRNA family. Generally, miRNA genes are transcribed into primary miRNAs (pri-miRNAs) by RNA polymerase II. These pri-miRNAs are subsequently processed into precursor miRNAs (pre-miRNAs) by the nucleases Drosha and its cofactor DGCR8 (19). Pre-miRNAs are then transported from the nucleus to the cytoplasm by the Exportin-5 complex. In the cytoplasm, the enzyme Dicer cleaves pre-miRNAs, yielding mature double-stranded miRNAs. These mature miRNAs are loaded onto the Argonaute protein, forming the miRNA-induced silencing complexes (19). Within these complexes, one strand of the miRNA duplex is rapidly degraded, while the other strand—the functional mature miRNA—bind to the 3’ untranslated regions (3’ UTRs) of target mRNAs (Figure 1). This binding regulates gene expression post-transcriptionally by either inhibiting translation or inducing degradation of the target mRNAs. Thus, miRNAs play critical roles in various cellular biological processes.
The functional role of miRNAs primarily depends on their ability to bind to the 3’ UTRs of target mRNAs, thereby regulating gene expression (20). Numerous studies have demonstrated that miRNAs are widely distributed across various human tissues and organs, significantly affecting essential biological processes such as proliferation, apoptosis, and differentiation (21, 22). In addition to their roles in normal physiology, miRNAs are pivotal in various pathological conditions, such as cancer, pulmonary fibrosis, and diabetes (23). Notably, the expression levels of specific miRNAs can be modulated to regulate target gene expression, thus influencing normal cellular activities (24). Furthermore, miRNAs function within regulatory networks, as individual miRNAs may target multiple genes, and individual genes can be regulated by multiple miRNAs, highlighting the complexity of their regulatory roles (25).
3 The exosomal miRNAs linked to OP
OP, a prevalent and serious bone disease, is characterized by reduced bone mineral density and increased susceptibility to fractures. It primarily results from an imbalance between osteoblast-mediated bone formation and osteoclast-mediated bone resorption (26). However, the pathogenesis of OP extends beyond bone remodeling disruptions to involve factors such as estrogen deficiency, oxidative stress, and inflammation (27, 28).
Emerging evidence indicates that dysregulated microRNA (miRNA) expression can significantly influence osteoblast differentiation and activity, contributing to bone remodeling imbalances and the progression of OP (29). The multifaceted nature of OP underscores the need to comprehensively understand its underlying mechanisms and to identify novel therapeutic targets.
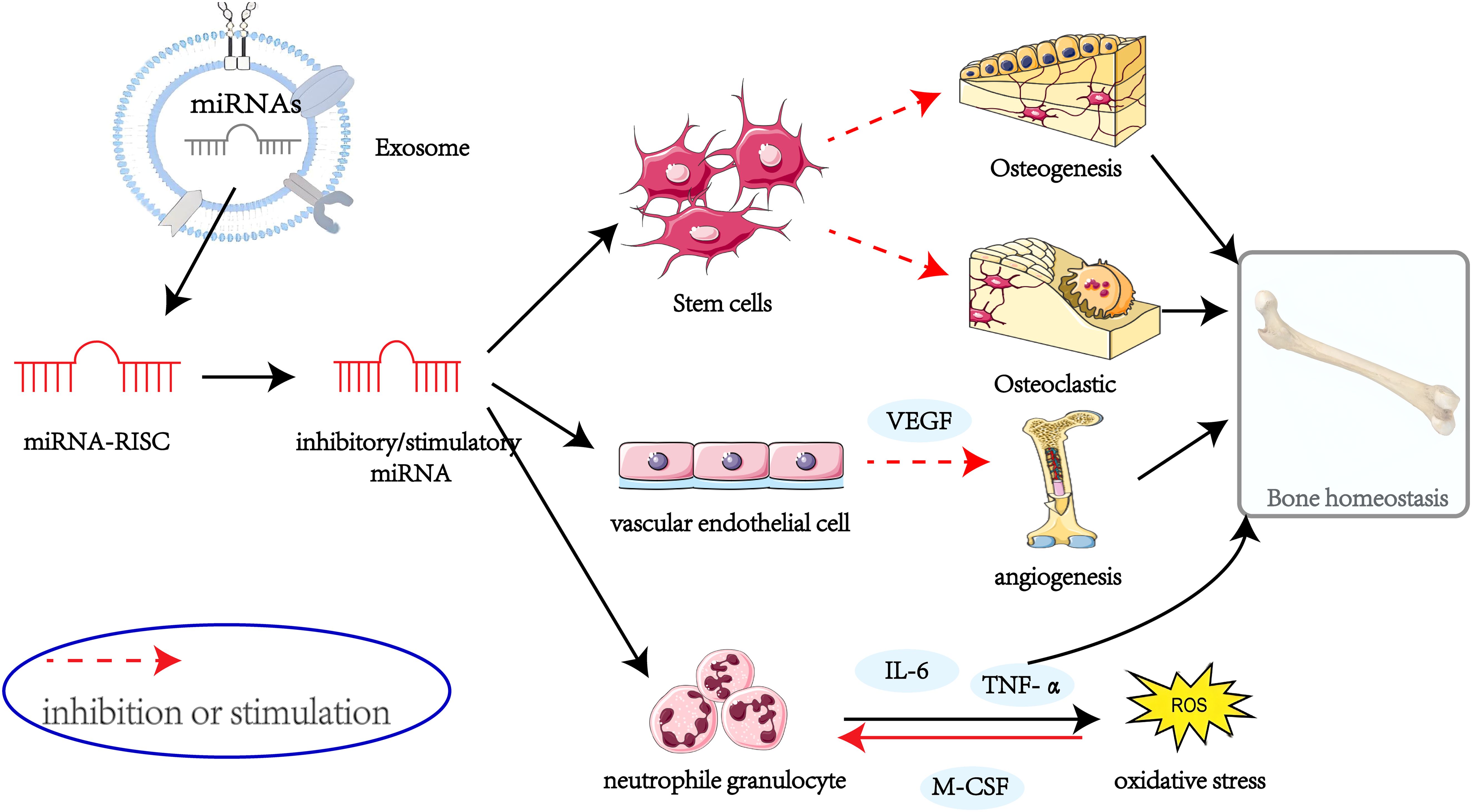
In recent years, the regulatory roles of miRNAs in OP - particularly those encapsulated within exosomes - have attracted considerable attention (30–32). These miRNAs play critical roles in modulating osteoblast and osteoclast proliferation and differentiation, thereby maintaining the delicate equilibrium between bone formation and resorption (Figure 2) (33). Bioinformatics analyses have revealed distinct miRNA expression patterns associated with postmenopausal osteoporosis (34). Notably, a comparative study of patients with osteoporotic versus non-osteoporotic hip fractures identified five miRNAs significantly elevated in the serum and bone tissue of osteoporotic patients (35). Additionally, numerous studies have highlighted the pivotal role of exosomal miRNAs in OP pathogenesis (36–38). For example, research investigating miRNA levels in serum samples from postmenopausal women with osteoporosis identified 331 differentially expressed miRNAs, including 122 upregulated and 209 downregulated miRNAs compared to controls (39). Collectively, these findings illustrate the intricate relationship between miRNAs and OP pathogenesis, highlighting potential therapeutic targets. This review therefore aims to further elucidate these miRNA-disease connections and facilitate the development of innovative treatment strategies.
4 The regulatory roles of miRNAs in OP
4.1 Role of miRNAs in regulating bone formation
Bone formation is a critical process within bone remodeling, involving the transformation of bone marrow mesenchymal stem cells (BMSCs) into osteoblasts. These osteoblasts are essential for secreting collagen fibers and facilitating bone matrix mineralization (40). Exosomal miRNAs, as remarkable regulators, fine-tune the expression of factors associated with bone formation, guiding osteoblast differentiation and function while influencing the intricate process of bone reconstruction (41). Dysregulated miRNA expression has been identified as a significant pathological factor impairing bone formation. miRNAs regulate osteogenic differentiation and bone formation through key signaling pathways, including the transforming growth factor β (TGF-β)/bone morphogenetic protein (BMP) pathway, the Wingless/Int-1 (Wnt)/β-catenin pathway, and the Notch signaling pathway (42).
Consistent with these roles, accumulating evidence highlights the sophisticated role of miRNAs in orchestrating osteogenesis (Table 1). miRNAs achieve this by either regulating key transcription factors through complex signaling cascades or directly targeting osteoblast proliferation and differentiation (43), emphasizing their potential as crucial mediators in bone health and promising therapeutic targets for bone-related disorders.
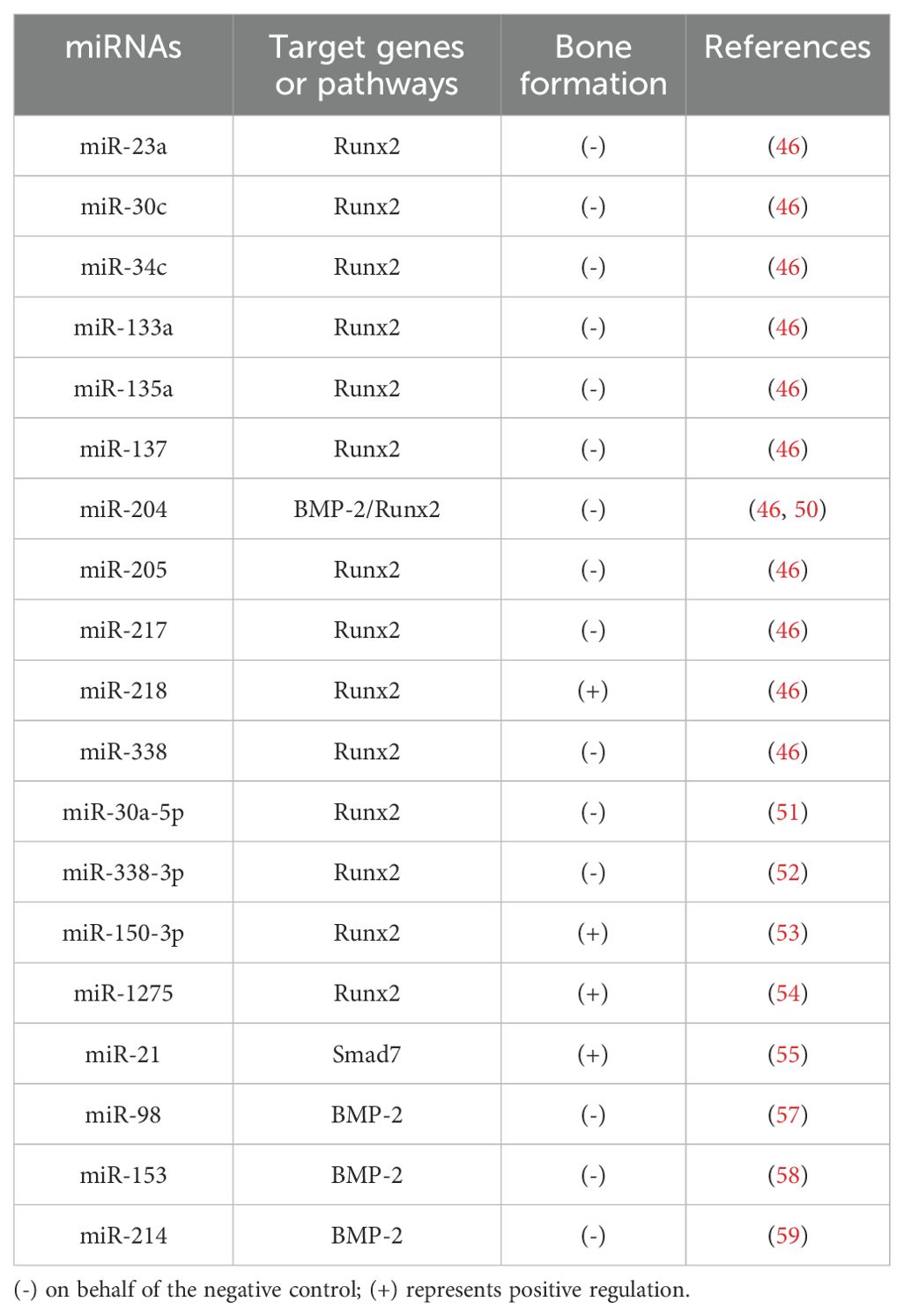
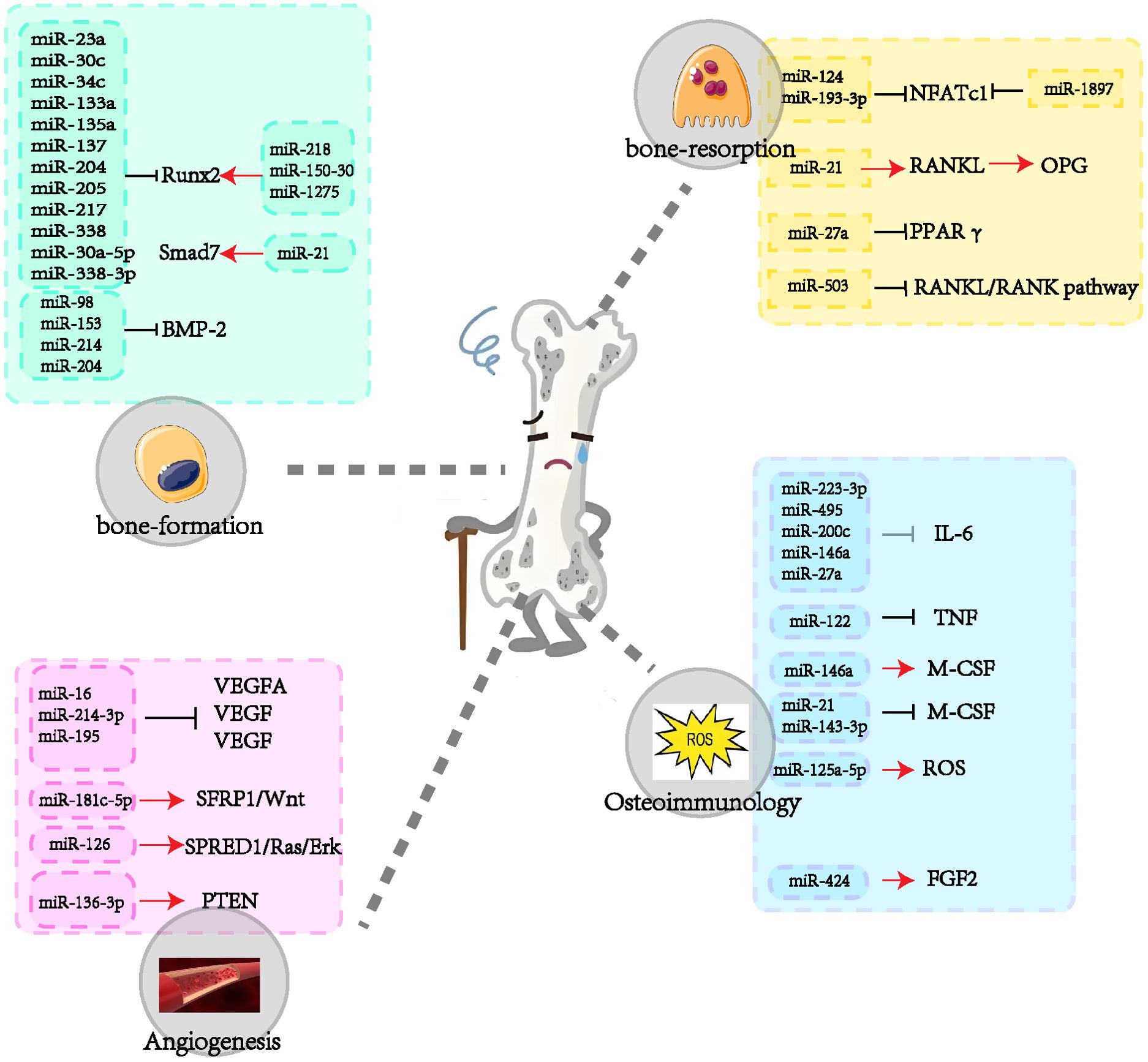
The Runt-related transcription factor (Runx) family is highly conserved and plays a vital role in organ development, cell metabolism, and stem cell differentiation (44). This family includes Runx1, Runx2, and Runx3, with Runx2 being essential for bone development by regulating osteoblast-mediated bone formation via various signaling pathways (45). Zhang et al. identified a set of 11 Runx2-targeted miRNAs, including miR-23a, miR-30c, miR-34c, miR-133a, miR-135a, miR-137, miR-204, miR-205, miR-217, miR-218, and miR-338, which exhibit lineage-specific expression patterns in mesenchymal cells. Among these, all except miR-218 showed a negative correlation with Runx2 expression (46). Most of these miRNAs have been previously shown to influence osteogenic differentiation (47–50). Notably, Zhang et al. also identified Runx2 as a downstream target of miR-30a-5p, where long non-coding RNA (lncRNA)-XIST promoted osteogenic differentiation in BMSCs by competitively binding miR-30a-5p and subsequently upregulating Runx2 expression (51). Additionally, Liu et al. observed increased miR-338-3p expression in an ovariectomy-induced rat osteoporosis model, reporting that miR-338-3p inhibited osteogenic differentiation by targeting both Runx2 and fibroblast growth factor receptor 2 (FGFR2) (52). Moreover, studies have demonstrated that specific exosomal miRNAs (e.g., miR-150-3p, miR-1275, and miR-21) enhance osteoblast differentiation and bone formation by upregulating Runx2 expression (53–55). These findings underscore the critical regulatory network involving Runx2 and miRNAs in bone biology.
Bone morphogenetic protein 2 (BMP-2) plays a crucial role in guiding mesenchymal stem cells (MSCs) towards becoming osteoblasts. BMP-2 achieves this by interacting with enzyme receptors on target cells, regulating the Smad signaling pathway, and ultimately activating osteogenic genes, thereby facilitating the formation of new bone (56). Studies have demonstrated that miRNAs such as miR-98 and miR-153 inhibit osteoblast proliferation and differentiation by directly targeting BMP-2, consequently influencing bone formation processes (57, 58). In human bone marrow mesenchymal stem cells (hBM-MSCs), miR-214 down-regulates the binding of BMP-2 expression to 3’ UTRs, and silencing miR-214 enhances osteogenic differentiation (59). Similarly, miR-204’s direct interaction with BMP-2 mRNA significantly impairs the differentiation of rat bone marrow MSCs (50). Maintaining a balance between osteogenic and adipogenic differentiation of MSCs is crucial for bone homeostasis (60). Inhibiting adipogenic differentiation, thus favoring osteogenic differentiation, represents a strategy to mitigate bone loss and enhance bone mass. MiR-146b-5p inhibits adipogenic differentiation of BMSCs from children with aplastic anemia by targeting SIAH2 and reducing PPARγ stability (61). Furthermore, scholars have found that miR-140 expression is downregulated in the serum of neonatal patients with developmental dysplasia, implying a potential role in neonatal bone formation, although its specific mechanism remains unreported (62).
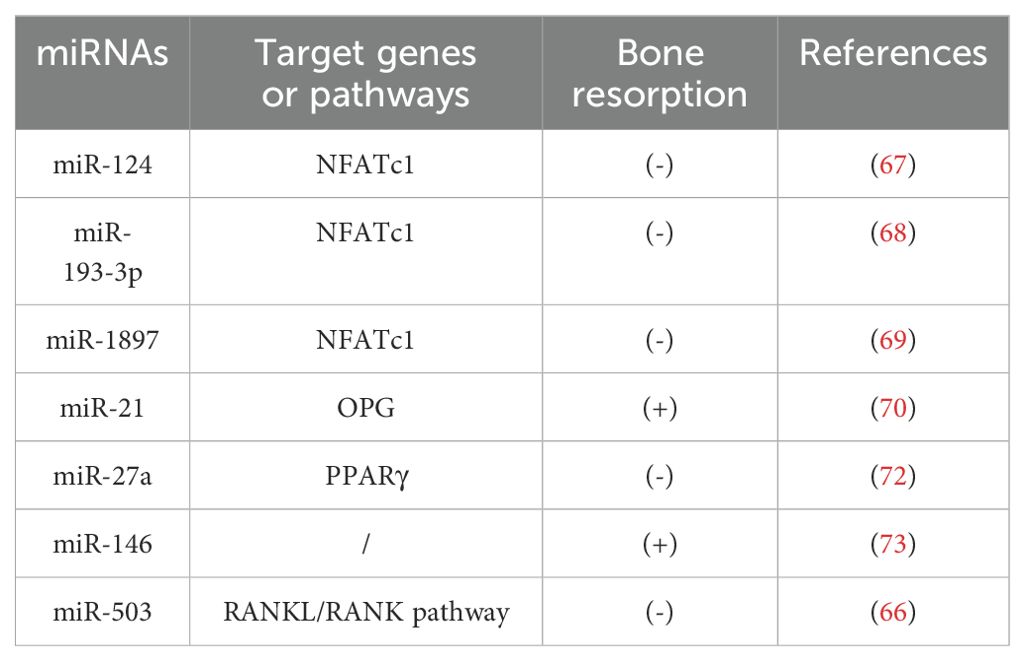
4.2 Role of miRNAs in regulating bone-resorption
Previous research has demonstrated that the receptor activator of nuclear factor κB ligand (RANKL)/receptor activator of nuclear factor κB (RANK)/osteoprotegerin (OPG) signaling pathway plays a pivotal role in regulating bone metabolism. RANKL, a key initiator of osteoclast differentiation, promotes the transformation of macrophages into osteoclasts by stimulating the expression of the transcription factor nuclear factor of activated T-cells cytoplasmic 1 (NFATc1). In contrast, OPG acts as a decoy receptor, binding to RANKL and thus inhibiting the RANKL/RANK interaction, which mitigates osteoclast activation and differentiation (63–65).
A study has demonstrated that the overexpression of miR-503 in ovariectomized mice directly inhibits the RANKL/RANK signaling pathway, reducing osteoclast activity (66). Several microRNAs, such as miR-124, regulate osteoclast differentiation by targeting NFATc1 mRNA. Specifically, miR-124 suppresses NFATc1 expression, affecting osteoclast differentiation through both RANKL-dependent and RANKL-independent pathways (67). In another study, Li et al. identified NFATc1 as a direct target of miR-193-3p. Overexpression of miR-193-3p inhibited NFATc1 expression, leading to reduced bone resorption in ovariectomized (OVX) mice (68). Additionally, recent research revealed that lncRNA-MIRG, acting as a competing endogenous RNA for miR-1897, inhibits miR-1897 expression. This inhibition enhances NFATc1 expression, promoting osteoclast differentiation from monocytic macrophages and exacerbating bone resorption in osteoporosis patients (69). Experimental data confirmed that increased miR-21 expression correlates with higher RANKL levels, reduced OPG concentrations, an increased RANKL/OPG ratio, and accelerated bone resorption, ultimately contributing to the progression of osteoporosis (70). Furthermore, another study demonstrated that knocking down miR-21 in mice reduced osteoclast function and number, resulting in increased trabecular bone volume and decreased bone resorption (71). These findings underscore the critical regulatory role of miRNAs in the RANKL/RANK/OPG signaling pathway and their potential as therapeutic targets in OP management. Moreover, miR-27a modulates estrogen-related processes by targeting the expression of PPARγ, thereby inhibiting osteoclast differentiation and bone resorption (72). Similarly, miR-146a has been identified as a key regulator in osteoclast formation. Deletion of miR-146a impairs osteoclast-mediated bone resorption, offering protection against OVX-induced bone loss (73). Together, these findings highlight the therapeutic potential of exosomal miRNAs for targeting osteoclast function in osteoporosis treatment. Future research is essential to further explore miRNA-dependent pathways that regulate osteoclast function, offering deeper insights into their role in bone diseases and paving the way for novel therapeutic strategies (Table 2).
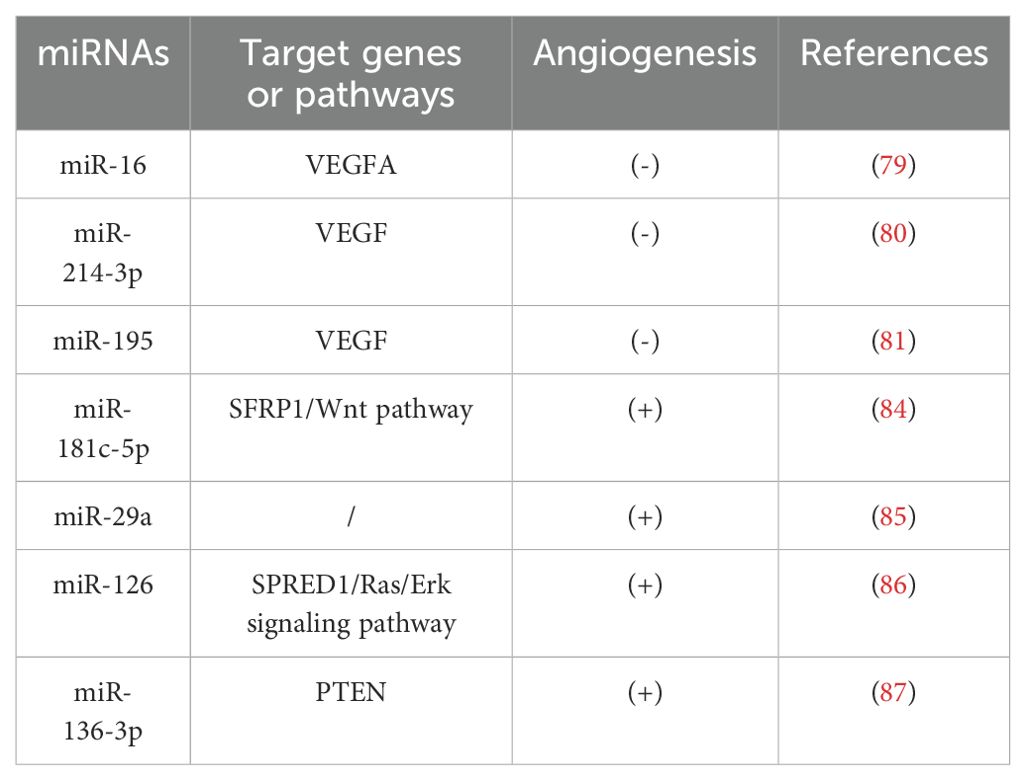
4.3 Role of miRNAs in regulating angiogenesis
Bone formation and angiogenesis are closely interconnected processes. Angiogenesis plays a critical role in promoting bone formation and maintaining bone homeostasis, particularly during bone development and fracture healing, where angiogenesis and osteogenesis are coupled (74). Vascular endothelial growth factor (VEGF) secreted by osteoblasts is an important regulator of the coupling of osteogenesis and angiogenesis; in particular, VEGFA, as a major pro-angiogenic factor, can attract endothelial cells to bone tissue and directly regulate the differentiation of osteoblasts and osteoclasts, thereby affecting bone metabolism (75). Dysregulated miRNA expression can lead to abnormal angiogenesis, therefore miRNAs can be used as potential targets to regulate angiogenesis and thus participate in the regulation of bone remodeling.
Several studies have shown that VEGFA plays an important regulatory role in OP (76, 77). Duan et al. found that osteogenic differentiation was decreased by miR-16 upregulation and increased by miR-16 downregulation (78). To further determine the regulatory role of miR-16 in OP, Yu et al. identified miR-16-5p as a potential miRNA targeting VEGFA mRNA using TargetScanHuman and DIANA software, and found that miR-16-5p can able to inhibit osteogenic differentiation by downregulating VEGFA expression (79). In addition, both miR-214-3p and miR-195 were found to be negative regulators of angiogenesis (80, 81). Differently, miR-214-3p was able to inhibit angiogenesis by downregulating VEGF expression and releasing negatively regulated angiogenic signals, whereas miR-195 inhibited bone-derived differentiation and angiogenesis in MSCs by decreasing the paracrine effect of MSCs on angiogenesis. Similarly, miR-181c-5p, an anti-angiogenic miRNA, is involved in regulating bone remodeling process by targeting and regulating the expression of Frizzled-related protein-1 (SFRP1), a negative regulator of osteoblasts, and activating the Wnt/β-catenin signaling pathway (82–84).
In addition, there is evidence that miR-29a, miR-126 and miR-136-3p all have positive effects on angiogenesis (85–87). Among these, miR-29a can effectively promote angiogenesis and osteogenesis in mice, while miR-126 and miR-136-3p are able to promote angiogenesis to accelerate the process of bone formation by triggering the production of a response signal in human umbilical vein endothelial cells (HUVEC), providing a new therapeutic for OP. These miRNAs represent promising therapeutic targets for the treatment of osteoporosis (Table 3).
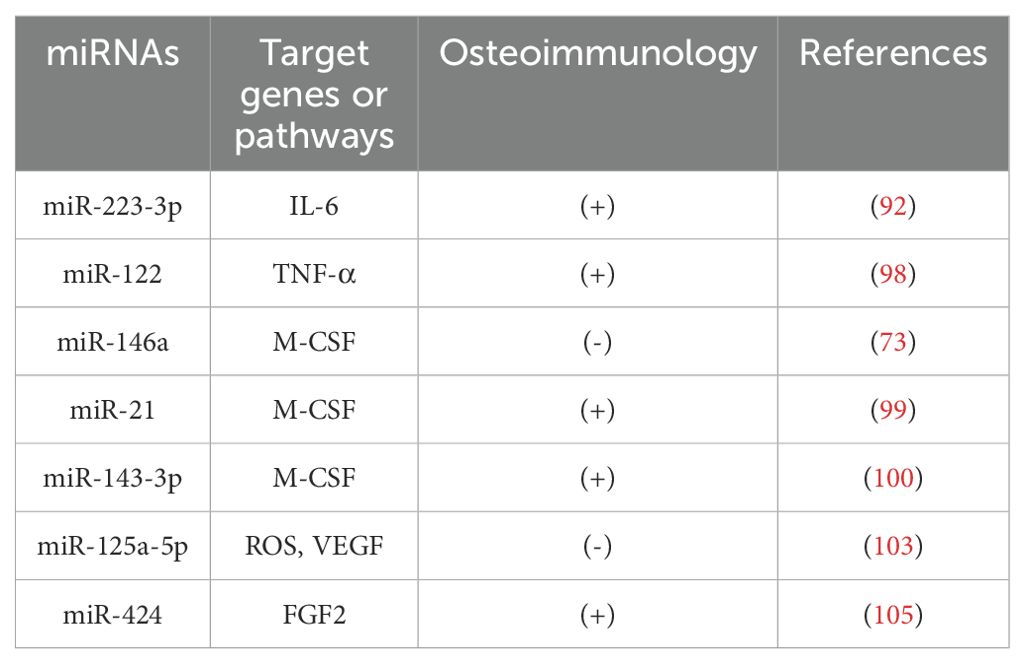
4.4 Role of miRNAs in regulating osteoimmunology
OP is increasingly recognized as an inflammatory bone disease characterized by a close interplay between immune cells and skeletal tissues (88, 89). In addition to inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and macrophage colony-stimulating factor (M-CSF), immune cells produce high levels of reactive oxygen species (ROS), which activate osteoclastogenic bone resorption (90). ROS are a major cause of oxidative stress (OS), which exacerbates injury (91). Taken together, inflammatory cytokines and immune cell-derived ROS interact directly or indirectly with osteoblasts, leading to an inflammatory response that drives the development of OP and regulates communication between the skeletal and immune systems (Table 4).
Cheng et al. found that IL-6 is a direct target gene of miR-223-3p, which inhibits the persistent pro-inflammatory response by suppressing IL-6 expression, thereby improving the bone microenvironment and regulating bone metabolism (92). Other studies have shown that miRNAs such as miR-495, miR-200c, miR-146a, miR-27a can promote bone formation by directly or indirectly down-regulating IL-6 (93–96). TNF-α inhibits osteoclast activity and stimulates osteoblast proliferation and differentiation at certain stages of differentiation (97), an effect which is improved by transfection of miR-122 mimics, reducing TNF-α stimulation in the organism, and reducing apoptosis (98). In addition, M-CSF acts as a regulator of osteoclasts and is able to induce osteoclast differentiation. miR-21, miR-143-3p and inhibition of miR-146a reduce osteoclast activity and inhibit osteoclast differentiation by reducing the amount of M-CSF in the bone microenvironment, thereby reducing bone loss (73, 99, 100).
Moreover, oxidative stress and exosome-derived miRNAs significantly influence OP pathogenesis (101, 102). Ye et al. found that miR-125a-5p and ROS were upregulated during osteogenic induction of hADSCs in vitro, suggesting that miR-125a-5p may reduce osteoblasts by exacerbating ROS damage and inhibiting VEGF expression, thereby reducing osteoblast sexual bone formation (103). Notably, forkhead box O1 (FoxO1), an important protein that protects bone from oxidative damage, is able to inhibit osteogenic differentiation by reducing ROS levels in cells (104). Furthermore, FoxO1 inhibited miR-424 expression and promoted cell proliferation and osteogenic differentiation, in part through the miR-424/FGF2 pathway (105).
5 Discussion
OP is a complex systemic metabolic disease characterized by multiple interacting mechanisms and pathways, as well as intricate communication between mesenchymal stromal cells, immune cells, and other biological cell types. This communication occurs either through direct cell-to-cell contact or via secreted factors, which are often transported by extracellular vesicles such as exosomes. Current treatments for OP primarily focus on inhibiting osteoclast proliferation and activation to reduce the rate of bone resorption. However, although drugs like bisphosphonates, denosumab, and estrogens are commonly used, long-term administration of these agents is associated with significant adverse effects and limited efficacy; moreover, the fundamental pathophysiological mechanisms of osteoporosis are not yet fully understood (106). As a result, there is a pressing need for further research into the molecular mechanisms regulating bone metabolism. Identifying low-toxicity, highly efficient drug targets that promote bone health could provide innovative strategies and methods for the prevention and treatment of OP.
miRNAs have the potential to serve as an early diagnostic biomarker for OP as well as a means of detecting the progression of this disease (64). Utilizing miRNAs that regulate osteoporosis pathogenesis could represent an effective therapeutic approach. For instance, miR-375 has been identified in the serum of postmenopausal women with an elevated risk of osteoporosis, serving as a potential marker of disease progression (107). Furthermore, prolonged administration of bisphosphonates has been associated with inhibited bone formation due to the overexpression of miR-30a-5p (108). It is noteworthy that, due to their multi-pathway and multi-target regulatory ability, the same miRNAs may regulate different targets, and multiple miRNAs may regulate the same or different mechanisms through the same or different targets. Consequently, the identification of specific miRNAs and their molecular targets and regulatory mechanisms involved in bone metabolism is an essential preliminary step in the development of clinical applications.
The role of miRNAs in exosomes in the aetiology and progression of OP has become a focus of research in recent years. These miRNAs regulate the proliferation and differentiation of osteoblasts and osteoclasts, influence angiogenesis, and participate in processes such as bone immunology. The present review provides a comprehensive overview of the role of multiple miRNAs in the regulation of osteoporosis genesis mechanisms (Figure 3). However, as there is no one-to-one correspondence between miRNAs and genes, specific miRNAs may affect multiple genes, which may result in potential side effects. Consequently, miRNA-based pharmaceutical agents for the management of osteoporosis have yet to be subjected to clinical trials.
The discovery of miRNAs and their regulatory roles in bone metabolism is closely linked to the development of new diagnostic and therapeutic techniques for OP. To translate these insights into clinical practice, comprehensive basic and clinical research is required to develop novel and more effective osteoporosis treatments. In the era of precision medicine, exploring exosomal miRNAs and their functions offers a unique approach to unraveling the molecular mechanisms of OP. miRNAs, as the main active components secreted by exosomes, have the unique advantage of being fine and precise, which will make this a reliable, sensitive and advanced technology for the treatment of OP in future studies.
Author contributions
FM: Writing – review & editing, Writing – original draft. CY: Investigation, Writing – review & editing. NL: Writing – review & editing. WH: Writing – original draft, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China Youth Science Fund, grant number 82004087.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yang J, Jiang T, Xu G, and Liu W. Bibliometrics analysis and visualization of sarcopenia associated with osteoporosis from 2000 to 2022. J Pain Res. (2023) 16:821–37. doi: 10.2147/jpr.S403648
2. Zhao H, Li X, Zhang D, Chen H, Chao Y, Wu K, et al. Integrative bone metabolomics-lipidomics strategy for pathological mechanism of postmenopausal osteoporosis mouse model. Sci Rep. (2018) 8:16456. doi: 10.1038/s41598-018-34574-6
3. Liu H, Song P, Zhang H, Zhou F, Ji N, Wang M, et al. Synthetic biology-based bacterial extracellular vesicles displaying BMP-2 and CXCR4 to ameliorate osteoporosis. J Extracell Vesicles. (2024) 13:e12429. doi: 10.1002/jev2.12429
4. Foundation IO. Epidemiology of osteoporosis and fragility fractures[EB/OL] (2025). Available online at: https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fracturesref_bottom_6 (Accessed June 16, 2025).
5. Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, and Khaltaev N. A reference standard for the description of osteoporosis. Bone. (2008) 42:467–75. doi: 10.1016/j.bone.2007.11.001
6. Yao Y, Jiang Y, Song J, Wang R, Li Z, Yang L, et al. Exosomes as potential functional nanomaterials for tissue engineering. Adv Healthc Mater. (2023) 12:e2201989. doi: 10.1002/adhm.202201989
7. Krylova SV and Feng D. The machinery of exosomes: biogenesis, release, and uptake. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24021337
8. Bottani M, Banfi G, and Lombardi G. Perspectives on miRNAs as epigenetic markers in osteoporosis and bone fracture risk: A step forward in personalized diagnosis. Front Genet. (2019) 10:1044. doi: 10.3389/fgene.2019.01044
9. Hensley AP and McAlinden A. The role of microRNAs in bone development. Bone. (2021) 143:115760. doi: 10.1016/j.bone.2020.115760
10. Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinf. (2015) 13:17–24. doi: 10.1016/j.gpb.2015.02.001
11. Yue B, Yang H, Wang J, Ru W, Wu J, Huang Y, et al. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Prolif. (2020) 53:e12857. doi: 10.1111/cpr.12857
12. Zeng ZL and Xie H. Mesenchymal stem cell-derived extracellular vesicles: a possible therapeutic strategy for orthopaedic diseases: a narrative review. Biomater Transl. (2022) 3:175–87. doi: 10.12336/biomatertransl.2022.03.002
13. Kim O, Tran PT, Gal M, Lee SJ, Na SH, Hwangbo C, et al. RAS−stimulated release of exosomal miR−494−3p promotes the osteolytic bone metastasis of breast cancer cells. Int J Mol Med. (2023) 52. doi: 10.3892/ijmm.2023.5287
14. Liu M, Sun Y, and Zhang Q. Emerging role of extracellular vesicles in bone remodeling. J Dent Res. (2018) 97:859–68. doi: 10.1177/0022034518764411
15. Rupaimoole R and Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. (2017) 16:203–22. doi: 10.1038/nrd.2016.246
16. Rivera-Barahona A, Pérez B, Richard E, and Desviat LR. Role of miRNAs in human disease and inborn errors of metabolism. J Inherit Metab Dis. (2017) 40:471–80. doi: 10.1007/s10545-017-0018-6
17. Nazri HM, Greaves E, Quenby S, Dragovic R, Tapmeier TT, and Becker CM. The role of small extracellular vesicle-miRNAs in endometriosis. Hum Reprod. (2023) 38:2296–311. doi: 10.1093/humrep/dead216
18. Lee RC, Feinbaum RL, and Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. (1993) 75:843–54. doi: 10.1016/0092-8674(93)90529-y
19. Hill M and Tran N. miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. (2021) 14. doi: 10.1242/dmm.047662
20. Desvignes T, Sydes J, Montfort J, Bobe J, and Postlethwait JH. Evolution after whole-genome duplication: teleost microRNAs. Mol Biol Evol. (2021) 38:3308–31. doi: 10.1093/molbev/msab105
21. Panda AC, Abdelmohsen K, and Gorospe M. SASP regulation by noncoding RNA. Mech Ageing Dev. (2017) 168:37–43. doi: 10.1016/j.mad.2017.05.004
22. Crippa S, Cassano M, and Sampaolesi M. Role of miRNAs in muscle stem cell biology: proliferation, differentiation and death. Curr Pharm Des. (2012) 18:1718–29. doi: 10.2174/138161212799859620
23. Patel V and Noureddine L. MicroRNAs and fibrosis. Curr Opin Nephrol Hypertens. (2012) 21:410–6. doi: 10.1097/MNH.0b013e328354e559
24. O'Brien J, Hayder H, Zayed Y, and Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). (2018) 9:402. doi: 10.3389/fendo.2018.00402
25. Xu P, Wu Q, Yu J, Rao Y, Kou Z, Fang G, et al. A Systematic Way to Infer the Regulation Relations of miRNAs on Target Genes and Critical miRNAs in Cancers. Front Genet. (2020) 11:278. doi: 10.3389/fgene.2020.00278
26. Chen R, Liao X, Chen F, Wang B, Huang J, Jian G, et al. Circulating microRNAs, miR-10b-5p, miR-328-3p, miR-100 and let-7, are associated with osteoblast differentiation in osteoporosis. Int J Clin Exp Pathol. (2018) 11:1383–90.
27. Wu D, Cline-Smith A, Shashkova E, Perla A, Katyal A, and Aurora R. T-cell mediated inflammation in postmenopausal osteoporosis. Front Immunol. (2021) 12:687551. doi: 10.3389/fimmu.2021.687551
28. Mohamad NV, Ima-Nirwana S, and Chin KY. Are oxidative stress and inflammation mediators of bone loss due to estrogen deficiency? A review of current evidence. Endocr Metab Immune Disord Drug Targets. (2020) 20:1478–87. doi: 10.2174/1871530320666200604160614
29. Iantomasi T, Romagnoli C, Palmini G, Donati S, Falsetti I, Miglietta F, et al. Oxidative stress and inflammation in osteoporosis: molecular mechanisms involved and the relationship with microRNAs. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24043772
30. Li QC, Li C, Zhang W, Pi W, and Han N. Potential effects of exosomes and their microRNA carrier on osteoporosis. Curr Pharm Des. (2022) 28:899–909. doi: 10.2174/1381612828666220128104206
31. de Oliveira MC, Heredia JE, da Silva FRF, and Macari S. Extracellular vesicles in bone remodeling and osteoporosis. Adv Exp Med Biol. (2023) 1418:155–68. doi: 10.1007/978-981-99-1443-2_11
32. Sun Y, Chen P, and Zhao B. Role of extracellular vesicles associated with microRNAs and their interplay with cuproptosis in osteoporosis. Noncoding RNA Res. (2024) 9:715–9. doi: 10.1016/j.ncrna.2024.03.002
33. Cui Q, Xing J, Yu M, Wang Y, Xu J, Gu Y, et al. Mmu-miR-185 depletion promotes osteogenic differentiation and suppresses bone loss in osteoporosis through the Bgn-mediated BMP/Smad pathway. Cell Death Dis. (2019) 10:172. doi: 10.1038/s41419-019-1428-1
34. Gu H, Wu L, Chen H, Huang Z, Xu J, Zhou K, et al. Identification of differentially expressed microRNAs in the bone marrow of osteoporosis patients. Am J Transl Res. (2019) 11:2940–54.
35. Seeliger C, Karpinski K, Haug AT, Vester H, Schmitt A, Bauer JS, et al. Five freely circulating miRNAs and bone tissue miRNAs are associated with osteoporotic fractures. J Bone Miner Res. (2014) 29:1718–28. doi: 10.1002/jbmr.2175
36. Sun Z, Shi J, Yang C, Chen X, Chu J, Chen J, et al. Identification and evaluation of circulating exosomal miRNAs for the diagnosis of postmenopausal osteoporosis. J Orthop Surg Res. (2023) 18:533. doi: 10.1186/s13018-023-04020-z
37. Lu J, Wu H, Jin H, He Z, Shen L, Ma C, et al. The influence of modified Qing E Formula on the differential expression of serum exosomal miRNAs in postmenopausal osteoporosis patients. Front Pharmacol. (2024) 15:1467298. doi: 10.3389/fphar.2024.1467298
38. Li Y, Wang X, Pan C, Yuan H, Li X, Chen Z, et al. Myoblast-derived exosomal Prrx2 attenuates osteoporosis via transcriptional regulation of lncRNA-MIR22HG to activate Hippo pathway. Mol Med. (2023) 29:54. doi: 10.1186/s10020-023-00649-y
39. Li Y, Shi Z, and Feng S. Systematic analysis of miRNAs in patients with postmenopausal osteoporosis. Gynecol Endocrinol. (2020) 36:997–1001. doi: 10.1080/09513590.2020.1785420
40. Šromová V, Sobola D, and Kaspar P. A brief review of bone cell function and importance. Cells. (2023) 12. doi: 10.3390/cells12212576
41. Gao M, Gao W, Papadimitriou JM, Zhang C, Gao J, and Zheng M. Exosomes-the enigmatic regulators of bone homeostasis. Bone Res. (2018) 6:36. doi: 10.1038/s41413-018-0039-2
42. Loh HY, Norman BP, Lai KS, Cheng WH, Nik Abd Rahman NMA, Mohamed Alitheen NB, et al. Post-transcriptional regulatory crosstalk between microRNAs and canonical TGF-β/BMP signalling cascades on osteoblast lineage: A comprehensive review. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24076423
43. Peng S, Gao D, Gao C, Wei P, Niu M, and Shuai C. MicroRNAs regulate signaling pathways in osteogenic differentiation of mesenchymal stem cells (Review). Mol Med Rep. (2016) 14:623–9. doi: 10.3892/mmr.2016.5335
44. Mevel R, Draper JE, Lie ALM, Kouskoff V, and Lacaud G. RUNX transcription factors: orchestrators of development. Development. (2019) 146. doi: 10.1242/dev.148296
45. Komori T. Molecular mechanism of runx2-dependent bone development. Mol Cells. (2020) 43:168–75. doi: 10.14348/molcells.2019.0244
46. Zhang Y, Xie RL, Croce CM, Stein JL, Lian JB, van Wijnen AJ, et al. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci U.S.A. (2011) 108:9863–8. doi: 10.1073/pnas.1018493108
47. Li T, Li H, Wang Y, Li T, Fan J, Xiao K, et al. microRNA-23a inhibits osteogenic differentiation of human bone marrow-derived mesenchymal stem cells by targeting LRP5. Int J Biochem Cell Biol. (2016) 72:55–62. doi: 10.1016/j.biocel.2016.01.004
48. Deng L, Hu G, Jin L, Wang C, and Niu H. Involvement of microRNA-23b in TNF-α-reduced BMSC osteogenic differentiation via targeting runx2. J Bone Miner Metab. (2018) 36:648–60. doi: 10.1007/s00774-017-0886-8
49. Liu G, Lu Y, Mai Z, Liu R, Peng Z, Chen L, et al. Suppressing microRNA-30b by estrogen promotes osteogenesis in bone marrow mesenchymal stem cells. Stem Cells Int. (2019) 2019:7547506. doi: 10.1155/2019/7547506
50. Jiang X, Zhang Z, Peng T, Wang G, Xu Q, and Li G. miR−204 inhibits the osteogenic differentiation of mesenchymal stem cells by targeting bone morphogenetic protein 2. Mol Med Rep. (2020) 21:43–50. doi: 10.3892/mmr.2019.10791
51. Zhang HL, Du XY, and Dong QR. LncRNA XIXT promotes osteogenic differentiation of bone mesenchymal stem cells and alleviates osteoporosis progression by targeting miRNA-30a-5p. Eur Rev Med Pharmacol Sci. (2019) 23:8721–9. doi: 10.26355/eurrev_201910_19266
52. Liu H, Sun Q, Wan C, Li L, Zhang L, and Chen Z. MicroRNA-338-3p regulates osteogenic differentiation of mouse bone marrow stromal stem cells by targeting Runx2 and Fgfr2. J Cell Physiol. (2014) 229:1494–502. doi: 10.1002/jcp.24591
53. Qiu M, Zhai S, Fu Q, and Liu D. Bone marrow mesenchymal stem cells-derived exosomal microRNA-150-3p promotes osteoblast proliferation and differentiation in osteoporosis. Hum Gene Ther. (2021) 32:717–29. doi: 10.1089/hum.2020.005
54. Zou Z, Dai R, Deng N, Su W, and Liu P. Exosomal miR-1275 secreted by prostate cancer cells modulates osteoblast proliferation and activity by targeting the SIRT2/RUNX2 cascade. Cell Transplant. (2021) 30:9636897211052977. doi: 10.1177/09636897211052977
55. Arumugam B, Balagangadharan K, and Selvamurugan N. Syringic acid, a phenolic acid, promotes osteoblast differentiation by stimulation of Runx2 expression and targeting of Smad7 by miR-21 in mouse mesenchymal stem cells. J Cell Commun Signal. (2018) 12:561–73. doi: 10.1007/s12079-018-0449-3
56. Halloran D, Durbano HW, and Nohe A. Bone morphogenetic protein-2 in development and bone homeostasis. J Dev Biol. (2020) 8. doi: 10.3390/jdb8030019
57. Zhang Y, Wei QS, Ding WB, Zhang LL, Wang HC, Zhu YJ, et al. Increased microRNA-93-5p inhibits osteogenic differentiation by targeting bone morphogenetic protein-2. PloS One. (2017) 12:e0182678. doi: 10.1371/journal.pone.0182678
58. Cao Y, Lv Q, and Lv C. MicroRNA-153 suppresses the osteogenic differentiation of human mesenchymal stem cells by targeting bone morphogenetic protein receptor type II. Int J Mol Med. (2015) 36:760–6. doi: 10.3892/ijmm.2015.2275
59. Wang CG, Liao Z, Xiao H, Liu H, Hu YH, Liao QD, et al. LncRNA KCNQ1OT1 promoted BMP2 expression to regulate osteogenic differentiation by sponging miRNA-214. Exp Mol Pathol. (2019) 107:77–84. doi: 10.1016/j.yexmp.2019.01.012
60. Hoshiba T, Kawazoe N, and Chen G. The balance of osteogenic and adipogenic differentiation in human mesenchymal stem cells by matrices that mimic stepwise tissue development. Biomaterials. (2012) 33:2025–31. doi: 10.1016/j.biomaterials.2011.11.061
61. Li H, Xu X, Wang D, Zeng L, Li B, Zhang Y, et al. miR-146b-5p regulates bone marrow mesenchymal stem cell differentiation by SIAH2/PPARγ in aplastic anemia children and benzene-induced aplastic anemia mouse model. Cell Cycle. (2020) 19:2460–71. doi: 10.1080/15384101.2020.1807081
62. Luo W, Dong Y, Hu T, Liu D, Wei X, Ma W, et al. 25(OH)D status and expression of miR-140 in the serum of patients with developmental dysplasia of the hip. Nutrition. (2021) 81:110896. doi: 10.1016/j.nut.2020.110896
63. Jiang T, Xia T, Qiao F, Wang N, Jiang Y, and Xin H. Role and regulation of transcription factors in osteoclastogenesis. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms242216175
64. Hu H, He X, Zhang Y, Wu R, Chen J, Lin Y, et al. MicroRNA alterations for diagnosis, prognosis, and treatment of osteoporosis: A comprehensive review and computational functional survey. Front Genet. (2020) 11:181. doi: 10.3389/fgene.2020.00181
65. Dinesh P, Kalaiselvan S, Sujitha S, and Rasool M. miR-506-3p alleviates uncontrolled osteoclastogenesis via repression of RANKL/NFATc1 signaling pathway. J Cell Physiol. (2020) 235:9497–509. doi: 10.1002/jcp.29757
66. Chen C, Cheng P, Xie H, Zhou HD, Wu XP, Liao EY, et al. MiR-503 regulates osteoclastogenesis via targeting RANK. J Bone Miner Res. (2014) 29:338–47. doi: 10.1002/jbmr.2032
67. Lee Y, Kim HJ, Park CK, Kim YG, Lee HJ, Kim JY, et al. MicroRNA-124 regulates osteoclast differentiation. Bone. (2013) 56:383–9. doi: 10.1016/j.bone.2013.07.007
68. Li X, Yang L, and Guo Z. miR-193-3p ameliorates bone resorption in ovariectomized mice by blocking NFATc1 signaling. Int J Clin Exp Pathol. (2019) 12:4077–86.
69. Ling L, Hu HL, Liu KY, Ram YI, Gao JL, and Cao YM. Long noncoding RNA MIRG induces osteoclastogenesis and bone resorption in osteoporosis through negative regulation of miR-1897. Eur Rev Med Pharmacol Sci. (2019) 23:10195–203. doi: 10.26355/eurrev_201912_19654
70. Pitari MR, Rossi M, Amodio N, Botta C, Morelli E, Federico C, et al. Inhibition of miR-21 restores RANKL/OPG ratio in multiple myeloma-derived bone marrow stromal cells and impairs the resorbing activity of mature osteoclasts. Oncotarget. (2015) 6:27343–58. doi: 10.18632/oncotarget.4398
71. Hu CH, Sui BD, Du FY, Shuai Y, Zheng CX, Zhao P, et al. miR-21 deficiency inhibits osteoclast function and prevents bone loss in mice. Sci Rep. (2017) 7:43191. doi: 10.1038/srep43191
72. Guo L, Chen K, Yuan J, Huang P, Xu X, Li C, et al. Estrogen inhibits osteoclasts formation and bone resorption via microRNA-27a targeting PPARγ and APC. J Cell Physiol. (2018) 234:581–94. doi: 10.1002/jcp.26788
73. Zhao J, Huang M, Zhang X, Xu J, Hu G, Zhao X, et al. MiR-146a deletion protects from bone loss in OVX mice by suppressing RANKL/OPG and M-CSF in bone microenvironment. J Bone Miner Res. (2019) 34:2149–61. doi: 10.1002/jbmr.3832
74. Kusumbe AP, Ramasamy SK, and Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. (2014) 507:323–8. doi: 10.1038/nature13145
75. Portal-Núñez S, Lozano D, and Esbrit P. Role of angiogenesis on bone formation. Histol Histopathol. (2012) 27:559–66. doi: 10.14670/hh-27.559
76. Wang Z, Ge X, Wang Y, Liang Y, Shi H, and Zhao T. Mechanism of dexmedetomidine regulating osteogenesis-angiogenesis coupling through the miR-361-5p/VEGFA axis in postmenopausal osteoporosis. Life Sci. (2021) 275:119273. doi: 10.1016/j.lfs.2021.119273
77. Toti P, Sbordone C, Martuscelli R, Califano L, Ramaglia L, and Sbordone L. Gene clustering analysis in human osteoporosis disease and modifications of the jawbone. Arch Oral Biol. (2013) 58:912–29. doi: 10.1016/j.archoralbio.2013.02.013
78. Duan L, Zhao H, Xiong Y, Tang X, Yang Y, Hu Z, et al. miR-16-2* Interferes with WNT5A to regulate osteogenesis of mesenchymal stem cells. Cell Physiol Biochem. (2018) 51:1087–102. doi: 10.1159/000495489
79. Yu T, You X, Zhou H, He W, Li Z, Li B, et al. MiR-16-5p regulates postmenopausal osteoporosis by directly targeting VEGFA. Aging (Albany NY). (2020) 12:9500–14. doi: 10.18632/aging.103223
80. Wang X, Li X, Li J, Zhai L, Liu D, Abdurahman A, et al. Mechanical loading stimulates bone angiogenesis through enhancing type H vessel formation and downregulating exosomal miR-214-3p from bone marrow-derived mesenchymal stem cells. FASEB J. (2021) 35:e21150. doi: 10.1096/fj.202001080RR
81. Almeida MI, Silva AM, Vasconcelos DM, Almeida CR, Caires H, Pinto MT, et al. miR-195 in human primary mesenchymal stromal/stem cells regulates proliferation, osteogenesis and paracrine effect on angiogenesis. Oncotarget. (2016) 7:7–22. doi: 10.18632/oncotarget.6589
82. Morrison KR, Solly EL, Shemesh T, Psaltis PJ, Nicholls SJ, Brown A, et al. Elevated HDL-bound miR-181c-5p level is associated with diabetic vascular complications in Australian Aboriginal people. Diabetologia. (2021) 64:1402–11. doi: 10.1007/s00125-021-05414-6
83. Amjadi-Moheb F, Hosseini SR, Kosari-Monfared M, Ghadami E, Nooreddini H, and Akhavan-Niaki H. A specific haplotype in potential miRNAs binding sites of secreted frizzled-related protein 1 (SFRP1) is associated with BMD variation in osteoporosis. Gene. (2018) 677:132–41. doi: 10.1016/j.gene.2018.07.061
84. Yu X, Rong PZ, Song MS, Shi ZW, Feng G, Chen XJ, et al. lncRNA SNHG1 induced by SP1 regulates bone remodeling and angiogenesis via sponging miR-181c-5p and modulating SFRP1/Wnt signaling pathway. Mol Med. (2021) 27:141. doi: 10.1186/s10020-021-00392-2
85. Lu GD, Cheng P, Liu T, and Wang Z. BMSC-derived exosomal miR-29a promotes angiogenesis and osteogenesis. Front Cell Dev Biol. (2020) 8:608521. doi: 10.3389/fcell.2020.608521
86. Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z, et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. (2020) 103:196–212. doi: 10.1016/j.actbio.2019.12.020
87. Chen Y, Yu H, Zhu D, Liu P, Yin J, Liu D, et al. miR-136-3p targets PTEN to regulate vascularization and bone formation and ameliorates alcohol-induced osteopenia. FASEB J. (2020) 34:5348–62. doi: 10.1096/fj.201902463RR
88. Fischer V and Haffner-Luntzer M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin Cell Dev Biol. (2022) 123:14–21. doi: 10.1016/j.semcdb.2021.05.014
89. Hardy R and Cooper MS. Bone loss in inflammatory disorders. J Endocrinol. (2009) 201:309–20. doi: 10.1677/joe-08-0568
90. Zupan J, Jeras M, and Marc J. Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem Med (Zagreb). (2013) 23:43–63. doi: 10.11613/bm.2013.007
91. Manoharan RR, Prasad A, Pospíšil P, and Kzhyshkowska J. ROS signaling in innate immunity via oxidative protein modifications. Front Immunol. (2024) 15:1359600. doi: 10.3389/fimmu.2024.1359600
92. Cheng N, Liu C, Li Y, Gao S, Han YC, Wang X, et al. MicroRNA-223-3p promotes skeletal muscle regeneration by regulating inflammation in mice. J Biol Chem. (2020) 295:10212–23. doi: 10.1074/jbc.RA119.012263
93. Du W, Yin L, Tong P, Chen J, Zhong Y, Huang J, et al. MiR-495 targeting dvl-2 represses the inflammatory response of ankylosing spondylitis. Am J Transl Res. (2019) 11:2742–53.
94. Akkouch A, Zhu M, Romero-Bustillos M, Eliason S, Qian F, Salem AK, et al. MicroRNA-200c attenuates periodontitis by modulating proinflammatory and osteoclastogenic mediators. Stem Cells Dev. (2019) 28:1026–36. doi: 10.1089/scd.2019.0027
95. Si YT, Song JH, Fang Z, Han XZ, and Jiang SY. MicroRNA-146a regulates the production of cytokines in lymphocytes stimulated by Porphyromonas gingivalis lipopolysaccharide. Hua Xi Kou Qiang Yi Xue Za Zhi. (2021) 39:26–31. doi: 10.7518/hxkq.2021.01.004
96. Zhang FQ, Wang Z, Zhang H, Liu L, Luo XL, and Liu WW. MiR-27a alleviates osteoarthritis in rabbits via inhibiting inflammation. Eur Rev Med Pharmacol Sci. (2019) 23:89–95. doi: 10.26355/eurrev_201908_18634
97. Luo G, Li F, Li X, Wang ZG, and Zhang B. TNF−α and RANKL promote osteoclastogenesis by upregulating RANK via the NF−κB pathway. Mol Med Rep. (2018) 17:6605–11. doi: 10.3892/mmr.2018.8698
98. Scott KM, Cohen DJ, Boyan BD, and Schwartz Z. miR-122 and the WNT/β-catenin pathway inhibit effects of both interleukin-1β and tumor necrosis factor-α in articular chondrocytes in vitro. J Cell Biochem. (2022) 123:1053–63. doi: 10.1002/jcb.30244
99. Li HW and Zeng HS. Regulation of JAK/STAT signal pathway by miR-21 in the pathogenesis of juvenile idiopathic arthritis. World J Pediatr. (2020) 16:502–13. doi: 10.1007/s12519-019-00268-w
100. Jiang B, Yuan C, Han J, Shen M, Zhou X, and Zhou L. miR-143-3p inhibits the differentiation of osteoclast induced by synovial fibroblast and monocyte coculture in adjuvant-induced arthritic rats. BioMed Res Int. (2021) 2021:5565973. doi: 10.1155/2021/5565973
101. Lu J, Zhang Y, Liang J, Diao J, Liu P, and Zhao H. Role of exosomal microRNAs and their crosstalk with oxidative stress in the pathogenesis of osteoporosis. Oxid Med Cell Longev. (2021) 2021:6301433. doi: 10.1155/2021/6301433
102. Davis C, Dukes A, Drewry M, Helwa I, Johnson MH, Isales CM, et al. MicroRNA-183-5p increases with age in bone-derived extracellular vesicles, suppresses bone marrow stromal (Stem) cell proliferation, and induces stem cell senescence. Tissue Eng Part A. (2017) 23:1231–40. doi: 10.1089/ten.TEA.2016.0525
103. Ye Y, Liu Q, Li C, and He P. miR-125a-5p regulates osteogenic differentiation of human adipose-derived mesenchymal stem cells under oxidative stress. BioMed Res Int. (2021) 2021:6684709. doi: 10.1155/2021/6684709
104. Liao L, Su X, Yang X, Hu C, Li B, Lv Y, et al. TNF-α Inhibits FoxO1 by Upregulating miR-705 to Aggravate Oxidative Damage in Bone Marrow-Derived Mesenchymal Stem Cells during Osteoporosis. Stem Cells. (2016) 34:1054–67. doi: 10.1002/stem.2274
105. Li L, Qi Q, Luo J, Huang S, Ling Z, Gao M, et al. FOXO1-suppressed miR-424 regulates the proliferation and osteogenic differentiation of MSCs by targeting FGF2 under oxidative stress. Sci Rep. (2017) 7:42331. doi: 10.1038/srep42331
106. Khosla S and Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. (2017) 5:898–907. doi: 10.1016/s2213-8587(17)30188-2
107. Kerschan-Schindl K, Hackl M, Boschitsch E, Föger-Samwald U, Nägele O, Skalicky S, et al. Diagnostic performance of a panel of miRNAs (OsteomiR) for osteoporosis in a cohort of postmenopausal women. Calcif Tissue Int. (2021) 108:725–37. doi: 10.1007/s00223-020-00802-3
Keywords: osteoporosis, bone remodeling, miRNAs, mechanism, exosomes
Citation: Meng F, Yang C, Li N and Wang H (2025) Progress in understanding the role and mechanism of miRNAs in osteoporosis. Front. Endocrinol. 16:1544944. doi: 10.3389/fendo.2025.1544944
Received: 20 February 2025; Accepted: 23 July 2025;
Published: 19 August 2025; Corrected: 14 October 2025.
Edited by:
Alberto Falchetti, Ospedale Santa Maria della Misericordia di Udine, ItalyReviewed by:
Gustavo Roberto Cointry, National University of Rosario, ArgentinaSuma Uday, Birmingham Women’s and Children’s Hospital, United Kingdom
Yan Kai Dong, Lishui University, China
Copyright © 2025 Meng, Yang, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaxin Wang, d2h4MjE1QDE2My5jb20=
 Feifei Meng
Feifei Meng Changwei Yang1
Changwei Yang1 Huaxin Wang
Huaxin Wang