- 1Department of Diabetology & Endocrinology, Kanazawa Medical University, Uchinada, Japan
- 2Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan
- 3Caress Sapporo Hokko Memorial Clinic, Sapporo, Japan
- 4Hiramitsu Heart Clinic, Nagoya, Japan
- 5Keiyukai Yoshida Hospital, Asahikawa, Japan
- 6Department of Clinical Pharmacology and Therapeutics, University of the Ryukyus, Nishihara, Japan
- 7Department of Social and Environmental Medicine, Kanazawa Medical University, Uchinada, Japan
Background: Fatty liver index (FLI) calculated by using body mass index, waist circumference and levels of triglycerides and γ-glutamyl transpeptidase is a noninvasive biomarker for diagnosis of metabolic dysfunction-associated steatotic liver disease (MASLD), which is one of the high-risk conditions of atherosclerotic cardiovascular diseases. To compare the effects of pemafibrate and omega-3 fatty acid ethyl on FLI, we conducted a sub-analysis study of the Pemafibrate Reduction of triglyceride-rich lipoproteins compared with Omega-3 fatty acid ethyl for Unmet needs in Dyslipidemic patients on target to apoB-48 (PROUD48) study.
Methods: 57 participants in the pemafibrate 0.4 mg per day treatment group (PEMA, men/women: 37/20, mean 64 years) and 60 participants in the omega-3 fatty acid ethyl 4 g per day treatment group (OMEGA-3, men/women: 35/25, mean 63 years) in the PROUD48 study were included in the present study. Changes in FLI and prevalence of MASLD from baseline to week 16 in PEMA and OMEGA-3 were investigated.
Results: Median FLI was significantly decreased by both PEMA (69.7 to 47.6, P < 0.001) and OMEGA-3 (64.8 to 59.5, P < 0.001). There was a significant difference in change in FLI between PEMA and OMEGA-3 (-18.3 ± 14.1 vs. -5.5 ± 9.4, P < 0.001). The proportions of MASLD estimated by FLI (baseline/week 16) in PEMA and OMEGA-3 were 93.0/68.4% (P = 0.002) and 90.0/85.0% (P = 0.582), respectively.
Conclusions: Pemafibrate is superior to omega-3 fatty acid ethyl in lowering effects of FLI and MASLD in patients with dyslipidemia receiving statin treatment, suggesting that pemafibrate is a beneficial agent for hypertriglyceridemia and reduction of the risk for MASLD.
1 Introduction
To prevent atherosclerotic cardiovascular disease (ASCVD), there is no doubt that low-density lipoprotein cholesterol (LDL-C)-lowering therapy is of paramount importance. A reduction of 1 mmol/L in LDL-C level by treatment with statins has been shown to reduce the incidence of major vascular events by 25% in individuals without prior ASCVD (1). However, the remaining > 70% incidence rate is known as the residual ASCVD risk, which includes a high level of triglycerides (TG) and a low level of high-density lipoprotein cholesterol (HDL-C) (2, 3).
Recently, we prospectively compared the lowering effects of pemafibrate, a selective peroxisome proliferator-activated receptor α (PPARα) modulator, and omega-3 fatty acid ethyl, polyunsaturated fatty acids, on levels of fasting apolipoprotein B-48 (apoB-48), a surrogate marker that reflects postprandial hypertriglyceridemia, in the Pemafibrate Reduction of triglyceride-rich lipoproteins compared with Omega-3 fatty acid ethyl for Unmet needs in Dyslipidemic patients on target to apoB-48 (PROUD48) study (4). The PROUD48 study demonstrated that pemafibrate was superior to omega-3 fatty acid ethyl in a lowering effect of fasting apoB-48 and also provided a new clinical insight that pemafibrate was a better option for pharmacotherapy of hypertriglyceridemia to reduce the residual ASCVD risk in patients with dyslipidemia receiving statin treatment (5).
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, in which global prevalence is approximately 25% and its incidence has been increasing (6). Some patients with simple steatosis in NAFLD progress to a more severe form nonalcoholic steatohepatitis (NASH) and develop liver cirrhosis and hepatocellular carcinoma. In recent years, NAFLD has also been considered to be a high-risk condition for ASCVD (7, 8).
An international panel of experts recently proposed metabolic dysfunction-associated fatty liver disease (MAFLD) independent of alcohol consumption as a new concept for fatty liver to replace NAFLD (9). The criteria for MAFLD are based on evidence of hepatic steatosis in addition to one of the following criteria: overweight/obesity, type 2 diabetes, and evidence of metabolic dysregulation. Subsequently, in 2023, three large multinational liver associations proposed to replace the term NAFLD with metabolic dysfunction-associated steatotic liver disease (MASLD) (10). They also proposed to choose metabolic dysfunction-associated steatohepatitis (MASH) as an alternative name to NASH. Furthermore, previous studies demonstrated that 95~99% of patients with NAFLD met the criteria of MASLD (11, 12).
Although the gold standard for diagnosis of NAFLD/MASLD is liver biopsy, several noninvasive diagnostic markers for NAFLD/MASLD have been established (9, 13). Fatty liver index (FLI), a biomarker for detection of hepatic steatosis proposed by Bedogni et al. in 2006, is calculated by using body mass index (BMI), waist circumference (WC) and levels of TG and γ-glutamyl transpeptidase (γ-GTP) (14). Previous studies showed that FLI closely corresponds to findings of histology and imaging modalities of NAFLD/MASLD (12, 15–17).
A previous study conducted in Japan showed that treatment with pemafibrate significantly improved levels of alanine aminotransferase (ALT), γ-GTP and alkaline phosphatase (ALP) in patients with NAFLD compared with placebo (18). Interestingly, the PROUD48 study also revealed that pemafibrate was superior to omega-3 fatty acid ethyl in lowering levels of ALT, γ-GTP, and ALP (5). Considering the relationship between MASLD and ASCVD risk, the favorable effect of pemafibrate on liver function is expected to have an additional impact on the reduction of ASCVD risk. However, the effects on MASLD have not been verified yet. To elucidate the effects of pemafibrate and omega-3 fatty acid ethyl on MASLD, change in FLI and the prevalence of MASLD based on the detection of hepatosteatosis by FLI were investigated in dyslipidemic patients with statin treatment as a sub-analysis of the PROUD48 study.
2 Methods
2.1 Study design
This study is a sub-analysis of the PROUD48 study, which was a prospective, multicenter, open-label, randomized, parallel group, comparative trial to compare the effects of pemafibrate and omega-3 fatty acid ethyl on the fasting apoB-48 level, a surrogate marker for postprandial hypertriglyceridemia in patients with dyslipidemia. The detailed rationale, design, and protocol of the PROUD48 study were previously described (4, 5). The PROUD48 study was registered in the Japan Registry of Clinical Trials (jRCT) on the 28th of April 2020 (No. jRCTs071200011).
2.2 Study participants
Participants were recruited from the PROUD48 study. The PROUD48 study was conducted in Japanese patients with dyslipidemia in accordance with the principles of the Declaration of Helsinki and its amendments. Participants in the PROUD48 study were ambulatory patients who presented to Asahikawa Medical University Hospital (Asahikawa, Hokkaido), Caress Sapporo Hokko Memorial Clinic (Sapporo, Hokkaido), Hiramitsu Heart Clinic (Nagoya, Aichi, Japan) and Keiyukai Yoshida Hospital (Asahikawa, Hokkaido). The inclusion criteria were ambulatory patients with dyslipidemia receiving statin treatment for more than 4 weeks, with fasting TG levels of ≥ 177 mg/dL (2 mmol/L); aged 20–79 years; and those who provided written informed consent. The exclusion criteria were fasting TG levels ≥ 500 mg/dL (5.7 mmol/L); diabetic patients with HbA1c levels ≥ 9% and who need insulin treatment; type 1 diabetes; serum creatinine levels ≥ 1.5 mg/dL or higher; patients who used fibrates and nicotinic acids within 4 weeks; patients who used polyunsaturated fatty acids including supplements within 24 weeks; symptomatic cardiovascular and cerebrovascular disorders; severe infections; acute hepatitis or liver cirrhosis; cancer; patients before or after surgery; women with pregnancy or during breastfeeding; patients who need lipid management with proprotein convertase subtilisin/kexin type 9 inhibitors or microsomal triglyceride transfer protein inhibitors; patients who have contraindications for pemafibrate and omega-3 fatty acid ethyl (4, 5). Written informed consent for participation was obtained from all participants prior to randomization. The study protocol was approved by the Certified Review Board of the University of the Ryukyus for Clinical Research Ethics (No. CRB7200001). Among 129 participants who were enrolled in the PROUD48 study, a total of 117 participants were eventually included in the present study.
2.3 Randomization and intervention
The randomization and intervention were also described in our previous reports (4, 5). The participants were randomly allocated to the pemafibrate treatment group (PEMA) or omega-3 fatty acid ethyl treatment group (OMEGA-3) in a 1:1 ratio. Participants in the PEMA were given pemafibrate at a dose of 0.2 mg orally twice a day for 16 weeks with continuing statin treatment. Participants in the OMEGA-3 were given omega-3 fatty acid ethyl at a dose of 2 g orally twice a day for 16 weeks with continuing statin treatment. During the study, the addition of new drugs, discontinuation, or dose changes of all drugs including statins, pemafibrate, and omega-3 fatty acid ethyl were not permitted. Based on the Japan Atherosclerosis Society guidelines, all participants were on a diet with an optimized total energy intake based on their ideal body weight and daily activity to maintain an appropriate body weight during the study (19).
2.4 Measurements
To profile the study population, clinical characteristics potentially associated with MASLD including age, sex, comorbidities, habits, WC, BMI, lipids-related parameters (total cholesterol, TG, LDL-C, and HDL-C), glycemic control-related parameters (fasting plasma glucose, fasting immunoreactive insulin [IRI], homeostasis model assessment insulin resistance [HOMA-IR], and HbA1c), and other blood biochemical parameters (aspartate aminotransferase [AST], ALT, γ-GTP, ALP, creatinine, and estimated glomerular filtration rate [eGFR]) were extracted and reiterated. Levels of total cholesterol, TG, LDL-C, HDL-C, IRI, and HbA1c were measured at a central clinical laboratory (SRL, Hachioji, Japan). Other clinical parameters were measured at each institution. HOMA-IR was calculated based on a previous report (20), and eGFR was calculated with serum creatinine, sex, and age using the established equation for Japanese subjects (21).
FLI was calculated by using BMI, WC and levels of TG and γ-GTP (14). Although the cutoff value for steatotic liver disease (SLD) was originally reported as FLI ≥ 60 in Italian subjects, FLI ≥ 35 for Japanese men and FLI ≥ 16 for Japanese women were used for the detection of SLD for diagnosis of MASLD as previously reported (12, 17).
MASLD was diagnosed by the absence of other discernible causes for hepatic steatosis and the presence of SLD with at least one of five cardiometabolic risk factors including 1) BMI ≥ 23 kg/m2 or WC > 90/80 cm in Asian men and women; 2) fasting glucose ≥ 100 mg/dL, 2-h post-load glucose levels ≥ 140 mg/dL (no measurement in the present study), HbA1c ≥ 5.7%, type 2 diabetes mellitus, or treatment for type 2 diabetes mellitus; 3) blood pressure ≥ 130/85 mmHg or specific antihypertensive drug treatment; 4) plasma TG ≥ 150 mg/dL or lipid-lowering treatment; and 5) plasma HDL-C ≤ 40 mg/dL for men and ≤ 50 mg/dL for women or lipid-lowering treatment (10). The category of MASLD and increased alcohol intake (MetALD) diagnosed by the presence of MASLD and average alcohol intake of 140–350 g/week (20–50 g/day) for women and 210–420 g/week (30–60 g/day) for men was also included (10).
2.5 Statistical analysis
Continuous and categorical variables were presented as means ± standard deviations (SDs), medians (interquartile ranges [IQR] or min-max values [Min-Max]) and frequencies with percentages. The Shapiro-Wilk test was used to assess the normality of data. Changes in FLI from baseline to week 16 were compared by using unpaired t test. The others were analyzed using the paired t test or Wilcoxon’s signed-rank test for intragroup comparisons and the unpaired t test or Mann-Whitney U test for comparisons between two groups. The baseline characteristics of the participants in the two groups were compared using the chi-square test or Fisher’s exact test for categorical variables and t test or Mann-Whitney U test for continuous variables. All P-values were two-sided with P < 0.05 taken to indicate statistical significance. All statistical analyses were performed by the study statistician (M. Sakurai) at the data center (Nexis, Fukuoka, Japan) using SPSS ver. 26 (IBM Corp., Armonk, NY, USA).
3 Results
3.1 Participants and baseline characteristics
As previously shown in the PROUD48 study (5), a total of 129 participants were recruited and assessed for eligibility. Three participants were excluded because of withdrawal of consent and failure to visit. The remaining 126 participants were randomly assigned to the PEMA group (n = 63) and the OMEGA-3 group (n = 63). After exclusion of patients who discontinued the treatment, 58 participants in the PEMA group and 61 participants in the OMEGA-3 were followed-up. Eventually, 57 participants (men/women: 37/20, mean 64 years) in the PEMA group and 60 participants (men/women: 35/25, mean 63 years) in the OMEGA-3 group completed the follow-up by May 22, 2021 (5) and were included in the present study.
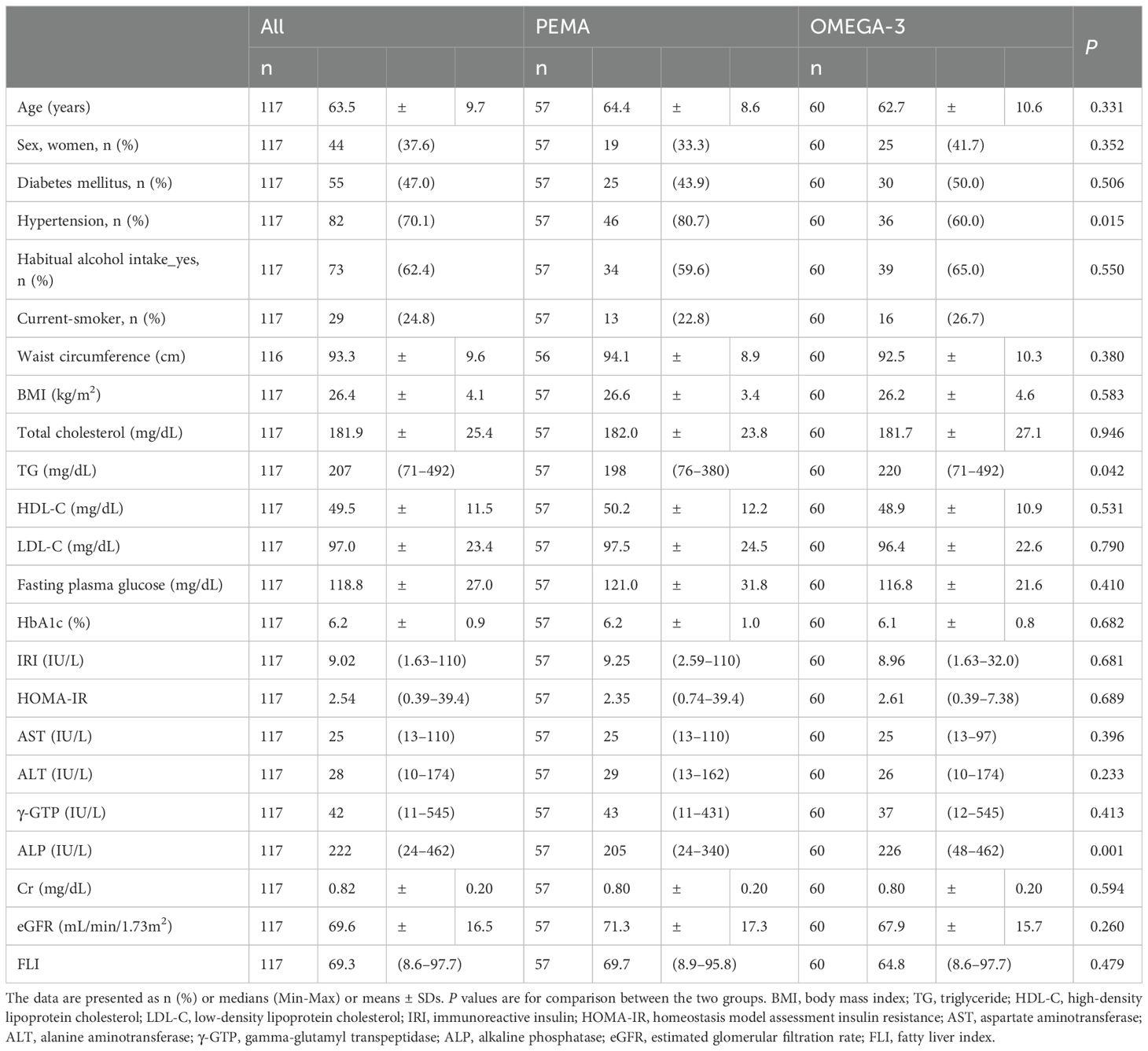
Baseline characteristics were well balanced between the two groups except for the prevalence of hypertension and levels of TG and ALP (Table 1). The median levels of FLI in the PEMA group and the OMEGA-3 group at baseline were 69.7 and 64.8, respectively.
3.2 Changes in FLI from baseline to week 16
From baseline to week 16, FLI was significantly decreased in both the PEMA group (69.7 [IQR 51.2, 80.4] to 47.6 [IQR 22.6, 67.1], P < 0.001) and the OMEGA-3 group (64.8 [IQR 35.8, 81.5] to 59.5 [IQR 27.6, 80.3], P < 0.001) (Figure 1A). The change in FLI in the PEMA group and that in the OMEGA-3 group were -18.3 ± 14.1 and -5.5 ± 9.4 (P < 0.001), respectively (Figure 1B). The percentage change in FLI in the PEMA group and that in the OMEGA-3 group were -32.3 ± 24.3% and -11.3 ± 22.7 (P < 0.001), respectively (Figure 1C).
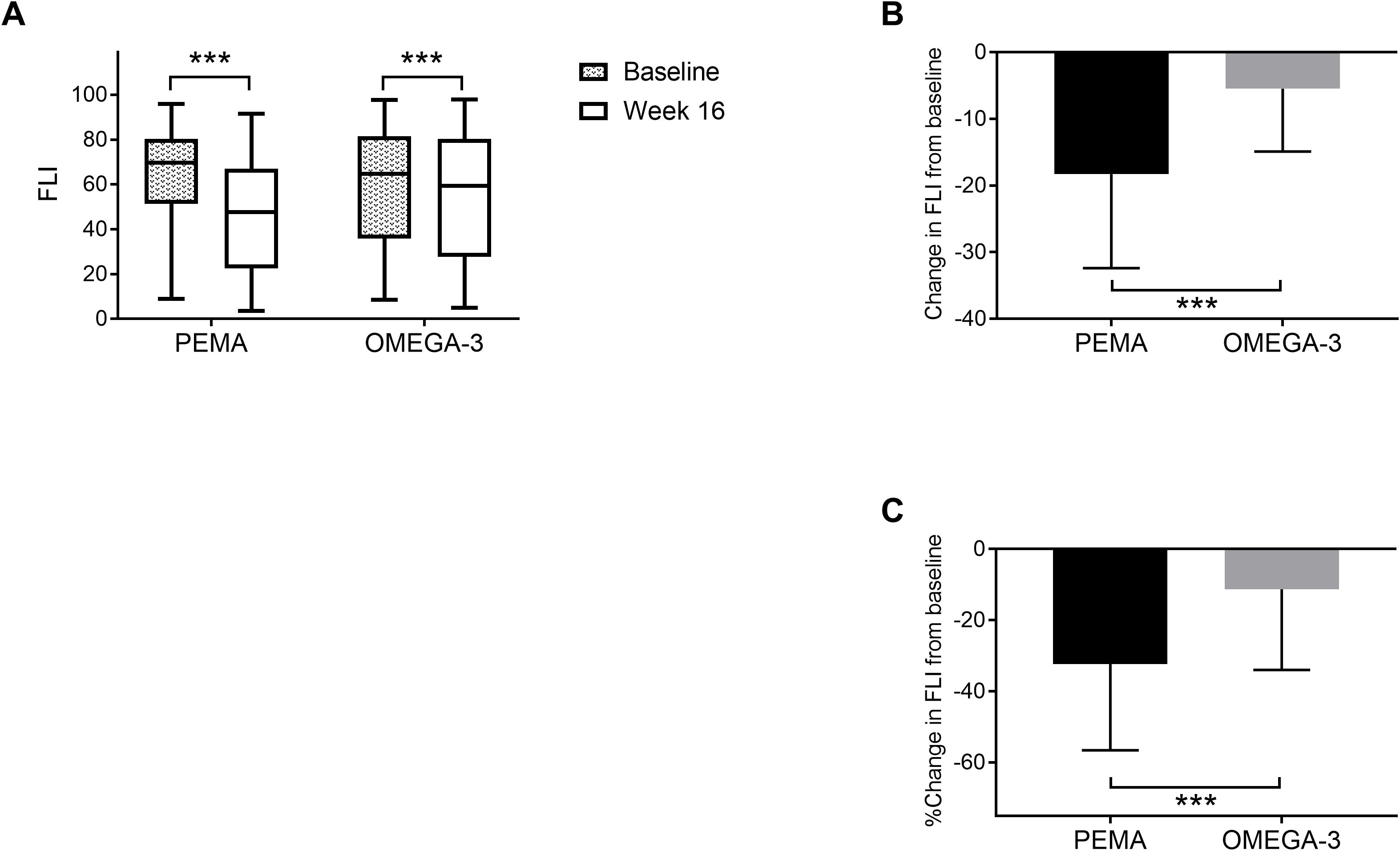
Figure 1. Change in FLI from baseline to week 16. (A) FLI at baseline and week 16. (B) Change in FLI. (C) Percentage change in FLI. The data are presented as medians (Min-Max) (A) or means ± SDs (B, C). The boxes indicate the interquartile ranges (A). ***P < 0.001 for comparisons between baseline and week 16 (A). ***P < 0.001 for comparisons between PEMA and OMEGA-3 (B, C). FLI, fatty liver index; PEMA, pemafibrate treatment group; OMEGA-3, omega-3 fatty acid ethyl treatment group.
Figure 2 shows changes in FLI from baseline to week 16 in both treatment groups divided by sex. In men, FLI was significantly decreased in both the PEMA group (71.7 [IQR 56.4, 80.4] to 47.9 [IQR 30.3, 65.9], P < 0.001) and the OMEGA-3 group (70.2 [IQR 44.6, 88.6] to 63.7 [IQR 39.4, 85.4], P = 0.005) (Figure 2A). The change in FLI in the PEMA group and that in the OMEGA-3 group were -20.9 ± 12.8 and -4.6 ± 9.1 (P < 0.001), respectively (Figure 2B). The percentage change in FLI in the PEMA group and that in the OMEGA-3 group were -33.3% ± 22.1 and -8.4% ± 20.4 (P < 0.001), respectively (Figure 2C).
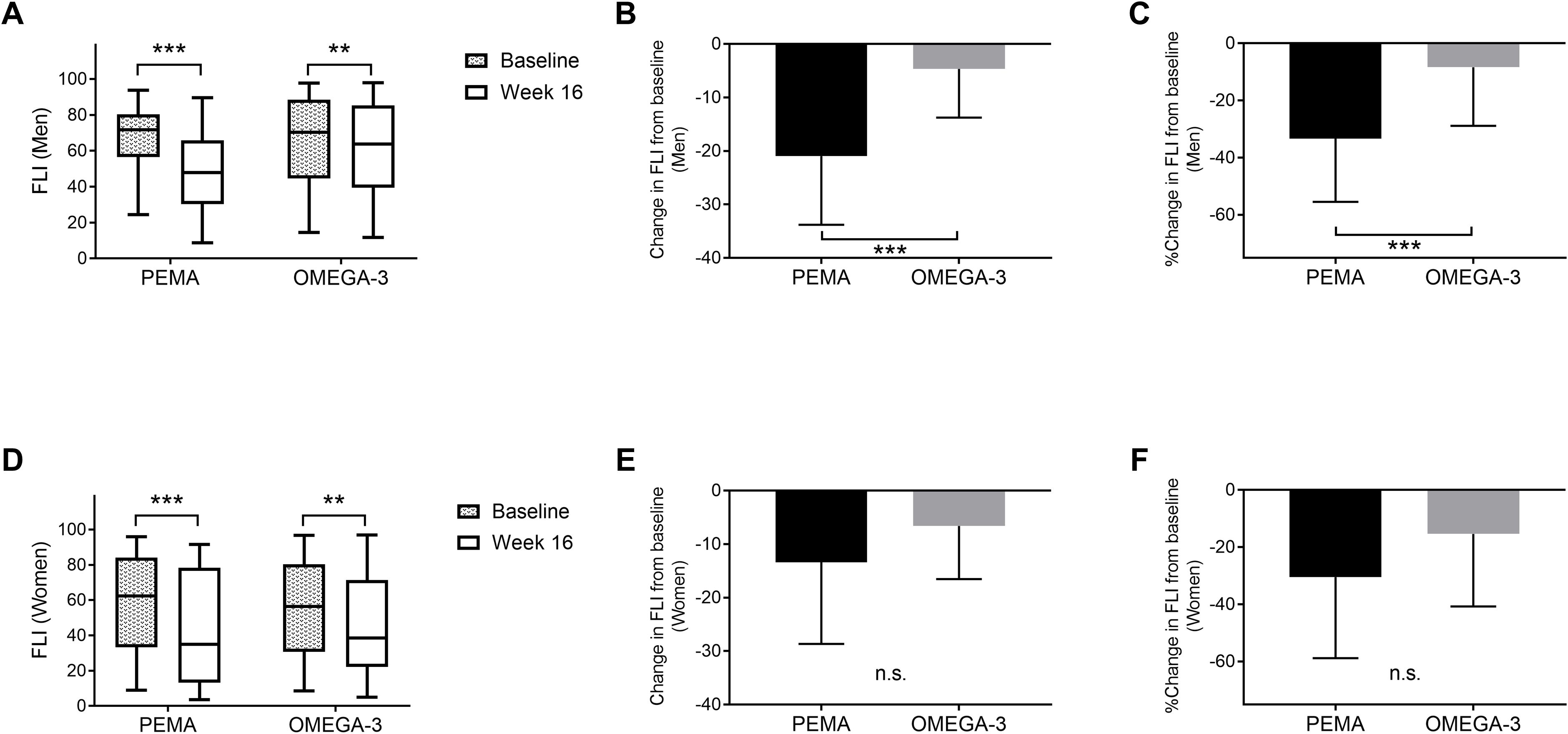
Figure 2. Change in FLI from baseline to week 16 divided by sex. (A) FLI at baseline and week 16 in men. (B) Change in FLI in men. (C) Percentage change in FLI in men. (D) FLI at baseline and week 16 in women. E: Change in FLI in women. F: Percentage change in FLI in women. The data are presented as medians (Min-Max) (A, D) or means ± SDs (B, C, E, F). The boxes indicate the interquartile ranges (A, D). **P < 0.01, ***P < 0.001 for comparisons between baseline and week 16 (A, D). ***P < 0.001 for comparisons between PEMA and OMEGA-3 (B, C). FLI, fatty liver index; PEMA, pemafibrate treatment group; OMEGA-3, omega-3 fatty acid ethyl treatment group; n.s., not significant.
In women, similar to the results in men, FLI was significantly decreased from baseline to week 16 in both the PEMA group (62.4 [IQR 33.3, 84.1] to 34.8 [IQR 13.2, 78.3], P < 0.001) and the OMEGA-3 group (56.4 [IQR 30.7, 80.3] to 38.6 [IQR 22.2, 71.5], P = 0.004) (Figure 2D). However, in women, there was no significant difference in the change in FLI or the percentage change in FLI between the treatment groups (Figures 2E, F).
3.3 The prevalence of MASLD
In the PEMA group, the proportion of MASLD was significantly decreased from baseline to week 16 (93.0% to 68.4%, P = 0.002) (Figure 3A). When the patients were divided by sex, the proportion of MASLD was significantly decreased from baseline to week 16 in men (94.6% to 64.9%, P = 0.003) but not in women (90.0% to 75.0%, P = 0.408) (Figures 3B, C). On the other hand, in the OMEGA-3 group, there was no significant difference in the proportion of MASLD between the points of baseline (90.0%) and week 16 (85.0%) (P = 0.582) (Figure 3D). Even when the patients were divided by sex, there was no significant difference in proportion of MASLD between the points of baseline and week 16 (Figures 3E, F).
Figure 3. The prevalence of MASLD. (A) The proportion of patients with MASLD at baseline and week 16 in PEMA. (B) The proportion of male patients with MASLD at baseline and week 16 in PEMA. (C) The proportion of female patients with MASLD at baseline and week 16 in PEMA. (D) The proportion of patients with MASLD at baseline and week 16 in OMEGA-3. (E) The proportion of male patients with MASLD at baseline and week 16 in OMEGA-3. (F) The proportion of female patients with MASLD at baseline and week 16 in OMEGA-3. The data and bars are presented as percentages. **P < 0.01 for comparisons between baseline and week 16 (A, B). MASLD: metabolic dysfunction-associated steatotic liver disease; PEMA, pemafibrate treatment group; OMEGA-3, omega-3 fatty acid ethyl treatment group; n.s., not significant.
4 Discussion
To the best of our knowledge, this study for the first time showed direct comparison of the effects of two TG-lowering agents on FLI in patients with dyslipidemia receiving statin treatment. We revealed that both pemafibrate and omega-3 fatty acid ethyl significantly decreased FLI from baseline to week 16 and that the decrease in FLI was significantly greater in patients treated with pemafibrate than in patients treated with omega-3 fatty acid ethyl. In addition to previous findings in the PROUD48 study showing that pemafibrate was superior to omega-3 fatty acid ethyl in lowering effect of ALT, γ-GTP, and ALP levels, we newly demonstrated that pemafibrate was also superior to omega-3 fatty acid ethyl in the lowering effect of FLI, a surrogate marker for detection of MASLD, in this sub-analysis study of the PROUD48 study. In addition, a significant reduction in the prevalence of MASLD was found in patients treated with pemafibrate but not in those treated with omega-3 fatty acid ethyl. Taken together, the findings obtained in the present study further highlight the possibility that pemafibrate is a useful agent for not only hypertriglyceridemia but also NAFLD/MASLD as a potential ASCVD risk.
A recent review Jump et al. (22) summarized various clinical findings including randomized trials regarding the effects of omega-3 fatty acid ethyl on NAFLD/NASH assessed by lipids, liver function, surrogate markers, and imaging findings such as ultrasound and magnetic resonance imaging (MRI) (23–29). Some studies verified that omega-3 fatty acid ethyl improved histological findings in the liver in patients with NAFLD/NASH (30, 31). On the other hand, although pemafibrate is a new TG-lowering agent, single-arm preliminary studies conducted in Japan showed that pemafibrate decreased levels of ALT, γ-GTP and ALP and improved surrogate markers for liver fibrosis and cirrhosis (32–36). In addition, a recent double-blind, placebo-controlled, randomized phase 2 trial conducted in Japanese patients with NAFLD showed that pemafibrate significantly improved MRI-based liver stiffness and reduced levels of ALT and surrogate markers for liver fibrosis compared with placebo, although treatment with pemafibrate failed to reduce MRI-based liver fat content (18). Thus, both two agents are potentially useful for the treatment of NAFLD/MASLD.
In the present study, pemafibrate was superior to omega-3 fatty acid ethyl in the lowering effect of FLI. Additionally, a reduction in the prevalence of MASLD was observed in only patients treated with pemafibrate. It has recently reported that pemafibrate was superior to omega-3 fatty acid ethyl in not only lowering ALT, the primary endpoint, but also improving other liver enzymes, lipid profiles, and hepatic fibrosis biomarkers in the PORTRAIT study, which prospectively compared the effects of pemafibrate and omega-3 fatty acid ethyl on liver function in patients with hypertriglyceridemia complicated by MASLD (37). Although backgrounds of participants, including the prevalence of MASLD, liver function, proportion of statin treatment, and the dose and treatment period, were different between the PROUD48 study and PORTRAIT study, these two studies provided an important evidence that pemafibrate is a promising drug for improving MASLD compared to omega-3 fatty acid ethyl.
Intrahepatic lipid content is normally regulated by a balance between lipid uptake and disposal in the liver (38). In NAFLD/MASLD, pathways of hepatic lipid metabolism are dysregulated as follows: 1) increased hepatic lipid uptake, 2) increased de novo lipogenesis, 3) decreased fatty acid oxidation, and 4) increased VLDL production, resulting in hepatic lipid accumulation (38). It has been reported that both pemafibrate and omega-3 fatty acid ethyl increase fatty acid oxidation in the liver, thereby reducing the fatty acid pool that is the source of hepatic lipid accumulation (39, 40). Treatment with omega-3 fatty acid ethyl has also been reported to reduce fatty acid pool by suppressing de novo lipogenesis in the liver (40). These effects of the two agents on hepatic lipid metabolism should be effective against NAFLD/MASLD.
In NAFLD/MASLD, both an increase in dietary fatty acids due to overnutrition and an increase in fatty acids due to enhanced lipolysis in peripheral adipose tissue are associated with insulin resistance, leading to increased hepatic lipid uptake (38). The latter has been reported to be the major source of lipid accumulation in the liver in patients with NAFLD (41). In the PROUD48 study, the median levels of HOMA-IR at baseline in the PEMA group and the OMEGA-3 group were 2.34 and 2.68, respectively, suggesting that the participants in both groups were mildly insulin resistant (5). Although there was no significant difference in change in HOMA-IR level from baseline to week 16 between the PEMA group and the OMEGA-3 group, HOMA-IR level was significantly decreased by only the treatment with pemafibrate (5). The improvement in insulin resistance by pemafibrate may result in the suppression of lipolysis in adipose tissue, leading to reduction of the fatty acid pool in the liver and subsequent improvement of NAFLD/MASLD. In addition, the favorable impact of pemafibrate on insulin resistance may be also involved in the reduction of dietary fatty acids via its effect on lipoprotein lipase (38, 42). Furthermore, we previously showed that pemafibrate was superior to omega-3 fatty acid ethyl in lowering effect of fasting apoB-48 as a surrogate marker that reflects postprandial hypertriglyceridemia (5). The greater effect of pemafibrate in lowering postprandial TG-rich lipoproteins may lead to a decrease in the absolute amount of TG derived from the gut, which accounts for most dietary lipids, and a reduction in its mobilization to the liver as dietary fatty acids (43).
In the present study, the median levels of FLI at baseline in the PEMA group and the OMEGA-3 group were 69.7 and 64.8, respectively. Since the levels of FLI in both groups were ≥ 60, which met the originally reported cutoff level for diagnosis of NAFLD in Italian subjects (14), it would be acceptable to consider most of the participants in the present study to have NAFLD/MASLD. It has been reported that the optimal FLI for predicting NAFLD was lower in Japanese subjects than in Italian subjects and that there was a sex difference in the cutoff level of FLI (≥ 35 for men and ≥ 16 for women) (17). Therefore, in the present study, we adopted the cutoff values for Japanese subjects in the detection of MASLD. Interestingly, the present study showed that the beneficial effects of pemafibrate compared with omega-3 fatty acid ethyl on MASLD were predominant in men. A previous study using a rodent model showed that hepatic PPARα expression was predominant in male rats compared with female rats (44). This report may help us understand why pemafibrate may be more beneficial than omega-3 fatty acid ethyl for NAFLD/MASLD. However, a recent retrospective study using Japanese patients with NAFLD showed that the beneficial effects of pemafibrate on NAFLD were predominant in women (45). This issue of possible sex difference in the efficacies of pemafibrate remains controversial and requires further investigation.
This study has several limitations. First, although FLI has been shown to correspond to histological findings of NAFLD/MASLD, it is nothing more than a surrogate marker for NAFLD. It is possible that changes in FLI as well as the prevalence of MASLD observed in this study merely reflected changes in parameters that constitute the equation of FLI. In fact, in the PROUD48 study, pemafibrate significantly reduced levels of TG and γ-GTP compared to omega-3 fatty acid ethyl (5). To compare the true effect of these two agents on MASLD, prospective comparative trial should be conducted preferably with histological outcomes rather than surrogate markers or biomarkers. Second, although FLI is an established biomarker for the detection and prediction of NAFLD/MASLD (12, 15–17), the change in FLI has not been validated as a clinical indicator reflecting alterations in the pathophysiology of NAFLD/MASLD. Therefore, certain caution is required in interpreting the results observed in this study. Third, there were concerns about the diagnostic process for MASLD. The diagnosis of MASLD requires the exclusion of other discernible causes for hepatic steatosis (10). Although we interviewed all participants and reviewed their medical records to confirm the existence of comorbid liver diseases, we were unable to perform liver imaging or testing for the presence of hepatitis viruses in all participants. Therefore, we could not exclude the possibility that some participants in this study had SLD associated with other etiologies. Fourth, since the present study was a sub-analysis of the PROUD48 study, the participants were patients with dyslipidemia. Therefore, some of the patients did not have diagnosis of MASLD. Fifth, approximately half of the participants had diabetes, and some of them were treated with glucose-lowering agents including sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists, which may affect hepatic steatosis. Sixth, as this study was an open-label trial, there were concerns about potential bias and its impact on the results. Finally, the lack of placebo or combination treatment group was also a limitation in the present study.
In conclusion, pemafibrate is superior to omega-3 fatty acid ethyl in lowering effects of FLI and the prevalence of MASLD estimated by FLI in patients with dyslipidemia receiving statin treatment. Pemafibrate would be a beneficial TG-lowering agent for reducing risk of ASCVD as well as MASLD compared to omega-3 fatty acid ethyl.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Certified Review Board of the University of the Ryukyus for Clinical Research Ethics (No. CRB7200001). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YT: Conceptualization, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. MF: Conceptualization, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. IS: Conceptualization, Investigation, Methodology, Project administration, Validation, Writing – review & editing. SH: Conceptualization, Investigation, Methodology, Project administration, Validation, Writing – review & editing. MO: Conceptualization, Investigation, Methodology, Project administration, Validation, Writing – review & editing. SU: Conceptualization, Investigation, Methodology, Project administration, Validation, Writing – review & editing. NK: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing. MS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This trial was funded by Kowa, Tokyo, Japan, including journal article processing charges. The design of the study, collection, analysis and interpretation of all data and drafting of the manuscript were conducted solely by the authors.
Acknowledgments
We thank all of the study participants.
Conflict of interest
MF received research grants and honoraria from Kowa. IS received an honorarium for a lecture from GlaxoSmithKline, Bayer Yakuhin and Kowa and research funding from Kowa. SH has received honoraria for lectures from Bayer Yakuhin, Daiichi Sankyo, Kowa, Mitsubishi Tanabe Pharma, MSD, Novartis Pharma, Otsuka Pharmaceutical and Takeda Pharmaceutical. SU has received honoraria for lectures from Daiichi Sankyo, Kowa and Taiho Pharmaceutical and grants from Bayer Yakuhin, Bristol-Myers Squibb and Kowa. NK received honoraria for lectures from Sumitomo Pharma, MSD and Novo Nordisk Pharma and research grants from Sumitomo Pharma and Terumo and research funding from Kowa.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cholesterol Treatment Trialists’ (CTT) Collaborators, Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. (2012) 380:581–90. doi: 10.1016/S0140-6736(12)60367-5
2. Bainton D, Miller NE, Bolton CH, Yarnell JW, Sweetnam PM, Baker IA, et al. Plasma triglyceride and high density lipoprotein cholesterol as predictors of ischaemic heart disease in British men. The Caerphilly and Speedwell Collaborative Heart Disease Studies. Br Heart J. (1992) 68:60–6. doi: 10.1136/hrt.68.7.60
3. Carey VJ, Bishop L, Laranjo N, Harshfield BJ, Kwiat C, Sacks FM. Contribution of high plasma triglycerides and low high-density lipoprotein cholesterol to residual risk of coronary heart disease after establishment of low-density lipoprotein cholesterol control. Am J Cardiol. (2010) 106:757–63. doi: 10.1016/j.amjcard.2010.05.002
4. Takeda Y, Sakuma I, Hiramitsu S, Okada M, Ueda S, Sakurai M. Study protocol of the PROUD48 study comparing the effects of pemafibrate and omega-3 fatty acid ethyl esters on apoB-48 in statin-treated patients with dyslipidaemia: A prospective, multicentre, open-label, randomised, parallel group trial in Japan. BMJ Open. (2022) 12:e061360. doi: 10.1136/bmjopen-2022-061360
5. Takeda Y, Sakuma I, Hiramitsu S, Okada M, Ueda S, Sakurai M. The effects of pemafibrate and omega-3 fatty acid ethyl on apoB-48 in dyslipidemic patients treated with statin: A prospective, multicenter, open-label, randomized, parallel group trial in Japan (PROUD48 study). Front Cardiovasc Med. (2023) 10:1094100. doi: 10.3389/fcvm.2023.1094100
6. Younossi ZM, Koeing AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
7. Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:948–63. doi: 10.1016/j.jacc.2018.11.050
8. Yoneda M, Yamamoto T, Honda Y, Imajo K, Ogawa Y, Kessoku T, et al. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: a retrospective nationwide claims database study in Japan. J Gastroenterol. (2021) 56:1022–32. doi: 10.1007/s00535-021-01828-6
9. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
10. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. (2023) 79:1542–56. doi: 10.1016/j.jhep.2023.06.003
11. Hagström H, Vessby J, Ekstedt M, Shang Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J Hepatol. (2024) 80:e76–7. doi: 10.1016/j.jhep.2023.08.026
12. Mori K, Akiyama Y, Tanaka M, Sato T, Endo K, Hosaka I, et al. Deciphering metabolic dysfunction-associated steatotic liver disease: insights from predictive modeling and clustering analysis. J Gastroenterol Hepatol. (2024) 39:1382–93. doi: 10.1111/jgh.16552
13. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. (2019) 156:1264–81. doi: 10.1053/j.gastro.2018.12.036
14. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. doi: 10.1186/1471-230X-6-33
15. Otgonsuren M, Estep MJ, Hossain N, Younossi E, Frost S, Henry L, et al. Single non-invasive model to diagnose non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). J Gastroenterol Hepatol. (2014) 29:2006–13. doi: 10.1111/jgh.2014.29.issue-12
16. Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. (2014) 40:1209–22. doi: 10.1111/apt.2014.40.issue-10
17. Takahashi S, Tanaka M, Higashiura Y, Mori K, Hanawa N, Ohnishi H, et al. Prediction and validation of nonalcoholic fatty liver disease by fatty liver index in a Japanese population. Endocr J. (2022) 69:463–71. doi: 10.1507/endocrj.EJ21-0563
18. Nakajima A, Eguchi Y, Yoneda M, Imajo K, Tamaki N, Suganami H, et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Phaemacol Ther. (2021) 54:1263–77. doi: 10.1111/apt.v54.10
19. Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, et al. Japan atherosclerosis society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. (2018) 25:846–984. doi: 10.5551/jat.GL2017
20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–9. doi: 10.1007/BF00280883
21. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. (2009) 53:982–92. doi: 10.1053/j.ajkd.2008.12.034
22. Jump DB, Lytle KA, Depner CM, Tripathy S. Omega-3 polyunsaturated fatty acids as a treatment strategy for nonalcoholic fatty liver disease. Pharmacol Ther. (2018) 181:108–25. doi: 10.1016/j.pharmthera.2017.07.007
23. Capanni M, Calella F, Biagini MR, Genise S, Raimondi L, Bodogni G, et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther. (2006) 23:1143–51. doi: 10.1111/j.1365-2036.2006.02885.x
24. Zhu FS, Liu S, Chen XM, Huang ZG, Zhang DW. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol. (2008) 14:6395–400. doi: 10.3748/wjg.14.6395
25. Spadaro L, Magliocco O, Spampinato D, Piro S, Oliveri C, Alagona C, et al. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis. (2008) 40:194–9. doi: 10.1016/j.dld.2007.10.003
26. Cussons AJ, Watts GF, Mori TA, Stuckey BG. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metab. (2009) 94:3842–8. doi: 10.1210/jc.2009-0870
27. Scorletti E, Bhatia L, McCormick KG, Clough GF, Nash K, Hodson L, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the Welcome study. Hepatology. (2014) 60:1211–21. doi: 10.1002/hep.27289
28. Argo CK, Patrie JT, Lackner C, Henry TD, de Lange EE, Weltman AL, et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol. (2015) 62:190–7. doi: 10.1016/j.jhep.2014.08.036
29. Qin Y, Zhou Y, Chen SH, Zhao XL, Ran L, Zeng XL, et al. Fish oil supplements lower serum lipids and glucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin E2 in nonalcoholic fatty liver disease associated with hyperlipidemia: A randomized clinical trial. PloS One. (2015) 10:e0133496. doi: 10.1371/journal.pone.0133496
30. Li YH, Yang LH, Sha KH, Liu TG, Zhang LG, Liu XX. Efficacy of poly-unsaturated fatty acid therapy on patients with nonalcoholic steatohepatitis. World J Gastroenterol. (2015) 21:7008–13. doi: 10.3748/wjg.v21.i22.7008
31. Nogueira MA, Oliveira CP, Alves VA, Stefano JT, Rodrigues LS, Torrinhas RS, et al. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled trial. Clin Nutr. (2016) 35:578–86. doi: 10.1016/j.clnu.2015.05.001
32. Ikeda S, Sugihara T, Kihara T, Matsuki Y, Nagahara T, Takata T, et al. Pemafibrate ameliorates liver dysfunction and fatty liver in patients with non-alcoholic fatty liver disease with hypertriglyceridemia: A retrospective study with the outcome after a mid-term follow-up. Diagn (Basel). (2021) 11:2316. doi: 10.3390/diagnostics11122316
33. Hatanaka T, Kakizaki S, Saito N, Nakano Y, Nakano S, Hazama Y, et al. Impact of pemafibrate in patients with hypertriglyceridemia and metabolic dysfunction-associated fatty liver disease pathologically diagnosed with non-alcoholic steatohepatitis: A retrospective, single-arm study. Intern Med. (2021) 60:2167–74. doi: 10.2169/internalmedicine.6574-20
34. Hatanaka T, Kosone T, Saito N, Takakusagi S, Tojima H, Naganuma A, et al. Effect of 48-week pemafibrate on non-alcoholic fatty liver disease with hypertriglyceridemia, as evaluated by the FibroScan-aspartate aminotransferase score. JGH Open. (2021) 5:1183–89. doi: 10.1002/jgh3.12650
35. Shinozaki S, Tahara T, Lefor AK, Ogura M. Pemafibrate decreases markers of hepatic inflammation in patients with non-alcoholic fatty liver disease. Clin Exp Hepatol. (2020) 6:270–4. doi: 10.5114/ceh.2020.99528
36. Shinozaki S, Tahara T, Lefor AK, Ogura M. Pemafibrate improves hepatic inflammation, function and fibrosis in patients with non-alcoholic fatty liver disease: a one-year observational study. Clin Exp Hepatol. (2021) 7:172–7. doi: 10.5114/ceh.2021.106864
37. Sumida Y, Toyoda H, Yasuda S, Kimoto S, Sakamoto K, Nakade Y, et al. Comparison of efficacy between pemafibrate and omega-3-acid ethyl ester in the liver: the PORTRAIT study. J Atheroscler Thromb. (2024) 31:1620–33. doi: 10.5551/jat.64896
38. Wang Z, Ye M, Zhang XJ, Zhang P, Cai J, Li H, et al. Impact of NAFLD and its pharmacotherapy on lipid profile and CVD. Atherosclerosis. (2022) 355:30–44. doi: 10.1016/j.atherosclerosis.2022.07.010
39. Yamashita S, Rizzo M, Su TC, Masuda D. Novel selective PPARα Modulator pemafibrate for dyslipidemia, nonalcoholic fatty liver disease (NAFLD), and atherosclerosis. Metabolites. (2023) 13:626. doi: 10.3390/metabo13050626
40. Spooner MH, Jump DB. Nonalcoholic fatty liver disease and omega-3 fatty acids: mechanisms and clinical use. Annu Rev Nutr. (2023) 43:199–223. doi: 10.1146/annurev-nutr-061021-030223
41. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. (2005) 115:1343–51. doi: 10.1172/JCI23621
42. Toth PP. Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc Health Risk Manag. (2016) 12:171–83. doi: 10.2147/VHRM.S104369
43. Masuda D, Sakai N, Sugimoto T, Kitazume-Taneike R, Yamashita T, Kawase R, et al. Fasting serum apolipoprotein B-48 can be a marker of postprandial hyperlipidemia. J Atheroscler Thromb. (2011) 18:1062–70. doi: 10.5551/jat.10470
44. Jalouli M, Carlsson L, Améen C, Lindén D, Ljungberg A, Michalik L, et al. Sex difference in hepatic peroxisome proliferator-activated receptor alpha expression: influence of pituitary and gonadal hormones. Endocrinology. (2003) 144:101–9. doi: 10.1210/en.2002-220630
Keywords: fatty liver index, atherosclerotic cardiovascular disease, residual risk, hypertriglyceridemia, nonalcoholic fatty liver disease, metabolic dysfunction-associated fatty liver disease, metabolic dysfunction-associated steatotic liver disease
Citation: Takeda Y, Furuhashi M, Sakuma I, Hiramitsu S, Okada M, Ueda S, Kumashiro N and Sakurai M (2025) Comparison of the effects of pemafibrate and omega-3 fatty acid ethyl on fatty liver index in patients with dyslipidemia treated with statin: a sub-analysis from the PROUD48 study. Front. Endocrinol. 16:1549687. doi: 10.3389/fendo.2025.1549687
Received: 21 December 2024; Accepted: 02 April 2025;
Published: 01 May 2025.
Edited by:
Hiroshi Yoshida, Jikei University Kashiwa Hospital, JapanReviewed by:
Daisaku Masuda, Rinku General Medical Center, JapanShojiro Sawada, Tohoku Medical and Pharmaceutical University, Japan
Minoru Takemoto, International University of Health and Welfare, Japan
Copyright © 2025 Takeda, Furuhashi, Sakuma, Hiramitsu, Okada, Ueda, Kumashiro and Sakurai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasutaka Takeda, eWt0YWtlNUBrYW5hemF3YS1tZWQuYWMuanA=; Masato Furuhashi, ZnVydWhhc2lAc2FwbWVkLmFjLmpw
 Yasutaka Takeda
Yasutaka Takeda Masato Furuhashi
Masato Furuhashi Ichiro Sakuma
Ichiro Sakuma Shinya Hiramitsu4
Shinya Hiramitsu4 Masaru Sakurai
Masaru Sakurai