- 1Department of Cardiology, Kunming Children's Hospital, Kunming, China
- 2Department of Respiratory Medicine, Kunming Children’s Hospital, Kunming, China
- 3Department of Cardiology, The First Affiliated Hospital of Kunming Medical University, Kunming, China
Background: Turner syndrome (TS), a chromosomal disorder (45, X) affecting approximately 1 in 2,000 female births, results in multisystem morbidity. This study aims to report global trends in the prevalence and disability-adjusted life years (DALYs) of childhood TS from 1990 to 2021.
Methods: This study analyzed the prevalence and DALYs of TS in children aged 0–14 years using data from the Global Burden of Disease (GBD) database. Prevalence and DALYs were calculated per 100,000 population with 95% uncertainty intervals (UI). A log-transformed linear regression model was applied to estimate the average annual percentage change (EAPC) and evaluate temporal trends.
Findings: Globally, the prevalence of TS in children in 2021 was 240598.45 cases (95% UI, 185491.24,318611.47), with 174,186.30 DALYs (95% UI, 127,104.64–223,265.92). From 1990 to 2021, the prevalence increased by 12.81% (95% UI, 11.37% to 14.05%), and DALYs decreased by 66.13% (95% UI, -79.97% to -44.24%). Among the five Sociodemographic Index (SDI) regions, the highest EAPCs were observed in the High SDI regions for prevalence (0.03; 95% CI, 0.01–0.05) and DALYs (0.03; 95% CI, 0.01–0.05). Regionally, the largest decline in prevalence rate occurred in Andean Latin America (EAPC = –0.44; 95% CI, –0.45 to –0.43), and the greatest increase was recorded in the Caribbean (EAPC = 0.05; 95% CI, 0.00–0.10). At the national level, India had the highest number of TS cases in 2021, with 45,941.86 cases (95% UI, 35,104.78–61,429.72).
Interpretation: Overall, these findings provide a critical foundation for shaping public health strategies and policy decisions aimed at reducing the global burden of pediatric TS through improved diagnostic practices, comprehensive care, and targeted interventions.
Introduction
Turner Syndrome (TS) represents the most frequent sex chromosomal abnormality in girls and women, caused by the missing part or entirety of an X chromosome (1). It is the only viable monosomy syndrome in humans, with an incidence of approximately 1 in 4,000 to 1 in 2,500 live female births (2). TS is primarily caused by the complete or partial loss of one X chromosome, with the most common karyotype being 45, X (3). The clinical manifestations of TS are highly heterogeneous, but the typical phenotype includes short stature, ovarian insufficiency, and congenital cardiovascular malformations (4–6). Studies have reported that about 25% of TS patients are diagnosed prenatally or at birth due to characteristic features such as a webbed neck and other fetal lymphoedema signs (7). Additional primary clinical features of TS include thyroid autoimmune abnormalities (8), congenital cardiovascular anomalies (9), liver abnormalities (10), hypertension (11), hearing loss (12), and renal anomalies (13). The age at diagnosis is predominantly distributed across three periods: under 1 year (14.9%), between 10–17 years (33.2%), and adulthood (38.5%), with a median age of diagnosis at 15.1 years, indicating a significant delay in diagnosis. Despite an increasing prevalence, the incidence rate of TS remains stable (14). Previous research has demonstrated that individuals with TS have a reduced life expectancy compared to the general population, with a difference of up to 12.5 years (15). In summary, TS presents clinicians with a multitude of challenges, including genetic, developmental, endocrine, cardiovascular, psychosocial, and reproductive issues. Patients may experience various complications throughout their lifespans, affecting not only their physical health but also leading to psychological and social impairments, thereby increasing the societal burden.
Currently, there is a paucity of research on the global burden and regional disparities of TS. Understanding the differences in disease burden across various regions can aid in formulating targeted health policies and promoting the rational allocation of medical resources. This study utilizes data from the Global Burden of Disease (GBD) study spanning from 1990 to 2021 to analyze the prevalence and Disability-Adjusted Life Years (DALYs) of children with TS aged 0–14 years at global, regional, and national levels, stratified by location and type. The aim is to assess the impact of socioeconomic factors and healthcare accessibility on the health burden of TS. This will provide references for healthcare professionals to develop new prevention and treatment strategies and offer insights for improving patient care and public health measures.
Methods
Overview and methodological details
Directed by the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, the GBD data source aims to quantify health losses caused by various diseases, injuries, and risk factors. It is recognized as one of the most comprehensive and systematic global epidemiological efforts (16). The GBD database was utilized to collect available data on individuals aged 0–14 years diagnosed with TS. Specifically, the dataset encompasses prevalence rates and cases at global, regional, and national levels, as well as DALY rates and cases. Additionally, trends in case numbers from 1990 to 2021 are included. All data employed in this study are accessible for download from the Global Health Data Exchange (GHDx) website (https://vizhub.healthdata.org/gbdresults/). Specifically, in the GHDx query tool, we chose “Cause” as “Turner syndrome”. Due to the lack of racial and ethnic data within the GBD database, analyses of these factors were not performed. This cross-sectional investigation entails examining and characterizing disease data across different timeframes and regions, ensuring that no identifiable personal information is included. As a result, the Ethics Committee of Kunming Children’s Hospital approved a waiver for the informed consent process. The study fully complied with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (17).
DALYs
It is a way to quantify the burden of diseases, injuries, and risk factors on a population. The concept combines years of life lost (YLL) due to premature mortality and years lived with disability (YLD) for incident cases of a disease. These metrics are calculated using the following equations:
Disability weights, determined through expert consensus, scale disease severity on a continuum from 0 (optimal health) to 1 (equivalent to mortality).
Socio-demographic index
The Socio-Demographic Index (SDI) is a comprehensive measure of socio-economic development for countries and regions, encompassing factors such as economic structure and scale, educational attainment, living standards, and social welfare and protection (18). SDI values range from 0 to 1, with higher values indicating more advanced socio-economic conditions. Utilizing data from the GBD database, countries and regions are categorized into five SDI tiers: low, lower-middle, middle, upper-middle, and high. This stratification facilitates the analysis of how socio-economic status and geographical disparities impact the burden of TS in children.
Statistical analysis
Prevalence and DALYs rates per 100,000 population, along with their 95% uncertainty intervals, were extracted from the GBD database. A log-transformed linear regression model was employed to estimate the average annual percentage change (EAPC) and its confidence interval (CI), facilitating the analysis of temporal trends in the prevalence and DALYs of TS in children from 1990 to 2021. The EAPC is particularly valuable for assessing long-term trends, as it captures the overall increase or decrease in prevalence rates over time, independent of short-term fluctuations. An EAPC with a lower 95% CI bound exceeding 0 indicates a significant upward trend in the respective metric, whereas an EAPC with an upper 95% CI bound below 0 signifies a significant downward trend. Additionally, fitted curves were utilized to examine the relationship between disease burden indicators and the SDI (19). All analyses were conducted using R version 4.3.3, with statistical significance determined at p < 0.05.
Result
Global burden trends
Prevalence
In 2021, the global prevalence of TS in children was 240,598.45 (95% UI, 185,491.24–318,611.47), representing a 12.81% (95% UI, 11.37–14.05) increase compared to 1990, when the prevalence was 213,271.59 (95% UI, 164,243.71–282,625.41). The global prevalence rate in 2021 was 11.96 (95% UI, 9.22–15.84), slightly lower than the 1990 rate of 12.26 (95% UI, 9.44–16.25). The estimated annual percentage change (EAPC) was -0.03 (95% CI, -0.06 to -0.00) (Table 1). In 2021, the prevalence rates by age groups were as follows: for children under 1 year, 17.75 (95% UI, 13.30–23.84); for children aged 1–2 years, 16.38 (95% UI, 13.32–21.95); for children aged 2–4 years, 14.12 (95% UI, 10.72–19.03); for children aged 5–9 years, 11.31 (95% UI, 8.68–15.18); and for children aged 10–14 years, 9.37 (95% UI, 7.19–12.42) (Supplementary Figure S1A). The prevalence rate of TS in children under 1 year of age is the highest, accounting for 25.7% of all children with TS in 2021, whereas children aged 10–14 years accounted for 13.6% of the total TS cases in 2021 (Figure 1A).
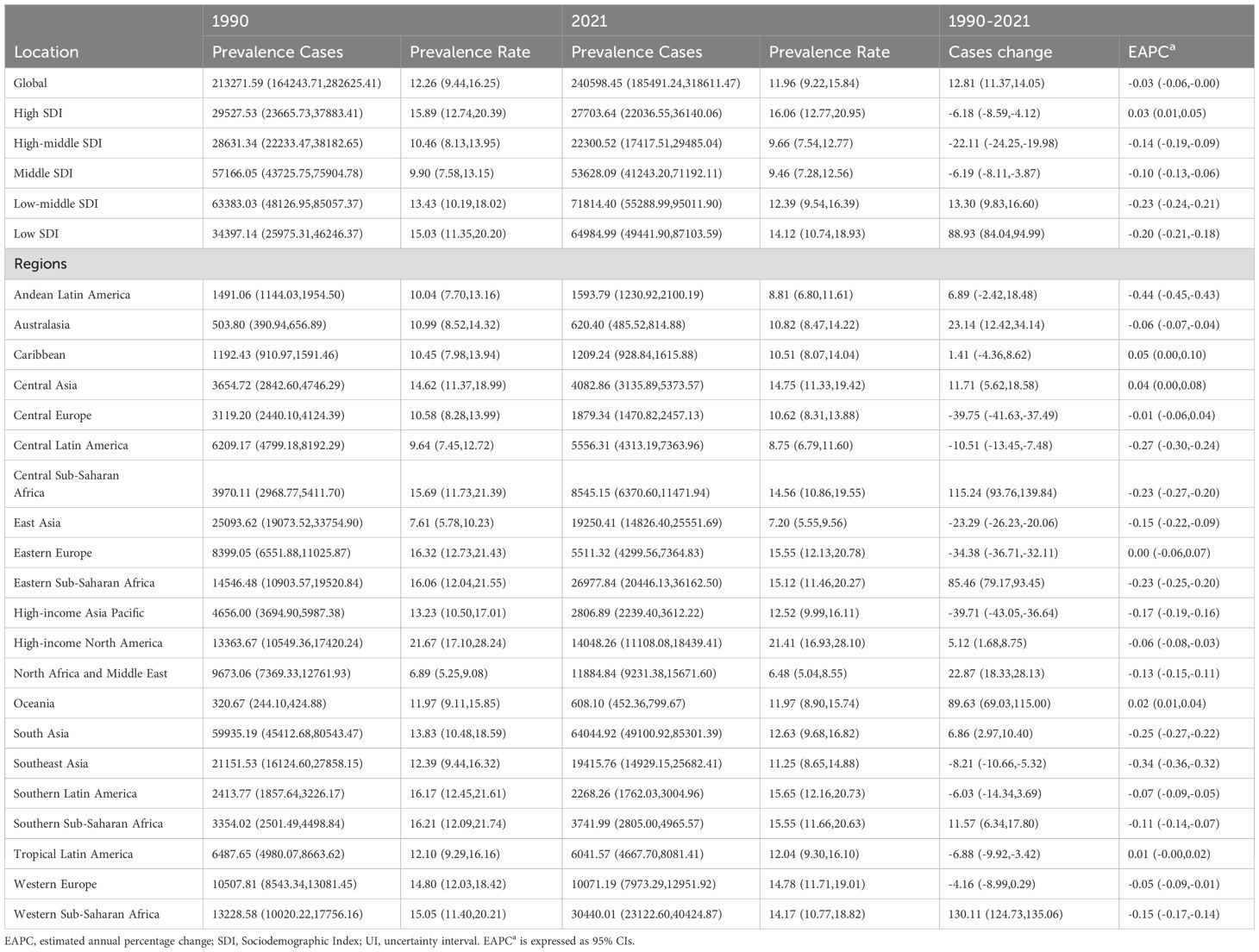
Table 1. Prevalence of turner syndrome in children between 1990 and 2021 at the global and regional level.
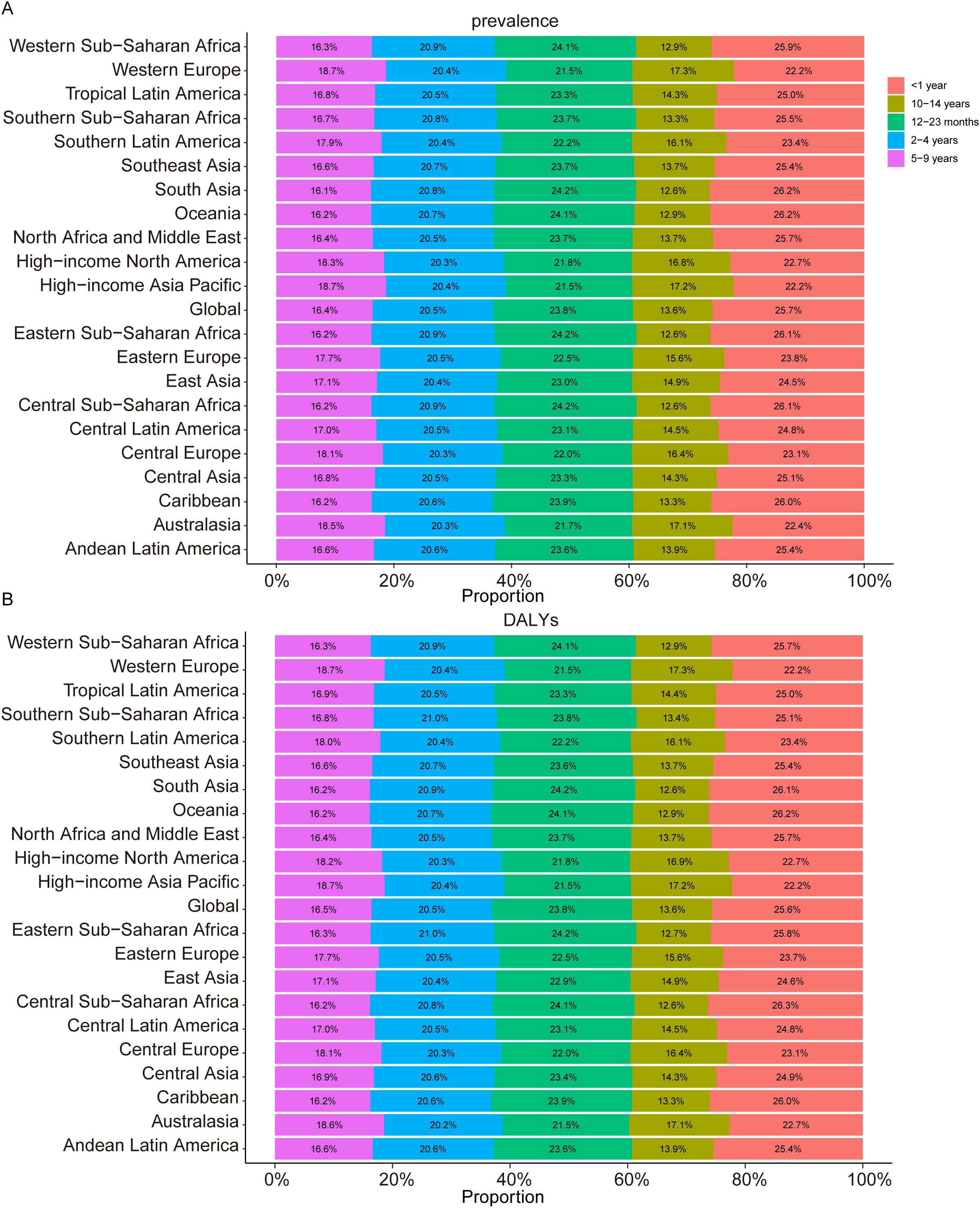
Figure 1. Age-specific Percentages of Childhood Turner Syndrome Prevalence and Disability-Adjusted Life Years (DALYs) in 2021. (A) Prevalence. (B) DALYs.
DALYs
In 2021, the global DALYs for TS in children was 3,328.35 (95% UI, 1,344.90–6,251.47), reflecting a decrease of 66.13 (95% UI, -79.97 to -44.24) compared to 1990, when the DALYs amounted to 2,948.47 (95% UI, 1,198.17–5,551.18). The DALY rate in 2021 was 0.17 (95% UI, 0.07–0.31), almost identical to the rate of 0.17 (95% UI, 0.07–0.32) in 1990. The EAPC for DALYs was -0.02 (95% CI, -0.05 to 0.00) (Table 1). The DALY rates by age group in 2021 were as follows: for children under 1 year, 0.24 (95% UI, 0.10–0.46); for children aged 1–2 years, 0.23 (95% UI, 0.09–0.43); for children aged 2–4 years, 0.20 (95% UI, 0.08–0.40); for children aged 5–9 years, 0.16 (95% UI, 0.06–0.29); and for children aged 10–14 years, 0.13 (95% UI, 0.05–0.25) (Supplementary Figure S1B). In 2021, children under 1 year of age had the highest DALY rate from TS, accounting for 25.6% of total TS-related DALYs among children, whereas those aged 10–14 contributed 13.6% (Figure 1B).
TS in children: regional trends by SDI
Prevalence
In 1990, the Low-middle SDI region had the highest number of pediatric TS cases, with 63,383.03 (95% UI, 48,126.95–85,057.37), while the High-middle SDI region had the fewest, with 28,631.34 (95% UI, 22,233.47–38,182.65). By 2021, the Low-middle SDI region still had the highest number of cases at 71,814.40 (95% UI, 55,288.99–95,011.90), and the High-middle SDI region again recorded the fewest at 22,300.52 (95% UI, 17,417.51–29,485.04). Additionally, in 2021, the High SDI region had the highest prevalence rate of pediatric TS (16.06 per 100,000; 95% UI, 12.77–20.95), whereas the Middle SDI region had the lowest (9.46 per 100,000; 95% UI, 7.28–12.56) (Table 1, Figure 2A).
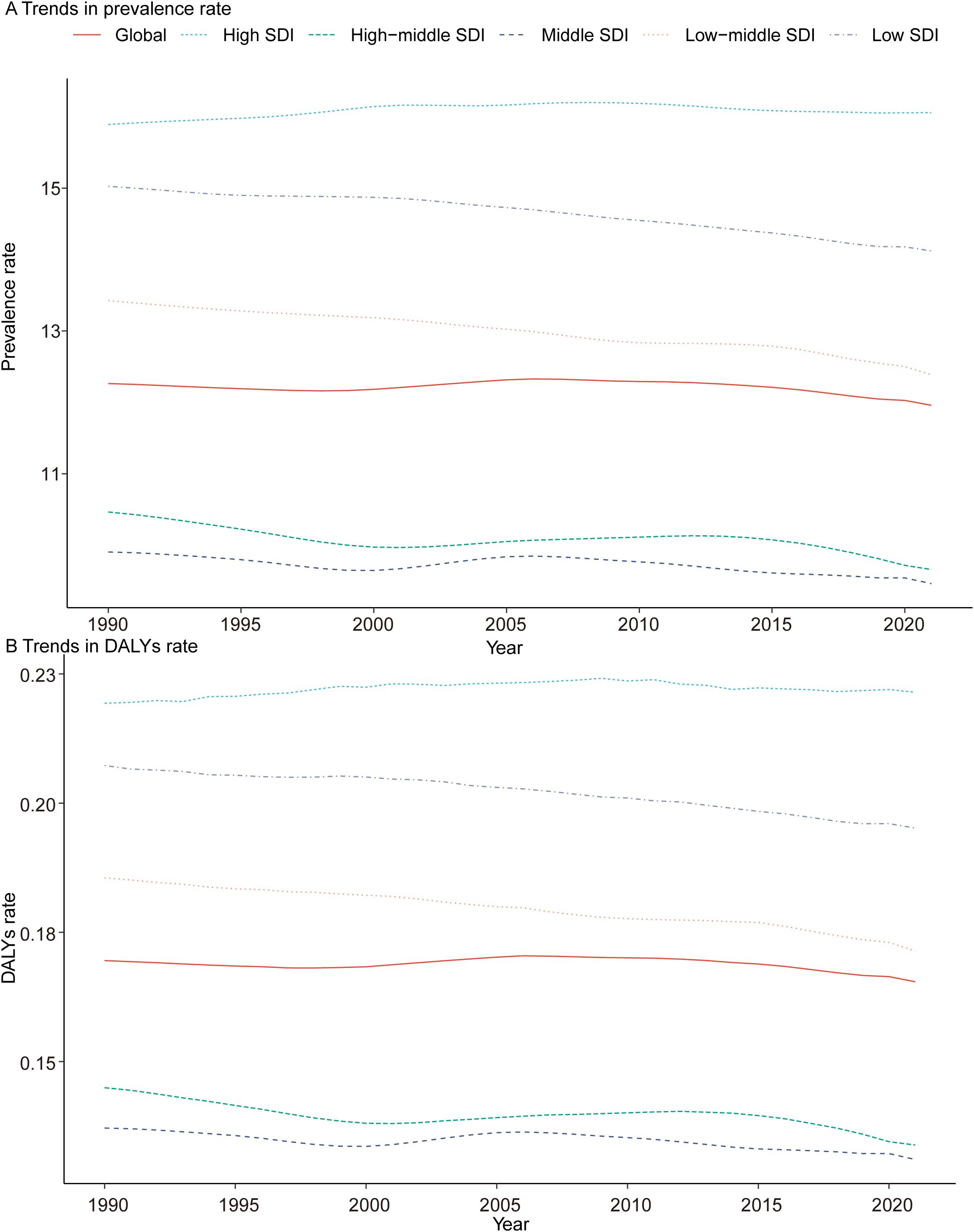
Figure 2. Epidemiologic Trends of Prevalence, and Disability-Adjusted Life-Years (DALYs) Rates in 5 Sociodemographic Index (SDI) Regions of Turner Syndrome in Children From 1990 to 2021. (A) Trends in prevalence rate. (B) Trends in DALYs rate.
DALYs
In 2021, the Low-middle SDI region exhibited the highest number of DALYs for pediatric TS at 993.76 (95% UI, 403.70–1,897.16), while the High-middle SDI region had the fewest at 309.05 (95% UI, 126.28–573.39). The High SDI region recorded the highest DALY rate (0.22 per 100,000; 95% UI, 0.09–0.40), while the High-middle and Middle SDI regions had the lowest rates (both at 0.13 per 100,000). Between 1990 and 2021, among all five SDI regions, only the Low SDI region showed a rise in DALYs for pediatric TS, increasing from 474.50 (95% UI, 192.10–900.91) in 1990 to 898.27 (95% UI, 358.27–1,710.59) in 2021. Notably, only the High SDI region exhibited a positive EAPC (0.03; 95% CI: 0.01–0.05) (Table 2, Figure 2B).
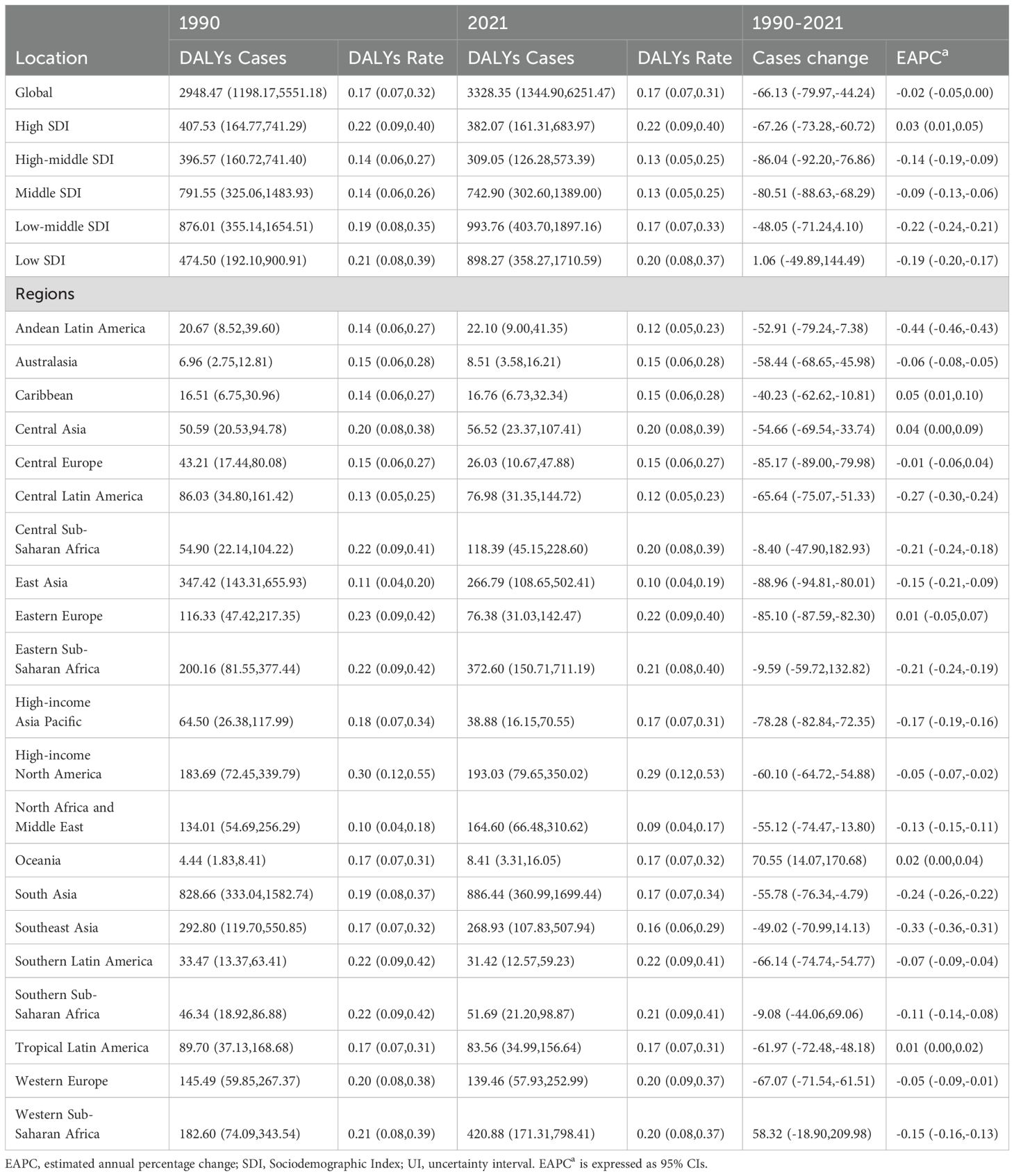
Table 2. DALYs of turner syndrome in children between 1990 and 2021 at the global and regional level.
TS in children: geographic regional trends
Prevalence
In 1990, Oceania reported the lowest number of pediatric TS cases (320.67; 95% UI, 244.10–424.88), whereas South Asia recorded the highest (59,935.19; 95% UI, 45,412.68–80,543.47). By 2021, Oceania remained the region with the fewest cases (608.1; 95% UI, 452.36–799.67), and South Asia continued to show the greatest burden (64,044.92; 95% UI, 49,100.92–85,301.39). In 2021, the lowest TS prevalence rate was observed in North Africa and the Middle East (6.48; 95% UI, 5.04–8.55), while High-income North America exhibited the highest rate (21.41; 95% UI, 16.93–28.10). Between 1990 and 2021, the largest decline in prevalence rate occurred in Andean Latin America (EAPC = –0.44; 95% CI, –0.45 to –0.43), and the greatest increase was recorded in the Caribbean (EAPC = 0.05; 95% CI, 0.00–0.10) (Table 1). In 2021, the global SDI was 0.67. Eight regions had pediatric TS prevalence rates below the global average, whereas thirteen exceeded it (Figure 3A).
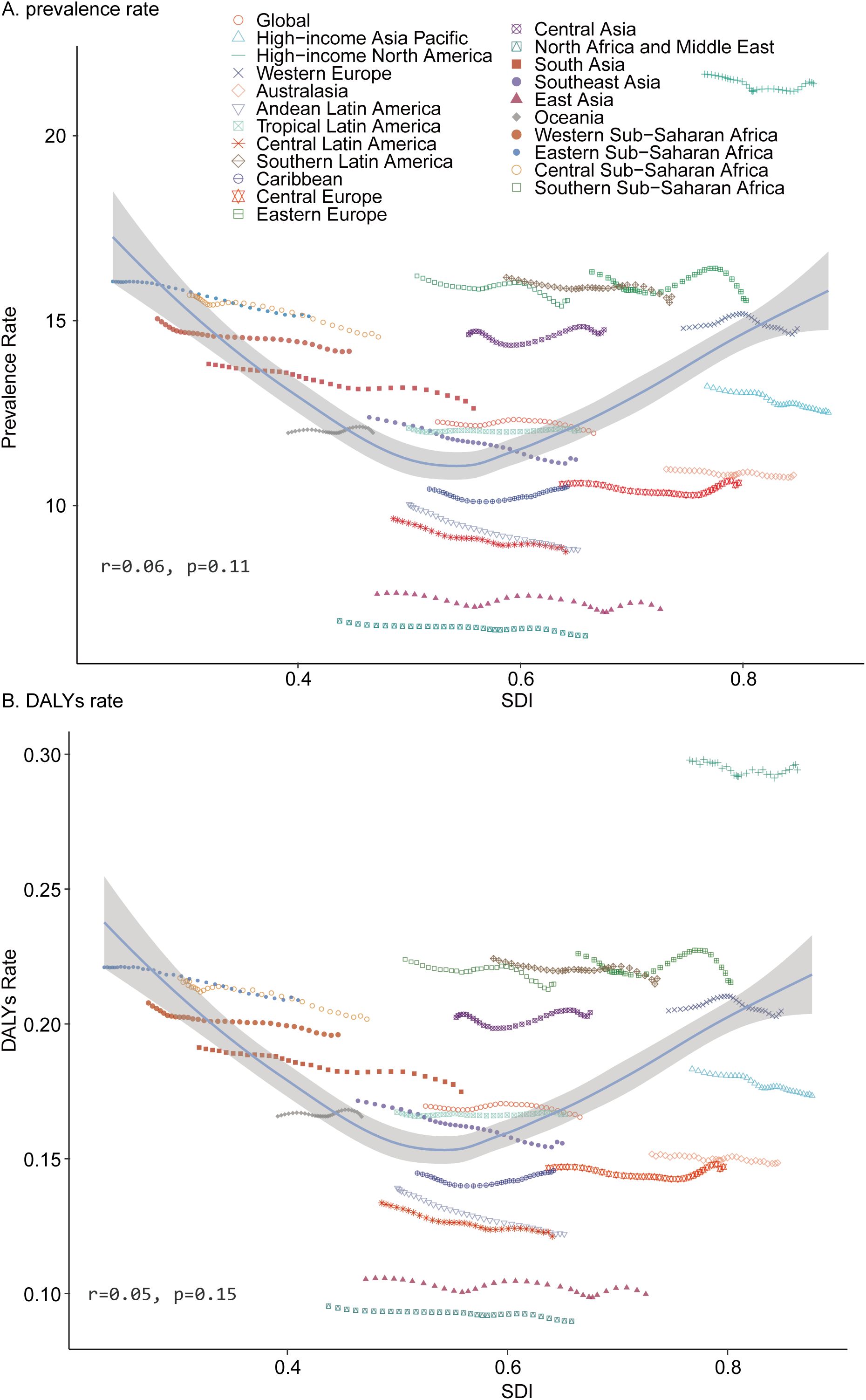
Figure 3. Prevalence and Disability-Adjusted Life-Years (DALYs) Rates for Childhood Turner Syndrome From 1990 to 2021. (A) Prevalence rate. (B) DALYs rate.
DALYs
In 2021, Oceania recorded the fewest DALYs for pediatric TS (8.41; 95% UI, 3.31–16.05), while South Asia experienced the highest DALYs (886.44; 95% UI, 360.99–1,699.44). The lowest DALYs rate was found in North Africa and the Middle East (0.09; 95% UI, 0.04–0.17), and the highest in High-income North America (0.29; 95% UI, 0.12–0.53). From 1990 to 2021, Andean Latin America showed the greatest reduction in DALYs rate (EAPC = –0.44; 95% CI, –0.46 to –0.43), whereas the Caribbean exhibited the largest increase (EAPC = 0.05; 95% CI, 0.01–0.10) (Table 2). Eight regions with a global SDI of 0.67 in 2021 had DALY rates below the global average, and thirteen exceeded it (Figure 3B).
TS in children: national trends
Prevalence
In 2021, among 204 countries surveyed, Niue recorded the lowest number of children with TS (0.04 [95% UI, 0.03–0.05]), whereas India reported the highest (45,941.86 [95% UI, 35,104.78–61,429.72]) (Supplementary Table S2, Figure 4A). Kuwait exhibited the lowest prevalence of pediatric TS (4.71 [95% UI, 3.59–6.20]), while New Zealand had the highest (23.60 [95% UI, 18.38–31.00]) (Supplementary Table S2, Figure 4B). Between 1990 and 2021, the largest decline in pediatric TS cases was observed in the United States Virgin Islands (case change = –61.72 [95% UI, –67.17 to –56.18]), whereas Qatar displayed the greatest increase (case change = 257.12 [95% UI, 214.03–315.98]) (Supplementary Table S2, Figure 4C). In terms of prevalence trends during this period, Equatorial Guinea experienced the steepest decrease (EAPC = –0.96 [95% CI, –1.04 to –0.89]), whereas France showed the largest rise (EAPC = 0.20 [95% CI, –0.05 to 0.45]) (Supplementary Table S2). Globally, the pediatric TS prevalence in 2021 reached 11.96 (95% UI, 9.22–15.84) per 100,000 individuals; among the 204 countries assessed, 99 fell below this global benchmark, while the remainder exceeded it.
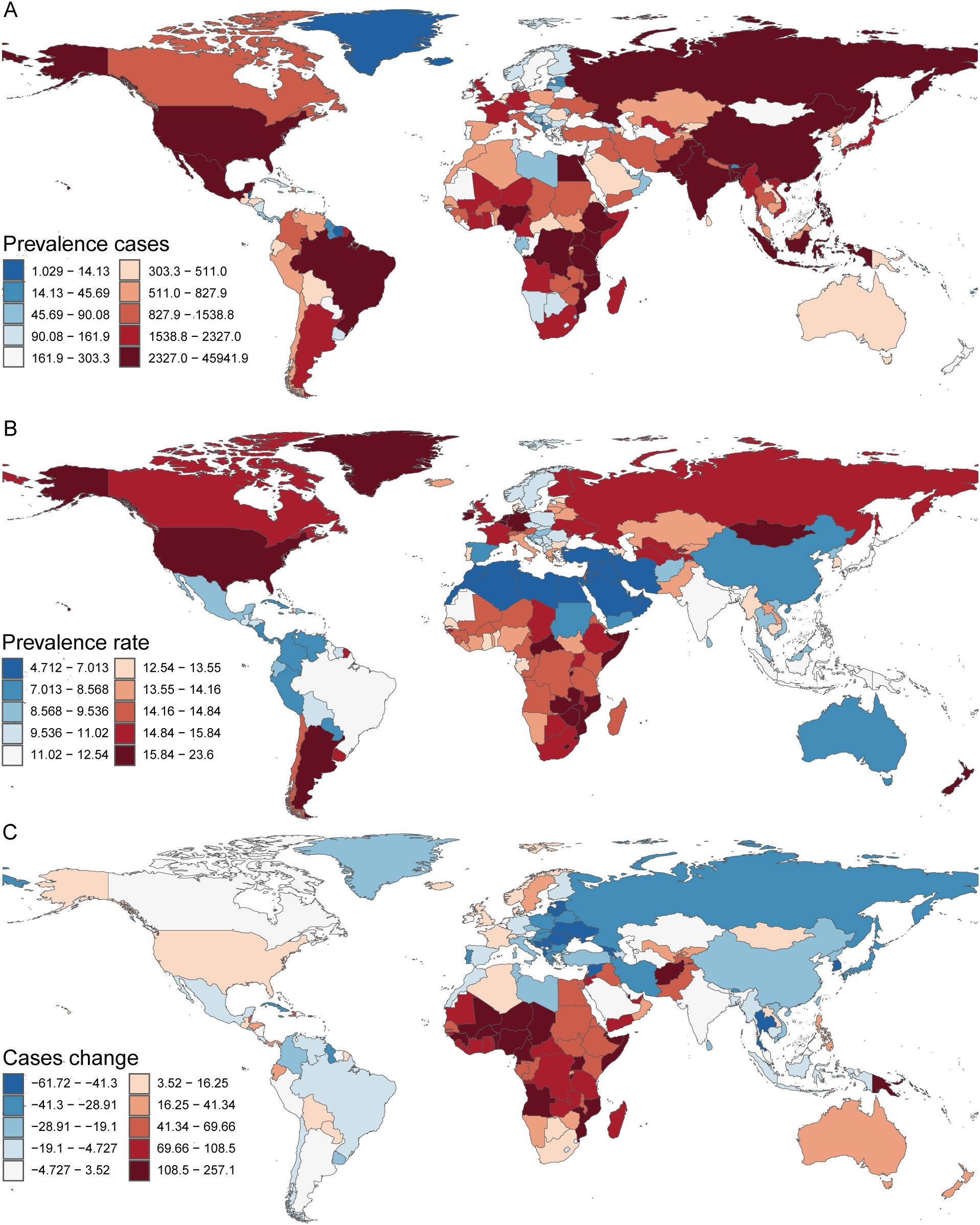
Figure 4. Prevalence of Turner Syndrome in Children in 204 Countries and Territories. (A) Prevalence cases. (B) Prevalence rate. (C) Change in Prevalence cases.
DALYs
In 2021, India recorded the highest number of DALYs attributable to pediatric TS, at 635.79 (95% UI, 255.73–1220.19), whereas New Zealand exhibited the highest DALY rate, reaching 0.32 (95% UI, 0.12–0.63). Over the period 1990–2021, the largest decrease in DALYs was observed in the United States Virgin Islands (case change = –61.69 [95% UI, –67.17 to –56.18]), while Qatar showed the greatest increase (case change = 257.07 [95% UI, 214.03–315.98]) (Supplementary Table S1, Supplementary Figures S2A–C). Concerning the DALY rate, Equatorial Guinea experienced the most pronounced decline (EAPC = –0.93 [95% CI, –1.00 to –0.86]), whereas France had the largest rise (EAPC = 0.20 [95% CI, –0.06 to 0.45]) (Supplementary Table S1). Globally, the DALY rate for pediatric TS in 2021 was 0.17 (95% UI, 0.07–0.31). Among the 204 countries analyzed, 98 had DALY rates below this global average, 13 were on par with the global rate, and the remainder exceeded it.
Discussion
TS is a rare genetic disorder primarily caused by the deletion or structural abnormalities of the X chromosome, making it one of the typical sex chromosome abnormalities (20). Although the global prevalence of TS is low (approximately 1 in 2,500 female live births (21)), many cases remain undiagnosed due to the subtlety of early symptoms or delayed diagnosis, resulting in a discrepancy between the number of confirmed cases and the actual prevalence (22). Studies have indicated that in countries where Down syndrome screening is routinely performed, the detection rate of TS fetuses is relatively higher (23, 24). This may be attributed to screening technologies that not only identify common chromosomal abnormalities such as Trisomy 21 but also detect other sex chromosome anomalies (25). Additionally, the comprehensive enhancement of screening protocols has led to an increased identification rate of various chromosomal abnormalities. In recent years, the diagnosis rate of TS mosaics has been steadily rising, primarily due to the continuous improvement of screening methods, including the application of high-throughput sequencing and non-invasive prenatal testing (NIPT) technologies (26), as well as an increase in the rate of elective terminations, which partly reflects the rise in early diagnostic interventions. Patients with TS often present with multiple comorbidities, including congenital heart and renal malformations, endocrine dysfunctions, gastrointestinal issues, and psychosocial disorders (27). These comorbidities not only significantly impact the quality of life of patients but also increase the demand for medical resources, thereby imposing a substantial burden on public health systems.
The epidemiological trends of TS from 1990 to 2021 highlight stark disparities between high- and low-SDI regions, driven by population dynamics, healthcare access, and systemic inequities. Globally, TS prevalence cases increased by 12.81%, primarily due to population growth, particularly in the low SDI region, where cases surged by 88.93% alongside rapid demographic expansion. However, declining prevalence rates (e.g., 15.03 to 14.12 per 100,000 in the low SDI region) and stable DALY rates (0.17 per 100,000 globally) suggest improved survival and partial diagnostic advances, albeit overshadowed by persistent management gaps. In high-SDI regions, widespread prenatal screening and multidisciplinary care reduced both prevalence cases (-6.18%) and DALYs (-67.26%), demonstrating the efficacy of early interventions. Conversely, low SDI regions face an interesting phenomenon: improved neonatal survival increases TS cases, but inadequate access to therapy, cardiac monitoring, and psychosocial support exacerbates long-term disability burdens, reflected in rising DALY cases (+1.06%) despite marginally declining rates. Further, accurately estimating the prevalence of TS requires distinguishing clinically diagnosed cases from the true disease burden, as many affected individuals remain undetected. International studies analyzing umbilical cord blood chromosomes in newborns (Denmark, Canada, Japan, etc.) report a biological prevalence of ~64 per 100,000 females (28–30)—a rate nearly double that observed in population-based registries from high-income countries (17–35 per 100,000) (25, 31, 32). This discrepancy likely reflects underdiagnosis due to delayed recognition, early mortality, or atypical presentations. Supporting this, genomic analyses of the UK Biobank cohort (n=245,000) identified numerous undiagnosed mosaic TS cases (45, X/46, XX) through SNP-array profiling (18), demonstrating that standard clinical practices miss subtle or mosaic forms of TS, even in well-resourced settings. These inequities underscore the interplay of population growth, fragmented healthcare systems, and cultural barriers, necessitating global collaboration to prioritize affordable screening tools, subsidized therapies, and integrated TS management within primary healthcare frameworks to bridge the gap between survival and quality of life.
As TS increasingly becomes a significant global public health concern, early diagnosis and intervention are of paramount importance. Early treatments, particularly the use of growth hormone, have been shown to markedly improve patients’ growth and overall prognosis (33). Effective screening programs can enhance the sensitivity and specificity of diagnoses, reduce the time to diagnosis, and facilitate early interventions. This, in turn, alleviates the health burden on patients and their families and optimizes the allocation of medical resources. Therefore, the refinement and widespread implementation of efficient screening technologies are crucial for improving the early diagnostic rates of TS and enhancing patient outcomes.
Despite leveraging the comprehensive GBD database, this study has several inherent limitations. Firstly, data quality and completeness vary across regions and countries within the GBD dataset. In particular, low-income countries often report less accurate or incomplete data, which may compromise the precision of the estimated disease burden for TS in these areas. Secondly, the clinical diagnosis and treatment of TS exhibit significant regional disparities. In low-income settings, the scarcity of diagnostic tools and limited access to healthcare resources can lead to delayed diagnoses or misdiagnoses, thereby affecting the reliability of prevalence and DALYs estimates. Additionally, early comorbidities associated with TS, such as cardiovascular issues or hearing loss, may not be adequately captured in the GBD database. This underreporting can result in an underestimation of both mortality and disability rates related to TS. Furthermore, while this study explores the impact of socioeconomic factors and healthcare accessibility on the disease burden of TS, the measurement of these variables in the GBD database is relatively coarse. The analysis does not account for specific health policies, variations in healthcare systems, or cultural factors within each region, which can significantly influence health outcomes. Lastly, many comorbid conditions associated with TS, including cardiovascular diseases and diabetes, typically manifest in adulthood. Consequently, the burden of these long-term complications is not fully reflected in the assessment of disease burden during childhood (ages 0–14 years). This limitation suggests that the overall impact of TS may be underestimated when focusing solely on the pediatric population. Future research should aim to incorporate more granular data, particularly from low-income regions, and consider longitudinal studies that track the long-term health outcomes of individuals with TS. Additionally, integrating detailed information on healthcare policies and cultural contexts could provide a more nuanced understanding of the factors influencing the disease burden of TS globally.
Conclusions
Our study thoroughly examines the global and regional burden of pediatric TS from 1990 to 2021. While the total number of TS cases rose worldwide, the overall prevalence and DALY rates changed only slightly, and large differences were observed across various regions and countries. High-SDI regions generally showed higher TS rates and DALYs, while low-SDI regions had the greatest cases. Notably, only the low SDI region experienced an increase in TS-related DALYs, reflecting potential challenges in healthcare access and early detection. Meanwhile, some regions (like Andean Latin America) showed significant decreases in prevalence and DALYs, suggesting that effective healthcare measures can help lower the TS burden. At the national level, India had the most TS cases, while New Zealand showed the highest prevalence and DALY rates, highlighting differences in population size, screening practices, and diagnostic availability. Some countries made progress in reducing the TS burden, but others still face rising cases and DALYs. These findings stress the importance of early intervention, better public health strategies, and sufficient healthcare resources to lessen the global impact of pediatric TS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
FD: Conceptualization, Data curation, Investigation, Software, Writing – original draft. JLX: Conceptualization, Data curation, Formal Analysis, Writing – original draft. JXX: Formal Analysis, Methodology, Project administration, Writing – original draft. QL: Conceptualization, Data curation, Formal Analysis, Resources, Writing – original draft. ZC: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LD: Funding acquisition, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1552300/full#supplementary-material
Supplementary Figure 1 | Age-Specific Rate of Childhood Turner Syndrome Prevalence and DALYs in 2021. (A) Prevalence. (B) DALYs.
Supplementary Figure 2 | Disability-Adjusted Life Years (DALYs) of Turner Syndrome in Children in 204 Countries and Territories. (A) DALYs cases. (B) DALYs rate. (C) Change in DALYs cases.
References
1. Cook KD, Shpargel KB, Starmer J, Whitfield-Larry F, Conley B, Allard DE, et al. T follicular helper cell-dependent clearance of a persistent virus infection requires T cell expression of the histone demethylase UTX. Immunity. (2015) 43(4):703–14. doi: 10.1016/j.immuni.2015.09.002
2. Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. (2017) 177(3):G1–g70. doi: 10.1530/eje-17-0430
3. de Souza AF, Bressan FF, Pieri NCG, Botigelli RC, Revay T, Haddad SK, et al. Generation of primordial germ cell-like cells from iPSCs derived from turner syndrome patients. Cells. (2021) 10(11). doi: 10.3390/cells10113099
4. Peek R, Schleedoorn M, Smeets D, van de Zande G, Groenman F, Braat D, et al. Ovarian follicles of young patients with Turner’s syndrome contain normal oocytes but monosomic 45,X granulosa cells. Hum Reprod. (2019) 34(9):1686–96. doi: 10.1093/humrep/dez135
5. Turner H and Jackson L. Evidence for penetrance in patients without a family history of disease: a systematic review. Eur J Hum Genet. (2020) 28(5):539–50. doi: 10.1038/s41431-019-0556-5
6. Wang H, Zhu H, Zhu W, Xu Y, Wang N, Han B, et al. Bioinformatic analysis identifies potential key genes in the pathogenesis of turner syndrome. Front Endocrinol (Lausanne). (2020) 11:104. doi: 10.3389/fendo.2020.00104
7. Schleedoorn MJ, Mulder BH, Braat DDM, Beerendonk CCM, Peek R, Nelen W, et al. International consensus: ovarian tissue cryopreservation in young Turner syndrome patients: outcomes of an ethical Delphi study including 55 experts from 16 different countries. Hum Reprod. (2020) 35(5):1061–72. doi: 10.1093/humrep/deaa007
8. Mohamed SOO, Elkhidir IHE, Abuzied AIH, Noureddin A, Ibrahim GAA, Mahmoud AAA, et al. Prevalence of autoimmune thyroid diseases among the Turner Syndrome patients: meta-analysis of cross sectional studies. BMC Res Notes. (2018) 11(1):842. doi: 10.1186/s13104-018-3950-0
9. Essandoh M, Castellon-Larios K, Zuleta-Alarcon A, Portillo JG, and Crestanello JA. Unicuspid aortic stenosis in a patient with turner syndrome: A case report. Front Cardiovasc Med. (2014) 1:14. doi: 10.3389/fcvm.2014.00014
10. Calanchini M, Moolla A, Tomlinson JW, Cobbold JF, Grossman A, Fabbri A, et al. Liver biochemical abnormalities in Turner syndrome: A comprehensive characterization of an adult population. Clin Endocrinol (Oxf). (2018) 89(5):667–76. doi: 10.1111/cen.13811
11. Gravholt CH, Juul S, Naeraa RW, and Hansen J. Morbidity in turner syndrome. J Clin Epidemiol. (1998) 51(2):147–58. doi: 10.1016/s0895-4356(97)00237-0
12. Bois E, Nassar M, Zenaty D, Léger J, Van Den Abbeele T, Teissier N, et al. Otologic disorders in Turner syndrome. Eur Ann Otorhinolaryngol Head Neck Dis. (2018) 135(1):21–4. doi: 10.1016/j.anorl.2017.08.006
13. Hamza RT, Shalaby MH, Hamed LS, Abdulla DBA, Elfekky SM, Sultan OM, et al. Renal anomalies in patients with turner syndrome: Is scintigraphy superior to ultrasound? Am J Med Genet A. (2016) 170a(2):355–62. doi: 10.1002/ajmg.a.37425
14. Stochholm K, Juul S, Juel K, Naeraa RW, and Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab. (2006) 91(10):3897–902. doi: 10.1210/jc.2006-0558
15. Price WH, Clayton JF, Collyer S, De Mey R, and Wilson J. Mortality ratios, life expectancy, and causes of death in patients with Turner’s syndrome. J Epidemiol Community Health. (1986) 40(2):97–102. doi: 10.1136/jech.40.2.97
16. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study. Lancet. (2021) 403(10440):2133–61. doi: 10.1016/s0140-6736(24)00757-8
17. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370(9596):1453–7. doi: 10.1016/s0140-6736(07)61602-x
18. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study. Lancet. (2019) 396(10258):1204–22. doi: 10.1016/s0140-6736(20)30925-9
19. Cao G, Liu J, and Liu M. Global, regional, and national incidence and mortality of neonatal preterm birth, 1990-2019. JAMA Pediatr. (2022) 176(8):787–96. doi: 10.1001/jamapediatrics.2022.1622
20. Berglund A, Stochholm K, and Gravholt CH. The epidemiology of sex chromosome abnormalities. Am J Med Genet C Semin Med Genet. (2020) 184(2):202–15. doi: 10.1002/ajmg.c.31805
21. Johnston L, Allen R, Hall Barrientos P, Mason A, and Kazakidi A. Hemodynamic abnormalities in the aorta of turner syndrome girls. Front Cardiovasc Med. (2021) 8:670841. doi: 10.3389/fcvm.2021.670841
22. Gravholt CH, Viuff M, Just J, Sandahl K, Brun S, van der Velden J, et al. The changing face of turner syndrome. Endocr Rev. (2023) 44(1):33–69. doi: 10.1210/endrev/bnac016
23. Boyd PA, Loane M, Garne E, Khoshnood B, and Dolk H. Sex chromosome trisomies in Europe: prevalence, prenatal detection and outcome of pregnancy. Eur J Hum Genet. (2011) 19(2):231–4. doi: 10.1038/ejhg.2010.148
24. Viuff MH, Stochholm K, Uldbjerg N, Nielsen BB, and Gravholt CH. Only a minority of sex chromosome abnormalities are detected by a national prenatal screening program for Down syndrome. Hum Reprod. (2015) 30(10):2419–26. doi: 10.1093/humrep/dev192
25. Berglund A, Viuff MH, Skakkebæk A, Chang S, Stochholm K, Gravholt CH, et al. Changes in the cohort composition of turner syndrome and severe non-diagnosis of Klinefelter, 47,XXX and 47,XYY syndrome: a nationwide cohort study. Orphanet J Rare Dis. (2019) 14(1):16. doi: 10.1186/s13023-018-0976-2
26. Wit JM, Kamp GA, and Oostdijk W. Towards a rational and efficient diagnostic approach in children referred for growth failure to the general paediatrician. Horm Res Paediatr. (2019) 91(4):223–40. doi: 10.1159/000499915
27. Ranke MB and Saenger P. Turner’s syndrome. Lancet. (2001) 358(9278):309–14. doi: 10.1016/s0140-6736(01)05487-3
28. Nielsen J and Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet. (1991) 87(1):81–3. doi: 10.1007/bf01213097
29. Bochkov NP, Kuleshov NP, Chebotarev AN, Alekhin VI, and Midian SA. Population cytogenetic investigation of newborns in Moscow. Humangenetik. (1974) 22(2):139–52. doi: 10.1007/bf00278453
30. Hamerton JL, Canning N, Ray M, and Smith S. A cytogenetic survey of 14,069 newborn infants. I. Incidence of chromosome abnormalities. Clin Genet. (1975) 8(4):223–43. doi: 10.1111/j.1399-0004.1975.tb01498.x
31. Schoemaker MJ, Swerdlow AJ, Higgins CD, Wright AF, and Jacobs PA. Mortality in women with turner syndrome in Great Britain: a national cohort study. J Clin Endocrinol Metab. (2008) 93(12):4735–42. doi: 10.1210/jc.2008-1049
32. Ji J, Zöller B, Sundquist J, et al. Risk of solid tumors and hematological Malignancy in persons with Turner and Klinefelter syndromes: A national cohort study. Int J Cancer. (2016) 139(4):754–8. doi: 10.1002/ijc.30126
Keywords: turner syndrome, GBD (global burden disease), prevalence, DALYs - disability-adjusted life years, children
Citation: Ding F, Xu J, Xiong J, Li Q, Cheng Z and Deng L (2025) Epidemiological analysis of turner syndrome in children aged 0–14 years: global, regional, and national perspectives (1990-2021). Front. Endocrinol. 16:1552300. doi: 10.3389/fendo.2025.1552300
Received: 27 December 2024; Accepted: 09 April 2025;
Published: 30 April 2025.
Edited by:
Sally Radovick, Rutgers, The State University of New Jersey, United StatesReviewed by:
Monika Obara-Moszynska, Poznan University of Medical Sciences, PolandAgustini Utari, Diponegoro University, Indonesia
Copyright © 2025 Ding, Xu, Xiong, Li, Cheng and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zugen Cheng, Y3pnMTg3MDI2OTM4NjJAb3V0bG9vay5jb20=; Lili Deng, Njc2MjUzMzU0QHFxLmNvbQ==
†These authors have contributed equally to this work
 Fang Ding
Fang Ding Jiaoli Xu
Jiaoli Xu Jingxuan Xiong
Jingxuan Xiong Qinhong Li
Qinhong Li Zugen Cheng
Zugen Cheng Lili Deng
Lili Deng