- Department of Minimally Invasive Laparoscopy, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
Objective: Thyroid hormones (THs) play a pivotal role in regulating metabolism, and their sensitivity may influence the risk of metabolic syndrome (MetS). This study aimed to investigate the association of impaired sensitivity to THs with MetS and MetS severity score (MetSSS) in Chinese euthyroid adults.
Methods: A cross-sectional analysis was conducted involving 17,272 health check-up participants. THs sensitivity indices, including Thyroid Feedback Quantile-Based Index (TFQI), Parametric Thyroid Feedback Quantile-Based Index (PTFQI), TSH Index (TSHI), Thyrotropin Thyroxine Resistance Index (TT4RI), and free triiodothyronine/free thyroxine (FT3/FT4) ratio were assessed. Multivariable regression and restricted spline cubic analyses were conducted to explore the association between THs sensitivity indices and MetS and MetSSS. Subgroup analysis was also performed to examine this association stratified by sex and age.
Results: Multivariable logistic regression analysis indicated that MetS risk was positively associated with all impaired THs sensitivity indices (per SD increase) (TFQI: OR=1.20, 95%CI: 1.15-1.25); PTFQI: OR=1.28, 95%CI: 1.23-1.33; TSHI: OR=1.35, 95%CI: 1.29-1.42; TT4RI: OR=1.57, 95%CI: 1.47-1.67; FT3/FT4: OR=1.17, 95%CI: 1.12-1.23)(all P-value<0.001). After adjusting for confounders, compared with the lowest group of MetSSS, individuals in the highest group of MetSSS were positively associated with all impaired THs sensitivity indices (per SD increase) (TFQI: OR=1.16, 95%CI: 1.07-1.21 PTFQI: OR=1.12, 95%CI: 1.06-1.17; TSHI: OR=1.13, 95%CI: 1.06-1.19; TT4RI: OR=1.25, 95%CI: 1.15-1.35; FT3/FT4: OR=1.82, 95%CI: 1.72-1.93). Nonlinear associations were found between THs sensitivity indicators and MetS (P for non-linear<0.001). Subgroup analysis indicated that all thyroid hormones sensitivity indices were positively associated with MetS by gender (male/female) and age (<60 years/≥60 years).
Conclusion: Impaired sensitivity to THs is associated with an increased risk of MetS and MetSSS in Chinese euthyroid adults. Future research should consider thyroid hormones sensitivity indices in the assessment of MetS risk.
Introduction
Metabolic syndrome (MetS) is a complex metabolic disorder characterized by the presence of central obesity, high blood sugar levels, abnormal lipid levels, and high blood pressure (1). Central obesity, with excess visceral fat, affects insulin sensitivity and vascular health. Elevated blood sugar indicates insulin resistance, leading to atherosclerosis and microvascular damage. Dyslipidemia increases plaque formation risk, while hypertension, tied to insulin resistance and obesity, worsens vascular issues. Collectively, these components boost the risk of cardiovascular diseases and type 2 diabetes (2). The prevalence of MetS has become a major global health concern, with the International Diabetes Federation estimating that one in four people worldwide are affected (2, 3). In China, the situation is equally troubling, as there has been a noticeable rise in the prevalence of MetS in recent years, highlighting the need for further research in this area (4).
Thyroid hormones (THs) are crucial for regulating development, metabolism, homeostasis in multiple organ systems and various physiological functions including energy balance. The two primary thyroid hormones are thyroxine (T4) and triiodothyronine (T3). T4, mainly secreted by the thyroid, acts as a prohormone, while T3, generated via tissue-specific deiodination of T4, is the biologically active form that binds to nuclear thyroid hormone receptors (TRs) to regulate gene transcription (genomic effects) (5).
Emerging evidence highlights the significance of thyroid hormone metabolites, which were once considered inactive byproducts. These metabolites include reverse T3 (rT3), tetraiodothyroacetic acid (Tetrac), triiodothyroacetic acid (Triac), diiodothyronines (e.g., 3,5-T2 and 3,3’-T2), and thyronamines (e.g., 3-T1AM). They exhibit distinct biological activities, particularly through non-genomic pathways. For example, 3,5-T2 modulates mitochondrial energy expenditure and lipid metabolism, while 3-T1AM influences thermoregulation and neuronal signaling. These metabolites often function independently of classical TR-mediated mechanisms, playing roles in fine-tuning physiological processes such as metabolic rate, cardiovascular function, and central nervous system activity (6). Both hypothyroidism and hyperthyroidism can result in insulin resistance and have a detrimental impact on glucose and lipid metabolism, thereby being associated with the development of MetS (7, 8). Understanding the diversity and functional interplay of thyroid hormones and their metabolites is essential for elucidating their contributions to health and disease, particularly in metabolic disorders where traditional TH signaling may be dysregulated.
The study’s biochemical basis is the complex link between thyroid hormones and metabolism. Thyroid hormones are key to energy balance, glucose and lipid metabolism, and cardiovascular function, with both genomic and non-genomic effects (9). Genomically, they bind to nuclear receptors, affecting genes related to glucose and lipid metabolism, which impacts insulin sensitivity and lipid profiles (10). For example, they boost insulin secretion and receptor expression. Non-genomically, thyroid hormones interact with cell membranes and cytoplasmic proteins, rapidly modulating ion channels and enzymes involved in energy and glucose metabolism (11). They also stimulate mitochondrial biogenesis and uncoupling proteins, influencing energy use and lipid oxidation. Moreover, thyroid hormones interact with other hormones like insulin and adrenaline, affecting metabolic regulation. The hypothalamus-pituitary-thyroid feedback loop controls thyroid hormone levels, and its disruption can cause metabolic issues (12).
Previous research has shown conflicting results when it comes to the relationship between thyroid function and MetS. Some studies have suggested a connection between normal levels of thyroid-stimulating hormone (TSH) and the presence of MetS (13, 14). while others have not found any association or have pointed to FT4 instead (15, 16). These discrepancies could be due to differences in study populations, methodologies, and the failure to consider potential confounding factors like gender, which has been shown to impact the prevalence of MetS components and their correlation with THs (17–19). Additionally, simply measuring TSH, FT3, and FT4 levels in individuals with normal thyroid function may not be enough to accurately assess thyroid function status. It is important to recognize that thyroid hormone balance may not be stable even if these markers fall within the normal range.
Recently, there has been a growing interest in the idea that reduced sensitivity to THs in the general population could play a role in metabolic disorders (20). This concept of THs sensitivity considers both FT4 and TSH levels. In cases of THs resistance syndrome, elevated levels of both FT4 and TSH are present, indicating issues with energy regulation. Normally, there is a negative correlation between THs and TSH due to the feedback loop of the hypothalamic-pituitary-thyroid axis (21, 22). However, individuals with mild resistance to THs may have high levels of both hormones. Researchers have developed various indices, such as the thyroid feedback quantile-based index (TFQI), parametric thyroid feedback quantile-based index (PTFQI) (20), thyrotrophic thyroxine resistance index (TT4RI) (23), and thyroid-stimulating hormone index (TSHI) (24), to quantify the relationship between thyroid function and metabolic factors. These indices help to clarify conflicting findings regarding the link between THs and MetS.
Previous studies have demonstrated a direct link between sensitivity to THs and certain health issues like prediabetes, decreased kidney function, and higher risk of cardiovascular disease (25–27). Yet, there has been a lack of investigation into the relationship between THs sensitivity and MetS in individuals with normal thyroid function. This cross-sectional study seeks to investigate the link between sensitivity to THs and MetS, as well as its severity score, in a sizable group of Chinese euthyroid adults. Through the evaluation of various THs sensitivity indices, we aim to gain a more detailed understanding of the association between sensitivity to THs and MetS in euthyroid Chinese adults. The results could shed light on potential risk factors for MetS and aid in the creation of personalized prevention and treatment plans for Chinese adults.
Materials and methods
Study population and design
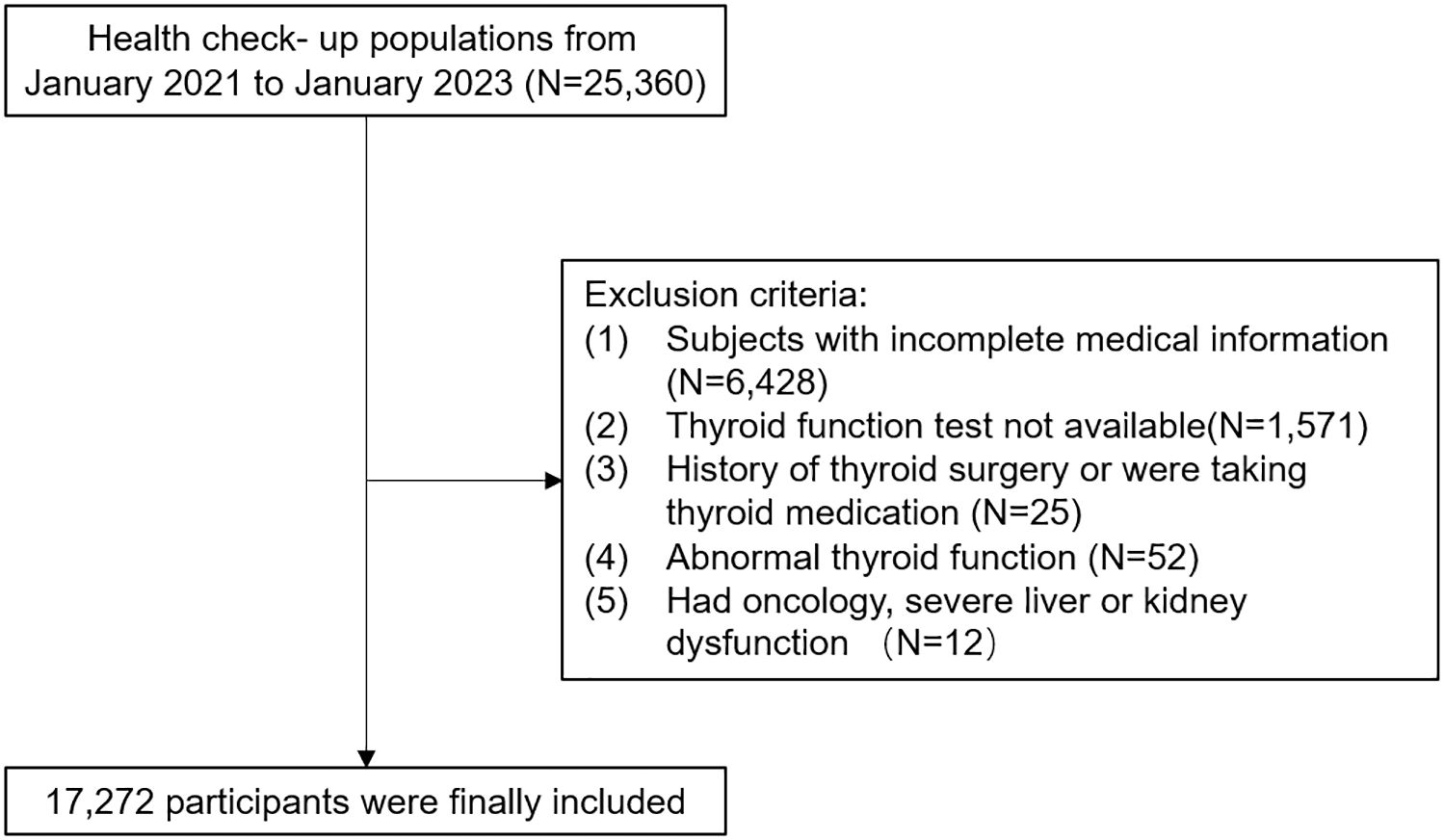
The study included adults over 18 years old who had undergone annual health examinations at the health check-up center of People’s Hospital in Wuxi city, affiliated with Nanjing Medical University. Initially, a total of 25,360 individuals were part of this retrospective study. Participants were excluded if they had incomplete medical information, lacked blood parameters for thyroid function tests, had a history of thyroid surgery or were taking thyroid medication, were not euthyroid, or had oncology, severe liver, or kidney dysfunction. After excluding these individuals, the study included 17,272 participants, comprising 10,442 males and 6,830 females aged 18 to 89 years. This retrospective study was approved by the Health Examination Center of People’s Hospital in Wuxi city (approval number: not applicable), affiliated with Nanjing Medical University, following the principles of the Declaration of Helsinki. Patient data was anonymized to ensure confidentiality, and statistical analysis was conducted securely for scientific research purposes. Therefore, informed consent was waived.
Data collection
We used a standard questionnaire to gather information on participants’ age, gender, and use of cigarettes and alcohol. Smoking was defined as consuming three or more cigarettes daily for a year, while alcohol consumption was defined as drinking at least three times a week for twelve months. Participants provided fasting venous blood samples after a 12-hour overnight fast. Levels of fasting plasma glucose (FPG), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), neutrophils (NE), and lymphocytes (LY) were measured using an automatic hematology analyzer. The neutrophil to lymphocyte ratio (NLR) was calculated. Strict quality control procedures were followed in the laboratory.
In addition, we gathered information on individuals’ health, such as whether they had been previously diagnosed with hypertension or diabetes, and if they were currently taking any medications. Diabetes was defined as having fasting blood glucose levels of 7.0 mmol/L or higher, being prescribed insulin or oral hypoglycemic agents, or self-reporting a history of the condition (28). Hypertension was determined by having a systolic blood pressure of 140 mmHg or higher, or a diastolic blood pressure of 90 mmHg or higher, and currently using antihypertensive medications (29). We used the electrochemiluminescence immunoassay method to measure the concentrations of thyroid-stimulating hormone (TSH), free triiodothyronine (FT3), and free thyroxine (FT4). The reference ranges for FT3, FT4, and TSH were 3.10 to 6.80 pmol/L, 12.00 to 22.00 pmol/L, and 0.27 to 4.20 mIU/L, respectively. Euthyroid was defined as having serum TSH and FT4 levels within the normal ranges and not using thyroid hormone medication.
The physical examination included measuring height (in centimeters), weight (in kilograms), waist circumference (in centimeters), and blood pressure (in mmHg). BMI was calculated by dividing weight in kilograms by height in meters squared. Systolic and diastolic blood pressure were measured on the right arm using a sphygmomanometer after at least 5 minutes of rest, and the average of two readings was recorded.
Metabolic syndrome and MetS severity score
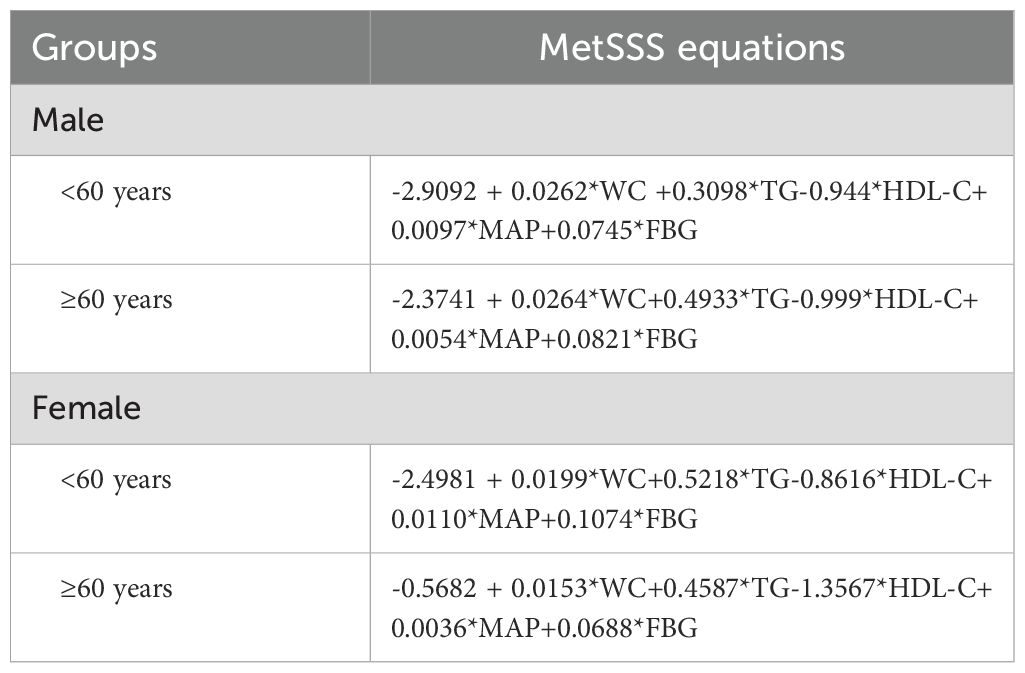
Metabolic syndrome (MetS) is defined according to the 2009 guidelines of the International Diabetes Federation (IDF) and the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) (30, 31). It includes three of the following five criteria: 1) elevated waist circumference, with specific measurements for men (≥90 cm) and women (≥80 cm); 2) elevated triglycerides (≥150 mg/dl) or use of medication for high triglyceride levels; 3) low HDL cholesterol (<40 mg/dl in men and <50 mg/dl in women) or use of medication for low HDL levels; 4) high blood pressure (systolic ≥130 mmHg and/or diastolic ≥85 mmHg) or use of antihypertensive medication; and 5) elevated fasting glucose (≥100 mg/dl) or use of medication for high glucose levels. Additionally, a Metabolic Syndrome Severity Score (MetSSS) is calculated based on specific equations for age, sex, and ethnicity (Table 1) (32).
Indices of thyroid hormone sensitivity
The participants’ central sensitivity to THs was assessed using Thyroid Feedback Quartile-Based index (TFQI), parametric thyroid feedback quantile-based index (PTFQI), TSH index (TSHI), and Thyrotroph T4 Resistance Index (TT4RI). Higher values of TFQI, PTFQI, TSHI, and TT4RI indicate lower central sensitivity to thyroid hormones. Peripheral THs sensitivity was evaluated using the FT3 to FT4 ratio (FT3/FT4), where higher values suggest higher sensitivity. The equations for calculation are provided with the following formulas (16, 19, 20): TFQI = cdf FT4 − (1 − cdf TSH); cdf: cumulative distribution function. PTFQI = φ((FT4-μFT4)/σFT4) - (1-φ ((ln TSH-μln TSH)/σlnTSH)), where μfT4 = 15.70, σfT4 = 1.80, μln TSH=0.62, and σln TSH=0.45 for the Chinese population. TSHI = ln TSH (mIU/L) + 0.1345 × FT4 (pmol/L). TT4RI = FT4 (pmol/L) × TSH (mIU/L). FT3/FT4 = FT3 (pmol/L)/FT4 (pmol/L).
Statistical analyses
The statistical analyses were conducted using SPSS 26.0 (Chicago, IL, USA) and R software (version 4.1). The normality of the variables was assessed using the Kolmogorov–Smirnov test. Normally distributed variables are presented as mean (standard deviation), skewed variables as median [interquartile range], and categorical variables as frequencies (proportions). One-way ANOVA test or Kruskal-Wallis H test were used to compare continuous variables, while the chi-square test was used for categorical variables. Multivariable logistic regression analysis was performed to assess the associations between MetS risk and thyroid hormone sensitivity indices, adjusting for potential confounding factors. Two models were used for adjustment: Model 1 included sex and age, while Model 2 included variables from Model 1 as well as smoking, drinking, BMI, and NLR. The relationship between thyroid hormone sensitivity indices and the risk of MetS and MetSSS in all euthyroid participants was examined using restricted cubic spline analysis. The model incorporated 4 knots placed at the 5th, 35th, 65th, and 95th percentiles of SII, with the p-value indicating the nonlinearity of the smooth curve fitting. Subgroup analysis was conducted to explore the correlation between thyroid hormone sensitivity indices and the risk of MetS and its components among different subgroups based on sex (males/females) and age (≥60 years/<60 years). A significance level of P < 0.05 (2-tailed) was considered statistically significant.
Results
Baseline characteristics
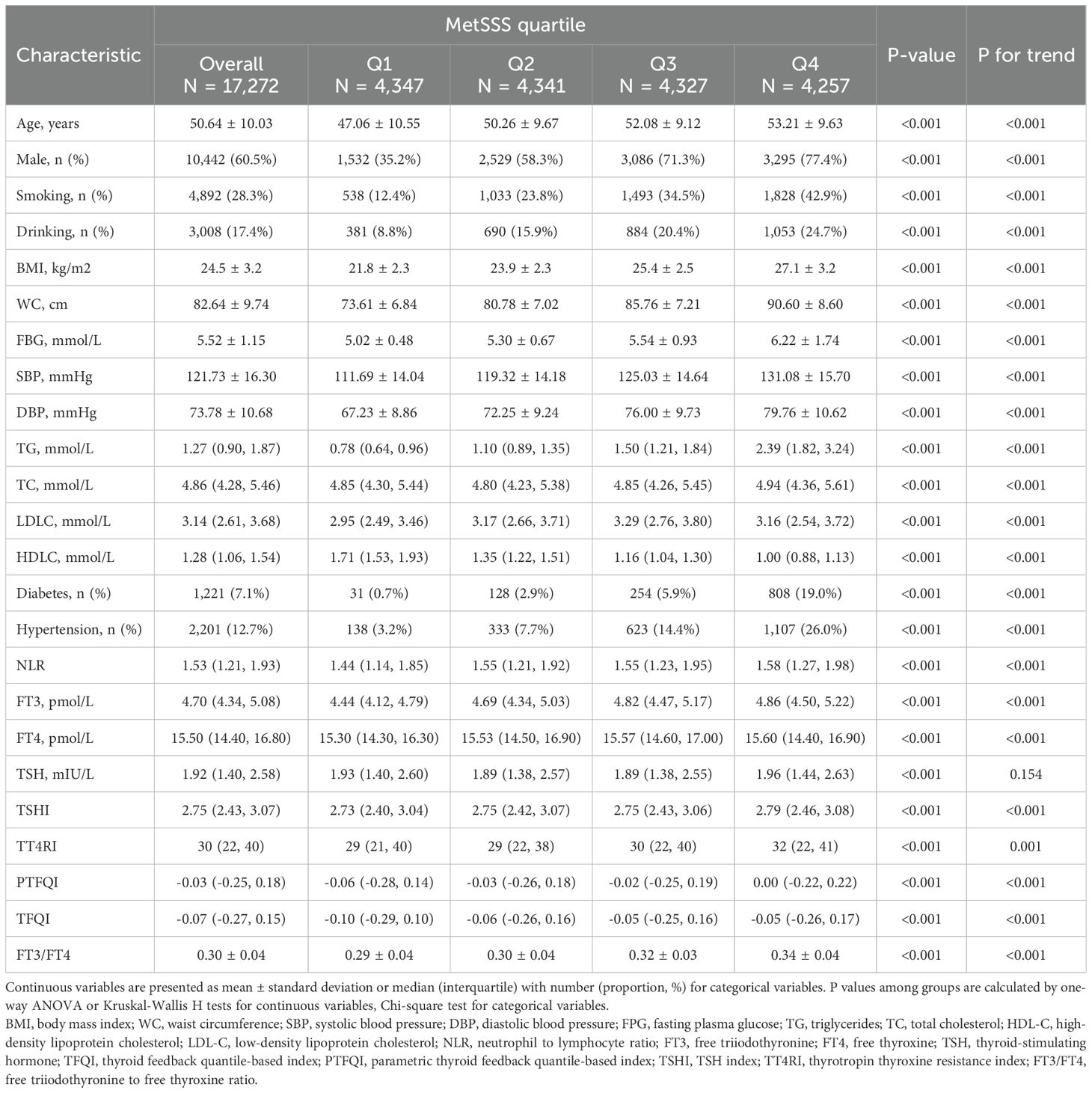
Table 2 presents the baseline characteristics of all participants categorized by quartiles of MetSSS. A total of 17,272 participants were included in the analysis, with 10,442 males (60.5%) and 6,830 females (39.5%) (Figure 1). There were significant differences in all baseline characteristics among the four quartiles of MetSSS (all P-values < 0.001). Individuals in the higher quartiles of MetSSS were more likely to be older, male, smokers, drinkers, and had higher rates of hypertension and diabetes (P for trend < 0.001). There were notable increases in BMI, WC, FBG, SBP, DBP, TG, TC, LDL-C, NLR, FT3, and FT4 across the quartiles of MetSSS, while HDLC decreased. Furthermore, participants in the higher quartiles of MetSSS exhibited higher levels of TFQI, PTFQI, TSHI, TT4RI, and FT3/FT4 (P for trend < 0.001).
Adjusted odds ratios for sensitivity to THs and risk of MetS and its components
Table 3 displays the adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the relationship between sensitivity to THs and the risk of MetS and its components. The analysis involved three models, including a crude model and two adjusted models controlling for various confounding factors. After adjusting for potential confounders such as age, sex, smoking, drinking, BMI, and NLR, the risk of MetS was found to be positively correlated with all sensitivity to THs [TFQI (+ 1 SD): OR=1.20, 95%CI: 1.15-1.25; PTFQI (+ 1 SD): OR=1.28, 95%CI: 1.23-1.33; TSHI (+ 1 SD): OR=1.35, 95%CI: 1.29-1.42; TT4RI (+ 1 SD): OR=1.57, 95%CI: 1.47-1.67; FT3/FT4 (+ 1 SD): OR=1.17, 95%CI: 1.12-1.23] (all P-value <0.001). Regarding MetS components, elevated waist circumference (WC) and low high-density lipoprotein cholesterol (HDL-C) did not show a significant association with thyroid sensitivity indices in the adjusted models. However, elevated blood pressure (BP), elevated triglycerides (TG), and elevated fasting plasma glucose (FPG) were positively associated with thyroid hormones sensitivity indices, with the strongest associations observed for FT3/FT4.
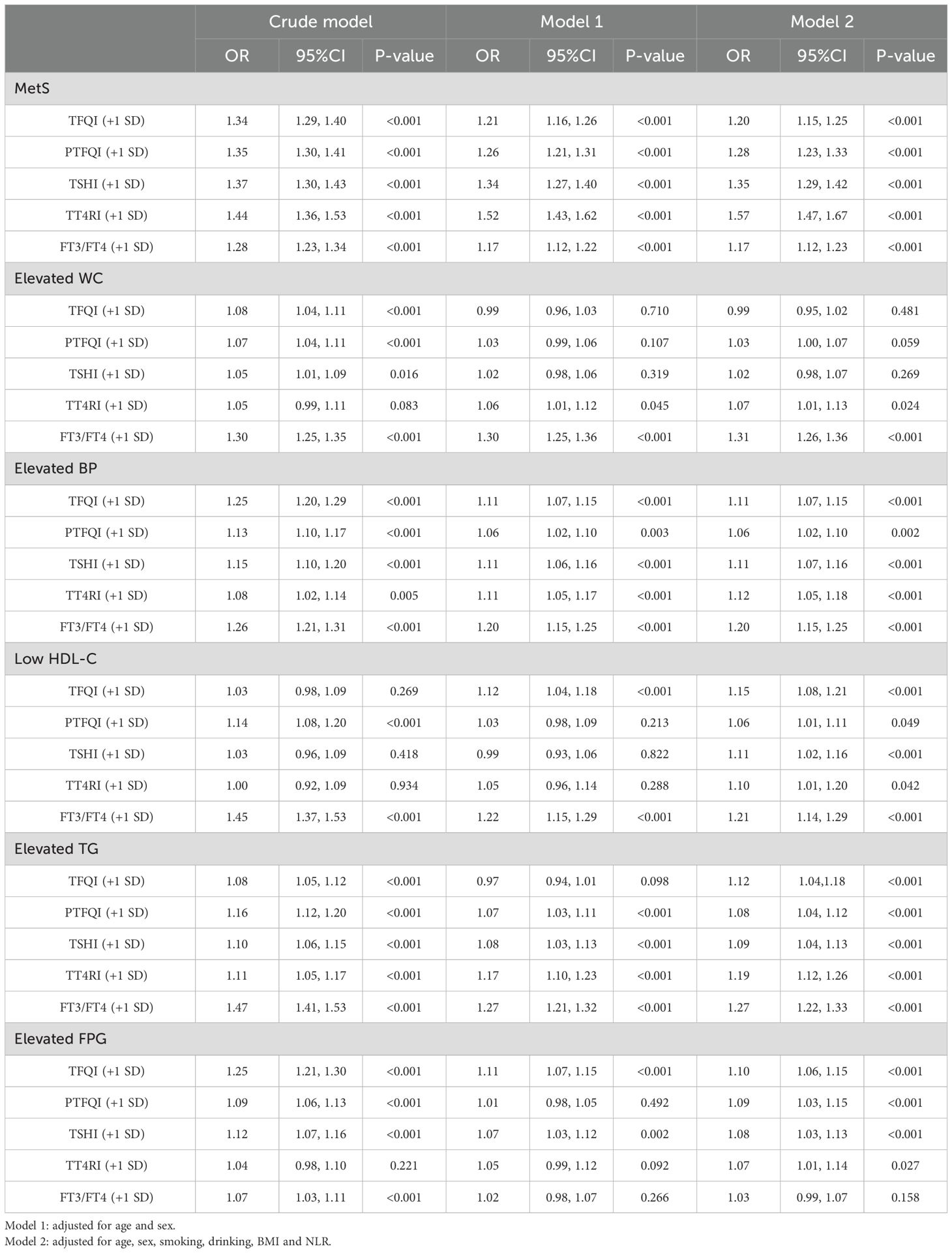
Table 3. Adjusted odds ratio (95% confidence interval) of sensitivity to THs and risk of MetS and its components.
Association of sensitivity to THs and MetSSS quartiles
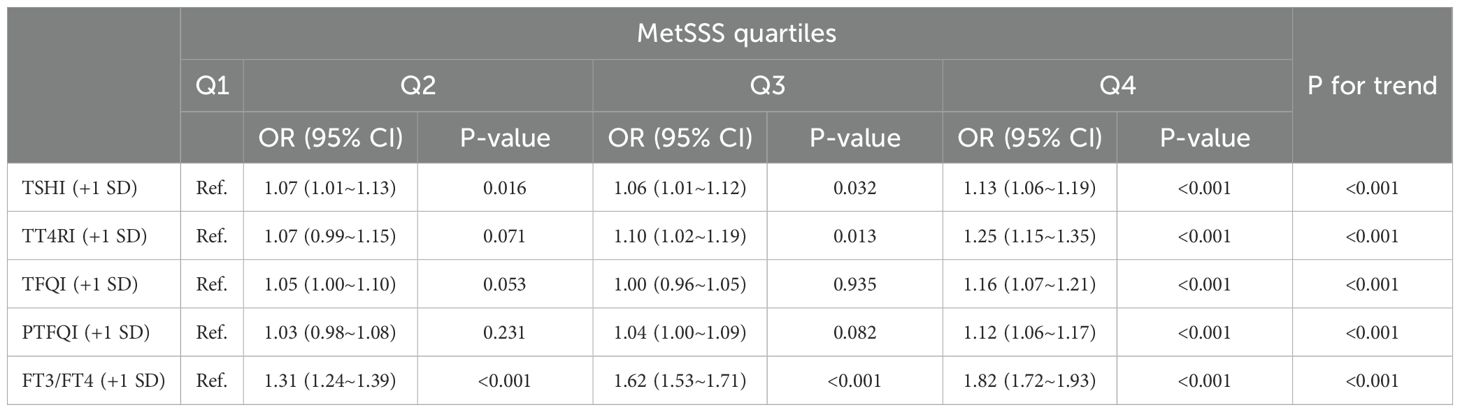
Table 4 shows the relationship between thyroid hormone sensitivity (per SD increase) and quartiles of MetSSS. The odds ratios for each quartile with 95% confidence intervals, adjusted for age, sex, smoking, drinking, BMI, and NLR, are presented. A significant positive trend was observed across MetSSS quartiles for TSHI and TT4RI (per SD increase), with the strongest association seen in the highest quartile (P for trend < 0.001). TFQI and PTFQI also demonstrated a positive trend, although less pronounced for the second quartile. The FT3/FT4 ratio showed a consistent and significant positive association across all quartiles, suggesting that higher peripheral thyroid hormone sensitivity may be related to a more severe MetS. Individuals in the highest quartile of MetSSS were more likely to have impaired THs sensitivity indices compared to those in the lowest quartile (TFQI: OR=1.16, 95%CI: 1.07-1.21; PTFQI: OR=1.12, 95%CI: 1.06-1.17; TSHI: OR=1.13, 95%CI: 1.06-1.19; TT4RI: OR=1.25, 95%CI: 1.15-1.35; FT3/FT4: OR=1.82, 95%CI: 1.72-1.93).
Exploration of nonlinear relationships
Based on the results of regression analysis, we conducted a restricted cubic spline analysis to investigate the dose-response relationship between indicators of THs sensitivity and MetS (Figure 2). After adjusting for potential confounders such as age, sex, smoking, drinking, and NLR, we found a significant association between sensitivity to thyroid hormones indices and MetS in all euthyroid participants (overall P<0.001). Non-linear relationships were observed between all indicators of thyroid hormone sensitivity and MetS (P for nonlinearity<0.001). Furthermore, both central and peripheral sensitivity to thyroid hormones were positively correlated with MetSSS) (overall P<0.05 (Figure 3). TSHI, TT4RI, TFQI, and PTFQI showed a linear association with MetSSS (P for nonlinearity>0.05), while FT3/FT4 exhibited a non-linear relationship with MetSSS (P for nonlinearity=0.001).
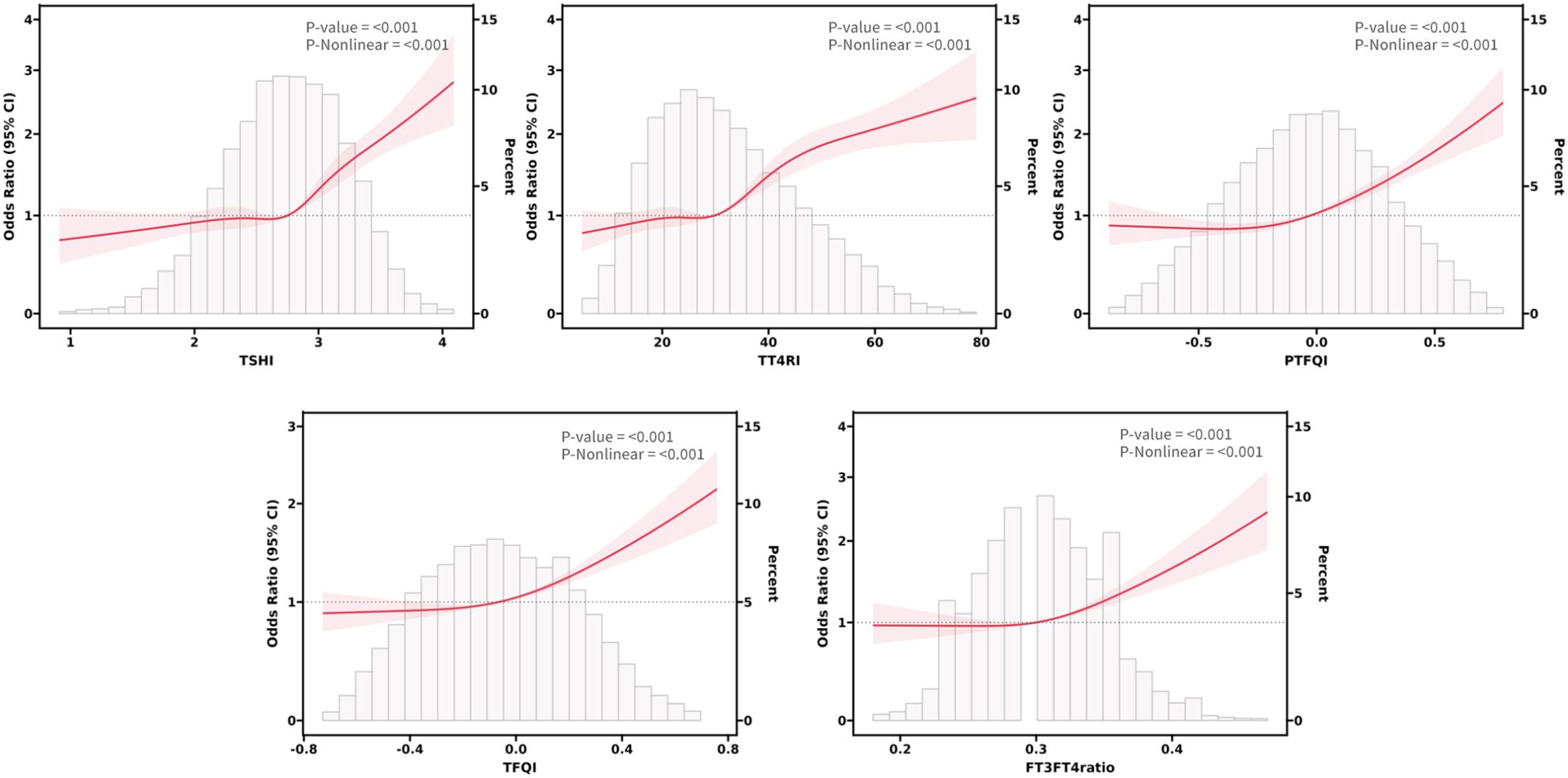
Figure 2. The smooth curve fitting between sensitivity to THs indicators and MetS. Solid red lines and red areas on both sides represent ORs and their 95%CI.
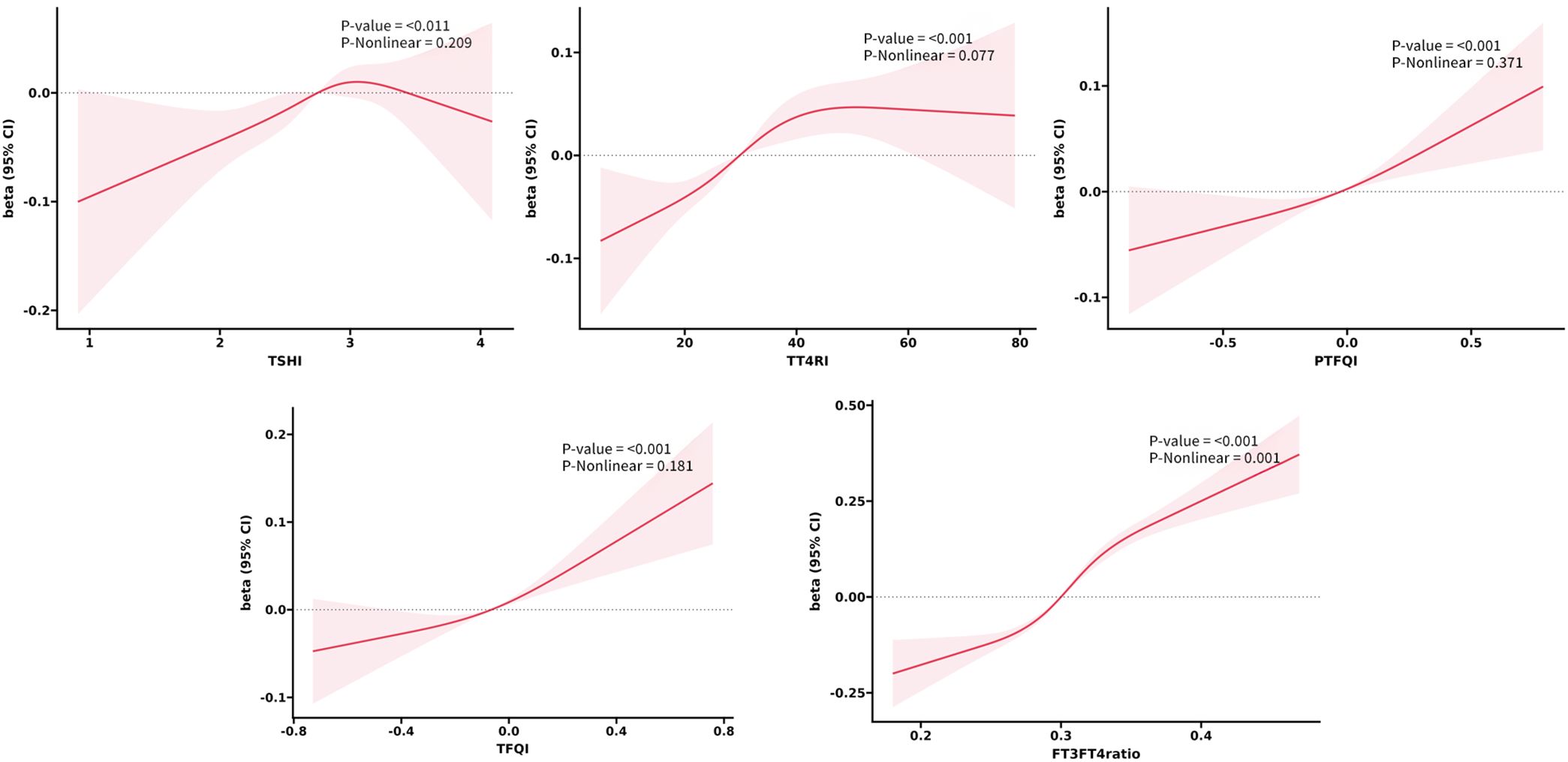
Figure 3. The smooth curve fitting between sensitivity to THs indicators and MetSSS. Solid red lines and red areas on both sides represent the estimated regression coefficient Beta and its 95% confidence interval.
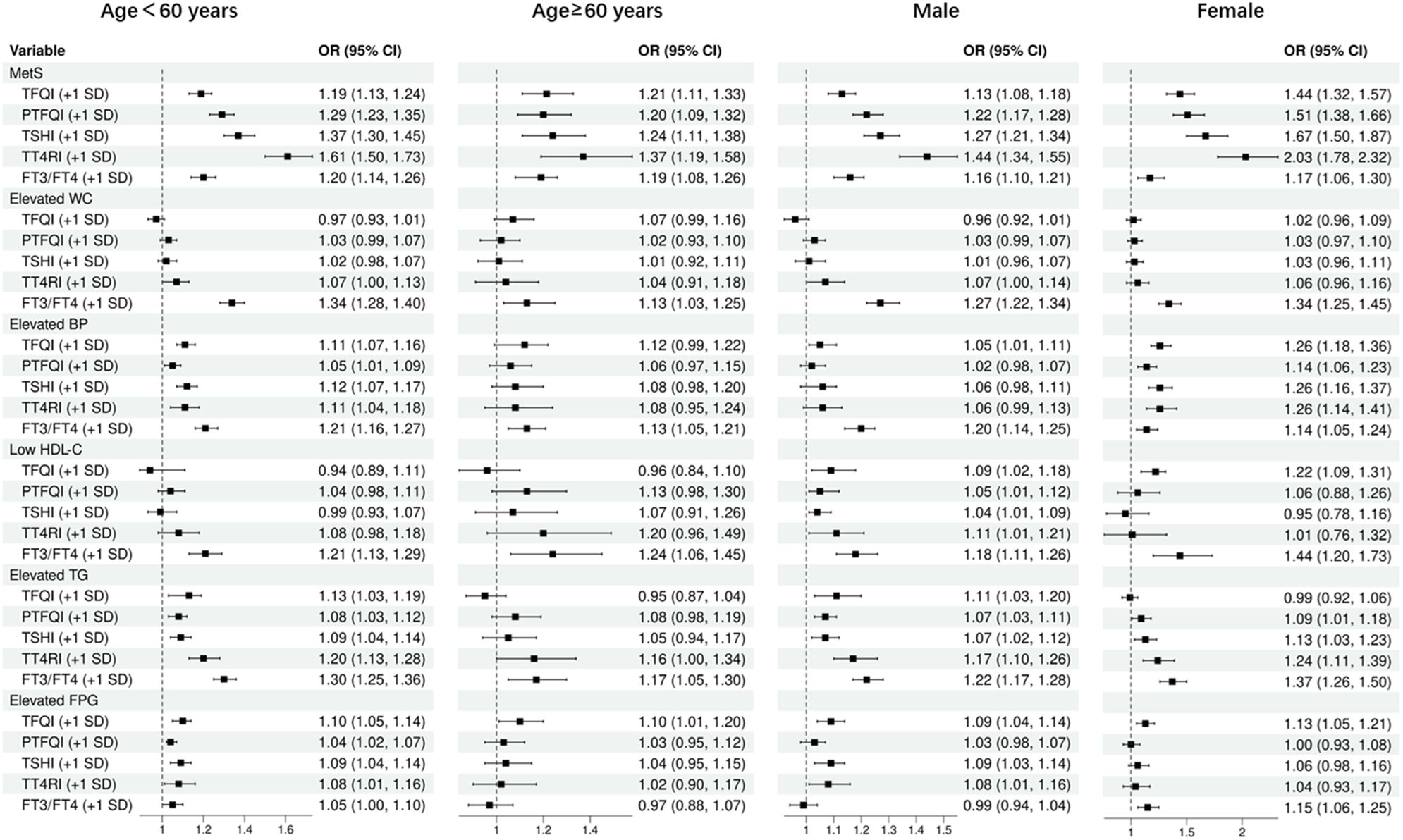
Subgroup analysis
As shown in Figure 4, after adjusting for potential confounders, the odds ratios (ORs) for the risk of MetS were found to increase with every 1 standard deviation increase in TFQI, PTFQI, TSHI, TT4RI, and FT3/FT4 ratio. These associations were observed in individuals under 60 years old and those over 60 years old, as well as in males and females. specifically, the ORs for MetS risk were 1.19 to 1.61 among individuals under 60 years old, 1.21 to 1.37 among those over 60 years old, 1.13 to 1.44 in males, and 1.44 to 2.03 in females. Additionally, the FT3/FT4 ratio was positively associated with all MetS components risk in all subgroups. however, most central sensitivities to THs were not significantly associated with elevated WC and low HDL-C. They were positively associated with elevated blood pressure (BP) and triglycerides (TG) in females and individuals under 60 years old, and all sensitivity to THs were positively associated with elevated FPG in subjects under 60 years old.
Discussion
To the best of our knowledge, this is the first large-sample cross-sectional study to evaluate the association between central and peripheral thyroid hormones sensitivity and risk of MetS and MetSSS in euthyroid Chinese adults. The results provide evidence that both decreased central sensitivity to THs (elevated TFQI, PTFQI, TSHI, and TT4RI) and increased peripheral sensitivity to THs (elevated FT3/FT4) were associated with an increased risk of MetS and MetSSS in euthyroid Chinese adults. Additionally, subgroup analysis showed that these relationships remain stable regardless of sex and age. This analysis goes beyond simply examining the absolute levels of FT3, FT4, and TSH, and provides insight into the resistance of THs within MetS and MetSSS.
THs have been found to play a significant role in all aspects of MetS through various mechanisms. These hormones can impact metabolic rate, appetite control, and sympathetic activity, which in turn affects adiposity (33). The stimulation of the sympathetic nervous system by THs also influences glucose and lipid metabolism, as well as cardiovascular regulation (34). However, fluctuations in THs levels within both normal and abnormal ranges have been linked to metabolic disorders. Previous studies have shown that levels of TSH or THs alone may not fully explain the relationship between the thyroid system and metabolic dysfunction (35). Therefore, comprehensive indices that reflect THs homeostasis may provide a more accurate understanding of this relationship. Laclaustra et al. have proposed the use of TFQI and PTFQI to quantify the relationship between thyroid function and metabolic indices (16). This approach, based on the theory of THs resistance, aims to explain contradictory findings in research and has opened up new avenues for studying the connection between thyroid function and metabolic disorders.
Numerous studies have investigated the relationship between sensitivity to THs and metabolic disorders. A recent study involving 31,678 patients with coronary heart disease found that impaired sensitivity to THs was significantly linked to dyslipidemia, regardless of gender, glucose levels, and blood pressure (35). Another recent cross-sectional study also showed a positive correlation between TFQI and FT3/FT4 levels with lipid levels (36). Our study revealed that all measures of THs sensitivity were positively linked to low HDL-C and high TG levels in euthyroid individuals. Research has demonstrated that TSH directly influences the expression of HMG-CoA reductase in the liver, leading to increased cholesterol synthesis (37, 38). TSH binds to its receptor and regulates protein kinase activation through the cyclic adenosine monophosphate/protein kinase A pathway, altering its inhibitory effect on the peroxisome proliferator-activated receptor-γ signaling pathway. This, in turn, triggers the activity of sterol regulatory element-binding protein (SREBP) 1c in the liver, promoting the expression of genes related to fat formation (39). SREBP is a transcription factor that positively regulates the expression of LDLR and cholesterol synthesis, with T3 mediating cholesterol synthesis through SREBP-1 and SREBP-2 (40, 41). These established mechanisms illustrate the close connection between THs sensitivity and dyslipidemia. Another study conducted on a cross-sectional basis found that the TFQI was linked to a higher prevalence of diabetes in both 2296 euthyroid adults in America and 8319 euthyroid adults in China (42). It has been noted that TSH can stimulate the secretion of leptin in human adipose tissue, which in turn reduces insulin secretion and synthesis in pancreatic β-cells. Therefore, the combined index TFQI, which takes into account both TSH and FT3, may offer a more accurate indication of the risk of developing diabetes compared to individual indices in individuals with normal TSH, FT3, and FT4 levels.
Furthermore, several studies have suggested that THs can directly and indirectly influence blood pressure. Insensitivity to THs can result in a decrease in the dilation of arterial smooth muscle, availability of nitric oxide, and endothelium-dependent vasodilation (43–45). A recent study also found a positive association between decreased central sensitivity to THs and visceral adipose tissue (46), which aligns with our findings. Disorders in THs sensitivity can disrupt the body’s metabolic balance, affecting the functioning of adipose tissue. While THs typically stimulate both brown and white adipose tissue, promoting thermogenesis and energy expenditure, impaired thyroid hormone sensitivity can lead to reduced activity in brown adipose tissue and impaired browning of white adipose tissue, resulting in decreased energy expenditure and a tendency for fat accumulation, particularly in the abdominal region (47, 48).
Previous studies examining the relationship between the FT3/FT4 ratio and MetS have produced conflicting results. A recent cross-sectional study found that lower FT3/FT4 levels were linked to MetS components,42 contradicting our findings. Greet et al. discovered a positive association between FT3, FT4, and the FT3/FT4 ratio with MetS components (49). Shon et al. observed a negative correlation between FT4 levels within the normal range and BMI (50). Roos et al. reported associations between low normal FT4 levels and increased insulin resistance and an unfavorable lipid profile in euthyroid adults (51). In a study involving 3,148 subjects, it was found that higher levels of FT4 were associated with higher levels of HDL cholesterol and lower levels of waist circumference, insulin resistance, and insulin (52). Additionally, previous studies have shown that higher levels of fT3 are linked to increased body fat mass and various metabolic parameters. A higher ratio of fT3 to fT4 has been associated with a less favorable metabolic profile and increased placental growth in pregnant women (53). Research has suggested that individuals with higher fat mass and/or less favorable metabolic profiles may have increased activity of deiodinase (DIO) 1 and/or 2, leading to higher conversion of fT4 to fT3 (54). This enhanced sensitivity to thyroid hormones can impact metabolic rate, glucose absorption and utilization, and lipid metabolism, potentially leading to fluctuations in blood sugar and lipid levels (55). It may also worsen insulin resistance, affect insulin signaling pathways, and increase cardiovascular activity, all of which are characteristics of metabolic syndrome. Essentially, an excessive response to normal thyroid hormone levels may increase the risk of developing metabolic abnormalities associated with MetS.
To further elaborate on the intricate relationship between thyroid function and metabolic regulation, it is essential to consider the role of circadian rhythms. Emerging evidence underscores the bidirectional interplay between thyroid hormones and circadian clocks (56). The hypothalamus-pituitary-thyroid axis demonstrates circadian oscillation, with thyroid hormone secretion orchestrated by both central and peripheral clocks. In turn, thyroid hormones modulate clock gene rhythmicity. Notably, triiodothyronine (T3) acts as a temporal cue for the central circadian clock by enhancing Bmal1 promoter activity, ensuring optimal metabolic regulation (57). Conversely, circadian disruptions, such as those in hypothyroidism, alter suprachiasmatic nucleus clock gene expression and impact metabolic parameters like oxygen consumption and body temperature, potentially contributing to metabolic syndrome (58). Importantly, aligning circadian rhythms through strategies like time-restricted feeding can restore metabolic health and enhance thyroid hormone signaling, underscoring the importance of circadian integrity in thyroid-metabolism axis regulation (59, 60). This interplay between circadian rhythms and thyroid function provides a more comprehensive framework for understanding metabolic regulation and suggests potential therapeutic avenues for addressing metabolic disorders.
The clinical importance of our study’s results lies in its potential to improve the understanding and management of MetS in euthyroid individuals. By identifying the association between thyroid hormone sensitivity and MetS risk, clinicians can consider evaluating thyroid function more comprehensively using indices like TFQI and FT3/FT4 ratio, rather than relying solely on traditional markers such as TSH, FT3, and FT4. This approach may allow for earlier identification of individuals at risk of MetS, enabling timely interventions to prevent or delay the onset of metabolic complications. Additionally, the findings highlight the need for further research into the therapeutic potential of modulating thyroid hormone sensitivity as a strategy for MetS management.
There were several limitations that should be acknowledged. Firstly, the cross-sectional design restricts the ability to establish a cause-and-effect relationship between thyroid hormone sensitivity indices and MetS. Additionally, the generalizability of the findings may be limited as the study was conducted in a Chinese population and may not be applicable to other ethnicities or regions. Lastly, the study did not consider all potential confounding factors, such as diet patterns or physical activity levels, that could impact the relationship between TH sensitivity and MetS.
Conclusion
In summary, this study presents evidence of a significant correlation between impaired thyroid hormone sensitivity and MetS, along with its severity, in euthyroid adults. The relationships with waist circumference, dyslipidemia, hyperglycemia, and hypertension emphasize the possible involvement of thyroid hormones in the pathogenesis of MetS. Future investigations should take into account thyroid hormone sensitivity indices when assessing MetS risk and further explore the mechanisms that drive these associations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by ethics committee of Health Examination Center of Wuxi people’s hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study is retrospectively designed.
Author contributions
XZ: Formal Analysis, Software, Validation, Writing – original draft. YZ: Funding acquisition, Resources, Writing – review & editing. ZL: Conceptualization, Methodology, Software, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the “The role and mechanism of Brix 1 in colorectal cancer” program (No. BJRC-9) from the city’s “double hundred” top plan.
Acknowledgments
We also thank all staff involved in this study for their painstaking efforts in conducting the data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
2. Ford ES, Li C, Zhao G, Pearson WS, and Mokdad AH. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes Care. (2008) 31:587–9. doi: 10.2337/dc07-1030
3. Castaneda G, Bhuket T, Liu B, and Wong RJ. Low serum high density lipoprotein is associated with the greatest risk of metabolic syndrome among U.S. adults. Diabetes Metab Syndr. (2018) 12:5–8. doi: 10.1016/j.dsx.2017.08.002
4. Li W, Song F, Wang X, Wang L, Wang D, Yin X, et al. Prevalence of metabolic syndrome among middle-aged and elderly adults in China: current status and temporal trends. Ann Med. (2018) 50:345–53. doi: 10.1080/07853890.2018.1464202
5. Sinha RA and Yen PM. Metabolic messengers: thyroid hormones. Nat Metab. (2024) 6:639–50. doi: 10.1038/s42255-024-00986-0
6. Gnocchi D, Steffensen KR, Bruscalupi G, and Parini P. Emerging role of thyroid hormone metabolites. Acta Physiol (Oxf). (2016) 217:184–216. doi: 10.1111/apha.12648
7. Mehran L, Amouzegar A, and Azizi F. Thyroid disease and the metabolic syndrome. Curr Opin Endocrinol Diabetes Obes. (2019) 26:256–65. doi: 10.1097/MED.0000000000000500
8. Iwen KA, Schroder E, and Brabant G. Thyroid hormones and the metabolic syndrome. Eur Thyroid J. (2013) 2:83–92. doi: 10.1159/000351249
9. Mullur R, Liu YY, and Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. (2014) 94:355–82. doi: 10.1152/physrev.00030.2013
10. Cheng SY, Leonard JL, and Davis PJ. Molecular aspects of thyroid hormone actions. Endocr. Rev. (2010) 31:139–70. doi: 10.1210/er.2009-0007
11. Ortiga-Carvalho TM, Sidhaye AR, and Wondisford FE. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol. (2014) 10:582–91. doi: 10.1038/nrendo.2014.143
12. Duntas LH and Brenta G. A renewed focus on the association between thyroid hormones and lipid metabolism. Front Endocrinol (Lausanne). (2018) 9:511. doi: 10.3389/fendo.2018.00511
13. Chang YC, Hua SC, Chang CH, Kao WY, Lee HL, Chuang LM, et al. High TSH level within normal range is associated with obesity, dyslipidemia, hypertension, inflammation, hypercoagulability, and the metabolic syndrome: A novel cardiometabolic marker. J Clin Med. (2019) 8(6):1–15. doi: 10.3390/jcm8060817
14. Ruhla S, Weickert MO, Arafat AM, Osterhoff M, Isken F, Spranger J, et al. A high normal TSH is associated with the metabolic syndrome. Clin Endocrinol (Oxf). (2010) 72:696–701. doi: 10.1111/j.1365-2265.2009.03698.x
15. Mehran L, Delbari N, Amouzegar A, Hasheminia Tohidi M, and Azizi MF. Reduced sensitivity to thyroid hormone is associated with diabetes and hypertension. J Clin Endocrinol Metab. (2022) 107:167–76. doi: 10.1210/clinem/dgab646
16. Kim BJ, Kim TY, Koh JM, Kim HK, Park JY, Lee KU, et al. Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clin Endocrinol (Oxf). (2009) 70:152–60. doi: 10.1111/j.1365-2265.2008.03304.x
17. Asvold BO, Bjoro T, and Vatten LJ. Association of serum TSH with high body mass differs between smokers and never-smokers. J Clin Endocrinol Metab. (2009) 94:5023–7. doi: 10.1210/jc.2009-1180
18. Nam JS, Cho M, Park JS, Ahn CW, Cha BS, Lee EJ, et al. Triiodothyronine level predicts visceral obesity and atherosclerosis in euthyroid, overweight and obese subjects: T3 and visceral obesity. Obes Res Clin Pract. (2010) 4:e247–342. doi: 10.1016/j.orcp.2010.08.003
19. Friedrich N, Rosskopf D, Brabant G, Volzke H, Nauck M, and Wallaschofski H. Associations of anthropometric parameters with serum TSH, prolactin, IGF-I, and testosterone levels: results of the study of health in Pomerania (SHIP). Exp Clin Endocrinol Diabetes. (2010) 118:266–73. doi: 10.1055/s-0029-1225616
20. Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, Mateo-Gallego R, Casasnovas JA, Guallar-Castillon P, et al. Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care. (2019) 42:303–10. doi: 10.2337/dc18-1410
21. Alkemade A. Central and peripheral effects of thyroid hormone signalling in the control of energy metabolism. J Neuroendocrinol. (2010) 22:56–63. doi: 10.1111/j.1365-2826.2009.01932.x
22. Bianco AC, Dumitrescu A, Gereben B, Ribeiro MO, Fonseca TL, Fernandes GW, et al. Paradigms of dynamic control of thyroid hormone signaling. Endocr Rev. (2019) 40:1000–47. doi: 10.1210/er.2018-00275
23. Yagi H, Pohlenz J, Hayashi Y, Sakurai A, and Refetoff S. Resistance to thyroid hormone caused by two mutant thyroid hormone receptors beta, R243Q and R243W, with marked impairment of function that cannot be explained by altered in vitro 3,5,3’-triiodothyroinine binding affinity. J Clin Endocrinol Metab. (1997) 82:1608–14. doi: 10.1210/jcem.82.5.3945
24. Jostel A, Ryder WD, and Shalet SM. The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH Index. Clin Endocrinol (Oxf). (2009) 71:529–34. doi: 10.1111/j.1365-2265.2009.03534.x
25. Liu B, Wang Z, Fu J, Guan H, Lyu Z, and Wang W. Sensitivity to thyroid hormones and risk of prediabetes: A cross-sectional study. Front Endocrinol (Lausanne). (2021) 12:657114. doi: 10.3389/fendo.2021.657114
26. Di Bonito P, Corica D, Marzuillo P, Di Sessa A, Licenziati MR, Faienza MF, et al. Sensitivity to thyroid hormones and reduced glomerular filtration in children and adolescents with overweight or obesity. Horm Res Paediatr. (2024) 97(4):383–7. doi: 10.1159/000534472
27. Sun Y, Teng D, Zhao L, Shi X, Li Y, Shan Z, et al. Impaired sensitivity to thyroid hormones is associated with hyperuricemia, obesity, and cardiovascular disease risk in subjects with subclinical hypothyroidism. Thyroid. (2022) 32:376–84. doi: 10.1089/thy.2021.0500
28. Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and treatment of diabetes in China, 2013-2018. JAMA. (2021) 326:2498–506. doi: 10.1001/jama.2021.22208
29. Wang JG, Zhang W, Li Y, and Liu L. Hypertension in China: epidemiology and treatment initiatives. Nat Rev Cardiol. (2023) 20:531–45. doi: 10.1038/s41569-022-00829-z
30. Bahar A, Kashi Z, Kheradmand M, Hedayatizadeh-Omran A, Moradinazar M, Ramezani F, et al. Prevalence of metabolic syndrome using international diabetes federation, National Cholesterol Education Panel- Adult Treatment Panel III and Iranian criteria: results of Tabari cohort study. J Diabetes Metab Disord. (2020) 19:205–11. doi: 10.1007/s40200-020-00492-6
31. de la Iglesia R, Lopez-Legarrea P, Abete I, Bondia-Pons I, Navas-Carretero S, Forga L, et al. A new dietary strategy for long-term treatment of the metabolic syndrome is compared with the American Heart Association (AHA) guidelines: the MEtabolic Syndrome REduction in NAvarra (RESMENA) project. Br J Nutr. (2014) 111:643–52. doi: 10.1017/S0007114513002778
32. Yang S, Yu B, Yu W, Dai S, Feng C, Shao Y, et al. Development and validation of an age-sex-ethnicity-specific metabolic syndrome score in the Chinese adults. Nat Commun. (2023) 14:6988. doi: 10.1038/s41467-023-42423-y
33. Mavromati M and Jornayvaz FR. Hypothyroidism-associated dyslipidemia: potential molecular mechanisms leading to NAFLD. Int J Mol Sci. (2021) 22(23):1–14. doi: 10.3390/ijms222312797
34. Koppeschaar HP, Meinders AE, and Schwarz F. Metabolic responses during modified fasting and refeeding. The role of sympathetic nervous system activity and thyroid hormones. Hum Nutr Clin Nutr. (1985) 39:17–28.
35. Liu Y, Ma M, Li L, Liu F, Li Z, Yu L, et al. Association between sensitivity to thyroid hormones and dyslipidemia in patients with coronary heart disease. Endocrine. (2023) 79:459–68. doi: 10.1007/s12020-022-03254-x
36. Sun H, Zhu W, Liu J, An Y, Wang Y, and Wang G. Reduced sensitivity to thyroid hormones is associated with high remnant cholesterol levels in chinese euthyroid adults. J Clin Endocrinol Metab. (2022) 108:166–74. doi: 10.1210/clinem/dgac523
37. Yuan C, Sun X, Liu Y, and Wu J. The thyroid hormone levels and glucose and lipid metabolism in children with type 1 diabetes: a correlation analysis. Transl Pediatr. (2021) 10:276–82. doi: 10.21037/tp-20-204
38. Lei Y, Yang J, Li H, Zhong H, and Wan Q. Changes in glucose-lipid metabolism, insulin resistance, and inflammatory factors in patients with autoimmune thyroid disease. J Clin Lab Anal. (2019) 33:e22929. doi: 10.1002/jcla.22929
39. Delitala AP, Delitala G, Sioni P, and Fanciulli G. Thyroid hormone analogs for the treatment of dyslipidemia: past, present, and future. Curr Med Res Opin. (2017) 33:1985–93. doi: 10.1080/03007995.2017.1330259
40. Cho S, Lee HJ, Shim JS, Song BM, and Kim HC. Associations between age and dyslipidemia are differed by education level: The Cardiovascular and Metabolic Diseases Etiology Research Center (CMERC) cohort. Lipids Health Dis. (2020) 19:12. doi: 10.1186/s12944-020-1189-y
41. Tognini S, Polini A, Pasqualetti G, Ursino S, Caraccio N, Ferdeghini M, et al. Age and gender substantially influence the relationship between thyroid status and the lipoprotein profile: results from a large cross-sectional study. Thyroid. (2012) 22:1096–103. doi: 10.1089/thy.2012.0013
42. Wan H, Yu G, He Y, Liu S, Chen X, Jiang Y, et al. Associations of thyroid feedback quantile-based index with diabetes in euthyroid adults in the United States and China. Ann Med. (2024) 56:2318418. doi: 10.1080/07853890.2024.2318418
43. Fernandez-Real JM, Lopez-Bermejo A, Castro A, Casamitjana R, and Ricart W. Thyroid function is intrinsically linked to insulin sensitivity and endothelium-dependent vasodilation in healthy euthyroid subjects. J Clin Endocrinol Metab. (2006) 91:3337–43. doi: 10.1210/jc.2006-0841
44. Gu Y, Zheng L, Zhang Q, Liu L, Meng G, Yao Z, et al. Relationship between thyroid function and elevated blood pressure in euthyroid adults. J Clin Hypertens (Greenwich). (2018) 20:1541–9. doi: 10.1111/jch.13369
45. Mehran L, Delbari N, Amouzegar A, Hasheminia M, Tohidi M, and Azizi F. Reduced sensitivity to thyroid hormone is associated with diabetes and hypertension. J Clin Endocrinol Metab. (2022) 107:167–76. doi: 10.1210/clinem/dgab646
46. Lv F, Cai X, Li Y, Zhang X, Zhou X, Han X, et al. Sensitivity to thyroid hormone and risk of components of metabolic syndrome in a Chinese euthyroid population. J Diabetes. (2023) 15:900–10. doi: 10.1111/1753-0407.13441
47. Petito G, Cioffi F, Magnacca N, de Lange P, Senese R, and Lanni A. Adipose tissue remodeling in obesity: an overview of the actions of thyroid hormones and their derivatives. Pharmaceuticals (Basel). (2023) 16(4):1–17. doi: 10.3390/ph16040572
48. Volke L and Krause K. Effect of thyroid hormones on adipose tissue flexibility. Eur Thyroid J. (2021) 10:1–9. doi: 10.1159/000508483
49. Roef GL, Rietzschel ER, Van Daele CM, Taes YE, De Buyzere ML, Gillebert TC, et al. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid. (2014) 24:223–31. doi: 10.1089/thy.2013.0314
50. Shon HS, Jung ED, Kim SH, and Lee JH. Free T4 is negatively correlated with body mass index in euthyroid women. Korean J Intern Med. (2008) 23:53–7. doi: 10.3904/kjim.2008.23.2.53
51. Roos A, Bakker SJ, Links TP, Gans RO, and Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. (2007) 92:491–6. doi: 10.1210/jc.2006-1718
52. Garduno-Garcia J, Alvirde-Garcia U, Lopez-Carrasco G, Padilla MM, Mehta R, Arellano-Campos O, et al. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol. (2010) 163:273–8. doi: 10.1530/EJE-10-0312
53. Bassols J, Prats-Puig A, Soriano-Rodriguez P, Garcia-Gonzalez MM, Reid J, Martinez-Pascual M, et al. Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J Clin Endocrinol Metab. (2011) 96:3717–23. doi: 10.1210/jc.2011-1784
54. Yang L, Sun X, Tao H, and Zhao Y. The association between thyroid homeostasis parameters and obesity in subjects with euthyroidism. J Physiol Pharmacol. (2023) 74(1):69–75. doi: 10.26402/jpp.2023.1.07
55. Corica D, Licenziati MR, Calcaterra V, Curro M, Di Mento C, Curatola S, et al. Central and peripheral sensitivity to thyroid hormones and glucose metabolism in prepubertal children with obesity: pilot multicenter evaluation. Endocrine. (2023) 80:308–11. doi: 10.1007/s12020-022-03276-5
56. Gnocchi D and Bruscalupi G. Circadian rhythms and hormonal homeostasis: pathophysiological implications. Biol (Basel). (2017) 6(1):1–20. doi: 10.3390/biology6010010
57. Gnocchi D, Custodero C, Sabba C, and Mazzocca A. Circadian rhythms: a possible new player in non-alcoholic fatty liver disease pathophysiology. J Mol Med (Berl). (2019) 97:741–59. doi: 10.1007/s00109-019-01780-2
58. Emrich F, Gomes BH, Selvatici-Tolentino L, Lopes RA, Secio-Silva A, Carvalho-Moreira JP, et al. Hypothyroidism alters the rhythmicity of the central clock, body temperature and metabolism: evidence of Bmal1 transcriptional regulation by T3. J Physiol. (2024) 602:4865–87. doi: 10.1113/JP286449
59. Helbling JC, Ginieis R, Mortessagne P, Ruiz-Gayo M, Bakoyiannis I, Ducourneau EG, et al. Time-restricted feeding prevents memory impairments induced by obesogenic diet consumption, via hippocampal thyroid hormone signaling. Mol Metab. (2024) 90:102061. doi: 10.1016/j.molmet.2024.102061
Keywords: thyroid hormones sensitivity, metabolic syndrome, severity, euthyroid, Chinese adults
Citation: Zhou X, Zhang Y and Li Z (2025) Impaired sensitivity to thyroid hormones is positively associated to metabolic syndrome severity in euthyroid Chinese adults as revealed by a cross-sectional study. Front. Endocrinol. 16:1552484. doi: 10.3389/fendo.2025.1552484
Received: 28 December 2024; Accepted: 22 April 2025;
Published: 15 May 2025.
Edited by:
Matthias Blüher, Leipzig University, GermanyReviewed by:
Anthony Martin Gerdes, New York Institute of Technology, United StatesDavide Gnocchi, University of Bari Medical School, Italy
Mostafa Vaghari-Tabari, Tabriz University of Medical Sciences, Iran
Copyright © 2025 Zhou, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zengyao Li, d3hsenkyM0AxNjMuY29t; Ye Zhang, emhhbmd5ZWtrd19jb2xvckAxMjYuY29t
 Xiong Zhou
Xiong Zhou Zengyao Li
Zengyao Li