- 1Department of Endocrinology, Taizhou Central Hospital (Taizhou University Hospital), Taizhou, China
- 2Department of Internal Medicine, Yuhuan Second People’s Hospital, Yuhuan, China
Background: Early-onset type 2 diabetes mellitus (T2DM) is closely associated with an increased risk of diabetic kidney disease (DKD), and obesity is a well-recognized contributing factor. Traditional measures like body mass index (BMI) have limitations in capturing visceral fat distribution. The A Body Shape Index (ABSI), a newer anthropometric indicator, may provide a more accurate assessment of central obesity. This study investigated the relationship between ABSI and DKD in individuals with early-onset T2DM.
Methods: This cross-sectional study analyzed data from 2,598 patients with early-onset T2DM enrolled at the National Metabolic Management Centers of Yuhuan Second People’s Hospital and Taizhou Central Hospital between 2017 and 2024. Multivariate logistic regression models were used to evaluate the association between ABSI and DKD, with additional analyses using restricted cubic splines (RCS) to explore dose-response patterns. Subgroup and interaction analyses were also performed.
Results: Of the participants, 1,030 (39.6%) were diagnosed with DKD. The prevalence of DKD increased across ABSI tertiles: 35.8% in the lowest tertile (T1), 38.5% in the middle tertile (T2), and 44.7% in the highest tertile (T3) (P < 0.001). Higher ABSI was independently associated with a greater risk of DKD (OR = 1.24; 95% CI: 1.05–1.50; P = 0.022) after adjusting for potential confounders. Patients in the highest ABSI tertile had a significantly higher risk of DKD than those in the lowest tertile (OR = 1.24; 95% CI: 1.01–1.52; P = 0.041). RCS analysis showed a positive linear relationship between ABSI and DKD risk (P for non-linearity = 0.139), and the findings were consistent across subgroups.
Conclusion: ABSI is positively and linearly associated with the risk of DKD in patients with early-onset T2DM. This metric may be useful for identifying individuals at higher risk and guiding early preventive strategies.
1 Introduction
Type 2 diabetes mellitus (T2DM) has traditionally been considered a condition affecting middle-aged and older adults (1). However, its incidence among younger populations has risen sharply in recent years (1). Early-onset T2DM, typically defined as a diagnosis before the age of 40, is associated with more severe clinical outcomes compared to late-onset T2DM (2). Individuals with early-onset T2DM face an elevated risk of cardiovascular disease (3), earlier mortality (4), and a faster progression of microvascular complications (5), including diabetic kidney disease (DKD) (6). The burden of DKD is particularly concerning in younger patients. Studies have shown that the cumulative incidence of end-stage kidney disease (ESKD) in T2DM is significantly higher in those diagnosed at younger ages, reaching 11.8% for diagnoses before age 30 and 9.3% for those diagnosed between ages 30 and 39 (6). These findings highlight the urgent need to understand better the risk factors contributing to DKD in early-onset T2DM and to improve risk stratification and prevention strategies in this vulnerable population.
Obesity is a well-established risk factor for DKD (7, 8), and body mass index (BMI) remains the most commonly used measure for assessing obesity (9). However, BMI has several limitations (10). It does not reflect fat distribution and cannot distinguish between fat and lean mass. To address these shortcomings, Krakauer and colleagues proposed the A Body Shape Index (ABSI), which incorporates waist circumference, height, and weight to better estimate central obesity (11). ABSI has been shown to capture visceral fat more accurately and has demonstrated associations with various metabolic disorders, including cardiovascular disease (12, 13), non-alcoholic fatty liver disease (14), metabolic syndrome (15), and diabetes (16). Recent studies have also reported significant associations between ABSI and kidney function impairment (17–19). However, data examining the relationship between ABSI and DKD in individuals with early-onset T2DM remains scarce.
To fill this gap, the present study analyzed data from 2,598 Chinese adults with early-onset T2DM to investigate the potential association between ABSI and the risk of developing DKD.
2 Materials and methods
2.1 Study design and participants
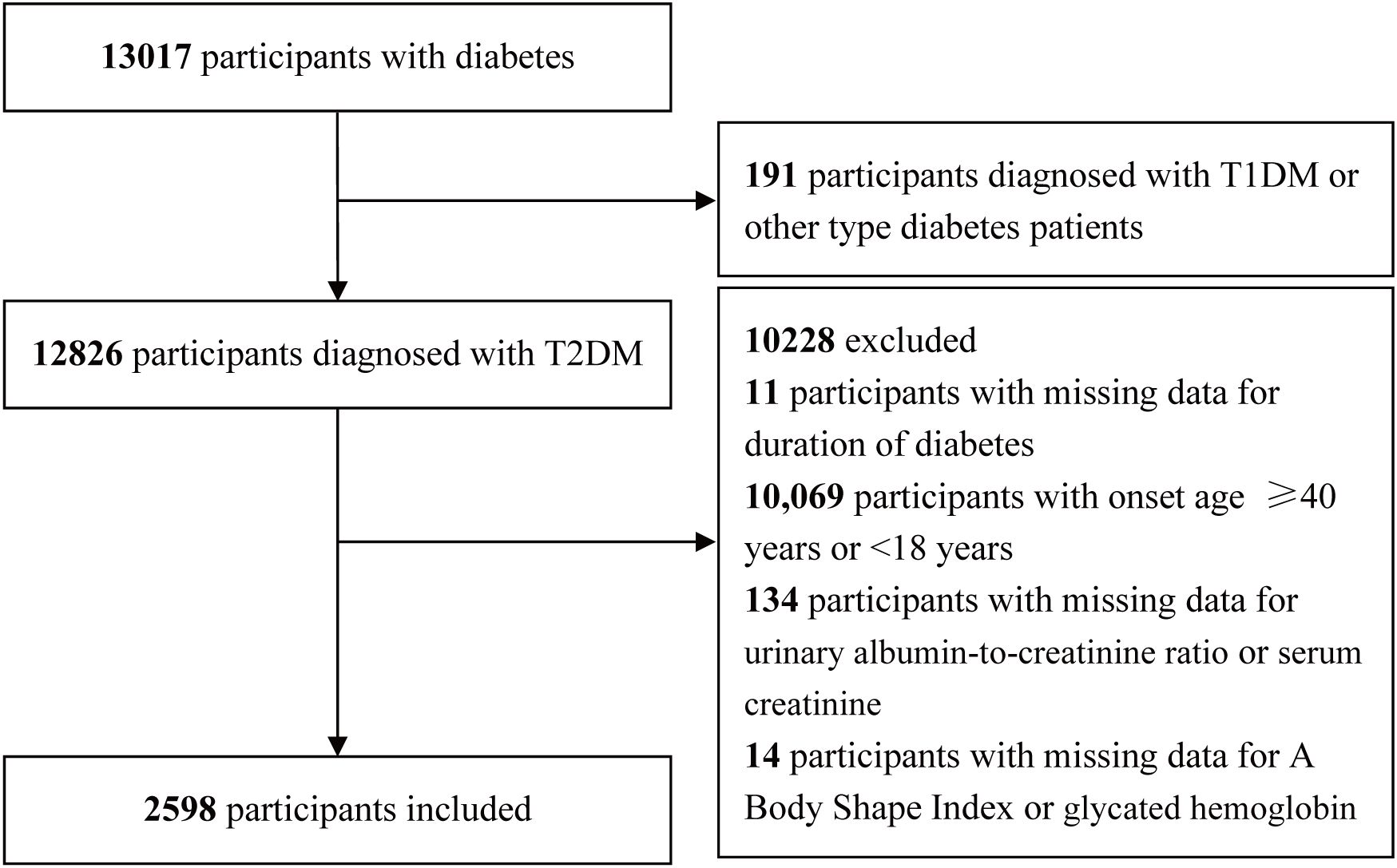
This cross-sectional analysis made use of information from the National Metabolic Management Center (MMC) collected between September 2017 and May 2024. A comprehensive description of the MMC program can be found in previous publications (20–22). The Clinical Research Ethics Committees of Yuhuan Second People’s Hospital and Taizhou Central Hospital (Taizhou University Hospital) approved the study protocol, and written informed consent was obtained from all participants in a manner consistent with the Declaration of Helsinki. A comprehensive overview of the inclusion and exclusion criteria is presented in Figure 1. In total, 13,017 participants with diabetes were initially included. During the screening process, 191 participants diagnosed with other types of diabetes, such as type 1 diabetes mellitus (T1DM), 11 participants with missing data on diabetes duration, 10,069 participants with an onset age of diabetes ≥40 years or <18 years, 134 participants with missing data on the urinary albumin-to-creatinine ratio (UACR) or serum creatinine (Scr), 14 participants with missing data on anthropometric and biochemical parameters, including the ABSI or glycated hemoglobin (HbA1c), were excluded. A final cohort of 2,598 participants was retained.
2.2 Data collection and analysis
Patient data were collected with an independent digital medical record system detailed in our prior study (20–22). Collected data included age, sex, education level, smoking and drinking status, diabetes duration, family history of diabetes, hypertension, hyperlipidemia, coronary heart disease, stroke, blood pressure (diastolic and systolic), anthropometric measurements (weight, height, waist circumference), and laboratory values including fasting blood glucose (FBG), fasting serum C peptide (FCp), HbA1c, triglycerides (TG), total cholesterol (TC), low- and high-density lipoprotein cholesterol (LDL-c and HDL-c), urea nitrogen (UN), Scr, uric acid (UA), and UACR. Homeostasis model assessment of insulin resistance (HOMA-IR) calculations were performed with the following formula: 1.5 + (FCp [pmol/L] × FBG[mmol/L])/2800 (23). Estimated glomerular filtration rate (eGFR) calculations made use of the Chronic Kidney Disease Epidemiology Collaboration equation (24). BMI was measured as weight (kg) divided by height squared (m²). DKD was identified based on a UACR ≥30 mg/g and/or eGFR <60 mL/min per 1.73 m2 (25). The ABSI was calculated using the formula (11): WC(mm)*Weight(kg)-2/3*Height(m)5/6.
2.3 Statistical analyses
Continuous data are shown as mean ± standard deviation or median (minimum, maximum) when normally distributed and skewed, respectively. Categorical data are given as frequencies or percentages. These three respective data types were compared with t-tests, Mann-Whitney U tests, and chi-square tests. To evaluate the association between ABSI and diabetic kidney disease (DKD), logistic regression models were utilized to assess odds ratios (OR) with corresponding 95% (CIs). ABSI tertiles were used to establish three participant groups, with the first tertile serving as the reference group for all odds ratio calculations. Additionally, the trend in odds ratios across ABSI tertiles was assessed by treating ABSI as an ordinal variable. Four models were utilized to assess the relationship between ABSI and DKD: The crude model did not adjust for any variables, Model 1 involved adjustments for age and sex, while Model 2 included additional adjustments for diabetes duration, HbA1c, and smoking/drinking status, and Model 3 was additionally adjusted for hyperlipidemia, coronary heart disease, hypertension, and stroke. The nonlinearity of the association between ABSI and DKD was explored via restricted cubic spline (RCS) regression using knots at the 5th, 35th, 65th, and 95th ABSI percentiles. This analysis assessed both the linearity of the relationship and the dose-response pattern following adjustment for variables in Model 3. Interaction and stratified analyses were conducted according to sex (male or female), age (<40y or ≥40), education (below high school or high school and above), HbA1c (<7.5% or ≥7.5%), because levels above 7.5% indicating poor glycemic control in diabetic patients (26, 27), hypertension (yes or no), and hyperlipidemia (yes or no). Stratified logistic regression was utilized for subgroup analyses, with the likelihood ratio test being used to detect interactions among these subgroups. R 4.2.2 (http://www.R-project.org, The R Foundation) and Free Statistics software version 2.0 were used for all analyses, with a two-sided P < 0.05 being considered significant.
3 Results
3.1 Characteristics of participants
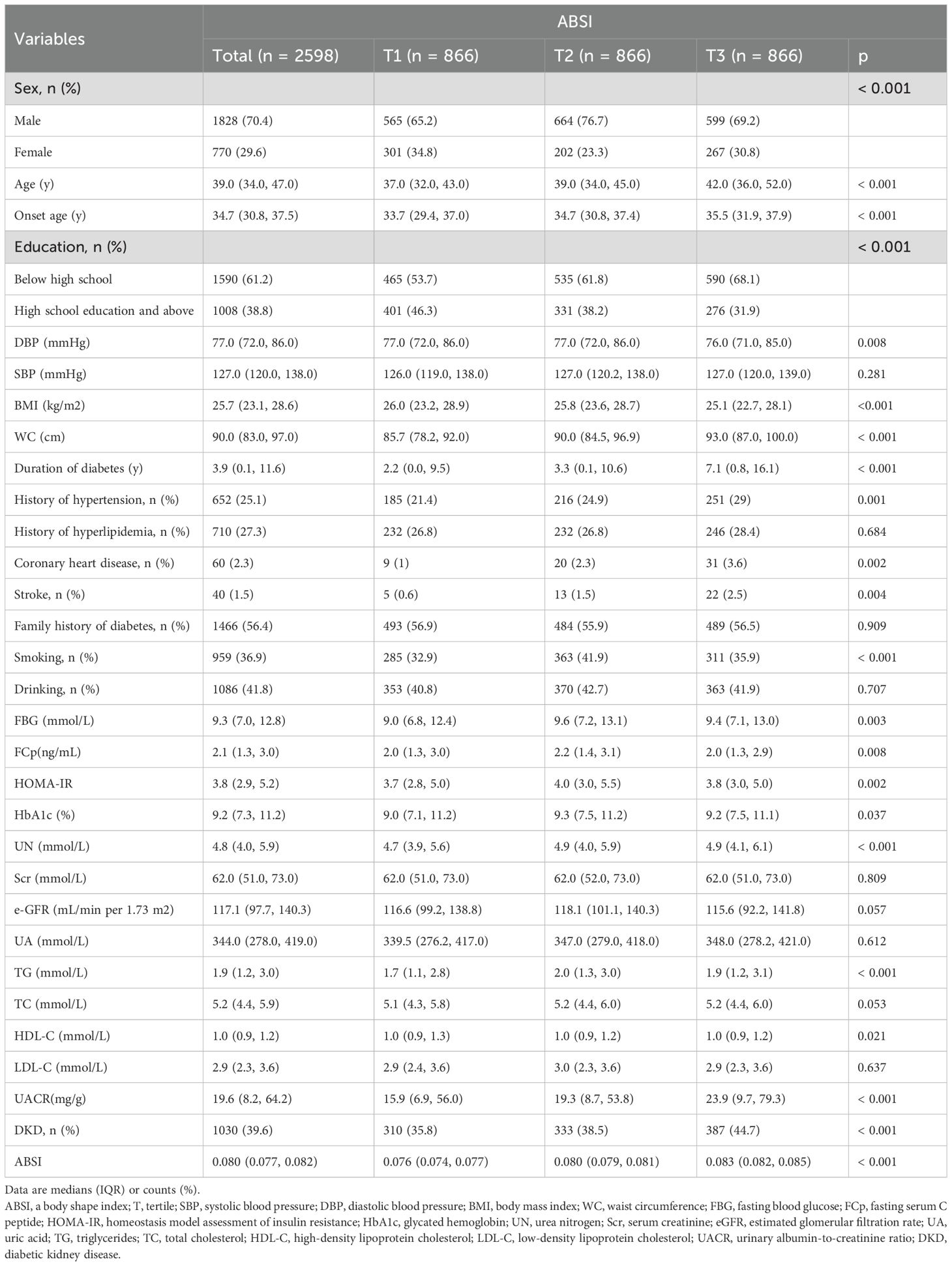
Overall, 2,598 participants were analyzed based on ABSI tertiles. Demographic, clinical, and metabolic factors differed significantly across the tertiles (Table 1). Males constituted the majority of the cohort (70.4%), with the proportion of males significantly different across tertiles (p < 0.001). Those in the highest tertile (T3) were significantly older relative to those in the first tertile (T1) (p < 0.001). In terms of anthropometric measures, WC increased across tertiles, while the opposite was observed for BMI (p < 0.001). Participants in the T3 group exhibited poorer glycemic control and higher insulin resistance, reflected by elevated FBG (p = 0.003), HbA1c (p = 0.037), and HOMA-IR (p = 0.002). UACR was also significantly greater in T3 relative to T1 (p < 0.001). DKD prevalence increased significantly from 35.8% in T1 to 44.7% in T3 (p < 0.001).
3.2 Association between ABSI and DKD in early-onset T2DM
RCS analyses revealed that ABSI was linearly related to OR of DKD (P for non-linearity = 0.139). The odds of DKD increased significantly with higher ABSI values (Figure 2).
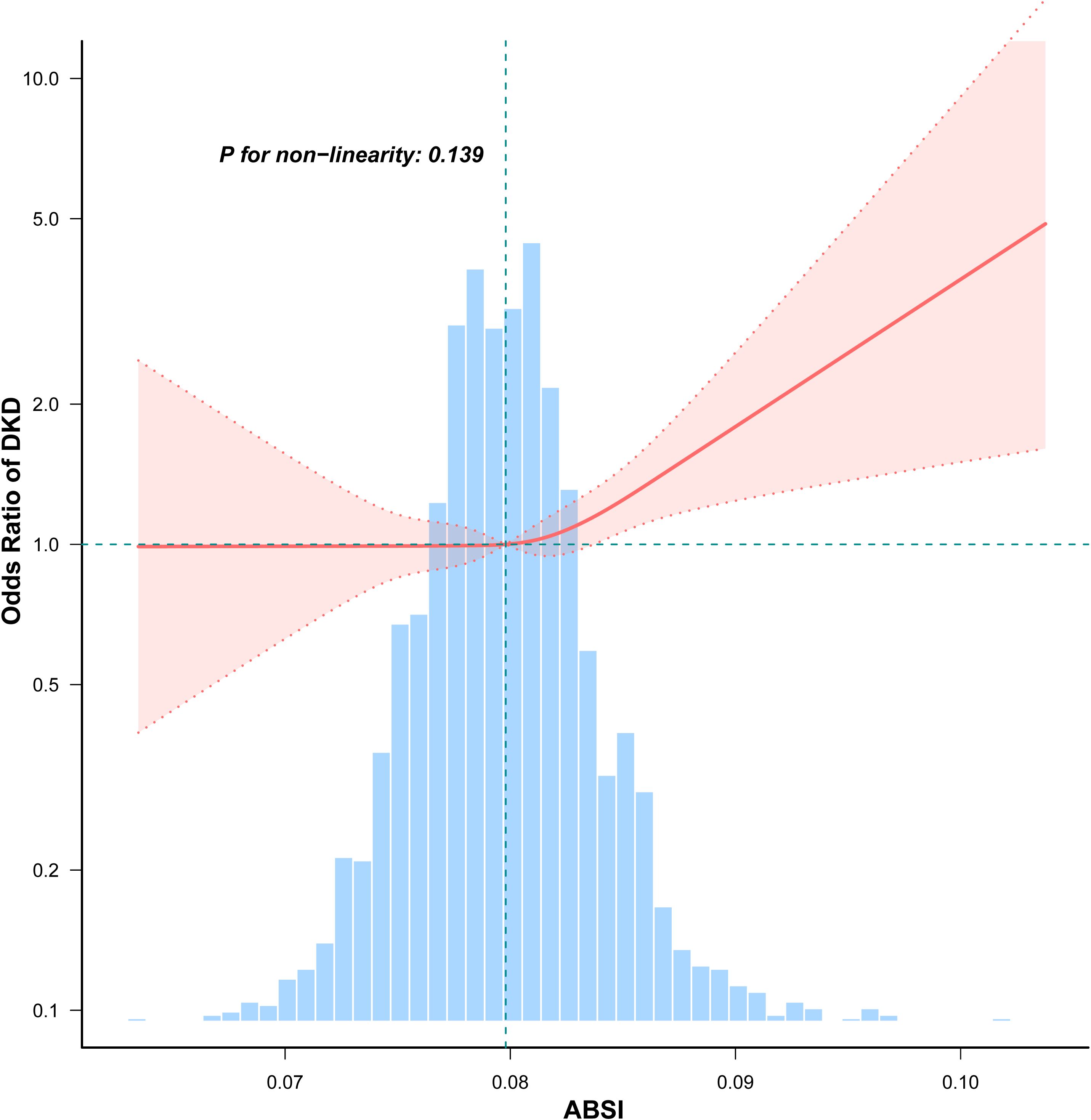
Figure 2. Non-linear association between ABSI and DKD in participants with early-onset T2DM. Adjusted for age, sex, duration of diabetes, HbA1c, drinking, smoking, hypertension, hyperlipidemia, coronary heart disease, and stroke, unless those were the variables used for stratification. The red solid line represents the fitted odds ratio estimated using restricted cubic spline regression. The shaded red area indicates the 95% CI. The histogram below illustrates the distribution of ABSI values among participants.
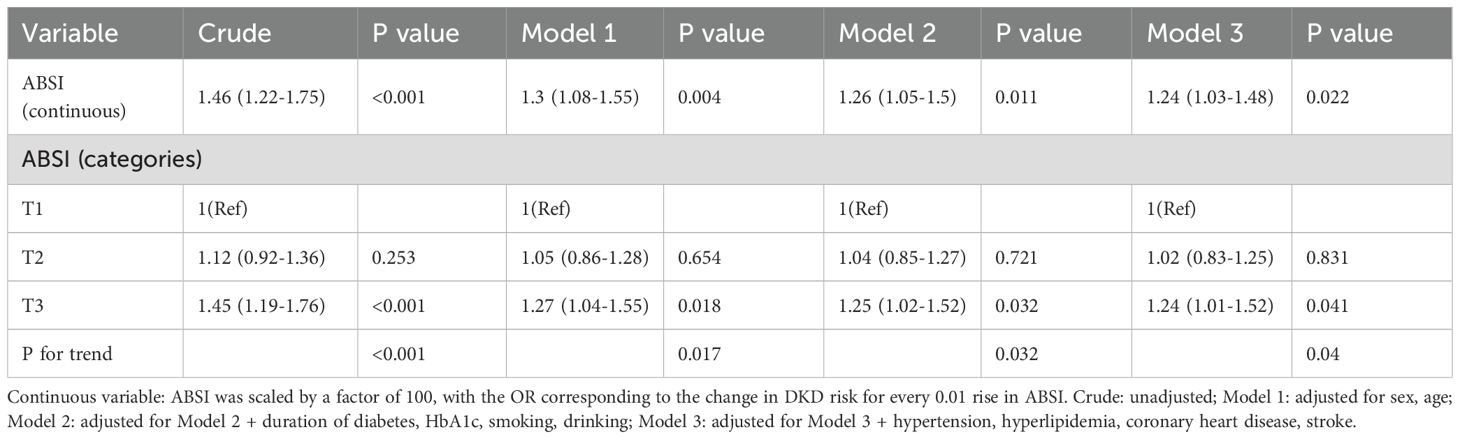
The association between ABSI and DKD was examined in a series of models adjusted for various factors (Table 2). When ABSI was analyzed as continuous variable, in the crude model, a 0.01-unit rise in ABSI was associated with a 46% rise in DKD risk(OR = 1.46, 95% CI: 1.22-1.75, P < 0.001). The significance of this relationship was retained after adjustments for basic demographic factors such as sex and age (Model 1: OR = 1.30, 95% CI: 1.08-1.55, P = 0.004). When adjusting for diabetes duration, HbA1c, smoking, and drinking (Model 2), the OR for DKD decreased slightly (OR = 1.26, 95% CI: 1.05-1.50, P = 0.011). When the model underwent full adjustment (Model 3) to account for comorbidities such as hypertension, hyperlipidemia, coronary heart disease, and stroke, this relationship remained significant (OR = 1.24, 95% CI: 1.03-1.48, P = 0.022). Further analysis by ABSI tertiles reinforced this finding. Cases in the top ABSI tertile (T3) had a 24% greater risk of DKD relative to cases in T1 under Model 3 (OR = 1.24, 95% CI: 1.01-1.52, P = 0.041). The trend across tertiles was significant (P for trend = 0.004).
Subgroup analyses were also undertaken to evaluate the relationship between ABSI and DKD in various subgroups, including sex, age, education, HbA1c, hypertension, and hyperlipidemia (Figure 3). The analyses revealed that higher ABSI values were consistently linked with elevated DKD risk across all subgroups. No significant interactions were found in any subgroups.
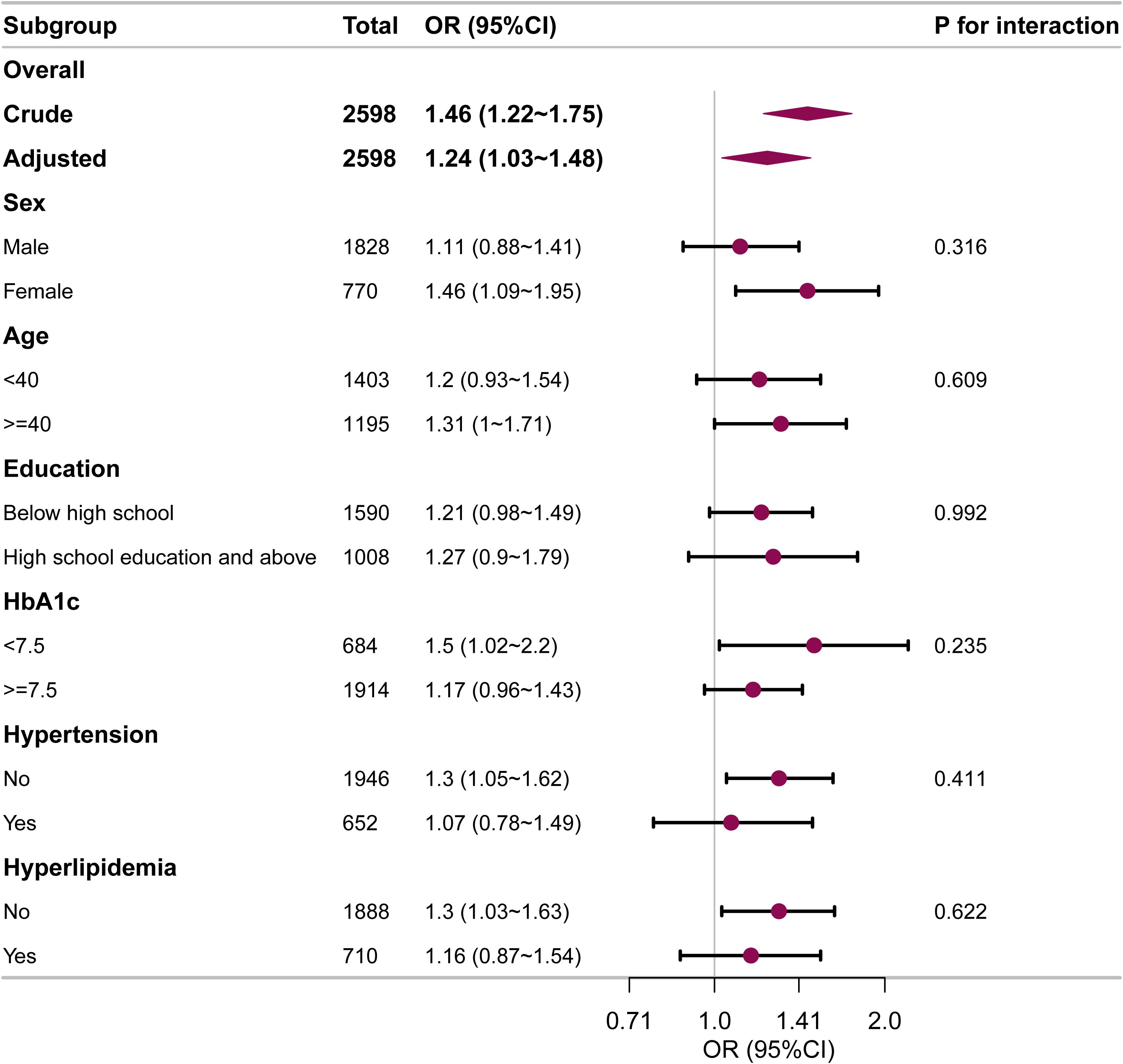
Figure 3. Subgroup and interaction analyses for the relationship between ABSI and DKD in early-onset T2DM. Adjustments were made for age, sex, diabetes duration, HbA1c, drinking, smoking, hypertension, hyperlipidemia, coronary heart disease, and stroke, unless those were the variables used for stratification. OR, odds ratio; CI, confidence interval.
4 Discussion
In this study, we conducted a multivariable logistic regression analysis to assess the association between ABSI values and the risk of DKD in patients with early-onset T2DM, while controlling for potential confounding factors. The results of the logistic regression indicated that higher ABSI values were significantly associated with an increased risk of DKD. RCS curves revealed that ABSI and DKD were positively associated with one another in a linear relationship, and these findings remained robust in subgroup analyses.
Compared to BMI, ABSI is considered a more accurate predictor of health risks associated with abdominal obesity because it more effectively captures the distribution pattern of visceral fat. In recent years, research has found a significant relationship between ABSI and kidney disease. Kim et al. (28) showed that ABSI was more strongly related to CKD than BMI was. ABSI is better at distinguishing different stages of CKD than BMI. ABSI also has a higher predictive ability for moderate to severe CKD compared to BMI. Sun et al. (17) determined that ABSI was positively correlated with UACR. Su et al. (29) determined that high ABSI was related to rapid renal functional decline. Zhang et al. (19) found that following adjustment for confounding factors, ABSI quartiles were positively related to elevated UACR (OR [95% CI] Q4 vs. Q1: 1.183 [1.080, 1.295], p for trend < 0.001). Our study focuses on the relationship between ABSI and DKD, and a positive correlation was also identified. A Mendelian randomization study (18) showed that a rise of one standard deviation in the value of genetically predicted ABSI led to increased UACR in women (β = 0.039 [95% CI: 0.016, 0.063] log [UACR], P = 0.001 for ABSI), but not in men. Yu et al. (30) also found that in women, ABSI values in those with CKD were markedly elevated relative to non-CKD cases, whereas the same was not true for men. Stratified analysis further revealed that as ABSI increased, elevated UACR was more likely to occur in populations characterized by older age, male gender, and hypertension. However, the study by Cosoreanu et al. (31) found that among White patients, ABSI was related to an extremely high risk of CKD progression, while no such association was found in Romani patients. The subgroup analyses in this study demonstrated stable results, with no significant interaction effects detected across the subgroups.
CKD is more common and progresses faster in obese diabetic patients compared to those with normal weight (32). This is a primary factor explaining the higher cumulative CKD incidence in T2DM patients as compared to those with T1DM (33). Obesity has a negative effect on risk factors for CKD, such as glycemic control, hypertension, and lipid levels, contributing to insulin resistance. It can also directly impact the kidneys, altering glomerular hemodynamics, increasing sympathetic nervous activity, and contributing to altered growth factor expression, systemic inflammatory activity, endothelial dysfunction, hypertension, and visceral fat-related kidney compression (34). Even without comorbid diabetes, obesity has been linked to greater proteinuria frequency and severity, with many reports of obesity-related glomerular lesions to date (35).
Our study revealed that ABSI and DKD are positively related to one another in patients with early-onset T2DM, suggesting that ABSI may be an important biomarker for screening the risk of developing DKD. Our study included a large participant population, and our data analysis strategy was comprehensive and methodologically rigorous. We employed multivariable regression with adjustment for potential confounding factors, ensuring that the observed link between ABSI and DKD was not influenced by other variables. Additionally, the use of generalized additive models and smooth curve fitting enabled explorations of potential nonlinear relationships between ABSI and DKD, providing a more nuanced understanding of this association. The robustness of the subgroup analysis further supports the stability of our results. Overall, the combination of a large, well-defined cohort and complex statistical techniques strengthens the credibility of our study and its potential implications for clinical practice. Given the increasing prevalence of T2DM and DKD in younger populations, our findings provide unique insights into the relationship between ABSI and DKD, filling a gap in the current literature, as there is limited research on anthropometric indicators as predictors of early-onset T2DM-related DKD. Future studies can further explore the mechanisms behind the ABSI-DKD relationship, particularly through longitudinal studies, to better understand its predictive value and how it can be integrated into broader public health strategies to reduce the burden of diabetes-related complications.
This study has limitations. First, the study involved only two centers, potentially limiting the generalizability of the findings. Therefore, future studies should be conducted in a broader multi-center setting to validate the generalizability and applicability of our findings. Second, as an observational study, while we made efforts to control for measurable confounding variables in the data analysis, the potential impact of unmeasured or unrecorded confounders cannot be excluded. For instance, in the absence of genetic testing, we cannot entirely rule out the possibility of maturity-onset diabetes of the young (MODY) in the participants. Third, the study analyzed a Chinese population, and extending the findings to any other ethnicities warrants caution. Differences in metabolism, genetics, and lifestyle factors across populations could potentially affect the relationship between ABSI and DKD. Finally, since this study is cross-sectional, the results should be interpreted cautiously as causal inferences cannot be drawn. Additional prospective studies are crucial to evaluate the predictive ability of ABSI for DKD risk.
5 Conclusions
In summary, ABSI and DKD were herein found to be significantly and positively linearly related to one another early-onset type 2 diabetes. ABSI may offer utility for establishing which individuals face a high risk of DKD, enabling earlier interventions. However, further prospective studies will be necessary to confirm causality and explore the long-term clinical impact.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Clinical Research Ethics Committees of Yuhuan Second People’s Hospital and Taizhou Central Hospital (Taizhou University Hospital). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MC: Funding acquisition, Writing – original draft, Writing – review & editing. YW: Writing – review & editing. PF: Writing – review & editing. LW: Writing – review & editing. HL: Writing – review & editing. YL: Writing – review & editing. MY: Writing – review & editing. QZ: Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study is supported by grants from the Science and Technology Plan Project of Taizhou (No. 24ywb44), Science and Technology Plan Project of Yuhuan (202349), Chen Xiao-ping Foundation for the Development of Science and Techonology of Hubei Province(CXPJJH122012-011). Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0508100).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Misra S, Ke C, Srinivasan S, Goyal A, Nyriyenda MJ, Florez JC, et al. Current insights and emerging trends in early-onset type 2 diabetes. Lancet Diabetes Endocrinol. (2023) 11:768–82. doi: 10.1016/S2213-8587(23)00225-5
2. Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, and Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. (2018) 6:69–80. doi: 10.1016/S2213-8587(17)30186-9
3. Zhao M, Song L, Sun L, Wang M, Wang C, Yao S, et al. Associations of type 2 diabetes onset age with cardiovascular disease and mortality: the kailuan study. Diabetes Care. (2021) 44:1426–32. doi: 10.2337/dc20-2375
4. Sattar N, Rawshani A, Franzén S, Rawshani A, Svensson AM, Rosengren A, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation. (2019) 139:2228–37. doi: 10.1161/CIRCULATIONAHA.118.037885
5. Rodriquez IM and O’Sullivan KL. Youth-onset type 2 diabetes: burden of complications and socioeconomic cost. Curr Diabetes Rep. (2023) 23:59–67. doi: 10.1007/s11892-023-01501-7
6. Morton JI, Liew D, McDonald SP, Shaw JE, and Magliano DJ. The association between age of onset of type 2 diabetes and the long-term risk of end-stage kidney disease: A national registry study. Diabetes Care. (2020) 43:1788–95. doi: 10.2337/dc20-0352
7. Yun C, Tang F, and Lou Q. Construction of risk prediction model of type 2 diabetic kidney disease based on deep learning (Diabetes metab J 2024;48:771-9). Diabetes Metab J. (2024) 48:1008–11. doi: 10.4093/dmj.2024.0490
8. Yamada K, Ohsugi M, Ito Y, Uchida H, Lee T, Ueki K, et al. Retrospective database study on risk factors for diabetic retinopathy and diabetic kidney disease in Japanese patients with diabetes mellitus. J Diabetes Investig. (2024) 16:120–8. doi: 10.1111/jdi.14341
9. Man REK, Gan ATL, Fenwick EK, Gupta P, Wong MYZ, Wong TY, et al. The relationship between generalized and abdominal obesity with diabetic kidney disease in type 2 diabetes: A multiethnic asian study and meta-analysis. Nutrients. (2018) 10:1685. doi: 10.3390/nu10111685
10. Nakanishi S, Hirukawa H, Shimoda M, Tatsumi F, Kohara K, Obata A, et al. Comparison of HbA1c levels and body mass index for prevention of diabetic kidney disease: A retrospective longitudinal study using outpatient clinical data in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. (2019) 155:107807. doi: 10.1016/j.diabres.2019.107807
11. Krakauer NY and Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. (2012) 7:e39504. doi: 10.1371/journal.pone.0039504
12. Kajikawa M, Maruhashi T, Kishimoto S, Yamaji T, Harada T, Saito Y, et al. A body shape index as a simple anthropometric marker of abdominal obesity and risk of cardiovascular events. J Clin Endocrinol Metab. (2024) 109:3272–81. doi: 10.1210/clinem/dgae282
13. Ikeue K, Kusakabe T, Yamakage H, Ishii K, and Satoh-Asahara N. A body shape index is useful for BMI-independently identifying Japanese patients with obesity at high risk of cardiovascular disease. Nutr Metab Cardiovasc Dis. (2024) 34:387–94. doi: 10.1016/j.numecd.2023.09.008
14. Kuang M, Sheng G, Hu C, Lu S, Peng N, and Zou Y. The value of combining the simple anthropometric obesity parameters, Body Mass Index (BMI) and a Body Shape Index (ABSI), to assess the risk of non-alcoholic fatty liver disease. Lipids Health Dis. (2022) 21:104. doi: 10.1186/s12944-022-01717-8
15. Sugiura T, Dohi Y, Takagi Y, Yokochi T, Yoshikane N, Suzuki K, et al. A body shape index could serve to identify individuals with metabolic syndrome and increased arterial stiffness in the middle-aged population. Clin Nutr ESPEN. (2021) 46:251–8. doi: 10.1016/j.clnesp.2021.10.001
16. Zhao W, Tong JJ, Cao YT, and Li JH. A linear relationship between a body shape index and risk of incident type 2 diabetes: A secondary analysis based on a retrospective cohort study in Japan. Diabetes Metab Syndr Obes. (2020) 13:2139–46. doi: 10.2147/DMSO.S256031
17. Sun Y, Yan Y, Liao Y, Chu C, Guo T, Ma Q, et al. The new visceral adiposity index outperforms traditional obesity indices as a predictor of subclinical renal damage in Chinese individuals: a cross-sectional study. BMC Endo Dis. (2023) 23:78. doi: 10.1186/s12902-023-01330-5
18. Kjaergaard AD, Krakauer J, Krakauer N, Teumer A, Winkler TW, and Ellervik C. Allometric body shape indices, type 2 diabetes and kidney function: A two-sample Mendelian randomization study. Diabetes Obes Metab. (2023) 25:1803–12. doi: 10.1111/dom.15037
19. Zhang Y, Gao W, Li B, Liu Y, Chen K, Wang A, et al. The association between a body shape index and elevated urinary albumin-creatinine ratio in Chinese community adults. Front Endocrinol (Lausanne). (2022) 13:955241. doi: 10.3389/fendo.2022.955241
20. Zhang Y, Wang W, and Ning G. Metabolic Management Center: An innovation project for the management of metabolic diseases and complications in China. J Diabetes. (2019) 11:11–3. doi: 10.1111/jdb.2019.11.issue-1
21. Chen M, Wang Y, Feng P, Liang Y, Liu Q, Yang M, et al. Association between age at type 2 diabetes onset and diabetic retinopathy: A double-center retrospective study. J Diabetes Res. (2023) 2023:5919468. doi: 10.1155/2023/5919468
22. Chen M, Feng P, Liang Y, Ye X, Wang Y, Liu Q, et al. The relationship between age at diabetes onset and clinical outcomes in newly diagnosed type 2 diabetes: A real-world two-center study. Diabetes Metab Syndr Obes. (2024) 17:4069–78. doi: 10.2147/DMSO.S485967
23. Li X, Zhou ZG, Qi HY, Chen XY, and Huang G. Replacement of insulin by fasting C-peptide in modified homeostasis model assessment to evaluate insulin resistance and islet β cell function. J Cent South Univ(Med Sci). (2004) 29:0419–05.
24. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
25. Alicic RZ, Rooney MT, and Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. (2017) 12:2032–45. doi: 10.2215/CJN.11491116
26. Şen GA, Tanrıkulu S, Beşer B, Akçakalem Ş, Çakır S, Dinççağ N, et al. Effects of prediabetes and type 2 diabetes on cognitive functions. Endocrine. (2024) 85:190–5. doi: 10.1007/s12020-024-03720-8
27. Murphy ME, Byrne M, Galvin R, Boland F, Fahey T, Smith SM, et al. Improving risk factor management for patients with poorly controlled type 2 diabetes: a systematic review of healthcare interventions in primary care and community settings. BMJ Open. (2017) 7:e015135. doi: 10.1136/bmjopen-2016-015135
28. Kim B, Kim G, Kim E, Park J, Isobe T, Sakae T, et al. The A body shape index might be a stronger predictor of chronic kidney disease than BMI in a senior population. Int J Env Res Public Health. (2021) 18:12874. doi: 10.3390/ijerph182412874
29. Su WY, Chen IH, Gau YC, Wu PY, Huang JC, Tsai YC, et al. Metabolic syndrome and obesity-related indices are associated with rapid renal function decline in a large Taiwanese population follow-up study. Biomedicines. (2022) 10:1744. doi: 10.3390/biomedicines10071744
30. Yu P, Meng X, Kan R, Wang Z, and Yu X. Association between metabolic scores for visceral fat and chronic kidney disease: A cross-sectional study. Front Endocrinol (Lausanne). (2022) 13:1052736. doi: 10.3389/fendo.2022.1052736
31. Cosoreanu A, Rusu E, Rusu F, Stanciu S, Enache G, and Radulian G. Progression of chronic kidney disease to dialysis in the roma population with type 2 diabetes mellitus in comparison with caucasian patients. Cureus. (2024) 16:e62118. doi: 10.7759/cureus.62118
32. Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. New Engl J Med. (2012) 366:2247–56. doi: 10.1056/NEJMoa1109333
33. Yokoyama H, Okudaira M, Otani T, Sato A, Miura J, Takaike H, et al. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int. (2000) 58:302–11. doi: 10.1046/j.1523-1755.2000.00166.x
34. Sharma K. The link between obesity and albuminuria: adiponectin and podocyte dysfunction. Kidney Int. (2009) 76:145–8. doi: 10.1038/ki.2009.137
Keywords: a body shape index, visceral obesity, type 2 diabetes, diabetic kidney disease, early-onset
Citation: Chen M, Wang Y, Feng P, Wu L, Liu H, Liang Y, Yang M and Zheng Q (2025) The association between a body shape index and diabetic kidney disease in early-onset type 2 diabetes: evidence from a two-center study. Front. Endocrinol. 16:1553890. doi: 10.3389/fendo.2025.1553890
Received: 31 December 2024; Accepted: 28 May 2025;
Published: 19 June 2025.
Edited by:
Evan P Nadler, George Washington University, United StatesReviewed by:
Saarah Fg Davids, Cape Peninsula University of Technology, South AfricaRaushan Kumar, ERA’s Lucknow Medical College, India
Alexandre Martini, University of Brasilia, Brazil
Copyright © 2025 Chen, Wang, Feng, Wu, Liu, Liang, Yang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qidong Zheng, emhlbmdxaWRvbmd5aXNoZW5nQDE2My5jb20=
 Mengdie Chen
Mengdie Chen Yiyun Wang
Yiyun Wang Ping Feng1
Ping Feng1 Lijing Wu
Lijing Wu Hanying Liu
Hanying Liu Qidong Zheng
Qidong Zheng