- 1Department of Endocrinology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, China
- 2Center for Clinical Evidence-Based and Translational Medicine, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, China
- 3Department of Metabolism, Digestion and Reproduction, Imperial College London, London, United Kingdom
Background: Cross-sectional studies have revealed that steatotic liver disease (SLD) is associated with prevalent diabetic microvascular complications, but longitudinal evidence in large samples is insufficient. We aimed to prospectively investigate the association between SLDs and the risk of microvascular complications in patients with type 2 diabetes (T2D), and to explore whether glycemic control played a mediating role in this association.
Methods: The population-based cohort, which was based on the UK Biobank study, included 25,630 T2D patients at baseline. SLD was defined as a fatty liver index ≥ 60. A glycated hemoglobin level ≥ 7% (53 mmol/mol) was considered poor glycemic control. The primary outcome was total incident diabetic microvascular complications, defined as the first occurrence of diabetic nephropathy, diabetic neuropathy, and/or diabetic retinopathy. The cox proportional hazard regression model was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for diabetic microvascular complications. Mediation analysis was applied to explore whether the association between SLDs and diabetic microvascular complications was mediated by glycemic control.
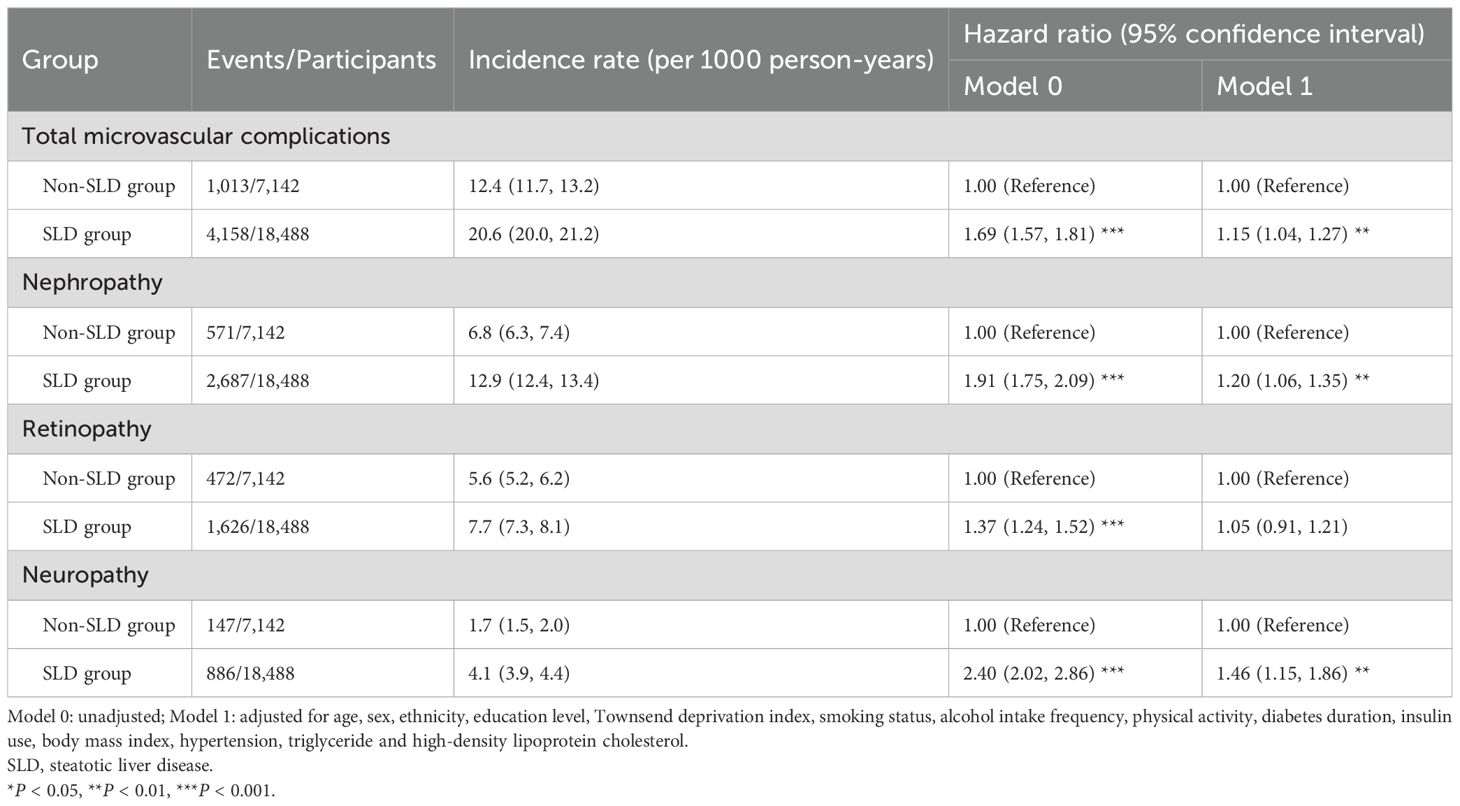
Results: The mean age of the study participants was 59.6 years, and 58.1% of them were males. During a median follow-up period of 12.1 years, 5,171 participants were diagnosed with microvascular complications. Compared with non-SLD participants, SLD participants had a HR of 1.15 (95% CI: 1.04, 1.27) for total microvascular complications, a HR of 1.20 (95% CI: 1.06, 1.35) for diabetic nephropathy, a HR of 1.05 (95% CI: 0.91, 1.21) for diabetic retinopathy, and a HR of 1.46 (95% CI: 1.15, 1.86) for diabetic neuropathy. The results of the mediation analysis revealed that the mediating proportion of glycemic control in the association between the SLD group and total diabetic microvascular complications was 22.5% (95% CI: 10.4%, 91.0%).
Conclusions: SLD was associated with an increased risk of microvascular complications, especially diabetic nephropathy and diabetic neuropathy, in T2D patients. Glycemic control partially mediated the association between SLDs and diabetic microvascular complications.
Introduction
Type 2 diabetes (T2D) is a common chronic disease with increasing prevalence worldwide and poses a major public health challenge (1). T2D patients may experience a variety of chronic complications, and microvascular complications are of particular concern because they greatly reduce patients’ quality of life and can lead to disability or premature death (2). While glycemic control is a known influencing factor, evidence also suggests that lipid metabolism substantially impacts the development of chronic diabetic complications (3).
Steatotic liver disease (SLD) is characterized by abnormal accumulation of fat in the liver, and is chosen as an overarching term to cover the various causes of steatosis (such as alcoholic and metabolic associated steatosis) according to the latest delphi consensus statement (4). SLD is common in T2D patients; for example, the prevalence of nonalcoholic fatty liver disease is estimated to be as high as 65% (5). Furthermore, in T2D patients, SLD is associated with an increased risk of macrovascular complications and mortality (6, 7). However, whether SLD increases the risk of diabetic microvascular complications in this population has not yet been systematically investigated. To date, studies have predominantly explored the associations between SLDs and individual microvascular complications (such as diabetic nephropathy, diabetic retinopathy, or diabetic neuropathy) through cross-sectional or case-control designs of small samples (8–13), which may be underpowered in terms of statistical efficiency.
The liver plays a critical role in maintaining glucose homeostasis, and abnormal increases in gluconeogenesis and impaired glycogen metabolism are observed in the SLD (14). Considering that microvascular complications are driven mainly by chronic hyperglycemia (15), we hypothesized that SLDs in T2D patients may influence the occurrence of microvascular complications by affecting glycemic control. Therefore, we aimed to investigate whether the SLD was associated with an increased risk of microvascular complications in T2D patients, and to explore whether glycemic control played a mediating role in this association in the UK Biobank cohort.
Materials and methods
Study design and population
Our data were sourced from the UK Biobank, which is a large cohort that included approximately 500,000 middle-aged and elderly volunteers at baseline between March 2006 and July 2010 (16). The follow-up of participants involved linkage with hospital admission records from England, Scotland, and Wales, as well as the national death register. Further details about the database can be found elsewhere (17).
A total of 28,078 participants aged between 40 and 72 years at the baseline survey were identified with T2D by using the International Classification of Diseases, 10th edition (ICD-10) code, blood glucose and glycated haemoglobin levels (18). Participants who met the following criteria were excluded: those with missing data for SLD (n = 1,746); and those with diabetic microvascular diseases at baseline (n = 702). Finally, 25,630 participants were included in the main analysis (Supplementary Figure 1).
Ascertainment of the baseline SLD
With no liver biopsy data available and only a small number of T2D patients having proton density fat fraction data in the UK Biobank (19), our study adopted a well-established index, the fatty liver index (FLI), to evaluate hepatic steatosis. In brief, FLI is a simple and noninvasive predictor of hepatic steatosis, with an accuracy of 0.84 (95% confidence interval (CI): 0.81, 0.87). The FLI is derived through a specific formula that incorporates body mass index (BMI), waist circumference, triglyceride, and gamma-glutamyl transpeptidase, and is calculated as:
(20).
The FLI score ranges from 0 to 100, and a FLI score ≥ 60 is defined as SLD, which has been widely used in large cohort studies (19, 21).
Ascertainment of outcome
The primary outcome was total incident diabetic microvascular complications, which was defined as the first occurrence of diabetic nephropathy, diabetic neuropathy, and/or diabetic retinopathy. The secondary outcomes included the incidence of each subtype of diabetic microvascular complications. The occurrence of each outcome was identified through linkage to the National Health Service in England, the Information and Statistics Division in Scotland, and the Secure Anonymised Information Linkage in Wales. The diagnosis was documented via the following ICD-10 codes: diabetic nephropathy (E11.2, E14.2, N08.3, N18.0, N18.1, N18.2, N18.3, N18.4, N18.5, N18.8, N18.9); diabetic retinopathy: (E11.3, E14.3, H28.0, H36.0); and diabetic neuropathy (E11.4, E14.4, G59.0, G62.9, G63.2, G99.0) (22, 23).
Ascertainment of covariates
The participants’ baseline information was obtained via the UK Biobank via touch-screen questionnaires, physical measurements or linkages with other authorities, etc. Information on age, sex and the Townsend deprivation index was acquired from local NHS primary care trust registries. The Townsend deprivation index is a measure of material deprivation, with a higher score indicating greater poverty (24).
Additional sociodemographic information (i.e. ethnicity and education level) and lifestyle information were collected through touch-screen questionnaires. Smoking status was classified into never smoking, former smoking, current smoking, and unwilling to answer. Alcohol intake frequency was stratified into seven groups, ranging from never drinking to daily or almost daily drinking, plus those unwilling to answer. Physical activity was categorized into high, medium, and low groups on the basis of International Physical Activity Questionnaire guidelines (25). Medication information was obtained with the following question: “Do you regularly take any of the following medications? (you can select more than one answer)”. Diabetes duration was the time span from the diagnosis of T2D to engagement in the baseline survey.
Height, weight and waist circumference were measured by trained nurses. BMI was calculated as BMI (kg/m2) = weight (kg)/height2 (m2) and was divided into three groups: normal weight (BMI < 25 kg/m2), overweight (25 ≤ BMI < 30 kg/m2) and obesity (BMI ≥ 30 kg/m2), according to the World Health Organization standard (26). Blood pressure (mmHg) was recorded as the average of two readings from the Omron device. The platelet count (10^9 cells/l) was the number of thrombocytes derived from the platelet histogram. Blood glucose (mmol/l), lipids (mmol/l) and several enzymes that reflect liver function (u/l) were measured via a Beckman Coulter AU5800. Glycated haemoglobin was measured via a Bio-Rad VARIANT II Turbo, and a value equal to or greater than 7% (53 mmol/mol) was considered poor glycemic control (27).
Statistical analysis
All analyses were performed using the Stata 17.0 (Stata Corp, College Station, TX, USA) and RStudio (version 2023.09.1) software. A P value < 0.05 was considered statistically significant. Continuous variables are presented as means ± standard deviations or medians (interquartile ranges), and categorical variables are presented as numbers (percentages). Differences were assessed using the t-tests, Mann-Whitney U tests and Chi-square tests. The missing covariates were addressed by multiple imputation method (28).
The cox proportional hazard regression model was used to calculate hazard ratios (HRs) and corresponding 95% CIs of diabetic microvascular complications in the SLD group compared with the non-SLD group. The follow-up time was calculated in calendar years from the baseline participation date to the date of outcome or death or September 30, 2021. A Schoenfeld residual test was performed to validate the proportional hazards assumption, and the P-value (> 0.05) indicated no violation of the assumption. We also categorized the FLI score into quartile groups or as a continuous variable (19), and assessed the presence of liver fibrosis to explore the association between the degree of hepatic steatosis and the risk of diabetic microvascular complications. The fibrosis-4 index (FIB-4) was used to define advanced liver fibrosis and was calculated as FIB-4 = (age * aspartate aminotransferase)/(platelet * alanine aminotransferase ^1/2). The low cut-off point and high cut-off point of the FIB-4 score were 1.30 and 2.67, respectively (29). In addition, we explored whether the association between the SLD group and microvascular complications in T2D patients was partially mediated by glycemic control through mediation analysis (30).
We investigated the association between the SLD group and the risk of diabetic microvascular complications stratified by age (< 65 years and ≥ 65 years), sex (male and female), ethnicity (White and non-white), diabetes duration (< 1 year, 1-5 years, and > 5 years), and BMI (< 25 kg/m2 and ≥ 25 kg/m2). Several sensitivity analyses were performed to test the stability of the results. First, we excluded participants whose outcomes occurred within two years after baseline to avoid possible reverse causality. Second, we excluded participants with missing covariates to examine the impact of missing data on the results. Third, we re-analyzed the data by using FLI tripartite classification (FLI ≥ 60, SLD group; 30-59, intermediate group; and FLI < 30, no-SLD group) to reduce potential misclassification bias (6, 20).
Results
Participant characteristics
The study involved 25,630 T2D patients with a mean age of 59.6 years, and 58.1% were males. Among them, 18,488 had SLD, accounting for 72.1%. Compared with T2D patients with non-SLD, those with SLD were more likely to be males, have a higher Townsend deprivation Index, be current smokers, and have low levels of physical activity, and they were less likely to have a college or higher education level (P < 0.001). T2D patients with SLD also showed a longer duration of diabetes, larger BMI and waist circumference, higher blood pressure, and higher levels of blood glucose, glycated haemoglobin, triglycerides and enzymes that reflect liver function, compared to those with non-SLD (P < 0.001) (Table 1).
Association of the SLD with diabetic microvascular complications
After a median follow-up of 12.1 years, 5,171 diabetic microvascular complications were observed. Among these patients, 3,258 had diabetic nephropathy, 2,098 had diabetic retinopathy, and 1,033 had diabetic neuropathy. According to the results of the log-rank test, the cumulative incidence of diabetic microvascular complications was significantly higher in the SLD group than in the non-SLD group (P < 0.001) (Supplementary Figure 2).
Compared with non-SLD participants, SLD participants had a HR of 1.15 (95% CI: 1.04, 1.27) for total microvascular complications, a HR of 1.20 (95% CI: 1.06, 1.35) for diabetic nephropathy, a HR of 1.05 (95% CI: 0.91, 1.21) for diabetic retinopathy, and a HR of 1.46 (95% CI: 1.15, 1.86) for diabetic neuropathy (Table 2). Compared with participants in the lowest quartile of FLI, participants in the highest quartile of FLI had a HR of 1.68 (95% CI: 1.46, 1.92) for total microvascular complications. Compared with non-SLD participants, SLD participants with FIB-4 ≥ 2.67 also had a 38% (HR: 1.38, 95% CI: 1.17, 1.63) higher risk of total microvascular complications. The results of the restricted cubic spline curve indicated a significant nonlinear association between FLI scores and the risk of total microvascular complications (P for nonlinear < 0.001) (Figure 1).
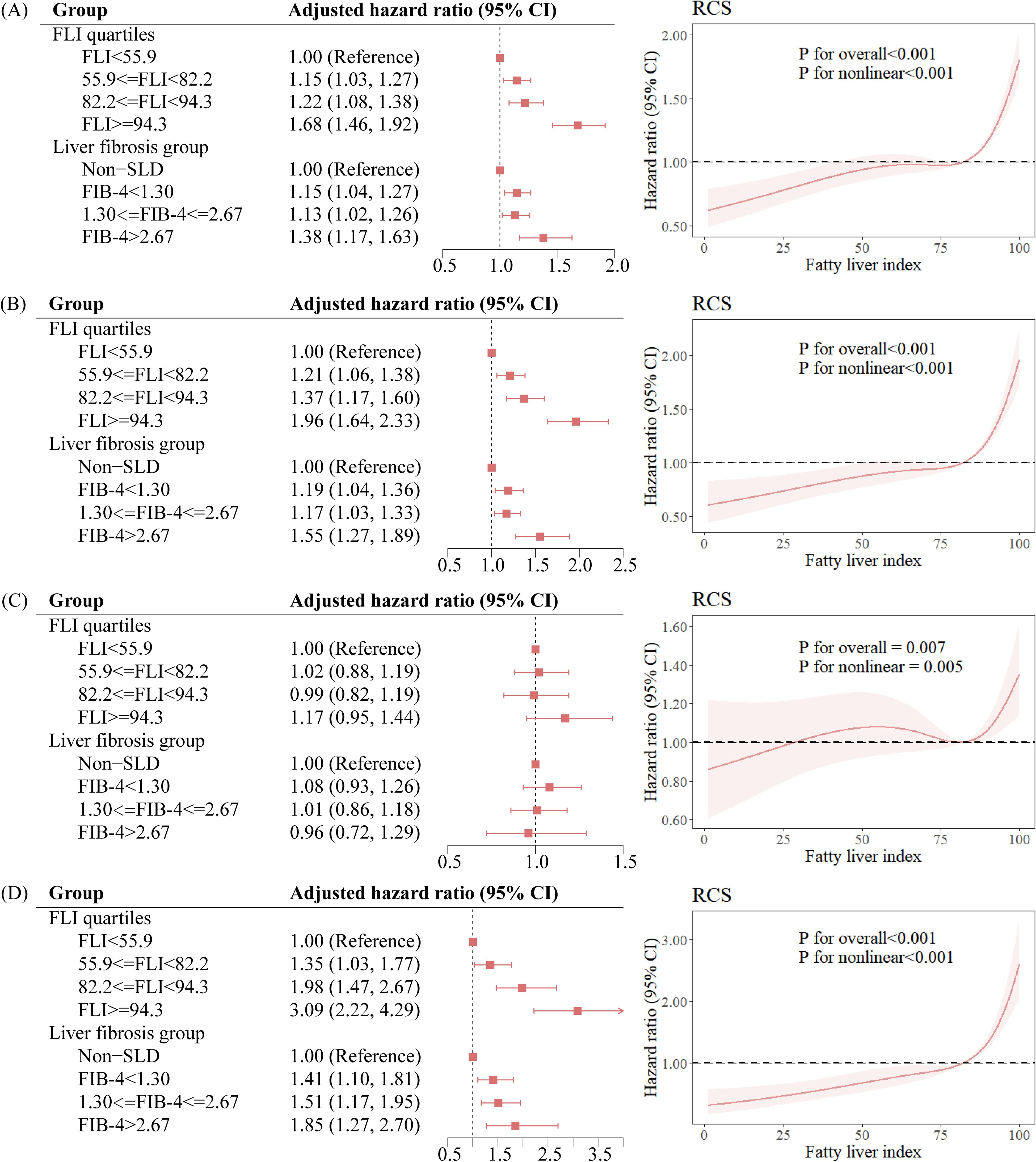
Figure 1. Association between the degree of hepatic steatosis and the risk of diabetic microvascular complications. (A) Total microvascular complications, (B) Nephropathy, (C) Retinopathy, (D) Neuropathy. FLI, fatty liver index; SLD, steatotic liver disease; CI, confidence interval; RCS, restricted cubic spline.
Mediation analysis
The results of the mediation analysis revealed that glycemic control played a mediating role in the association between the SLD group and diabetic microvascular complications, and the mediating proportion was 22.5% (95% CI: 10.4%, 91.0%) for total microvascular complications (Figure 2). The association between the SLD group and glycemic control as well as the association between glycemic control and diabetic microvascular complications was shown in Supplementary Table 1.
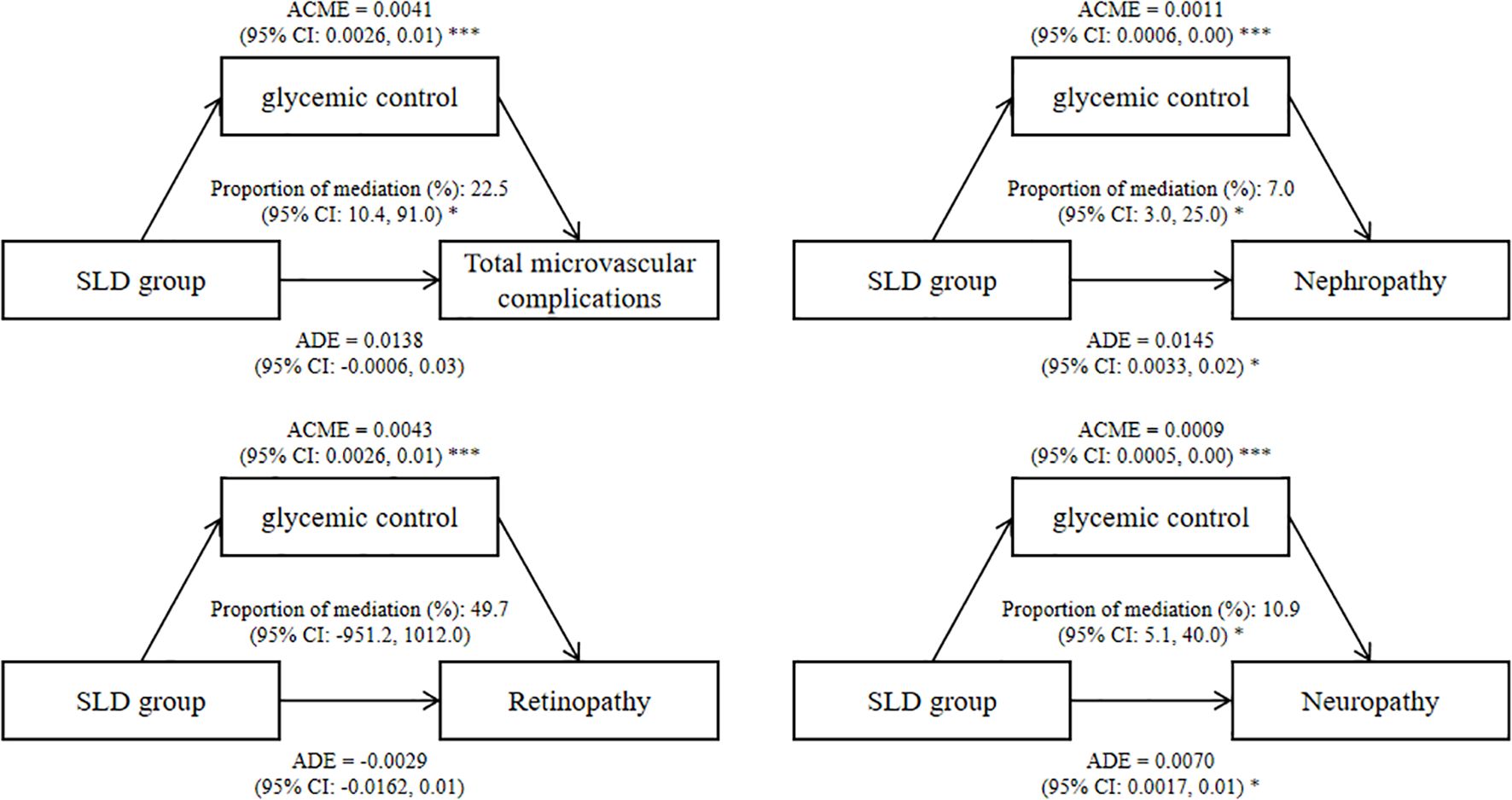
Figure 2. Mediating effects of SLD group on diabetic microvascular complications by glycemic control. Effect and proportion estimations were adjusted for age, sex, ethnicity, education level, Townsend deprivation index, smoking status, alcohol intake frequency, physical activity, diabetes duration, insulin use, body mass index, hypertension, triglyceride and high-density lipoprotein cholesterol. ADE, average direct effects; ACME, average causal mediation effects; CI, confidence interval; SLD, steatotic liver disease. *P < 0.05, ***P < 0.001.
Subgroup analysis and sensitivity analysis
Subgroup analysis revealed that in T2D patients who were under 65 years of age (HR: 1.25, 95% CI: 1.09, 1.43), female (HR: 1.24, 95% CI: 1.05, 1.45), White (HR: 1.15, 95% CI: 1.03, 1.28), had a diabetes duration of over 5 years (HR: 1.21, 95% CI: 1.00, 1.45), and were overweight or obese (HR: 1.39, 95% CI: 1.27, 1.53), SLD was significantly associated with an increased risk of total microvascular complications, while no significant association was found in the remaining subgroups (Supplementary Table 2). Sensitivity analysis indicated no significant changes in the direction or magnitude of the association between the SLD group and diabetic microvascular complications (Supplementary Table 3).
Discussion
Our study used data of 25,630 T2D patients from the UK Biobank to systematically explore the association between SLD and the risk of diabetic microvascular complications. There were three key findings: Firstly, SLD was associated with a 15% increased risk of total microvascular complications compared to non-SLD, with a significant association with diabetic nephropathy and diabetic neuropathy (P < 0.01). Secondly, increased degree of hepatic steatosis, as assessed by the FLI score and liver fibrosis, was associated with increased risks of microvascular complications. Lastly, glycemic control mediated the association between the SLD group and total microvascular complications by 22.5%.
Hepatic steatosis is an important risk factor for macrovascular complications and mortality in T2D patients (6, 31), but its association with microvascular complications is inconsistent, and there are few studies on total microvascular complications (32, 33). One case-control study explored the effect of nonalcoholic fatty liver disease on total diabetic microvascular complications, and did not find a significant association (odds ratio:0.53, 95% CI: 0.11, 2.49) (32). Notably, only 935 T2D patients were included in this study, which may lead to imprecise estimates of effects and lower confidence in the results. Another cohort study found that nonalcoholic fatty liver disease was independently and positively associated with the development of microvascular diseases (adjusted HR: 1.45, 95% CI: 1.28, 1.63) (33), which was consistent with our findings.
In terms of individual microvascular complications, we found that SLD was positively associated with the risk of diabetic nephropathy and diabetic neuropathy. The harmful effects of hepatic steatosis on chronic kidney disease have been extensively studied in the general population (34, 35), and a positive association was also observed in patients with diabetes (13, 36, 37), which was in line with our findings. Additionally, recent evidence highlights the role of cholemic nephropathy, a condition characterized by renal dysfunction secondary to hyperbilirubinemia, in subclinical renal injury. For instance, a study suggested that even acute mild hyperbilirubinemia may induce early renal tubular damage in the absence of alterations of the normal parameters used in clinical practice (38). While our study focused on SLD-associated metabolic disturbances, future research may explore whether elevated bilirubin levels in SLD patients contribute to diabetic nephropathy via cholemic mechanisms. This could further elucidate the hepatic-renal axis in T2D patients and refine risk stratification strategies. The results regarding the association between SLDs and diabetic neuropathy were consistent with the findings of a prospective study conducted in Iran (13) and several cross-sectional studies (10). In this prospective study, the incidence risk of diabetic neuropathy was significantly increased in patients with nonalcoholic fatty liver disease (odds ratio: 1.34, 95% CI: 1.09, 1.64) (13).
Current findings on the association between SLD and diabetic retinopathy are contradictory. Our study did not find a significant association between the two, which is consistent with a prior cohort study (3,123 Iranians) and cross-sectional study (5,963 Americans) reporting non-significant odds ratios of 0.90 (95% CI: 0.71, 1.13) and 0.77 (95% CI: 0.47, 1.26) for nonalcoholic fatty liver disease and diabetic retinopathy, respectively (13, 39). Two cross-sectional studies reported a lower prevalence of diabetic retinopathy in T2D patients with nonalcoholic fatty liver disease (12, 40). These discrepant results may be due to differences in study design, sample size and participants’ ethnicity. The two studies had small sample sizes from Asian countries (929 Koreans and 411 Chinese). In contrast, Targher et al. suggested that nonalcoholic fatty liver disease was associated with an increased prevalence of proliferative/laser-treated retinopathy in T2D patients in Italy (HR: 1.75, 95% CI: 1.10, 3.70), but no significant association was found with non-proliferative retinopathy (HR: 1.19, 95% CI: 0.80, 1.70), suggesting that the type of diabetic retinopathy may also influence the association (11).
Liver fibrosis is a more serious disease state than hepatic steatosis. Several studies have explored its association with diabetic microvascular complications, most of which were consistent with our findings (41–43). For example, Rosa Lombardi et al. reported that significant liver fibrosis was independently associated with the presence of microvascular complications (adjusted odds ratio: 4.20, 95% CI: 1.50, 11.40) (42). However, in the study by Niloofar Deravi et al., liver fibrosis assessed by the FIB-4 was not significantly associated with diabetic microvascular complications (13). This may be because that the reference group in this study was the first tertile of FIB-4, which may include patients with SLD, thus reducing the difference between groups.
Previous literature has shown that patients with SLD have abnormally increased gluconeogenesis and impaired glycogen metabolism (14), which is related to the occurrence of diabetic microvascular complications (44). In our study, we quantified the role that glycemic control played in the association between SLDs and microvascular complications, mediated by 22.5%. This may suggest that T2D patients with hepatic steatosis have worse glycemic control than T2D patients alone, which needs to be considered in the clinical treatment and management of patients with diabetes and hepatic steatosis. The remaining approximately 77% of the effect may be related to inflammation, lipid metabolism and other pathways. Hepatic steatosis is closely associated with chronic low-grade inflammation, characterized by elevated pro-inflammatory cytokines such as TNF-α, IL-6, and C-reactive protein (45). These inflammatory mediators may directly impair endothelial function, promote oxidative stress, and exacerbate insulin resistance, thereby contributing to microvascular damage (46). Furthermore, altered lipid metabolism in SLD, including increased free fatty acid flux, hepatic overproduction of triglyceride-rich lipoproteins, and ectopic lipid deposition in peripheral tissues, may induce lipotoxicity and mitochondrial dysfunction in microvascular cells (47).
Strengths and limitations
The strengths of this study lie in the utilization of a large population-based sample and a long follow-up period. On the basis of investigating the association between SLDs and diabetic microvascular complications, we further explored the possible mediating effect of glycemic control. However, some limitations should be acknowledged. Firstly, the gold standard for diagnosing hepatic steatosis is liver biopsy, but this is difficult to implement in large epidemiological studies, and there is no available data on liver biopsy in the UK Biobank. Therefore, we utilized a well-established and validated index to assess hepatic steatosis (20). Secondly, despite our efforts to adjust for potential confounders, the possibility of residual confounding may remain. Thirdly, microvascular complications in our study were identified by using ICD-10 codes linked with hospital records. This may result in underdiagnosis and undercoding of microvascular complications, potentially leading to an underestimation of its incidence. Fourthly, the wide confidence interval of the mediating effect may reflect the sample size limitation in mediation analyses and biological variability of this study, which need cautious interpretation and validation in large-scale longitudinal studies. Lastly, the UK Biobank cohort predominantly comprises data from participants across the UK. Caution is needed when extending our results to other ethnic groups, as the demographics may differ.
Conclusions
SLD was associated with an increased risk of microvascular complications, especially diabetic nephropathy and diabetic neuropathy, in T2D patients. Glycemic control partially mediated the association between SLDs and diabetic microvascular complications. In the course of clinical diagnosis and treatment, it is advisable to routinely screen T2D patients for hepatic steatosis and inform them about the potential increased risk of microvascular complications. Priority should be given to GLP-1 agonists or SGLT2 inhibitors in T2D patients with SLD to treat both hyperglycemia and hepatic steatosis. Future studies may explore other biological pathways mediating the association between SLDs and diabetic microvascular complications to provide a basis for precise interventions in T2D patients.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ukbiobank.ac.uk.
Ethics statement
The studies involving humans were approved by the North West Multicenter Research Ethics Committee (ethical approval number: 21/NW/0157). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LL: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. QY: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. WY: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing. AL: Writing – review & editing. YW: Writing – review & editing. JZ: Writing – review & editing. MF: Writing – review & editing. BC: Writing – review & editing. WF: Writing – review & editing. SX: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was partly supported by the Key project of Hubei Natural Science Foundation Innovation and Development Joint Fund (Grant number 2023AFD031); the Science and Technology Research Key Project of Education Department of Hubei Province China (Grant number D20212602); and the Hubei Natural Science Foundation Program (Youth Project) (Grant number 2023AFB211).
Acknowledgments
We thank the UK Biobank participants and research staff for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1554798/full#supplementary-material
References
1. GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. (2023) 402:203–34. doi: 10.1016/S0140-6736(23)01301-6
2. Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, Schnell O, et al. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. (2019) 26:25–32. doi: 10.1177/2047487319878371
3. Eid S, Sas KM, Abcouwer SF, Feldman EL, Gardner TW, Pennathur S, et al. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. (2019) 62:1539–49. doi: 10.1007/s00125-019-4959-1
4. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. (2023) 79:1542–56. doi: 10.1016/j.jhep.2023.06.003
5. Elina En Li C, Chong Zhe A, Jingxuan Q, Clarissa Elysia F, Lincoln Kai En L, Zane En Qi H, et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Gut. (2023) 72:2138. doi: 10.1136/gutjnl-2023-330110
6. Park J, Kim G, Kim B-S, Han K-D, Kwon SY, Park SH, et al. The associations of hepatic steatosis and fibrosis using fatty liver index and BARD score with cardiovascular outcomes and mortality in patients with new-onset type 2 diabetes: a nationwide cohort study. Cardiovasc Diabetol. (2022) 21:53. doi: 10.1186/s12933-022-01483-y
7. Adams LA, Harmsen S, St Sauver JL, Charatcharoenwitthaya P, Enders FB, Therneau T, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. (2010) 105:1567–73. doi: 10.1038/ajg.2010.18
8. Mantovani A, Turino T, Lando MG, Gjini K, Byrne CD, Zusi C, et al. Screening for non-alcoholic fatty liver disease using liver stiffness measurement and its association with chronic kidney disease and cardiovascular complications in patients with type 2 diabetes. Diabetes Metab. (2020) 46:296–303. doi: 10.1016/j.diabet.2019.11.004
9. Li Y, Zhu S, Li B, Shao X, Liu X, Liu A, et al. Association between non-alcoholic fatty liver disease and chronic kidney disease in population with prediabetes or diabetes. Int Urol Nephrol. (2014) 46:1785–91. doi: 10.1007/s11255-014-0796-9
10. Greco C, Nascimbeni F, Carubbi F, Andreone P, Simoni M, and Santi D. Association of nonalcoholic fatty liver disease (NAFLD) with peripheral diabetic polyneuropathy: A systematic review and meta-analysis. J Clin Med. (2021) 10:4466. doi: 10.3390/jcm10194466
11. Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. (2008) 51:444–50. doi: 10.1007/s00125-007-0897-4
12. Kim BY, Jung CH, Mok JO, Kang SK, and Kim CH. Prevalences of diabetic retinopathy and nephropathy are lower in Korean type 2 diabetic patients with non-alcoholic fatty liver disease. J Diabetes Investig. (2014) 5:170–5. doi: 10.1111/jdi.2014.5.issue-2
13. Deravi N, Dehghani Firouzabadi F, Moosaie F, Asadigandomani H, Arab Bafrani M, Yoosefi N, et al. Non-alcoholic fatty liver disease and incidence of microvascular complications of diabetes in patients with type 2 diabetes: a prospective cohort study. Front Endocrinol (Lausanne). (2023) 14:1147458. doi: 10.3389/fendo.2023.1147458
14. Scoditti E, Sabatini S, Carli F, and Gastaldelli A. Hepatic glucose metabolism in the steatotic liver. Nat Rev Gastroenterol Hepatol. (2024) 21:319–34. doi: 10.1038/s41575-023-00888-8
15. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:S151–s67. doi: 10.2337/dc21-S011
16. Introduction to the UK Biobank showcase. Available online at: https://www.ukbiobank.ac.uk/ (Accessed March 18, 2024).
17. Caleyachetty R, Littlejohns T, Lacey B, Bešević J, Conroy M, Collins R, et al. United Kingdom Biobank (UK Biobank): JACC focus seminar 6/8. J Am Coll Cardiol. (2021) 78:56–65. doi: 10.1016/j.jacc.2021.03.342
18. He P, Li H, Ye Z, Liu M, Zhou C, Wu Q, et al. Association of a healthy lifestyle, life’s essential 8 scores with incident macrovascular and microvascular disease among individuals with type 2 diabetes. J Am Heart Assoc. (2023) 12:e029441. doi: 10.1161/JAHA.122.029441
19. Wu S, Yuan C, Yang Z, Liu S, Zhang Q, Zhang S, et al. Non-alcoholic fatty liver is associated with increased risk of irritable bowel syndrome: a prospective cohort study. BMC Med. (2022) 20:262. doi: 10.1186/s12916-022-02460-8
20. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. doi: 10.1186/1471-230X-6-33
21. Amadou C, Nabi O, Serfaty L, Lacombe K, Boursier J, Mathurin P, et al. Association between birth weight, preterm birth, and nonalcoholic fatty liver disease in a community-based cohort. Hepatol. (2022) 76:1438–51. doi: 10.1002/hep.32540
22. Wu Y, Xiong T, Tan X, and Chen L. Frailty and risk of microvascular complications in patients with type 2 diabetes: a population-based cohort study. BMC Med. (2022) 20:473. doi: 10.1186/s12916-022-02675-9
23. Geng T, Zhu K, Lu Q, Wan Z, Chen X, Liu L, et al. Healthy lifestyle behaviors, mediating biomarkers, and risk of microvascular complications among individuals with type 2 diabetes: A cohort study. PloS Med. (2023) 20:e1004135. doi: 10.1371/journal.pmed.1004135
24. Ye X, Wang Y, Zou Y, Tu J, Tang W, Yu R, et al. Associations of socioeconomic status with infectious diseases mediated by lifestyle, environmental pollution and chronic comorbidities: a comprehensive evaluation based on UK Biobank. Infect Dis Poverty. (2023) 12:5. doi: 10.1186/s40249-023-01056-5
25. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
26. Obesity and overweight 2021. Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (Accessed March 18, 2024).
27. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. (2020) 44:S73–84. doi: 10.2337/dc21-S006
28. Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. (2016) 4:30. doi: 10.3978/j.issn.2305-5839.2015.12.63
29. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, and Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2009) 7:1104–12. doi: 10.1016/j.cgh.2009.05.033
30. Tofighi D and MacKinnon DP. RMediation: an R package for mediation analysis confidence intervals. Behav Res Methods. (2011) 43:692–700. doi: 10.3758/s13428-011-0076-x
31. Kim KS, Hong S, Han K, and Park CY. Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study. Bmj. (2024) 384:e076388. doi: 10.1136/bmj-2023-076388
32. Afarideh M, Aryan Z, Ghajar A, Ganji M, Ghaemi F, Saadat M, et al. Association of non-alcoholic fatty liver disease with microvascular complications of type 2 diabetes. Prim Care Diabetes. (2019) 13:505–14. doi: 10.1016/j.pcd.2019.03.009
33. Ebert T, Widman L, Stenvinkel P, and Hagström H. Increased risk for microvascular outcomes in NAFLD-A nationwide, population-based cohort study. J Intern Med. (2023) 294:216–27. doi: 10.1111/joim.v294.2
34. Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Schattenberg JM, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut. (2022) 71:156–62. doi: 10.1136/gutjnl-2020-323082
35. Chen S, Pang J, Huang R, Xue H, and Chen X. Association of MAFLD with end-stage kidney disease: a prospective study of 337,783 UK Biobank participants. Hepatol Int. (2023) 17:595–605. doi: 10.1007/s12072-023-10486-0
36. Jia G, Di F, Wang Q, Shao J, Gao L, Wang L, et al. Non-alcoholic fatty liver disease is a risk factor for the development of diabetic nephropathy in patients with type 2 diabetes mellitus. PloS One. (2015) 10:e0142808. doi: 10.1371/journal.pone.0142808
37. Targher G, Chonchol M, Bertolini L, Rodella S, Zenari L, Lippi G, et al. Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J Am Soc Nephrol. (2008) 19:1564–70. doi: 10.1681/ASN.2007101155
38. Scilletta S, Leggio S, Di Marco M, Miano N, Musmeci M, Marrano N, et al. Acute hyperbilirubinemia determines an early subclinical renal damage: Evaluation of tubular biomarkers in cholemic nephropathy. Liver Int. (2024) 44:2341–50. doi: 10.1111/liv.16005
39. Lin TY, Chen YJ, Chen WL, and Peng TC. The relationship between nonalcoholic fatty liver disease and retinopathy in NHANES III. PloS One. (2016) 11:e0165970. doi: 10.1371/journal.pone.0165970
40. Zhang M, Li L, Chen J, Li B, Zhan Y, and Zhang C. Presence of diabetic retinopathy is lower in type 2 diabetic patients with non-alcoholic fatty liver disease. Med (Baltimore). (2019) 98:e15362. doi: 10.1097/MD.0000000000015362
41. Mantovani A, Morieri ML, Aldigeri R, Palmisano L, Masulli M, Bonomo K, et al. MASLD, hepatic steatosis and fibrosis are associated with the prevalence of chronic kidney disease and retinopathy in adults with type 1 diabetes mellitus. Diabetes Metab. (2024) 50:101497. doi: 10.1016/j.diabet.2023.101497
42. Lombardi R, Airaghi L, Targher G, Serviddio G, Maffi G, Mantovani A, et al. Liver fibrosis by FibroScan(®) independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. (2020) 40:347–54. doi: 10.1111/liv.14274
43. Mikolasevic I, Rahelic D, Turk-Wensween T, Ruzic A, Domislovic V, Hauser G, et al. Significant liver fibrosis, as assessed by fibroscan, is independently associated with chronic vascular complications of type 2 diabetes: A multicenter study. Diabetes Res Clin Pract. (2021) 177:108884. doi: 10.1016/j.diabres.2021.108884
44. Škrha J, Šoupal J, Škrha J Jr., and Prázný M. Glucose variability, HbA1c and microvascular complications. Rev Endocr Metab Disord. (2016) 17:103–10. doi: 10.1007/s11154-016-9347-2
45. Duan Y, Pan X, Luo J, Xiao X, Li J, Bestman PL, et al. Association of inflammatory cytokines with non-alcoholic fatty liver disease. Front Immunol. (2022) 13:880298. doi: 10.3389/fimmu.2022.880298
46. Tang L, Xu GT, and Zhang JF. Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy. Neural Regener Res. (2023) 18:976–82. doi: 10.4103/1673-5374.355743
Keywords: steatotic liver disease, type 2 diabetes, microvascular complications, cohort, glycemic control
Citation: Liu L, You Q, Yu W, Lett AM, Wu Y, Zeng J, Fan M, Chen B, Fu W and Xu S (2025) Association between steatotic liver disease and microvascular complications in individuals with type 2 diabetes: a cohort study in the UK Biobank. Front. Endocrinol. 16:1554798. doi: 10.3389/fendo.2025.1554798
Received: 03 January 2025; Accepted: 06 May 2025;
Published: 28 May 2025.
Edited by:
Alexander Akhmedov, University of Zurich, SwitzerlandReviewed by:
Giosiana Bosco, University of Catania, ItalySrividya Velagapudi, University of Zurich, Switzerland
Tamer A. Addissouky, University of Menoufia, Egypt
Copyright © 2025 Liu, You, Yu, Lett, Wu, Zeng, Fan, Chen, Fu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoyong Xu, eW9qaV94dUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Longfu Liu
Longfu Liu Qiqi You2†
Qiqi You2† Jingjing Zeng
Jingjing Zeng Bo Chen
Bo Chen Wan Fu
Wan Fu Shaoyong Xu
Shaoyong Xu