- Shanghai Key Laboratory of Maternal Fetal Medicine, Shanghai Institute of Maternal-Fetal Medicine and Gynecologic Oncology, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University, Shanghai, China
Background: Gestational diabetes mellitus (GDM) and thyroid dysfunction share demographic overlap in at-risk populations, both exerting adverse effects on pregnancy. Their combined influence on pregnancy outcomes and complications requires further investigation through large-scale clinical studies.
Objective: This study aimed to compare trimester-specific thyroid function in a GDM population with a euthyroid population, and to examine the impact of maternal thyroid function on pregnancy outcomes after adjusting for GDM diagnosis status.
Methods: The retrospective cohort study involved singleton pregnant women registered between 2013 and 2020 in Shanghai, China. Maternal and infant biometrics were extracted from the electronic system at Shanghai First Maternity and Infant Hospital. The primary statistical methods in the study include logistic regression model and parallel mediation analyses.
Results: Of the 81,488 pregnancies included, 8,868 had GDM. Compared to the population without GDM, the GDM population exhibited lower free thyroxine (FT4) and different elevated thyroid-stimulating hormone (TSH) levels in specific trimesters. Mid-pregnancy thyroid function correlated with risks of preterm birth [for FT4<2.5th percentile while TSH>97.5th, adjusted odds ratio (OR)=2.471, 95% confidence interval (CI): 1.234−4.478] and fetal overgrowth (for FT4<2.5th percentile, adjusted OR=1.551, 95% CI: 1.271–1.874). Late-pregnancy low FT4 was associated with hypertensive disorders (for FT4<2.5th percentile while TSH>97.5th, adjusted OR=3.279, 95% CI: 1.221−7.375; for isolated FT4<2.5th percentile, adjusted OR=2.010, 95% CI: 1.260−3.057). GDM amplified all these risks. Moreover, maternal ferritin was a primary mediator in thyroid-neonatal weight associations, particularly in late pregnancy (mediation proportion: 22.1%).
Conclusion: This study highlighted the increased risk of adverse outcomes associated with thyroid dysfunction in GDM pregnancies, underscoring the necessity for combined thyroid function and glucose metabolism screening, which facilitates timely interventions to mitigate risks of preterm birth, hypertensive disease, and fetal overgrowth.
1 Introduction
As a common chronic disease during pregnancy, gestational diabetes mellitus (GDM) poses an increasing threat to the health of pregnant women worldwide. Substantial evidence demonstrates that GDM adversely impacts maternal health through inducing metabolic abnormalities (1–3) and higher risks of adverse pregnancy outcomes, including macrosomia, large for gestational age (LGA) infants (4), cesarean delivery, and preterm birth (5).
Remarkably, glucose homeostasis during pregnancy may be associated with the physiological adaptation of maternal thyroid function, though this relationship requires further investigation (6). In addition to fetal neurodevelopment, adequate maternal thyroid function is also essential for fetal growth (7), and thyroid dysfunction in pregnant women can lead to increased risk of adverse outcomes, including gestational hypertension or preeclampsia, preterm birth, and fetal growth abnormalities (8, 9) Thyroid dysfunction, especially hypothyroidism-related diseases (Hypo-RD), such as isolated maternal hypothyroxinemia (IMH), subclinical hypothyroidism, and clinical hypothyroidism, and GDM represent the most prevalent endocrinopathies in pregnancy, constituting key focus areas in obstetric endocrinology. Disordered glucose metabolism and thyroid dysfunction co-occur frequently (10, 11), due to the intricate relationship between thyroid function and glucose metabolism regulation (12, 13), and the shared risk factors include advanced conception age, overweight or obesity, and autoimmune predisposition (14).
While the increased risk of glucose metabolism disorders in pregnant women with thyroid dysfunction is well-established (15), the precise characteristics of thyroid function alterations in GDM remain incompletely characterized. There are existing studies reporting elevated thyroid-stimulating hormone (TSH) levels, higher thyroid peroxidase antibody (TPOAb) positivity rates, and decreased free thyroxine (FT4) concentrations in patients with GDM (11, 16). However, given the dynamic changes in thyroid function across gestation, each trimester exhibits distinct physiological patterns and clinical implications (17). Critically, thyroid dysfunction manifests differential clinical impacts on maternal-fetal outcomes depending on gestational timing (17), while few studies have focused on the association between second- and third-trimester disturbances and obstetric complications. These trimester-specific risk profiles necessitate validation in large cohorts with rigorous trimester stratification. The diagnostic criteria for GDM (18–20) and thyroid disorders (21, 22) keep evolving over time, and thus previous related studies are outdated and no longer align with the characteristics of the current population.
In this large-scale retrospective cohort study of Chinese pregnant women, we systematically characterized trimester-specific variations in thyroid function between GDM and non-GDM pregnancies and quantified the synergistic effects of concurrent thyroid dysfunction and GDM on adverse pregnancy outcomes. Our findings provide robust clinical evidence for understanding how glucose metabolism status modifies thyroid function-outcome relationships, offering critical insights for developing targeted screening protocols and timely therapeutic interventions in high-risk pregnancies.
2 Materials and methods
2.1 Study design, participants, and ethics approval
This historical cohort research study was conducted at Shanghai First Maternity and Infant Hospital in China. We analyzed the electronic medical records of singleton pregnancies in women aged 19–40 years registered between January 2013 and December 2020. Exclusion criteria included: 1) Pre-existing hypertension or thyroid surgery history; 2) Other metabolic disorders (polycystic ovary syndrome, hyperlipidemia); 3) Implausible gestational age estimates; 4) Assistant reproductive technique (ART) conception or multifetal pregnancy reduction; 5) Autoimmune disease (e.g., systemic lupus erythematosus); 6) Incident maternal thyroid dysfunction during pregnancy (excluding Hypo-RD); 7) Therapeutic termination. After removing duplicate pregnancy records, 81,488 eligible cases were included (Figure 1). Demographic and clinical data were prospectively collected through standardized clinical documentation, including age at conception, residential origin (divided into Shanghai/non-Shanghai), parental family history of hypertension/diabetes, ethnicity (Han/non-Han), employment status (employed/unemployed), obstetric history (abortion types, preterm deliveries, parity), pre-pregnancy anthropometrics (height, weight), and calculated body mass index [BMI = weight(kg)/height(m)²]. Gestational age was determined by last menstrual period. Delivery outcomes (mode of delivery) and neonatal parameters (sex, birth weight, Apgar scores) were retrieved from electronic records. This study received approval from the Ethics Committee of the Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University (Approval Number: KS1998).
2.2 Diagnostic classification and outcome definitions
TPOAb positivity was defined as >60 IU/mL. According to the American Thyroid Association (ATA) guideline (22), trimester-specific FT4 and TSH reference ranges were established after excluding TPOAb-positive cases and those with thyroid disease history (Supplementary Table S1). Pregnancy was stratified into trimesters: first (within 13 weeks), second (13–27+6 weeks), and third (≥28 weeks). The diagnosis of GDM depended on the American Diabetes Association guidelines (23). GDM was diagnosed by excessive fasting plasma glucose, 1-hour glucose, or 2-hour glucose. Hypertensive disorders of pregnancy (HDP) were identified using the guidelines of the Chinese Society of Obstetrics and Gynecology during the study period (24). Data on fetal macrosomia (birth weight ≥4 kg) and LGA were extracted from discharge records.
2.3 Laboratory procedures
Fasting venous blood samples were centrifuged (3,000 rpm ×10 min) and stored at -80°C until analysis. Thyroid function tests (FT4, TSH, TPOAb) were performed using chemiluminescent immunoassays (ADVIA Centaur XP, Siemens). Serum ferritin was quantified with Beckman Coulter kits on UniCel DxI 800 analyzers. 25-hydroxyvitamin D levels were measured via chemiluminescent microparticle immunoassay (Architect i2000SR, Abbott). For each participant, we calculated the average values of blood tests (FT4, TSH, ferritin, 25-hydroxyvitamin D) for each trimester based on all available results within that period.
2.4 Covariate classification
Pre-pregnancy BMI was categorized according to the Chinese standard: underweight (<18.5 kg/m2), normal (≥18.5, <24 kg/m2), overweight/obesity (≥24 kg/m2). Advanced maternal age was defined as ≥35 years. Delivery modes were dichotomized as spontaneous vaginal delivery (with/without instrumentation) versus cesarean section. Missing data were excluded from the analysis.
2.5 Statistical analyses
Continuous variables underwent normality assessment using Kolmogorov–Smirnov tests. Non-normally distributed data were expressed as median (interquartile range), and categorical variables as counts (percentages). Group comparisons employed Mann–Whitney U tests (non-normal), Student’s t-tests (normal), χ² tests (expected frequencies ≥5), or Fisher’s exact tests. Multivariable logistic regression models were adjusted for clinically relevant confounders identified through univariate screening. Parallel mediation analyses using the SPSS PROCESS macro (Model 4) examined the ferritin/vitamin D-mediated relationships between thyroid parameters (TSH/FT4) and birth weight. Bootstrap resampling (5,000 iterations) generated 95% confidence intervals for direct/indirect effects. Mediation proportion was calculated as (indirect effect/total effect)×100%. Analyses were stratified by trimester with a significance threshold at p<0.05. All analyses were performed using IBM SPSS 26.0 (Armonk, NY).
3 Results
3.1 Basic characteristic of the study population
A total of 102,115 cases were enrolled, and ultimately, 81,488 were included to establish a historical cohort. Records with a discharge diagnosis of “gestational diabetes mellitus” were assigned to the GDM group (totaling 8,868), while the remaining records were divided into the non-GDM group (totaling 72,620) (Figure 1). Table 1 presents the baseline characteristics of the participants stratified by GDM diagnosis status. As expected, the participants in the GDM group were more likely to be ≥30 years and have a family history of hypertension or diabetes. The prevalence of overweight or obesity was also significantly higher in the GDM group compared to the non-GDM group. Additionally, gestational age at delivery was shorter in the participants in the GDM group, and the rate of spontaneous vaginal delivery (without instrumental assistance) was significantly lower (57.4% vs. 63.9%). Notably, the prevalence of TPOAb positivity was similar between the two groups.
3.2 The GDM population exhibited distinct thyroid function and nutritional marker profiles
The prenatal examination results stratified by GDM status are presented in Table 2. Significant differences were observed in thyroid function markers (TSH, FT4) and nutritional indicators (ferritin, vitamin D) between the GDM and non-GDM groups across pregnancy stages. In the GDM group, TSH and FT4 levels showed significant differences during the first and second trimesters. Specifically, in the first trimester, the GDM group exhibited higher TSH levels (p = 0.006) and lower FT4 levels (p < 0.001) compared to the non-GDM group. In the second trimester, while TSH levels increased and FT4 levels decreased in both groups, the GDM group maintained lower levels of both markers (TSH: p = 0.001; FT4: p < 0.001). No significant differences in TSH or FT4 levels were observed between the groups in late pregnancy.

Table 2. Distribution of thyroid function indicators and incidence of hypothyroid diseases in the participants in the GDM and non-GDM groups.
Additionally, maternal serum vitamin D levels progressively increased throughout pregnancy, whereas ferritin levels declined. Notably, the patients with GDM demonstrated consistently higher vitamin D and ferritin levels compared to the non-GDM patients across all gestational stages (Table 2).
3.3 Trimester-specific analysis of maternal thyroid dysfunction and pregnancy outcomes
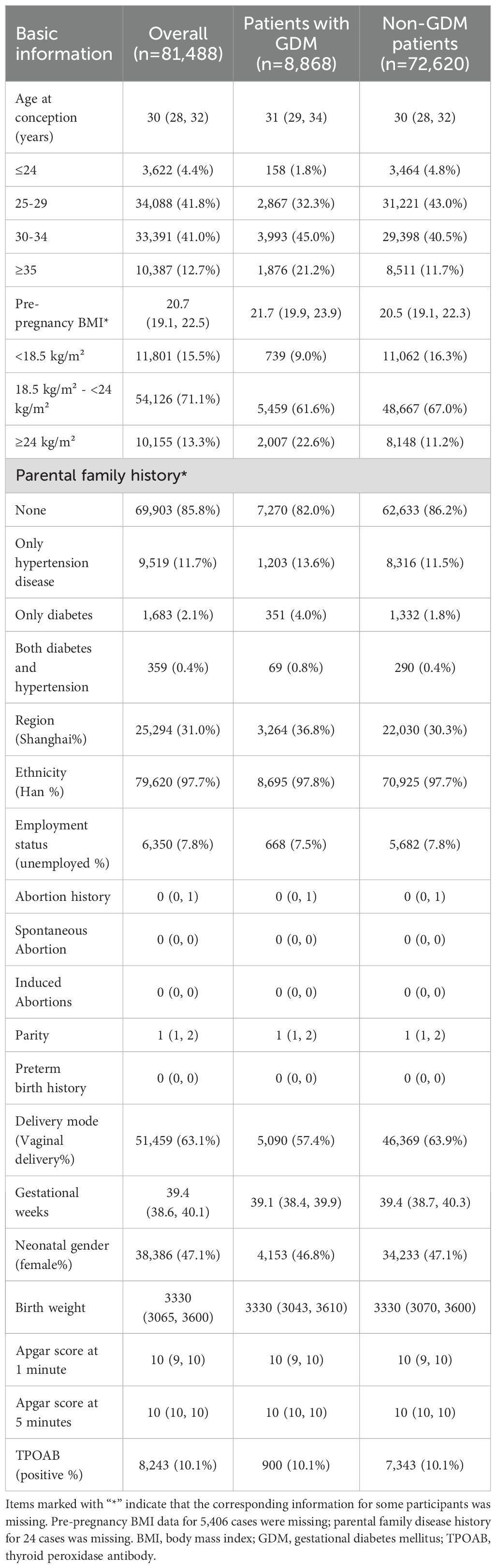
We identified adverse pregnancy outcomes that were significantly associated with maternal thyroid dysfunction during specific gestational trimesters, setting p<0.01 as the significance level. The primary exposure of thyroid dysfunction was classified according to trimester-specific reference ranges (Supplementary Table S1) into three categories: (1) isolated hypothyroxinemia (FT4<2.5th percentile [P2.5]), (2) isolated elevated TSH [>97.5th percentile (P97.5)], and (3) combined dysfunction (FT4<P2.5 and TSH>P97.5), as illustrated in the forms of a table and forest plot in Figures 2-4. The association of the three types of thyroid dysfunction with each outcome exhibited distinct trimester-specific patterns. During the first trimester, no significant relation was found between TSH, FT4, and adverse outcomes (Figure 2). The second-trimester analysis revealed that isolated hypothyroxinemia increased LGA/macrosomia risk by 55.1% [adjusted odds ratio (OR)=1.551, 95% confidence interval (CI): 1.271–1.874], while isolated TSH elevation showed the opposite effect (adjusted OR=0.689, 95% CI: 0.527–0.882). Notably, combined thyroid dysfunction in the second trimester demonstrated a strong association with preterm birth (adjusted OR=2.471, 95%CI: 1.234–4.478), after adjusting for confounders (Figure 3). The third-trimester analysis revealed particularly strong associations between thyroid dysfunction and HDP. The highest risk was observed in women with combined hypothyroxinemia and elevated TSH (FT4<P2.5% and TSH>P97.5%), demonstrating a 3.3-fold increased risk (adjusted OR=3.279, 95% CI: 1.221–7.375). Isolated hypothyroxinemia was associated with a 2.0-fold increased HDP risk (adjusted OR=2.010, 95% CI:1.260–3.057). Though it was not significant, isolated TSH elevation still showed a 1.4-fold risk increase (adjusted OR=1.420, 95% CI:1.059–1.865, p=0.015). All associations remained statistically significant after adjustment for potential confounders. The results demonstrated a robust trimester-specific relationship between thyroid dysfunction and pregnancy outcomes.
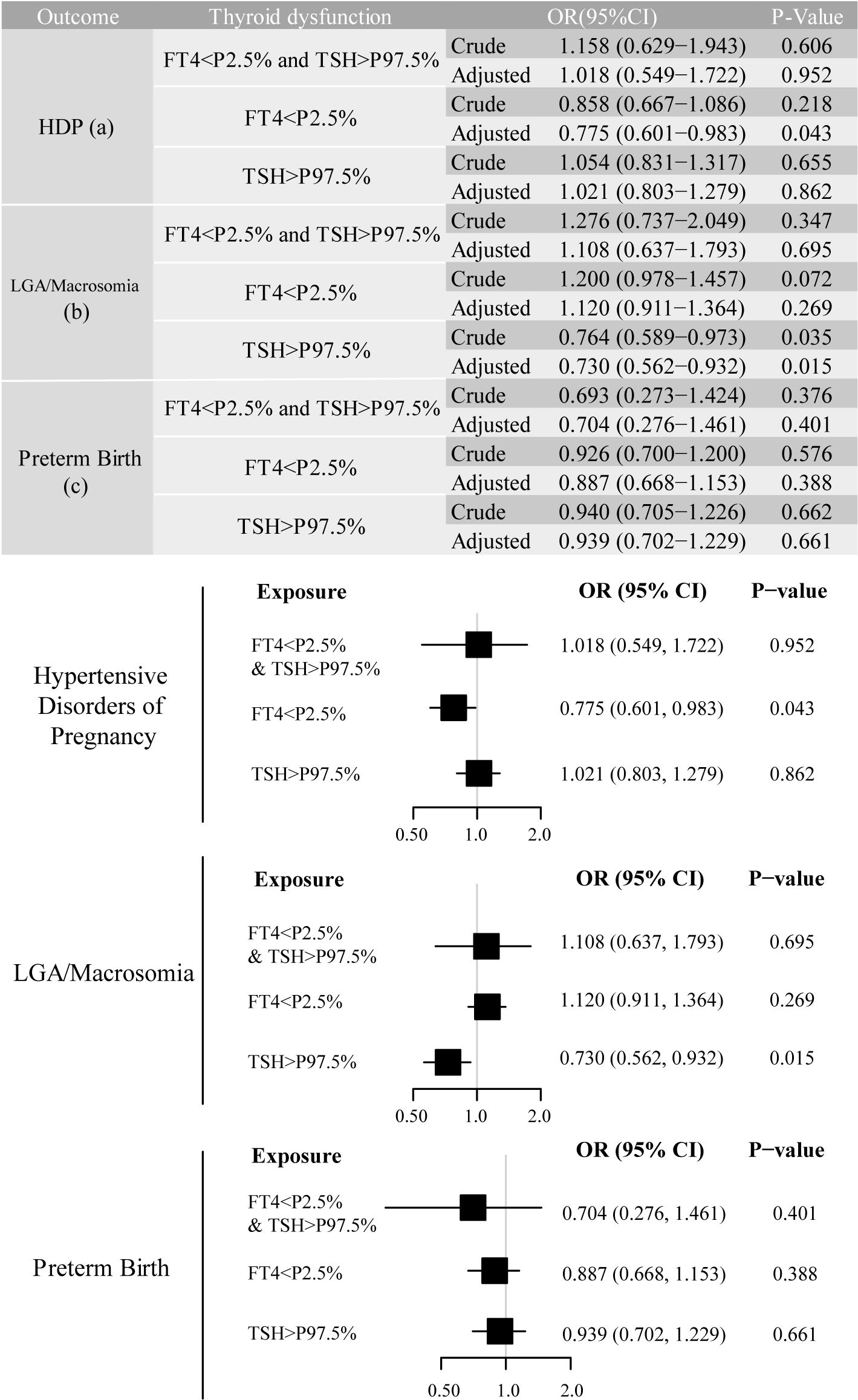
Figure 2. Adjusted odds ratios (95% CI) for pregnancy outcomes by first-trimester maternal thyroid function. The results were derived from logistic regression with outcome-specific covariate adjustments: hypertensive disorders of pregnancy (adjusted for maternal age, pre-pregnancy BMI, and delivery mode); large for gestational age/macrosomia (adjusted for newborn sex, pre-pregnancy BMI, and delivery mode), and preterm birth (adjusted for maternal age, newborn sex, pre-pregnancy BMI, and delivery mode), and the adjusted ORs are specifically depicted in forest plots. The regression analysis included three exposure groups divided by first-trimester FT4 and TSH levels: (1) FT4 < 2.5th percentile (P2.5); (2) TSH > 97.5th percentile (P97.5); (3) concurrent FT4 < P2.5 and TSH > P97.5. The reference group consisted of euthyroid pregnancies in this trimester. Statistical significance was set at P-value ≤ 0.01. CI, confidence interval; HDP, hypertensive disorders of pregnancy; LGA, large for gestational age; OR, odds ratio.

Figure 3. Adjusted odds ratios (95% CI) for pregnancy outcomes by second-trimester maternal thyroid function. The results were derived from logistic regression with outcome-specific covariate adjustments, as aforementioned. The adjusted ORs are specifically depicted in forest plots. The regression analysis included three exposure groups divided by second-trimester FT4 and TSH levels: (1) FT4 < P2.5; (2) TSH > P97.5; (3) concurrent FT4 < P2.5 and TSH > P97.5. The reference group consisted of euthyroid pregnancies in this trimester. Significant associations (P-value ≤ 0.01) are shown in red in the forest plots. CI, confidence interval; HDP, hypertensive disorders of pregnancy; LGA, large for gestational age; OR, odds ratio.
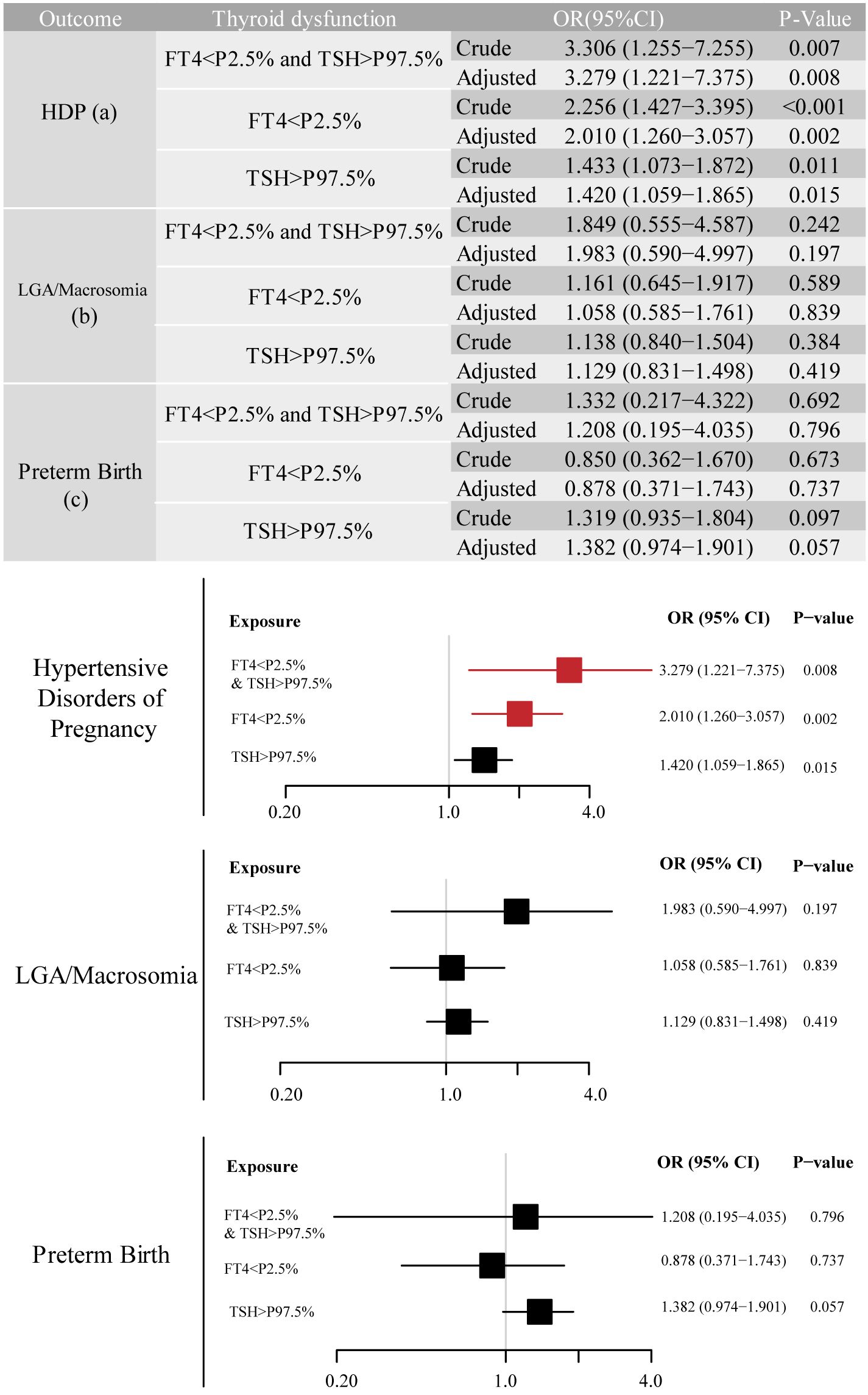
Figure 4. Adjusted odds ratios (95% CI) for pregnancy outcomes by third-trimester maternal thyroid function. The results were derived from logistic regression with outcome-specific covariate adjustments, as aforementioned. The adjusted ORs are specifically depicted in forest plots. The regression analysis included three exposure groups divided by third-trimester FT4 and TSH levels: (1) FT4 < P2.5; (2) TSH > P97.5; (3) concurrent FT4 < P2.5 and TSH > P97.5. The reference group consisted of euthyroid pregnancies in this trimester. Significant associations (P-value ≤ 0.01) are shown in red in the forest plots. CI, confidence interval; HDP, hypertensive disorders of pregnancy; LGA, large for gestational age; OR, odds ratio.
3.4 Comprehensive analysis of the impact of GDM and thyroid dysfunction on pregnancy outcomes
Prior to the comprehensive analyses, we also analyzed the relationship between isolated GDM status and adverse pregnancy outcomes (Supplementary Table S2). GDM was associated with elevated risks of HDP (adjusted OR=1.227, 95% CI:1.116–1.349) and preterm birth (adjusted OR=1.141, 95% CI:1.013–1.286). Before confounder adjustment, the OR for LGA/macrosomia in the patients with GDM was 1.223 (1.113, 1.343) compared to the non-GDM cohort, while the OR was insignificant after adjustment (adjusted OR=0.997, 95% CI: 0.905–1.098).
Based on the study cohort, we revealed that mid- and late-pregnancy thyroid function parameters demonstrated stronger associations with adverse perinatal outcomes compared to first-trimester measures. Thus, logistic regression models to evaluate the comprehensive effects of second/third-trimester maternal thyroid function, GDM, and other confounders on pregnancy outcomes were constructed. Across all the outcomes and complications, Model 1 assessed thyroid function markers alone (TSH and FT4 distribution, serving as continuous or categorical variables), Model 2 incorporated GDM status, and Model 3 included full adjustment for confounders.
According to Figure 5, continuous FT4 levels were inversely associated with LGA/macrosomia risk in all the models (fully-adjusted OR=0.936 per unit increase, 95% CI: 0.913–0.959, p<0.001). Isolated hypothyroxinemia (FT4<P2.5%) demonstrated progressively attenuated but persistently significant effects across models: crude OR=1.560 (95% CI: 1.294–1.880, p<0.001), GDM-adjusted OR=1.547 (95% CI: 1.284–1.865, p<0.001), and fully-adjusted OR=1.464 (95% CI: 1.211–1.768, p<0.001), while isolated TSH elevation showed reversed effects (OR=0.639, 95% CI: 0.499–0.819). In mid-pregnancy, for all forms of thyroid function, GDM diagnosis initially appeared to be a significant covariate, but its effect became non-significant after full adjustment. Moreover, second-trimester combined dysfunction (FT4<P2.5% and TSH>P97.5%) was associated with a 2.039-fold increased preterm birth risk (95% CI: 1.105–3.763, p=0.023), remaining significant in the fully-adjusted model (Model 3 OR=2.208, 95% CI: 1.174–4.153, p=0.014). A GDM diagnosis further increased preterm birth risk by 13.9% (95% CI: 1.010–1.283, p=0.034).
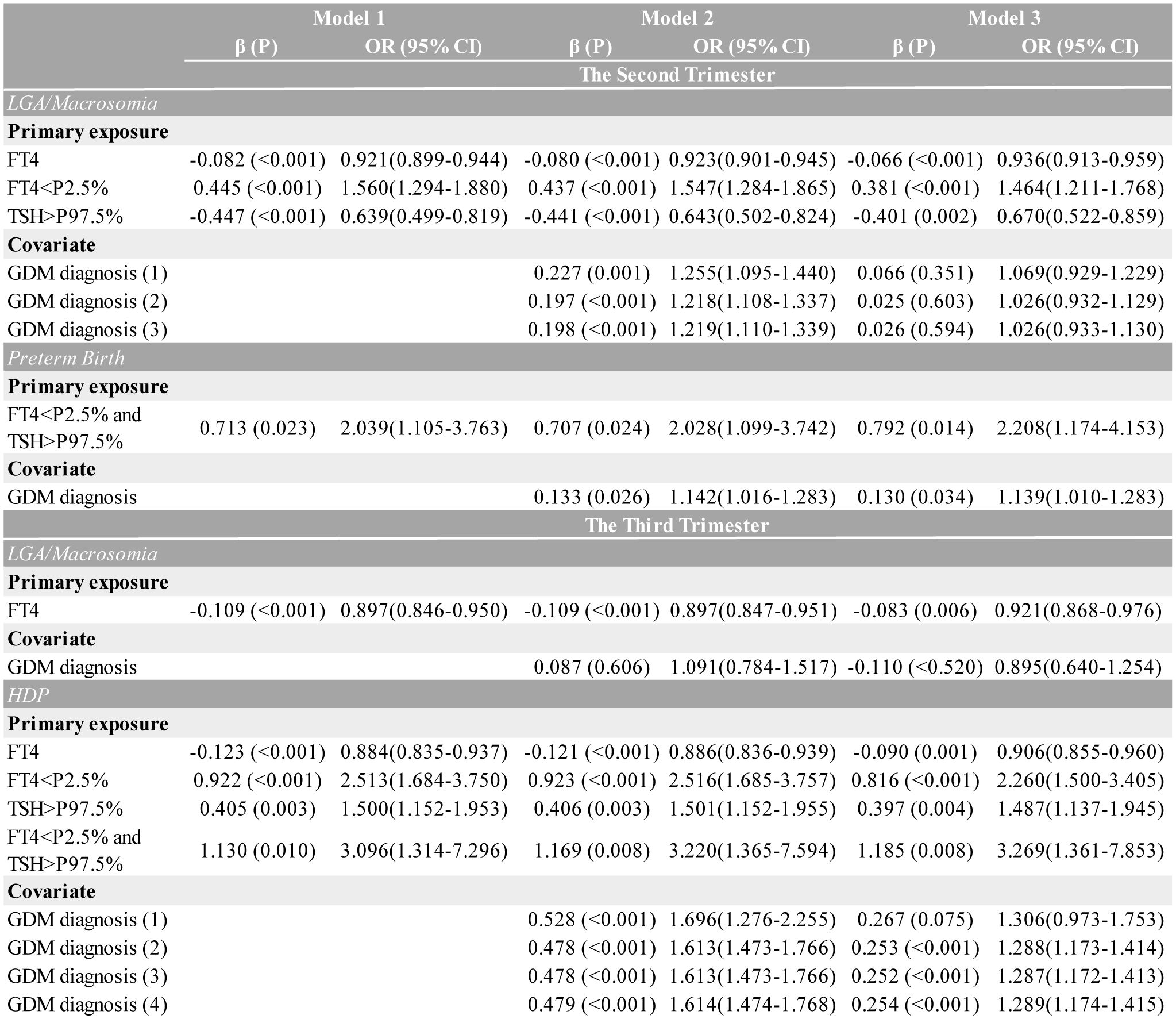
Figure 5. Multivariate logistic regression models presenting the association between second/third-trimester thyroid function and different outcomes, adjusted for GDM diagnosis. Three multivariable models were constructed: Model 1 examined thyroid function (using FT4 as a continuous variable; isolated low FT4, isolated high TSH, and combined low FT4/high TSH as categorical variables) alone. Model 2 added gestational diabetes (GDM) diagnosis; the superscripts (1)–(4) stand for distinct adjustments applied sequentially to each thyroid variable (listed top-to-bottom in the sub-table of each disease). Model 3 was fully adjusted for outcome-specific perinatal factors, including hypertensive disorders of pregnancy (adjusted for maternal age, pre-pregnancy BMI, and delivery mode), large for gestational age/macrosomia (adjusted for newborn sex, pre-pregnancy BMI, and delivery mode), preterm birth (adjusted for maternal age, newborn sex, pre-pregnancy BMI, and delivery mode), thyroid function, and GDM diagnosis. Only GDM ORs are displayed for covariates. CI, confidence interval; FT4, free thyroxine; OR, odds ratio.
The third-trimester analyses revealed significant associations between thyroid dysfunction and HDP risk across multiple modeling approaches. As a continuous variable, each unit increase in FT4 was associated with a 9.4% reduction in HDP risk (fully-adjusted OR=0.906, 95% CI: 0.855–0.960, p=0.001). When analyzed categorically, isolated hypothyroxinemia showed progressively attenuated but persistently significant effects: crude OR=2.513 (95% CI: 1.684–3.750, p<0.001), GDM-adjusted OR=2.516 (95% CI: 1.685–3.757, p<0.001), and fully-adjusted OR=2.260 (95% CI: 1.500–3.405, p<0.001). Similarly, isolated TSH elevation maintained significant associations throughout model adjustments (crude OR=1.500, 95% CI: 1.152–1.953; fully-adjusted OR=1.487, 95% CI: 1.137–1.945). The strongest association was observed for combined dysfunction, with risk estimates increasing from the crude OR=3.096 (95% CI: 1.314–7.296) to the fully-adjusted OR=3.269 (95% CI: 1.361–7.853). While GDM diagnosis showed initial strong associations (Model 2 OR=1.613, 95% CI: 1.473–1.766), these effects were attenuated but remained significant after full adjustment (OR=1.287, 95% CI: 1.172–1.413). Notably, the predictive value of continuous FT4 levels remained stable regardless of GDM adjustment.
3.5 Ferritin potentially mediates thyroid-birth weight association
According to the prior result, we found a relationship between second-trimester thyroid function and LGA/macrosomia risk (as illustrated in Figures 3 and 5). Given the observed elevation of both vitamin D and ferritin levels in patients with GDM (Table 2), we performed parallel mediation analyses to examine their potential mediating roles in the thyroid function-birth weight relationship.
Figure 6 demonstrates the consistent negative correlations of maternal serum ferritin levels with neonatal birth weight across all gestational stages, and it shows the positive association with FT4 levels and negative association with TSH levels throughout pregnancy. This finding provides a potential mechanism explaining the inconsistent relationship between maternal TSH level and neonatal birthweight, especially the reduced macrosomia risk associated with an increased second-trimester isolated TSH level. The mediating effect of ferritin was most pronounced during the third trimester, where it mediated 22.12% of the total effect of third-trimester FT4 on birth weight, and the third trimester is also a critical period with the most fetal weight gain. However, no significant evidence supported vitamin D as an effective mediating factor in the relationship between maternal thyroid function and neonatal birth weight during any stage of pregnancy.
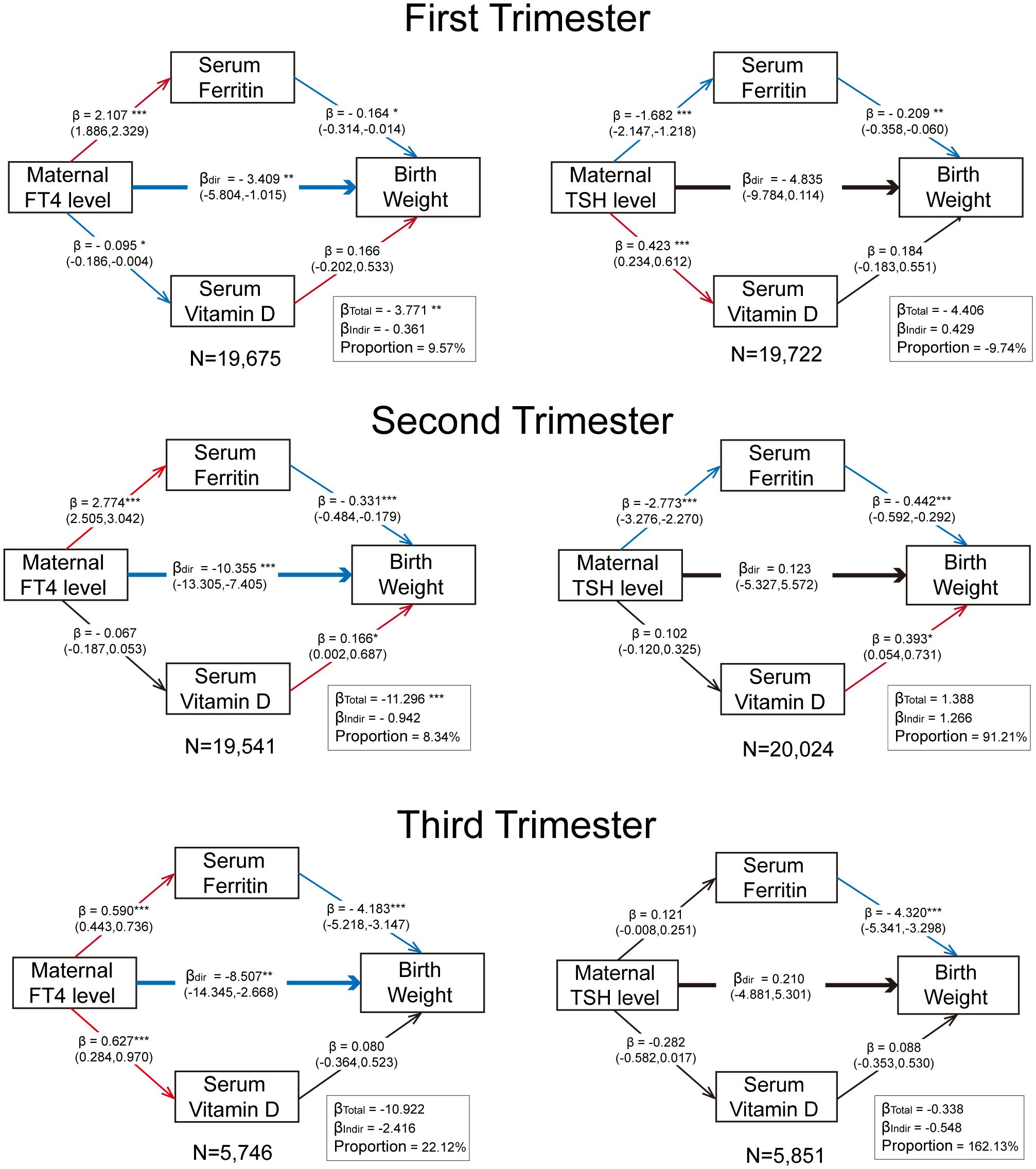
Figure 6. Trimester-specific mediation effect of maternal ferritin and vitamin D levels on the relationship between maternal thyroid function and birthweight. All models were adjusted for pre-pregnancy BMI and age at conception. The values in parentheses represent 95% CIs. The significance levels are indicated as follows: “*” for p < 0.05, “**” for p < 0.01, and “***” for p < 0.001. The proportion refers to the ratio of the indirect effect to the total effect. Red arrows denote significant positive correlations (p < 0.05), blue arrows denote significant negative correlations, and black arrows indicate non-significant relationships. The sample size for each analysis is displayed following “N =“.
4 Discussion
The role of thyroid hormones in glucose metabolism is well-established, with demonstrated effects on hepatic glucose metabolism, insulin secretion, and β-cell function. These mechanisms link thyroid function to hyperglycemia and insulin resistance, even in euthyroid individuals (25–28). While clinical studies frequently associate GDM with thyroid disorders, particularly autoimmune thyroid diseases (29, 30), the underlying mechanisms remain poorly understood beyond known comorbid factors like obesity. Intriguingly, in this cohort, TPOAb positivity rates were comparable between the GDM and non-GDM groups, yet thyroid function differences persisted. This discrepancy may reflect the necessity of removing cases with pre-existing type 1 or type 2 diabetes, which helped to exclude the confounding effect of heightened autoimmunity.
Emerging evidence implicates thyroid dysfunction in increased macrosomia/LGA risk, especially hypothyroidism-related conditions (17, 31, 32). LGA fetuses frequently exhibit metabolic disturbances, the reduced placental transfer of thyroid hormones triggers compensatory fetal hypothalamic-pituitary-thyroid axis activation and increased endogenous fetal thyroid hormone production. This results in accelerated fetal metabolism with enhanced lipolysis and altered glucose utilization, promoting excessive growth (33). Concurrently, elevated fetal leptin levels and insulin-like growth factor (IGF) axis dysregulation further contribute to macrosomia (34). The metabolic profile includes increased oxidative stress and pro-inflammatory cytokine activation, which may impair placental vascular function and nutrient transport efficiency (35). These metabolic adaptations predispose to neonatal hypoglycemia and altered adipokine profiles, with potential long-term implications for obesity risk and metabolic dysfunction in offspring (36, 37). However, our findings suggest that assessing fetal growth solely by neonatal birth weight may lead to the neglect of metabolic issues in offspring of pregnant women with concurrent GDM and thyroid dysfunction, especially in those with normal pre-pregnancy weight.
After adjusting for confounders (such as pre-pregnancy BMI), the difference in LGA/macrosomia rates among the women with GDM and the non-GDM women became statistically insignificant, indicating that it was probably caused by the oversupply of maternal fatty acids and amino acids due to higher BMI, or the genetic and gender characteristics of the infant, more than GDM itself (38). Meanwhile, it was well-established in our study that the GDM cohort exhibited elevated maternal serum ferritin, while high level of ferritin was simultaneously accompanied by high FT4 and low TSH levels. As for ferritin, it exhibits a dual role of both iron reserves and an inflammation indicator. High ferritin levels in patients with GDM can be due to chronic inflammation (IL-6, TNF-α-driven ferritin synthesis) and insulin resistance (enhanced intestinal iron absorption) contributes to paradoxical iron overload (39), which may impair placental function via oxidative stress (ROS) (40) and provide a possible explanation for the negative relationship between ferritin level and neonatal birthweight. The low ferritin levels in low-FT4 individuals and the elevated ferritin levels in those with GDM may have counteracting effects, potentially maintaining neonatal birth weight within the normal range. This phenomenon could mask the metabolic abnormalities in offspring associated with these two maternal conditions.
Furthermore, the study also confirmed a significant positive association between GDM and both HDP and preterm delivery after confounder adjustment. The potential mechanism may involve diabetes-related polyhydramnios, leading to overpressure on the cervix (41) and elevated inferior abdominal pressure.
The identification of trimester-specific thyroid function patterns in patients with GDM highlights the necessity for dynamic monitoring of thyroid hormones during pregnancy, especially in high-risk populations. Additionally, our findings suggest that maternal ferritin levels may act as a mediator in the association between GDM/thyroid dysfunction and neonatal weight, offering a potential biomarker for risk stratification. Clinically, these results advocate for integrated screening protocols that simultaneously evaluate thyroid function and glucose homeostasis in pregnant women, enabling timely interventions to mitigate the risks of preterm birth, HDP, and fetal overgrowth. Future research should explore mechanistic links and intervention strategies to optimize pregnancy management in high-risk populations.
Several limitations should be acknowledged. The retrospective design may introduce selection bias and unaccounted confounders, such as dietary habits and other lifestyle factors. Incomplete follow-up data, particularly for third-trimester thyroid function tests, could compromise the accuracy of late-pregnancy risk assessments. The lack of detailed treatment records for the patients with GDM precludes an analysis of how glycemic control or medication use may modify outcomes. Moreover, the single-center Chinese cohort may limit generalizability to other populations.
5 Conclusion
This study demonstrates that trimester-specific maternal thyroid dysfunction differentially influences pregnancy outcomes, with the effects amplified by GDM status and partially mediated by ferritin levels. The relationship between thyroid hormones and glucose metabolism underscores the necessity of comprehensive endocrine evaluations during pregnancy. Our findings advocate for personalized antenatal care that integrates thyroid function monitoring with metabolic and nutritional assessments, particularly in women with GDM or obesity. By addressing these interconnected pathways, clinicians may better predict and prevent complications, ultimately improving maternal and neonatal health outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University. The studies were conducted in accordance with the local legislation and institutional requirements. In this study, due to its retrospective nature, all data were sourced from existing medical records and databases, involving no new patient interventions or data collection. Therefore, written informed consent was not required as approved by the ethics committee. The use of all data complied with relevant privacy protection and data security regulations, ensuring the confidentiality and anonymity of patient information.
Author contributions
CZ: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft. QS: Supervision, Writing – review & editing. YY: Writing – review & editing. YL: Writing – review & editing. QD: Conceptualization, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the National Natural Science Foundation (grant No.82371693), Shanghai Municipal Health Commission (grant No.202340113), Pudong New Area Health Commission (grant No. PW2023E-04), and Open Project of Shanghai Key Laboratory of Maternal and Fetal Medicine (grant No. mfmkf202202).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1555409/full#supplementary-material
References
1. Moon JH and Jang HC. Gestational diabetes mellitus: diagnostic approaches and maternal-offspring complications. Diabetes Metab J. (2022) 46:3–14. doi: 10.4093/dmj.2021.0335
2. Wang YX, Mitsunami M, Manson JE, Gaskins AJ, Rich-Edwards JW, Wang L, et al. Association of gestational diabetes with subsequent long-term risk of mortality. JAMA Internal Med. (2023) 183:1204–13. doi: 10.1001/jamainternmed.2023.4401
3. Oğlak SC, Yılmaz EZ, and Budak M. Abdominal subcutaneous fat thickness combined with a 50-g glucose challenge test at 24–28 weeks of pregnancy in predicting gestational diabetes mellitus. J Obstet Gynaecol: J Institute Obstet Gynaecol. (2024) 44:2329880. doi: 10.1080/01443615.2024.2329880
4. Pauza AG, Thakkar P, Tasic T, Felippe I, Bishop P, Greenwood MP, et al. GLP1R attenuates sympathetic response to high glucose via carotid body inhibition. Circ Res. (2022) 130:694–707. doi: 10.1161/circresaha.121.319874
5. Ye W, Luo C, Huang J, Li C, Liu Z, and Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ (Clinical Res ed). (2022) 377:e067946. doi: 10.1136/bmj-2021-067946
6. Amin A, Robinson S, and Teoh TG. Endocrine problems in pregnancy. Postgrad Med J. (2011) 87:116–24. doi: 10.1136/pgmj.2008.078048
7. Derakhshan A, Peeters RP, Taylor PN, Bliddal S, Carty DM, Meems M, et al. Association of maternal thyroid function with birthweight: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. (2020) 8:501–10. doi: 10.1016/s2213-8587(20)30061-9
8. Toloza FJK, Derakhshan A, Männistö T, Bliddal S, Popova PV, Carty DM, et al. Association between maternal thyroid function and risk of gestational hypertension and pre-eclampsia: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. (2022) 10:243–52. doi: 10.1016/s2213-8587(22)00007-9
9. Sankoda A, Suzuki H, Imaizumi M, Yoshihara A, Kobayashi S, Katai M, et al. Effects of levothyroxine treatment on fertility and pregnancy outcomes in subclinical hypothyroidism: A systematic review and meta-analysis of randomized controlled trials. Thyroid: Off J Am Thyroid Assoc. (2024) 34:519–30. doi: 10.1089/thy.2023.0546
10. Pinto S, Croce L, Carlier L, Cosson E, and Rotondi M. Thyroid dysfunction during gestation and gestational diabetes mellitus: a complex relationship. J Endocrinol Invest. (2023) 46:1737–59. doi: 10.1007/s40618-023-02079-3
11. Yanachkova V and Kamenov Z. The relationship between thyroid dysfunction during pregnancy and gestational diabetes mellitus. Endokrynol Polska. (2021) 72:226–31. doi: 10.5603/EP.a2021.0016
12. Yan Y, Niu Z, Sun C, Li P, Shen S, Liu S, et al. Hepatic thyroid hormone signalling modulates glucose homeostasis through the regulation of GLP-1 production via bile acid-mediated FXR antagonism. Nat Commun. (2022) 13:6408. doi: 10.1038/s41467-022-34258-w
13. Eom YS, Wilson JR, and Bernet VJ. Links between thyroid disorders and glucose homeostasis. Diabetes Metab J. (2022) 46:239–56. doi: 10.4093/dmj.2022.0013
14. Kahaly GJ and Frommer L. Polyglandular autoimmune syndromes. J Endocrinol Invest. (2018) 41:91–8. doi: 10.1007/s40618-017-0740-9
15. Biondi B, Kahaly GJ, and Robertson RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocr Rev. (2019) 40:789–824. doi: 10.1210/er.2018-00163
16. Guo J, Zhou Y, Li Q, and Zhang P. A meta-analysis of differences in thyroid and cardiac function between women with normal pregnancies and gestational diabetes mellitus. Altern Ther Health Med. (2024) 30:66–70.
17. Liu X, Zhang C, Lin Z, Zhu K, He R, Jiang Z, et al. Association of maternal mild hypothyroidism in the first and third trimesters with obstetric and perinatal outcomes: a prospective cohort study. Am J Obstet Gynecol. (2025) 232:480. doi: 10.1016/j.ajog.2024.08.047
18. Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes–a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. (2012) 12:23. doi: 10.1186/1471-2393-12-23
19. Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, and Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Internal Med. (2013) 159:123–9. doi: 10.7326/0003-4819-159-2-201307160-00661
20. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. (2023) 46(Suppl 1):S19–S40. doi: 10.2337/dc23-S002
21. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid: Off J Am Thyroid Assoc. (2011) 21:1081–125. doi: 10.1089/thy.2011.0087
22. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the american thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid: Off J Am Thyroid Assoc. (2017) 27:315–89. doi: 10.1089/thy.2016.0457
23. American Diabetes Association Professional Practice Committee: ElSayed NA, Aleppo G, Bannuru RR, Bruemmer D, Collins BS, Ekhlaspour L, et al.Management of diabetes in pregnancy: standards of care in diabetes-2024. Diabetes Care. (2024) 47:S282–s94. doi: 10.2337/dc24-S015
24. Wu J, Lu AD, Zhang LP, Zuo YX, and Jia YP. Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi. (2019) 40:52–7. doi: 10.3760/cma.j.issn.0253-2727.2019.01.010
25. Wang D, Wan S, Liu P, Meng F, Zhang X, Ren B, et al. Relationship between excess iodine, thyroid function, blood pressure, and blood glucose level in adults, pregnant women, and lactating women: A cross-sectional study. Ecotoxicol Environ Safety. (2021) 208:111706. doi: 10.1016/j.ecoenv.2020.111706
26. Chauhan A and Patel SS. Thyroid hormone and diabetes mellitus interplay: making management of comorbid disorders complicated. Hormone Metab Res = Hormon und Stoffwechselforschung = Hormones Metabo. (2024) 56:845–58. doi: 10.1055/a-2374-8756
27. He J, Lai Y, Yang J, Yao Y, Li Y, Teng W, et al. The relationship between thyroid function and metabolic syndrome and its components: A cross-sectional study in a Chinese population. Front Endocrinol. (2021) 12:661160. doi: 10.3389/fendo.2021.661160
28. Pavan Kumar J and UM A. Comparison of thyroid function tests among type 2 diabetes patients with and without diabetic nephropathy and controls. Cureus. (2024) 16:e70462. doi: 10.7759/cureus.70462
29. Konar H, Sarkar M, and Roy M. Association of thyroid dysfunction and autoimmunity in pregnant women with diabetes mellitus. J Obstet Gynaecol India. (2018) 68:283–8. doi: 10.1007/s13224-017-1033-0
30. Alotaibi EA, AlHaidar AM, Alotaibi SA, Alshehri NA, Alotaibi RA, Bashumeel YY, et al. Assessment of thyroid dysfunction among pregnant women with pre-existing diabetes mellitus or gestational diabetes mellitus. Cureus. (2023) 15:e44390. doi: 10.7759/cureus.44390
31. Sankoda A, Arata N, Sato S, Umehara N, Morisaki N, Ito Y, et al. Association of isolated hypothyroxinemia and subclinical hypothyroidism with birthweight: A cohort study in Japan. J Endocr Soc. (2023) 7:bvad045. doi: 10.1210/jendso/bvad045
32. Zhu YD, Han Y, Huang K, Zhu BB, Yan SQ, Ge X, et al. The impact of isolated maternal hypothyroxinaemia on the incidence of large-for-gestational-age infants: the Ma’anshan Birth Cohort study. BJOG: an Int J Obstet Gynaecol. (2018) 125:1118–25. doi: 10.1111/1471-0528.15107
33. Harris SE, De Blasio MJ, Zhao X, Ma M, Davies K, Wooding FBP, et al. Thyroid deficiency before birth alters the adipose transcriptome to promote overgrowth of white adipose tissue and impair thermogenic capacity. Thyroid: Off J Am Thyroid Assoc. (2020) 30:794–805. doi: 10.1089/thy.2019.0749
34. Ambler G. Overgrowth. Best Pract Res Clin Endocrinol Metab. (2002) 16:519–46. doi: 10.1053/beem.2002.0207
35. Monaco-Brown M and Lawrence DA. Obesity and maternal-placental-fetal immunology and health. Front Pediatr. (2022) 10:859885. doi: 10.3389/fped.2022.859885
36. Dumolt J, Powell TL, Jansson T, and Rosario FJ. Normalization of maternal adiponectin in obese pregnant mice prevents programming of impaired glucose metabolism in adult offspring. FASEB J: Off Publ Fed Am Societies Exp Biol. (2022) 36:e22383. doi: 10.1096/fj.202200326R
37. Al Bekai E, Beaini CE, Kalout K, Safieddine O, Semaan S, Sahyoun F, et al. The hidden impact of gestational diabetes: unveiling offspring complications and long-term effects. Life (Basel Switzerland). (2025) 15(3):440. doi: 10.3390/life15030440
38. Castillo-Castrejon M and Powell TL. Placental nutrient transport in gestational diabetic pregnancies. Front Endocrinol (Lausanne). (2017) 8:306. doi: 10.3389/fendo.2017.00306
39. Li T, Zhang J, and Li P. Ferritin and iron supplements in gestational diabetes mellitus: less or more? Eur J Nutr. (2024) 63:67–78. doi: 10.1007/s00394-023-03250-5
40. Yang N, Wang Q, Ding B, Gong Y, Wu Y, Sun J, et al. Expression profiles and functions of ferroptosis-related genes in the placental tissue samples of early- and late-onset preeclampsia patients. BMC Pregnancy Childbirth. (2022) 22:87. doi: 10.1186/s12884-022-04423-6
Keywords: gestational diabetes mellitus, hypothyroidism, birth weight, hypertensive disorder, preterm birth
Citation: Zou C, Shen Q, Yang Y, Liao Y and Du Q (2025) Association of maternal thyroid function and gestational diabetes with pregnancy outcomes: a retrospective cohort study. Front. Endocrinol. 16:1555409. doi: 10.3389/fendo.2025.1555409
Received: 04 January 2025; Accepted: 16 May 2025;
Published: 06 June 2025.
Edited by:
Marco António Campinho, University of Algarve, PortugalReviewed by:
Cemil Oğlak, Diyarbakır Gazi Yaşargil Training and Research Hospital, TürkiyeSatyajit Kulkarni, Gujarat Ayurved University, India
Copyright © 2025 Zou, Shen, Yang, Liao and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiaoling Du, cWxkdTIwMDRAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Chang Zou
Chang Zou Qinxin Shen†
Qinxin Shen† Qiaoling Du
Qiaoling Du