- 1Department of Orthopedics, Guilin Municipal Hospital of Traditional Chinese Medicine, Guilin, Guangxi, China
- 2Graduate College, Guangxi University of Chinese Medicine, Nanning, Guangxi, China
Background: Postmenopausal women are at an increased risk of bone density reduction, with multiple factors implicated, including folate, a B vitamin whose impact on bone health is gaining attention. The purpose of this research was to examine the association between red blood cell (RBC) folate levels and lumbar bone mineral density (BMD) in postmenopausal women.
Methods: We performed a cross-sectional study to investigate the association between postmenopausal women’s lumbar BMD and RBC folate levels, using the data from the 2009–2018 National Health and Nutrition Examination Survey (NHANES). Participants were categorized into quartiles based on RBC folate levels (Q1-Q4). Univariate and multivariate regression models assessed the association between RBC folate levels and lumbar BMD, with threshold effect analysis performed.
Results: A total of 1315 postmenopausal women were included. RBC folate levels were positively associated with lumbar BMD. The trend analysis across the quartiles of RBC folate indicated a statistically significant trend in all models (P for trend: Model 1 = 0.020; Model 2 = 0.015; Model 3 = 0.037), suggesting that higher RBC folate levels are associated with increased lumbar BMD. In the unadjusted model 1, a 10 nmol/L increase in RBC folate was associated with a 0.0002 g/cm² increase in lumbar BMD (P=0.002509). The correlation was still significant (P=0.0006) even after age and race were taken into account (model 2). Further adjustment for multiple variables (model 3) showed a 0.0002 g/cm² increase in lumbar BMD per 10 nmol/L increase in RBC folate (P=0.0212). Threshold effect analysis revealed a breakpoint at 92.4 nmol/dL, suggesting a nonlinear relationship between RBC folate levels and lumbar BMD.
Conclusions: Postmenopausal women’s RBC folate levels had a positive association with their lumbar BMD. Maintaining appropriate RBC folate levels may help preserve bone density and offer a fresh approach to avoiding osteoporosis in postmenopausal women.
1 Introduction
The symptoms of osteoporosis, a systemic skeletal illnesses, involve decreased bone mass, altered bone microstructure, increased bone fragility, and a higher risk of fractures (1, 2). Predominantly affecting postmenopausal women, this condition often stems from estrogen deficiency, which is linked to decreased bone density (3, 4). The impact of osteoporosis on the quality of life is substantial, particularly among women who have undergone menopause (5, 6). It is the most prevalent disease in women and ranks second among men, with postmenopausal women having a higher incidence of hip fractures than men (7). Nutritional factors are recognized as pivotal in influencing bone density (8). Folate, or vitamin B9, is a crucial dietary component for skeletal health maintenance (9). Elevated homocysteine (Hcy) levels are identified as an independent risk factor for osteoporosis, with folate deficiency correlating to increased Hcy levels (10). Research indicates that micronutrient supplementation containing folate can modulate Hcy levels in older adult individuals (11–13). Folate supplementation is primarily achieved through balanced diets and supplements. While most research have focused on the association between folate intake and bone mineral density (BMD) or osteoporosis, fewer have studied the link between red blood cell folate and bone density (14–16). A study has demonstrated a positive correlation between serum folate levels and BMD (17). However, serum folate levels are less stable than red blood cell folate levels, necessitating further research into the relationship between red blood cell folate and BMD in postmenopausal women. Such studies could potentially enhance our understanding of bone density improvement and osteoporosis prevention, which may also contribute to the development of osteoporosis screening strategies for postmenopausal women.
2 Materials and methods
2.1 Study population
The National Health and Nutrition Examination Survey (NHANES) database, which encompasses comprehensive health and nutrition data on the US population, served as the source of data for this study’s analysis. The survey findings have been instrumental in shaping health programs and services, enhancing health awareness, and assessing the prevalence of significant diseases and illness risk factors. For our analysis, we integrated data from five consecutive two-year cycles of the NHANES, conducted from 2009 to 2018. All procedures were approved by the National Centre for Health Statistics Research Ethics Review Board, and participants presented their informed consent for the use of anonymized data in research.
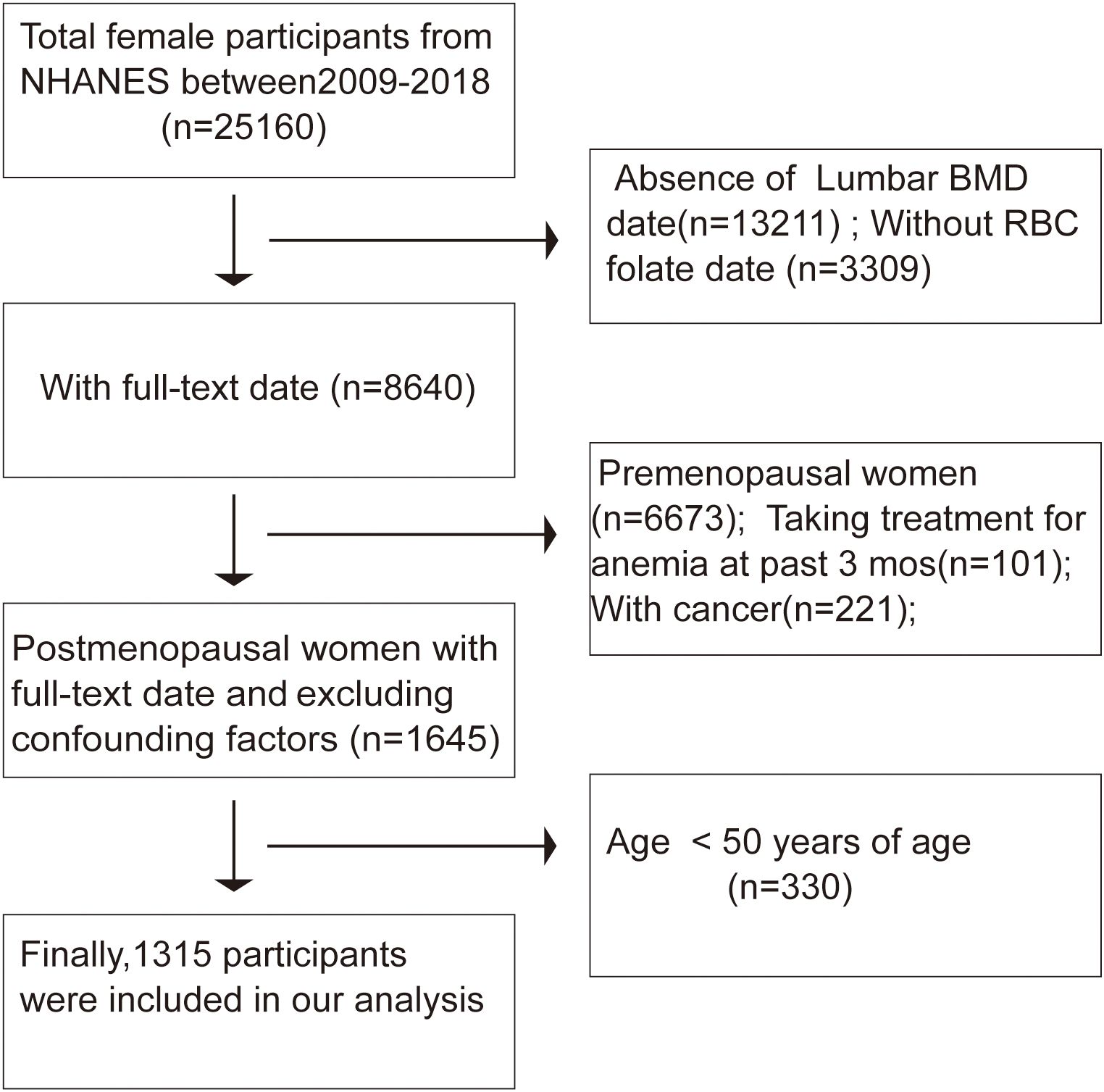
With the complete de-identified dataset, the current study was deemed exempt by the institutional review committee of the author’s institution. The study was carried out in accordance with the Helsinki Declaration. The participants in our research had to be at least 50 years old, specifically postmenopausal women, with the NHANES database yielding 1315 eligible patients during the 2009–2018 period. Out of 25,160 female participants in the survey, those lacking lumbar bone mineral density (BMD) data (n = 13,211) and those with uncertain RBC folate status (n = 3,309) were excluded. Menopausal status was ascertained based on responses to a reproductive health questionnaire. Participants were initially asked, “Have you had regular periods in the past 12 months?” Those who answered “no” were further inquired, “What is the reason for not having regular periods? (Options: Menopause/change of life; Pregnancy; Breastfeeding; Medical conditions/treatments; other)” Ultimately, 1,967 postmenopausal women were included in enrollment. Additionally, individuals who had received anemia treatment within the past three months (n = 101) or those with missing cancer values (n = 221) were not included. Consistent with previous studies (17), participants under 50 years old (n = 330) were also excluded. After screening, our analysis included data from 1,315 participants (Figure 1). A synopsis of the NHANES survey’s data-gathering techniques is available at www.cdc.gov/nchs/nhanes/. The Mobile Examination Center was used for physical and medical examinations, as well as the collecting of urine and blood samples. Data on lifestyle, health, and demographics was gathered through in-home interviews.
2.2 Folate status
Folate status in the population was assessed differently across the NHANES cycles from 2009 to 2018. For the 2009–2010 NHANES, a microbiologic assay was employed to measure whole-blood and serum folate levels, from which red blood cell folate (RBC folate) was estimated. In contrast, the folate status assessment for the 2011–2018 NHANES cycles integrated two analytical methods: serum folate levels were determined using isotope-dilution high-performance liquid chromatography- tandem mass spectrometry (LC-MS/MS), while whole-blood folate levels were ascertained through microbiologic testing. By synthesizing data from these two methodologies, RBC folate concentrations were subsequently calculated. {whole blood folate−[serum blood folate∗(1.0 − hematocrit/100)]}/(hematocrit/100) is the equation for red blood cell folate.
2.3 Covariates
The NHANES database provided additional information on potential confounders that were accounted for in our analysis. A Hologic QDR-4500A densitometer was used to measure lumbar bone mineral density (BMD), and qualified radiology technologists used established procedures. Continuous variables included age, Poverty Income Ratio (PIR), serum iron, calcium, vitamin D, total protein, total cholesterol, blood urea nitrogen, serum creatinine, and total bilirubin. Categorical variables were classified as follows: race, smoking status (defined as having smoked 100 cigarettes or more over one’s lifetime), intensity of work activity, educational level, alcohol intake (defined as having had at least four drinks in one’s life), marital status, and body mass index (BMI), which was categorized into three categories: “normal” (BMI < 25 kg/m2), “overweight” (25 ≤ BMI < 30 kg/m2), and “obese” (BMI ≥ 30 kg/m2). For continuous variables with missing values, we used mean substitution. For categorical variables, we created a separate category for those with many missing values and merged those with few missing values into an “other” category. Further details on the collection methods for RBC folate status, lumbar BMD, and other variables can be obtained on the NHANES website(http://www.cdc.gov/nchs/nhanes/).
2.4 Statistical analysis
Statistical analyses adhered to NHANES guidelines, accounting for the survey’s complex sampling design to mitigate biases from sample selection, oversampling, and nonresponse. Unweighted multiple regression analyses were conducted to describe the association between RBC folate and LBMD, with the aim of identifying the corresponding β coefficient and its 95% confidence interval (CI). To facilitate a more comprehensive analysis, RBC folate levels were processed in two distinct ways: by categorizing them into four quartiles (Q1-Q4) and by performing logarithmic transformation to treat them as a continuous variable. For continuous variables, presented as weighted means ± standard, weighted t-tests were utilized to examine differences in RBC folate levels among participants across the different quartiles. In the analysis of categorical variables, presented as weighted percentages, weighted chi-square tests were employed. For inter-group comparisons, the first quartile (Q1) was chosen as the baseline characteristics group. Additionally, to ensure the robustness of our findings, the second quartile (Q2) was considered as a back-up baseline feature. In constructing the regression models, adherence to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) standards was strictly observed (18). Three regression models were designed as follows: Model 1 was the unadjusted base model; Model 2 incorporated adjustments for race and age; and Model 3 included comprehensive adjustments for all other covariates. To further explore potential moderating factors, subgroup analyses were conducted, stratifying the data by race, educational level, and smoking status. Additionally, we employed a piecewise linear regression model to determine possible inflection points. All statistical analyses were performed using EmpowerStats (version 5.0) and R (version 3.4.3), with a p-value of less than 0.05 considered statistically significant.
3 Results
3.1 Baseline characteristics
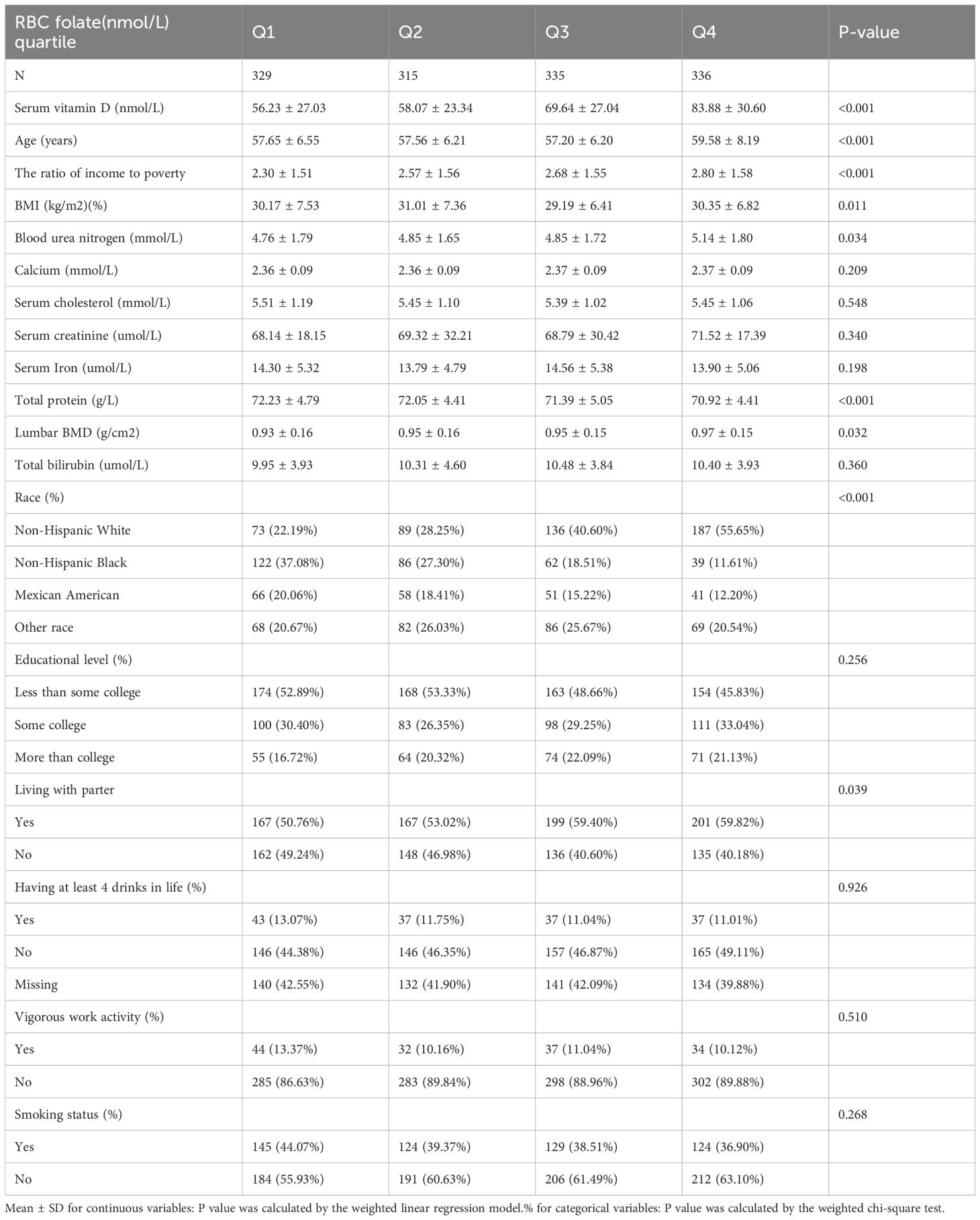
Table 1 provides specifics on the baseline characteristics of the 1,315 research participants. Participants’ red blood cell folate levels were divided into four quartiles. Compared to the first quartile (Q1), individuals in the higher quartile groups exhibited significantly higher levels of serum vitamin D, blood urea nitrogen, and PIR. Additionally, a larger proportion of participants in these quartiles identified as Non-Hispanic White. Furthermore, a greater percentage of participants in the upper quartiles reported never having smoked 100 cigarettes or more over one’s lifetime.
3.2 Associations of RBC folate status with LBMD
As presented in Table 2, the trend analysis across quartiles of RBC folate levels revealed a statistically significant increasing trend in all models for the association with lumbar bone mineral density (BMD) (P for trend: Model 1 = 0.020; Model 2 = 0.015; Model 3 = 0.037). This suggests that higher RBC folate levels are linked to greater lumbar BMD. However, when Q2 was used as the reference category instead of Q1, the association’s significance was altered.
Subgroup analyses by race, educational level, and smoking status were conducted, and the results were consistent, as shown in Table 3. In the unadjusted model, lumbar BMD increased by 0.0002 g/cm² for every 10 nmol/L rise in RBC folate (P = 0.0025). When age and race were taken into account, this relationship was still significant (Adjust I model, P = 0.0006). Further adjustment for additional variables (Adjust II model) retained the significance, with a 0.0002 g/cm² increase in lumbar BMD for each 10 nmol/L increase in RBC folate (P = 0.0212), although the statistical significance was somewhat reduced.
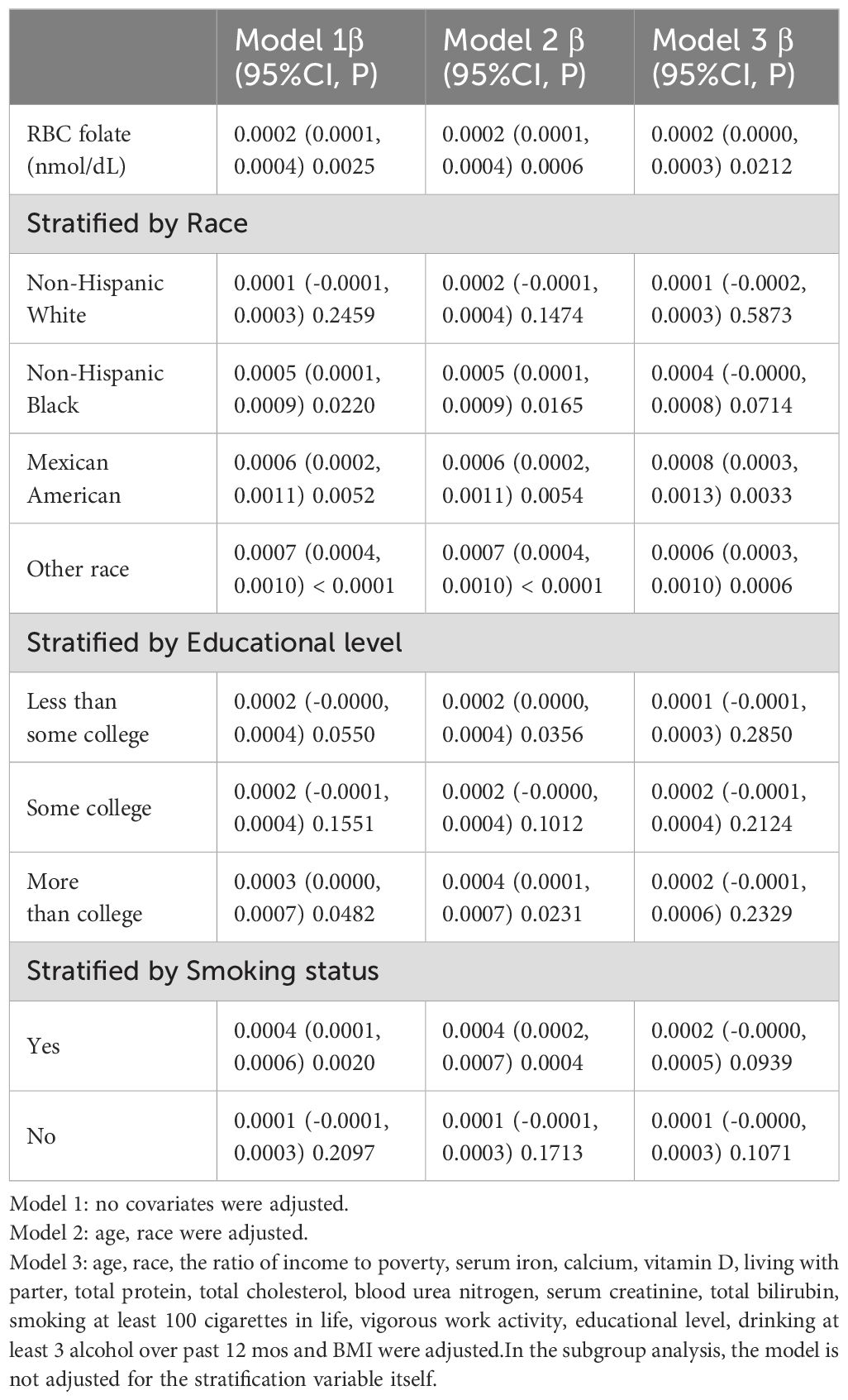
Table 3. Association of RBC folate with lumbar BMD,stratified by race, educational level or smoking.
Furthermore, the lumbar BMD of postmenopausal women showed a U-shaped association with erythrocyte folate levels (Figure 2). To explore the potential non-linear relationship between erythrocyte folate levels and lumbar BMD, we utilized smooth curve fitting techniques. The analysis identified a breakpoint at 92.4 nmol/dL, with effects of 0.0011 (P = 0.0015) below this threshold and 0.0002 (P = 0.1083) above it. These findings suggest a potential non-linear effect of RBC folate levels on lumbar BMD, as detailed in Table 4.
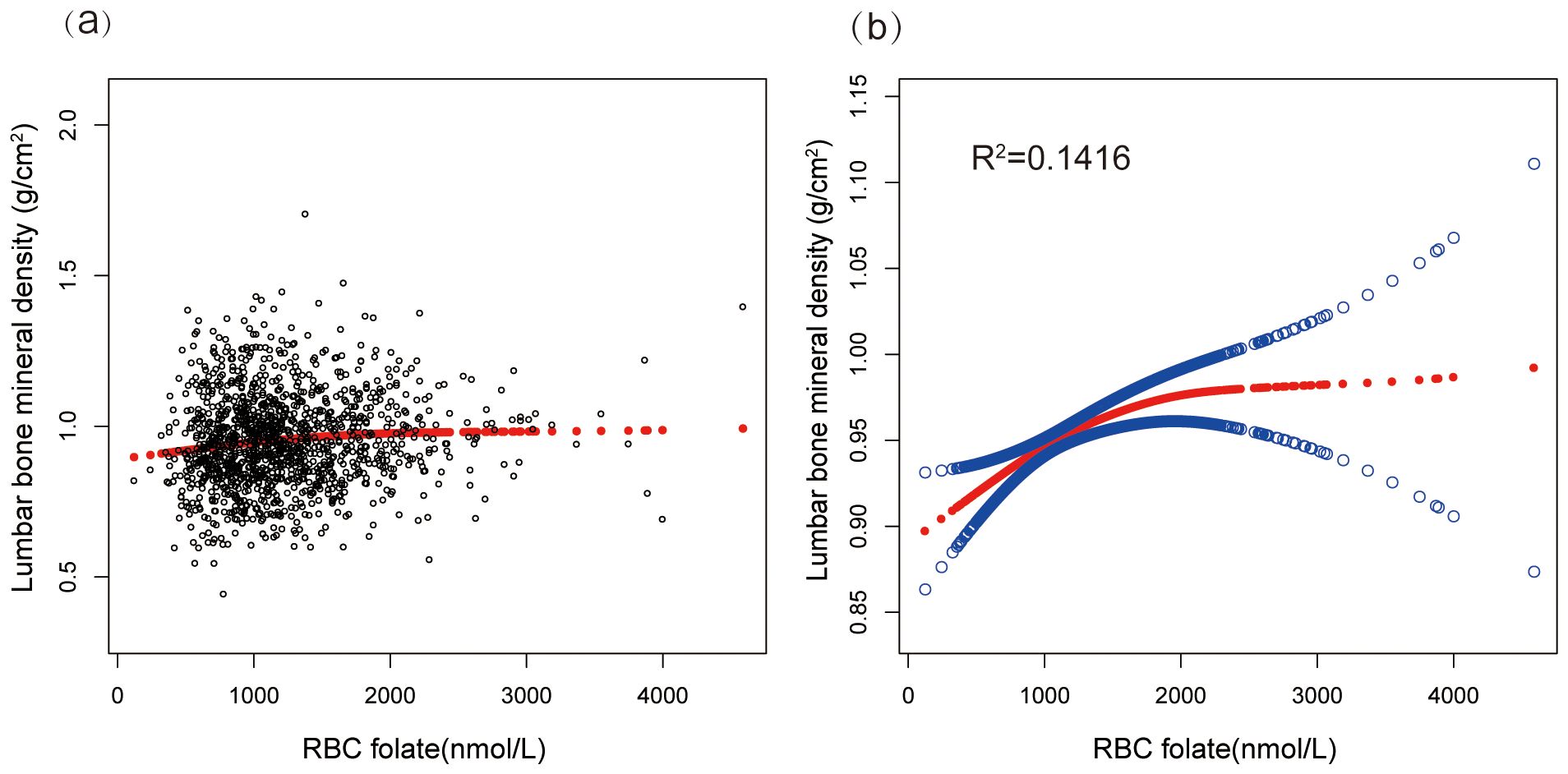
Figure 2. A scatter plot and smooth curve fitting illustrated the association between serum RBC folate level and lumbar bone mineral density. (a) Each black point represents a sample. (b) Solid red line represents the smooth curve fit between variables (R2=0.1416). Blue bands represents the 95% of confidence interval from the fit. Adjusted for age, race, the ratio of income to poverty, serum iron, calcium, vitamin D, total protein, total cholesterol, blood urea nitrogen, serum creatinine, living with parter, total bilirubin, smoking at least 100 cigarettes in life, vigorous work activity, educational level, drinking at least 3 alcohol over past 12 mos and BMI.
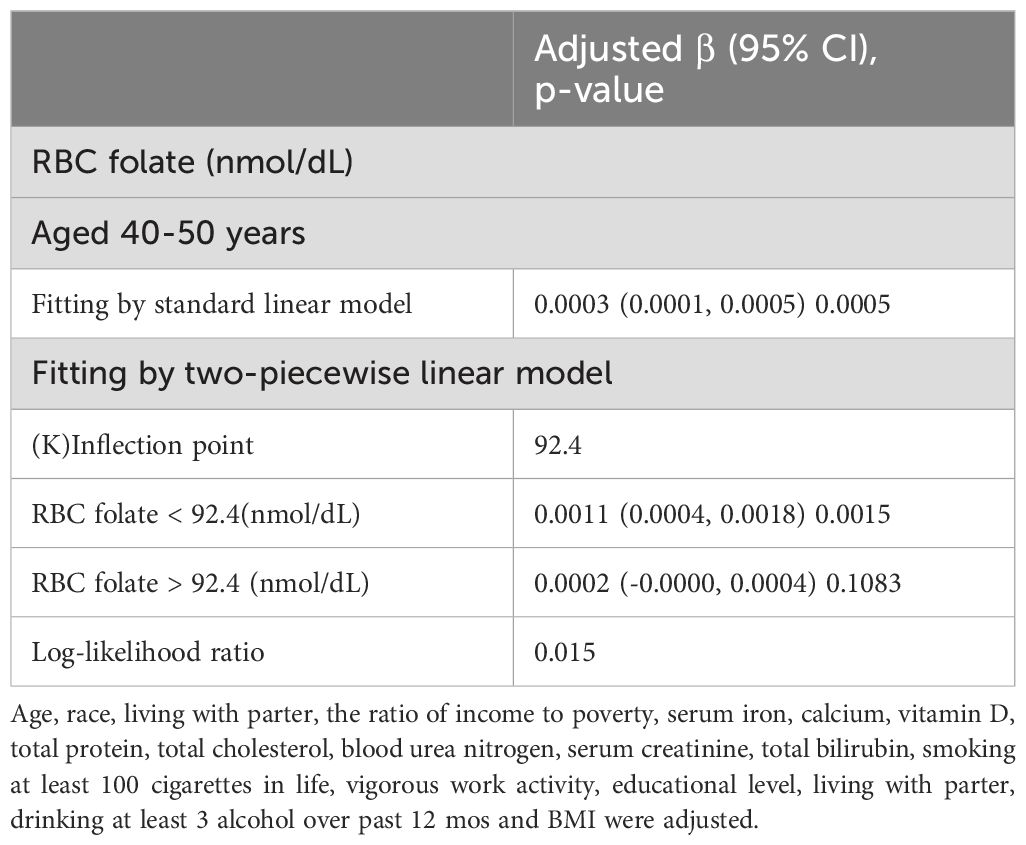
Table 4. Threshold effect analysis between RBC folate and lumbar BMD in postmenopausal women using a two-piecewise linear regression model.
4 Discussion
This study provides clear evidence that lumbar BMD in postmenopausal women is positively correlated with RBC folate levels. Our findings indicate that elevated RBC folate levels are linked to higher lumbar BMD, a relationship that remains significant even after accounting for various confounding factors. Although the significance of some associations was attenuated after multivariable adjustments, the overall trend suggests a potential role for folate in influencing bone mineral density. Additionally, a threshold effect study showed a nonlinear connection with an inflection point at 92.4 nmol/dL, suggesting that the impact of RBC folate on BMD may vary depending on the content of folate. These results underscore the importance of adequate folate levels for bone health and hint at the possibility that interventions to increase folate intake could be particularly beneficial for postmenopausal women with lower RBC folate levels.
Osteoporosis is a significant global health concern, especially among postmenopausal women, due to the heightened risk of fractures and the associated morbidity and mortality (19). Identifying modifiable dietary factors like folate that impact bone health is crucial for developing effective prevention strategies. Folate, an essential B vitamin, is involved in numerous physiological processes, including DNA synthesis, methylation, and homocysteine (Hcy) metabolism, all of which may influence bone health (20). Red blood cell folate content, which reaches a median equilibrium of 9 months following the initiation of folic acid supplementation, allows for a more comprehensive assessment of folate consumption over time (21). RBC folate are regarded to be a trustworthy indication of tissue folate levels (22, 23). This study found a significant positive association between RBC folate levels and lumbar BMD in postmenopausal women, particularly at lower folate levels. The identified inflection point at 92.4 nmol/dL suggests that while folate is beneficial for bone health, its protective effects may plateau or diminish at higher concentrations. These findings stress the importance of maintaining sufficient folate levels for bone health in postmenopausal women and suggest that interventions aimed at increasing folate intake could be especially beneficial for those with lower RBC folate levels.
Folate’s role in bone health has been increasingly recognized, particularly in postmenopausal women who are more susceptible to osteoporosis. Studies suggest that folate may protect bones through several molecular mechanisms, including Hcy regulation and oxidative stress reduction (24, 25). Hcy, a sulfur-containing amino acid and an intermediate in methionine metabolism, has been implicated in bone loss and increased fracture risk when present at elevated levels, a condition known as hyperhomocysteinemia (26). When methionine synthase catalyzes the remethylation of Hcy to methionine with vitamin B12 as a cofactor, folate is an essential component (24, 25). This process lowers circulating Hcy levels, thereby reducing its detrimental effects on bone. Elevated Hcy levels have been shown to induce oxidative stress and apoptosis in osteoblasts, the cells responsible for bone formation, while promoting osteoclastogenesis, leading to increased bone resorption (27, 28). Additionally, by decreasing nitric oxide bioavailability and elevating oxidative stress, Hcy causes endothelial dysfunction, which hinders blood flow to bone tissue and may jeopardize bone remodeling and repair (29).
Adequate folate levels are essential for maintaining low Hcy concentrations. Studies have shown that folate supplementation can significantly reduce plasma Hcy levels, thereby protecting against bone loss (30). A study by He et al. (2021) demonstrated that folic acid supplementation in a high-fat diet-induced osteoporosis mouse model reduced Hcy levels and improved bone microarchitecture through the activation of the AMPK signaling pathway (27). Clinical trials have also reported that folate supplementation, in combination with vitamin B12, effectively lowers Hcy levels and improves BMD in postmenopausal women (31). Another mechanism may involve reducing oxidative stress. Particularly in postmenopausal women, oxidative stress which is defined as an imbalance between the generation of reactive oxygen species (ROS) and antioxidant defenses is a significant cause of bone loss (25). ROS can directly damage bone cells and the extracellular matrix, as well as disrupt signaling pathways involved in bone remodeling. The antioxidant qualities of folate may be essential for reducing oxidative stress and maintaining bone health. The balance between osteoblasts (bone creation) and osteoclasts (bone resorption) is upset by excessive ROS generation because it causes osteoblast death and activates the RANKL signaling pathway, which encourages osteoclast development and activity and increases bone resorption (32, 33). ROS also degrades type I collagen and other components of the bone matrix, compromising bone strength and integrity (34). Folate contributes to oxidative stress reduction through glutathione synthesis, regulation of antioxidant enzymes, and mitochondrial protection (30). Folate is involved in the synthesis of glutathione, a major intracellular antioxidant that neutralizes ROS and protects bone cells from oxidative damage (35). Supplementing with folate has been demonstrated to increase the expression of antioxidant enzymes that scavenge reactive oxygen species (ROS) and lessen oxidative stress, including catalase and superoxide dismutase (SOD) (36). By regulating ROS generation and preserving mitochondrial membrane potential, folate maintains mitochondrial function and inhibits osteoblast apoptosis (37). Folate treatment decreased oxidative stress indicators and increased bone mineral density (BMD) in a model of postmenopausal osteoporosis, according to a research by Asbaghi et al. (38). The authors attributed these effects to enhanced glutathione synthesis and reduced RANKL expression (39). Clinical data from postmewer levels of oxidative stress biomarkers and improved bone health outcomes (40). The interplanopausal women have also shown that higher dietary folate intake is associated with loy between Hcy regulation and oxidative stress reduction highlights the multifaceted role of folate in bone health. Despite these possibilities, further research is needed to elucidate the underlying molecular mechanisms for the association between RBC folate and BMD and to develop effective interventions to prevent and treat osteoporosis.
This study has several strengths, including its nationally representative sample, the relatively stable folate status assessed, and the investigation of non-linear correlations using smooth curve fits. Nonetheless, it is critical to acknowledge some limitations. First, we cannot completely rule out the possibility of residual confounding that may affect RBC folate levels, even though we accounted for the majority of known confounders. Second, the absence of weighted regression analysis may mean that our results do not accurately reflect the relationship between red blood cell folate and lumbar BMD. Third, in line with other published research (41), we excluded patients whose age at the onset of osteoporosis was less than 50 years old, which may have introduced some statistical bias. Fourth, we only selected lumbar spine bone density for data comparison, which may not be comprehensive (42). However, many studies have made comparisons in this manner (43, 44). Fifth, causality between RBC folate levels and BMD cannot be established due to the cross-sectional character of the study, and longitudinal investigations are required to demonstrate the directionality of this connection (45, 46). Folate status, influenced by dietary intake, supplementation, and genetic factors, can vary significantly over an individual’s lifetime (47). Lastly, although the study controlled for multiple confounders, other factors such as genetic predispositions, specific dietary patterns, and gut microbiota composition may also influence the relationship between folate and BMD (48).
5 Conclusion
In conclusion, postmenopausal women’s RBC folate levels had a positive association with their lumbar BMD. Maintaining appropriate RBC folate levels may help preserve bone density and offer a fresh approach to avoiding osteoporosis in postmenopausal women.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The requirement of ethical approval was waived by Centers for Disease Control and Prevention for the studies involving humans. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because all procedures were approved by the National Centre for Health Statistics Research Ethics Review Board, and participants presented their informed consent for the use of anonymized data in research.
Author contributions
HW: Methodology, Writing – original draft, Writing – review & editing. ZJ: Data curation, Writing – review & editing. LZ: Validation, Writing – original draft. GT: Validation, Writing – original draft. SC: Formal Analysis, Writing – review & editing. XC: Writing – review & editing. YT: Methodology, Writing – review & editing. WZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study received funding from the Guilin Talent Small Highland Medical and Nursing Integration Project and Project of Guangxi University of Traditional Chinese Medicine (2022MS0067).
Acknowledgments
The authors appreciate the NHANES research participants and staff for their valuable contributions and supporting fund projects.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1559043/full#supplementary-material
Supplementary Table 1 | Association of RBC folate with lumbar BMD, stratified by gender.
Supplementary Table 2 | Association of RBC folate with lumbar BMD,stratified by age.
Supplementary Table 3 | Association of RBC folate with lumbar BMD adjusting serum vitamin D.
Supplementary Table 4 | Association of RBC folate with serum vitamin D.
Supplementary Table 5 | Association of RBC folate with lumbar BMD,stratified by Vigorous work activity.
Supplementary Table 6 | Association of RBC folate with lumbar BMD in different segments.
Abbreviations
NHANES, National Health and Nutrition Examination Survey; RBC, red blood cell; BMD, Bone Mineral Density; PIR, Poverty Income Ratio; BMI, body mass index; CI, confidence interval; DNA, deoxyribo nucleic acid; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; Hcy, Homocysteine; ROS, Reactive oxygen species; AMPK, AMP-activated protein kinase; RANKL, Receptor Activator of Nuclear Factor-kappa B Ligand; SOD, Superoxide dismutase.
References
1. Kamel HK. Postmenopausal osteoporosis: etiology, current diagnostic strategies, and nonprescription interventions. J managed Care pharm: JMCP. (2006) 12:S4–9. doi: 10.18553/jmcp.2006.12.S6-A.S4
2. McClung MR, Pinkerton JV, Blake J, Cosman FA, Lewiecki EM, Shapiro M, et alManagement of osteoporosis in postmenopausal women: the 2021 position statement of The North American Menopause Society. Menopause (New York NY). (2021) 28:973–97. doi: 10.1097/GME.0000000000001831
3. Rentzeperi E, Pegiou S, Tsakiridis I, Kalogiannidis I, Kourtis A, Mamopoulos A, et al. Diagnosis and management of osteoporosis: A comprehensive review of guidelines. Obstet gynecol survey. (2023) 78:657–81. doi: 10.1097/OGX.0000000000001181
4. Gao S, Zhao Y. Quality of life in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Qual Life Res. (2023) 32:1551–65. doi: 10.1007/s11136-022-03281-1
5. Lippuner K, Golder M, Greiner R. Epidemiology and direct medical costs of osteoporotic fractures in men and women in Switzerland. Osteop Int. (2005) 16 Suppl 2:S8–s17. doi: 10.1007/s00198-004-1696-0
6. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteop Int. (2005) 16 Suppl 2:S3–7. doi: 10.1007/s00198-004-1702-6
7. Mornar M, Novak A, Bozic J, Vrdoljak J, Kumric M, Vilovic T, et al. Quality of life in postmenopausal women with osteoporosis and osteopenia: associations with bone microarchitecture and nutritional status. Qual Life Res. (2024) 33:561–72. doi: 10.1007/s11136-023-03542-7
8. Bahtiri E, Islami H, Rexhepi S, Qorraj-Bytyqi H, Thaçi K, Thaçi S, et al. Relationship of homocysteine levels with lumbar spine and femur neck BMD in postmenopausal women. Acta reumatol portuguesa. (2015) 40:355–62. doi: 10.1016/j.actr.2015.07.001
9. Chen KJ, Pan WH, Yang FL, Wei IL, Shaw NS, Lin BF. Association of B vitamins status and homocysteine levels in elderly Taiwanese. Asia Pacific J Clin Nutr. (2005) 14:250–5.
10. Savic-Hartwig M, Kerlikowsky F, van de Flierdt E, Hahn A, Schuchardt JP. A micronutrient supplement modulates homocysteine levels regardless of vitamin B biostatus in elderly subjects. Int J vit Nutr Res Intern Z fur Vitamin- und Ernahrungsforschung J Int vit Nutr. (2024) 94:120–32. doi: 10.1024/0300-9831/a000777
11. Lewerin C, Nilsson-Ehle H, Matousek M, Lindstedt G, Steen B. Reduction of plasma homocysteine and serum methylmalonate concentrations in apparently healthy elderly subjects after treatment with folic acid, vitamin B12 and vitamin B6: a randomised trial. Eur J Clin Nutr. (2003) 57:1426–36. doi: 10.1038/sj.ejcn.1601707
12. Zheng Z, Luo H, Xu W, Xue Q. Association between dietary folate intake and bone mineral density in a diverse population: a cross-sectional study. J orthop Surg Res. (2023) 18:684. doi: 10.1186/s13018-023-04188-4
13. Koehler KM, Pareo-Tubbeh SL, Romero LJ, Baumgartner RN, Garry PJ. Folate nutrition and older adults: challenges and opportunities. J Am Diet Assoc. (1997) 97:167–73. doi: 10.1016/S0002-8223(97)00044-8
14. Rejnmark L, Vestergaard P, Hermann AP, Brot C, Eiken P, Mosekilde L. Dietary intake of folate, but not vitamin B2 or B12, is associated with increased bone mineral density 5 years after the menopause: results from a 10-year follow-up study in early postmenopausal women. Calcified Tissue Int. (2008) 82:1–11. doi: 10.1007/s00223-007-9087-0
15. Zhou L, Deng W, Wu Q, Pan Y, Huang H. Association between dietary folate intake and the risk of osteoporosis in adults: a cross-sectional study. BMC musculoskel Disord. (2024) 25:487. doi: 10.1186/s12891-024-07605-9
16. Li X, Liu X. Associations of serum vitamins levels with bone mineral density in the different race-ethnicities US adults. BMC musculoskel Disord. (2021) 22:137. doi: 10.1186/s12891-021-03997-0
17. Wang K, Wu J, Deng M, Tao F, Li Q, Luo X, et al. Associations of healthy eating index-2015 with osteoporosis and low bone mass density in postmenopausal women: a population-based study from NHANES 2007-2018. Front Nutr. (2024) 11:1388647. doi: 10.3389/fnut.2024.1388647
18. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLOS Med. (2007) e296. doi: 10.1371/journal.pmed.0040296
19. Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteop Int. (2019) 30:3–44. doi: 10.1007/s00198-018-4704-5
20. Li HH, Li XQ, Sai LT, Cui Y, Xu JH, Zhou C, et al. Association of homocysteine with ankylosing spondylitis: a systematic review and meta-analysis. Meta-Anal Adv Rheumatol. (2021) 61:17. doi: 10.1186/s42358-021-00175-7
21. Zhou L, Huang H, Wen X, Chen Y, Liao J, Chen F, et al. Associations of serum and red blood cell folate with all-cause and cardiovascular mortality among hypertensive patients with elevated homocysteine. Front Nutr. (2022) 9:849561. doi: 10.3389/fnut.2022.849561
22. Liwinski T, Lang UE. Folate and its significance in depressive disorders and suicidality: A comprehensive narrative review. Nutrients. (2023) 15:3859. doi: 10.3390/nu15173859
23. Mason JB. Biomarkers of nutrient exposure and status in one-carbon (methyl) metabolism. J Nutr. (2003) 133 Suppl 3:941s–7s. doi: 10.1093/jn/133.3.941S
24. Bailey RL, Looker AC, Lu Z, Fan R, Eicher-Miller HA, Fakhouri TH, et al. B-vitamin status and bone mineral density and risk of lumbar osteoporosis in older females in the United States. Am J Clin Nutr. (2019) 102:687–94. doi: 10.3945/ajcn.115.108787
25. Herrmann M, Schmidt JP, Umanskaya N, Wagner A, Taban-Shomal O, Widmann T, et al. The role of hyperhomocysteinemia as well as folate, vitamin B6, and B12 deficiencies in osteoporosis: a systematic review. Clin Chem Lab Med. (2020) 58:1621–32. doi: 10.1515/cclm-2020-1684
26. Wang YC, Chiang JH, Hsu HC, Tsai CH. Decreased fracture incidence with traditional Chinese medicine therapy in patients with osteoporosis: a nationwide population-based cohort study. BMC Complem Altern Med. (2019) 19:42. doi: 10.1186/s12906-019-2446-3
27. He H, Zhang Y, Sun Y, Zhang Y, Xu J, Yang Y, et al. Folic acid supplementation in a high-fat diet-induced osteoporosis mouse model reduced homocysteine levels and improved bone microarchitecture through the activation of the AMPK signaling pathway. Bone Res. (2021) 9:16. doi: 10.3389/fcell.2021.791880
28. Lucock M, Yates Z. Folic acid fortification: a double-edged sword. Curr Opin Clin Nutr Metab Care. (2023) 12:555–64. doi: 10.1097/MCO.0b013e32833192bc
29. Zhao J, Fu S, Chen Q. Association between the serum vitamin D level and prevalence of obesity/abdominal obesity in women with infertility: a cross-sectional study of the National Health and Nutrition Examination Survey data. Gynecol Endocrinol. (2023) 39:2217251. doi: 10.1080/09513590.2023.2217251
30. Shen J, Fu S, Song Y. Relationship of fibroblast growth factor 23 (FGF-23) serum levels with low bone mass in postmenopausal women. J Cell Biochem. (2017) 118:4454–9. doi: 10.1002/jcb.v118.12
31. Golbahar J, Aminzadeh MA, Hamidi SA, Omrani GR. Association of red blood cell 5-methyltetrahydrofolate with bone mineral density in postmenopausal Iranian women. Osteop Int. (2023) 16:1894–8. doi: 10.1007/s00198-005-1961-x
32. McCabe L, Britton RA, Parameswaran N. Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Curr Osteop Rep. (2015) 13:363–71. doi: 10.1007/s11914-015-0292-x
33. Weaver CM. Diet, gut microbiome, and bone health. Curr Osteop Rep. (2015) 13:125–30. doi: 10.1007/s11914-015-0257-0
34. Yan J, Charles JF. Gut Microbiome and Bone: to Build, Destroy, or Both? Curr Osteop Rep. (2017) 15:376–84. doi: 10.1007/s11914-017-0382-z
35. Ejtahed HS, Soroush AR, Angoorani P, Larijani B, Hasani-Ranjbar S. Gut microbiota as a target in the pathogenesis of metabolic disorders: a new approach to novel therapeutic agents. Horm Metab Res. (2016) 48:349–58. doi: 10.1055/s-0042-107792
36. Whisner CM, Martin BR, Nakatsu CH, McCabe GP, McCabe LD, Peacock M, et al. Soluble maize fibre affects short-term calcium absorption in adolescent boys and girls: a randomised controlled trial using dual stable isotopic tracers. Br J Nutr. (2014) 112:446–56. doi: 10.1017/S0007114514000981
37. Lu L, Chen X, Liu Y, Yu X. Gut microbiota and bone metabolism. FASEB J. (2021) 35:e21627. doi: 10.1096/fj.202100451R
38. Asbaghi O, Ghanavati M, Ashtary-Larky D, Bagheri R, Rezaei Kelishadi M, Nazarian B, et al. Effects of folic acid supplementation on oxidative stress markers: A systematic review and meta-analysis of randomized controlled trials. Antioxid (Basel). (2021) 10:871. doi: 10.3390/antiox10060871
39. Nakamura K, Saito T, Kobayashi R, Oshiki R, Kitamura K, Watanabe Y. Physical activity modifies the effect of calcium supplements on bone loss in perimenopausal and postmenopausal women: subgroup analysis of a randomized controlled trial. Arch Osteop. (2019) 14:17. doi: 10.1007/s11657-019-0575-4
40. Akpolat V, Bilgin HM, Celik MY, Erdemoglu M, Isik B. An evaluation of nitric oxide, folate, homocysteine levels, and lipid peroxidation in postmenopausal osteoporosis. Adv Clin Exp Med. (2013) 22:403–9.
41. Tang Y, Peng B, Liu J, Liu Z, Xia Y, Geng B. Systemic immune-inflammation index and bone mineral density in postmenopausal women: A cross-sectional study of the national health and nutrition examination survey (NHANES) 2007-2018. Front Immunol. (2022) 13:975400. doi: 10.3389/fimmu.2022.975400
42. Ichchou L, Allali F, Rostom S, Bennani L, Hmamouchi I, Abourazzak FZ, et al. Relationship between spine osteoarthritis, bone mineral density and bone turnover markers in postmenopausal women. BMC Womens Health. (2010) 10:25. doi: 10.1186/1472-6874-10-25
43. Nemtoi A, Nemtoi A, Fochi A, Sirghe AE, Preda C, Earar K, et al. CBCT evaluation of the mandibular bone quality in relation to skeletal status after treatment with strontium renelate in diabetic patients. Rev Chimie. (2019) 70:4113–8. doi: 10.37358/RC.19.11.7714
44. Cho M, Kim HS, Choi BS, Lee JH. Effects of abdominal aortic calcification and facet joint arthritis on lumbar bone mineral density using dual-energy X-ray absorptiometry. Res Square. (2021) 55. doi: 10.21203/rs.3.rs-1032910/v1
45. Ota S, Chiba D, Sasaki E, Kumagai G, Yamamoto Y, Nakaji S, et al. Symptomatic bone marrow lesions induced by reduced bone mineral density in middle-aged women: a cross-sectional Japanese population study. Arthritis Res Ther. (2019) 21:113. doi: 10.1186/s13075-019-1900-4
46. Hsieh YC, Chou LS, Lin CH, Wu HC, Li DJ, Tseng PT. Serum folate levels in bipolar disorder: a systematic review and meta-analysis. BMC Psychiatry. (2019) 19:305. doi: 10.1186/s12888-019-2269-2
47. Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, McDowell MA, Hornick RB, et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003-2006. Am J Clin Nutr. (2010) 91:231–7. doi: 10.3945/ajcn.2009.28427
Keywords: lumbar BMD, RBC folate, osteoporosis, postmenopausal female, a cross-sectional study, NHANES
Citation: Wei H, Jin Z, Zhou L, Tang G, Chai S, Che X, Tan Y and Zeng W (2025) Association between RBC folate and lumbar bone mineral density in postmenopausal women, a cross-sectional study from NHANES 2009–2018. Front. Endocrinol. 16:1559043. doi: 10.3389/fendo.2025.1559043
Received: 21 January 2025; Accepted: 03 April 2025;
Published: 28 April 2025.
Edited by:
Guanwu Li, Shanghai University of Traditional Chinese Medicine, ChinaReviewed by:
Fangjun Xiao, Guangzhou University of Chinese Medicine, ChinaShivmurat Yadav, University of Oklahoma Health Sciences Center, United States
Copyright © 2025 Wei, Jin, Zhou, Tang, Chai, Che, Tan and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiqing Zeng, eWl6aGFueGluZ2tvbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Hua Wei1†
Hua Wei1† Gangjian Tang
Gangjian Tang Weiqing Zeng
Weiqing Zeng