- Department of Urology, 940 Hospital of the Joint Service Support Force of the Chinese People’s Liberation Army, Lanzhou, Gansu, China
Background: Hyperuricemia, a prevalent metabolic disorder, is witnessing a global annual increase in incidence. The gout it triggers and its link to other chronic diseases pose a severe threat to human health. The unique natural environment of high-altitude regions, characterized by low oxygen partial pressure and cold climate, may exert a distinctive influence on human metabolism, thereby impacting the onset and progression of hyperuricemia.
Methods: This study recruited 284 plateau migrants undergoing physical examinations at Ritu County Hospital from June to December 2024. Among them, 224 had hyperuricemia and 60 had normal uric acid levels. It collected various indicators of all subjects, including general demographic information, blood routine parameters, and biochemical markers. For univariate analysis, the t-test was used for continuous variables and the chi-square test for categorical variables to screen potential factors related to hyperuricemia. Then, a multicollinearity analysis was done on the univariate factors. After excluding variables with a VIF greater than 5, the remaining ones were put into the multivariate logistic regression model to identify the independently related factors of hyperuricemia.
Result: The incidence of hyperuricemia in the study population was 78.87%. Variables such as gender, age, red blood cell count and creatinine were found to be independently associated with hyperuricemia.
Conclusion: This study revealed an elevated incidence of hyperuricemia in high-altitude area migrants and identified its independent related factors, offering a crucial foundation for the prevention and treatment of hyperuricemia in these regions.
1 Introduction
Hyperuricemia, a common metabolic disorder, has been experiencing a rising prevalence worldwide year by year (1), drawing extensive public health attention. According to relevant statistics, the prevalence of hyperuricemia in the general population can reach approximately 10% - 20% (2, 3), and in certain specific regions or populations, this proportion may be even higher (4). In high-altitude areas, the unique geographical environment and climatic conditions render the prevalence of this disease more complex. Data from the Tibet Autonomous Region Center for Disease Control and Prevention show that the prevalence of hyperuricemia among residents aged ≥18 years in Tibet is 16.86%, far higher than the national average in China (5). Plateau migrants exhibit more severe hyperuricemia due to their bodies’ maladaptation to hypoxic environments (6, 7).
Previous research has indicated that factors such as low oxygen partial pressure, low temperature, and intense ultraviolet radiation in high altitude environments may disrupt the normal metabolic processes of the human body (8, 9), potentially affecting uric acid metabolism in migrants (10–12). Studies have found that individuals traveling to high-altitude areas, acutely exposed to high altitude, or long-term residents of high-altitude regions exhibit elevated uric acid levels in serum or urine (13), and there is a significant correlation between uric acid levels and red blood cell count as well as creatinine (6).
Notably, while foundational research exists on hyperuricemia among high-altitude indigenous populations, the landscape of hyperuricemia in high-altitude migrant populations remains poorly characterized. In-depth exploration of hyperuricemia-related factors in high-altitude migrants is thus of critical importance. This study utilizes physical examination data from high-altitude migrants at Ritu County Hospital (June–December 2024) to analyze hyperuricemia-related factors in this population.
2 Materials and methods
2.1 Research subjects
The population who underwent physical examinations at Ritu County Hospital from June to December 2024 were selected as the research subjects. All subjects were migrants who moved from low-altitude areas to work on the plateau. As the average altitude of Ritu County exceeds 4,000 meters and the annual outdoor working period is approximately 8–10 months, it was difficult to collect data on plateau residence durations exceeding 1 year. Therefore, the study population was defined as individuals with a plateau residence duration of 6 months or more.
2.2 Inclusion and exclusion criteria
Inclusion and exclusion criteria were strictly applied to ensure the homogeneity of the study population: (1) age > 18 years; (2) migration from low-altitude areas (altitude < 1,500 m) to high-altitude regions with ≥6 months of continuous residence; (3) no use of uric acid-regulating drugs (e.g., allopurinol, febuxostat) within the prior 3 months.
2.3 Research variables
General demographic information (including gender, age), blood routine indicators (white blood cell count, lymphocyte count, red blood cell count, etc.), biochemical markers (aspartate aminotransferase, alanine aminotransferase, total protein, albumin, etc.), and other relevant parameters (such as creatinine, urea, triglyceride, etc.) of the plateau migrant subjects were collected.
2.4 Research methods
Univariate analysis: For continuous variables, the t-test was used to compare the differences between the hyperuricemia group and the normal group. For categorical variables, the chi-square test was applied to analyze their correlation with hyperuricemia in high-altitude area migrants. A p-value less than 0.05 was considered statistically significant.
Multicollinearity analysis: Multicollinearity can lead to inaccurate model estimations, such as an increase in the standard error of coefficient estimation and the coefficient sign not conforming to the actual situation. Removing such variables helps improve the stability and interpretability of the model. The variance inflation factor (VIF) of each variable was calculated. Variables with a VIF greater than 5 were regarded as having serious collinearity and were excluded from subsequent analyses.
Multivariate logistic regression analysis: The remaining variables after multicollinearity analysis were included in the multivariate logistic regression model, with hyperuricemia as the dependent variable (yes = 1, no = 0). This was done to determine the factors independently related to hyperuricemia in plateau migrants and calculate the odds ratio (OR) and its 95% confidence interval (CI).
3 Results
3.1 Characteristics statistics of participants
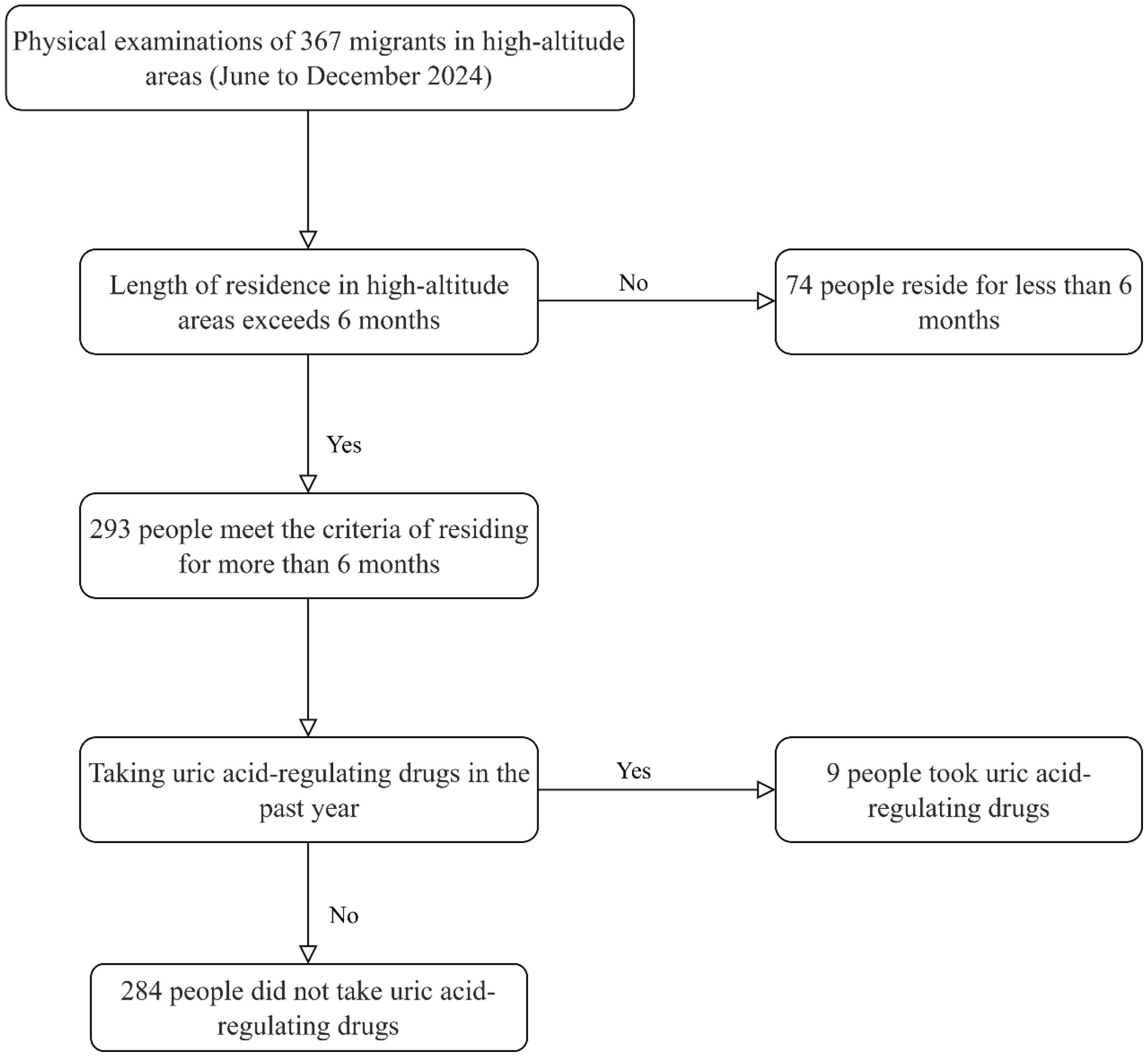
The screening process is as follows: A total of 367 plateau migrants who came for physical examinations from June to December 2024 were initially included. First, 74 individuals who had resided in high-altitude areas for less than 6 months were excluded, leaving 293 people who met the requirement of a residence duration of 6 months or more. Subsequently, 9 individuals who had taken uric acid-regulating drugs in the past year were excluded, resulting in a final inclusion of 284 plateau migrant participants who had not taken uric acid-regulating drugs. Among them, 224 were diagnosed with hyperuricemia, and 60 had normal uric acid levels. Specific screening process is shown in Figure 1.
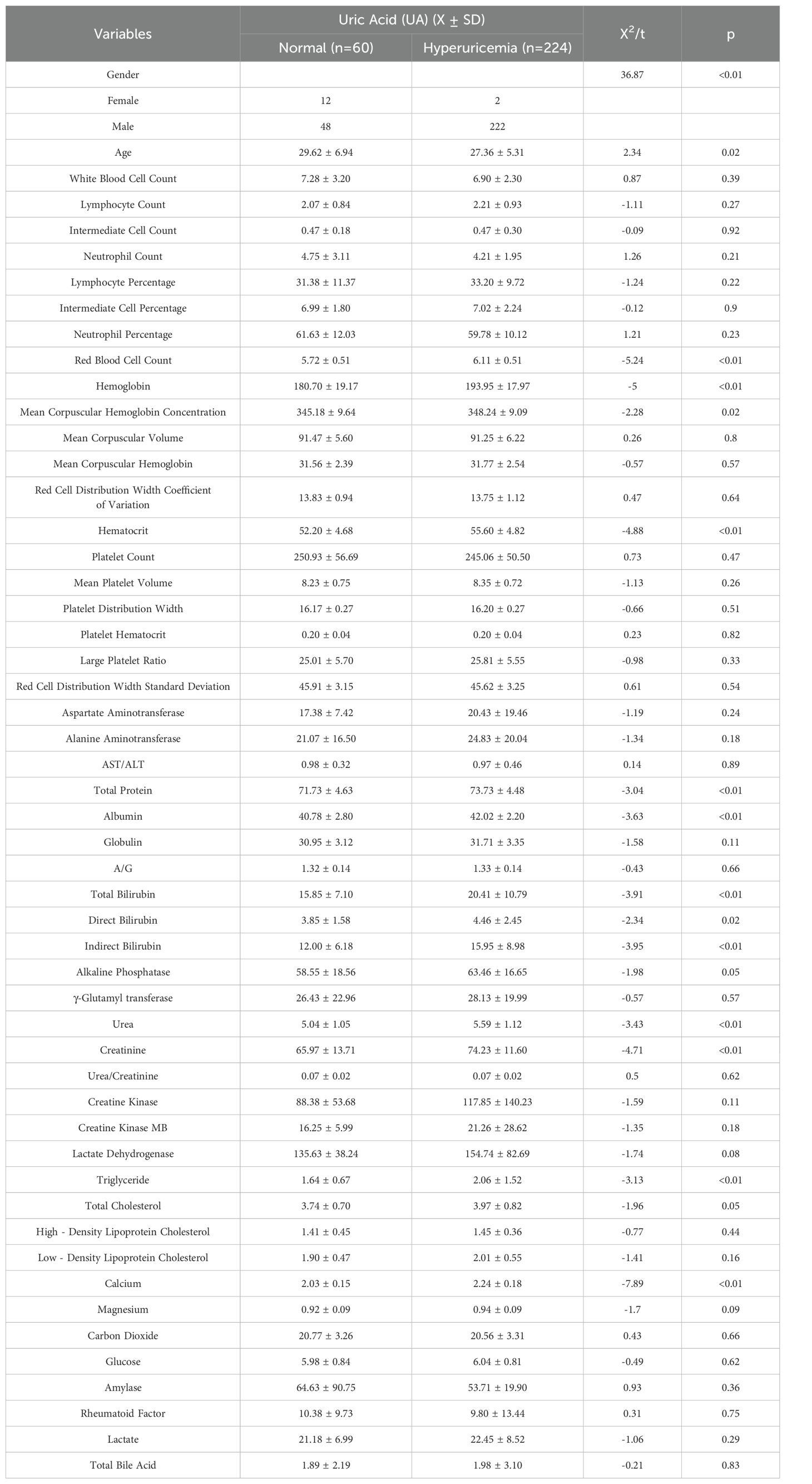
A comprehensive and detailed comparative analysis was conducted on the various indicators of these plateau migrants. The detailed data of this comparative analysis are presented in Table 1 below. In terms of gender distribution, the proportion of males in the hyperuricemia population was significantly higher than that in the normal population. The average age of the normal population was 29.62 ± 6.94 years old, while the average age of the hyperuricemia population was 27.36 ± 5.31 years old. The calculated t-value was 2.34, and the corresponding p-value was 0.02 (p < 0.05), indicating a significant age difference between the two groups, with the hyperuricemia population having a relatively lower average age. Further analysis revealed extremely significant differences between the two groups in key indicators such as red blood cell count, hemoglobin, urea, creatinine, and triglyceride.
3.2 Multicollinearity analysis
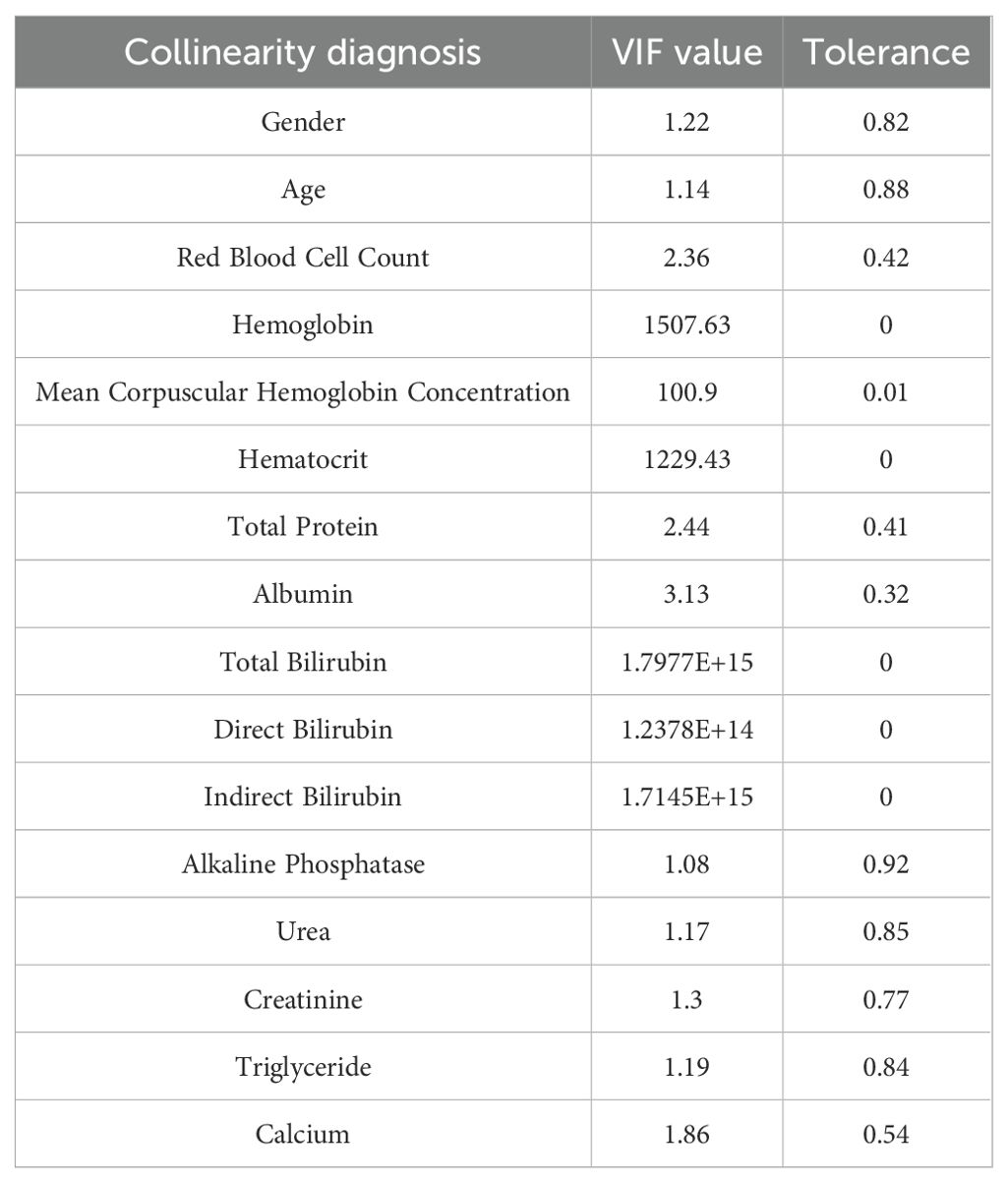
To ensure that the multiple regression model was not affected by collinearity issues, a multicollinearity analysis was performed on the independent variables that were significantly related to hyperuricemia in the univariate analysis of plateau migrants. The results of the multicollinearity analysis are shown in Table 2 as follows. The results showed that 6 variables, namely hemoglobin, mean cell hemoglobin concentration, hematocrit, total bilirubin, direct bilirubin, and indirect bilirubin, had a VIF greater than 5, indicating serious collinearity. These variables were removed from subsequent analyses.
3.3 Results of multivariate logistic regression analysis
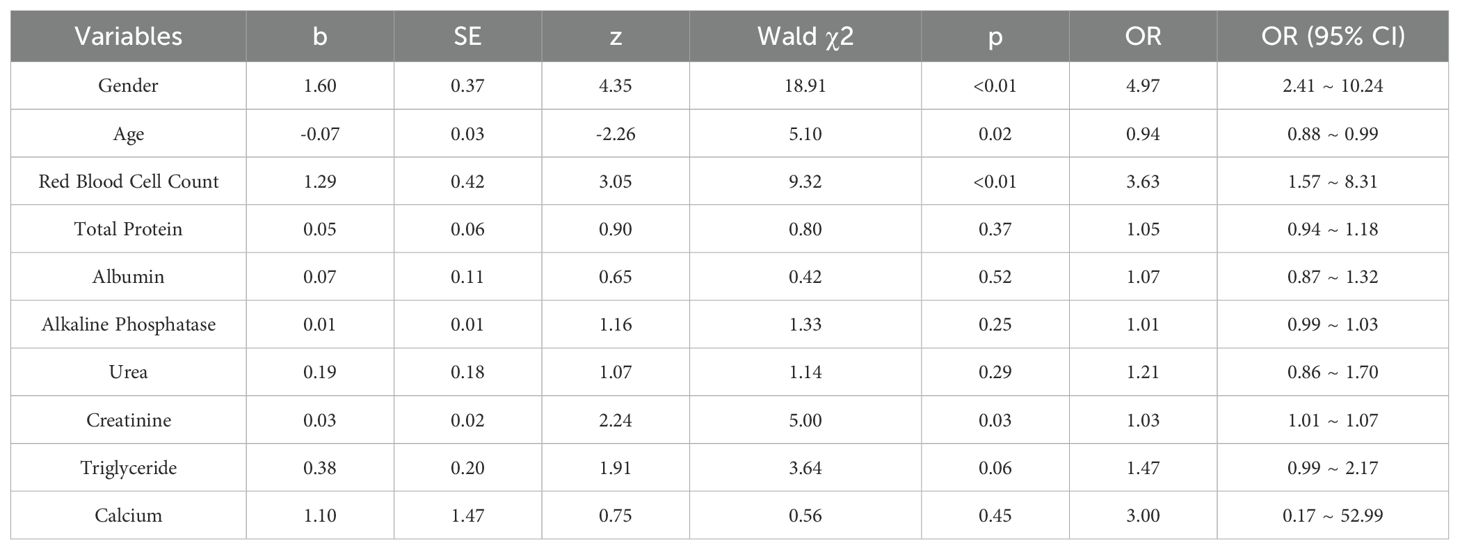
The variables finally included in the model and the results are presented in Table 3. The results indicate that variables such as gender, age, red blood cell count, and creatinine are independently related to hyperuricemia in plateau migrants. The risk of men developing hyperuricemia is 6.73 times that of women. For every 1-year increase in age, the risk of developing hyperuricemia decreases by about 7%. For every 1-unit increase in red blood cell count, the risk of developing hyperuricemia increases by about 2.51 times. For every 1-unit increase in creatinine, the risk of developing hyperuricemia increases by about 4%.
4 Discussion
This study demonstrated that factors such as gender, age, red blood cell count, and creatinine are independently correlated with hyperuricemia in high-altitude area migrants, providing crucial insights into the pathogenesis of hyperuricemia in this specific population.
The incidence rate of hyperuricemia in plateau migrants is as high as 78.87%, which is much higher than that in plain areas (14) and is the result of the joint action of multiple factors. Among the environmental factors, the hypoxia condition is particularly crucial. The hypoxic environment in high-altitude regions has a profound impact on the human metabolic system of migrants. On the one hand, continuous hypoxia prompts the body to initiate a series of stress responses in order to ensure the oxygen supply to vital organs (15). Among them, hypoxia-inducible factors are activated in large quantities. It not only further stimulates the excessive secretion of erythropoietin (16), triggering a sharp proliferation of red blood cells in plateau migrants (17), leading to a significant increase in blood viscosity and a remarkable slowdown in blood flow velocity (18). Several studies have shown that long-term exposure to high altitudes (ranging from 2,800 to 5,800 meters) can increase hematocrit levels in migrants (19–22). This increase is accompanied by an elevation in hemoglobin levels and blood viscosity. Due to the increase in hematocrit, renal plasma flow is reduced (23, 24). Under such a low-perfusion state, the uric acid excretion pathway of the kidneys is blocked, and the excretion capacity is severely restricted (25). On the other hand, hypoxia can also directly interfere with the intracellular energy metabolism pathway, forcing cells to rely more on anaerobic glycolysis for energy supply (26). During this process, the de novo synthesis pathway of purine nucleotides is abnormally activated (27), generating a large number of purines continuously, and the uric acid produced by subsequent metabolism also increases dramatically (28).
The incidence of hyperuricemia in men is much higher than that in women in high-altitude area migrants, which is mainly attributed to multiple factors. At the physiological level, men have higher androgen levels, while estrogen in women helps excrete uric acid (29). Notably, testosterone can stimulate the activity of isolated xanthine oxidase and increase circulating uric acid levels (30), further explaining the gender disparity in hyperuricemia prevalence. In terms of lifestyle, men among plateau migrants are more likely to drink alcohol to cope with the cold in high-altitude areas. Alcohol can impede uric acid excretion and compete with uric acid for excretion channels (31). At the same time, they consume more high-purine foods. In addition, due to differences in environmental adaptation, the oxygen is thin in high-altitude areas. Men’s stronger erythropoiesis ability leads to more uric acid precursors produced by red blood cell metabolism (32). Moreover, men have higher muscle mass, and the metabolic process also contributes to increased uric acid production (33).
In high-altitude area migrants, the incidence of hyperuricemia among young people is relatively high, which is the result of the combined action of multiple factors. Young individuals generally consume more high-purine foods than their older counterparts. Such dietary patterns directly lead to increased uric acid production (34). At the environmental adaptation level, the hypoxic environment at high altitudes prompts the young people’s bodies to produce more red blood cells (35, 36), whose metabolism generates more uric acid precursors.
The association between red blood cell count and hyperuricemia is likely closely related to the special environment in high altitude areas for migrants. Stimulated by the low oxygen environment at high altitudes, the body increases red blood cell production to meet the oxygen demand. In the process of circulation, the increased number of red blood cells will have more cellular metabolic activities. Red blood cells contain a large amount of phosphoribosyl pyrophosphate, which is an important raw material for purine synthesis (37–39). During the metabolism of red blood cells, more purines will be produced. After metabolism, purines will generate uric acid, thus increasing the production of uric acid.
Creatinine is a key indicator reflecting renal function. It mainly comes from muscle metabolism and has a relatively stable production rate. It is filtered by the glomerulus and hardly reabsorbed by the renal tubules, so it can accurately reflect the filtration function of the kidneys. Once the level of creatinine increases, it indicates that the glomerular filtration rate of the kidneys has declined and the ability of the kidneys to remove metabolic wastes has weakened (40, 41). As a result, creatinine accumulates in the body and its level in the blood rises. The excretion of uric acid highly depends on the kidneys. The dynamic balance of blood uric acid is maintained through glomerular filtration, renal tubular reabsorption and secretion. When the renal function is impaired and the filtration rate decreases, the excretion pathway of uric acid will be blocked. Unable to be excreted normally, uric acid will accumulate continuously in the blood, leading to an increase in the content of uric acid in the blood (42).
However, this study has several notable limitations. First, the research data are solely derived from the physical examination population at Ritu County Hospital, potentially leading to selection bias as the sample only includes high-altitude migrants and excludes indigenous populations, making it impossible to compare hyperuricemia characteristics or risk factors between migrant and indigenous groups. This limitation restricts the ability to distinguish whether hyperuricemia in migrants is driven by environmental adaptation or genetic/epigenetic factors inherent in indigenous populations, thus limiting the understanding of disease heterogeneity in high-altitude regions. Second, key clinical variables were underrepresented, including detailed migration duration, body mass index (BMI), smoking status, and oxygen uptake levels. The absence of these variables may obscure potential confounding effects or dose-response relationships. In our study, as the included population was plateau migrant workers, females accounted for a relatively small proportion in physical labor at high altitudes. This resulted in a low proportion of females in the study (only 2 females in the hyperuricemia group and 12 in the normal group), which may limit the conclusion that gender is a related factor for hyperuricemia. The small sample size of females may overestimate the association between male gender and hyperuricemia, and insufficient female data hinder accurate evaluation of estrogen’s protective effect and gender-specific risks, leading to male-biased conclusions with limited generalizability to females.
5 Conclusion
This study investigated the related factors of hyperuricemia in high-altitude area migrants using the physical examination population at Ritu County Hospital as the research object. The results showed that the incidence of hyperuricemia in this migrant population was 78.87%. Through a series of analyses, gender, age, red blood cell count, and creatinine were identified as independently related to hyperuricemia in plateau migrants. Men had a higher risk of developing the disease, the risk decreased with age, and an increase in red blood cell count and creatinine increased the risk of developing the disease. These findings are of great significance for both clinical practice and public health among high-altitude area migrants. Clinically, they assist doctors in early diagnosis and treatment of key populations and the formulation of personalized treatment plans for migrants.
Data availability statement
The datasets generated during the current study are available from the corresponding author upon reasonable request.
Ethics statement
The study was approved by the Medical Ethics Committee of Joint Logistics Support Force 940 Hospital (No. 2022KYLL061). Written informed consent was obtained from all participants.
Author contributions
DM: Investigation, Writing – original draft. YK: Supervision, Writing – original draft. DC: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li Q, Li X, Wang J, Liu H, Kwong JS, Chen H, et al. Diagnosis and treatment for hyperuricemia and gout: a systematic review of clinical practice guidelines and consensus statements. BMJ Open. (2019) 9:e026677. doi: 10.1136/bmjopen-2018-026677
2. Chen-Xu M, Yokose C, Rai SK, Pillinger MH, and Choi HK. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the national health and nutrition examination survey, 2007-2016. Arthritis Rheumatol. (2019) 71:991–9. doi: 10.1002/art.40807
3. Liu R, Han C, Wu D, Xia X, Gu J, Guan H, et al. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: A systematic review and meta-analysis. BioMed Res Int. (2015) 2015:762820. doi: 10.1155/2015/762820
4. Bardin T, Magnat E, Clerson P, Richette P, and Rouchon B. Epidemiology of gout and hyperuricemia in New Caledonia. Joint Bone Spine. (2022) 89:105286. doi: 10.1016/j.jbspin.2021.105286
5. Shen Y, Wang Y, Chang C, Li S, Li W, and Ni B. Prevalence and risk factors associated with hyperuricemia among working population at high altitudes: a cross-sectional study in Western China. Clin Rheumatol. (2019) 38:1375–84. doi: 10.1007/s10067-018-4391-9
6. Cui D, Huang R, Yongzong D, Lin B, Huang X, Ciren Q, et al. Gender-specific association between blood cell parameters and hyperuricemia in high-altitude areas. Front Public Health. (2024) 12:1336674. doi: 10.3389/fpubh.2024.1336674
7. Song Z, Zhang A, Luo J, Xiong G, Peng H, Zhou R, et al. Prevalence of high-altitude polycythemia and hyperuricemia and risk factors for hyperuricemia in high-altitude immigrants. High Alt Med Biol. (2023) 24:132–8. doi: 10.1089/ham.2022.0133
8. León-Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, et al. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol. (2005) 6:147–57. doi: 10.1089/ham.2005.6.147
9. He C, Zhu B, Gao W, Wu Q, and Zhang C. Study on allele specific expression of long-term residents in high altitude areas. Evol Bioinform Online. (2024) 20:11769343241257344. doi: 10.1177/11769343241257344
10. Du Y, Qi M, Wang W, and Chen B. Effect of high-altitude hypoxia environment on uric acid excretion, desmin protein level in podocytes, and na+-K+- ATPase activity. Cell Mol Biol (Noisy-le-grand). (2022) 68:84–91. doi: 10.14715/cmb/2022.68.6.14
11. Su Q, Li YC, Zhuang DH, Liu XY, Gao H, Li D, et al. Rewiring of uric acid metabolism in the intestine promotes high-altitude hypoxia adaptation in humans. Mol Biol Evol. (2024) 41:msae233. doi: 10.1093/molbev/msae233
12. Leighton S, Kok LF, Halliday GM, and Byrne SN. Inhibition of UV-induced uric acid production using allopurinol prevents suppression of the contact hypersensitivity response. Exp Dermatol. (2013) 22:189–94. doi: 10.1111/exd.12096
13. Gonzales GF and Tapia V. Increased levels of serum γ-glutamyltransferase and uric acid on metabolic, hepatic and kidney parameters in subjects at high altitudes. J Basic Clin Physiol Pharmacol. (2015) 26:81–7. doi: 10.1515/jbcpp-2013-0162
14. Song J, Jin C, Shan Z, Teng W, and Li J. Prevalence and risk factors of hyperuricemia and gout: A cross-sectional survey from 31 provinces in mainland China. J Transl Int Med. (2022) 10:134–45. doi: 10.2478/jtim-2022-0031
15. Richalet JP, Hermand E, and Lhuissier FJ. Cardiovascular physiology and pathophysiology at high altitude. Nat Rev Cardiol. (2024) 21:75–88. doi: 10.1038/s41569-023-00924-9
16. Li X, Zhang J, Liu G, Wu G, Wang R, and Zhang J. High altitude hypoxia and oxidative stress: The new hope brought by free radical scavengers. Life Sci. (2024) 336:122319. doi: 10.1016/j.lfs.2023.122319
17. Villafuerte FC, Simonson TS, Bermudez D, and León-Velarde F. High-altitude erythrocytosis: mechanisms of adaptive and maladaptive responses. Physiol (Bethesda). (2022) 37:0. doi: 10.1152/physiol.00029.2021
18. Grenier JMP, El Nemer W, and De Grandis M. Red blood cell contribution to thrombosis in polycythemia vera and essential thrombocythemia. Int J Mol Sci. (2024) 25:1417. doi: 10.3390/ijms25031417
19. Thron CD, Chen J, Leiter JC, and Ou LC. Renovascular adaptive changes in chronic hypoxic polycythemia. Kidney Int. (1998) 54:2014–20. doi: 10.1046/j.1523-1755.1998.00186.x
20. Singh MV, Salhan AK, Rawal SB, Tyagi AK, Kumar N, Verma SS, et al. Blood gases, hematology, and renal blood flow during prolonged mountain sojourns at 3500 and 5800 m. Aviat Space Environ Med. (2003) 74:533–6.
21. Al-Hashem FH, Alkhateeb MA, Shatoor AS, Khalil MA, and Sakr HF. Chronic exposure of rats to native high altitude increases in blood pressure via activation of the renin-angiotensin-aldosterone system. Saudi Med J. (2012) 33:1169–76.
22. Zouboules SM, Lafave HC, O’Halloran KD, Brutsaert TD, Nysten HE, Nysten CE, et al. Renal reactivity: acid-base compensation during incremental ascent to high altitude. J Physiol. (2018) 596:6191–203. doi: 10.1113/JP276973
23. Wang SY, Gao J, and Zhao JH. Effects of high altitude on renal physiology and kidney diseases. Front Physiol. (2022) 13:969456. doi: 10.3389/fphys.2022.969456
24. Hurtado A, Escudero E, Pando J, Sharma S, and Johnson RJ. Cardiovascular and renal effects of chronic exposure to high altitude. Nephrol Dial Transplant. (2012) 27 Suppl 4:iv11–6. doi: 10.1093/ndt/gfs427
25. Park JH, Jo YI, and Lee JH. Renal effects of uric acid: hyperuricemia and hypouricemia. Korean J Intern Med. (2020) 35:1291–304. doi: 10.3904/kjim.2020.410
26. Xie Y, Shi X, Sheng K, Han G, Li W, Zhao Q, et al. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol Med Rep. (2019) 19:783–91. doi: 10.3892/mmr.2018.9713
27. Becker MA and Kim M. Regulation of purine synthesis de novo in human fibroblasts by purine nucleotides and phosphoribosylpyrophosphate. J Biol Chem. (1987) 262:14531–7. doi: 10.1016/S0021-9258(18)47828-3
28. Camici M, Garcia-Gil M, Allegrini S, Pesi R, Bernardini G, Micheli V, et al. Inborn errors of purine salvage and catabolism. Metabolites. (2023) 13:787. doi: 10.3390/metabo13070787
29. Posadzy-Malaczynska A, Rajpold K, Woznicka-Leskiewicz L, and Marcinkowska J. Reversal of an unfavorable effect of hydrochlorothiazide compared to angiotensin converting enzyme inhibitor on serum uric acid and oxypurine levels by estrogen-progestin therapy in hypertensive postmenopausal women. Curr Med Res Opin. (2019) 35:1687–97. doi: 10.1080/03007995.2019.1612656
30. Yahyaoui R, Esteva I, Haro-Mora JJ, Almaraz MC, Morcillo S, Rojo-Martínez G, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab. (2008) 93:2230–3. doi: 10.1210/jc.2007-2467
31. Towiwat P and Li ZG. The association of vitamin C, alcohol, coffee, tea, milk and yogurt with uric acid and gout. Int J Rheum Dis. (2015) 18:495–501. doi: 10.1111/1756-185X.12622
32. Murphy WG. The sex difference in haemoglobin levels in adults - mechanisms, causes, and consequences. Blood Rev. (2014) 28:41–7. doi: 10.1016/j.blre.2013.12.003
33. Miller SG, Matias C, Hafen PS, Law AS, Witczak CA, and Brault JJ. Uric acid formation is driven by crosstalk between skeletal muscle and other cell types. JCI Insight. (2024) 9:e171815. doi: 10.1172/jci.insight.171815
34. Beyl RN Jr, Hughes L, and Morgan S. Update on importance of diet in gout. Am J Med. (2016) 129:1153–8. doi: 10.1016/j.amjmed.2016.06.040
35. Su F, Cao L, Ren X, Hu J, Tavengana G, Wu H, et al. Age and sex trend differences in hemoglobin levels in China: a cross-sectional study. BMC Endocr Disord. (2023) 23:8. doi: 10.1186/s12902-022-01218-w
36. Ji C, Su Y, Zhang C, and Huang W. Reference intervals for hemoglobin and age- and gender-related trends in the population of southwest China. Clin Lab. (2015) 61:1831–6. doi: 10.7754/clin.lab.2015.150423
37. Hardwell TR, Braven J, Shaw S, and Whittaker M. Phosphoribosyl pyrophosphate synthetase and glutathione reductase in erythrocytes from hyperuricaemic and gout patients. Clin Chim Acta. (1982) 126:217–26. doi: 10.1016/0009-8981(82)90295-9
38. Sperling O, Eilam G, Sara-Persky-Brosh, and De Vries A. Accelerated erythrocyte 5-phosphoribosyl-1-pyrophosphate synthesis. A familial abnormality associated with excessive uric acid production and gout. Biochem Med. (1972) 6:310–6. doi: 10.1016/0006-2944(72)90017-8
39. Berman PA and Human L. Regulation of 5-phosphoribosyl 1-pyrophosphate and of hypoxanthine uptake and release in human erythrocytes by oxypurine cycling. J Biol Chem. (1990) 265:6562–8. doi: 10.1016/S0021-9258(19)39184-7
40. Levey AS, Perrone RD, and Madias NE. Serum creatinine and renal function. Annu Rev Med. (1988) 39:465–90. doi: 10.1146/annurev.me.39.020188.002341
41. Becker J and Friedman E. Renal function status. AJR Am J Roentgenol. (2013) 200:827–9. doi: 10.2214/AJR.12.9872
Keywords: high altitude area, hyperuricemia, multivariate logistic regression, creatinine, red blood cell count, plateau migrants
Citation: Meng D-D, Kang Y-D and Chang D-H (2025) Research on related factors of hyperuricemia in high altitude area migrant population. Front. Endocrinol. 16:1559260. doi: 10.3389/fendo.2025.1559260
Received: 12 January 2025; Accepted: 12 June 2025;
Published: 07 July 2025.
Edited by:
Shuogui Xu, Second Military Medical University, ChinaReviewed by:
Yachen Si, No. 944 Hospital of Joint Logistics Support Force, ChinaKai Tao, Chengdu Military General Hospital, China
Copyright © 2025 Meng, Kang and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin-Dong Kang, bWFnaWNib3VsZXZhcmRra0AxNjMuY29t; De-Hui Chang, Y2hkaHVpQDE2My5jb20=
 Dong-Dong Meng
Dong-Dong Meng Yin-Dong Kang*
Yin-Dong Kang*