- 1Ultrasound in Cardiac Electrophysiology and Biomechanics Key Laboratory of Sichuan Province, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Cardiovascular Ultrasound & Noninvasive Cardiology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
Background: Blood urea nitrogen (BUN), one of the recognized indicators of renal function, is a key marker of metabolic diseases, but there are few data on the association of BUN levels with hyperuricemia (HUA) in the general adult population. The aim of the study is to explore the relationship between BUN and HUA in the general population and the potential impact of gender on this relationship.
Methods: This study was conducted involving 17,846 adults from the National Health and Nutrition Examination Survey (NHANES) between 1999-2020. Data on age, gender, race, marital status, education level, height, weight, body mass index (BMI), waist circumference (WC), systolic blood pressure (SBP), diastolic blood pressure (DBP), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting plasma glucose (FPG), hemoglobin A1c (HbA1C), serum uric acid (SUA), BUN, creatinine, and albumin were collected from all participants. Multivariate logistic regression, curve fitting and subgroup analyses were employed to investigate the associations between BUN and HUA stratified by sex.
Results: After weighted analysis, the results of this study represented approximately 164.42 million U.S. adults. The overall prevalence of HUA was 18.22%, and 20.72% in males and 15.82% in females. In the fully adjusted model, there was a positive association between BUN and HUA and this positive association remained significantly stratified by sex. Smoothed curve-fitting analysis revealed that the dose-response relationship between BUN and the risk of developing HUA was linear in men and nonlinear in women. There was evidence of an interaction between BUN levels and gender status that increased the risk of HUA and the OR for the association between BUN and HUA was higher in females than in males. Subgroup analyses showed that the association between BUN and the risk of developing HUA remained consistently positive across all subgroups in both male and female participants.
Conclusions: This study confirmed that BUN were positively associated with HUA among U.S. adults that remained significant when stratified by sex, but there were gender differences in the form and extent of this positive correlation.
Introduction
Hyperuricemia (HUA), a metabolic disorder characterized by dysregulated uric acid homeostasis involving either overproduction or impaired excretion, has been pathophysiologically linked to gout pathogenesis and chronic kidney disease progression (1). Contemporary epidemiological studies have established significant associations between HUA and other metabolic disorders such as obesity, hypertension, dyslipidemia, and nonalcoholic fatty liver disease (2–7). The etiology of HUA is multifactorial, involving a complex interplay of genetic predisposition and environmental influences. Unhealthy lifestyle habits, such as poor diet and sedentary behavior, are known to exacerbate the risk of developing HUA (8, 9). HUA can affect patients of all ages and genders, and its prevalence is on the rise globally; as of 2016, the global prevalence of HUA had reached 21%, with its prevalence varying by geographic region. Nationally representative surveys revealed concerning temporal trends: NHANES 2015–2016 documented 20% prevalence among U.S. adults (10), while Chinese adult populations demonstrated accelerated prevalence increases from 11.1% (2015-16) to 14.0% (2018-19) (11). Importantly, longitudinal cohort studies have shown that individuals with HUA are at a higher risk of mortality (12, 13). Therefore, gaining a comprehensive understanding of HUA and its associated factors is crucial for effective prevention and management strategies, ultimately improving the prognosis and quality of life for individuals with HUA. BUN serves as a clinically validated biomarker of renal filtration efficiency, quantitatively reflecting circulating urea concentrations derived from exogenous protein catabolism and endogenous nitrogen homeostasis. Its serum levels are physiologically modulated by multifactorial determinants including dietary protein intake, proteolytic metabolic pathways, hydration homeostasis, hepatic urea cycle enzymatic activity, and renal excretory capacity, thereby constituting a critical diagnostic parameter for both nephrological evaluation and nutritional status assessment (14). As research related to BUN has intensified, many studies have found that high levels of BUN are associated with a poor prognosis in a number of acute or critical illnesses, including severe cardiovascular and cerebrovascular events [e.g., acute coronary syndromes (15), acute aortic coarctation (16), cardiogenic shock (17), and acute ischemic strokes (18)], neonatal sepsis (19), critical limb ischemia, acute exacerbation of chronic obstructive pulmonary disease (20), and primary pulmonary hypertension (21). Recently, there has been interest and attempts to investigate the relationship between BUN and metabolic diseases [including diabetes mellitus (DM) (22), hyperlipidemia (23), hypertension and fatty liver (24, 25)], as well as the potential role of BUN in the diagnosis and treatment of metabolic diseases. Notably, a critical knowledge gap persists regarding BUN-HUA interrelationships within general adult populations, particularly in the United States. Therefore, this study aims to explore the relationship between BUN and HUA in the general population, as well as the potential impact of gender on this relationship.
Materials and methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is an open comprehensive program of research studies aimed at evaluating the health and nutritional status of both adults and children across the United States. This initiative is overseen by the Centers for Disease Control and Prevention (CDC). What sets NHANES apart is its distinctive approach, which integrates both personal interviews and thorough physical examinations. Each participant provided written informed consent, and all procedures were sanctioned by the NCHS Research Ethics Review Board.
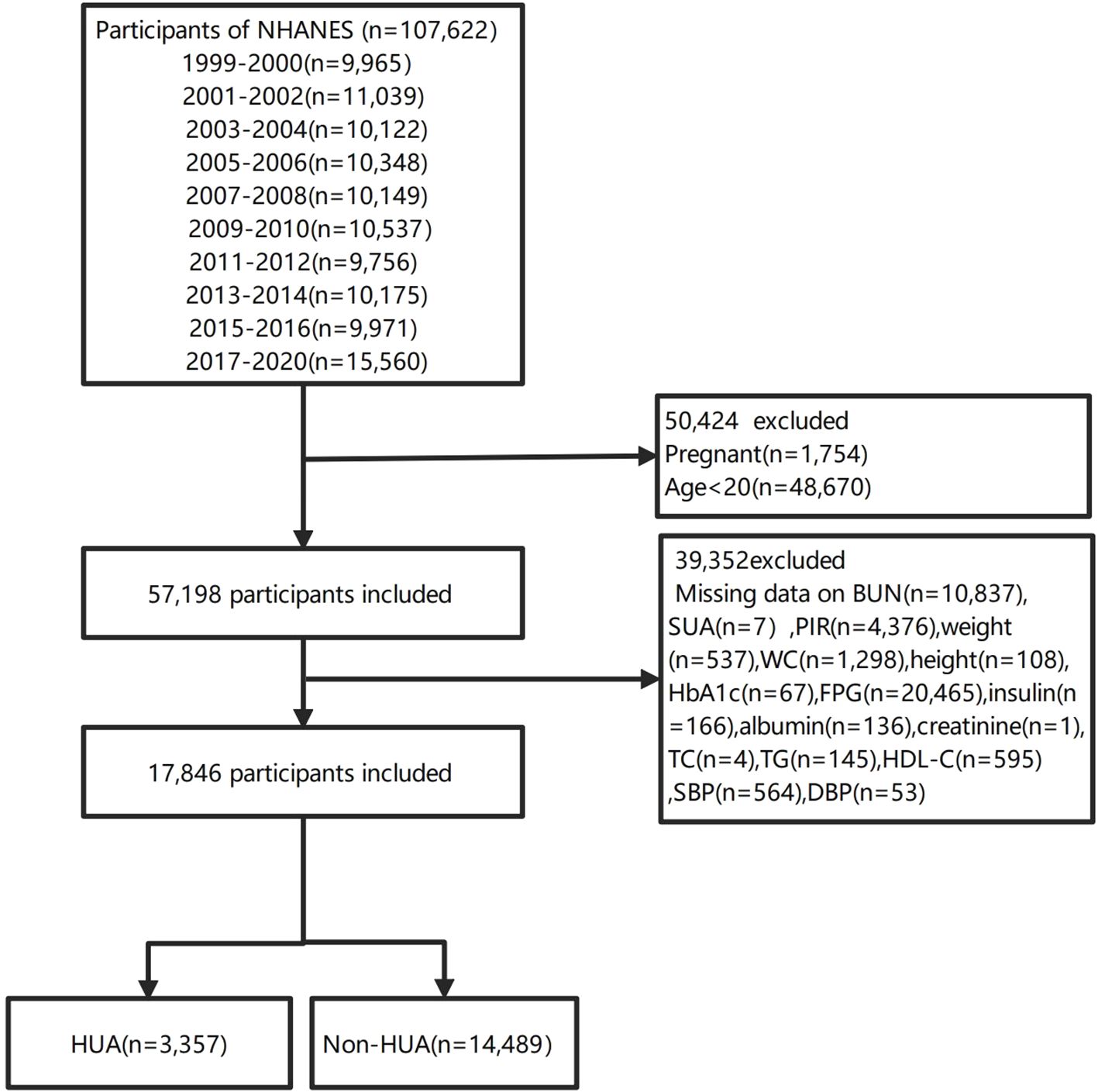
In our investigation, we compiled data from ten NHANES cycles from 1999 to 2020.3, which encompassed a total of 107,622 individuals. To maintain the focus of our study, we omitted pregnant women (n=1754), individuals under the age of 20(n=48670). To ensure the precision and uniformity of our data, we further excluded participants with missing BUN(n=10837), SUA(n=7), family poverty income ratio (PIR) data (n=4376), weight(n=537), WC(n=1298), height(n=108), HbA1c(n=67), FPG(n=20465), insulin(n=166), albumin(n=136), creatinine(n=1), TC(n=4), TG(n=145), HDL-C(n=595), SBP(n=564) and DBP(n=53). Consequently, our analysis was conducted on a cohort of 17,846 participants, comprising 3,357 with HUA and 14,489 without HUA (non-HUA). The participant enrollment flowchart is depicted in Figure 1.
Definition of HUA
HUA is characterized by overproduction or under-excretion of uric acid. In this study, HUA was quantified using SUA measurements from the NHANES data, defined as 420umol/L (7.0 mg/dl) for men and 360umol/L (6.0 mg/dl) for women (26).
Covariates
The sociodemographic covariates included age, sex, race, marital status, and education level. Health-related covariates included height, weight, WC, BMI, SBP, DBP, as well as smoking and drinking habits. Venous blood samples were obtained to determine the serum concentrations of TG, TC, HDL-C, LDL-C, FPG, HbA1c, SUA, BUN, creatinine, and albumin.
A range of potential covariates, including age, sex, race, marital status, education level, family income, smoking and drinking habits, hypertension, hyperlipidemia and DM, were evaluated on the basis of literature. Age was categorized according to the World Health Organization (WHO) into four groups: young individuals (≤44 years), middle-aged individuals (45–59 years), younger elderly (60–74 years), and older elderly (75–89 years).The race categories included Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other races. Marital status was categorized as married/living with a partner, widowed/divorced/separated, or never married. Educational attainment was divided into three groups: less than high school, high school diploma (including GED), and more than high school. A US government report classified family income into low-income (PIR ≤ 1.3), middle-income (PIR>1.3–3.5), and high-income (PIR≥3.5) (27). Individuals who answered “yes” to the question “Have you smoked at least 100 cigarettes in your entire life?” were classified as “yes” in terms of smoking status, and subjects who answered “no” or refused to answer the question were categorized as “no” smoking status. Individuals who answered “Yes” to the question “Had you had at least 12 alcohol drinks/1 yr?” were classified as “Yes” of drinking status, and subjects who answered “no” or refused to answer the question were categorized as “No” drinking status.
The outcome of hypertension was defined as a mean SBP of ≥130 mmHg, a mean DBP of ≥80 mmHg, a self-reported hypertension diagnosis, or the use of an antihypertensive drug (28). Adult Treatment Panel III (ATP 3) of the National Cholesterol Education Program (NCEP) classified hyperlipidemia as TC≥200 mg/dL, TG≥150 mg/dL, HDL-C (40<mg/dL in men and <50mg/dL in women) or LDL-C≥130 mg/dL (29). Alternately, persons who reported using cholesterol-lowering drugs were also classified as having hyperlipidemia. According to the ADA’s DM diagnostic criteria, DM was defined by self-reported diagnosis, the use of insulin or oral hypoglycemic medication, a FPG ≥ 126 mg/dL or an HbA1c level ≥ 6.5% (30).
Statistical analysis
The study was a secondary analysis of publicly available datasets. We used weights for the weighted analysis. For the combined analyses of NHANES 1999–2000 and 2001–2002.3 data, a four-year fasting subsample weight (WTSAF4YR) set was used; for 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, and 2015–2016, a two-year fasting subsample weight (WTSAF2YR) set was used; and for 2017–2020.3, the special fasting subsample weight (WTSAFPRP) set was used. The sampling weights for 1999–2020.3 were calculated as follows:1999–2000 and 2001–2002 weights were WTSAF4YRx2/10.625, 2003–2004,2005–2006,2007–2008, 2009–2010, 2011–2012, 2013–2014, and 2015–2016 weights were WTSAF2YRx1/10.625, and the 2017–2020.3 weight was WTSAFPx1.625/10.625.
The characteristics of the participants were described as the means (standard deviations, SDs) for continuous variables and numbers and percentage frequencies (%) for categorical variables. Comparison of continuous variables among groups was performed with the use of the independent samples Student’s t-test or Mann-Whitney U-test depending on the normality of the distribution, and categorical data were compared by chi-square or Fisher’s exact test as appropriate.
BUN was categorized into three quantiles (Q1-Q3) and calculated the p for trend in order to verify the results of BUN as the continuous variable, and to examine the possibility of nonlinearity. Multivariate logistic regression analyses were performed via four models to examine the associations between BUN and HUA, with the lowest quantile used as the reference category, and the results were presented as ORs with 95% CIs. On the basis of clinical judgment and previous scientific literature, four models were constructed as follows: Model 1: unadjusted; Model 2: age, race, gender, marital status and education level; Model 3, adjusted for age, race, gender, marital status, education level, WC, BMI, insulin, albumin and creatinine; Model 4, adjusted for age, race, gender, marital status, education level, WC, BMI, insulin, albumin, hypertension status, DM status, smoking status, drinking status and hyperlipidemia status.
A generalized additive model (GAM) and restricted cubic spine (RCS) model were used to examine the possible nonlinear dose-response associations between BUN and HUA by sex. Besides, we performed interaction and subgroup analyses according to age group, race, marital status, education level, smoking status, drinking status, hypertension status, hyperlipidemia status and DM status via logistic regression models by sex.
Participants’ BUN levels and risk of HUA were compared among male and female subjects. The multivariate logistic regression model was used to perform subgroup analysis based on different gender. Interactions between subgroups were examined by likelihood ratio testing.
Statistical testing was two-sided with a level of significance set at p = 0.05. All analyses were performed with the Free Statistics platform (Version 1.9, Beijing, China, http://www.clinicalscientists.cn/freestatistics) and the statistical software packages R (http://www.R-project.org, The R Foundation).
Results
Baseline characteristics of the participants
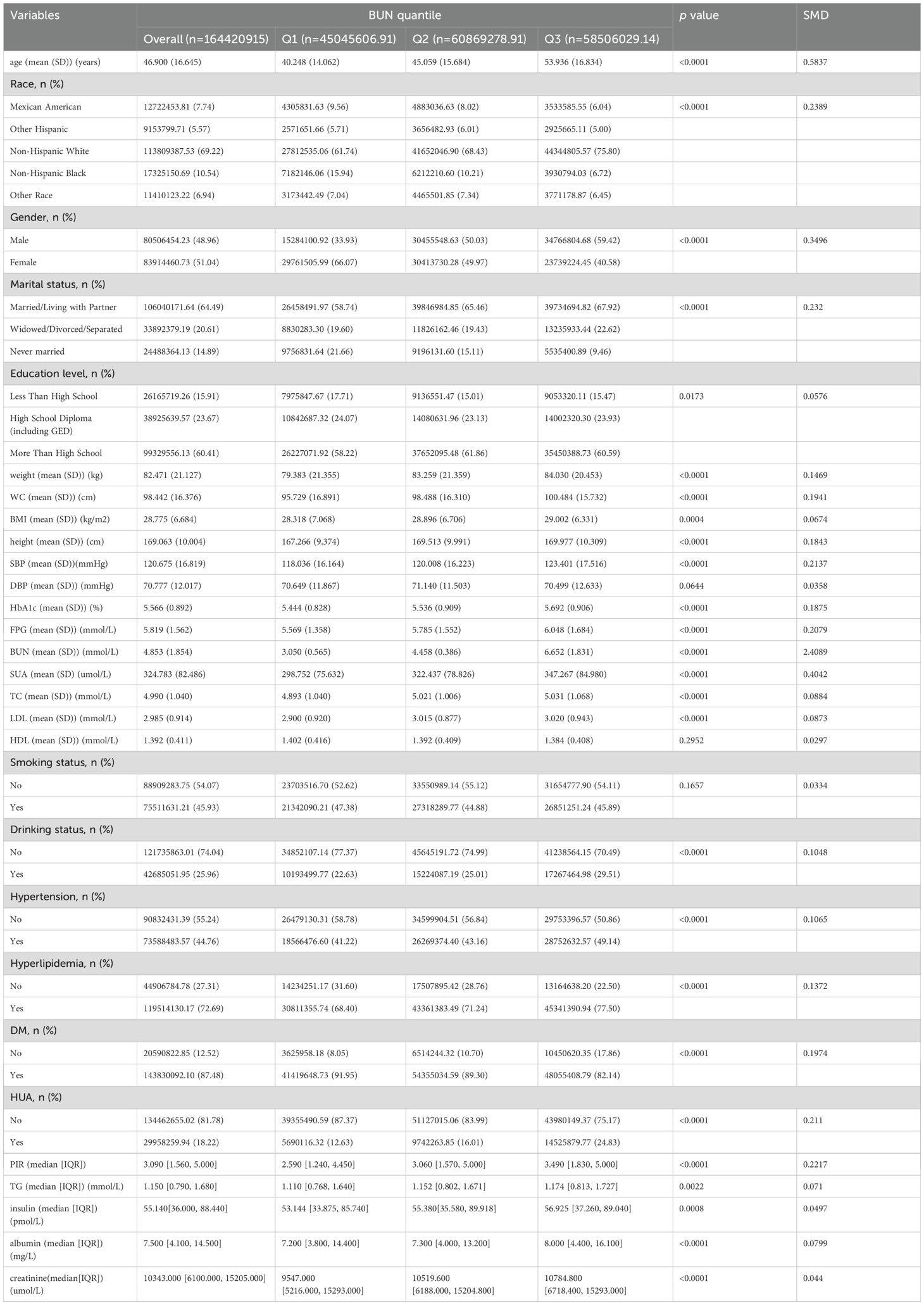
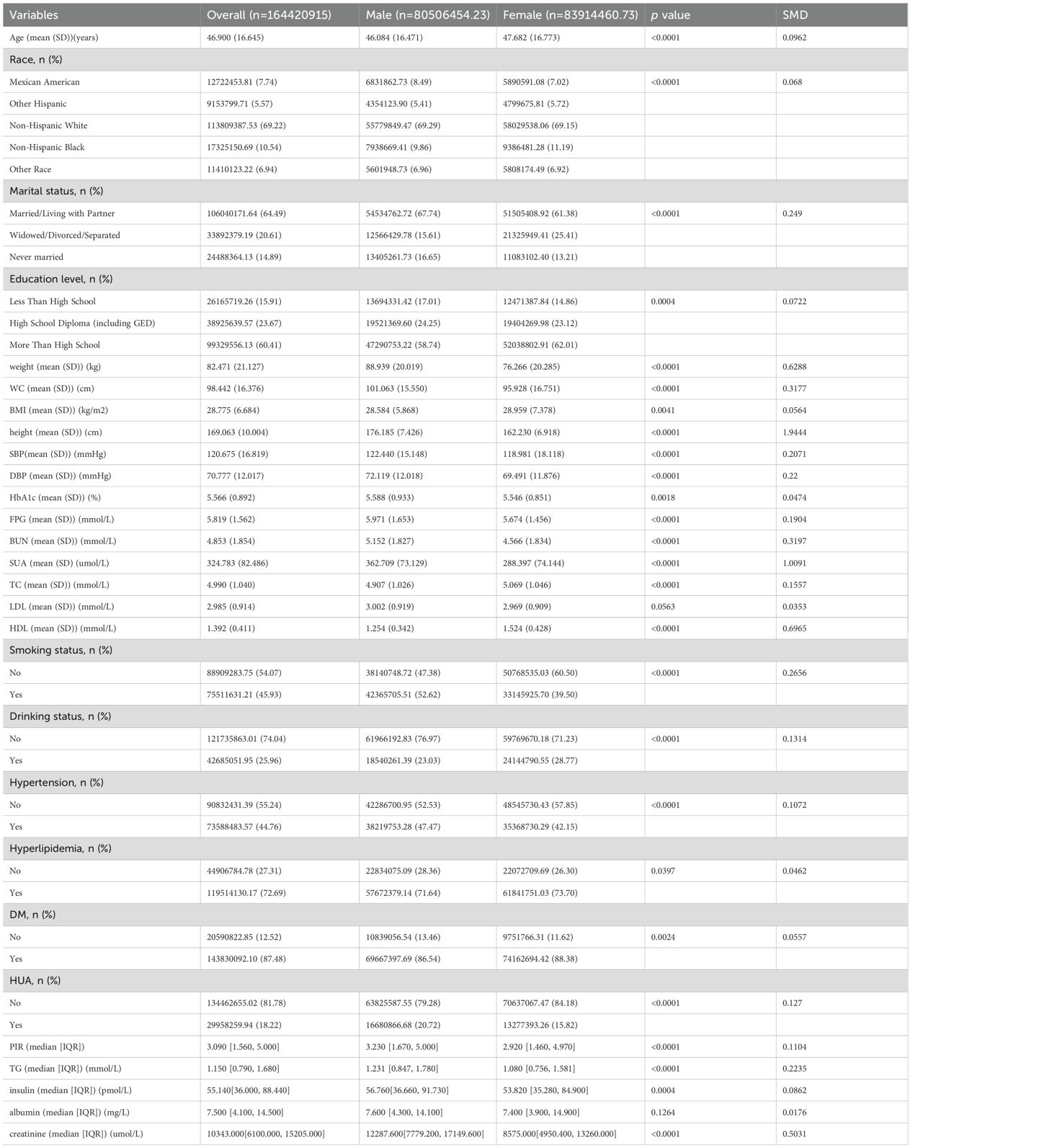
Among all participants,3357(18.8%) participants were diagnosed with HUA, and14489(81.2%) did not have HUA (Figure 1). This study included 17,846 participants with available data for analysis are shown in Tables 1 and 2, representing approximately 164.42 million US adults aged ≥20 years after weighted analysis. The baseline characteristics of the participants were presented by BUN quantiles as follows: Q1 ≤ 3.93 mmol/L; Q2: 3.93~5.36 mmol/L; Q3≥5.36 mmol/L. The mean (SD) BUN was 4.853 mmol/L (SD:1.854). The weighted mean age was 46.900 ± 16.645 years, and 51.04% of the participants were female.
Among the quantiles of the BUN, there were significant between-group differences in all baseline characteristics except for DBP, HDL and smoking status(p>0.05). Participants in the highest quantile.
of the BUN were more likely to be male, be Non-Hispanic White, be widowed/divorced/separated and be drinkers; had the highest age, weight, WC, BMI, height, SBP, HbA1c, FPG, BUN, SUA, TC, LDL, creatinine, insulin, TG, albumin and PIR; and had the highest prevalence of HUA, hypertension and hyperlipidemia (all p < 0.05). In addition, participants in the group with the lowest BUN were more likely to be non-Hispanic Black and never married; had the lowest proportion of smokers and the lowest prevalence of DM (p <0.01; Table 1).
Compared to the female group, male participants were typically younger, more likely to be Mexican American, more likely to be married/living with a partner or never married, less than high school education, smokers, hypertensive, and HUA; higher PIR, weight, WC, height, SBP, DBP, HbA1c, FPG, BUN, SUA,TG, insulin, and creatinine; lower BMI, TC, and HDL; and lower incidence of alcohol abuse, DM and hyperlipidemia (p <0.05). Nevertheless, both female and male populations had similar levels of LDL and albumin (all p >0.05; Table 2).
Risk factors for HUA in the selected population
As shown in Table 3. By univariate analysis, we found that age, race, sex, marital status, education level, weight, WC, BMI, height, SBP, DBP, HbA1c, FPG, insulin, albumin, creatinine, BUN, TC, TG, LDL, HDL, DM status, smoking status, hypertension status and hyperlipidemia status were associated with the risk of developing HUA (all p < 0.05). However, PIR and drinking status were not related to HUA incidence (Table 3).
Effect of the BUN on HUA incidence
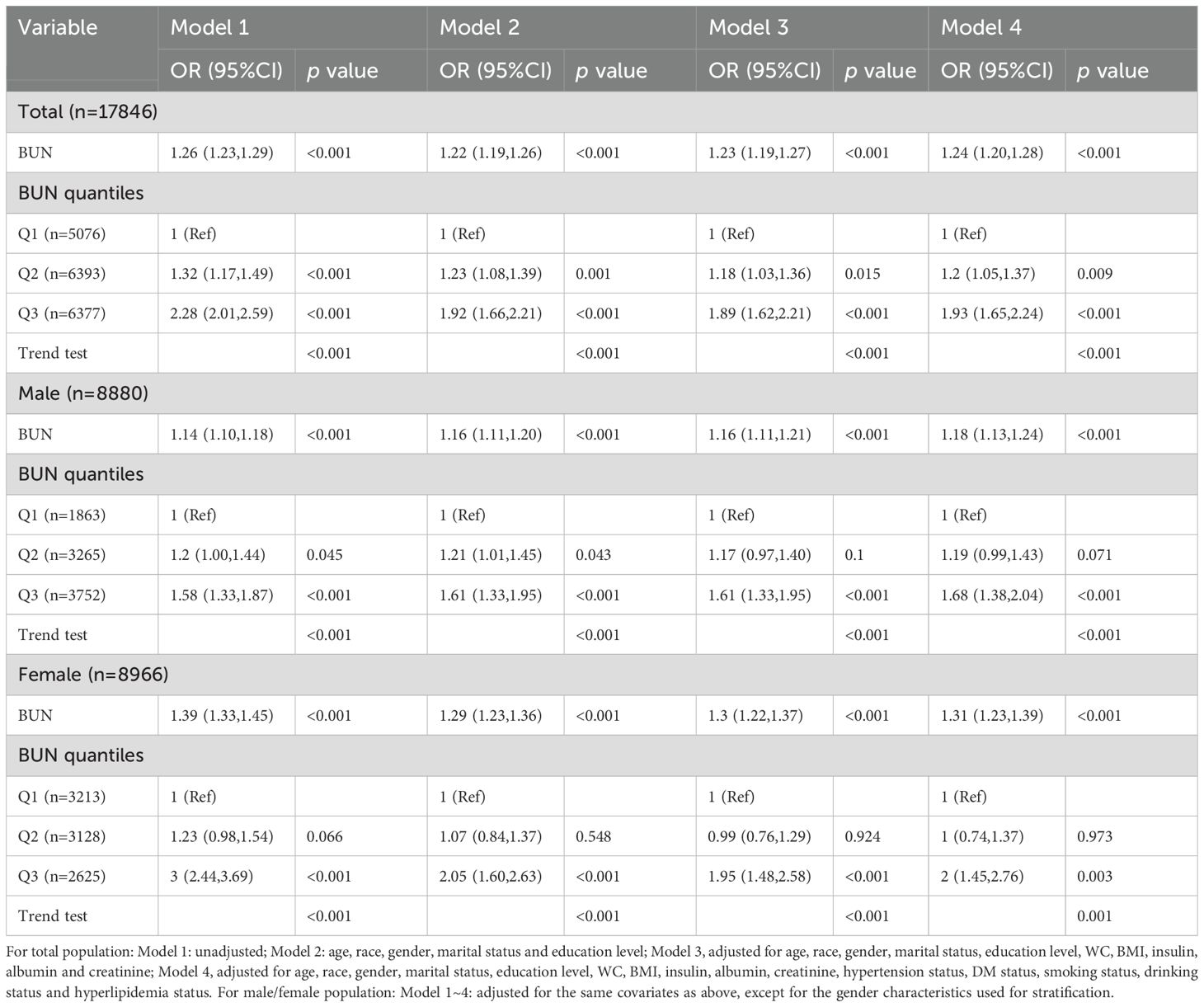
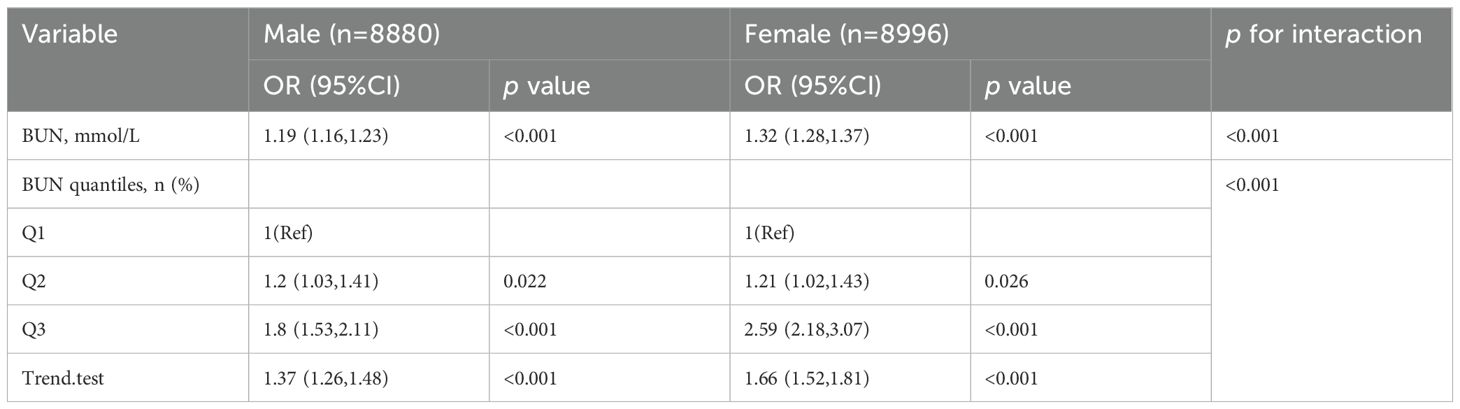
The associations between BUN and HUA were analyzed using multivariate logistic regression and subgroup analyses stratified by sex, and the results are shown in Table 4. In the fully adjusted model (Model 4) adjusted for age, race, gender, marital status, education level, WC, BMI, insulin, albumin, hypertension status, DM status, smoking status, drinking status, hyperlipidemia status, the positive relationships between the BUN and the risk of HUA were found as a quantile variable, Q3 vs. Q1, OR (95%CI): 1.93(1.65~2.24), p < 0.05, p for trend < 0.001; as a continuous variable, per 1 mmol/L increment, OR (95%CI): 1.24(1.20~1.28), p < 0.001.Besides, among men, per 1 mmol/L increment in BUN, the risk of HUA increased by 18% [OR (95%CI): 1.18(1.13~1.24)] in the fully adjusted model. When the BUN was divided into three groups according to quantile, we compared the Q1 with the adjusted OR of Q2 and Q3, which were 1.19 (95% CI: 0.99~1.43) and 1.68 (95% CI: 1.38~2.04) in model 4. Among women, per 1 mmol/L in BUN, the risk of HUA increased by 31% [OR (95%CI): 1.31 (1.23~1.39)] in the fully adjusted model. The multivariable-adjusted OR for HUA compared Q1 with Q2 and Q3, which were 1 (95% CI: 0.74~1.37) and 2 (95% CI: 1.45~2.76). The highest level of BUN was associated with an increased risk of HUA.
Subgroup analyses
Stratified subgroup analysis was conducted in the study population to evaluate whether the relationship between BUN and HUA remained consistent or differed across different demographic characteristics and disease status groups.
We observed that the positive relationship between the BUN and the risk of developing HUA remained consistent across all subgroup variables in male and female population (Figures 2, 3). Interestingly, we also found an interaction effect in the female subgroup, whereby the association between BUN and HUA was stronger in the non-hypertensive group (OR=1.36, 95% CI: 1.25 ~1.47) (p=0.048) (Figure 3).
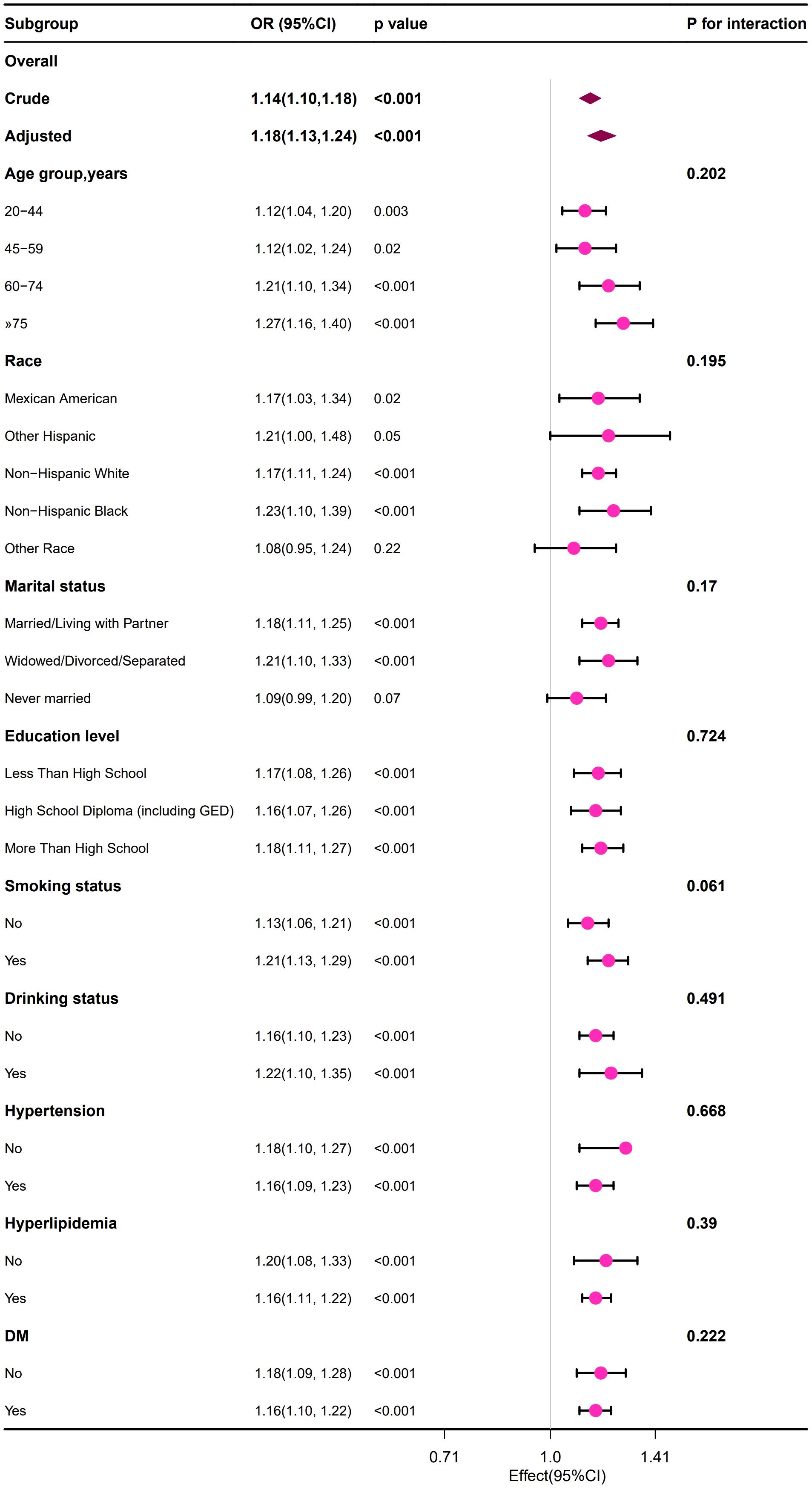
Figure 2. Forest plot of the association between the BUN and the risk of developing HUA in male group.
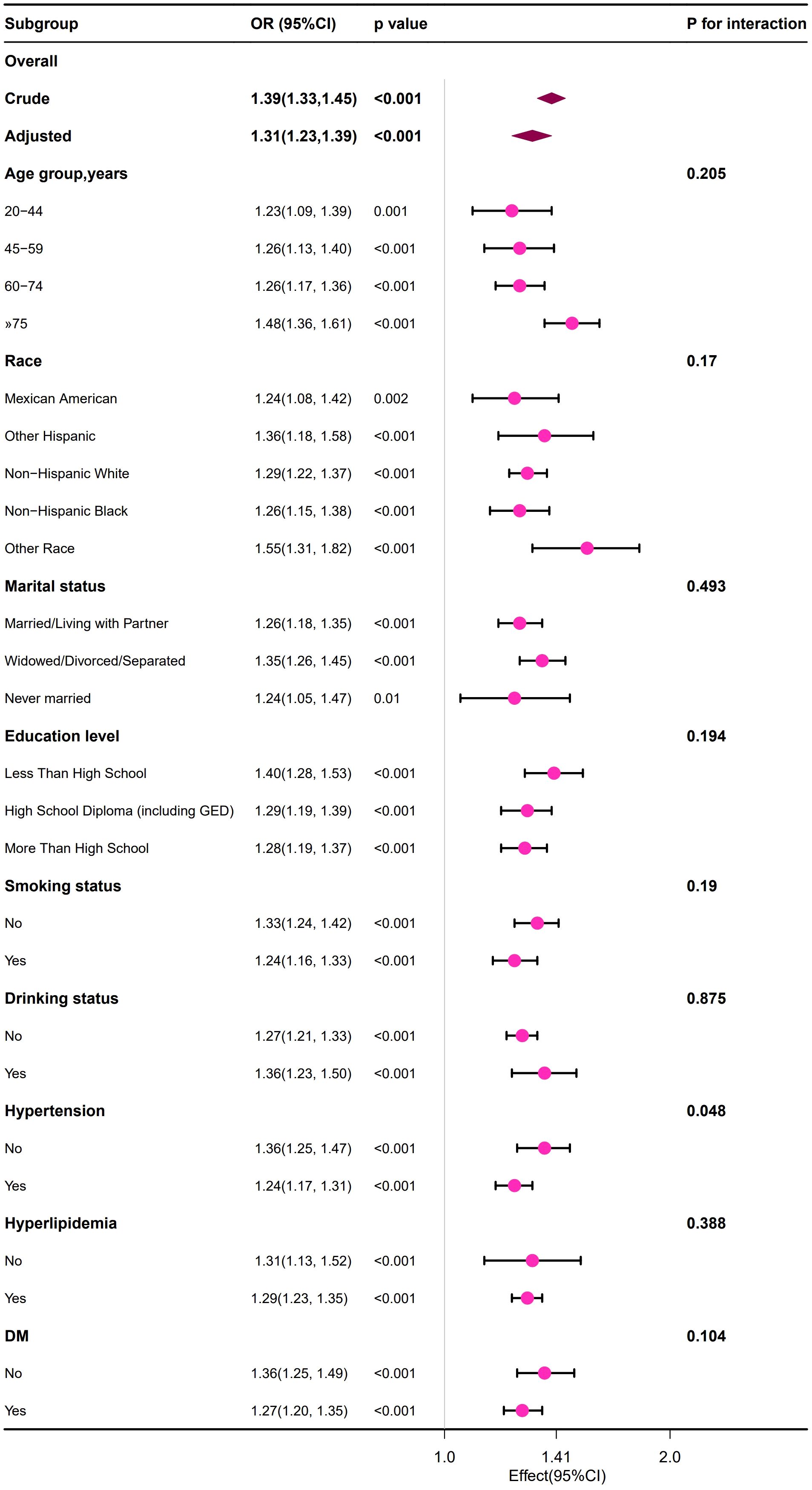
Figure 3. Forest plot of the association between the BUN and the risk of developing HUA in female group.
Interaction of sex with the association between BUN and HUA
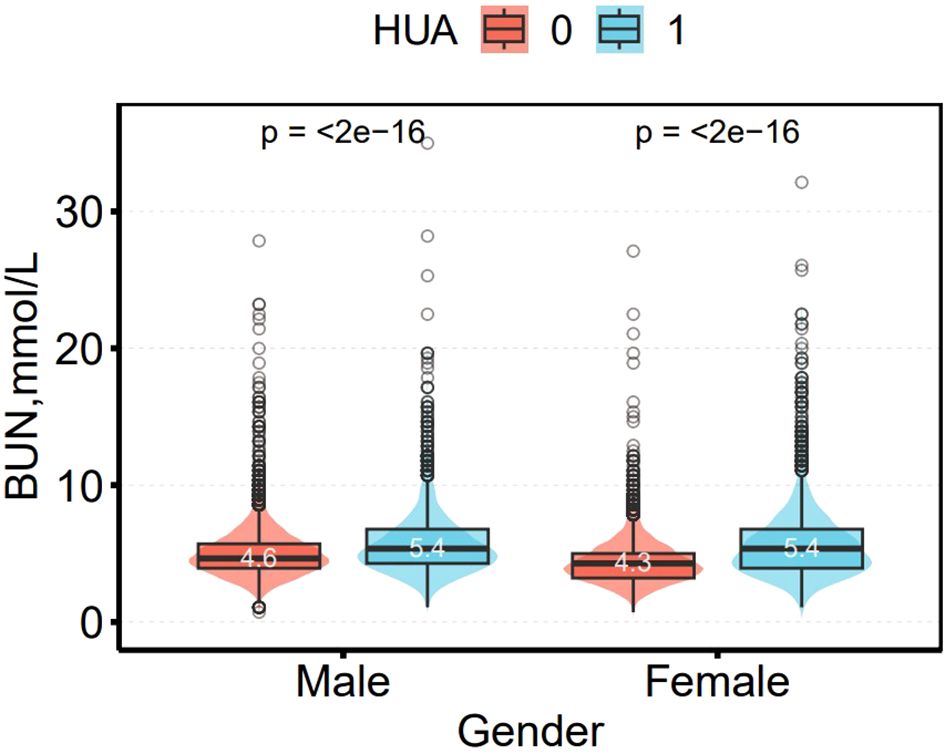
Figure 4 shows the difference in BUN between non-HUA and HUA participants. Among male and female participants, BUN levels were significantly higher in the HUA group than in the non-HUA group, (4.6 vs. 5.4 mmol/L) and (4.3 vs. 5.4 mmol/L (all p < 0.01), respectively.
After adjusting for age, race, gender, marital status, education level, WC, BMI, insulin, albumin, hypertension status, DM status, smoking status, drinking status, hyperlipidemia status, as BUN increased, the risk of HUA increased significantly in both men (OR: 1.19, 95% Cl:1.16~1.23) and women (OR:1.32, 95% Cl:1.32 (1.28~1.37)(all p < 0.001). The interaction between gender status and the prevalence of BUN and HUA was significant (p for interaction likelihood ratio test <0.01). This interaction remained significant when converting BUN to categorical variables (p for interaction likelihood ratio test <0.01) (Table 5).
The nonlinear relationship between BUN and HUA
After adjusting according to Model 4, the fitting curves of the BUN and the risk of developing HUA were drawn to better explain their relationships. The results showed that there was a straightforward linear relationship between the BUN and the risk of developing HUA in males (p for nonlinearity = 0.076) (Figure 5), while the dose-response relationship between the BUN and HUA in females was positive in a nonlinear manner (p for nonlinearity = 0.012) (Figure 6).
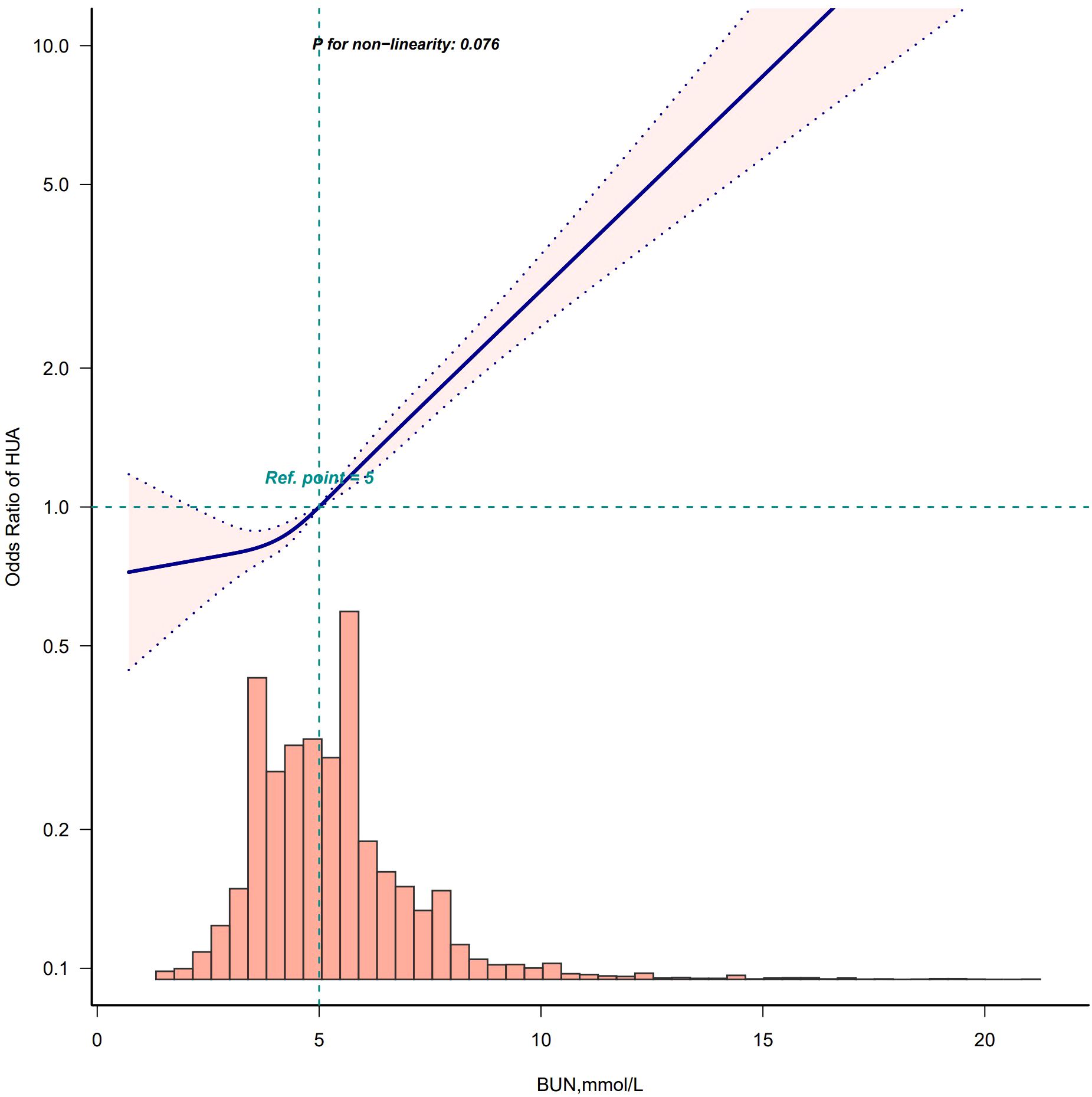
Figure 5. Restricted cubic spline model of the odds ratios of BUN with HUA in male. Adjusted for Model 4: age, race, marry status, education level, WC, BMI, insulin, albumin, creatinine, hypertension, DM, smoke, drink, hyperlipidemia.
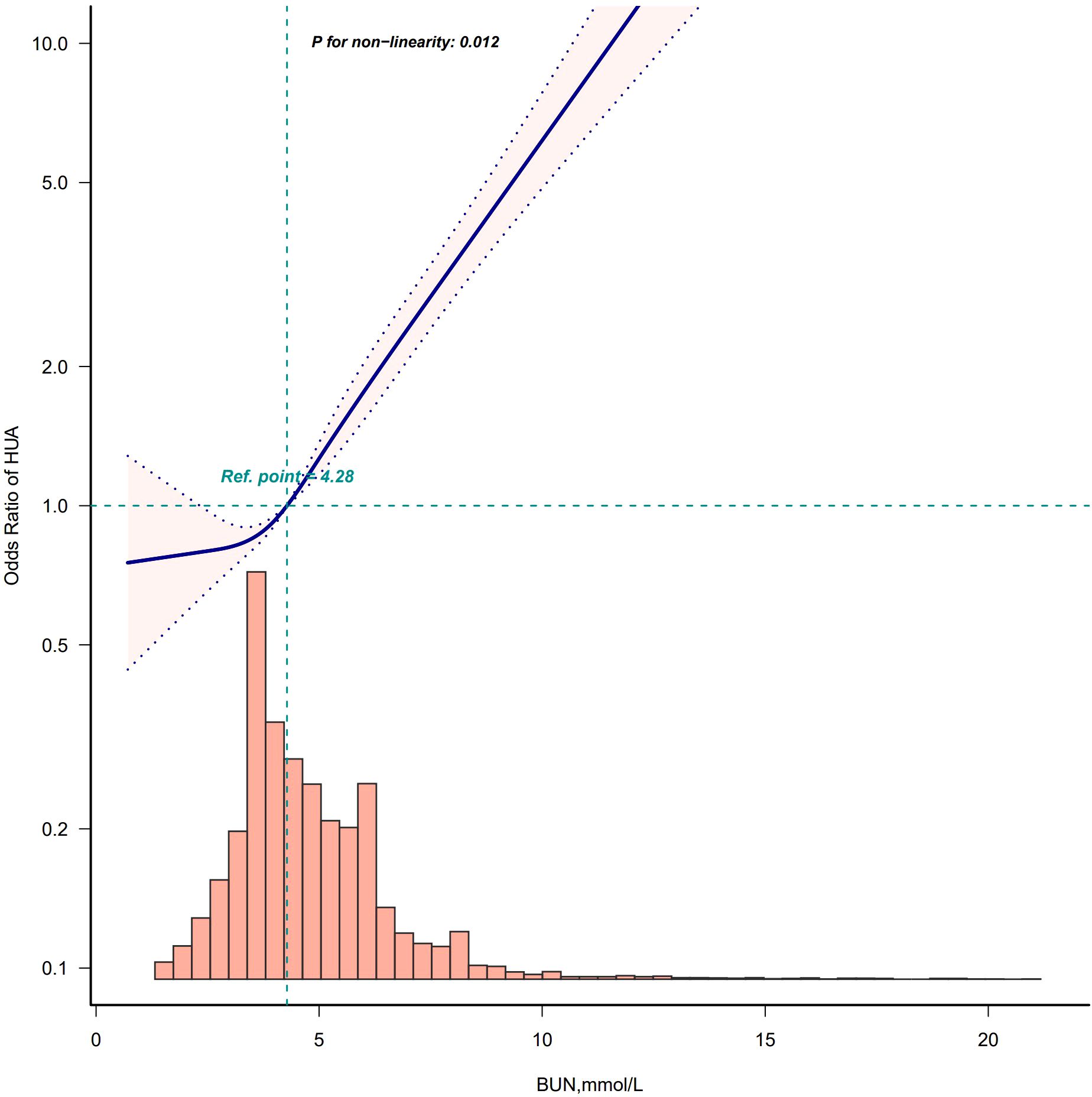
Figure 6. Restricted cubic spline model of the odds ratios of BUN with HUA in female. Adjusted for Model 4: age, race, marry status, education level, WC, BMI, insulin, albumin, creatinine, hypertension, DM, smoke, drink, hyperlipidemia.
Discussion
In this extensive population-based cross-sectional study, we found that BUN was significantly and positively associated with HUA in U.S. adults, after full adjustment for all potential confounders. Additional assessment of gender differences between BUN and HUA prevalence showed a stronger correlation in female subjects and a nonlinear correlation in females. To our knowledge, this is the first study describing the association of BUN with the development of HUA in the general population of the United States and gender differences. These findings have important clinical implications for HUA management strategies in Western populations, particularly given the escalating HUA prevalence and associated multi-organ morbidity.
Previous studies have shown that BUN is strongly associated with a variety of metabolic diseases, such as DM (22, 31, 32), diabetic retinopathy (33), hyperlipidemia (23) and fatty liver (25). However, few studies have evaluated the relationship between BUN and the risk of HUA development in the general adult population, particularly in the United States. In this study, we used data from NHANES, which, after weighted analysis, found that BUN was positively associated with HUA in U.S. adults (OR,1.24; 95%CI: 1.20-1.28) after adjusting for age, race, sex, marital status, education level, BMI, insulin, albumin, hypertensive status, DM status, smoking status, drinking status, and hyperlipidemia status. Besides, in this study we performed curve-fitting analysis and subgroup analysis to further confirm the correlation between BUN and HUA. In this study, we used data from NHANES, which, after weighted analysis, found that BUN was positively associated with HUA in U.S. adults (OR,1.24; 95%CI: 1.20-1.28) after adjusting for age, race, sex, marital status, education level, BMI, insulin, albumin, hypertensive status, DM status, smoking status, drinking status, and hyperlipidemia status. Besides, in this study we performed curve-fitting analysis and subgroup analysis to further confirm the correlation between BUN and HUA.
To the best of our knowledge, only one cross-sectional study in China has shown that BUN is a risk factor for HUA (34). The study showed that among low-income adults in rural North China, high BUN levels were significantly associated with a higher risk of developing HUA. The results were consistent with our findings. However, the study involved fewer logistic regression-adjusted variable models, a small sample size, no further subgroup or curve-fitting analyses, and a study population of middle-aged and older adults (aged >50 years) in rural northern China, which limited its external validity. With a focus on the US population, our study may complement the findings of previous research. Because we used an NHANES design to obtain estimates that were nationally representative of the US, our results should be generalizable to adults in the United States.
The biological mechanisms underlying the association between the BUN and the risk of HUA have not been elucidated, with some of the following potential explanations. Elevated BUN: (i) usually reflects abnormal kidney function, which may include chronic kidney disease or acute kidney injury. When kidney function is impaired, the ability to excrete urea and uric acid may be reduced, resulting in higher concentrations of urea and uric acid in the blood (35); (ii) associated with a high-protein diet. High-protein diets may lead to elevated BUN and impaired renal function (36–38), and also increase uric acid production because uric acid is metabolized from purines, and high-protein foods are usually high in purines; (iii)associated with increased insulin resistance. Researchers have now found that urea can directly act on pancreatic β-cells, thus affecting insulin secretion and inducing insulin resistance through various mechanisms such as oxidative stress and inducing post-translational modifications (e.g., O-GlcNAcylation) (39), and insulin resistance is closely related to HUA (40–43); (iv)related to insufficient renal tubular reabsorption. Elevated BUN may lead to impaired renal tubular function, which affects excretion and reabsorption of uric acid, leading to elevated uric acid concentration in the blood. These mechanisms may lead to increased production or decreased excretion of uric acid in the body, ultimately leading to the development of HUA.
In this study, the overall prevalence of HUA in U.S. adults from 1999 to 2020 was 18.22%, with 20.72% in men and 15.82% in women. Our study was in general agreement with the findings of Che et al., who showed that the overall prevalence of HUA in the United States from 2007–2018 was 19.0% (5841/30819), with prevalence rates of 21.04% (3179/15112) and 16.95% (2662/15707) in men and women, respectively (12). However, Singh et al. found that the overall prevalence of HUA in U.S. adults was 14.6% from 2007–08 to 2015–16 (1) and Chen-Xu et al. found that the prevalence of HUA in adult men and women in the United States in 2015–2016 was 20.2% and 20.0%, respectively (10). The results of these two U.S. population studies was somewhat different from ours, and possible reasons for this were considered to be that the diagnostic criteria for HUA were not the same as the year of the study population. In our and the other U.S. population studies mentioned above, the sex differences in the prevalence of HUA were not very dramatic. However, this is not the case in East Asian countries. Zhang et al. found that the prevalence of HUA in Chinese adult males and females was 19.3% and 2.8% respectively in 2015-2016, while in 2018–2019 the prevalence was 24.4% in males and 3.6% in females (11). Pia et al. found that in 2015–2017 prevalence of HUA in Chinese adult males and females was 21.2% and 8.5%, respectively (44). Kawano et al. showed that the prevalence of HUA in Japanese adult males and females was 4.87% (551/11324) and 0.78% (146/18683), respectively (45). Koo et al. found that the prevalence of HUA in Korean adult males and females was 13.33% and 0.82%, respectively (46). Sex differences in the prevalence of HUA are more pronounced in East Asian countries, possibly due to marked differences in lifestyle and dietary habits between men and women within these countries, and genetic factors may also be a contributing factor.
The present study further investigated gender differences in the correlation between BUN and HUA and found that the correlation between BUN and HUA may be stronger in females than in males, and that the correlation between BUN and HUA in females was nonlinear, whereas the correlation between BUN and HUA in males was linear. Furthermore, in the female group only, the correlation was even higher in non-hypertensive than in hypertensive patients. These gender differences may primarily involve three mechanisms: (i) sex hormones regulating uric acid metabolism, (ii) renal clearance disparities, and (iii) lifestyle factors’ varied impacts. Sex hormone regulation may play a pivotal role. Experimental studies showed that estrogen enhances renal urate excretion through downregulation of urate transporter 1 and glucose transporter 9 transporters, while testosterone upregulated these reabsorption channels (47–50). This hormonal dichotomy may explain why premenopausal women exhibit lower SUA levels than age-matched males. Interestingly, the nonlinear correlation pattern in females may reflect the dynamic estrogen fluctuations during menstrual cycles and menopausal transition. The results of the present study corroborated with previous studies: Li et al. (51) found a nonlinear dose-response relationship between estrogen levels and the risk of HUA in women, whereas Cho et al. (52) reported a significant increase in the prevalence of HUA from the late menopausal transition stage, which was highly compatible with the gender-differentiated features observed in the present study. Renal clearance disparities warrant particular attention. Although males generally have higher glomerular filtration rates, gender-specific urea handling mechanisms may exist. An animal study confirmed the presence of the Urea Transporter B2 in the rumen of deer rumen and significantly higher levels of Urea Transporter B2 in adult females than in adult males, suggesting a potential pathway for gender-specific urea-urate regulation (53). Furthermore, estrogen’s modulatory effects on the renin-angiotensin-aldosterone system could create sex-dimorphic renal hemodynamic environments affecting both BUN and SUA homeostasis. Lifestyle factors also show significant gender differences, broadly defined to include dietary patterns, physical activity, and psychological and social behaviors. Liu et al. found that for men, dietary habits had a greater impact on the likelihood of developing HUA, whereas for women, HUA was more associated with physical activity-related lifestyle choices (e.g., type of work, commute, and exercise) (54). Feraco et al. showed that men preferred to consume meat and to participate in strength and endurance sports, whereas women consumed more vegetables and were less physically active than men (55). New evidence suggests that these sex-specific lifestyle differences may arise from biological mechanisms (e.g., metabolic differences regulated by sex hormones) (56) and evolutionary adaptations shaped by energy allocation strategies and reproductive priorities (57). Although the exact mechanisms are unknown and further research is needed, gender factors need to be emphasized in the early management of HUA.
BUN has the advantages of easy and inexpensive detection and has the basic conditions to be used as a large-scale screening marker, but its level is easily affected by a variety of factors such as protein intake, dehydration, renal blood flow, etc., and its accuracy is not high and its application alone has some limitations. Our findings reveal positive correlation between BUN levels and HUA risk, indicating that BUN might be used as a risk stratification tool in clinical practice. However, the current study provides only preliminary evidence of correlation and a comprehensive risk stratification model has not yet been developed; more data need to be collected at a later stage and risk assessment models need to be constructed and validated in combination with other risk factors.
This study has several strengths: (i) it explored the relationship between BUN and HUA in the general U.S. population with gender-stratified analysis;(ii) the data for this study were obtained from a national population-based prospective survey, providing a representative sample of the population and increased the credibility of the study; (iii) the data were subjected to weighted statistical analysis, which enabled us to generalize the results to a larger sample size of the population. Nevertheless, there are some limitations in this study: (i) due to the cross-sectional design of NHANES, the temporal relationship between exposure and outcome cannot be established, limiting our ability to infer causality; thus, the association between BUN and the risk of developing HUA needs to be clarified by further cohort studies; (ii) although we adjusted for a variety of confounders in the multiple regression analysis, other potential confounders may still exist. Despite these limitations, these data effectively examine the relationship between BUN and the development of HUA, providing additional evidence on this topic and revealing possible sex differences in the association.
Conclusion
The current study shows that elevated BUN is proportionally associated with an increased risk of developing HUA in the general adult population in the United States, with gender differences. These results suggest that BUN may be an independent risk factor for HUA, and possible gender differences warrant attention in the management of HUA prevention. However, further prospective studies are needed to elucidate the causality and potential mechanisms of the above associations to break through the limitation that cross-sectional studies cannot determine causality.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LC: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. LY: Conceptualization, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This study used data from the National Health and Nutrition Examination Survey (NHANES). We are grateful to all of the NHANES research team for their time and effort in the NHANES project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singh G, Lingala B, and Mithal A. Gout and hyperuricaemia in the USA: prevalence and trends. Rheumatol (Oxford). (2019) 58:2177–80. doi: 10.1093/rheumatology/kez196
2. Kim YJ, Kim S, Seo JH, and Cho SK. Prevalence and associations between metabolic syndrome components and hyperuricemia by race: findings from US population, 2011-2020. Arthritis Care Res (Hoboken). (2024) 76:1195–202. doi: 10.1002/acr.25338
3. Tian S, Liu Y, Feng A, and Zhang S. Sex-specific differences in the association of metabolically healthy obesity with hyperuricemia and a network perspective in analyzing factors related to hyperuricemia. Front Endocrinol (Lausanne). (2020) 11:573452. doi: 10.3389/fendo.2020.573452
4. Zhang Y, Zhang M, Yu X, Wei F, Chen C, Zhang K, et al. Association of hypertension and hypertriglyceridemia on incident hyperuricemia: an 8-year prospective cohort study. J Transl Med. (2020) 18:409. doi: 10.1186/s12967-020-02590-8
5. Son M, Seo J, and Yang S. Association between dyslipidemia and serum uric acid levels in Korean adults: Korea National Health and Nutrition Examination Survey 2016-2017. PloS One. (2020) 15:e0228684. doi: 10.1371/journal.pone.0228684
6. Xu Y, Dong H, Zhang B, Zhang J, Ma Q, and Sun H. Association between dyslipidaemia and the risk of hyperuricaemia: a six-year longitudinal cohort study of elderly individuals in China. Ann Med. (2022) 54:2402–10. doi: 10.1080/07853890.2022.2118368
7. Sun Q, Zhang T, Manji L, Liu Y, Chang Q, Zhao Y, et al. Association between serum uric acid and non-alcoholic fatty liver disease: an updated systematic review and meta-analysis. Clin Epidemiol. (2023) 15:683–93. doi: 10.2147/CLEP.S403314
8. Zhang T, Bian S, Gu Y, Meng G, Zhang Q, Liu L, et al. Sugar-containing carbonated beverages consumption is associated with hyperuricemia in general adults: A cross-sectional study. Nutr Metab Cardiovasc Dis. (2020) 30:1645–52. doi: 10.1016/j.numecd.2020.05.022
9. Zhang T, Liu W, and Gao S. Exercise and hyperuricemia: an opinion article. Ann Med. (2024) 56:2396075. doi: 10.1080/07853890.2024.2396075
10. Chen-Xu M, Yokose C, Rai SK, Pillinger MH, and Choi HK. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the national health and nutrition examination survey, 2007-2016. Arthritis Rheumatol. (2019) 71:991–9. doi: 10.1002/art.40807
11. Zhang M, Zhu X, Wu J, Huang Z, Zhao Z, Zhang X, et al. Prevalence of hyperuricemia among chinese adults: findings from two nationally representative cross-sectional surveys in 2015–16 and 2018-19. Front Immunol. (2022) 12:791983. doi: 10.3389/fimmu.2021.791983
12. Che J, Tong J, Kuang X, Zheng C, He N, and Liu Z. Hyperuricemia and gout enhanced the risk of long-term mortality in hypertension: insights from the National Health and Nutrition Examination Survey 2007-2018. J Hypertens. (2024) 42:1390–8. doi: 10.1097/HJH.0000000000003744
13. Otaki Y, Konta T, Ichikawa K, Fujimoto S, Iseki K, Moriyama T, et al. Possible burden of hyperuricaemia on mortality in a community-based population: a large-scale cohort study. Sci Rep. (2021) 11:8999. doi: 10.1038/s41598-021-88631-8
14. Evans RDR, Hemmila U, Mzinganjira H, Mtekateka M, Banda E, Sibale N, et al. Diagnostic performance of a point-of-care saliva urea nitrogen dipstick to screen for kidney disease in low-resource settings where serum creatinine is unavailable. BMJ Glob Health. (2020) 5:e002312. doi: 10.1136/bmjgh-2020-002312
15. Kirtane AJ, Leder DM, Waikar SS, Chertow GM, Ray KK, Pinto DS, et al. Serum blood urea nitrogen as an independent marker of subsequent mortality among patients with acute coronary syndromes and normal to mildly reduced glomerular filtration rates. J Am Coll Cardiol. (2005) 45:1781–6. doi: 10.1016/j.jacc.2005.02.068
16. Liu J, Sun LL, Wang J, and Ji G. Blood urea nitrogen in the prediction of in-hospital mortality of patients with acute aortic dissection. Cardiol J. (2018) 25:371–6. doi: 10.5603/CJ.a2017.0075
17. Liu EQ and Zeng CL. Blood urea nitrogen and in-hospital mortality in critically ill patients with cardiogenic shock: analysis of the MIMIC-III database. BioMed Res Int. (2021) 2021:5948636. doi: 10.1155/2021/5948636
18. Peng R, Liu K, Li W, Yuan Y, Niu R, Zhou L, et al. Blood urea nitrogen, blood urea nitrogen to creatinine ratio and incident stroke: The Dongfeng-Tongji cohort. Atherosclerosis. (2021) 333:1–8. doi: 10.1016/j.atherosclerosis.2021.08.011
19. Li X, Li T, Wang J, Dong G, Zhang M, Xu Z, et al. Higher blood urea nitrogen level is independently linked with the presence and severity of neonatal sepsis. Ann Med. (2021) 53:2192–8. doi: 10.1080/07853890.2021.2004317
20. Du J, Niu J, Ma L, Sui Y, and Wang S. Association between blood urea nitrogen levels and length of stay in patients with pneumonic chronic obstructive pulmonary disease exacerbation: A secondary analysis based on a multicentre, retrospective cohort study. Int J Chron Obstruct Pulmon Dis. (2022) 17:2847–56. doi: 10.2147/COPD.S381872
21. Hu B, Xu G, Jin X, Chen D, Qian X, Li W, et al. Novel prognostic predictor for primary pulmonary hypertension: focus on blood urea nitrogen. Front Cardiovasc Med. (2021) 8:724179. doi: 10.3389/fcvm.2021.724179
22. Du J, Zhang W, Niu J, and Wang S. Association between blood urea nitrogen levels and the risk of diabetes mellitus in Chinese adults: secondary analysis based on a multicenter, retrospective cohort study. Front Endocrinol (Lausanne). (2024) 15:1282015. doi: 10.3389/fendo.2024.1282015
23. Shen J, Wang Z, Liu Y, Wang T, Wang XY, Qu XH, et al. Association of blood urea nitrogen with all-cause and cardiovascular mortality in hyperlipidemia: NHANES 1999-2018. Lipids Health Dis. (2024) 23:164. doi: 10.1186/s12944-024-02158-1
24. Erçin CN, Doğru T, Çelebi G, Gürel H, Genç H, Sertoğlu E, et al. The relationship between blood urea nitrogen levels and metabolic, biochemical, and histopathologic findings of nondiabetic, nonhypertensive patients with nonalcoholic fatty liver disease. Turk J Med Sci. (2016) 46:985–91. doi: 10.3906/sag-1502-144
25. Imamura Y, Mawatari S, Oda K, Kumagai K, Hiramine Y, Saishoji A, et al. Changes in body composition and low blood urea nitrogen level related to an increase in the prevalence of fatty liver over 20 years: A cross-sectional study. Hepatol Res. (2021) 51:570–9. doi: 10.1111/hepr.13631
26. Borghi C, Domienik-Karłowicz J, Tykarski A, Filipiak KJ, Jaguszewski MJ, Narkiewicz K, et al. Expert consensus for the diagnosis and treatment of patients with hyperuricemia and high cardiovascular risk: 2023 update. Cardiol J. (2024) 31:1–14. doi: 10.5603/cj.98254
27. Agricultural ruralural Research Service and US Department of Agriculture. What we eat in america: data tables . Available online at: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/ (Accessed 7 March 2021).
28. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. (2018) 138:e426–83. doi: 10.1161/CIR.0000000000000597
29. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. (2002) 106:3143–421. doi: 10.1161/circ.106.25.3143
30. Zou X, Zhou X, Zhu Z, and Ji L. Novel subgroups of patients with adult-onset diabetes in Chinese and US populations. Lancet Diabetes Endocrinol. (2019) 7:9–11. doi: 10.1016/S2213-8587(18)30316-4
31. Li SN, Cui YF, Luo ZY, Lou YM, Liao MQ, Chen HE, et al. Association between blood urea nitrogen and incidence of type 2 diabetes mellitus in a Chinese population: a cohort study. Endocr J. (2021) 68:1057–65. doi: 10.1507/endocrj.EJ20-0794
32. Feng P, Wang G, Yu Q, Zhu W, and Zhong C. First-trimester blood urea nitrogen and risk of gestational diabetes mellitus. J Cell Mol Med. (2020) 24:2416–22. doi: 10.1111/jcmm.14924
33. Du K and Luo W. Association between blood urea nitrogen levels and diabetic retinopathy in diabetic adults in the United States (NHANES 2005-2018). Front Endocrinol (Lausanne). (2024) 15:1403456. doi: 10.3389/fendo.2024.1403456
34. Qi D, Liu J, Wang C, Wang L, Zhang X, Lin Q, et al. Sex-specific differences in the prevalence of and risk factors for hyperuricemia among a low-income population in China: a cross-sectional study. Postgrad Med. (2020) 132:559–67. doi: 10.1080/00325481.2020.1761133
35. Fathallah-Shaykh SA and Cramer MT. Uric acid and the kidney. Pediatr Nephrol. (2014) 29:999–1008. doi: 10.1007/s00467-013-2549-x
36. Frank H, Graf J, Amann-Gassner U, Bratke R, Daniel H, Heemann U, et al. Effect of short-term high-protein compared with normal-protein diets on renal hemodynamics and associated variables in healthy young men [published correction appears in Am J Clin Nutr. 2010 Feb;91(2):494. Graf, Juliane [corrected to Graf, Julia. Am J Clin Nutr. (2009) 90:1509–16. doi: 10.3945/ajcn.2009.27601
37. Kalantar-Zadeh K, Kramer HM, and Fouque D. High-protein diet is bad for kidney health: unleashing the taboo. Nephrol Dial Transplant. (2020) 35:1–4. doi: 10.1093/ndt/gfz216
38. Farhadnejad H, Asghari G, Emamat H, Mirmiran P, and Azizi F. Low-carbohydrate high-protein diet is associated with increased risk of incident chronic kidney diseases among tehranian adults. J Ren Nutr. (2019) 29:343–9. doi: 10.1053/j.jrn.2018.10.007
39. Allison SJ. Diabetes: Urea inhibits insulin secretion in CKD. Nat Rev Nephrol. (2016) 12:581. doi: 10.1038/nrneph.2016.131
40. Han T, Lan L, Qu R, Xu Q, Jiang R, Na L, et al. Temporal relationship between hyperuricemia and insulin resistance and its impact on future risk of hypertension. Hypertension. (2017) 70:703–11. doi: 10.1161/HYPERTENSIONAHA.117.09508
41. McCormick N, O'Connor MJ, Yokose C, Merriman TR, Mount DB, Leong A, et al. Assessing the causal relationships between insulin resistance and hyperuricemia and gout using bidirectional mendelian randomization. Arthritis Rheumatol. (2021) 73:2096–104. doi: 10.1002/art.41779
42. Sun H, Chang X, Bian N, An Y, Liu J, Leng S, et al. Adipose tissue insulin resistance is positively associated with serum uric acid levels and hyperuricemia in northern chinese adults. Front Endocrinol (Lausanne). (2022) 13:835154. doi: 10.3389/fendo.2022.835154
43. Liu XZ, Xu X, Zhu JQ, and Zhao DB. Association between three non-insulin-based indexes of insulin resistance and hyperuricemia. Clin Rheumatol. (2019) 38:3227–33. doi: 10.1007/s10067-019-04671-6
44. Piao W, Zhao L, Yang Y, Fang H, Ju L, Cai S, et al. The prevalence of hyperuricemia and its correlates among adults in China: results from CNHS 2015-2017. Nutrients. (2022) 14:4095. doi: 10.3390/nu14194095
45. Kawano K, Ueno T, Maeda T, Nohara C, Maki K, Iwanaga K, et al. Relationship between abdominal circumference and the incidence of hyperuricemia in the general Japanese population. Sci Rep. (2024) 14:4573. doi: 10.1038/s41598-024-55008-6
46. Koo BS, Jeong HJ, Son CN, Kim SH, Kim HJ, Kim GH, et al. Distribution of serum uric acid levels and prevalence of hyper- and hypouricemia in a Korean general population of 172,970. Korean J Intern Med. (2021) 36:S264–72. doi: 10.3904/kjim.2020.116
47. Matsubayashi M, Sakaguchi YM, Sahara Y, Nanaura H, Kikuchi S, Asghari A, et al. 27-Hydroxycholesterol regulates human SLC22A12 gene expression through estrogen receptor action. FASEB J. (2021) 35:e21262. doi: 10.1096/fj.202002077R
48. Takiue Y, Hosoyamada M, Kimura M, and Saito H. The effect of female hormones upon urate transport systems in the mouse kidney. Nucleosides Nucleotides Nucleic Acids. (2011) 30:113–9. doi: 10.1080/15257770.2010.551645
49. Hosoyamada M, Takiue Y, Shibasaki T, and Saito H. The effect of testosterone upon the urate reabsorptive transport system in mouse kidney. Nucleosides Nucleotides Nucleic Acids. (2010) 29:574–9. doi: 10.1080/15257770.2010.494651
50. Halperin Kuhns VL and Woodward OM. Sex differences in urate handling. Int J Mol Sci. (2020) 21:4269. doi: 10.3390/ijms21124269
51. Li GY, Qian XD, Ma CM, and Yin FZ. The dose-response relationship between sex hormones and hyperuricemia in different gender: NHANES 2013-2016. Front Endocrinol (Lausanne). (2022) 13:1035114. doi: 10.3389/fendo.2022.1035114
52. Cho SK, Winkler CA, Lee SJ, Chang Y, and Ryu S. The prevalence of hyperuricemia sharply increases from the late menopausal transition stage in middle-aged women. J Clin Med. (2019) 8:296. doi: 10.3390/jcm8030296
53. Zhong C, Griffin LL, Heussaff O, O'Dea R, Whelan C, and Stewart G. Sex-related differences in UT-B urea transporter abundance in fallow deer rumen. Vet Sci. (2022) 9:73. doi: 10.3390/vetsci9020073
54. Liu L, Lou S, Xu K, Meng Z, Zhang Q, and Song K. Relationship between lifestyle choices and hyperuricemia in Chinese men and women. Clin Rheumatol. (2013) 32:233–9. doi: 10.1007/s10067-012-2108-z
55. Feraco A, Gorini S, Camajani E, Filardi T, Karav S, Cava E, et al. Gender differences in dietary patterns and physical activity: an insight with principal component analysis (PCA). J Transl Med. (2024) 22:1112. doi: 10.1186/s12967-024-05965-3
56. Park DY, Kim YS, Ryu SH, and Jin YS. The association between sedentary behavior, physical activity and hyperuricemia. Vasc Health Risk Manag. (2019) 15:291–9. doi: 10.2147/VHRM.S200278
Keywords: hyperuricemia, blood urea nitrogen, risk, gender medicine, NHANES
Citation: Chen L and Yin L (2025) Sex-specific associations between blood urea nitrogen and risk of hyperuricemia in U.S. adults: the NHANES 1999-2020. Front. Endocrinol. 16:1560738. doi: 10.3389/fendo.2025.1560738
Received: 17 January 2025; Accepted: 22 May 2025;
Published: 05 June 2025.
Edited by:
Deise Carla Almeida Leite Dellova, University of São Paulo, BrazilReviewed by:
Maria Franco, Federal University of São Paulo, BrazilRosa Maria Marcusso, Institute of Infectology Emílio Ribas, Brazil
Copyright © 2025 Chen and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixue Yin, eWlubGl4dWVfY2FyZGlhY0AxNjMuY29t
 Lingling Chen
Lingling Chen Lixue Yin
Lixue Yin