- 1Epidemiology and Population Health Department, Faculty of Health Sciences, American University of Beirut, Beirut, Lebanon
- 2Department of Biochemistry and Molecular Genetics, Faculty of Medicine, American University of Beirut, Beirut, Lebanon
Background: Liver cancer has seen a concerning rise in incidence, currently ranked as the sixth most prevalent cancer. Diabetes, along with indices of social, biological, and behavioral determinants of health, was linked to the risk of liver cancer.
Aim: We aim to determine the effect of diabetes and selected indices of determinants of health on liver cancer.
Methods: Our quantitative study is based on a sample of 239,006 US participants adopted from the BRFSS-2022 data. Our results are summarized using frequency distributions and weighted percentages. Weighted logistic regressions were employed to determine the associations with liver cancer.
Results: In this sample population, 73 individuals experienced liver cancer, 12.17% (n=33,776) had diabetes, with a four-fold increase in the odds of liver cancer for individuals with diabetes (OR: 4.27, CI: 1.73-10.57). Employment status, educational level, urban/rural living, smoking status were determinants of health associated with liver cancer as well. Our subgroup analysis focusing exclusively on those diagnosed with liver cancer following their diabetes diagnosis confirmed diabetes as risk factor for liver cancer (OR: 5.44, 95%CI: 1.58-18.70), along with marital status and other determinants of health.
Conclusion: Effective diabetes management and addressing key health determinants are crucial for reducing liver cancer risk and improving prevention and treatment outcomes.
1 Introduction
Primary liver cancer is the sixth most common cancer and the third leading cause of cancer-related deaths (1). It arises from malignant tumor cells forming in liver tissues (1). Liver cancer can be classified into several types based on the site of its origin. Hepatocellular Carcinoma (HCC) is the predominant type of liver cancer originating in hepatocytes and accounts for about 80 to 90% of liver cancer cases (2). Cholangiocarcinoma, stemming from bile duct cells, accounts for about 10 to 20% of liver cancer cases, while angiosarcoma, a rare type, constitutes less than 2% of liver cancer cases, and originates in blood vessels, mainly affects adults over 70 years of age (3).
Cirrhosis of the liver is an essential risk factor associated with liver cancer. Cirrhosis develops when scar tissues replace the damaged liver cells. Individuals who suffer from cirrhosis face heightened susceptibility to liver cancer. Remarkably, the majority of liver cancer cases, up to 90% in the States (US), stem from pre-existing cirrhosis (4, 5).
The incidence of liver cancer has been increasing over the past several years, rising from 2.641 cases per 100,000 person-years in 1975 to 8.657 cases per 100,000 person-years in 2017 (6). In 2020, around 905,677 individuals were diagnosed with liver cancer, globally, marking an age-standardized incidence rate of 9.5 per 100,000 individuals (7).
Social determinants of health (SDOH), defined as the social and economic factors that shape health outcomes, are known to be associated with liver cancer risk. For instance, race is a significant risk factor for liver cancer, with its incidence varying across different racial groups (8). African Americans have a higher incidence rate ratio (1.4) compared to White Americans (8), and are more likely to develop advanced liver cancers with less advanced liver disease (9). Moreover, according to the OHSU Knight Cancer Institute, Native Americans and Native Alaskan adults are at a higher risk of developing liver cancer among other racial and ethnic groups (10). These findings underscore the role of racial and ethnic disparities, along with broader social determinants of health (SDOHs) in influencing liver cancer risk. A recent study in the European Economic Area and the United Kingdom concluded that individuals with low socioeconomic status (SES), who reside in disadvantaged areas with low educational levels, face elevated death rates from cirrhosis and context-related increased risk of liver cancer (11). Particularly, they may face barriers such as discrimination and inadequate healthcare access in liver cancer care (11), highlighting the impact of socioeconomic factors on liver cancer risk.
Employment and occupational types can also affect the risk of certain types of cancer. In this regard, a recent study, included over 12,000 individuals analyzing their employment history from age 16 to 65, revealed that certain employment trajectories were associated with varying cancer risks and suggested that employment history can influence cancer development (12). In addition, certain occupations and workplace exposures were found to be associated with an increased risk of liver cancer that are due to contact with specific chemicals and toxins (13–15). Aflatoxin, for example, accounts for approximately 20% of liver cancer cases worldwide, with a high proportion occurring in sub-Saharan Africa, mainly among farmers and textile workers engaged in agricultural activities (14), whereas exposure to endotoxin may be protective against liver cancer risk (16), underscoring the impact of socioeconomic and occupational factors on liver cancer risk.
Interestingly, liver cancer prognosis has also been shown to be affected by partner status, with patients who are married or with partners having positive prognosis and better survival rates compared to single, unmarried or widowed participants (17–19). This link may be explained by the emotional and social support a person living with cancer can receive from a partner which can help alleviate the psychological distress triggered by their cancer diagnosis (20–22). This suggests that marital status, a key index of social determinant of health, may also play a role in influencing liver cancer risk.
Certain biologic characteristics were also identified as potential correlates of liver cancer. In this respect, it was reported that the age-standardized incidence rate for liver cancer is 14.1 per 100,000 for males and 5.2 per 100,000 for females (23). According to estimates by the American Cancer Society for 2023, primary liver cancer and intrahepatic Cholangiocarcinoma (ICC) are projected to affect approximately 41,630 individuals in the US. Among these cases, about 28,000 are expected to affect men and 13,630 to affect women (5), showing that men are at a higher risk of developing liver cancer. A recent study in China concluded that about 45% of liver cancer cases were among adults aged 60–79 years and 37% were among 45–59 years adults suggesting that comorbidities associated with aging, and older ages elevate liver cancer risk (23). Furthermore, obesity, classified as having Body Mass Index (BMI) >35 Kg/m2 is also identified as a risk factor for liver cancer (24–26). For instance, there is a 39% higher risk of HCC for every 5-unit rise in BMI in a meta-analysis of 21 prospective studies that included 17,624 cases of primary liver cancer (27). Moreover, a recent systematic review and meta-analysis of 28 prospective cohort studies concluded that an increased BMI, particularly obesity, is associated with a three times higher risk of developing primary liver cancer (28). These findings highlight the role of biological factors, such as age, gender, and BMI, on the risk of this type of malignancy.
Behavioral and lifestyle factors such as alcohol consumption, smoking, and exercise can also affect liver cancer. For instance, findings from the World Health Organization’s International Agency for Research on Cancer (WHO-IARC) have established alcohol consumption as a causal factor in the development of liver cancer (29), accounting for 32% of HCC cases in Italy (30) and 45% in the US (31). A recent meta-analysis further confirmed these findings, indicating that heavy alcohol consumption is associated with double the risk of developing two specific types of liver cancer: HCC and ICC (32). Additionally, the Liver Cancer Pooling Project, consisting of 14 cohort studies in the US, revealed an 87% increased risk of HCC among heavy alcohol drinkers compared to non-drinkers (33). Furthermore, several studies confirmed that liver cancer risk decreases by 6% to 7% after cessation of alcohol consumption, emphasizing the significant impact of lifestyle and behavioral factors on liver cancer risk (31). In addition, alcohol consumption is a recognized risk factor for liver cirrhosis, with a dose-response relationship (34, 35). A prospective study of 401,806 women in the UK, conducted over 15 years of follow-up, revealed that the incidence of liver cirrhosis increases with higher alcohol intake. Compared to consuming 1 or 2 drinks per week, drinking 15 or more drinks weekly increases the relative risk of cirrhosis nearly 3 times (35). Along the same lines, studies have highlighted an elevated risk of HCC among current smokers (33, 36), with a smoker who consumes more than 25 cigarettes per day facing a 55% increased risk of HCC (33). Furthermore, in recent studies, exercise interventions have been linked to improving health parameters for liver cancer patients (37), and liver cirrhosis patients significantly (38). A meta-analysis of 10 prospective cohort studies concluded that engaging in vigorous-intensity physical exercise in high amounts reduces liver cancer risk by 54% (39). These findings underscore the importance of lifestyle choices on the liver cancer risk.
In addition to the biological, social and behavioral factors, diabetes is a comorbid disease that also contributes to elevated liver cancer risk (40). Diabetes is a highly prevalent chronic disease that affects about 37 million Americans, including youth and adults (41). Individuals with type 2 diabetes face a heightened 2 to 4 times risk of developing liver cancer compared to non-diabetics, suggesting an increased susceptibility to severe liver diseases such as HCC in diabetic patients (42, 43). Furthermore, a study conducted among patients with cirrhosis found that, in the multivariable analysis, diabetes was associated with a significantly increased risk of HCC, with a hazard ratio of 4.2 (44). The increased incidence and the advancement of chronic liver disease among individuals with diabetes may be linked to an increased risk of liver cancer, with several factors involved, such as viral infections and excessive alcohol consumption (40). In addition, diabetes is strongly influenced by various social determinants of health (SDOHs), socio-demographic factors, and behavioral patterns (45–47). Therefore, it is essential to examine the association between diabetes and liver cancer within the context of these diverse variables.
Due to the rising incidence of liver cancer cases, it’s critical to identify and address the causes of this increase. Several factors, such as diabetes and other social, biological, and behavioral variables, fall within our sphere of influence. However, current research lacks a comprehensive understanding of all the contributing factors. That’s where our study comes in: we seek to fill this gap by investigating how diabetes, biological, social health determinants, and behavioral choices can influence liver cancer risk. In this study, we aim to explore how various indices of social determinants of health (SDOH), including race, employment, education, marital status, and others, are correlated with liver cancer, alongside behavioral factors such as smoking, alcohol consumption, and exercise, and biological factors encompassing age, sex, and BMI. The novelty of our study lies in the inclusion of diabetes, a highly prevalent clinical condition, alongside a wide array of non-clinical characteristics to assess their relationships comprehensively with this specific type of malignancy.
2 Methods
2.1 Study population and sampling
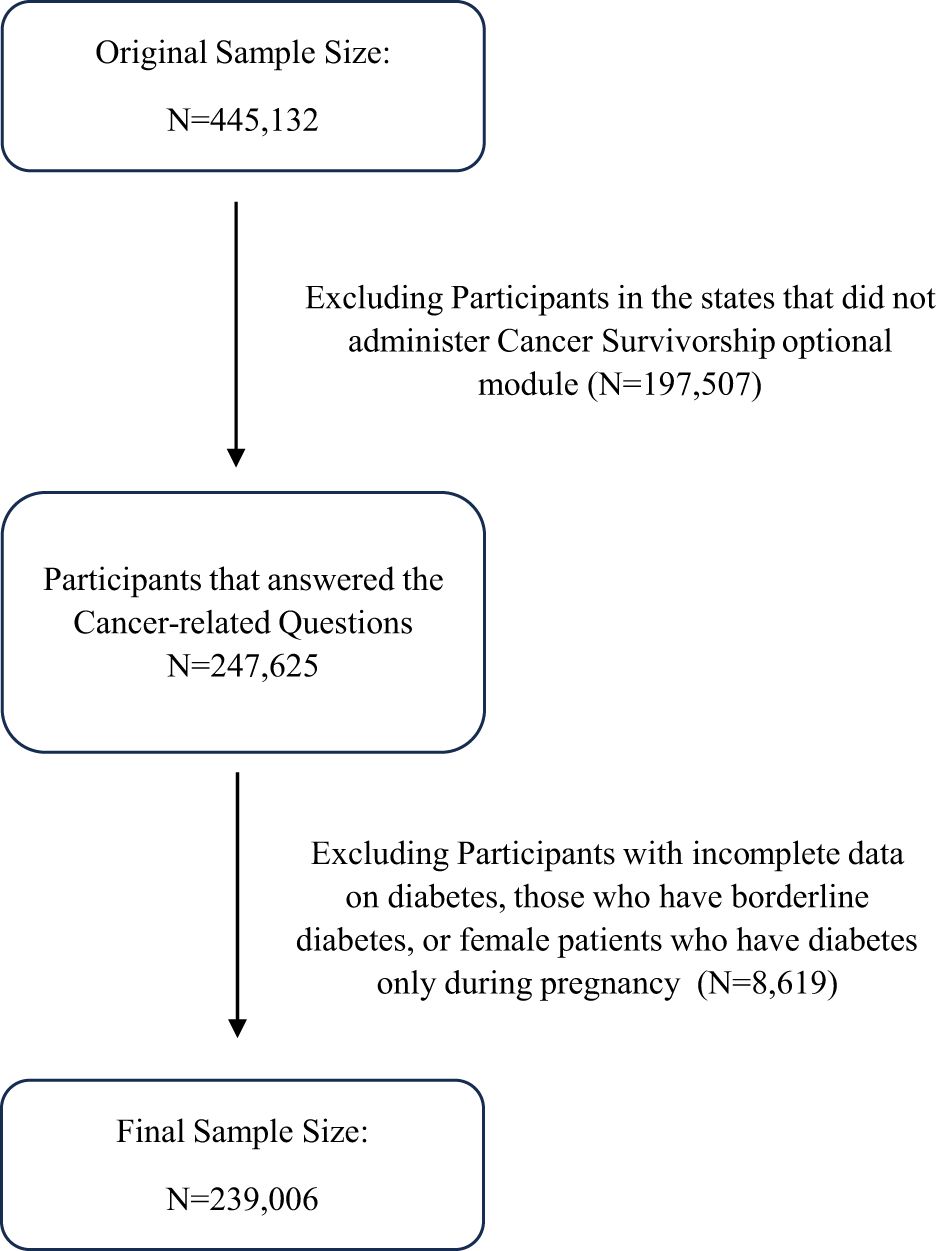
Our quantitative study is based on secondary data retrieved from the Behavioral Risk Factor Surveillance System (BRFSS) collected cross-sectionally in the year 2022. The BRFSS, administered by the Centers for Disease Control and Prevention (CDC), serves as the primary health-related telephone survey system in the US. It aims to gather state-specific data on various health-related risk behaviors, chronic health conditions, and the use of preventive services among U.S. residents. This survey system included noninstitutionalized adults aged 18 years or older residing in 50 US states and participating regions, totaling 445,132 participants. Not all states administered the “Cancer Survivorship: type of cancer” optional module; accordingly, 30 states were included in this study. After excluding states without responses to cancer-related questions, the sample size decreased to 247,625 individuals. Diabetes serves as the primary predictor in our study, so it is important to exclude participants with incomplete data on diabetes. As a result, the final sample size for our analysis is 239,006 participants, presented in the flowchart depicted in Figure 1.
The BRFSS selects participants randomly for interviews, using a multistage cluster design, resulting in a nationally representative sample. Aiming to adequately represent smaller and geographically distinct regions of interest, a weighted sampling approach was used to collect data disproportionately.
2.2 Sub-analysis study population
To ensure that liver cancer occurred after diabetes, we excluded participants whose diabetes was diagnosed after their liver cancer. Specifically, we focused on participants with only one type of cancer, liver cancer, and created a new variable representing the age difference between the diagnosis of cancer and diabetes (cancer diagnosis age - diabetes diagnosis age). Participants with a negative age difference, indicating that cancer was diagnosed before diabetes, were excluded from the study. As a result, 42 observations were removed, and the final sample size is 238,964 for this sub-analysis.
2.3 Concepts and measures
The outcome of interest in our study is liver cancer which was classified as a binary yes-no question. Participants who reported being diagnosed with cancer were further asked about the specific type they had, and those indicating liver cancer were considered the cases in our study.
Diabetes, the primary predictor in our quantitative analysis, was dichotomously classified based on participants’ self-reported diabetes status. Other predictors, encompassing SDOHs, include variables such as race dichotomized into white and non-white, home ownership, marital status, employment status, income level, place of residence, educational level, and race.
In addition, several biological and behavioral determinants of health were also considered in our analysis. These factors included age, sex, body mass index (BMI), exercise, smoking status, and alcohol consumption. Participants who reported consuming at least one alcoholic drink in the past 30 days were categorized as alcohol drinkers and assigned a value of “Yes” for the alcohol use variable. BMI was categorized according to the CDC classification into four groups: underweight (BMI less than 18.5 kg/m²), normal weight (BMI between 18.5 kg/m² and 25.0 kg/m²), overweight (BMI between 25.0 kg/m² and 30.0 kg/m²), and obese (BMI more than 30.0 kg/m²). Data on smoking were collected using two questions. First, participants were asked, “Have you smoked at least 100 cigarettes in your entire life?” Those who answered “No” were classified as never smokers, while those who answered “Yes” were further categorized. To distinguish between former and current smokers, a follow-up question was asked: “Do you now smoke cigarettes every day, some days, or not at all?” Participants who answered every day or some days were classified as current smokers, while those who answered not at all were classified as former smokers.
Further details about the distribution and categories of our variables can be found in the descriptive table (Table 1).
2.4 Statistical analysis
Accounting for the complex sampling weighted approach in the BRFSS data collection process, our analysis required weighted descriptive and regression analyses. Our analysis was initiated by generating summary statistics, including counts and weighted percentages reported for all variables in the study to determine the distribution of the different levels of the variables among participants. The crude associations were evaluated using weighted simple logistic regression computing unadjusted odds ratios (ORs) along with their 95% confidence intervals (CIs) for the associations between diabetes, SDOH, biologic and behavioral factors, and liver cancer. A p-value that is less than or equal to 0.05 reveals a significant predictor for liver cancer risk. Subsequently, we built our multivariable model using weighted multiple logistic regression, and including most of the variables that were significant at the unadjusted level, yielding adjusted ORs along with their 95% CIs, and p-values. Our multivariable models were built gradually by adding each of the covariates one at a time while monitoring the changes in the covariates’ magnitude and direction. This gradual approach in model building helped us avoid issues of multicollinearity among predictors whilst ensuring that measures of multicollinearity such as the variance inflation factor remained low and below the conservative cutoff point of 2.5 assumed for logistic regression. We have employed the statistical package StataSE 18 for analysis of the data.
3 Results
3.1 Descriptive table
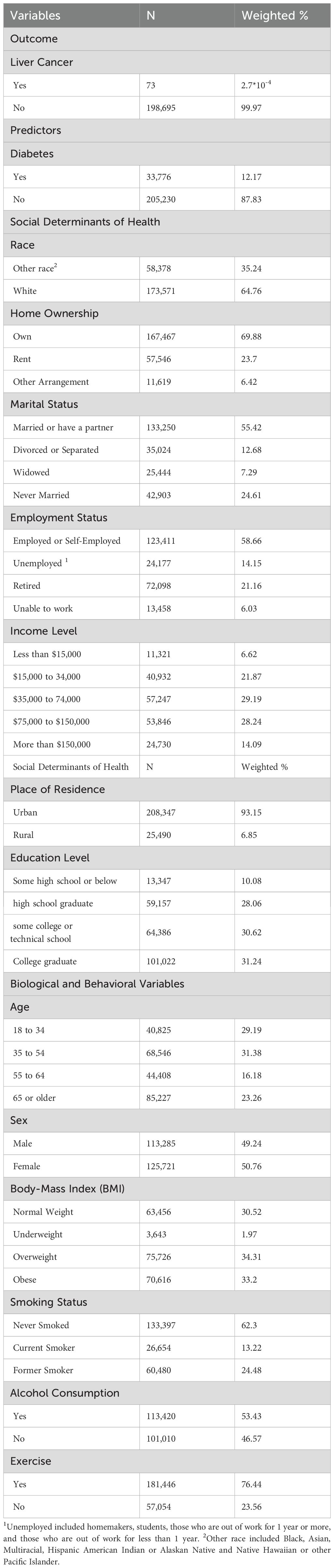
The characteristics of our study population are summarized in Table 1. The total number of participants in our study is 239,006, among whom 73 individuals experienced the outcome of interest, liver cancer. Diabetes is prevalent among 12.17% of the participants (n=33,776).
The majority of participants in our study reside in urban areas (93.15%, n=208,347), own homes (69.88%, n=167,467), and are either married or have a partner (55.42%, n=144,250). A balanced distribution of income is observed, with 21.87% of the population earning between $15,000 and $34,000, 29.19% between $35,000 and $74,000, and around 28.24% between $75,000 and $150,000. A small percentage (6%) have income levels below $15,000, while 14% have incomes exceeding $150,000.
Most of the participants are employed or self-employed (58.66%, n=123,411), while 14.15% (n=24,177) are unemployed, including homemakers, students, and those out of work. Educational levels vary, with around 10% having some high school education or below. The rest are distributed among high school graduates (28.06%), those with some college or technical school attendance (30.62%), and college graduates (31.24%).
Regarding race, the majority are identified as white (64.76%, n=173,571), with the remaining participants falling into the “other race” category, including Black, Asian, Multiracial, Hispanic, American Indian or Alaskan Native, and Native Hawaiian or other Pacific Islander. Gender distribution is nearly equal, with 50.76% females (n=125,721) and 49.24% males (n=113,285).
Concerning the biological factors, around 60% of participants fall within the younger age group of 18 to 54 (n=109,371), while approximately 40% (n=129,635) constitutes the older age group of 55 years and above.
BMI categories, particularly normal weight, overweight, and obese, exhibit a relatively even distribution, each comprising approximately one-third of the study cohort, with underweight participants accounting for a minimal prevalence of about 2%. In terms of behavioral factors, the majority of participants reported never smoking (62.3%, n=133,397), engaging in regular physical exercise (76.44%, n=181,446), and consuming alcohol in the 30 days before data collection (53.43%, n=113,420).
3.2 Liver cancer, diabetes, and SDOHs: unadjusted and adjusted associations
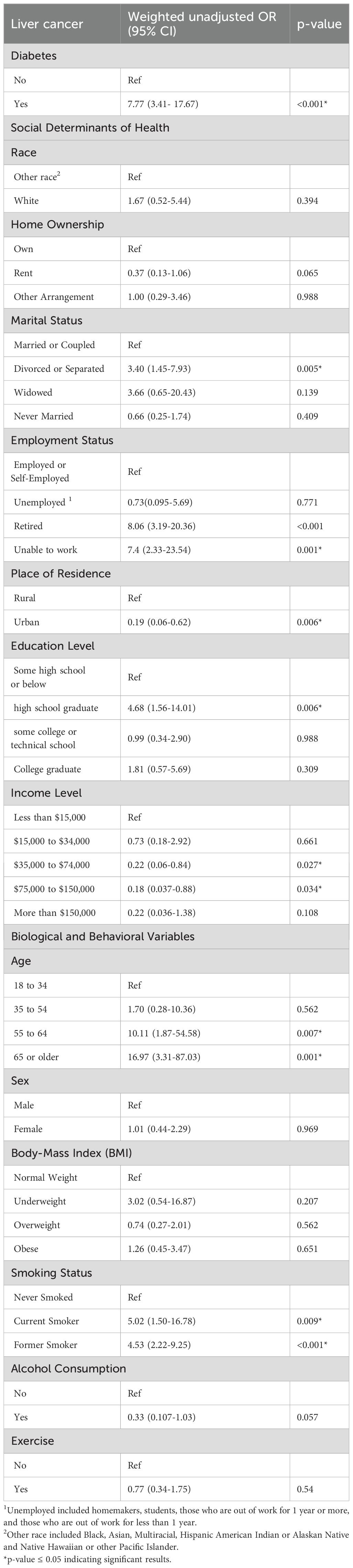
Table 2 presents the weighted unadjusted ORs of the associations between liver cancer and all the predictors considered in our study to examine the crude associations. The purpose of these associations is to identify significant predictors, defined by a p-value less than 0.05, for inclusion in the subsequent multivariable model, Table 3.
Results from the unadjusted analysis indicate that diabetes, marital status, employment status, place of residence, educational level, income level representing indices of SDOH, along with age and smoking which represent the biologic and behavioral determinants of heath respectively are significantly associated with liver cancer risk. As such, some of these variables were incorporated in Table 3 to construct the multivariable model. Recognizing that both income and employment status are components of SDOH and are correlated with financial status, and noting that employment status can also be affected by age, combining all these covariates together in one model may not be feasible given their strong interconnectivity. Consequently, employment status was included in our multivariable model excluding income and age.
The findings of the full model underscore a significant association between diabetes and liver cancer, indicating a four-fold increase in the odds of liver cancer for individuals with versus without diabetes (OR: 4.27, CI: 1.73-10.57, p-value: 0.002) (Table 3).
With respect to the SDOH, marital status, initially significant in the unadjusted analysis, had an increase in p-value and became borderline significant for the divorced/separated category compared to the married or coupled participants (OR: 2.28, CI: (0.96-5.41, p-value: 0.061). However, employment status emerges as a crucial factor, with unemployment showing a protective association with liver cancer (OR: 0.01; 95% CI: 0.002-0.13, p-value: 0.000). Conversely, being retired or unable to work appears to be linked with increased odds of liver cancer by approximately three and four times, respectively, compared to employed individuals. Educational level had a significant association with liver cancer, with high school graduates exhibiting a seven-fold increase in the odds of liver cancer compared to those with some schooling or below (OR: 7.04, 95% CI: 1.94-25.51, p-value: 0.003). Living in an urban area emerges as a protective factor, that is significantly associated with reduced risk of liver cancer (OR: 0.24, 95% CI: 0.08-0.71, p-value: 0.01).
Regarding the behavioral factors, smoking status was significantly associated with liver cancer in the full model indicating that former smokers had twice the odds of liver cancer compared to those who never smoked (OR: 2.55, 95% CI: 1.20-5.42, p-value: 0.015) (Table 3).
3.3 Sub-analysis: characteristics of the participants with liver cancer diagnosed after preexisting diabetes and the weighted adjusted associations with diabetes, and the other variables in this subpopulation (n=238,964)
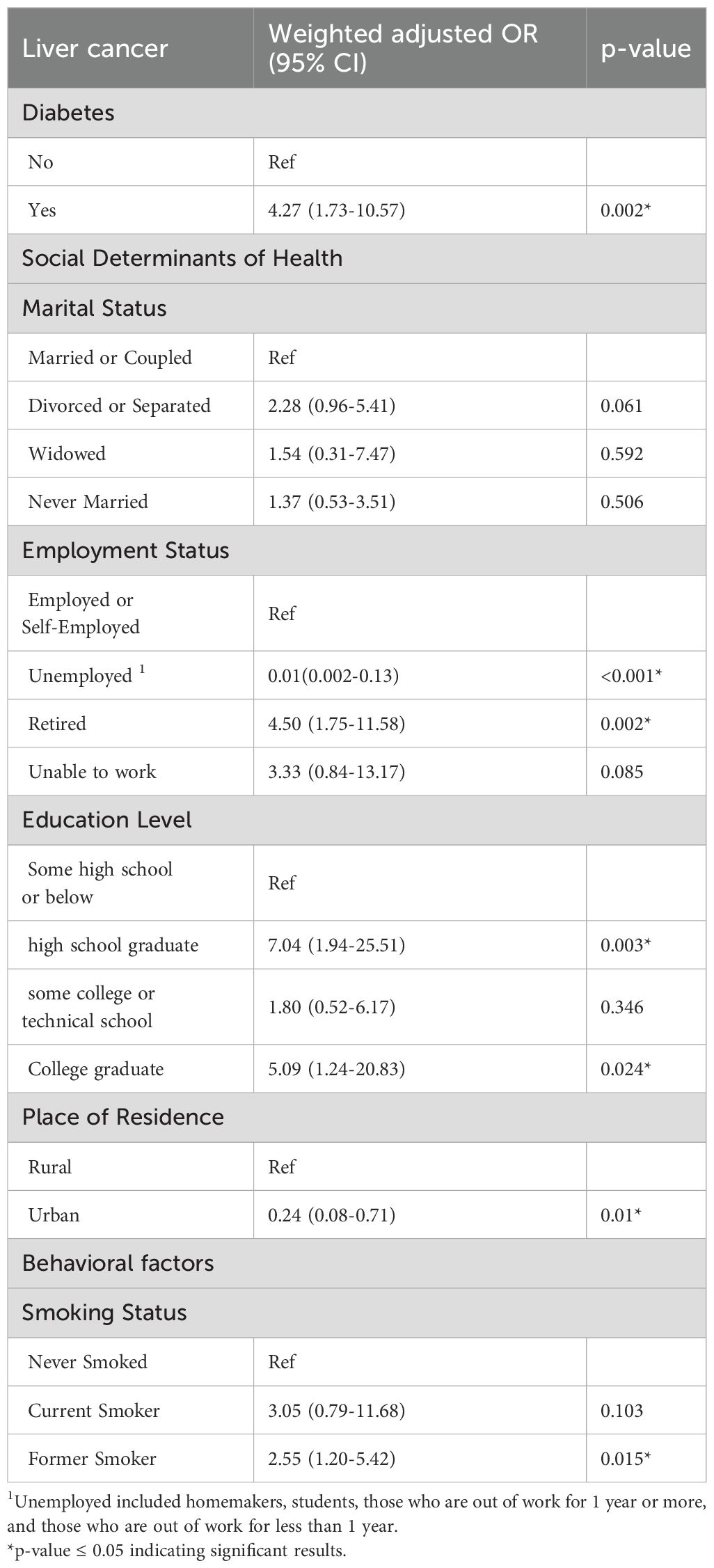
After excluding participants diagnosed with diabetes after their liver cancer, the number of liver cancer cases decreased to 31 within the subpopulation of 238,964. In this group, the prevalence of diabetes is 12.16% (n=33,754) (Table 4). Similar to our initial approach, we conducted unadjusted analyses to identify significant variables and build our new model. Diabetes, marital status, employment status, place of residence, and smoking status were the variables included in our adjusted model of the sub-group. Our adjusted sub-analysis presented in Table 5 showed a 5-fold increase in liver cancer risk for diabetics (OR: 5.44; 95% CI (1.58-18.70)), compared to non-diabetics. Moreover, employment status was also associated with liver cancer among participants who had diabetes before liver cancer. Specifically, compared to employed participants, those who were retired had a higher risk of liver cancer (OR: 3.30; 95% CI: 1.00-10.86), while unemployed participants appeared to be protected against liver cancer (OR: 0.02; 95% CI: 0.002-0.18). Marital status was also linked to liver cancer. In particular, widowed participants had a six-fold higher risk (OR: 6.37; 95% CI: 1.17-34.58), while those who never married had a four-fold higher risk (OR: 4.24; 95% CI: 1.14-15.72) compared to participants who were married or in a couple. Lastly, place of resident was also associated with liver cancer risk whereby residing in urban areas was linked to an 84% reduced risk (OR:0.16; 95%CI (0.032-0.81)). These results confirm once more diabetes and social determinants of health as significant associates of liver cancer.
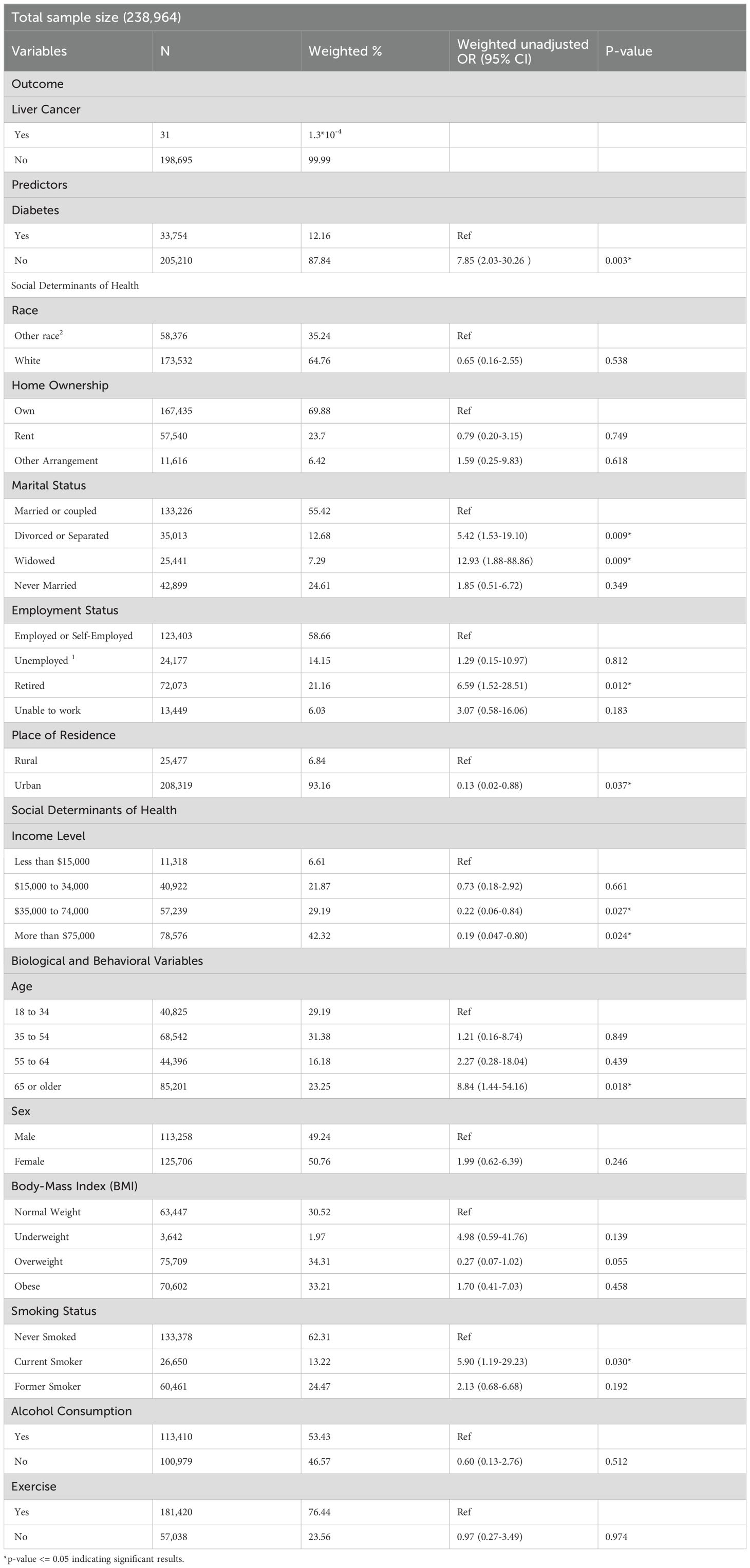
Table 4. Characteristics of the participants with liver cancer diagnosed after preexisting diabetes and the weighted unadjusted associations with diabetes, and the other variables in this subpopulation.
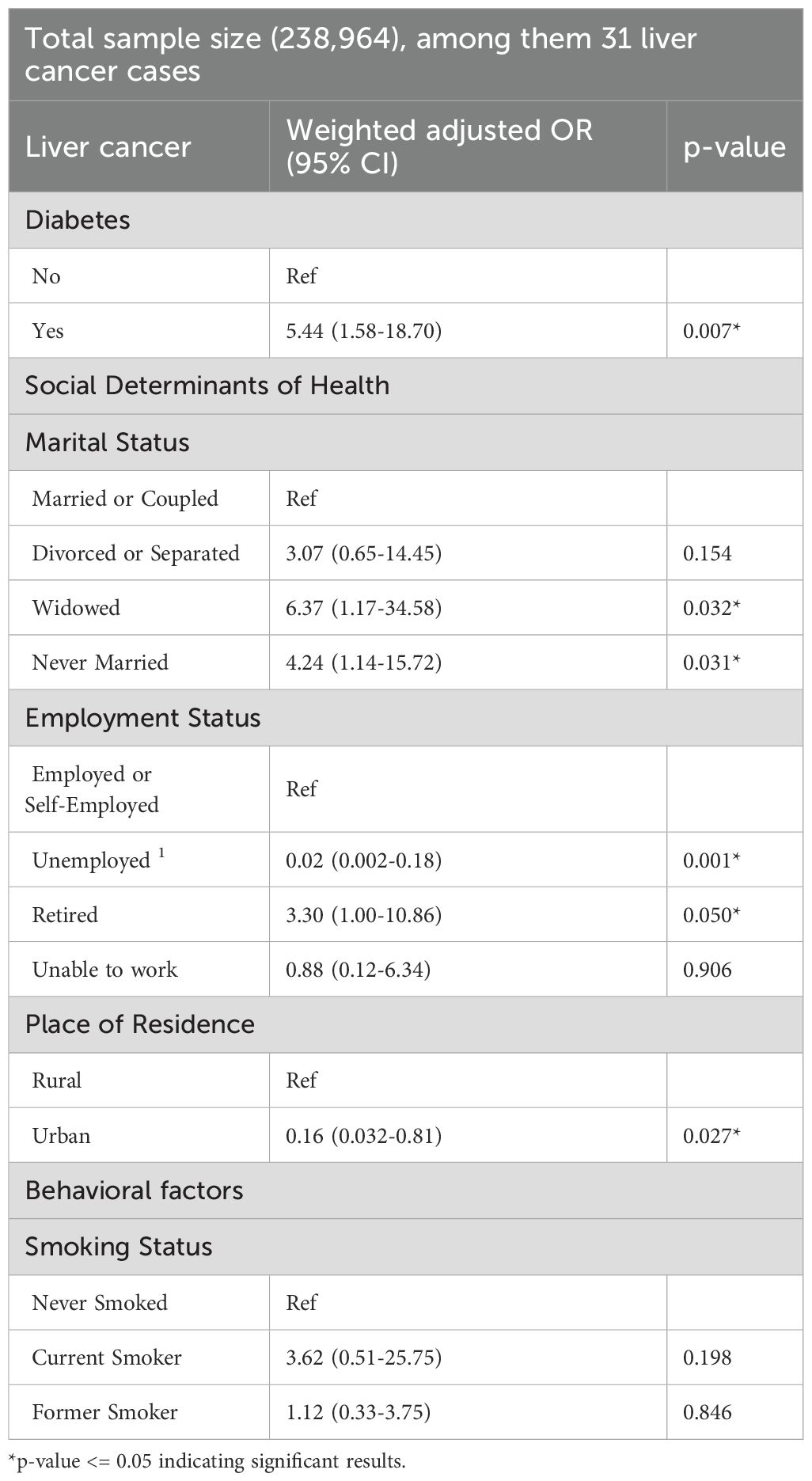
Table 5. Adjusted associations: liver cancer, diabetes, SDOH, and other covariates among participants with liver cancer diagnosed after preexisting diabetes.
4 Discussion
We present here a study that focuses on identifying the risk factors for liver cancer using nationally representative population-based BRFSS data. We have examined diabetes in relation to liver cancer in a comprehensive model that included a broad set of SDOH, biological, and behavioral correlates of liver cancer.
Our findings indicated that diabetes, employment status, educational level, place of residence, smoking status, and marital status were significantly associated with liver cancer. Specifically, individuals with diabetes, currently employed or retired adults, high school or college graduates, residents of rural areas, former smokers, and widowed or never married, have an increased risk of liver cancer.
4.1 Liver cancer risk and diabetes
Previous research has identified diabetes as a risk factor for various cancer types, including liver cancer (48). Consistent with this, our study findings revealed that individuals with diabetes face a four-fold increase in the odds of liver cancer compared to those without diabetes. In a six-year cohort study consisting of 10,794 Type 2 diabetes cases, 59 primary liver cancer cases were diagnosed, indicating an incidence rate of 54.66 per 10,000 individuals (49). Similar to our results, in a hospital-based case-control study, individuals with diabetes were found to have a four times higher risk of HCC in their multivariable model (50). They also suggested that as Type 2 diabetes progresses, the risk of liver cancer increases significantly (50). Specifically, in the presence of viral hepatitis B and C and heavy alcohol intake, the risk of liver cancer was observed to increase ten times among type 2 diabetics (50), indicating that the effect of diabetes on liver cancer risk is highly impacted by other coexisting conditions. Studies showed that some metabolic conditions, such as non-alcoholic fatty liver disease (NAFLD), might mediate the associations between diabetes and the increased liver cancer risk (51, 52). NAFLD is a condition in which there is an excess fat build-up on the liver that is not related to the excess in alcohol use (53). More than 70% of individuals with diabetes have NAFLD, often due to insulin resistance (43). In individuals with NAFLD, diabetes contributes to liver cancer through complex mechanisms involving liver inflammation (54). In response to diabetes, the liver undergoes chronic inflammation characterized by the release of proinflammatory cytokines and the generation of reactive oxygen species (ROS) (54). These inflammatory mediators induce cellular stress and disrupt normal cellular homeostasis by activating intracellular signaling pathways (54). Moreover, a major consequence of diabetes-induced inflammation is the induction of genomic instability, marked by the accumulation of DNA damage and mutations within hepatocytes. This genetic instability predisposes liver cells to malignant transformation and the development of HCC (54).
Identifying diabetes as an important risk factor for liver cancer highlights the potential influence of diabetes management on reducing the risk of liver cancer. The use of metformin, a medication for diabetes management, appears to lower liver cancer risk, highlighting the significance of diabetes control in impacting its risk (31). A study indicated that metformin may be associated with a 62% decrease in the risk of liver cancer among individuals with type 2 diabetes (32). Therefore, diabetes regulation is a critical preventive measure against liver cancer, and further exploration of different other medications may be essential in this regard.
4.2 Liver cancer risk and social determinants of health
Our study delves into the impact of SDOHs, the non-medical conditions influencing health outcomes. Among these determinants, employment status, educational level, place of residence, and marital status emerge as significant factors associated with liver cancer risk.
Our findings indicate that unemployed individuals had 0.01 lower odds of being affected by liver cancer compared to their employed counterparts, suggesting that unemployment is a protective factor against liver cancer. Thus, being employed rather than unemployed could be associated with an increased odds of liver cancer.
Noting that the unemployed category encompasses students, homemakers, and individuals currently out of work, these often represent a younger age group in good health but facing joblessness due to their living conditions. In contrast, the retired participants face 4.5 times higher risks of developing liver cancer, compared to the employed group. This discrepancy may be partially attributed to the fact that the retired category predominantly includes individuals from an older age group. This prediction was supported by our supplementary analysis, presented in Supplementary Table 1, which showed that 55.83% of the unemployed participants fall in the younger age group (18 to 34 years), and about 83% of the retired participants are in the older age group, 65 or older. This aligns with a recent study that established an increase in the prevalence of certain liver cancer conditions with age, and an increased prevalence of advanced liver diseases among older individuals compared to their younger counterparts (55). Moreover, advanced age was found to be the primary determinant for complications and progression to end-stage liver cancer, as well as presenting challenges in the use of targeted therapeutic interventions in older adults (55).
The age distribution among the employment status may in part explain the detected association between employment and liver cancer risk. However, other factors can also contribute to the increase in the risk of cancer among previously employed and currently retired individuals, or among those who are currently employed. These factors include exposure to certain occupational carcinogens and hazardous materials such as asbestos, lead, pesticides, among other chemicals and substances, and sun exposure (56). Moreover, increased risk of cancer can also be induced by work-related stress and shift work especially the night shifts which can cause a disruption in certain hormone levels (57). For instance, a study has shown that full-time working women were at higher risk of having cancer than staying home women who were full-time caretakers of their households (12). Several explanations were proposed for these findings including social and psychological stressors, less time to health promoting behaviors, in addition to the exposure to hazardous environments (12, 58).
Another crucial SDOH significantly associated with liver cancer risk is the place of residence, particularly whether in a rural or urban area. Our study’s findings suggest that living in an urban area is a protective factor compared to rural areas. This protective effect may result from increased accessibility to healthcare resources in urban settings, facilitating regular checkups, immediate treatments, and efficient diagnosis in well-established hospitals (59). Our research aligns with the results of a Chinese study examining the incidence of liver cancer, which found that rural areas had a higher incidence rate compared to urban areas based on geographical analysis (60). Specifically, the crude incidence rates were 35.78 per 100,000 in rural areas and 21.64 per 100,000 in urban areas (60). Furthermore, after adjusting for age, the incidence rates remained higher in rural areas, with 34.34 per 100,000 in rural and 15.72 per 100,000 in urban areas (60). Moreover, in a nationwide study conducted from 2000 to 2016, targeting adults newly diagnosed with HCC, the focus was on disease manifestation and prognosis disparities across rural and urban regions (59). The findings showed that individuals residing in rural and suburban areas in the US were more likely to be diagnosed with HCC at a later stage and less likely to receive treatment (59). Additionally, the socioeconomic and geographic disparities were highlighted by a study of regional variations among patients with ICC in a Texas cancer registry (61). The analysis indicated that individuals residing in low-income regions were less likely to receive treatment, suggesting that residing in rural areas heightens liver cancer risk, leading to poorer outcomes and survival rates (61). Furthermore, environmental exposures may help explain the elevated liver cancer risk among rural residents. For example, a case-control study among California residents in agriculturally intensive areas found that exposure to organochlorine pesticides was associated with increased HCC risk, even after adjusting for liver disease and diabetes, particularly in men, with an odds ratio of 2.76 (95% CI: 1.58–4.82) (62). Similarly, a meta-analysis investigating HCC risk factors highlighted that agricultural work, common in rural settings, and pesticide exposure are significant contributors to HCC development. These findings emphasize the importance of educating rural populations on the safe use of pesticides and promoting access to lower-toxicity alternatives to improve health outcomes and reduce liver cancer risk (63).
Educational level emerged as a significant predictor for liver cancer in our study, with high school graduates and college graduates having seven-fold and five-fold higher risk, respectively, compared to those with some high school or below, the reference category. A possible explanation of this association may be reflective of the link between higher educational attainment, improved healthcare access, and regular health screenings, which can contribute to early detection and increase diagnosis rates (64). For instance, a study revealed that highly educated individuals are more likely to participate in cancer screening at their own initiative (65), a finding that suggests that better education fosters greater health awareness, potentially elevating cancer diagnosis rates (66). However, it is important to distinguish here between the true risk differences and detection-related disparities. In this regard, a study examining temporal trends in liver cancer mortality by educational attainment in the US over 15 years showed that the rise in liver cancer death rates has primarily affected individuals with lower levels of education (67). This finding may be attributed to lower detection rate and delayed diagnosis in the group of individuals with lower educational levels, leading to poorer prognosis and decreased chances of survival (68, 69).
Nevertheless, our findings are in contrast to a study examining the impact of socioeconomic status on HCC, which revealed that patients with lower levels of education experienced worse short-term and long-term outcomes, resulting in elevated HCC mortality rates (70). However, it is noteworthy that the less educated patients in the latter study were characterized as older, low-income individuals residing in rural areas, exhibiting a more advanced tumor burden, and receiving fewer curative treatments and regional therapies (70). These factors may explain the underlying increased risk of liver cancer associated with low educational attainment, thereby opposing the results observed in our study.
To further explore this elevated liver cancer risk among more educated individuals in our sample, we analyzed educational attainment in relation to the place of residence (rural vs. urban) as detailed in Supplementary Table 2. Among rural residents, the largest proportion (35.45%) were high school graduates, followed by individuals with some college education (32.45%) and college graduates (18.56%). This suggests that living in rural areas may partly explain the increased odds of liver cancer seen among more educated individuals in our dataset.
Marital status was also identified as a risk factor for liver cancer in our analysis, with widowed individuals having the highest risk, followed by those who never married, compared to those who were married. Consistent with our findings, a recent study of 4,933 participants concluded that marriage appears to be protective against liver cancer, with non-married individuals having an elevated risk (HR: 1.15) and a worse prognosis compared to married individuals (18). Moreover, survival outcomes for liver cancer patients have been found to be better among married individuals. For example, a retrospective study using population-based data revealed that married participants had a higher 5-year HCC cause-specific survival rate of 46.7%, compared to 37.8% for unmarried participants, with widowed individuals showing the poorest survival rate at 29.4% (19). These associations may be explained by the greater social and emotional support that married individuals tend to receive compared to their non-married counterparts (22). Moreover, unmarried individuals are at higher risk for psychological distress, which is linked to poorer cancer prognosis (17, 21, 71).
4.3 Liver cancer risk and smoking
Our study highlighted that the risk of liver cancer was approximately 3 times higher among former smokers compared to individuals who have never smoked, highlighting smoking as a significant and enduring predictor for liver cancer even after cessation. Our results are consistent with findings from a population-based case-control study conducted among men in the US, which identified a link between cigarette smoking and the likelihood of primary liver cancer development (72). However, the strength of the association in their findings was comparatively weaker than in ours, with a reported OR of 1.85 for former smokers compared to non-smokers. Furthermore, their study concluded that an increased duration of smoking and a higher number of packs smoked per day were associated with an increased liver cancer risk (72). Likewise, a recent prospective cohort study conducted in China reached a similar conclusion, indicating that former smokers exhibit an increased liver cancer risk compared to non-smokers. The study reported a multivariable-adjusted hazard ratio of 1.42 for former smokers in comparison to non-smokers, and a 49% elevated risk of liver cancer for individuals with a smoking history exceeding 40 years (73). Our dataset lacks information regarding the duration and intensity of smoking among participants. This lack of information encompasses individuals who may have recently quit smoking, posing a limitation in our study. A plausible explanation for the association between liver cancer and smoking is provided by a study indicating that carcinogenic compounds in cigarette smoke stimulate the progression of liver cancer by inducing inflammation and fibrosis (36, 74). For instance, N-nitrosodimethylamine is recognized for its role in promoting liver fibrosis and subsequent cancerous transformations. Similarly, 4-Aminobiphenyl undergoes metabolic breakdown within the liver, primarily facilitated by hepatic CYP1A2 enzymes (74). This compound forms DNA adducts, which are abnormal chemical structures created when a compound binds to DNA. These adducts can disrupt normal cellular processes, thus increasing the likelihood of cancer development within liver cells (74).
4.4 Limitations
This study provided valuable insights into liver cancer risk factors, including diabetes, specific SDOHs, biological and behavioral factors. However, several limitations warrant acknowledgment. Our analysis is based on data from the BRFSS, where information is self-reported and lacks objective diagnosis by healthcare professionals, potentially impacting data accuracy. Additionally, as mentioned previously, not all states responded to the cancer-related questions, leading to missing data from these states. Moreover, the lack of specific questions on diabetes medications, alcohol and smoking duration, type of occupation, exposure to occupation-related hazardous substances, as well as the type of liver cancer, presents additional limitations to our study. Additionally, the BRFSS does not collect data on key known risk factors for liver cancer, such as viral hepatitis B and C or non-alcoholic fatty liver disease (NAFLD), which could have provided valuable insights for our analysis. Furthermore, although our sub-analysis provided initial insights into the ages of diagnosis of diabetes and liver cancer and their potential correlation, the small number of liver cancer cases utilized in this sub-group analysis limited the robustness and generalizability of these findings, making it difficult to draw firm conclusions about diabetes as a risk factor.
5 Conclusions and implications
In this comprehensive study that explored potential risk factors for liver cancer, we found that diabetes was significantly associated with an increased risk of liver cancer, whilst adjusting for the effect of a diverse array of social, biological, and behavioral variables. These findings suggest that prioritizing diabetes prevention and management efforts may play an important role in reducing liver cancer risk at the population level.
Our study also identified a higher risk of liver cancer among rural residents, which may be explained by limited access to healthcare and resources in these areas. Furthermore, we observed increased liver cancer risk among certain demographic groups, including widowed or never married, employed or retired individuals, and those with higher education. These findings emphasize the importance of considering socioeconomic factors as part of SDOHs in our comprehension of liver cancer risk, which necessitates directing more attention toward these specific demographic groups to address the underlying disparities effectively.
In conclusion, this study offers valuable preliminary insights into the range of factors potentially associated with liver cancer risk. However, confirming these associations as true risk factors will require future cohort studies with adequate number of liver cancer cases, comprehensive data collection, and careful attention to the limitations outlined in this study. Such research is essential to strengthen the evidence base and guide effective prevention and intervention efforts.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/brfss/annual_data/annual_2022.html.
Ethics statement
Given that the data is from a secondary source and publicly available, ethical approval from the Institutional Review Board (IRB) from the American University of Beirut (AUB) was not needed. All participants provided informed consent, and appropriate measures were taken to safeguard the privacy and confidentiality of their data through de-identification methods.
Author contributions
MS: Formal analysis, Software, Visualization, Writing – original draft. AJ: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing. MJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1562854/full#supplementary-material
Supplementary Table 1 | Weighted percentage (row percent) of the distribution of the participants based on their age and their employment status.
Supplementary Table 2 | Weighted percentage (column percent) of the distribution of the participants based on their educational level and their place of residence.
Abbreviations
AUB, American University of Beirut; BMI, Body Mass Index; BRFSS, Behavioral Risk Factor Surveillance System; CDC, Centers for Disease Control and Prevention; CIs, Confidence Intervals; HCC, Hepatocellular Carcinoma; ICC, Intrahepatic Cholangiocarcinoma; IRB, Institutional Review Board; NAFLD, non-alcoholic fatty liver disease; ORs, Odds Ratios; ROS, reactive oxygen species; SES, socioeconomic status; SDOHs, Social Determinants of Health; US, United States.
References
1. Liver Cancer Causes, Risk Factors, and Prevention (2023). Available online at: https://www.cancer.gov/types/liver/what-is-liver-cancer/causes-risk-factors#:~:text=Worldwide%2C%20liver%20cancer%20is%20the,deaths%20in%20the%20United%20States (Accessed February 24, 2025).
2. Llovet Josep M, Kelley Robin Kate, Villanueva Augusto, Singal Amit G, Pikarsky Eli, Roayaie Sasan, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7:6. doi: 10.1038/s41572-020-00240-3
3. Liver Cancer (2023). Available online at: https://www.cancer.org.au/cancer-information/types-of-cancer/liver-cancer (Accessed February 12, 2025).
4. Pinter Matthias, Trauner M, Peck-Radosavljevic M, and Sieghart W. Cancer and liver cirrhosis: implications on prognosis and management. ESMO Open. (2016) 1:e000042. doi: 10.1136/esmoopen-2016-000042
5. Global Cancer Facts & Figures (2018). Available online at: https://www.cancer.org/cancer/types/liver-cancer/about/what-is-key-statistics.html#:~:text=The%20American%20Cancer%20Society's%20estimates,will%20die%20of%20these%20cancers (Accessed February 11, 2025).
6. Yao Z, Dai C, Yang J, Xu M, Meng H, Hu X, et al. Time-trends in liver cancer incidence and mortality rates in the U.S. from 1975 to 2017: a study based on the Surveillance, Epidemiology, and End Results database. J Gastrointest Oncol. (2023) 14:312–24. doi: 10.21037/jgo-23-25
7. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
8. Muhimpundu S, Conway RBN, Warren Andersen S, Lipworth L, Steinwandel MD, Blot WJ, et al. Racial differences in hepatocellular carcinoma incidence and risk factors among a low socioeconomic population. Cancers (Basel). (2021) 13:1–15 . doi: 10.3390/cancers13153710
9. Winters AC, Shaltiel T, Sarpel U, and Branch AD. Liver cancer has a distinctive profile in black patients: current screening guidelines may be inadequate. Hepatol Commun. (2022) 6:8–11. doi: 10.1002/hep4.1771
10. Understanding Liver Cancer . Available online at: https://www.ohsu.edu/knight-cancer-institute/understanding-liver-cancer#:~:text=Age%3A%20More%20than%2085%25%20of,foods)%2C%20can%20increase%20risk.
11. Kondili LA, Lazarus JV, Jepsen P, Murray F, Schattenberg JM, Korenjak M, et al. Inequities in primary liver cancer in Europe: The state of play. J Hepatol. (2024). 50:645–60doi: 10.1016/j.jhep.2023.12.031
12. Cullati S, Sieber S, Gabriel R, Studer M, Chiolero A, and van der Linden BWA. Lifetime employment trajectories and cancer. Sci Rep. (2024) 14:20224. doi: 10.1038/s41598-024-70909-2
13. Rapisarda V, Loreto C, Malaguarnera M, Ardiri A, Proiti M, Rigano G, et al. Hepatocellular carcinoma and the risk of occupational exposure. World J Hepatol. (2016) 8:573–90. doi: 10.4254/wjh.v8.i13.573
14. Barsouk A, Thandra KC, Saginala K, Rawla P, and Barsouk A. Chemical risk factors of primary liver cancer: an update. Hepatic Medicine: Evidence Res. (2021) 12:179–88. doi: 10.2147/HMER.S278070
15. Uccello M, Malaguarnera G, Corriere T, Biondi A, Basile F, and Malaguarnera M. Risk of hepatocellular carcinoma in workers exposed to chemicals. Hepatitis Monthly. (2012) 12:1–9. doi: 10.5812/hepatmon.5943
16. Chang CK, Astrakianakis G, Thomas DB, Seixas NS, Ray RM, Gao DL, et al. Occupational exposures and risks of liver cancer among Shanghai female textile workers—a case–cohort study. Int J Epidemiol. (2005) 35:361–9. doi: 10.1093/ije/dyi282
17. He XK, Lin ZH, Qian Y, Xia D, Jin P, and Sun LM. Marital status and survival in patients with primary liver cancer. Oncotarget. (2017) 8:64954–63. doi: 10.18632/oncotarget.11066
18. Liang Y, Wu X, Lu C, and Xiao F. Impact of marital status on the prognosis of liver cancer patients without surgery and the critical window. Ann Palliative Med. (2021) 10:2990–9. doi: 10.21037/apm-20-1885
19. Wu C, Chen P, Qian JJ, Jin SJ, Yao J, Wang XD, et al. Effect of marital status on the survival of patients with hepatocellular carcinoma treated with surgical resection: an analysis of 13,408 patients in the surveillance, epidemiology, and end results (SEER) database. Oncotarget. (2016) 7:79442–52. doi: 10.18632/oncotarget.12722
20. Kaiser NC, Hartoonian N, and Owen JE. Toward a cancer-specific model of psychological distress: population data from the 2003–2005 National Health Interview Surveys. J Cancer Surviv. (2010) 4:291–302. doi: 10.1007/s11764-010-0120-3
21. Goldzweig G, Andritsch E, Hubert A, Brenner B, Walach N, Perry S, et al. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol. (2010) 21:877–83. doi: 10.1093/annonc/mdp398
22. Baine Michael, Sahak Freshta, Lin Chi, Chakraborty Subhankar, Lyden Elizabeth, and Batra Surinder K.. Marital status and survival in pancreatic cancer patients: A SEER based analysis. PloS One. (2011) 6:e21052. doi: 10.1371/journal.pone.0021052
23. Li Q, Cao M, Lei L, Yang F, Li H, Yan X, et al. Burden of liver cancer: From epidemiology to prevention. Chin J Cancer Res. (2022) 34:554–66. doi: 10.21147/j.issn.1000-9604.2022.06.02
24. Calle EE, Rodriguez C, Walker–Thurmond K, and Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. Adults New Engl J Med. (2003) 348:1625–38. doi: 10.1056/NEJMoa021423
25. Gupta A, Das A, Majumder K, Arora N, Mayo HG, Singh PP, et al. Obesity is independently associated with increased risk of hepatocellular cancer-related mortality: A systematic review and meta-analysis. Am J Clin Oncol. (2018) 41:874–81. doi: 10.1097/COC.0000000000000388
26. Befort CA and Weinman SA. Obesity and risk of liver and biliary tract cancer: does timing and trajectory matter? JNCI Cancer Spectr. (2022) 6:1–2. doi: 10.1093/jncics/pkac057
27. Wang Y, Wang B, Shen F, Fan J, and Cao H. Body mass index and risk of primary liver cancer: a meta-analysis of prospective studies. Oncologist. (2012) 17:1461–8. doi: 10.1634/theoncologist.2012-0066
28. Sohn W, Lee HW, Lee S, Lim JH, Lee MW, Park CH, et al. Obesity and the risk of primary liver cancer: A systematic review and meta-analysis. Clin Mol Hepatol. (2021) 27:157–74. doi: 10.3350/cmh.2020.0176
30. Schütze M, Boeing H, Pischon T, Rehm J, Kehoe T, Gmel G, et al. Alcohol attributable burden of incidence of cancer in eight European countries based on results from prospective cohort study. Bmj. (2011) 342:d1584. doi: 10.1136/bmj.d1584
31. Testino G, Leone S, and Borro P. Alcohol and hepatocellular carcinoma: a review and a point of view. World J Gastroenterol. (2014) 20:15943–54. doi: 10.3748/wjg.v20.i43.15943
32. Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. (2015) 112:580–93. doi: 10.1038/bjc.2014.579
33. Petrick JL, Campbell PT, Koshiol J, Thistle JE, Andreotti G, Beane-Freeman LE, et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br J Cancer. (2018) 118:1005–12. doi: 10.1038/s41416-018-0007-z
34. Corrao G, Bagnardi V, Zambon A, and La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. (2004) 38:613–9. doi: 10.1016/j.ypmed.2003.11.027
35. Simpson RF, Hermon C, Liu B, Green J, Reeves GK, Beral V, et al. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million Women Study. Lancet Public Health. (2019) 4:e41–8. doi: 10.1016/S2468-2667(18)30230-5
36. Jain Divya, Chaudhary Priya, Varshney Nidhi, Razzak Khandaker Sabit Bin, Verma Devret, Zahra Tasnim Reza Khan, et al. Tobacco smoking and liver cancer risk: potential avenues for carcinogenesis. J Oncol. (2021) 2021:5905357. doi: 10.1155/2021/5905357
37. Chen H, Zhou H, Wu B, Lu H, Zhang J, Zhang Y, et al. Physical activity and exercise in liver cancer. Liver Res. (2024) 8:22–33. doi: 10.1016/j.livres.2024.03.001
38. Vuille-Lessard É and Berzigotti A. Exercise interventions for cirrhosis. Curr Treat Options Gastroenterol. (2022) 20:336–50. doi: 10.1007/s11938-022-00393-y
39. Lee J. Associations between physical activity and liver cancer risks and mortality: A systematic review and meta-analysis. Int J Environ Res Public Health. (2020) 17:8943. doi: 10.3390/ijerph17238943
40. Onikanni SA, Lawal B, Bakare OS, Ajiboye BO, Ojo OA, Farasani A, et al. Cancer of the Liver and its Relationship with Diabetes mellitus. Technol Cancer Res Treat. (2022) 21:15330338221119743. doi: 10.1177/15330338221119743
41. National Diabetes Month: November 2023 (2023). Available online at: https://www.census.gov/newsroom/stories/diabetes-month.html#:~:text=%E2%80%9CDiabetes%20is%20a%20disease%20that,to%20some%20types%20of%20cancer (Accessed November 2, 2023).
42. Can diabetes cause liver cancer (2022). Available online at: https://www.riversideonline.com/patients-and-visitors/healthy-you-blog/blog/c/diabetes-and-liver-cancer#:~:text=%E2%80%9CIndividuals%20with%20type%202%20diabetes,gastroenterologist%20with%20Riverside%20Gastroenterology%20Specialists (Accessed January 27, 2022).
43. Mantovani A and Targher G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: spotlight on nonalcoholic fatty liver disease. Ann Transl Med. (2017) 5:270. doi: 10.21037/atm.2017.04.41
44. Yang JD, Ahmed F, Mara KC, Addissie BD, Allen AM, Gores GJ, et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology. (2020) 71:907–16. doi: 10.1002/hep.30858
45. Hill-Briggs F, Adler NE, Berkowitz SA, Chin MH, Gary-Webb TL, Navas-Acien A, et al. Social determinants of health and diabetes: A scientific review. Diabetes Care. (2020) 44:258–79. doi: 10.2337/dci20-0053
46. Spruijt-Metz D, O'Reilly GA, Cook L, Page KA, and Quinn C. Behavioral contributions to the pathogenesis of type 2 diabetes. Curr Diabetes Rep. (2014) 14:475. doi: 10.1007/s11892-014-0475-3
47. Dey S, Mukherjee A, Pati MK, Kar A, Ramanaik S, Pujar A, et al. Socio-demographic, behavioural and clinical factors influencing control of diabetes and hypertension in urban Mysore, South India: a mixed-method study conducted in 2018. Arch Public Health. (2022) 80:234. doi: 10.1186/s13690-022-00996-y
48. Shahid RK, Ahmed S, Le D, and Yadav S. Diabetes and cancer: risk, challenges, management and outcomes. Cancers (Basel). (2021) 13:1–21. doi: 10.3390/cancers13225735
49. Su Q, Sun F, Li J, Zhang H, Wang M, Zhou H, et al. The correlation analysis of primary liver cancer with Type 2 diabetes. Indian J Cancer. (2015) 52:e148–52. doi: 10.4103/0019-509X.186557
50. Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. (2002) 36:1206–13. doi: 10.1053/jhep.2002.36780
51. Wainwright P, Scorletti E, and Byrne CD. Type 2 diabetes and hepatocellular carcinoma: risk factors and pathogenesis. Curr Diabetes Rep. (2017) 17:20. doi: 10.1007/s11892-017-0851-x
52. Bosetti C, Turati F, and La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. (2014) 28:753–70. doi: 10.1016/j.bpg.2014.08.007
53. NIDDK. Definition & Facts of NAFLD & NASH (2021). Available online at: https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/definition-facts#:~:text=Nonalcoholic%20fatty%20liver%20disease%20(NAFLD)%20is%20a%20condition%20in%20which,called%20alcohol%2Dassociated%20liver%20disease.
54. Teng PC, Huang DQ, Lin TY, Noureddin M, and Yang JD. Diabetes and risk of hepatocellular carcinoma in cirrhosis patients with nonalcoholic fatty liver disease. Gut Liver. (2023) 17:24–33. doi: 10.5009/gnl220357
55. Georgieva M, Xenodochidis C, and Krasteva N. Old age as a risk factor for liver diseases: Modern therapeutic approaches. Exp Gerontol. (2023) 184:112334. doi: 10.1016/j.exger.2023.112334
56. Cancer Research UK. Cancer risks in the workplace (2023). Available online at: https://www.cancerresearchuk.org/about-cancer/causes-of-cancer/cancer-risks-in-the-workplace#:~:text=What%20types%20of%20job%20might,the%20risk%20of%20certain%20cancers (Accessed June 30, 2023).
57. Yang T, Qiao Y, Xiang S, Li W, Gan Y, and Chen Y. Work stress and the risk of cancer: A meta-analysis of observational studies. Int J Cancer. (2019) 144:2390–400. doi: 10.1002/ijc.v144.10
58. Chida Y, Hamer M, Wardle J, and Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. (2008) 5:466–75. doi: 10.1038/ncponc1134
59. Zhou K, Pickering TA, Gainey CS, Cockburn M, Stern MC, Liu L, et al. Presentation, management, and outcomes across the rural-urban continuum for hepatocellular carcinoma. JNCI Cancer Spectr. (2021) 5:1–8 . doi: 10.1093/jncics/pkaa100
60. Gao J, Xie L, Chen WQ, Zhang SW, Wu QJ, Yang Y, et al. Rural–urban, sex variations, and time trend of primary liver cancer incidence in China, 1988–2005. Eur J Cancer Prev. (2013) 22:448–54. doi: 10.1097/CEJ.0b013e32835de82a
61. Kneuertz PJ, Kao LS, Ko TC, and Wray CJ. Regional disparities affect treatment and survival of patients with intrahepatic cholangiocarcinoma–a Texas Cancer Registry analysis. J Surg Oncol. (2014) 110:416–21. doi: 10.1002/jso.23664
62. VoPham T, Brooks MM, Yuan JM, Talbott EO, Ruddell D, Hart JE, et al. Pesticide exposure and hepatocellular carcinoma risk: A case-control study using a geographic information system (GIS) to link SEER-Medicare and California pesticide data. Environ Res. (2015) 143:68–82. doi: 10.1016/j.envres.2015.09.027
63. Lu W, Zheng F, Li Z, Zhou R, Deng L, and Xiao W. Association between environmental and socioeconomic risk factors and hepatocellular carcinoma: A meta-analysis. Front Public Health. (2022) 10:2022. doi: 10.3389/fpubh.2022.741490
64. Fletcher JM and Frisvold DE. Higher education and health investments: does more schooling affect preventive health care use? J Hum Cap. (2009) 3:144–76. doi: 10.1086/645090
65. Willems B and Bracke P. Participants, Physicians or Programmes: Participants’ educational level and initiative in cancer screening. Health Policy. (2018) 122:422–30. doi: 10.1016/j.healthpol.2018.02.001
66. Sarveswaran G and Mathur P. Educational interventions to improve participation of communities in cancer screening programs. Cancer Research Statistics Treat. (2023) 6:443–5. doi: 10.4103/crst.crst_224_23
67. Ma J, Siegel RL, Islami F, and Jemal A. Temporal trends in liver cancer mortality by educational attainment in the United States, 2000-2015. Cancer. (2019) 125:2089–98. doi: 10.1002/cncr.32023
68. Sivaranjini K, Oak A, Cheulkar S, Maheshwari A, Mahantshetty U, and Dikshit R. Role of education and income on disparities of time-to-treatment initiation and its impact on cervical cancer survival. Indian J Public Health. (2023) 67:235–9. doi: 10.4103/ijph.ijph_1299_22
69. Grau-Pérez M, Cabello C, González-Martín JM, Borrego L, and Carretero G. Low educational attainment is still associated with late melanoma diagnosis: A cross-sectional study from a European setting with universal healthcare. Cancer Epidemiol. (2019) 62:101576. doi: 10.1016/j.canep.2019.101576
70. Shen Y, Guo H, Wu T, Lu Q, Nan KJ, Lv Y, et al. Lower education and household income contribute to advanced disease, less treatment received and poorer prognosis in patients with hepatocellular carcinoma. J Cancer. (2017) 8:3070–7. doi: 10.7150/jca.19922
71. Zhou R, Yan S, and Li J. Influence of marital status on the survival of patients with gastric cancer. J Gastroenterol Hepatol. (2016) 31:768–75. doi: 10.1111/jgh.2016.31.issue-4
72. Zhu K, Moriarty C, Caplan LS, and Levine RS. Cigarette smoking and primary liver cancer: a population-based case–control study in US men. Cancer Causes Control. (2007) 18:315–21. doi: 10.1007/s10552-006-0105-8
73. Zhang Y, Li ZY, Shen QM, Tuo JY, Tan JY, Tan YT, et al. A prospective cohort study of cigarette smoking, alcohol drinking and liver cancer incidence in Chinese men. J Dig Dis. (2022) 23:527–34. doi: 10.1111/1751-2980.13136
Keywords: Behavioral Risk Factor Surveillance System (BRFSS) 2022, behavioral, biologic and social determinants of health, diabetes, liver cancer, weighted logistic regression analysis
Citation: Shouman M, Jaffa AA and Jaffa MA (2025) The impact of diabetes and social, biologic and behavioral determinants of health on liver cancer risk. Front. Endocrinol. 16:1562854. doi: 10.3389/fendo.2025.1562854
Received: 18 January 2025; Accepted: 05 June 2025;
Published: 27 June 2025.
Edited by:
Chiara Liverani, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Abdullah Shaito, Qatar University, QatarGeeta Yadav, Vardhman Mahavir Medical College and Safdarjung Hospital, India
Copyright © 2025 Shouman, Jaffa and Jaffa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayad A. Jaffa, YWoyNEBhdWIuZWR1Lmxi; Miran A. Jaffa, bXMxNDhAYXViLmVkdS5sYg==
 Marwa Shouman1,2
Marwa Shouman1,2 Ayad A. Jaffa
Ayad A. Jaffa Miran A. Jaffa
Miran A. Jaffa