- Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, National Clinical Research Center for Metabolic Diseases (Shanghai), Ruijin Yangtze River Delta Health Institute, Research Unit of Clinical and Basic Research on Metabolic Diseases of Chinese Academy of Medical Sciences, Wuxi Branch of Ruijin Hospital, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Aims: The purpose of this study was to evaluate the relationship between night sleep duration, daytime napping, and diabetic retinopathy (DR) in type 2 diabetes (T2D) patients and to explore the potential mediating role of metabolic factors.
Methods: In this cross-sectional, retrospective study, night sleep and napping were assessed according to the standardized questionnaire. The metabolic factors in the examination were systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), and HbA1c. Multivariate logistic regression and stratified and conjoint analysis were carried out. In addition, causal mediation analysis was performed to explore the mediating role.
Results: A total of 2,433 patients [mean (SD) age, 55.82 (11.66) years; 40.07% women] were included in the final analysis. The prevalence of DR was 15.95%. Compared with reference groups, patients with long sleep [odds ratio (OR), 1.31, 95% confidence interval (CI), 1.01–1.70] and long nap (1.09, 1.04–1.23) were both associated with DR, and stratified analysis showed that this association varied among different sex and diabetes duration groups. Conjoint analysis showed that patients with both long sleep and long naps had a significantly increased risk of DR (1.75, 1.13–2.71). Mediation analysis showed that metabolic factors partially mediated this association between night sleep, naps, and DR, contributing to 9.8% and 16.3% of the total effects, respectively.
Conclusions: Long sleep and long nap were associated with DR, and male patients with T2D with a shorter course (<5 years) especially need to be vigilant. The effects of night sleep and naps on DR could be superimposed, and metabolic factors partially explain the underlying mechanism.
Introduction
Diabetes, one of the most common chronic diseases, is gaining more and more attention. The International Diabetes Federation (IDF) reported that 8.3% of adults, approximately 382 million, worldwide have diabetes, and its incidence continues to increase. It is expected that in 2030, the number of patients with diabetes will exceed 500 million globally, among which the most common type is type 2 diabetes (T2D), accounting for 85.0%–95.0% (1–3). Among complications, diabetic retinopathy (DR), the leading cause of preventable blindness in adults, affects approximately 40% of all patients, seriously reducing their quality of daily life and bringing heavy burden to their families and society (4, 5).
Previous studies have shown that anomalous sleep duration is associated with higher risk of T2D (6, 7). However, limited studies investigated the relationship between sleep phenotypes, including night sleep and daytime napping with DR, and results were not consistent. A cross-sectional survey showed that long sleep independently associated with DR (8); a Korean study has highlighted gender differences (9), while other studies did not find a correlation (10, 11). Critically, prior research often examined night sleep and daytime napping in isolation, neglecting their potential additive or synergistic effects on DR risk. This gap is noteworthy because disrupted circadian rhythms from prolonged nighttime sleep abnormalities may drive compensatory daytime napping, collectively exacerbating metabolic dysregulation (e.g., insulin resistance and sympathetic activation) that accelerates microvascular damage in DR (12–14). Furthermore, daytime napping could reflect poor sleep quality at night or fragmented sleep architecture, which may independently contribute to retinal hypoxia and inflammation (15). In addition, the mechanisms underlying sleep-associated DR are not fully understood, and modifiable behavioral factors, including metabolic factors, physical activity, and diet, are the possible risk factors (16–18). Among them, blood pressure, body mass index (BMI), and HbA1c have been found to be closely related to sleep and DR (19, 20).
Based on these observations, our study aimed to investigate the potential relationship between sleep phenotypes, including night sleep duration and daytime napping, and DR among Chinese patients with T2D. In addition, metabolic factors (blood pressure, BMI, and HbA1c) were also included in the analysis to identify potential mediators.
Materials and methods
Study population and design
The study population is composed of the 2,634 patients with T2D who were assessed at the National Metabolic Management Center (MMC) in Ruijin Hospital (Shanghai, China) between June 2017 and June 2021. As described in our previous research (21), MMC has built standard services both inside and out of the hospital and patients can enjoy one-stop care to receive a comprehensive series of services from registration, tests, evaluation, prescriptions, to health education. The study was approved by the Ethics Committee of Ruijin Hospital and obtained written informed consent from each participant.
The inclusion criteria were as follows: (1) adults diagnosed with T2D aged 18 years and older and (2) complete clinical data. The exclusion criteria were as follows: (1) lack of sleep data and (2) lack of evaluation and documentation of DR.
Data collection and covariates
Data collection was performed by MMC medical staff according to a standard protocol. Detailed information on demographic characteristics, medical record, and lifestyle behaviors was obtained. Specifically, age, sex, systolic blood pressure (SBP), diastolic blood pressure (DBP), BMI, and duration of diabetes were gathered based on the MMC system. Glycated hemoglobin (HbA1c) levels were performed in the central clinical laboratory of Ruijin Hospital by automated analyzers (22, 23). Smoking was defined as current smoking or quitting smoking for not more than 12 months and nondrinker was defined as a person who never drank alcohol or quit. The covariates included in the regression model include the above indicators, which are based on theoretical evidence of previous studies (8–11, 18–20) and statistical significance.
Assessment of night sleeping duration, napping, and DR
Habitual nocturnal sleep duration and daytime napping duration were obtained by trained medical staff according to a standardized process of MMC through a validated questionnaire at the initial visit. The specific questions regarding sleep phenotypes are as follows: (1) “How many hours do you take a daytime napping every day at noon?“; (2) “How many hours do you sleep every night (Record the time of falling asleep and waking up)?”. Time recording adopts a 24-h system, and the answer to this question is accurate to the minute and all results of sleep duration have been converted into hours (e.g., 8 h 30 min = 8.5 h). Based on the night sleep duration, we divided the patients into three groups: short (<7 h), middle (7–8 h), and long (>8 h) sleep. Based on the napping duration, we also divided the patients into three groups: no nap (0 h), short nap (>0–<1 h), and long nap (≥1 h). According to the previous studies, middle sleep and no nap were used as references (24, 25).
Combined with fundus examination and the international DR grading standard in 2003 (22, 26), DR was defined as moderate non-proliferative diabetic retinopathy (NPDR) or worse, or clinical events including vitreous/preretinal hemorrhage, clinically significant macular edema, treatment with laser or anti-vascular endothelial growth factor therapy, vitrectomy, and diabetes-related blindness and controls were defined as without DR, or with mild NPDR. DR was confirmed or excluded with combined diagnostic tools as direct fundus examination under slit lamp, fundus photography, optical coherence tomography (Triton DRI-OCT, Topcon, Inc., Tokyo, Japan), and fundus fluorescence angiography (FFA) or OCT angiography (OCTA). At least one standard non-dilated pupil, macula-centered color, and non-stereoscopic retinal fundus image with 45° visual field was obtained from each participant per eye. The integrated results were evaluated by experienced and trained ophthalmologists and finally reviewed by fundus experts.
Statistical analysis
All statistical analyses were conducted using R software (version 4.1.2) and IBM SPSS Statistics 24.0 (SPSS Inc., Chicago, IL, United States). Continuous variables were expressed as mean with SD, and categorical variables were presented as numbers (proportions). General characteristics of the participants were shown by groups of night sleep duration. For continuous variables, ANOVA was conducted, and p-values were used to assess group differences. For categorical variables, chi-square tests were employed. For variables with missing values (≤3% missingness), median imputation was applied to continuous variables, while mode imputation was used for categorical variables. Multivariate logistic regression analyses were applied to examine the associations of night sleep duration, daytime napping, and DR. Odds ratios (ORs) were adjusted for age, sex, smoking, drinking, SBP, DBP, BMI, HbA1c, and duration of diabetes. The R package “mediation” was used for mediation analysis. Mediation effects were analyzed using the mediation package (v4.5.0) in R 4.1.2. The quasi-Bayesian Monte Carlo method with 5,000 simulations was adopted to obtain the estimates for the average causal mediation effect, average direct effect, and total effect of potential mediators. In bilateral tests, p < 0.05 indicated statistically significance.
Results
Patient characteristics
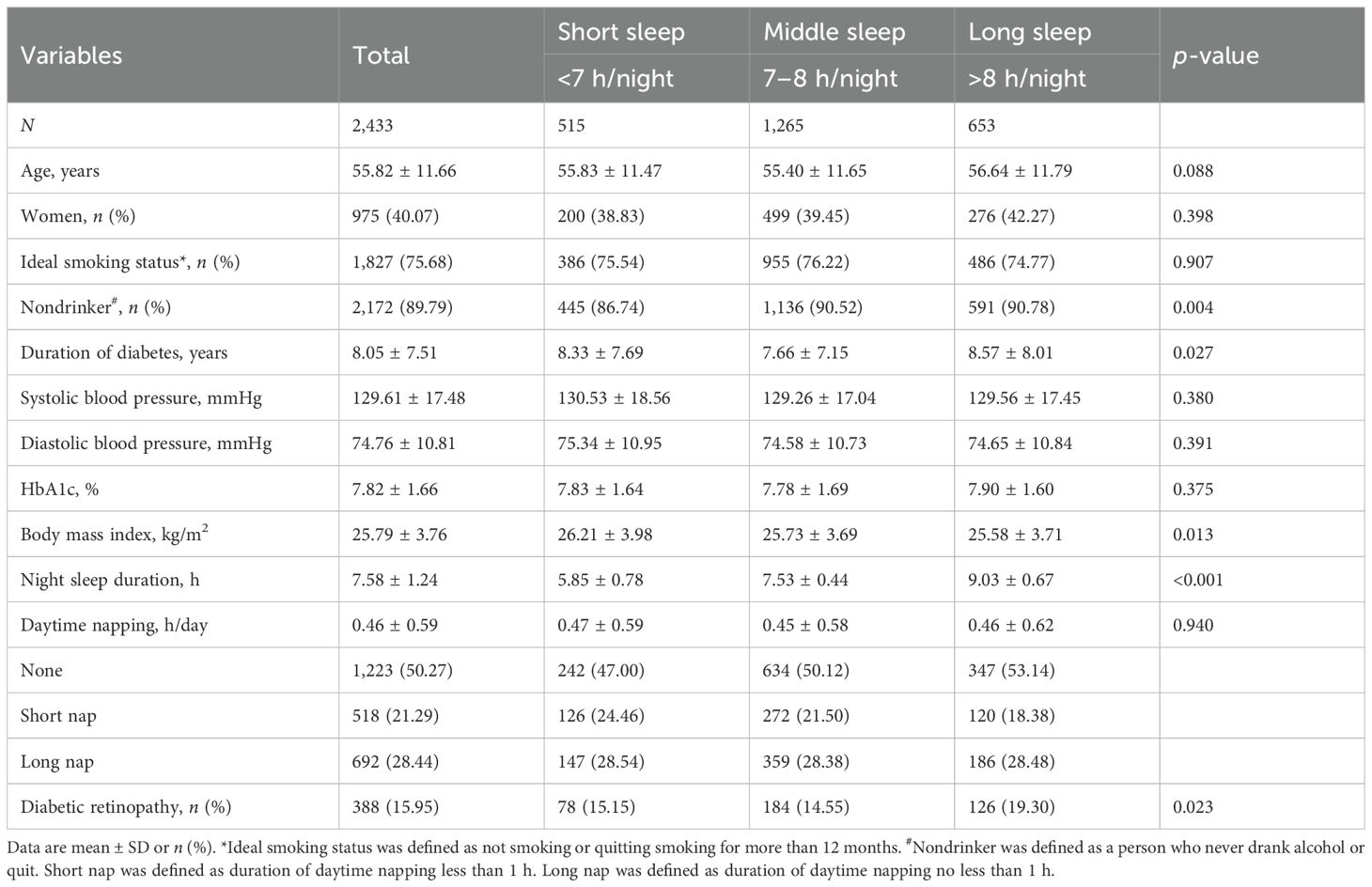
After excluding missing values for night sleep duration and records of DR, a total of 2,433 patients were included in this study and general characteristics are shown in Table 1. Among all participants, there were 1,458 men (59.93%) and 975 women (40.07%). Average age of visit and duration of diabetes were 55.82 and 8.05 years, respectively. Of the total participants, 89.79% were nondrinkers and a total of 15.95% of patients presented with DR; 21.17%, 51.99%, and 26.84% reported night sleeping for <7, 7–8, and >8 h, respectively. The long sleep group was more likely to be nondrinkers (90.78%), have a longer course of diabetes (8.57 years), and have a higher proportion of DR (19.30%), compared with the middle and short sleep group. In addition, there were no significant differences in age of visit, smoking, blood pressure, and HbA1c among the three groups of patients.
Associations of sleep phenotypes with DR events
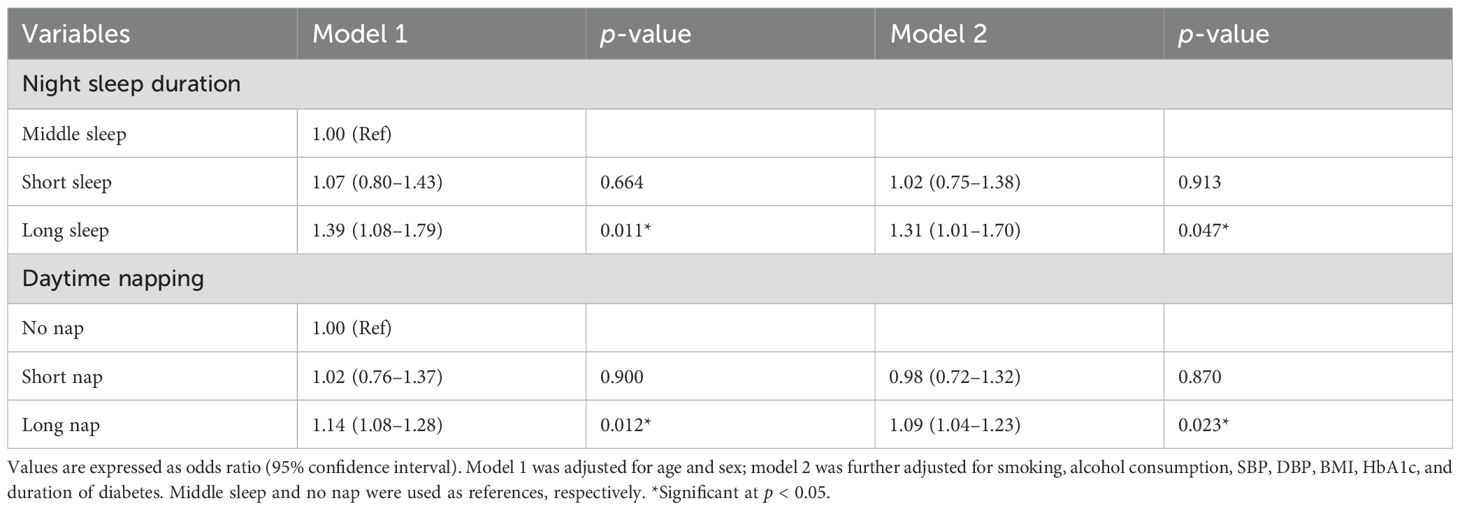
Table 2 shows the multivariate logistic regression analysis with DR as dependent variable in patients with T2D. According to the sleep groups, compared with those with middle sleep, patients with long sleep had a higher risk of DR (OR 1.39, 95% CI 1.08–1.79) when adjusted for age and sex (model 1). Moreover, such a relationship was still established when further adjusted for smoking, alcohol consumption, SBP, DBP, BMI, HbA1c, and duration of diabetes (1.31, 1.01–1.70, model 2). In comparison, there was no correlation between short sleep and DR (1.02, 0.75–1.38, p = 0.913). As for daytime napping, long nap was associated with the risk of DR (model 1, 1.14, 1.08–1.28; model 2, 1.09, 1.04–1.23) after adjusting variables, and there was no correlation between short nap and DR (0.98, 0.72–1.32, p = 0.870), compared with the reference group.
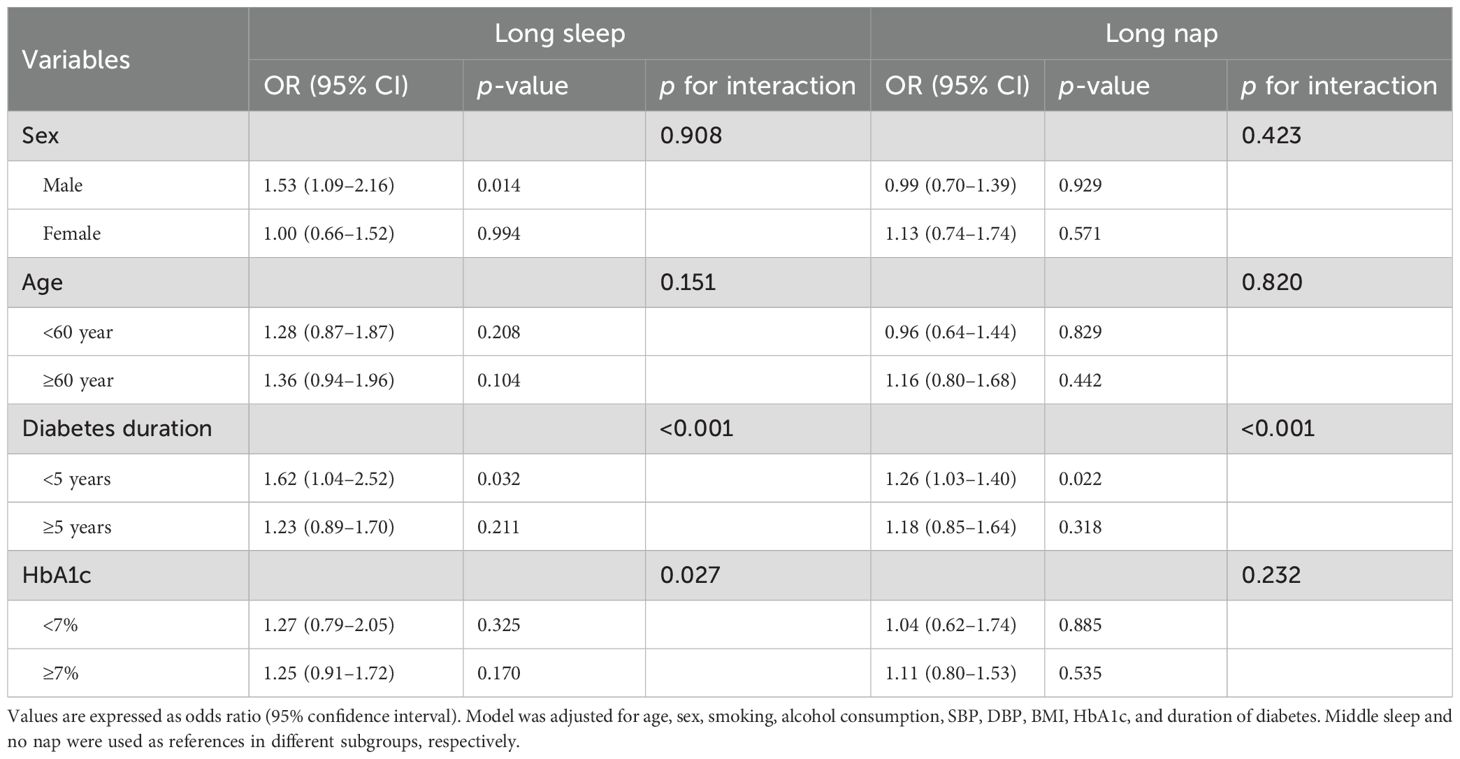
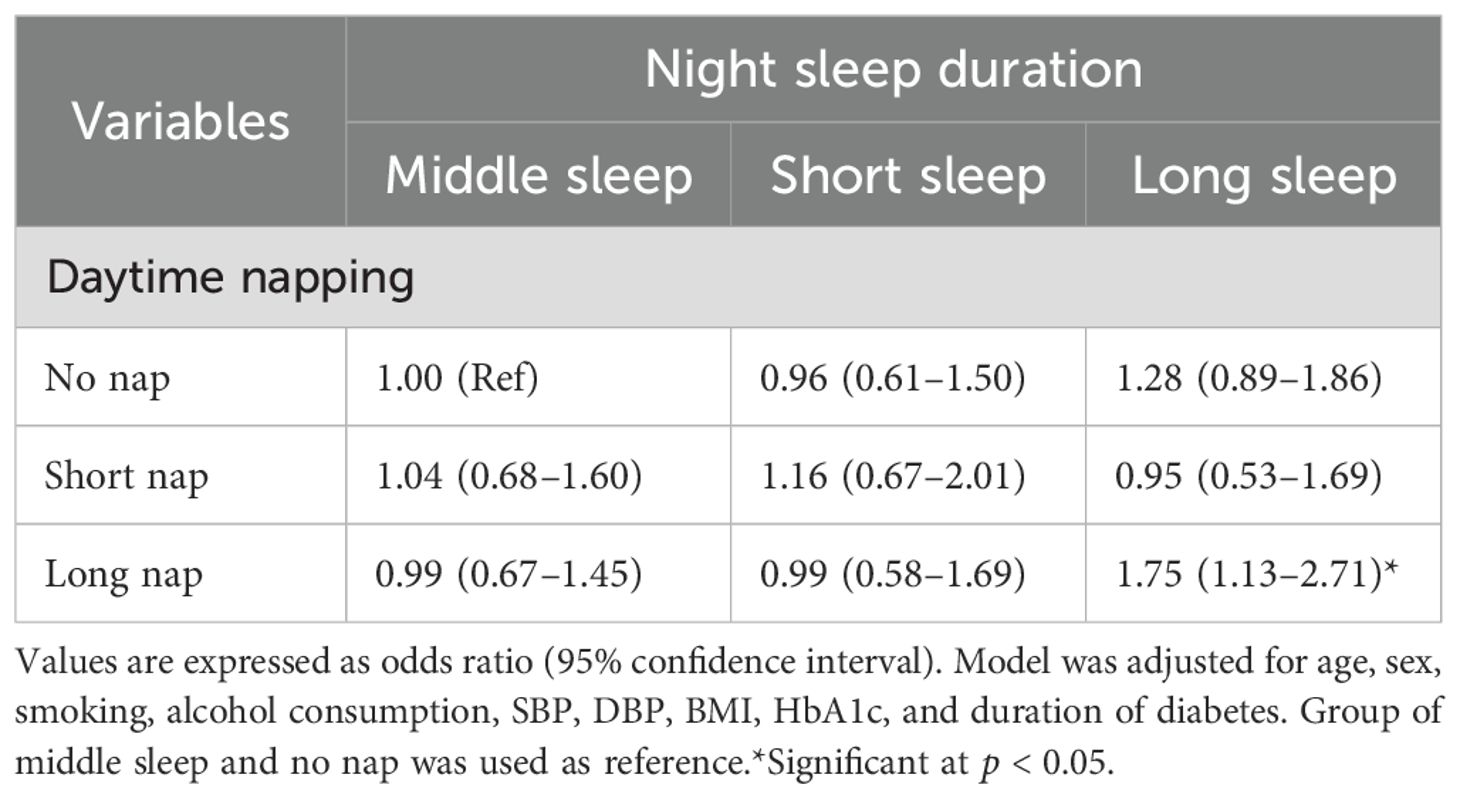
Table 3 shows stratified analysis of the association of long sleep and long nap with DR. When the independent variable was night sleep, diabetes duration <5 years and male groups were positively associated with the risk of DR (1.62, 1.04–2.52; 1.53, 1.09–2.16), while there was no statistical significance between the groups of age and HbA1c after adjusting for age, sex, smoking, alcohol consumption, SBP, DBP, BMI, HbA1c, and duration of diabetes. When the independent variable was long nap, only diabetes duration <5 years was positively associated with the risk of DR (1.26, 1.03–1.40). In addition, the results of the conjoint analysis showed that patients with long sleep and long nap were positively associated with DR (1.75, 1.13–2.71, Table 4) compared with patients with no nap and middle sleep.
Mediation effects of metabolic factors on night sleep, napping–DR risk
Figure 1 shows the mediating role of metabolic factors in the relationship between night sleep duration and DR. Model was adjusted for age, sex, smoking, alcohol consumption, SBP, DBP, BMI, HbA1c, and duration of diabetes. When a metabolic factor itself acts as a mediator in turn, excluding itself as a covariate. All four metabolic factors significantly mediated the association between long sleep and DR, with SBP, DBP, BMI, and HbA1c explaining 5.5%, 0.4%, 2.0%, and 1.9% of the association, respectively (both p < 0.05). In addition, the relationship between daytime napping and DR was partially mediated by SBP, DBP, and HbA1c, accounting for 3.6%, 1.1%, and 11.6%, respectively, while the mediating role of BMI tended to be non-significant (Figure 2).
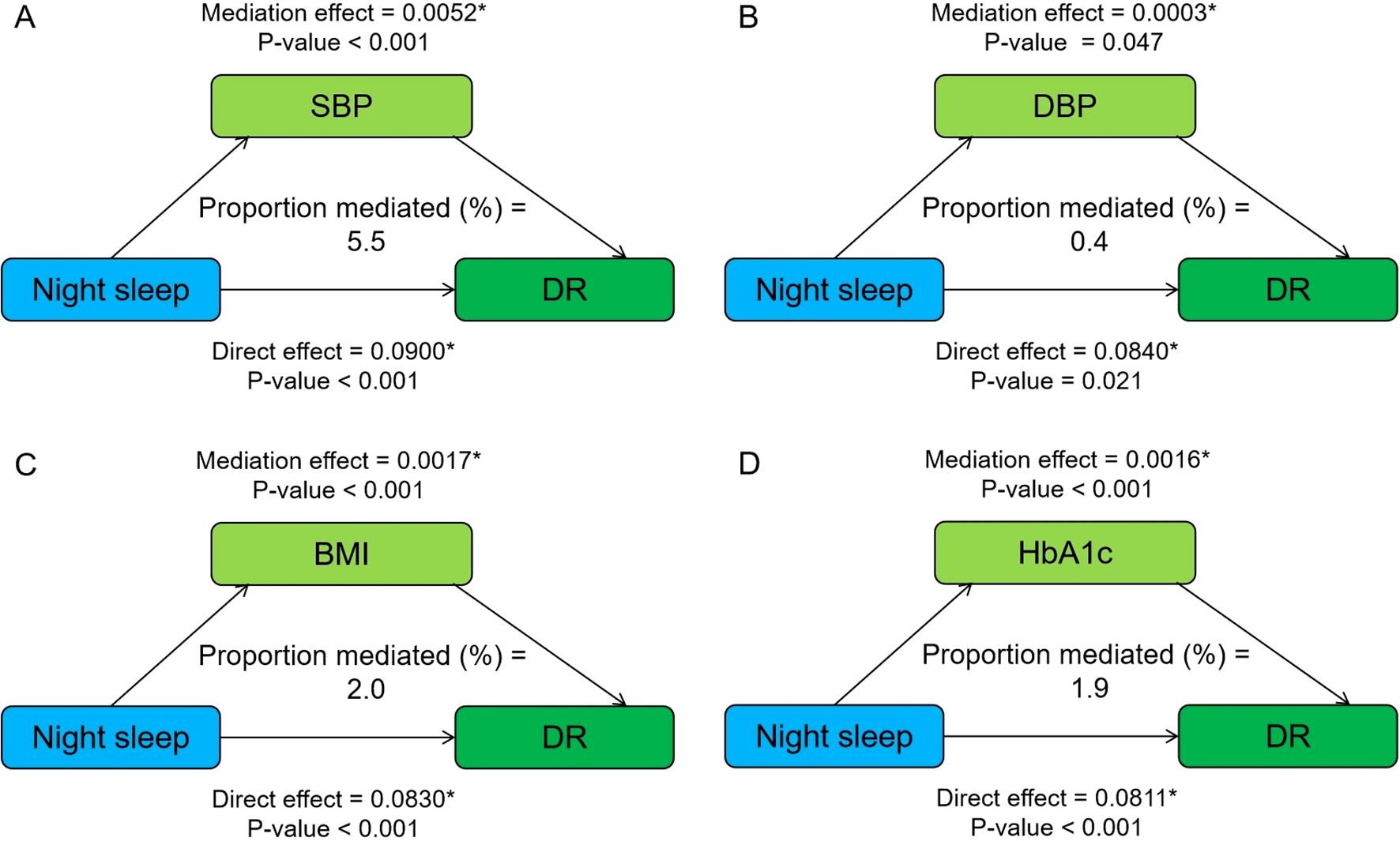
Figure 1. Mediation analysis of metabolic factors, including SBP (A), DBP (B), BMI (C), and HbA1c (D) on the relationship between night sleep duration and DR. Model was adjusted for age, sex, smoking, alcohol consumption, SBP, DBP, BMI, HbA1c, and duration of diabetes. When a metabolic factor itself acts as a mediator in turn, excluding itself as a covariate. * Significant at p < 0.05.
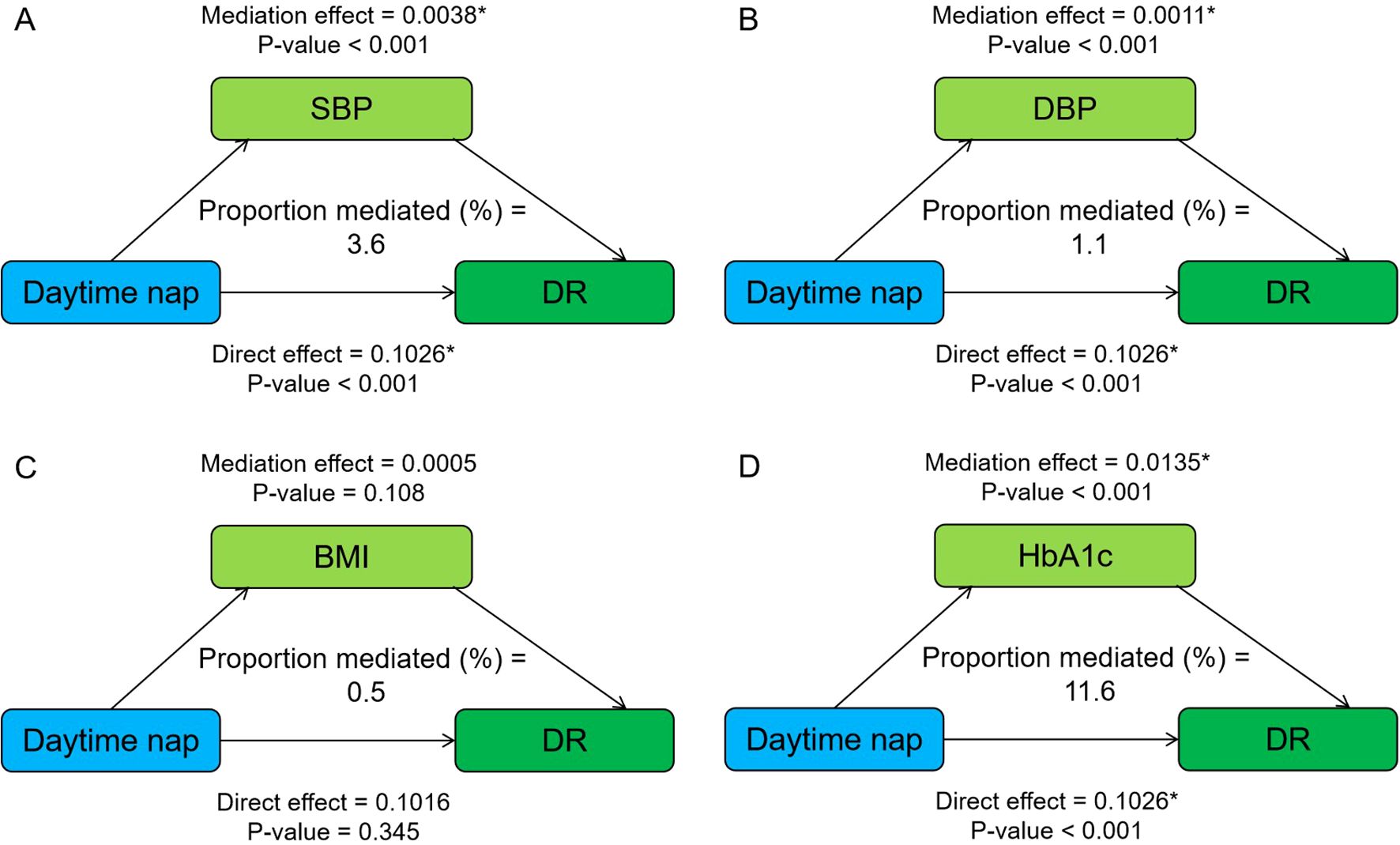
Figure 2. Mediation analysis of metabolic factors, including SBP (A), DBP (B), BMI (C), and HbA1c (D) on the relationship between daytime napping and DR. Model was adjusted for age, sex, smoking, alcohol consumption, SBP, DBP, BMI, HbA1c, and duration of diabetes. When a metabolic factor itself acts as a mediator in turn, excluding itself as a covariate. * Significant at p < 0.05.
Discussion
In this study, associations between night sleep, daytime napping, and DR in patients with T2D were investigated. Our study indicated that long sleep and long nap were associated with DR, and this association was more significant in men and in the diabetes duration < 5 years group. The results of the joint analysis showed that the effects of night sleep and nap on DR could be superimposed, and that patients with long sleep and long naps were positively associated with DR. In addition, metabolic factors, including SBP, DBP, BMI, and HbA1c, partially explain the mechanism between night sleep, daytime napping, and DR.
In the present study, 15.95% of patients suffered from DR, and among them, 40.07% were female, which was similar to previous studies (27–29). In recent years, a growing number of studies have confirmed that sleep was closely related to DR (12, 30–33). The vast majority of research focused on the impact of night sleep duration and sleep quality on DR, with substantial heterogeneity observed across studies. Long sleep was associated with the risk of DR, while short sleep was controversial. There are many sources of heterogeneity, and differences in race, age, BMI, and sleep assessment methods may partly explain it. A meta-analysis involving six studies showed that association between short sleep and DR was significant in those ≥60 years, while there was no significance in the BMI subgroup analysis (12). It should be acknowledged that the sample size of short sleep patients in previous studies and our research is relatively small, and further research is needed to confirm our results. In our study, long sleep duration was positively associated with DR, and this association was more significant in men and in the diabetes duration < 5 years group. The results of a nationwide population-based cohort study of patients with T2D in Korea (34) showed that sex and diabetes duration exerted interactive effects with DR status in increasing the insomnia risk, and the youngest age group (<40 years) and male patients were most vulnerable to insomnia risk, which is similar to our research findings.
Few studies have focused on the effects of daytime napping on DR, not to mention the combined effects of night sleep and daytime napping on DR. To the best of our knowledge, our study is the first to analyze the separate and combined effects of night sleep and nap on DR among patients with T2D. Patients with both long sleep and long naps exhibited a higher prevalence of DR, which implies superimposed effect on the risk of DR. It is of positive significance to clarify the causal relationship between them and study the potential mechanism, and the findings of our study provide a foundation for future research to build upon. Further studies incorporating longitudinal designs, intervention approaches, detailed metabolic assessments, and a focus on specific patient subgroups will be crucial in advancing our understanding of the complex relationships between sleep and DR in T2D and ultimately translating these findings into improved clinical care and prevention strategies.
The mechanisms between long sleep, long nap, and DR are not fully understood, and the possible mechanisms are as follows: melatonin dysregulation, mainly manifested as low production or mistimed (12, 35); long sleep duration and long nap have been associated with increases in markers of systemic inflammation and adipocytokines, including CRP, IL-6, visfatin, and leptin, which can promote the formation of new blood vessels, increase vascular permeability in the retina, and potentially accelerate the progression of DR by exacerbating retinal ischemia and hemorrhage (36–38); prolonged napping and sleeping can reduce daily energy expenditure, increase appetite, and contribute to obesity (16, 39). Oxidative stress is another key factor linking long sleep and napping to DR. Prolonged sleep and napping may disrupt the balance between reactive oxygen species (ROS) production and antioxidant defenses, leading to oxidative stress. This process could amplify advanced glycation end products (AGEs) accumulation, thickening retinal capillary basement membranes and impairing nutrient diffusion, and exacerbate oxidative damage in the retina (40). Also, these sleep habits might affect retinal microcirculation by altering blood rheology and vascular tone, thereby exacerbating retinal ischemia and hypoxia, and promoting the development of DR (40, 41). In addition, increased insulin resistance, changes in beta-cell function, poor sleep quality, and decreased physical activity are also some of the possible mechanisms (16–18). Given the potential mechanisms mentioned above, exercise, weight loss, increasing anti-inflammatory diets including goji berries, and maintaining a balanced lifestyle overall may be effective treatment options (42–44). In our study, metabolic factors, including SBP, DBP, BMI, and HbA1c, partially explain the mechanism between night sleep, daytime napping, and DR. Previous epidemiological studies have demonstrated that intensive blood glucose and blood pressure control, and improved obesity status could improve the long-term outcomes of patients with T2D, including reducing the risk of DR (45–47). Our study provides novel cross-sectional evidence that prolonged napping and sleeping are positively associated with DR, with observed relationships potentially mediated by blood pressure alterations, obesity indices, and suboptimal glycemic control. These findings highlight the importance of considering sleep patterns in clinical assessments and suggest potential pathways for targeted interventions.
We comprehensively assessed single and overall effects of night sleep and daytime napping on DR. Additionally, our study explored the potential mediating role of metabolic factors on them. However, our study has several limitations. Firstly, duration of sleep and napping is based on patients’ self-report, which may have recall bias and result in potential misclassification. Considering the randomness and non-interference of this issue, we believe that such error would be expected to result toward the null, although this would lead to an underestimation of the risk estimates. Secondly, being limited to a retrospective cross-sectional study, our study failed to explore the longitudinal effects of changes in sleep patterns on DR and further research is needed. Thirdly, our study did not account for potential underlying illness or sleep disturbances (e.g., obstructive sleep apnea and depression) that may influence both sleep patterns and DR. The observed associations between prolonged sleep duration and DR could partially reflect residual confounding from these unmeasured comorbidities and excessive daytime napping might serve as a clinical marker for undiagnosed sleep disorders or systemic inflammation. This potential confounding by indication should be acknowledged when interpreting the findings. Fourthly, it is crucial to recognize that the mediation models assume no unmeasured confounding between the exposure, mediator, and outcome. This assumption is difficult to meet, especially in observational data, and we included mediators in the main model, which may weaken the association. These inherent limitations underscore the need for caution in causal interpretation of mediation effects. In addition, while our study was adjusted for covariates through multivariable regression and performed stratified and conjoint analysis, unmeasured possible confounding variables such as socioeconomic status, genetic predisposition, or dietary habits might introduce residual confounding, which also suggests that future research can further improve the control of these factors.
In summary, long sleep and long nap were associated with DR, and this association was more significant in men and in the diabetes duration < 5 years group. In addition, the effects of night sleep and nap on DR could be superimposed, and that patients with both long sleep and long naps exhibited a higher prevalence of DR. Metabolic factors partially explain the mechanism between night sleep, daytime napping, and DR.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Ruijin Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LX: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft. XS: Methodology, Software, Writing – original draft. ZF: Methodology, Software, Writing – original draft. YC: Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1565508/full#supplementary-material
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Li C, Bishop TRP, Imamura F, Sharp SJ, Pearce M, Brage S, et al. Meat consumption and incident type 2 diabetes: an individual-participant federated meta-analysis of 1·97 million adults with 100 000 incident cases from 31 cohorts in 20 countries. Lancet Diabetes Endocrinol. (2024) 12:619–30. doi: 10.1016/S2213-8587(24)00179-7
3. Taylor R. Understanding the cause of type 2 diabetes. Lancet Diabetes Endocrinol. (2024) 12:664–73. doi: 10.1016/S2213-8587(24)00157-8
4. Lyssenko V and Vaag A. Genetics of diabetes-associated microvascular complications. Diabetologia. (2023) 66:1601–13. doi: 10.1007/s00125-023-05964-x
5. Pei X, Huang D, and Li Z. Genetic insights and emerging therapeutics in diabetic retinopathy: from molecular pathways to personalized medicine. Front Genet. (2024) 15:1416924. doi: 10.3389/fgene.2024
6. Reutrakul S and Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. (2018) 84:56–66. doi: 10.1016/j.metabol.2018.02.010
7. Sondrup N, Termannsen AD, Eriksen JN, Hjorth MF, Færch K, Klingenberg L, et al. Effects of sleep manipulation on markers of insulin sensitivity: A systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. (2022) 62:101594. doi: 10.1016/j.smrv.2022.101594
8. Meng LL, Liu Y, Geng RN, Tang YZ, and Li DQ. Association of diabetic vascular complications with poor sleep complaints. Diabetol Metab Syndr. (2016) 8:80. doi: 10.1186/s13098-016-0195-8
9. Jee D, Keum N, Kang S, and Arroyo JG. Sleep and diabetic retinopathy. Acta Ophthalmol. (2017) 95:41–7. doi: 10.1111/aos.13169
10. Raman R, Gupta A, Venkatesh K, Kulothungan V, and Sharma T. Abnormal sleep patterns in subjects with type II diabetes mellitus and its effect on diabetic microangiopathies: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS, report 20). Acta Diabetol. (2012) 49:255–61. doi: 10.1007/s00592-010-0240-2
11. Dutta S, Ghosh S, and Ghosh S. Association of sleep disturbance with diabetic retinopathy. Eur J Ophthalmol. (2022) 32:468–74. doi: 10.1177/1120672120974296
12. Simonson M, Li Y, Zhu B, McAnany JJ, Chirakalwasan N, Sutabutr Vajaranant T, et al. Multidimensional sleep health and diabetic retinopathy: Systematic review and meta-analysis. Sleep Med Rev. (2024) 74:101891. doi: 10.1016/j.smrv.2023.101891
13. Koren D and Taveras EM. Association of sleep disturbances with obesity, insulin resistance and the metabolic syndrome. Metabolism. (2018) 84:67–75. doi: 10.1016/j.metabol.2018.04.001
14. Olivares MJ, Toledo C, Ortolani D, Ortiz FC, Díaz HS, Iturriaga R, et al. Sleep dysregulation in sympathetic-mediated diseases: implications for disease progression. Sleep. (2022) 45:zsac166. doi: 10.1093/sleep/zsac166
15. Liu H, Zhu H, Lu Q, Ye W, Huang T, Li Y, et al. Sleep features and the risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Med. (2025) 57:2447422. doi: 10.1080/07853890.2024.2447422
16. Tan X, Chapman CD, Cedernaes J, and Benedict C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: A review of possible mechanisms. Sleep Med Rev. (2018) 40:127–34. doi: 10.1016/j.smrv.2017.11.001
17. Huang ZG, Gao JW, Zhang HF, You S, Xiong ZC, Wu YB, et al. Cardiovascular health metrics defined by Life’s Essential 8 scores and subsequent macrovascular and microvascular complications in individuals with type 2 diabetes: A prospective cohort study. Diabetes Obes Metab. (2024) 26:2673–83. doi: 10.1111/dom.15583
18. Maimaitituerxun R, Chen W, Xiang J, Xie Y, Xiao F, Wu XY, et al. Sleep quality and its associated factors among patients with type 2 diabetes mellitus in Hunan, China: a cross-sectional study. BMJ Open. (2024) 14:e078146. doi: 10.1136/bmjopen-2023-078146
19. Ohkuma T, Fujii H, Iwase M, Kikuchi Y, Ogata S, Idewaki Y, et al. Impact of sleep duration on obesity and the glycemic level in patients with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetes Care. (2013) 36:611–7. doi: 10.2337/dc12-0904
20. Au CT, Ho CK, Wing YK, Lam HS, and Li AM. Acute and chronic effects of sleep duration on blood pressure. Pediatrics. (2014) 133:e64–72. doi: 10.1542/peds.2013-1379
21. Liu J and Bloomgarden Z. The Chinese metabolic management centers. J Diabetes. (2022) 14:362–4. doi: 10.1111/1753-0407.13290
22. Zhang Y, Shi J, Peng Y, Zhao Z, Zheng Q, Wang Z, et al. Artificial intelligence-enabled screening for diabetic retinopathy: a real-world, multicenter and prospective study. BMJ Open Diabetes Res Care. (2020) 8:e001596. doi: 10.1136/bmjdrc-2020-001596
23. Xu C, Li L, Shi J, Ji B, Zheng Q, Wang Y, et al. Kidney disease parameters, metabolic goal achievement, and arterial stiffness risk in Chinese adult people with type 2 diabetes. J Diabetes. (2022) 14:345–55. doi: 10.1111/1753-0407.13269
24. Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. Life’s essential 8: updating and enhancing the american heart association’s construct of cardiovascular health: A presidential advisory from the American heart association. Circulation. (2022) 146:e18–43. doi: 10.1161/CIR.0000000000001078
25. Sun J, Ma C, Zhao M, Magnussen CG, and Xi B. Daytime napping and cardiovascular risk factors, cardiovascular disease, and mortality: A systematic review. Sleep Med Rev. (2022) 65:101682. doi: 10.1016/j.smrv.2022.101682
26. Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. (2003) 110:1677–82. doi: 10.1016/S0161-6420(03)00475-5
27. Alshahrani AM, Alshahrani AM, Al-Boqami BAH, Alqahtani AA, Alzahrani B, Bassi Y, et al. Prevalence and predictors of diabetic retinopathy in Saudi Arabia: insights from a systematic review and meta-analysis. Biomolecules. (2024) 14:1486. doi: 10.3390/biom14121486
28. Wondmeneh TG and Mohammed JA. Prevalence of diabetic retinopathy and its associated risk factors among adults in Ethiopia: a systematic review and meta-analysis. Sci Rep. (2024) 14:28266. doi: 10.1038/s41598-024-78596-9
29. Cai K, Liu YP, and Wang D. Prevalence of diabetic retinopathy in patients with newly diagnosed type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab Res Rev. (2023) 39:e3586. doi: 10.1002/dmrr.3586
30. Wang Z, Wu M, Li H, and Zheng B. Association between rest-activity rhythm and diabetic retinopathy among US middle-age and older diabetic adults. Front Endocrinol (Lausanne). (2024) 15:1440223. doi: 10.3389/fendo.2024.1440223
31. Li B, Zhou C, Gu C, Cheng X, Wang Y, Li C, et al. Modifiable lifestyle, mental health status and diabetic retinopathy in U.S. adults aged 18-64 years with diabetes: a population-based cross-sectional study from NHANES 1999-2018. BMC Public Health. (2024) 24:11. doi: 10.1186/s12889-023-17512-8
32. Sun XJ, Zhang GH, Guo CM, Zhou ZY, Niu YL, Wang L, et al. Associations between psycho-behavioral risk factors and diabetic retinopathy: NHANES (2005-2018). Front Public Health. (2022) 10:966714. doi: 10.3389/fpubh.2022.966714
33. Zheng Z, Wang C, Li C, Wu Q, Chen X, Chen H, et al. Meta-analysis of relationship of sleep quality and duration with risk of diabetic retinopathy. Front Endocrinol (Lausanne). (2022) 13:922886. doi: 10.3389/fendo.2022.922886
34. Um YH, Kim TW, Jeong JH, Hong SC, Seo HJ, and Han KD. Association between diabetic retinopathy and insomnia risk: A nationwide population-based study. Front Endocrinol (Lausanne). (2022) 13:939251. doi: 10.3389/fendo.2022.939251
35. Ba-Ali S, Brøndsted AE, Andersen HU, Sander B, Jennum PJ, and Lund-Andersen H. Assessment of diurnal melatonin, cortisol, activity, and sleep-wake cycle in patients with and without diabetic retinopathy. Sleep Med. (2019) 54:35–42. doi: 10.1016/j.sleep.2018.10.018
36. Irwin MR, Olmstead R, and Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. (2016) 80:40–52. doi: 10.1016/j.biopsych.2015.05.014
37. Janmohammadi P, Raeisi T, Zarei M, Nejad MM, Karimi R, Mirali Z, et al. Adipocytokines in obstructive sleep apnea: A systematic review and meta-analysis. Respir Med. (2023) 208:107122. doi: 10.1016/j.rmed.2023.107122
38. Liu H, Wu Y, Zhu H, Wang P, Chen T, Xia A, et al. Association between napping and type 2 diabetes mellitus. Front Endocrinol (Lausanne). (2024) 15:1294638. doi: 10.3389/fendo.2024.1294638
39. Jiang H, Zhang H, Wen Z, Yuan J, Wang H, and Zhang X. Association of sleep duration with obesity in older adults: A systematic review and meta-analysis. Geriatr Gerontol Int. (2024) 24:1269–82. doi: 10.1111/ggi.15004
40. Papachristoforou E, Lambadiari V, Maratou E, and Makrilakis K. Association of glycemic indices (Hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and diabetic complications. J Diabetes Res. (2020) 2020:7489795. doi: 10.1155/2020/7489795
41. Davinelli S, Medoro A, Savino R, and Scapagnini G. Sleep and oxidative stress: current perspectives on the role of NRF2. Cell Mol Neurobiol. (2024) 44:52. doi: 10.1007/s10571-024-01487-0
42. Golmohammadi M, Samadi M, Salimi Y, Nachvak SM, and Ebrahimzadeh Attari V. The association of dietary inflammatory index with sleep outcomes: A systematic review. Health Promot Perspect. (2024) 14:136–47. doi: 10.34172/hpp.42595
43. Jiang C, Chen Z, Liao W, Zhang R, Chen G, Ma L, et al. The medicinal species of the lycium genus (Goji berries) in east asia: A review of its effect on cell signal transduction pathways. Plants (Basel). (2024) 13:1531. doi: 10.3390/plants13111531
44. Simó R and Hernández C. What else can we do to prevent diabetic retinopathy? Diabetologia. (2023) 66:1614–21. doi: 10.1007/s00125-023-05940-5
45. Chen P, Sun Q, Xu L, Li F, and Liu H. Patients with type 2 diabetes who achieve reduced postprandial glucose levels during insulin intensive therapy may have a better recovery of β-cell function. Diabetes Res Clin Pract. (2024) 215:111805. doi: 10.1016/j.diabres.2024.11180534
46. Crawford AL and Laiteerapong N. Type 2 diabetes. Ann Intern Med. (2024) 177:ITC81–96. doi: 10.7326/AITC202406180
47. Kunutsor SK, Balasubramanian VG, Zaccardi F, Gillies CL, Aroda VR, Seidu S, et al. Glycaemic control and macrovascular and microvascular outcomes: A systematic review and meta-analysis of trials investigating intensive glucose-lowering strategies in people with type 2 diabetes. Diabetes Obes Metab. (2024) 26:2069–81. doi: 10.1111/dom.15511
Keywords: sleep, napping, type 2 diabetes, diabetic retinopathy, metabolic factors
Citation: Xi L, Sun X, Feng Z and Cao Y (2025) Associations of night sleep duration and daytime napping with diabetic retinopathy in patients with type 2 diabetes. Front. Endocrinol. 16:1565508. doi: 10.3389/fendo.2025.1565508
Received: 23 January 2025; Accepted: 04 June 2025;
Published: 24 June 2025.
Edited by:
Tomislav Bulum, Medical School University of Zagreb, CroatiaReviewed by:
James David Adams, Independent Researcher, Benicia, CA, United StatesMarquis Samuel Hawkins, University of Pittsburgh, United States
Copyright © 2025 Xi, Sun, Feng and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanan Cao, eWFuYW5jYW9Ac2p0dS5lZHUuY24=; Y2FveWFuYW5AdmlwLnNpbmEuY29t
†ORCID: Yanan Cao, orcid.org/0000-0002-3848-7040
 Lei Xi
Lei Xi Xiaohui Sun
Xiaohui Sun Zhimin Feng
Zhimin Feng Yanan Cao
Yanan Cao