- 1Department of Reproductive Medicine, Hebei Medical University Third Hospital, Shijiazhuang, Hebei, China
- 2Department of Reproductive Medicine, Bethune International Peace Hospital, Shijiazhuang, Hebei, China
Objective: To analyze the expression levels of the oocyte-secreted factors growth differentiation factor-9 (GDF-9) and bone morphogenetic protein-15 (BMP-15) in cumulus cells (CCs) under different controlled ovarian stimulation (COS) protocols and their association with oocyte maturity and embryo developmental potential.
Methods: This study included 76 patients requiring intracytoplasmic sperm injection (ICSI) due to severe oligoasthenoteratozoospermia or previous ICSI treatment, resulting in the collection of 749 CC samples. Patients were divided into four groups based on COS protocols: short-acting luteal phase (14 patients, 168 CCs), long-acting follicular phase (21 patients, 189 CCs), micro-stimulation (12 patients, 86 CCs) and antagonist (29 patients, 306 CCs). The mRNA was extracted from cumulus granulosa cells, and the relative levels of GDF-9 and BMP-15 were measured using real-time quantitative PCR (Q-PCR). The expression levels of GDF-9 and BMP-15 were compared across different ovarian stimulation protocols, while oocyte maturation, fertilization, cleavage, and blastocyst formation were assessed. The expression levels of GDF-9 and BMP-15 were compared across protocols, and oocyte maturation, fertilization, cleavage and blastocyst formation were assessed.
Results: GDF-9 and BMP-15 levels were substantially higher in MII oocytes than in MI and GV oocytes and were also elevated in the normal fertilization group, high-quality cleavage embryos and high-quality blastocysts. Growth differentiation factor-9 expression was higher in the short-acting luteal phase protocol than in the antagonist protocol, whereas BMP-15 expression was higher in both the short-acting luteal phase and long-acting follicular phase protocols compared with the micro-stimulation and antagonist groups.
Conclusion: GDF-9 and BMP-15 are reliable indicators of oocyte developmental potential. The long-acting follicular phase and short-acting luteal phase protocols enhance oocyte maturity and embryo development, whereas the micro-stimulation and antagonist protocols appear less favorable.
1 Introduction
With advancements in society and increasing competitive pressures, many women are delaying childbirth, leading to a growing proportion of older couples seeking to conceive and a rising incidence of infertility (1). In vitro fertilization and embryo transfer (IVF-ET) has become a primary assisted reproductive technology, with treatment protocols gradually becoming more individualized to address various aetiologies (2). A key determinant of IVF success is the choice of controlled ovarian stimulation protocol, which substantially influences oocyte quality and embryo developmental potential (3).
Common ovarian stimulation protocols include the long-acting follicular phase protocol, short-acting luteal phase protocol, antagonist protocol and micro-stimulation protocol (4). Despite their widespread clinical application, the efficacy and suitability of these protocols remain debated due to the heterogeneity of patient populations and individual differences in response to stimulation (5). Although numerous clinical studies compare the therapeutic outcomes of these protocols, laboratory-based research specifically examining their impact on oocyte-secreted factor expression remains limited.
Oocyte-secreted factors (OSFs) play a crucial role in oocyte development, with growth differentiation factor-9 (GDF-9) and bone morphogenetic protein-15 (BMP-15) being key OSFs (6–8). These factors regulate granulosa cell proliferation, apoptosis and metabolism, thereby influencing oocyte maturation and quality, which ultimately determine embryo developmental potential (9–11). Although studies have demonstrated a close association between GDF-9 and BMP-15 expression and oocyte quality (12), limited research has investigated how different ovarian stimulation protocols affect their expression.
This study aims to examine the impact of various ovarian stimulation protocols on the expression levels of GDF-9 and BMP-15 in oocyte granulosa cells and to analyze the relationship between these factors, oocyte maturity and embryo developmental potential. By addressing this research gap, we aim to establish a theoretical foundation for optimizing ovarian stimulation protocols in IVF and provide more precise guidance for individualized treatment strategies.
2 Materials and methods
2.1 Study participants
This prospective cohort study was conducted on patients undergoing intracytoplasmic sperm injection (ICSI) treatment at Hebei Medical University Third Hospital and Bethune International Peace Hospital between January 2020 and June 2024. Eligible participants included those requiring ICSI due to severe oligoasthenoteratozoospermia in individuals with testes or a history of failed or low fertilization in IVF-ET. All patients received routine clinical treatment, and those who met the inclusion criteria but did not fulfill the exclusion criteria were enrolled for data collection.
Intracytoplasmic sperm injection technology enables the direct selection of a single sperm with normal morphology and high motility for injection into the oocyte, thereby minimizing the potential impact of sperm quality on embryonic development during natural fertilization (study flow shown in Figure 1).
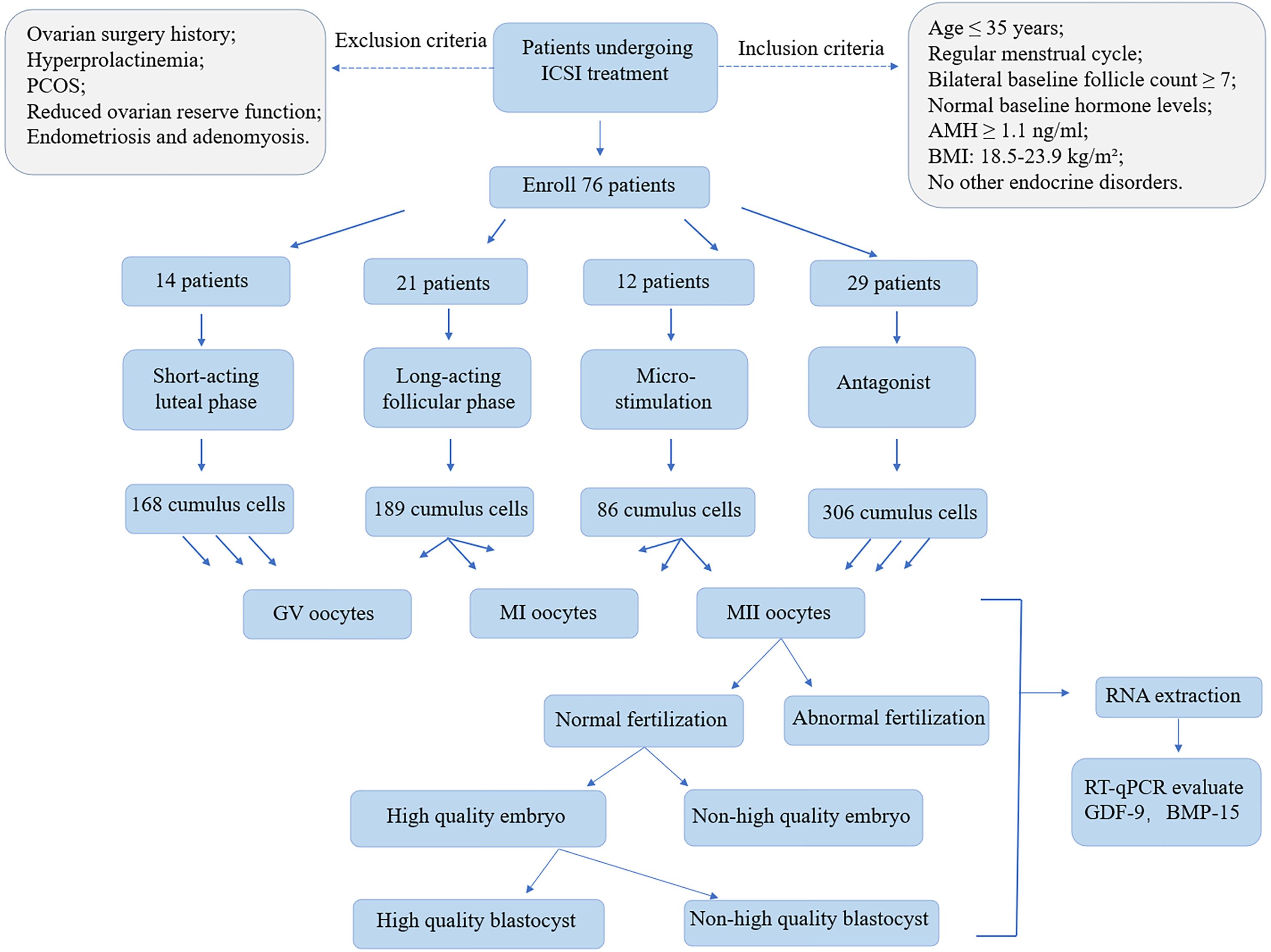
Figure 1. The study flow diagram. The whole design includes patient enrollment, cumulus cells collection, oocyte collection. RNA was extracted from both GV oocytes, MI oocytes, MII oocytes to evaluate GDF-9, BMP-15 expression levels. MII oocytes further divided into different group and RNA was extracted from all groups to evaluate GDF-9, BMP-15 expression levels.
The inclusion criteria were as follows: Age ≤ 35 years, regular menstrual cycle (28 ± 7 days), bilateral baseline follicle count ≥ 7, normal baseline hormone levels, anti-Müllerian hormone (AMH) ≥ 1.1 ng/ml, body mass index (BMI) between 18.5 and 23.9 kg/m² and no other endocrine disorders.
The exclusion criteria were as follows: History of ovarian surgery, hyperprolactinaemia, polycystic ovary syndrome, diminished ovarian reserve, endometriosis and adenomyosis.
This study was approved by the ethics committee of the Hebei Medical University Third Hospital (W2021-007-1).
2.2 Grouping method
Following standard clinical practices in IVF-ET and the European Society of Human Reproduction and Embryology (ESHRE) guidelines on ovarian stimulation for IVF (13), participants were categorized into four groups: Group A (short-term long protocol during the luteal phase), Group B (long-term long protocol during the follicular phase), Group C (micro-stimulation protocol) and Group D (antagonist protocol).
Based on oocyte maturation, cumulus cells (CCs) were classified into GV, MI and MII stages. The MII group was further divided into normal and abnormal fertilization groups. The normal fertilization group was subsequently categorized into high-quality and non-high-quality embryos based on Day 3 embryo scoring. Embryos were then cultured to assess blastocyst formation on Days 5 and 6 and classified into high-quality and non-high-quality blastocyst groups.
2.3 Controlled ovarian stimulation medication protocols
2.3.1 Short-term GnRH-a long protocol (luteal phase)
Administer short-term GnRH-a (Dabigatran, Bayer, Germany) at a dosage of 0.1 mg daily, starting 7 days after ovulation or when five to seven contraceptive pills remain in the combined oral contraceptive cycle. After 16–18 days, initiate gonadotropin (Gn) (Gonal-f, Merck Serono, Switzerland) at 150–300 IU/day for ovulation induction once downregulation is confirmed. Trigger ovulation with 250 µg of hCG (Merck Serono, Italy) when follicles reach ≥18 mm (at least two follicles or ≥ three follicles measuring ≥17 mm). Oocyte retrieval is performed 36 hours later.
2.3.2 Long-term GnRH-a long protocol (follicular phase)
Administer long-acting GnRH-a (leuprolide acetate, Shanghai Livzon Pharmaceutical) at a dosage of 3.75 mg on the 2nd or 3rd day of menstruation for downregulation. Initiate Gn at 150–300 IU/day for ovulation induction 28–40 days later. Trigger ovulation with 250 µg of hCG once follicular criteria are met, followed by oocyte retrieval 36 hours later.
2.3.3 Micro-stimulation protocol
Administer letrozole (Jiangsu Hengrui) at 5 mg/day and human menopausal gonadotropin (Guangzhou Lizhu Group) at 75–150 IU/day, starting on the 2nd or 3rd day of menstruation. After 5 days, replace letrozole with clomiphene citrate (Cyprus Gout Pharmaceutical Co., Ltd) at 50 mg/day. Adjust the Gn dosage based on ovarian response. If LH levels reach ≥15 U/L, administer Cetrorelix (Cetrotide, Merck Serono) at 0.125–0.25 mg/day to suppress the LH surge. Trigger ovulation with 250 µg of hCG (Merck Serono, Italy) or a combination of 0.2 mg Triptorelin (Dabigatran, Bayer, Germany) and 2,000 IU of hCG (Injectable Chorionic Gonadotropin, Shanghai Lizhu Group), followed by oocyte retrieval.
2.3.4 Antagonist protocol
Initiate recombinant human FSH at 150–300 IU/day on the 2nd or 3rd day of menstruation. Introduce cetrorelix (cetrotide, Merck Serono, Switzerland) at 0.25 mg/day when follicles reach 12–14 mm and/or E2 levels exceed 1,468 pmol/L, with LH >5 IU/L. Trigger ovulation with 250 µg of hCG (Merck Serono, Italy), followed by oocyte retrieval 36 hours later.
2.4 Collection of cumulus granulosa cell mass
Transvaginal oocyte retrieval under ultrasound guidance is performed 36 hours after the trigger. The cumulus-oocyte complex is placed in individual microdrops of embryo processing solution. Between 40 and 42 hours post-trigger, the CCs surrounding the oocyte are treated with hyaluronidase and mechanically denuded.
The remaining cumulus granulosa cells in the microdrop are transferred into small EP tubes, repeatedly washed with PBS (pH 7.4) and centrifuged. The PBS is then removed, leaving only the granulosa cells at the bottom of the tube. These cells are frozen at −80°C and labelled to ensure a one-to-one correspondence between the oocyte and the collected CCs.
2.5 Cumulus-oocyte complex maturity assessment, fertilization, cleavage stage and blastocyst grading
The expression levels of OSFs are observed in GV, MI and MII stage oocytes. All MII stage oocytes undergo ICSI fertilization, with continuous monitoring of fertilization, cleavage and blastocyst formation. Pronuclear assessment is performed 16–18 hours post-fertilization, where the presence of two pronuclei indicates normal fertilization, while 0, 1 or ≥3 pronuclei indicate abnormal fertilization. Intracytoplasmic sperm injection fertilization and culture in microdrops are conducted 40–42 hours post-trigger.
Embryo evaluation follows the ESHRE guidelines, with fertilization confirmed approximately 16–18 hours post-ICSI. Normal fertilization is indicated by the presence of 2PN, whereas other outcomes denote abnormal fertilization. Day 3 embryos are assessed using the Peter cleavage stage embryo scoring system (14), where high-quality embryos are defined as those with 6–10 blastomeres of equal or slightly unequal sizes and ≤20% fragmentation. The specific laboratory criteria for high-quality embryos include 7–9 blastomeres, regular arrangement and ≤20% fragmentation.
On Day 5, blastocysts are graded using the Gardner blastocyst scoring system (15, 16). High-quality blastocysts are classified as those with grades ≥4AA, 4AB, 4BA or 4BB.
2.6 RNA extraction and reverse transcription reaction
Cells at different developmental stages, including oocytes, embryos and blastocysts, are collected for RNA isolation. RNA extraction is performed using Trizol. RNA samples are treated with DNase I to degrade contaminating DNA. Extracted RNA is assessed for quality using agarose gel electrophoresis, followed by reverse transcription (RT) according to the instructions provided with the Reverse Transcription Reaction kit (Promega Biotech Co., Ltd.). The RT reaction is carried out under the following conditions: 70°C for 10 minutes, 42°C for 50 minutes and 95°C for 5 minutes.
2.7 Real-time fluorescent quantitative polymerase chain reaction
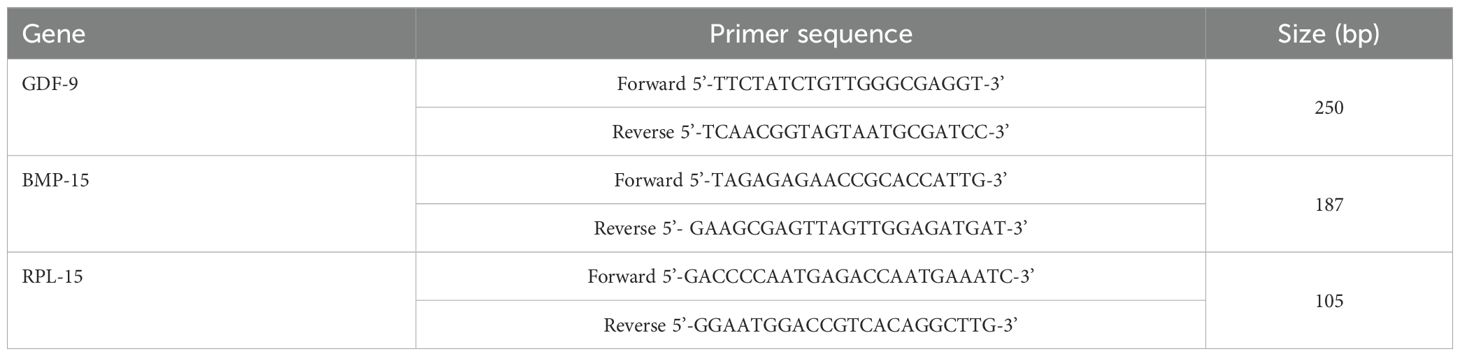
RPL-15 is used as the reference gene due to its stable expression in CCs under experimental conditions. Primers for the target genes (GDF-9 and BMP-15) and the reference gene (RPL-15) are designed using PrimerPremier 5.0 software (see Table 1). Primers with optimal melting temperatures (Tm) and minimal secondary structures are selected for amplification. The reaction is conducted following the instructions of the QuantiFast® SYBR® Green PCR kit. To ensure PCR quality control, no-template controls are included to detect non-specific amplification, whereas no-RT controls are used to identify DNA contamination. Each sample is run in three technical replicates to minimize variability. The mean value of duplicate runs is used for statistical analysis.
2.8 Statistical methods
The relative expression levels of GDF-9 and BMP-15 RNA in each group are analyzed using real-time PCR. The obtained Ct values are calculated using the formula 2ΔCt (17), where ΔCt = Ct (target gene) − Ct (reference gene).
Statistical analyses are conducted using SPSS 25.0 (IBM, Armonk, NY, USA). Normally distributed data are presented as mean ± standard deviation ( ± s) and compared using independent sample t-tests. Non-normally distributed data are expressed as median (P25, P75) and analyzed using Mann-Whitney U tests, with P < 0.05 indicating statistical significance. Comparisons between different protocol groups are performed using F-tests, with P < 0.05 considered statistically significant.
3 Results
3.1 Comparison of general conditions among the four groups
There were no statistically significant differences in age, infertility duration, BMI, AMH levels or baseline hormone levels among the four groups (all P > 0.05), indicating comparability between groups. Although no significant differences were observed in the number of Gn days, there were significant differences in total Gn dosage. The microstimulation protocol required a significantly lower Gn dose than the other three groups (P = 0.000), whereas no significant differences were found among the remaining three groups (Table 2).
3.2 Relationship between the relative expression of GDF-9 in CCs and oocyte maturity and developmental potential
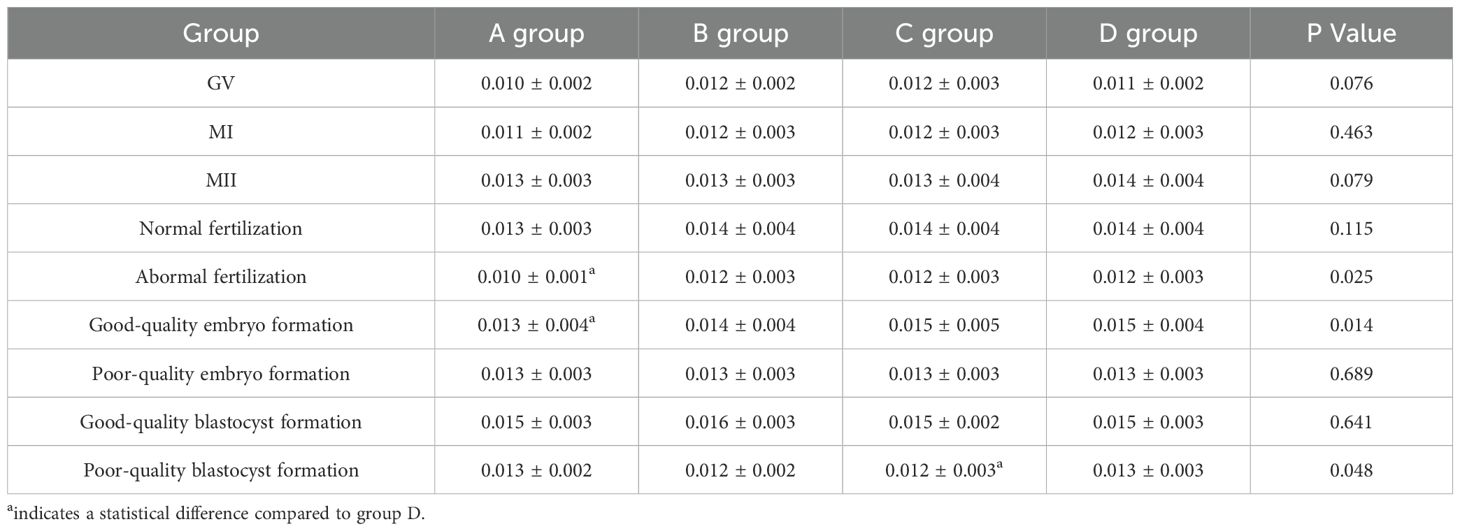
As shown in Tables 3–6, across the four ovarian stimulation protocols, the relative expression levels of GDF-9 were significantly higher in MII oocytes compared with MI oocytes (fold change > 2, P = 0.037; P = 0.029; P = 0.016). Additionally, expression levels in MI oocytes were significantly higher than in GV oocytes (fold change > 1.5, P = 0.046; P = 0.014; P = 0.026).
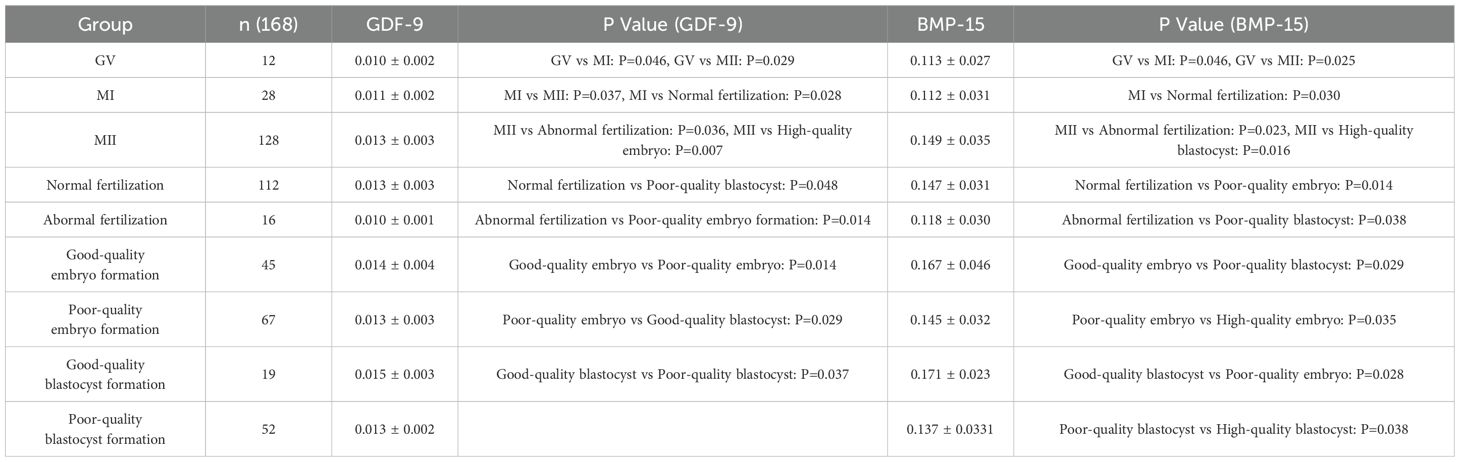
Table 3. Expression levels of GDF-9 and BMP-15 in the short-term GnRH-a long protocol during the luteal phase ( ± s).
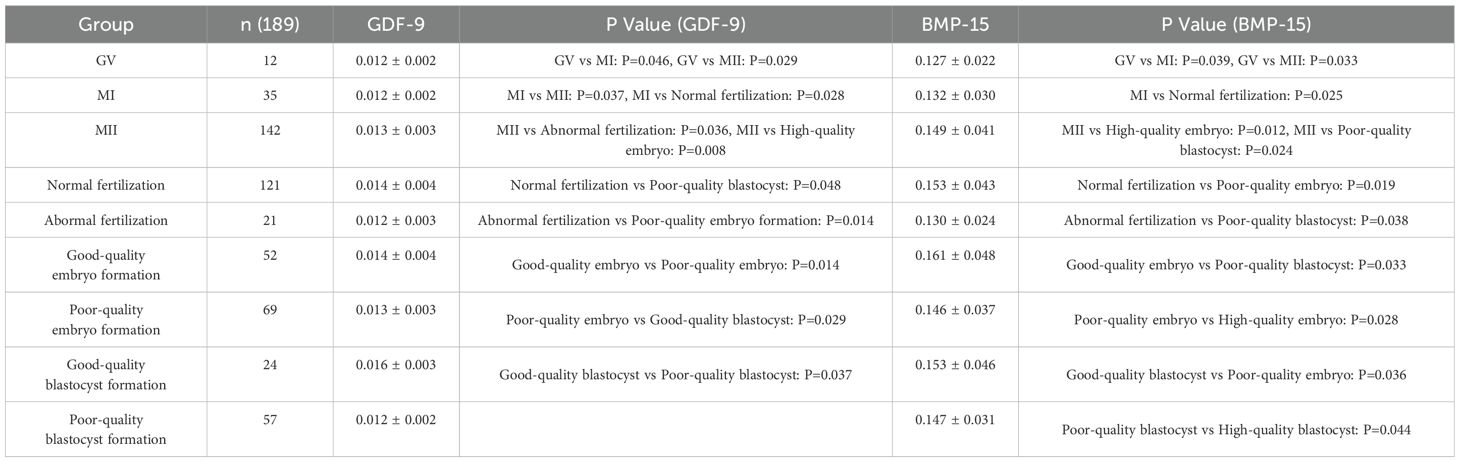
Table 4. Expression levels of GDF-9 and BMP-15 in the long-term GnRH-a Long protocol during the follicular phase ( ± s).
Significant differences in GDF-9 expression were observed between the normal and abnormal fertilization groups (fold change > 1.5, P = 0.001; P = 0.036; P = 0.028). Expression levels in high-quality embryo groups were significantly higher than in non-high-quality embryo groups (fold change > 1.8, P = 0.007; P = 0.014; P = 0.026). Similarly, expression levels in high-quality blastocyst groups were significantly higher than in non-high-quality blastocyst groups (fold change > 2, P = 0.048; P = 0.029; P = 0.016).
3.3 Relationship between the relative expression of BMP-15 in CCs and oocyte maturity and developmental potential
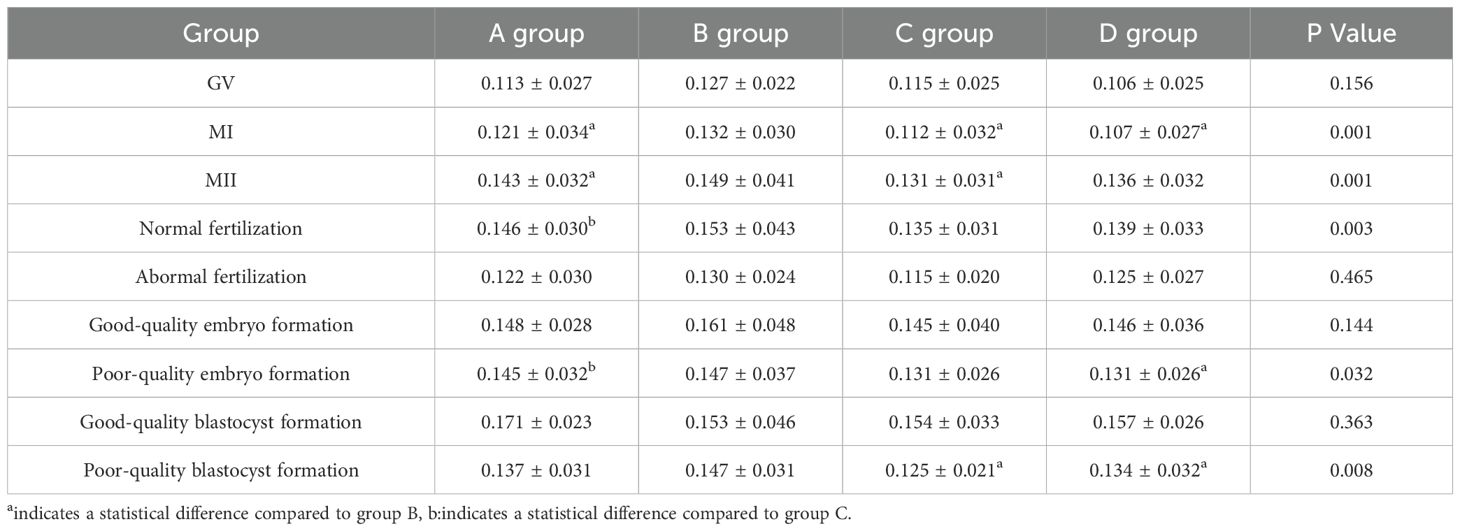
Across the four ovarian stimulation protocols, the relative expression of BMP-15 in MII oocytes was significantly higher than in MI oocytes (P = 0.046; P = 0.025; P = 0.049) and expression in MI oocytes was significantly higher than in GV oocytes (P = 0.000; P = 0.038; P = 0.044). Expression levels in normal fertilization groups were significantly higher than in abnormal fertilization groups (P = 0.012; P = 0.034; P = 0.024). Additionally, expression levels in high-quality embryo formation groups were significantly higher than in non-high-quality embryo groups (P = 0.015; P = 0.002; P = 0.040). Statistically significant differences in BMP-15 expression were also observed between high-quality blastocyst groups and non-high-quality blastocyst groups (P = 0.040; P = 0.049; P = 0.037).
3.4 Comparison of relative expression levels of GDF-9 and BMP-15 in four ovulation induction protocols
There were no significant statistical differences in general clinical data among the four ovulation induction protocols: luteal phase standard long protocol, follicular phase long protocol, microstimulation protocol and antagonist protocol. However, Gn amounts were significantly lower in the microstimulation protocol compared with the luteal phase long protocol, follicular phase long protocol and antagonist protocol (P = 0.000).
There were statistically significant differences in the relative expression of GDF-9 during the fertilization stage between the luteal phase standard long protocol and antagonist protocol (P = 0.007) and during the cleavage stage between the luteal phase standard long protocol and antagonist protocol (P = 0.002). Statistically significant differences were also observed during the blastocyst formation stage between the microstimulation protocol and antagonist protocol (P = 0.038) (Figure 2, Table 7).

Figure 2. Expression levels of GDF-9 in four ovarian stimulation protocols. The different ovarian stimulation regiments are represented by different colored column. Group A is short-term long protocol during the luteal phase, displayed in red column; Group B is long-term long protocol during the follicular phase, displayed in yellow column; Group C is micro-stimulation protocol, displayed in green column; Group D is antagonist protocol, displayed in blue column. The Y-axis represents the mRNA expression level of GDF-9 obtained by RT-qpcr, n=3, * indicates p < 0.05. The X-axis indicates the different stages of RT-qpcr detection.
The relative expression of BMP-15 in MI oocytes was significantly different in the follicular phase long protocol compared with the other three groups (P = 0.011, P = 0.030, P = 0.000). In MII oocytes, expression levels were significantly different in the luteal phase standard long protocol compared with the follicular phase long protocol and microstimulation protocol (P = 0.033, P = 0.043).
There were statistically significant differences in the relative expression of BMP-15 during the fertilization stage between the luteal phase standard long protocol and follicular phase long protocol (P = 0.007). During the cleavage stage, statistically significant differences were found between the luteal phase standard long protocol and both the microstimulation protocol and antagonist protocol (P = 0.033, P = 0.035), as well as between the follicular phase long protocol and microstimulation protocol (P = 0.034) (Figure 3, Table 8). During the blastocyst formation stage, expression levels differed significantly between the luteal phase standard long protocol and both the microstimulation protocol and antagonist protocol (P = 0.001, P = 0.013) (Tables 1, 7 and 8 for details).
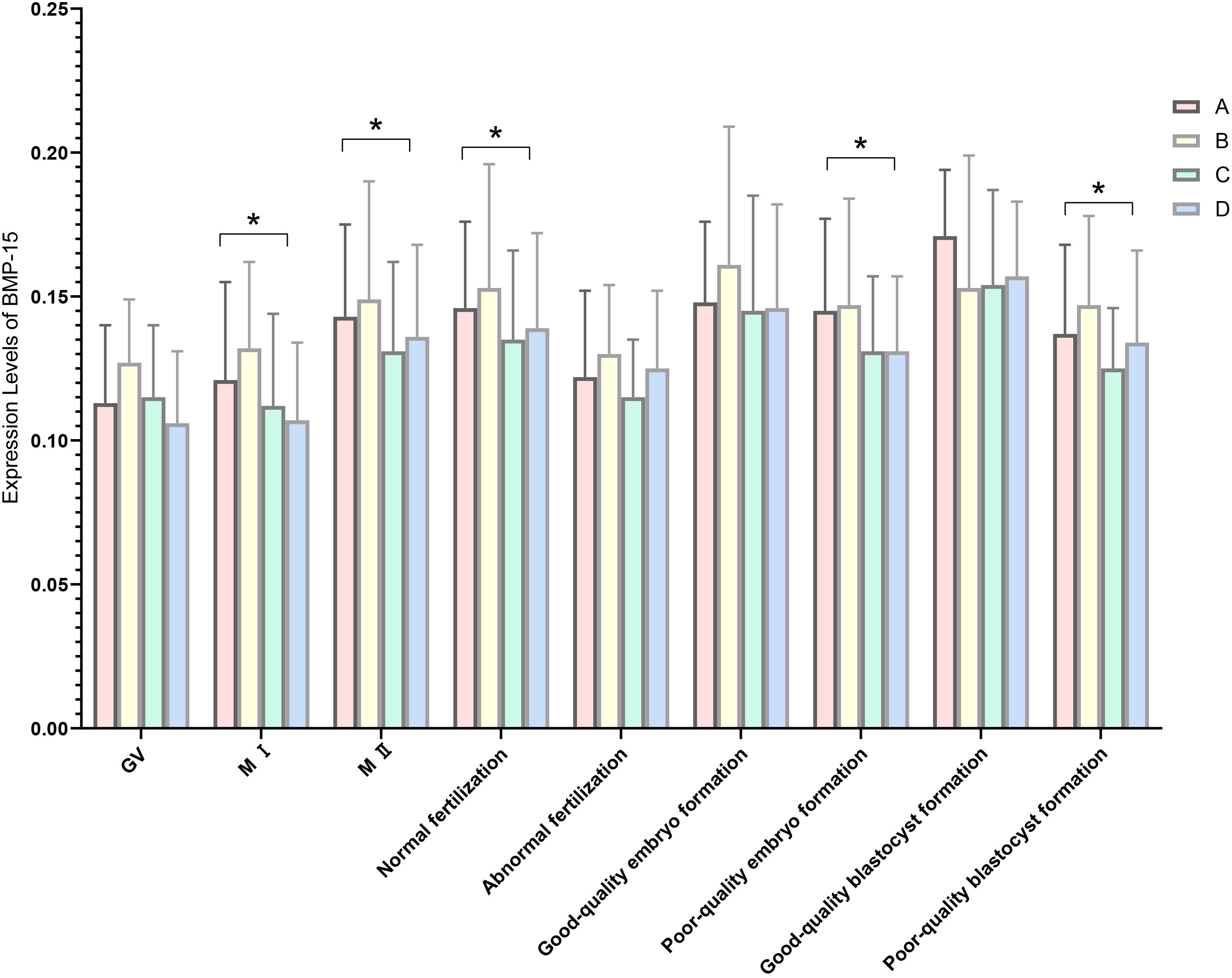
Figure 3. Expression levels of BMP-15 in four ovarian stimulation protocols. The different ovarian stimulation regiments are represented by different colored column. Group A is short-term long protocol during the luteal phase, displayed in red column; Group B is long-term long protocol during the follicular phase, displayed in yellow column; Group C is micro-stimulation protocol, displayed in green column; Group D is antagonist protocol, displayed in blue column. The Y-axis represents the mRNA expression level of BMP-15 obtained by RT-qpcr, n=3, * indicates p < 0.05. The X-axis indicates the different stages of RT-qpcr detection.
4 Discussion
This study aimed to investigate the expression levels of GDF-9 and BMP-15 across different ovarian stimulation protocols and their potential impact on oocyte maturation and developmental potential. Our results demonstrate that GDF-9 and BMP-15 exhibit distinct expression patterns depending on the ovarian stimulation protocol, and these patterns appear to be closely associated with oocyte maturation and embryo development. However, there are several limitations to this study, and the findings should be interpreted within the context of these shortcomings.
Our results indicate that the expression of GDF-9 and BMP-15 is substantially higher in MII oocytes compared with GV and MIical difference compared to gro oocytes, which is consistent with findings from previous studies (18, 19). Additionally, we observed that normal fertilization groups exhibited higher levels of GDF-9 and BMP-15 expression than abnormal fertilization groups, aligning with the established role of these molecules in folliculogenesis and oocyte maturation (20). Given that GDF-9 and BMP-15 regulate granulosa cell function, follicle development and oocyte quality (21), their expression could serve as a valuable biomarker for assessing oocyte developmental competence.
Despite these substantial findings, the molecular mechanisms underlying the regulation of these molecules in different ovarian stimulation protocols remain unclear. Future studies utilizing gene-editing techniques or animal models are warranted to elucidate how these molecules specifically influence oocyte maturation and embryo development in response to varying ovarian stimulation conditions (22).
Although this study provides valuable insights into the role of GDF-9 and BMP-15 in oocyte development, several limitations must be considered:
1. Sample Size and Diversity: Although the results are statistically significant within the sample size used, the overall sample size remains relatively small, particularly in the abnormal fertilization and poor-quality blastocyst formation groups. Expanding the sample size and including a more diverse cohort – encompassing patients of different age groups, ovarian reserves and underlying conditions – would enhance the generalizability of our findings (22).
2. Limited Range of Ovarian Stimulation Protocols: This study focused on four commonly used ovarian stimulation protocols (A, B, C, D). Expanding the analysis to include additional stimulation regimens and patients with varying ovarian responses (e.g. poor responders) would help determine whether GDF-9 and BMP-15 serve as universal biomarkers across different stimulation protocols (19, 21, 23).
3. Short-Term Follow-Up: The study primarily assessed oocyte maturation and early embryo development. Conducting a long-term follow-up to evaluate clinical pregnancy rates and live birth outcomes in relation to GDF-9 and BMP-15 expression would provide a clearer understanding of the role these molecules play in successful IVF outcomes (24, 25).
4. Mechanistic Insights: Although this study analyzed the expression levels of GDF-9 and BMP-15, the specific molecular pathways through which these factors regulate oocyte maturation and embryo development were not explored. Future research should investigate the underlying signaling pathways, particularly the TGF-β and SMAD pathways, which are known to be critical in ovarian function (21, 26).
5. Method of cumulus cells isolation: Although the cumulus cell isolation method follows the clinical practice, the cell heterogeneity and operation standardization still need to be optimized. Nevertheless, we have ensured internal consistency of the data through strict experimental procedures such as harmonization of enzymatic hydrolysis times and standardization of mechanical stripping.
6. Single center: The single-center design may limit the external validity of the results, and it is necessary to expand the sample diversity through multi-center cooperation in the future. Follow-up studies will combine single-cell techniques with multicenter cohorts to further validate the potential of GDF-9/BMP-15 as a marker of efficacy for ovarian stimulation.
Our findings align with previous studies demonstrating the involvement of GDF-9 and BMP-15 in oocyte maturation and embryo quality (27, 28). However, unlike some studies, we did not observe substantial differences in BMP-15 expression between certain groups, which may be attributed to variations in sample size or experimental conditions (29). This underscores the need for further research with larger sample sizes and a broader range of ovarian stimulation protocols to validate the role of these molecules in oocyte maturation.
Furthermore, the expression patterns of GDF-9 and BMP-15 in our study were consistent with the known influence of the TGF-β superfamily on follicular growth and oocyte quality (21, 26). Although the role of these growth factors in regulating granulosa cell proliferation and oocyte competence is well documented (30, 31), the precise mechanisms by which GDF-9 and BMP-15 mediate their effects in response to different ovarian stimulation protocols remain unclear.
Building on the limitations and findings of this study, several key research directions should be pursued. First, increasing the sample size and incorporating a more diverse cohort are essential to enhance the generalizability of our findings and strengthen the robustness of the results. Additionally, exploring different ovarian stimulation protocols, including newer approaches such as dual stimulation, could provide valuable insights into how GDF-9 and BMP-15 influence oocyte maturation and embryo development across varied patient populations.
Furthermore, future research should investigate the molecular mechanisms regulating GDF-9 and BMP-15 during oocyte maturation. Advanced techniques such as CRISPR-Cas9 gene editing and RNA sequencing could offer a deeper understanding of the specific signaling pathways involved. Finally, long-term outcome studies are crucial for assessing clinical pregnancy rates and live birth outcomes in relation to GDF-9 and BMP-15 expression. These studies would help establish their predictive value as biomarkers for IVF success, ultimately contributing to more personalized fertility treatments.
5 Conclusion
In conclusion, this study offers valuable insights into the expression patterns of GDF-9 and BMP-15 in response to different ovarian stimulation protocols. Our findings suggest that GDF-9 and BMP-15 play a crucial role in regulating oocyte maturation and embryo development. However, the study’s limitations, including sample size, protocol diversity and lack of long-term follow-up, highlight the need for further investigation. Addressing these limitations in future research could help validate the role of these molecules as biomarkers for improving IVF outcomes and contribute to more personalized treatment strategies in assisted reproductive technology.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by The Third Hospital of Hebei Medical University (W2021-007-1). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LC: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. JZ: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. DL: Investigation, Methodology, Project administration, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. PX: Data curation, Investigation, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. SL: Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. RG: Conceptualization, Investigation, Project administration, Validation, Writing – original draft, Writing – review & editing. XW: Formal analysis, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. LZ: Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen X, Giles J, Yao Y, Yip W, Meng Q, Berkman L, et al. The path to healthy ageing in China: a Peking University-Lancet Commission. Lancet. (2022) 400:1967–2006. doi: 10.1016/S0140-6736(22)01546-X
2. Tu X, You B, Jing M, Lin C, and Zhang R. Progestin-primed ovarian stimulation versus mild stimulation protocol in advanced age women with diminished ovarian reserve undergoing their first in vitro fertilization cycle: A retrospective cohort study. Front Endocrinol (Lausanne). (2022) 12:801026. doi: 10.3389/fendo.2021.801026
3. Rodriguez-Purata J, Gomez-Cuesta MJ, and Cervantes-Bravo E. Association of ovarian stimulation and embryonic aneuploidy in in vitro fertilization cycles with preimplantation genetic testing: A narrative systematic review. JBRA Assist Reprod. (2022) 26:348–61. doi: 10.5935/1518-0557.20210069
4. Alper MM and Fauser BC. Ovarian stimulation protocols for IVF: is more better than less? Reprod BioMed Online. (2017) 34:345–53. doi: 10.1016/j.rbmo.2017.01.010
5. Kong P, Yin M, Tang C, Zhu X, Bukulmez O, Chen M, et al. Effects of early cumulus cell removal on treatment outcomes in patients undergoing in vitro fertilization: A retrospective cohort study. Front Endocrinol (Lausanne). (2021) 12:669507. doi: 10.3389/fendo.2021.669507
6. Xie J, Xu X, and Liu S. Intercellular communication in the cumulus-oocyte complex during folliculogenesis: A review. Front Cell Dev Biol. (2023) 11:1087612. doi: 10.3389/fcell.2023.1087612
7. Cena H, Chiovato L, and Nappi RE. Obesity, polycystic ovary syndrome, and infertility: A new avenue for GLP-1 receptor agonists. J Clin Endocrinol Metab. (2020) 105:e2695–709. doi: 10.1210/clinem/dgaa285
8. Li R, Norman RJ, Armstrong DT, and Gilchrist RB. Oocyte-secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol Reprod. (2000) 63:839–45. doi: 10.1095/biolreprod63.3.839
9. Demiray SB, Goker ENT, Tavmergen E, Yilmaz O, Calimlioglu N, Soykam HO, et al. Differential gene expression analysis of human cumulus cells. Clin Exp Reprod Med. (2019) 46:76–86. doi: 10.5653/cerm.2019.46.2.76
10. Turathum B, Gao EM, and Chian RC. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells. (2021) 10:2292. doi: 10.3390/cells10092292
11. Aguila L, Treulen F, Therrien J, Felmer R, Valdivia M, and Smith LC. Oocyte selection for in vitro embryo production in bovine species: noninvasive approaches for new challenges of oocyte competence. Anim (Basel). (2020) 10:2196. doi: 10.3390/ani10122196
12. Liu MN, Zhang K, and Xu TM. The role of BMP15 and GDF9 in the pathogenesis of primary ovarian insufficiency. Hum Fertil (Camb). (2021) 24:325–32. doi: 10.1080/14647273.2019.1672107
13. Ovarian Stimulation TEGGO, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI†. Hum Reprod Open. (2020) 2020:hoaa009. doi: 10.1093/hropen/hoaa009
14. Zhu HB, Zhang ZH, Fadlalla E, Wang RX, Geng DF, and Liu RZ. Culturing surplus poor-quality embryos to blastocyst stage have positive predictive value of clinical pregnancy rate. Iran J Reprod Med. (2014) 12:609–16.
15. Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. (2011) 26:1270–83. doi: 10.1093/humrep/der037
16. Gardner DK, Lane M, Stevens J, Schlenker T, and Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. (2000) 73:1155–8. doi: 10.1016/s0015-0282(00)00518-5
17. Schmittgen TD and Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. (2008) 3:1101–8. doi: 10.1038/nprot.2008.73
18. Cadenas J, Pors SE, Kumar A, Kalra B, Kristensen SG, Andersen CY, et al. Concentrations of oocyte secreted GDF9 and BMP15 decrease with MII transition during human IVM. Reprod Biol Endocrinol. (2022) 20:126. doi: 10.1186/s12958-022-01000-6
19. Rossetti R, Ferrari I, Bestetti I, Moleri S, Brancati F, Petrone L, et al. Fundamental role of BMP15 in human ovarian folliculogenesis revealed by null and missense mutations associated with primary ovarian insufficiency. Hum Mutat. (2020) 41:983–97. doi: 10.1002/humu.23988
20. Montgomery GW. Genetic regulation of ovulation rate and multiple births. Reprod Fertil Dev. (2024) 36:RD24083. doi: 10.1071/RD24083
21. Belli M and Shimasaki S. Molecular aspects and clinical relevance of GDF9 and BMP15 in ovarian function. Vitam Horm. (2018) 107:317–48. doi: 10.1016/bs.vh.2017.12.003
22. Yang R, Guan Y, Perrot V, Ma J, and Li R. Comparison of the long-acting gnRH agonist follicular protocol with the gnRH antagonist protocol in women undergoing in vitro fertilization: A systematic review and meta-analysis. Adv Ther. (2021) 38:2027–37. doi: 10.1007/s12325-020-01612-7
23. Ahmad HI, Liu G, Jiang X, Edallew SG, Wassie T, Tesema B, et al. Maximum-likelihood approaches reveal signatures of positive selection in BMP15 and GDF9 genes modulating ovarian function in mammalian female fertility. Ecol Evol. (2017) 7:8895–902. doi: 10.1002/ece3.3336
24. Huang TH, Chen FR, Zhang YN, Chen SQ, Long FY, Wei JJ, et al. Decreased GDF9 and BMP15 in follicle fluid and granulosa cells and outcomes of IVF-ET among young patients with low prognosis. J Assist Reprod Genet. (2023) 40:567–76. doi: 10.1007/s10815-023-02723-0
25. Gong Y, Li-Ling J, Xiong D, Wei J, Zhong T, and Tan H. Age-related decline in the expression of GDF9 and BMP15 genes in follicle fluid and granulosa cells derived from poor ovarian responders. J Ovarian Res. (2021) 14:1. doi: 10.1186/s13048-020-00757-x
26. Sanfins A, Rodrigues P, and Albertini DF. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet. (2018) 35:1741–50. doi: 10.1007/s10815-018-1268-4
27. Kadoura S, Alhalabi M, and Nattouf AH. Conventional GnRH antagonist protocols versus long GnRH agonist protocol in IVF/ICSI cycles of polycystic ovary syndrome women: a systematic review and meta-analysis. Sci Rep. (2022) 12:4456. doi: 10.1038/s41598-022-08400-z
28. Lin G, Zhong X, Li S, Liu X, and Xu L. The clinical value of progestin-primed ovarian stimulation protocol for women with diminished ovarian reserve undergoing IVF/ICSI: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1232935. doi: 10.3389/fendo.2023.1232935
29. Deveci ŞD. Alterations in follicular fluid BMP-15 RNA expression in women undergoing controlled ovarian hyperstimulation. Turk J Med Sci. (2020) 50:1247–53. doi: 10.3906/sag-2002-208
30. Yang J, Zhang Y, Xu X, Li J, Yuan F, Bo S, et al. Transforming growth factor-β is involved in maintaining oocyte meiotic arrest by promoting natriuretic peptide type C expression in mouse granulosa cells. Cell Death Dis. (2019) 10:558. doi: 10.1038/s41419-019-1797-5
Keywords: oocyte-secreted factors, granulosa cells, oocyte maturation, controlled ovarian stimulation, embryo development
Citation: Cheng L, Zhang J, Li D, Xu P, Liu S, Guo R, Wang X and Zhang L (2025) The impact of different ovarian stimulation protocols on the expression levels of GDF-9 and BMP-15 in cumulus cells of follicles. Front. Endocrinol. 16:1572388. doi: 10.3389/fendo.2025.1572388
Received: 07 February 2025; Accepted: 18 April 2025;
Published: 04 June 2025.
Edited by:
Richard Ivell, University of Nottingham, United KingdomReviewed by:
Patricia Rodrigues, Lusofona University, PortugalMuhjah Falah, University of Kerbala, Iraq
Copyright © 2025 Cheng, Zhang, Li, Xu, Liu, Guo, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Cheng, Y2hlbmdsaWxpX2NsODhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Lili Cheng
Lili Cheng Jie Zhang2†
Jie Zhang2†