- 1Department of Endocrinology and Metabology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong First Medical University, Shandong Key Laboratory of Rheumatic Diseaseand Translational Medicine, Shandong Institute of Nephrology, Jinan, China
- 2College of First Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
Homocysteine (Hcy) is an important intermediate product in methionine metabolism which plays a key role in the methylation of DNA, RNA and proteins. High level of Hcy can induce endothelial cell damage, promote the release of inflammatory factors, stimulate oxidative stress and inhibit the fibrinolytic system. Numerous studies have confirmed the close relationship between hyperhomocysteinemia (HHcy) and the occurrence/development of various diseases such as cardiovascular diseases, neurological disorders, thrombotic diseases, and tumors. With the rising incidence of thyroid diseases, the relationship between Hcy and thyroid diseases has attracted widespread attention. It has been found that HHcy may be directly or indirectly associated with the development of hypothyroidism, but the findings with hyperthyroidism, chronic lymphocytic thyroiditis and reduced thyroid hormone sensitivity are controversial. This article reviews the research progress of Hcy and thyroid diseases, with a view to providing new ideas for the prevention and clinical treatment of diseases.
1 Introduction
In the 1930s, Nobel Prize-winning chemist Vincent du Vigneaud isolated homocysteine (Hcy) from bladder stones. Hcy is an intermediate metabolite of the methionine cycle which is a naturally occurring sulfur-containing amino acid with important roles in nucleic acid synthesis, DNA methylation, amino acid homeostasis, epigenetic maintenance, and redox processes. Normally, about 80% of Hcy is bound to plasma proteins such as albumin by disulfide bonds, and the rest of Hcy is mostly bound to thiols in the form of cystine or other forms, while only a small portion is free. Higher than normal level of Hcy in the blood is called hyperhomocysteinemia (HHcy). The Expert Consensus on the Diagnosis and Treatment of Hyperhomocysteinemia sets the diagnostic standard for HHcy at 10 umol/L. The consensus also points out that the median Hcy for adults in China is in the range of 13–14 umol/L, exceeding the diagnostic standard by more than 30% (1). With the gradual deepening understanding of Hcy, researchers have found that HHcy is closely related to the occurrence/development of cardiovascular diseases, stroke, diabetes mellitus complications, chronic kidney disease, pregnancy disorders, tumors and other diseases (1–3). Although the relationship between Hcy and thyroid diseases is not complete, changes in Hcy level are still considered by many scholars to be the cause or consequence of thyroid diseases. This article reviews the relationship between Hcy and thyroid diseases with the aim of providing new ideas for disease prevention, diagnosis and control.
2 Source and metabolism of Hcy
Hcy is a product of demethylation during the methionine cycle, which is the only source of Hcy. Hcy is metabolized mainly through the remethylation pathway and the transsulfuration pathway (4). The two pathways coexist and complement but cannot completely replace each other. Only when the two metabolic pathways are in balance can Hcy be kept within the normal range (Figure 1). (1) Remethylation can occur in cells of a variety of tissues. Hcy meets with the methyl group provided by 5-Methyltetrahydrofolic acid (derived from folic acid) or betaine to produce methionine catalyzed by methionine synthase (MS). (2) Transsulfuration which is the production of cysteine by Hcy with the assistance of vitamin B6, catalyzed by cystathionine-β-synthase (CBS). Cysteine can form disulfide bonds which have the effect of stabilizing the spatial structure of peptide chains.
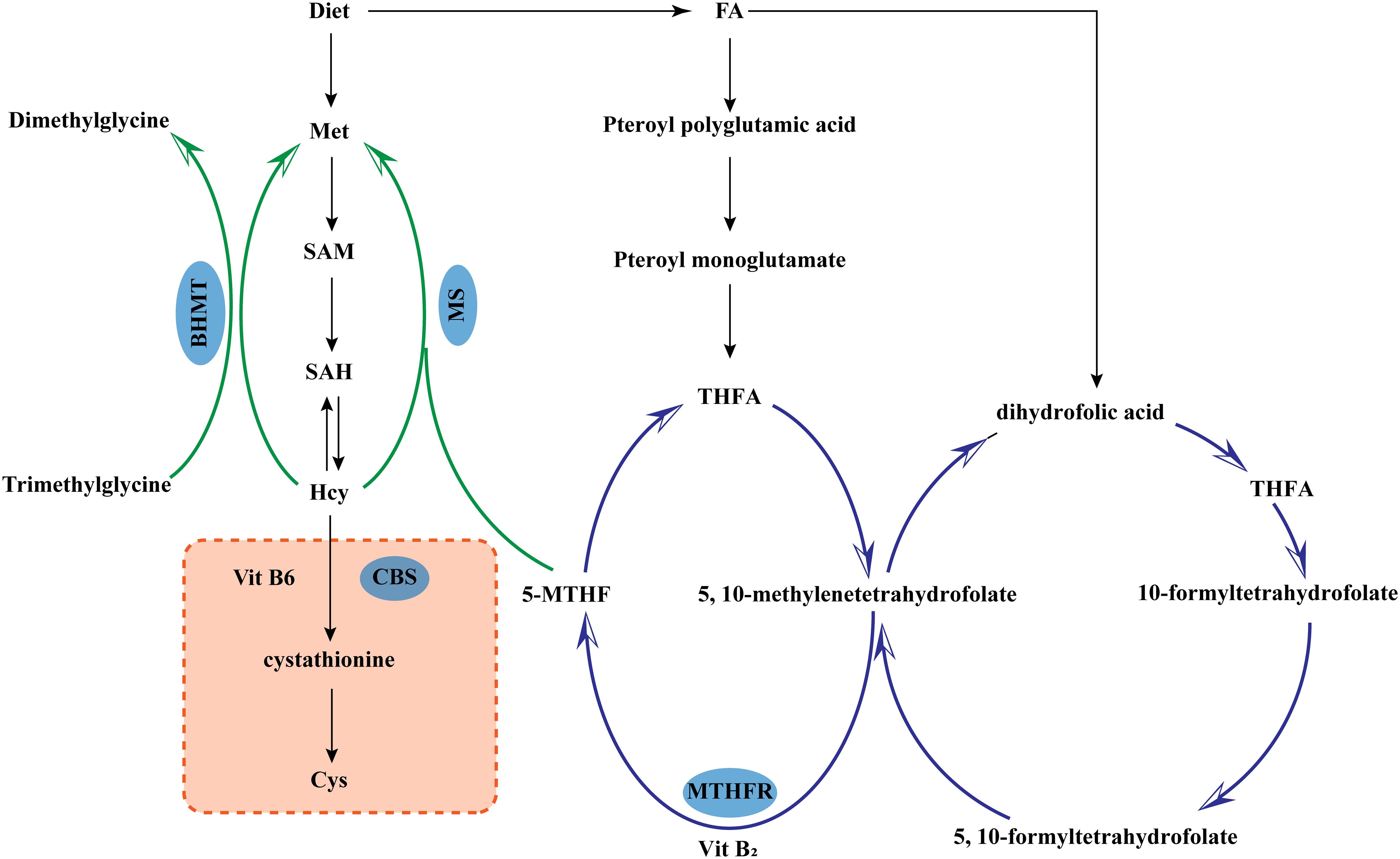
Figure 1. Metabolism of homocysteine. 5-MTHF, 5-Methyltetrahydrofolic acid; BHMT, Betaine-homocysteine methyltransferase; CBS, Cystathionine-β-synthase; Cys, L-Cysteine; FA, Folic acid; Hcy, Homocysteine; Met, Methionine; MS, Methionine synthase; SAM, S-adenosylmethionine; SAH, S-Adenosyl-L-homocysteine; MTHFR, Methylenetetrahydrofolate Reductase; THFA, Tetrahydro folic Acid.
3 Influencing factors of Hcy level
3.1 Genetic factors
Genetic errors or deficiencies in key enzymes of Hcy metabolism can lead to HHcy. Methylenetetrahydrofolate reductase (MTHFR) C677T mutation is the most common cause. The mutations can lead to a decrease in the activity and heat resistance of the enzyme, which in turn affects the cycling of folic acid (FA). It was found that in CT and TT populations, the activity of MTHFR was only 65% and 30% of that in CC populations. On the other hand, Hcy levels were increased by 20%-70% in TT populations compared to CC populations (5, 6) (Supplementary Table 1).
3.2 Age and gender
Conventional opinion is that Hcy level increases with age (7). But in recent years, studies have found that there is a trend towards a younger age for HHcy, which has become an independent risk factor for cardiovascular and cerebrovascular diseases in young and middle-aged people (8). Hcy levels are significantly higher in men than in women due to the important role of sex hormones in methionine metabolism (9).
3.3 Dietary and nutritional factors
Long-term consumption of protein leads to excessive intake of methionine which in turn raises Hcy level, while eating large amounts of vegetables and fruits can indirectly reduce Hcy level by increasing vitamin content. Bad lifestyles such as prolonged heavy drinking, coffee consumption or smoking can lead to elevated Hcy level by reducing intake or increasing consumption of B vitamins and FA.
(4) Diseases: Chronic kidney disease, severe anemia, hypothyroidism, malignancy and drugs that affect the absorption of B vitamins and FA have been shown to increase Hcy level (10).
4 Hcy and thyroid hormones
Thyroid hormone (TH) is a major regulator of cellular metabolism and maintains metabolic homeostasis by coordinating networks of carbohydrate, lipid and protein metabolism. In tissues, thyroid hormone action is mediated by transmembrane transporters, deiodinases and thyroid hormone receptors. The selenoprotein family of iodothyronine deiodinases consists of three enzymes, D1, D2 and D3, which are specifically distributed in tissues and regulate the activation and inactivation of TH: type 1 and 2 deiodinases (D1/D2) catalyse the conversion of the inactive precursor thyroxine (T4) to the active form, triiodothyronine (T3), while type 3 deiodinases (D3) terminate the hormone’s action by degradation (11, 12). Studies have shown that homocysteine (Hcy) can impair deiodinase activity through oxidative stress and inflammatory mechanisms. In addition, studies have highlighted a biochemical link between Hcy metabolism and glutamine metabolism that may be mediated by stress-related pathways affecting cellular function (13). The critical role of thyroid hormones (THs) in regulating glutamine metabolism has been demonstrated (14). Together, these findings suggest that THs may constitute an important metabolic regulatory network involved in stress response and cellular function through direct regulation of glutamine metabolism, which may in turn indirectly influence Hcy metabolic pathways.
5 Hcy and thyroid diseases
5.1 Hcy and thyroid nodules
In recent decades, with the widespread availability of ultrasound technology, thyroid nodules have become a common clinical disease. The prevalence of thyroid nodules with a diameter of more than 0.5 cm in Chinese adults is as high as 20.43% (15). Current studies have found that the development of thyroid nodules is associated with genetic factors, gender, age, iodine intake, autoimmunity and insulin resistance. However, Hcy and thyroid nodules have been less studied (16–19). By analyzing the serological indicators of 48 patients with thyroid nodules and 52 healthy people, Li et al. found that the Hcy levels of patients with thyroid nodules were significantly higher (P < 0.05) (16). Clinical studies have found a positive correlation between Hcy level and the prevalence of thyroid nodules, which is an independent risk factor for the development of thyroid nodules (19, 20). In patients with type 2 diabetes mellitus (T2DM), high level of Hcy increases the incidence of thyroid nodules by 1.055-1.475 times (17). The reason for this phenomenon may be related to the fact that Hcy affects cell methylation and regulates cell proliferation and apoptosis (21). On the other hand, Hcy is associated with autoimmune response and insulin resistance, which are also important etiological factors that induce thyroid nodules (22, 23). In conclusion, Hcy may be an important indicator for thyroid nodule surveillance. Thyroid ultrasound screening of patients with high level of Hcy may facilitate early detection of thyroid nodules.
5.2 Hcy and hyperthyroidism
Hyperthyroidism is a group of clinical syndromes in which increased thyroid hormones by the thyroid gland cause accelerated metabolism in the body. In a hypermetabolic state, the body releases large amount of reactive oxygen species (ROS), which can damage thyroid follicular cells, release intracellular antigens to activate the immune system and induce autoimmune deterioration. Nedrebø et al. reported no significant difference in Hcy levels between hyperthyroid patients and controls, whereas Demirbaş et al. found reduced serum Hcy levels in untreated hyperthyroid patients (24, 25). This difference may be attributed to increased renal clearance of Hcy due to increased glomerular filtration rate in hyperthyroid states. Another important factor is the regulation of several enzyme systems by thyroid hormones, which may affect the activity of enzymes involved in the Hcy metabolic pathway. Moreover, A study by Nechiporuk found that hyperthyroidism elevated the activities of CBS, cysteine dioxygenase (CDO), sulfite oxidase (SO) in brain and cysteine aminotransferase (CAT) in heart, which accelerated the process of transsulfuration (26). This seems to explain why researchers often discover the phenomenon that Hcy levels are reduced in patients with hyperthyroidism (27–31). Interestingly, findings regarding changes in Hcy levels before and after treatment for hyperthyroidism patients have not always been consistent. Studies by Pawilojc and Nedrebø both found that Hcy level gradually rebounded as the disease resolved (32, 33). On the contrary, in Colleran’s study, Hcy levels were further reduced in Graves’ disease patients after treatment with methimazole (34). Why it occurs is unclear and may be related to the immunomodulatory properties of methimazole as well as organismal immune recovery (35, 36). The results of current studies point to a reduction of Hcy level in patients with hyperthyroidism, but relationships between the condition and the change in Hcy level remain divergent and require more researchs.
5.3 Hcy and hypothyroidism
5.3.1 Hcy and clinical hypothyroidism
Elevated Hcy levels in patients with clinical hypothyroidism have been generally recognized by researchers at home and abroad (37–40), and some researchers have suggested that Hcy can be used as a diagnostic reference indicator for hypothyroidism. Impaired Hcy conversion due to decreased activity of metabolically key enzymes such as MTHFR and reduced absorption of nutrients from the gastrointestinal tract are the main reasons for elevated Hcy level (41, 42). On the other hand, lowered glomerular filtration rate or even possible immune complex nephritis that reduces Hcy clearance could also explain the elevated Hcy (43). Hcy is an important risk factor for cardiovascular diseases. The risk of developing coronary heart disease was found to increase by 40% for every 4 umol/L increase in Hcy (44). A study by Dang et al. found that elevated Hcy was associated with the development of myocardial injury and left ventricular hypoplasia in patients with hypothyroidism (37), which may be related to the activation of mitochondrial matrix metalloproteinases by Hcy. Hcy increases oxidized low-density lipoprotein (OxLDL) and causes endothelial cell dysfunction, which in turn accelerates arterial inflammation and fat deposition (39, 40, 45). From another perspective of lipid metabolism, Hcy inhibits the expression and function of apolipoprotein A-I to the extent that circulating high-density lipoprotein (HDL) level is reduced (46–49). Altogether, Hcy can participate in the atherosclerotic process through various mechanisms such as inducing mitochondrial dysfunction and promoting lipid peroxidation. This implies that patients with hypothyroidism who have a combination of elevated Hcy are at a substantially higher risk of cardiovascular diseases, and that attention to Hcy level can be useful in assessing disease risk. Hormone replacement therapy is a routine treatment strategy for patients with hypothyroidism. Several studies have confirmed that levothyroxine (L-T4) can reduce Hcy level (50). In addition, Catargi et al. reported lower levels of folic acid in hypothyroid patients and recommended supplementation of folic acid along with thyroid therapy (51). Amir Ziaee also noted that the combination of LT4 and folic acid is more effective in lowering serum homocysteine levels (52). Therefore, clinical hypothyroid patients should be actively tested with Hcy and treated in a timely manner.
5.3.2 Hcy and subclinical hypothyroidism
Only thyroid stimulating hormone (TSH) level is elevated, while free triiodothyronine (FT3) and free tetraiodothyronine (FT4) level remain within the normal range are important features of subclinical hypothyroidism (SCH). Epidemiological data showed that the global prevalence of SCH was approximately 1.3%-10%. The incidence of SCH increases progressively with age. The prevalence of SCH in the elder group can be as high as 5.7%-20% (53). Studies on the natural history of the disease suggest that SCH is more likely to progress to clinical hypothyroidism when combined with thyroid autoantibody positivity or TSH >10 mU/L, and thus SCH is also considered to be a precursor to clinical hypothyroidism.
Based on the correlation between Hcy and clinical hypothyroidism, more researchers have shifted their attention to the relationship between Hcy and SCH with a series of clinical studies (54–57), but the results of these studies are not the same. Aldasouqi et al. tested Hcy levels in 47 patients with SCH and did not find correlations (55). In another study, researchers divided SCH patients into two groups based on TSH levels and found that only SCH patients with TSH >10 mU/L had high Hcy levels (57). Data from Wang et al. suggested that regardless of the extent of SCH disease, Hcy levels were elevated in patients compared to the normal group (P < 0.05), and Hcy levels were higher in patients with severe disease compared to those with mild (P < 0.05) (54). A Meta-analysis of 12 observational studies conducted by Zhang showed that SCH patients had high Hcy levels compared to subjects with normal thyroid function (58). The study also pointed out that differences in testing techniques could affect research on the correlation between Hcy and subclinical hypothyroidism, which may also account for the different results of numerous studies. On the other hand, Hcy is also an independent risk factor for SCH which is involved in the occurrence and development of SCH (59). Thyroid peroxidase (TPO) is an autoantigen that catalyzes thyroid hormones (60, 61). Hcy increases the expression of thyroid peroxidase antibody (TPOAb), which inhibits thyroid function by decreasing TPO activity and exacerbates SCH (62).
In addition to exacerbating cardiovascular burden, Hcy is directly related to insulin resistance (IR) by inducing secretion and expression of resistin (63, 64). The prolonged high Hcy state of the body aggravates the risk of diabetes mellitus in SCH patients (65, 66). Considering that Hcy level is closely related to the severity and natural prognosis of SCH, some scholars believe that Hcy is a predictor of SCH patients’ prognosis. An observational study that included 104 patients with SCH found that Hcy level can predict the prognosis of SCH patients with area under curve (AUC) as high as 0.946 by ROC curve plotting (54). Hcy levels were positively correlated with SCH condition and SCH patients with HHcy had a higher risk of progressing to clinical hypothyroidism. Similar to the effect of the intervention in patients with clinical hypothyroidism, Hcy levels in patients with SCH can be significantly reduced after L-T4 replacement therapy (40).
Although the change of Hcy level in SCH patients remains controversial, high level of Hcy is often seen in SCH patients. Increased Hcy level can be both a consequence of SCH and a risk factor for SCH. The changes of Hcy level is associated with the development of other diseases such as cardiovascular disease and insulin resistance in patients with SCH, which can be used as a prognostic indicator for SCH. Hcy should be monitored dynamically in SCH patients.
5.3.3 Hcy and hypothyroidism in pregnancy
Data from epidemiological surveys showed the prevalence of clinical hypothyroidism in pregnancy was 0.3%-1.9% and the prevalence of subclinical hypothyroidism in pregnancy was 1.5%-5% (67, 68). While analysis of epidemiological data in China revealed that the prevalence of clinical hypothyroidism in pregnancy and subclinical hypothyroidism in pregnancy were respectively 1% and 5.27% (69, 70). Increased iodine demand, increased renal iodine clearance, compensatory enlargement of the thyroid gland and change in sex hormone are important causes of hypothyroidism in pregnancy (71, 72). Moreover, other studies have found that the activated immune system of the maternal body during pregnancy induces the production of TPOAb, which damages the thyroid gland and induces hypothyroidism (73). Hypothyroidism in pregnancy is not only strongly associated with many complications such as gestational hypertension and gestational diabetes mellitus, but also raises the risk of adverse pregnancy outcomes such as miscarriage, preterm labor, intrauterine growth and developmental abnormalities in the fetus (74–76).
Hcy levels in healthy pregnant women begin to decline in early stage and can fall to 50%-60% of pre-pregnancy levels by 20–32 weeks, remaining relatively stable or rising slightly in late pregnancy (77). This may be related to changes in hormones, increased blood volume, increased glomerular filtration rate, increased amino acid requirements of the pregnant woman and fetus during pregnancy. Low level of Hcy maintains the integrity and function of maternal vascular endothelial cells, which helps to regulate the adaptation to pregnancy. On the other hand, it also ensures the normal development of multiple organs or systems, including nervous system.
Several studies have found that Hcy levels are significantly elevated in women with hypothyroidism in pregnancy which is positively correlated with TSH levels (78–82). In addition to the reasons already analyzed before, the increased demand for vitamins and FA by pregnant women and changes in hormones are also key reasons for the rise in Hcy. A study by Zhang et al. found a significant negative correlation between folate level and Hcy level (83), which supported the idea that a relative deficiency of FA was responsible for elevated Hcy level. HHcy is strongly linked to adverse pregnancy outcomes through its engagement in vasculopathy processes such as placental vascular micro thrombosis and induction of abnormal fetal growth and development (80, 81, 84, 85). Meanwhile, HHcy promotes the release of inflammatory factors, stimulates oxidative stress and increases the maternal cardiovascular burden. Obvious improvements in thyroid function and Hcy were seen in hypothyroidism patients with pregnancy who were treated with L-T4 (86). However, its effect on pregnancy outcomes is uncertain. A study of infertile women with SCH showed that L-T4 treatment before and after assisted reproductive technology could reduce the rate of miscarriage and increase the rates of embryo implantation (87). A Meta-analysis by Rao et al. in 2018 found that L-T4 treatment only reduced the miscarriage rate, with no significant effect on the clinical pregnancy rate, live birth rate or preterm birth rate (88). This is probably related to the fact that only four randomized controlled trials were included in the study by Rao. The timing of thyroid function tests and LT4 treatment varied from the different studies.
Although there are conflicting views on the benefits of L-T4 supplementation in hypothyroidism patients with pregnancy, it is generally considered to be useful in reducing the rate of miscarriage. Women with hypothyroidism during pregnancy should be monitored for changes in Hcy, folic acid and vitamins.
5.4 Hcy and chronic lymphocytic thyroiditis
The incidence of CLT has been increasing with the widespread of screening tests. However, epidemiological data on CLT are still limited due to its atypical clinical symptoms in that a large number of patients who have not yet developed to thyroid function abnormalities are not diagnosed. There is a bidirectional link between Hcy and immune inflammation. In the first place, Hcy is an initiator of autoimmune and inflammatory, which induces the transcription of multiple cytokines and proinflammatory mediators. Alternatively, elevated Hcy is a consequence of immune and inflammation, which stimulates oxidative stress and increases antioxidant depletion.
The study by Cicone included patients with acute medical hypothyroidism and analyzed their Hcy levels. Hcy levels were found to be markedly higher in patients with Hashimoto’s thyroiditis than non-Hashimoto’s thyroiditis patients (89). Fewer studies have been reported on Hcy in CLT patients with thyroid dysfunction. Only a part of the studies has seen elevated Hcy levels in thyroglobulin antibody (TgAb)-positive or TPOAb-positive subjects (90). These findings suggest that alterations in Hcy levels of CLT patients may be related to autoimmunity. The concentration of TPOAb is directly tied to the degree of lymphocyte infiltration within the thyroid gland (91, 92). Even if the disease has not progressed to thyroid dysfunction, the presence of TPOAb can influence Hcy level through constantly stimulating the immune-inflammatory system. Previous studies have found that thyroid autoantibodies are associated with nutrients such as FA and vitamins (90, 93), so some researchers have also hypothesized that the relationship between thyroid autoantibodies and Hcy may be mediated by FA and vitamins. Similar to the findings in SCH, elevated Hcy can damage the thyroid gland by enhancing the activity of TPOAb. At the same time, higher Hcy level is directly related to the risk of several diseases, including cardiovascular diseases (94).
There are still no precise conclusions about the relationship between Hcy and CLT without thyroid dysfunction, which still needs to be confirmed by large sample and mechanistic studies.
5.5 Hcy and thyroid neoplasms
Thyroid cancer is the most common tumor of the endocrine system. The new incidence rate of thyroid cancer in China ranked the third highest among all cancer types in 2022 (95). The study of the pathogenesis of thyroid cancer has been the focus of many researchers. Hcy accumulated in the body synthesizes methionine through the remethylation pathway, which provides methyl for methylation of DNA, RNA and proteins. DNA methylation has been found to be an important contributor to the increased risk of thyroid cancer (96). In addition to affecting DNA methylation, high levels of Hcy upregulate histone H3K79Hcy, which regulates the expression levels of BMP7, CTNNB1, GLI2, NOTCH1, and RXRA genes (97). High expression of these genes has been reported to be associated with the development and progression of thyroid cancer (98–102). In addition, polymorphisms in the MTHFR gene, a key enzyme involved in folate metabolism, increase the risk of thyroid cancer (103). On the contrary, rapid proliferation of metabolic tumor cells increases the consumption of folic acid and vitamins, and the lack of folic acid and vitamins in the body will cause the elevation of Hcy, resulting in a vicious circle. Hcy can activate immunoinflammation, and immunoinflammation induces the development and progression of various tumors, so the specific mechanism of the role of Hcy in the development and progression of thyroid cancer needs to be further researched.
5.6 Hcy and reduced thyroid hormone sensitivity
Reduced responsiveness of tissue cells to thyroid hormones is seen in some normal populations in clinical work, with the coexistence of high FT4 combined with high TSH as the main manifestation, which may be related to impaired thyroid hormone sensitivity and can further develop into thyroid dysfunction (hyperthyroidism or hypothyroidism). A study by Ding et al. revealed for the first time that impaired thyroid hormone sensitivity can lead to elevated Hcy levels, and that reduced thyroid hormone sensitivity increases the risk of atherosclerosis, even during phases of normal thyroid function (104).
Thyroid hormones are involved in maintaining the activity of riboflavin metabolizing enzymes (especially riboflavin kinase). Flavin adenine dinucleotide is a cofactor of MTHFR, so thyroid hormones can influence the process of Hcy metabolism (105). When tissue thyroid hormone sensitivity is impaired, MTHFR activity is limited, which in turn increases Hcy levels. On the other hand, high levels of Hcy can regulate the expression of thyroid-related genes in the brain and thus affect the thyroid hormone network, further exacerbating thyroid hormone resistance (81).
The relationship between thyroid function and Hcy is not described in detail in this article because thyroid disorders are often combined with abnormal thyroid hormone sensitivity, the results of studies on the relationship between thyroid function and Hcy are conflicting, and we believe that the association between the two needs to be considered individually. The relationship between thyroid hormone sensitivity and Hcy needs to be confirmed by studies with large sample data.
6 Conclusion
There is a bidirectional link between thyroid disease and Hcy levels. Thyroid diseases tend to cause changes in Hcy levels, which in turn exacerbate the progression of the disease. High levels of Hcy have been found to be associated with the incidence of thyroid nodules, hypothyroidism, and thyroid cancer; hypothyroidism in turn can cause patients’ Hcy levels to rise further, increasing the risk of cardiovascular and cerebral vascular disease, forming a vicious cycle; while Hcy levels commonly fall in patients with hyperthyroidism. Clinically, thyroid function and ultrasound should be performed in a timely manner in people with changes in Hcy levels for early detection of disease and intervention. Further experimental and clinical studies are needed to confirm the role and biological mechanism of Hcy in the development of uncomplicated thyroid dysfunction CLT and thyroid cancer.
Author contributions
LC: Writing – original draft. FW: Writing – original draft. CL: Investigation, Writing – original draft. FL: Methodology, Writing – original draft. HW: Writing – review & editing. JZ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the National Natural Science Foundation of China [grant number 82303031], Shandong Provincial Natural Science Foundation of China Grants [grant number ZR2019PH025], Projects of medical and health technology development program in Shandong province [grant number 2016WS0499], Bethune Public Welfare Foundation [grant number Z04JKM2022E036].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1572997/full#supplementary-material
References
1. Kong J. The expert consensus on the diagnosis and treatment of hyperhomocysteinemia. J Electronic J Oncol Metab Nutr. (2020) 7:283–8. doi: 10.16689/j.cnki.cn11-9349/r.2020.03.007
2. Schaffer A, Verdoia M, Cassetti E, Marino P, Suryapranata H, and De Luca G. Relationship between homocysteine and coronary artery disease. Results from a large prospective cohort study. Thromb Res. (2014) 134:288–93. doi: 10.1016/j.thromres.2014.05.025
3. Shah H, Jan MU, Altaf A, and Salahudin M. Correlation of hyper-homocysteinemia with coronary artery disease in absence of conventional risk factors among young adults. J Saudi Heart Assoc. (2018) 30:305–10. doi: 10.1016/j.jsha.2018.04.002
4. Azzini E, Ruggeri S, and Polito A. Homocysteine: its possible emerging role in at-risk population groups. Int J Mol Sci. (2020) 21(4):1421. doi: 10.3390/ijms21041421
5. Crider KS, Yang TP, Berry RJ, and Bailey LB. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv Nutr (Bethesda Md). (2012) 3:21–38. doi: 10.3945/an.111.000992
6. Iacobazzi V, Infantino V, Castegna A, and Andria G. Hyperhomocysteinemia: related genetic diseases and congenital defects, abnormal DNA methylation and newborn screening issues. Mol Genet Metab. (2014) 113:27–33. doi: 10.1016/j.ymgme.2014.07.016
7. Morris MS, Jacques PF, Selhub J, and Rosenberg IH. Total homocysteine and estrogen status indicators in the third national health and nutrition examination survey. Am J Epidemiol. (2000) 152:140–8. doi: 10.1093/aje/152.2.140
8. Zhang H and Yan F. Correlation between homocysteine level and ischemic stroke. J J Southeast Univ (Medical Edition). (2019) 38:77–80.
9. Zha K, Ge SH, Cai J, Zhou Y, Ying R, Gu T, et al. Thyroid function and blood uric acid correlation with hyperhomocysteinemia in patients with type 2 diabetes mellitus. J Chin J Diabetes Mellitus. (2023) 31:688–92.
10. Cao X. Analysis of triggers of hyperhomocysteinemia and its pathophysiological significance. J Smart Health. (2020) 5:47–8. doi: 10.19335/j.cnki.2096-1219.2020.15.024
11. Cicatiello AG, Di Girolamo D, and Dentice M. Metabolic effects of the intracellular regulation of thyroid hormone: old players, new concepts. Front Endocrinol (Lausanne). (2018) :474. doi: 10.3389/fendo.2018.00474
12. McAninch EA and Bianco AC. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann N Y Acad Sci. (2014) 1311:77–87. doi: 10.1111/nyas.12374
13. Tchantchou F, Miller C, Goodfellow M, Puche A, and Fiskum G. Hypobaria-induced oxidative stress facilitates homocysteine transsulfuration and promotes glutathione oxidation in rats with mild traumatic brain injury. J Cent Nerv Syst Dis. (2021) 13:1179573520988193. doi: 10.1177/1179573520988193
14. Cicatiello AG, Sagliocchi S, Nappi A, Di Cicco E, Miro C, Murolo M, et al. Thyroid hormone regulates glutamine metabolism and anaplerotic fluxes by inducing mitochondrial glutamate aminotransferase GPT2. Cell Rep. (2022) 38:110562. doi: 10.1016/j.celrep.2022.110562
15. Chinese Society of Endocrinology, Chinese Society of Surgery, Thyroid and Metabolic Surgery Group, Head and Neck Tumor Committee of the Chinese Anti-Cancer Society, Chinese Society of Nuclear Medicine, Thyroid Cancer Committee of the Chinese Anti-Cancer Society, Thyroid Surgery Committee of the Surgeons’ Branch of the Chinese Physicians’ Association, et al. Guidelines on the diagnosis and treatment of thyroid nodules and differentiated thyroid cancer (2nd ed.). J Chinese Endocrinol Metab. (2023) 39:181–226.
16. Li X, Sun T, Feng Y, Niu L, Xie X, An Y, et al. Factors contributing to the development of thyroid nodules and the association of hcy with agr and thyroid autoantibodies in adults. J Basic Med Clinics. (2024) 44:1133–6. doi: 10.16352/j.issn.1001-6325.2024.08.1133
17. Gao X and Ye S. Correlation analysis of serum hcy and cysc and met levels with the occurrence of thyroid nodules in patients with type 2 diabetes mellitus. J J Trop Med. (2024) 24:551–5.
18. Chang X, Zhang Y, Liu J, Miao S, and Liu J. Correlation between hhcy, Hua and the prevalence of thyroid nodules in Han Chinese middle-aged and elderly women with hypothyroidism in Wuwei City, Gansu Province, China. J J Lanzhou Univ (Medical Edition). (2023) 49:57–62. doi: 10.13885/j.issn.1000-2812.2023.09.010
19. Chen X, Zhu W, and Ye S. Correlation between hyperhomocysteinemia and the occurrence of thyroid nodules in adults. J China Endemic Dis Control. (2021) 36:495–8.
20. Qiu X and Zhang Z. Clinical analysis of the relationship between homocysteine and thyroid nodules. J Fujian Med J. (2018) 40:81–3.
21. Hasan T, Arora R, Bansal AK, Bhattacharya R, Sharma GS, and Singh LR. Disturbed homocysteine metabolism is associated with cancer. Exp Mol Med. (2019) 51:1–13. doi: 10.1038/s12276-019-0216-4
22. Wang J. Relationship between uric acid and homocysteine and insulin resistance in patients with new-onset type 2 diabetes mellitus. J Jilin Med. (2020) 41:2999–3001.
23. Yin L and Xiao W. Hyperhomocysteinemia and inflammatory response diseases. J Int J Lab Med. (2020) 41:228–32.
24. Nedrebø BG, Ericsson UB, Nygård O, Refsum H, Ueland PM, Aakvaag A, et al. Plasma total homocysteine levels in hyperthyroid and hypothyroid patients. Metabolism. (1998) 47:89–93. doi: 10.1016/s0026-0495(98)90198-6
25. Demirbas B, Ozkaya M, Cakal E, Culha C, Gulcelik N, Koc G, et al. Plasma homocysteine levels in hyperthyroid patients. Endocr J. (2004) 51:121–5. doi: 10.1507/endocrj.51.121
26. Nechiporuk V, Mel’nyk A, Korda M, Pentiuk N, and Kachula S. Influence of chronic hyperhomocysteinemia on metabolism of sulfur containing amino acids in the rats’ Heart and brain on the background of hyperthyreosis and hypothyreosis. Georgian Med News. (2019) 295):127–32.
27. Yang F, Dan Z, Gao S, Liu X, He L, and Yang L. Expression and correlation of Il-8, lipocalin and Hcy in patients with t2dm combined with hyperthyroidism. J Label Immunoassay Clinics. (2023) 30:615–9+56.
28. Xie X and Wang M. The value of serum tsh and hcy monitoring in the diagnosis of thyroid dysfunction. J China Urban Rural Enterprise Hyg. (2023) 38:141–2. doi: 10.16286/j.1003-5052.2023.11.053
29. Orzechowska-Pawilojc A, Sworczak K, Lewczuk A, and Babinska A. Homocysteine, Folate and Cobalamin Levels in Hypothyroid Women before and after Treatment. Endocr J. (2007) 54:471–6. doi: 10.1507/endocrj.k06-112
30. Nedrebø BG, Nygård O, Ueland PM, and Lien EA. Plasma total homocysteine in hyper- and hypothyroid patients before and during 12 months of treatment. Clin Chem. (2001) 47:1738–41. doi: 10.1093/clinchem/47.9.1738
31. Diekman MJ, van der Put NM, Blom HJ, Tijssen JG, and Wiersinga WM. Determinants of changes in plasma homocysteine in hyperthyroidism and hypothyroidism. Clin Endocrinol. (2001) 54:197–204. doi: 10.1046/j.1365-2265.2001.01170.x
32. Orzechowska-Pawilojc A, Siekierska-Hellmann M, Syrenicz A, and Sworczak K. Homocysteine, Folate, and Cobalamin Levels in Hyperthyroid Women before and after Treatment. Endokrynol Polska. (2009) 60:443–8.
33. Nedrebø BG, Hustad S, Schneede J, Ueland PM, Vollset SE, Holm PI, et al. Homocysteine and Its Relation to B-Vitamins in Graves’ Disease before and after Treatment: Effect Modification by Smoking. J Internal Med. (2003) 254:504–12. doi: 10.1046/j.1365-2796.2003.01222.x
34. Colleran KM, Romero LA, Upton DA, and Burge MR. Methimazole-induced hypothyroidism paradoxically decreases homocysteine. Metab: Clin Exp. (2005) 54:460–5. doi: 10.1016/j.metabol.2004.10.013
35. Bandyopadhyay U, Biswas K, and Banerjee RK. Extrathyroidal actions of antithyroid thionamides. Toxicol Lett. (2002) 128:117–27. doi: 10.1016/s0378-4274(01)00539-2
36. Lechpammer M, Lukac J, Lechpammer S, and Kusić Z. Antithyroid drug-induced immunomodulation in graves’ Disease patients. Acta Med Croatica: casopis Hravatske akademije medicinskih znanosti. (2002) 56:21–6.
37. Dang Q and Kang J. Relationship between serum 25 hydroxyvitamin d and hcy levels and left ventricular function in hypothyroid patients. J Hebei Med. (2021) 27:1440–3.
38. Jiang X. Homocysteine and thyroid-stimulating hormone for the diagnosis of hypothyroidism. J China Health Standard Manag. (2019) 10:99–101.
39. Kumar Singh N, Suri A, Kumari M, and Kaushik P. A study on serum homocysteine and oxidized ldl as markers of cardiovascular risk in patients with overt hypothyroidism. Hormone Mol Biol Clin Invest. (2022) 43:329–35. doi: 10.1515/hmbci-2021-0029
40. Dong X, Yao Z, Hu Y, Yang N, Gao X, Xu Y, et al. Potential harmful correlation between homocysteine and low-density lipoprotein cholesterol in patients with hypothyroidism. Medicine. (2016) 95:e4291. doi: 10.1097/md.0000000000004291
41. Avila MA, Berasain C, Prieto J, Mato JM, García-Trevijano ER, and Corrales FJ. Influence of impaired liver methionine metabolism on the development of vascular disease and inflammation. Curr Medicinal Chem Cardiovasc Hematol Agents. (2005) 3:267–81. doi: 10.2174/1568016054368197
42. Nechyporuk V, Korda M, Pentiuk L, Dmytrenko I, and Bulko I. The impact of B vitamins on the functioning of methylation cycle in the liver and the kidneys of hyper- and hypothyroid rats. Polski merkuriusz lekarski: Organ Polskiego Towarzystwa Lekarskiego. (2020) 48:55–9.
43. de Montmollin M, Feller M, Beglinger S, McConnachie A, Aujesky D, Collet TH, et al. L-thyroxine therapy for older adults with subclinical hypothyroidism and hypothyroid symptoms: secondary analysis of a randomized trial. Ann Internal Med. (2020) 172:709–16. doi: 10.7326/m19-3193
44. Boushey CJ, Beresford SA, Omenn GS, and Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. Jama. (1995) 274:1049–57. doi: 10.1001/jama.1995.03530130055028
45. Wald DS, Law M, and Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ (Clinical Res ed). (2002) 325:1202. doi: 10.1136/bmj.325.7374.1202
46. Yang N, Yao Z, Miao L, Liu J, Gao X, Xu Y, et al. Homocysteine diminishes apolipoprotein a-I function and expression in patients with hypothyroidism: A cross-sectional study. Lipids Health Dis. (2016) 15:123. doi: 10.1186/s12944-016-0293-5
47. Miyazaki A, Sagae N, Usami Y, Sato M, Kameda T, Yoshimoto A, et al. N-Homocysteinylation of Apolipoprotein a-I Impairs the Protein’s Antioxidant Ability but Not Its Cholesterol Efflux Capacity. Biol Chem. (2014) 395:641–8. doi: 10.1515/hsz-2013-0262
48. Liao D, Tan H, Hui R, Li Z, Jiang X, Gaubatz J, et al. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein a-I protein synthesis and enhancing hdl cholesterol clearance. Circ Res. (2006) 99:598–606. doi: 10.1161/01.Res.0000242559.42077.22
49. Mikael LG, Genest J Jr., and Rozen R. Elevated homocysteine reduces apolipoprotein a-I expression in hyperhomocysteinemic mice and in males with coronary artery disease. Circ Res. (2006) 98:564–71. doi: 10.1161/01.RES.0000204825.66410.0b
50. Han L, Ma Y, Liang Z, and Chen D. Laboratory characteristics analysis of the efficacy of levothyroxine on subclinical hypothyroidism during pregnancy: A single-center retrospective study. Bioengineered. (2021) 12:4183–90. doi: 10.1080/21655979.2021.1955589
51. Catargi B, Parrot-Roulaud F, Cochet C, Ducassou D, Roger P, and Tabarin A. Homocysteine, hypothyroidism, and effect of thyroid hormone replacement. Thyroid. (1999) 9:1163–6. doi: 10.1089/thy.1999.9.1163
52. Ziaee A, Hajibagher Tehrani N, Hosseinkhani Z, Kazemifar A, Javadi A, and Karimzadeh T. Effects of folic acid plus levothyroxine on serum homocysteine level in hypothyroidism. Caspian J Intern Med. (2012) 3:417–20.
53. Biondi B, Cappola AR, and Cooper DS. Subclinical hypothyroidism: A review. Jama. (2019) 322:153–60. doi: 10.1001/jama.2019.9052
54. Wang Y, Zhou F, and Yi Z. Serum hcy, D-D, Fib, Pai-1 and t-Pa levels and clinical significance in Sch patients. J J Mol Diagnosis Ther. (2022) 14(11):1946–9. doi: 10.19930/j.cnki.jmdt.2022.11.040
55. Aldasouqi S, Nkansa-Dwamena D, Bokhari S, Alzahrani AS, Khan M, Al-Reffi A, et al. Is subclinical hypothyroidism associated with hyperhomocysteinemia? Endocr Pract: Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. (2004) 10:399–403. doi: 10.4158/ep.10.5.399
56. Xiong L, Luo C, Liu Y, Wu Y, and Wen Y. Changes of plasma homocysteine and peripheral arterial stiffness in patients with subclinical hypothyroidism. J West China Med. (2015) 30:2205–7.
57. Yin D, Tan Y, Zhang Y, Zhu C, Jin X, and Gao Y. Clinical study on the influence of subclinical hypothyroidism on cardiovascular diseases. J Chongqing Med. (2015) 44:4227–8+31.
58. Zhang SF, Li LZ, Zhang W, Guo JR, Liu FF, Ma K, et al. Association between plasma homocysteine levels and subclinical hypothyroidism in adult subjects: A meta-analysis. Hormone Metab Res Hormon und Stoffwechselforschung Hormones Metabolisme. (2020) 52:625–38. doi: 10.1055/a-1199-2633
59. Zhao L, Liang T, Li X, Liao Z, and Zhang H. Study on the correlation between cerebral infarction combined with subclinical hypothyroidism and serum thyroid-stimulating hormone, homocysteine and lipoprotein a. J Clin Military Med J. (2024) 52:712–4. doi: 10.16680/j.1671-3826.2024.07.14
60. Zhang X, Xu S, Wang M, Zhu L, Yuan N, Xue J, et al. Expression and correlation of serum tsh Mpo ferritin in hypothyroidism. J Hebei Med. (2022) 28:619–24.
61. Peng G, Qiu M, and Lin R. Changes in serum igf-1 Igfbp-3 and hcy levels in children with congenital hypothyroidism and their relationship with physical development. J Hebei Med. (2022) 28:1479–84.
62. Yin G, Ma R, and Pan J. Diagnostic value of total cholesterol and homocysteine in hypothyroidism. J Chin Foreign Med Res. (2024) 22:73–6. doi: 10.14033/j.cnki.cfmr.2024.03.019
63. Schachter M, Raziel A, Friedler S, Strassburger D, Bern O, and Ron-El R. Insulin resistance in patients with polycystic ovary syndrome is associated with elevated plasma homocysteine. Hum Reprod (Oxford England). (2003) 18:721–7. doi: 10.1093/humrep/deg190
64. Li Y, Jiang C, Xu G, Wang N, Zhu Y, Tang C, et al. Homocysteine upregulates resistin production from adipocytes in vivo and in vitro. Diabetes. (2008) 57:817–27. doi: 10.2337/db07-0617
65. Ebrahimpour A, Vaghari-Tabari M, Qujeq D, Moein S, and Moazezi Z. Direct correlation between serum homocysteine level and insulin resistance index in patients with subclinical hypothyroidism: does subclinical hypothyroidism increase the risk of diabetes and cardio vascular disease together? Diabetes Metab Syndr. (2018) 12:863–7. doi: 10.1016/j.dsx.2018.05.002
66. Yang N, Yao Z, Miao L, Liu J, Gao X, Fan H, et al. Novel clinical evidence of an association between homocysteine and insulin resistance in patients with hypothyroidism or subclinical hypothyroidism. PloS One. (2015) 10:e0125922. doi: 10.1371/journal.pone.0125922
67. Lee SY. Subclinical hypothyroidism in pregnancy may have long-term effects on metabolic parameters. J Clin Endocrinol Metab. (2020) 105:e2628–9. doi: 10.1210/clinem/dgaa198
68. Li N, Yang J, Chen X, Huang J, Lai M, Fang F, et al. Postpartum follow-up of patients with subclinical hypothyroidism during pregnancy. Thyroid: Off J Am Thyroid Assoc. (2020) 30:1566–73. doi: 10.1089/thy.2019.0714
69. Li P, Cui JH, Li L, Chen XJ, Ouyang LP, Fan JH, et al. Relationship between thyrotropin compliance and the development of gestational diabetes mellitus in hypothyroid women during early pregnancy. J Chin J Diabetes Mellitus. (2021) 13:1075–80.
70. Jia S, Wang Y, Li T, Yang H, Ding W, Kang Y, et al. Observations on the outcome of hypothyroidism in pregnant women after 10 years. J China Endemic Dis Control. (2021) 36:101–3.
71. Wu M, Chi C, Yang Y, Guo S, Li T, Gu M, et al. Dynamics of gut microbiota during pregnancy in women with tpoab-positive subclinical hypothyroidism: A prospective cohort study. BMC Pregnancy Childbirth. (2022) 22:592. doi: 10.1186/s12884-022-04923-5
72. Wang S, Liu X, and Yang C. Relationship between serum 25 hydroxyvitamin d expression and pregnancy outcome in pregnant women with gestational diabetes mellitus combined with hypothyroidism. J Chin J Eugenics Genet. (2021) 29:359–62. doi: 10.13404/j.cnki.cjbhh.20210810.004
73. Yang Y, Liu R, and Yin H. Impact of tpo-Ab-positive subclinical hypothyroidism on pregnancy outcome. J Chin Clin J Obstet Gynecol. (2021) 22:108–10. doi: 10.13390/j.issn.1672-1861.2021.01.045
74. Ceyhan ST, Beyan C, Atay V, Yaman H, Alanbay I, Kaptan K, et al. Serum vitamin B12 and homocysteine levels in pregnant women with neural tube defect. Gynecol Endocrinol: Off J Int Soc Gynecol Endocrinol. (2010) 26:578–81. doi: 10.3109/09513591003632183
75. Dhobale M, Chavan P, Kulkarni A, Mehendale S, Pisal H, and Joshi S. Reduced folate, increased vitamin B(12) and homocysteine concentrations in women delivering preterm. Ann Nutr Metab. (2012) 61:7–14. doi: 10.1159/000338473
76. Lindblad B, Zaman S, Malik A, Martin H, Ekström AM, Amu S, et al. Folate, vitamin B12, and homocysteine levels in South Asian women with growth-retarded fetuses. Acta Obstet Gynecol Scand. (2005) 84:1055–61. doi: 10.1111/j.0001-6349.2005.00876.x
77. Luo C, Wan B, Wu X, and Liu G. Investigation of serum homocysteine levels in normal pregnant women. J Lab Med. (2014) 29:723–6.
78. Yan LL, Yang ZY, Teng Y, and Wang H. Changes in serum tsh, Hcy, 25(Oh)D3 levels and clinical significance in patients with hypothyroidism in pregnancy. Changes in serum tsh, Hcy, 25(Oh)D3 levels and clinical significance in patients with hypothyroidism combined with pregnancy. J J Mol Diagnosis Ther. (2024) 16:881–4+9. doi: 10.19930/j.cnki.jmdt.2024.05.020
79. Zhang L, Deng M, Liao N, and Liu J. Correlation analysis of folic acid utilization capacity with serum homocysteine and vitamin b_(12) in pregnant women with subclinical hypothyroidism during pregnancy. J Pract Gynecol Endocrinol Electronic J. (2022) 9:5–7.
80. Wei Z, Zhao Z, Meng T, Ye Q, Wan Y, and Liu Y. Changes in serum vitamin b12 and Hcy levels in patients with hypothyroidism during pregnancy and their effects on pregnancy outcome. J Chin J Family Planning. (2022) 30:1720–5.
81. Gu YH, Zhang Q, Guo J, Wang F, Bao Y, Qiu Z, et al. Higher serum homocysteine and lower thyroid hormone levels in pregnant women are associated with neural tube defects. J Trace Elements Med Biol: Organ Soc Minerals Trace Elements (GMS). (2021) 68:126814. doi: 10.1016/j.jtemb.2021.126814
82. Barjaktarovic M, Steegers EAP, Jaddoe VWV, de Rijke YB, Visser TJ, Korevaar TIM, et al. The association of thyroid function with maternal and neonatal homocysteine concentrations. J Clin Endocrinol Metab. (2017) 102:4548–56. doi: 10.1210/jc.2017-01362
83. Zhang D, Zhou M, Wu D, and Wang K. Correlation between serum folate and Hcy levels and thyroid function in patients with gestational sch. J Chin Prescript Drugs. (2021) 19:158–9.
84. Ma Z, Lin T, and Chen Q. Analysis of serum hcy and tpo-Ab levels in relation to pregnancy outcome in patients with subclinical hypothyroidism during pregnancy. J Chin Prescript Drugs. (2023) 21:179–82.
85. Lin Q, Huang Z, and Chen W. Effect of serum hcy and tpo-Ab levels on pregnancy outcome in patients with subclinical hypothyroidism during pregnancy. J Chin Foreign Med Res. (2022) 20:165–9. doi: 10.14033/j.cnki.cfmr.2022.13.044
86. Jiang S, Hua F, and Wei YB. Serum ferritin and homocysteine levels in pregnant women with subclinical hypothyroidism during pregnancy and the effect of levothyroxine replacement therapy. J Chin J Family Plann. (2023) 31:358–61+65.
87. Abdel Rahman AH, Aly Abbassy H, and Abbassy AA. Improved in vitro fertilization outcomes after treatment of subclinical hypothyroidism in infertile women. Endocr Pract: Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. (2010) 16:792–7. doi: 10.4158/ep09365.Or
88. Rao M, Zeng Z, Zhao S, and Tang L. Effect of levothyroxine supplementation on pregnancy outcomes in women with subclinical hypothyroidism and thyroid autoimmuneity undergoing in vitro fertilization/intracytoplasmic sperm injection: an updated meta-analysis of randomized controlled trials. Reprod Biol Endocrinol: RB&E. (2018) 16:92. doi: 10.1186/s12958-018-0410-6
89. Cicone F, Santaguida MG, My G, Mancuso G, Papa A, Persechino R, et al. Hyperhomocysteinemia in acute iatrogenic hypothyroidism: the relevance of thyroid autoimmunity. J Endocrinol Invest. (2018) 41:831–7. doi: 10.1007/s40618-017-0811-y
90. Wang YP, Lin HP, Chen HM, Kuo YS, Lang MJ, and Sun A. Hemoglobin, iron, and vitamin B12 deficiencies and high blood homocysteine levels in patients with anti-thyroid autoantibodies. J Formosan Med Assoc Taiwan yi zhi. (2014) 113:155–60. doi: 10.1016/j.jfma.2012.04.003
91. Mikoś H, Mikoś M, Obara-Moszyńska M, and Niedziela M. The role of the immune system and cytokines involved in the pathogenesis of autoimmune thyroid disease (Aitd). Endokrynol Polska. (2014) 65:150–5. doi: 10.5603/ep.2014.0021
92. Czarnocka B, Janota-Bzowski M, McIntosh RS, Asghar MS, Watson PF, Kemp EH, et al. Immunoglobulin G kappa antithyroid peroxidase antibodies in Hashimoto’s thyroiditis: epitope-mapping analysis. J Clin Endocrinol Metab. (1997) 82:2639–44. doi: 10.1210/jcem.82.8.4124
93. Caplan RH, Davis K, Bengston B, and Smith MJ. Serum folate and vitamin B12 levels in hypothyroid and hyperthyroid patients. Arch Internal Med. (1975) 135:701–4. doi: 10.1001/archinte.1975.00330050075013
94. An J, Wang X, Ren J, Na R, Wu X, Wang S, et al. Effects of 25-hydroxyvitamin d_3, low-density lipoprotein cholesterol, and homocysteine on carotid artery plaque stability in patients with Hashimoto’s thyroiditis. J J Vasc Endoluminal Vasc Surg. (2024) 10:667–71. doi: 10.19418/j.cnki.issn2096-0646.2024.06.06
95. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
96. Ozdemir S, Silan F, Hasbek Z, Uludag A, Atik S, Erselcan T, et al. Increased T-allele frequency of 677 C>T polymorphism in the methylenetetrahydrofolate reductase gene in differentiated thyroid carcinoma. Genet Test Mol Biomarkers. (2012) 16:780–4. doi: 10.1089/gtmb.2011.0347
97. Zhang Q, Bai B, Mei X, Wan C, Cao H, Dan L, et al. Elevated H3k79 homocysteinylation causes abnormal gene expression during neural development and subsequent neural tube defects. Nat Commun. (2018) 9:3436. doi: 10.1038/s41467-018-05451-7
98. Franzén A and Heldin NE. Bmp-7-induced cell cycle arrest of anaplastic thyroid carcinoma cells via P21(Cip1) and P27(Kip1). Biochem Biophys Res Commun. (2001) 285:773–81. doi: 10.1006/bbrc.2001.5212
99. Kordestani Z, Sanjari M, Safavi M, Mashrouteh M, Asadikaram G, Abadi MFS, et al. Enhanced beta-catenin expression is associated with recurrence of papillary thyroid carcinoma. Endocr Pract: Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. (2018) 24:411–8. doi: 10.4158/ep171983.Or
100. Yu XM, Jaskula-Sztul R, Ahmed K, Harrison AD, Kunnimalaiyaan M, and Chen H. Resveratrol induces differentiation markers expression in anaplastic thyroid carcinoma via activation of Notch1 signaling and suppresses cell growth. Mol Cancer Ther. (2013) 12:1276–87. doi: 10.1158/1535-7163.Mct-12-0841
101. Ma J, Huang X, Li Z, Shen Y, Lai J, Su Q, et al. Foxe1 supports the tumor promotion of Gli2 on papillary thyroid carcinoma by the Wnt/B-catenin pathway. J Cell Physiol. (2019) 234:17739–48. doi: 10.1002/jcp.28399
102. Zhang R, Li H, Zhang S, Zhang Y, Wang N, Zhou H, et al. Rxrα Provokes tumor suppression through P53/P21/P16 and Pi3k-Akt signaling pathways during stem cell differentiation and in cancer cells. Cell Death Dis. (2018) 9:532. doi: 10.1038/s41419-018-0610-1
103. Fard-Esfahani P, Fard-Esfahani A, Saidi P, Fayaz S, Mohabati R, and Majdi M. An increased risk of differentiated thyroid carcinoma in Iran with the 677c→T homozygous polymorphism in the mthfr gene. Cancer Epidemiol. (2011) 35:56–8. doi: 10.1016/j.canep.2010.10.001
104. Ding X, Wang Y, Liu J, and Wang G. Impaired sensitivity to thyroid hormones is associated with elevated homocysteine levels in the euthyroid population. J Clin Endocrinol Metab. (2022) 107:e3731–e7. doi: 10.1210/clinem/dgac371
Keywords: homocysteine, hyperhomocysteinemia, hypothyroidism, hyperthyroidism, MTHFR
Citation: Cui L, Wang F, Li C, Liu F, Wang H and Zhao J (2025) Homocysteine and thyroid diseases. Front. Endocrinol. 16:1572997. doi: 10.3389/fendo.2025.1572997
Received: 08 February 2025; Accepted: 25 June 2025;
Published: 10 July 2025.
Edited by:
Terry Francis Davies, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Morteza Gholami, Golestan University, IranLucia Acampora, University of Naples Federico II, Italy
Copyright © 2025 Cui, Wang, Li, Liu, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haipeng Wang, MTM1ODkxMjkwMzBAMTYzLmNvbQ==; Junyu Zhao, emhhb2p1bnl1QHNkZm11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Lili Cui
Lili Cui Fei Wang1†
Fei Wang1† Chunyu Li
Chunyu Li Fan Liu
Fan Liu Junyu Zhao
Junyu Zhao