- Dietetic Department, Great Ormond Street Hospital for Children National Health Service (NHS) Foundation Trust, London, United Kingdom
Context: Hyperinsulinism is characterized by dysregulated insulin secretion and is typically associated with reduced fasting tolerance. Long-term hyperinsulinism management includes nutrition and medication to normalize plasma glucose levels. Management of enteral feeds and oral intake is integral to the clinical management strategy in children with hyperinsulinism. A new generation of commercial Food Based Enteral Formulas (FBF) consists of rehydrated food, whole‐food protein sources and a mixture of soluble and insoluble fiber.
Objective: Review the tolerance of a food-based enteral formula for the nutritional management of children with hyperinsulinism.
Methods: A single-center retrospective case series to explore the dietetic practice of prescribing a commercially available FBF (Compleat® pediatric, Nestlé Health Science) in children with congenital hyperinsulinism (CHI) or postprandial hypoglycemia (PPH). Data were collected on demographics, gastrointestinal symptoms, anthropometrics, percentage glucose management indicators (GMI%) and hypo/hyperglycemic episodes.
Results: Data were collected on eight children; six were diagnosed with CHI and two children had PPH. The mean age was 1.4 years (0.4SD). All children had a gastrostomy for enteral nutrition. Six of the eight children were prescribed proton pump inhibitors or stool softeners to manage gastroesophageal reflux and/or constipation symptoms. Within one month of children being prescribed FBF dietitians documented an 80% improvement in gastrointestinal symptoms. Dexcom glucose data was available for six children. Six months after FBF was prescribed, Dexcon Inc. data reported that five children had an improvement or stable GMI% and all six children had a reduction in hyperglycemic episodes (chi-square =5.8, p-value 0.02). Children prescribed FBF were less likely to require a glucose polymer (Chi-square = 4.9, p-value = 0.02).
Conclusion: Our retrospective case series suggests that FBF is well tolerated in children with hyperinsulinism and may mitigate gut motility issues associated with hyperinsulinism, especially in relation to gastro-esophageal reflux and constipation symptoms. Furthermore, FBF may help reduce the dependency on glucose polymers and reduce the likelihood of a hyperglycemic episode. A larger sample size and longer follow-up study are necessary to substantiate the potential beneficial impact of FBF’s on glucose control in children with hyperinsulinism.
Highlights
● FBF are a new generation of enteral formulas with limited real-world evidence
● FBF may improve gastroesophageal reflux and constipation in children with CHI
● FBF may reduce the dependency on glucose polymers
1 Introduction
Hyperinsulinism is characterized by unregulated and inappropriate insulin secretion from pancreatic β-cells. It results in hypoglycemia and is the most common cause of persistent hypoglycemia in neonates (1). For this case series, we will specifically focus on Congenital hyperinsulinism (CHI) and post-prandial hypoglycemia (PPH). CHI is a genetically and morphologically heterogeneous group of disorders associated with persistent dysregulated insulin secretion, associated with a significant risk of epilepsy, causing permanent brain damage. The molecular basis involves defects in key genes. The most common mutations affect the potassium adenosine triphosphate (K-ATP) channel genes ABCC8 and KCNJ11, which regulate insulin secretion. Rapid genetic analysis, imaging with 18F-DOPA-PET/CT scan, and development in surgical techniques have improved the management and outcome of the disease (2). Reactive hypoglycemia is a condition of PPH that occurs 2–5 hours after food intake. Elevated insulin levels usually account for hypoglycemia. Some patients rarely show increased insulin sensitivity (3).
The long-term management of hyperinsulinism involves medication to manage the inappropriate over-secretion of insulin through drugs like diazoxide, octreotide, and nifedipine (4). Treatment strategies are individualized based on the specific type and cause of hyperinsulinism, with the aim to normalize plasma glucose levels, provide an age-adjusted fasting tolerance and avoid neurological symptoms associated with hypoglycemia (5). Nutritional management is a crucial component in the treatment of hyperinsulinism, particularly in preventing and managing hypoglycemic episodes. The nutritional strategies may include frequent feeding with high-energy carbohydrate feeds and reducing the frequency and severity of hypoglycemic episodes (2). Children may require additional carbohydrates, which can be delivered as glucose polymers, high-energy formulas, and energy-dense foods. It is recommended that children requiring additional oral carbohydrates to prevent hypoglycemia should be referred to an experienced pediatric dietitian (6).
The current evidence supports the use of dietary fiber in enteral feeding formulas as a first-line therapy for children who need nutritional support (7). Soluble fiber products and fiber from natural foods are effective in improving glycemic control and insulin sensitivity. The magnitude of blood glucose control depends on the fiber type, dose, and intervention length (8). Furthermore, it is recognized that specific dietary components, such as certain dietary fibers, can increase the abundance of specific gut bacteria, which is beneficial for glucose regulation. Dietary fiber improves glucose metabolism and is associated with an increased abundance of the gut microbiome that protects against Bacteroides, which can induce glucose intolerance (9, 10).
Commercial food‐based enteral formulas are rapidly growing in popularity and may help with gastrointestinal symptoms such as constipation, reflux, and loose stools. A new generation of commercial Food Based Formulas (FBFs) is made with a variety of whole‐food protein sources, such as pea protein and chicken, and a mixture of soluble and insoluble fiber (1g per 100ml enteral formula). The development of commercial FBFs has outpaced research evaluating their composition, benefits, safety, and clinical outcomes (11). This single-center retrospective case series aimed to monitor gastrointestinal tolerance, glucose control, and weight change in children with hyperinsulinism who were prescribed FBF.
2 Materials and methods
2.1 Study design and patient population
This single-center retrospective case series reviewed the dietetic practice of prescribing a commercially available FBF (Compleat® pediatric, Nestlé Health Science), in children (licensed for children one year old and over) with CHI and PPH. Compleat Pediatric is 1.2kcal/ml with 14% food‐derived ingredients containing rehydrated chicken, peas and green beans, with peach puree and orange juice; the fiber is from rehydrated vegetables 4.3% (pea fiber 3.8%, green beans 0.54%), acacia gum, fructo-oligosaccharide and inulin. Compleat Pediatric is not suitable for children with cow’s milk allergies or vegetarians.
2.2 Definition
Children who had been reviewed at our tertiary endocrine service with refractory hypoglycemia were included in this review. CHI diagnosis was based on documented hypoglycemia, in the presence of an intravenous glucose infusion rate of > 8 mg/kg/min, and/or detectable insulin or C-peptide levels with low free fatty acids and low ketone bodies (6). PPH was diagnosed with a positive mixed meal (liquid solution containing equal amounts of carbohydrate, protein and fat) and/or oral glucose tolerance test, which either elicited hypoglycemic symptoms or serum glucose levels reduced below 3.1mmol/L (55mg/dl) (6).
2.3 Data collection
Data was collected for all children prescribed an FBF via an enteral feeding tube at our specialist pediatric hospital between March 2022 and December 2024 (the timeframe FBF has been available at our specialist center). Parental consent was obtained from all participants, and the study was approved by the Great Ormond Street Hospital Audit, Quality Improvement and Service Evaluation Committee (registration number GOSH2024/4044). Children’s clinical and dietetic information was collected from the hospital’s electronic records (EPIC, Madison, WI, USA) and Electronic Dietetics Manager (MicroMan2000 Ltd) on demographics (age, sex ethnicity and primary diagnosis), feeding regimen (formula types and routes, carbohydrate intake, protein intake, fiber intake and protein-to-energy ratio), anthropometrics (weight and height-for-age Z-scores), hyperinsulinism related medication and glucose control. Data were collected one month before and one month after an FBF was prescribed. Data was extracted retrospectively, so we were only able to use documented medical and dietetic information – this consisted of carer-reported changes along with medical/dietetic observations.
2.4 Outcome measures
2.4.1 Gastrointestinal symptoms
All children received nutritional assessments from a pediatric specialist endocrine dietitian. If gastrointestinal symptoms had been documented before commencing FBF we measured gastrointestinal symptoms as either improved, no change or worsened after FBF was prescribed on key markers of tolerance: (gastro-esophageal reflux, vomiting, constipation and loose stools). Constipation was defined by the Rome IV Criteria as less than three defecations a week and painful and hard stools. Loose stools were defined as more than one loose stool a day lasting longer than 7 days (12). Reflux was defined as the parental observation of the passage of gastric contents into the esophagus causing regurgitation, posseting, or vomiting, which leads to troublesome symptoms that affect daily functioning (13).
2.4.2 Glucose monitoring
Dexcom Inc. Continuous Glucose Monitoring System© (CGM) is a company that develops, manufactures, produces, and distributes continuous glucose monitoring systems for ambulatory glucose profiles. Continuous glucose monitoring allows close monitoring of glucose levels via a tiny electrochemical sensor electrode inserted under the skin (14). The sensor is connected to a transmitter, which sends this information to a detector, such as a smartphone, or a continuous subcutaneous insulin infusion (CSII) device and provides information about current and previous glucose levels, glucose trends and anticipated future glycemic status.
Dexcom generates a short report with statistical and graphic information, including glucose level statistics (e.g. hypoglycemia) and percentage glucose management indicator (GMI%). GMI% reports the patient’s approximate hemoglobin A1C level, based on the average glucose level from CGM readings for 14 or more days. In general, each 25 mg/dL (1.4mmol/L) increase in mean glucose corresponds to a GMI increase of 0.6%, e.g., a mean glucose of 150 mg/dL (8.3mmol/L) corresponds to a GMI of 6.9%. A healthy GMI target is below 7.5%. Dexcom also records the number of hypoglycemic events <63mg/dl (3.5mmol/L) and hyperglycemic events > 180mg/dl (10mmol/L/L) (15).
2.4.3 Anthropometric measurements
Weight and height were extracted from EPIC electronic growth charts. Assessment of height reflects adequate growth and nutritional status but can be challenging in children with malformations. The nutrition status (weight-for-age and height-for-age) was assessed using Z-scores. Moderate overweight was identified if the Z-scores were between +2 and +3 standard deviations (SD) and severe overweight was identified if the Z-scores were above +3 (SD). Height and weight for age Z-scores were determined using the World Health Organization (WHO) growth standards, which describe the optimal growth of healthy children in a supportive environment (16).
2.5 Statistical analysis
Continuous data were tabulated using descriptive statistics (mean and standard deviation [SD]). To assess gastrointestinal tolerance of FBF, medical and dietetic reports of upper and lower gastrointestinal symptoms before starting FBF were compared with medical and dietetic reports of upper and lower GI symptoms within one month of FBF prescribed. Chi-square statistic was used to measure the probability of children being prescribed glucose polymer before and after FBF was prescribed; and to assess hypo and/or hyperglycemic episodes before and after FBF was prescribed. Comparative analysis was used to compare changes before and after (6 months and 1 month) the formula changed to FBF for weight-for-age Z-score, and nutritional composition. Statistical significance was based on an alpha level of 0.05. Data were analyzed using RStudio R version 4.3.2.
3 Results
3.1 Demographics
Data were collected on eight children, six were diagnosed with CHI and two were diagnosed with post-prandial hyperinsulinemia. Six children were female and two were male. The mean age when FBF was prescribed was 29 months (6.0SD). Six children were white British/European, and two children were Asian British (Pakistan). Five of the six children with CHI had the ABCC8 gene. All children had a gastrostomy (size 9 Freka - Fresenius Kabi) for enteral nutrition; case study 3 had a gastrostomy with a jejunal extension for the management of severe gastroesophageal reflux. Five of the eight children had some oral intake, which accounted for less than 20% of their estimated nutritional requirements (Table 1).
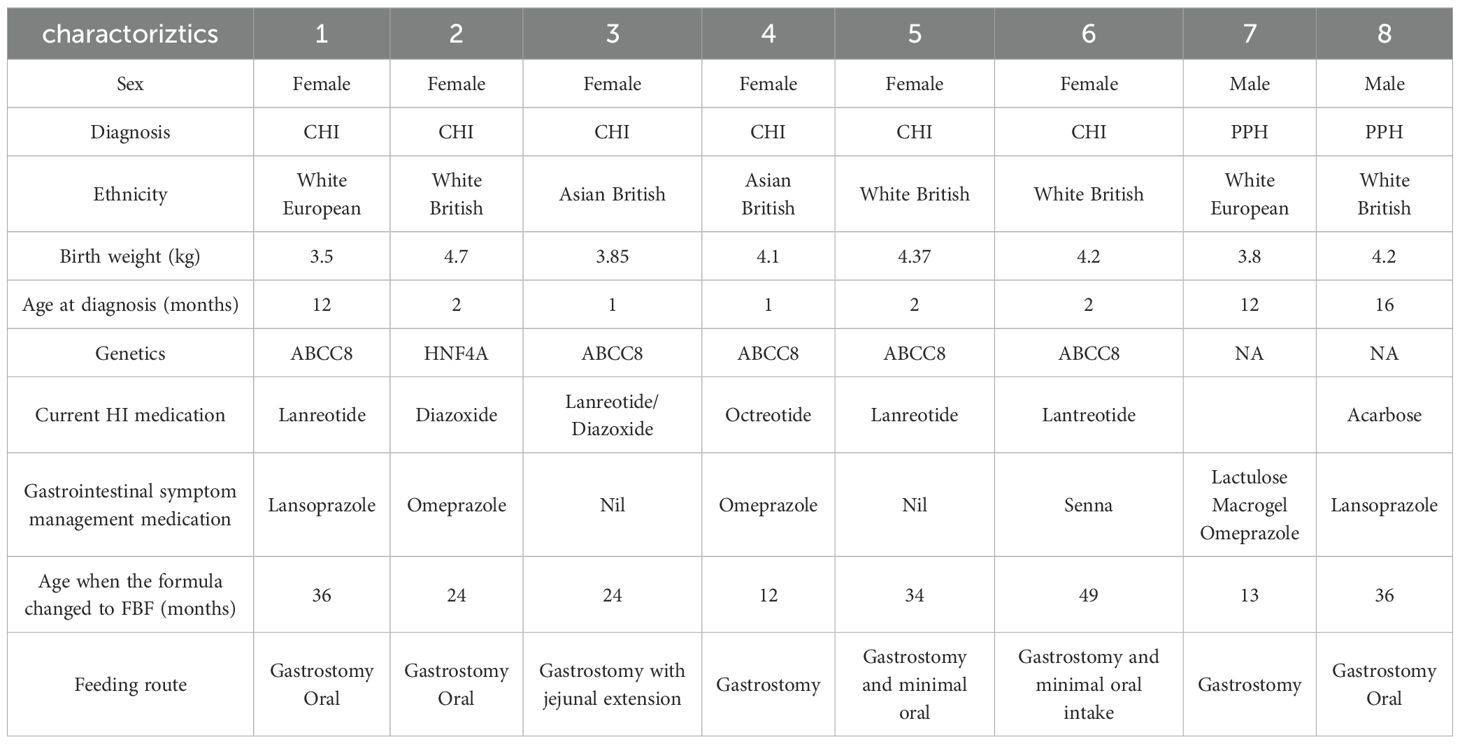
Table 1. Demographic, clinical and anthropometric data for children with hyperinsulinemia prescribed Food-based formula (FBF).
3.2 Nutritional composition and feeding regimens
There was no significant difference before and after the formula changed to FBF for total fluids, kcal/day, carbohydrates (grams)/day, protein (grams)/day or Protein to Energy Ratio (Table 2). However, there was a significant difference in the amount of fiber intake before and after the formula changed to FBF, 95% CI -10, -0.9: p-value 0.03. (Table 2) Additionally, there was no significant difference before and after the formula changed to FBF for total fluids (Table 2); furthermore, feeding regimens were not altered after formula change – all children were on continuous feeds to optimize glucose control. Of note, there were no reported feeding tube blockages after the formula switch to FBF.
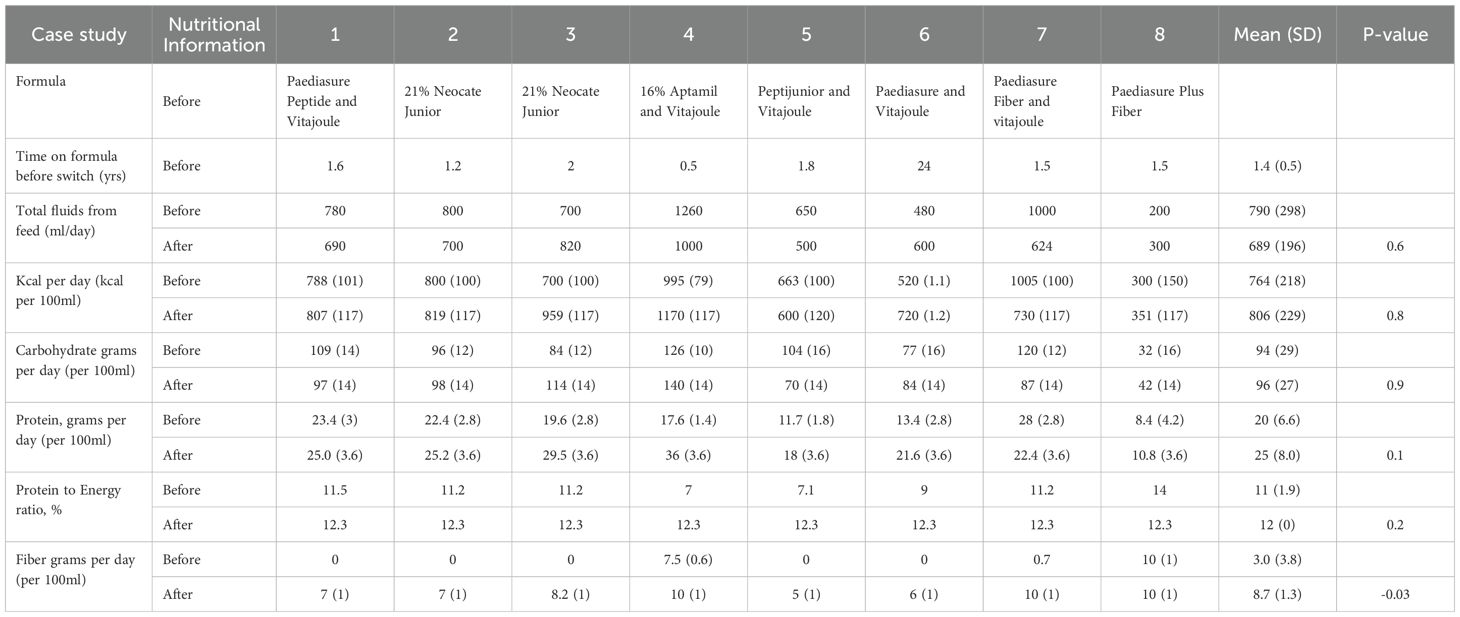
Table 2. Nutritional composition of enteral formula before and after Food based formula was prescribed.
Before the formula changed to a FBF, five children (cases 1, 4, 5, 6 and 7) were on additional glucose polymers to maintain blood glucose levels. After FBF commenced, all five children discontinued glucose polymers. The was a significant probability that children on FBF were less likely to require additional glucose polymer (Chi-square = 4.9, p-value = 0.02).
Five children’s feeding regimens were simplified after the formula changed to FBF; no longer requiring the need to add glucose polymer and/or transferred from a powdered amino acid/partially hydrolyzed formula to a ‘ready to feed’ formula (Table 2).
There were no changes to the oral intake in the four children who were able to tolerate solid food over the 6 months after FBF was commenced.
3.3 Gastrointestinal symptoms
All children had documented gastrointestinal symptoms before starting FBF, and of these six were prescribed proton pump inhibitors and/or stool softeners to mitigate gastroesophageal reflux and constipation symptoms (Table 1). Before FBF was prescribed, two children were on an amino acid formula and two children were on a partially hydrolyzed (peptide) formula due to upper gastrointestinal issues. The other three children were receiving a whole protein formula. Within one month of commencing FBF, the dietitians had reported an improvement in constipation symptoms in 4 of the eight children and an improvement in gastroesophageal reflux in 5 of the 8 children.
3.4 Anthropometrics
The mean weight-for-age Z-score when the FBF was prescribed = 0.18 (1.5 SD). Six months after the FBF was prescribed, the weight-for-age Z-score was 0.15 (1.6SD) (p-value 0.4). The mean weight (kg) increased from 12.3kg to 13.1kg over the 6 months children were prescribed FBF, p-value 0.08 (Table 3).
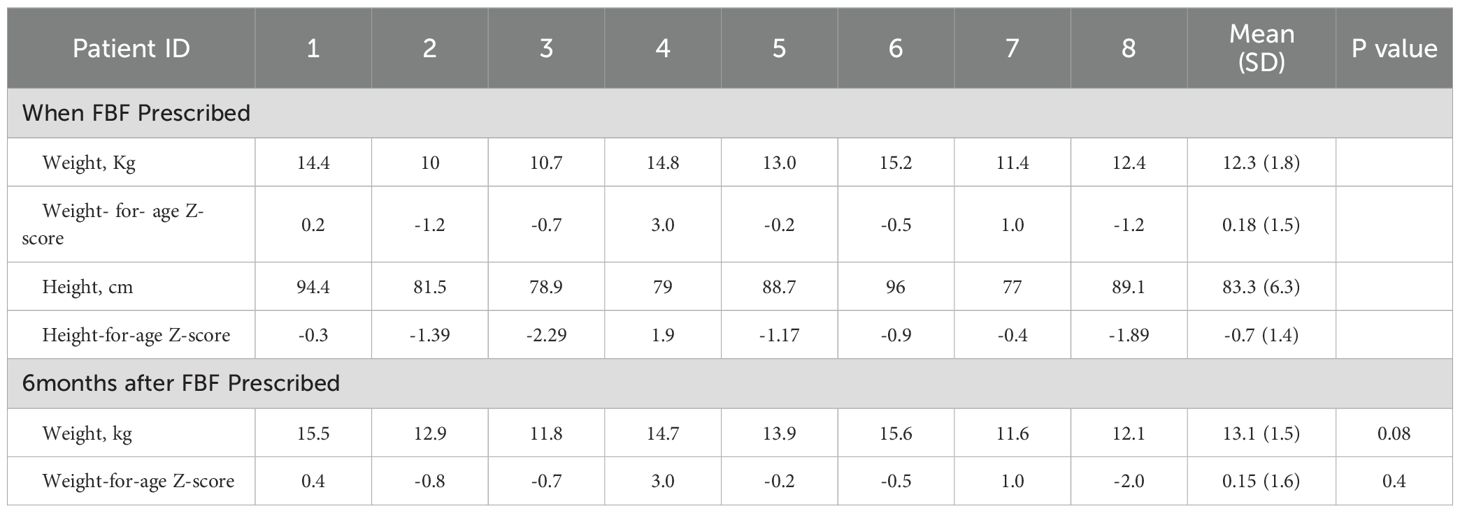
Table 3. Anthropometric changes from when FBF was prescribed and 6 months after commencing the Food-based formula (FBF).
3.5 Glucose monitoring
Continuous glucose monitoring data were available for six of the eight children; of these, five reported an improvement or stable GMI% one month after commencing FBF. Case studies 1 and 4 reported the greatest reduction in GMI%, 0.3% and 0.2%, respectively. Before the FBF was prescribed, both children were receiving additional glucose polymer. Case study 7 reported no change in GMI% before and after FBF was prescribed: this child was prescribed a high-energy whole protein formula with fiber before FBF. Case study 3 reported a 0.2% increase in GMI, before the formula change, this patient was receiving an amino acid formula (Figure 1). After 6 months on FBF case study 1,4 and 7 continued to report improved GMI%.
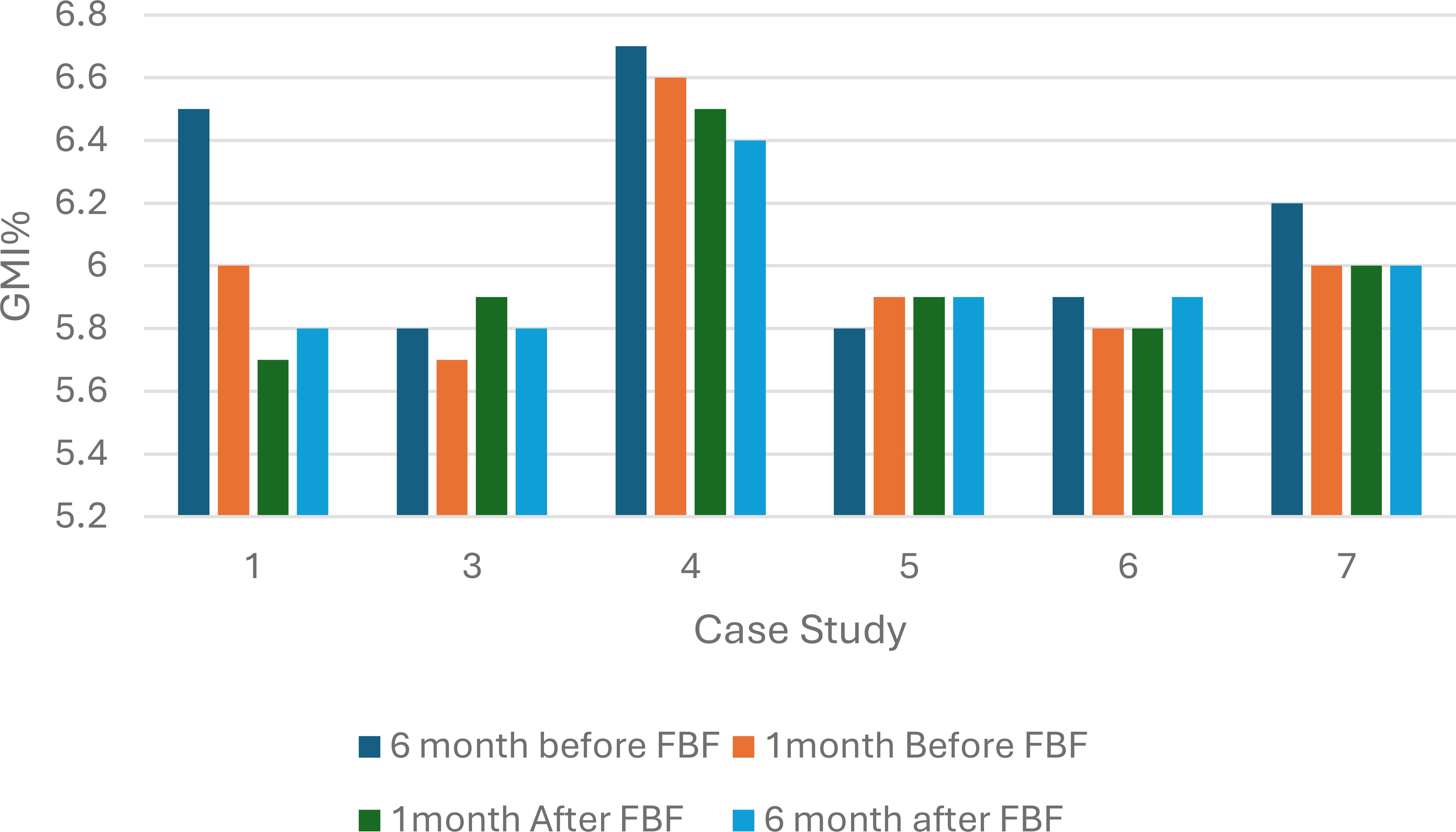
Figure 1. Percentage glucose monitoring indicator (%GMI) 6 months and 1 month before and after Food Based Enteral Formula prescribed in children with hyperinsulinism (n=6). FBF, Food Based Enteral Formula; %GMI, percentage glucose management indicator.
All six children had a decrease in the number of hyperglycemic episodes within one month after commencing FBF compared to one month before FBF was prescribed. This reduction in hyperglycemic episodes continued 6 months after the formula was switched to a FBF. The probability of having a hyperglycemic episode after FBF was prescribed was significantly reduced (chi-square statistic =5.8, p-value 0.02) (Figure 2).
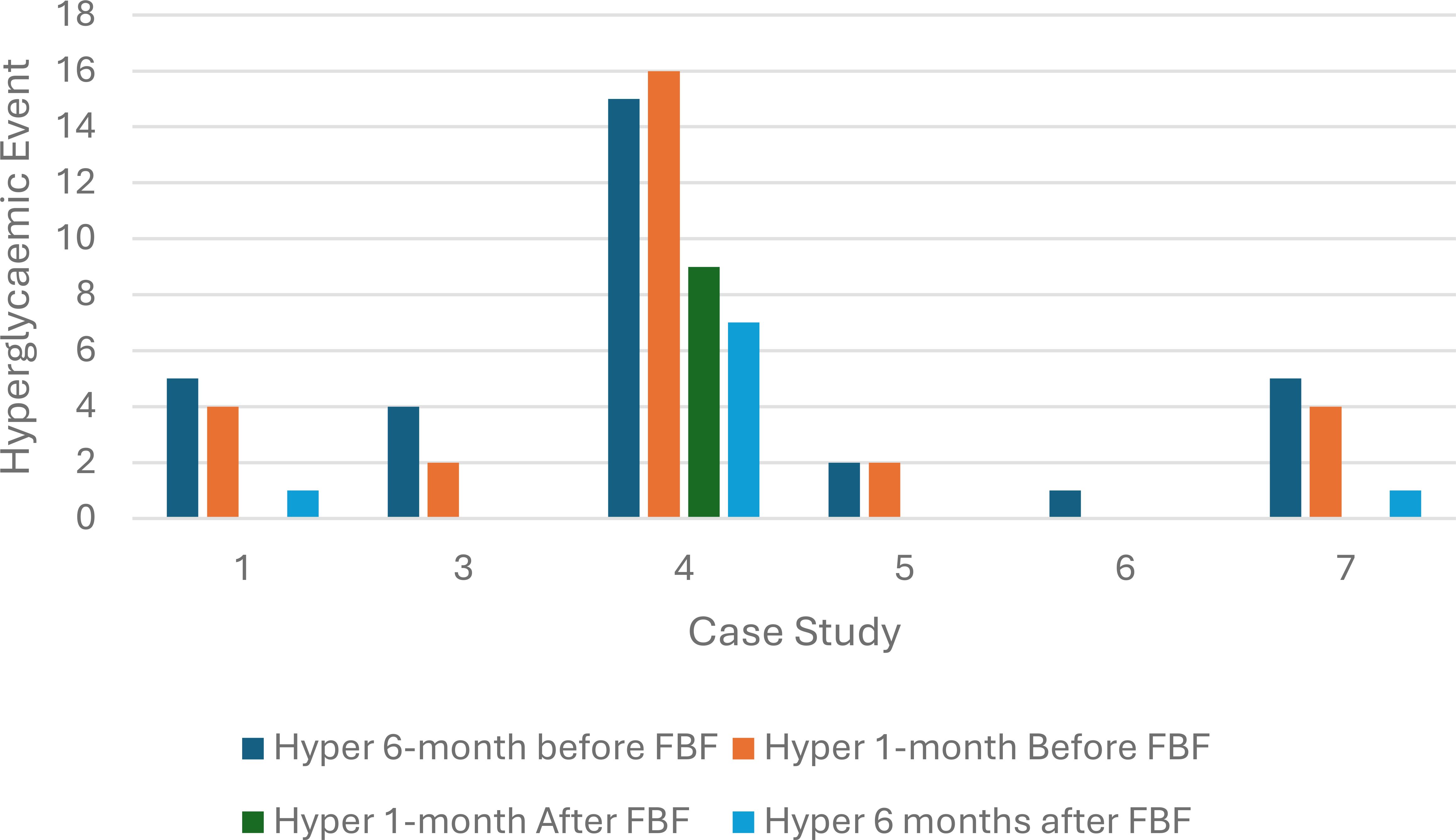
Figure 2. Hyperglycemic episodes at six months and one month before and after food-based formula was prescribed (n=6).
Three children (case study 1, 3 and 5) had a reduction in hypoglycemic events, and two children had no change, while one child had an increase in hypoglycemia event. The probability of a hypoglycemic episode remained unchanged after FBF was prescribed (p-value =0.6) (Figure 3).
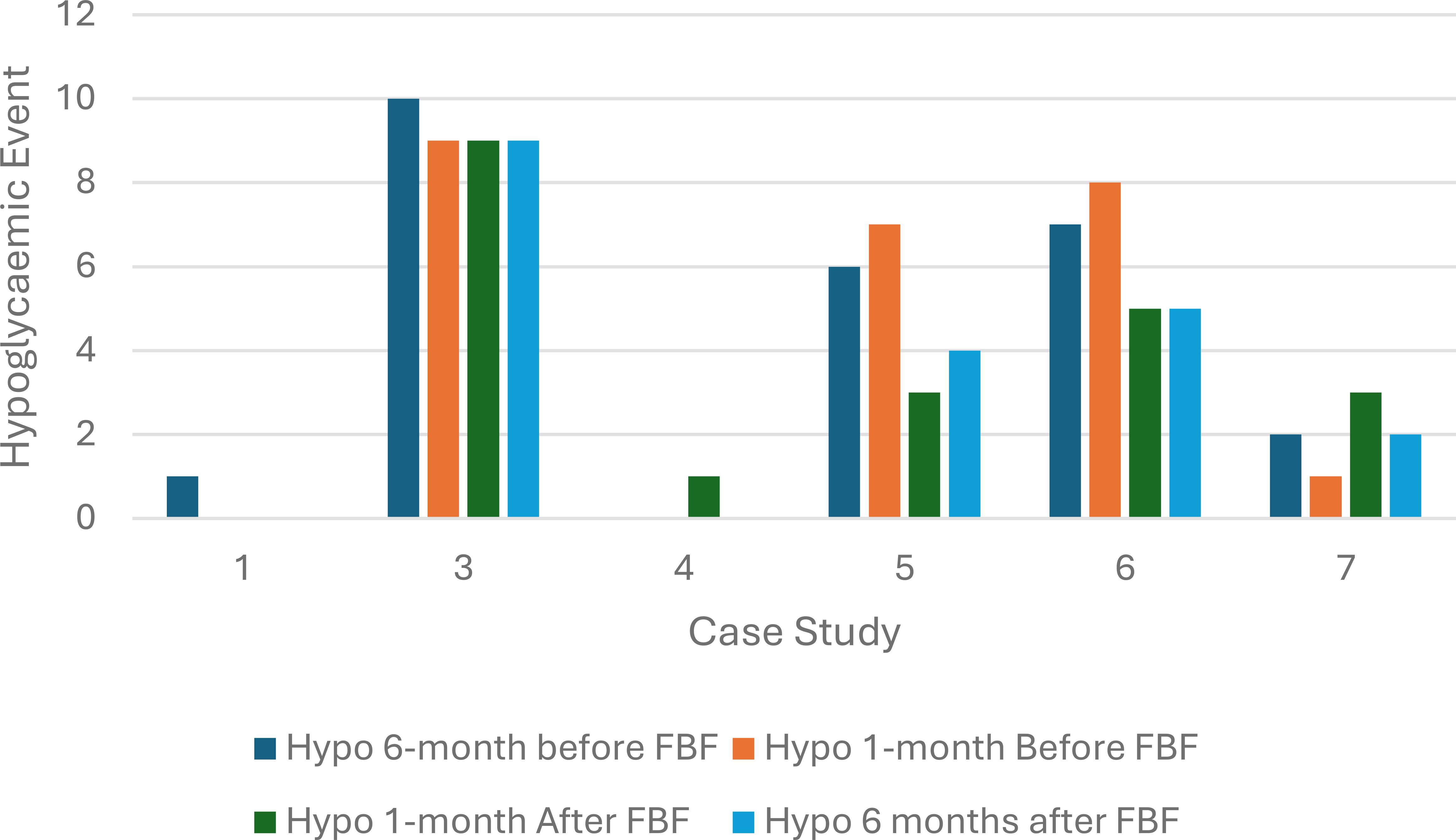
Figure 3. Hypoglycemic episodes at 6 and 1 month before and after food-based formula was prescribed (n=6).
4 Discussion
Our retrospective case series found that children with gastrointestinal symptoms (reflux and constipation) who were previously on an amino acid/hydrolyzed formula had improved symptoms after transitioning to a whole protein FBF. Also, children had a reduced reliance on glucose polymers when prescribed FBF. All children prescribed FBF had a significant reduction in hyperglycemic episodes. There was a significant increase in fiber intake after children were prescribed FBF.
Before commencing FBF, all eight children had documented gastrointestinal symptoms, with six children receiving medication to mitigate gastroesophageal reflux and constipation symptoms. Gastrointestinal symptoms were reported to have improved after the formula was changed to FBF. Previous retrospective studies that monitored the impact of FBF on gastrointestinal symptoms also reported a significant improvement in reflux and constipation (17, 18). In a community-focused review by Raskin et al. (2022), focused on increasing the understanding of the experience of living with CHI, families shared their own stories. Chronic constipation and stomach pain were commonly reported issues in children with CHI (19). Hyperinsulinism can cause gastric and intestinal motility deceleration, associated with reduced ghrelin and cholecystokinin levels, disturbing brain-gut axis functioning and may be responsible for gastric motility deceleration and subsequent constipation (20). Hyperinsulinism impairs gastrointestinal motility in both the postabsorptive and postprandial states - this effect is combined with delayed carbohydrate absorption. Therefore, hyperinsulinism may lead to alterations in carbohydrate absorption and can also contribute to gastrointestinal disturbances (21).
The average fiber intake in our case series significantly increased when the formula was changed to FBF, which may have contributed to the improved GMI% and reduction in hyperglycemic events. Of note, there was no difference in carbohydrate intake before and after the formula was changed to FBF. Dietary fiber is an essential component of the human diet and a major determinant of digestive health. Non-digestible dietary fiber undergoes fermentation by the intestinal microbiota to produce short-chain fatty acids (SCFAs), which positively impact the local and systemic immune system. A study by Xi et al. (2017) reported episodes of hypoglycemia significantly improved with the addition of fiber supplementation (22). Of note, a systematic review of randomized controlled trials investigating the clinical effects of fiber-supplemented enteral nutrition versus placebo in critically ill patients concluded that evidence suggests that fiber-supplemented enteral nutrition has clinical benefits, and recommends larger multi-center studies should be delivered to substantiate findings (23).
Carbohydrate metabolism of gut microbiomes has been proposed to contribute up to 10% of the host’s overall energy extraction (24). Fecal carbohydrates, particularly host-accessible monosaccharides, are increased in individuals with insulin resistance and are associated with microbial carbohydrate metabolisms and host inflammatory cytokines. A study by Takeuchi et al. (2023) investigated the relationship between gut microbiomes and glucose control using a comprehensive multi-omics strategy in humans. The authors concluded that gut bacteria were associated with insulin resistance and insulin sensitivity, which showed a distinct pattern of carbohydrate metabolism (25).
Although the exact mechanisms as to why FBF are better tolerated in some children remain unclear. One theory that has been postulated is associated with the amount and mixture of fiber and the subsequent beneficial impact on the gut microbiome (26). The diversity of the gut microbiome is influenced by the variety of the diet; a diet solely of commercial enteral feeds has been implicated in reducing the diversity of microbial species in the gut microbiome (27). Therefore, enteral formulas containing fiber may support normal digestive health. Commercial tube feeds devoid of fiber appear to negatively alter children’s gut microbiome (7).
After FBF was prescribed, all five children who were prescribed glucose polymers discontinued and three children transitioned from a partially hydrolyzed or amino acid powder formula to a whole protein ready-to-feed FBF. An estimated cost was calculated to assess the financial impact of transferring to an FBF. The average daily cost for 21% Amino acid formula with additional glucose polymer to 16% concentration at 1000ml/day = £40 per day (including additional ancillaries). Compared to 2 x 500ml ready-to-hang FBF bottles = £16/day. Transitioning to a FBF can equate to a cost saving to the health provider of £8760 per year. Furthermore, the reduced time for feed preparation after FBF was prescribed (estimated 15–20 minutes/day), reducing the disease burden of this complex disorder. Furthermore, the total feed volume decreased by an average of 100ml per day. This equates to a reduction of 1-1.5 hours on a feeding pump each day. A joint consensus guideline on gastroesophageal reflux reported evidence from a comparative study, which suggested that compared with larger volume feeds, smaller volumes are associated with fewer reflux episodes (13). This reduced time on the feeding pump may positively impact the child and family’s quality of life, with more time off the feeding pump and more time to play. Furthermore, reducing the feed volume may promote an increase in solid food, which can mitigate gastroesophageal reflux (28).
It is important to highlight that any child who has been tube-fed from birth will not have been through a weaning process and exposed to common allergens. Therefore, we would advise clinicians and parents to start by adding 30ml FBF with Food-derived ingredients to their current formula. Gradually building the volumes to tolerance and nutritional requirements. Detailed guidance is outlined in the British Dietetic Association’s Practice Toolkit: The Use of Blended Diet with Enteral Feeding Tubes, November 2021: Section 4.7.2 (29).
4.1 Limitations
Retrospective case series have several limitations owing to their design, which are dependent on the review of records and documentation and therefore the results are ungeneralizable rather than stating causation, we can only allude to a potential association that an FBF may improve gastrointestinal symptoms and glucose control in children with hyperinsulinism. The Glucose Management Indicator estimates Hemoglobin A1c (HbA1c) from continuous glucose monitoring data and is used in diabetes management, but it was not primarily created or validated for all people with diabetes or other blood glucose disorders. The standard GMI equation was developed from trials in adults with type 1 diabetes, and its performance, including its accuracy in predicting HbA1c, is not consistently well-characterized across different populations (30).
5 Conclusion
Our retrospective case series suggests that FBF is well tolerated in children with hyperinsulinism and may mitigate gut motility issues associated with hyperinsulinism, especially in relation to gastro-esophageal reflux and constipation symptoms. Furthermore, FBF may help reduce the dependency on glucose polymers and reduce the likelihood of a hyperglycemic episode. A larger sample size and longer follow-up study are necessary to substantiate the potential beneficial impact of FBF’s on glucose control in children with hyperinsulinism.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Great Ormond Street Hospital Audit, Quality Improvement and Service Evaluation Committee (registration number GOSH2024/4044). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from Blood results collected from patients routine clinical care. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
GO’C: Conceptualization, Data curation, Formal Analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. LC: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. AC: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Nestle Health Science covered the open-access fees.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shah P, Rahman SA, Demirbilek H, Güemes M, and Hussain K. Hyperinsulinaemic hypoglycaemia in children and adults. Lancet Diabetes Endocrinol. (2017) 5:729–42. doi: 10.1016/S2213-8587(16)30323-0
2. Demirbilek H and Hussain K. Congenital hyperinsulinism: diagnosis and treatment update. J Clin Res Pediatr Endocrinol. (2017) 9:69–87.
3. Aynsley-Green A, Hussain K, Hall J, Saudubray JM, Nihoul-Fékété C, De Lonlay-Debeney P, et al. Practical management of hyperinsulinism in infancy. Arch Dis Child Fetal Neonatal Ed. (2000) 82:F98–f107. doi: 10.1136/fn.82.2.F98
4. Welters A, Lerch C, Kummer S, Marquard J, Salgin B, Mayatepek E, et al. Long-term medical treatment in congenital hyperinsulinism: a descriptive analysis in a large cohort of patients from different clinical centers. Orphanet J Rare Dis. (2015) 10:150. doi: 10.1186/s13023-015-0367-x
5. Banerjee I, Raskin J, Arnoux JB, De Leon DD, Weinzimer SA, Hammer M, et al. Congenital hyperinsulinism in infancy and childhood: challenges, unmet needs and the perspective of patients and families. Orphanet J Rare Dis. (2022) 17:61. doi: 10.1186/s13023-022-02214-y
6. Shaikh MG, Lucas-Herald AK, Dastamani A, Salomon Estebanez M, Senniappan S, Abid N, et al. Standardised practices in the networked management of congenital hyperinsulinism: a UK national collaborative consensus. Front Endocrinol (Lausanne). (2023) 14:1231043. doi: 10.3389/fendo.2023.1231043
7. Lionetti P, Wine E, Ran Ressler R, Minor GJ, Major G, Zemrani B, et al. Use of fiber-containing enteral formula in pediatric clinical practice: an expert opinion review. Expert Rev Gastroenterol Hepatol. (2023) 17:665–75. doi: 10.1080/17474124.2023.2217355
8. Tsitsou S, Athanasaki C, Dimitriadis G, and Papakonstantinou E. Effects of dietary fiber on glycemic control and insulin sensitivity in patients with type 2 diabetes: A systematic review and meta-analysis. J Funct Foods. (2021) 82:104500. doi: 10.1016/j.jff.2021.104500
9. Crudele L, Gadaleta RM, Cariello M, and Moschetta A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. eBioMedicine. (2023) 97. doi: 10.1016/j.ebiom.2023.104821
10. Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab. (2015) 22:971–82. doi: 10.1016/j.cmet.2015.10.001
11. Murayi JA, Evenson E, Britton C, Gehred A, and Goday PS. Clinical effects of pediatric commercial food-based formulas: A systematic review. J Pediatr Gastroenterol Nutr. (2025) 80:501–9. doi: 10.1002/jpn3.12450
12. Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and rome IV. Gastroenterology. (2016) 19:S0016-5085(16)00223-7. doi: 10.1053/j.gastro.2016.02.032
13. Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the north american society for pediatric gastroenterology, hepatology, and nutrition and the european society for pediatric gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr. (2018) 66:516–54. doi: 10.1097/MPG.0000000000001889
14. Mian Z, Hermayer KL, and Jenkins A. Continuous glucose monitoring: review of an innovation in diabetes management. Am J Med Sci. (2019) 358:332–9. doi: 10.1016/j.amjms.2019.07.003
15. Danne T, Bergenstal RM, Amiel SA, Beck R, and Biester T. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. (2019) 42:1593–603. doi: 10.2337/dci19-0028
16. Cole TJ, Wright CM, and Williams AF. Designing the new UK-WHO growth charts to enhance assessment of growth around birth. Arch Dis Child Fetal Neonatal Ed. (2012) 97:F219–22. doi: 10.1136/adc.2010.205864
17. O'Connor G, Watson M, Van Der Linde M, Bonner RS, Hopkins J, Saduera S, et al. Monitor gastrointestinal tolerance in children who have switched to an “enteral formula with food-derived ingredients”: A national, multicenter retrospective chart review (RICIMIX study). Nutr Clin Pract. (2022) 37:929–34. doi: 10.1002/ncp.10812
18. O’Connor G, Velandia AC, and Capriles ZH. The impact of an enteral formula with food-derived ingredients on dietetic practice at a specialist children’s hospital in the UK: Retrospective study. J Hum Nutr Dietetics. (2025) 38:e13374.
19. Raskin J, Pasquini TLS, Bose S, Tallis D, and Schmitt J. Congenital hyperinsulinism international: A community focused on improving the lives of people living with congenital hyperinsulinism. Front Endocrinol (Lausanne). (2022) 13:886552. doi: 10.3389/fendo.2022.886552
20. Ihana-Sugiyama N, Nagata N, Yamamoto-Honda R, Izawa E, Kajio H, Shimbo T, et al. Constipation, hard stools, fecal urgency, and incomplete evacuation, but not diarrhea is associated with diabetes and its related factors. World J Gastroenterol. (2016) 22:3252–60. doi: 10.3748/wjg.v22.i11.3252
21. Eliasson B, Björnsson E, Urbanavicius V, Andersson H, Fowelin J, Attvall S, et al. Hyperinsulinaemia impairs gastrointestinal motility and slows carbohydrate absorption. Diabetologia. (1995) 38:79–85. doi: 10.1007/BF02369356
22. Xi F, Xu X, Tan S, Gao T, Shi J, Kong Y, et al. Efficacy and safety of pectin-supplemented enteral nutrition in intensive care: a randomized controlled trial. Asia Pac J Clin Nutr. (2017) 26:798–803. doi: 10.6133/apjcn.082016.07
23. Koch JL, Lew CCH, Kork F, Koch A, Stoppe C, Heyland DK, et al. The efficacy of fiber-supplemented enteral nutrition in critically ill patients: a systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Crit Care. (2024) 28:359. doi: 10.1186/s13054-024-05128-2
24. McNeil NI. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. (1984) 39:338–42. doi: 10.1093/ajcn/39.2.338
25. Takeuchi T, Kubota T, Nakanishi Y, Tsugawa H, Suda W, Kwon AT, et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature. (2023) 621:389–95. doi: 10.1038/s41586-023-06466-x
26. O'Connor G. An open-label pilot single-subject study to monitor the impact of a Food-Based enteral formula on faecal short-chain fatty acid concentrations in children admitted to intensive care with sepsis. Clin Nutr Open Sci. (2024) 53:1–10. doi: 10.1016/j.nutos.2023.12.002
27. Tanes C, Bittinger K, Gao Y, Friedman ES, Nessel L, Paladhi UR, et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe. (2021) 29:394–407.e5. doi: 10.1016/j.chom.2020.12.012
28. Rybak A, Pesce M, Thapar N, and Borrelli O. Gastro-esophageal reflux in children. Int J Mol Sci. (2017) 18(8):1671. doi: 10.3390/ijms18081671
29. BDA. British dietetic associations practice toolkit: the use of blended diet with enteral feeding tubes. (2021). Available online at: https://www.bda.uk.com/resource-report/the-use-of-blended-diet-with-enteral-feeding-tubes.html. (Accessed September 15, 2025).
Keywords: hyperinsulinism, food-based enteral formula, gastrointestinal tolerance, food-derived ingredients, glucose control, fiber
Citation: O’Connor G, Cheng L and Cunjamalay A (2025) Case Report: Food-based enteral formula in the nutritional management of children with hyperinsulinism: single center retrospective case series. Front. Endocrinol. 16:1574093. doi: 10.3389/fendo.2025.1574093
Received: 10 February 2025; Accepted: 08 September 2025;
Published: 25 September 2025.
Edited by:
Klaus Mohnike, University Hospital Magdeburg, GermanyReviewed by:
Rajmohan Dharmaraj, University of New Mexico, United StatesTai Ls Pasquini, Congenital Hyperinsulinism International, United States
Copyright © 2025 O’Connor, Cheng and Cunjamalay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Graeme O’Connor, Z3JhZW1lLm9jb25ub3JAbmhzLm5ldA==
 Graeme O’Connor
Graeme O’Connor Lauren Cheng
Lauren Cheng Annaruby Cunjamalay
Annaruby Cunjamalay