- 1Center of Reproductive Medicine, Yulin Maternal and Child Health Hospital, Yulin, China
- 2Pediatric Surgery, The People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
- 3Qinzhou Maternity and Child Health Care Hospital, Guangxi, China
Background: The incidence of thin endometrium in assisted reproductive technology (ART) is between 1% and 2.5%, yet its treatment options are varied and often show limited efficacy. There is an urgent need to delineate the relative effectiveness of various interventions to guide clinical practice.
Objective: This study aims to systematically compare the efficacy of different interventions for treating thin endometrium via a network meta-analysis, focusing on improvements in endometrial thickness and clinical pregnancy rates.
Methods: A systematic search was conducted in PubMed, The Cochrane Library, EMBASE, CBM, and CNKI databases, covering literature until December 31, 2024. Randomized controlled trials (RCTs) evaluating treatments for thin endometrium were included and assessed for quality using the Cochrane Risk of Bias Tool. Stata 17.0 software was used for the network meta-analysis, employing Bayesian methods to construct network diagrams and calculate the surface under the cumulative ranking curve (SUCRA).
Results: Eighteen RCTs involving six interventions (oral aspirin, Ding Kun Dan, intrauterine infusion of platelet-rich plasma [PRP], intrauterine infusion of granulocyte-macrophage colony-stimulating factor [G-CSF], intramuscular injection of recombinant human growth hormone [rhGH], and neuromuscular electrical stimulation [NMES]) were included. The network meta-analysis revealed: 1) Endometrial thickness: All intervention groups showed varying degrees of effectiveness in increasing thickness compared to the control group. The top three ranked in effectiveness were G-CSF (SUCRA = 78.48%), aspirin (SUCRA = 70.89%), and PRP (SUCRA = 68.14%). 2) Clinical pregnancy rate: PRP ranked highest in improving pregnancy rates (SUCRA = 80.12%), followed by aspirin (SUCRA = 70.29%) and Ding Kun Dan (SUCRA = 62.79%). Overall, PRP showed significant advantages in both increasing endometrial thickness and improving clinical pregnancy rates, making it the most promising intervention.
Conclusion: PRP demonstrates the best clinical application potential in treating thin endometrium, particularly in improving clinical pregnancy rates. Future high-quality RCTs are necessary to further validate and optimize intervention strategies.
1 Background
In the successful implementation of assisted reproductive technology (ART), high-quality embryos and adequate endometrial receptivity are two indispensable key factors. Endometrial thickness (EMT) serves as an important morphological indicator for assessing endometrial receptivity, and its clinical significance is widely recognized (1). Currently, there is no consensus on the definition of “thin endometrium,” but most studies consider an EMT of less than 7 mm as thin (2). The pathological mechanisms of thin endometrium have not been fully elucidated. Current evidence suggests that they may be attributed to several factors: reduced expression of estrogen receptors in the endometrium; high vascular resistance in the uterine spiral arteries, resulting in slow glandular epithelial growth; low or absent expression of vascular endothelial growth factor (VEGF) and integrin β3; fibrosis and adhesions leading to impaired angiogenesis in the endometrium; downregulation of metabolic and antioxidant gene expression; and polymorphisms in estrogen receptor genes α and β. These pathological changes result in a significant decrease in endometrial receptivity, subsequently affecting the embryo implantation process (3, 4). The study by Von Wolff et al. indicates patients with EMT ≤ 7 mm have a pregnancy rate of only 7.4%, significantly lower than the 30.8% rate for those with EMT > 7 mm (P = 0.03) (5).This research underscores the importance of optimizing endometrial thickness, as an adequate endometrial thickness facilitates successful embryo implantation.
In clinical practice, the management of thin endometrium remains a significant challenge. Currently, no single therapy has been proven to consistently and significantly improve pregnancy outcomes in this patient population. This situation forces many patients to confront the repeated cancellation of embryo transfer cycles or to endure multiple failures of in vitro fertilization and embryo transfer (IVF-ET). Existing treatment strategies primarily encompass the following approaches: Hormonal Therapy: Appropriate supplementation of estrogen (either orally or trans dermally) may be considered; however, its efficacy can vary among individuals, and long-term use may increase the risk of thrombosis. Medications to Improve Endometrial Blood Flow: Agents such as sildenafil (a phosphodiesterase-5 inhibitor), enteric-coated aspirin primarily act by vasodilation to enhance microcirculation within the endometrium, thereby promoting its growth. Platelet-Rich Plasma (PRP) Treatment: Rich in various growth factors, studies suggest that PRP may facilitate endometrial regeneration (6–8). Biologic Therapies: Granulocyte colony-stimulating factor (G-CSF) intrauterine infusion may exert effects by activating endometrial stem cells, although further research is needed to assess its efficacy in patients with severe intrauterine adhesions (9). Physical Therapy: Bioelectric stimulation can improve the condition of the endometrium by modulating local blood flow and neuroendocrine function. Stem Cell Therapy: Preliminary studies indicate that stem cells may repair damaged endometrium via paracrine mechanisms; however, more evidence from well-designed studies is required to validate their efficacy and safety (10, 11). Regarding the selection of treatment protocols, a network meta-analysis conducted by Li et al. compared four commonly used intrauterine infusion regimens. The findings suggest that hCG, along with subcutaneous or intrauterine CSF (SG-CSF), may represent the current optimal choice (12). However, the limitations of this study include a limited number of randomized controlled trials (RCTs) included and insufficient consideration of patient-specific factors affecting treatment efficacy. Therefore, this study aims to incorporate high-quality RCTs of various interventions and employ a network meta-analysis approach to systematically evaluate the relative efficacy of each treatment strategy, with the goal of providing more reliable evidence for accurately tailoring individualized treatment plans.
2 Methods
2.1 Data and methods
2.1.1 Literature search
A comprehensive search of PubMed, The Cochrane Library, EMBASE, CBM, and CNKI was conducted, with a cut-off date of December 31, 2024. Keywords included “thin endometrium” and “randomized controlled trials,” combining subject headings and free-text terms. Studies were limited to English and Chinese languages, and references were traced for additional relevant studies.
2.1.2 Study inclusion criteria
Inclusion criteria: (i) studies involving patients with thin endometrium; (ii) RCTs with complete data; (iii) outcome measures including endometrial thickness or clinical pregnancy rate; (iv) studies published in English or Chinese.
Exclusion criteria: (i) animal experiments or case reports; (ii) studies with missing or unusable data; (iii) duplicate publications.
2.1.3 Study selection and data extraction
Two researchers independently screened the literature, extracted data, and cross-checked analyses. Initial screening involved reading titles and abstracts to exclude studies not meeting inclusion criteria, followed by full-text review for final inclusion determination.
Risk of bias in included studies was assessed using the Cochrane Risk of Bias Tool, evaluating aspects such as selection (random sequence generation and allocation concealment), performance (blinding of participants and personnel), detection (blinding of outcome assessment), attrition (completeness of outcome data), reporting (selective reporting), and other biases. Each criterion was rated as “low risk,” “high risk,” or “unclear.”
2.1.4 Statistical analysis
RevMan 5.4 software was used for bias assessment. Bayesian network meta-analysis was conducted using Stata 17.0, with effect sizes expressed as standard mean differences (SMD) or odds ratios (OR) and 95% credible intervals (CrI) for statistical significance assessment. SUCRA was used to rank treatment efficacy.
2.1.5 Ethical statement
This study only utilized publicly available data, thus ethical approval was not required. The study was registered with PROSPERO, registration number CRD42025637150.
3 Results
3.1 Literature search results
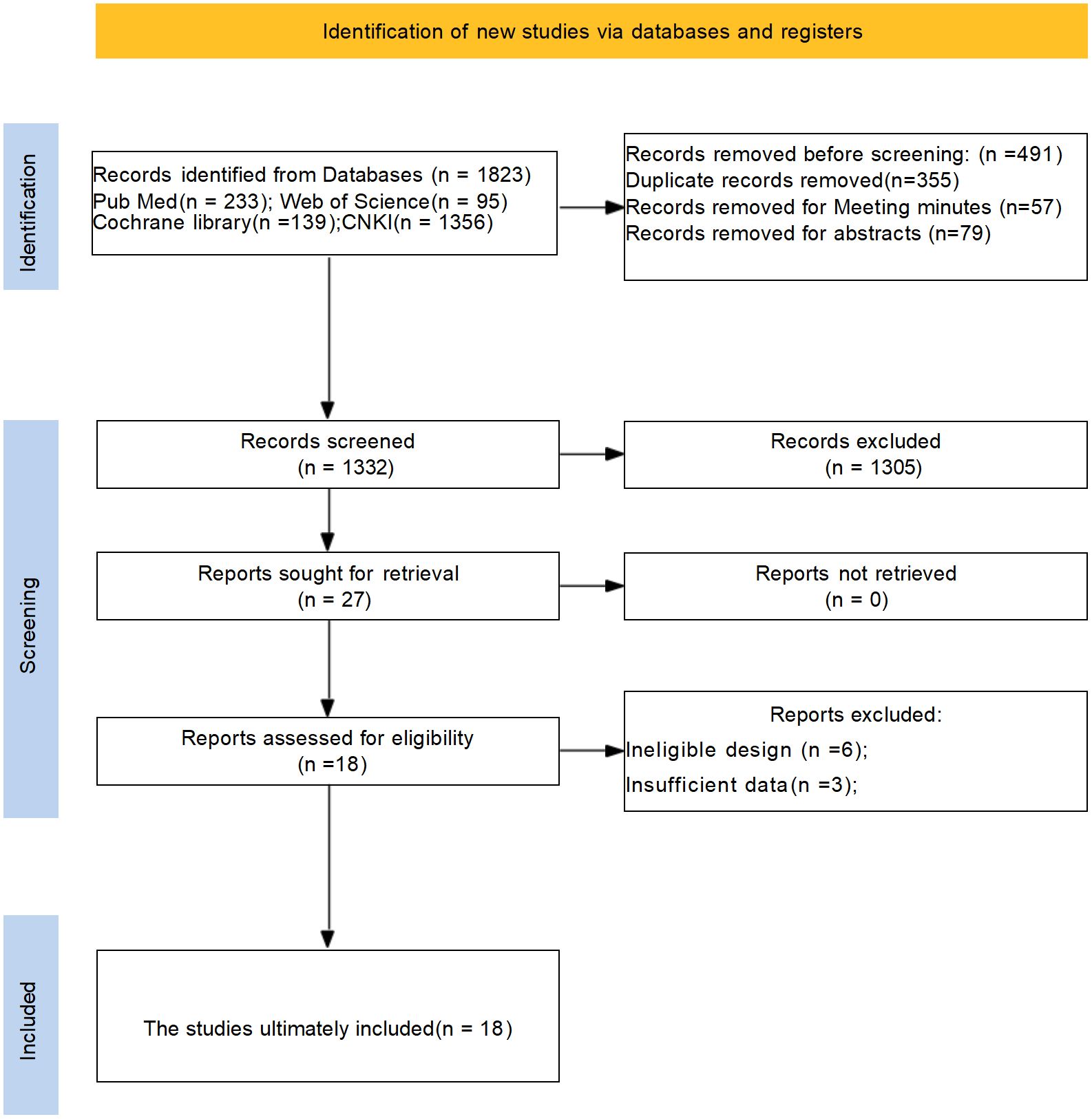
The PRISMA flow diagram clearly outlines the process of literature screening in this systematic review and meta-analysis, adhering to standardized reporting requirements. An initial search yielded 1823 relevant studies. After screening titles, abstracts, and full texts, duplicate and non-qualifying studies were excluded, resulting in the inclusion of 18 rigorously selected studies, ensuring high quality of the included literature. The literature search process and results are depicted in Figure 1.
3.2 Characteristics of included studies
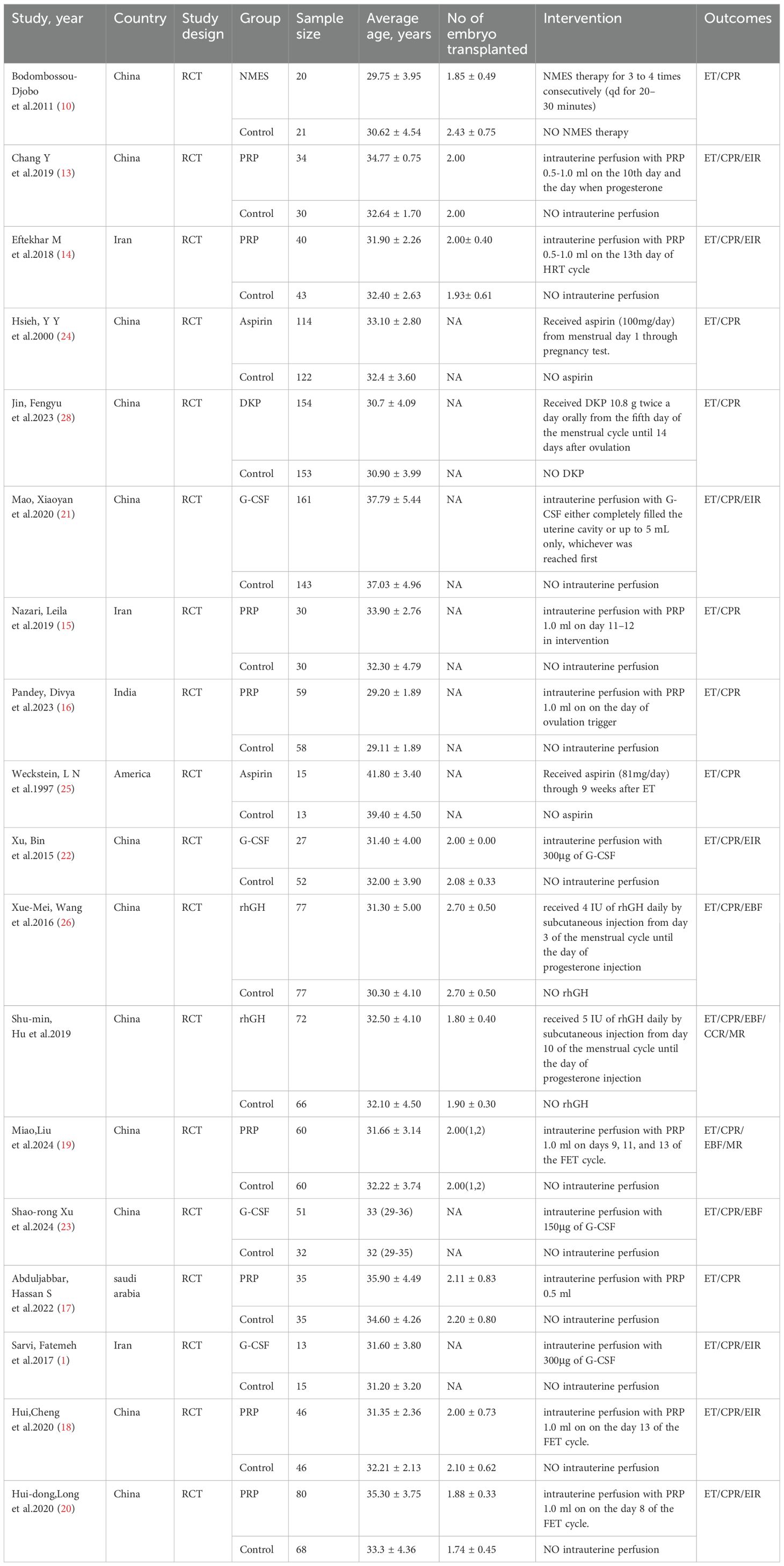
Eighteen clinical studies were included, with sample sizes ranging from 28 to 307, totaling 2152 patients. Of these, 1088 participants were randomly assigned to treatment groups, and 1064 to control groups. The studies included 8 on intrauterine PRP infusion (13–20), 4 on intrauterine G-CSF infusion (1, 21–23), 2 on oral aspirin (24, 25), 2 on intramuscular rhGH (26, 27), 1 on oral Ding Kun Dan (28), and 1 on NMES (10), all RCTs. Study characteristics are detailed in Table 1.
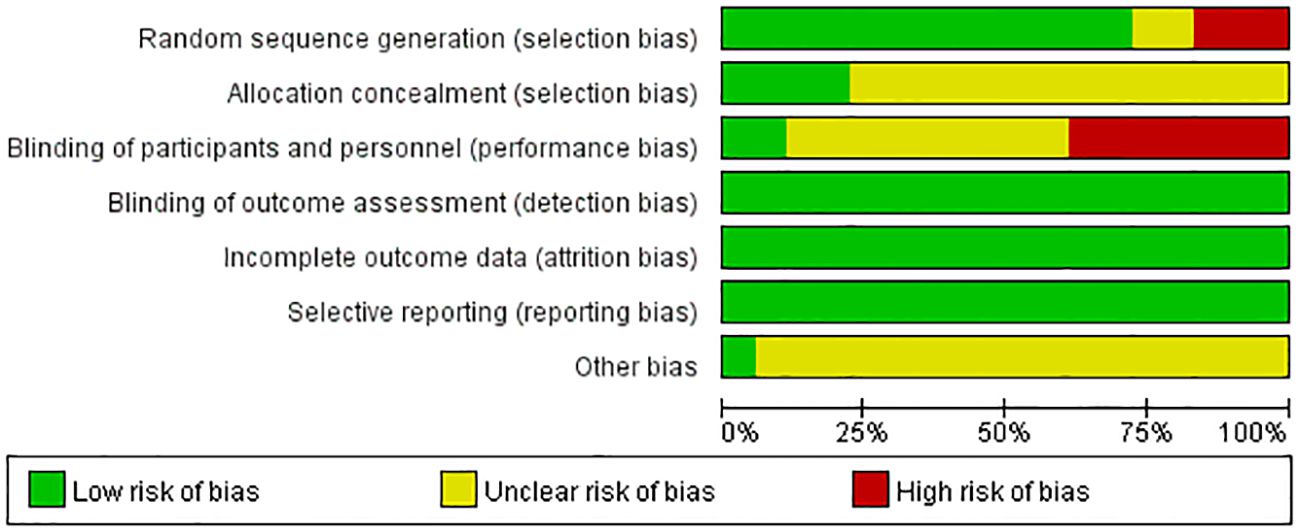
3.3 Risk of bias assessment
Figure 2 displays the risk of bias assessment results for all studies. The quality of included studies was high in terms of random sequence generation and outcome data completeness, but there was a lack of blinding, attributed to the specificity of treatment interventions.
3.4 Meta-analysis results
3.4.1 Endometrial thickness results
Figure 3 presents the results of the subgroup analysis on the impact of various treatment regimens on endometrial thickness, illustrated in a forest plot format. The heterogeneity analysis indicates significant variability in the subgroups receiving oral aspirin and intrauterine growth hormone. This heterogeneity may stem from the limited number of included studies. Based on these findings, we employed a random-effects model for subsequent analyses to more accurately estimate the overall effect size.
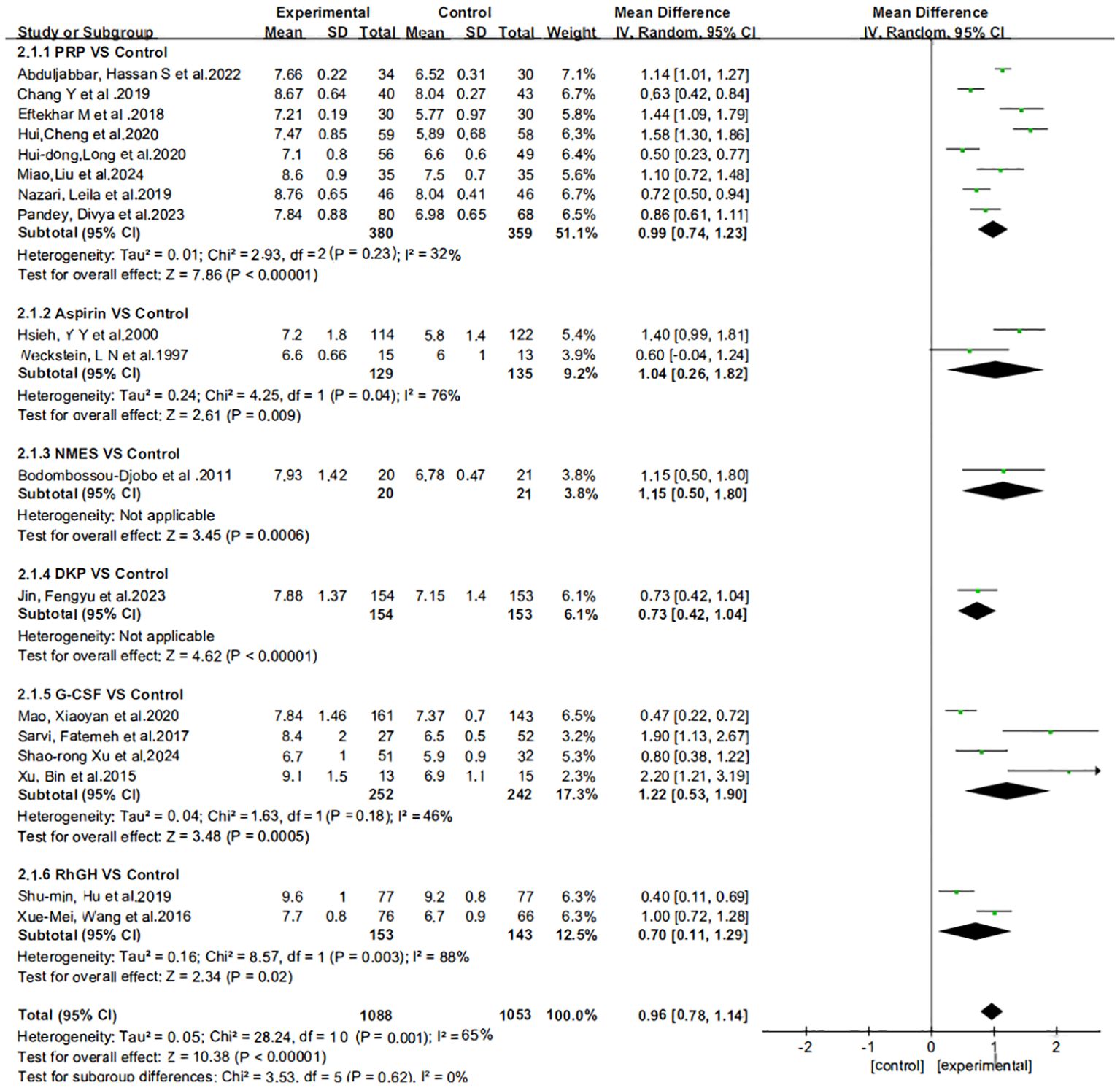
Figure 3. Subgroup analysis and heterogeneity assessment of different treatment regimens on endometrial thickness.
The effect estimates (Mean Difference, MD) for the six interventions (Aspirin, DKP, G-CSF, NMES, PRP, rhGH) relative to the control group in improving endometrial thickness are presented with 95% credible intervals (CrI). Aspirin vs. Control: MD = 1.05, 95% CrI = [0.217, 1.85]. DKP vs. Control: MD = 0.73, 95% CrI = [−0.341, 1.81]. G-CSF vs. Control: MD = 1.12, 95% CrI = [0.576, 1.77]. NMES vs. Control: MD = 0.611, 95% CrI = [−0.628, 1.83]. PRP vs. Control: MD = 0.989, 95% CrI = [0.612, 1.36]. rhGH vs. Control: MD = 0.503, 95% CrI = [−0.253, 1.26]. Summary: Aspirin, G-CSF, and PRP showed statistically significant positive effects. DKP, NMES, and rhGH did not show statistically significant effects, as their credible intervals crossed zero, as shown in Figure 4.
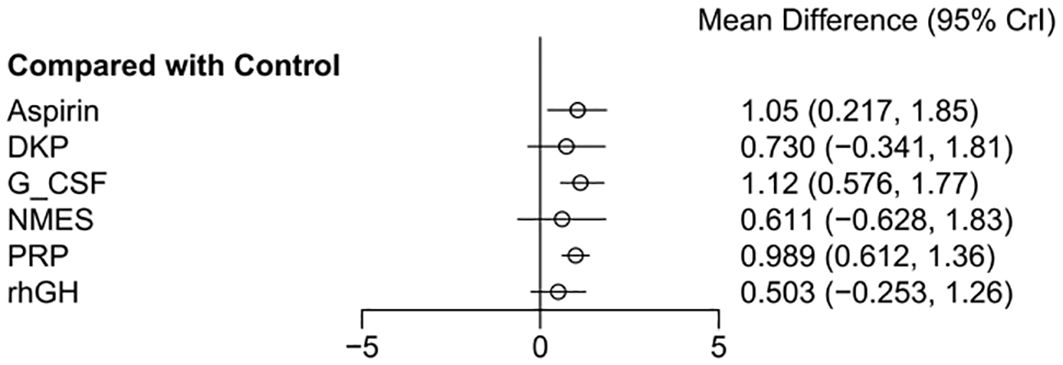
Figure 4. Meta-analysis results of six interventions compared to control group in improving endometrial thickness.
3.4.2 Clinical pregnancy rate results
Figure 5 displays the results of the subgroup analysis regarding the clinical pregnancy outcomes associated with different treatment approaches. The heterogeneity analysis reveals low variability among the studies. Given the low level of heterogeneity detected, we ultimately opted for a fixed-effects model for the analysis.
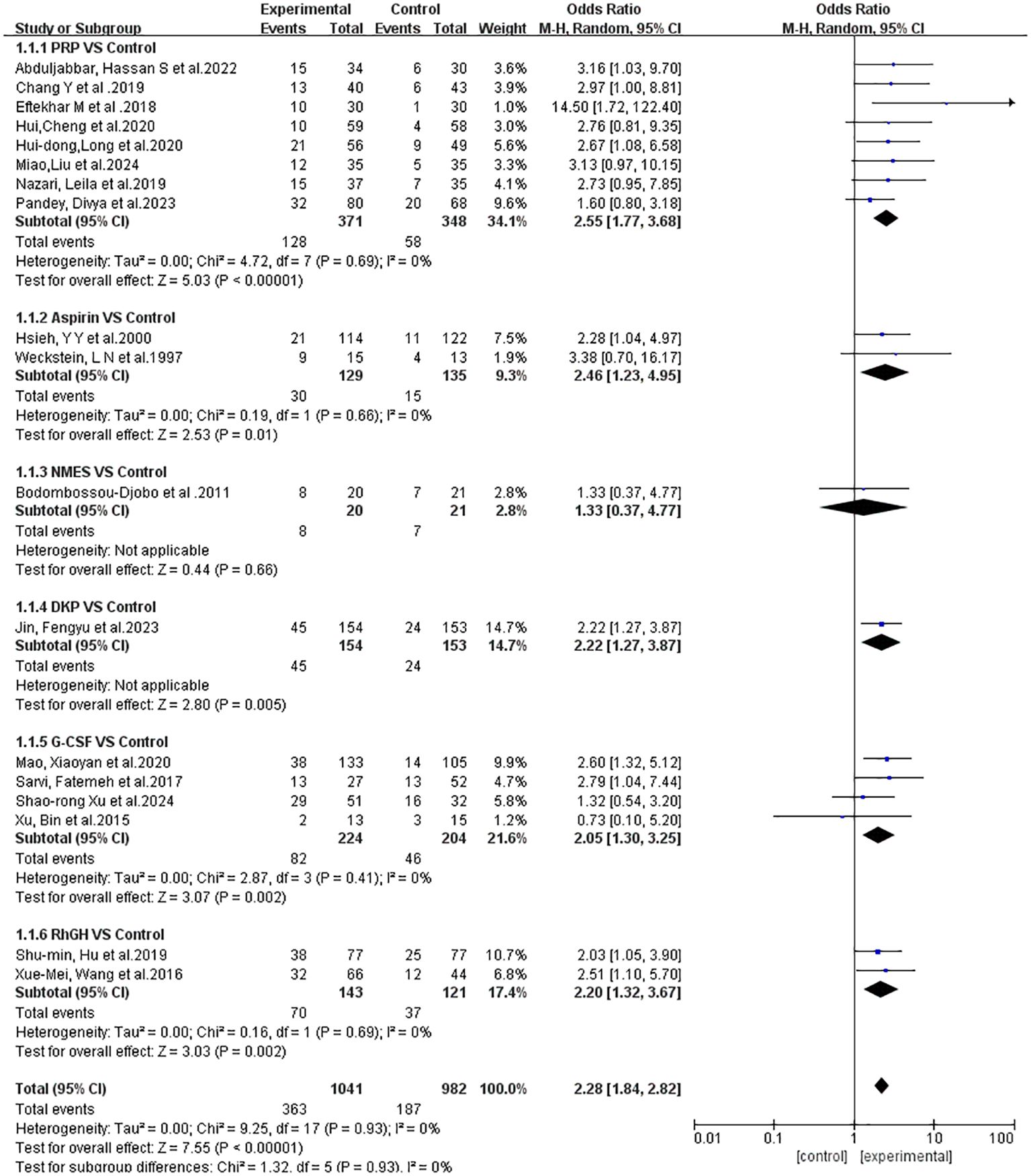
Figure 5. Subgroup analysis and heterogeneity evaluation of various treatment approaches on clinical pregnancy outcomes.
The odds ratios (OR) for the six interventions (Aspirin, DKP, G-CSF, NMES, PRP, rhGH) relative to the control group in improving clinical pregnancy rates are presented with 95% credible intervals (CrI). Aspirin vs. Control: OR = 2.51, 95% CrI = [1.17, 6.09]. DKP vs. Control: OR = 2.27, 95% CrI = [1.02, 5.06]. G-CSF vs. Control: OR = 2.06, 95% CrI = [1.15, 3.53]. NMES vs. Control: OR = 1.39, 95% CrI = [0.350, 5.66]. PRP vs. Control: OR = 2.77, 95% CrI = [1.86, 4.32]. rhGH vs. Control: OR = 1.62, 95% CrI = [0.813, 3.15]. Summary: Interventions with significant effects included Aspirin, DKP, G-CSF, and PRP, as their credible intervals did not cross one. NMES and rhGH did not show significant effects, as shown in Figure 6.
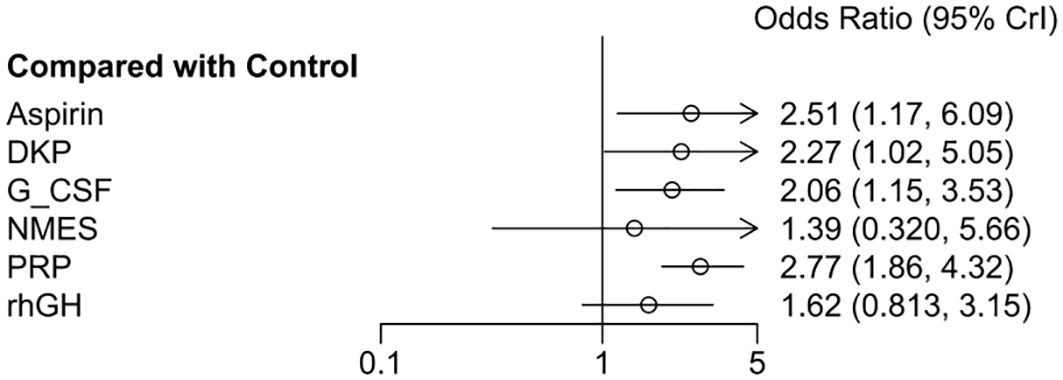
Figure 6. Meta-analysis results of six interventions compared to control group in increasing clinical pregnancy rate.
3.4.3 Network evidence of different interventions
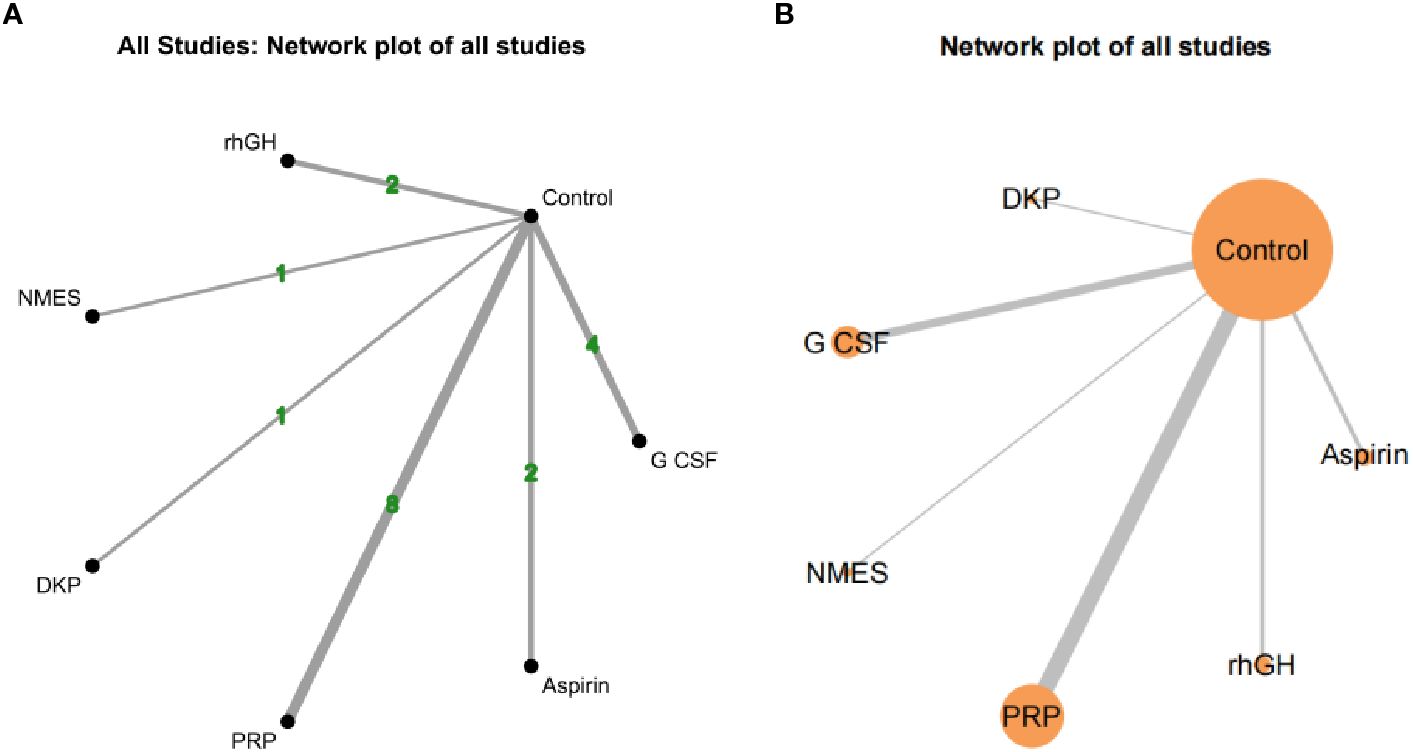
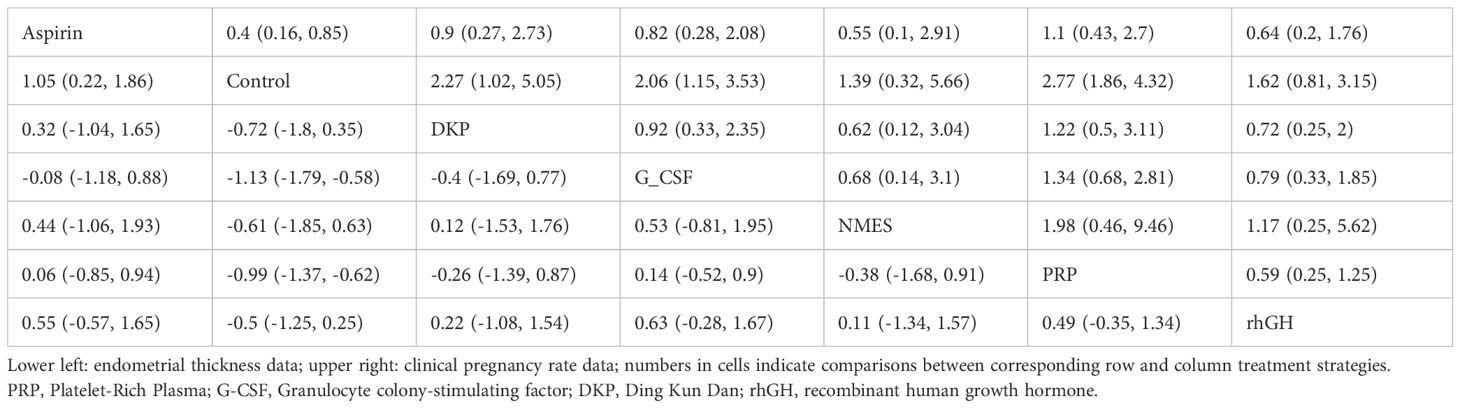
Both network diagrams show the same structure, with Control as the central node directly compared to all interventions. Figure 7A emphasizes the number of direct comparisons, while Figure 7B highlights the weight of sample sizes and direct evidence. The strongest direct evidence exists between PRP and Control (most studies and largest sample size), leading to more reliable conclusions about PRP’s effectiveness. NMES and DKP have the weakest direct evidence (few studies and small sample sizes), making their results potentially unstable and reliant on indirect evidence credibility. As no closed loops were formed between studies, consistency analysis was not conducted, as shown in Figure 7.
3.4.4 Network meta-analysis results
Rows and columns represent different treatment strategies, including Aspirin, Control (placebo), DKP, G-CSF, NMES, PRP, and rhGH. Each cell displays the comparison results between the row and column strategies. Lower left area: comparisons for endometrial thickness. Upper right area: comparisons for clinical pregnancy rate.
Regarding endometrial thickness: Aspirin was significantly better than Control (MD = 0.9, 95% CrI = [0.4, 1.5]), DKP (MD = 1.0, 95% CrI = [0.5, 1.6]), and NMES (MD = 1.2, 95% CrI = [0.6, 1.8]). There was no significant difference between Aspirin and PRP (MD = −0.1, 95% CrI = [−0.6, 0.5]). PRP was significantly better than Control (MD = 1.0, 95% CrI = [0.4, 1.6]) and NMES (MD = 1.2, 95% CrI = [0.6, 1.9]). No significant differences were found between PRP and Aspirin or rhGH. rhGH was significantly better than Control (MD = 1.0, 95% CrI = [0.2, 1.7]) and NMES (MD = 1.3, 95% CrI = [0.5, 2.1]). No significant differences were found between rhGH and Aspirin or PRP.
Regarding clinical pregnancy rate: PRP was significantly better than Control (OR = 2.2, 95% CrI = [1.2, 4.0]), DKP (OR = 2.4, 95% CrI = [1.3, 4.4]), and NMES (OR = 2.9, 95% CrI = [1.5, 5.8]). There was no significant difference between PRP and rhGH (OR = 1.3, 95% CrI = [0.7, 2.6]). rhGH was significantly better than Control (OR = 2.0, 95% CrI = [1.0, 3.9]) and NMES (OR = 2.6, 95% CrI = [1.3, 5.4]). No significant differences were found between rhGH and PRP, Aspirin, or G-CSF. Control was significantly inferior to PRP and rhGH. See Table 2.
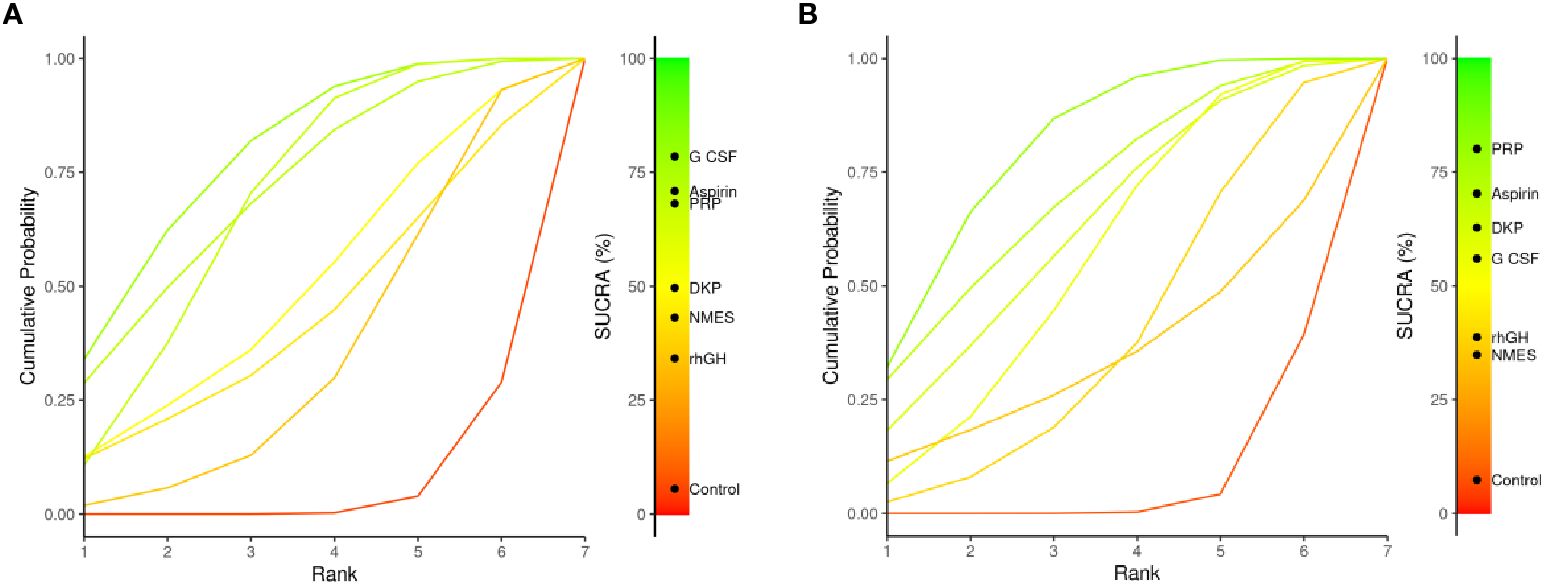
3.4.5 SUCRA analysis
The SUCRA plots (Surface Under the Cumulative Ranking Curve) compare the relative ranking probability distribution of multiple interventions for a particular outcome. A curve closer to the top-left corner indicates a higher overall ranking for that intervention in the outcome measure. Figure 8A shows endometrial thickness: G-CSF’s curve is notably closer to the top-left corner, indicating it is the best-ranked intervention. Aspirin and PRP also have high SUCRA values and rank as the next best. SUCRA ranking for interventions: G-CSF > Aspirin > PRP > DKP > NMES > rhGH > Control. Figure 8B shows clinical pregnancy rate: PRP’s curve is closest to the top-left corner, indicating it is the best-ranked intervention. Aspirin and DKP also have high SUCRA values and rank as the next best. SUCRA ranking for interventions: PRP > Aspirin > DKP > G-CSF > rhGH > NMES > Control. Summary: PRP shows significant advantages in both outcome measures, suggesting it as a comprehensive and effective intervention to be prioritized in clinical practice for treating thin endometrium.
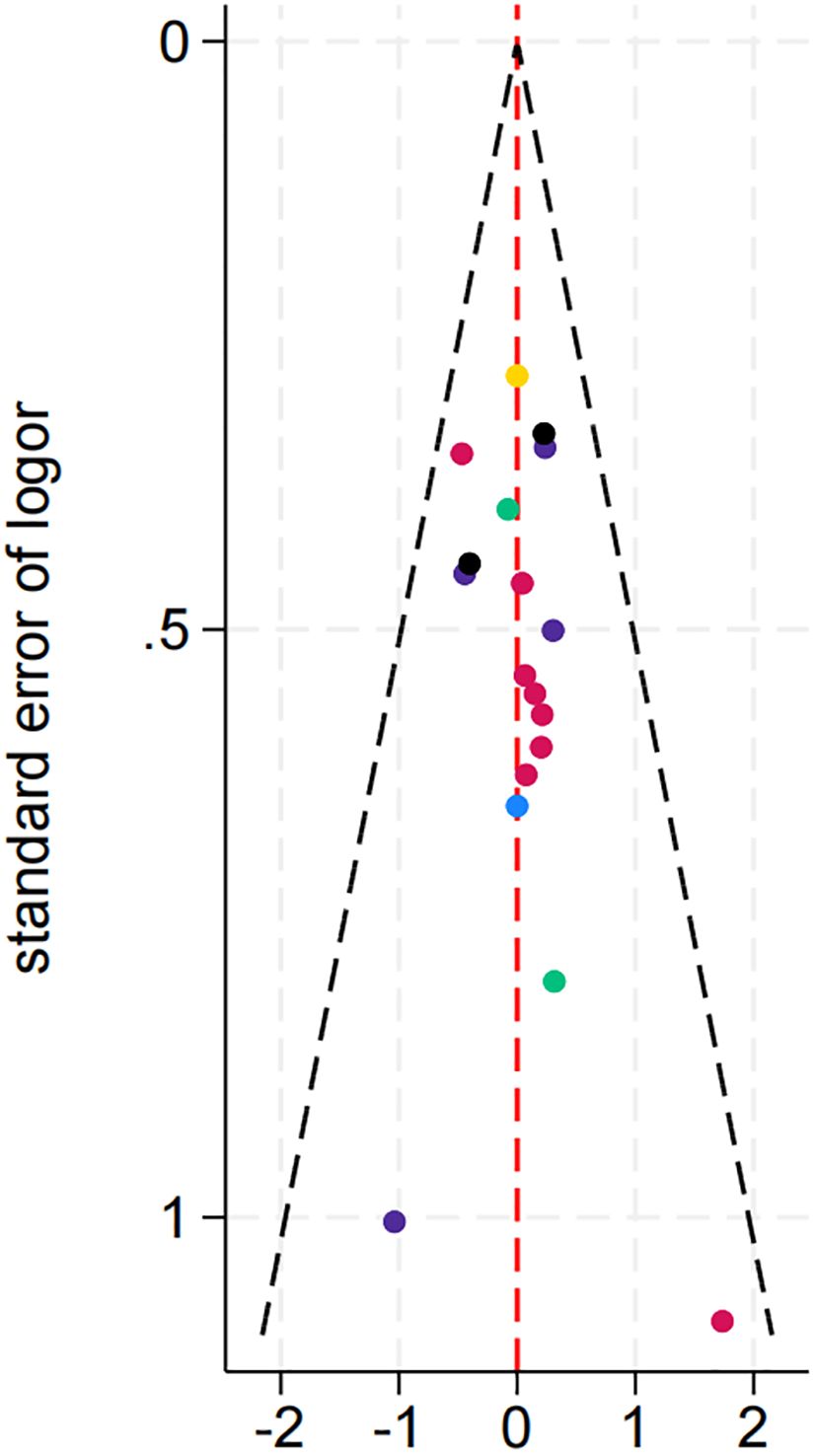
3.4.6 Publication bias
As shown in the Figure 9, the effect sizes and standard errors of the studies are symmetrically distributed, suggesting minimal publication bias or other forms of systematic bias.
3.5 Discussion
This study incorporated 18 randomized controlled trials (RCTs) that met the inclusion criteria, involving a total of 2,152 participants. While the cumulative probability plots indicated that granulocyte colony-stimulating factor (G-CSF) had a higher SUCRA value for improving endometrial thickness, ranking the interventions as G-CSF > Aspirin > Platelet-Rich Plasma (PRP), direct meta-analysis revealed no significant difference between PRP and Aspirin in terms of enhancing endometrial thickness. However, in direct comparisons and cumulative probability plots for improving clinical pregnancy rates, PRP significantly outperformed other interventions. Thus, although G-CSF may hold an advantage in enhancing endometrial thickness, PRP demonstrates superior efficacy in both improving endometrial thickness and increasing clinical pregnancy rates, suggesting it may be the optimal treatment for patients with thin endometrium.
Despite the variety of treatment options available for thin endometrium, each method has its advantages and disadvantages, and their efficacy can vary significantly. Estrogen primarily acts by activating estrogen receptor pathways, inducing the transformation of endometrial epithelial cells and promoting the mitosis of both endometrial and stromal cells, thereby facilitating the repair of the endometrium. However, the effects of estrogen can differ among individuals, and long-term use may increase the risks of endometrial hyperplasia, thrombosis, and breast cancer (29). Additionally, high doses of estrogen may lead to increased secretion of inflammatory and adhesion factors in the endometrium, which can cause stromal fibrosis and ultimately hinder the repair of the endometrium. Aspirin, as a cyclooxygenase (COX) inhibitor, exerts its effects by inhibiting the synthesis of thromboxane A2, thereby promoting the release of prostacyclin 2 from endothelial cells. This mechanism results in vasodilation, reduced platelet aggregation, and decreased vascular resistance, leading to improved endometrial thickness (EMT) and microcirculation, which enhances endometrial receptivity. However, studies have indicated that COX-2 plays a crucial role in regulating the implantation window of the endometrium and the process of embryo implantation, and the use of aspirin may inhibit COX-2 synthesis, potentially affecting successful embryo implantation (30). Stem cell therapy, which involves primitive undifferentiated cells with self-renewal and differentiation potential, has shown promise in effectively promoting the regeneration and proliferation of the endometrium. Its mechanisms of action primarily include inducing cell differentiation and immune modulation. However, the complexity involved in obtaining and preparing stem cells, along with stringent ethical regulations, poses significant challenges to their clinical application (31, 32). Although granulocyte-colony stimulating factor (G-CSF) has demonstrated some efficacy in clinical treatments, the quality of existing research regarding the dosing and safety of G-CSF is relatively low. Therefore, there is an urgent need for further exploration of optimal dosing regimens and potential risks associated with its use (33, 34).
Platelet-rich plasma (PRP) is a biologic derived from centrifuged autologous peripheral venous blood, rich in platelets and associated cytokines (35). PRP contains 4 to 8 times the platelet concentration found in whole blood and releases various growth factors and cytokines upon activation, including platelet-derived growth factor, transforming growth factor-beta, insulin-like growth factor, epidermal growth factor, fibroblast growth factor, and vascular endothelial growth factor. These autologous growth factors and cytokines promote cell proliferation, differentiation, chemotaxis, and angiogenesis (36–38). During platelet formation, platelets accumulate numerous molecules from the culture medium through endocytosis, including lipid mediators like sphingosine-1-phosphate (SPP), phosphatidic acid, and lysophosphatidic acid, which exhibit anti-apoptotic effects on endothelial cells, participate in chemotaxis, and promote capillary formation. Sphingosine acts as a ligand for endothelial differentiation gene (EDG) receptors, playing a crucial role in angiogenesis, while overexpression of vascular endothelial growth factor receptor 2 sensitizes endothelial cells to vascular endothelial growth factor, initiating angiogenic processes (39, 40). Additionally, PRP can function as an immunomodulator to mitigate immune responses and reduce the release of pro-inflammatory factors such as IL-6, IL-1β, and IL-8 within the endometrium, thereby attracting macrophages and neutrophils (41). Consequently, PRP may enhance endometrial receptivity by modulating the cellular immune microenvironment. In 2015, Chang et al. first applied intrauterine infusion of PRP in frozen embryo transfer (FET) cycles, improving the endometrial conditions of patients with thin endometrium and facilitating successful clinical pregnancies (42). In recent years, studies on PRP treatment for thin endometrium have surged. For instance, Einfeldt et al. conducted a randomized trial showing that PRP injection significantly increased endometrial thickness and local microcirculation, enhancing cell proliferation and angiogenesis to support embryo implantation (43). Additionally, a double-blind randomized study demonstrated higher chemical and clinical pregnancy rates in the PRP group compared to controls, indicating significant efficacy in patients with refractory thin endometrium (15). While PRP improves uterine conditions and pregnancy rates, positioning itself as a new strategy in assisted reproductive technology (ART), it faces clinical application challenges: (i) PRP preparation lacks standardization, and its mechanism of action, optimal concentration, activity, and treatment regimen for different conditions remain unclear; (ii) the relatively low concentration of growth factors necessitates repeated treatments. Further basic research and large-scale, multicenter RCTs are required to validate the long-term efficacy of PRP in ART.
4 Study limitations
This study has several limitations: (i)This analysis was restricted to randomized controlled trials (RCTs) published in English or Chinese, and certain treatment options (e.g., Ding Kun Dan and neuromuscular electrical stimulation) had only one eligible study each, which may introduce selection bias and compromise the accuracy and reliability of the findings; (ii)Although data from multiple countries were included, the majority originated from China, potentially limiting the generalizability of the results due to regional variations in patient demographics and clinical practices; (iii) Some of the included studies did not adequately implement randomization or blinding procedures, which may affect the internal validity and robustness of the pooled results.
5 Conclusions
In conclusion, the network meta-analysis of the included studies indicates that PRP is the most effective among common treatments for thin endometrium in enhancing endometrial thickness and improving clinical pregnancy rates. However, further high-quality research is needed to substantiate this finding. In clinical practice, we advise careful consideration of these results, with treatment selection based on specific clinical contexts.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
FK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ZZ: Data curation, Methodology, Writing – original draft, Writing – review & editing. ZL: Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. LY: Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing. LX: Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. LD: Data curation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. ZJ: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. LL: Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. WL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Guangxi Autonomous Region Health Committee Self-Financed Fund Project (Project No.: Z-K20231788).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sarvi F, Arabahmadi M, Alleyassin A, Aghahosseini M, and Ghasemi M. Effect of increased endometrial thickness and implantation rate by granulocyte colony-stimulating factor on unresponsive thin endometrium in fresh in vitro fertilization cycles: A randomized clinical trial. Obstet Gynecol Int. (2017) 2017:3596079. doi: 10.1155/2017/3596079
2. Zhao J, Huang G, Sun H, Liqing F, Yun F, Huan S, et al. Chinese expert consensus on the diagnosis and treatment of abnormal endometrium in assisted reproductive technology. J Reprod Med. (2018) 27:1057–64. doi: 10.3969/.issn.10043845.2018.11.003
3. Miwa I, Tamura H, Takasaki A, Yamagata Y, Shimamura K, and Sugino N. Pathophysiologic features of “thin” endometrium. Fertil Steril. (2009) 91:998–1004. doi: 10.1016/j.fertnstert.2008.01.029
4. Yuan R and Le AW. A study on the estrogen receptor α gene polymorphism and its expression in thin endometrium of unknown etiology. Gynecol Obstet Invest. (2012) 74:13–20. doi: 10.1159/000334174
5. Von Wolff M, Fäh M, Roumet M, Mitter V, Stute P, Griesinger G, et al. Thin endometrium is also associated with lower clinical pregnancy rate in unstimulated menstrual cycles: A study based on natural cycle IVF. Front Endocrinol (Lausanne). (2018) 9:776. doi: 10.3389/fendo.2018.00776
6. Chen MJ, Yang JH, Peng FH, Chen SU, Ho HN, and Yang YS. Extended estrogen administration for women with thin endometrium in frozen-thawed in-vitro fertilization programs. J Assist Reprod Genet. (2006) 23:337–42. doi: 10.1007/s10815-006-9053-1
7. Zinger M, Liu JH, and Thomas MA. Successful use of vaginal sildenafil citrate in two infertility patients with Asherman’s syndrome. J Womens Health (Larchmt). (2006) 15:442–4. doi: 10.1089/jwh.2006.15.442
8. Lédée-Bataille N, Olivennes F, Lefaix JL, Chaouat G, Frydman R, and Delanian S. Combined treatment by pentoxifylline and tocopherol for recipient women with a thin endometrium enrolled in an oocyte donation programme. Hum Reprod. (2002) 17:1249–53. doi: 10.1093/humrep/17.5.1249
9. Zhao J, Xu B, Xie S, Zhang Q, and Li YP. Whether G-CSF administration has beneficial effect on the outcome after assisted reproductive technology? A systematic review and meta-analysis. Reprod Biol Endocrinol. (2016) 14:62. doi: 10.1186/s12958-016-0197-2
10. Bodombossou-Djobo MM, Zheng C, Chen S, and Yang D. Neuromuscular electrical stimulation and biofeedback therapy may improve endometrial growth for patients with thin endometrium during frozen-thawed embryo transfer: a preliminary report. Reprod Biol Endocrinol. (2011) 9:122. doi: 10.1186/1477-7827-9-122
11. Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, et al. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum Reprod. (2016) 31:2723–9. doi: 10.1093/humrep/dew235
12. Li L, Kou Z, Zhao F, Wang Y, and Zhang X. Network meta-analysis of four common immunomodulatory therapies for the treatment of patients with thin endometrium. Gynecol Endocrinol. (2024) 40:2360072. doi: 10.1080/09513590.2024.2360072
13. Chang Y, Li J, Wei LN, Pang J, Chen J, and Liang X. Autologous platelet-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Med (Baltimore). (2019) 98:e14062. doi: 10.1097/MD.0000000000014062
14. Eftekhar M, Neghab N, Naghshineh E, and Khani P. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwan J Obstet Gynecol. (2018) 57:810–3. doi: 10.1016/j.tjog.2018.10.007
15. Nazari L, Salehpour S, Hoseini S, Zadehmodarres S, and Azargashb E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: A double-blind RCT. Int J Reprod BioMed. (2019) 17:443–8. doi: 10.18502/ijrm.v17i6.4816
16. Pandey D, Bajaj B, Kapoor G, and Bharti R. Intrauterine instillation of autologous platelet-rich plasma in infertile females with thin endometrium undergoing intrauterine insemination: an open-label randomized controlled trial. AJOG Glob Rep. (2023) 3:100172. doi: 10.1016/j.xagr.2023.100172
17. Abduljabbar HS, Hashim H, Abduljabar HH, Elnaeim AA, and Abduljabar NH. The effect of autologous platelet-rich plasma treatment on in vitro fertilization/intracytoplasmic sperm injection and its impact on the endometrium and clinical pregnancy rate. Cureus. (2022) 14:e27913. doi: 10.7759/cureus.27913
18. Hui C, Kepeng L, Yuan L, and Yaqiong G. The effect of platelet-rich plasma on pregnancy outcomes in patients with thin endometrium undergoing cryopreservation cycles. Modern Obstetrics and Gynecology Progress. (2020) 29(6):450–2. doi: 10.13283/j.cnki.xdfckjz.2020.06.008
19. Liu M, Wei X, Zhou Z, Huixia X, and Qun L. Efficacy analysis of platelet-rich plasma infusion treatment in patients with thin endometrium. J Pract Clin Med. (2024) 21:127–31.
20. Long H, Wang F, Wu D, Weifen D, Yuqun H, and Yubin L. Comparative study on the efficacy of two intrauterine infusion treatments in patients with thin endometrium. J Reprod Med. (2020) 29:1421–6. doi: 10.3969/j.issn.1004-3845.2020.11.005
21. Mao X, Zhang J, Cai R, Tao Y, Gao H, Kuang Y, et al. Therapeutic role of granulocyte macrophage colony-stimulating factor (GM-CSF) in patients with persistent thin endometrium: A prospective and randomized study. Int J Gynaecol Obstet. (2020) 150:194–9. doi: 10.1002/ijgo.13152
22. Xu B, Zhang Q, Hao J, Xu D, and Li Y. Two protocols to treat thin endometrium with granulocyte colony-stimulating factor during frozen embryo transfer cycles. Reprod BioMed Online. (2015) 30:349–58. doi: 10.1016/j.rbmo.2014.12.006
23. Xu S, Ma Q, Zhang Y, An Y, He W, Ma Y, et al. Effect of intrauterine perfusion of granulocyte colony-stimulating factor on endometrium and blood flow parameters in patients with thin endometrium: A prospective controlled clinical trial. Sichuan Da Xue Xue Bao Yi Xue Ban. (2024) 55:574–9. doi: 10.12182/20240560504
24. Hsieh YY, Tsai HD, Chang CC, Lo HY, and Chen CL. Low-dose aspirin for infertile women with thin endometrium receiving intrauterine insemination: a prospective, randomized study. J Assist Reprod Genet. (2000) 17:174–7. doi: 10.1023/A:1009474307376
25. Weckstein LN, Jacobson A, Galen D, Hampton K, and Hammel J. Low-dose aspirin for oocyte donation recipients with a thin endometrium: prospective, randomized study. Fertil Steril. (1997) 68:927–30. doi: 10.1016/S0015-0282(97)00330-0
26. Xue-Mei W, Hong J, Wen-Xiang Z, and Yang L. The effects of growth hormone on clinical outcomes after frozen-thawed embryo transfer. Int J Gynaecol Obstet. (2016) 133:347–50. doi: 10.1016/j.ijgo.2015.10.020
27. Shumin H, Yifu L, Linlin M, Yanjiao Z, Yang G, Xulei S, et al. Clinical research on the application of growth hormone in endometrial preparation during frozen-thawed embryo transfer cycles in patients with thin endometrium. Chin J Reprod Med Contraception. (2019) 39(12):963–7. doi: 10.3760/cma.j.issn.2096-2916.2019.12.002
28. Jin F, Ruan X, Qin S, Xu X, Yang Y, and Gu M. Traditional Chinese medicine Dingkun pill to increase fertility in women with a thin endometrium-a prospective randomized study. Front Endocrinol (Lausanne). (2023) 14:1168175. doi: 10.3389/fendo.2023.1168175
29. Luo J and Liu D. Does GPER really function as a G protein-coupled estrogen receptor in vivo? Front Endocrinol (Lausanne). (2020) 11:148. doi: 10.3389/fendo.2020.00148
30. Tian J and Ling X. Research progress on the treatment of thin endometrium in assisted reproductive technology. Chin J Clin New Med. (2023) 16:746–50. doi: 10.3969/j.issn.1674-3806.2023.07.22
31. Lee YJ and Yi KW. Bone marrow-derived stem cells contribute to regeneration of the endometrium. Clin Exp Reprod Med. (2018) 45:149–53. doi: 10.5653/cerm.2018.45.4.149
32. Hu J, Song K, Zhang J, Zhang Y, and Tan BZ. Effects of menstrual blood−derived stem cells on endometrial injury repair. Mol Med Rep. (2019) 19:813–20.
33. Ding J, Wang J, Cai X, Yin T, Zhang Y, Yang C, et al. Granulocyte colony-stimulating factor in reproductive-related disease: Function, regulation and therapeutic effect. BioMed Pharmacother. (2022) 150:112903. doi: 10.1016/j.biopha.2022.112903
34. Fu LL, Xu Y, Yan J, Zhang XY, Li DD, and Zheng LW. Efficacy of granulocyte colony-stimulating factor for infertility undergoing IVF: a systematic review and meta-analysis. Reprod Biol Endocrinol. (2023) 21:34. doi: 10.1186/s12958-023-01063-z
35. Collins T, Alexander D, and Barkatali B. Platelet-rich plasma: a narrative review. EFORT Open Rev. (2021) 6:225–35. doi: 10.1302/2058-5241.6.200017
36. Yuan T, Guo SC, Han P, Zhang CQ, and Zeng BF. Applications of leukocyte- and platelet-rich plasma (L-PRP) in trauma surgery. Curr Pharm Biotechnol. (2012) 13:1173–84. doi: 10.2174/138920112800624445
37. Opneja A, Kapoor S, and Stavrou EX. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb Res. (2019) 179:56–63. doi: 10.1016/j.thromres.2019.05.001
38. Alves R and Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. (2018) 4:18–24. doi: 10.1159/000477353
39. Molina A, Sánchez J, Sánchez W, and Vielma V. Platelet-rich plasma as an adjuvant in the endometrial preparation of patients with refractory endometrium. JBRA Assist Reprod. (2018) 22:42–8. doi: 10.5935/1518-0557.20180009
40. Paiva P, Hannan NJ, Hincks C, Meehan KL, Pruysers E, Dimitriadis E, et al. Human chorionic gonadotrophin regulates FGF2 and other cytokines produced by human endometrial epithelial cells, providing a mechanism for enhancing endometrial receptivity. Hum Reprod. (2011) 26:1153–62. doi: 10.1093/humrep/der027
41. Hajipour H, Farzadi L, Latifi Z, Keyhanvar N, Navali N, Fattahi A, et al. An update on platelet-rich plasma (PRP) therapy in endometrium and ovary related infertilities: clinical and molecular aspects. Syst Biol Reprod Med. (2021) 67:177–88. doi: 10.1080/19396368.2020.1862357
42. Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, et al. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. (2015) 8:1286–90.
Keywords: thin endometrium, endometrial thickness, clinical pregnancy rate, network meta-analysis, treatment
Citation: Keng F, Ling W, Zhao Z, Yudi L, Xiang L, Derong L, Junjie Z, Liuping L and Lingling Z (2025) Network meta-analysis on the efficacy of different interventions for treating thin endometrium. Front. Endocrinol. 16:1575248. doi: 10.3389/fendo.2025.1575248
Received: 12 February 2025; Accepted: 21 July 2025;
Published: 20 August 2025.
Edited by:
Zhice Xu, Wuxi Maternity and Child Health Care Hospital, ChinaReviewed by:
Hilma Putri Lubis, Universitas Sumatera Utara, IndonesiaKarina Sequeira, Shady Grove Fertility Center, United States
Tian Xia, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, China
Copyright © 2025 Keng, Ling, Zhao, Yudi, Xiang, Derong, Junjie, Liuping and Lingling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhu Lingling, NTI1OTgwNDg1QHFxLmNvbQ==
†These authors share first authorship
 Feng Keng
Feng Keng Wu Ling2†
Wu Ling2† Luo Yudi
Luo Yudi