- 1Department of Endocrinology, Endocrine and Metabolic Disease Medical Center, Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, Nanjing, China
- 2Branch of National Clinical Research Center for Metabolic Diseases, Nanjing, China
- 3Department of General Surgery, Drum Tower Hospital Affiliated to Nanjing Medical University, Nanjing, China
- 4Department of Endocrinology, Endocrine and Metabolic Disease Medical Center, Affiliated Drum Tower Hospital, Medical School, Nanjing University, Nanjing, China
Background: Liraglutide effectively manages mild obesity, but individual weight loss outcomes vary significantly. We aimed to identify clinical predictors influencing differential treatment responses in patients with mild obesity.
Methods: A retrospective analysis was conducted on 64 adults (BMI 28–32.5 kg/m²) undergoing a 12-week liraglutide intervention. Participants were categorized based on therapeutic success: those achieving composite endpoints (≥5% total weight loss [TWL] and BMI normalization to <28 kg/m²) versus suboptimal responders. Comprehensive biometric and biochemical assessments were performed, and multivariate predictive modeling was applied.
Results: Responders (n=37, 75.7% female) showed significantly better metabolic outcomes than non-responders (n=27, 77.8% female), with notable differences in %TWL (11.0 ± 3.6% vs 4.2 ± 2.6%), total weight loss (9.04 ± 3.32 kg vs 3.55 ± 2.20 kg), and BMI reduction (3.3 ± 1.1 vs 1.4 ± 0.9 kg/m²) (all p’s <.01). Responders also demonstrated improved glucolipid metabolism, and reduced metabolic-associated fatty liver disease (p <.05). Regression analysis identified a history metabolic surgery (MS) and a baseline BMI ≥30.5 kg/m² as significant negative predictors of success. Adjusted odds ratios indicated strong inverse associations, with MS history showing an OR of 6.78 (95% CI: 1.95–23.61; p <.01) and elevated BMI (≥30.5 kg/m²) yielding an OR of 4.79 (95% CI: 1.46–15.71; p <.01).
Conclusion: A history of MS significantly affects liraglutide’s responsiveness in patients with mild obesity, emphasizing the need for personalized therapeutic strategies in post-surgical patients. These findings highlight the importance of a comprehensive medical history in guiding obesity pharmacotherapy.
1 Introduction
Mild obesity (body mass index [BMI] 28–32.5 kg/m²) is a significant contributor to the obesity epidemic in China, posing risks for metabolic complications such as metabolic dysfunction-associated steatotic liver disease (MASLD), cardiometabolic disorders, and progression to moderate-severe obesity (BMI ≥32.5 kg/m²) if untreated (1). Cohort studies suggest that a 5–10% total weight loss (%TWL) in this group can significantly reduce obesity-related complications, making weight management the first-line therapeutic approach (1, 2).
Glucagon-like peptide-1 receptor agonists (GLP-1RAs), including liraglutide, have revolutionized obesity management through their therapeutic efficacy, achieving an average of 8.0% weight loss in phase III clinical trials (3). However, individual weight loss outcomes vary (3–5), and the factors influencing this variability are not fully understood. Identifying predictors of liraglutide’s weight-loss efficacy could optimize its therapeutic use.
For patients with moderate to severe obesity, metabolic surgery offers significant benefits by promoting substantial weight loss and improving metabolic health. These procedures typically function by restricting food intake, altering nutrient absorption, or both (6, 7).The most commonly performed procedure is sleeve gastrectomy (SG), which involves removing approximately 80% of the stomach to create a tubular “sleeve,” thereby reducing stomach capacity and decreasing hunger hormone (ghrelin) production. Another prevalent procedure is the Roux-en-Y gastric bypass (RYGB), which creates a small stomach pouch and reroutes the small intestine to limit both food intake and nutrient absorption. biliopancreatic diversion with duodenal switch (BPD-DS) combines a sleeve gastrectomy with a significant bypass of the small intestine, leading to substantial weight loss and metabolic improvements, particularly in type 2 diabetes management. However, BPD-DS carries a higher risk of nutritional deficiencies, making it less commonly performed (6–8). SG is associated with a lower perioperative complication rate compared to RYGB and demonstrates comparable efficacy in weight loss and improvement in metabolic indicators within the initial years post-surgery (8). Consequently, SG has become increasingly popular, representing a growing proportion of metabolic surgeries (8). However, long-term outcomes may favor RYGB concerning sustained weight loss and metabolic benefits (6–8). A newer, simplified procedure, the single-anastomosis duodenoileal bypass with sleeve gastrectomy (SADI-S), which utilizes a single intestinal connection to reduce surgical complexity while maintaining efficacy. Studies indicate that SADI-S achieves comparable or superior weight loss outcomes to RYGB and SG, with fewer long-term complications (9). These surgical options are tailored based on individual patient profiles, considering factors such as BMI, comorbidities, and previous surgical history.
With the growing prevalence of metabolic surgery (MS), a significant number of patients post-MS experienced weight regain, leading to a recurrence of mild to moderate-severe obesity if not managed promptly. Adjunctive weight loss therapies are increasingly needed for this patient group (10). A meta-analysis of 16 studies (N=881) showed that liraglutide treatment (ranging from 3 months to 4 years) resulted in a mean weight reduction of 16.03 kg in patients with ≥ 5 years post-MS (11). In patients exhibiting persistent/recurrent type 2 diabetes mellitus (T2DM) post-MS and a baseline BMI ≥37.0 kg/m², liraglutide achieved a mean weight reduction of 5.26 kg over 26 weeks (12). Comparative analyses demonstrated comparable weight reduction between patients post-MS and non-surgical counterparts undergoing liraglutide therapy. Notably, these findings were observed in cohorts with a baseline BMI ≥35 kg/m², a threshold exceeding current therapeutic guidelines for anti-obesity medications (12, 13). Although patients with mild obesity constitute part of the indicated population for liraglutide therapy, it remains unclear whether prior MS influences the observed heterogeneity in weight loss outcomes.
To fill this knowledge gap, we initiated the current research to analyze the response of patients with mild obesity to liraglutide, including those post-MS. The findings indicate that previous MS reduces GLP-1RA efficacy, underscoring the need for tailored therapeutic strategies in this rapidly expanding patient population.
2 Materials and methods
2.1 Study design
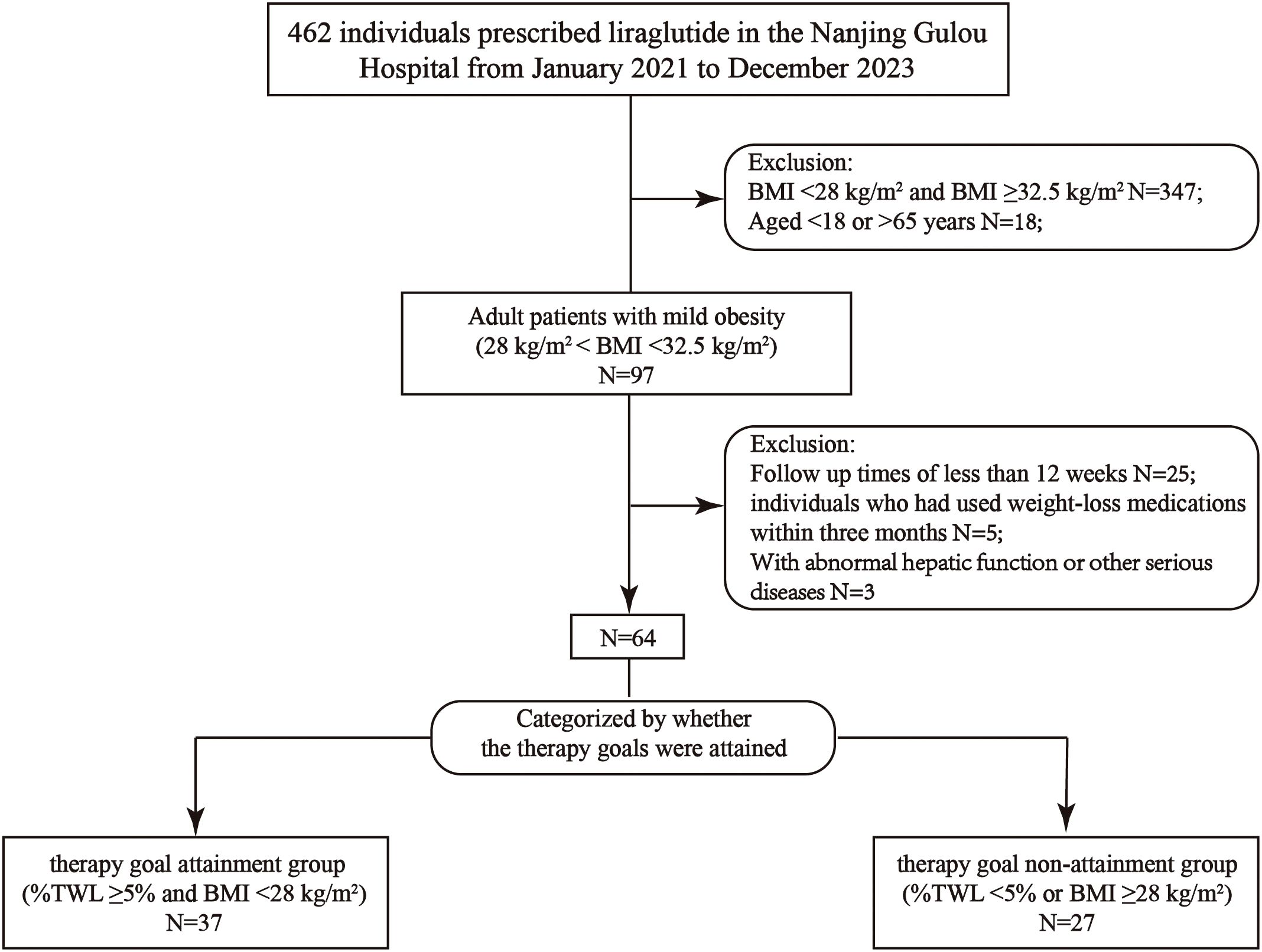
This retro-cohort study was carried out from February 2021 to December 2023 at Drum Tower Hospital, affiliated with Nanjing University Medical School in Nanjing, China. Adult patients with mild obesity (28 kg/m2 ≤ BMI <32.5 kg/m2) who completed 12 weeks of liraglutide treatment were enrolled in the present study. Participants were grouped according to the attainment of goals or not after treatment: the goal attainment group (achieved treatment targets of ≥5% total weight loss [%TWL] and BMI < 28 kg/m²) and the goal non-attainment group (did not meet the targets) (Figure 1).
Liraglutide dosing 0.6 mg per day in the initial week, increasing to 1.2 mg on week 2, and 1.8 mg per day in the 3rd week until completion of treatment. Weight reduction and alleviation of the obesity-related complications from the two groups were analyzed. Personalized guidance on diet and physical activity was provided to all patients. The dietary intervention targeted an approximate daily caloric reduction of 500 kcal, along with a recommended exercise regimen comprising 150 minutes of moderate-intensity aerobic activity and 60 minutes of resistance training per week. These recommendations were adjusted during follow-up visits based on each patient’s progress and clinical feedback. However, adherence to the prescribed dietary and exercise protocols was not quantitatively assessed.
This study was approved by the ethics committee of Nanjing Drum Tower Hospital (2023-507) and in accordance with the Declaration of Helsinki. All participants had written informed consensus.
2.2 Study population
Adult patients with mild obesity who completed 12 weeks of liraglutide treatment were enrolled and received follow-up visits every 4 weeks from the start of treatment with nutritional and physical activity counseling (14).
Patients were excluded if they had used weight-affecting drugs such as GLP-1RAs, sodium-glucose cotransporter protein 2 inhibitors, metformin, orlistat within 3 months before treatment. Additionally, exclusion of individuals with glomerular filtration rate (eGFR) <60 ml/min/1.73 m2, alanine aminotransferase (ALT) >100 U/L, aspartate aminotransferase (AST) >100 U/mL, serum procalcitonin levels above the limit of normal, or suffering from cardio-cerebral, psychiatric disorders, malignancies, pancreatitis, severe gastrointestinal disorders, or acute infections.
Type 2 diabetes mellitus (T2DM) was diagnosed following the criteria of the World Health Organization. Remission of T2DM was defined as glycated hemoglobin (HbA1C) level <6.5%, without any anti-diabetes medications for at least 3 months (15, 16).
Hypertension is defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, and/or using antihypertensive medications. A blood pressure of 120/80 mmHg was considered remission of hypertension when not on antihypertensive medication (17). MASLD was diagnosed using abdominal ultrasound, and assessed for lipid deposition and hepatic fibrosis by controlled attenuation parameter (CAP) and liver stiffness measurements (LSM) (18). Hyperuricemia is diagnosed by serum uric acid (UA) level ≥420 mmol/L or receiving anti-hyperuricemic drugs. Dyslipidemia is defined by fasting total cholesterol ≥5.2 mmol/L, low-density lipoprotein (LDL) cholesterol (LDL-C) ≥3.4 mmol/L, high-density lipoprotein (HDL) cholesterol (HDL-C) <1.04 mmol/L, triglycerides ≥1.7 mmol/L, in addition to previous lipid-lowering medication therapy. (19). Relief of dyslipidemia and hyperuricemia was defined as normalization of serum lipid and uric acid levels in biochemical values when not on medication (20).
2.3 Data collection
Data covering weight, height, blood pressure, comorbidities, and medications from patients at baseline and every 4 weeks were collected. Lab data consisting of liver and kidney functionality, blood fat levels, glycometabolic markers, and nutrients were measured at baseline and week 12. Static model assessment of insulin resistance (HOMA-IR) on the formula: HOMA-IR = (fasting insulin level × FBG level)/22.5 was calculated at baseline and 12 weeks. (21). Measurement of controlled attenuation parameters and liver stiffness utilizing a FibroTouch® (Haskell Medical Technology Co., Ltd., Wuxi, China). Visceral fat area and body composition on the InBody 720 (BioSpace Co., Ltd., Seoul, Korea) were evaluated.
All participants’ %TWL, Δweight, BMI, and ΔBMI at each follow-up visit for assessment of weight loss on liraglutide. Calculations based on the following formulas: %TWL = ([baseline weight - weight at follow-up]/baseline weight) × 100%; Δweight = (weight at follow-up) - (baseline weight); BMI = body weight (kg)/height2 (m2); and ΔBMI = (BMI at follow-up) - (baseline BMI) (22).
2.4 Statistical analyses
Statistics were analyzed by IBM SPSS Statistics version 26.0 for Windows (IBM Corp.). Categorical variables are described by frequency, and analyzed with Pearson’s χ2 test and Fisher’s exact test. Continuous variables are compared by independent samples t-test. All types of variables are presented by percentages and means ± SD. Linear regression models based on baseline were used to analyze the differences. Differences before and after the intervention between groups were evaluated with paired t-tests. Variations in weight, BMI, and %TWL were examined using repeated-measures ANOVA, and tests for homogeneity of variance were performed prior to conducting ANOVA. To identify factors affecting the weight loss efficacy of liraglutide, multiple stepwise logistic regression was performed.
Paired comparisons were made by post hoc tests. Statistical analysis of outcomes was conducted with the t-test and Mann-Whitney U-test. Differences in comorbidities were evaluated by the chi-square or Fisher’s exact test, with standardized residuals for multiple comparisons. Additionally, ordinal logistic regression and multivariate logistic models were employed to determine the factors. Diagnostic accuracy was evaluated with receiver operating characteristic curves (ROCs). A p <0.05 was considered statistically significant for all hypothetical tests.
3 Results
3.1 Baseline characteristics
A total of 64 patients were included in the study. The mean age of the participants was 35.6 ± 8.2 years, and the mean BMI was 30.0 ± 1.7 kg/m2. Of these, 37 were in the goal attainment group (28 females, age: 35.5 ± 8.9 years) and 27 were in the goal non-attainment group (21 females, age: 35.6 ± 7.5 years).
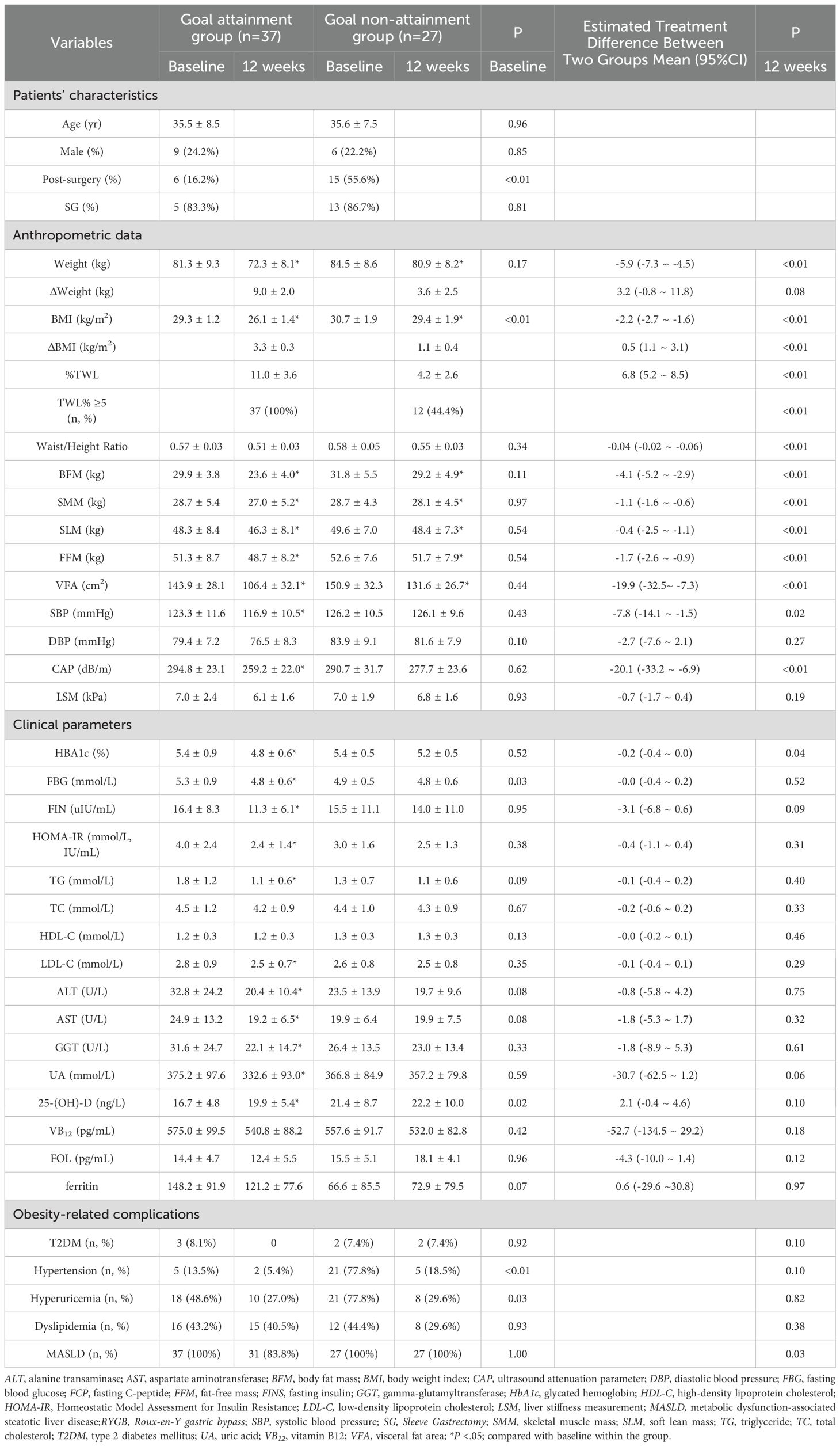
At baseline, the goal attainment group had a lower BMI (29.3 ± 1.2 vs 30.7 ± 1.9 kg/m2, P <.01), lower serum 25-(OH)-D (16.7 ± 4.8 vs. 21.4 ± 8.7 ng/L, P <.05), lower prevalence of hypertension (13.5 vs 77.8%, P <.01) and lower prevalence of hyperuricemia (48.6 vs 77.8%, P <.05). Additionally, a smaller proportion of patients in the goal attainment group had a history of MS (16.2 vs 55.6%, P <.01), and they exhibited higher serum FBG levels (5.3 ± 0.9 vs. 4.8 ± 0.6 mmol/L, P <.05) compared to the goal non-attainment group. Other baseline characteristics remained similar in the two groups (Table 1).
3.2%TWL, Weight loss, ΔBMI, and Body Composition
After 12 weeks treatment, the goal attainment group showed a higher %TWL compared to the goal non-attainment group (11.0 ± 3.6% vs 4.2 ± 2.6%), with an adjusted mean difference of 6.8% (95% confidence interval [CI], (5.2 ~ 8.5)%, P<.01). Only 44.4% patients in the goal non-attainment group achieved a %TWL ≥5% (Table 1 and Figures 2a).
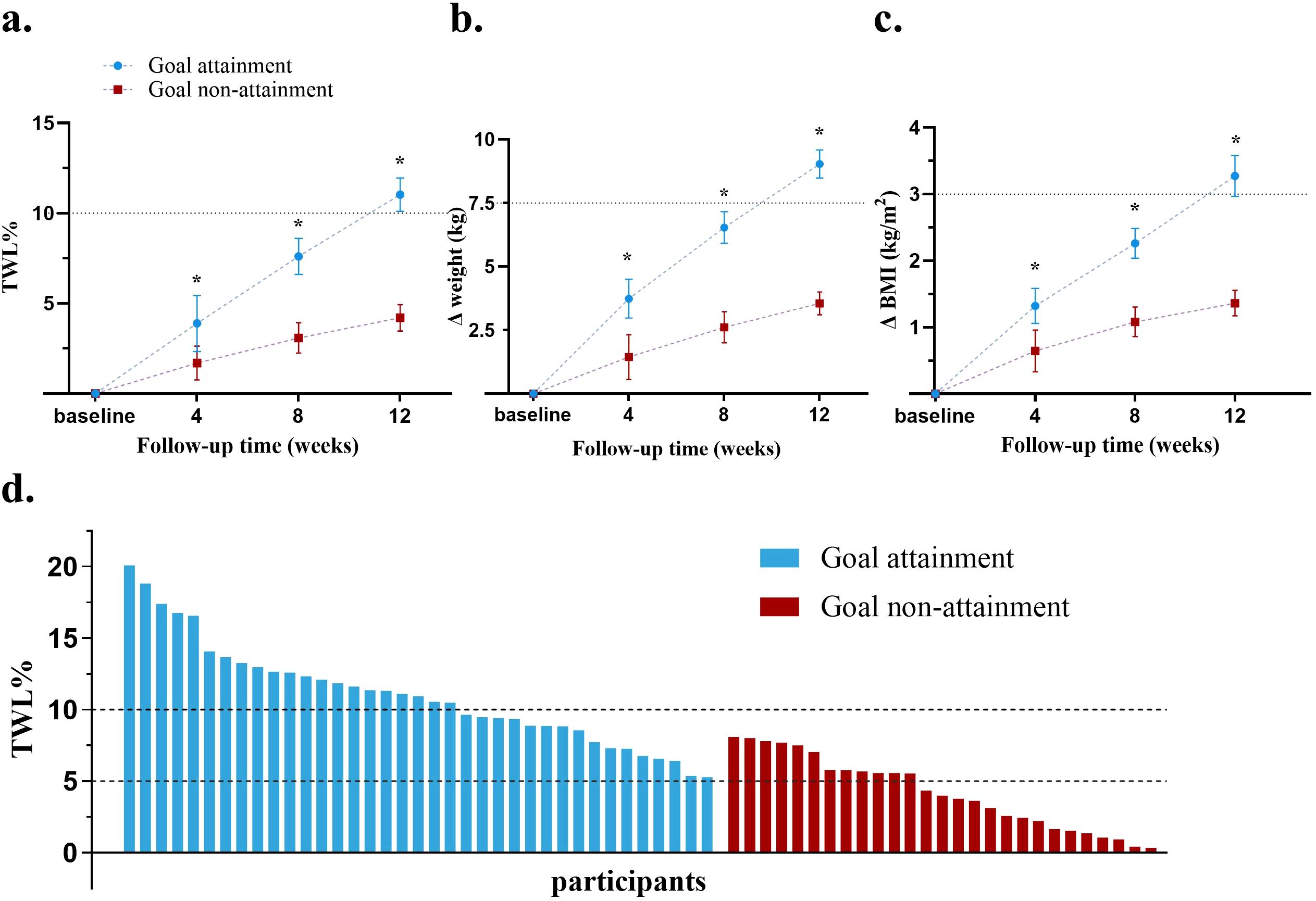
Figure 2. Comparison of weight loss effect in the two groups. (a) Changes in mean %TWL at each follow-up; (b) Changes in mean weight loss at each follow-up; (c) Changes in mean BMI at each follow-up; (d) %TWL of each patient after therapy; BMI, body mass index; TWL, total weight loss. *P<0.05.
Both groups showed reductions in weight and BMI from baseline (P<.05 vs baseline). The goal attainment group experienced a significant collective weight loss of 9.0 ± 2.0 kg and a BMI decrease of 3.3 ± 0.3 kg/m2. In contrast, the goal non-attainment group showed a weight reduction of 3.6 ± 2.5 kg, resulting in a BMI reduction of 1.1 ± 0.4 kg/m2, with no significant change compared with weight and BMI at baseline. The adjusted mean differences in the weight and BMI reductions between the goal attainment and goal non-attainment groups were -5.9kg (95% CI, -7.3 to -4.5, P<0.01) and -2.2 kg/m2 (95% CI, -2.7 to -1.6, P<0.01), respectively (Table 1).
Greater %TWL, weight loss, and ΔBMI were observed at week 4 in the goal attainment group compared to the goal non-attainment group, which continued to increase over time at weeks 8 and 12 (all P<.05) (Figures 2a). Waterfall plots illustrated the %TWL for each patient in both groups (Figure 2).
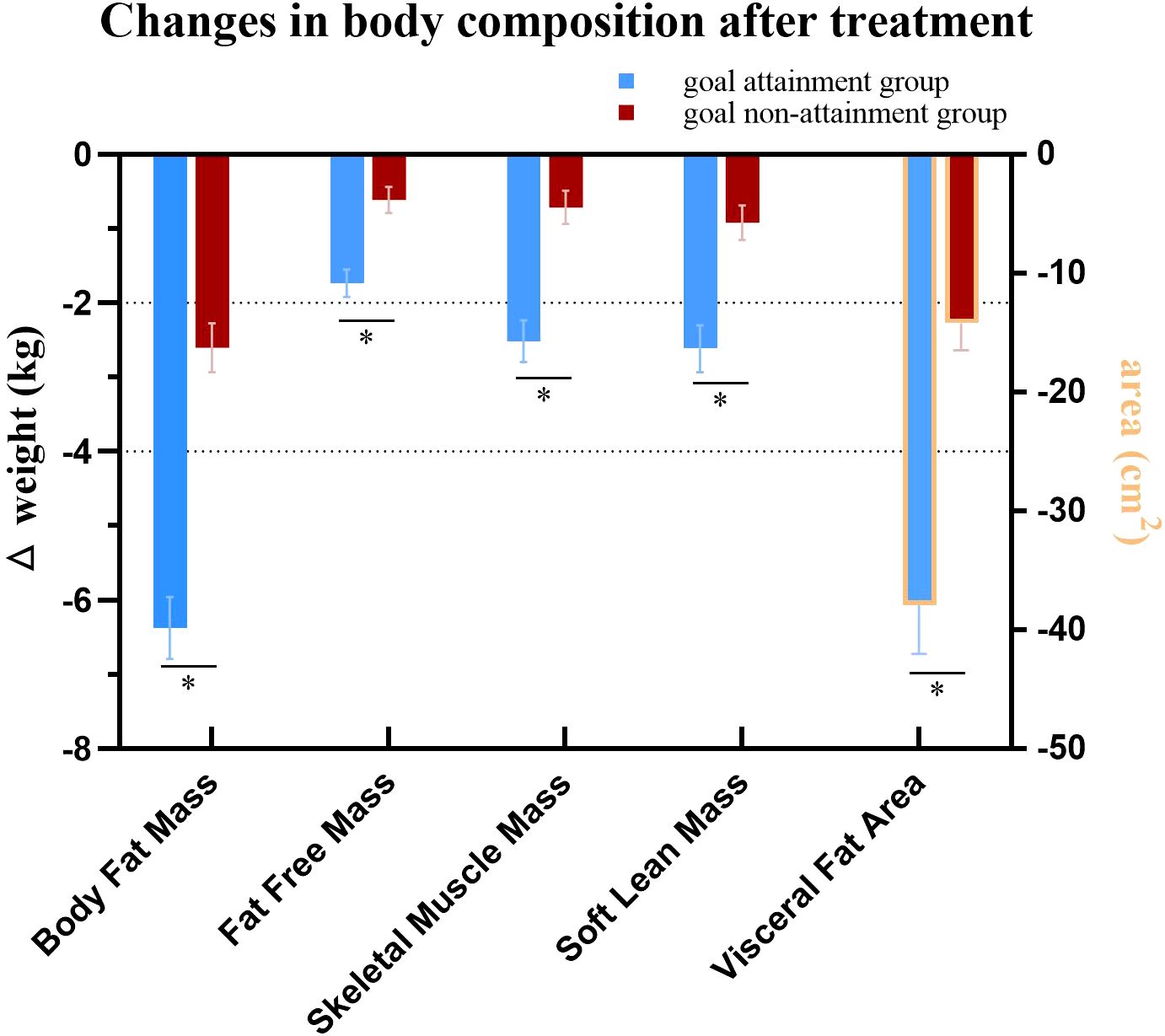
Body composition analysis showed reductions in BFM, SMM, SLM, FFM, and VFA in both groups (all P<.05). However, the goal attainment group demonstrated greater reductions in these parameters compared to the goal non-attainment group after 12 weeks of liraglutide treatment (Table 1, Figure 3).
3.3 Remission of obesity-related co-morbidities
Few patients in either group had T2DM. After 3 months of liraglutide treatment, HbA1c, FBG, FIN, and HOMA-IR decreased significantly in the goal achievement group (all P<.05), with no changes observed in the goal non-attainment group. Remission of T2DM could not be defined for any patients using liraglutide (Table 1). In the goal attainment group, reductions were observed in SBP, serum TG, LDL-C, and UA levels, as well as in the prevalence of MASLD and MASLD-related indicators, including CAP, serum ALT, AST, and GGT levels (all P<.05, Table 1).
3.4 Factors influencing the weight loss efficacy of liraglutide
Relevant disparities, including baseline BMI, FBG, 25-(OH)-D, MS history prevalence of hypertension, and hyperuricemia were analyzed as variables. The results indicated that BMI and history of MS were significant factors influencing the weight loss efficacy of liraglutide (P <.01, Table 1). The baseline BMI cutoff of 30.5 kg/m2 was calculated from the ROC curve. Forest plots revealed that patients with a history of MS and those with a baseline BMI >30.5 kg/m2 were less likely to achieve treatment targets (Figure 4).
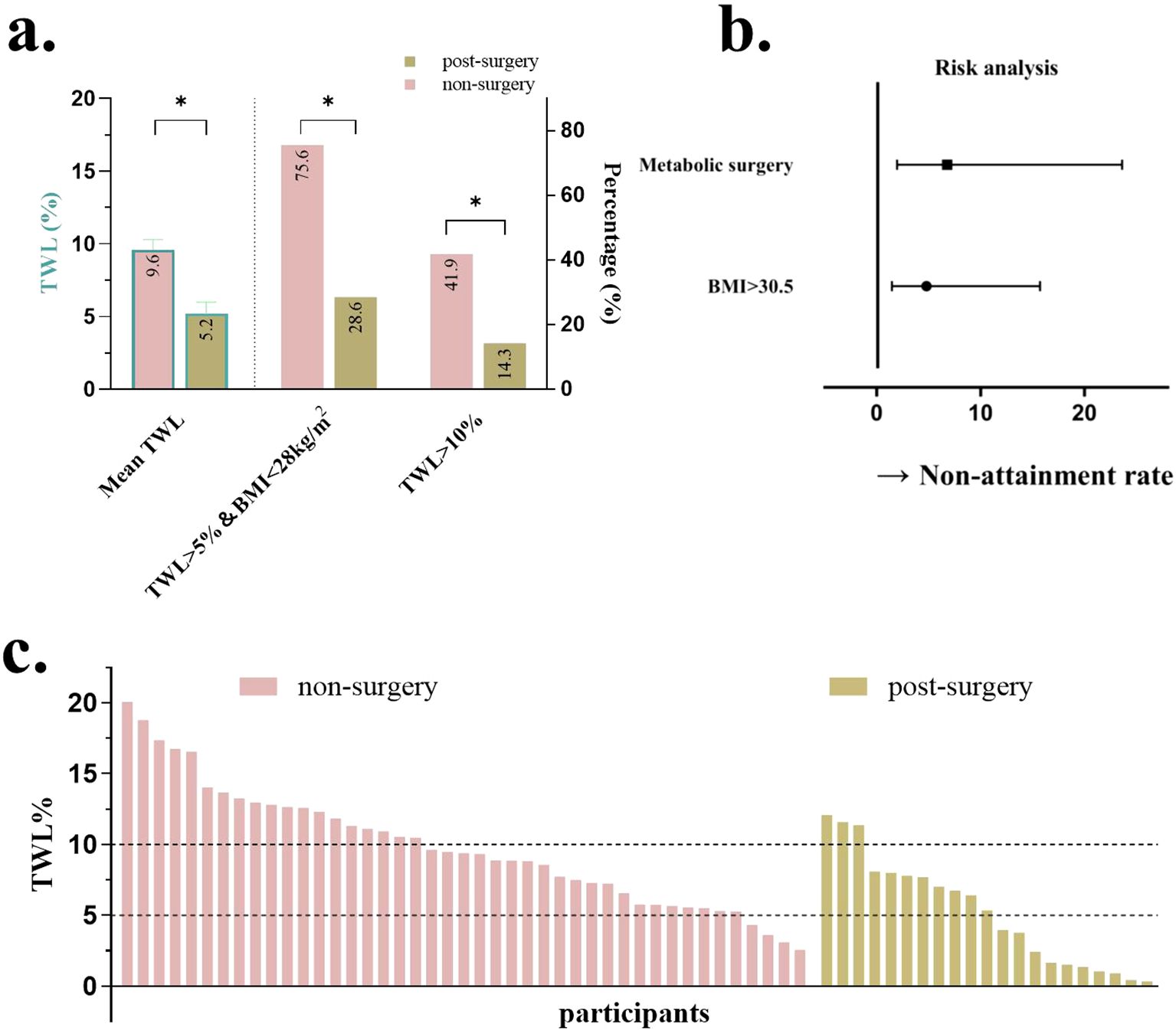
Figure 4. Comparison when grouped by a history of metabolic surgery. (a) %TWL after therapy and proportion of patients achieving %TWL>10%, %TWL >5%, and BMI <28; (b) Forest plots for the impact of baseline BMI and history of metabolic surgery; (c) %TWL of each patient after therapy. TWL, total weight loss; BMI, body mass index. *P<0.05.
The area under the ROC curve was 0.715 (95% CI, 0.579–0.851) and 0.697 (95% CI, 0.561–0.832) for baseline BMI and history of MS, respectively (Supplementary Figure S1). A baseline BMI cutoff of 30.5 kg/m² was derived from the ROC analysis. As shown in the forest plots, patients with a history of MS and a baseline BMI >30.5 kg/m² were significantly less likely to achieve treatment targets. Adjusted odds ratios (ORs) demonstrated strong inverse associations: a history of MS was associated with an OR of 6.78 (95% CI, 1.95–23.61; P<.01), while elevated BMI (≥30.5 kg/m²) was associated with an OR of 4.79 (95% CI, 1.46–15.71; P<.01) (Figure 4).
Patients were then divided into two groups based on whether or not they had an MS history. Patients without MS had a greater %TWL compared to those who were post-MS (9.6 ± 0.7% vs 5.2 ± 0.8%, P<.01, Figure 4). More patients without MS history got ≥5% TWL, a BMI <28kg/m2, and %TWL ≥10% (75.6% vs 28.6% and 41.9% vs 14.3%, respectively, both P<.01, Figure 4). Waterfall plots illustrated the %TWL for each patient in groups with and without MS (Figure 4).
4 Discussion
While achieving %TWL ≥5% serves as a primary therapeutic target in obesity management, our findings suggest that for patients with mild obesity, the dual endpoint of %TWL ≥5% combined with a BMI reduction below 28 kg/m² may better reflect the pharmacological efficacy of anti-obesity medications. In this 12-week liraglutide intervention study, 37 out of 64 participants (57.8%) reached this composite endpoint. Notably, those who achieved the endpoints demonstrated superior therapeutic outcomes compared to non-achievers, including double the %TWL (11.0% vs. 4.2%), nearly three times greater absolute weight reduction (9.0 kg vs. 3.3 kg), and more pronounced improvements in body composition, metabolic parameters, and MASLD.
Multivariate analysis identified a history of MS and a baseline BMI ≥30.5 kg/m² as independent negative predictors of treatment response, potentially due to altered postprandial GLP-1 and PYY secretion patterns in post-MS patients (23). Notably, the diminished efficacy of liraglutide in this population contrasts with previous reports describing enhance GLP-1R effects following bariatric procedures (23). This apparent paradox may reflect adaptive GLP-1R desensitization or downstream signaling alterations resulting from post-surgical metabolic remodeling (24, 25). Furthermore, the type of metabolic surgery performed may modulate GLP-1RA responsiveness by reshaping intestinal anatomy and the spatial distribution of enteroendocrine cells (EECs). As emphasized by Nwako and McCauley (2024), EECs exhibit region-specific and crypt–villus axis-dependent patterns of hormone expression, which are influenced by local signaling gradients and structural remodeling. Surgical procedures such as RYGB, SG, and SADI-S impose distinct anatomical and physiological changes to the gastrointestinal tract, potentially leading to differential enrichment or depletion of GLP-1-, PYY-, or GIP-secreting EECs within the exposed mucosa (26). Although direct assessment of GLP-1R expression was not conducted in this study, prior evidence supports the development of receptor desensitization in response to chronically elevated endogenous GLP-1 levels. Rubio-Herrera et al. observed attenuated pharmacological responses to GLP-1RAs in post-bariatric patients despite high circulating GLP-1 concentrations, likely due to altered receptor density or function (27). Clinical evidence from Shah et al. further demonstrated that GLP-1R blockade using exendin (9-39) markedly reduced endogenous GLP-1 activity within five years post-surgery (24). Supporting this, preclinical models of diet-induced obesity showed blunted GLP-1R-mediated weight loss following experimental MS (25). Taken together, both receptor-level adaptations and region-specific alterations in EEC distribution may collectively contribute to the diminished liraglutide response observed in post-MS individuals.
These findings underscore critical clinical considerations: anatomical and neurohormonal adaptations following MS may require optimized dosing strategies for GLP-1RAs. This concept is consistent with tirzepatide trials (a GLP-1/GIP dual agonist), where activation of the GIP receptor overcame reduced efficacy in treatment-resistant populations (28). Discrepancies with Suliman et al.’s report of comparable liraglutide 3.0 mg/day efficacy between surgical and non-surgical cohorts (13) may stem from differences in baseline BMI (29.8 vs. 37.5 kg/m²), surgical technique (predominantly SG vs. RYGB), and dosing (1.8 mg/day vs. 3.0 mg/day). Our cohort’s lower baseline BMI likely constrained absolute weight loss potential, while subtherapeutic dosing may have further limited responses. Notably, 85.7% of patients in our post-MS patiennts underwent SG, contrasting with Suliman et al.’s cohort where RYGB was the predominant procedure - an important distinction given RYGB’s typically stronger association with weight loss outcomes (13). These differences underscore how post-surgical metabolic adaptations (e.g., sustained appetite suppression and energy expenditure changes) may diminish pharmacological additive benefits (23).
The clinical uncertainty regarding dose escalation to 3.0 mg/day for overcoming metabolic adaptations in mild obesity remains unresolved. This highlights the need for prospective studies to evaluate optimized therapeutic approaches in this population. Clinically, we suggest a practical framework: initiate liraglutide at 0.6 mg/day and titrate weekly to 3.0 mg/day, where approved. Re-evaluate efficacy after 12 weeks at full dose. If <5% TWL is observed, consider next-generation therapies such as semaglutide or tirzepatide (5, 28). Monthly monitoring of weight, body composition (e.g., visceral fat area), and metabolic labs is recommended for response assessment. Prolonged treatment (>24 weeks) may also enhance outcomes via sustained receptor reprogramming (29). Key practical implications include the following: 1) Post-MS patients may require prolonged treatment or higher liraglutide doses to match non-surgical outcomes, consistent with 56-week data showing 3.0 mg’s superiority over 1.8 mg (30); and 2) Dual incretin agonists (e.g., GLP-1/GIP co-agonists) may circumvent reduced GLP-1R sensitivity, as evidenced by recent trials (31).
Our therapeutic success definition (≥5% TWL + BMI <28 kg/m²) revealed baseline BMI ≥30.5 kg/m² as a negative predictor—a finding concordant with global data showing 8.0% mean weight loss with liraglutide 3.0 mg/day in patients without diabetes versus 6.0–4.7% in overweight/obese T2D populations (30, 32). Achieving BMI <28 kg/m² appears particularly challenging with liraglutide 1.8 mg/day in patients with baseline BMI ≥30.5 kg/m². A tiered approach is recommended as follows: begin with dose escalation to 3.0 mg/day for enhanced GLP-1R activation (5); follow with next-generation agents targeting multiple pathways (GLP-1/GIP/glucagon) to bypass metabolic adaptation (27); and consider extended treatment duration (>24 weeks) to maximize neuroendocrine remodeling (33).
The ROC-derived BMI threshold of 30.5 kg/m², slightly exceeding the World Health Organization’s obesity criteria, may mark a pathophysiological inflection point where adiposity begins impairing GLP-1RA pharmacodynamics. Mechanistically, higher BMI is associated with visceral adiposity, chronic inflammation, and potential GLP-1R desensitization (34, 35). Future studies should incorporate body composition metrics (e.g., visceral fat area and waist-hip ratio) to refine stratification (34, 35).
Notably, the goal attainment group showed significant improvements in glucolipid metabolism and MASLD, consistent with the pleiotropic benefits of GLP-1RAs beyond weight loss (36). The metabolic differences between groups may be related to baseline variations in insulin sensitivity, a known modulator of GLP-1RA response (29). However, our regression analysis specifically identified a history of MS, rather than baseline metabolic parameters, as the primary predictor of liraglutide efficacy, emphasizing the importance of considering clinical history in therapeutic decision-making.
This study had some limitations. First, its retrospective design and single-center Chinese cohort limit statistical power and generalizability. Second, local regulatory restrictions capped the liraglutide dose at 1.8 mg/day, only 60% of the approved 3.0 mg/day, which may have reduced efficacy—particularly in post-MS patients with altered incretin signaling. Higher dosing or off-label use may be needed in select populations. Third, adherence-related factors such as social support, care accessibility, and follow-up were not systematically assessed. Fourth, since most patients underwent SG, comparisons across surgical types were not feasible. Given anatomical and physiological differences among SG, RYGB, and SADI-S—which influence enteroendocrine responses and GLP-1RA pharmacodynamics—this is a notable limitation. Lastly, the lack of data on lifestyle and socioeconomic variables, including diet, exercise, and financial constraints, may confound outcome interpretation.In mild obesity post-MS, liraglutide 3.0 mg/day or GLP-1/GIP dual agonists may be required for optimal results. Longer follow-up (≥6 months) is also needed to assess treatment durability.
In conclusion, the majority of patients with mild obesity achieved significant weight loss after 12 weeks of liraglutide 1.8 mg treatment. Our findings support a personalized approach to obesity management, where MS history and baseline BMI ≥ 30.5kg/m2 guide GLP-1RA dosing and monitoring. As metabolic procedures and incretin-based therapies increasingly intersect, understanding their bidirectional interactions is essential for optimizing long-term outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YO: Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. XX: Investigation, Writing – original draft. XH: Data curation, Investigation, Writing – original draft. XC: Resources, Supervision, Validation, Writing – review & editing. WT: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. WF: Conceptualization, Data curation, Project administration, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Support for the research was provided by: Natural Science Foundation of Jiangsu Province (BK20201115), Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0508105), National Natural Science Foundation of China (82030026, 82270883, and 81970704), Major Programs in Clinical Medicine of Nanjing City, Key R&D Program of Jiangsu Province, China (BE2022666), Key Medical Discipline Cultivation Unit of Jiangsu Province (JSDW202201), PRC Key National Research and Development Program (2021YFC2501600 and 2022YFC2505306) and the Special Fund for Health Science and Technology Development of Nanjing City (YKK23072). The fee for the journal’s service charge would be financed by the writer.
Acknowledgments
Thanks to the participants in this study
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1580159/full#supplementary-material
Supplementary Figure 1 | ROC curve for the impact of baseline BMI and history of metabolic surgery.
Abbreviations
%TWL, Percent Total Weight Loss; BMI, Body Mass Index; MASLD, Metabolic Dysfunction-Associated Steatotic Liver Disease; MS, Metabolic Surgery; GLP-1RA, Glucagon-Like Peptide-1 Receptor Agonist; SG, Sleeve Gastrectomy; RYGB, Roux-en-Y gastric bypass; BPD-DS, biliopancreatic diversion with duodenal switch; SADI-S, single-anastomosis duodenoileal bypass with sleeve gastrectomy; GIP, Glucose-Dependent Insulinotropic Polypeptide; SMM, Skeletal Muscle Mass; SLM, Skeletal Lean Mass; FFM, Fat-Free Mass; VFA, Visceral Fat Area; FBG, Fasting Blood Glucose; FIN, Fasting Insulin; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; TG, Triglycerides; LDL-C, Low-Density Lipoprotein Cholesterol; CAP, Controlled Attenuation Parameter; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; GGT, Gamma-Glutamyl Transferase.
References
1. Department of Medical Administration NHCotPsRoC. Chinese guidelines for the clinical management of obesity (2024 edition). Med J Peking Union Med Coll Hosp. (2025) 16:90–108. doi: 10.12290/xhyxzz.2024-0918
2. Chen K, Shen Z, Gu W, Lyu Z, Qi X, Mu Y, et al. Prevalence of obesity and associated complications in China: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obesity Metab. (2023) 25:3390–9. doi: 10.1111/dom.v25.11
3. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New Engl J Med. (2015) 373:11–22. doi: 10.1056/NEJMoa1411892
4. Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of pharmacological treatments for obesity with weight loss and adverse events. Jama. (2016) 315(22):2424–34. doi: 10.1001/jama.2016.7602
5. Wadden TA, Tronieri JS, Sugimoto D, Lund MT, Auerbach P, Jensen C, et al. Liraglutide 3.0 mg and intensive behavioral therapy (IBT) for obesity in primary care: the SCALE IBT randomized controlled trial. Obesity. (2020) 28:529–36. doi: 10.1002/oby.22726
6. Colquitt JL, Pickett K, Loveman E, and Frampton GK. Surgery for weight loss in adults. Cochrane Database systematic Rev. (2014) 2014:Cd003641. doi: 10.1002/14651858.CD003641.pub4
7. Arterburn DE, Telem DA, Kushner RF, and Courcoulas AP. Benefits and risks of bariatric surgery in adults: A review. Jama. (2020) 324:879–87. doi: 10.1001/jama.2020.12567
8. Maroun J, Li M, Oyefule O, Badaoui JE, McKenzie T, Kendrick M, et al. Ten year comparative analysis of sleeve gastrectomy, Roux-en-Y gastric bypass, and biliopancreatic diversion with duodenal switch in patients with BMI ≥ 50 kg/m(2). Surg Endosc. (2022) 36:4946–55. doi: 10.1007/s00464-021-08850-y
9. Yashkov Y, Bordan N, Torres A, Malykhina A, and Bekuzarov D. SADI-S 250 vs roux-en-Y duodenal switch (RY-DS): results of 5-year observational study. Obesity surgery. (2021) 31:570–9. doi: 10.1007/s11695-020-05031-z
10. Istfan NW, Lipartia M, Anderson WA, Hess DT, and Apovian CM. Approach to the patient: management of the post–bariatric surgery patient with weight regain. J Clin Endocrinol Metab. (2021) 106:251–63. doi: 10.1210/clinem/dgaa702
11. de Moraes FCA, Morbach V, Sano VKT, Fernandes LR, Kreuz M, and Kelly FA. Liraglutide for the treatment of weight regain after bariatric surgery: A systematic review and meta-analysis. Obesity surgery. (2024) 34:2844–53. doi: 10.1007/s11695-024-07384-1
12. Haase CL, Serratore Achenbach MG, Lucrezi G, Jeswani N, Maurer S, and Egermann U. Use of liraglutide 3.0 mg for weight management in a real-world setting in Switzerland. Obesity Facts. (2021) 14:568–76. doi: 10.1159/000518325
13. Suliman M, Buckley A, Al Tikriti A, Tan T, le Roux CW, Lessan N, et al. Routine clinical use of liraglutide 3 mg for the treatment of obesity: Outcomes in non-surgical and bariatric surgery patients. Diabetes Obesity Metab. (2019) 21:1498–501. doi: 10.1111/dom.2019.21.issue-6
14. Grunvald E, Shah R, Hernaez R, Chandar AL, Pickett-Blakely O, Teigen LM, et al. AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity. Gastroenterology. (2022) 163(5):1198–225. doi: 10.1053/j.gastro.2022.08.045
15. Chinese Diabetes Society. National Office for Primary Diabetes Care. Zhonghua Nei Ke Za Zhi. (2022) 61(3):249–62. doi: 10.3760/cma.j.cn112138-20220120-000063
16. Forouhi NG, Misra A, Mohan V, Taylor R, and Yancy W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ. (2018) 361:k2234. doi: 10.1136/bmj.k2234
17. Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, et al. Status of hypertension in China: results from the China hypertension survey, 2012-2015. Circulation. (2018) 137:2344–56. doi: 10.1161/CIRCULATIONAHA.117.032380
18. Qu Y, Song YY, Chen CW, Fu QC, Shi JP, Xu Y, et al. Diagnostic performance of fibroTouch ultrasound attenuation parameter and liver stiffness measurement in assessing hepatic steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Clin Transl Gastroenterol. (2021) 12:e00323. doi: 10.14309/ctg.0000000000000323
19. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41:111–88. doi: 10.1093/eurheartj/ehz455
20. Wu J, Qiu L, Cheng XQ, Xu T, Wu W, Zeng XJ, et al. Hyperuricemia and clustering of cardiovascular risk factors in the Chinese adult population. Sci Rep. (2017) 7:5456. doi: 10.1038/s41598-017-05751-w
21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, and Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–9. doi: 10.1007/BF00280883
22. Brethauer SA, Kim J, El Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg. (2015) 25:587–606. doi: 10.1007/s11695-015-1645-3
23. Madsbad S, Dirksen C, and Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes endocrinology. (2014) 2:152–64. doi: 10.1016/S2213-8587(13)70218-3
24. Shah M LJ, Micheletto F, Sathananthan M, Dalla Man C, Cobelli C, Rizza RA, et al. Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes. (2014) 63:483–93. doi: 10.2337/db13-0954
25. Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol. (2014) 306:R352–62. doi: 10.1152/ajpregu.00491.2013
26. Nwako JG and McCauley HA. Enteroendocrine cells regulate intestinal homeostasis and epithelial function. Mol Cell Endocrinol. (2024) 593:112339. doi: 10.1016/j.mce.2024.112339
27. Rubio-Herrera MA, Mera-Carreiro S, Sánchez-Pernaute A, and Ramos-Levi AM. Impact of Treatment with GLP1 Receptor Agonists, Liraglutide 3.0 mg and Semaglutide 1.0 mg, While on a Waiting List for Bariatric Surgery. Biomedicines. (2023) 11:2785. doi: 10.3390/biomedicines11102785
28. Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. (2022) 387:205–16. doi: 10.1056/NEJMoa2206038
29. Alfaris N, Waldrop S, Johnson V, Boaventura B, Kendrick K, and Stanford FC. GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: a narrative review. EClinicalMedicine. (2024) 75:102782. doi: 10.1016/j.eclinm.2024.102782
30. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. Jama. (2015) 314:687–99. doi: 10.1001/jama.2015.9676
31. Jamal M, Alhashemi M, Dsouza C, Al-Hassani S, Qasem W, Almazeedi S, et al. Semaglutide and tirzepatide for the management of weight recurrence after sleeve gastrectomy: A retrospective cohort study. Obesity surgery. (2024) 34:1324–32. doi: 10.1007/s11695-024-07137-0
32. Garvey WT, Birkenfeld AL, Dicker D, Mingrone G, Pedersen SD, Satylganova A, et al. Efficacy and safety of liraglutide 3.0 mg in individuals with overweight or obesity and type 2 diabetes treated with basal insulin: the SCALE insulin randomized controlled trial. Diabetes Care. (2020) 43:1085–93. doi: 10.2337/dc19-1745
33. Imam A, Alim H, Binhussein M, Kabli A, Alhasnani H, Allehyani A, et al. Weight loss effect of GLP-1 RAs with endoscopic bariatric therapy and bariatric surgeries. J Endocrine Soc. (2023) 7:bvad129. doi: 10.1210/jendso/bvad129
34. Neeland IJ, Marso SP, Ayers CR, Lewis B, Oslica R, Francis W, et al. Effects of liraglutide on visceral and ectopic fat in adults with overweight and obesity at high cardiovascular risk: a randomised, double-blind, placebo-controlled, clinical trial. Lancet Diabetes endocrinology. (2021) 9:595–605. doi: 10.1016/S2213-8587(21)00179-0
35. Blüher M, Aras M, Aronne LJ, Batterham RL, Giorgino F, Ji L, et al. New insights into the treatment of obesity. Diabetes obesity Metab. (2023) 25:2058–72. doi: 10.1111/dom.15077
Keywords: glucagon-like peptide-1 receptor agonist, metabolic surgery, weight loss variability, body mass index, metabolic adaptation
Citation: Ouyang Y, Xiang X, Hu X, Chu X, Tang W and Feng W (2025) Prior metabolic surgery attenuates the weight-loss efficacy of liraglutide in patients with mild obesity. Front. Endocrinol. 16:1580159. doi: 10.3389/fendo.2025.1580159
Received: 20 February 2025; Accepted: 30 April 2025;
Published: 22 May 2025.
Edited by:
Xiaodong Sun, Affiliated Hospital of Shandong Second Medical University, ChinaReviewed by:
Anapaula Sommer Vinagre, Federal University of Rio Grande do Sul, BrazilHua Bian, Fudan University, China
Zhe Dai, Wuhan University, China
Copyright © 2025 Ouyang, Xiang, Hu, Chu, Tang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenhuan Feng, ZmVuZ3doNTAxQDE2My5jb20=
†These authors have contributed equally to this work
 Yuqin Ouyang
Yuqin Ouyang Xinyue Xiang1,2†
Xinyue Xiang1,2† Wenhuan Feng
Wenhuan Feng