- Department of Reproductive Medicine, Lanzhou University Second Hospital, Lanzhou, China
Objective: Recurrent pregnancy loss (RPL) is a significant clinical challenge, with many cases remaining unexplained, and existing risk prediction models often lacking objective evaluation. This study aims to systematically review and evaluate the published risk prediction models for pregnancy outcomes in RPL.
Methods: Literature search was conducted in August 2024 using PubMed, Embase, Web of Science, CNKI, and CMAJ databases to identify studies that reported the development and/or validation of clinical prediction models for RPL pregnancy outcomes. Pregnancy outcomes included pregnancy loss, ongoing pregnancy, and live birth. Data were extracted using the CHARMS checklist. Risk of bias and applicability were evaluated with PROBAST.
Results: A total of 1,112 records were identified, with 15 studies ultimately included, encompassing 22 risk prediction models for evaluating RPL patients’ pregnancy outcomes. The majority were retrospective cohort studies (13/15), and logistic regression was the predominant modeling method (14/15). Sample sizes ranged from 85 to 789, with the number of predictors per model varying from 2 to 18 (median=5). In total, 65 distinct predictors were identified, including five categories: patient-related, imaging-related, thrombophilia-related, metabolic/endocrinologic, and immunological factors, most frequently maternal age (n=10) and number of previous pregnancy losses (n=9). Among the 20 models that reported discriminative performance by the area under the receiver operating characteristics (ROC) curve (AUC), 13 achieved AUC above 0.80 (range: 0.809–0.97). Notably, 7 studies did not perform any form of validation, and only 3 studies conducted external validation. Despite the models reported a good predictive performance, they were all appraised to have high risk of bias in applicability due to methodological deficiencies.
Conclusion: The findings suggest that current risk prediction models for RPL pregnancy outcomes have a high risk of bias in clinical applications, primarily due to methodological flaws in development and validation processes. Future research should focus on data quality, sample diversity, and model transparency to ensure broad applicability across different populations, providing more reliable and effective tools for clinical practice.
Systematic review eegistration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024570481, identifier CRD42024570481.
1 Introduction
Recurrent pregnancy loss (RPL) is commonly referred to two or more spontaneous abortions (1–4). Known factors contributing to RPL include maternal age, previous pregnancy loss, parental chromosomal abnormalities, uterine anatomical abnormalities, endocrine disorders, and immune disorders (5). Despite thorough surveys, the cause of RPL remains unexplained in about 50% of cases (6). Research shows that in nulligravidae, the risk of pregnancy loss escalates with each additional loss, rising from around 11% after three losses to approximately 40% (7). Therefore, developing a tool to predict pregnancy outcomes for RPL women is crucial for better risk assessment and personalized treatment. Clinical risk prediction models utilize medical data and statistical methods to estimate a patient’s future risk of having a certain disease or experiencing an event (8). For RPL, current research primarily focuses on diagnosing and prognosticating outcomes to enhance clinical management (9–11). Despite progress in existing research, many models still lack objective and unbiased validation, particularly external cohort validation, which limits their widespread application and practical value in clinical decision-making. Therefore, this study aims to systematically review the published RPL risk prediction models, aiming to identify the strengths and limitations of existing models and provide recommendations for developing more clinically applicable risk prediction tools in the future.
2 Materials and methods
The review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (12). The protocol of this systematic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42024570481).
2.1 Data sources and search strategy
A comprehensive search was conducted in both English and Chinese databases, including PubMed, Web of Science, Embase, China National Knowledge Infrastructure (CNKI), and China Medical Association Journals (CMAJ), covering studies published up to August 2, 2024. The articles were restricted to Chinese- or English-language literature, as these languages are spoken by large population, and focusing on the most widely used databases in both language communities ensures a comprehensive understanding of the relevant research. Search terms were a combination of controlled vocabulary (Medical Subject Heading [MeSH] terms) and free-text terms, including “abortion, habitual[MeSH terms]”, “recurrent miscarriage”, “recurrent pregnancy loss”, “nomograms[MeSH terms]”, “machine learning[MeSH terms]”, “risk assessment[MeSH terms]”, “risk prediction”, “risk model”, “predictive model”, and “scoring system”. The full search strategies for all databases are provided in Supplementary Table 1. Additionally, we screened the reference lists of the included studies and relevant reviews to identify any additional eligible studies that may have been missed during the database search.
2.2 Inclusion and exclusion criteria
We included literature that met the following criteria: (1) patients diagnosed with RPL; (2) studies focused on the development and/or validation of risk prediction models for RPL; (3) the prediction model contained at least two predictors; and (4) the pregnancy outcomes limited to pregnancy loss, ongoing pregnancy, or live birth. We excluded the following types of literature: (1) studies that analyzed risk factors without constructing a risk prediction model; and (2) gray literature such as preprints, conference abstracts, reviews, systematic reviews, meta-analyses, editorials, or letters to the editor.
2.3 Study selection and data extraction
Two independent researchers, trained prior to the article selection to ensure understanding and consistent application of screening criteria, conducted a step-by-step method to screen each article from the systematic search after removing duplicates. First, the titles and abstracts were used for initial screening. Second, full texts were reviewed to further assess the records that might meet the eligibility criteria. Records that did not match the pre-established criteria were excluded. Any disagreements at each step should be reached through discussion or consultation with a third researcher to reach consensus. Inter-rater agreement will be calculated using Cohen’s kappa (κ) at the title/abstract screening and full-text review stages. After finalizing the included studies, data were extracted using a standardized form based on the checklist for Critical Appraisal And Data Extraction For Systematic Reviews Of Prediction Modeling Studies (CHARMS) by two independent investigators (13). The extracted data included first author, year of publication, study type, study population, study period, sample size, number of events, outcome prediction, candidate and final predictors, missing data, model construction and validation methods, model performance (discrimination and calibration), and model’s final presentation format.
2.4 Model risk of bias and applicability assessment
The risk of bias (ROB) and applicability of the included studies were assessed by two other independent investigators trained in the prediction Model Risk Of Bias Assessment Tool (PROBAST) (14, 15), with a third researcher resolving any disagreements. PROBAST assesses ROB across four domains of “Participant Selection,” “Predictors,” “Outcomes,” and “Analysis” using 20 signaling questions. Each question is answered as “yes,” “probably yes,” “no,” “probably no,” or “no information,” and each domain is rated as “high,” “low,” or “unclear” ROB (15). The overall ROB for each study was classified as low, high, or unclear based on these ratings. For instance, a study was rated as having low overall ROB if all domains were rated “low”, while a high ROB was assigned if any domain was rated “high”. If one or more domains were “unclear” but all other domains were rated as “low”, the study was considered to have an unclear ROB.
3 Results
A total of 1,112 papers were identified, 167 of which were duplicates and removed. After screening the 945 records’ titles and abstracts, 42 articles were selected for full-text review. Of these, 17 studies were excluded due to their focus on diagnostic models, 6 studies were excluded because no predictive model was developed, 3 studies only used a single predictor, and 1 study included both RPL and recurrent implantation failure (RIF) patients without providing separate data for RPL cases. Consequently, 15 studies were included in this study (16–30). There was a good inter-rater agreement between the two reviewers for the title/abstract screening (κ = 0.878) and full-text review stage (κ = 0.847). Figure 1 illustrates the literature screening flowchart.
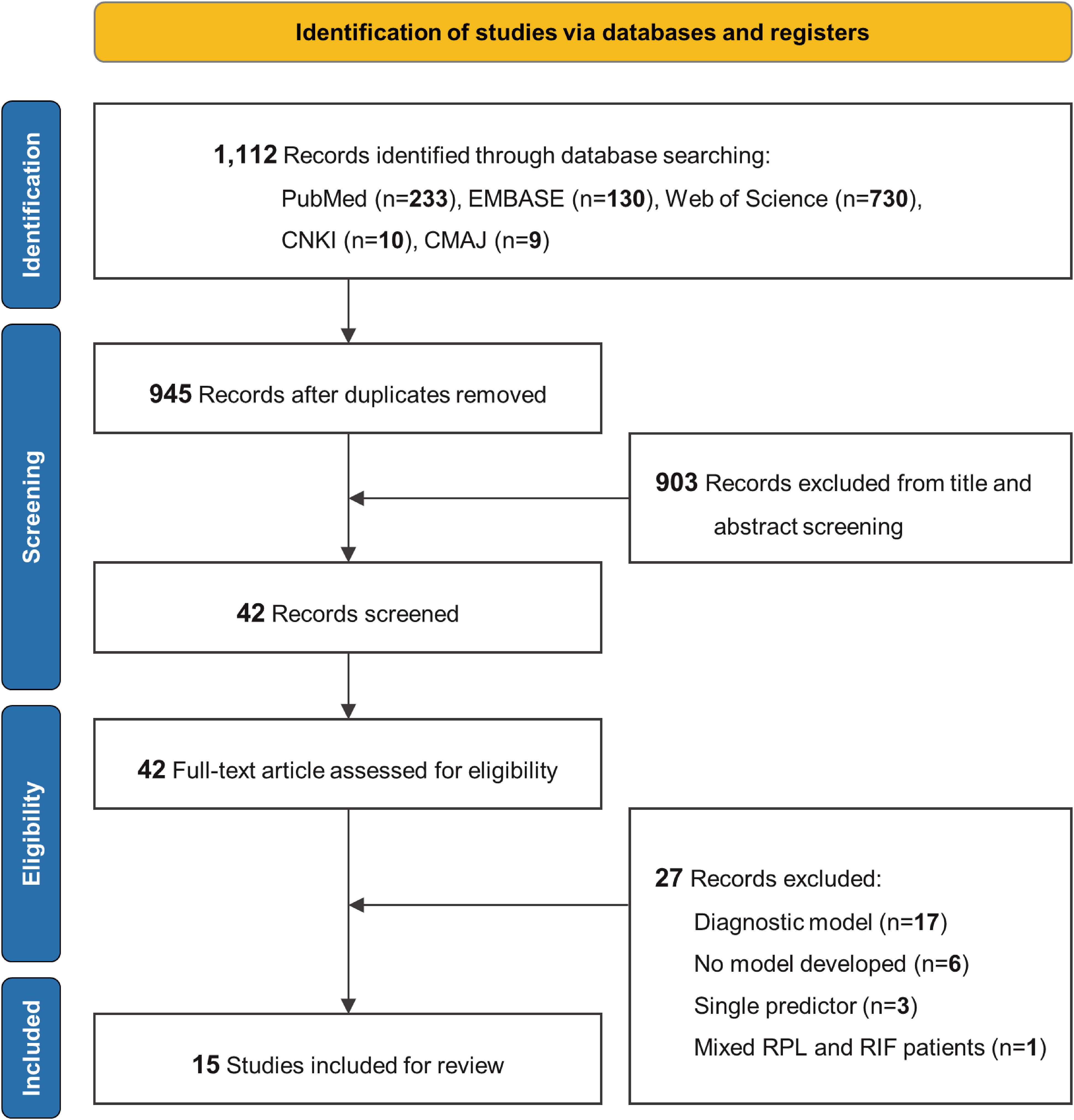
Figure 1. Literature screening process. EMBASE, Excerpta Medica Database; CNKI, China National Knowledge Infrastructure; CMAJ, China Medical Association Journals; RPL, recurrent pregnancy loss; RIF, recurrent implantation failure.
3.1 Basic characteristic
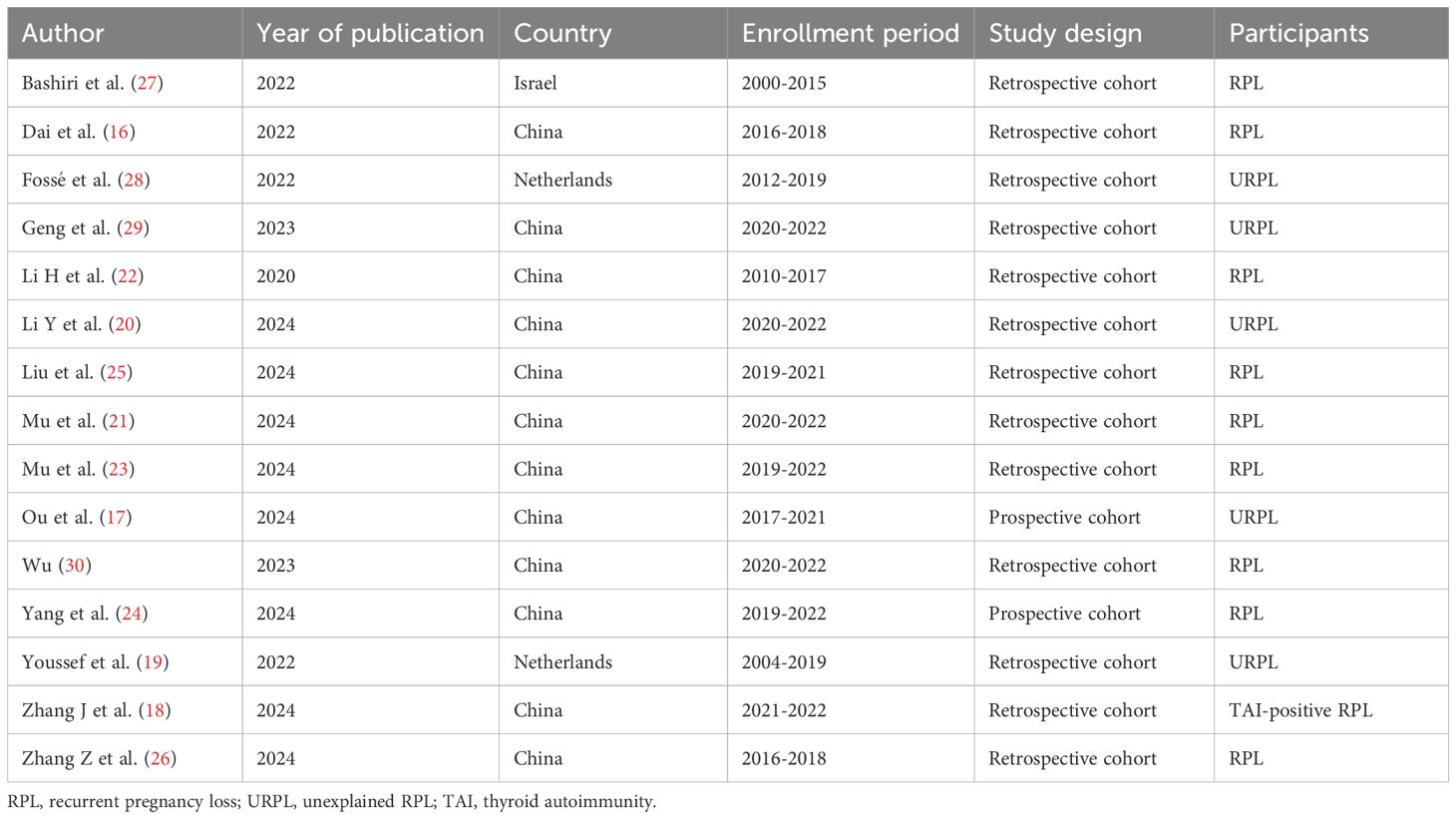
Table 1 summarizes the characteristics of the 15 included studies. Of these, two were in Chinese (29, 30) and 13 in English (16–28), with all studies were published between 2020 and 2024. In terms of study designs, 13 were retrospective cohort studies (16, 18–23, 25–30), and two were prospective cohort studies (17, 24). The enrollment periods varied, with the longest spanning 15 years (2000~2015) (19, 27) and the most frequent being from 2020 to 2022 (20, 21, 29, 30). The 15 included studies originated from three countries: China (n=12) (16–18, 20–26, 29, 30), the Netherlands (n=2) (19, 28), and Israel (n=1) (27), indicating a predominance of research from China. (Supplementary Figure 1).
3.2 Population and outcomes
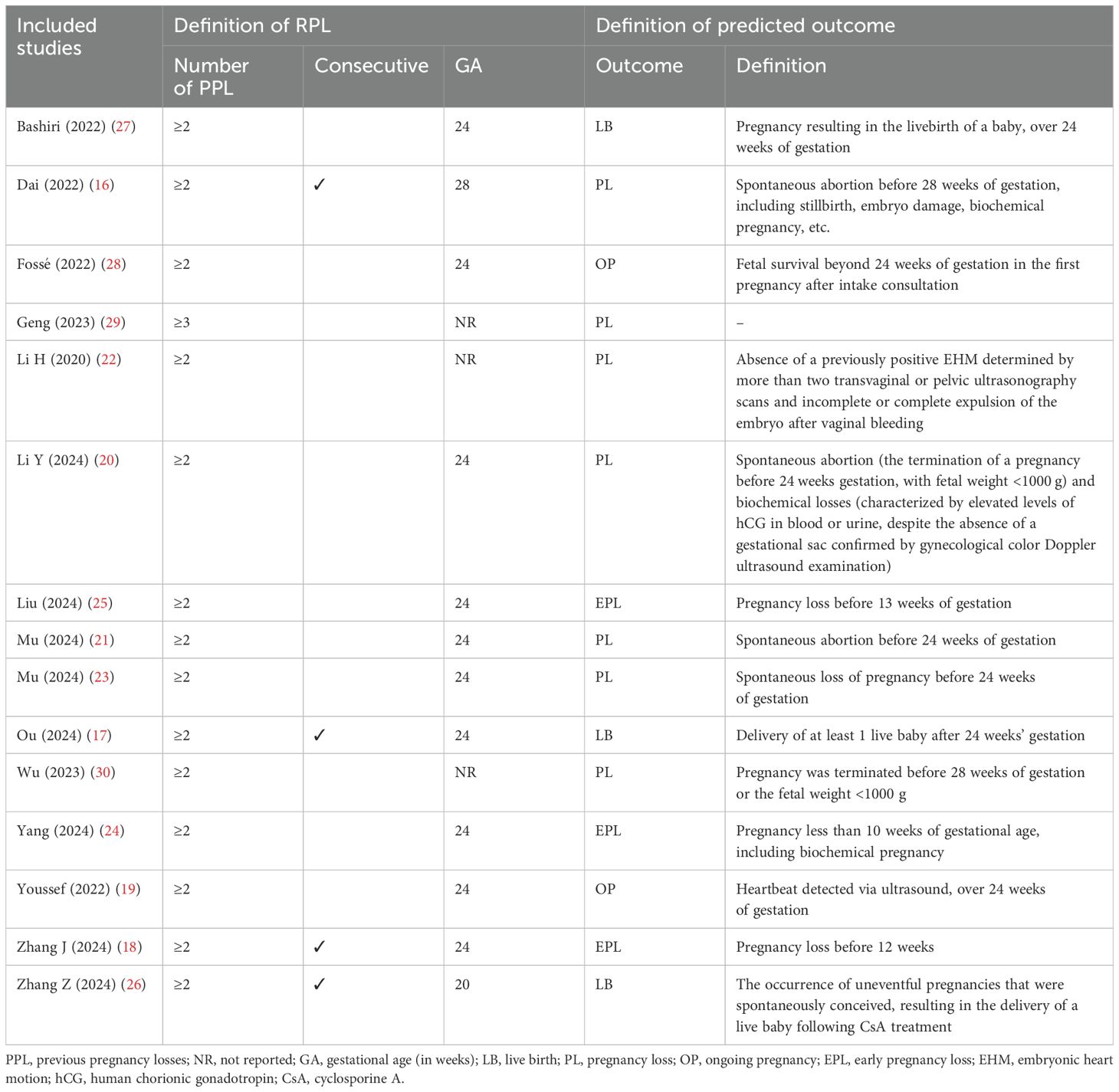
As shown in Table 2, almost all studies defined RPL as two or more pregnancy losses (16–28, 30), except one study required at least three or more losses (29). The discrepancies in the definitions of RPL mainly regarding the gestational age and whether losses needed to be consecutive. Specifically, 10 studies consistently reported a gestational age of 24 weeks (17–21, 23–25, 27, 28). Two studies used different gestational ages of 28 (16) and 20 weeks (26), while three did not report (22, 29, 30). Furthermore, four studies required consecutive losses, with gestational ages of 28 (16), 24 (17, 18), and 20 weeks (26). The remaining studies did not specify consecutive losses, with most reporting a gestational age of 24 weeks. Supplementary Table 2 shows participant inclusion and exclusion criteria. For the predictive outcomes, two-third studies (10/15) developed models for pregnancy loss risk, including “pregnancy loss” (16, 20–23, 29, 30) and “early pregnancy loss” (18, 24, 25); one-third studies constructed for “ongoing pregnancy” (19, 28) and “live birth” (17, 26, 27). Notably, the definitions of predicted outcomes varied significantly, with details presented in Table 2.
3.3 Predictors
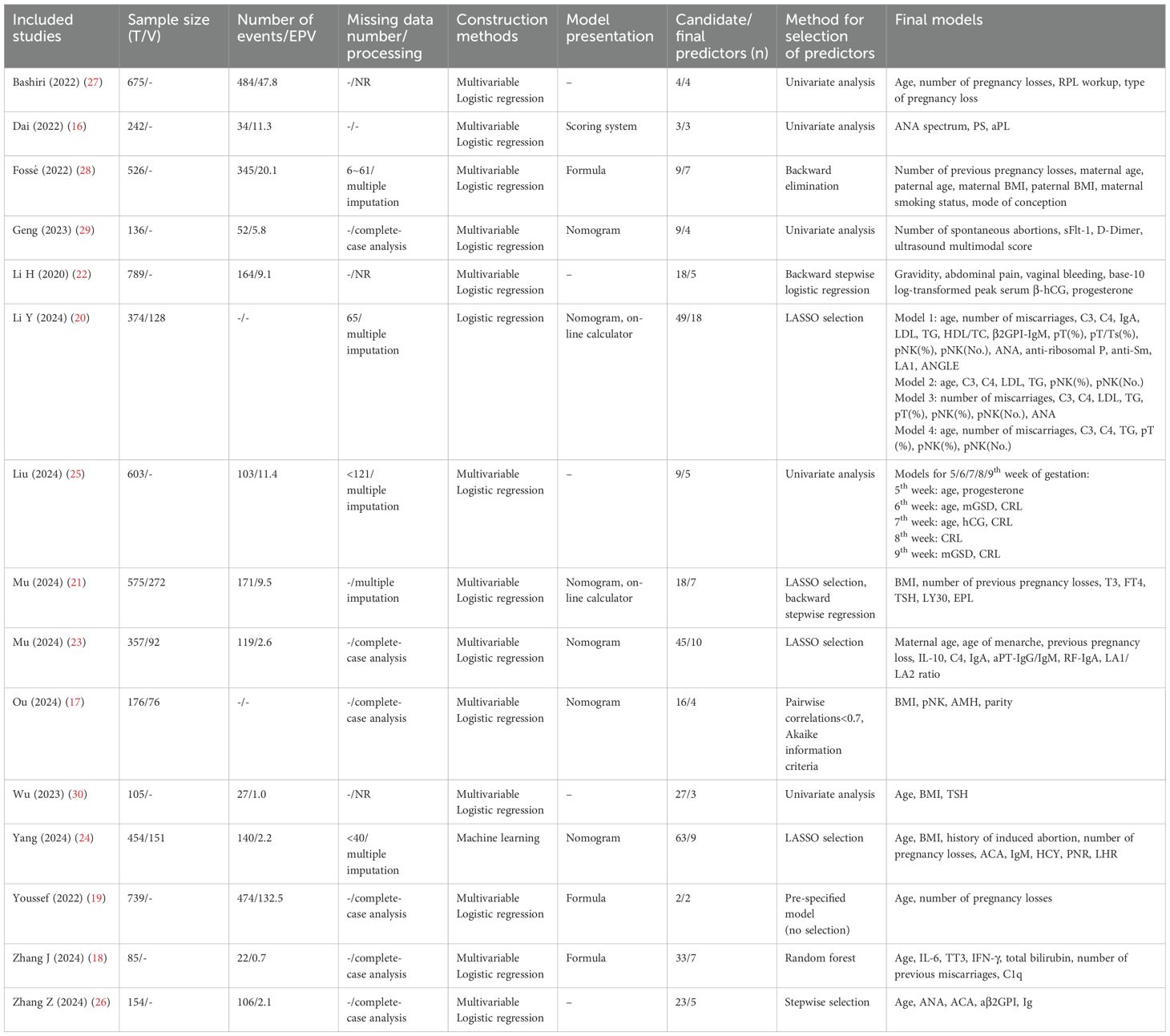
Table 3 shows the information on the models’ construction. Fourteen studies reported their method for selecting predictors, with common methods including the Least Absolute Shrinkage And Selection Operator (LASSO) selection (20, 21, 23, 24), backward stepwise logistic regression (21, 22, 26, 28), and univariate analysis (16, 25, 27, 29, 30). The number of predictors in the final model ranged from 2 to 18, with a median of 5.
A total of 65 distinct predictors were identified, categorized into five categories: (1) Patient-related (e.g., maternal/paternal age, maternal body mass index [BMI], number of pregnancy losses); (2) Imaging-related (e.g., mean of the gestational sac diameter, crown-rump length); (3) Thrombophilia-related (e.g., D-dimer, protein S); (4) Metabolic/endocrinologic (e.g., progesterone, thyroid stimulating hormone [TSH], anti-Müllerian hormone); and (5) Immunological factors (e.g., antinuclear antibody [ANA], anti-cardiolipin antibody [ACA], complement 4 [C4]). A full list of predictors is presented in Supplementary Figures 2, 3.
The most frequently identified predictors included the model were patient-related: maternal age (n=10), number of pregnancy losses (n=9), maternal BMI (n=5); followed by metabolic/endocrinologic (n=2: progesterone, TSH) and immunological factors (n=2: ANA, ACA, C4, immunoglobulin A [IgA], percentage of peripheral natural killer [pNK] cells) (Figure 2).
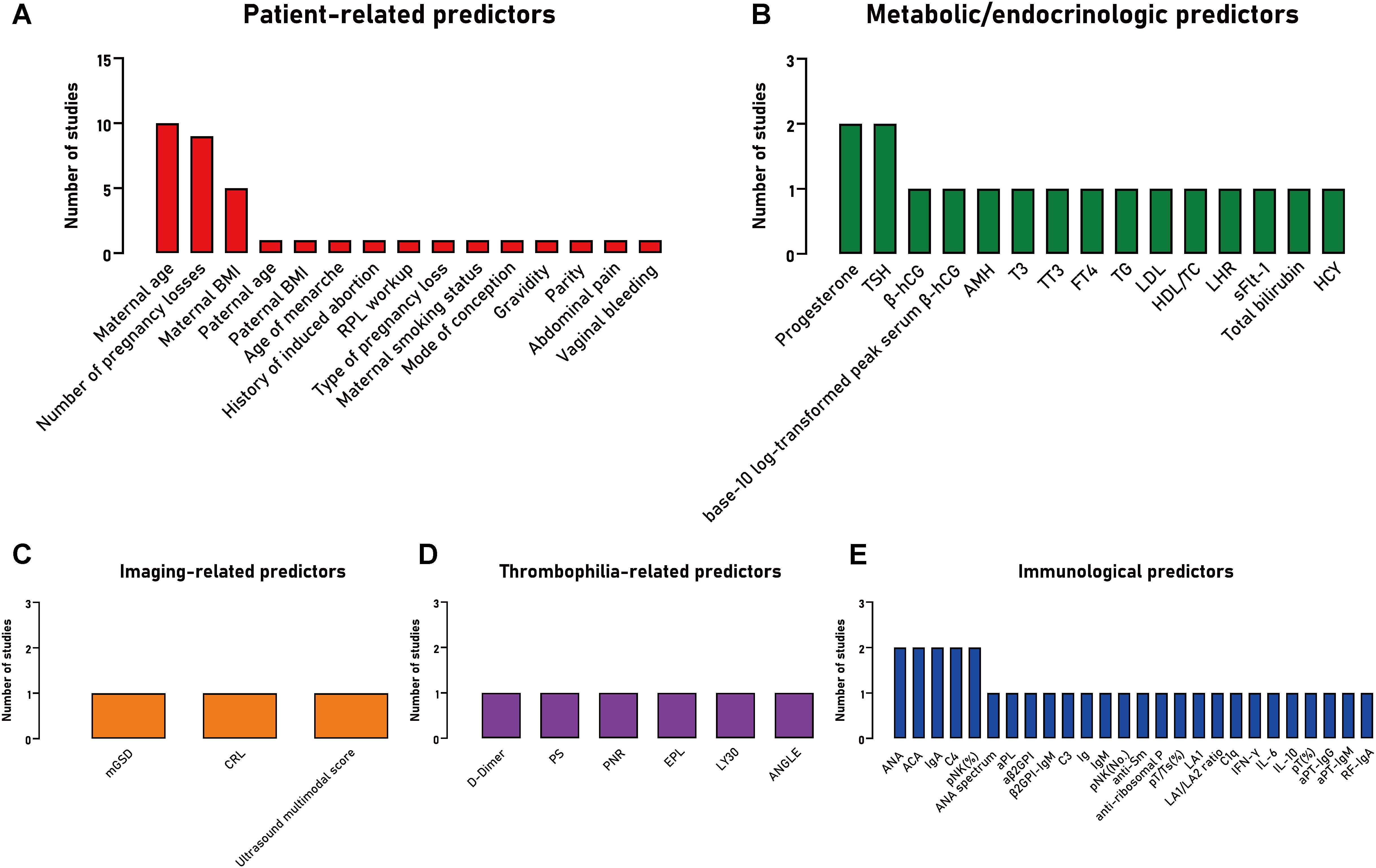
Figure 2. The frequency of predictors included in studies developing a clinical prediction model for the prognosis of patients with recurrent pregnancy loss, stratified by predictor type. (A) Patient-related predictors; (B) Metabolic/endocrinologic predictors; (C) Imaging-related predictors; (D) Thrombophilia-related predictors; (E) Immunological predictors. BMI, body mass index; RPL, recurrent pregnancy loss; TSH, thyroid stimulating hormone; β-hCG, β human chorionic gonadotropin; AMH, anti-Müllerian hormone; T3, triiodothyronine; TT3, total triiodothyronine; FT4, free thyroxine; TG, triglyceride; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TC, cholesterol; LHR, low-density lipoprotein to high-density lipoprotein ratio; sFlt-1, soluble vascular endothelial growth factor receptor-1; HCY, homocysteine; mGSD, mean of the gestational sac diameter; CRL, crown-rump length; PS, protein S; PNR, platelet to neutrophilic ratio; EPL, an estimated percentage of clot lysis at 30 minutes after the maximum amplitude on TEG tracing; LY30, an actual percentage of clot lysis at 30 minutes after the maximum amplitude on TEG tracing; ANGLE, angle Alpha; ANA, antinuclear antibody; ACA, anti cardiolipin antibody; IgA, immunoglobulin A; C4, complement 4; pNK, peripheral natural killer cells; pNK (%), percentage of pNK; pNK (No.), number of pNK; aPL, anti-phospholipid antibody; β2GPI, β2 glycoprotein I antibody; aβ2GPI, anti-β2 glycoprotein I antibody; C3, complement 3; Ig, immunoglobulin; IgM, immunoglobulin M; IgG, immunoglobulin G; pT, peripheral T cells; Ts, suppressor T cells; pT (%), percentages of peripheral T cells; LA1, lupus anticoagulant screening test; LA2, lupus anticoagulant confirmatory test; C1q, complement component 1q; IFN-γ, interferon-γ; IL-6, interleukin-6; IL-10, interleukin-10; aPT, anti-prothrombin antibody; RF, rheumatoid factor.
3.4 Missing data
Fourteen studies acknowledged the presence of missing data during the development of their risk prediction models (Table 3). In specific, 5 studies handled missing data using multiple imputations (20, 21, 24, 25, 28), while 6 studies performed complete case analysis by excluding individuals with missing data (17–19, 23, 26, 29). However, 3 studies reported missing data without specifying the handling method (22, 27, 30), and one study did not report any information regarding missing data (16). Since statistical software defaults to complete case analysis when the handling of missing data is unspecified (31), we assumed that these studies employed complete-case analysis.
3.5 Model construction
Fifteen studies encompassed 22 prediction models, with candidate predictors ranging from 2 to 63 and sample sizes between 85 and 789. The events per variable (EPV) varied from 0.7 to 132.5. Logistic regression was the primary approach (16–23, 25–30), while one study employed machine learning algorithms (24). For model presentation, one model used a risk scoring system, six used nomograms, three presented logistic regression equations, and two were online calculators. It should be noted that in some studies containing multiple models (20, 25), these models shared the same dataset, candidate variable selection process, and construction procedures, differing only through distinct predictor combinations to achieve prediction objectives. Details are in Table 3.
3.6 Model evaluation
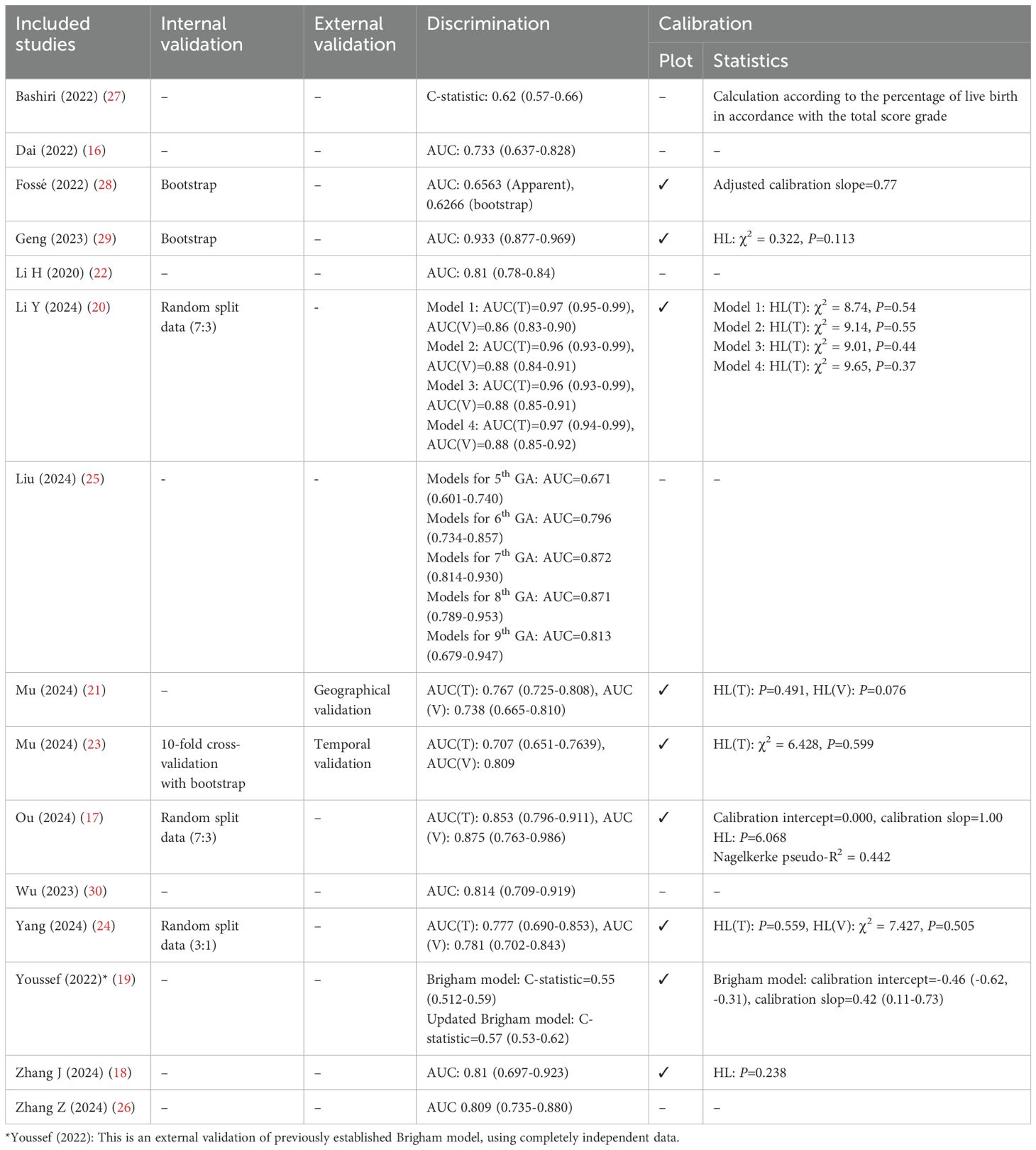
The discriminative ability was assessed with the area under the receiver operating characteristics (ROC) curve (AUC) in 13 studies and C-statistics in 2 studies (Table 4). Among the 20 models that provided AUC values, 13 of them exhibited an AUC exceeding 0.80 (ranges from 0.809~0.97), while the remainder indicated moderate discrimination, ranging from 0.62 to 0.796. Additionally, 10 studies assessed how well the predicted risks compared to the observed risks (calibration), with 7 conducting the Hosmer-Lemeshow goodness-of-fit test (17, 18, 20, 21, 23, 24, 29), 9 presenting calibration plots (17–21, 23, 24, 28, 29), and 3 employing the calibration slope (17, 19, 28).
3.7 Model validation
Model validation methods varied across studies (Table 4). Internal validation was the most common, including random splitting data (n=3) (17, 20, 24), bootstrapping (n=2) (28, 29), and cross-validation (n=1) (23). Among these, 3 studies demonstrated high consistency in AUC between training and validation datasets (difference ≤ 0.02) (17, 24, 28), while one study showed significant discrepancy (difference 0.09~0.11) (20). External validation was reported in only 3 studies (13%) (19, 21, 23), with 2 demonstrating similar or improved performance in the validation set (21, 23). However, one study found poor performance even after updating the model (19). Notably, 7 studies did not perform validation, which could affect model reliability and generalizability.
3.8 ROB assessment
All models assessed by PROBAST showed high ROB (Supplementary Table 3), indicating significant methodological shortcomings in development/validation, raising concerns about their real-world performance. In the predictor domain, 7 studies had high ROB (16, 19–23, 28), with one study having unclear risk (26) due to missing details on predictors. In the outcome domain, 11 studies showed high ROB (16, 18, 19, 21–23, 25–29) due to lack of blinding in outcomes/predictors evaluations. In the analysis domain, 11 studies had high ROB, with 9 failing to meet the EPV criterion due to small sample sizes (17, 18, 21–24, 26, 29, 30). Also, 7 studies showed issues with overfitting, underfitting, and performance optimism, and lacked internal/external validation (16, 18, 19, 22, 26, 27, 30). For applicability, 12 studies indicated low ROB. However, 3 studies had unclear applicability, with 2 in the predictor domain (16, 26), and one in the outcome domain due to unclear definition of ‘pregnancy loss’ (29).
4 Discussion
This systematic review identified 15 studies describing 22 RPL risk prediction models. Despite the models showed moderate to excellent predictive performance, they were all assessed to have high ROB due to methodological deficiencies.
Utilizing the PROBAST tool, this study found that the model exhibited low applicability concerns (e.g., good alignment with the target population, predictors, and outcomes) but carried a high overall ROB, primarily reflected in domains such as predictor selection, outcome definition, and analysis methods. This contradictory result suggests that, although the models are theoretically suitable for the current clinical setting in its design, methodological flaws during their development may lead to deviations of predicted values from true risks, potentially affecting the models’ decision-support value in practice. It is also important to note that the PROBAST assessments relies on reporting completeness. Despite our efforts to reconstruct model development details through data verification, there may still be unidentified sources of bias (e.g., unrecorded measurement errors in variables).
Additionally, inconsistencies in defining the RPL population further challenge model generalizability. Most studies included in this review defined RPL as two or more pregnancy losses, but there were differences regarding gestational age and the requirement for consecutive pregnancy losses. The primary goal of prediction models in the medical field is to support informed decision-making. Therefore, it is important to clearly define the target population in order to evaluate the performance of the developed model and ensure users understand its applicability. Moreover, 10 studies were deemed to have high ROB due to model overfitting. Overfitting is a common challenge in constructing multivariable prediction models. The coexistence of high AUCs and high ROB may stem from overfitting in small samples, where models capture noise rather than true biological signals, inflating performance in training sets but failing in external validation (32). To mitigate these risks, widely accepted guidelines recommend a minimum of 10 EPV to reduce overfitting and improve generalization, thereby minimizing bias (33, 34). However, 8 studies reported EPV<10 (18, 21–24, 26, 29, 30), and 2 studies were unable to calculate EPV (17, 20). Importantly, low EPV can lead to inaccurate effect estimates and statistical instability, which threatens model validity and may result in poor performance on new datasets (35, 36). Therefore, restricting their application to narrowly defined subpopulations could mitigate misclassification risks until methodological refinements are achieved. Future research should adhere established guidelines for constructing models to enhance the model’s predictive power and generalizability.
In this review, the most frequently included predictive variables were maternal age, number of previous pregnancy losses, and maternal BMI, which have been consistently incorporated into RPL prediction models over the past five years. Their association with RPL abortion has been extensively validated. Advanced maternal age is associated with increased risk of chromosomal abnormalities and reduced endometrial receptivity (37). Elevated maternal BMI has been linked to insulin resistance, chronic inflammation, and impaired implantation (38). A greater number of pregnancy losses often reflects persistent underlying risk factors, such as anatomical, immunological, or coagulation-related issues, which could affect the success rate of subsequent pregnancies and embryo implantation (39). In addition to these strong patient-related factors, a growing number of studies have explored immunological and metabolic/endocrine predictors. For instance, ANA and ACA suggest underlying autoimmune activation that could interfere with embryo implantation or promote placental thrombosis (40, 41). Increased concentrations of C4 could indicate an overly active immune reaction, which may result in harm to the fetal-placental unit (42). On the endocrine side, abnormal levels of progesterone or TSH could indicate luteal phase deficiency or subclinical hypothyroidism, both of which are associated with adverse pregnancy outcomes (43). These findings highlight the multifactorial nature of RPL and the importance of integrating diverse predictor domains in model development.
Furthermore, one model included paternal factors (age and BMI) and the mode of conception as predictors for assessing RPL pregnancy outcomes (28). These couple-related predictors are rarely explored but may be important contributors to reproductive outcomes. For example, while natural conception is commonly influenced by basic health factors like age and BMI, patients undergoing assisted reproductive technology (ART) face additional challenges such as ovarian dysfunction and embryo quality (44, 45). However, over 70% of included studies did not specify whether pregnancies were naturally conceived or ART-mediated. Only two studies reported inclusion of ART conceptions, but these were analyzed together with natural conceptions without reporting pregnancy outcomes separately (17, 22). Given the rising prevalence of ART, future research should explore how conception methods interact with risk factors and should clearly report and stratify by conception method. This would help refine RPL risk models, improve their clinical applicability, and support more precise prognostic assessments.
Assessing the performance of a risk prediction model is crucial after its development. In this review, only 6 studies performed internal validation, and just 2 performed external validation. Although many studies demonstrated good discrimination in their training datasets, the lack of proper validation contributed to a high risk of bias, particularly in the analysis domain. This limitation undermines the reliability and clinical applicability of these models and highlights the need for improved data quality, validation strategies, and transparent reporting.
To our knowledge, this is the first systematic review to evaluate the risk prediction models for RPL pregnancy outcomes. Our review highlighted the methodological deficiencies in these studies, which could inform future research in constructing more robust, reliable models applicable across various clinical settings. Unlike previous reviews that primarily focus on English-language sources, our study also includes Chinese-language literature, offering a broader evaluation of existing RPL risk prediction models. However, due to heterogeneity across studies, such as differences in predictor variables, pregnancy outcome use, data sources, and analytical methods, only a narrative systematic review was conducted. Additionally, most of the included studies (12/15) were conducted in China, with only 2 from the Netherlands and 1 from Israel. This concentration in a single region raises the possibility of regional bias. Differences in population characteristics, healthcare practices, and resource availability across countries may influence both model performance and applicability. As a result, these models may not be directly applicable to broader or more heterogeneous populations. Future research should consider validating or adapting existing models in more diverse clinical settings to improve their generalizability and clinical utility.
Collectively, to translate these findings into clinical utility, future research must prioritize immediate and longer-term goals. Immediate priorities should focus on both internal and external validation of existing models across diverse populations, standardization of outcome definitions (e.g., gestational age), and adherence to EPV criteria and TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis) statement (46) during model development. These steps are critical to confirm generalizability, reduce measurement bias, and prevent overfitting—issues that directly undermine current models’ reliability. Simultaneously, longer-term strategies could explore adaptive model architectures capable of dynamically integrating emerging data, thereby maintaining relevance as clinical practices evolve. This approach would enhance accuracy while reducing reliance on manual recalibration. By addressing these challenges, the developed risk prediction models can better support clinical practice in managing patients with RPL, ultimately leading to more personalized, timely, and effective interventions that improve patient outcomes.
5 Conclusions
The findings suggest that current risk prediction models for RPL pregnancy outcomes have a high ROB in clinical applications. Future research should prioritize rigorous model construction and validation processes to provide more reliable and effective tools for clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
QJ: Conceptualization, Visualization, Writing – original draft. FM: Data curation, Formal Analysis, Writing – original draft. KW: Formal Analysis, Validation, Writing – original draft. FW: Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1582156/full#supplementary-material
References
1. Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss: an update in 2022. Hum Reprod Open. (2023) 2023:hoad002. doi: 10.1093/hropen/hoad002
2. Medicine PCotASfR. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertility sterility. (2012) 98:1103–11. doi: 10.1016/j.fertnstert.2012.06.048
3. Regan L, Rai R, Saravelos S, and Li TC. Recurrent miscarriageGreen-top guideline no. 17. BJOG: an Int J obstetrics gynaecology. (2023) 130:e9–e39. doi: 10.1111/1471-0528.17515
4. Obstetrics Subgroup CSoOaG, Abortion CMACECGoDaMoRS. Chinese expert consensus on diagnosis and management of recurrent spontaneous abortion (2022). Chin J Obstetrics Gynecology. (2022) 57:653–67. doi: 10.3760/cma.j.cn112141-20220421-00259
5. van Dijk MM, Kolte AM, Limpens J, Kirk E, Quenby S, van Wely M, et al. Recurrent pregnancy loss: diagnostic workup after two or three pregnancy losses? A systematic review of the literature and meta-analysis. Hum Reprod update. (2020) 26:356–67. doi: 10.1093/humupd/dmz048
6. Alijotas-Reig J and Garrido-Gimenez C. Current concepts and new trends in the diagnosis and management of recurrent miscarriage. Obstet Gynecol Surv. (2013) 68:445–66. doi: 10.1097/OGX.0b013e31828aca19
7. Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, and Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ (Clinical Res ed). (2019) 364:l869. doi: 10.1136/bmj.l869
8. Wynants L, van Smeden M, McLernon DJ, Timmerman D, Steyerberg EW, and Van Calster B. Three myths about risk thresholds for prediction models. BMC medicine. (2019) 17:192. doi: 10.1186/s12916-019-1425-3
9. Mora-Sánchez A, Aguilar-Salvador DI, and Nowak I. Towards a gamete matching platform: using immunogenetics and artificial intelligence to predict recurrent miscarriage. NPJ digital medicine. (2019) 2:12. doi: 10.1038/s41746-019-0089-x
10. Wu Y, Xin M, Han Q, Wang J, Yin X, He J, et al. Extensive serum biomarker analysis in the prethrombotic state of recurrent spontaneous abortion. J Cell Mol medicine. (2021) 25:6679–94. doi: 10.1111/jcmm.v25.14
11. Bruno V, D'Orazio M, Ticconi C, Abundo P, Riccio S, Martinelli E, et al. Machine Learning (ML) based-method applied in recurrent pregnancy loss (RPL) patients diagnostic work-up: a potential innovation in common clinical practice. Sci reports. (2020) 10:7970. doi: 10.1038/s41598-020-64512-4
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg (London England). (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
13. Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PloS medicine. (2014) 11:e1001744. doi: 10.1371/journal.pmed.1001744
14. Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: A tool to assess the risk of bias and applicability of prediction model studies. Ann Internal medicine. (2019) 170:51–8. doi: 10.7326/M18-1376
15. Moons KG, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Internal Med. (2019) 170:W1–W33. doi: 10.7326/M18-1377
16. Dai Y-F, Lin L-Z, Lin N, He D-Q, Guo D-H, Xue H-L, et al. APA scoring system: a novel predictive model based on risk factors of pregnancy loss for recurrent spontaneous abortion patients. J Obstetrics Gynaecology. (2022) 42:2069–74. doi: 10.1080/01443615.2021.2021507
17. Ou M, Luo L, Yang Y, Yan N, Yan X, Zhong X, et al. Decrease in peripheral natural killer cell level during early pregnancy predicts live birth among women with unexplained recurrent pregnancy loss: a prospective cohort study. Am J obstetrics gynecology. (2024) 230:675.e1–.e13. doi: 10.1016/j.ajog.2023.10.042
18. Zhang J, Song Z, Yuan H, and Cai ZH. The effects of metabolic indicators and immune biomarkers on pregnancy outcomes in women with recurrent spontaneous abortion: a retrospective study. Front endocrinology. (2024) 14:1297902. doi: 10.3389/fendo.2023.1297902
19. Youssef A, van der Hoorn MLP, Dongen M, Visser J, Bloemenkamp K, van Lith J, et al. External validation of a frequently used prediction model for ongoing pregnancy in couples with unexplained recurrent pregnancy loss. Hum Reprod (Oxford England). (2022) 37:393–9. doi: 10.1093/humrep/deab264
20. Li Y, Wu IXY, Wang X, Song J, Chen Q, and Zhang W. Immunological parameters of maternal peripheral blood as predictors of future pregnancy outcomes in patients with unexplained recurrent pregnancy loss. Acta obstetricia gynecologica Scandinavica. (2024) 103:1444–56. doi: 10.1111/aogs.v103.7
21. Mu F, Huo H, Wang C, Hu N, and Wang F. A new prognostic model for recurrent pregnancy loss: assessment of thyroid and thromboelastograph parameters. Front endocrinology. (2024) 15:1415786. doi: 10.3389/fendo.2024.1415786
22. Li H, Qin S, Xiao F, Li Y, Gao Y, Zhang J, et al. Predicting first-trimester outcome of embryos with cardiac activity in women with recurrent spontaneous abortion. J Int Med Res. (2020) 48:300060520911829. doi: 10.1177/0300060520911829
23. Mu F, Wang M, Zeng X, and Wang F. Predicting risk of subsequent pregnancy loss among women with recurrent pregnancy loss: An immunological factor-based multivariable model. Am J Reprod Immunol (New York NY: 1989). (2024) 91:e13837. doi: 10.1111/aji.13837
24. Yang X, Wang R, Zhang W, Yang Y, and Wang F. Predicting risk of the subsequent early pregnancy loss in women with recurrent pregnancy loss based on preconception data. BMC women's Health. (2024) 24:381. doi: 10.1186/s12905-024-03206-9
25. Liu C, Wei X, and Wang F. The predictive value of ultrasound markers for pregnancy outcomes in recurrent pregnancy loss: a retrospective study. Sci reports. (2024) 14:16657. doi: 10.1038/s41598-024-67744-w
26. Zhang Z-M, Zhang N, and Wang X-F. Prognostic model on pregnancy outcomes for women with recurrent spontaneous abortions treated with cyclosporin A: A single-institution experience. Clinics. (2024) 79:100349. doi: 10.1016/j.clinsp.2024.100349
27. Bashiri A, Giliutin M, Ziedenberg H, Plakht Y, and Baumfeld Y. A proposed prognostic prediction tool for a live birth among women with recurrent pregnancy loss. J Maternal-Fetal Neonatal Medicine. (2022) 35:3736–42. doi: 10.1080/14767058.2020.1839877
28. du Fossé NA, van der Hoorn MP, de Koning R, Mulders A, van Lith JMM, le Cessie S, et al. Toward more accurate prediction of future pregnancy outcome in couples with unexplained recurrent pregnancy loss: taking both partners into account. Fertility sterility. (2022) 117:144–52. doi: 10.1016/j.fertnstert.2021.08.037
29. Geng L, Qiu J, Zhang W, Xu J, Yuan F, and Liu Y. Prediction of pregnancy outcome of unexplained recurrent abortion by Ultrasonic multimodal score and sFlt-1 and D-Dimer [in Chinese. J Xinjiang Med Univ. (2023) 46:1490–5. doi: 10.3969/j.issn.1009-5551.2023.11.014
30. Wu Y. Study on the distribution of traditional Chinese medicine syndrome types and influencing factors of pregnancy outcome in recurrent spontaneous abortion. Guangzhou, Guangdong Province, China: Guangzhou University of Chinese Medicine (2023).
31. Gachau S, Quartagno M, Njagi EN, Owuor N, English M, and Ayieko P. Handling missing data in modelling quality of clinician-prescribed routine care: Sensitivity analysis of departure from missing at random assumption. Stat Methods Med Res. (2020) 29:3076–92. doi: 10.1177/0962280220918279
32. Pavlou M, Ambler G, Qu C, Seaman SR, White IR, and Omar RZ. An evaluation of sample size requirements for developing risk prediction models with binary outcomes. BMC Med Res methodology. (2024) 24:146. doi: 10.1186/s12874-024-02268-5
33. Peduzzi P, Concato J, Feinstein AR, and Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin epidemiology. (1995) 48:1503–10. doi: 10.1016/0895-4356(95)00048-8
34. Concato J, Peduzzi P, Holford TR, and Feinstein AR. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J Clin epidemiology. (1995) 48:1495–501. doi: 10.1016/0895-4356(95)00510-2
35. Peduzzi P, Concato J, Kemper E, Holford TR, and Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin epidemiology. (1996) 49:1373–9. doi: 10.1016/S0895-4356(96)00236-3
36. Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosomatic medicine. (2004) 66:411–21. doi: 10.1097/01.psy.0000127692.23278.a9
37. Li J, Fan W, Wang X, Hou X, Chen Z, and Lv M. Mental health in early pregnancy and spontaneous abortion risk: A prospective cohort study. Alpha Psychiatry. (2024) 25:648–55. doi: 10.5152/alphapsychiatry.2024.241682
38. Howell KR and Powell TL. Effects of maternal obesity on placental function and fetal development. Reprod (Cambridge England). (2017) 153:R97–r108. doi: 10.1530/REP-16-0495
39. Karavani G, Alexandroni H, Sheinin D, Dior UP, Simon A, Ben-Meir A, et al. Endometrial thickness following early miscarriage in IVF patients - is there a preferred management approach? Reprod Biol endocrinology: RB&E. (2021) 19:93. doi: 10.1186/s12958-021-00780-7
40. Deng T, Liao X, and Zhu S. Recent advances in treatment of recurrent spontaneous abortion. Obstet Gynecol Surv. (2022) 77:355–66. doi: 10.1097/OGX.0000000000001033
41. Valeff NJ, Ventimiglia MS, Diao L, and Jensen F. Lupus and recurrent pregnancy loss: the role of female sex hormones and B cells. Front endocrinology. (2023) 14:1233883. doi: 10.3389/fendo.2023.1233883
42. Sugiura-Ogasawara M, Nozawa K, Nakanishi T, Hattori Y, and Ozaki Y. Complement as a predictor of further miscarriage in couples with recurrent miscarriages. Hum Reprod (Oxford England). (2006) 21:2711–4. doi: 10.1093/humrep/del229
43. Kaur R and Gupta K. Endocrine dysfunction and recurrent spontaneous abortion: An overview. Int J Appl basic Med Res. (2016) 6:79–83. doi: 10.4103/2229-516X.179024
44. Tian J, Zhang Z, Mei J, Kong N, Yan Y, Shen X, et al. Dysregulation of endometrial stromal serotonin homeostasis leading to abnormal phosphatidylcholine metabolism impairs decidualization in patients with recurrent implantation failure. Hum Reprod Open. (2024) 2024:hoae042. doi: 10.1093/hropen/hoae042
45. Vlaisavljevic V, Apter S, Capalbo A, D'Angelo A, Gianaroli L, Griesinger G, et al. The Maribor consensus: report of an expert meeting on the development of performance indicators for clinical practice in ART. Hum Reprod Open. (2021) 2021:hoab022. doi: 10.1093/hropen/hoab022
Keywords: recurrent pregnancy loss, prediction model, pregnancy outcomes, systematic review, general practice
Citation: Jian Q, Mu F, Wang K and Wang F (2025) Risk prediction models for pregnancy outcomes in recurrent pregnancy loss: a narrative systematic review. Front. Endocrinol. 16:1582156. doi: 10.3389/fendo.2025.1582156
Received: 24 February 2025; Accepted: 13 May 2025;
Published: 29 May 2025.
Edited by:
Duan Xing, Southeast University, ChinaReviewed by:
Hong-Hui Wang, Weihai Second Municipal Hospital of Qingdao University, ChinaQunying Fang, University of Science and Technology of China, China
Copyright © 2025 Jian, Mu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Wang, ZXJ5X2Z3YW5nQGx6dS5lZHUuY24=
 Qiliang Jian
Qiliang Jian Fangxiang Mu
Fangxiang Mu Kexin Wang
Kexin Wang Fang Wang
Fang Wang