- 1Department of Reproductive Medical Center, Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2The Second Clinical Medicine college, Henan University of Chinese Medical, Zhengzhou, China
Purpose: Our aim was to explore the clinical outcomes of a single blastocyst frozen–thawed transfer (single blastocyst frozen–thawed transfer (singleton frozen embryo transfer, sFET) derived from low-quality day 3 (D3) embryos.
Methods: This retrospective cohort study was conducted at the Reproductive Health Center of the Third Affiliated Hospital of Zhengzhou University. All data on sFET were collected between March 2016 and September 2022. Blastocysts derived from good-quality and low-quality D3 embryos were designated as the good-quality group and the low-quality group, respectively. Patients were divided into three groups according to age: <35 group, 35–39 group, and ≥40 group. Based on whether preimplantation genetic testing (PGT) was performed or not, the blastocysts derived from low-quality embryos were divided into the PGT group and the non-PGT group, respectively.
Results: After adjusting for female age, male age, infertility duration, and other potential confounders, the difference in the clinical pregnancy rate and the live birth rate in the good quality and low-quality groups maintained statistical significance [adjusted odds ratio adjusted odds ratio (aOR) = 0.32 and 0.35, p < 0.001]. When adjusting for embryo quality, the clinical pregnancy rate and the live birth rate in the <35 and 35–39 groups were significantly higher than those in the ≥40 group (OR = 3.02 and 3.56, p < 0.001; OR = 1.89 and 1.84, p < 0.001). Embryo quality significantly affected the clinical pregnancy rate and the live birth rate (p < 0.001). The clinical pregnancy rate and the live birth rate in the PGT group were higher than those in the non-PGT group (40.0% vs. 29.3% and 40.0% vs. 22.0%, respectively).
Conclusion: D3 embryos with low score/low quality can still obtain a certain live birth rate after further culturing to blastocysts with PGT.
Introduction
In the process of in vitro fertilization embryo transfer (IVF-ET) treatment, the majority of reproductive centers select high-quality or high-morphological-score embryos for transplantation or freezing after performing morphological scoring on the third day (D3) after fertilization. Low-quality embryos with poor developmental potential would be discarded after informed consent. However, it is still controversial whether low-quality D3 embryos have clinical value or not (1). Emerging evidence indicates that vitrified–thawed blastocysts originating from poor-quality D3 embryos are capable of establishing viable pregnancies and delivering healthy offspring (2, 3). Stecher et al. demonstrated that culturing low-quality D3 embryos to blastocysts prior to vitrification could improve the utilization rate of embryos and the cumulative pregnancy rate of cycles (4). The above studies indicate that even low-quality D3 embryos may still show better developmental potential during blastocyst culture.
Studies have shown that a large proportion of embryos with high morphological scores may be aneuploid, while some low-quality D3 embryos may also be euploid (5, 6). However, in the majority of cases, the correlations between aneuploidy and the morphologies of embryos have been weak (5).
Clinically, due to advanced age, decreased ovarian reserve, and other reasons, some patients do not have high-quality embryos. For these patients, the use of embryos with low quality and poor development potential will be of great significance. This study aimed to explore the clinical pregnancy outcomes of a single blastocyst frozen–thawed transfer derived from low-quality D3 embryos.
Materials and methods
Participants
This study was conducted at the Reproductive Health Center of the Third Affiliated Hospital of Zhengzhou University. All data on single blastocyst frozen–thawed transfer (singleton frozen embryo transfer, sFET) were collected between March 2016 and September 2022.
The inclusion criteria were as follows: 1) infertile couples who had experienced IVF-ET due to female tubal factors and male factors, among others, and 2) sFET. The exclusion criteria were as follows: 1) patients who donated sperm or oocytes; 2) patients with incomplete medical records; and 3) patients who had experienced recurrent implantation failure.
All sFETs derived from low-quality D3 embryos were divided into the pre-implantation genetic testing (PGT) group and the non-PGT group based on whether PGT was performed.
Methods
Ovarian stimulation program
Based on the woman’s age and ovarian reserve function, the clinician would formulate an appropriate scheme for ovulation promotion (7). Oocyte retrieval was performed under ultrasound guidance at 36–38 h after human chorionic gonadotropin (hCG) injection.
In vitro fertility and embryo culture
Based on oocyte maturity and sperm quality on the day of oocyte retrieval, the oocytes were inseminated via in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) at 38–40 h after hCG injection. After 16–18 h, the appearance of two evident pronuclei indicates fertility. The zygotes were cultured in the cleavage medium (G-1 PLUS; Vitrolife, Gothenburg, Sweden).
The embryos were transferred from the cleavage medium into the blastocyst medium (G-2; Vitrolife, Gothenburg, Sweden) on day 3 after insemination for development into blastocysts. Subsequently, they were cultured until day 7 after insemination in humidified air maintained at 37°C under a 6% CO2 and 5% O2 atmosphere. The development of blastocysts was observed and scored during this period.
In non-PGT treatment cycles, the blastocysts were either transferred in the fresh cycle or cryopreserved for subsequent FET based on the clinical indications and patient-specific factors. For the PGT cycles, once the blastocysts had developed, three to five trophoblast cells were taken for genetic testing, and then the blastocysts were cryopreserved. The decision to perform the transplantation.
Embryo score
D3 embryos were scored according to the following criteria: grade I—blastomere number (BL) of 6–10, of equal size, and fragmentation (FR) = 0%-5%; grade II—BL = 6–10, slightly equal in size, and FR = 5%–24%; grade III—BL = 6–10, unequal in size, and FR = 25%–49% or BL = 4–5 or >10; and grade IV—severely unequal-sized blastomeres, or FR > 50%, or embryo arrest. Grades I, II, and III indicate good-quality embryos, of which grades I and II are top-quality, and grade IV indicates low-quality embryos.
In our center, dependent on the situation of the patients, one or two good-quality embryos on D3 were chosen for freezing or transfer, while the others were cultured and frozen when they developed into blastocysts.
The blastocysts were observed and scored according to Gardner (8) on D5, D6, and D7 after insemination. Blastocysts at stage 3 or higher with an inner cell mass (ICM) score ≥B were considered for transfer or freezing. Blastocysts that scored 4BB or higher were considered top-quality blastocysts. Blastocysts derived from good-quality D3 embryos and bad-quality D3 embryos were defined as the good-quality group and the low-quality group, respectively.
Vitrification and warming of blastocysts
Vitrification and warming of the blastocysts were carried out according to the instructions in the Vit Kit (Kitazato Biopharma, Shizuoka, Japan). Before vitrification, the Vit Kits were stored at room temperature for at least 30 min. First, the blastocysts were incubated for 10 min in an equilibration solution, followed by a vitrification solution for 60 s. Subsequently, the blastocysts were placed in a carrier before being loaded into a cannula in liquid nitrogen. During warming, the cannula was taken off, the carrier end was rapidly immersed in a thawing solution (TS) at 37°C, and the blastocyst was kept there for 1 min. Then, the blastocyst was transferred to a diluent solution for 3 min, followed by washing solutions 1 and 2 for 3 min. Finally, the blastocyst was placed in blastocyst medium (G-2; Vitrolife, Gothenburg, Sweden) for transfer.
Endometrial preparation
The routine scheme of our center (9) was adopted for the endometrial preparation scheme of the FET cycle, which is selected according to the specific situation of the patient. Currently, the natural cycle, artificial cycle, and stimulation cycle are often used. For patients with regular menstruation and normal ovulation, natural cycles are adopted. Artificial cycles were used for patients with anovulation, luteal insufficiency, and a thin endometrium. Stimulation cycles are used for patients with follicular dysplasia, ovulation disorders, polycystic ovarian syndrome (PCOS), or contraindications to estrogen use.
Evaluation of pregnancy outcome
A serum β-hCG level ≥50 IU/L on day 14 after transfer, along with a gestational sac observed in the intrauterine cavity on day 35 after transfer, indicated clinical pregnancy. According to the American Society for Reproductive Medicine (ASRM), a miscarriage is defined as a termination of pregnancy at <20 weeks of gestation with a fetal weight of less than 500 g. A live birth is defined as a pregnancy reaching 28 weeks of gestation and resulting in the delivery of a live neonate.
Methods for calculating clinical indicators
The clinical indicators were determined as follows: Clinical pregnancy rate = count of clinical pregnancy cycles/count of transfer cycles × 100%; live birth rate = count of live birth cycles/count of transfer cycles × 100%; Abortion rate = count of abortion cycles/count of transfer cycles × 100%; and euploidy rate = count of euploid embryos/count of embryos with PGT.
Statistical analysis
All data were analyzed using SPSS 25.0. Measurement data are indicated as mean ± standard deviation (SD), and continuous variables were analyzed using a t-test. Count data are shown as percentages. Chi-square analysis was used to compare the rates between groups. Logistic regression was applied to control for confounding factors. A p-value less than 0.05 was considered statistically significant.
Results
Comparison of the basic clinical data and the clinical outcomes between the two groups
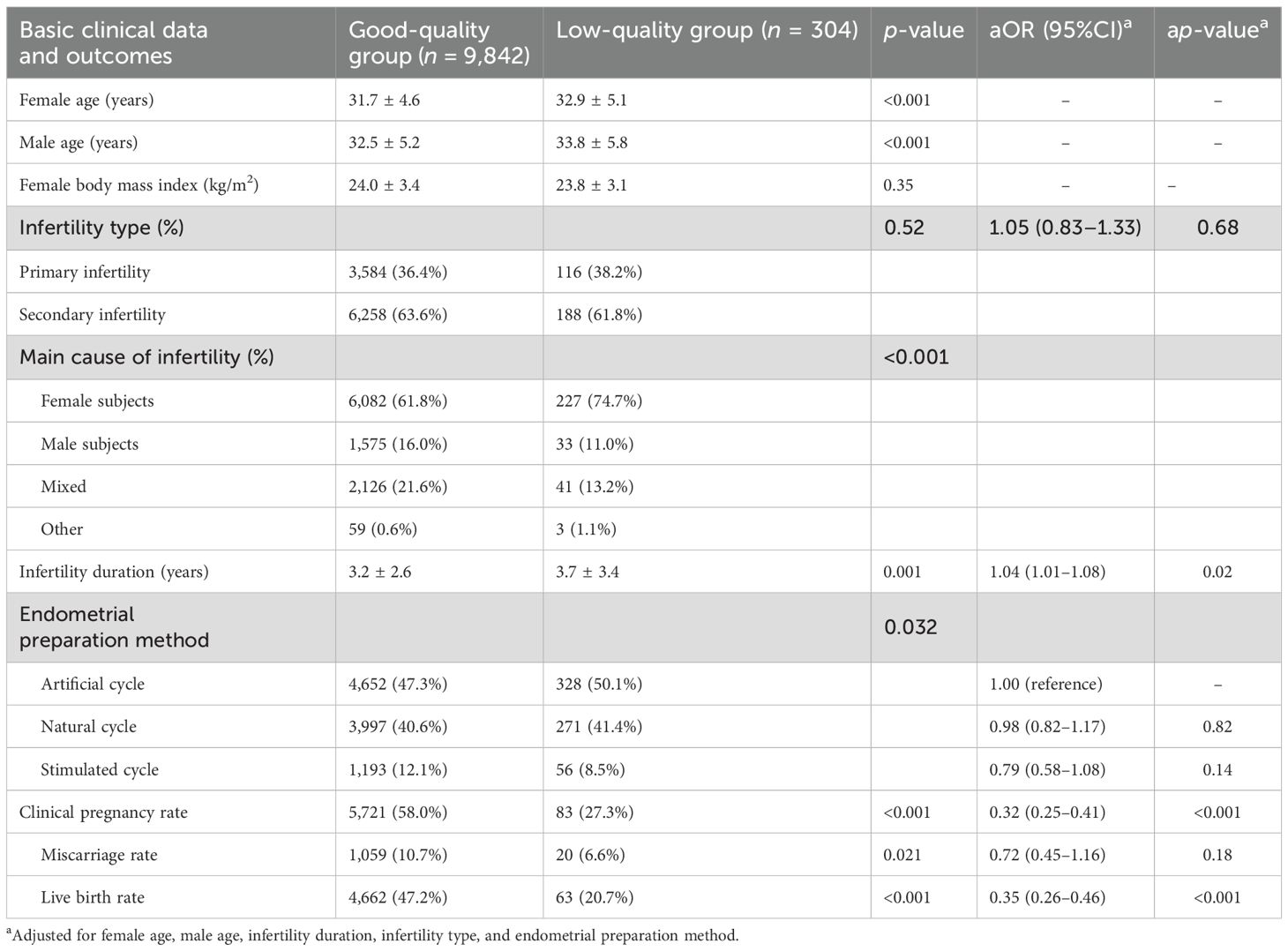
A total of 10,146 sFET cycles were compared in this study, of which 9,842 were in the good quality group and 304 were in the low-quality group. Female age, male age, and infertility duration in the good-quality group were all significantly lower than those in the low-quality group (p < 0.05). In addition, compared with the good-quality group, the clinical pregnancy rate and the live birth rate were significantly lower in the low-quality group (p < 0.001). After adjusting for female age, male age, infertility duration, and other potential confounders, the difference maintained statistical significance [adjusted odds ratio (aOR) = 0.32 and 0.35, p < 0.001] (Table 1).
Effect of age on the pregnancy outcomes from sFET in the good-quality and low-quality groups
Patients were divided into three different age groups: <35 years old (the <35 group), 35–39 years old (the 35–39 group), and ≥40 years old (the ≥40 group). The effects of age on the pregnancy outcomes from sFET in the good-quality and low-quality groups were compared.
A comparative analysis of the blastocyst quality scores between the two groups was performed. The analysis revealed a significantly higher proportion of top-quality blastocysts in the good-quality group compared with the low-quality group (57.1% vs. 12.5%, p < 0.01). Moreover, the rate of bad-quality blastocysts in the low-quality group was higher than that of the good-quality group (42.9% vs. 87.5%, p < 0.01) (Figure 1).
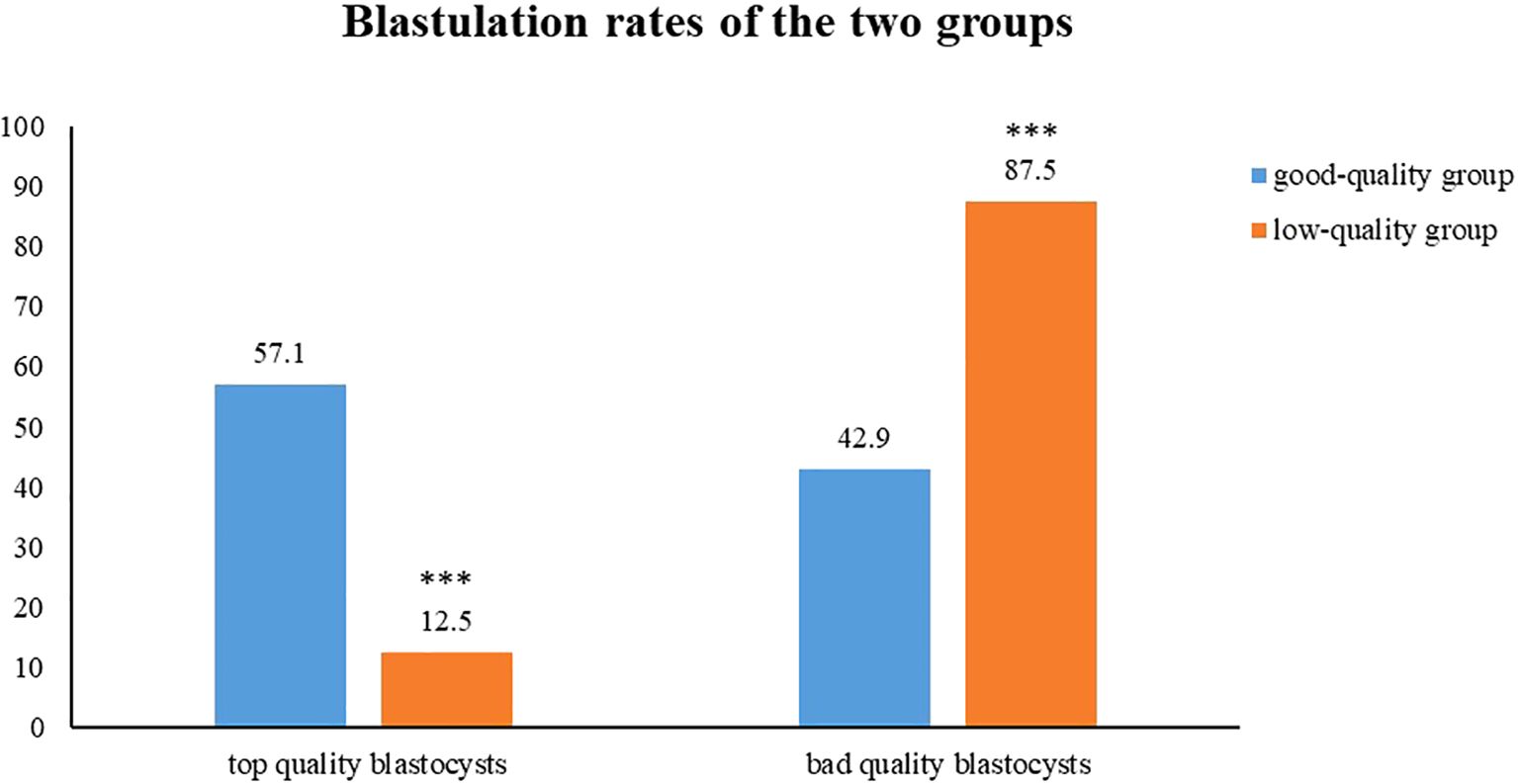
Figure 1. The comparison of the proportions of high-quality blastocysts and low-quality blastocysts between the two groups. Top quality blastocysts shows 57.1% in the good-quality group (blue) and 12.5% for the low-quality group (orange). Bad quality blastocysts shows 42.9% for good-quality and 87.5% for low-quality.
In the same age group, the clinical pregnancy rate and the live birth rate of the good quality group were higher than those in the low-quality group, and the difference between the <35 group and the 35–39 group was statistically significant (p < 0.05) (Table 2).
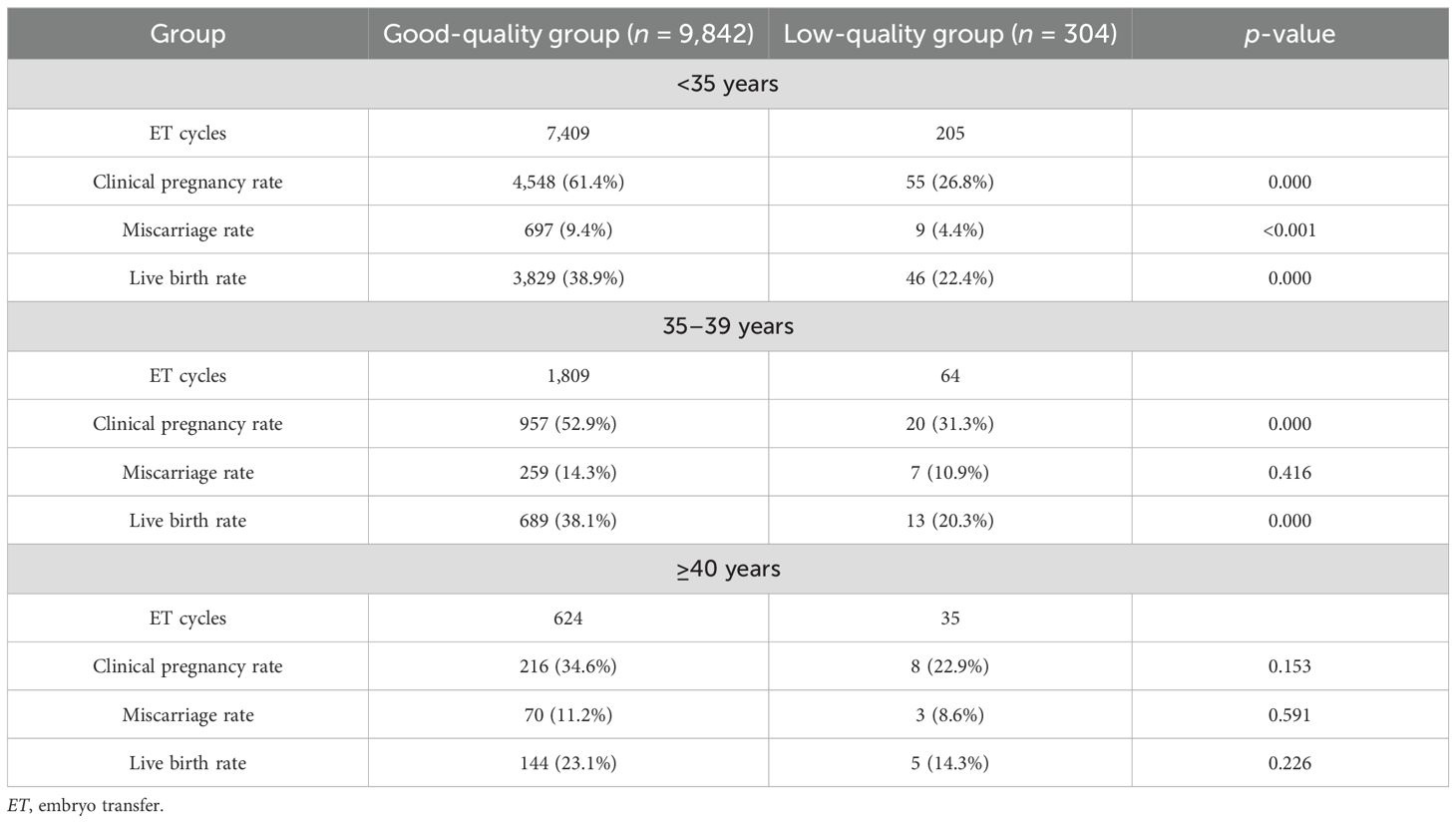
Table 2. Comparison of pregnancy outcomes between good-quality and low-quality groups within the same age range.
Multivariate regression analysis was performed to control for confounding factors (embryo quality and age). After adjusting for embryo quality, the clinical pregnancy rate and the live birth rate in the <35 group were significantly higher than those in the ≥40 group (OR = 3.02 and 3.56, p < 0.001). The pregnancy and live birth rates in the 35–39 group were still higher than those in the ≥40 group, but the odds ratios decreased (OR = 1.89 and 1.84, p < 0.001). Embryo quality significantly affected the clinical pregnancy rate and the live birth rate (p < 0.001) (Table 3; Supplementary Table S1).
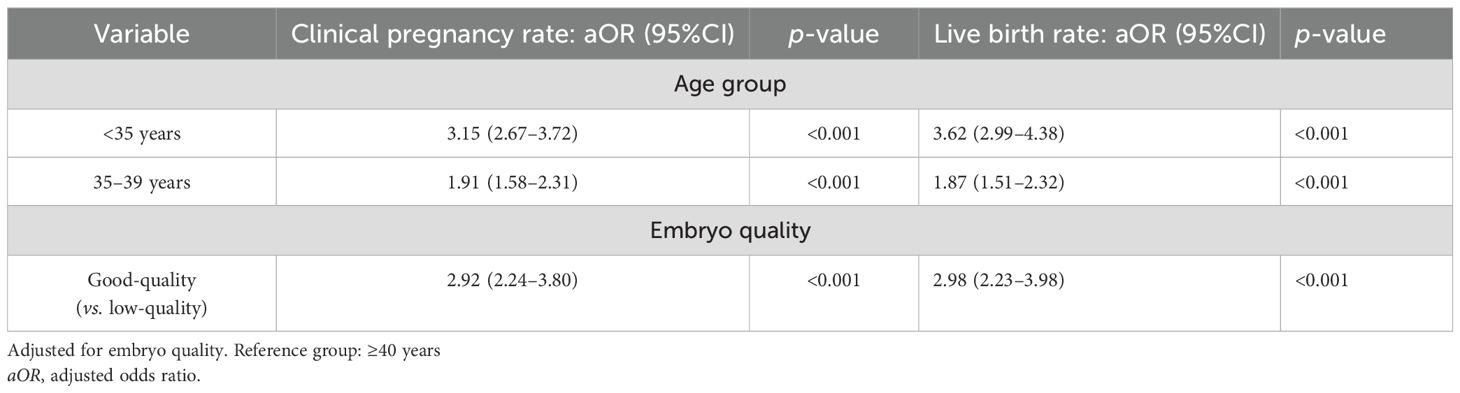
Table 3. Comparison of pregnancy outcomes of blastocyst transfer from the good-quality group and the low-quality group in different age groups.
Euploidy rates of the good-quality group and the low-quality group in the PGT cycles
The results of the 650 cycles of sFET with PGT from March 2016 to September 2022 were included. The euploidy rate of the blastocysts from the low-quality group was higher than that of the good-quality group; however, the difference was not statistically significant (p = 0.561) (Table 4).
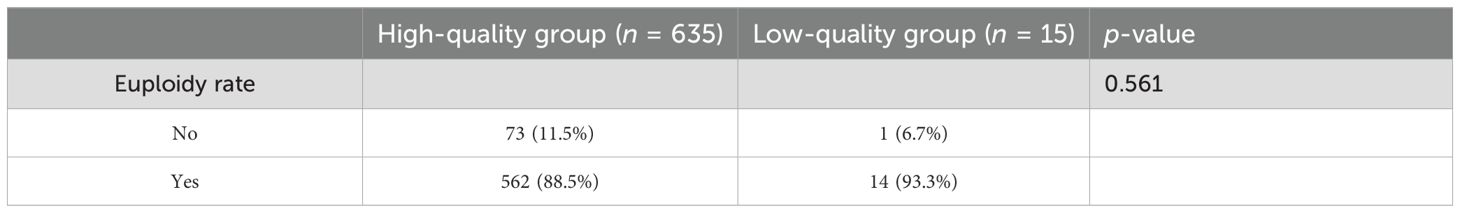
Table 4. Comparison of the euploidy rates in the good-quality group and the low-quality group in pre-implantation genetic testing (PGT) cycles.
Basic clinical data and outcomes of the sFET cycles derived from low-quality embryos in the PGT and non-PGT groups
There were 15 and 413 sFET cycles derived from low-quality embryos in the PGT and non-PGT groups, respectively. Compared with the non-PGT group, the clinical pregnancy rate and the live birth rate in the PGT group were higher; moreover, the miscarriage rate in the PGT group was lower. No significant differences were found (p > 0.05) (Table 5).
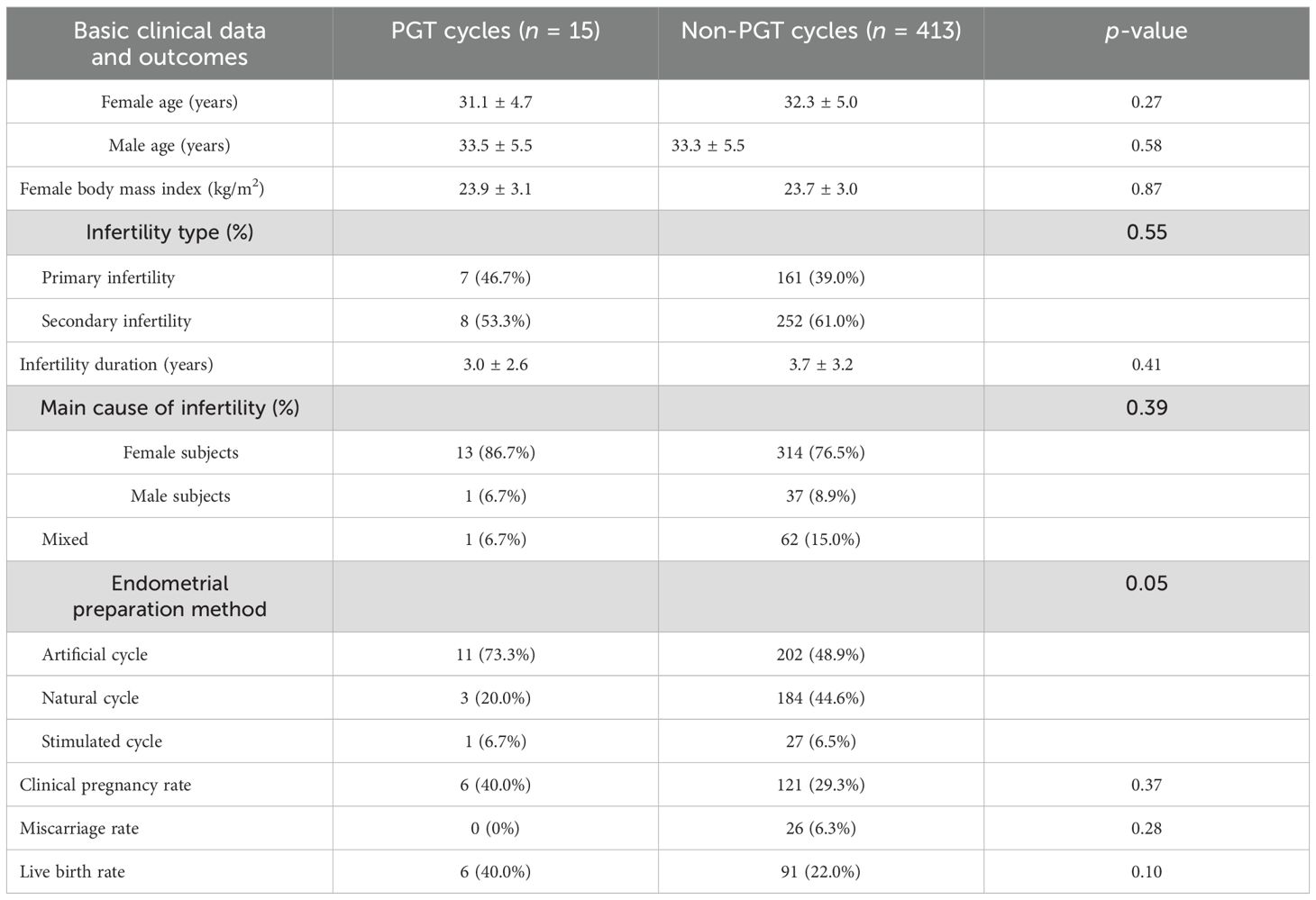
Table 5. Basic clinical data and outcomes of single blastocyst frozen–thawed transfer (sFET) cycles derived from low-quality embryos of pre-implantation genetic testing (PGT) and non-PGT.
Discussion
Morphological assessment continues to serve as the primary method for evaluation of embryo development potential. However, it has several limitations. Morphological scoring relies on visual assessment, which can be subjective. Additionally, morphology does not detect chromosomal abnormalities (aneuploidy), which are a major cause of implantation failure and miscarriage (10). Furthermore, high morphological scores do not always correlate with successful implantation or live birth (11, 12). In addition, many embryos may receive the same high score, making it difficult to choose the single best one for transfer.
In assisted reproductive technology, embryos with poor morphological scores are generally considered to have lower developmental potential. However, emerging research has demonstrated that some low-morphological-score embryos may undergo self-repair mechanisms to restore normal development and even achieve successful pregnancies (13, 14). Embryos with significant fragmentation (>25%) can undergo intrinsic repair processes during blastocyst development. These self-repair mechanisms, including lysosomal degradation of cellular fragments, enable certain fragmented embryos to achieve morphological normalization and to develop into viable blastocysts for transfer (15). It has been demonstrated that, during the development of D3 embryos into blastocysts, with the activation of the embryo genome, embryos with genetic and metabolic defects will be naturally eliminated, and a portion of these embryos appear to be able to repair themselves and eventually develop into blastocysts (16–18). Furthermore, with the development of blastocyst culturing and freezing technology, low-quality embryos will still have the potential to develop into blastocysts, even into high-quality blastocysts when cultured in vitro (18). Studies have shown that the blastocysts derived from low-quality embryos have the potential to deliver healthy babies successfully after freezing and thawing (3). In this study, it was found that the clinical pregnancy rate and the live birth rate were significantly lower in the low-quality group than in the high-quality group. Although the results revealed that embryo quality is an independent predictor of pregnancy outcomes, live birth rates in patients who underwent freeze–thaw transfer of single blastocysts derived from low-quality D3 embryos accounted for 20.7%, which may be related to the self-repair function of the embryo (19).
Age is another independent factor affecting assisted reproductive technology pregnancy outcomes. In this study, it was found that the parental ages in the low-quality group were significantly higher than those in the high-quality D3 group, suggesting that the advanced age of couples can affect the embryo quality in the cleavage stage. Consistent with a previous study, we found that whether the transplanted blastocysts were from the good-quality or the low-quality D3 group, the clinical pregnancy rate and the live birth rate in the <35-year-old group were highest (20). In the same age group, the live birth rate of the good-quality D3 group was higher than that of the low-quality group. In addition, age is an independent factor affecting live birth rates. With advancing age, the aneuploidy rate of embryos increases by 10%, which is also the main cause of embryo implantation failure and abortion in elderly (≥40 years old) patients during IVF cycles (21, 22).
Aneuploidy is the main cause of spontaneous abortion. Munne et al. reported that the aneuploidy rate of embryos was 63% and that the chromosome aneuploidy rate of embryos in the low-quality group was higher than that of the good-quality group in women aged 35–37 years (23). However, Lee et al. reported that the most obvious association between chromosomes and morphology concerned embryo gender rather than aneuploidy (24). In this study, there was no statistically significant association between the morphological score and the euploidy rate (p = 0.561). Nevertheless, the small sample size in the low-quality PGT group limited the robustness of our findings; thus, this analysis should be considered hypothesis-generating rather than conclusive. More studies with larger sample sizes are needed to confirm these findings. Our findings were also consistent with the conclusion of Lee et al., who used a different scoring system.
For those patients who do not have any embryos, low-quality embryos can be cultured into blastocysts, which can give them a chance for transfer and even a successful pregnancy. Moreover, we compared the clinical outcomes in PGT and non-PGT cycles of the blastocysts from the low-quality group. It was found that the clinical pregnancy rate and the live birth rate of the blastocysts from the low-quality group in the PGT cycles were higher than those in the non-PGT cycles. These results indicate that biopsy and PGT significantly enhance blastocyst utilization efficiency in the low-quality group while reducing unnecessary embryo transfers.
This study did not evaluate neonatal outcomes (such as birth defects and preterm birth), which is a significant limitation. Although there is controversy in the existing literature (25) regarding the association between embryo quality and perinatal outcomes, low-quality D3 embryos may be subjected to additional stress when cultured in vitro to the blastocyst stage, theoretically increasing the risk (26, 27). Future research should verify this hypothesis through the design of birth cohort studies (such as follow-up until infancy).
In summary, although embryos with development potential can be screened by further cultivation to blastocysts, the aneuploidy rate of blastocysts from low-quality embryos is still high. Thus, for infertile couples without good-quality D3 embryos, blastocyst culture of low-quality embryos and PGT can be performed to obtain euploid blastocysts, which can improve the clinical pregnancy and live birth rates.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
This research was approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XZ: Data curation, Funding acquisition, Writing – original draft. QZ: Supervision, Writing – review & editing. YG: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a grant from the Joint Project of Medical Disciplines of Henan Province (LHGJ20230381), the Natural Science Foundation of Henan Province (242300420405), and the PhD research startup foundation of the Third Affiliated Hospital of Zhengzhou University (2021083).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1583779/full#supplementary-material
References
1. Cai J, Liu L, Chen J, Liu Z, Jiang X, Chen H, et al. Day-3-embryo fragmentation is associated with singleton birth weight following fresh single blastocyst transfer: A retrospective study. Front Endocrinol. (2022) 13:919283. doi: 10.3389/fendo.2022.919283
2. Shaw-Jackson C, Bertrand E, Becker B, Colin J, Beaudoin-Chabot C, Rozenberg S, et al. Vitrification of blastocysts derived from fair to poor quality cleavage stage embryos can produce high pregnancy rates after warming. J assisted Reprod Genet. (2013) 30:1035–42. doi: 10.1007/s10815-013-0037-7:10.1007/s10815-013-0037-7
3. Kaartinen N, Das P, Kananen K, Huhtala H, and Tinkanen H. Can repeated IVF-ICSI-cycles be avoided by using blastocysts developing from poor-quality cleavage stage embryos? Reprod biomedicine Online. (2015) 30:241–7. doi: 10.1016/j.rbmo.2014.11.016:10.1016/j.rbmo.2014.11.016
4. Lerou PH, Yabuuchi A, Huo H, Miller JD, Boyer LF, Schlaeger TM, et al. Derivation and maintenance of human embryonic stem cells from poor-quality in vitro fertilization embryos. Nat Protoc. (2008) 3:923–33. doi: 10.1038/nprot.2008.60:10.1038/nprot.2008.60
5. Alfarawati S, Fragouli E, Colls P, Stevens J, Gutiérrez-Mateo C, Schoolcraft WB, et al. chromosomal abnormality, and embryo gender. Fertility sterility. (2011) 95:520–4. doi: 10.1016/j.fertnstert.2010.04.003:10.1016/j.fertnstert.2010.04.003
6. Dang TT, Phung TM, Le H, Nguyen TB, Nguyen TS, Nguyen TL, et al. Preimplantation genetic testing of aneuploidy by next generation sequencing: association of maternal age and chromosomal abnormalities of blastocyst. Open Access Macedonian J Med Sci. (2019) 7:4427–31. doi: 10.3889/oamjms.2019.875:10.3889/oamjms.2019.875
7. Liu J, Kong H, Yu X, Zhou M, Liu X, Liu X, et al. The role of endometrial thickness in predicting ectopic pregnancy after in vitro fertilization and the establishment of a prediction model. Front Endocrinol. (2022) 13:895939. doi: 10.3389/fendo.2022.895939
8. Gardner DK, Lane M, Stevens J, Schlenker T, and Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertility sterility. (2000) 73:1155–8. doi: 10.1016/s0015-0282(00)00518-5:10.1016/s0015-0282(00)00518-5
9. Du M, Zhang J, Li Z, Liu X, Li J, Liu W, et al. Comparison of the cumulative live birth rates of progestin-primed ovarian stimulation and flexible gnRH antagonist protocols in patients with low prognosis. Front Endocrinol. (2021) 12:705264. doi: 10.3389/fendo.2021.705264
10. Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertility sterility. (2019) 112:1071–9.e7. doi: 10.1016/j.fertnstert.2019.07.1346:10.1016/j.fertnstert.2019.07.1346
11. Bori L, Meseguer F, Valera MA, Galan A, Remohi J, and Meseguer M. The higher the score, the better the clinical outcome: retrospective evaluation of automatic embryo grading as a support tool for embryo selection in IVF laboratories. Hum Reprod (Oxford England). (2022) 37:1148–60. doi: 10.1093/humrep/deac066:10.1093/humrep/deac066
12. Valera MA, Aparicio-Ruiz B, Pérez-Albalá S, Romany L, Remohí J, and Meseguer M. Clinical validation of an automatic classification algorithm applied on cleavage stage embryos: analysis for blastulation, euploidy, implantation, and live-birth potential. Hum Reprod (Oxford England). (2023) 38:1060–75. doi: 10.1093/humrep/dead058:10.1093/humrep/dead058
13. Shen X, Long H, Gao H, Guo W, Xie Y, Chen D, et al. The valuable reference of live birth rate in the single vitrified-warmed BB/BC/CB blastocyst transfer: the cleavage-stage embryo quality and embryo development speed. Front Physiol. (2020) 11:1102. doi: 10.3389/fphys.2020.01102
14. Kirillova A, Lysenkov S, Farmakovskaya M, Kiseleva Y, Martazanova B, Mishieva N, et al. Should we transfer poor quality embryos? Fertility Res Pract. (2020) 6:2. doi: 10.1186/s40738-020-00072-5
15. Xu J, Zhang M, Niu W, Yao G, Sun B, Bao X, et al. Genome-wide uniparental disomy screen in human discarded morphologically abnormal embryos. Sci Rep. (2015) 5:12302. doi: 10.1038/srep12302
16. Balaban B, Urman B, Alatas C, Mercan R, Aksoy S, and Isiklar A. Blastocyst-stage transfer of poor-quality cleavage-stage embryos results in higher implantation rates. Fertility sterility. (2001) 75:514–8. doi: 10.1016/s0015-0282(00)01756-8:10.1016/s0015-0282(00)01756-8
17. Nagai H, Okada M, Nagai Y, Sakuraba Y, Okae H, Suzuki R, et al. Abnormal cleavage is involved in the self-correction of bovine preimplantation embryos. Biochem Biophys Res Commun. (2021) 562:76–82. doi: 10.1016/j.bbrc.2021.05.028:10.1016/j.bbrc.2021.05.028
18. Majumdar G, Majumdar A, Verma IC, and Upadhyaya KC. Relationship between morphology, euploidy and implantation potential of cleavage and blastocyst stage embryos. J Hum Reprod Sci. (2017) 10:49–57. doi: 10.4103/0974-1208.204013:10.4103/0974-1208.204013
19. Coticchio G, Barrie A, Lagalla C, Borini A, Fishel S, Griffin D, et al. Plasticity of the human preimplantation embryo: developmental dogmas, variations on themes and self-correction. Hum Reprod Update. (2021) 27:848–65. doi: 10.1093/humupd/dmab016:10.1093/humupd/dmab016
20. Sun YF, Zhang J, Xu YM, Luo ZY, Sun Y, Hao GM, et al. Effects of age on pregnancy outcomes in patients with simple tubal factor infertility receiving frozen-thawed embryo transfer. Sci Rep. (2020) 10:18121. doi: 10.1038/s41598-020-75124-3:10.1038/s41598-020-75124-3
21. La Marca A, Capuzzo M, Imbrogno MG, Donno V, Spedicato GA, Sacchi S, et al. The complex relationship between female age and embryo euploidy. Minerva obstetrics gynecology. (2021) 73:103–10. doi: 10.23736/s2724-606x.20.04740-1:10.23736/s2724-606x.20.04740-1
22. Farfalli VI, Magli MC, Ferraretti AP, and Gianaroli L. Role of aneuploidy on embryo implantation. Gynecologic obstetric Invest. (2007) 64:161–5. doi: 10.1159/000101741:10.1159/000101741
23. Munné S, Chen S, Fischer J, Colls P, Zheng X, Stevens J, et al. Preimplantation genetic diagnosis reduces pregnancy loss in women aged 35 years and older with a history of recurrent miscarriages. Fertility sterility. (2005) 84:331–5. doi: 10.1016/j.fertnstert.2005.02.027:10.1016/j.fertnstert.2005.02.027
24. Lee A and Kiessling AA. Early human embryos are naturally aneuploid-can that be corrected? J assisted Reprod Genet. (2017) 34:15–21. doi: 10.1007/s10815-016-0845-7:10.1007/s10815-016-0845-7
25. Gao R, Wang C, Gao Y, Xiu W, Chen J, Kou X, et al. Inhibition of aberrant DNA re-methylation improves post-implantation development of somatic cell nuclear transfer embryos. Cell Stem Cell. (2018) 23:426–35.e5. doi: 10.1016/j.stem.2018.07.017:10.1016/j.stem.2018.07.017
26. Li X, Zeng Y, Zhu L, Yang Z, Luo Y, and Jia JL. The association between pregnancy outcomes and frozen-thawed embryo transfer cycles based on D3 cell count in high-quality blastocysts. Front Endocrinol. (2024) 15:1464313. doi: 10.3389/fendo.2024.1464313
27. Martins WP, Nastri CO, Rienzi L, van der Poel SZ, Gracia CR, and Racowsky C. Obstetrical and perinatal outcomes following blastocyst transfer compared to cleavage transfer: a systematic review and meta-analysis. Hum Reprod (Oxford England). (2016) 31:2561–9. doi: 10.1093/humrep/dew244:10.1093/humrep/dew244
Keywords: low-quality embryos, morphological score, frozen-thawed transfer, single blastocyst, clinical outcomes
Citation: Zhao X, Zhou Q and Guan Y (2025) Clinical outcomes of frozen–thawed single blastocyst transfer derived from low-quality day 3 embryos: A retrospective cohort study. Front. Endocrinol. 16:1583779. doi: 10.3389/fendo.2025.1583779
Received: 26 February 2025; Accepted: 03 July 2025;
Published: 28 July 2025.
Edited by:
Bo Huang, Huazhong University of Science and Technology, ChinaReviewed by:
Xiushan Feng, Fujian Medical University, ChinaPanagiotis Cherouveim, Massachusetts General Hospital and Harvard Medical School, United States
Hong-Hui Wang, Weihai Second Municipal Hospital of Qingdao University, China
Copyright © 2025 Zhao, Zhou and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinyan Zhao, MTM4OTUwMDk3NDBAMTYzLmNvbQ==
 Xinyan Zhao
Xinyan Zhao Qiongge Zhou2
Qiongge Zhou2