- 1Faculty of Pharmacy, Middle East University, Amman, Jordan
- 2Division of Genomics and Translational Biomedicine, College of Health and Life Sciences, Hamad Bin Khalifa University, Doha, Qatar
- 3Department of Biochemistry and Molecular Genetics, American University of Beirut, Beirut, Lebanon
Introduction: Vitamin D is a pleiotropic hormone essential for bone health and overall physiological function. Despite its significance, vitamin D deficiency remains widespread and is often influenced by genetic factors.
Methods: This study investigates the role of SDR42E1, a gene encoding a short-chain dehydrogenase/reductase enzyme, in vitamin D regulation and sterol metabolism. Using CRISPR/Cas9 gene-editing, we generated an SDR42E1 knock-in model in HCT116 colorectal cells, which exhibit high endogenous SDR42E1 expression, harboring a nonsense variant associated with vitamin D deficiency.
Results: Integrated transcriptomic and proteomic analyses revealed significant dysregulation of sterol absorption and metabolism (fold change (FC) = 1.8, P = 0.007) and cancer-related signaling pathways (FC = −1.7, P = 0.02). Notably, key differentially expressed genes included upregulated LRP1B and ABCC2, alongside downregulated WNT16 and SLC7A5. Proteomic profiling confirmed alterations in cell proliferation-related proteins, including reduced ALDOA expression (FC = −0.37, P = 0.0005). Functionally, SDR42E1 deficiency reduced cell viability by 53% (P = 0.0001), an effect reversed by transient SDR42E1 overexpression with restoring ABCC2 expression.
Conclusion: These findings establish SDR42E1 as a key modulator of vitamin D-related pathways and highlight its potential as a therapeutic target for addressing vitamin D deficiency and associated pathologies, including cancer.
1 Introduction
Vitamin D is a vital fat-soluble nutrient crucial for calcium and phosphorus homeostasis, bone health, and immune function. Despite the availability of dietary sources and sunlight exposure (1), deficiencies can arise due to impaired absorption and metabolism. These processes are orchestrated by a network of critical proteins, like ATP-binding cassette B member 2 (ABCB2) and solute carrier family proteins, including SLC7A5 (2, 3). In circulation, vitamin D binds to vitamin D-binding protein (VDBP) and undergoes hydroxylation in the liver by cytochrome P450 enzymes such as CYP2R1, CYP11A1, and CYP27A1, which hydroxylate vitamin D, followed by activation in the kidney by CYP27B1. The vitamin D receptor (VDR) mediates its biological effects, while CYP24A1 regulates its inactivation (4). Both VDR and CYP24A1 influence these processes, although key mechanistic gaps remain incompletely understood (5).
Short-chain Dehydrogenase/Reductase 42E member 1 (SDR42E1), a member of the short-chain dehydrogenase/reductase enzyme family plays a potentially significant role in lipid and steroid metabolism (6–8), potentially functioning as an oxidoreductase and steroid delta-isomerase (9, 10). The largest genome-wide association study (GWAS) on vitamin D deficiency (11), identified a nonsense variant (rs11542462) on chromosome 16q23 that replaces glutamine with a termination codon at position 30 (p.Q30*), producing a truncated, non-functional SDR42E1 enzyme (12–14). Interestingly, this variant correlates with elevated serum levels of 8-dehydrocholesterol (8-DHC) and 7-dehydrocholesterol (7-DHC), precursors in vitamin D synthesis (15). Lately, our in silico studies identified these sterols, along with vitamin D3 and 25-hydroxyvitamin D (25(OH)D), as potential substrates of the SDR42E1 (9); however, its precise role in vitamin D metabolism remains unclear.
Our recent research, utilizing clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) gene-editing technology in skin keratinocytes, demonstrated that SDR42E1 depletion disrupts the steroid biosynthesis, leading to accumulation of 7-DHC and a concomitant reduction in vitamin D levels. Additionally, SDR42E1 mRNA showed elevated expression in intestinal epithelial cells and the analogous colorectal HCT116 cell line, underscoring its putative role in vitamin D homeostasis within the gastrointestinal tract (16). Building on these observations, this study seeks to elucidate the functional role of SDR42E1 in regulating intestinal vitamin D absorption and sterol metabolism. To achieve this, we employed CRISPR/Cas9-mediated gene editing in HCT116 cells to introduce a nonsense variant of SDR42E1 previously associated with vitamin D deficiency. Comprehensive transcriptomic and proteomic analyses were then performed to characterize downstream molecular alterations of SDR42E1 disruption. Through this integrative approach, we aim to advance mechanistic understanding of SDR42E1’s contribution to vitamin D homeostasis and to explore its broader implications in metabolic regulation and disease pathogenesis.
2 Materials and methods
2.1 Cell culture
The human colorectal carcinoma cell line, HCT116 (CCL-247, Research Resource Identifier (RRID): CVCL_029), was sourced from the American Type Culture Collection (Manassas, VA, United States). Cells were cultured in Dulbecco’s modified Eagle’s medium-high D-glucose (4.5 g/L), glutamine (2 mM), and sodium pyruvate (1 mM), and further supplemented with 10% fetal bovine serum and (1X) antibiotic-antimycotic (Thermo Fisher Gibco, United States). To maintain sterility and optimal growth conditions, the culture medium was refreshed every two days. Cells were allowed to reach 70-80% confluency before being detached using 0.25% trypsin-ethylenediaminetetraacetic acid (Sigma-Aldrich), washed with Dulbecco’s phosphate-buffered saline (DPBS, Gibco), and then centrifuged at 900 revolutions per minute (rpm) for 3 minutes. The cells were then subcultured at a 1:6 ratio. The cultures were maintained in a sterile environment at 37°C, in a 5% CO2 and 95% humidity atmosphere, ensuring monolayer growth. A summary of key resources for materials and reagents used is presented in Supplementary Table S1.
2.2 Plasmid construction and cloning
To assess the potential role of the SDR42E1 protein, wild-type SDR42E1 (pcDNA3.1+/N-HA) plasmid was procured from GenScript (USA). Wild-type SDR42E1 was transiently overexpressed using a pair of primers (forward: GGGCTACACATTCCCGTCTA, reverse: AACCATTCCACTGCTTCCTG, amplicon size: 231 base pair, primer length: 20 nucleotides). Clones were amplified in DH5α competent Escherichia coli (E. coli) and validated by Sanger sequencing and Western blotting analyses. HCT116 cells, at 40-60% confluency, were transiently transfected with the wild-type plasmids using Lipofectamine 3000 (Thermo Fisher, USA), for 24 hours per manufacturer’s instructions.
2.3 Creation of SDR42E1 knock-in cells using CRISPR/Cas9
A loss-of-function p.Q30* nonsense mutation in the SDR42E1 gene, recapitulating the variant observed in patients, was introduced into HCT116 cells using CRISPR/Cas9 technology (GenScript Biotech, https://www.genscript.com/, USA, Order ID: U864NGG050). This was achieved by inserting a G>A substitution into exon 3 using a guide RNA (gRNA) and Cas9 protein delivered via the eSpCas9-2A-GFP (PX458) plasmid (Supplementary Figure S1). For this process, HCT116 cells were co-transfected with a donor DNA template containing the target gene and a puromycin resistance cassette to promote DNA repair via homologous recombination. Following 2 weeks of puromycin selection, positive clones were selected from the cell pools through polymerase chain reaction (PCR) for the Puro-GFP insert and validated by Sanger sequencing.
The overall CRISPR editing process, including gRNA design, donor synthesis, and screening of up to 100 single-cell clones, was carried out by GenScript. Editing efficiency in the initial cell pool was confirmed by Sanger sequencing, with successful targeting further validated by Western blot and immunofluorescence to confirm loss of SDR42E1 protein expression (Figure 1). GenScript’s quality control criteria included MycoAlert™ mycoplasma testing, cell viability assessment, and full allelic validation by sequencing.
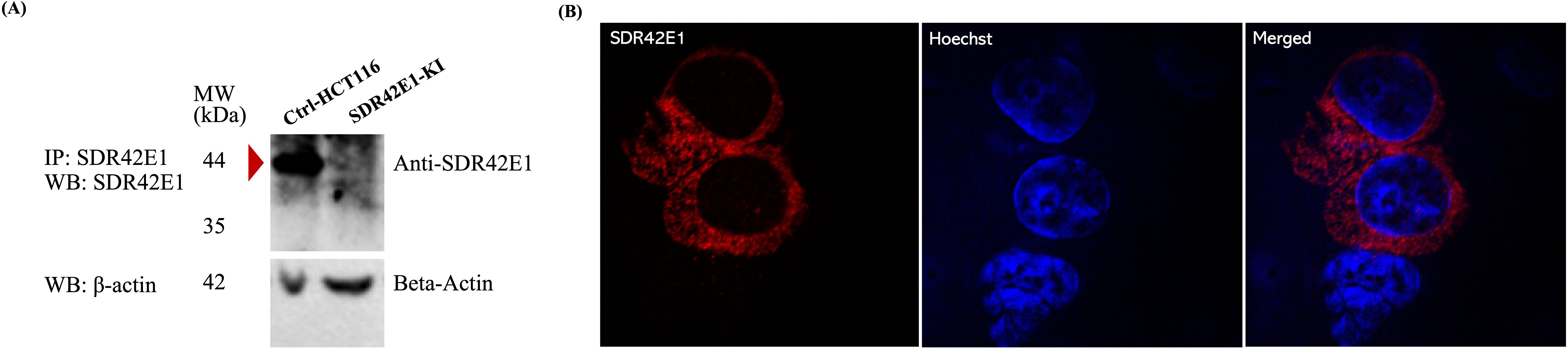
Figure 1. Localization and expression of SDR42E1 protein in HCT116 cells. (A) HCT116 cells, expressing wild-type SDR42E1 (green), were transiently transfected with 10 µg of SDR42E1-HA plasmid and stained overnight with a rabbit SDR42E1-tag polyclonal antibody (1:100 dilution). Nuclear staining was done by Hoechst staining (blue). (B) Western blot (WB) of immunoprecipitated (IP) SDR42E1 from wild-type control HCT116 cells (Ctrl-HCT116) detected a 44 kDa SDR42E1 protein, indicated with a red arrow, which was absent in the SDR42E1-knockin model (SDR42E1-Ki) using a rabbit polyclonal antibody against SDR42E1 (PA5-53156, Invitrogen). Before conducting the IP, a fraction (one-tenth) of the total lysates were analyzed via WB as input controls with a monoclonal anti-beta-actin mouse antibody (A5441, Sigma, 1:5000 dilution). All experimental conditions were analyzed with three independent biological replicates (n = 3 per group).
2.4 RNA extraction and RNA sequencing
Total RNA was extracted from the SDR42E1-gene edited cells through 1 mL of TRIzol reagent (Invitrogen), following the protocol provided by the manufacturer. The integrity and quantity of the extracted RNA were assessed using agarose gel electrophoresis and a NanoDrop 8000 spectrophotometer (Thermo Scientific). Following this, 2 μg of the total RNA was converted into complementary DNA (cDNA) utilizing the High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, United States), adhering to the manufacturer’s instructions.
To explore differentially expressed genes within relevant pathways, equal quantities of high-quality RNA pools (100–200 ng) were precipitated using 75% ethanol and sent to Macrogen Inc. (http://macrogen.com, Korea) for constructing RNA-sequencing libraries and performing next-generation sequencing. The TruSeq Stranded Total RNA Library Prep Kit (Illumina) was used to prepare the RNA sequencing libraries, according to the manufacturer’s guidelines. After PCR enrichment, the integrity and quantity of the libraries were assessed before sequencing on the Illumina NovaSeq 6000 platform, which produced 101 bp paired end reads. The raw sequence data underwent trimming to remove PCR duplicates, contaminants, and adapter sequences. Low-quality reads (below Q20) were filtered out utilizing CLC Genomics Workbench version 22.0.2, which was also used for assembling and aligning the reads to the latest human genome release (Homo sapiens, GRCh38). Genes showing average raw counts below 10 were discarded.
Differential gene expression analysis between knock-in and control samples identified significant changes characterized by at least a 2-fold increase or decrease in expression and an adjusted P-value of less than 0.05. This analysis was conducted using the R package DESeq2 version 1.34 with default settings (17). Heatmaps of gene expression data were generated using log2 fold-change maximum likelihood estimate (lfcMLE) values and −log10 of false discovery rate (FDR) values, and visualized using the R package pheatmap (version 1.0.2). The Pathview R package facilitated the visualization of KEGG pathways involving the differentially expressed genes (DEG) (18). GSE was carried out on human gene sets from the Enrichment map, ordered by the unshrunk log2 fold change values provided by DESeq2, as previously described (19). ClusterProfiler version 4.2.2, a tool known for its robust visualization capabilities, was used for functional annotation analysis utilizing standard parameters, including Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway, and enrichment analyses (20).
Validation of the RNA-sequencing results was performed through a comparative analysis between the RNA-sequencing data from the knock-in and overexpression of SDR42E1 in HCT116 cells and genes linked to vitamin D traits in the NHGRI-EBI GWAS Catalog (EFO_0004631), with the catalog version released on July 08, 2024, and accessed on July 25, 2024 (21). This analysis focused on genetic markers within a 250-kilobase region of the GWAS signals and a statistical significance threshold of a P-value < 5.0 e−8. The aim was to thoroughly identify significant genes linked to vitamin D in our knock-in model, thereby enhancing the robustness of our research findings.
2.5 Cell lysis and Western blot of immunoprecipitation
To verify the loss of SDR42E1 protein expression in gene-edited HCT116 cells, cells were lysed in ice-cold radioimmunoprecipitation assay (RIPA) buffer containing a protease inhibitor cocktail (UD282713, Thermo Fisher Scientific), dithiothreitol (1M), and phenylmethylsulfonyl fluoride (1 mM). The lysates were incubated for 30 minutes at 4°C with rotation, then centrifuged at 10,000 Xg for 15 minutes at 4°C. The clear supernatant was stored at −80°C. Protein concentrations were measured using a bicinchoninic acid assay kit (Pierce, Thermo Fisher Scientific).
To prepare for immunoprecipitation, cell extracts were mixed with an anti-SDR42E1 rabbit monoclonal antibody (WG3329739B, Invitrogen, 1:500 dilution) and rotated overnight at 4°C. Protein samples were then incubated with Protein A/G magnetic beads (Pierce, 88802, Thermo Scientific) for 2 hours at 4°C, followed by magnetic separation to remove unbound material. The beads were washed thrice with cold RIPA buffer and the proteins were eluted by heating in 5X SDS-sample buffer at 37°C for 30 minutes.
For Western blot analysis, an equal quantity of proteins was separated on a Novex NuPAGE 4-12% Bis-Tris SDS-polyacrylamide gel (Invitrogen) and wet-transferred onto a nitrocellulose membrane (Millipore). The membrane was blocked with 5% bovine serum albumin (BSA, Tocris, United Kingdom) for one hour at room temperature, then probed with the same anti-SDR42E1 antibody for three days at 4°C. Beta-actin was used as a loading control, detected with a monoclonal anti-beta-actin mouse antibody (1:5000 dilution, A5441, Sigma-Aldrich). After washing with Tris Buffered Saline with Tween 20 (TBST), the bands were visualized using a chemiluminescent substrate (Pierce ECL, Thermo Scientific) after application of peroxidase IgG fraction monoclonal anti-mouse IgG (H+L) secondary antibody HRP conjugate (1:5000 dilution, 62-6520, Invitrogen) or anti-rabbit IgG light chain specific (1:5000 dilution, 211-032-171, Jackson ImmunoResearch, United Kingdom).
2.6 Proteomics
For preparation, protein samples were incubated at 37°C for 30 minutes after the whole-cell extraction described earlier. Each sample, comprising 50 μg of protein from three biological replicates of the SDR42E1 knock-in and HCT116 control samples, was then loaded onto a Novex NuPAGE 4-12% Bis-Tris SDS-polyacrylamide gel and electrophoresed at 100 volts for about 60 minutes. Overnight staining of the proteins was performed using PageBlue Protein Staining Solution at 4°C, and the gel was subsequently washed for 10 minutes with sterilized distilled water. Each gel lane was excised, placed in individual tubes, and stored at 4°C for subsequent mass spectrometry analysis as detailed in a previous study (22).
Protein samples were treated with dithioerythritol and s-carbamidomethylation with iodoacetamide as reducing agents prior to in-gel tryptic digestion. The gel pieces were washed and rehydrated with sequencing-grade modified porcine trypsin and incubated overnight at 37°C. The peptides were then extracted, desalted, and loaded onto a nanoflow ultra-performance liquid chromatography (UPLC) system, where they were separated using a gradient elution. Analysis of the resulting peptides was performed through an Orbitrap Fusion Tribrid mass spectrometer (Thermo Scientific). The relative abundance of proteins was determined by measuring the areas of precursor ions from unique, non-conflicting peptides. Data was then analyzed by R packages Limma (Linear Models for Microarray Data) version 3.56.2, Mascot Daemon (version 2.6.0, Matrix Science), and Progenesis QI (Version 2.2., Waters). Benjamini–Hochberg approach was utilized to convert Student’s t-tests-derived P-values to multiple test-corrected q-values, with a cut-off of <0.05. Functional annotations and enrichment Analysis were conducted on the ranked proteins based on P-values against Homo sapiens protein sets through ClusterProfiler.
2.7 Cell viability assay
To assess the cell viability of SDR42E1 knock-in compared to wild-type HCT116, cells (2e4 cells/mL) were seeded in 96-well plates with a complete medium. Two-fold serial dilutions were performed, yielding a final concentration of around 6000 cells/mL, with control wells containing only a complete medium. Viability was assessed at 24–48 and 72 hours of incubation using the CellTiter-Glo® Luminescent Cell Viability (Promega Corporation, G755A, USA) per manufacturer’s protocol. The assay, measuring ATP levels, provides a luminescent signal proportional to the percentage of living cells.
2.8 Immunofluorescence
Wild-type HCT116 cells were seeded on poly-L-lysine-coated coverslips within a 12-well plate. Once the cells reached 40-60% confluency, they were transiently transfected with 5 micrograms of SDR42E1-HA tag plasmids (Addgene) through Lipofectamine 3000 (2293283, Thermo Fisher Scientific), following the provided guidelines for 24 hours. Post-transfection, the cells were fixed with 4% paraformaldehyde and then blocked with 5% BSA in DPBS with 0.1% Tween-20 for 60 minutes at room temperature. After washing with DPBS, the cells were incubated with primary antibodies overnight at 4°C, followed by repeated washes in DPBS and 60-minute incubations with both secondary and tertiary antibodies sequentially at room temperature.
Primary antibodies, including recombinant rabbit HSP60 (Heat shock protein 60)-mitochondrial marker monoclonal antibody (HSPD1-2206R, Thermo Scientific), rabbit calreticulin-endoplasmic reticulum marker IgG polyclonal antibody (PA3900, Thermo Scientific), and mouse monoclonal anti-Golgi 58K antibody (NB600-41, Invitrogen), were used at a 1:100 dilution. Secondary antibodies, including biotinylated goat anti-rabbit IgG (H+L) (65-6140, Thermo Scientific) and biotinylated goat anti-mouse IgG (BA-9200, Vector), and tertiary antibody, Streptavidin Alexa Fluor 488 conjugate (S11223, Thermo Scientific), were applied at a 1:250 dilution. The nuclei were stained using Hoechst (33258, Invitrogen) at 1 µg/ml, and slides were mounted using ProlongTM Antifade Histomount (Thermo Fisher Scientific). Confocal microscopy images were conducted at 100X magnification using consistent imaging parameters.
2.9 Confocal microscopy
Microscope images were captured utilizing the Nikon A1R confocal fluorescence microscope with motor arm set at 60 μm, 0.3 mm, and 6.0 mm to ensure consistent field visualization. Image reconstruction and analysis were conducted through Fiji-Image J software (23). All images received uniform treatment to eliminate any potential bias.
2.10 Statistical analyses
Statistical analyses were conducted through GraphPad Prism 10. Two-group comparisons were evaluated using two-tailed unpaired or paired Student’s t-test, while multiple group comparisons utilized Kruskal-Wallis one-way ANOVA with Dunn’s post hoc test. Statistical significance was identified at a P-value threshold of 0.05. Findings are expressed as mean ± standard deviation or ± standard error of the mean (SEM), with a minimum of three independent biological replicates per experiment.
3 Results
3.1 Localization of SDR42E1 to the cytoplasmic membrane in HCT116 cells
To analyze the expression and cellular distribution of the wild-type SDR42E1, we transiently introduced a custom-constructed plasmid containing the full SDR42E1 coding sequence into HCT116 cells. Western blot confirmed the SDR42E1 expression at the expected 44 kDa molecular weight (Figure 1A), and cellular localization assessments revealed a major presence in the cytoplasm and on the cell membrane (Figure 1B), with no detection in the endoplasmic reticulum, Golgi apparatus, and mitochondria (data not shown).
3.2 Transcriptomic profiling reveals vitamin D regulation mechanisms in SDR42E1 knock-in model
To investigate the functional role of SDR42E1 in HCT116 cells, a homozygous SDR42E1 knock-in model was generated using the CRISPR/Cas9 gene-editing technology, incorporating the rs11542462 non-sense mutation. Prior to the functional investigations, the complete absence of SDR42E1 expression was confirmed through Western blotting on immunoprecipitated SDR42E1 in whole cell lysates (Figure 1A).
RNA-sequencing analysis using DESeq2/R revealed 4,663 DEG among 19,285 total reads, with an adjusted P-value FDR below 0.05 compared to wild-type controls (Figure 2). Principal component analysis (PCA) and multidimensional scaling (MDS) successfully distinguished the SDR42E1 knock-in samples from the wild-type cells (Figure 2A, Supplementary Figure S2a), a distinction further supported by a heatmap showcasing the top 100 DEG (Figure 2B).
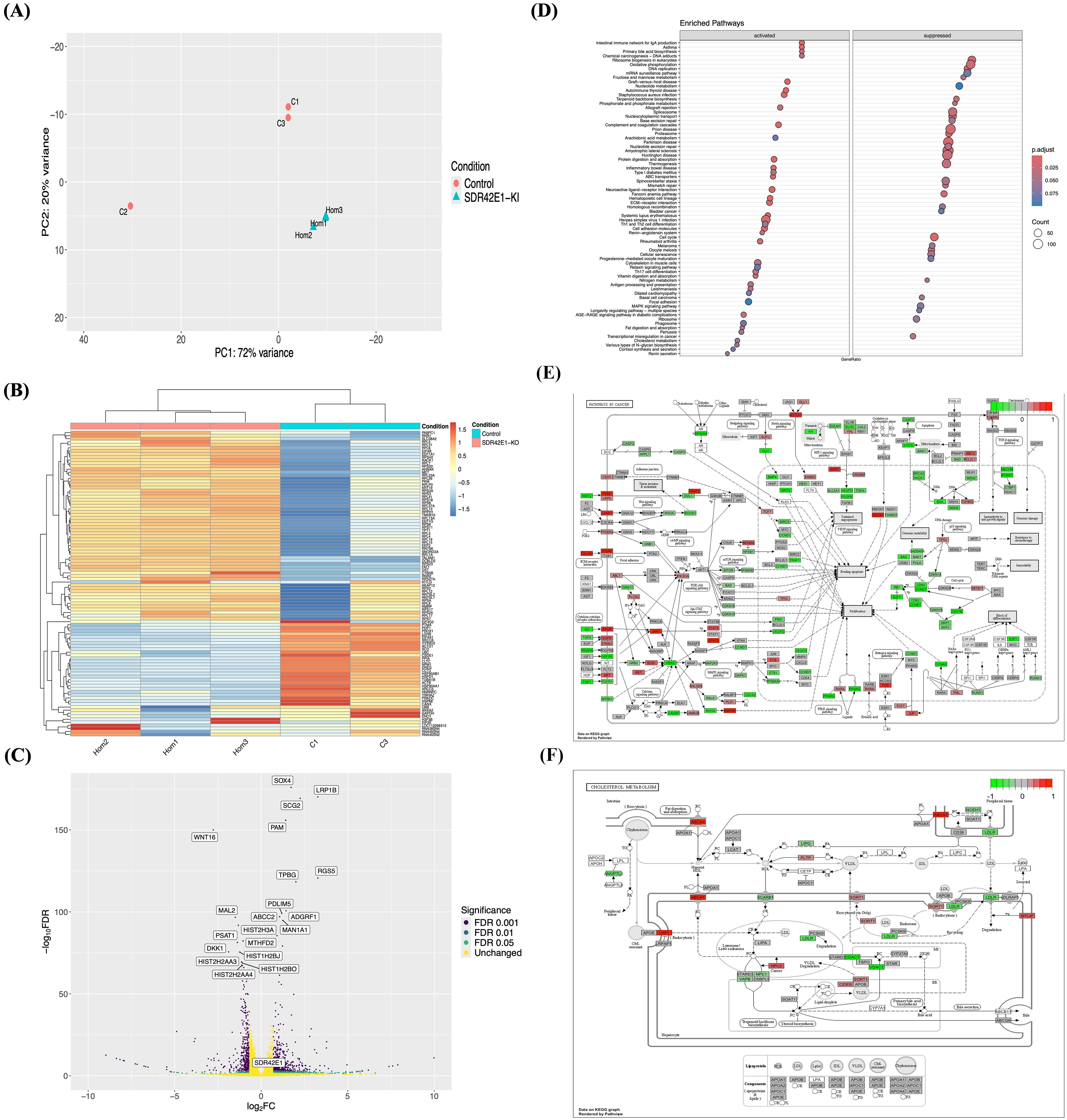
Figure 2. Transcriptomic reveals vitamin D regulation mechanisms in SDR42E1 knock-in model. (A) PCA plot shows clustering of wild-type HCT116 controls (C; in rose) and SDR42E1 knock-in (Hom, in blue) biological replicates, along the major principal components of the regularized log-transformed counts. (B) Heatmap of top 100 DEGs displays the Z-scores of regularized log-transformed counts with lower (blue) and higher (red) expressions. The X- and Y-axes are labeled with sample names and DEGs, respectively. (C) Volcano plot of significant gene expression changes in SDR42E1 knock-in compared to wild-type controls. The X-axis represents the log2 fold change (FC), with upregulated genes to the right and downregulated genes to the left with Log2FC > 2 or < −2, while the Y-axis shows the false discovery rate (FDR) < 0.05, with the top 20 most significantly altered genes labeled. (D) Dot plot shows KEGG pathway enrichment of DEGs, with KEGG pathways on the Y-axis and gene ratio on the X-axis. KEGG pathways diagram shows genes expression profiles (E) in pathways of cancer, and (F) in cholesterol metabolism with upregulated (red) and downregulated (green) genes. All analyses were performed and visualized using R/DESeq2, Pheatmap, or Pathview. All experimental conditions were analyzed with three independent biological replicates (n = 3 per group).
A volcano plot demonstrated approximately 56% of DEGs were upregulated with a log2 fold change (FC) ≥ 0.3 (Figure 2C), including SRY-box transcription factor 4 (SOX4, FC = 1.7, FDR < 1.0 e−170), low-density lipoprotein receptor-related protein 1B (LRP1B, FC = 3.3, FDR < 1.0e−160), regulator of G protein signaling 5 (RGS5, FC = 3.3, FDR < 1.0e−100), and ATP-binding cassette C2 (ABCC2, FC = 1.10, FDR < 1.0e−90). Further, 525 DEG were efficiently downregulated with a log2 FC of ≤ −0.3, including wingless-type MMTV integration site 16 (WNT16, FC = −2.8, FDR < 1.0e−140), histone cluster 2H3A (HIST2H3A, FC = −1.31, FDR < 1.0e−80), SLC7A5 (FC = −1.07, FDR < 1.0e−50), and aldolase A (ALDOA, FC = −0.37, FDR < 1.0e−04) (Supplementary Table S2).
Functional enrichment analysis of DEG revealed the biological processes influenced by SDR42E1 knock-in in HCT116 cells. KEGG pathway analysis through the ClusterProfiler/R identified several activated pathways, including immunity disorders (FC = 2.6, adjusted P-value < 1.0e−04), vitamin digestion and absorption (FC = 2.0, adjusted P-value < 1.0e−03), ABC transporters (FC = 1.8, adjusted P-value < 1.00e−02), inflammatory bowel disease (FC = 1.8, adjusted P-value < 1.0e−02), and primary bile acid biosynthesis (FC = 1.6, adjusted P-value < 1.0e−02), while pathways related to DNA repair and replication (FC = −2.4, adjusted P-value < 1.0e−04), cell cycle regulation (FC = −2.5, adjusted P-value < 1.0e−04), transcriptional misregulation in cancer (FC = −1.7, adjusted P-value < 1.0e−02), and cellular senescence (FC = −1.5, adjusted P-value < 1.0e−02) were deactivated (Figure 2D).
Specific gene alterations in cancer and cellular senescence were noted, including downregulation of wingless-type MMTV integration site 2 (WNT2, FC = −2.8, FDR < 1.0e−140) and heat shock protein 90kDa alpha (cytosolic) A1 (HSP90AA1, FC = −0.67, FDR < 2.0e−20), and upregulation in signal transducer and activator of transcription 2 (STAT2, FC = 0.6, FDR < 5.0e−08) and human leukocyte antigens A (HLA-A, FC = 0.38, FDR < 3.0e−05) (Figure 2F, Supplementary Figure S2b). Additionally, low-density lipoprotein receptor (LDLR, FC = −0.56, FDR < 2.0e−11), cytochrome P450 enzymes such as CYP51A1 (FC = −0.44, FDR = 3.0e−09), and 24-dehydrocholesterol reductase (DHCR24, FC = −0.6, FDR < 1.0e−14) were downregulated, while lipoprotein receptor related protein (LRP1, FC = 3.3, FDR < 5.0e−160) and ABCA1 (FC = 1.5, FDR < 1.0e−06) were upregulated in cholesterol metabolism and steroid biosynthesis (Figure 2E, Supplementary Figure S2c).
Moreover, gene set enrichment function analysis (GSE) confirmed pathways linked to protein-lipid complex remodeling (FC = 1.9, adjusted P-value < 2.0e−03), sterol transfer and transporter activities (FC = 1.8, adjusted P-value < 5.0e−03), intestinal lipid and sterol absorption (FC = 1.9, adjusted P-value < 5.0e−03), lipid digestion (FC = 1.7, adjusted P-value < 1.0e−02), ATPase-coupled lipid transmembrane transporter activity (FC = 1.6, adjusted P-value < 1.0e−02), and cholesterol metabolic process (FC = 1.5, adjusted P-value < 4.0e−02). Downregulated pathways included sterol biosynthesis (FC = −1.9, adjusted P-value < 1.1e−02), osteoblast development (FC = −1.6, adjusted P-value < 1.0e−02), and NADH dehydrogenase activity (FC = −1.7, adjusted P-value < 4.0e−02) (Supplementary Figure S2d).
To validate our findings, we conducted a comparative analysis of DEG from the SDR42E1 knock-in model with vitamin D-related GWAS Catalog data (21). This analysis revealed that 50 out of 304 genes were replicated in both datasets. Among these, key genes such as transcription factor Dp-2 (TFDP2), carbamoyl-phosphate synthase 1 (CPS1), farnesyl diphosphate synthase (FDPS), CYP24A1, and LDLR showed significant correlations, with P-values ranging from 2e−342 to 5e−08 (Table 1).
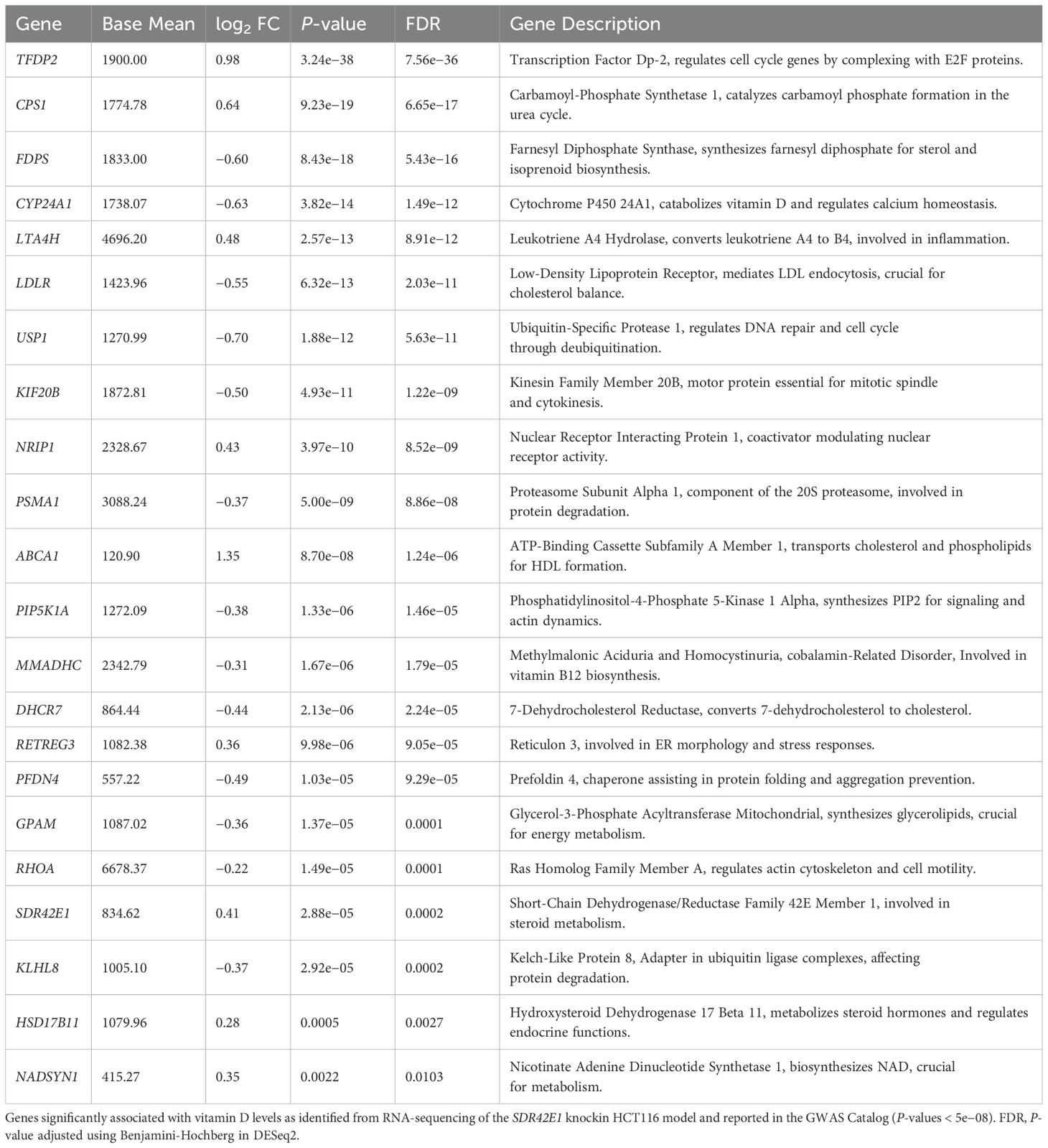
Table 1. Replication of differentially expressed genes reported in GWAS catalog for vitamin D in the SDR42E1 knock-in model.
3.3 Proteomic insights validate extensive pathway modifications upon SDR42E1 depletion
To validate the transcriptomic profile and identify differentially expressed proteins in HCT116 cells harboring the homozygous SDR42E1 knock-in non-functional variant, three replicates of SDR42E1 knock-in and wild-type cells underwent label-free LC-MS/MS analysis. Differential protein expression was assessed using a Student’s t-test in the Limma R package, with a significance threshold of P-value < 0.05. Proteomic analysis revealed substantial differences in protein profiles between SDR42E1 knock-in and wild-type HCT116 cells, as demonstrated by the PCA analysis (Figure 3A). This finding was further supported by heatmap and volcano plots (Figures 3B, C), highlighting 140 differentially expressed proteins out of 1,320 analyzed in the knock-in model (Supplementary Table S3).
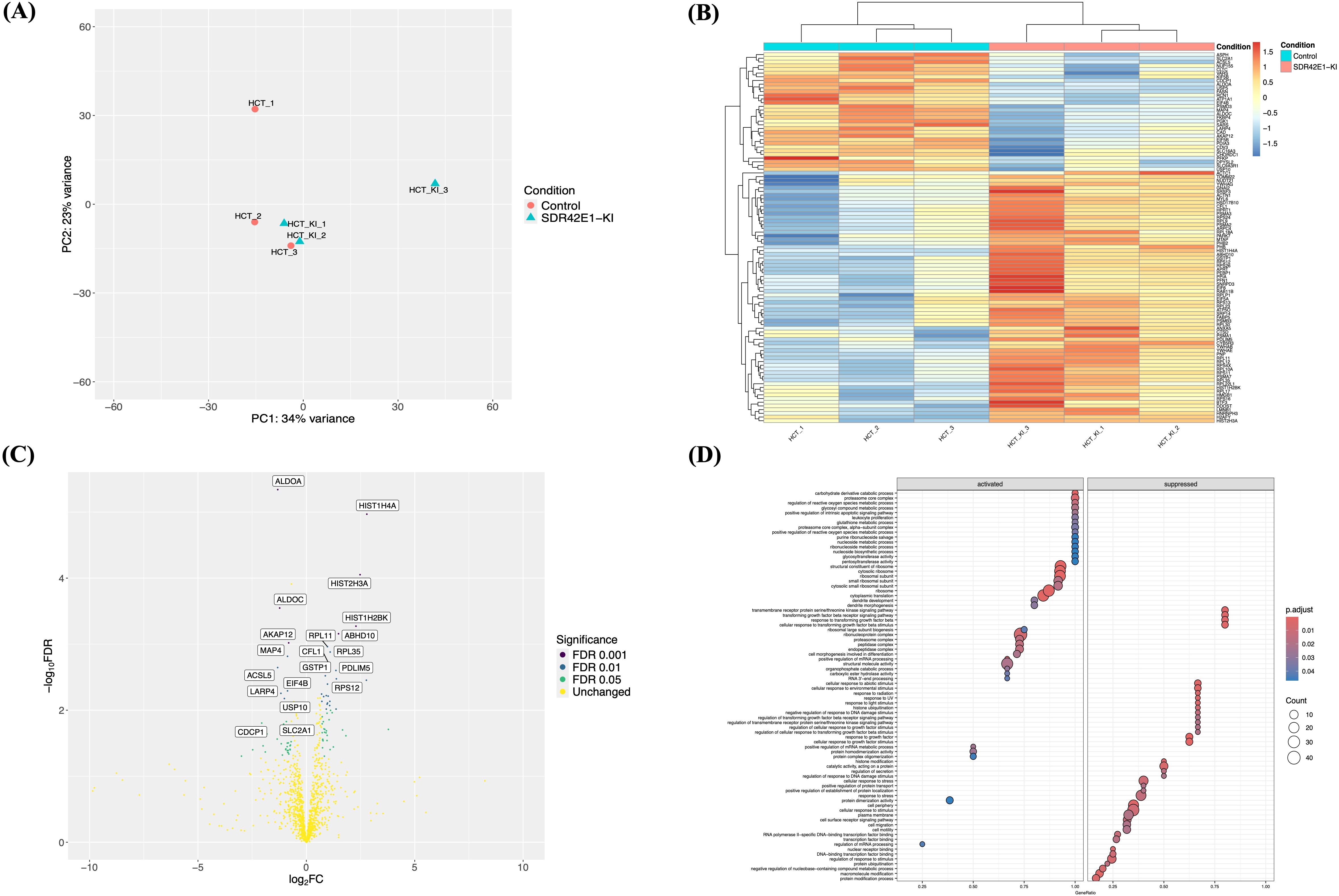
Figure 3. Proteomic insights validate extensive pathway modifications upon SDR42E1 depletion. (A) PCA plot of wild-type HCT116 controls (HCT; in rose) and SDR42E1 knock-in (HCT_KI, in blue) based on the major components of regularized log-transformed counts. (B) Heatmap of top 100 differentially expressed proteins displays the Z-scores of log-transformed counts, with lower (blue) and higher (red) expression. Axes are labeled with sample names and proteins. (C) Volcano plot of proteomic data comparing SDR42E1 knock-in to wild-type HCT116 cells. The X-axis shows log2 fold change (FC), with significant upregulation to the right (greater than 2) and downregulation to the left (less than −2), while the Y-axis shows the −log of the false discovery rate (FDR), marking significance below 0.05. The top 20 significantly altered proteins are labeled. (D) GSE dot plot of pathway enrichment analysis of the differentially expressed proteins in SDR42E1 knock-in. These analyses were conducted and visualized using R/Limma, Pheatmap, and ClusterProfiler. All experimental conditions were analyzed with three independent biological replicates (n = 3 per group).
Several proteins showed consistent regulation with RNA-sequencing findings. Specifically, downregulated proteins included aldolase fructose-bisphosphate A (ALDOA, P-value = 4.56e−06, Log2FC = −1.32), fatty acid synthase (FASN, P-value = 4.56e−04, Log2FC = −1.32), aldolase fructose-bisphosphate C (ALDOC, P-value = 2.80e−04, Log2FC = −1.23), A kinase (PRKA) anchor protein 12 (AKAP12, P-value = 9.50e−04, Log2FC = −0.90), and acyl-CoA synthetase long chain family 5 (ACSL5, P-value = 2.20e−03, Log2FC = −1.32). Upregulated proteins, included histone 1 H4A (HIST1H4A, P-value = 1.07e−05, Log2FC = 2.80), HIST2H3A (P-value = 8.90e−05, Log2FC = 2.50), abhydrolase domain containing 10 (ABHD10, P-value = 6.90e−04, Log2FC = 2.80), ribosomal protein L11 (RPL11, P-value = 1.00e−03, Log2FC = 2.80), and H2A Histone Family, Member Y (H2AFY, P-value = 3.50e−03, Log2FC = 2.76) (Figure 3C).
Pathway enrichment analysis highlighted several key processes, including the upregulation of ribosomal structure (FC = 2.14, adjusted P-value = 2.70e−04), carbohydrate derivative catabolic process (FC = 1.8, adjusted P-value = 6.00e−04), cytoplasmic translation (FC = 1.90, adjusted P-value = 1.30e−03), metabolic process (FC = 1.71, adjusted P-value = 8.40e−03), and reactive oxygen species process (FC = 1.69, adjusted P-value = 8.90e−03). Downregulated pathways involved response to growth factor (FC = −2.06, adjusted P-value = 7.50e−04), cell periphery (FC = 2.14, adjusted P-value = 2.60e−03), transmembrane receptor protein serine/threonine kinase signaling pathway (FC = −1.90, adjusted P-value = 2.80e−03), and DNA repair (FC = −2.28, adjusted P-value = 9.10e−03) (Figure 3D).
3.4 Transcriptomic profiling unveils divergent pathway modifications upon SDR42E1 overexpression
To understand the role of SDR42E1 in cellular metabolism, we established a comparative baseline model through transiently overexpressing the SDR42E1 gene in HCT116 cells and examined its impact on biological profiles and sterol pathways. The overexpression was achieved using a wild-type plasmid tagged with HA (Figure 1A) and was further confirmed via RNA-sequencing analysis. This analysis revealed significant transcriptional deregulations, with 1,483 DEG out of 19,285 total reads at an FDR < 0.05 compared to the control group, as demonstrated by PCA and MDS analysis (Figure 4A, Supplementary Figure S3a). This distinction was further corroborated by a heatmap showing the top 100 DEG (Figure 4B).
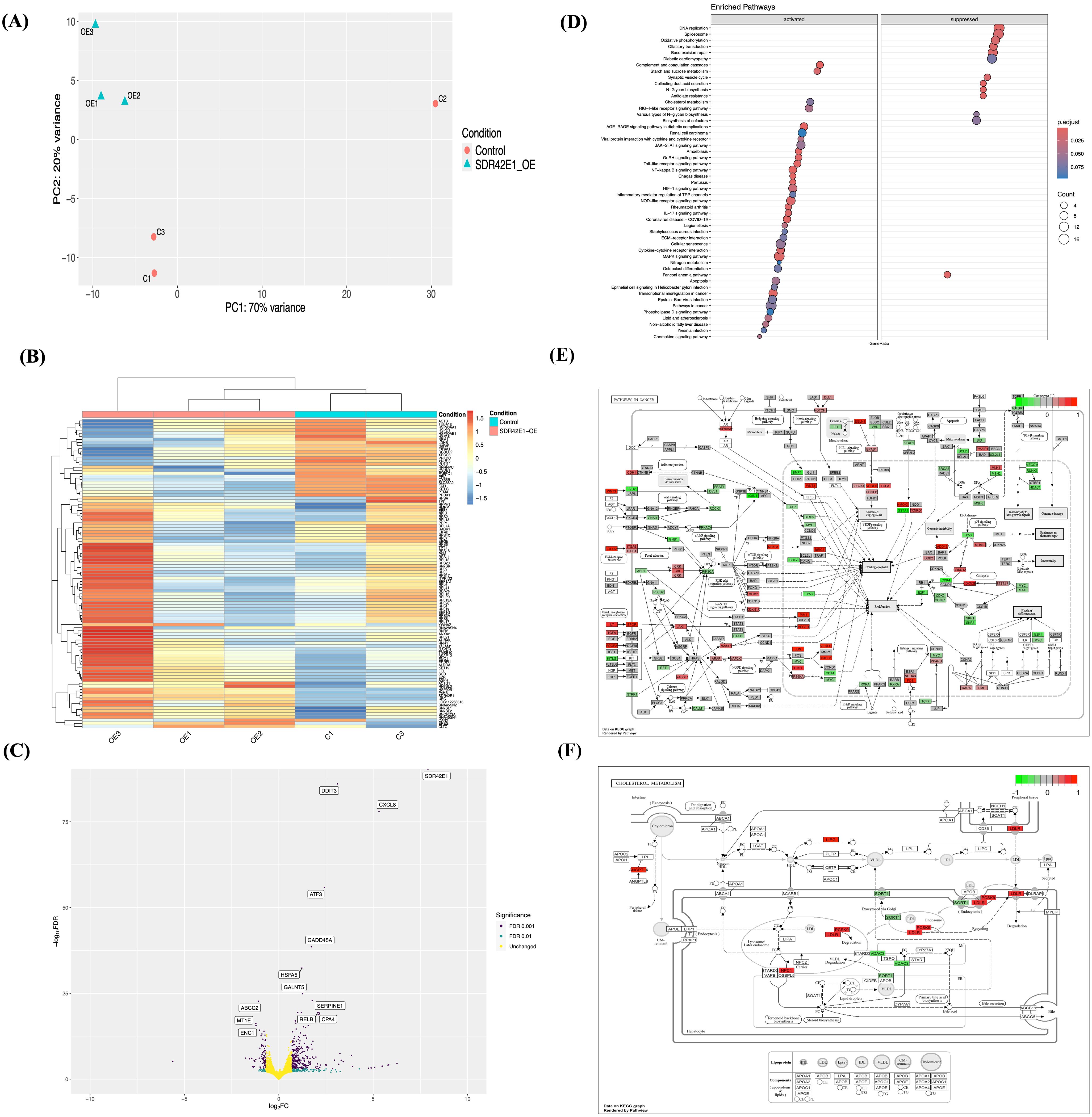
Figure 4. Transcriptomic unveils divergent pathway modifications upon SDR42E1 overexpression. (A) PCA plot of wild-type HCT116 controls (C; in rose) and SDR42E1 overexpression (Hom; in blue) replicates, with main principal components derived from regularized log-transformed counts. (B) Heatmap of top 100 DEGs shows Z-scores of regularized log-transformed counts, with blue indicating lower expression and red indicating higher expression distinguishing sample conditions. The X- and Y-axes are annotated with sample names and DEGs, respectively. (C) Volcano plot of significant gene expression changes in SDR42E1 overexpression compared to wild-type controls. The X-axis represents log2 fold change (FC), with upregulated genes on the right and downregulated genes on the left (Log2FC > 2 or < −2), while the Y-axis indicates the false discovery rate (FDR) < 0.05, with the top 20 most significantly altered genes labeled. (D) Dot plot of KEGG pathway enrichment for DEGs, with the Y-axis showing KEGG pathways and the X-axis indicating genes ratio. KEGG pathway shows gene expression profiles involved (E) in cancer pathways, and (F) in cholesterol metabolism, with upregulated (red) and downregulated (green) genes. All analyses were performed and visualized using R/DESeq2, Pheatmap, and Pathview. All experimental conditions were analyzed with three independent biological replicates (n = 3 per group).
The majority of these genes, approximately 343 in total, showed increased expression levels, including SDR42E1 (FC = 8.0, FDR < 2.2e−308), DNA damage-inducible transcript 3 (DDIT3, FC = 3.1, FDR = 6.12e−83), C-X-C motif chemokine ligand 8 (CXCL8, FC = 5.3, FDR = 4.1e−75), growth arrest and DNA damage-inducible Alpha (GADD45A, FC = 1.7, FDR = 2.7e−39), heat shock protein A (Hsp70) 5 (HSPA5, FC = 1.2, FDR = 9.85e−30), and serine protease inhibitor E1 (SERPINE1, FC = 1.7, FDR = 2.30e−20). Additionally, 95 genes exhibited decreased expression, including ABCC2 (FC = −1.10, FDR = 3.05e−20), metallothionein 1E (MT1E, FC = −1.22, FDR = 4.35e−14), ectodermal-Neural Cortex 1 (ENC1, FC = −1.21, FDR = 1.49e−13), inhibitor of DNA binding 3 (ID3, FC = −1.03, FDR = 2.70e−12), and SRY-box transcription factor 4 (SOX4, FC = −0.78, FDR = 5.23e−12) (Figure 4C, Supplementary Table S4).
To explore the biological functions influenced by DEG in the SDR42E1 overexpression model, we conducted a KEGG enrichment analysis that revealed activation in pathways related to nuclear factor kappa B (NF-kB) signaling pathway (FC = 2.2, adjusted P-value = 1.3e−04), transcriptional misregulation in cancer pathways (FC = 2.02, adjusted P-value = 1.8e−03), immune response pathway (FC = 1.9, adjusted P-value = 2.0e−03), cellular senescence (FC = 1.7, adjusted P-value = 2.0e−02), atherosclerosis and lipid (FC = 1.7, adjusted P-value = 3.0e−02), and cholesterol metabolism (FC = 1.6, adjusted P-value = 3.0e−02). In contrast, there was downregulation in the DNA repair and replication (FC = −2.2, adjusted P-value = 2.0e−03), collecting duct acid secretion (FC = −1.9, adjusted P-value = 1.7e−03), N-glycan biosynthesis (FC = −1.9, adjusted P-value = 3.0e−04), and antifolate resistance (FC = −1.9, adjusted P-value = 5.0e−03) (Figure 4D).
Notable genetic alterations were found within the KEGG pathways related to cancer, cellular senescence, cholesterol metabolism, and steroid biosynthesis. Critical genes in the cancer and cellular senescence, encompassing elevated expression of CXCL8, GADD45, jun proto-oncogene (JUN, FC = 1.3, adjusted P-value = 3.2e−13), and laminin beta 3 (LAMB3, FC = 1.4, adjusted P-value = 6.6e−11), as well as decreased tyrosine-kinase receptor G (KITG, FC = −0.57, adjusted P-value = 3.1e−06), retinoid X receptor alpha (RXRA, FC = −0.57, adjusted P-value = 1.4e−05), and axis inhibition protein 2 (AXIN2, FC = −0.93, adjusted P-value = 2.0e−05) (Figure 4E, Supplementary Figure S3b). In cholesterol metabolism and steroid biosynthesis, low-density lipoprotein receptor (LDLR, FC = 0.7, FDR = 4.1e−07), 7-dehydrocholesterol reductase (DHCR7, FC = 0.5, adjusted P-value = 1.1e−03), proprotein convertase subtilisin/kexin type 9 (PCSK9, FC = 0.6, FDR = 1.0e−03), and lipase G (LIPG, FC = 0.89, adjusted P-value = 1.7e−03) show upregulation, while CYP24A1 (FC = −0.9, adjusted P-value = 7.0e−14), voltage-dependent anion channel (VDAC3, FC = −0.4, adjusted P-value = 4.3e−03), and sortilin (SORT1, FC = −0.4, adjusted P-value = 1.7e−03) exhibit downregulation (Figure 4F, Supplementary Figure S3c).
GSE function analysis also highlighted several enriched categories, including the activation of the inflammatory response (FC = 2.1, adjusted P-value = 1.1e−04), negative regulation of catalytic activity and molecular function (FC = 2.0, adjusted P-value = 2.0e−04), defense response (FC = 3.1, adjusted P-value = 2.0e−04), regulation of cell population proliferation (FC = 1.7, adjusted P-value = 5.0e−04), regulation of angiogenesis and vasculature development (FC = 2.1, adjusted P-value = 5.9e−04), steroid biosynthetic and metabolic processes (FC = 2.1, adjusted P-value = 1.0e−03), steroid dehydrogenase activity (FC = 1.7, adjusted P-value = 2.3e−03), and intracellular sterol transport (FC = 1.8, adjusted P-value = 4.0e−03). Deactivated categories included a response to lectin (FC = −2.24, adjusted P-value = 2.5e−04), condensed chromosome-centromeric region (FC = −2.5, adjusted P-value = 5.1e−04), mitochondrial respiratory chain complex assembly (FC = −2.29, adjusted P-value = 6.3e−04), folic acid binding and metabolic process (FC = −1.9, adjusted P-value = 1.4e−03), and NADH dehydrogenase activity (FC = −1.7, adjusted P-value = 4.0e−02) (Supplementary Figure S3d).
The replication of common genes was examined by comparing the DEG from the SDR42E1 overexpressed HCT116 RNA-sequencing data with those associated with vitamin D in the GWAS Catalog. Our analysis identified a significant overlap, with 29 out of 304 genes showing replication in the SDR42E1 overexpressed dataset. Prominent genes such as SDR42E1, C-X-C motif chemokine ligand 8 (CXCL8), CYP24A1, LDLR, PCSK9, and LIPG were prominently featured, showing strong associations in both datasets, with P-values spanning from 2e−342 to 5e−08 (Table 2).
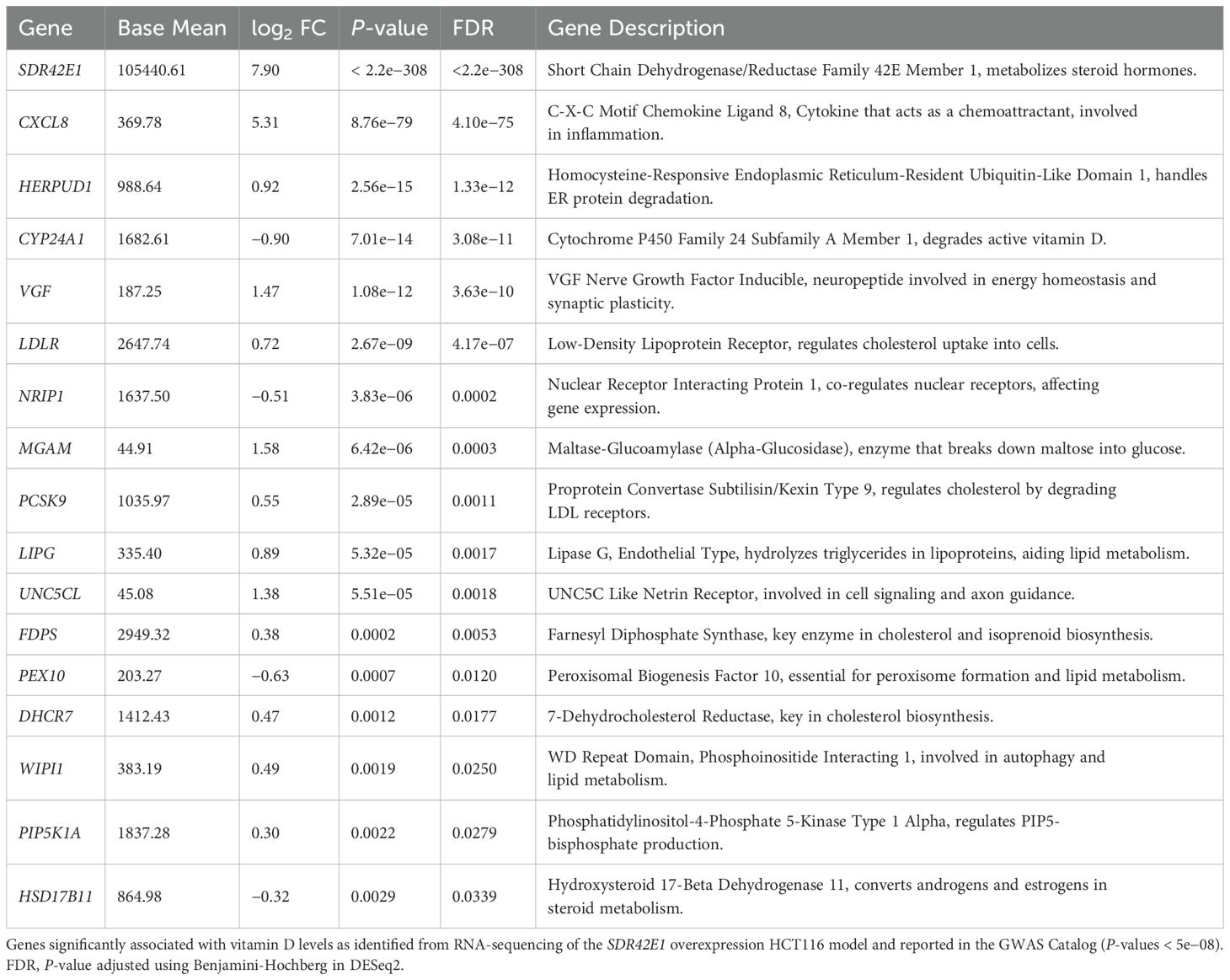
Table 2. Replication of differentially expressed genes reported in the GWAS catalog for vitamin D in the SDR42E1 overexpression model.
3.5 Pronounced reduction in cell viability upon SDR42E1 depletion
To explore the role of the SDR42E1 gene in cell viability, the survival of stable gene-edited HCT116 cells was assessed compared to wild-type controls using the CellTiter-Glo luminescent cell viability assay. The results indicated a significant decrease in cell viability of the SDR42E1 knock-in HCT116 cells by 53%, with a P-value less than 1.0e−04 (Figure 5).
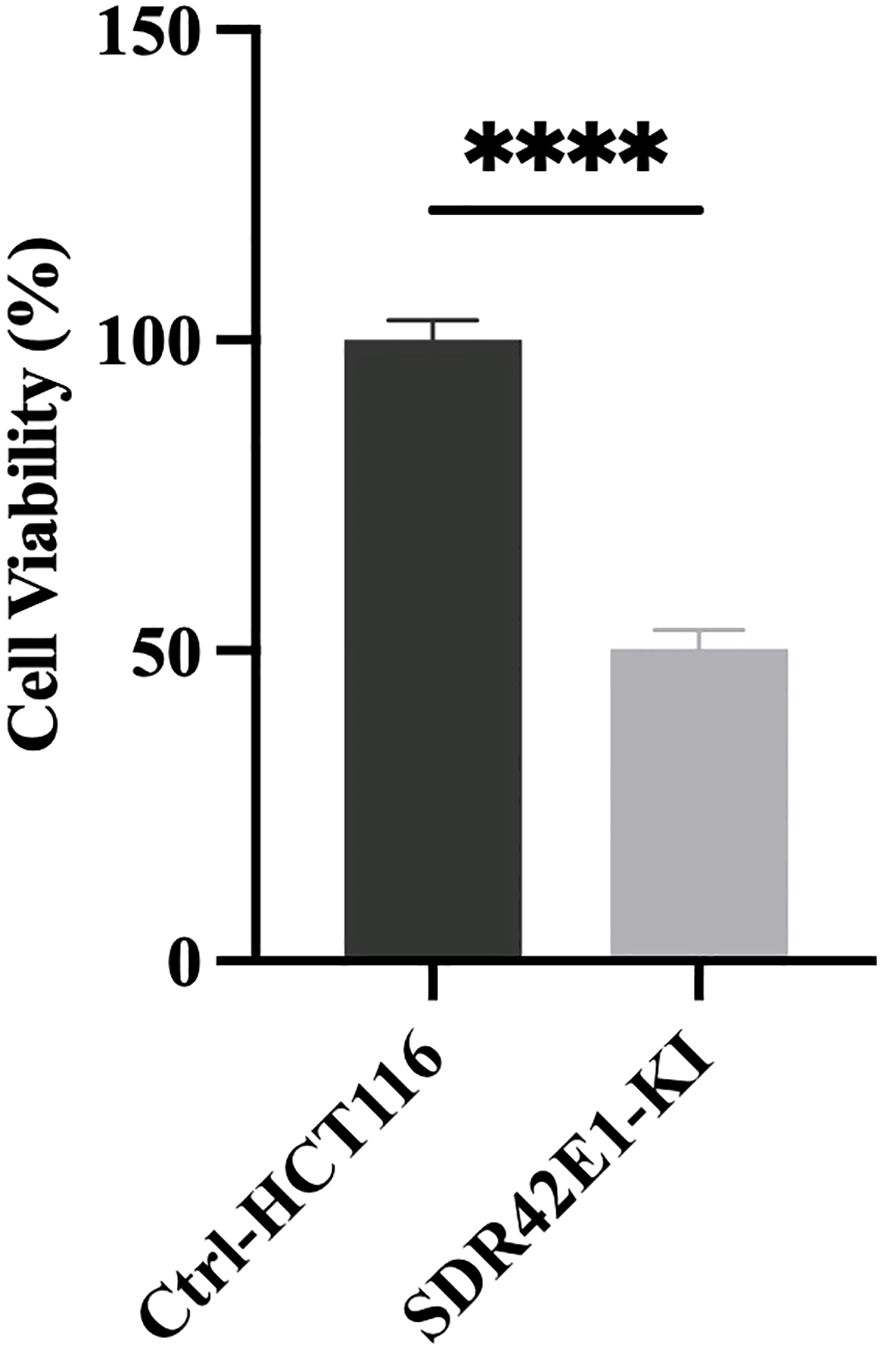
Figure 5. Pronounced reduction in cell viability upon SDR42E1 depletion. Cell viability of the SDR42E1 knock-in model was assessed using a CellTiter-Glo Luminescent Cell Viability (ATP) assay. Gene knock-in reduced viability compared to wild-type HCT116 cells among three replicate experiments (****P-value < 0.0001). All experimental conditions were analyzed with three independent biological replicates (n = 3 per group).
4 Discussion
Vitamin D deficiency is a widespread health concern, contributing to health disorders such as osteoporosis, rickets, cardiovascular diseases, and immune dysfunction (1). Understanding the complex mechanisms governing vitamin D absorption and metabolism is crucial for effective interventions. The absorption of lipids and steroids involves intricate processes, including bile acid micelles and intestinal transport proteins. Any defects or inefficiencies in these processes exacerbate the deficiency (24), emphasizing the importance of studying related genes and pathways.
Our study highlights SDR42E1 as a key player in vitamin D absorption, a gene recently linked to serum 25(OH)D levels through GWAS research (12–14). SDR42E1 is implicated in multiple metabolic processes that potentially affect lipid metabolism and steroid synthesis (9, 10). While SDR family proteins localize to diverse cellular compartments (9, 25), recent research has identified SDR42E1 in the plasma membrane and cytoplasm of keratinocytes, a crucial site for vitamin D biosynthesis (16). We confirmed significant cytoplasmic membrane localization of SDR42E1 in intestinal HCT116 cells, supporting its role in lipid and steroid metabolism. Although our focus has been on genomic pathways, vitamin D also exerts rapid non-genomic effects through membrane-associated VDRs and calcium signaling cascades (26). Given SDR42E1’s predominant membrane localization, it is plausible that it modulates these non-canonical pathways—such as membrane-initiated rapid responses and calcium-mediated signaling—highlighting a broader functional impact that warrants further investigation.
CRISPR/Cas9-mediated SDR42E1 nonsense mutation and the transient overexpression induced considerable transcriptomic changes in the HCT116 models. LRP1B and ABCC2 were upregulated upon SDR42E1 depletion but downregulated in overexpression models, highlighting their role in vitamin D and sterols absorption. LRP1B, a low-density lipoprotein receptor family member, facilitates the endocytosis of lipid-based vitamins (27). Similarly, ABCC2, an ATP-binding cassette transporter, facilitates the transport and uptake of vitamin D metabolites (28–30), with genetic variations linked to altered vitamin D3 bioavailability and deficiency (31, 32). Recent studies also identified the hepatic vitamin D3 metabolite, 25OHD3-3-O-glucuronide, as a bona fide substrate of related intestinal ABC transporters (33). These observations support our earlier in silico study, demonstrating SDR42E1’s strong substrate affinity for vitamin D3 (9), underlining its potential role in vitamin D homeostasis and metabolism.
In alignment with the Hanahan and Weinberg framework (34), functional enrichment analysis indicates that SDR42E1 disruption intersects multiple oncogenic hallmarks, particularly those related to colorectal cancer progression and chemotherapy response. The upregulation of LRP1B, RGS5, STAT2, and HLA-A in SDR42E1-depleted cells suggests enhanced apoptotic singling, tumor-immune crosstalk, and immune recognition—aligning with vitamin D’s immunomodulatory roles and potentially increasing chemotherapy sensitivity (35–37). Simultaneously, the downregulation of WNT16, cell cycle regulators such as SLC7A5, and histone genes, like HIST2H3A and HIST1H2BJ, points to disruptions in the Wnt signaling and chromatin remodeling, processes central to cancer cell proliferation and epigenetic regulation (5, 38–41). Additionally, transcriptional repression of DNA repair and senescence-related genes implies that SDR42E1 may influence replicative stress and cell cycle arrest— mechanisms frequently dysregulated in malignancy (34). The concurrent downregulation of NADH dehydrogenase activity further implicates impaired redox homeostasis, consistent with vitamin D’s role in protecting against oxidative DNA damage (42). Together with observed reduction in HCT116 cell viability, these findings support a role for SDR42E1 in modulating cell survival and senescence, both associated with poor cancer prognosis (43).
Conversely, overexpression of SDR42E1 induced transcriptional changes consistent with stress response and apoptotic resistance. Notably, the upregulation of HSPA5, CXCL8, and SERPINE1, indicates activation of pro-survival and pro-metastatic pathways (44, 45). Elevated levels of SOX4, DDIT3, and GADD45A—genes implicated in stress response, DNA repair, and cancer stem cell maintenance—further suggest engagement of transcriptional mechanisms linked to cancer progression, therapy resistance, and recurrence (46, 47). Additionally, the downregulation of CYP24A1, a key enzyme in vitamin D catabolism and a biomarker of aggressive cancers, may sensitize cells to vitamin D-based therapies and conventional chemotherapy (48). Collectively, these findings highlight SDR42E1 as a potential regulator of tumor biology and promising therapeutic target for improving cancer outcomes. These observations warrant further experimental validation through assays, such as Annexin V staining, apoptosis and cell cycle analysis—and mechanistic studies exploring the roles of chromatin remodeling and non-coding RNAs in mediating SDR42E1’s effects on vitamin D signaling and cancer-related pathways.
Consistent with transcriptomic data, proteomic profiling confirmed that SDR42E1 knock-in influences key proteins alterations, showing downregulation of ALDOA, ALDOC, ABCC2, and FASN. These proteins regulate energy metabolism and lipid biosynthesis in liver and intestinal cell proliferation, potentially affecting cell viability (49). ALDOA, a glycolytic enzyme implicated in cancer (50), may link SDR42E1 to glucose metabolism and vitamin D regulation (51, 52). Given vitamin D deficiency’s association with muscle disorders and ALDOA elevation (53), further exploration of SDR42E1-ALDOA interactions is warranted.
Comparison of DEG in SDR42E1-depleted and -overexpressed models with GWAS catalog data (21) validated key regulators of vitamin D metabolism, including CYP24A1, LIPE-AS1, and PCSK (54–56). Additionally, DHCR7 and FDPS, essential for synthesizing vitamin D precursors, were implicated (9, 57). Consistent with our previous findings, replicated alterations in LDLR, LIPG, SLC7A5, and ABCA1 (16, 54) further support SDR42E1’s role in vitamin D absorption. These insights enhance our understanding of vitamin D homeostasis and SDR42E1’s to these intricate molecular processes.
Despite these valuable findings, certain limitations must be acknowledged. While HCT116 cells are relevant and well-characterized model for gene expression and molecular pathway analysis (58, 59), they may not fully reproduce the structural and functional complexity of vitamin D regulation in normal human intestinal or epithelial tissues. Their cancerous origin and limited differentiation potential could influence the generalizability of findings related to vitamin D absorption and metabolism. Nevertheless, their high endogenous expression of SDR42E1 (16), along with key vitamin D-related genes, such as ABCC2, CYP24A1, and CYP27B1 (60), supports their suitability for modeling SDR42E1-related molecular mechanisms in intestinal-like contexts. Moreover, although the CRISPR/Cas9 gene-editing approach enables precise genomic alterations, the potential for off-target effects remains a consideration. The lack of complementary functional assays—such as apoptosis, migration, or vitamin D rescue experiments— also constrains mechanistic interpretation. To address these limitations, further studies should include validation in primary human intestinal cells and in vivo models to better assess the physiological and therapeutic relevance of SDR42E1 in vitamin D homeostasis and disease.
5 Conclusion
Overall, our study elucidates valuable insights into the role of SDR42E1 in vitamin D metabolism and sterol processing, as well as its broader implications in cancer-related pathways. Through transcriptomic, proteomic, and functional analyses, we demonstrate that SDR42E1 disruption affects key regulators of vitamin D absorption, cell proliferation, and metabolic homeostasis. The findings advance our understanding of the molecular mechanisms underpinning vitamin D deficiency and highlight SDR42E1 as a potential molecular target. Importantly, this work opens avenues for exploring SDR42E1 modulation as a therapeutic strategy to enhance vitamin D bioavailability and potentially counteract tumorigenic processes associated with sterol dysregulation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
NH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. GN: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Open access publication funding was supported by the Qatar National Library. The work was partially funded by the Qatar Research, Development, and Innovation Council (QRDI) under the Academic Research Grant (ARG02-0430-240403).
Acknowledgments
The York Centre of Excellence in Mass Spectrometry was established with significant investment through Science City York, backed by Yorkshire Forward using funds from the Northern Way Initiative. Ongoing support has been provided by EPSRC (EP/K039660/1; EP/M028127/1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1585859/full#supplementary-material
References
1. Mendoza A, Takemoto Y, Cruzado KT, Masoud SS, Nagata A, Tantipanjaporn A, et al. Controlled lipid beta-oxidation and carnitine biosynthesis by a vitamin D metabolite. Cell Chem Biol. (2022) 29:660–9 e12. doi: 10.1016/j.chembiol.2021.08.008
2. Kiourtzidis M, Kuhn J, Brandsch C, and Stangl GI. Vitamin D status of mice deficient in scavenger receptor class B type 1, cluster determinant 36 and ATP-binding cassette proteins G5/G8. Nutrients. (2020) 12(8):E2295. doi: 10.3390/nu12082169
3. Zhang Y, Li CN, Jiang WD, Wu P, Liu Y, Kuang SY, et al. An emerging role of vitamin D(3) in amino acid absorption in different intestinal segments of on-growing grass carp (Ctenopharyngodon idella). Anim Nutr. (2022) 10:305–18. doi: 10.1016/j.aninu.2022.05.004
4. Ma R, Gu Y, Zhao S, Sun J, Groome LJ, and Wang Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol Endocrinol Metab. (2012) 303:E928–35. doi: 10.1152/ajpendo.00279.2012
5. Hendi NN and Nemer G. Epigenetic regulation of vitamin D deficiency. Epigenomics. (2023) 15:653–5. doi: 10.2217/epi-2023-0246
6. Persson B, Kallberg Y, Bray JE, Bruford E, Dellaporta SL, Favia AD, et al. (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chem Biol Interact. (2009) 178:94–8. doi: 10.1016/j.cbi.2008.10.040
7. Bouhouche A, Albaroudi N, El Alaoui MA, Askander O, Habbadi Z, El Hassani A, et al. Identification of the novel SDR42E1 gene that affects steroid biosynthesis associated with the oculocutaneous genital syndrome. Exp Eye Res. (2021) 209:108671. doi: 10.1016/j.exer.2021.108671
8. Chen S, Liu W, Yang C, Li X, Shen X, Jiang D, et al. Gonadotropin inhibitory hormone downregulates steroid hormone secretion and genes expressions in duck granulosa cells. Anim Reprod. (2021) 18:e20210036. doi: 10.1590/1984-3143-ar2021-0036
9. Hendi NN and Nemer G. In silico characterization of the novel SDR42E1 as a potential vitamin D modulator. J Steroid Biochem Mol Biol. (2023) 238:106447. doi: 10.1016/j.jsbmb.2023.106447
10. Loveland JL, Lank DB, and Kupper C. Gene expression modification by an autosomal inversion associated with three male mating morphs. Front Genet. (2021) 12:641620. doi: 10.3389/fgene.2021.641620
11. Ogunmwonyi I, Adebajo A, and Wilkinson JM. The genetic and epigenetic contributions to the development of nutritional rickets. Front Endocrinol (Lausanne). (2022) 13:1059034. doi: 10.3389/fendo.2022.1059034
12. Sinnott-Armstrong N, Tanigawa Y, Amar D, Mars N, Benner C, Aguirre M, et al. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat Genet. (2021) 53:185–94. doi: 10.1038/s41588-020-00757-z
13. Revez JA, Lin T, Qiao Z, Xue A, Holtz Y, Zhu Z, et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat Commun. (2020) 11:1647. doi: 10.1038/s41467-020-15421-7
14. Qiu S, Zheng K, Hu Y, and Liu G. Genetic correlation, causal relationship, and shared loci between vitamin D and COVID-19: A genome-wide cross-trait analysis. J Med Virol. (2023) 95:e28780. doi: 10.1002/jmv.28780
15. Stiles AR, Kozlitina J, Thompson BM, McDonald JG, King KS, and Russell DW. Genetic, anatomic, and clinical determinants of human serum sterol and vitamin D levels. Proc Natl Acad Sci U S A. (2014) 111:E4006–14. doi: 10.1073/pnas.1413561111
16. Hendi NN, Bengoechea-Alonso MT, Ericsson J, and Nemer G. Functional characterization of the SDR42E1 reveals its role in vitamin D biosynthesis. Heliyon. (2024) 10:e36466. doi: 10.1016/j.heliyon.2024.e36466
17. Love MI, Huber W, and Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. (2014) 15:550. doi: 10.1186/s13059-014-0550-8
18. Luo W and Brouwer C. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics. (2013) 29:1830–1. doi: 10.1093/bioinformatics/btt285
19. Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb). (2021) 2:100141. doi: 10.1016/j.xinn.2021.100141
20. Yoo M, Shin J, Kim J, Ryall KA, Lee K, Lee S, et al. DSigDB: drug signatures database for gene set analysis. Bioinformatics. (2015) 31:3069–71. doi: 10.1093/bioinformatics/btv313
21. Sollis E, Mosaku A, Abid A, Buniello A, Cerezo M, Gil L, et al. The NHGRI-EBI GWAS Catalog: knowledgebase and deposition resource. Nucleic Acids Res. (2023) 51:D977–D85. doi: 10.1093/nar/gkac1010
22. Dekker J, Larson T, Tzvetkov J, Harvey VL, Dowle A, Hagan R, et al. Spatial analysis of the ancient proteome of archeological teeth using mass spectrometry imaging. Rapid Commun Mass Spectrom. (2023) 37:e9486. doi: 10.1002/rcm.v37.8
23. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. (2012) 9:676–82. doi: 10.1038/nmeth.2019
24. Setchell KD, Heubi JE, Shah S, Lavine JE, Suskind D, Al-Edreesi M, et al. Genetic defects in bile acid conjugation cause fat-soluble vitamin deficiency. Gastroenterology. (2013) 144:945–55 e6; quiz e14-5. doi: 10.1053/j.gastro.2013.02.004
25. He XY, Merz G, Yang YZ, Mehta P, Schulz H, and Yang SY. Characterization and localization of human type10 17beta-hydroxysteroid dehydrogenase. Eur J Biochem. (2001) 268:4899–907. doi: 10.1046/j.0014-2956.2001.02421.2421.x
26. Zmijewski MA. Nongenomic activities of vitamin D. Nutrients. (2022) 14(23):4953. doi: 10.3390/nu14235104
27. Beenken A, Cerutti G, Brasch J, Guo Y, Sheng Z, Erdjument-Bromage H, et al. Structures of LRP2 reveal a molecular machine for endocytosis. Cell. (2023) 186:821–36 e13. doi: 10.1016/j.cell.2023.01.016
28. Sodani K, Patel A, Kathawala RJ, and Chen ZS. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer. (2012) 31:58–72. doi: 10.5732/cjc.011.10329
29. Alam C, Aufreiter S, Georgiou CJ, Hoque MT, Finnell RH, O’Connor DL, et al. Upregulation of reduced folate carrier by vitamin D enhances brain folate uptake in mice lacking folate receptor alpha. Proc Natl Acad Sci U S A. (2019) 116:17531–40. doi: 10.1073/pnas.1907077116
30. Guo YX, He LY, Zhang M, Wang F, Liu F, and Peng WX. 1,25-Dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Abeta1–40 brain-to-blood efflux and peripheral uptake transport. Neuroscience. (2016) 322:28–38. doi: 10.1016/j.neuroscience.2016.01.041
31. Claro da Silva T, Hiller C, Gai Z, and Kullak-Ublick GA. Vitamin D3 transactivates the zinc and manganese transporter SLC30A10 via the Vitamin D receptor. J Steroid Biochem Mol Biol. (2016) 163:77–87. doi: 10.1016/j.jsbmb.2016.04.006
32. Fan J, Liu S, Du Y, Morrison J, Shipman R, and Pang KS. Up-regulation of transporters and enzymes by the vitamin D receptor ligands, 1alpha,25-dihydroxyvitamin D3 and vitamin D analogs, in the Caco-2 cell monolayer. J Pharmacol Exp Ther. (2009) 330:389–402. doi: 10.1124/jpet.108.149815
33. Gao C, Liao MZ, Han LW, Thummel KE, and Mao Q. Hepatic transport of 25-hydroxyvitamin D(3) conjugates: A mechanism of 25-hydroxyvitamin D(3) delivery to the intestinal tract. Drug Metab Dispos. (2018) 46:581–91. doi: 10.1124/dmd.117.078881
34. Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
35. Kong P, Wang X, Gao YK, Zhang DD, Huang XF, Song Y, et al. RGS5 maintaining vascular homeostasis is altered by the tumor microenvironment. Biol Direct. (2023) 18:78. doi: 10.1186/s13062-023-00437-y
36. Xu C, Li YM, Sun B, Zhong FJ, and Yang LY. ATE1 inhibits liver cancer progression through RGS5-mediated suppression of wnt/beta-catenin signaling. Mol Cancer Res. (2021) 19:1441–53. doi: 10.1158/1541-7786.MCR-21-0027
37. Anderson TS, McCormick AL, Daugherity EA, Oladejo M, Okpalanwaka IF, Smith SL, et al. Listeria-based vaccination against the pericyte antigen RGS5 elicits anti-vascular effects and colon cancer protection. Oncoimmunology. (2023) 12:2260620. doi: 10.1080/2162402X.2023.2260620
38. Sun Y, Zhu D, Chen F, Qian M, Wei H, Chen W, et al. SFRP2 augments WNT16B signaling to promote therapeutic resistance in the damaged tumor microenvironment. Oncogene. (2016) 35:4321–34. doi: 10.1038/onc.2015.494
39. Salhia B, Kiefer J, Ross JT, Metapally R, Martinez RA, Johnson KN, et al. Integrated genomic and epigenomic analysis of breast cancer brain metastasis. PloS One. (2014) 9:e85448. doi: 10.1371/journal.pone.0085448
40. Naldi M, Andrisano V, Fiori J, Calonghi N, Pagnotta E, Parolin C, et al. Histone proteins determined in a human colon cancer by high-performance liquid chromatography and mass spectrometry. J Chromatogr A. (2006) 1129:73–81. doi: 10.1016/j.chroma.2006.06.100
41. Najumudeen AK, Ceteci F, Fey SK, Hamm G, Steven RT, Hall H, et al. The amino acid transporter SLC7A5 is required for efficient growth of KRAS-mutant colorectal cancer. Nat Genet. (2021) 53:16–26. doi: 10.1038/s41588-020-00753-3
42. Dwivedi S, Singh V, Sen A, Yadav D, Agrawal R, Kishore S, et al. Vitamin D in disease prevention and cure-part I: an update on molecular mechanism and significance on human health. Indian J Clin Biochem. (2024). doi: 10.1007/s12291-024-01251-7
43. Liu S, Dong Q, and Wang E. Rsf-1 overexpression correlates with poor prognosis and cell proliferation in colon cancer. Tumour Biol. (2012) 33:1485–91. doi: 10.1007/s13277-012-0399-y
44. Li SJ, Wei XH, Zhan XM, He JY, Zeng YQ, Tian XM, et al. Adipocyte-derived leptin promotes PAI-1 -mediated breast cancer metastasis in a STAT3/miR-34a dependent manner. Cancers (Basel). (2020) 12(12):3741. doi: 10.3390/cancers12123864
45. Wang Q, Ke S, Liu Z, Shao H, He M, and Guo J. HSPA5 promotes the proliferation, metastasis and regulates ferroptosis of bladder cancer. Int J Mol Sci. (2023) 24(6):5475. doi: 10.3390/ijms24065144
46. Liu J, Jiang G, Mao P, Zhang J, Zhang L, Liu L, et al. Down-regulation of GADD45A enhances chemosensitivity in melanoma. Sci Rep. (2018) 8:4111. doi: 10.1038/s41598-018-22484-6
47. Berastegui N, Ainciburu M, Romero JP, Garcia-Olloqui P, Alfonso-Pierola A, Philippe C, et al. The transcription factor DDIT3 is a potential driver of dyserythropoiesis in myelodysplastic syndromes. Nat Commun. (2022) 13:7619. doi: 10.1038/s41467-022-35192-7
48. Kamiya S, Nakamori Y, Takasawa A, Takasawa K, Kyuno D, Ono Y, et al. Suppression of the vitamin D metabolizing enzyme CYP24A1 provides increased sensitivity to chemotherapeutic drugs in breast cancer. Oncol Rep. (2023) 49(5):85. doi: 10.3892/or.2023.8522
49. Votava JA, John SV, Li Z, Chen S, Fan J, and Parks BW. Mining cholesterol genes from thousands of mouse livers identifies aldolase C as a regulator of cholesterol biosynthesis. J Lipid Res. (2024) 65:100525. doi: 10.1016/j.jlr.2024.100525
50. Pirovich DB, Da’dara AA, and Skelly PJ. Multifunctional fructose 1,6-bisphosphate aldolase as a therapeutic target. Front Mol Biosci. (2021) 8:719678. doi: 10.3389/fmolb.2021.719678
51. Alvarez JA and Ashraf A. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. (2010) 2010:351385. doi: 10.1155/2010/351385
52. Tang H, Li D, Li Y, Zhang X, Song Y, and Li X. Effects of vitamin D supplementation on glucose and insulin homeostasis and incident diabetes among nondiabetic adults: A meta-analysis of randomized controlled trials. Int J Endocrinol. (2018) 2018:7908764. doi: 10.1155/2018/7908764
53. Rasheed K, Sethi P, and Bixby E. Severe vitamin d deficiency induced myopathy associated with rhabydomyolysis. N Am J Med Sci. (2013) 5:334–6. doi: 10.4103/1947-2714.112491
54. Warren T, McAllister R, Morgan A, Rai TS, McGilligan V, Ennis M, et al. The interdependency and co-regulation of the vitamin D and cholesterol metabolism. Cells. (2021) 10(8):2007. doi: 10.3390/cells10082007
55. Thunen A, La Placa D, Zhang Z, and Shively JE. Role of lncRNA LIPE-AS1 in adipogenesis. Adipocyte. (2022) 11:11–27. doi: 10.1080/21623945.2021.2013415
56. Sachan V, Le Devehat M, Roubtsova A, Essalmani R, Laurendeau JF, Garcon D, et al. PCSK7: A novel regulator of apolipoprotein B and a potential target against non-alcoholic fatty liver disease. Metabolism. (2024) 150:155736. doi: 10.1016/j.metabol.2023.155736
57. Guarana WL, Lima CAD, Barbosa AD, Crovella S, and Sandrin-Garcia P. Farnesyl diphosphate synthase gene associated with loss of bone mass density and alendronate treatment failure in patients with primary osteoporosis. Int J Mol Sci. (2024) 25(11):5623. doi: 10.3390/ijms25115623
58. Wierzbicka JM, Binek A, Ahrends T, Nowacka JD, Szydlowska A, Turczyk L, et al. Differential antitumor effects of vitamin D analogues on colorectal carcinoma in culture. Int J Oncol. (2015) 47:1084–96. doi: 10.3892/ijo.2015.3088
59. Hibler EA, Jacobs ET, Stone AD, Sardo CL, Galligan MA, and Jurutka PW. Associations between vitamin D-binding protein isotypes, circulating 25(OH)D levels, and vitamin D metabolite uptake in colon cancer cells. Cancer Prev Res (Phila). (2014) 7:426–34. doi: 10.1158/1940-6207.CAPR-13-0269
Keywords: vitamin D regulation, SDR42E1, endocrine disorders, genetics in endocrinology, precision medicine
Citation: Hendi NN and Nemer G (2025) SDR42E1 modulates vitamin D absorption and cancer pathogenesis: insights from an in vitro model. Front. Endocrinol. 16:1585859. doi: 10.3389/fendo.2025.1585859
Received: 01 March 2025; Accepted: 27 May 2025;
Published: 18 July 2025.
Edited by:
Benjamin Udoka Nwosu, Hofstra University, United StatesReviewed by:
Shailendra Dwivedi, All India Institute Of Medical Sciences Gorakhpur, IndiaAkhileshwar Namani, Sri Shankara Cancer Hospital and Research Centre, India
Komal Wakle, Trainee, Wockhardt Research Center, India
Copyright © 2025 Hendi and Nemer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georges Nemer, Z25lbWVyQGhia3UuZWR1LnFh
†ORCID: Nagham Nafiz Hendi, orcid.org/0000-0003-2737-0565
Georges Nemer, orcid.org/0000-0003-2157-5279
 Nagham Nafiz Hendi
Nagham Nafiz Hendi Georges Nemer
Georges Nemer