- Department of Gynaecology and Obstetrics, The First People’s Hospital of Shangqiu, Clinical College of Xuzhou Medical University, Shang Qiu, Henan, China
Background: Gestational diabetes mellitus (GDM) is a prevalent condition during pregnancy, and macrosomia is a recognized risk associated with it. However, the specific relationship between fasting blood glucose (FBG) levels and the risk of macrosomia in GDM, particularly any potential thresholds for this relationship, remains unclear.
Methods: This retrospective cohort study analyzed data from 7,957 pregnant women who underwent antenatal care and delivered at The First People’s Hospital of Shangqiu between February 1, 2018, and December 30, 2022. Participants were stratified into three groups based on FBG levels: <5.1 mmol/L, 5.1–7 mmol/L, and ≥7 mmol/L. Multivariable logistic regression analyses were performed to assess the association between FBG levels and the risk of macrosomia. Two-piecewise regression models were applied to identify a threshold for the FBG-macrosomia relationship.
Results: The prevalence of macrosomia increased substantially with increasing FBG levels (P < 0.001). The adjusted multivariable logistic regression analyses revealed that compared to women with FBG levels <5.1 mmol/L, those with FBG levels of 5.1–7 mmol/L and ≥7 mmol/L had 4.69 (95% CI: 4.06-5.42) and 8.65 (95% CI: 7.31-10.23) times higher risk of macrosomia, respectively (both P < 0.001). Two-piecewise regression models identified a threshold of 8.037 mmol/L. Below this threshold, each unit increase in FBG was associated with a 1.93-fold increase in the odds of macrosomia (95% CI: 1.83-2.04, P < 0.001). Above this threshold, the association was no longer statistically significant (OR = 1.04, 95% CI: 0.90-1.21, P = 0.587). Furthermore, the stratified analysis also showed a positive association between FBG level and macrosomia.
Conclusion: There is a nonlinear relationship between FBG levels during pregnancy and the risk of macrosomia in GDM women, with a potential threshold effect at 8.037 mmol/L. Below the threshold, macrosomia prevalence markedly rises with elevated FBG levels, whereas above it, the association loses significance, implying a potential saturation at very high glucose levels.
Introduction
Gestational diabetes mellitus (GDM) is a significant metabolic disorder occurring during pregnancy, posing substantial risks to both the mother and the developing fetus (1, 2). One of the most concerning complications associated with GDM is macrosomia, or excessive fetal growth, which can lead to various adverse outcomes such as difficult delivery, shoulder dystocia, and neonatal hypoglycemia (3, 4). Controlling fasting blood glucose (FBG) levels during pregnancy is crucial in managing GDM and preventing macrosomia.
While previous investigations have established a general correlation between elevated FBG levels and macrosomia risk (5, 6), critical knowledge gaps persist. A landmark study by Landon et al. demonstrated linear associations between maternal glycemia and fetal overgrowth, yet their diagnostic frameworks primarily relied on single-timepoint measurements rather than longitudinal glycemic patterns (7). Subsequent studies by Zhang et al. and Tong et al. attempted to establish a correlation between FBG and birth weight, but their findings showed marked geographical variability, potentially constrained by heterogeneous diagnostic criteria and inconsistent measurement protocols (8, 9). Notably, none of these studies systematically evaluated the specific FBG threshold for GDM patients with the risk of macrosomia, especially when FBG is taken from the GDM average measured three times after treatment.
It is well-established that managing gestational diabetes emphasizes stringent glycemic targets during pregnancy, yet not all patients are able to maintain their FBG levels within the optimal range (10, 11). McIntyre et al. found that there is a lack of uniform worldwide consensus on the hyperglycemic threshold levels that warrant a diagnosis of GDM and subsequent treatment during pregnancy, optimal management of both mother and infant during long-term follow-up remains challenging (12). Persistent discrepancies exist between major international guidelines, particularly between the National Institute for Health and Care Excellence and the World Health Organization diagnostic protocols, this inconsistency stems from fundamental methodological variations across studies. There is marked population heterogeneity in existing studies, most thresholds are derived from European cohorts and lack validation in Asian populations. Moreover, there’s inconsistency in the diagnostic gold standard. Guidelines vary in their emphasis on postprandial versus fasting glucose levels (13, 14). These challenges underscore the urgent need to establish clinically relevant FBG thresholds through robust epidemiological evidence. In this investigation, we conducted longitudinal monitoring of FBG levels from GDM diagnosis through delivery, employing triplicate measurements to enhance glycemic status assessment reliability. Our analysis focuses on elucidating the quantitative relationship between third-trimester FBG control and macrosomia risk, aiming to establish evidence-based glycemic targets for optimizing neonatal outcomes in this high-risk population.
Materials and methods
Study design and population
This retrospective cohort study included 7,957 pregnant women who received antenatal care and delivered at The First People’s Hospital of Shangqiu between February 1, 2018, and December 30, 2022. Pregnant women diagnosed with GDM during their antenatal care visits were eligible for inclusion. Data were collected from electronic medical records and antenatal care charts. The following variables were recorded for each participant: age, education level, number of pregnancies, pre-pregnancy body mass index (BMI), smoking status, FBG levels during pregnancy, and the occurrence of macrosomia. Macrosomia was defined as a birth weight exceeding 4000 grams. The study protocol was consistent with ethical principles and received approval from the Ethics Committee of The First People’s Hospital of Shangqiu (No: SYQ1033992). Given the retrospective nature of the study, the Ethical Committee of the First People’s Hospital of Shangqiu waived the requirement of informed consent. The present study is in compliance with the Declaration of Helsinki.
Inclusion Criteria: Pregnant women with a confirmed diagnosis of GDM based on the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria during the second trimester of pregnancy; complete antenatal records including at least three FBG measurements from the time of GDM diagnosis until delivery; singleton pregnancies with a known birth outcome. Exclusion Criteria: Women with pre-existing type 1 or type 2 diabetes prior to pregnancy; multiple gestations; incomplete antenatal records or missing FBG measurements; fetal anomalies or congenital malformations. The technical roadmap is detailed in Figure 1.
GDM diagnosis
GDM was diagnosed according to the IADPSG criteria, which define GDM as one or more of the following: fasting plasma glucose ≥ 5.1 mmol/L, 1-hour plasma glucose ≥ 10.0 mmol/L, or 2-hour plasma glucose ≥ 8.5 mmol/L during a 75-gram oral glucose tolerance test performed between 24 and 28 weeks of gestation (15).
FBG measurement
For each woman included in the study, three FBG measurements were obtained during the period from GDM diagnosis to delivery. The average of these three measurements was calculated and used to stratify women into three groups: FBG < 5.1 mmol/L, 5.1–7 mmol/L, and ≥ 7 mmol/L.
Statistical analysis
Statistical analysis was performed with SPSS version 26.0 (IBM, Chicago, USA) and R (version 4.3.2). Descriptive statistics were used to summarize the baseline characteristics of the study population. Categorical data were reported as percentages, The chi-square test was applied to assess statistical differences for categorical variables. Multivariable logistic regression analyses were performed to assess the association between FBG levels and the risk of macrosomia, with adjustments for potential confounders including age, BMI, education level, number of pregnancies, and smoking status. Following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations, three models were tested: a crude model without other covariates, Model 1 adjusted for age and BMI, and Model 2 adjusted for age, BMI, education, number of pregnancies, and smoking status.
To identify potential thresholds for the FBG-macrosomia relationship, two-piecewise regression models were applied. These models evaluated the relationship between FBG levels and macrosomia risk below and above a threshold value, which was determined based on statistical significance and goodness of fit. Subgroup analyses were conducted to explore the association between FBG levels and the risk of macrosomia within specific demographic and clinical strata, including age, education, BMI, and smoking status. P-value < 0.05 was considered statistically significant.
Results
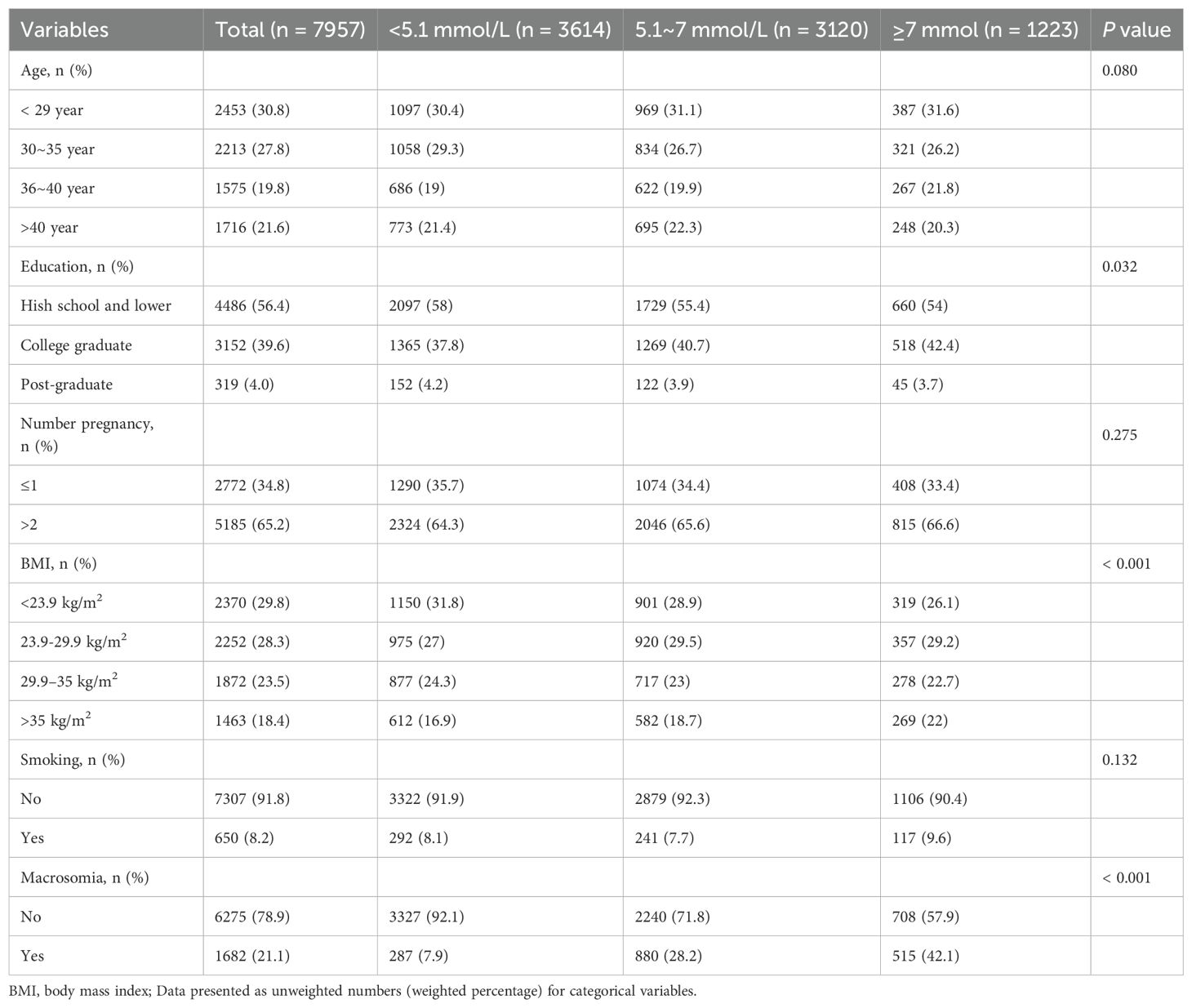
This study analyzed data from 7,957 pregnant women who underwent antenatal care and delivered at The First People’s Hospital of Shangqiu between February 1, 2018, and December 30, 2022. The study population was stratified into three groups based on FBG levels: <5.1 mmol/L (n=3614), 5.1–7 mmol/L (n=3120), and ≥7 mmol/L (n=1223). There were significant differences in education level (p=0.032) and BMI (p<0.001) across the three FBG groups, but age, number of pregnancies, and smoking status did not significantly differ. A substantial increase in the prevalence of macrosomia was observed with increasing FBG levels. Specifically, the prevalence of macrosomia was 7.9% in the <5.1 mmol/L group, 28.2% in the 5.1–7 mmol/L group, and 42.1% in the ≥7 mmol/L group, resulting in a highly significant difference (P < 0.001) (Table 1).
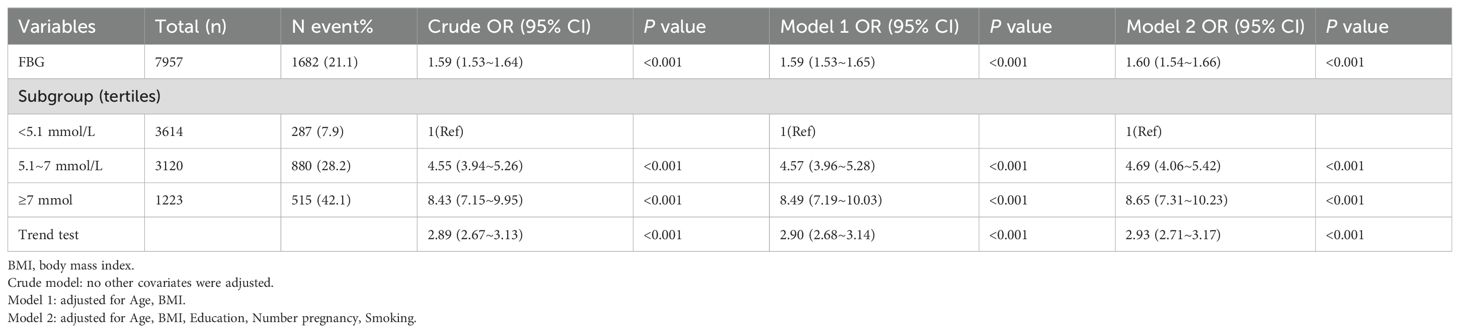
Multivariable logistic regression analyses consistently demonstrated a positive relationship between higher FBG levels and the risk of macrosomia. In the fully adjusted model (Model 2), compared to women with FBG levels <5.1 mmol/L, those with FBG levels of 5.1–7 mmol/L and ≥7 mmol/L had 4.69 (95% CI: 4.06-5.42) and 8.65 (95% CI: 7.31-10.23) times higher risk of macrosomia, respectively (both P < 0.001). A trend test confirmed a linear increase in the risk of macrosomia with rising FBG levels (P < 0.001) (Table 2).
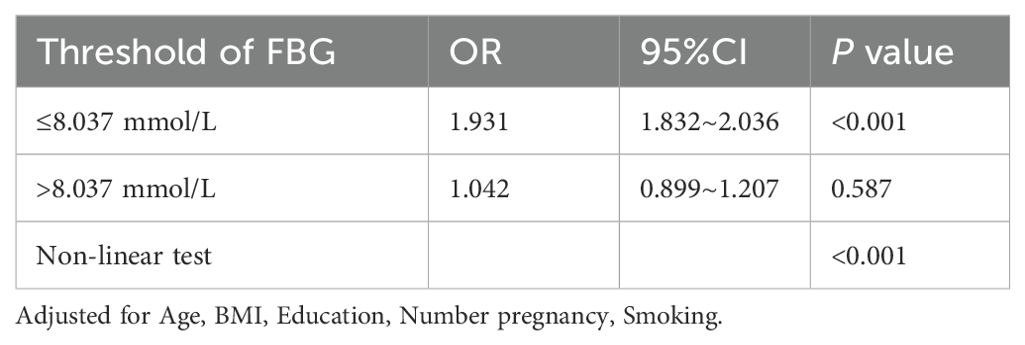
To identify a potential threshold for the FBG-macrosomia relationship, two-piecewise regression models were applied (Figure 2). The analysis identified a significant threshold at 8.037 mmol/L, beyond which the risk of macrosomia did not further increase significantly. Below this threshold, each unit increase in FBG was associated with a 1.93-fold increase in the odds of macrosomia (95% CI: 1.83-2.04, P < 0.001). Above this threshold, the association was no longer statistically significant (OR = 1.04, 95% CI: 0.90-1.21, P = 0.587), suggesting a potential plateau or saturation effect (Table 3).
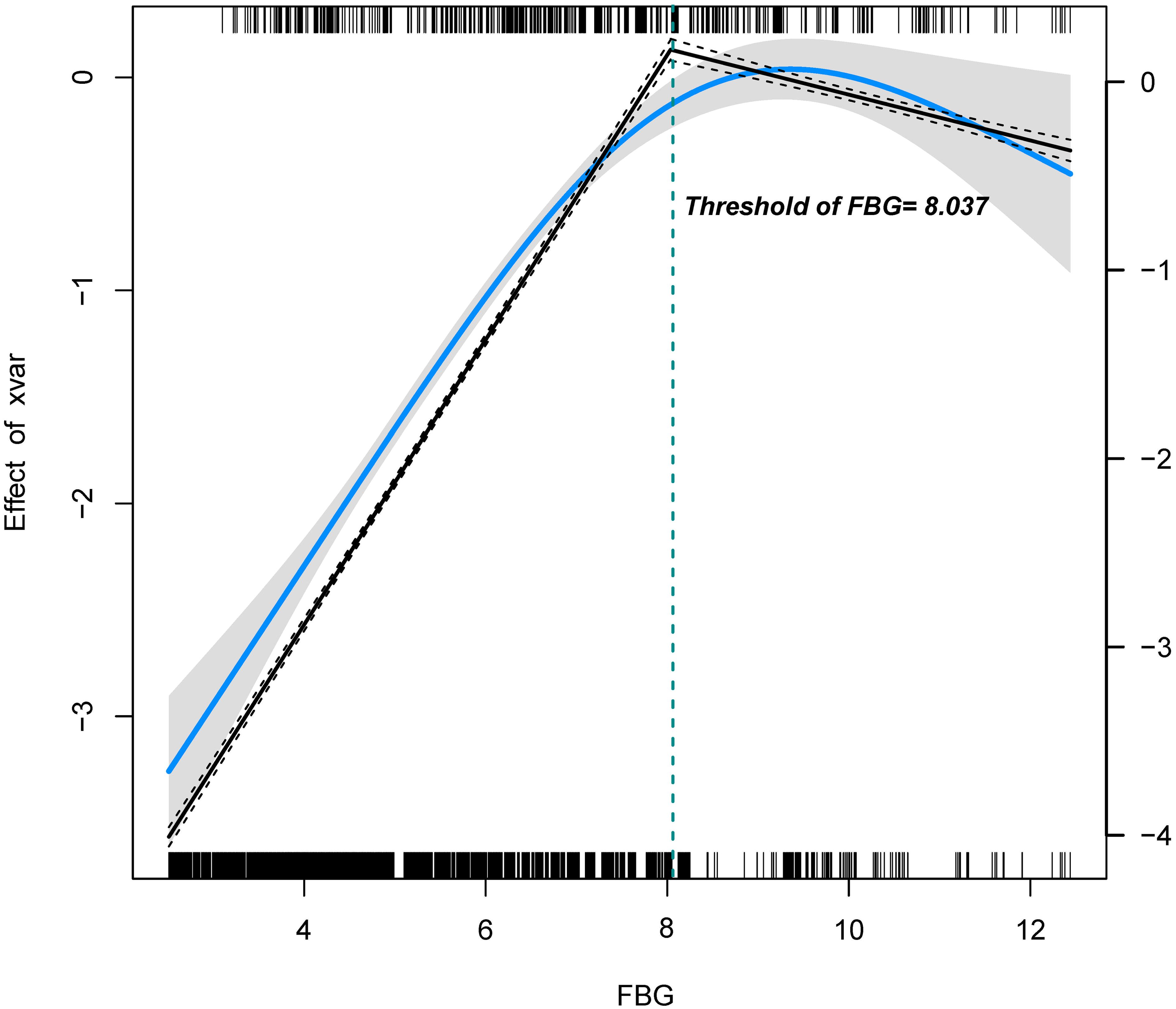
Figure 2. Association between FBG and macrosomia using two-piecewise regression. The solid blue line indicates the predicted value and the grey areas indicate the 95% CI. The restricted cubic spline model was adjusted for Age, BMI, Education, Number of pregnancies, and Smoking.
Stratified analyses by age, education, BMI, and smoking status revealed that the risk of macrosomia increased significantly with higher FBG levels across all subgroups. Notably, smokers had a markedly higher prevalence of macrosomia even within the lowest FBG group (<5.1 mmol/L), highlighting the importance of considering smoking status in clinical management (Figure 3).
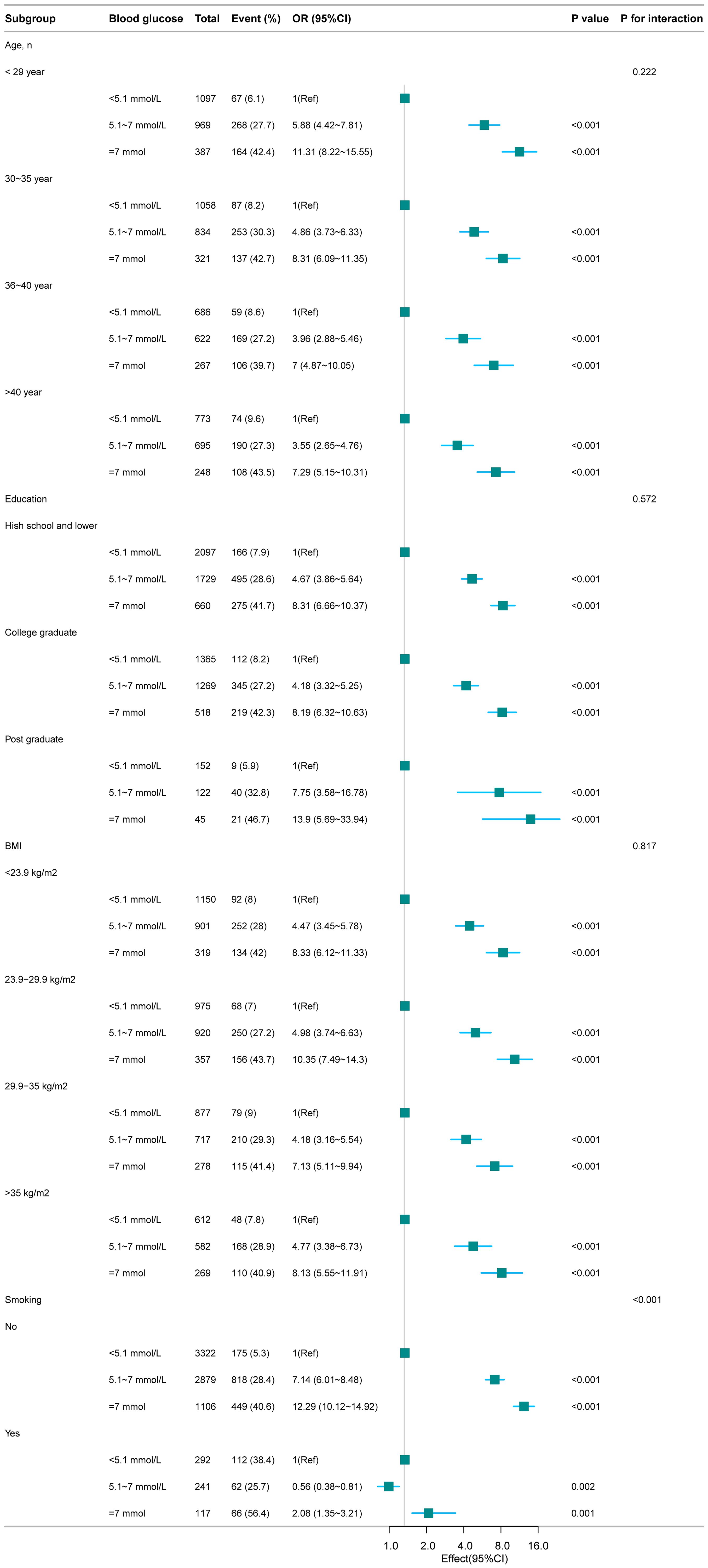
Figure 3. Association between FBG and macrosomia according to the subgroup analyses. Except for the stratification factor itself, the stratifications were adjusted for all variables (Age, BMI, Education, Smoking).
Discussion
The incidence of GDM is notably high, and its implications are profound (16). Studies have shown that inadequate control of FBG levels is associated with an increased risk of macrosomia (17, 18), a condition characterized by excessive fetal growth. This complication not only poses significant challenges to the maternal delivery process, including difficult labor and shoulder dystocia, but also threatens the fetus, potentially leading to neonatal hypoglycemia and other adverse outcomes (19, 20). Therefore, it is imperative to prioritize effective FBG control in GDM management to mitigate these risks and optimize maternal and fetal health outcomes. Our study revealed a nonlinear relationship between fasting FBG levels during pregnancy and the risk of macrosomia in women with gestational diabetes mellitus, with a potential threshold effect observed at 8.037 mmol/L. Specifically, below this threshold, each unit increase in FBG was associated with a 1.93-fold increase in the odds of macrosomia. Conversely, above this threshold, the association between FBG and macrosomia became statistically insignificant, suggesting a potential saturation effect or the involvement of other mitigating factors at extremely high glucose levels.
Our findings emphasize a substantial rise in the prevalence of macrosomia with increasing FBG levels (P < 0.001), aligning with prior research that established a connection between hyperglycemia and fetal overgrowth, corroborating the observations made by Zhang et al (8). Additionally, the two-piecewise regression models identified a threshold of 8.037 mmol/L, this novel discovery contributes to the existing literature by indicating that FBG levels reaching a specific threshold may not further exacerbate the risk of macrosomia. The exact mechanism by which hyperglycemia leads to macrosomia in GDM women remains elusive. Nevertheless, several studies have postulated that hyperglycemia may induce fetal hyperinsulinemia, thereby stimulating fetal growth and adiposity (21, 22). Furthermore, hyperglycemia may facilitate increased nutrient transport to the fetus, further promoting fetal growth (23). Our study supports these mechanisms by demonstrating a dose-response relationship between FBG levels and the risk of macrosomia. However, the absence of a significant association above the identified threshold hints at the possibility of additional mechanisms or compensatory responses operative at very high glucose levels, necessitating further investigation.
Consensus on optimal glycemic control targets for GDM pregnant women remains unsettled. In 2013, the American Association of Clinical Endocrinologists’ clinical practice guidelines suggested controlling fasting plasma glucose to <5.0 mmol/L while avoiding hypoglycemia (24). In 2015, the National Institute for Health and Care Excellence (NICE) guidelines in the UK recommended controlling 2-hour postprandial glucose to <6.4 mmol/L (25). Regarding the definition of hyperglycemia, the IADPSG utilized data from the HAPO study to establish a diagnostic threshold for GDM at FBG levels that elevate the risk of adverse pregnancy outcomes by 75% compared to average levels, with a fasting plasma glucose cutoff of 5.1 mmol/L (26). Studies examining FBG fluctuations in normal pregnant women have revealed that their peak postprandial glucose levels during weeks 28 to 38 of pregnancy remain below 5.8 mmol/L (27, 28). For GDM pregnant women, the ADA and FIGO currently recommend glycemic control targets of fasting, pre-meal, or bedtime glucose <5.3 mmol/L, 1-hour postprandial <7.8 mmol/L, or 2-hour postprandial <6.7 mmol/L (15, 29). These discrepancies are likely attributable to heterogeneous clinical objectives, divergent patient demographics, and methodological inconsistencies across validation studies. Specifically, the NICE guidelines prioritize diagnostic sensitivity for early detection in predominantly UK/European cohorts, whereas the ADA recommendations emphasize specificity optimization to minimize false-positive diagnoses in advanced-stage US populations. Despite these nuanced differences, both sets of guidelines consistently advocate for stringent FBG control in pregnant women with GDM, as elevated FBG levels during pregnancy are strongly associated with adverse perinatal outcomes.
Our findings align with the glycemic control targets recommended by the ADA and FIGO guidelines, demonstrating a significant association between maternal FBG levels during pregnancy and neonatal outcomes in GDM patients. Notably, the present study revealed a positive correlation between FBG levels below the identified threshold of 8.037 mmol/L and macrosomia incidence. However, this association lost statistical significance when FBG exceeded this critical value, though this observation should not be misinterpreted as justifying glycemic negligence. Substantial evidence confirms that suboptimal glycemic control in gestational diabetes predisposes to adverse perinatal outcomes (30–32). Elevated glucose levels in pregnant women traverse the placenta, stimulating fetal pancreatic β-cell proliferation and insulin secretion, which can adversely impact fetal health (33, 34). Moreover, high glucose levels heighten the risk of reproductive tract infections, intrauterine infections, and subsequent inflammatory cytokine production, potentially leading to preterm birth (35). This preterm birth phenomenon associated with excessive FBG levels might partially explain the attenuated correlation between supra-threshold hyperglycemia and macrosomia. Hyperglycemia is frequently accompanied by insulin resistance, and elevated insulin levels stimulate sympathetic nerve excitation, simultaneously, high FBG levels damage endothelial cells, leading to vasoconstriction and elevated blood pressure (12, 36). Pregnant women with high FBG levels are thus at an increased risk of adverse perinatal outcomes, including macrosomia, preterm birth, and preeclampsia.
One of the strengths of our study lies in its large sample size, which enabled us to stratify participants into three groups based on FBG levels and conduct subgroup analyses stratified by age, education, BMI, and smoking status to assess the association between FBG levels and the risk of macrosomia. Furthermore, the employment of two-piecewise regression models facilitated the identification of a potential threshold for the FBG-macrosomia relationship. However, our study is constrained by its retrospective design, which relies on existing data and may be prone to bias and confounding variables. Additionally, our study population was confined to women who delivered at a single hospital, potentially limiting the generalizability of our findings. Moreover, gestation represents a multifactorial biological phenomenon wherein various determinants may substantially impact perinatal outcomes. While our analysis incorporated adjustments for key covariates including maternal age, educational attainment, and BMI, residual confounding may persist due to unaccounted variables such as pharmacotherapeutic interventions, habitual physical activity patterns, and nutritional exposures, which could potentially influence the observed associations.
In conclusion, our findings underscore the significance of glycemic control in preventing macrosomia. There is a nonlinear relationship between FBG levels during pregnancy and the risk of macrosomia in GDM women, with a potential threshold effect at 8.037 mmol/L. Below the threshold, macrosomia prevalence markedly rises with elevated FBG levels, whereas above it, the association loses significance, implying a potential saturation at very high glucose levels. Our study provides novel insights by identifying a distinct threshold for this risk, thereby contributing to a more nuanced understanding of the intricate relationship between FBG levels and fetal growth in GDM.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study protocol was consistent with the ethical principles and received approval from the Ethics Committee of The First People’s Hospital of Shangqiu (No: SYQ1033992). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the retrospective nature of the study.
Author contributions
YL: Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Data curation, Project administration, Resources, Software, Writing – original draft. WZ: Data curation, Formal analysis, Project administration, Writing – review & editing. YG: Formal analysis, Software, Writing – review & editing. RH: Data curation, Methodology, Writing – original draft. TY: Project administration, Supervision, Writing – original draft. YC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (U1904165), the Key Science and Technology Foundation of Henan Province (LHGJ20240822), the Medical Education Research Project of Henan Province (WJLX2024138), The Science and Technology Development Project of Shangqiu (2024013 and 2024107), Hospital fund of the First People’s Hospital of Shangqiu City (YJ202302001).
Acknowledgments
The authors thank the patients who participated in this study and all the physicians and nurses who participated in this study, for their support in collecting the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nabi T, Rafiq N, Charak G, and Mishra S. Maternal and neonatal outcomes in women with recurrent gestational diabetes mellitus. Diabetes Metab syndrome. (2022) 16:102420. doi: 10.1016/j.dsx.2022.102420
2. Hod M, Kapur A, and McIntyre HD. Evidence in support of the International Association of Diabetes in Pregnancy study groups’ criteria for diagnosing gestational diabetes mellitus worldwide in 2019. Am J Obstet Gynecol. (2019) 221:109–16. doi: 10.1016/j.ajog.2019.01.206
3. Bukhari I, Iqbal F, and Thorne RF. Research advances in gestational, neonatal diabetes mellitus and metabolic disorders. Front Endocrinol (Lausanne). (2022) 13:969952. doi: 10.3389/fendo.2022.969952
4. Antoniou MC, Gilbert L, Gross J, Rossel JB, Fumeaux CJF, Vial Y, et al. Main fetal predictors of adverse neonatal outcomes in pregnancies with gestational diabetes mellitus. J Clin Med. (2020) 9(8):2409. doi: 10.3390/jcm9082409
5. Ringholm L, Damm P, and Mathiesen ER. Improving pregnancy outcomes in women with diabetes mellitus: modern management. Nat Rev Endocrinol. (2019) 15:406–16. doi: 10.1038/s41574-019-0197-3
6. Corcillo A, Quansah DY, Kosinski C, Benhalima K, and Puder JJ. Impact of risk factors on short and long-term maternal and neonatal outcomes in women with gestational diabetes mellitus: A prospective longitudinal cohort study. Front Endocrinol (Lausanne). (2022) 13:866446. doi: 10.3389/fendo.2022.866446
7. Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New Engl J Med. (2009) 361:1339–48. doi: 10.1056/NEJMoa0902430
8. Zhang Y, Chen L, Zhang L, Wu Y, and Li L. Fasting plasma glucose and fetal ultrasound predict the occurrence of neonatal macrosomia in gestational diabetes mellitus. BMC pregnancy childbirth. (2023) 23:269. doi: 10.1186/s12884-023-05594-6
9. Tong JN, Chen YX, Guan XN, Liu K, Yin AQ, Zhang HF, et al. Association between the cut-off value of the first trimester fasting plasma glucose level and gestational diabetes mellitus: a retrospective study from southern China. BMC pregnancy childbirth. (2022) 22:540. doi: 10.1186/s12884-022-04874-x
10. Ikemoto Sato AK, Zerbinatti Pereira R, Moreira Dos Santos PH, Mazzo A, Zajdenverg L, and Negrato CA. Barriers and interventions for postpartum reclassification of glycemic status in women with gestational diabetes mellitus: A scoping review. Diabetes Metab syndrome. (2022) 16:102552. doi: 10.1016/j.dsx.2022.102552
11. Matikainen N and Meri S. Do multiparous women need to work or exercise extra hard to control gestational diabetes? J sport Health Sci. (2022) 11:550–1. doi: 10.1016/j.jshs.2022.03.006
12. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, and Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. (2019) 5:47. doi: 10.1038/s41572-019-0098-8
13. Broughton C and Douek I. An overview of the management of diabetes from pre-conception, during pregnancy and in the postnatal period. Clin Med (Lond). (2019) Sep;19(5):399–402. doi: 10.7861/clinmed.2018-0288
14. Colagiuri S, Falavigna M, Agarwal MM, Boulvain M, Coetzee E, Hod M, et al. Strategies for implementing the WHO diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Diabetes Res Clin Pract. (2014) 103:364–72. doi: 10.1016/j.diabres.2014.02.012
15. He Y, Ma RCW, McIntyre HD, Sacks DA, Lowe J, Catalano PM, et al. Comparing IADPSG and NICE diagnostic criteria for GDM in predicting adverse pregnancy outcomes. Diabetes Care. (2022) 45:2046–54. doi: 10.2337/dc22-0579
16. Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. (2012) 35:780–6. doi: 10.2337/dc11-1790
17. Bernea EG, Uyy E, Mihai DA, Ceausu I, Ionescu-Tirgoviste C, Suica VI, et al. New born macrosomia in gestational diabetes mellitus. Exp Ther Med. (2022) 24:710. doi: 10.3892/etm.2022.11646
18. Song X, Shu J, Zhang S, Chen L, Diao J, Li J, et al. Pre-pregnancy body mass index and risk of macrosomia and large for gestational age births with gestational diabetes mellitus as a mediator: A prospective cohort study in central China. Nutrients. (2022) 14(5):1072. doi: 10.3390/nu14051072
19. Giouleka S, Tsakiridis I, Ralli E, Mamopoulos A, Kalogiannidis I, Athanasiadis A, et al. Diagnosis and management of macrosomia and shoulder dystocia: A comprehensive review of major guidelines. Obstetrical gynecological survey. (2024) 79:233–41. doi: 10.1097/OGX.0000000000001253
20. Pénager C, Bardet P, Timsit J, and Lepercq J. Determinants of the persistency of macrosomia and shoulder dystocia despite treatment of gestational diabetes mellitus. Heliyon. (2020) 6:e03756. doi: 10.1016/j.heliyon.2020.e03756
21. Cilvik SN, Wesolowski SR, Anthony RV, Brown LD, and Rozance PJ. Late gestation fetal hyperglucagonaemia impairs placental function and results in diminished fetal protein accretion and decreased fetal growth. J Physiol. (2021) 599:3403–27. doi: 10.1113/tjp.v599.13
22. Jenner ZB, O’Neil Dudley AE, Mendez-Figueroa H, Ellis VS, Chen HY, and Chauhan SP. Morbidity associated with fetal macrosomia among women with diabetes mellitus. Am J perinatology. (2018) 35:515–20. doi: 10.1055/s-0037-1608811
23. Araujo Júnior E, Peixoto AB, Zamarian AC, Elito Júnior J, and Tonni G. Macrosomia. Best Pract Res Clin obstetrics gynaecology. (2017) 38:83–96. doi: 10.1016/j.bpobgyn.2016.08.003
24. Blumer I, Hadar E, Hadden DR, Jovanovič L, Mestman JH, Murad MH, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2013) 98:4227–49. doi: 10.1210/jc.2013-2465
25. National Collaborating Centre for Ws, Children’s H. National institute for health and care excellence: clinical guidelines. In: Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. National Institute for Health and Care Excellence (UK, London (2015).
26. Weinert LS. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: comment to the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes Care. (2010) 33:e97; author reply e98. doi: 10.2337/dc10-0544
27. Sesmilo G, Prats P, Garcia S, Rodríguez I, Rodríguez-Melcón A, Berges I, et al. First-trimester fasting glycemia as a predictor of gestational diabetes (GDM) and adverse pregnancy outcomes. Acta diabetologica. (2020) 57:697–703. doi: 10.1007/s00592-019-01474-8
28. Sweeting A, Wong J, Murphy HR, and Ross GP. A clinical update on gestational diabetes mellitus. Endocr Rev. (2022) 43:763–93. doi: 10.1210/endrev/bnac003
29. Hod M, Pretty M, and Mahmood T. Joint position statement on universal screening for GDM in Europe by FIGO, EBCOG and EAPM. Eur J Obstet Gynecol Reprod Biol. (2018) 228:329–30. doi: 10.1016/j.ejogrb.2018.05.037
30. Murphy HR. Maternal glycemia and fetal well-being: continuous glucose monitoring and continuous cardiotocography. Diabetes Technol Ther. (2015) 17:603–4. doi: 10.1089/dia.2015.0167
31. Martinez MP, Lin J, Chow T, Chung J, Wang X, and Xiang AH. Maternal gestational diabetes and type 2 diabetes during pregnancy and risk of childhood asthma in offspring. J Pediatr. (2020) 219:173–179.e171. doi: 10.1016/j.jpeds.2019.12.053
32. Bradshaw JM, Ensor SJA, and Lorenz HAL. Gestational diabetes and childhood obesity. Jama. (2019) 321:708. doi: 10.1001/jama.2018.19750
33. Malhotra R, Jakhar B, Bisht K, Kant R, Singh A, Khoiwal K, et al. A comparative morphometric and histological study of human fetus and fetal pancreas in hyperglycemic and normoglycemic mothers. Cureus. (2022) 14:e33008. doi: 10.7759/cureus.33008
34. Lowe WL Jr., Bain JR, Nodzenski M, Reisetter AC, Muehlbauer MJ, Stevens RD, et al. Maternal BMI and glycemia impact the fetal metabolome. Diabetes Care. (2017) 40:902–10. doi: 10.2337/dc16-2452
35. Gupta R, Khoury JC, Altaye M, Jandarov R, and Szczesniak RD. Assessing the relationship between gestational glycemic control and risk of preterm birth in women with type 1 diabetes: A joint modeling approach. J Diabetes Res. (2020) 2020:3074532. doi: 10.1155/2020/3074532
Keywords: gestational diabetes mellitus, fasting blood glucose, macrosomia, threshold effect, pregnancy
Citation: Li Y, Zhu Y, Zheng W, Gao Y, Huang R, Yang T and Chen Y (2025) The threshold effect of fasting blood glucose levels on the risk of delivering macrosomia in gestational diabetes mellitus patients. Front. Endocrinol. 16:1587306. doi: 10.3389/fendo.2025.1587306
Received: 04 March 2025; Accepted: 13 May 2025;
Published: 29 May 2025.
Edited by:
Jan Tesarik, MARGen Clinic, SpainReviewed by:
Cosmin Mihai Vesa, University of Oradea, RomaniaMartin Ming Him Wong, University College London, United Kingdom
Copyright © 2025 Li, Zhu, Zheng, Gao, Huang, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Chen, Y2ljaTE4NzM2ODBAMTYzLmNvbQ==
 Yuan Li
Yuan Li Yumin Zhu
Yumin Zhu Tingting Yang
Tingting Yang