- 1Department of Metabolic Medicine, Graduate School of Medicine, The University of Osaka, Yamadaoka, Suita, Osaka, Japan
- 2Department of Pediatrics, The University of Osaka Graduate School of Medicine, Yamadaoka, Suita, Osaka, Japan
- 3Laboratory of Children’s Health and Genetics, Division of Health Sciences, The University of Osaka Graduate School of Medicine, Yamadaoka, Suita, Osaka, Japan
- 4Department of Genetic Counseling, The University of Osaka Hospital, Yamadaoka, Suita, Osaka, Japan
- 5Department of Adipose Management, Graduate School of Medicine, The University of Osaka, Yamadaoka, Suita, Osaka, Japan
Glial cells missing transcription factor 2 (GCM2) is one of the genes responsible for isolated hypoparathyroidism. Most cases of hypoparathyroidism caused by GCM2 pathogenic variants result from homozygous or compound heterozygous loss-of-function variants, with only a limited number of heterozygous variants reported. A 24-year-old woman with recurrent tonic convulsions was admitted to our hospital. Laboratory findings revealed severe hypocalcemia (1.28 mmol/L), normophosphatemia (1.36 mmol/L), and low intact parathyroid hormone levels (0.84 pmol/L). Based on this, hypoparathyroidism was diagnosed. Comprehensive gene analysis using next-generation sequencing revealed a novel heterozygous frameshift pathogenic variant (c.1366delG, p.Ala456ProfsTer75) in the GCM2 gene (NM_004752.4). Sanger sequencing of the patient and parents confirmed de novo occurrence. This variant is predicted to exert a dominant-negative effect by impairing GCM2 function. This case provides further evidence that heterozygous GCM2 variants can lead to hypoparathyroidism. Additionally, it underscores the importance of genetic testing for hypoparathyroidism of unknown etiology even in adults.
Introduction
Hypoparathyroidism is a rare endocrine disorder characterized by hypocalcemia and hyperphosphatemia caused by insufficient parathyroid hormone (PTH). It has several causes, such as neck surgery, radiotherapy, autoimmune disorders, or hypomagnesemia (1, 2). Genetic abnormalities are rare causes of hypoparathyroidism and are generally categorized into syndromic forms, such as 22q11.2 deletion syndrome, and a non-syndromic form, namely isolated hypoparathyroidism (3, 4). Several genes associated with the development or function of the parathyroid gland, including PTH and the calcium-sensing receptor (CASR), have been implicated in the pathogenesis of isolated hypoparathyroidism, which can follow autosomal dominant, autosomal recessive, or X-linked inheritance patterns (3, 4). One of the genes responsible for isolated hypoparathyroidism is glial cells missing transcription factor 2 (GCM2), which is located on chromosome 6p24.2 (3–5). GCM2 is comprised of five exons and encodes a zinc finger-type transcription factor consisting of 506 amino acids (5). This protein includes a DNA-binding domain, transactivation domain 1, an inhibitory domain, and transactivation domain 2 (TAD2), which are organized sequentially from the N-terminal region (5, 6). GCM2 plays a critical role in the development and maintenance of the parathyroid gland (7–10), and several pathogenic variants associated with isolated hypoparathyroidism have been identified. Most cases of hypoparathyroidism caused by GCM2 pathogenic variants result from homozygous or compound heterozygous loss-of-function variants, with a limited number of heterozygous variants reported (11–14). Herein, we present the case of a patient diagnosed with isolated hypoparathyroidism with a novel de novo heterozygous frameshift pathogenic variant of the GCM2 gene.
Case presentation
A 24-year-old woman was diagnosed with hypoparathyroidism and admitted to our hospital. She was born full term with no perinatal or infantile developmental abnormalities. At 12 years of age, QT prolongation was observed on an electrocardiogram during a medical checkup; however, further examination was not performed. Since 19 years of age, she has experienced recurrent painful muscle spasms in cold weather and numbness in the extremities following episodes of diarrhea. At 22 years of age, she often experienced difficulty in moving because of numbness and rigidity, although these symptoms resolved spontaneously within a short time. At 24 years of age, she was urgently admitted to a local hospital with repeated tonic convulsions following a viral infection. Laboratory findings revealed severe hypocalcemia (1.28 mmol/L [ref: 2.15–2.57 mmol/L]), normophosphatemia (1.36 mmol/L [ref: 0.94–1.55 mmol/L]), mild hypomagnesemia (0.62 mmol/L [ref: 0.74–0.99 mmol/L]), and a low intact PTH level (0.84 pmol/L [ref: 2.72–7.97 pmol/L]). The patient’s renal function was normal (estimated glomerular filtration rate, 85.1 mL/min/1.73 m2). She was diagnosed with primary hypoparathyroidism and started on oral and intravenous electrolyte supplementation. Subsequently, she was transferred to our hospital for further evaluation and treatment.
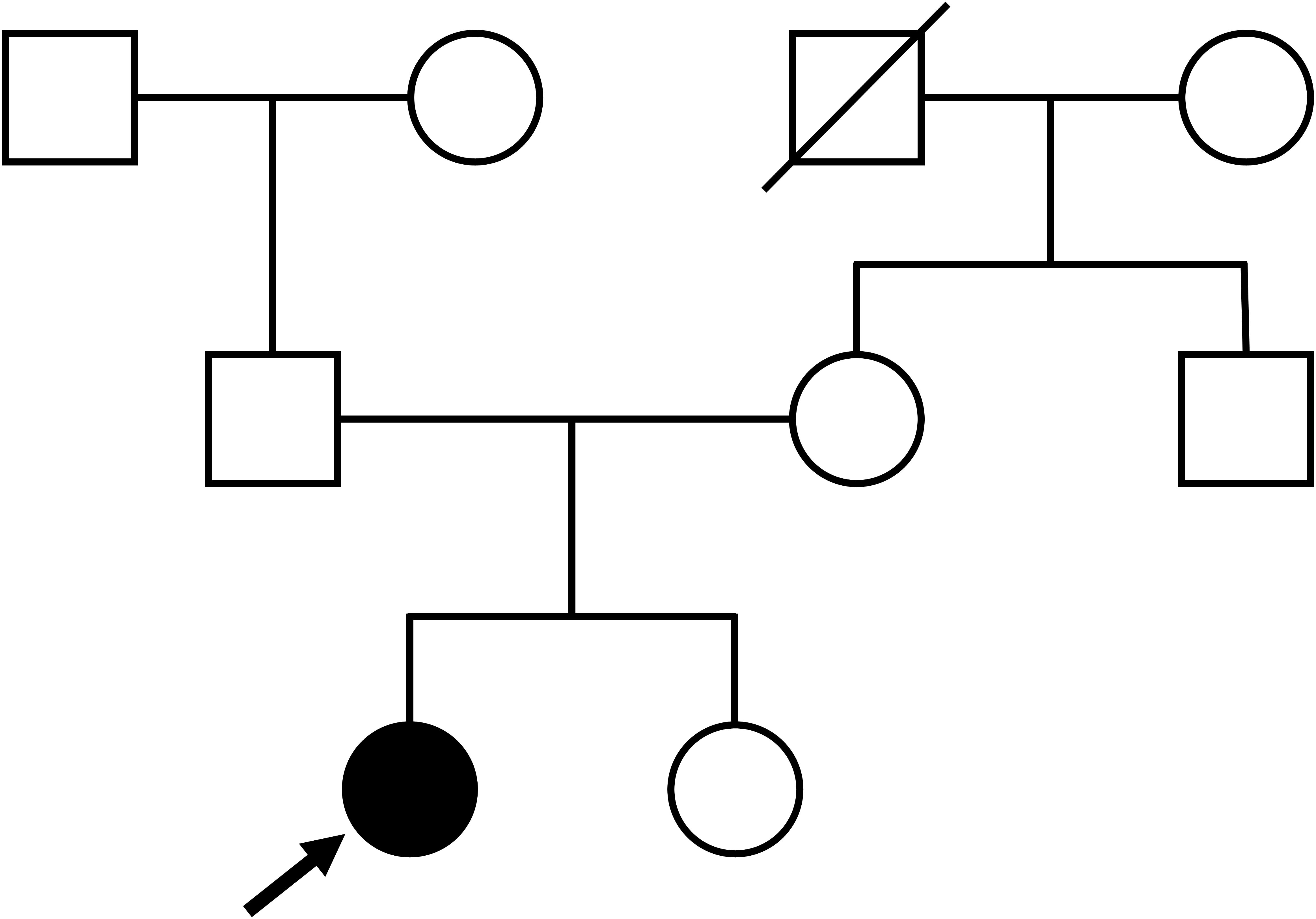
On admission, the patient’s height, weight, and body mass index were 149.0 cm, 58.0 kg, and 26.1 kg/m2, respectively. She had no medical history of neck surgery or autoimmune disease and had not consumed alcohol. The pedigree diagram is shown in Figure 1. The patient had one younger sister. She had no family history of calcium metabolism or other endocrine disorders. Physical examination revealed no abnormal findings, such as abnormal facial appearance or hearing loss. Computed tomography (CT) of the head revealed ectopic calcifications in the bilateral caudate nucleus, putamen, globus pallidus, and cerebellar dentate nucleus. Abdominal CT showed no renal malformations or nephrocalcinosis. Bone mineral density was normal (T-score: 1.2 at the lumbar spine, 1.9 at the femoral neck). Hypocalcemia persisted despite the normalization of serum magnesium levels, eliminating hypomagnesemia as the cause of hypoparathyroidism. Although her 1,25-dihydroxyvitamin D levels were within the reference range (62.4 pmol/L [ref: 48.0–144.0 pmol/L]), low 25-hydroxyvitamin D (42.2 nmol/L [ref: >74.9 mmol/L]) was indicative that vitamin D deficiency may have worsened the hypocalcemia. Trends in treatment and laboratory data are presented in Figure 2. We continued to supplement the patient with calcium, magnesium, and vitamin D, which resulted in appropriate serum calcium levels. She was discharged with calcium carbonate 500 mg/day, magnesium oxide 660 mg/day, and alfacalcidol 2 μg/day. Alfacalcidol was chosen as the vitamin D analog because of its longer half-life and less frequent dosing, which improved adherence compared with that by calcitriol. Following further adjustments to her supplementation regimen, her serum calcium levels remained stable near the lower limit of the reference range without hyperphosphatemia, hypercalciuria, renal impairment, or symptom recurrence.
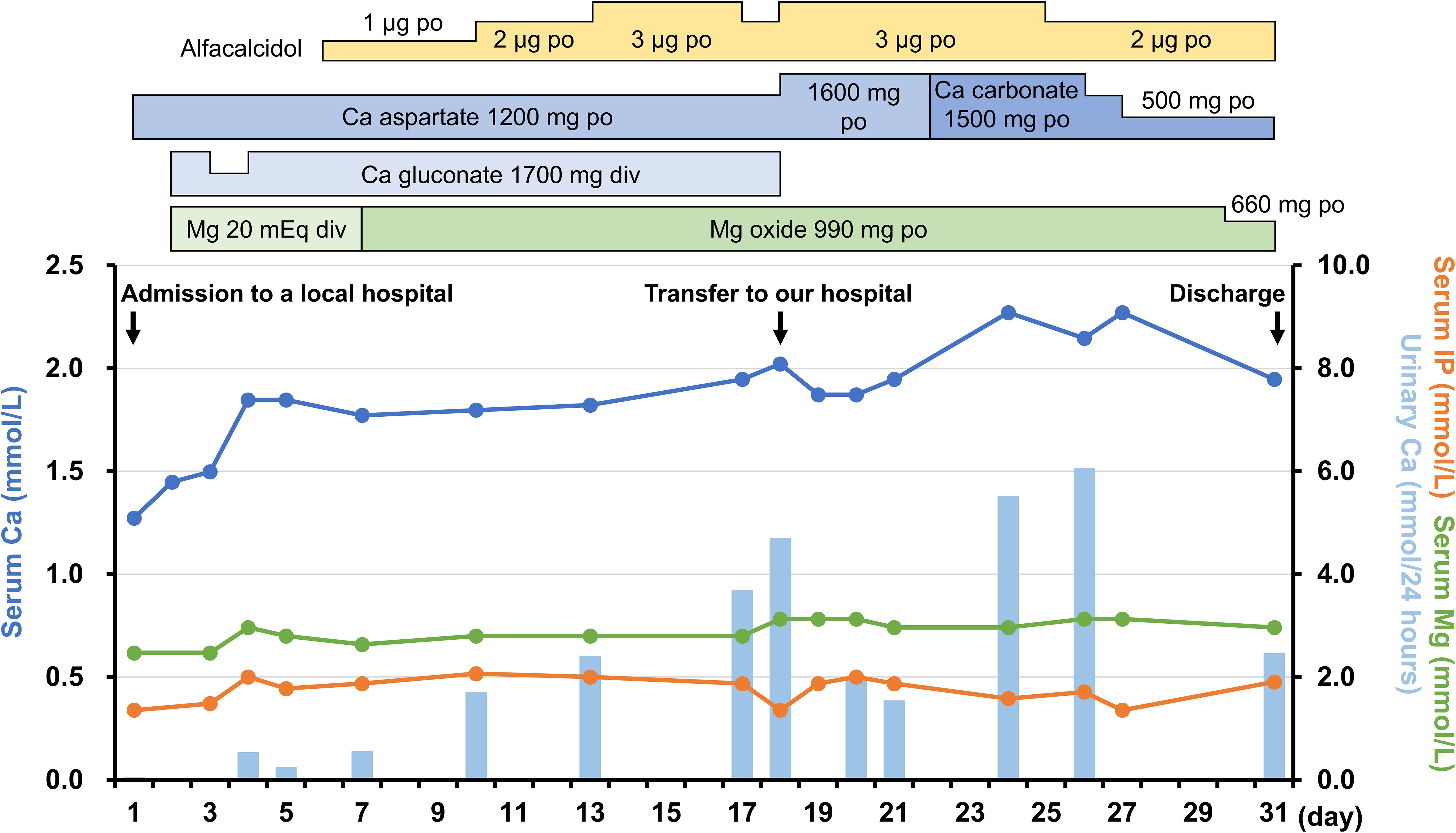
Figure 2. Trends in treatment and laboratory data from the previous hospital admission to discharge at our hospital. The 24-hour urinary calcium excretion was estimated using spot urine samples based on urinary creatinine levels. Ca, calcium; IP, phosphorus; Mg, magnesium.
Although she had no family history of hypoparathyroidism or syndromic features, the presence of QT prolongation and muscle symptoms during her youth suggests that genetic abnormalities may have contributed to the hypoparathyroidism. The coding and splicing regions of 22 major genes associated with calcium metabolism disorders were analyzed using the NextSeq Sequencing System (Illumina, San Diego, CA, USA) at the Kazusa DNA Research Institute (Kisarazu, Japan). The genes analyzed included CASR, GNA11, GCM2, TBX1, TBX2, NEBL, CHD7, SEMA3E, GATA3, TBCE, FAM111A, PTH, SOX3, AIRE, NLRP5, HADHA, HADHB, ACADM, DHCR7, CLDN16, CLDN19, and TRPM6. As a result, we identified a novel heterozygous frameshift variant (c.1366delG, p.Ala456ProfsTer75) of the GCM2 gene (NM_004752.4). The variant was located within the TAD2 region of exon 5 and caused a frameshift, replacing the C-terminal end of the putative TAD2 with 75 unrelated amino acid residues. This variant has not been previously reported in the literature, ClinVar, or the Genome Aggregation Database. Sanger sequencing confirmed the variant in the patient, but it was absent in the parents, who exhibited normal calcium levels, indicating that it was de novo (Figure 3). According to the American College of Medical Genetics and Genomics guidelines (15), this heterozygous GCM2 frameshift variant was classified as pathogenic (PVS1+PS2+PM2) and determined to be the cause of hypoparathyroidism in this patient.
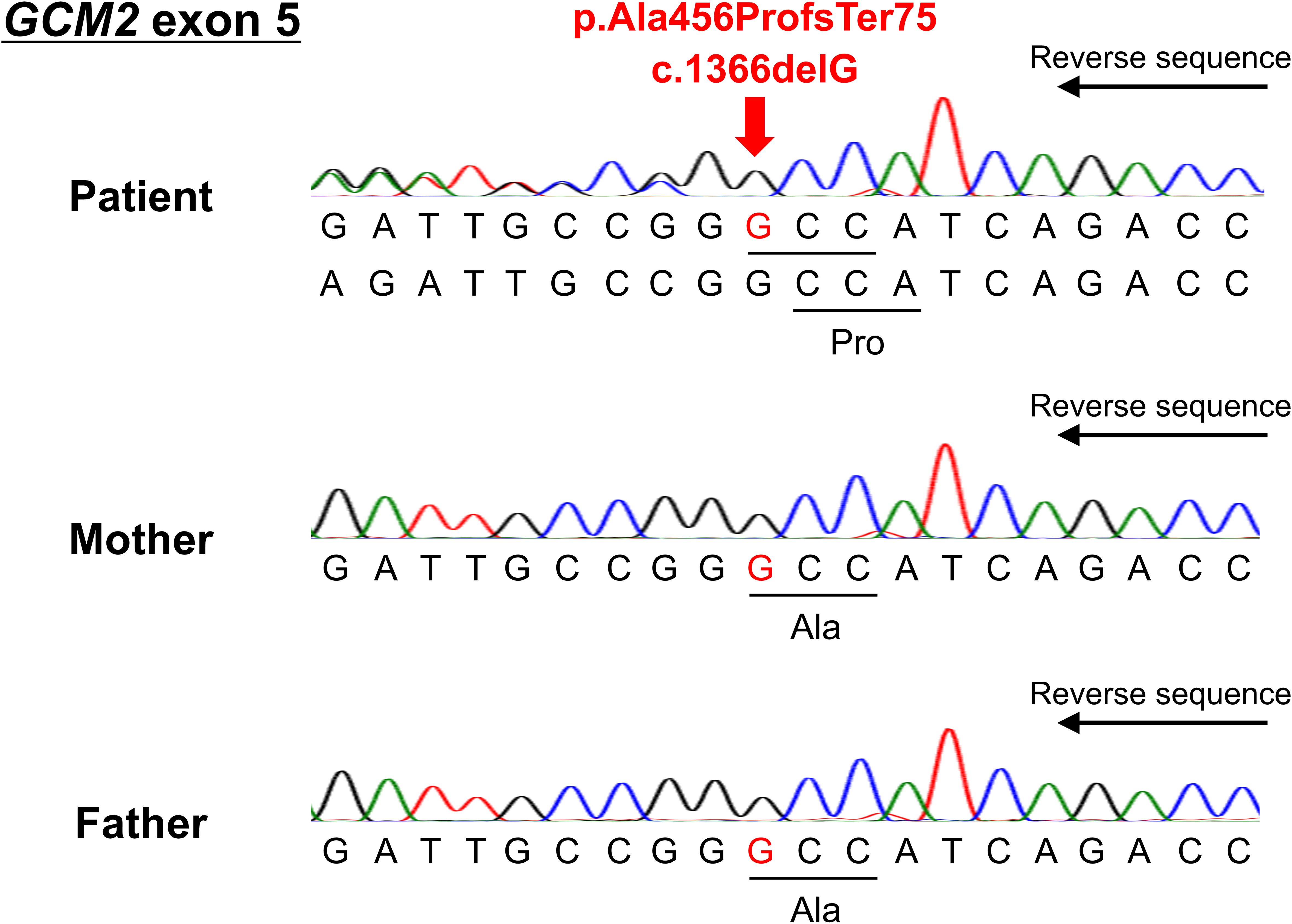
Figure 3. Reverse sequencing results of exon 5 of the GCM2 gene for the patient and her parents. The deletion of guanine at position 1366 resulted in a frameshift after amino acid 456 (arrow).
Discussion
Herein, we present the case of a woman diagnosed with hypoparathyroidism. Although the patient had no family history of the disease or other features suggestive of genetic abnormalities, genetic testing revealed a novel de novo heterozygous frameshift pathogenic variant of GCM2 that contributed to the development of hypoparathyroidism.
In 1999, GCM2 was first identified in humans as an ortholog of the Drosophila glial cells missing gene (5). In mammals, GCM2 is specifically expressed in developing and mature PTH-secreting cells of the parathyroid glands, and Gcm2 knockout mice lack parathyroid glands (7). Additionally, conditional Gcm2 knockout in adult mice inhibited cell proliferation, increased cell death in the parathyroid glands, and caused hypoparathyroidism (8). GCM2 contributes to the maintenance of parathyroid function by regulating CASR and PTH gene expression (9, 10). These findings highlight that GCM2 plays a critical role in the development and maintenance of the parathyroid glands.
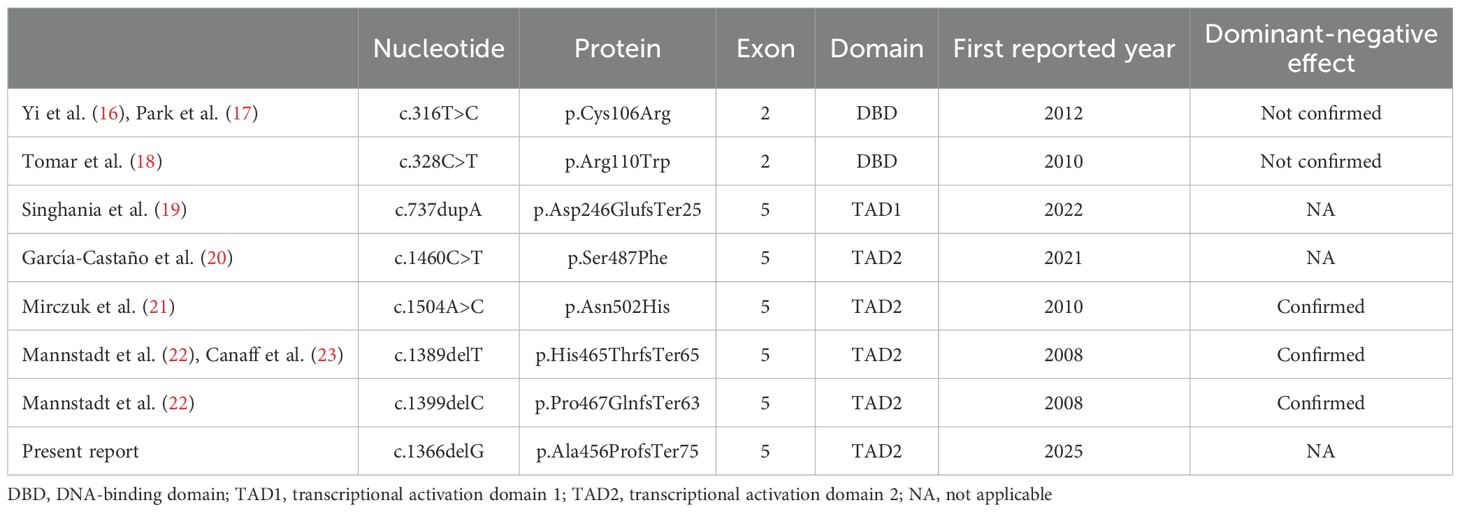
Hypoparathyroidism caused by GCM2 pathogenic variants was first reported in 2001 (11). Thereafter, several pathogenic variants were identified with no specific variant hotspots. Most reported cases involve homozygous or compound heterozygous loss-of-function variants of GCM2, leading to autosomal recessive inheritance (11–14). This observation is consistent with findings in heterozygous Gcm2 knockout mice, which exhibit a normal phenotype (7), suggesting that simple haploinsufficiency may not be sufficient to cause disease. In contrast, seven heterozygous GCM2 variants associated with hypoparathyroidism have been reported to date (Table 1) (16–23). Since no parent-specific genetic imprinting has been reported for the GCM2 gene, heterozygous variants must exert a dominant-negative effect to cause the disease. However, two heterozygous missense variants in the DNA-binding domain (c.316T>C [p.Cys106Arg] and c.328C>T [p.Arg110Trp]) have not been shown to exert dominant-negative effects in in vitro assays (16–18). Similarly, two other heterozygous variants (c.737dupA [p.Asp246GlufsTer25] and c.1460C>T [p.Ser487Phe]) have yet to be evaluated for these effects (19, 20). Therefore, these four variants may require additional alterations in another allele (compound heterozygosity) or other genes (digenic/polygenic inheritance) to manifest a pathogenic phenotype, although haploinsufficiency cannot be completely ruled out. Consequently, only three heterozygous variants have been confirmed to cause hypoparathyroidism via dominant-negative effects, as demonstrated by luciferase reporter assays (Table 1) (21–23). All three pathogenic variants were located in the TAD2 region, which is critical for GCM2 transcriptional activity. Two of these variants are frameshifts that replace approximately 50% of the C-terminus of putative TAD2 with unrelated amino acid residues, similar to the variant identified in the present patient (Figure 4) (22, 23). Although the variant in our patient was not evaluated using an in vitro assay, its high similarity to previously reported pathogenic variants strongly suggests that the variant protein exerts a dominant-negative effect, impairing GCM2 function and leading to hypoparathyroidism. The absence of this variant in the patient’s normocalcemic parents and the lack of abnormalities in other candidate genes further support the pathogenicity of this heterozygous variant.
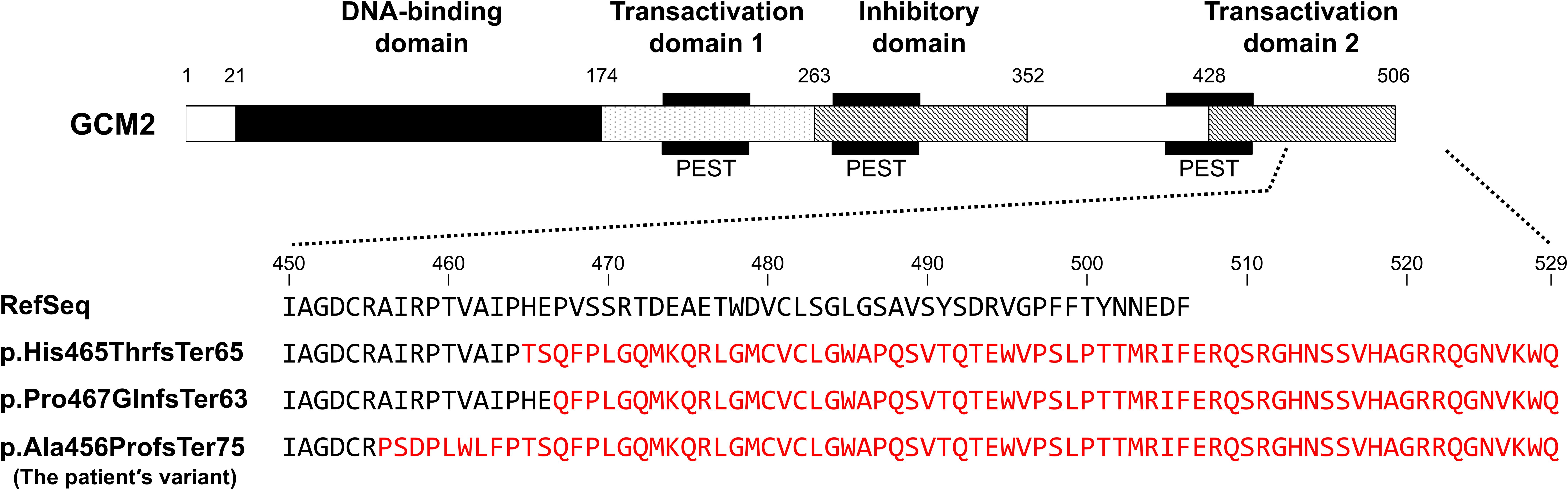
Figure 4. A schematic diagram of the GCM2 protein and comparison of GCM2 amino acid sequences, including the reference sequence (RefSeq), previously reported frameshift pathogenic variants (p.His465ThrfsTer65 [reference: (22, 23)] and p.Pro467GlnfsTer63 [reference (22):]) with confirmed dominant-negative effects and the patient’s variant (p.Ala456ProfsTer75). Mutated amino acids are highlighted in red.
In contrast, gain-of-function GCM2 variants have been reported to cause autosomal dominant hyperparathyroidism 4 (24), suggesting that distinct GCM2 variants can lead to opposing phenotypes via different molecular mechanisms. This underscores the critical role of GCM2 in regulating parathyroid function and the importance of understanding how different variants affect its activity.
Most patients with hypoparathyroidism without a family history, diagnosed during adulthood, may not undergo genetic testing in clinical practice. However, patients with isolated hypoparathyroidism exhibit a wide spectrum of clinical features, ranging from asymptomatic mild hypocalcemia to symptomatic severe hypocalcemia, even among individuals with the same pathogenic variant (4). As a result, while many cases of isolated hypoparathyroidism are diagnosed in early childhood owing to typical symptoms, some cases may remain undiagnosed until adulthood because of mild or nonspecific symptoms (4). Consequently, genetic abnormalities may have been under-diagnosed in some adult patients classified as having “idiopathic” hypoparathyroidism. Currently, there are no standardized criteria for patient selection or preferred methodologies for genetic testing. However, precise genetic testing is essential not only to identify the underlying cause of unknown etiology, but also to provide appropriate genetic counseling, particularly for patients of reproductive age. Therefore, genetic testing should be considered in patients with hypoparathyroidism of unknown etiology, including adults. Furthermore, a comprehensive analysis of the responsible genes using next-generation sequencing, as was performed in the present patient, may be especially valuable for uncovering the genetic causes of the disease.
This study has certain limitations. First, we did not assess whether the variant in our patient exerts a dominant-negative effect using an in vitro assay. Second, we cannot entirely rule out the presence of large deletions or structural variations in another allele or other genes, which may be challenging to detect using next-generation sequencing.
In summary, we report a case of isolated hypoparathyroidism in an adult woman, caused by a novel de novo heterozygous frameshift pathogenic variant of the GCM2 gene. This case provides further evidence that heterozygous GCM2 variants can lead to hypoparathyroidism. Additionally, it underscores the importance of genetic testing for hypoparathyroidism of unknown etiology even in adults.
Data availability statement
The datasets presented in this article are not publicly available due to privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The University of Osaka Hospital Ethical Review Board (approval number 700-14). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AO: Data curation, Investigation, Visualization, Writing – original draft. YoO: Conceptualization, Methodology, Project administration, Visualization, Writing – original draft. TH: Conceptualization, Data curation, Investigation, Writing – review & editing. MF: Formal analysis, Investigation, Resources, Writing – review & editing. YaO: Formal analysis, Investigation, Resources, Writing – review & editing. YT: Data curation, Investigation, Writing – review & editing. SK: Data curation, Investigation, Writing – review & editing. KMu: Methodology, Writing – review & editing. KMi: Methodology, Writing – review & editing. KY: Formal analysis, Resources, Writing – review & editing. TK: Formal analysis, Resources, Writing – review & editing. AF: Supervision, Writing – review & editing. IS: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by a Health and Labour Sciences Research Grant for Research on Intractable Diseases from the Ministry of Health, Labour, and Welfare of Japan (grant no. 24FC1015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fukumoto S, Namba N, Ozono K, Yamauchi M, Sugimoto T, Michigami T, et al. Causes and differential diagnosis of hypocalcemia–recommendation proposed by expert panel supported by ministry of health, labour and welfare, Japan. Endocr J. (2008) 55:787–94. doi: 10.1507/endocrj.k08e-076
2. Pasieka JL, Wentworth K, Yeo CT, Cremers S, Dempster D, Fukumoto S, et al. Etiology and pathophysiology of hypoparathyroidism: a narrative review. J Bone Miner Res. (2022) 37:2586–601. doi: 10.1002/jbmr.4714
3. Gordon RJ and Levine MA. Genetic disorders of parathyroid development and function. Endocrinol Metab Clin North Am. (2018) 47:809–23. doi: 10.1016/j.ecl.2018.07.007
4. Mannstadt M, Cianferotti L, Gafni RI, Giusti F, Kemp EH, Koch CA, et al. Hypoparathyroidism: genetics and diagnosis. J Bone Miner Res. (2022) 37:2615–29. doi: 10.1002/jbmr.4667
5. Kanemura Y, Hiraga S, Arita N, Ohnishi T, Izumoto S, Mori K, et al. Isolation and expression analysis of a novel human homologue of the Drosophila glial cells missing (gcm) gene. FEBS Lett. (1999) 442:151–6. doi: 10.1016/s0014-5793(98)01650-0
6. Tuerk EE, Schreiber J, and Wegner M. Protein stability and domain topology determine the transcriptional activity of the mammalian glial cells missing homolog, GCMb. J Biol Chem. (2000) 275:4774–82. doi: 10.1074/jbc.275.7.4774
7. Günther T, Chen ZF, Kim J, Priemel M, Rueger JM, Amling M, et al. Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature. (2000) 406:199–203. doi: 10.1038/35018111
8. Yamada T, Tatsumi N, Anraku A, Suzuki H, Kamejima S, Uchiyama T, et al. Gcm2 regulates the maintenance of parathyroid cells in adult mice. PLoS One. (2019) 14:e0210662. doi: 10.1371/journal.pone.0210662
9. Mizobuchi M, Ritter CS, Krits I, Slatopolsky E, Sicard G, and Brown AJ. Calcium-sensing receptor expression is regulated by glial cells missing-2 in human parathyroid cells. J Bone Miner Res. (2009) 24:1173–9. doi: 10.1359/jbmr.090211
10. Kawahara M, Iwasaki Y, Sakaguchi K, Taguchi T, Nishiyama M, Nigawara T, et al. Involvement of GCMB in the transcriptional regulation of the human parathyroid hormone gene in a parathyroid-derived cell line PT-r: effects of calcium and 1,25(OH)2D3. Bone. (2010) 47:534–41. doi: 10.1016/j.bone.2010.05.031
11. Ding C, Buckingham B, and Levine MA. Familial isolated hypoparathyroidism caused by a mutation in the gene for the transcription factor GCMB. J Clin Invest. (2001) 108:1215–20. doi: 10.1172/JCI13180
12. Bowl MR, Mirczuk SM, Grigorieva IV, Piret SE, Cranston T, Southam L, et al. Identification and characterization of novel parathyroid-specific transcription factor Glial Cells Missing Homolog B (GCMB) mutations in eight families with autosomal recessive hypoparathyroidism. Hum Mol Genet. (2010) 19:2028–38. doi: 10.1093/hmg/ddq084
13. Doyle D, Kirwin SM, Sol-Church K, and Levine MA. A novel mutation in the GCM2 gene causing severe congenital isolated hypoparathyroidism. J Pediatr Endocrinol Metab. (2012) 25:741–6. doi: 10.1515/jpem-2012-0080
14. Thambundit A, Martinez-Agosto JA, Kianmahd Shamshoni J, Winer KK, and Mittelman SD. Three siblings with familial isolated hypoparathyroidism: a diagnostic journey from CASR to novel GCM2 variant. JCEM Case Rep. (2024) 2:luae185. doi: 10.1210/jcemcr/luae185
15. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
16. Yi HS, Eom YS, IeB P, Lee S, Hong S, Jüppner H, et al. Identification and characterization of C106R, a novel mutation in the DNA-binding domain of GCMB, in a family with autosomal-dominant hypoparathyroidism. Clin Endocrinol (Oxf). (2012) 76:625–33. doi: 10.1111/j.1365-2265.2011.04256.x
17. Park SY, Eom YS, Choi B, Yi HS, Yu SH, Lee K, et al. Genetic and clinical characteristics of korean patients with isolated hypoparathyroidism: from the Korean hypopara registry study. J Korean Med Sci. (2013) 28:1489–95. doi: 10.3346/jkms.2013.28.10.1489
18. Tomar N, Bora H, Singh R, Gupta N, Kaur P, Chauhan SS, et al. Presence and significance of a R110W mutation in the DNA-binding domain of GCM2 gene in patients with isolated hypoparathyroidism and their family members. Eur J Endocrinol. (2010) 162:407–21. doi: 10.1530/EJE-09-0303
19. Singhania P, Ghosh A, Das D, Bhattacharjee R, Roy A, and Chowdhury S. Glial cell missing homolog 2 mutation causing severe hypoparathyroidism: report of two cases with novel mutations. J Endocr Soc. (2022) 7:bvac166. doi: 10.1210/jendso/bvac166
20. García-Castaño A, Madariaga L, Gómez-Conde S, Cordo CLR, López-Iglesias M, Garcia-Fernández Y, et al. Five patients with disorders of calcium metabolism presented with GCM2 gene variants. Sci Rep. (2021) 11:2968. doi: 10.1038/s41598-021-82661-y
21. Mirczuk SM, Bowl MR, Nesbit MA, Cranston T, Fratter C, Allgrove J, et al. A missense glial cells missing homolog B (GCMB) mutation, Asn502His, causes autosomal dominant hypoparathyroidism. J Clin Endocrinol Metab. (2010) 95:3512–16. doi: 10.1210/jc.2009-2532
22. Mannstadt M, Bertrand G, Muresan M, Weryha G, Leheup B, Pulusani SR, et al. Dominant-negative GCMB mutations cause an autosomal dominant form of hypoparathyroidism. J Clin Endocrinol Metab. (2008) 93:3568–76. doi: 10.1210/jc.2007-2167
23. Canaff L, Zhou X, Mosesova I, Cole DE, and Hendy GN. Glial cells missing-2 (GCM2) transactivates the calcium-sensing receptor gene: effect of a dominant-negative GCM2 mutant associated with autosomal dominant hypoparathyroidism. Hum Mutat. (2009) 30:85–92. doi: 10.1002/humu.20827
Keywords: hypoparathyroidism, GCM2, heterozygous, novel variant, case report
Citation: Onishi A, Obata Y, Hayakawa T, Fujiwara M, Ohata Y, Tamura Y, Kawata S, Mukai K, Miyashita K, Yamamoto K, Kubota T, Fukuhara A and Shimomura I (2025) A novel heterozygous frameshift pathogenic variant in GCM2 gene causing isolated hypoparathyroidism: a case report. Front. Endocrinol. 16:1589182. doi: 10.3389/fendo.2025.1589182
Received: 07 March 2025; Accepted: 10 June 2025;
Published: 27 June 2025.
Edited by:
Robert Daniel Blank, Garvan Institute of Medical Research, AustraliaReviewed by:
Fang Liu, The 980th Hospital of the People’s Liberation Army Joint Service Support Force, ChinaMichael Bennett, St. Vincent’s Health Network Sydney (SVHNS), Australia
Copyright © 2025 Onishi, Obata, Hayakawa, Fujiwara, Ohata, Tamura, Kawata, Mukai, Miyashita, Yamamoto, Kubota, Fukuhara and Shimomura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshinari Obata, b2JhdGEteW9zaGluYXJpQGVuZG1ldC5tZWQub3Nha2EtdS5hYy5qcA==
 Ayano Onishi1
Ayano Onishi1 Yoshinari Obata
Yoshinari Obata Tomoaki Hayakawa
Tomoaki Hayakawa Yasuhisa Ohata
Yasuhisa Ohata Satoshi Kawata
Satoshi Kawata Kosuke Mukai
Kosuke Mukai Kenichi Yamamoto
Kenichi Yamamoto Takuo Kubota
Takuo Kubota Atsunori Fukuhara
Atsunori Fukuhara Iichiro Shimomura
Iichiro Shimomura