- 1Department of Pharmacology and Therapeutics, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
- 2Metabolic, Cardiovascular, and Aging Cluster, Indonesia Medical Education and Research Institute (IMERI), Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
- 3Master Program in Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
- 4Department of Cardiovascular Aging, National Cerebral and Cardiovascular Center Research Institute, Osaka, Japan
- 5Institute of Medical Education and Research Indonesia, Jakarta, Indonesia
- 6Department of Medical Pharmacy, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
- 7Drug Development Research Center (DDRC), Indonesian Medical Education and Research Institute, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
- 8Doctoral Program in Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
- 9Clinical Pharmacology Specialist Study Program, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Insulin Resistance (IR) is a complication that frequently occurs in obesity. The inflammation-mediated senescence in White Adipose Tissue (WAT) is important in obesity-induced IR. Centella asiatica (CA) is a potential medicinal plant with anti-aging and anti-obesity properties. Here, we explored the effect of CA on obesity-mediated IR in mice fed with a High Fat-High Fructose (HFHF) diet and treated simultaneously with CA at 150 mg/kgBW (CA150) or 300 mg/kgBW (CA300). The total body mass and visceral WAT weight in both CA groups decreased in comparison with HFHF group alone. We demonstrated that HFHF-diet mice treated with CA300 improved insulin sensitivity and enhanced Irs-1 activation in WAT. CA300, but not CA150, prevented the senescence phenotype in WAT, represented by decreased Senescence-associated beta-galactosidase (SA-β-Gal) activity and diminished Cdkn2a and Cdkn1a expression levels at mRNA level. Mechanistically, CA300 prevented the enhancement of Il6 and Il1b mRNA expression levels and macrophage activity in the immunostaining analysis of WAT. In vitro, RAW264.7 cells stimulated with high glucose and low dose of Lipopolysaccharides (LPS) also confirmed that CA 200 μg/ml alleviated the expression levels of M1 markers such as Ccl2, Il6, Il1b, and Tnf at mRNA level. Our data indicated that CA has therapeutic potential for obesity-mediated IR by suppressing proinflammatory M1 macrophages and preventing inflammation-induced senescence in WAT.
1 Introduction
Obesity and its complications significantly contribute to an increase in morbidity and a decrease in quality of life (1). Furthermore, the mortality rate increased by 27% and 93% for grade 1 and 2 obesity, respectively (2). Obesity is a condition of chronic low-grade inflammation that leads to numerous metabolic disorders, such as insulin resistance (3). Various mechanisms contribute to the development of insulin resistance, including increased concentrations of fatty acids, glycerol, hormonal imbalances, and inflammatory cytokines (4).
Obesity, often associated with a high-fat diet, leads to excessive accumulation of triglycerides (TG). TG can build up within adipocytes, resulting in adipocyte hypertrophy and the increased pro-inflammatory cytokines, such as Il-1b, Il6, and Ccl2. Adipocyte hypertrophy exhibited elevated oxygen consumption, which contributes to adipocyte extracellular matrix remodeling and fibrosis. Altogether, these changes lead to a vicious cycle of adipose tissue dysfunction (5, 6). Adipose tissue dysfunction and insulin resistance are key contributors to cellular senescence. Both conditions can lead to increased reactive oxygen species (ROS) production, activation of the p53/p21 signaling pathway, and elevated Tnf/Il6 secretion and β-galactosidase activity (7). Specifically, cellular senescence, a cell with a proliferative arrest state, secretes various of pro-inflammatory cytokines, including Il-1b and Il6, chemokines, and growth factors to alter tissue microenvironments (8). We previously revealed that senescent endothelial cells induce adipocyte senescence in WAT, reducing Irs1 expression-mediating systemic insulin resistance through Il1a-mediating SASP secretion in EC-specific progeroid mice (9).
Centella asiatica (CA), or Indian pennywort, has been traditionally used to increase brain function, lower blood sugar levels, improve blood circulation, and for other uses (10). CA has four main triterpenes (madecassoside, asiaticoside, madecassic acid, and asiatic acid) that exhibit anti-inflammatory, anti-oxidative, and anti-apoptotic properties (11). These effects have been observed in various clinical trials, including in venous hypertension (12), diabetic neuropathy (13), and vascular cognitive impairment (14). Moreover, CA antioxidant properties could prevent aging in human dermal fibroblasts (15). In this study, we explored the effects of CA supplementation on the metabolic phenotypes in obesity-induced insulin-resistant mouse models. We revealed the benefits of dietary CA on this model by alleviating the senescence phenotype.
2 Methods and analysis
2.1 Diets and CA preparation
Research diets® (D12492) (Rodent Diet with 60% kcal% fat) and D12450J (Rodent Diet With 10 kcal% Fat) from Research Diets, USA were used as a high-fat diet and normal (control) diet, respectively. Diet composition can be seen in Supplementary Figure 1. Fructose 25% was made by diluting 25 mg fructose powder (Sweet Food Supply, Indonesia) in 100 mL of drinking water. CA ethanol extract was bought from PT. Plamed (China) and produced following the manufacturer’s procedures. The extraction process of CA began with the crushing of raw plant material into fine powder. Then, the powder was stirred in boiling water and filtered using a plate and frame filter press to separate the solids. The liquid extract was then purified using column chromatography with ethanol as the eluent. The ethanol fraction was concentrated to collect the final extract, which was used for further analysis. CA extract with a concentration of 7.5 mg/mL was made by diluting 22.5 mg of CA ethanol extract into 3 mL of distilled water. The detailed extraction procedure can be found in Supplementary Figure 2.
2.2 Animal preparations
This study adheres to the principles and standards of animal experiments and has been approved by our institution’s ethics committee with ethical clearance number KET-562/UN2.F1/ETIK/PPM.00.02/2022. Twelve-week DDY strain mice (Biofarma, Indonesia) were housed at a room temperature of 250C and a 12-hour light and dark cycle. The mice were divided into four groups of eight, six of which were used for an in vivo study. This study was conducted for 15 weeks which the mice were divided into a control group that was fed with a standard diet and water ad libitum; the negative control (HFHF) group was fed with a HFHF diet; treatment group 1 was fed with a HFHF diet and 150 mg/kgBW/day of CA (CA150) via oral gavage; and treatment group 2 was fed with a HFHF diet and 300 mg/kgBW/day of CA (CA300) via oral gavage.150 mg/kgBW/day and 300 mg/kgBW/day were chosen based on a previous reference of CA in D-galactose-induced senescence mice (16).
2.3 Body weight and white adipose tissue weight
The weight of each mouse from every group was measured following a 15-week intervention period. At the end of the experiment, the mice were euthanized by anesthesia using intraperitoneal cocktail injection of ketamine at 100 mg/kg and xylazine at 10 mg/kg. Adipose tissues, skeletal muscle tissues, and liver tissues were obtained for analysis. Afterwards, the white adipose tissues located in the inguinal, retroperitoneal, mesenteric, and gonadal regions were individually assessed in terms of their weight.
2.4 Insulin tolerance test and glucose tolerance test
At the end of 13th week of the experiment, an intraperitoneal ITT was conducted by administering 5 IU/kgBW human insulin intraperitoneally. At the end of the 14th week of the experiment, a GTT was conducted by administering 5 µL/gBW glucose (3 g of glucose in 10 mL of PBS) following an 8-hour fasting period. In both procedures, blood glucose levels were measured at different interval time points, such as baseline, 15, 30, 60, 90, and 120 minutes after insulin or glucose induction, via cutting off the tip of the tail.
2.5 Real-time quantitative reverse transcription polymerase chain reaction analysis
Mice were sacrificed immediately after the GTT test. Adipose tissue was harvested, homogenized by ultra turrax tissue homogenizer (T-25 Janke and Kullkel, UK) and centrifuged by H-103N centrifugator (Kokusan H-103N, Japan). Total RNA was isolated from adipose by the “Direct Zol RNA midiprep Plus with TRI Reagent” kit. cDNA synthesis reaction was performed by ReverTra Ace® qPCR RT Master Mix with gDNA Remover (Toyobo, Japan). All primer sequences can be seen in Supplementary Table 1. mRNA relative expressions were examined by RT-qPCR, using Rplp0 as the housekeeping gene. The mRNA relative levels were calculated using the Livak Method according to our previous research (17).
2.6 Histopathology examination
WAT and liver tissues were fixed with 4% paraformaldehyde (PFA) (Wako Pure Chemical) for 24 h prior to dehydration and paraffin embedding, followed by cutting into 3 μm sections for liver and 5 μm sections for WAT. The liver and WAT sections were stained with hematoxylin and eosin (H&E) to evaluate their structural differences, such as adipocyte diameter for WAT and fatty liver pathology for liver. For the fatty liver phenotype, the assessment staging was performed as previously explained (18). Based on a scoring methodology, the histological pathologics were divided into four stages, as follows: 0: no pathological detected; 1: moderate fatty liver (pathological detected limited in one zone only, such as periportal, midzonar, or centrolobular); 2: severe fatty liver (at least two zones are affected); 3: highly severe fatty liver (all zones are affected). In addition, WAT tissues were stained with F4/80 macrophage antibody immunohistochemical staining (Serotec, Raileigh, NC) at a 1:100 dilution to evaluate the crown-like structure. The analysis was performed by calculating F4/80-positive cells using Image J® software in every field of view (19). Leica DM750 digital microscope (Leica) was used to capture images of the liver and WAT sections at 5 random fields in each sample with a total magnification of 100x.
2.7 SA-β-Gal staining
Fresh WAT was pre-washed with PBS and incubated in the SA-β-Gal staining solution (Cell Signaling Technology, USA) at 37°C. The WAT was observed every 30 minutes until a cyanish (blue-green) stain appeared. The density of cyan color per area was determined by photographing the five stained WAT in each group to evaluate SA-β-gal activity and analyze cyan color density using Image J® software (9).
2.8 RAW264.7 cell and RT-qPCR
RAW264.7 cells, a mouse macrophage cell line (Riken BRC, Japan), were cultured in low glucose-DMEM medium containing 1.0 g/L D-glucose (Sigma, #D6046) or high glucose-DMEM medium containing 4.5 g/L D-glucose (Gibco, #12430-054), both supplemented with 10% FBS and 1% PS (20). We treated the cells with either DMEM medium supplemented with low glucose (5.5 mM) or high glucose (22 mM) for seven days and subsequently stimulated with lipopolysaccharides (LPS) 0.1 μg/mL for 24 hours to induce pro-inflammatory response from macrophages (21). The cells were pretreated with CA (diluted in DMSO <0.01%) at 200 μg/μL 30 minutes before LPS stimulation in both low and high glucose medium (20). Our detailed experimental design for RAW264.7 cells are displayed in Supplementary Figure 3. After 24 hours of LPS exposure, the RNA of the cells was extracted, and RT-qPCR was performed. We analyzed the expressions of Ccl2, Il6, Il1b, and Tnf as M1-proinflammatory markers in macrophages, and Il10, Cd206, and Mgl1 as M2-anti-inflammatory markers (22).
2.9 Statistical analysis
The statistical analyses were conducted using SPSS version 26. The data were presented as the mean value ± the standard error of the mean (SEM). The Shapiro-Wilk test was used to assess data normality. For normally distributed data, one-way ANOVA was applied, followed by Tukey’s post hoc test. If the data were not normally distributed, the Kruskal-Wallis test was used instead, followed by Dunn’s multiple comparisons test. The ITT and GTT data were analyzed using two-way ANOVA, with Dunnett’s post hoc test for multiple comparisons. Statistical significance was denoted as *p<0.05 and **p<0.01. The graphics were generated using GraphPad Prism version 9.5.1.
3 Results
3.1 Centella asiatica supplementation improves the metabolic phenotype in the obese mice model
The mice in the HFHF-diet group exhibited a significant increase in body weight compared to the control group. CA150 and CA300 groups showed a reduction in body weight compared to the HFHF-diet group, although the reduction was not statistically significant (Figure 1a). Similarly, the weight of WAT showed a significant increase in the gonadal (Figure 1b), inguinal (Figure 1c), retroperitoneal (Figure 1d), and mesenteric (Figure 1e) regions from the HFHF-diet group than the control group, but no statistically significant difference between the HFHF-diet, CA150, and CA300 groups. Histopathological analysis of adipose tissue revealed that adipocyte diameter was larger in mice fed with the HFHF-diet compared to those in the other groups, while smaller adipocyte sizes were observed in the control and CA300 groups (Figure 1f).
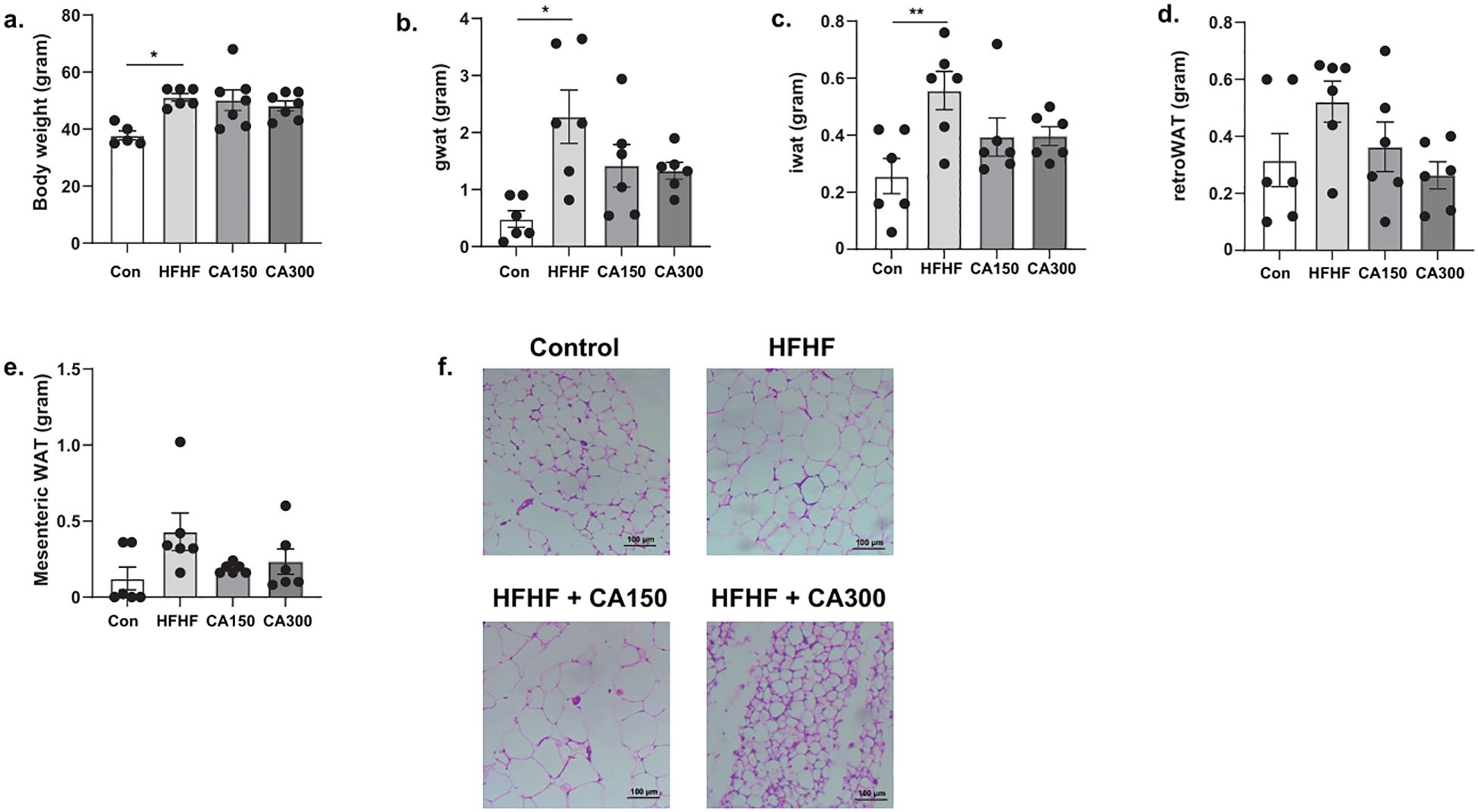
Figure 1. The effects of Centella asiatica (CA) supplementation on (a) body weight, (b) gonadal white adipose tissue (gWAT) weight, (c) inguinal white adipose tissue (iWAT) weight, (d) retroperitoneal white adipose tissue (retroWAT) weight, (e) mesenteric WAT weight, and (f) adipocyte diameter based on adipose tissue histopathology analysis at total magnification of 100x (n=5 each group); top left: Con, top right: HFHF, bottom left: HFHF + CA150, bottom right: HFHF + CA300. Con: Control mice, HFHF: High Fat-High Fructose, CA150: 150 mg of Centella asiatica, CA300: 300 mg of Centella asiatica. *: p<0.05, **: p<0.01.
HFHF-diet groups exhibited insulin resistance as indicated by the ITT (Figure 2a) and GTT results (Figure 2b). Moreover, mice fed with HFHF-diet also showed a reduction in Irs1 expression in WAT isolated. Of note, CA300, but not CA150 groups, significantly enhanced Irs1 mRNA expression level (Figure 2c). Additionally, both CA150 and CA300 treatments significantly prevented the fatty liver phenotype induced by HFHF based on liver histopathology analysis (Figures 2d, e). These data indicate that CA supplementation was able to improve the metabolic impairment in the HFHF-treated mice model.
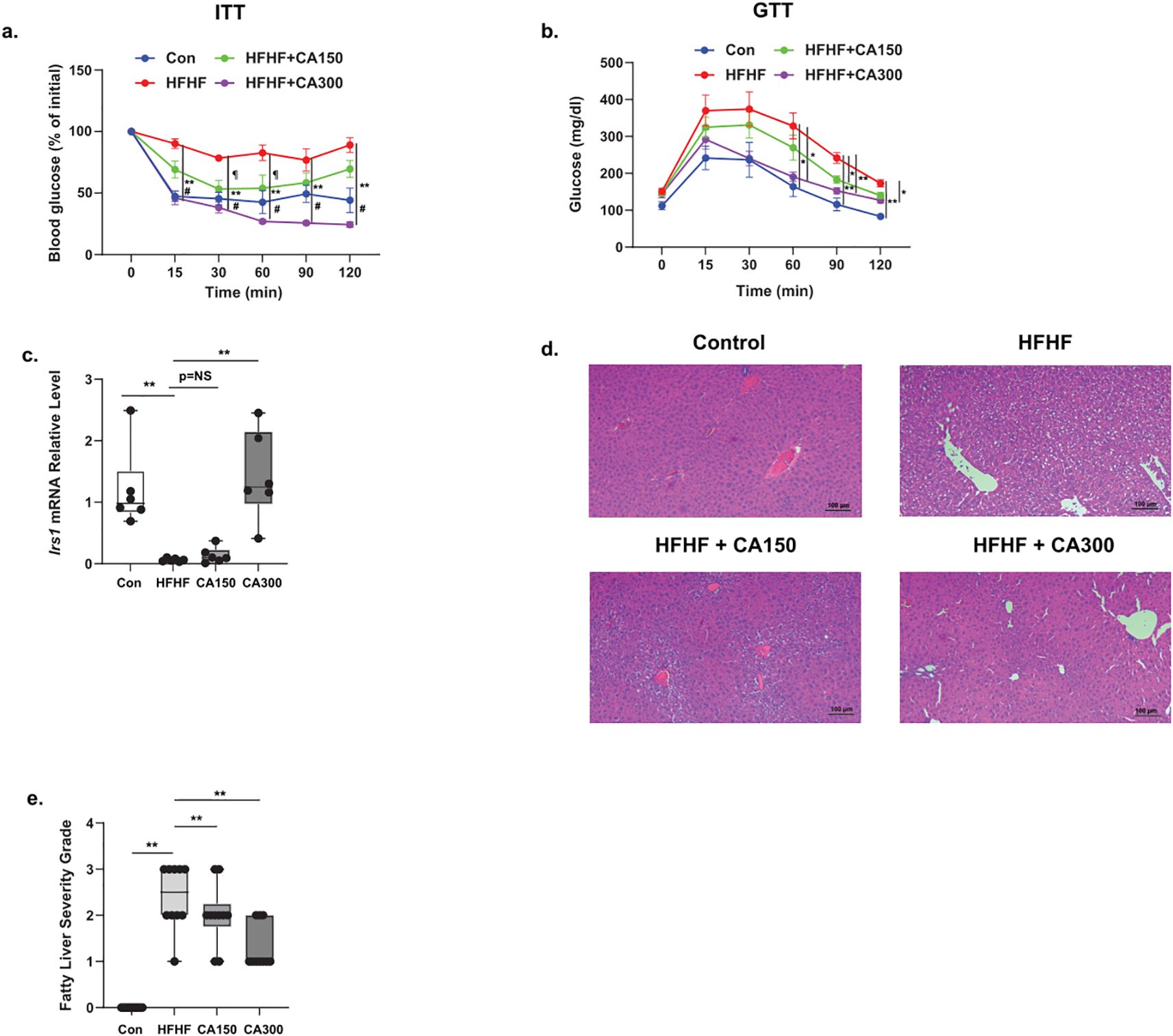
Figure 2. The effect of Centella asciatica (CA) supplementation on metabolic properties. (a) Intra-peritoneal Insulin Tolerance Test (ITT) and (b) Intra-peritoneal Glucose Tolerance Test (GTT). (c) IRS-1 mRNA expression level from WAT (n=5 each group). Representative photomicrographs (d) and the quantification (e) of hematoxylin and eosin (H&E) staining of liver tissues at 100x total magnification (n=5 each group); top left: N, top right: HFHF, bottom left: HFHF + CA150, bottom right: HFHF + CA300. Con: Control mice, HFHF: High Fat-High Fructose, CA150: 150 mg of Centella asiatica, CA300: 300 mg of Centella asiatica. In (a, b) #: p<0.01 between N vs. HFHF groups, **: p<0.01 between HFHF vs. CA300 groups, ¶: p<0.05 between HFHF vs. CA150 groups. In (c, e, f, h), **: p<0.01, NS, Not Significant.
3.2 Centella asiatica treatment prevents the senescence phenotype in white adipose tissue isolated from HFHF-diet groups
We previously showed that senescence phenotype in WAT may induce insulin resistance in mice (9). Based on our findings, SA-β-Gal activity was detected in WAT of the HFHF-diet group. CA300 supplementation significantly suppressed the activity of SA-β-Gal, as indicated by a decrease in the “cyanish” appearance in WAT compared to the HFHF-diet group (Figures 3a, b). In the HFHF-diet group, the mRNA expression levels of Cdkn2a (Figure 3c) and Cdkn1a (Figure 3d), the Cyclin Dependent Kinase (CDK) inhibitors, were consistently increased, while CA300 treatment significantly reduced the expression of both CDK inhibitors. These data suggest CA treatment may potentially inhibit the senescence progression in WAT following HFHF administration, with CA300 treatment appearing to be potentially more effective.
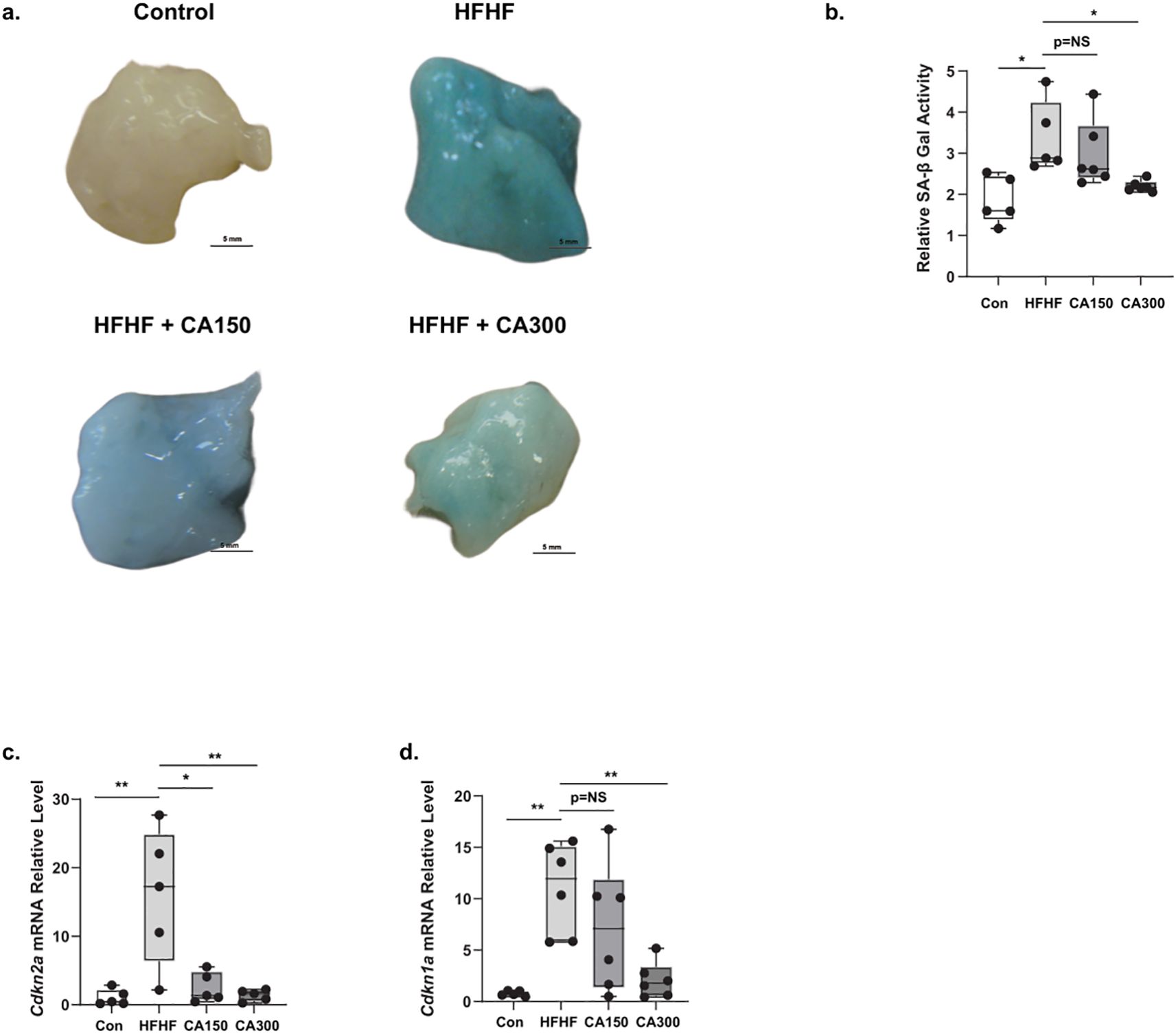
Figure 3. The effect of Centella asciatica (CA) on senescence phenotype and factors. SA-β Gal Staining (a) and its quantification (b) in WAT isolated from normal diet (Control) (top-left), High Fat High Fructose (HFHF) diet (top-right), Centella asciatica 150 mg (CA150) + HFHF (bottom-left), and CA 300 mg (CA300) + HFHF (bottom-right) (n=5 each group). (c) The effects of CA supplementation on the Cdkn2a mRNA expression level, and (d) Cdkn1a mRNA expression level (n=5 each group). Each bar represents the mean relative mRNA expression ± SEM. Con: Control mice, HFHF: High Fat-High Fructose, CA150: 150 mg of Centella asiatica, CA300: 300 mg of Centella asiatica. *: p<0.05, **: p<0.01, NS, Not Significant.
3.3 Centella asiatica treatment alleviates inflammation phenotype in white adipose tissue after HFHF administration
Pro-inflammatory cytokines, including Interleukin 1 Beta (Il1b) and Interleukin 6 (Il6), are commonly found in WAT and are characteristic indicators of senescence (9).These cytokines are often associated with the development of insulin resistance in obesity. We observed an increase in the expression of both Il1b (Figure 4a) and Il6 (Figure 4b) in WAT isolated from the HFHF diet. Both the treatments of CA150 and CA300 significantly decrease the amplification of inflammatory markers following HFHF exposure, but only the CA300 group showed significant downregulation of Il6 mRNA relative expression levels. Likewise, crown-like structures were observed in WAT isolated from the HFHF-diet group, and both CA 150 and CA300 administration significantly diminished these (Figures 4c, d). Collectively, these data strongly suggest that CA treatment suppresses the inflammation phenotype in HFHF-diet mice.
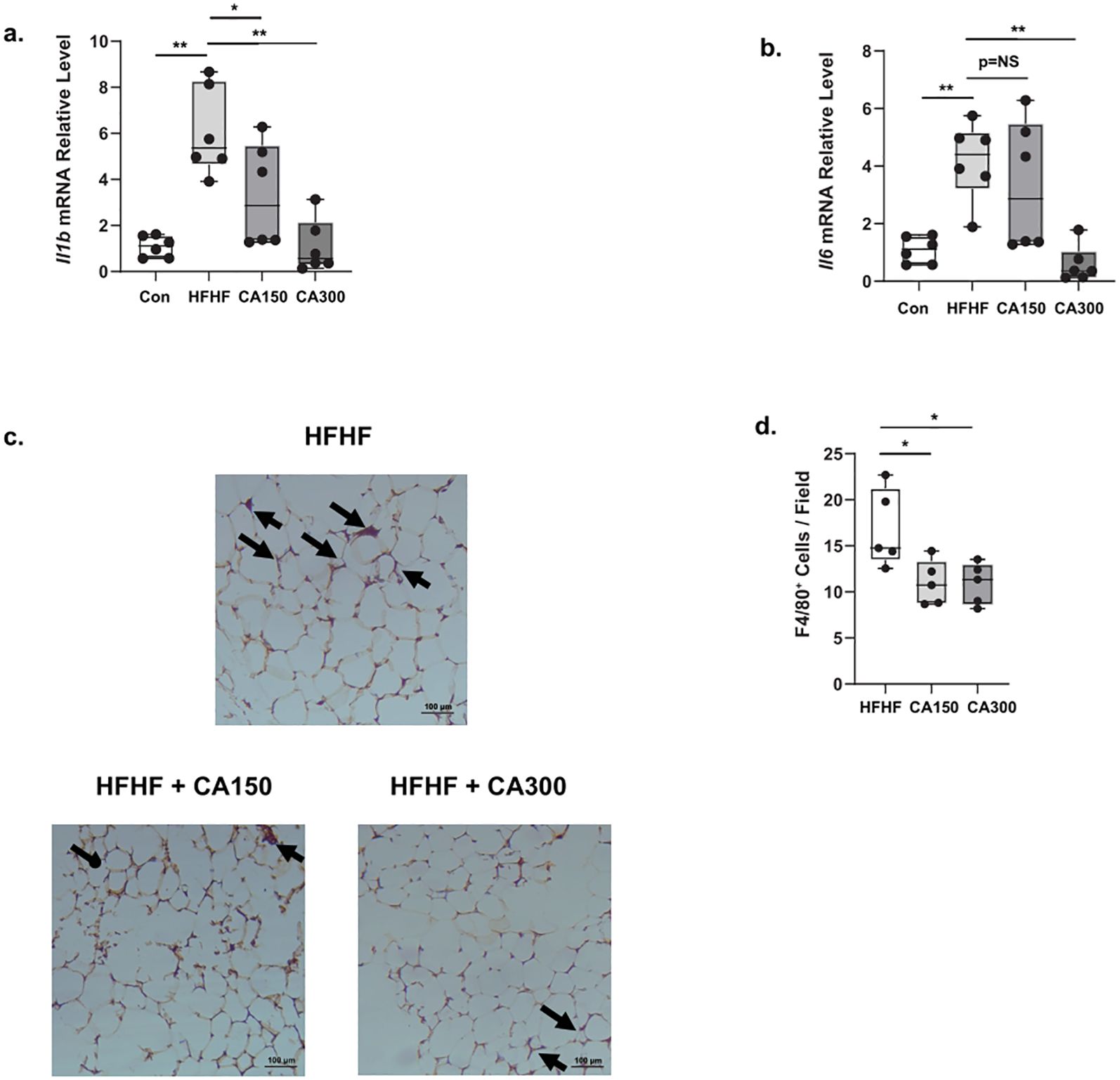
Figure 4. The effect of Centella asciatica (CA) on inflammation phenotype in White Adipose Tissue (WAT); n=5 each group. (a) The effects of CA supplementation on the Il1b mRNA expression level. (b) The effects of CA supplementation on the Il6 mRNA expression level. Representative photomicrographs (c) and the quantification (d) of F4/80-stained white adipose tissues at 100x total magnification; top: HFHF, Bottom left: HFHF + CA150, bottom right: HFHF + CA300. Con: Control mice, HFHF: High Fat-High Fructose, CA150: 150 mg of Centella asiatica, CA300: 300 mg of Centella asiatica. *: p<0.05, **: p<0.01, NS, Not Significant.
3.4 Centella asiatica treatment suppresses proinflammatory M1 macrophage polarization in vitro
To further elucidate the inflammatory molecular mechanism of CA in macrophage polarization, we analyzed both M1-proinflammatory macrophage markers, such as Ccl2, Il6, Il1b, and Tnf, and, M2-antiinflammatory macrophage markers, including Il10, Mgl1, and Cd206 in RAW264.7 mouse macrophage cells stimulated with low (5.5 mM) or high glucose (22 mM) in combination with a low dose of LPS (0.1 μg/mL). CA treatment at 200 μg/ml (CA200) only affected the Il10 mRNA relative expression levels after LPS stimulation under low glucose condition (Supplementary Figure 4). However, when RAW cells were exposed with LPS and high glucose condition, CA200 treatment alleviated the mRNA relative expression levels of M1-associated markers such as Ccl2 (Figure 5a), Il6 (Figure 5b), and Il1b (Figure 5c), but not in Tnf (Figure 5d). In M2 macrophage analysis, the Il10 (Figure 5e) and Mgl1 (Figure 5f) mRNA expression levels were comparable between LPS and LPS+CA groups. Additionally, the expression of Cd206 (Figure 5g) showed inconsistency, as it was found to be elevated after CA200 treatment.
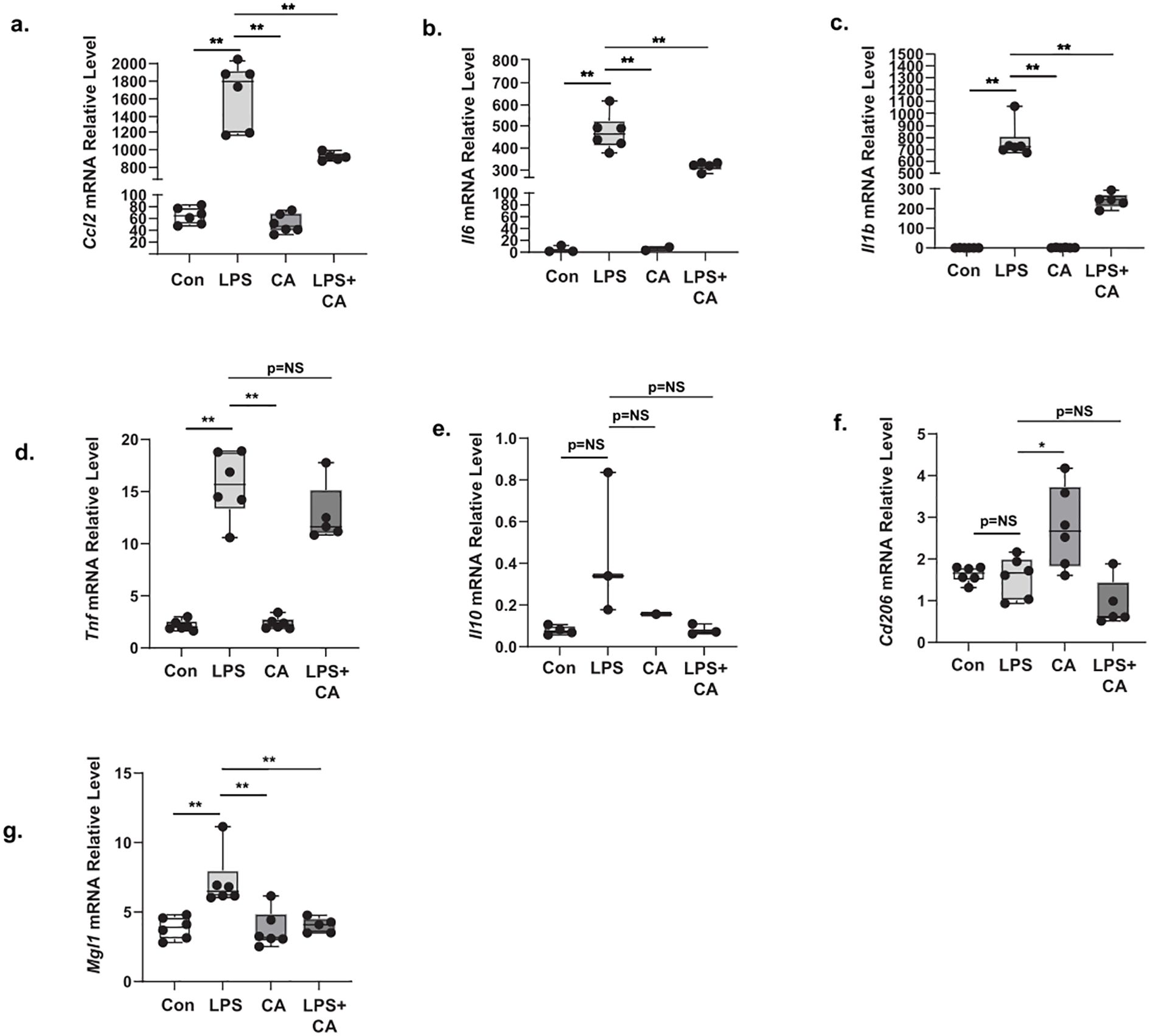
Figure 5. The effect of Centella asciatica (CA) on the mRNA expression level of M1 and M2 macrophage markers in RAW264.7 cell stimulated with high glucose and LPS (n=5 each group). (a) The effects of CA supplementation on the Ccl2 mRNA expression level. (b) The effects of CA supplementation on the Il6 mRNA expression level. (c) The effects of CA supplementation on the Il1b mRNA expression level. (d) The effects of CA supplementation on the Tnf mRNA expression level. (e) The effects of CA supplementation on the Il10 mRNA expression level. (f) The effects of CA supplementation on the Cd206 mRNA expression level. (g) The effects of CA supplementation on the Mgl1 mRNA expression level. Con: Control cells, LPS: LPS-treated cells, CA: CA-treated cells, LPS+CA: LPS and CA-treated cells. *: p<0.05, **: p<0.01, NS, Not Significant.
4 Discussion
In this study, we suggest that CA supplementation may contribute to preventing the pathogenesis of obesity-related metabolic disorders by suppressing senescence-mediated inflammation in WAT. The HFHF-diet mouse model was selected for this study due to its reliability and effectiveness in closely mimicking the metabolic syndromes observed in humans (23). Our model has successfully induced increases in body weight, visceral fat weight, and adipocyte diameter. Moreover, it impaired the insulin sensitivity and downregulated the Irs1 gene expression in WAT. These results are consistent with previous findings analyzing metabolic parameters in the HFHF-diet mouse model (24, 25). We focused on WAT in our research because obesity is associated with an increased accumulation of senescent adipocytes in WAT (26). These senescent adipocytes may induce senescence-associated secretory phenotype (SASP) to produce a chronic sterile inflammatory microenvironment that results in local and systemic metabolic dysfunction (9). Therefore, pharmaceutical interventions that target senescent cells have been known to prevent organ damage associated with obesity (27).
Previous research demonstrated that CA at doses of 300 and 600 mg/kgBW can reduce body fat accumulation in the HFHF-diet model (28). Previous studies also showed that different doses of CA (50, 100, and 200 mg/kgBW) and asiatic acid (50 mg/kgBW), an active compound found in CA, effectively decreased ITT and GTT in the diabetic mouse model (29, 30). To the best of our knowledge, our study is the first study to investigate ITT and GTT parameters in the HFHF-diet mouse model. We selected CA150 and CA300 because CA300 has demonstrated efficacy in improving metabolic parameters in the HFHF-diet model, while doses below 300 mg/kgBW have proven effective in the diabetic model. We aimed to investigate whether a lower CA dosage would also be effective in the HFHF-diet mouse model. Based on our results, even though CA150 and CA300 treatments failed to reduce body weight, the levels of visceral fat were reduced, and improved insulin and glucose tolerance as indicated by ITT and GTT. At the molecular level, Irs1 mRNA expression level was significantly upregulated in CA300 groups. Reduced levels of Irs1 in adipocytes have been identified as a predictor of insulin resistance in human studies (31). CA300 exhibits a greater lowering of the inguinal and retroperitoneal WAT weights, as well as GTT and ITT results. These findings suggest that CA300 has the potential to enhance metabolic properties more effectively than CA150. CA has been known to have a moderate-to-severe hypoglycemic effect, with no published data showing an increase in counter-regulatory hormones, such as glucagon, adrenaline, and cortisol (32). However, those hormones were not analyzed in this manuscript, making it a limitation for this study. We showed the persistent hypoglycemic condition 120 minutes after insulin treatment in the ITT test, particularly in the control (normal-chow diet) and CA300 groups, respectively. This phenomenon was probably due to the relatively high insulin dose (5 IU/kgBW) that we administered to the mice, since we assumed this is a suitable dose in the HFHF model for inducing a decrease of at least 50% from baseline blood glucose levels. We used a 1 IU/kgBW insulin dose in normal-feeding diet mice for the ITT test in our previous study (9). Even this higher insulin dose could achieve that condition; some groups, including the group with Centella asiatica, had a persistent hypoglycemic effect.
Previous research showed that CA significantly decreased the expression of the Cdkn2a and Cdkn1a genes in human dermal fibroblasts and human hepatoma cells (33, 34). To the best of our knowledge, there is no previous in vivo study related to this topic. Our study showed that the expression of Cdkn2a and Cdkn1a in WAT was reduced by the administration of CA300. The upregulated of these gene expression accelerates the senescence process by affecting these core aging pathways, particularly p16INK4A/pRB, and p53/p21WAF1/CIP1 pathways (35). Consistent with our findings, a previous study demonstrated that asiaticoside, a key active compound of CA, inhibited Cdkn1a expression in UV-induced HaCat cells (36). Our SA-β-gal test conducted in WAT showed a “cyan-color” appearance in all the HFHF-diet group, including those treated with CA. Although the CA150 and CA300 groups showed a lesser cyanish color compared with the HFHF-diet group alone. This finding showed that CA can inhibit the increase of lysosomal activity, indicating the inhibition of the senescence process. Another previous in vitro study also showed a similar effect in human dermal fibroblasts (37).
Senescence cells disrupt metabolic states and lipid metabolism, leading to metabolic disease (38). Metabolic derangement has two important hallmarks, Il1b and Il6. Il1b is associated with the initiation and progression of obesity-induced insulin resistance (39), whereas Il6 plays a role in maintaining energy and metabolic balance through its involvement in energy metabolism (40). Therefore, we used these two biomarkers to evaluate the CA ability to prevent the inflammation caused by senescence. Our study demonstrated that the mRNA relative expression levels of Il1b and Il6 were reduced in the CA administration group compared to the HFHF-diet group, confirming the effect of CA in this pathway. This finding aligns with other studies conducted on mouse macrophages and a psoriasis-like skin inflammation mouse model (41, 42).
Our study also discovered that CA supplementation reduced adipocyte diameter on the histopathological analysis. One proven hypothesis is that CA can regulate lipid metabolism-related genes, such as peroxisome proliferator-activated receptor gamma (PPARγ), fatty acid synthase (FAS), cluster of differentiation 36 (CD36), 3- 3-hydroxyl-3-methylglutaryl CoA reductase (HMGCR), and stearoyl CoA desaturase 1 (SCD1) in the HFHF-diet model (28). We proposed an additional mechanism by which CA supplementation reduces adipocyte diameter by regulating Il1b and Il6, as these interleukins are involved in fat-liver crosstalk and adipogenesis, respectively (43, 44). Crown-like structure (CLS) is a characteristic feature of inflamed fat cells that is a persistent location for macrophage activation (45). The CLS macrophage activation mechanism is believed to occur through elevated levels of MAC-2, also known as galectin-3, a lectin that facilitates macrophage phagocytic and inflammatory responses (45, 46).
We found that CA decreased the number of CLS in WAT. Adipocyte hypertrophy can trigger low-grade inflammation, and CLS numbers are related to increased expression of M1 macrophages (47, 48). Under obesity-related metabolic pathological conditions, macrophages undergo a phenotypic shift towards the M1 pro-inflammatory macrophages. The M1 polarization will induce the secretion of pro-inflammatory cytokines, such as Il6, Il1b, Tnf, and Ccl2 (48). Therefore, we conducted an in vitro study on LPS-induced RAW264.7 cells to gain better insight into the mechanism of CA in macrophage inflammatory responses. CA reduced the expression of M1 macrophage markers, such as Ccl2, Il6, Il1b, and Tnf, in high glucose environments. Our results suggest that CA could suppress M1 macrophages. Another study showed that CA can decrease the expression of proinflammatory macrophages is related to the suppression of IL-1 receptor-associated kinase and transforming growth factor-β-activated kinase 1 (IRAK1-TAK1) pathways (42). This finding supports the hypothesis that one potential mechanism by which CA can reduce senescence markers and metabolic phenotypes is through its anti-inflammatory properties, particularly by modulating macrophage activity.
We discovered interesting characteristics in the IL-10 response in RAW264.7 cells, whereby IL-10 expression was upregulated following LPS stimulation under low glucose conditions but did not exhibit a substantial increase under high glucose conditions. In contrast, Cd206, another M2 macrophage marker, was downregulated after LPS induction. The increase in Il10 expression is consistent with findings from a previous study on the THP-1 macrophage cell line, which also reported elevated Il10 expression level following LPS stimulation. Additionally, the previous study also found that Cd206 expression was decreased after 24 hours of LPS exposure (49).
5 Conclusion
Centella asiatica shows anti-senescence effect in obesity-induced insulin resistance (IR) by preventing senescence induction in WAT of an obesity rodent model. This phenomenon effectively improves systemic insulin sensitivity and further suppresses M1-proinflammatory macrophage-induced inflammation. These findings highlight the role of Centella asiatica as a promising medicinal compound treatment for obesity-related metabolic disorders.
Data availability statement
The raw data supporting the conclusions of this article are available from the corresponding author upon reasonable request.
Ethics statement
The animal study was approved by Faculty of Medicine, University of Indonesia (ethical clearance number KET-562/UN2.F1/ 211 ETIK/PPM.00.02/2022). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Writing – original draft, Writing – review & editing. WA: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. ND: Investigation, Writing – review & editing. MS: Investigation, Writing – review & editing. IS: Investigation, Writing – review & editing. Y-TH: Investigation, Writing – review & editing. NS: Investigation, Writing – review & editing. RH: Investigation, Resources, Writing – review & editing. NK: Investigation, Writing – review & editing. HH: Formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by grants from PUTI Q1 UI Grant 2022 (NKB-444/UN2.RST/HKP.05.00/2022).
Acknowledgments
We thank Nur Amalina Fitria for her excellent technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplemental Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1589444/full#supplementary-material
References
1. Abdelaal M, le Roux CW, and Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. (2017) 5:161–1. doi: 10.21037/atm.2017.03.107
2. Xu H, Cupples LA, Stokes A, and Liu C-T. Association of obesity with mortality over 24 years of weight history. JAMA Netw Open. (2018) 1:e184587. doi: 10.1001/jamanetworkopen.2018.4587
3. Hildebrandt X, Ibrahim M, and Peltzer N. Cell death and inflammation during obesity: “Know my methods, WAT(son). Cell Death Differ. (2023) 30:279–92. doi: 10.1038/s41418-022-01062-4
4. Wondmkun YT. Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Diabetes Metab Syndr Obes. (2020) 13:3611–6. doi: 10.2147/DMSO.S275898
5. Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. (2019) 20:2358. doi: 10.3390/ijms20092358
6. Al-Mansoori L, Al-Jaber H, Prince MS, and Elrayess MA. Role of inflammatory cytokines, growth factors and adipokines in adipogenesis and insulin resistance. Inflammation. (2022) 45:31–44. doi: 10.1007/s10753-021-01559-z
7. Zhang Y-X, Ou M-Y, Yang Z-H, Sun Y, Li Q-F, and Zhou S-B. Adipose tissue aging is regulated by an altered immune system. Front Immunol. (2023) 14:1125395. doi: 10.3389/fimmu.2023.1125395
8. Kumari R and Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. (2021) 9:645593. doi: 10.3389/fcell.2021.645593
9. Barinda AJ, Ikeda K, Nugroho DB, Wardhana DA, Sasaki N, Honda S, et al. Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype. Nat Commun. (2020) 11:481. doi: 10.1038/s41467-020-14387-w
10. Das AJ. Review on Nutritional, Medicinal and Pharmacological Properties of Centella asiatica (Indian pennywort). J Biologically Active Products Nat. (2011) 1:216–28. doi: 10.1080/22311866.2011.10719089
11. Sun B, Wu L, Wu Y, Zhang C, Qin L, Hayashi M, et al. Therapeutic potential of centella asiatica and its triterpenes: A review. Front Pharmacol. (2020) 11:568032. doi: 10.3389/fphar.2020.568032
12. De Sanctis MT, Belcaro G, Incandela L, Cesarone MR, Griffin M, Ippolito E, et al. Treatment of edema and increased capillary filtration in venous hypertension with total triterpenic fraction of Centella asiatica: a clinical, prospective, placebo-controlled, randomized, dose-ranging trial. Angiology. (2001) 52 Suppl 2:S55–9. doi: 10.1177/000331970105202S11
13. Lou J-S, Dimitrova DM, Murchison C, Arnold GC, Belding H, Seifer N, et al. Centella asiatica triterpenes for diabetic neuropathy: a randomized, double-blind, placebo-controlled, pilot clinical study. Esper Dermatol. (2018) 20:12–22. doi: 10.23736/S1128-9155.18.00455-7
14. Farhana KM, Malueka RG, Wibowo S, and Gofir A. Effectiveness of gotu kola extract 750 mg and 1000 mg compared with folic acid 3 mg in improving vascular cognitive impairment after stroke. Evidence-Based Complementary Altern Med. (2016) 2016:1–6. doi: 10.1155/2016/2795915
15. Barinda AJ, Arozal W, and Yuasa S. A review of pathobiological mechanisms and potential application of medicinal plants for vascular aging: focus on endothelial cell senescence. Med J Indonesia. (2022) 31:132–40. doi: 10.13181/mji.rev.226064
16. Kumar A, Prakash A, and Dogra S. Centella asiatica attenuates D-galactose-induced cognitive impairment, oxidative and mitochondrial dysfunction in mice. Int J Alzheimers Dis. (2011) 2011:347569. doi: 10.4061/2011/347569
17. Barinda AJ, Arozal W, Hardi H, Dewi YR, Safutra MS, and Lee HJ. Water extracts of moringa oleifera leaves alter oxidative stress–induced neurotoxicity in human neuroblastoma SH-SY5Y cells. Sci World J. (2024) 2024:1–9. doi: 10.1155/2024/7652217
18. Gerspach C, Imhasly S, Klingler R, Hilbe M, Hartnack S, and Ruetten M. Variation in fat content between liver lobes and comparison with histopathological scores in dairy cows with fatty liver. BMC Vet Res. (2017) 13:98. doi: 10.1186/s12917-017-1004-9
19. Akakabe Y, Koide M, Kitamura Y, Matsuo K, Ueyama T, Matoba S, et al. Ecscr regulates insulin sensitivity and predisposition to obesity by modulating endothelial cell functions. Nat Commun. (2013) 4:2389. doi: 10.1038/ncomms3389
20. Wang Q, Yokoo H, Takashina M, Sakata K, Ohashi W, Abedelzaher LA, et al. Anti-inflammatory profile of levosimendan in cecal ligation-induced septic mice and in lipopolysaccharide-stimulated macrophages*. Crit Care Med. (2015) 43:e508–20. doi: 10.1097/CCM.0000000000001269
21. Suzuki T, Yamashita S, Hattori K, Matsuda N, and Hattori Y. Impact of a long-term high-glucose environment on pro-inflammatory responses in macrophages stimulated with lipopolysaccharide. Naunyn Schmiedebergs Arch Pharmacol. (2021) 394:2129–39. doi: 10.1007/s00210-021-02137-8
22. Yao Y, Xu X-H, and Jin L. Macrophage polarization in physiological and pathological pregnancy. Front Immunol. (2019) 10:792. doi: 10.3389/fimmu.2019.00792
23. Zhuhua Z, Zhiquan W, Zhen Y, Yixin N, Weiwei Z, Xiaoyong L, et al. A novel mice model of metabolic syndrome: the high-fat-high-fructose diet-fed ICR mice. Exp Anim. (2015) 64:435–42. doi: 10.1538/expanim.14-0086
24. Zhu A-Q, Luo N, Zhou X-T, Yuan M, Zhang C-M, Pan T-L, et al. Transcriptomic insights into the lipotoxicity of high-fat high-fructose diet in rat and mouse. J Nutr Biochem. (2024) 128:109626. doi: 10.1016/j.jnutbio.2024.109626
25. Lee JS, Jun DW, Kim EK, Jeon HJ, Nam HH, and Saeed WK. Histologic and metabolic derangement in high-fat, high-fructose, and combination diet animal models. Sci World J. (2015) 2015:1–9. doi: 10.1155/2015/306326
26. Pini M, Czibik G, Sawaki D, Mezdari Z, Braud L, Delmont T, et al. Adipose tissue senescence is mediated by increased ATP content after a short-term high-fat diet exposure. Aging Cell. (2021) 20:e13421. doi: 10.1111/acel.13421
27. Robbins PD, Jurk D, Khosla S, Kirkland JL, LeBrasseur NK, Miller JD, et al. Senolytic drugs: reducing senescent cell viability to extend health span. Annu Rev Pharmacol Toxicol. (2021) 61:779–803. doi: 10.1146/annurev-pharmtox-050120-105018
28. Chang YB, Ahn Y, Seo D, Bae S, Suh HJ, Hong YH, et al. Centella asiatica lowers body fat accumulation via regulating cholesterol homeostasis- and lipid metabolism-related genes in mice with high-fat, high-sugar diet-induced obesity. Appl Biol Chem. (2023) 66:88. doi: 10.1186/s13765-023-00846-7
29. Sun W, Xu G, Guo X, Luo G, Wu L, Hou Y, et al. Protective effects of asiatic acid in a spontaneous type 2 diabetic mouse model. Mol Med Rep. (2017) 16:1333–9. doi: 10.3892/mmr.2017.6684
30. Giribabu N, Karim K, Kilari EK, Nelli SR, and Salleh N. Oral administration of Centella asiatica (L.) Urb leave aqueous extract ameliorates cerebral oxidative stress, inflammation, and apoptosis in male rats with type-2 diabetes. Inflammopharmacology. (2020) 28:1599–622. doi: 10.1007/s10787-020-00733-3
31. Carvalho E, Jansson P, Axelsen M, Eriksson JW, Huang X, Groop L, et al. Low cellular IRS 1 gene and protein expression predict insulin resistance and NIDDM. FASEB J. (1999) 13:2173–8. doi: 10.1096/fasebj.13.15.2173
32. Kabir AU, Samad MB, D’Costa NM, Akhter F, Ahmed A, and Hannan J. Anti-hyperglycemic activity of Centella asiatica is partly mediated by carbohydrase inhibition and glucose-fiber binding. BMC Complement Altern Med. (2014) 14:31. doi: 10.1186/1472-6882-14-31
33. Kim YJ, Cha HJ, Nam KH, Yoon Y, Lee H, and An S. Centella asiatica extracts modulate hydrogen peroxide-induced senescence in human dermal fibroblasts. Exp Dermatol. (2011) 20:998–1003. doi: 10.1111/j.1600-0625.2011.01388.x
34. Chen J-Y, Xu Q-W, Xu H, and Huang Z-H. Asiatic Acid Promotes p21 WAF1/CIP1 Protein Stability through Attenuation of NDR1/2 Dependent Phosphorylation of p21 WAF1/CIP1 in HepG2 Human Hepatoma Cells. Asian Pacific J Cancer Prev. (2014) 15:963–7. doi: 10.7314/APJCP.2014.15.2.963
35. Wagner K-D and Wagner N. The Senescence Markers p16INK4A, p14ARF/p19ARF, and p21 in Organ Development and Homeostasis. Cells. (2022) 11:1966. doi: 10.3390/cells11121966
36. Jiang H, Zhou X, and Chen L. Asiaticoside delays senescence and attenuate generation of ROS in UV-exposure cells through regulates TGF-β1/Smad pathway. Exp Ther Med. (2022) 24:667. doi: 10.3892/etm.2022.11603
37. Haryanti S, Budiarti M, Farida S, Dewi APK, Supriyati N, Jokopriyambodo W, et al. The palm oil-based chlorophyll removal and the evaluation of antiaging properties on Centella asiatica ethanolic extract. IOP Conf Ser Earth Environ Sci. (2024) 1312:12041. doi: 10.1088/1755-1315/1312/1/012041
38. Wiley CD and Campisi J. The metabolic roots of senescence: mechanisms and opportunities for intervention. Nat Metab. (2021) 3:1290–301. doi: 10.1038/s42255-021-00483-8
39. Ballak DB, Stienstra R, Tack CJ, Dinarello CA, and van Diepen JA. IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine. (2015) 75:280–90. doi: 10.1016/j.cyto.2015.05.005
40. Ghanemi A and St-Amand J. Interleukin-6 as a “metabolic hormone. Cytokine. (2018) 112:132–6. doi: 10.1016/j.cyto.2018.06.034
41. Lin P, Shi H, Lu Y, and Lin J. Centella asiatica alleviates psoriasis through JAK/STAT3-mediated inflammation: An in vitro and in vivo study. J Ethnopharmacol. (2023) 317:116746. doi: 10.1016/j.jep.2023.116746
42. Cho Y-C, Vuong HL, Ha J, Lee S, Park J, Wibow AE, et al. Inhibition of Inflammatory Responses by Centella asiatica via Suppression of IRAK1-TAK1 in Mouse Macrophages. Am J Chin Med (Gard City N Y). (2020) 48:1103–20. doi: 10.1142/S0192415X20500548
43. Nov O, Shapiro H, Ovadia H, Tarnovscki T, Dvir I, Shemesh E, et al. Interleukin-1β Regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. PloS One. (2013) 8:e53626. doi: 10.1371/journal.pone.0053626
44. Almuraikhy S, Kafienah W, Bashah M, Diboun I, Jaganjac M, Al-Khelaifi F, et al. Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance. Diabetologia. (2016) 59:2406–16. doi: 10.1007/s00125-016-4031-3
45. Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. (2005) 46:2347–55. doi: 10.1194/jlr.M500294-JLR200
46. Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, et al. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. (2000) 165:2156–64. doi: 10.4049/jimmunol.165.4.2156
47. Püschel GP, Klauder J, and Henkel J. Macrophages, low-grade inflammation, insulin resistance and hyperinsulinemia: A mutual ambiguous relationship in the development of metabolic diseases. J Clin Med. (2022) 11:4358. doi: 10.3390/jcm11154358
48. Yao J, Wu D, and Qiu Y. Adipose tissue macrophage in obesity-associated metabolic diseases. Front Immunol. (2022) 13:977485. doi: 10.3389/fimmu.2022.977485
Keywords: Centella asiatica, inflammation, M1 pro-inflammatory macrophage, senescence, white adipose tissue, obesity, insulin resistance
Citation: Barinda AJ, Arozal W, Dwita NC, Safutra MS, Shimizu I, Hsiao YT, Sandora N, Hakim RW, Khatimah NG and Hardi H (2025) Centella asiatica extract improves senescence-associated metabolic dysfunction by targeting inflammation in adipose tissue and macrophage in obesity-induced insulin resistance mice. Front. Endocrinol. 16:1589444. doi: 10.3389/fendo.2025.1589444
Received: 14 March 2025; Accepted: 02 July 2025;
Published: 31 July 2025.
Edited by:
Laura J. Den Hartigh, University of Washington, United StatesReviewed by:
Lucas Grun, Federal University of Rio Grande do Sul, BrazilAnmol Bhandari, Guru Nanak Dev University, India
Copyright © 2025 Barinda, Arozal, Dwita, Safutra, Shimizu, Hsiao, Sandora, Hakim, Khatimah and Hardi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wawaimuli Arozal, d2F3YWltdWxpLmFyb3phbEB1aS5hYy5pZA==
 Agian Jeffilano Barinda
Agian Jeffilano Barinda Wawaimuli Arozal
Wawaimuli Arozal Nounik Cheri Dwita
Nounik Cheri Dwita Muhamad Sadam Safutra
Muhamad Sadam Safutra Ippei Shimizu
Ippei Shimizu Yung Ting Hsiao
Yung Ting Hsiao Normalina Sandora
Normalina Sandora Rani Wardani Hakim
Rani Wardani Hakim Nurul Gusti Khatimah
Nurul Gusti Khatimah Harri Hardi
Harri Hardi