- Reproductive Medical Center, The 924th Hospital of the Joint Logistic Support Force of the Chinese People's Liberation Army, Guilin, China
Purpose: Investigating whether increasing the dose of gonadotropins (Gn) in the second in vitro fertilization (IVF) cycle using the antagonist protocol could improve the cumulative live birth rate (CLBR) in POSEIDON Groups 1 and 2.
Methods: This retrospective study included 343 patients from POSEIDON Groups 1 and 2 who underwent two consecutive cycles of ovarian stimulation with an antagonist protocol between May 2018 and September 2022. Patients were divided into an Additive group (those who increased the Gn dosage in the second cycle) and a Control group (those who maintained or decreased the Gn dosage), with a 1:2 propensity score matching analysis. The primary outcome was the CLBR.
Results: In the second IVF cycle, the Additive group had higher initial (191.8 vs 183.4, P=0.135) and total (2161.7 vs 1770.6, P=0.461) Gn doses compared to the Control group. The Additive group also had a higher average number of retrieved oocytes and Metaphase II (MII) oocytes, a higher two pronuclei (2PN) fertilization rate (3.3 vs 2.6, P=0.065), and higher blastocyst formation rates (44.9% vs 44.2%, P=0.937) compared to the Control group; however, these differences were not statistically significant. The Control group had a slightly higher CLBR (31.5% vs 28.9%, P=0.8), which was also not statistically significant.
Conclusions: For POSEIDON Groups 1 and 2, increasing the dose of Gn under the antagonist protocol increased treatment costs but did not improve the CLBR. Routine increase of Gn dose was not recommended.
Introduction
The management of low-prognosis patients is a significant challenge in the field of in vitro fertilization (IVF). To better identify and manage POR, reproductive medicine experts introduced the Bologna criteria in 2011 (1). Subsequently, in 2016, to address the limitations of the Bologna criteria, the POSEIDON criteria were proposed (2). The POSEIDON criteria categorize low-prognosis patients into an “expected low-prognosis group” and an “unexpected low-prognosis group” based on age, antral follicle count (AFC), and anti-Müllerian hormone (AMH). The “unexpected POR group” includes Group 1, aged under 35 years, and Group 2, aged 35 years and above. A study by Esteves, S. C. et al. on 13,146 infertile women undergoing conventional ovarian stimulation showed that 43% of patients met the POSEIDON criteria, with 44% belonging to Group 1 and 36% to Group 2 (3). The unexpected low response in POSEIDON Groups 1 and 2 leads to a significant psychological gap for patients, which in turn increases the pressure on reproductive physicians in treatment. The issue of how to optimize ovarian stimulation protocols to improve treatment outcomes in subsequent IVF cycles remains controversial.
In ovarian stimulation for general IVF patients, increasing the dose of gonadotropins (Gn) is an effective means to increase the number of oocytes retrieved, and a higher initial gonadotropin dose is an independent protective factor against suboptimal response (4, 5). However, the therapeutic effect of increasing the Gn dose on POSEIDON Groups 1 and 2 currently lacks high-quality research evidence support. Existing studies lack consideration of individual variation factors in POSEIDON Groups 1 and 2 patients, and the assessment of the effectiveness of interventions for POSEIDON Groups 1 and 2 should include before-and-after comparisons in consecutive IVF cycles to exclude the interference of individual variation factors. In addition, the cumulative live birth rate (CLBR), as an important indicator for evaluating the effectiveness of IVF treatment (6), should be included in the scope of research outcomes. In the retrospective study by Parimala Chinta et al., increasing the dose of gonadotropins or changing the protocol can improve the live birth outcomes of POSEIDON Groups 1 and 2 patients (7). In the study by Alyssa Hochberg et al., through multivariate logistic regression analysis, it was found that in women with AMH values between 1.20 and 2.97 ng/mL and/or AFC between 5 and 12, increasing the dose of follicle-stimulating hormone (FSH) did not reduce the risk of suboptimal response (8). After analyzing 658,519 fresh autologous IVF cycles, Baker, V. L. et al. found that for patients with normal ovarian response, the live birth rate significantly decreased with the increase of FSH dose, a trend unrelated to the number of oocytes retrieved (9). For POSEIDON Groups 1 and 2 patients, the effectiveness of increasing the Gn dose in improving patients’ cumulative live birth rate still needs to be verified.
Therefore, we conducted a retrospective cohort study to perform a before-and-after comparison in two consecutive IVF cycles for patients in POSEIDON Groups 1 and 2, assessing the impact of increased Gn dosage during ovarian stimulation with the antagonist protocol on the CLBR of these groups.
Study design and population
This was a single-center, retrospective cohort study targeting Poseidon Groups 1 and 2, with participants from the southern province of China. The study included cycles of utilizing sperm that was either fresh or cryopreserved. The study period from May 2018 to September 2022, and included 343 patients from POSEIDON Groups 1 and 2 who underwent repeated IVF cycles using the antagonist protocol, grouped based on the change in Gn dosage during the second IVF cycle. The Additive group consisted of 109 patients, in whom the Gn dosage was increased relative to the first IVF cycle in the second cycle. The Control group included 234 patients, in whom the Gn dosage remained stable or decreased relative to the first IVF cycle in the second cycle. The retrospective study was approved by the hospital ethics committee, and patient treatment information was obtained from the electronic medical records system.
The inclusion criteria were as follows: 1. AFC≥5 and AMH≥1.2 ng/ml; 3. patients who underwent two ovarian stimulations using the antagonist protocol within a 6-month period. The exclusion criteria included: 1. severe uterine anomalies; 2. moderate to severe intrauterine adhesions; 3. adenomyosis and endometriosis; 4. women who had oocytes cryopreserved.
All patients’ AMH tests were conducted prior to the initiation of their IVF cycles. The AFC assessment was determined on the day of ovarian stimulation initiation using two-dimensional transvaginal ultrasound, with the AFC assessment criteria referring to the practical guide published in January 2018 (10). The study’s clinical procedures were in accordance with the prevailing clinical guidelines. Ovarian stimulation for all participants commenced on day 2 through day 5 of the menstrual cycle. The dosage of the antagonist protocol was determined based on factors such as the patient’s age, BMI, and AFC. The administration of GnRH antagonists was conducted according to either a fixed or flexible protocol. Triggering was considered when the mean diameter of a follicle exceeded 18 mm or when the mean diameters of two follicles exceeded 17 mm. The preferred method for triggering was using human chorionic gonadotropin (hCG); for patients at high risk of OHSS, gonadotropin-releasing hormone agonist (GnRH-a) was used for triggering. On the trigger day, blood was drawn to measure progesterone levels to assess suitability for fresh embryo transfer (ET). Oocyte retrieval was conducted via transvaginal ultrasound-guided follicular aspiration within 36 to 38 hours following the trigger.
Laboratory procedures
Following oocyte retrieval, the decision to use IVF or ICSI was based on sperm quality. After fertilization, embryos were individually cultured in medium with an oil overlay. All embryo culture dishes were placed in traditional incubators maintained at 37°C with an atmosphere of 5% oxygen (O2), 6% carbon dioxide (CO2), and 89% nitrogen (N2). Pronuclear morphology was observed 16 to 18 hours after fertilization, and embryos were evaluated daily until the day of transfer or cryopreservation. Throughout the study period, our laboratory procedures and the reagents and consumables used remained consistent.
The assessment of embryo quality was based on a scoring system that evaluated the embryos’ morphological characteristics (11). Day 3 (D3) embryos were classified into Grades I to IV based on the number and uniformity of blastomeres, and degree of fragmentation (12). Among them, Grade I and Grade II embryos were considered high-quality embryos. The Gardner grading system was employed to grade the blastocysts (13). Embryos graded as CA, CB, and CC were not subjected to transfer or cryopreservation.
For patients with multiple embryos, the sequence of embryo transfer was ascertained in accordance with the embryonic grading. For D3 embryos with identical scores, those with high pronuclei scores were prioritized for transfer. Regarding the order of blastocyst transfer, fully expanded blastocysts were prioritized over expanding ones, and the inner cell mass grade was more important than the trophectoderm grade; when the blastocysts scores were the same, the D3 embryo score was taken into account.
Fresh embryo transfers were conducted on D3 or D5 following oocytes retrieval. If fresh cycle embryo transfer was not performed, vitrification technology was used to cryopreserve the embryos. The vitrification cryoprotectant solution was primarily composed of 0.6M sucrose, 15% ethylene glycol, and 15% dimethyl sulfoxide. The principal causes for canceling fresh cycle embryo transfer encompassed the absence of transferable embryos on Day 5, preimplantation genetic testing (PGT) and OHSS. The frozen D3 embryos were not thawed for blastocyst culture.
Thawed embryo transfer
The endometrial preparation protocols for frozen embryo transfer (FET) encompassed the natural cycle and hormone replacement therapy (HRT). The natural cycle protocol mandated that patients have regular menstrual cycles. In the hormone replacement therapy, patients orally administered 2-6mg of estradiol daily and monitored the endometrial thickness via transvaginal ultrasound; when the thickness surpassed 8 millimeters and/or after 14 days of estradiol administration, endometrial transformation was induced with an intramuscular injection of 60mg of progesterone. In our IVF center, hormone replacement therapy was typically utilized to prepare the endometrium. The day of ovulation or the day of progesterone administration was designated as Day 0, with embryo transfer conducted on Day 3 or Day 5, and the thawing and revival of the embryos accomplished on the day of transfer.
Under the guidance of transabdominal ultrasound, the embryo transfer was performed using a transfer catheter from COOK company. No assisted hatching was applied to the embryos. The number of embryos transferred was determined based on factors such as the woman’s age and embryo quality. Typically, two Day 3 embryos or one high-quality blastocyst were transferred; when patients lacked high quality blastocysts, two average-quality blastocysts might have been transferred, with a maximum of two embryos per transfer.
Luteal support
There were mainly two approaches to post-transfer luteal support: 1. Micronized progesterone vaginal suppositories, Crinone® 8% at a dosage of 90 milligrams (Merck Serono); 2. Progesterone injection solution administered via daily intramuscular injection at a dosage of 40 to 60 milligrams. Luteal support was initiated on the day of oocyte retrieval or the day of endometrial transformation. A β-hCG test was conducted on the 14th day after transfer; if embryo implantation occurred, progesterone administration would continue until 6 weeks post-transfer.
Outcomes
The primary outcome of the study was the CLBR over two IVF cycles, which included the live birth outcomes from both fresh embryo transfers and FETs. The secondary outcomes were comprised of the clinical pregnancy rate, implantation rate, multiple pregnancy rate, pregnancy loss rate, and ectopic pregnancy rate. The criterion for clinical pregnancy was the sonographic visualization of a gestational sac. Pregnancy loss referred to the failure of a clinical pregnancy, excluding ectopic pregnancy. The live birth was defined by the presence of a heartbeat, respiration, and muscle movement in the newborn at the time of delivery. The CLBR was defined as the sum of live birth occurrences from both fresh transfers and frozen embryo transfers, with multiple births counted as one. The follow-up endpoints of the study included two scenarios: follow-up was terminated if the patient achieved a live birth; if no live birth was achieved, follow-up was terminated by November 30, 2024. From the date of the last oocyte retrieval, all patients who did not achieve live birth were followed up for over 26 months.
Statistical analysis
The propensity score matching (PSM) was performed using the “MatchIt” package in R software. The PSM model included several covariates such as age, obstetric history, basal bFSH, AFC, dose of gonadotropin, interval between two ovarian stimulations, number of metaphase II (MII) oocytes and high-quality Day 3 embryos to estimate propensity scores. Propensity scores for each subject were estimated using logistic regression. A 1:2 nearest neighbor matching method was applied to pair each subject in the Additive group with control subjects who had the closest propensity scores. The matching process used a caliper of 0.05 to restrict the maximum distance between matching pairs, ensuring balance of covariates within specific subgroups by limiting the maximum PS difference between the matched pairs.
The distribution characteristics of the data were assessed using the Kolmogorov-Smirnov test, and continuous variables were described using the mean ± standard deviation. Normally distributed data were compared using the student’s t-test, while non-normally distributed data were compared using the Mann-Whitney U test. Categorical data were presented as frequency counts and percentage distributions, with intergroup comparisons assessed via chi-square testing. Statistical significance was set at a P-value < 0.05. The R 4.3.3 software was utilized for all statistical analyses.
Results
Cohort characteristics
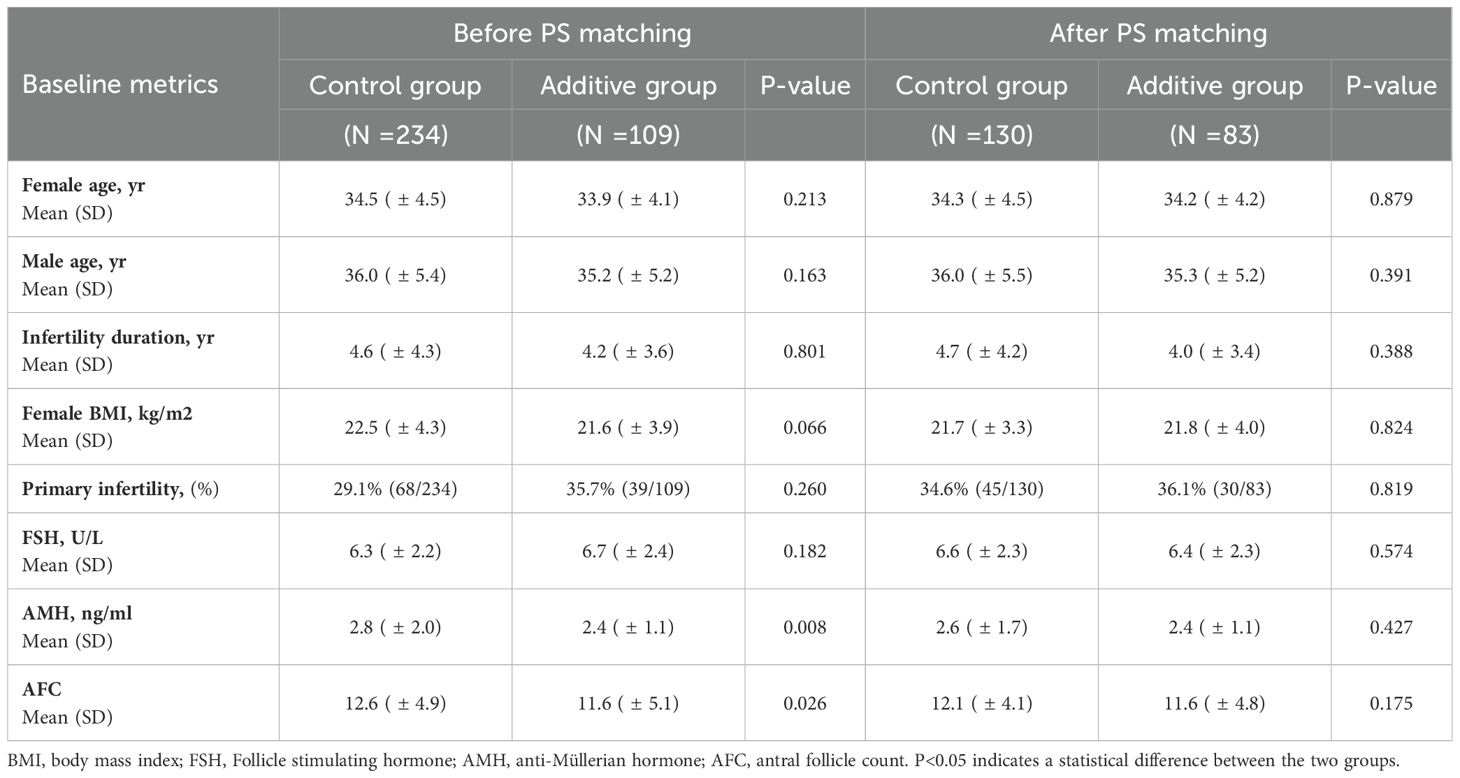
The screening process of this retrospective study cohort is shown in Figure 1. Initially, 364 patients from POSEIDON Groups 1 and 2 were included, and subsequently, 21 patients were excluded due to uterine anomalies, intrauterine adhesions, oocyte cryopreservation, and other reasons (Figure 1). Based on the inclusion and exclusion criteria of the cohort study, a total of 343 patients were enrolled. Table 1 summarizes the demographic characteristics of the cohort before and after propensity score (PS) matching. Prior to PS matching, the Control group had significantly higher AMH and AFC compared to the Additive group [2.8 (± 2.0) vs. 2.4 (± 1.1), P=0.008] and [12.6 (± 4.9) vs. 11.6 (± 5.1), P=0.026], respectively. Apart from these differences, no statistical differences were found in baseline data between the two groups, including mean age, duration of infertility, BMI, FSH, AMH, and age of male partners. After PS matching, 130 women in the Control group and 83 in the Additive group were included in the subsequent comparative analysis; no statistically differences were found in baseline indicators such as female age, infertility duration, BMI, FSH, AMH, and AFC between the two groups (Table 1).
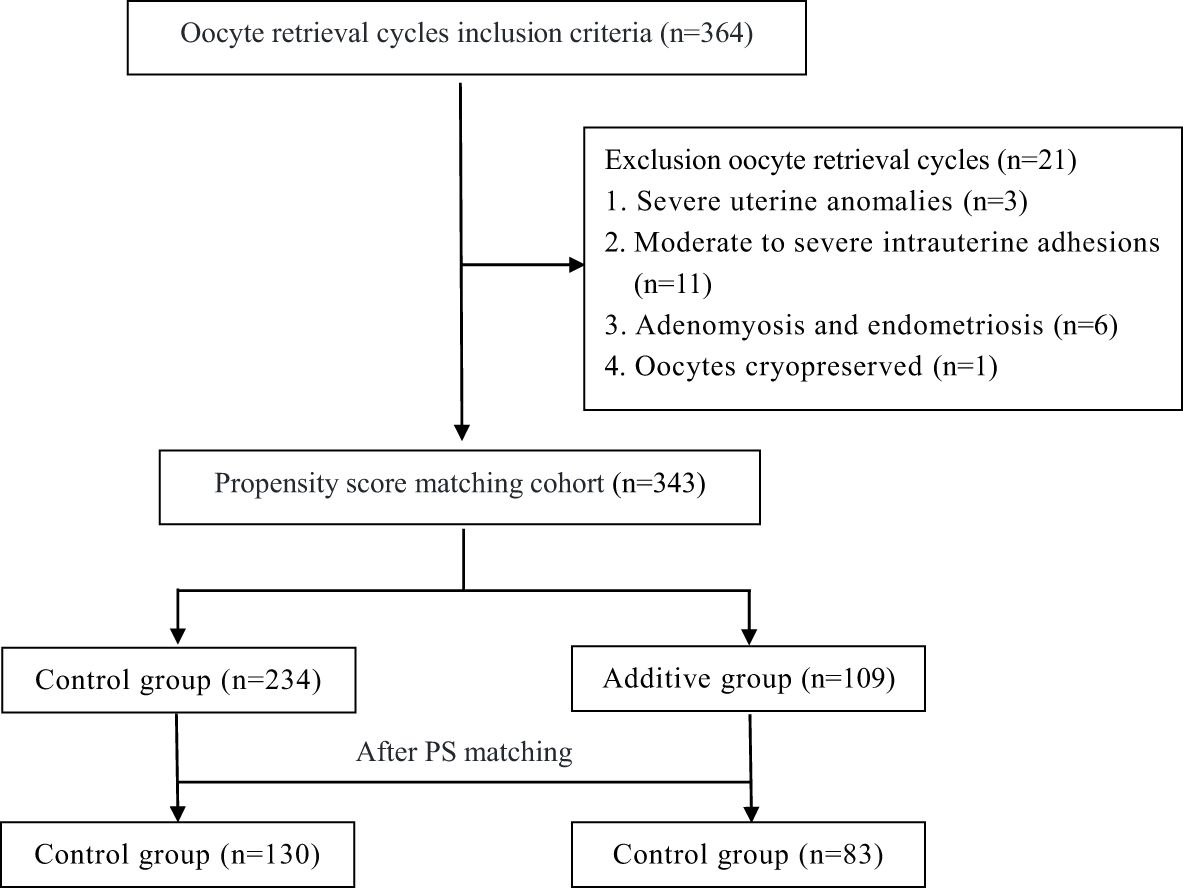
Figure 1. Flow chart showing the selection of the study cohort. Additive group: increased the Gn dosage in the second cycle, Control group: maintained or decreased the Gn dosage in the second cycle.
First IVF cycle
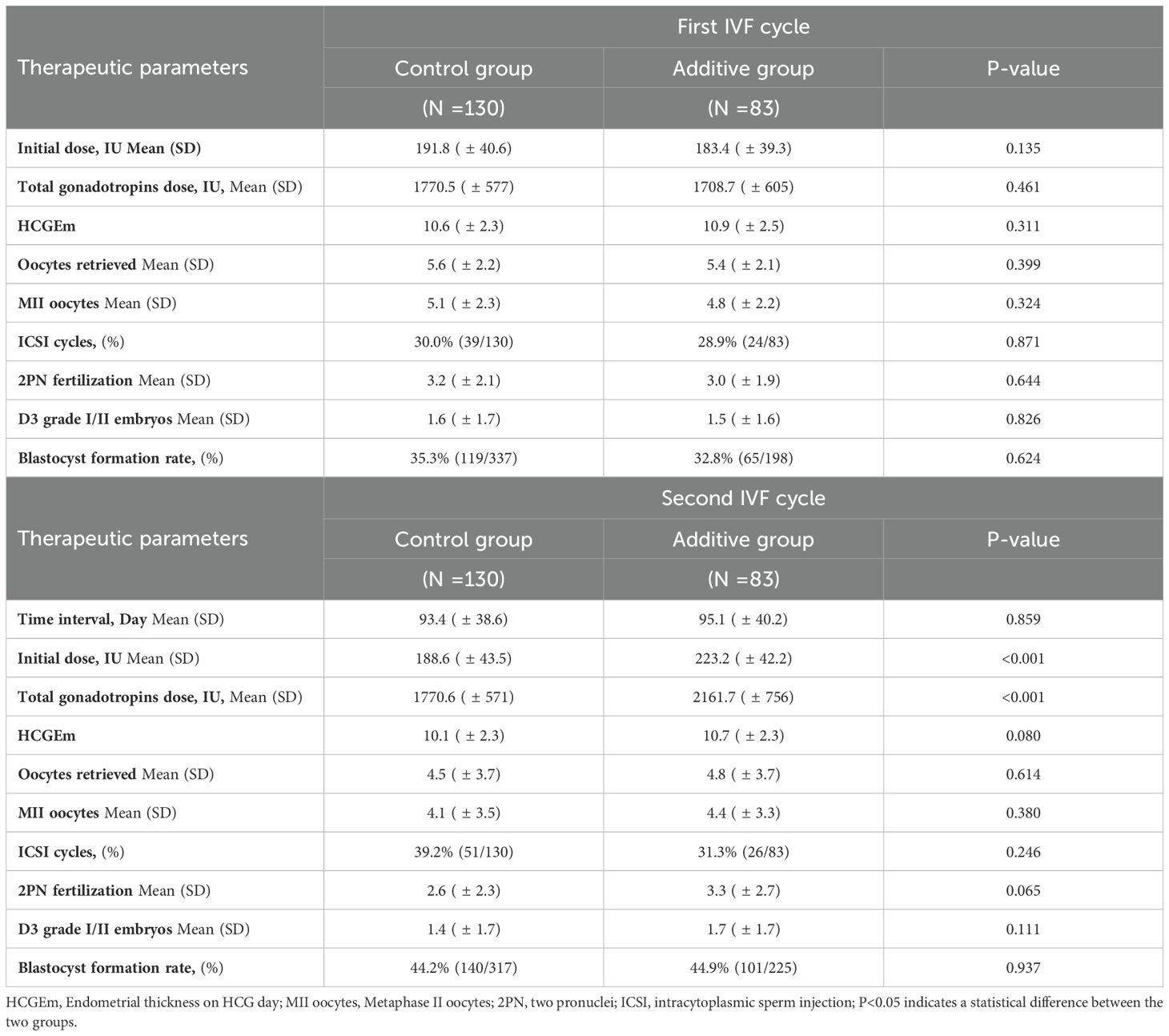
In the first IVF cycle, the Control group had a higher initial dose of gonadotropins (Gn) and a higher total gonadotropins dose compared to the Additive group (1770.5 vs 1708.7, P=0.461), but these differences were not significant. The endometrial thickness on the trigger day was slightly lower in the Control group (10.6 vs 10.9, P=0.311), and the average number of MII oocytes was slightly higher in the Control group (5.1 vs 4.8, P=0.324). The proportion of ICSI used was also slightly higher in the Control group (30.0% vs 28.9%, P=0.871). Ultimately, the Control group had a slightly higher rate of 2PN fertilization and the number of good quality embryos on day 3 compared to the Additive group, but these differences were not statistically significant. Similarly, the blastocyst formation rate was slightly higher in the Control group (35.3% vs 32.8%, P=0.624) (Table 2).
Second IVF cycle
In the second ovarian stimulation cycle, there was no statistically significant difference in the interval since the last ovulation stimulation between the two groups (93.4 vs 95.1, P=0.859). The Additive group had a higher initial dose of gonadotropins (Gn) compared to the Control group (191.8 vs 183.4, P=0.135), and a higher total gonadotropins dose (2161.7 vs 1770.6, P=0.461), as well as a thicker endometrial lining on the trigger day (10.6 vs 10.9, P=0.311), but these differences were not significant. The Additive group had a higher average number of retrieved oocytes (4.8 vs 4.5, P=0.614) and MII oocytes (4.4 vs 4.1, P=0.380) compared to Control group, but these differences were also not significant. The Control group had a higher proportion of ICSI fertilization compared to the Additive group (39.2% vs 31.3%, P=0.115). The Additive group had a higher 2PN fertilization rate (3.3 vs 2.6, P=0.065) and a higher number of good quality embryos on day 3 (1.7 vs 1.4, P=0.111) compared to the Control group, but these differences were not significant. Ultimately, the blastocyst formation rates were similar between the Additive group and the Control group (44.9% vs 44.2%, P=0.937), and both were higher than the blastocyst formation rates in the first IVF cycle (Table 2).
Fresh embryo transfer outcomes
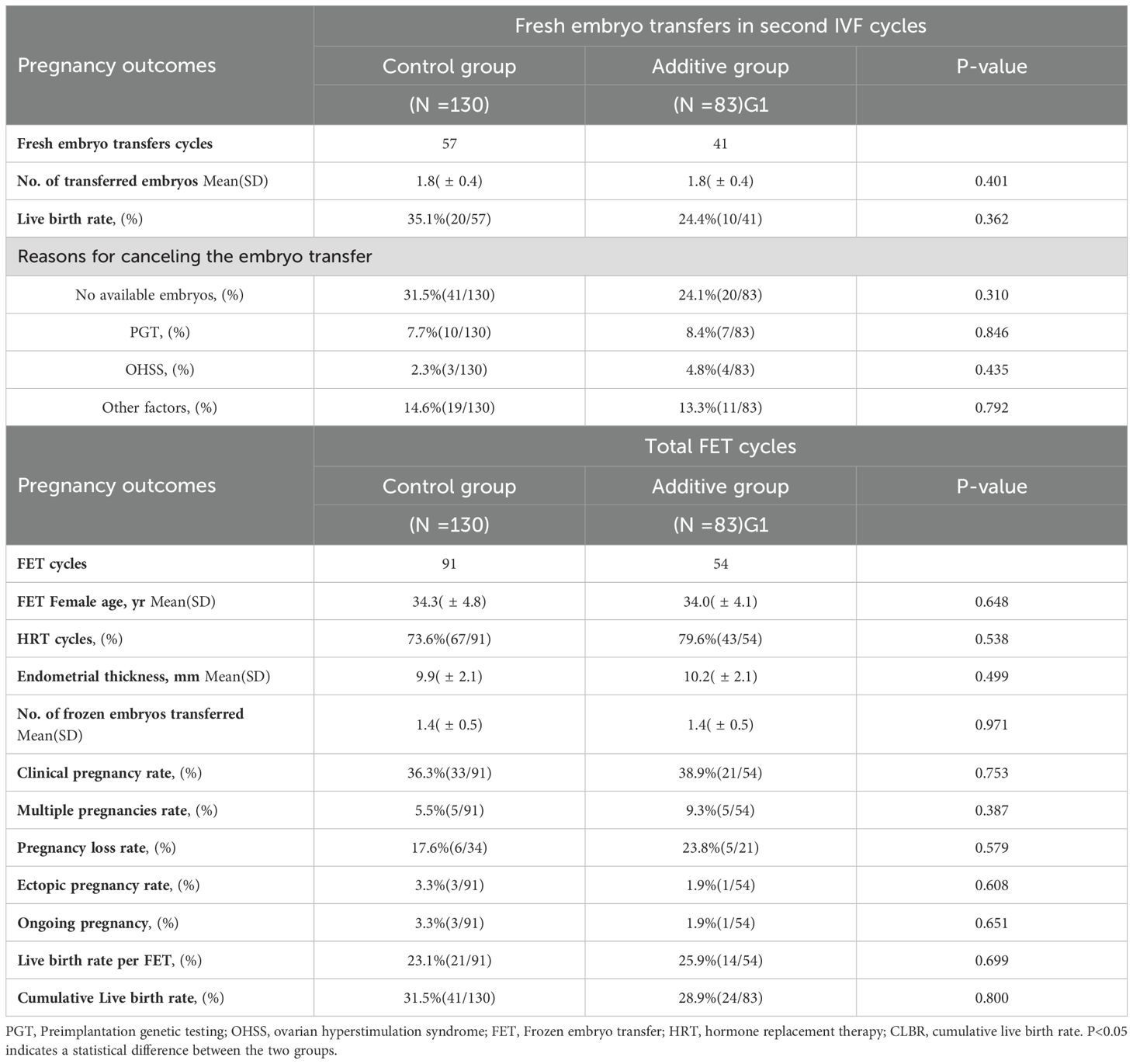
In the first IVF cycle, neither group had any live births following fresh ET. During the second IVF cycle, the Control group underwent 57 fresh embryo transfers, while the Additive group had 41. The average number of embryos transferred per patient was not significantly different between the two groups (1.8 vs 1.4, P=0.401). The live birth rate after fresh ET in the Additive group was lower than that in the Control group (35.1% vs 24.4%, P=0.362) (Table 3).
Frozen embryo transfer outcomes
In the comparison of frozen embryo transfer cycles, the Additive group underwent 91 embryo transfers, while the Control group had 54. Both groups primarily used hormone replacement therapy (HRT) for endometrial preparation, with no statistically significant difference. There was no statistical difference in the endometrial thickness before transfer or in the average number of embryos transferred. The clinical pregnancy rates between the Additive group and the Control group were not statistically different (36.3% vs 38.9%, P=0.753). The Control group had a higher rate of multiple pregnancies (9.3% vs 5.5%, P=0.387) and pregnancy loss (23.8% vs 5.5%, P=0.387) compared to the Additive group, but these differences were not statistically significant. The rates of ectopic pregnancy were also not statistically different between the two groups (3.3% vs 1.9%, P=0.651). The live birth rate following frozen embryo transfer in the Additive group was slightly lower than that in the Control group (23.1% vs 25.9%, P=0.699), without statistical significance. As of the study’s follow-up endpoint, a total of four patients in both groups were in ongoing pregnancies (Table 3).
Cumulative live birth rates
In the comparison of CLBR within two IVF cycles, the Control group had a slightly higher rate than the Additive group (31.5% vs. 28.9%, P=0.8), but this difference was not statistically significant.
Discussion
This study aimed to assess the impact of increased Gn dosage in the antagonist protocol on the cumulative live birth rate for patients in POSEIDON Groups 1 and 2. To reduce confounding factors, the study conducted a before-and-after comparison analysis of treatment outcomes in two consecutive IVF cycles. All enrolled patients initiated their second IVF cycle only after the failure of the first IVF attempt. Therefore, the CLBR in this study may have been lower than that of the general IVF population.
Ovarian reserve in women of reproductive age declines with age, and the time interval between two IVF cycles can affect treatment outcomes. Previous studies have included intervals of over one year or even several years between two IVF cycles, during which a longer interval can lead to a decrease in ovarian response due to aging (14–16). To minimize the impact of age on study outcomes, our retrospective analysis excluded cases where the time interval between two oocyte retrievals exceeded six months. Furthermore, to control for the influence of different ovarian stimulation protocols, the study only included patients who underwent both cycles with an antagonist protocol. After screening, a total of 343 infertile women were included. Following PS matching of baseline data, 130 patients in the Additive group and 83 in the Control group were analyzed. There were no statistically significant differences in baseline data between the two groups (Table 1). In the second IVF cycle, the initiating and total Gn doses in the Additive group were significantly higher than those in the Control group; however, there were no significant differences in the average number of retrieved oocytes, MII oocytes, 2PN fertilization, and blastocyst formation rate between the two groups. In the before-and-after comparison between the first and second IVF cycles, the number of retrieved oocytes and MII oocytes relatively decreased in the second IVF cycle, while the blastocyst formation rate and the number of blastocysts relatively increased after culture, the exact reasons for which are not yet fully understood. A possible explanation is that in the first and second groups of the POSEIDON classification, the follicles themselves have reduced sensitivity to FSH, and the number of follicles that respond to FSH is limited (17, 18), thus increasing the dose of Gn did not increase the number of retrieved oocytes and MII oocytes. However, the proportion of patients using ICSI fertilization in the second IVF cycle increased, which may be one of the reasons for the increased blastocyst formation rate and number of blastocysts. Finally, the antral follicles of the patients were recruited in a Follicular Waves pattern (19); the quality of the antral follicles recruited in the second IVF cycle may be superior to that in the first IVF cycle (20).
Eppsteiner EE et al. reported that in women with normal ovarian reserve undergoing repeated IVF cycles, increasing the gonadotropin dose was the only method that significantly increased oocyte yield (21). Studies by Out H. J and Drakopoulos P. et al. indicated that increasing the Gn dose in subsequent IVF cycles could result in a higher number of retrieved oocytes, regardless of whether the women had a normal ovarian response or a history of poor ovarian response (5, 22). In the second IVF cycle of our study, the group with increased Gn dose also obtained more MII oocytes and 2PN fertilized embryos. However, neither the studies by Drakopoulos P. nor Eppsteiner EE et al. tracked the CLBR, thus it remains unclear whether the increased oocyte yield could improve the CLBR (21, 22). Additionally, increasing the Gn dose poses additional risks to patients: excessive exogenous gonadotropins may increase the risk of OHSS (23, 24), potentially raise the incidence of embryo mosaicism (25, 26), and affect the endometrial receptivity in the fresh embryo transfer cycle of patients (27, 28); ultimately, the cumulative live birth rate of patients did not improve (29, 30).
In our retrospective study, the initiating dose and total dose of Gn in the Additive group were significantly higher than those in the Control group (P < 0.001), and the risk of moderate to severe OHSS was also higher in the Additive group. However, there was no statistically significant difference in the CLBR between the two groups. At the end of the follow-up period, 1 case in the Additive group and 3 cases in the Control group were still pregnant; however, regardless of the pregnancy outcomes, these did not affect the conclusions of the study. Therefore, increasing the dose of gonadotropin without indication not only increases the treatment cost for patients but also does not align with the modern IVF technology’s treatment philosophy of patient-friendliness and safety.
Limitations
The data for this retrospective study were sourced from a single in vitro fertilization (IVF) center. The study had limited male data collection. Although PS matching was used to control for some confounding factors and inconsistencies in treatment (31, 32), potential selection bias in patient ovarian stimulation might have affected the study outcomes and limited the applicability of our findings.
Conclusions
A comparative analysis of consecutive two IVF cycles in patients of POSEIDON Groups 1 and 2 indicated that increasing the dose of Gn under the antagonist protocol did not improve the cumulative live birth rate. Considering that increasing the dose of Gn would increase the treatment cost for patients and bring additional risks, it was not recommended to routinely increase the dose of Gn in subsequent IVF cycles for patients in POSEIDON Groups 1 and 2.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of the 924th Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HW: Software, Writing – original draft, Writing – review & editing. JD: Conceptualization, Data curation, Methodology, Writing – review & editing. SW: Data curation, Investigation, Writing – review & editing. BZ: Data curation, Writing – review & editing. HJ: Conceptualization, Data curation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the PLA Family Planning Special Project (Grant No. 20JSZ08).
Acknowledgments
The authors would like to thank all contributors to this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, and Gianaroli L. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod (Oxford England). (2011) 26:1616–24. doi: 10.1093/humrep/der092
2. Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertility sterility. (2016) 105:1452–3. doi: 10.1016/j.fertnstert.2016.02.005
3. Esteves SC, Yarali H, Vuong LN, Carvalho JF, Özbek İY, Polat M, et al. Low prognosis by the POSEIDON criteria in women undergoing assisted reproductive technology: A multicenter and multinational prevalence study of over 13,000 patients. Front Endocrinol. (2021) 12:630550. doi: 10.3389/fendo.2021.630550
4. Wang B, Liu W, Liu Y, Zhang W, Ren C, and Guan Y. What does unexpected suboptimal response during ovarian stimulation suggest, an overlooked group? Front Endocrinol. (2021) 12:795254. doi: 10.3389/fendo.2021.795254
5. Out HJ, Rutherford A, Fleming R, Tay CC, Trew G, Ledger W, et al. A randomized, double-blind, multicentre clinical trial comparing initial doses of 150 and 200 IU of recombinant FSH in women treated with the GnRH antagonist ganirelix for assisted reproduction. Hum Reprod (Oxford England). (2004) 19:90–5. doi: 10.1093/humrep/deh044
6. Vaiarelli A, Cimadomo D, Ubaldi N, Rienzi L, and Ubaldi FM. What is new in the management of poor ovarian response in IVF? Curr Opin obstetrics gynecology. (2018) 30:155–62. doi: 10.1097/GCO.0000000000000452
7. Chinta P, Antonisamy B, Mangalaraj AM, Kunjummen AT, and Kamath MS. POSEIDON classification and the proposed treatment options for groups 1 and 2: time to revisit? A retrospective analysis of 1425 ART cycles. Hum Reprod Open. (2021) 2021:hoaa070. doi: 10.1093/hropen/hoaa070
8. Hochberg A, Dahan MH, Yarali H, Vuong LN, and Esteves SC. Effect of follicle-stimulating hormone dose on the risk of being classified as suboptimal responders according to the POSEIDON criteria. J assisted Reprod Genet. (2024) 41(12):3387–98. doi: 10.1007/s10815-024-03296-2
9. Baker VL, Brown MB, Luke B, Smith GW, and Ireland JJ. Gonadotropin dose is negatively correlated with live birth rate: analysis of more than 650,000 assisted reproductive technology cycles. Fertility sterility. (2015) 104:1145–1152.e1141-1145. doi: 10.1016/j.fertnstert.2015.07.1151
10. Coelho Neto MA, Ludwin A, Borrell A, Benacerraf B, Dewailly D, da Silva Costa F, et al. Guerriero S et al: Counting ovarian antral follicles by ultrasound: a practical guide. Ultrasound obstetrics gynecology: Off J Int Soc Ultrasound Obstetrics Gynecology. (2018) 51:10–20. doi: 10.1002/uog.18945
11. Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod (Oxford England). (2011) 26:1270–83. doi: 10.1093/humrep/der037
12. Li D, Parmegiani L, Yang D, Vajta G, and Li R. Expert consensus on the morphological evaluation of human cleavage-stage embryos and blastocysts. Chin Med J. (2023) 136:1009–11. doi: 10.1097/CM9.0000000000002609
13. Gardner DK, Lane M, Stevens J, Schlenker T, and Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertility sterility. (2000) 73:1155–8. doi: 10.1016/S0015-0282(00)00518-5
14. Jain A, Robins JC, Williams DB, and Thomas MA. The effect of multiple cycles in oocyte donors. Am J obstetrics gynecology. (2005) 192:1382–4. doi: 10.1016/j.ajog.2004.12.038
15. Doldi N, Persico P, De Santis L, Rabellotti E, Papaleo E, and Ferrari A. Consecutive cycles in in vitro fertilization–embryo transfer. Gynecological endocrinology: Off J Int Soc Gynecological Endocrinol. (2005) 20:132–6. doi: 10.1080/09513590400021094
16. Polyzos NP, Nelson SM, Stoop D, Nwoye M, Humaidan P, Anckaert E, et al. Does the time interval between antimüllerian hormone serum sampling and initiation of ovarian stimulation affect its predictive ability in in vitro fertilization-intracytoplasmic sperm injection cycles with a gonadotropin-releasing hormone antagonist? A retrospective single-center study. Fertility sterility. (2013) 100:438–44. doi: 10.1016/j.fertnstert.2013.03.031
17. Esteves SC, Roque M, Bedoschi GM, Conforti A, Humaidan P, and Alviggi C. Defining low prognosis patients undergoing assisted reproductive technology: POSEIDON criteria-the why. Front Endocrinol. (2018) 9:461. doi: 10.3389/fendo.2018.00461
18. Simoni M, Nieschlag E, and Gromoll J. Isoforms and single nucleotide polymorphisms of the FSH receptor gene: implications for human reproduction. Hum Reprod Update. (2002) 8:413–21. doi: 10.1093/humupd/8.5.413
19. Baerwald AR, Adams GP, and Pierson RA. A new model for ovarian follicular development during the human menstrual cycle. Fertility sterility. (2003) 80:116–22. doi: 10.1016/S0015-0282(03)00544-2
20. Gu F, Ruan S, Luo C, Huang Y, Luo L, Xu Y, et al. Can repeat IVF/ICSI cycles compensate for the natural decline in fertility with age? an estimate of cumulative live birth rates over multiple IVF/ICSI cycles in Chinese advanced-aged population. Aging. (2021) 13:14385–98. doi: 10.18632/aging.203055
21. Eppsteiner EE, Sparks AE, Liu D, and Van Voorhis BJ. Change in oocyte yield in repeated in vitro fertilization cycles: effect of ovarian reserve. Fertility sterility. (2014) 101:399–402. doi: 10.1016/j.fertnstert.2013.10.049
22. Drakopoulos P, Santos-Ribeiro S, Bosch E, Garcia-Velasco J, Blockeel C, Romito A, et al. The effect of dose adjustments in a subsequent cycle of women with suboptimal response following conventional ovarian stimulation. Front Endocrinol. (2018) 9:361. doi: 10.3389/fendo.2018.00361
23. Toftager M, Bogstad J, Bryndorf T, Løssl K, Roskær J, Holland T, et al. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod (Oxford England). (2016) 31:1253–64. doi: 10.1093/humrep/dew051
24. Delbaere A, Smits G, Olatunbosun O, Pierson R, Vassart G, and Costagliola S. New insights into the pathophysiology of ovarian hyperstimulation syndrome. What makes the difference between spontaneous and iatrogenic syndrome? Hum Reprod (Oxford England). (2004) 19:486–9. doi: 10.1093/humrep/deh124
25. Shuai J, Liu W, Wan S, Chen Q, Zhang Q, Zhou D, et al. Total gonadotropin dose did not affect euploid blastocyst rates: an analysis of more than 19,000 oocytes. J assisted Reprod Genet. (2024) 41:2385–96. doi: 10.1007/s10815-024-03183-w
26. Sachdeva K, Upadhyay D, Discutido R, Varghese MM, Albuz F, Almekosh R, et al. Low gonadotropin dosage reduces aneuploidy in human preimplantation embryos: first clinical study in a UAE population. Genet testing Mol Biomarkers. (2018) 22:630–4. doi: 10.1089/gtmb.2018.0063
27. Shen X, Guo Y, Liu Y, Song W, Li G, and Jin H. Effects of total gonadotropin dose on embryo quality and clinical outcomes with AMH stratification in IVF cycles: a retrospective analysis of 12,588 patients. Eur J Med Res. (2024) 29:167. doi: 10.1186/s40001-024-01768-w
28. Munch EM, Sparks AE, Zimmerman MB, Van Voorhis BJ, and Duran EH. High FSH dosing is associated with reduced live birth rate in fresh but not subsequent frozen embryo transfers. Hum Reprod (Oxford England). (2017) 32:1402–9. doi: 10.1093/humrep/dex094
29. Jia ZC, Li YQ, Li R, Hou S, Xia QC, Yang K, et al. Comparison of two different initial dose of rhFSH in GnRH antagonist protocol for patients with normal ovarian reserve. Front Endocrinol. (2023) 14:1068141. doi: 10.3389/fendo.2023.1068141
30. Jayaprakasan K, Hopkisson J, Campbell B, Johnson I, Thornton J, and Raine-Fenning N. A randomised controlled trial of 300 versus 225 IU recombinant FSH for ovarian stimulation in predicted normal responders by antral follicle count. BJOG: an Int J obstetrics gynaecology. (2010) 117:853–62. doi: 10.1111/j.1471-0528.2010.02545.x
31. Connell MT, Richter KS, Devine K, Hill MJ, DeCherney AH, Doyle JO, et al. Larger oocyte cohorts maximize fresh IVF cycle birth rates and availability of surplus high-quality blastocysts for cryopreservation. Reprod biomedicine Online. (2019) 38:711–23. doi: 10.1016/j.rbmo.2018.12.007
Keywords: in vitro fertilization, POSEIDON, antagonist protocol, gonadotropin dosage, cumulative live birth rate
Citation: Wei H, Duan J, Wang S, Zhu B and Jiang H (2025) Gonadotropin dose selection for repeat IVF cycles in POSEIDON Groups 1 and 2. Front. Endocrinol. 16:1591743. doi: 10.3389/fendo.2025.1591743
Received: 11 March 2025; Accepted: 26 June 2025;
Published: 17 July 2025.
Edited by:
Suranga P. Kodithuwakku, University of Peradeniya, Sri LankaReviewed by:
Renato De Oliveira, Faculdade de Medicina do ABC, BrazilMünire Funda Cevher Akdulum, Gazi University, Türkiye
Copyright © 2025 Wei, Duan, Wang, Zhu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: HaiLing Jiang, NDkzNDUxNTkyQHFxLmNvbQ==
†ORCID: HaiLing Jiang, orcid.org/0009-0007-0127-3704
Hao Wei, orcid.org/0009-0004-1910-6144
 Hao Wei
Hao Wei JinLiang Duan
JinLiang Duan HaiLing Jiang
HaiLing Jiang