- Department of Ophthalmology, Xinqiao Hospital, Army Medical University, Chongqing, China
Purpose: To investigate the influence of renal function on the surgical outcomes of vitrectomy in patients with proliferative diabetic retinopathy (PDR).
Methods: A secondary analysis was conducted on data from a retrospective cohort study.
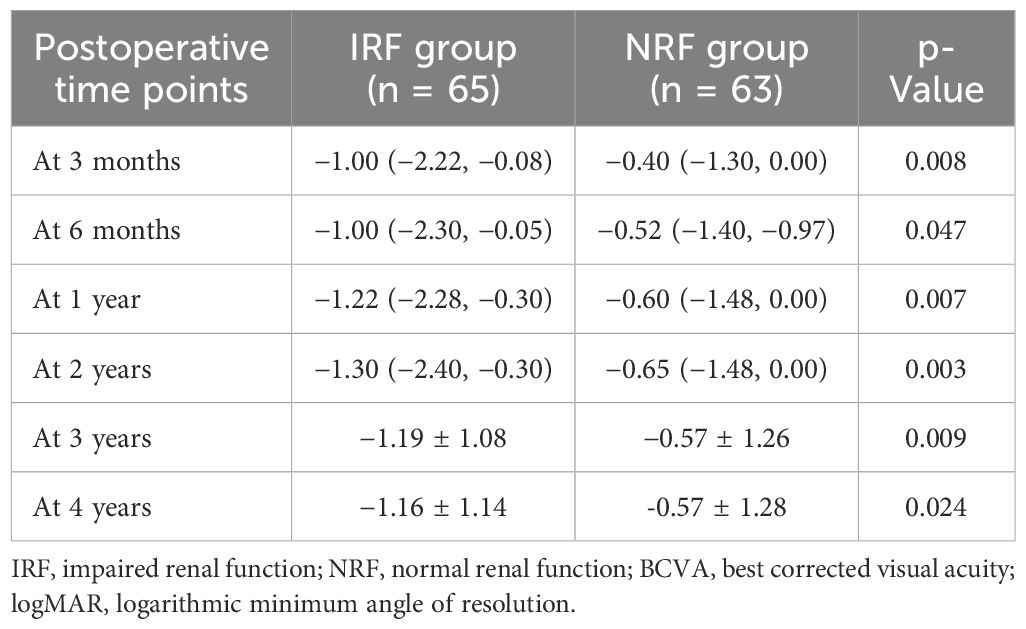
Results: A total of 128 eyes with PDR that underwent pars plana vitrectomy (PPV) and were followed up for at least 2 years were enrolled, including 65 eyes in the impaired renal function (IRF) group and 63 eyes in the normal renal function (NRF) group. No significant between-group differences were observed in the proportion of cataract surgery (p = 0.722), intraoperative retinal photocoagulation (p = 0.476), gas tamponade (p = 0.932), silicone oil tamponade (p = 0.254), retinal dialysis and/or iatrogenic retinal breaks (p = 0.447), and 23- or 25-gauge (G) microincision vitrectomy surgery (MIVS) (p = 0.160). Similarly, intergroup comparisons showed no significant differences in the proportion of reoperation (p = 0.883), postoperative vitreous hemorrhage (VH) and/or retinal detachment (RD) (p = 0.919), postoperative neovascular glaucoma (NVG) (p = 0.600), and postoperative diabetic macular edema (DME) (p = 0.794). Notably, the IRF group had worse baseline best corrected visual acuity (BCVA) (p = 0.039) and showed greater BCVA improvement at 3 months (p = 0.008), 6 months (p = 0.047), 1 year (p = 0.007), 2 years (p = 0.003), 3 years (p = 0.009), and 4 years (p = 0.024) after surgery. However, there was no significant difference in postoperative BCVA between the two groups at each follow-up time (all p > 0.05).
Conclusions: Renal insufficiency does not adversely affect the surgical outcomes of PPV in patients with PDR.
1 Introduction
Diabetes mellitus (DM) is one of the fastest-growing diseases in the world and is predicted to affect 6.93 billion adults by 2045 (1). Concomitant with the rising DM incidence, its complications, including diabetic retinopathy (DR) and diabetic nephropathy (DN), are increasingly prevalent. DR and DN not only dramatically decrease patients’ life quality but also impose an enormous socioeconomic burden (2–4).
As the most common vascular retinopathy in ophthalmology, DR has become the leading cause of blindness among working-age individuals globally (5). It was predicted that the number of patients with DR would rise to 1.91 billion by 2023 (6, 7). The primary pathophysiology of DR is the long-term effect of hyperglycemia on retinal microvasculature, resulting in increased vascular leakage, retinal ischemia, increased production of vasoactive factors, and finally, neovascularization (8). DR is clinically classified into non-proliferative DR (NPDR) and proliferative DR (PDR) stages. As a more advanced stage of DR, PDR is characterized by neovascularization, during which vision progressively deteriorates due to complications such as vitreous hemorrhage (VH) or retinal detachment (RD), requiring pars plana vitrectomy (PPV) surgery (9, 10).
DN, another severe microvascular complication of DM, affects approximately 40% of patients with DM. It is the primary cause of chronic kidney disease (CKD) and end-stage renal disease requiring dialysis or transplantation in the United States and worldwide (11–13). As both microvascular complications of DM, DN and DR exhibit similar pathophysiological mechanisms and close clinical interrelation (14, 15). Previous studies have demonstrated that hematuria, proteinuria, albumin–creatinine ratio, estimated glomerular filtration rate (eGFR), and glomerulopathy severity were significantly associated with the risk of DR (16, 17). DR severity and diabetic macular edema (DME) were reported to be positively correlated with renal function among southern Chinese patients (18). It was reported that CKD, high urine albumin to creatinine ratio, and low eGFR were associated with DR prevalence among type 2 diabetes mellitus (T2DM) populations (19). Kotlarsky P et al. demonstrated that the degree of renal impairment was directly proportional to the degree of ocular impairment—categorized as normal, mild NPDR, moderate NPDR, or PDR—in patients with T2DM (20). In addition, Fang J et al. revealed positive genetic associations between DR and DN and a significant causal link of DN with both NPDR and PDR (11). However, other studies have demonstrated that DR and DN do not always develop in parallel (21, 22). The relationship between these two microvascular complications may be influenced by obesity, ethnicity, and use of renin–angiotensin–aldosterone antagonists (23).
PPV is a major beneficial surgical treatment for PDR patients with vision-threatening lesions, including preretinal membrane, VH, tractional retinal detachment (TRD), and combined RD. Although most patients can achieve anatomical and visual improvement following PPV, some patients still experience disease progression and vision deterioration. Therefore, it is of great significance to identify factors influencing the surgical outcomes of vitrectomy for PDR. Given the association between DR and DN, renal function may be a vital influencing factor. A retrospective study has demonstrated that severe renal insufficiency may serve as a risk factor for patients with PDR requiring bilateral PPV (24). Additionally, renal dysfunction was reported as the most common cause of death during postoperative follow-up (25). However, limited studies have been conducted on the impact of renal function on PPV surgical outcomes, with a notable absence of long-term follow-up data and a lack of consistent conclusions across existing studies (26–29). In this study, we conducted a secondary analysis of data from a published paper by Nishi et al. (30), which investigated the factors associated with visual outcomes after a long follow-up in patients who underwent vitrectomy for PDR.
2 Methods
2.1 Study participants and procedures
This study represents a secondary analysis of data derived from a retrospective cohort study conducted by Nishi et al. (30). The original study design was thoroughly described in the primary article by Nishi et al. They retrospectively reviewed the medical records of PDR patients receiving PPV at Yamagata University Hospital between January 2008 and September 2012. They excluded cases with only DME. All patients received three-port 20-gauge (G) or microincision vitrectomy surgery (MIVS) (23-G or 25-G) PPV for persistent VH and TRD. Additionally, all the surgical procedures were performed by two vitreoretinal surgeons. Ultimately, 128 eyes from 100 PDR patients with a follow-up duration of at least 2 years were included. None of the patients received anti-vascular endothelial growth factor (anti-VEGF) therapy as a preoperative adjunct. Pan-retinal photocoagulation was cautiously performed either before or during PPV in all PDR patients. During postoperative follow-up, treatment was performed for progressed cataract, posterior capsular opacification, DME, neovascular glaucoma (NVG), and other vision-threatening lesions. Patients were followed up at 3 months, 6 months, 1 year, 2 years, and 4 years after surgery.
As Nishi et al. (30) stated in the original paper, this study was approved by the Ethics Committee of the Yamagata University Faculty of Medicine (approval number: H26-21) and conformed to the Declaration of Helsinki. Due to the retrospective nature of the study, the institutional review board waived the need for informed consent.
2.2 Outcome measures
The following baseline demographic and clinical characteristics were collected: age, gender, duration from awareness of visual loss to surgery, history of hypertension, DM duration, insulin treatment, oral antidiabetic medications, history of DN, history of coronary heart disease and/or stroke, and use of anticoagulant and/or antiplatelet drugs. Systemic factors included systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate, and blood biochemical parameters, such as preoperative eGFR, glycosylated hemoglobin (HbA1c), creatinine, blood urea nitrogen, uric acid, total cholesterol, triglyceride, and hemoglobin.
The preoperative ophthalmologic parameters included the following: visual acuity, history of intravitreal injection of triamcinolone acetonide, intraocular lens implantation, preoperative retinal photocoagulation, ocular hypertension (>21 mmHg), rubeosis iridis, posterior vitreous detachment, VH, fibrovascular membrane, RD, and macular detachment. The following intraoperative outcomes were included: cataract surgery, silicone oil or gas tamponade, retinal photocoagulation, intraoperative complications (retinal dialysis and iatrogenic retinal breaks), and MIVS application. Lastly, the postoperative outcomes included reoperation, postoperative complications (VH, RD, NVG, and DME), and best corrected visual acuity (BCVA) at each postoperative follow-up time point.
We allocated all patients into two groups according to eGFR: those with normal renal function (NRF) (CKD stage 1–2, eGFR ≥ 60 mL/min/1.73 m2) and those with impaired renal function (IRF) (CKD stage 3–4, eGFR < 60 mL/min per 1.73 m2) (28, 31). We compared baseline characteristics, intraoperative outcomes (including intraoperative retinal photocoagulation, gas tamponade, and silicone oil tamponade), and postoperative outcomes (reoperation rate, complications, and visual outcomes) between the two groups.
2.3 Statistical analyses
Statistical analyses were performed using the SPSS software (version 23.0, IBM Corp., Armonk, NY, USA). For continuous variables with normal distribution (verified by the Kolmogorov–Smirnov test and visual inspection of Q-Q plots), data were expressed as mean ± standard deviation (SD), and group comparisons were performed using an independent samples t-test. Non-normally distributed continuous variables were presented as median [interquartile range (IQR)], and the Mann–Whitney U test was used for two independent groups. Categorical variables were described as numbers and percentages (%) and analyzed using the chi-square test or with Fisher’s exact test when the expected cell counts were less than 5. Differences were regarded to be statistically significant at a two-sided alpha level of p < 0.05.
3 Results
3.1 Baseline characteristics
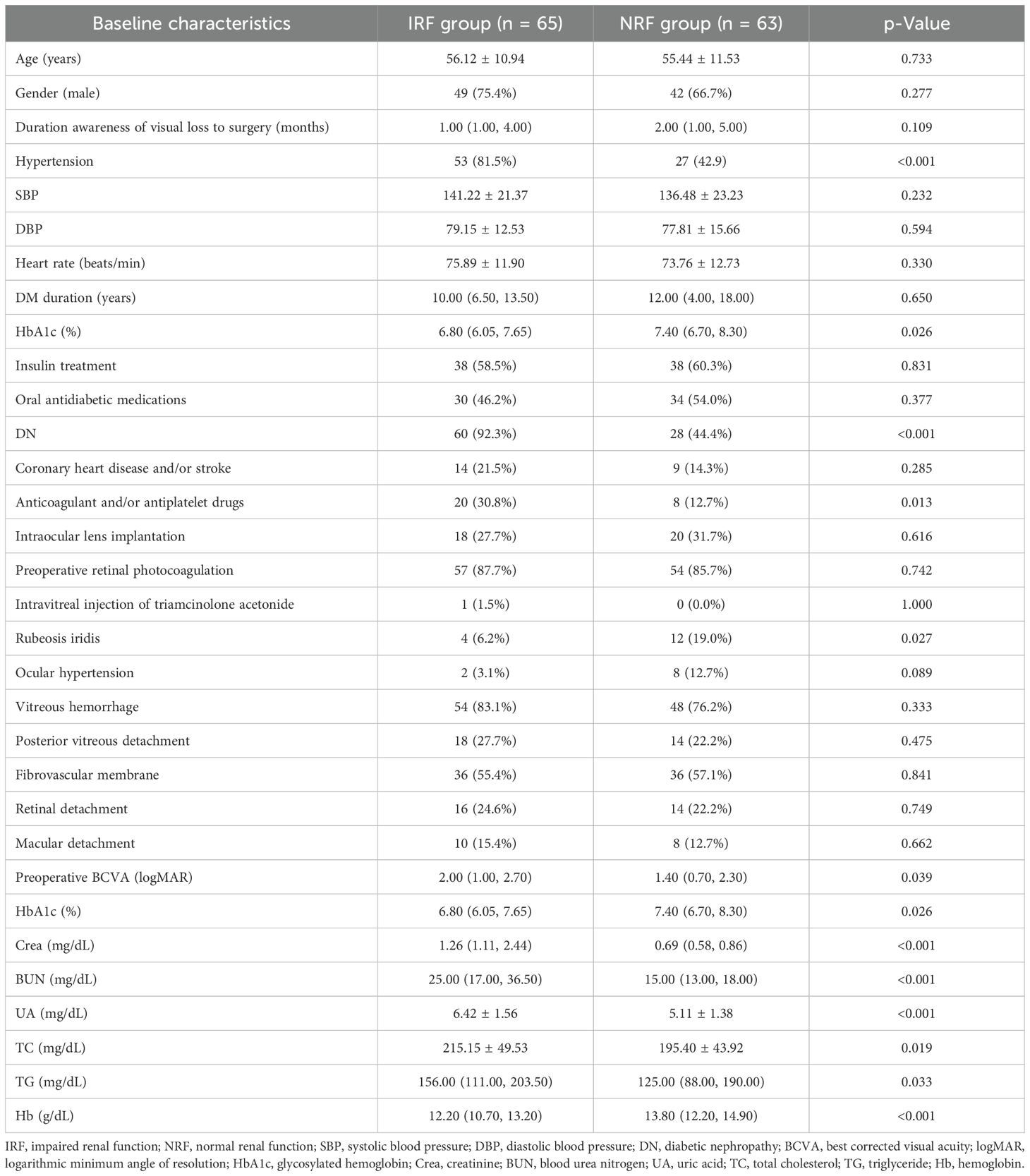
The baseline characteristics are shown in Table 1. A total of 128 eyes from PDR patients with a follow-up duration of at least 2 years after primary PPV were included in the final analysis, comprising 65 eyes in the IRF group and 63 eyes in the NRF group. Of these, 102 eyes (50 in IRF and 52 in NRF) completed 3-year follow-up, and 91 eyes (45 in IRF and 46 in NRF) completed 4-year follow-up. Compared with the NRF group, the IRF group had a higher percentage of hypertension (81.5% vs. 42.9%, p < 0.001), diabetic nephropathy (92.3% vs. 44.4%, p < 0.001), and anticoagulant and/or antiplatelet agent administration (30.8% vs. 12.7%, p = 0.013) but a lower percentage of rubeosis iridis (6.2% vs. 19.0%, p = 0.027). Additionally, the IRF group had worse baseline BCVA (2.00 logMAR vs. 1.40 logMAR, p = 0.039). For blood biochemical parameters, the IRF group exhibited significantly lower levels of HbA1c (%) (6.80 vs. 7.40, p = 0.026) and hemoglobin (12.20 vs. 13.80, p < 0.001) while showing higher levels of blood urea nitrogen (25.00 vs. 15.00, p < 0.001), creatinine (1.26 vs. 0.69, p < 0.001), uric acid (6.42 vs. 5.11, p < 0.001), total cholesterol (215.15 vs. 195.40, p = 0.019), and triglyceride (156.00 vs. 125.00, p = 0.033). There were no significant differences between the two groups in age, gender, duration from visual loss awareness to the primary surgery, or other baseline variables.
3.2 Intraoperative outcomes
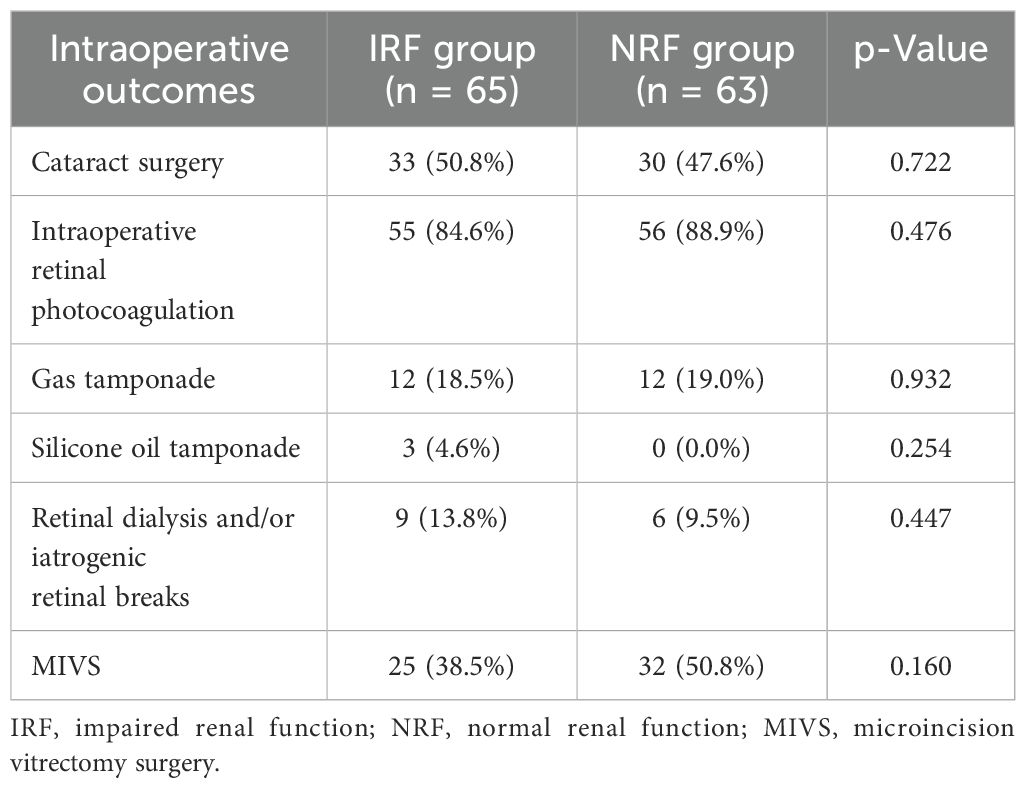
Intraoperative outcomes for the two groups are presented in Table 2. No significant group differences were found in the following parameters: the proportion of cataract surgery (50.8% vs. 47.6%, p = 0.722), intraoperative retinal photocoagulation (84.6% vs. 88.9%, p = 0.476), gas tamponade (18.5% vs. 19.0%, p = 0.932), silicone oil tamponade (4.6% vs. 0.0%, p = 0.254), intraoperative complications (retinal dialysis and/or iatrogenic retinal breaks) (13.8% vs. 9.5%, p = 0.447), and MIVS application (38.5% vs. 50.8%, p = 0.160).
3.3 Postoperative outcomes
3.3.1 Postoperative complications
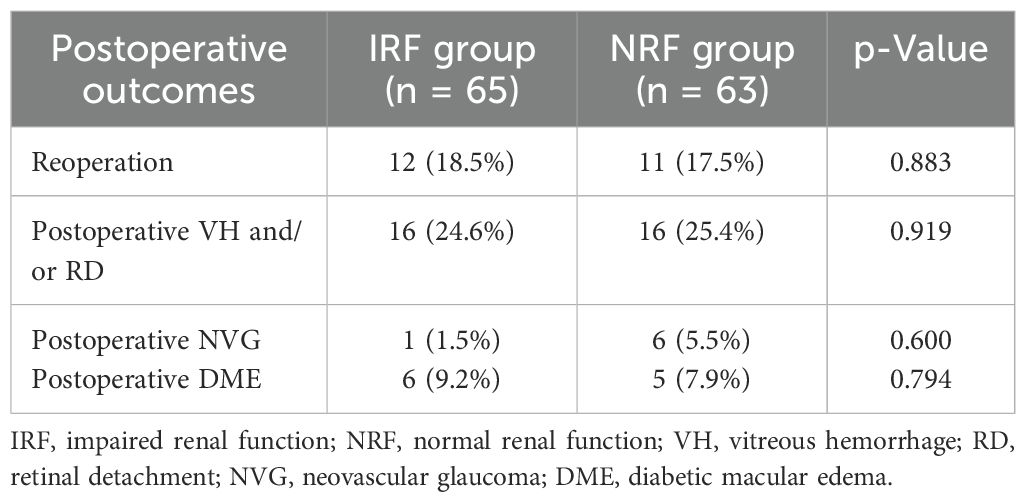
There were no significant differences between the two groups in terms of the rates of reoperation (18.5% vs. 17.5%, p = 0.883), postoperative VH and/or RD (24.6% vs. 25.4%, p = 0.919), postoperative NVG (1.5% vs. 5.5%, p = 0.600), and postoperative DME (9.2% vs. 7.9%, p = 0.794) (Table 3).
3.3.2 Postoperative visual outcomes
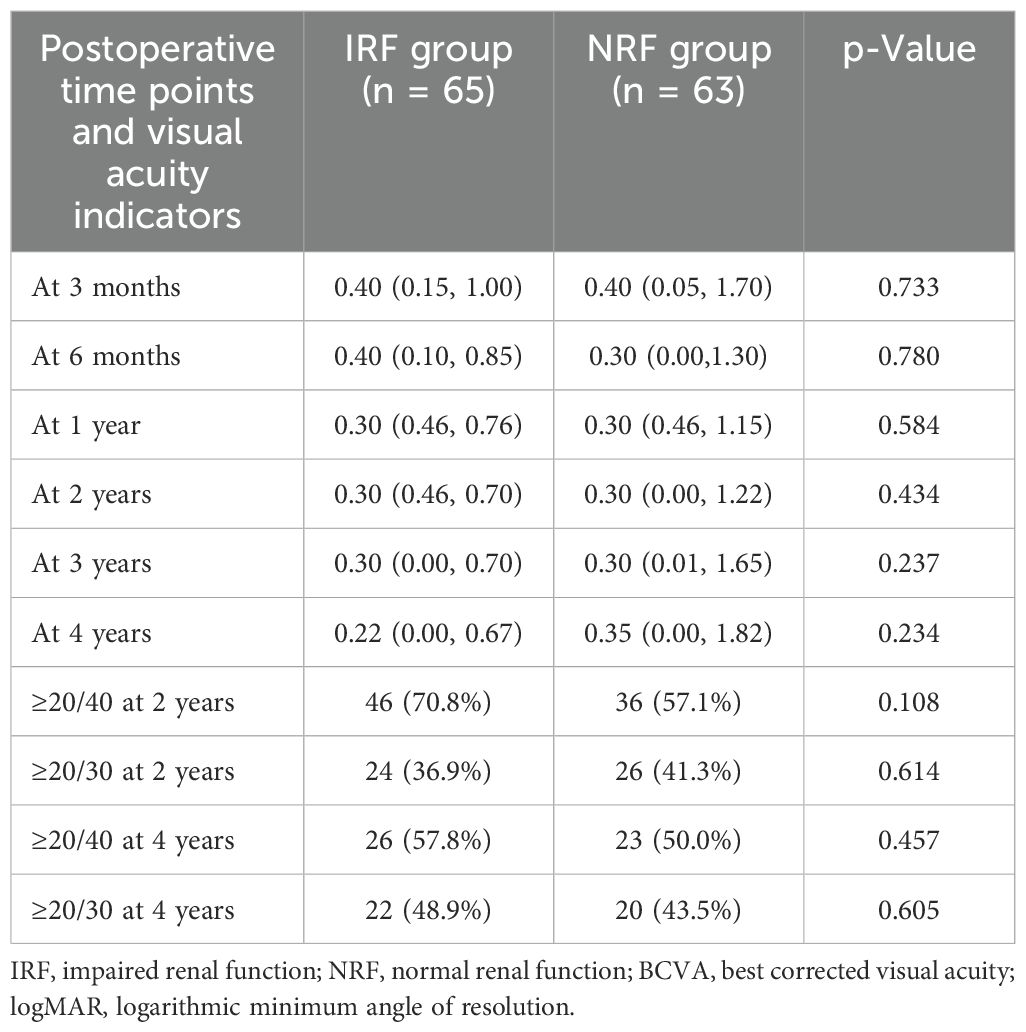
Table 4 presents the comparison of postoperative BCVA across different follow-up time points. As shown, the two groups demonstrated no significant differences in BCVA at 3 months (p = 0.733), 6 months (p = 0.780), 1 year (p = 0.584), 2 years (p = 0.434), 3 years (p = 0.237), and 4 years (p = 0.234) after PPV. Additionally, the percentage of postoperative BCVA ≥20/40 at 2 years (p = 0.108), ≥20/30 at 2 years (p = 0.614), ≥20/40 at 4 years (p = 0.457), and ≥20/30 at 4 years (p = 0.605) did not differ significantly between groups as well.
Table 5; Figure 1 show the comparison of postoperative BCVA (logMAR) improvement between the two groups. The IRF group demonstrated significantly greater BCVA improvement than the NRF group at 3 months (−1.00 logMAR vs. −0.40 logMAR, p = 0.008), 6 months (−1.00 logMAR vs. −0.52 logMAR, p = 0.047), 1 year (−1.22 logMAR vs. −0.60 logMAR, p = 0.007), 2 years (−1.30 logMAR vs. −0.65 logMAR, p = 0.003), 3 years (−1.19 logMAR vs. −0.57 logMAR, p = 0.009), and 4 years (−1.16 logMAR vs. −0.57 logMAR, p = 0.024).
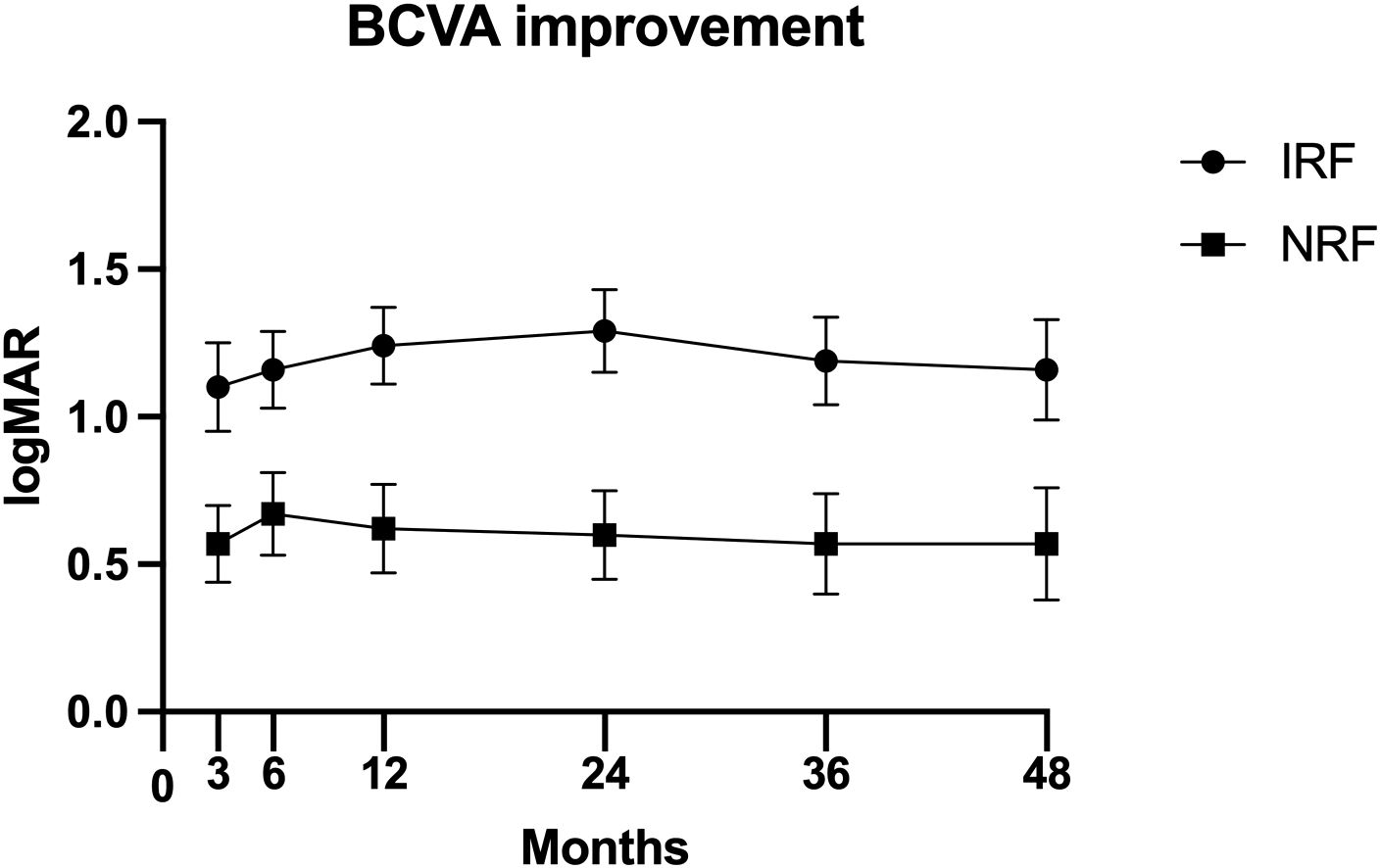
Figure 1. Postoperative best corrected visual acuity (BCVA) improvement [mean ± standard error of the mean (± SEM)] at 3 months, 6 months, 1 year, 2 years, 3 years, and 4 years after primary vitrectomy.
4 Discussion
As common microvascular complications of DM, DN and DR are reported to be closely related (20, 32). Despite the different functions of the kidney and the eye, these organs share similar molecular structure and developmental pathways, leading to substantial overlap in disease-causing risk factors, pathogenesis, and pathological changes between DR and DN (33). It has been reported that the severity of ocular damage in DR correlates with the severity of renal impairment in DN, and the two conditions exhibit reciprocal predictive effects (20). A cross-sectional study has demonstrated that DR, particularly PDR, serves as an independent predictor of DN (34). Additionally, subclinical DN can be predicted by eGFR in DR patients, even in the absence of proteinuria (35). However, as two different organs, the kidney and eye possess unique microenvironments and physiological processes, leading to different diabetic microangiopathic damage in the two. Furthermore, different susceptibility genes (36, 37) and involved cytokines have been found in the two diseases (38, 39). Additionally, DR is a neurovascular disease resulting from the destruction of the retinal neurovascular unit (40). Patients with DR do not necessarily have DN, and vice versa (41, 42). Thus, DR and DN demonstrate both parallel and non-parallel patterns in their disease onset and progression (17, 32).
In our study, 68.8% of PDR patients had DN, while 31.2% of PDR patients did not. Similarly, Agardh E et al. reported that 35% of 100 insulin-treated PDR patients showed no DN changes (43). Meanwhile, Bermejo S et al. conducted a multi-center and retrospective study in 832 diabetic patients with renal biopsy data. After removing missing data, they found that 65.6% of 221 DR patients had DN and 70.7% of 205 DN patients had DR (44). Additionally, Cao X et al. demonstrated that 64 of 98 (65.3%) Chinese patients with simple DN had DR (41). However, Dong Z at al. screened T2DM patients who underwent renal biopsy and found that DR prevalence was 82.3% in the DN group and 7.9% in the non-diabetic renal disease (NDRD) group, indicating that diabetic patients without DR were more likely to develop NDRD (42). The absence of DR in some DN patients may be due to two key factors: 1) renal biopsy was not performed in some clinically diagnosed DN patients, potentially misclassifying cases; 2) some NDRD (e.g., IgA nephropathy) are included in the DN group, which results in the underestimation of DR prevalence in DN. In addition, disparities in diagnostic means of DN and DR may also contribute. A study of 138 patients with insulin-dependent DM found that those without retinopathy rarely developed nephropathy, while retinopathy was common in those without nephropathy (45). This discrepancy may arise because the retina is more susceptible to diabetic damage than the kidney, and fundus examination, being less invasive than kidney biopsy, yields higher DR detection rates (46). However, most prior studies are retrospective with relatively small sample sizes. Large-scale prospective studies are needed in the future to clarify the association between these two diabetic microvascular complications.
Li J et al. reported a higher percentage of incomplete pan-retinal photocoagulation (64.8% vs. 42.3%), severe fibrovascular membrane (43.7% vs. 25.5%), macula-involved TRD (46.5% vs. 24.1%), and extensive retinal vascular closure (63.3% vs. 13.4%) in the IRF group. They found no significant difference in baseline corrected visual acuity (28). Our study demonstrated no significant difference in the percentage of preoperative retinal photocoagulation, fibrovascular membrane, and macular detachment, but worse baseline BCVA, in the IRF group. Additionally, the IRF group in our study had a lower percentage of rubeosis iridis (6.2% vs. 19.0%) than the NRF group. Similar to our results, Larrañaga-Fragoso P et al. reported worse baseline BCVA in the group with poor renal function (p = 0.039) (27). In contrast, Liu J et al. demonstrated that patients with renal dysfunction had better baseline BCVA (p = 0.006) (29). The discrepancies in these results may be due to different inclusion criteria, grouping methods, and sample sizes.
Our study showed no significant differences in intraoperative outcomes, including the rate of cataract surgery, intraoperative retinal photocoagulation, gas tamponade, silicone oil tamponade, intraoperative complications (retinal dialysis and/or iatrogenic retinal breaks), and MIVS application between the IRF and NRF groups. Similarly, Li et al. reported no significant differences in the rate of combined cataract surgery, the use of silicone oil tamponade, laser points, and operative time between the two groups. However, they recorded a higher percentage of severe intraoperative bleeding, intraocular subretinal fluid drainage, and perfluorocarbon liquids in the IRF group (28). Larrañaga-Fragoso P et al. detected no significant difference in the percentage of air and gas tamponade between groups categorized by renal function. Notably, they found that patients with poor renal function required less silicone. They postulated that it is because patients with poor renal function tend to present late with more fibrotic membranes (27). Additionally, the overall percentage of silicone tamponade in the study by Larrañaga-Fragoso P et al. was higher than ours (24.8% vs. 2.3%). This may be because all their participants had delamination or segmentation of pre-retinal membranes. Although 90 mL/min/1.73 m2 was used as the threshold for subgrouping, the study by Liu J et al. showed no significant differences between the IRF and NRF groups in terms of PPV time and the percentage of iatrogenic hole and tamponade (air, gas, and silicone oil) (all p > 0.05), which can be explained by the similar expression level of VEGF-A in the vitreous and aqueous humor and serum between the two groups (29).
Postoperative VH, NVG, and DME are common complications of PPV. Our study found no significant intergroup difference in the incidences of postoperative VH and/or RD, NVG, and DME. Similarly, Li J et al. demonstrated no significant difference in VH incidence (5.4% and 5.7%) and persistent DME (2.7% and 4.2%) between the IRF group and NRF groups. No cases of NVG developed during the 3-month follow-up period (28). However, Kameda Y et al. reported that the 6-month incidences of VH and NVG in the IRF group were significantly higher than those in the NRF group (43% vs. 10% and 17% vs. 2%, respectively). Additionally, patients with postoperative VH and NVG had lower eGFRs than those without (p < 0.001 and p = 0.007, respectively) (26). Larrañaga-Fragoso P et al. also showed that late VH occurred more frequently in patients with poor renal function (p = 0.003) (27). Liu J et al. reported a 60% overall DME rate at 3 months after surgery (50% in IRF and 68% in NRF) (29), which far exceeded our findings (8.6% in total, 9.3% in IRF, and 7.9% in NRF) and data reported by Li J et al. (28). The disparity may be due to their exclusion of patients who received preoperative anti-inflammatory therapy.
This study observed no significant between-group differences in postoperative BCVA at each follow-up point, consistent with previous studies’ results (27–29). Li J et al. reported no significant difference in the percentage of corrected visual acuity changes increased for more than two lines in patients with and without IRF (46.5% vs. 54.4%) (28). However, our data showed that the IRF group exhibited significantly greater BCVA improvement than the NRF group at all follow-up points. Similarly, Larrañaga-Fragoso P et al. demonstrated that patients with IRF achieved greater post-operative BCVA improvement than those with NRF at 6 months (27). In contrast, Liu J et al. found that the NRF group gained greater post-operative BCVA improvement at 3 months (29). The divergent findings across these studies may stem from the fact that the group with poorer baseline BCVA had greater room for visual improvement. Additionally, this study found no significant between-group differences in the proportion of postoperative BCVA ≥20/40 and ≥20/30 at both 2 and 4 years. Notably, Liu J et al. reported a lower proportion of patients in the IRF group with postoperative BCVA ≥ 6/12 at 3 months (6.7% vs. 13.3%), and Larrañaga-Fragoso P et al. similarly demonstrated a lower proportion of patients achieving BCVA ≥ 6/12 at 6 months in the IRF group (10.7% vs. 21.4%). This discrepancy may be attributed to the relatively short follow-up periods in the studies by Liu J et al. and Larrañaga-Fragoso P et al.
Our study is the first to investigate the association of renal function and long-term outcomes of PPV in patients with PDR. Patients in our cohort were followed up for at least 2 years and up to 4 years post-PPV. A comprehensive comparison was also conducted including baseline characteristics, intraoperative outcomes, postoperative complications, and postoperative visual outcomes. However, this study has several limitations. First, the retrospective design necessitates prospective studies to further confirm our findings. Second, all surgical procedures were performed by two surgeons, and subtle variations in surgical technique may affect anatomic and visual results. Third, although no statistical difference in the proportion of MIVS was observed between the two groups, the therapeutic effects of three-port 20-, 23-, and 25-G PPVs require further evaluation.
5 Conclusion
In conclusion, our secondary analysis demonstrated that renal insufficiency does not adversely affect the surgical outcomes of PPV for PDR. Thus, renal function alone should not be regarded as a prognostic indicator for PPV in patients with PDR.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Ethics Committee of the Yamagata University Faculty of Medicine (approval number: H26-21). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because Due to the retrospective nature of the study, the institutional review board waived the need of obtaining informed consent.
Author contributions
QY: Conceptualization, Data curation, Formal analysis, Project administration, Writing – original draft. ZY: Data curation, Formal analysis, Investigation, Writing – review & editing. WF: Formal analysis, Investigation, Writing – review & editing. XC: Investigation, Methodology, Project administration, Writing – review & editing. HZ: Methodology, Project administration, Software, Writing – review & editing. RY: Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank Nishi et al. for providing the raw data in their publication so that we can conduct this secondary analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1592618/full#supplementary-material
References
1. Cole JB and Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. (2020) 16:377–90. doi: 10.1038/s41581-020-0278-5
2. Morrish NJ, Wang SL, Stevens LK, Fuller JH, and Keen H. Mortality and causes of death in the who multinational study of vascular disease in diabetes. Diabetologia. (2001) 44 Suppl 2:S14–21. doi: 10.1007/pl00002934
3. Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet (London England). (2015) 385:1975–82. doi: 10.1016/s0140-6736(14)61601-9
4. da Rocha Fernandes J, Ogurtsova K, Linnenkamp U, Guariguata L, Seuring T, Zhang P, et al. Idf diabetes atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res Clin Pract. (2016) 117:48–54. doi: 10.1016/j.diabres.2016.04.016
5. Leasher JL, Bourne RR, Flaxman SR, Jonas JB, Keeffe J, Naidoo K, et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: A meta-analysis from 1990 to 2010. Diabetes Care. (2016) 39:1643–9. doi: 10.2337/dc15-2171
6. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. (2012) 35:556–64. doi: 10.2337/dc11-1909
7. López-Contreras AK, Martínez-Ruiz MG, Olvera-Montaño C, Robles-Rivera RR, Arévalo-Simental DE, Castellanos-González JA, et al. Importance of the use of oxidative stress biomarkers and inflammatory profile in aqueous and vitreous humor in diabetic retinopathy. Antioxidants (Basel Switzerland). (2020) 9:891. doi: 10.3390/antiox9090891
8. Kang Q and Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. (2020) 37:101799. doi: 10.1016/j.redox.2020.101799
9. Wang W and Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. (2018) 19:1816. doi: 10.3390/ijms19061816
10. Nawaz IM, Rezzola S, Cancarini A, Russo A, Costagliola C, Semeraro F, et al. Human vitreous in proliferative diabetic retinopathy: characterization and translational implications. Prog retinal eye Res. (2019) 72:100756. doi: 10.1016/j.preteyeres.2019.03.002
11. Fang J, Luo C, Zhang D, He Q, and Liu L. Correlation between diabetic retinopathy and diabetic nephropathy: A two-sample mendelian randomization study. Front Endocrinol. (2023) 14:1265711. doi: 10.3389/fendo.2023.1265711
12. Patel AB, Mistry K, and Verma A. Dapa-ckd: significant victory for ckd with or without diabetes. Trends Endocrinol metabolism: TEM. (2021) 32:335–7. doi: 10.1016/j.tem.2021.02.007
13. Scilletta S, Di Marco M, Miano N, Filippello A, Di Mauro S, Scamporrino A, et al. Update on diabetic kidney disease (Dkd): focus on non-albuminuric dkd and cardiovascular risk. Biomolecules. (2023) 13:752. doi: 10.3390/biom13050752
14. Mohandes S, Doke T, Hu H, Mukhi D, Dhillon P, and Susztak K. Molecular pathways that drive diabetic kidney disease. J Clin Invest. (2023) 133:e165654. doi: 10.1172/jci165654
15. Man RE, Sasongko MB, Wang JJ, MacIsaac R, Wong TY, Sabanayagam C, et al. The association of estimated glomerular filtration rate with diabetic retinopathy and macular edema. Invest Ophthalmol Visual Sci. (2015) 56:4810–6. doi: 10.1167/iovs.15-16987
16. Zhang J, Wang Y, Li L, Zhang R, Guo R, Li H, et al. Diabetic retinopathy may predict the renal outcomes of patients with diabetic nephropathy. Ren Fail. (2018) 40:243–51. doi: 10.1080/0886022x.2018.1456453
17. Tang S, An X, Sun W, Zhang Y, Yang C, Kang X, et al. Parallelism and non-parallelism in diabetic nephropathy and diabetic retinopathy. Front Endocrinol. (2024) 15:1336123. doi: 10.3389/fendo.2024.1336123
18. Zhuang X, Cao D, Yang D, Zeng Y, Yu H, Wang J, et al. Association of diabetic retinopathy and diabetic macular oedema with renal function in southern chinese patients with type 2 diabetes mellitus: A single-centre observational study. BMJ Open. (2019) 9:e031194. doi: 10.1136/bmjopen-2019-031194
19. Rodríguez-Poncelas A, Mundet-Tudurí X, Miravet-Jiménez S, Casellas A, Barrot-De la Puente JF, Franch-Nadal J, et al. Chronic kidney disease and diabetic retinopathy in patients with type 2 diabetes. PloS One. (2016) 11:e0149448. doi: 10.1371/journal.pone.0149448
20. Kotlarsky P, Bolotin A, Dorfman K, Knyazer B, Lifshitz T, and Levy J. Link between retinopathy and nephropathy caused by complications of diabetes mellitus type 2. Int Ophthalmol. (2015) 35:59–66. doi: 10.1007/s10792-014-0018-6
21. Zhao L, Ren H, Zhang J, Cao Y, Wang Y, Meng D, et al. Diabetic retinopathy, classified using the lesion-aware deep learning system, predicts diabetic end-stage renal disease in chinese patients. Endocr Pract. (2020) 26:429–43. doi: 10.4158/ep-2019-0512
22. Grunwald JE, Pistilli M, Ying GS, Daniel E, Maguire M, Xie D, et al. Association between progression of retinopathy and concurrent progression of kidney disease: findings from the chronic renal insufficiency cohort (Cric) study. JAMA Ophthalmol. (2019) 137:767–74. doi: 10.1001/jamaophthalmol.2019.1052
23. Mottl AK, Kwon KS, Garg S, Mayer-Davis EJ, Klein R, and Kshirsagar AV. The association of retinopathy and low gfr in type 2 diabetes. Diabetes Res Clin Pract. (2012) 98:487–93. doi: 10.1016/j.diabres.2012.09.041
24. Song YS, Nagaoka T, Omae T, Yokota H, Takahashi A, and Yoshida A. Systemic risk factors in bilateral proliferative diabetic retinopathy requiring vitrectomy. Retina (Philadelphia Pa). (2016) 36:1309–13. doi: 10.1097/iae.0000000000000886
25. Banerjee PJ, Moya R, Bunce C, Charteris DG, Yorston D, and Wickham L. Long-term survival rates of patients undergoing vitrectomy for proliferative diabetic retinopathy. Ophthalmic Epidemiol. (2016) 23:94–8. doi: 10.3109/09286586.2015.1089578
26. Kameda Y, Saeki T, Hanai K, Suzuki Y, Uchigata Y, Babazono T, et al. Is chronic kidney disease affecting the postoperative complications of vitrectomy for proliferative diabetic retinopathy? J Clin Med. (2021) 10:5309. doi: 10.3390/jcm10225309
27. Larrañaga-Fragoso P, Laviers H, McKechnie C, and Zambarakji H. Surgical outcomes of vitrectomy surgery for proliferative diabetic retinopathy in patients with abnormal renal function. Graefes Arch Clin Exp Ophthalmol. (2020) 258:63–70. doi: 10.1007/s00417-019-04532-7
28. Li J, Chandra A, Liu L, Zhang L, Xu J, and Zhao M. Ocular findings, surgery details and outcomes in proliferative diabetic retinopathy patients with chronic kidney disease. PloS One. (2022) 17:e0273133. doi: 10.1371/journal.pone.0273133
29. Liu J, Zhang W, Xie P, Yuan S, Jiang L, Liu Q, et al. The relationship between renal function and surgical outcomes of patients with proliferative diabetic retinopathy. Front Endocrinol (Lausanne). (2022) 13:984561. doi: 10.3389/fendo.2022.984561
30. Nishi K, Nishitsuka K, Yamamoto T, and Yamashita H. Factors Correlated with Visual Outcomes at Two and Four Years after Vitreous Surgery for Proliferative Diabetic Retinopathy. PloS One. (2021) 16:e0244281. doi: 10.1371/journal.pone.0244281
31. Jia W, Gao X, Pang C, Hou X, Bao Y, Liu W, et al. Prevalence and risk factors of albuminuria and chronic kidney disease in chinese population with type 2 diabetes and impaired glucose regulation: shanghai diabetic complications study (Shdcs). Nephrology dialysis transplantation: Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc. (2009) 24:3724–31. doi: 10.1093/ndt/gfp349
32. Li J, Cao Y, Liu W, Wang Q, Qian Y, and Lu P. Correlations among diabetic microvascular complications: A systematic review and meta-analysis. Sci Rep. (2019) 9:3137. doi: 10.1038/s41598-019-40049-z
33. Wong CW, Wong TY, Cheng CY, and Sabanayagam C. Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int. (2014) 85:1290–302. doi: 10.1038/ki.2013.491
34. El-Asrar AM, Al-Rubeaan KA, Al-Amro SA, Moharram OA, and Kangave D. Retinopathy as a predictor of other diabetic complications. Int Ophthalmol. (2001) 24:1–11. doi: 10.1023/a:1014409829614
35. Saini DC, Kochar A, and Poonia R. Clinical correlation of diabetic retinopathy with nephropathy and neuropathy. Indian J Ophthalmol. (2021) 69:3364–8. doi: 10.4103/ijo.IJO_1237_21
36. Skol AD, Jung SC, Sokovic AM, Chen S, Fazal S, Sosina O, et al. Integration of genomics and transcriptomics predicts diabetic retinopathy susceptibility genes. Elife. (2020) 9:e59980. doi: 10.7554/eLife.59980
37. Zhou TB, Drummen GP, Jiang ZP, and Li HY. Methylenetetrahydrofolate reductase (Mthfr) C677t gene polymorphism and diabetic nephropathy susceptibility in patients with type 2 diabetes mellitus. Ren Fail. (2015) 37:1247–59. doi: 10.3109/0886022x.2015.1064743
38. Wu CC, Sytwu HK, and Lin YF. Cytokines in diabetic nephropathy. Adv Clin Chem. (2012) 56:55–74. doi: 10.1016/b978-0-12-394317-0.00014-5
39. Obadă O, Pantalon AD, Rusu-Zota G, Hăisan A, Lupuşoru SI, Constantinescu D, et al. Aqueous humor cytokines in non-proliferative diabetic retinopathy. Medicina (Kaunas). (2022) 58:909. doi: 10.3390/medicina58070909
40. Ji L, Tian H, Webster KA, and Li W. Neurovascular regulation in diabetic retinopathy and emerging therapies. Cell Mol Life Sci. (2021) 78:5977–85. doi: 10.1007/s00018-021-03893-9
41. Cao X, Gong X, and Ma X. Diabetic nephropathy versus diabetic retinopathy in a chinese population: A retrospective study. Med Sci Monit. (2019) 25:6446–53. doi: 10.12659/msm.915917
42. Dong Z, Wang Y, Qiu Q, Zhang X, Zhang L, Wu J, et al. Clinical predictors differentiating non-diabetic renal diseases from diabetic nephropathy in a large population of type 2 diabetes patients. Diabetes Res Clin Pract. (2016) 121:112–8. doi: 10.1016/j.diabres.2016.09.005
43. Agardh E, Tallroth G, Bauer B, Cavallin-Sjöberg U, and Agardh CD. Retinopathy and nephropathy in insulin-dependent diabetics: an inconsistent relationship? Diabetes Med. (1987) 4:248–50. doi: 10.1111/j.1464-5491.1987.tb00873.x
44. Bermejo S, González E, López-Revuelta K, Ibernon M, López D, Martín-Gómez A, et al. The coexistence of diabetic retinopathy and diabetic nephropathy is associated with worse kidney outcomes. Clin Kidney J. (2023) 16:1656–63. doi: 10.1093/ckj/sfad142
45. Johansen J, Sjølie AK, Elbøl P, and Eshøj O. The relation between retinopathy and albumin excretion rate in insulin-dependent diabetes mellitus. From the funen county epidemiology of type 1 diabetes complications survey. Acta Ophthalmol (Copenh). (1994) 72:347–51. doi: 10.1111/j.1755-3768.1994.tb02771.x
Keywords: diabetes mellitus, proliferative diabetic retinopathy, renal function, surgical outcomes, vitrectomy
Citation: Yuan Q, Yang Z, Fan W, Chen X, Zou H and Yuan R (2025) The influence of renal function on surgical outcomes of vitrectomy in patients with proliferative diabetic retinopathy. Front. Endocrinol. 16:1592618. doi: 10.3389/fendo.2025.1592618
Received: 12 March 2025; Accepted: 24 June 2025;
Published: 16 July 2025.
Edited by:
Mayank Choubey, NYU Grossman Long Island School of Medicine, United StatesReviewed by:
Beatrice Gallo, Mid and South Essex NHS Foundation Trust, United KingdomAyusman Dash, University of Cincinnati, United States
Copyright © 2025 Yuan, Yang, Fan, Chen, Zou and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongdi Yuan, WXVhbnJvbmdkaUB0bW11LmVkdS5jbg==
 Qiongzhen Yuan
Qiongzhen Yuan Zhouquan Yang
Zhouquan Yang