- 1Department of Gynecology, The Affiliated Traditional Chinese Medicine Hospital, Southwest Medical University, Luzhou, Sichuan, China
- 2Department of Health Management Center, The Affiliated Hospital, Southwest Medical University, Luzhou, Sichuan, China
- 3Department of Reproductive Medicine Center, The Affiliated Hospital, Southwest Medical University, Luzhou, Sichuan, China
- 4Department of Pathology, The Affiliated Hospital, Southwest Medical University, Luzhou, China
Background: Poor ovarian response (POR) during in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) significantly compromises clinical pregnancy outcomes. This study evaluated the clinical characteristics and outcomes of IVF/ICSI cycles in poor ovarian responders, focusing specifically on maternal age and progesterone levels at the trigger day as predictors of clinical pregnancy.
Methods: This retrospective study included 652 poor ovarian responders treated with IVF/ICSI between January 2018 and December 2021 at a tertiary fertility center. POR was defined according to the Bologna criteria. Various ovarian stimulation protocols (antagonist, modified natural cycle, short agonist, and long agonist protocols) were employed based on individualized patient assessment. Demographic data, ovarian stimulation details, cycle outcomes, and hormonal levels at trigger day were analyzed. Multivariate logistic regression was performed to identify independent predictors of clinical pregnancy.
Results: Of the 652 patients analyzed, the clinical pregnancy rate was 5.5%. Age, body mass index (BMI), stimulation protocols, and other clinical variables showed no significant differences between pregnant and non-pregnant groups. Multivariate logistic regression analysis identified maternal age (adjusted OR: 1.035; 95% CI: 1.024–1.078; P=0.047) and progesterone levels at the trigger day (adjusted OR: 1.422; 95% CI: 1.380–1.564; P=0.034) as independent predictors of clinical pregnancy.
Conclusions: Maternal age and progesterone levels on the trigger day are independent predictors of clinical pregnancy in poor ovarian responders undergoing IVF/ICSI. Tailoring ovarian stimulation strategies according to these predictive factors may enhance clinical outcomes in this challenging patient population. Further studies exploring advanced techniques and individual patient characteristics are necessary to optimize management strategies for POR patients.
Introduction
Infertility, defined as the inability to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse, affects 10-15% of couples worldwide (1). Assisted reproductive technology (ART), including in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), has become the primary treatment option for couples struggling with infertility (2). Despite significant advances in ART, poor ovarian response (POR) in IVF/ICSI treatments remains a challenge for reproductive medicine (3).
POR is characterized by a reduced number of developing follicles and a consequent low number of retrieved oocytes following controlled ovarian stimulation (COS) (4). POR occurs in approximately 9-24% of IVF/ICSI cycles and is associated with reduced pregnancy rates and increased cycle cancellation rates (5). Several factors have been proposed to contribute to POR, including advanced maternal age, diminished ovarian reserve, and genetic factors (6, 7). However, the exact etiology of POR remains elusive, making it difficult to develop effective treatment strategies for this challenging patient population.
Various ovarian stimulation protocols have been developed to optimize treatment outcomes in poor ovarian responders, including the antagonist protocol, modified natural cycle (MNC), short agonist protocol, and long agonist protocol (8). Although these protocols have shown some benefits, the optimal stimulation strategy for poor ovarian responders is still a matter of debate (9). Moreover, existing studies on the clinical characteristics and treatment outcomes of poor ovarian responders have reported inconsistent results, and the identification of potential predictors for clinical pregnancy remains an unmet need (10, 11).
Maternal age has long been recognized as an important determinant of fertility and treatment outcomes in ART (12). As women age, the quantity and quality of their oocytes decline, leading to decreased pregnancy rates and increased miscarriage rates (13). Several studies have investigated the association between maternal age and treatment outcomes in poor ovarian responders undergoing IVF/ICSI, but the results have been inconclusive (14, 15). Another potential predictor of clinical pregnancy in poor ovarian responders is the progesterone level on the trigger day. Progesterone, a hormone produced by the corpus luteum, plays a crucial role in the establishment and maintenance of pregnancy (16). Elevated progesterone levels on the day of human chorionic gonadotropin (hCG) administration have been associated with decreased pregnancy rates in IVF/ICSI cycles, possibly due to impaired endometrial receptivity (17). However, the relationship between progesterone levels on the trigger day and treatment outcomes in poor ovarian responders remains unclear (18).
Given the limited and inconsistent evidence on the clinical characteristics and treatment outcomes of poor ovarian responders, as well as the potential predictors of clinical pregnancy, further research is needed to better understand this complex patient population and optimize their treatment strategies. In this retrospective study, we aimed to assess the clinical characteristics and treatment outcomes of poor ovarian responders treated with IVF/ICSI between January 2018 and December 2021, and identify potential predictors for clinical pregnancy, focusing on maternal age and progesterone levels on the trigger day. Understanding the impact of these factors on clinical pregnancy outcomes in poor ovarian responders could have important implications for personalized treatment strategies and improve the chances of pregnancy success for this challenging patient population. Furthermore, this study may help to shed light on the underlying mechanisms contributing to POR and guide future research efforts in the field of reproductive medicine.
Patients and methods
This retrospective study was conducted in a tertiary fertility center between January 2018 and December 2021. The study population comprised of poor ovarian responders undergoing IVF or ICSI treatments. Poor ovarian response was defined according to the Bologna criteria, which included at least two of the following three features: (1) advanced maternal age (≥ 40 years) or any other risk factor for poor ovarian response, (2) a previous poor ovarian response (≤ 3 oocytes with a conventional stimulation protocol), and (3) an abnormal ovarian reserve test, defined specifically as an antral follicle count (AFC) of less than 5-7, and/or an anti-Mullerian hormone (AMH) level of 0.5-1.1 ng/ml. Patients with missing data, preimplantation genetic testing, or oocyte or embryo cryopreservation were excluded from the study.
Patients were treated with various ovarian stimulation protocols, including the antagonist protocol, modified natural cycle (MNC), short agonist protocol, and long agonist protocol. The choice of protocol was based on the patient’s age, ovarian reserve, and previous response to ovarian stimulation. Data were collected from the electronic medical records and included patients’ demographic characteristics, stimulation details, and treatment outcomes. The primary outcome of interest was clinical pregnancy, defined as the presence of a gestational sac with fetal heartbeat observed on transvaginal ultrasound at 6–7 weeks of gestation.
Statistical analysis was performed as described in the “Statistical Analysis” section. Descriptive statistics were used to summarize the study population’s baseline characteristics, and comparative analyses were conducted to identify differences in outcomes between various subgroups. Multivariate logistic regression analysis was employed to evaluate the potential predictors of clinical pregnancy in poor ovarian responders.
This study was approved by the Institutional Review Board of the fertility center, and the requirement for informed consent was waived due to the retrospective nature of the study. All procedures were performed in accordance with the ethical standards of the institutional research committee and the Helsinki Declaration.
Statistical analysis
Statistical analysis for the current study was performed using the SPSS software package version 25 (SPSS Inc., Chicago, IL). Continuous variables were presented as means and standard deviations (SD), while categorical variables were presented as numbers and percentages. Differences in variables were statistically analyzed by Student’s t-test, Fisher exact test, and Pearson chi-square test, as appropriate. Normality of variables was assessed via the Shapiro-Wilks test of normality. To further investigate predictors for clinical pregnancy in poor ovarian responders, a multivariate logistic regression analysis was used, controlling for confounding effects that included the patients’ age, BMI, protocol type, day 3 FSH levels, duration of stimulation, total dose of gonadotropins, the number of follicles greater than 15 mm, leading follicle size, E2 levels at the trigger day, trigger type, and the number of retrieved oocytes. Two-sided p-values of < 0.05 were accepted as statistically significant.
Results
Baseline characteristics and cycle outcomes of poor ovarian responders undergoing IVF treatment
Table 1 presents the baseline characteristics and cycle outcomes of poor ovarian responders undergoing IVF treatment (N = 652). The overall mean age of the patients was 37.245 ± 7.4342 years, and the mean BMI was 27.257 ± 5.3277 kg/m². The distribution of the stimulation protocols was as follows: antagonist protocol in 483 cases (74.1%), modified natural cycle (MNC) in 118 cases (18.1%), short agonist protocol in 31 cases (4.8%), and long agonist protocol in 20 cases (3.1%). The mean day 3 FSH levels were 15.852 ± 9.0466 IU/L, and the mean duration of stimulation was 8.9632 ± 3.2088 days. The total dose of gonadotropins used was 4028.3 ± 2232.4 IU. Regarding the cycle outcomes, the mean number of follicles greater than 15 mm was 1.6994 ± 0.79077, and the mean leading follicle size was 18.277 ± 2.1674 mm. The mean E2 level at the trigger day was 1825.1 ± 913.15 pg/mL. The trigger distribution included dual trigger in 206 cases (31.6%) and hCG trigger in 446 cases (68.4%). The mean progesterone levels at the trigger day were 1.5337 ± 1.0309 ng/mL, and the mean number of retrieved oocytes was 1.6626 ± 1.1743. Concerning embryo quality, 269 cycles (41.3%) had one top-quality embryo, 369 cycles (56.6%) had none, and 14 cycles (2.1%) had two top-quality embryos. The pregnancy outcome was positive in 36 cases (5.5%), while 616 cases (94.5%) did not result in pregnancy.
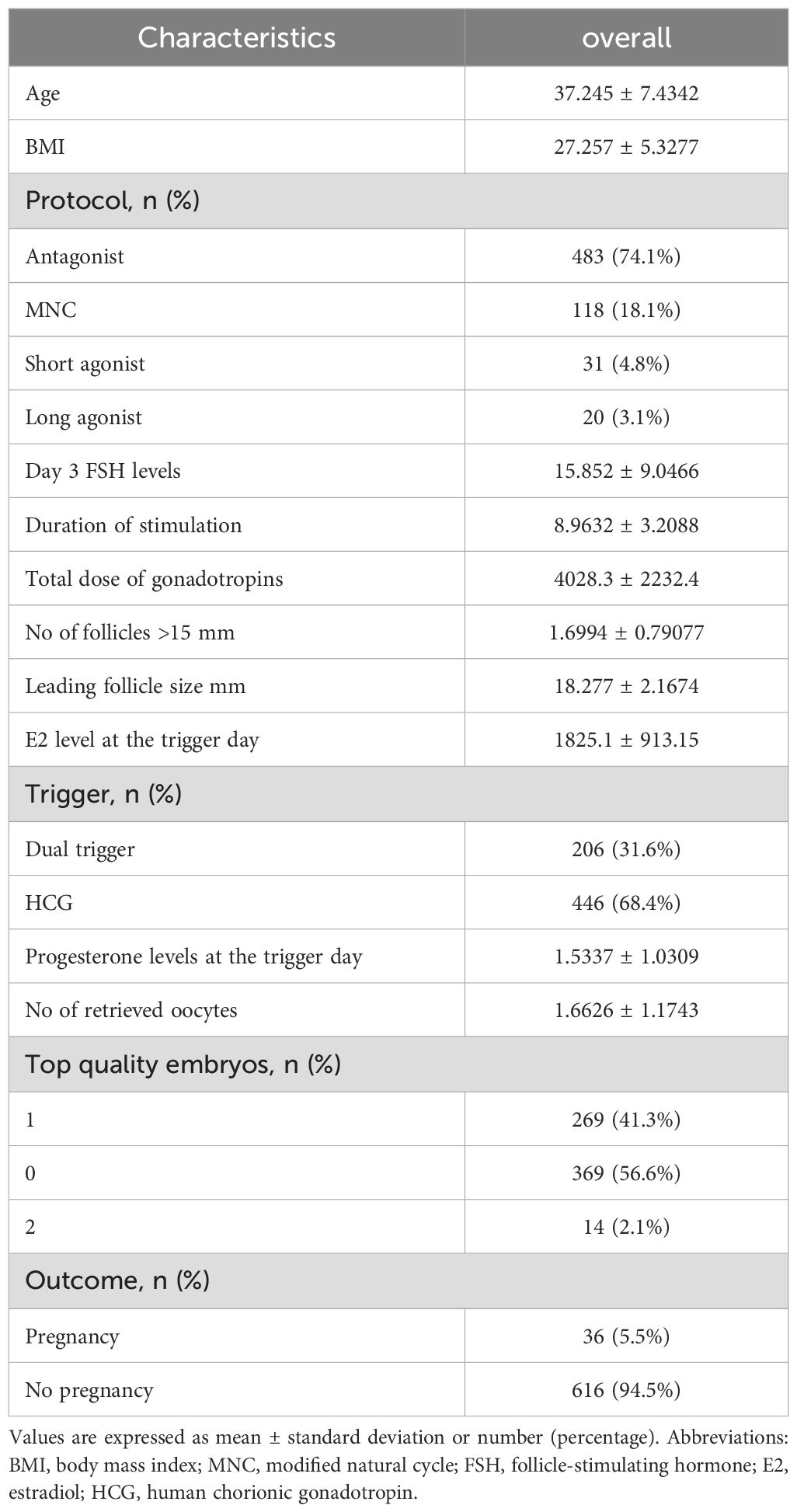
Table 1. Baseline characteristics and cycle outcomes of poor ovarian responders undergoing IVF/ICSI treatment.
Comparison of baseline characteristics and cycle outcomes in poor ovarian responders undergoing IVF treatment by age group (40 vs. ≥40 years)
Table 2 compares the baseline characteristics and cycle outcomes in poor ovarian responders undergoing IVF treatment by age group, specifically those younger than 40 years (n = 230) and those aged 40 years and older (n = 422). The mean age for the younger group was 28.552 ± 6.038 years, while the older group had a mean age of 41.983 ± 1.3567 years (P < 0.001). There was no significant difference in BMI between the two groups (26.768 ± 5.5099 kg/m² in the younger group and 27.524 ± 5.2131 kg/m² in the older group; P = 0.083). There were no significant differences in the distribution of stimulation protocols between the two age groups (P = 0.242). The mean day 3 FSH levels were significantly higher in the older group (16.372 ± 9.4863 IU/L) compared to the younger group (14.9 ± 8.1121 IU/L; P = 0.038). The duration of stimulation was also significantly longer in the older group (9.1682 ± 3.1649 days) compared to the younger group (8.587 ± 3.2614 days; P = 0.027). The total dose of gonadotropins used was significantly higher in the older group (4465.9 ± 2276.2 IU) compared to the younger group (3225.3 ± 1908.4 IU; P < 0.001). However, there were no significant differences in the number of follicles greater than 15 mm, leading follicle size, or E2 levels at the trigger day between the two age groups (P > 0.05 for all comparisons). The distribution of triggers and progesterone levels at the trigger day showed significant differences between the two age groups (P = 0.026). Regarding the number of retrieved oocytes and the distribution of top-quality embryos, there were no significant differences between the two age groups (P > 0.05 for all comparisons). However, the pregnancy outcome showed a significant difference, with the younger group having a higher percentage of pregnancies (3.1%) compared to the older group (2.5%; P = 0.009).
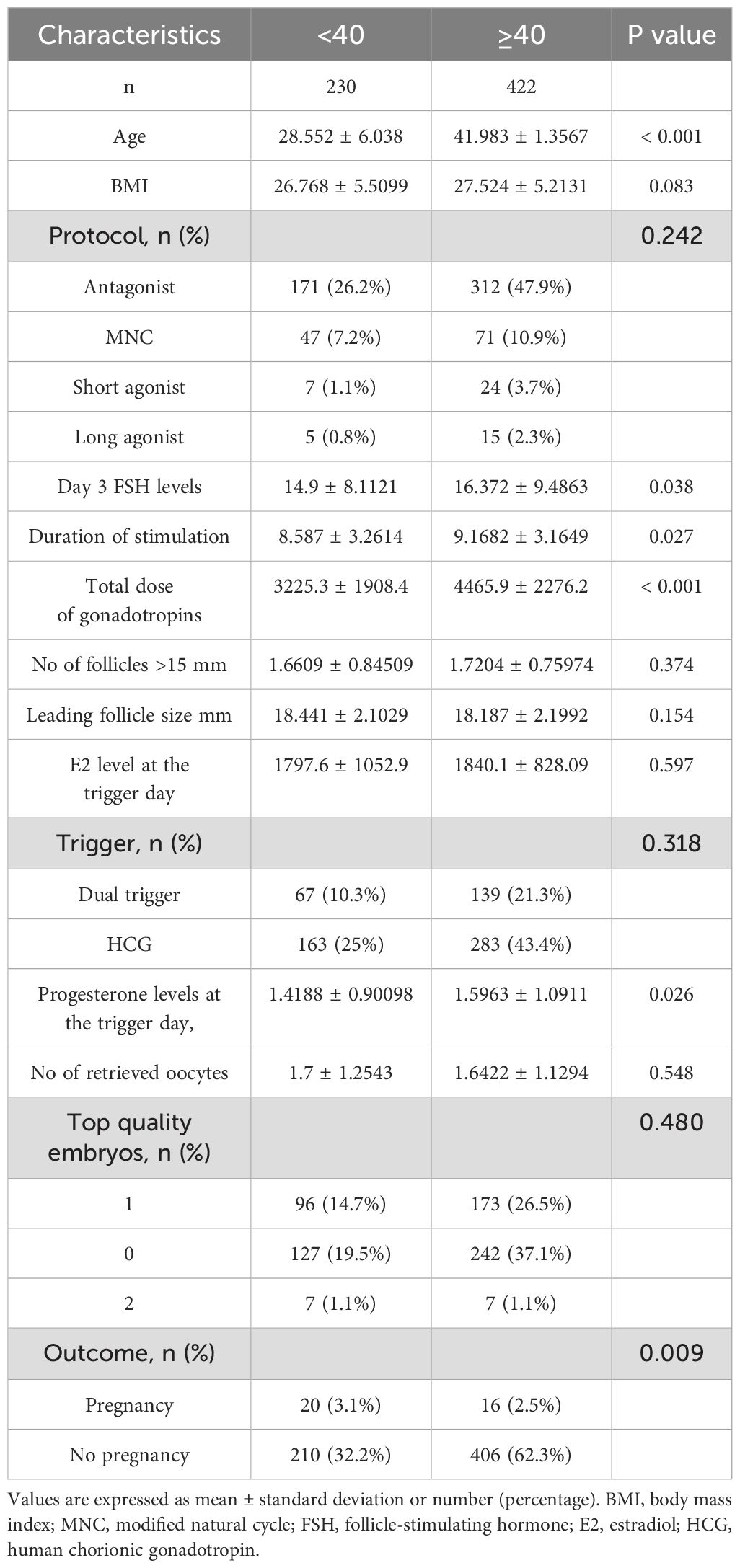
Table 2. Comparison of baseline characteristics and cycle outcomes in poor ovarian responders undergoing IVF/ICSI treatment by age group (<40 vs. ≥40 years).
Comparison of baseline characteristics and cycle outcomes between pregnant and non-pregnant poor ovarian responders undergoing IVF treatment
Table 3 presents a comparison of the baseline characteristics and cycle outcomes between poor ovarian responders who achieved pregnancy (n = 36) and those who did not (n = 616) following IVF treatment. The mean age was significantly lower in the pregnancy group (35.083 ± 7.2835 years) compared to the no pregnancy group (37.372 ± 7.4292 years; P = 0.043). However, there was no significant difference in BMI between the two groups (26.847 ± 4.6203 kg/m² in the pregnancy group and 27.281 ± 5.3685 kg/m² in the no pregnancy group; P = 0.635). The distribution of stimulation protocols between the two groups did not show any significant differences (P = 0.947). There were also no significant differences in day 3 FSH levels, duration of stimulation, total dose of gonadotropins, the number of follicles greater than 15 mm, leading follicle size, and E2 levels at the trigger day between the two groups (P > 0.05 for all comparisons). Regarding the triggers, there was no significant difference in the distribution between the pregnancy and no pregnancy groups (P = 0.333). The progesterone levels at the trigger day were higher in the no pregnancy group (1.5526 ± 1.0391 ng/mL) compared to the pregnancy group (1.209 ± 0.82418 ng/mL), but this difference did not reach statistical significance (P = 0.052). There was no significant difference in the number of retrieved oocytes between the two groups (1.8611 ± 1.2684 in the pregnancy group and 1.651 ± 1.1687 in the no pregnancy group; P = 0.297). However, the distribution of top-quality embryos showed a significant difference between the two groups (P < 0.001). In the pregnancy group, all patients had at least one top-quality embryo, while in the no pregnancy group, 56.6% had no top-quality embryos.
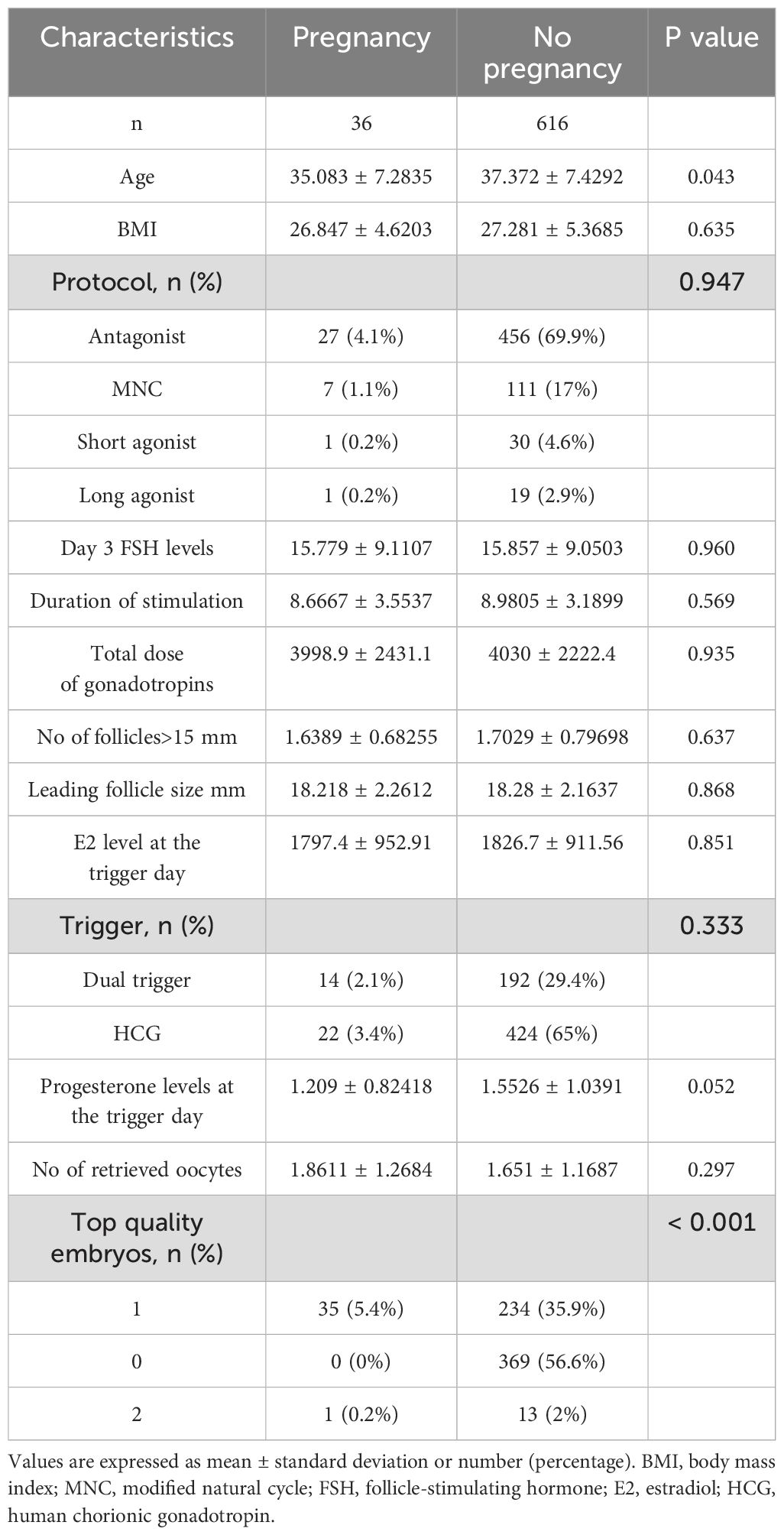
Table 3. Comparison of baseline characteristics and cycle outcomes between pregnant and non-pregnant poor ovarian responders undergoing IVF/ICSI treatment.
Univariate and multivariate logistic regression analysis of factors associated with pregnancy outcomes in poor ovarian responders undergoing IVF cycles
Table 4 displays the results of univariate and multivariate logistic regression analyses of factors potentially associated with clinical pregnancy in poor ovarian responders (total N = 652). In the univariate analysis, age was found to be significantly associated with clinical pregnancy, with an odds ratio (OR) of 1.037 (95% CI: 1.006 - 1.039; P = 0.026). However, BMI, stimulation protocol, day 3 FSH levels, duration of stimulation, total dose of gonadotropins, the number of follicles greater than 15 mm, leading follicle size, E2 levels at the trigger day, trigger type, and the number of retrieved oocytes were not significantly associated with clinical pregnancy (P > 0.05 for all comparisons). In the multivariate analysis, age remained significantly associated with clinical pregnancy, with an adjusted OR of 1.035 (95% CI: 1.024 - 1.078; P = 0.047). Progesterone levels at the trigger day also showed a significant association with clinical pregnancy, with an adjusted OR of 1.422 (95% CI: 1.380 - 1.564; P = 0.034). Other factors included in the analysis did not show significant associations with clinical pregnancy in the multivariate model.
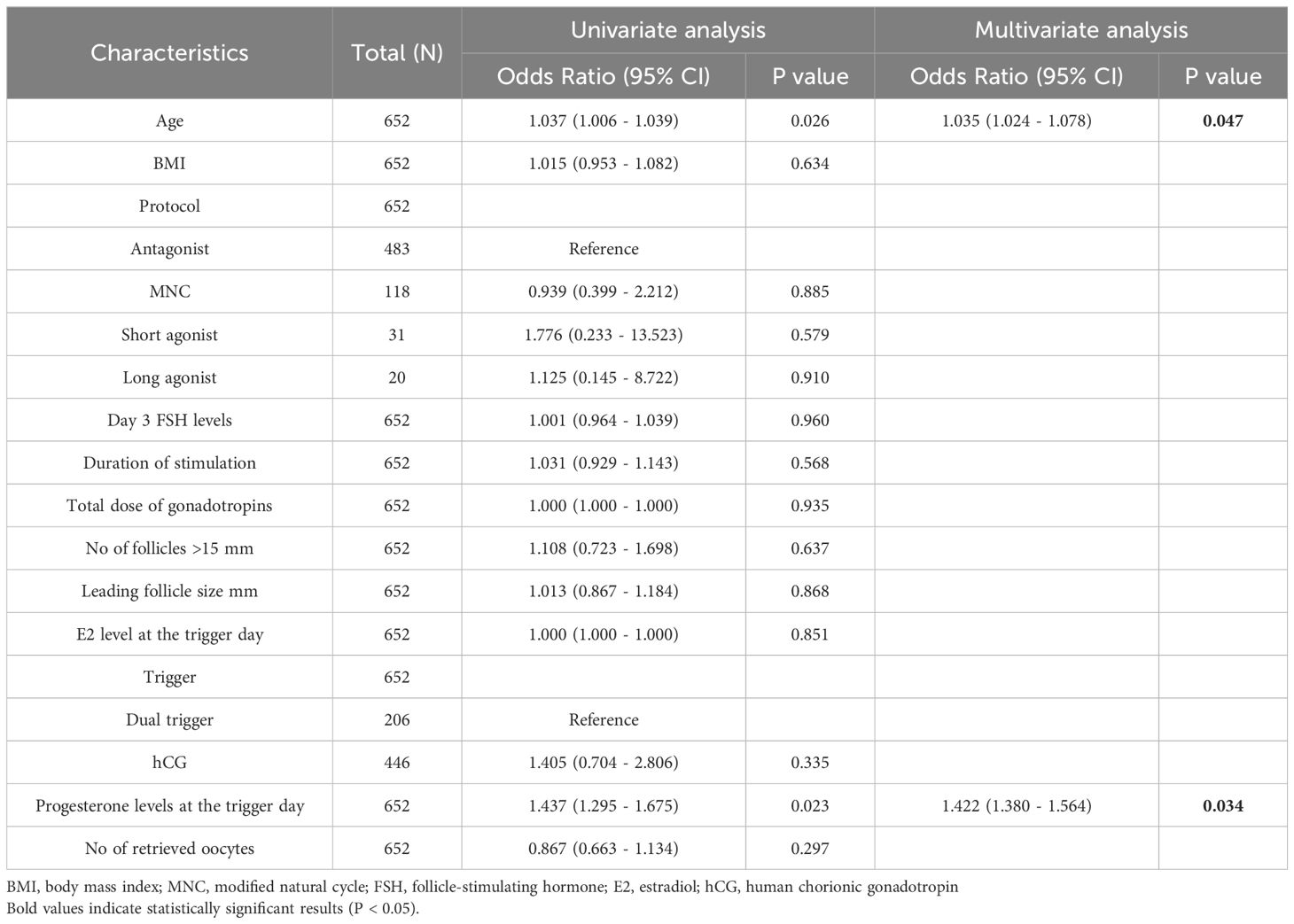
Table 4. Univariate and multivariate logistic regression analysis of factors associated with pregnancy outcomes in poor ovarian responders undergoing IVF/ICSI cycles.
Receiver operating characteristic analysis of age and progesterone levels at the trigger day, and the model for predicting clinical pregnancy in poor ovarian responders
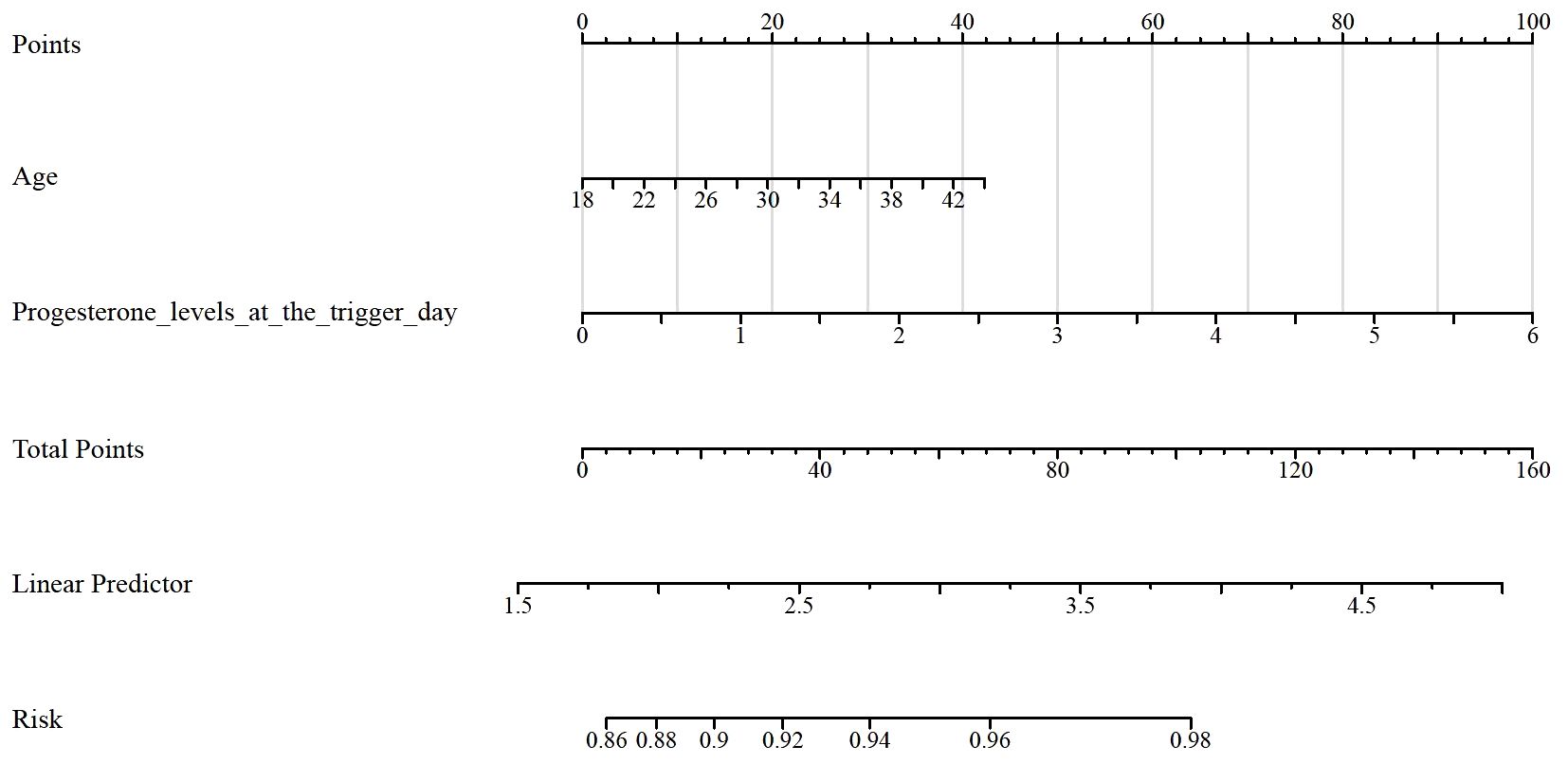
Table 5 shows the results of the Receiver Operating Characteristic (ROC) analysis for age, progesterone levels at the trigger day, and the combined model, in predicting clinical pregnancy among poor ovarian responders. The Area Under the Curve (AUC) for age was 0.604, with a 95% confidence interval (CI) ranging from 0.512 to 0.696, indicating a moderate predictive accuracy. The optimal cut-off value for age was found to be 39.5 years, with a specificity of 55.56% and a sensitivity of 65.91%. Progesterone levels at the trigger day presented an AUC of 0.588 (95% CI: 0.497 - 0.679), suggesting slightly less predictive accuracy than age. The optimal cut-off value for progesterone levels was determined to be 2.1756 ng/mL. This threshold, while offering a high sensitivity of 91.67%, was associated with a comparatively low specificity of 26.79%. The combined model, integrating both age and progesterone levels at the trigger day, offered an increased AUC of 0.632 (95% CI: 0.545 - 0.720). This illustrates the improved predictive power of the model, with the specificity and sensitivity being 69.44% and 53.57% respectively at an optimal cut-off value of 2.8728. More results can be found in Figures 1, 2. These findings underscore the potential utility of age and progesterone levels on the trigger day as predictors for clinical pregnancy in poor ovarian responders. Moreover, they highlight the increased predictive power achieved by considering both variables concurrently in a combined model. Furthermore, a nomogram was developed based on maternal age and progesterone levels at the trigger day to visually aid in predicting clinical pregnancy in poor ovarian responders (Figure 3).
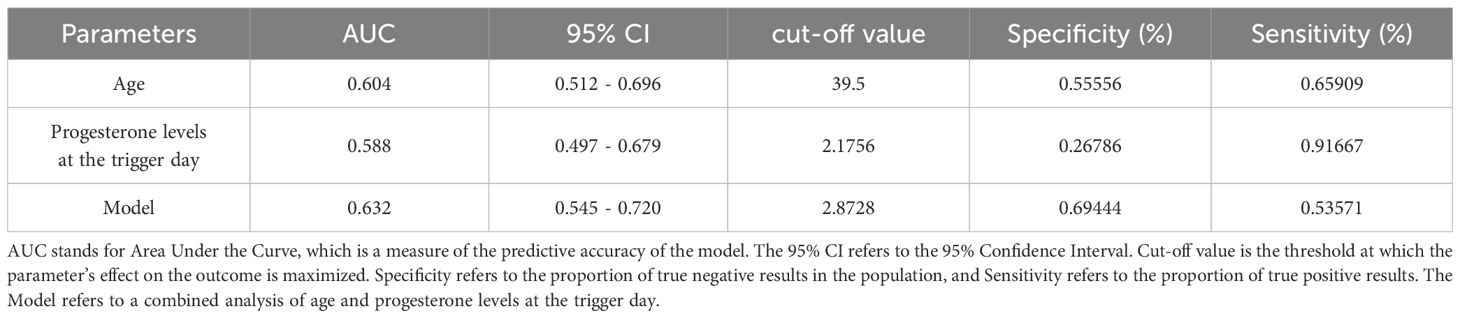
Table 5. Receiver operating characteristic (ROC) analysis of age and progesterone levels at the trigger day, and the model for predicting clinical pregnancy in poor ovarian responders.
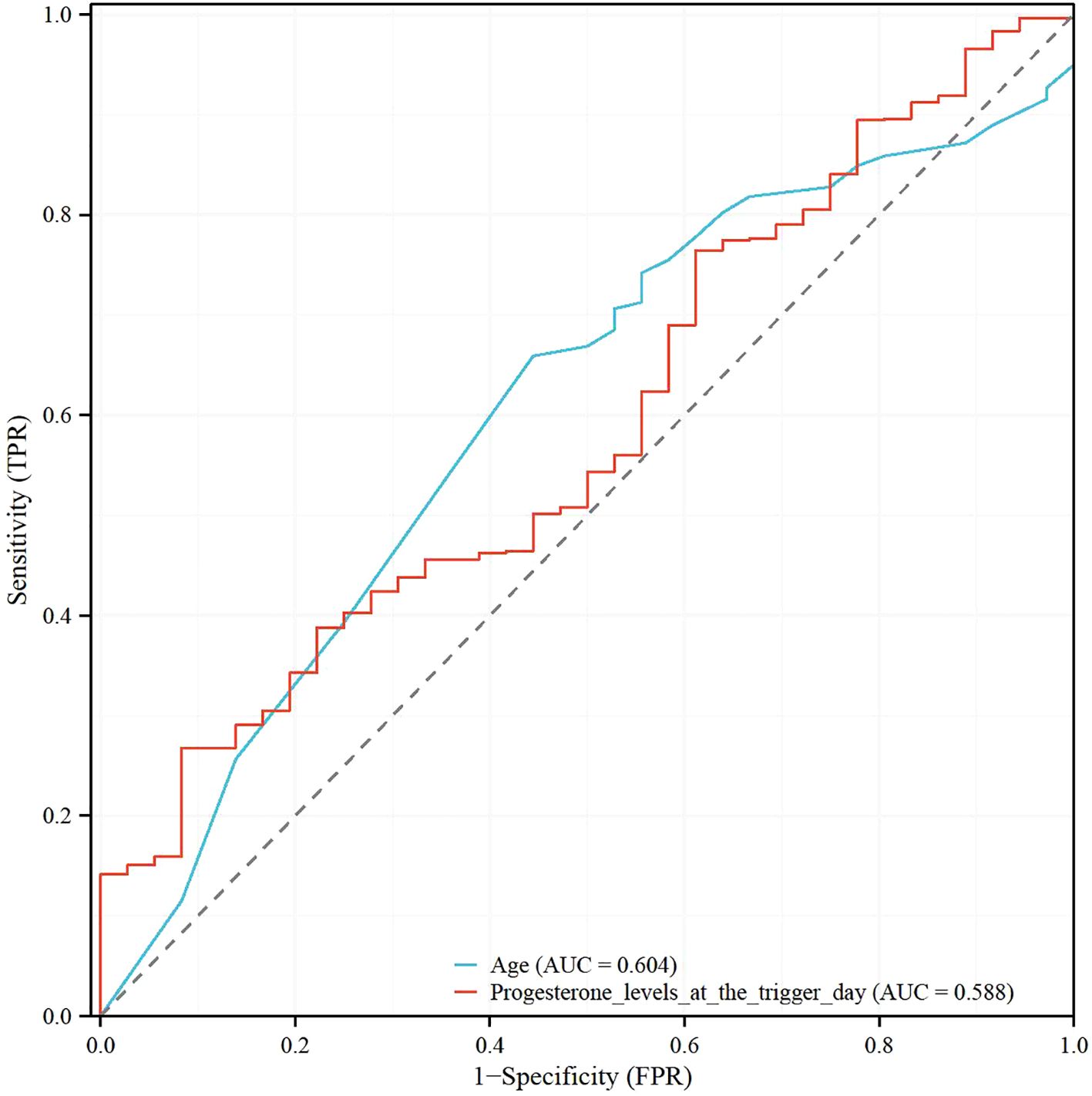
Figure 1. Receiver operating characteristic (ROC) analysis of age and progesterone levels at the trigger day in poor ovarian responders.
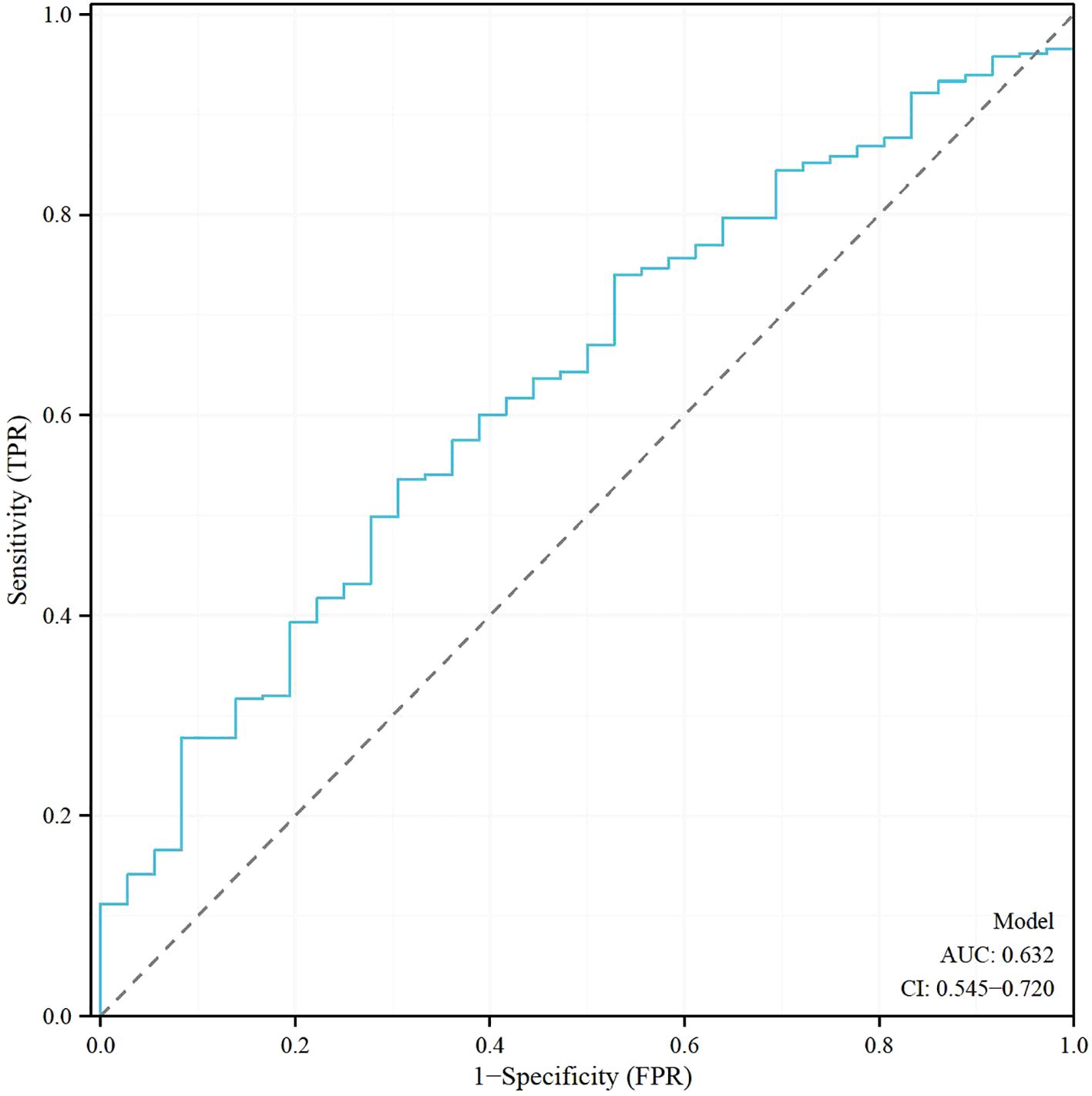
Figure 2. Receiver operating characteristic (ROC) Analysis of the model for predicting clinical pregnancy in poor ovarian responders.
Discussion
In this comprehensive retrospective analysis, we reviewed the clinical characteristics and treatment outcomes of 652 poor ovarian responders undergoing IVF/ICSI treatment. Our findings have led us to conclude that maternal age and progesterone levels on the day of the trigger emerged as independent predictors of clinical pregnancy in poor ovarian responders.
The overall pregnancy rate registered in our study was 5.5%, aligning with previous studies revealing decreased pregnancy rates in poor ovarian responders relative to normal responders (4, 5). This marked reduction underscores the need for a more profound understanding of this subgroup of patients and the requirement to optimize their treatment strategies.
The significant correlation of age with clinical pregnancy was evident in both univariate and multivariate analyses. This observation corroborates previous studies stating that advanced maternal age is a potent determinant affecting the success rates in IVF/ICSI cycles (9, 19). The age-associated decline in ovarian function and oocyte quality has been thoroughly investigated and is known to adversely impact IVF/ICSI success rates (6, 13). Some studies indicate that inositol supplementation might enhance oocyte quality and affect subsequent clinical outcomes (20). These findings underline the importance of considering maternal age and oocyte quality while devising personalized treatment plans for poor ovarian responders.
Complementing the age factor, our analysis revealed progesterone levels on the trigger day to be significantly associated with clinical pregnancy outcomes. This supports earlier research hinting that elevated progesterone levels might compromise endometrial receptivity and subsequently, pregnancy rates in IVF/ICSI cycles (21, 22). The specific mechanism responsible for this association is yet to be unraveled, thus necessitating further investigations to elucidate the role of progesterone levels on the trigger day in treatment outcomes for poor ovarian responders.
Contrary to expectations, no significant differences in pregnancy rates were observed among different stimulation protocols employed in our study population. This observation aligns with certain earlier studies reporting no notable differences in treatment outcomes among antagonist, MNC, short agonist, and long agonist protocols in poor ovarian responders (23, 24). Yet, some studies reported contrasting results, thereby fueling ongoing debates about the optimal stimulation strategy for poor ovarian responders (3, 5).
Our study has several limitations. First, its retrospective nature may introduce selection bias and limit the generalizability of our findings. Second, the relatively small sample size of pregnant patients might have affected the statistical power of the study. Third, our study did not consider potential confounding factors, such as genetic variables, lifestyle factors, the quality of the IVF laboratory, and thyroid dysfunction (25). Furthermore, another consideration is the psychological implications of participating in an IVF program, a stressor that significantly impacts couples on their fertility journey (26). Comprehensive care should, therefore, be multidimensional, encompassing both physical and psychological health. For patients undergoing cancer treatments like chemotherapy or radiotherapy, fertility preservation through methods such as oocyte vitrification is recommended (27). Comparing the safety of open versus closed vitrification systems merits further research in light of contrasting studies in the literature (28). Additionally, our study focused on clinical pregnancy as the primary outcome, whereas the live birth rate, a critical measure of success in IVF/ICSI treatments, was not fully assessed due to constraints on available data. Future studies should regard live birth rate as a key outcome to provide a more complete understanding of the factors affecting IVF/ICSI success in poor ovarian responders.
The potential long-term impact of IVF on neonatal outcomes also warrants consideration, including neuro-psycho-motor implications for children conceived through assisted reproductive techniques (29, 30). Research has revealed a potential increased risk of congenital heart diseases in children born through assisted reproductive techniques (20). The advent of non-invasive prenatal diagnostic techniques, such as cell-free fetal DNA, could facilitate early identification of chromosomopathies and pediatric monogenic diseases in children conceived via these treatments (31). In addition, new technologies like artificial intelligence could potentially enhance assisted reproduction outcomes, opening new avenues in oocyte or embryo selection, error reduction, and optimization of treatment strategies (32). Although our study did not directly explore this aspect, the potential of these technologies illustrates the ongoing evolution of the field and the importance of maintaining a broad perspective when considering strategies to improve clinical pregnancy outcomes in poor ovarian responders. Moreover, the emerging field of ovarian tissue cryopreservation and transplantation presents new potential treatment avenues for poor ovarian responders (33). Given the challenges associated with treating this patient group, the potential role of these techniques merits further investigation.
We also recognize the need to consider the broader implications of these treatments on the long-term health of children conceived through IVF. As mentioned before, there exists a potential increased risk of congenital heart diseases in children born through assisted reproductive techniques (20). Finally, our study underscores the criticality of considering maternal age and progesterone levels on the trigger day in the context of clinical pregnancy outcomes in poor ovarian responders undergoing IVF/ICSI treatment. It is envisaged that treatment strategies tailored to these factors may contribute to improved outcomes for this specific patient population. Nevertheless, it is of essence that further large-scale, prospective studies are undertaken to validate our findings and explore additional predictors of clinical pregnancy in poor ovarian responders.
In conclusion, while our study contributes to a greater understanding of factors influencing IVF/ICSI success rates in poor ovarian responders, it also highlights the need for comprehensive, multi-faceted strategies to improve outcomes. The need for a broader perspective in patient management, encompassing not only biological but also psychological considerations, is emphasized. Future research should take into account the potential of novel technologies and methods, as well as the long-term implications of IVF treatments on the health and wellbeing of resulting children.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
XS: Conceptualization, Validation, Software, Data curation, Writing – review & editing, Writing – original draft. LH: Visualization, Writing – original draft, Data curation, Conceptualization. LL: Writing – original draft, Supervision, Formal analysis, Investigation, Methodology. SW: Validation, Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors state that they conducted the research without any commercial or financial relationships that might be considered a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology. Fertil Steril. (2009) 92:1520–4. doi: 10.1016/j.fertnstert.2009.09.009
2. De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2014: results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod. (2018) 33:1586–601. doi: 10.1093/humrep/dey242
3. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. (2011) 26:1616–24. doi: 10.1093/humrep/der092
4. Polyzos NP and Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril. (2011) 96:1058–61.e7. doi: 10.1016/j.fertnstert.2011.09.048
5. Shanbhag S, Aucott L, Bhattacharya S, Hamilton MA, and McTavish AR. Interventions for ‘poor responders’ to controlled ovarian hyperstimulation (COH) in in-vitro fertilisation (IVF). Cochrane Database Syst Rev. (2007) 1):CD004379. doi: 10.1002/14651858.CD004379
6. Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, and Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. (2006) 12:685–718. doi: 10.1093/humupd/dml034
7. van Montfrans JM, Hoek A, van Hooff MH, de Koning CH, Tonch N, and Lambalk CB. Predictive value of basal follicle-stimulating hormone concentrations in a general subfertility population. Fertil Steril. (2000) 74:97–103. doi: 10.1016/s0015-0282(00)00560-4
8. Ubaldi F, Vaiarelli A, D’Anna R, and Rienzi L. Management of poor responders in IVF: is there anything new? BioMed Res Int. (2014) 2014:352098. doi: 10.1155/2014/352098
9. Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, and Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400–135 treatment cycles. Hum Reprod. (2011) 26:1768–74. doi: 10.1093/humrep/der106
10. Papathanasiou A, Searle BJ, King NM, and Bhattacharya S. Trends in ‘poor responder’ research: lessons learned from RCTs in assisted conception. Hum Reprod Update. (2016) 22:306–19. doi: 10.1093/humupd/dmw001
11. Jeve YB and Bhandari HM. Effective treatment protocol for poor ovarian response: A systematic review and meta-analysis. J Hum Reprod Sci. (2016) 9:70–81. doi: 10.4103/0974-1208.183515
12. Broekmans FJ, Soules MR, and Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. (2009) 30:465–93. doi: 10.1210/er.2009-0006
13. te Velde ER and Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. (2002) 8:141–54. doi: 10.1093/humupd/8.2.141
14. La Marca A and Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. (2014) 20:124–40. doi: 10.1093/humupd/dmt037
15. Patrizio P, Vaiarelli A, Levi Setti PE, Tobler KJ, Shoham G, Leong M, et al. How to define, diagnose and treat poor responders? Responses from a worldwide survey of IVF clinics. Reprod BioMed Online. (2015) 30:581–92. doi: 10.1016/j.rbmo.2015.03.002
16. Mesen TB and Young SL. Progesterone and the luteal phase: a requisite to reproduction. Obstet Gynecol Clin North Am. (2015) 42:135–51. doi: 10.1016/j.ogc.2014.10.003
17. Venetis CA, Kolibianakis EM, Bosdou JK, and Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60–000 cycles. Hum Reprod Update. (2013) 19:433–57. doi: 10.1093/humupd/dmt014
18. Huang Y, Wang EY, Du QY, Xiong YJ, Guo XY, Yu YP, et al. Progesterone elevation on the day of human chorionic gonadotropin administration adversely affects the outcome of IVF with transferred embryos at different developmental stages. Reprod Biol Endocrinol. (2015) 13:82. doi: 10.1186/s12958-015-0075-3
19. McLernon DJ, Harrild K, Bergh C, Davies MJ, de Neubourg D, Dumoulin JC, et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ. (2010) 341:c6945. doi: 10.1136/bmj.c6945
20. Gullo G, Scaglione M, Laganà AS, Perino A, Andrisani A, Chiantera V, et al. Assisted reproductive techniques and risk of congenital heart diseases in children: a systematic review and meta-analysis. Reprod Sci. (2023). doi: 10.1007/s43032-023-01252-6
21. Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, and Tarlatzis BC. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update. (2007) 13:343–55. doi: 10.1093/humupd/dmm007
22. Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. (2010) 25:2092–100. doi: 10.1093/humrep/deq125
23. Jeve YB and Bhandari HM. Effective treatment protocol for poor ovarian response: A systematic review and meta-analysis. J Hum Reprod Sci. (2016) 9:70–81. doi: 10.4103/0974-1208.183515
24. Hu L, Bu Z, Guo Y, Su Y, Zhai J, and Sun Y. Comparison of different ovarian hyperstimulation protocols efficacy in poor ovarian responders according to the Bologna criteria. Int J Clin Exp Med. (2014) 7:1128–34.
25. Medenica S, Abazovic D, Ljubić A, Vukovic J, Begovic A, Cucinella G, et al. The role of cell and gene therapies in the treatment of infertility in patients with thyroid autoimmunity. Int J Endocrinol. (2022) 2022:4842316. doi: 10.1155/2022/4842316
26. Burgio S, Polizzi C, Buzzaccarini G, Laganà AS, Gullo G, Perricone G, et al. Psychological variables in medically assisted reproduction: a systematic review. Prz Menopauzalny. (2022) 21:47–63. doi: 10.5114/pm.2022.114404
27. Zaami S, Stark M, Signore F, Gullo G, and Marinelli E. Fertility preservation in female cancer sufferers: (only) a moral obligation? Eur J Contracept Reprod Health Care. (2022) 27:335–40. doi: 10.1080/13625187.2022.2045936
28. Gullo G, Perino A, and Cucinella G. Open vs. closed vitrification system: which one is safer? Eur Rev Med Pharmacol Sci. (2022) 26:1065–7. doi: 10.26355/eurrev_202202_28092
29. Gullo G, Scaglione M, Cucinella G, Chiantera V, Perino A, Greco ME, et al. Neonatal outcomes and long-term follow-up of children born from frozen embryo, a narrative review of latest research findings. Medicina (Kaunas). (2022) 58:1218. doi: 10.3390/medicina58091218
30. Gullo G, Scaglione M, Cucinella G, Perino A, Chiantera V, D’Anna R, et al. Impact of assisted reproduction techniques on the neuro-psycho-motor outcome of newborns: a critical appraisal. J Obstet Gynaecol. (2022) 42:2583–7. doi: 10.1080/01443615.2022.2109953
31. Gullo G, Scaglione M, Buzzaccarini G, Laganà AS, Basile G, Chiantera V, et al. Cell-free fetal DNA and non-invasive prenatal diagnosis of chromosomopathies and pediatric monogenic diseases: A critical appraisal and medicolegal remarks. J Pers Med. (2022) 13:1. doi: 10.3390/jpm13010001
32. Medenica S, Zivanovic D, Batkoska L, Marinelli S, Basile G, Perino A, et al. The future is coming: artificial intelligence in the treatment of infertility could improve assisted reproduction outcomes-the value of regulatory frameworks. Diagnostics (Basel). (2022) 12:2979. doi: 10.3390/diagnostics12122979
33. Gullo G, Etrusco A, Cucinella G, Basile G, Fabio M, Perino A, et al. Ovarian tissue cryopreservation and transplantation in menopause: new perspective of therapy in postmenopausal women and the importance of ethical and legal frameworks. Eur Rev Med Pharmacol Sci. (2022) 26:9107–16. doi: 10.26355/eurrev_202212_30660
Keywords: poor ovarian response, IVF, ICSI, clinical pregnancy, maternal age, progesterone levels, predictors
Citation: Sun X, He L, Liu L and Wang S (2025) Maternal age and progesterone levels at trigger day as independent predictors of clinical pregnancy in poor ovarian responders undergoing IVF/ICSI: a retrospective analysis. Front. Endocrinol. 16:1593298. doi: 10.3389/fendo.2025.1593298
Received: 13 March 2025; Accepted: 09 June 2025;
Published: 23 June 2025.
Edited by:
Michio Kitajima, Takagi Hospital, JapanReviewed by:
Soobin Jang, Daegu Haany University, Republic of KoreaAtul Kumar, Columbia University, United States
Copyright © 2025 Sun, He, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaohua Wang, d2FuZ3NoYW9odWEyMDAwNkAxNjMuY29t
 Xingyu Sun
Xingyu Sun Lijuan He
Lijuan He Ling Liu
Ling Liu Shaohua Wang
Shaohua Wang