- Department of Fertility and Sterility, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
The success of assisted reproductive technology (ART) relies on the quality of embryos, particularly the euploid status, which is influenced by controlled ovarian hyperstimulation (COH) protocols. In recent years, the progesterone-primed ovarian stimulation (PPOS) protocol has gained popularity due to its potential benefits. However, the impact of PPOS on euploid embryo rates (EER) and reproductive outcomes remains incompletely understood. Therefore, we conducted this review to comprehensively assess this impact by comparing the PPOS with conventional COH protocols in PGT cycles. The results revealed that the PPOS protocol demonstrated comparable EER and reproductive outcomes to conventional COH protocols in the general population. Among patients with a good prognosis, EER and associated reproductive outcomes with PPOS may be less favorable. However, in individuals with a poor prognosis, PPOS showed comparable or even superior outcomes. Additionally, the timing of cycle initiation, whether in the follicular or luteal phase, had no significant impact on clinical outcomes in patients with diverse ovarian responses.
1 Introduction
Controlled ovarian hyperstimulation (COH) is fundamental in assisted reproductive technology (ART), with the primary objective of retrieving multiple oocytes to generate sufficient embryos for transfer. As the field of ART has evolved, the emphasis has gradually shifted from the quantity of embryos to their quality, particularly in achieving euploidy embryos (1). Various studies have reported associations between COH protocols and euploidy embryo rates (EER), presenting a significant challenge for reproductive specialists in determining the optimal COH strategy for patients (2).
The progesterone-primed ovarian stimulation (PPOS) approach, utilizes oral administration of exogenous progesterone to suppress the estradiol-induced luteinizing hormone (LH) surges, offering a viable alternative to traditional COH treatment. This protocol offers several notable advantages by mitigating the risk of ovarian hyperstimulation syndrome, facilitating convenient administration, and providing high cost-effectiveness (3).
In a large retrospective study involving 14,981 recipients of vitrified oocytes from 3,599 donors stimulated with PPOS protocol and 4,998 stimulated with GnRH antagonist protocol, the PPOS group demonstrated comparable clinical outcomes to the GnRH antagonist group in vitrified donor oocyte cycles (4). A meta-analysis encompassing 14 studies with 4,182 participants also revealed that the PPOS protocol improves in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) outcomes in women with diminished ovarian reserve (DOR) (5). However, some studies had contradictory results. In a propensity score-matched retrospective cohort study involving 6,520 infertile women aged 20 – 50 years, the GnRH antagonist protocol showed significantly higher cumulative live birth rates (CLBRs) and shorter time to live birth (TTLB) than the progestin-primed ovarian stimulation (PPOS) protocol in unselected IVF patients (6). In a prospective randomized controlled trial at a university-affiliated IVF center, 318 oocyte donors were randomized 1:1 to PPOS or GnRH antagonist protocols. While both groups yielded similar numbers of mature oocytes, retrospective analysis of recipient outcomes showed lower ongoing pregnancy and live birth rates in recipients of PPOS-stimulated donor oocytes (7). Nevertheless, the precise effects of progesterone administration during COH on embryo quality and the underlying mechanisms remain incompletely understood.
Euploidy status is a pivotal aspect in assessing embryo quality and has emerged as a crucial determinant in ART outcomes. As preimplantation genetic testing (PGT) enables the selection of euploid embryos (8), it becomes practical to evaluate the effects of PPOS protocol in PGT cycles. This review aims to comprehensively investigate the impact of PPOS protocol on both the EER and reproductive outcomes in PGT cycles by comparison with other COH protocols. By conducting subgroup analyses, we strive to identify preferences for different patient cohorts, ultimately contributing to the refinement and optimization of ART strategies.
2 Methods
2.1 Inclusion criteria
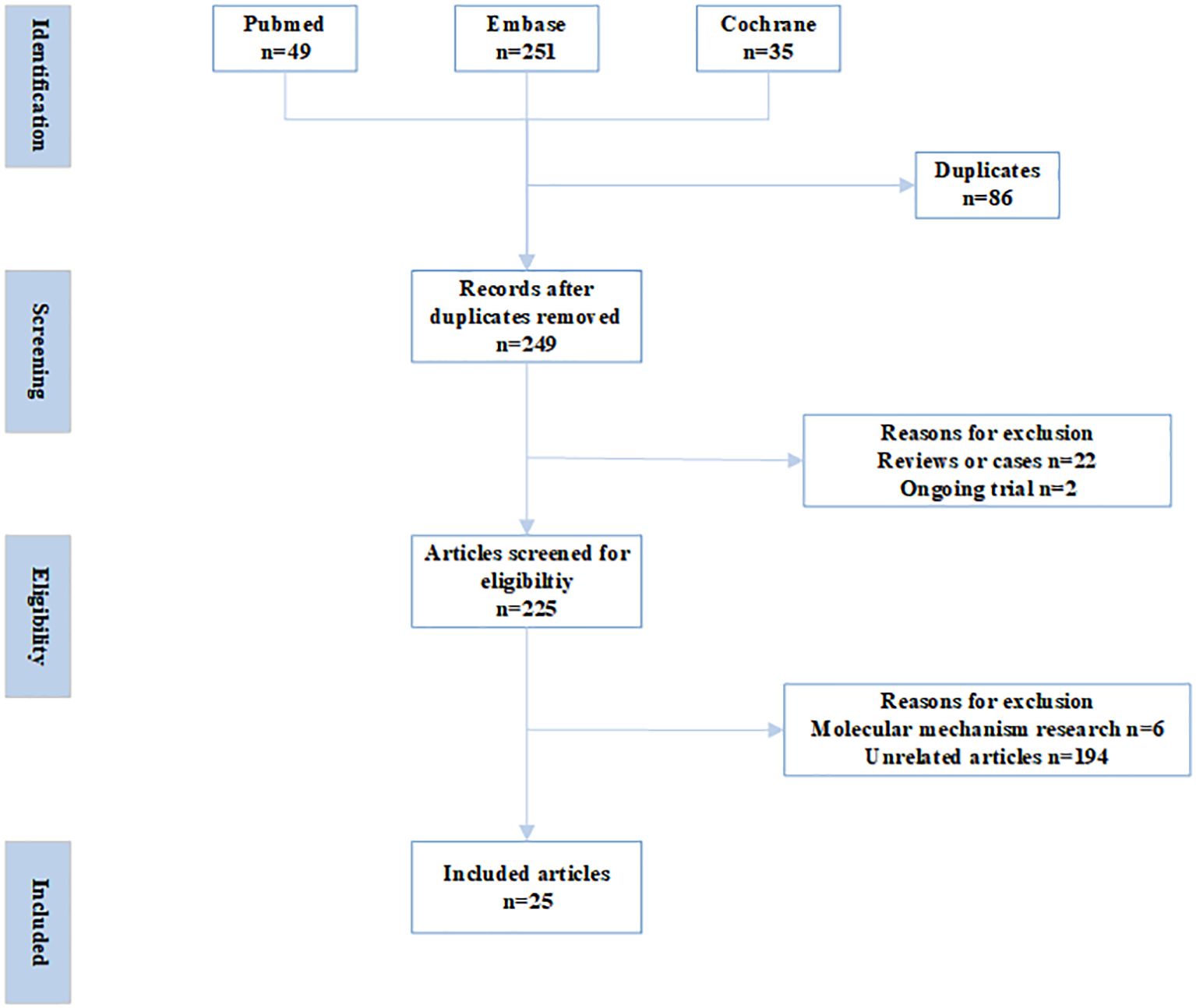
Databases of PubMed, Embase, and Cochrane Central Register of Controlled Trials were searched with the following terms: (preimplantation genetic diagnosis OR preimplantation genetic screening OR euploid OR preimplantation genetic test) AND (Controlled ovarian hyperstimulation OR COH OR Ovarian stimulation OR OS OR Controlled ovarian stimulation OR COS OR Ovarian hyperstimulation) AND (Progesterone-Primed Ovarian Stimulation OR PPOS OR progestin-primed ovarian stimulation OR dydrogesterone OR progesterone OR Norethisterone acetate OR Medroxyprogesterone OR MPA) from inception to December, 2024. The inclusion criteria were as follows. Published in English in peer-reviewed journals; irrespective of study-design; studies focusing on the impact of PPOS protocol on euploid embryo rates and reproductive outcomes in PGT cycles. Commentaries, letters, reviews, conference abstracts, and irrelevant studies were excluded (Figure 1).
2.2 Study selection, data extraction and comprehensive analysis
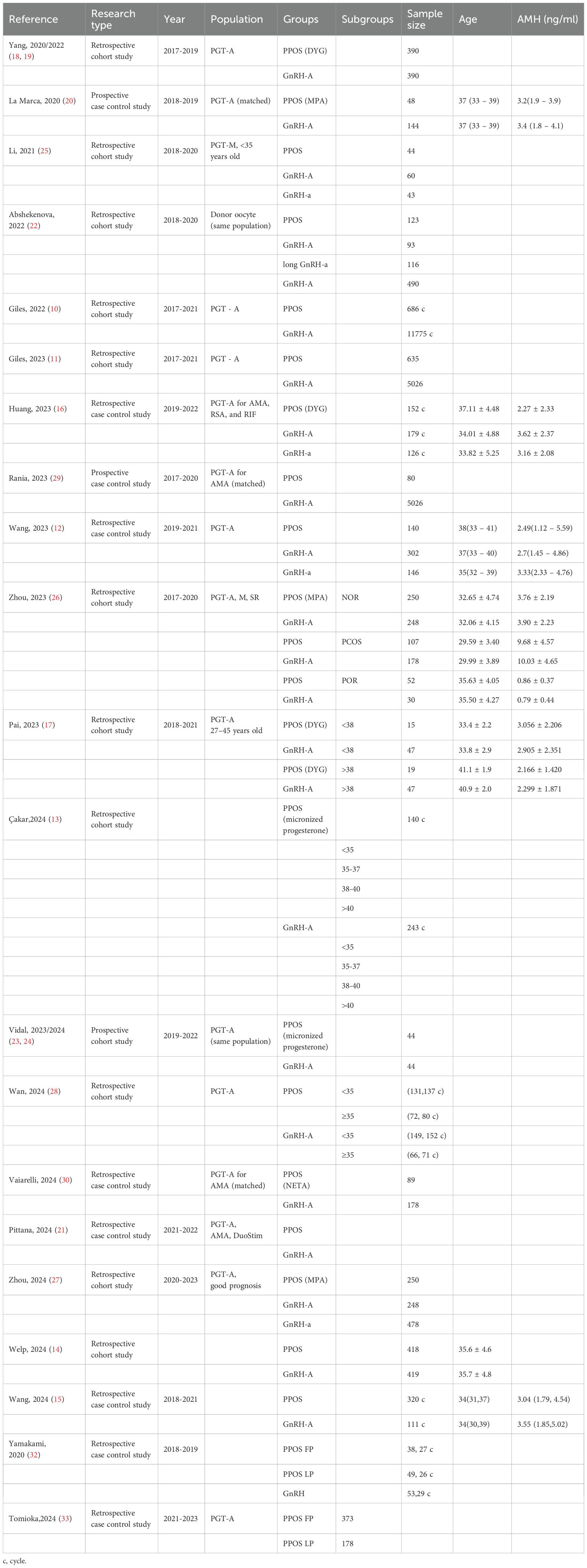
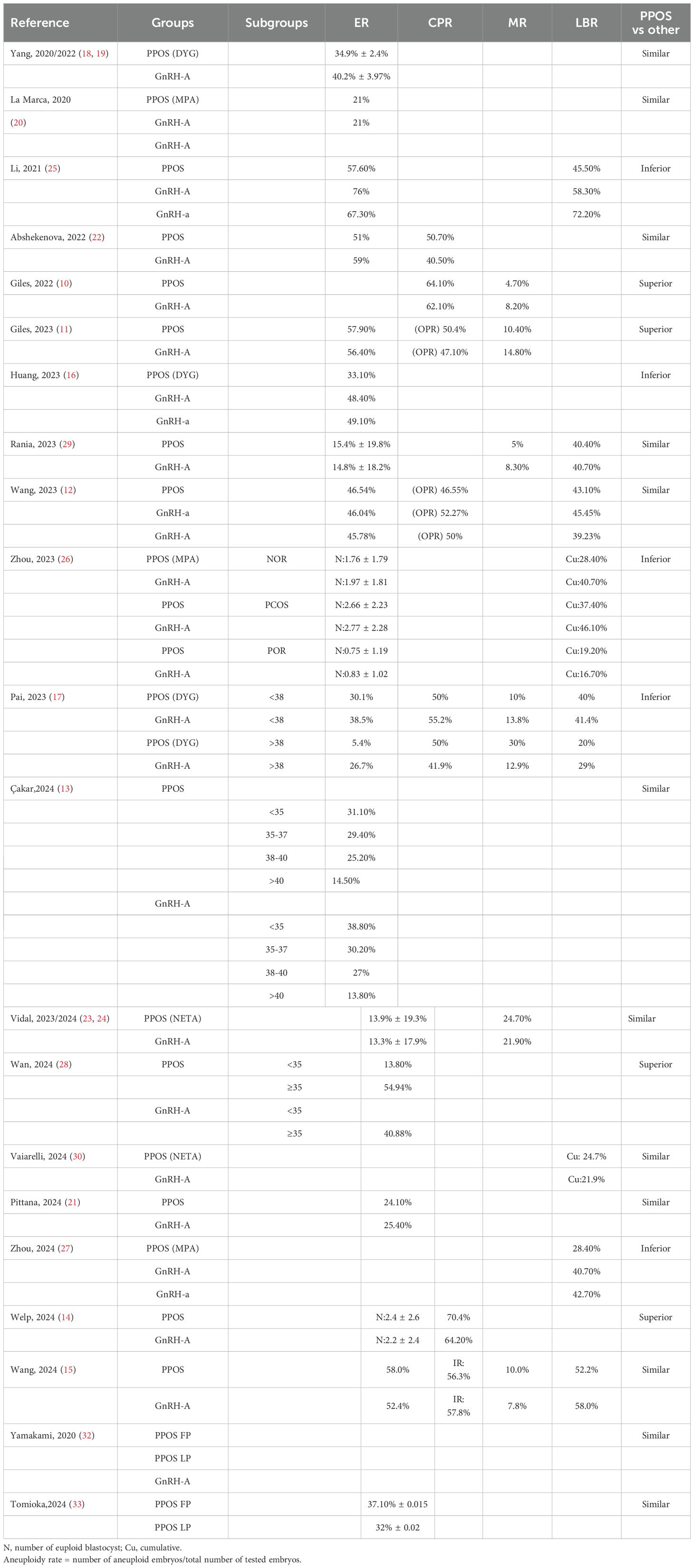
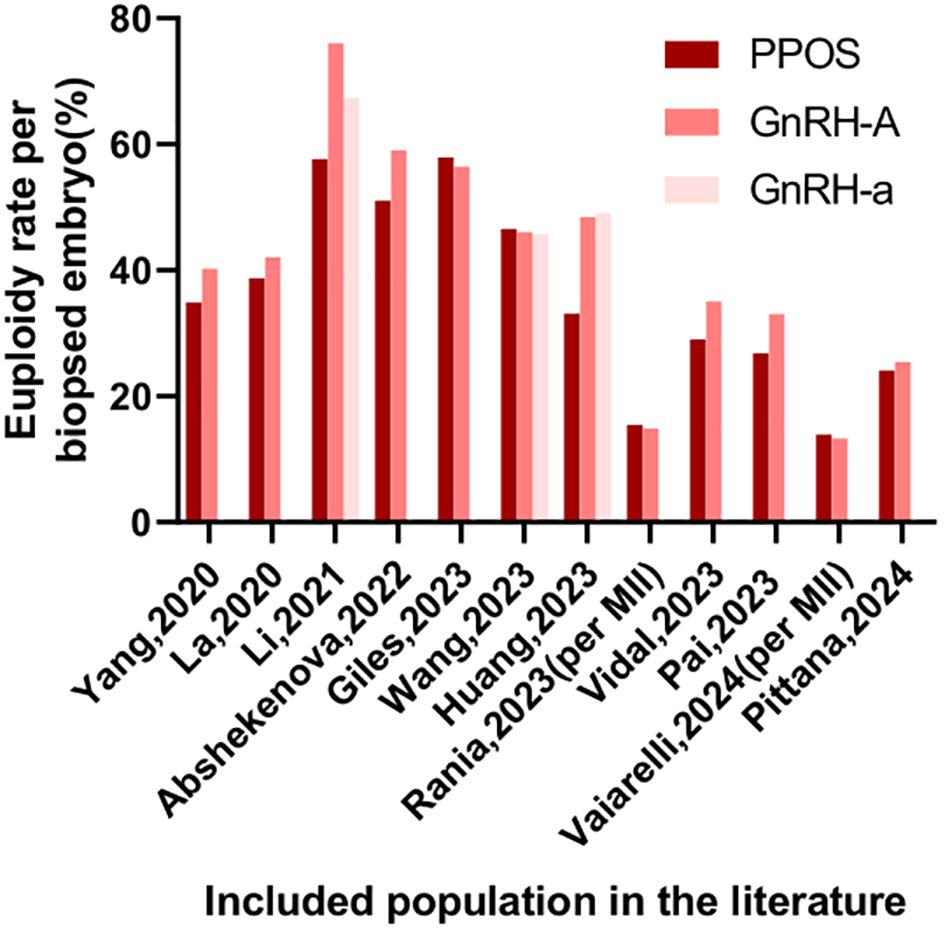
Two independent reviewers carried out the initial screening and full-text assessment to determine inclusion. In case of disagreement, consensus was reached through discussion with a third reviewer. The extracted information encompassed details like author names, publication year, study population characteristics, sample size, methodologies, interventions, euploidy embryo rates, and reproductive outcomes, with stratified analyses performed based on demographic features. Due to substantial heterogeneity among the studies, meta-analysis was excluded (Table 1, 2, Figures 2, 3). This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (9). Newcastle-Ottawa Scale (NOS) was used to assess the risk of bias of the included studies.
3 Results
3.1 In the general population
3.1.1 In the unclassified population
In 2022, Giles conducted a multicenter observational study encompassing 4,961 non-oncological fertility preservation cycles and 12,461 preimplantation genetic testing for aneuploidy (PGT-A) cycles. Notably, despite a reduced number of biopsied embryos in the PPOS group, the study revealed comparable aneuploidy rates, implantation rates (IR), and clinical pregnancy rates (CPR) to those of the gonadotropin-releasing hormone antagonist (GnRH-A) group. Moreover, the PPOS group exhibited significantly lower miscarriage rates (MR) (10). A subsequent multi-center retrospective observational cohort study by Giles analyzed 1652 social fertility preservation cycles and 5661 PGT-A cycles. The PPOS group had comparable metaphase II (MII) oocytes, biopsied embryos, EER, or ongoing pregnancy rate (OPR) and a lower clinical miscarriage rate compared to the GnRH-A group (11). Wang’s retrospective cohort study also had similar conclusions. The study included 608 PGT-A cycles, with 146 women in the PPOS group, 160 in the GnRH agonist (GnRH-a) group, 302 in the GnRH-A group, and 267 corresponding first frozen embryo transfer (FET) cycles were analyzed. The results demonstrated comparable EER per MII oocyte, with no significant differences in reproductive outcomes, including live birth rate (LBR) among the three groups (12). Recently, Çakar conducted a multicenter retrospective cohort study, examining 1,425 PGT-A-tested blastocysts derived from 383 cycles. The study revealed that there were no significant differences in EER per blastocyst tested or HCG positivity rates per transferred embryo between the PPOS and GnRH-A groups (13). In another cohort trial, Welp’s investigation demonstrated the efficacy of medroxyprogesterone acetate (MPA) in effectively inhibiting ovulation, while achieving comparable cycle outcomes and reproductive success rates. Furthermore, MPA conferred several patient-centered benefits, including cost savings, a reduction in the number of monitoring visits, and a decrease in the frequency of injections (14). More recently, Wang’s study, which encompassed a substantial sample size of 431 PGT-A cycles, provided additional insights into this area. Specifically, the study found no statistically significant differences in aneuploidy embryo rates, clinical pregnancy rates, or cumulative LBR between the PPOS protocol (n=320) and the GnRH-A protocol (n=111) (15).
Despite these favorable outcomes, differing perspectives existed. Huang observed that, although baseline characteristics were comparable, the average euploid blastocyst count and EER were lower in the PPOS group compared to the GnRH-a and GnRH-A groups in 457 PGT-A cycles. However, it is crucial to note that patients in the PPOS group had a significantly higher mean age than those in the other two groups (16). Similarly, a retrospective cohort study by Pai analyzed 128 PGT-A cycles at a university hospital-affiliated fertility center between 2018 and 2021. Among elderly patients (≥38 years old), the PPOS group showed lower blastocyst formation and euploidy rates compared to the GnRH-A group. However, the sample size was small (only 34 cases in the PPOS group), and the study was retrospective, necessitating cautious interpretation of the results (17). Collectively, the PPOS protocol yields reproductive outcomes non-inferior to those of GnRH-a and GnRH-A protocols in the unclassified population.
3.1.2 In the matched or self-controlled population
To address potential biases, several studies employed matched population. Yang conducted a single-center retrospective cohort study comparing 390 PGT-A cycles with oral dydrogesterone (DYG) to 390 cycles with the GnRH-A protocol among 780 initial PGT-A cycles. After propensity matching for age, body mass index (BMI), and Anti - Müllerian Hormone (AMH), the study found similar EER per biopsy between the two groups (18, 19). A prospective non-inferiority, age-matched case-control study by La Marca, involving 192 patients and 785 blastocysts from 1867 injected oocytes, demonstrated that the PPOS protocol achieved comparable euploid blastocyst formation rates per oocyte compared to GnRH-A protocol. The study also found similar percentages of patients with euploid embryos and total euploid blastocysts per patient, further supporting the effectiveness of the PPOS protocol (20). A recent retrospective study by Pittana compared 138 patients undergoing PPOS-DuoStim with 138 matched patients undergoing conventional-DuoStim protocol. The study found comparable EER per biopsied blastocyst between the PPOS-DuoStim group (24.1%) and the conventional-DuoStim group (25.4%) (21). In self-controlled population, the following studies had similar conclusions. In a retrospective cohort study by Abshekenova, which compared 201 IVF/ICSI cycles using self-matched donor oocytes, no significant differences were observed between the consecutive PPOS and GnRH-A groups in gonadotropin dosage, oocyte maturity, fertilization rates, blastocyst formation rates, blastocyst with top quality, EER, or pregnancy rates (22). Lastly, a prospective study by Vidal involving 44 women who underwent two consecutive ovarian stimulation protocols—GnRH-A and PPOS with oral micronized progesterone—within a six-month period revealed comparable euploid means between the two groups. Although there was a slight difference in EER per biopsied embryo (29% in PPOS vs 35% in GnRH-A), the PPOS group had more oocytes, MIIs, and 2 pronucleus (PNs) (23, 24). In summary, the PPOS protocol can achieve reproductive outcomes comparable to, if not superior to, those of the GnRH-a and GnRH-A protocol.
3.2 In specific population
3.2.1 In population with a good prognosis
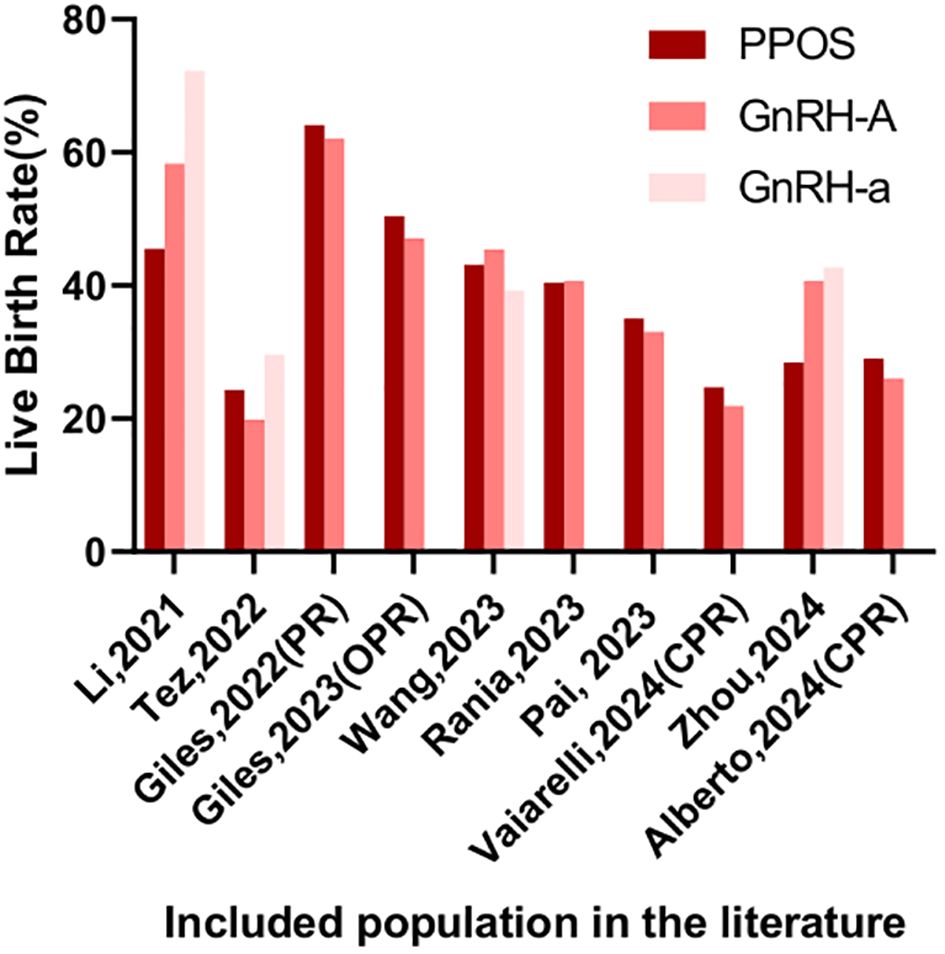
Li observed a notable decline in both the EER (57.6% vs 76.0% vs 67.3%) and LBR (45.5% vs 58.3% vs 72.2%) among young patients (<35 years old) utilizing PPOS protocol, in comparison to those using GnRH-A and GnRH-a, respectively (25). Another retrospective cohort study compared the cumulative LBR of PPOS and GnRH-A protocols in PGT cycles among 865 patients from three population (normal ovarian response (NOR), polycystic ovary syndrome (PCOS), poor ovarian response (POR)). Results showed that in NOR and seemingly in PCOS, PPOS had lower or seemingly lower cumulative LBR compared to GnRH-A, while in POR they were comparable (26). In the retrospective cohort study conducted by Zhou, comparing PPOS with GnRH analogues in patients with a good prognosis undergoing PGT cycles, the PPOS protocol was found to be adversely associated with both cumulative LBR and blastocyst quality. While perinatal outcomes were comparable across PPOS, GnRH-A, and GnRH-a groups, the time to live birth was longer with PPOS protocol compared to GnRH-A protocol (27). In summary, the PPOS protocol may be less effective than conventional COH approaches.
3.2.2 In population with a poor prognosis
In patients with a poor prognosis, the potential advantages of PPOS have gained significant attention. A recent study by Wan revealed that higher EER and a decreased mosaicism rate when compared to GnRH-A protocol within the older patient cohort. This disparity suggests that exogenous progesterone administration in the PPOS protocol may exert a positive influence on oocyte meiosis or early embryo mitosis in older patients. Intriguingly, among younger patients, no statistically significant differences were observed between PPOS and GnRH-A treatments, and no correlation was established between the ovarian stimulation protocol and ongoing pregnancy rates (28). Adding to this body of evidence, a matching case-control study conducted by Rania focused on advanced-maternal-age (AMA) women undergoing PGT-A. Across key performance indicators such as fertilization rates, blastulation rates, EER, and both MR and LBR, similar outcomes were reported for both PPOS and GnRH-A groups. Furthermore, cumulative LBR were also comparable between the two protocols (29). Vaiarelli et al. conducted a retrospective matched case-control study comparing oocyte competence between norethisterone acetate-primed ovarian stimulation and the conventional GnRH antagonist protocol in advanced maternal age (AMA) women undergoing preimplantation genetic testing for aneuploidy (PGT-A). Results revealed comparable EBR per MII oocytes, blastocyst morphology, developmental rates, clinical outcomes, and cumulative LBR between the PPOS and control groups (30). Expanding the scope of investigation, Vaiarelli’s another retrospective study compared PPOS-DuoStim with antagonist-DuoStim cycles in 444 couples undergoing PGT-A at a private center, with a mean age of 40. This study demonstrated comparable embryological and clinical outcomes, including similar euploid blastocyst rates per MII oocyte and cumulative LBR between the two protocols (31). It could be found that among those with poor prognoses, the PPOS protocol has shown comparable, even superior, euploid embryo rates and reproductive outcomes.
3.3 The timing of cycle initiation
Does the initiation of PPOS in the follicular phase versus the luteal phase matter? In 2020, Yamakami et al. conducted a retrospective study comparing different pituitary suppression regimens during ovarian stimulation. The results revealed that the administration of progestins, irrespective of whether initiated in the follicular phase (FP) or luteal phase (LP), did not exert any significant influence on chromosomal rearrangements, pregnancy outcomes, or endometrial priming. Notably, compared to GnRH-A (72.9%), both FP and LP progestin groups exhibited a substantial increase in fertilization rates (FP: 86.0% vs LP: 90.5%) (32). In 2024, Tomioka et al. performed a retrospective observational study encompassing 551 PGT-A cycles. This study found no remarkable differences in oocyte competence and embryonic ploidy between FP and LP cycles among patients undergoing PPOS, regardless of their ovarian response. Specifically, the rates of MII oocytes, fertilization, high-quality blastocysts, and EER were comparable between the two groups (33). It appears that the initiation of PPOS in the follicular phase versus the luteal phase does not affect reproductive outcomes.
4 Discussion
Our review may emerge as a pioneering endeavor, comprehensively evaluating the impact of PPOS protocol on EER and reproductive outcomes in PGT cycles. By conducting subgroup analyses, we have gained deeper insights into the performance of PPOS. Furthermore, this review underscores that the initiation of the PPOS protocol does not significantly impact EER or reproductive outcomes. This may broaden the potential applications of PPOS, particularly in patients requiring fertility preservation measures.
In the general population, the PPOS protocol has exhibited comparable reproductive outcomes to those attained through alternative COH protocols. This conclusion is further reinforced by findings from matched and self-controlled population. This is consistent with the conclusions drawn in non-PGT cycles. A recent meta-analysis emphasizes the comparable LBR or OPR per embryo transfer between cycles utilizing the PPOS protocol and those employing the GnRH-A protocol (34).
When stratifying analyses among patient subgroups, the picture changes. In patients with a good prognosis, several studies reported a potential underperformance of the PPOS protocol compared to traditional COH methods. The underlying mechanism may be attributed to the detrimental effects of elevated progesterone levels on oocyte quality. Studies have demonstrated that progesterone can impair blastocyst formation rates in bovine cumulus-oocyte complexes (35), inhibit meiosis resumption in mouse oocytes, and adversely affect oocyte maturation and quality (36). It was also reported that progesterone could suppress the rate of mitosis and apoptosis on granulosa cells via phosphatidylinositol-3 kinase/protein kinase B and mitogen-activated protein kinase pathway (37, 38). Notably, scRNA-seq analysis revealed significantly higher expression of 12 mitochondrial DNA genes in mural granulosa cells of the PPOS group compared with the GnRH antagonist group, which may mechanistically underlie the lower live birth rates observed in PPOS cycles (39).
Conversely, in patients with a poor prognosis, the PPOS protocol has demonstrated comparable or even superior euploid embryo rates and reproductive outcomes, aligning with findings from studies conducted in non-PGT cycles. A self-controlled study among infertile patients with diminished ovarian reserve (DOR) revealed that MPA effectively suppressed LH surges, leading to enhanced outcomes, including LBR (40). Furthermore, a meta-analysis encompassing 14 studies with 4,182 participants found statistically significant improvements in clinical pregnancy rate, optimal embryo rate, and cumulative pregnancy rate when PPOS was compared to clomiphene citrate/letrozole plus gonadotropin protocol for women with DOR (5). The reasons behind these benefits are multifaceted. Patients with a poor prognosis are prone to premature or occult LH surges, which adversely affect follicle quality. The PPOS protocol effectively mitigates these adverse effects by suppressing LH surges from the outset. This is supported by a study demonstrating that MPA significantly suppressed LH surges and improved reproductive outcomes compared to the clomiphene citrate protocol (40). Furtherly, a retrospective analysis of 102 patients in POSEIDON group 4 found lower LH levels on the trigger day and a higher proportion of MII oocytes in the PPOS group compared to the minimal stimulation group (41). At the molecular level, progesterone may improve the follicular microenvironment by regulating the expression of specific microRNAs, such as miR-4261 and miR-6869-5p, in granulosa cells (42). MPA could enhance ovulation rates by upregulating the messenger RNA expression of gap junction protein alpha 1 and vascular endothelial growth factor in follicles (43). Additionally, DYG has been shown to stimulate oocyte maturation and ovulation by increasing the concentrations of acylcarnitines, lysophospholipids, urea, putrescine, and free amino acids in the ovary, via purinergic signaling and arachidonic acid metabolism pathways (44).
From the aforementioned, it is evident that progesterone exerts both beneficial and detrimental effects on oocyte and embryo quality at the molecular level, with its primary role likely influenced by the surrounding microenvironment. This may explain why, in the general population, which likely includes a mix of patients with varying reproductive characteristics, the PPOS protocol demonstrates no significant difference in EER and reproductive outcomes compared to conventional COH protocols.
Nevertheless, it is critically important to recognize the divergent conclusions presented in numerous studies. Such discrepancies are likely attributable to variations in study designs, participant age, ovarian function, sample sizes, as well as differences in the formulations and dosages of progesterone used. Previous studies have indicated that within controlled ovarian hyperstimulation (COH) regimens, different doses and types of progesterone result in varying pregnancy outcomes (34, 45). Additionally, several key clinical variables—including causes of infertility, duration of infertility, male semen quality, and PGT testing methods—were not consistently reported across the studies. Therefore, these results should be interpreted with caution.
5 Conclusion
In conclusion, while PPOS has demonstrated comparable EBR and reproductive outcomes to conventional COH protocols in general population, its application in population with a poor prognosis yields comparable or even better reproductive outcomes, whereas cautious use is warranted in those with a good prognosis. The timing of PPOS cycle initiation, had no significant impact on clinical outcomes in patients with diverse ovarian responses. It is imperative to make individualized treatment plans based on patients’ characteristics and needs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
YM: Methodology, Formal Analysis, Data curation, Writing – original draft. YW: Formal Analysis, Writing – original draft, Data curation. LK: Data curation, Formal Analysis, Writing – original draft. YL: Conceptualization, Writing – review & editing. FW: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. It was used for language polishing.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviation
PPOS, progesterone-primed ovarian stimulation; PGT, preimplantation genetic testing; EER, euploidy embryo rate; COH, controlled ovarian hyperstimulation; ART, assisted reproductive technology; LH, luteinizing hormone; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection; PGT-A, preimplantation genetic testing for aneuploidy; IR, implantation rates; CPR, clinical pregnancy rates; MR, miscarriage rate; GnRH-A, gonadotropin-releasing hormone antagonist; MII, metaphase II; OPR, ongoing pregnancy rate; GnRH-a, GnRH agonist; FET, frozen embryo transfer; LBR, live birth rate; DYG, dydrogesterone; BMI, body mass index; AMH, Anti - Müllerian Hormone; NOR, normal ovarian response; PCOS, polycystic ovary syndrome; POR, poor ovarian response; AMA, advanced-maternal-age; MR, miscarriage rate; DOR, diminished ovarian reserve; MPA, medroxyprogesterone acetate.
References
1. Steward RG, Lan L, Shah AA, Yeh JS, Price TM, Goldfarb JM, et al. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril. (2014) 101:967–73. doi: 10.1016/j.fertnstert.2013.12.026
2. Rodriguez-Purata J and Martinez F. Ovarian stimulation for preimplantation genetic testing. Reproduction. (2019) 157:R127–42. doi: 10.1530/REP-18-0475
3. Kuang Y, Chen Q, Fu Y, Wang Y, Hong Q, Lyu Q, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. (2015) 104:62–70.e3. doi: 10.1016/j.fertnstert.2015.03.022
4. Murria L, Giles J, Bori L, Remohí J, and Cobo A. Progestin prime ovarian stimulation provides comparable outcomes to GnRH antagonist in donor cycles with vitrified oocytes. Fertil Steril. (2025) 20:S0015–0282(25)00442-X. doi: 10.1016/j.fertnstert.2025.05.154
5. Lin G, Zhong X, Li S, Liu X, and Xu L. The clinical value of progestin-primed ovarian stimulation protocol for women with diminished ovarian reserve undergoing IVF/ICSI: a systematic review and meta-analysis. Front Endocrinol. (2023) 14:1232935. doi: 10.3389/fendo.2023.1232935
6. Chen H, Teng XM, Sun ZL, Yao D, Wang Z, and Chen ZQ. Comparison of the cumulative live birth rates after 1 in vitro fertilization cycle in women using gonadotropin-releasing hormone antagonist protocol vs. progestin-primed ovarian stimulation: a propensity score-matched study. Fertil Steril. (2022) 118:701–12. doi: 10.1016/j.fertnstert.2022.06.012
7. Beguería R, García D, Vassena R, and Rodríguez A. Medroxyprogesterone acetate versus ganirelix in oocyte donation: a randomized controlled trial. Hum Reprod. (2019) 34:872–80. doi: 10.1093/humrep/dez034
8. Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. (2014) 101:656–663.e1. doi: 10.1016/j.fertnstert.2013.11.004
9. Moher D, Liberati A, Tetzlaff J, and Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
10. Giles J, Cruz M, Cobo A, Vidal C, Alama P, Requena A, et al. Is Medroxiprogesterone acetate (MPA) an adequate alternative to GnRH antagonist in oocyte vitrification for non oncological fertility preservation (FP) and preimplantation genetic test (PGT-A) cycles? Hum Reprod. (2022) 37:i85–6. doi: 10.1093/humrep/deac106.P-601
11. Giles J, Cruz M, Cobo A, Vidal C, Requena A, Remohi J, et al. Medroxyprogesterone acetate: an alternative to GnRH-antagonist in oocyte vitrification for social fertility preservation and preimplantation genetic testing for aneuploidy. Reprod BioMedicine Online. (2023) 47:103222. doi: 10.1016/j.rbmo.2023.04.013
12. Wang L, Wang J, Zhang Y, Qian C, Wang X, Bai J, et al. Analysis of euploidy rates in preimplantation genetic testing for aneuploidy cycles with progestin-primed versus GnRH agonist/antagonist protocol. Eur J Med Res. (2023) 28:28. doi: 10.1186/s40001-023-01000-1
13. Çakar A, Kalafat E, Benlioglu C, Keles I, Oktem O, Seyhan A, et al. Association of pituitary suppression method with euploid blastocyst yield in preimplantation genetic testing for aneuploidy (PGT-A) cycles. Hum Reprod. (2024) 39:i49. doi: 10.1093/humrep/deae108.095
14. Welp AM, Williams CD, Smith LP, Purcell S, and Goodman LR. Oral medroxyprogesterone acetate for the use of ovulation suppression in in vitro fertilization: a cohort trial. Fertil Steril. (2024) 121:806–13. doi: 10.1016/j.fertnstert.2024.01.026
15. Wang X, Chen B, Fang L, Wang J, Xu A, Xu W, et al. Aneuploidy rates and clinical pregnancy outcomes after preimplantation genetic testing for aneuploidy using the progestin-primed ovarian stimulation protocol or the gonadotropin-releasing hormone antagonist protocol. J Gynecol Obstet Hum Reprod. (2024) 54:102883. doi: 10.1016/j.jogoh.2024.102883
16. Huang B, Li H, Xu B, Li N, Wang X, Li Y, et al. Correlation between controlled ovarian stimulation protocols and euploid blastocyst rate in pre-implantation genetic testing for aneuploidy cycles. Reprod Biol Endocrinol. (2023) 21:118. doi: 10.1186/s12958-023-01166-7
17. Pai AHY, Sung YJ, Li CJ, Lin CY, and Chang CL. Progestin Primed Ovarian Stimulation (PPOS) protocol yields lower euploidy rate in older patients undergoing IVF. Reprod Biol Endocrinol. (2023) 21:72. doi: 10.1186/s12958-023-01124-3
18. Yang L, Luo K, Lu G, Lin G, and Gong F. Euploidy rates between either oral dydrogesterone (DYG) primed ovarian stimulation protocol or the GnRH antagonist (GnRH-ant) protocol in 780 patients in the first PGT-A cycle. Hum Reprod. (2020) 35:i238–9. doi: 10.1093/humrep/35.supplement_1.1
19. Yang L, Luo K, Lu G, Lin G, and Gong F. Euploidy rates among preimplantation genetic testing for aneuploidy cycles with oral dydrogesterone primed ovarian stimulation or GnRH antagonist protocol. Reprod BioMed Online. (2022) 45:721–6. doi: 10.1016/j.rbmo.2022.03.003
20. La Marca A, Capuzzo M, Sacchi S, Imbrogno MG, Spinella F, Varricchio MT, et al. Comparison of euploidy rates of blastocysts in women treated with progestins or GnRH antagonist to prevent the luteinizing hormone surge during ovarian stimulation. Hum Reprod. (2020) 35:1325–31. doi: 10.1093/humrep/deaa068
21. Pittana E, Cimadomo D, Innocenti F, Colamaria S, Argento C, Giuliani M, et al. Progesterone Primed Ovarian Stimulation (PPOS) for DuoStim combined with PGT-A in poor prognosis patients: a valuable strategy. Hum Reprod. (2024) 39:i175–6. doi: 10.1093/humrep/deae108.358
22. Abshekenova A, Karibayeva S, Lokshin V, Valiyev R, Rybina A, and Askar Y. Progestin primed ovarian stimulation: whether there is an effect on embryo euploidy and pregnancy rate in IVF programs with donor oocytes. Hum Reprod. (2022) 37:i505. doi: 10.1093/humrep/deac106.P-672
23. Vidal Segui M, Martinez F, Rodríguez I, and Polyzos NP. Embryo ploidy rates following PPOS or GnRH antagonist protocol. A prospective study with repeated ovarian stimulation. Hum Reprod. (2023) 38:i501–2. doi: 10.1093/humrep/dead093.988
24. Vidal MDM, Martínez F, Rodríguez I, and Polyzos NP. Ovarian response and embryo ploidy following oral micronized progesterone-primed ovarian stimulation versus GnRH antagonist protocol. A prospective study with repeated ovarian stimulation cycles. Hum Reprod. (2024) 39:1098–104. doi: 10.1093/humrep/deae047
25. Li Y, Zhao W, and Liang X. Pregnancy outcomes of progestin primed ovarian stimulation protocol, GnRH antagonist protocol and GnRH agonist protocol for young patients undergoing PGT-M. Hum Reprod. (2021) 36:i411. doi: 10.1093/humrep/deab130.601
26. Zhou R, Dong M, Huang L, Wang S, Fan L, Liang X, et al. Comparison of cumulative live birth rates between progestin-primed ovarian stimulation protocol and gonadotropin-releasing hormone antagonist protocol in different populations. Front Endocrinol (Lausanne). (2023) 14:1117513. doi: 10.3389/fendo.2023.1117513
27. Zhou R, Dong M, Huang L, Wang S, Wang Z, Xu L, et al. Comparison of cumulative live birth rates between progestin and gnRH analogues in preimplantation genetic testing cycles. J Clin Endocrinol Metab. (2024) 109:217–26. doi: 10.1210/clinem/dgad397
28. Wan L, Chen F, Xiong D, Chen S, Chen J, Qin J, et al. Comparison of aneuploidy for patients of different ages treated with progestin-primed ovarian stimulation or GnRH antagonist protocols. Reprod BioMedicine Online. (2024) 49:104349. doi: 10.1016/j.rbmo.2024.104349
29. Rania E, Vaiarelli A, Cimadomo D, Pittana E, Gallo C, Fiorenza A, et al. No impact of Progestin Primed Ovarian Stimulation (PPOS) on euploid blastocyst rate per cohort of metaphase-II oocytes during PGT-A cycles: a case-control study. Hum Reprod. (2023) 38:i491. doi: 10.1093/humrep/dead093.968
30. Vaiarelli A, Cimadomo D, Ruffa A, Rania E, Pittana E, Gallo C, et al. Oocyte competence is comparable between progestin primed ovarian stimulation with Norethisterone acetate (NETA-PPOS) and GnRH-antagonist protocols: A matched case-control study in PGT-A cycles. Eur J Obstetrics Gynecology Reprod Biol. (2024) 294:4–10. doi: 10.1016/j.ejogrb.2023.12.035
31. Vaiarelli A, Pittana E, Cimadomo D, Ruffa A, Colamaria S, Argento C, et al. A multicycle approach through DuoStim with a progestin-primed ovarian stimulation (PPOS) protocol: a valuable option in poor prognosis patients undergoing PGT-A. J Assisted Reprod Genet. (2025) 42(1):255–64. doi: 10.1007/s10815-024-03317-0
32. Yamakami L, Nakano M, Ramos F, Matsumoto L, Tomioka R, Duarte Filho O, et al. Progestins as an alternative to Gonadotropin-Releasing hormone analogues: A retrospective study comparing in vitro fertilization outcomes during follicular and luteal phase stimulation. Hum Reprod. (2020) 35:i4–5. doi: 10.1093/humrep/35.supplement_1.1
33. Tomioka RB, Yamakami L, Tiemi Higa TT, Kowes CL, Miyadahira EH, Polese Freitas LR, et al. Impact of progestin-primed ovarian stimulation on oocyte competence and embryonic ploidy in cycles started in the follicular and luteal phases according to ovarian responses. Fertility Sterility. (2024) 122:246. doi: 10.1016/j.fertnstert.2024.07.723
34. Yildiz S, Turkgeldi E, and Ata B. role and effectiveness of progestins in pituitary suppression during ovarian stimulation for assisted reproductive technology: a systematic review and a meta-analysis. Minerva Obstetrics Gynecology. (2023) 75:573–82. doi: 10.23736/S2724-606X.22.05176-4
35. Salehnia M and Zavareh S. The effects of progesterone on oocyte maturation and embryo development. Int J Fertil Steril. (2013) 7:74–81.
36. Borman SM, Chaffin CL, Schwinof KM, Stouffer RL, and Zelinski-Wooten MB. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol Reprod. (2004) 71:366–73. doi: 10.1095/biolreprod.104.030751
37. Peluso JJ. Progesterone receptor membrane component 1 and its role in ovarian follicle growth. Front Neurosci. (2013) 13:99. doi: 10.3389/fnins.2013.00099
38. Peluso JJ. Progesterone receptor membrane component 1 and its role in ovarian follicle growth. J Adv Res. (2021) 2:189–99. doi: 10.1016/j.jare.2021.02.008
39. Handa M, Takiuchi T, Kawaguchi S, Hon CC, Moody J, Okazaki Y, et al. Adverse effects of progestin-primed ovarian stimulation: combination of clinical study and single-cell analysis. Reprod BioMed Online. (2025) 20:104833. doi: 10.1016/j.rbmo.2025.104833
40. Yu CM, Wang Y-F, Gao T-T, Cao F, Xia X-Y, Chen L, et al. Progestin-primed ovarian stimulation improves the outcomes of IVF/ICSI cycles in infertile women with diminished ovarian reserve. J Chin Med Assoc. (2019) 82:845–8. doi: 10.1097/JCMA.0000000000000177
41. Zhao S and Wang C. Efficacy of progestin-primed ovarian stimulation (PPOS) versus minimal stimulation in women of advanced maternal age with poor ovarian response under the Patient-Oriented Strategies Encompassing Individualized Oocyte Number (POSEIDON) criteria. Ann Palliat Med. (2023) 12:133–40. doi: 10.21037/apm-22-1448
42. Yu J, Zhu D, Zeng C, Zhang Y, Yang H, and Xu Y. MicroRNA expression profiles in the granulosa cells of infertile patients undergoing progestin primed ovarian stimulation. Eur J Obstet Gynecol Reprod Biol. (2022) 276:228–35. doi: 10.1016/j.ejogrb.2022.08.001
43. Seekallu SV, Grazul-Bilska AT, Rawlings NC, and Toosi BM. Markers of ovarian antral follicular development in sheep: comparison of follicles destined to ovulate from the final or penultimate follicular wave of the estrous cycle. Reproduction. (2010) 140:559–68. doi: 10.1530/REP-10-0064
44. Jiang YX, Shi WJ, Ma DD, Zhang JN, Ying GG, Zhang H, et al. Dydrogesterone exposure induces zebrafish ovulation but leads to oocytes over-ripening: An integrated histological and metabolomics study. Environ Int. (2019) 128:390–8. doi: 10.1016/j.envint.2019.04.059
Keywords: progesterone-primed ovarian stimulation protocol, preimplantation genetic testing, euploidy embryo rate, reproductive outcomes, controlled ovarian hyperstimulation
Citation: Mei Y, Wang Y, Kuang L, Lin Y and Wang F (2025) Impact of the PPOS protocol on euploidy embryo rates and reproductive outcomes in preimplantation genetic testing cycles: a systematic review. Front. Endocrinol. 16:1595232. doi: 10.3389/fendo.2025.1595232
Received: 17 March 2025; Accepted: 19 August 2025;
Published: 05 September 2025.
Edited by:
Giorgio Ivan Russo, University of Catania, ItalyReviewed by:
Shuoping Zhang, Reproductive and Genetic Hospital of CITIC-Xiangya, ChinaIman Halvaei, Tarbiat Modares University, Iran
Fernando Prado Ferreira, Neo Vita Clinic, Brazil
Copyright © 2025 Mei, Wang, Kuang, Lin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghong Lin, bGlueWhjZDIwMTFAMTYzLmNvbQ==; Fang Wang, d2FuZ2ZhbmdjZDIwMjJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Youwen Mei
Youwen Mei Yacong Wang†
Yacong Wang† Yonghong Lin
Yonghong Lin Fang Wang
Fang Wang