- 1Division of Gynecology and Obstetrics, Peking University International Hospital, Beijing, China
- 2Department of Reproductive Medicine, Affiliated Hospital of Jining Medical University, Jining, China
- 3Department of Psychiatry, Shandong Daizhuang Hospital, Jining, Shandong, China
- 4Beijing Key Laboratory of Mental Disorders, National Clinical Research Center for Mental Disorders & National Center for Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, China
- 5Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, China
- 6Henan Key Laboratory of Medical Tissue Regeneration, School of Basic Medical Sciences, Xinxiang Medical University, Xinxiang, China
- 7School of Psychology and Mental Health, North China University of Science and Technology, Tangshan, Hebei, China
- 8Faculty of Psychology, Tianjin Normal University, Tianjin, China
Infertility poses considerable challenges to both the endocrine system and psychological well-being of affected women. In particular, those undergoing Assisted Reproductive Technology (ART) confront not only physiological demands but also significant psychological stress. Despite the potential interplay between endocrine factors and psychological status in ART outcomes, large-scale investigations remain limited. In this prospective study, we enrolled 493 women undergoing in vitro fertilization and embryo transfer (IVF-ET) or intracytoplasmic sperm injection (ICSI). We collected baseline demographic data, reproductive history, treatment parameters, sleep quality, and psychological status (via validated questionnaires). We then evaluated the relationships between these variables and clinical pregnancy outcomes. Our analysis revealed that perceived stress, as measured by the Perceived Stress Scale (PSS), was a significant predictor of pregnancy outcomes, whereby elevated perceived stress correlated with lower pregnancy rates. In addition, endocrine and clinical parameters—specifically, basal serum LH levels, endometrial thickness on the day of embryo transfer, number of transferred embryos, and the proportion of top-quality embryos—were strongly associated with clinical pregnancy outcomes. These findings underscore the critical interplay between endocrinology and psychology in ART treatment. Elevated perceived stress and specific endocrine factors each exerted a notable impact on IVF/ICSI success, emphasizing the need for integrative approaches that address both physiological and psychological aspects to enhance clinical pregnancy rates.
Introduction
Infertility is categorized as a disease by the World Health Organization and is defined as failure to achieve pregnancy within twelve months of unprotected intercourse or therapeutic donor insemination in women younger than 35 years or within six months in women who are older than 35 years (1, 2). In the last decades, the incidence of infertility is increasing. The trend of delaying marriage and childbearing has further exacerbated the burden of infertility. The prevalence of infertility ranges from 3.5 to 16.7% in high-resourced countries, and 6.9 to 9.3% in low-resourced countries. In the US, approximately 12.7% of reproductive age women seek treatment for infertility each year (2). And once recent research in China shows that the prevalence of infertility was 25% among couples of reproductive age, and more than half of the couples experiencing infertility would seek medical help (3).
Fertility treatments are complex, a significant number of couples resort to the Assisted Reproductive Technology (ART) (2). The use of ART has substantially improved the ability of couples with infertility to have biological children. ART has evolved rapidly since 1976, it includes intrauterine insemination (IUI),In Vitro fertilization and Embryo Transfer (IVF-ET), Intracytoplasmic Sperm Injection (ICSI), frozen Embryo Transfer (FET), Preimplantation genetic diagnosis (PGD)and etc. (4). Among ART, IVF-ET and ICSI is most widely used. In recent years, although many efforts have been made to improve the protocol of controlled ovarian hyperstimulation, optimize the laboratory culture system, and apply advanced molecular biology techniques, the current clinical pregnancy rate of IVF-ET is not satisfactory (5). In the year 2013, a survey involving 2,639 clinics in 75 participating countries with a global participation rate of 73.6% reported and estimated that 1,160,474 embryo transfers (ETs) were performed resulting in >344,317 babies (6). The IVF/ICSI combined delivery rates per fresh aspiration and frozen ET cycles were 24.2% and 22.8%, respectively. The cumulative delivery rate per aspiration was only 30.4% (6). That means that nearly 70% of the population have failed. Scientists and clinicians continue to explore the factors affecting clinical pregnancy, to achieve a high clinical pregnancy rate has been the common goal of doctors and patients.
Infertility, affecting millions globally, has significant physical, economic, and psychological implications on patients undergoing ART, including IVF. IVF is a multi-step procedure and will take much time and energy, and many factors can affect IVF failure and success rates (5). Previous studies on factors related to pregnancy outcome mainly focused on individual and clinical characteristics, for example, women's age and body mass index (BMI), genetics, serum levels of some hormones, sperm and egg characteristics, environmental and occupational factors and etc. (5, 7). Among them, the relationship between the psychological factor and infertility has been debated for years. There have been dozens of studies which have investigated the relationship between psychological symptoms prior to and during ART cycles and subsequent pregnancy rates, with conflicting results (8). While certain studies suggest no direct association between psychological symptoms and IVF clinical pregnancy rates, others emphasize the need for psychological evaluation and interventions for infertile couples, underlining the multifaceted nature of IVF treatments and outcomes. Moreover, Sleep disorders are prevalent in patients undergoing ART treatment (9–11) Although the sleep quality of females undergoing IVF/ICSI treatment is gaining more interest, sleep status prior to treatment is rarely assessed. The sample sizes of previous studies that investigated the incidence of sleep disorders before IVF/ICSI treatment were relatively small (11, 12). The relationship between the clinical features, the sleep quality and other psychosocial factors and the pregnancy outcome is limited. Our previous research has revealed that the sleep quality of infertile women before ovulation induction was poorer than that of normal adults (13). However, until now, the determinants that affect clinical pregnancy have not been fully revealed.
This study attempts to explore the effects of demographic characteristics, clinical characteristics, mental state and sleep conditions on the clinical pregnancy outcome of IVF-ET/ICSI, aim to provide clinical interventions before IVF/ICSI cycle for improving the clinical pregnancy rate.
Materials and methods
Participants and study design
This is a prospective cohort study. From April 2020 to July 2022, we recruited 493 females who planned to undergo IVF/ICSI treatment at the Reproductive Medicine Department, Affiliated Hospital of Jining Medical College, China. Inclusion criteria: a. Participants who had regular sexual intercourse without contraception for at least one year without pregnancy; b. Participants who planned to undergo IVF/ICSI; c. Age ≥20 years; d. Participants must be able to complete the questionnaire independently; e. No other serious physical illness. Exclusion criteria: a. History of major life events in the last 2 months; b. History of major mental illness. Each participant gave written informed consent, and all protocols were approved by the Medical Science Ethics Board of Affiliated Hospital of Jining Medical University (2020C081).
Data collection
The baseline questionnaire requested details regarding general demographic and socioeconomic status, physical measurements, lifestyle habits, such as birth date, height, weight, educational level, income, smoking, alcohol use, drinking tea or cola, marriage and bearing status (e.g. length of marriage and cohabitation, pregnancy history including information concerning live births, miscarriage, induced abortions and stillbirths), seeking of medical help(e.g. number of previous IVF/ICSI cycles). Validated standardized scales were used to assess participants' psychological states including depression, anxiety, perceived stress, and sleep quality. The clinical data of IVF/ICSI treatment were extracted from the hospital information system, which included basal serum hormonal level, ovarian stimulation protocol, endometrial thickness on the day of embryo transfer, the number of oocytes retrieved, oocyte retrieval rate, number of mature oocytes, number of top-quality embryos, fertilization rate, and clinical pregnancy. The primary endpoint was clinical pregnancy, characterized by the cessation of menstruation, elevated levels of serum human chorionic gonadotropin (HCG), and the ultrasonographic visualization of a gestational sac with embryocardia-beats.
Measures
One of the main challenges in assessing the mental state and sleep conditions in women with infertility is the accuracy of self-report measures. Face-to-face interviews were conducted by trained interviewers comprising psychiatrists and nurses. In the process of investigation, women were interviewed to recall information about their partners and themselves in private to assure the confidentiality of the information obtained.
Basic personal information questionnaire
The custom questionnaire collects demographic information such as age, body mass index, education level, economic status, lifestyle and fertility information such as duration of infertility, cause of infertility, infertility type, past pregnancy history and previous treatment.
Pittsburgh sleep quality index
Pittsburgh Sleep Quality Index (PSQI) was developed in 1989 by Buysse and colleagues and has been widely used to assess sleep quality (14, 15). PSQI consists of 19 items rated by self and 5 items rated by roommate or bedpartner. The 5 other-rated items were used for clinical information only and were not included in the scoring of PSQI. The 19 self-rated items are classified into seven components: subjective sleep quality, subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. Each component scores 0–3 and the scores of the seven components are added together to obtain a total PSQI score to measure global sleep quality. The Chinese version of PSQI showed great reliability and validity in previous study (16).
Beck depression inventory-short form
Beck Depression Inventory-Short Form (BDI-13) is widely used to evaluate the severity of depression. BDI consists of 13 items, and each item scores 0–3. The scoring assessment is as follows: 0–4 no depression, 5–7 mild depression, 8–15 moderate depression, 16–39 severe depression (17).
Self-rating anxiety scale
Zung's self-rating anxiety scale (SAS) assesses subjects' subjective feelings of anxiety (18). The scale includes 20 items that cover 4 groups of manifestations: cognitive, autonomic, motor, and central nervous system symptoms. Each item is rated on a Likert-type scale of 1–4, and the final score was equal to the integer part of the total score of 20 items multiplied by 1.25. Anxiety standard scores ≥ 50 were considered to be at risk for clinical anxiety (19).
Perceived stress scale
Perceived Stress Scale, which had demonstrated good reliability and validity (20), was developed in 1983 by American psychologist Cohent and contains two dimensions: sense of loss of control and tension. Each item is scored on a 5-point scale and the total score ranges from 0 to 56, with higher scores indicating greater perceived stress. The Cronbach's coefficient of the scale is 0.84–0.86, indicating good reliability (21, 22).
Statistical analysis
Normally distributed continuous variables were expressed as mean ± standard deviation and categorical data were expressed as frequency and proportion. To assess the influences of demographic and reproductive characteristics, as well as IVF treatment characteristics on the clinical pregnancy, χ2, multiple logistic regression or Wilcoxon rank sum test were used for categorical variables. For the number of oocytes retrieved, the number of mature oocytes, and the number of high-quality embryos, a negative binomial distribution and log link function were specified. For oocyte retrieval rate and fertilization rate, a linear function was specified. To explore the potential effects of psychological characteristics and sleep status on clinical pregnancy, we used a logistic regression which include depression, anxiety, perceived stress, and sleep quality as independent variables (continuous). Our results reported odds ratios (OR) and 95% CI. OR < 1 indicates a reduced chance of the target event occurrence. Data were organized using Excel 2016 and analyzed using SPSS 27.0. The significance level was set at a two-tailed 5%.
Results
Demographics and fertility characteristics
This investigation encompassed 493 participants, delineating 272 women who achieved clinical pregnancy and 221 who did not. The median ages for male and female partners were established at 32 (range 29-37) and 32 (range 30-36), respectively. Among the cohort, 299 individuals (60.6%) had attained an education level of high school or above, 479 participants (97.2%) were identified as non-smokers or infrequent smokers, and 450 (91.3%) were non-drinkers or infrequent drinkers. The study identified 167 cases (33.9%) of primary infertility. A majority, 442 patients (89.7%), never had sought IVF/ICSI treatment. Two or more embryos transferred in 389 patients (78.9%). Median follicle-stimulating hormone (FSH) levels were recorded at 7.78 (range 6.67-9.19), luteinizing hormone (LH) at 4.77 (range 3.46-6.41), and endometrial thickness on the day of embryo transfer at 11 mm (range 9.2-12.5). Median scores for the Beck Depression Inventory (BDI), Self-Rating Anxiety Scale (SAS), Perceived Stress Scale (PSS), and Pittsburgh Sleep Quality Index (PSQI) were 1 (range 0-5), 28 (range 25-32), 20 (range 15-26), and 4 (range 2-5.5), respectively.
Factor associated with IVF outcome
Demographic characteristic analysis (see Table 1) revealed no significant disparities between pregnant and non-pregnant women concerning BMI (p = 0.994), education level (p = 0.305), average monthly income (p = 0.632), smoking habits (p = 0.618), and alcohol consumption (p = 0.290). Conversely, differences in female and partner ages were statistically significant (p = 0.002 and p < 0.001, respectively), with habits of tea consumption showing marginal significance (p < 0.10). Given the important role of age, subgroup analyses were conducted to examine the predictive effects of age groups for both females and their male partners. Participants were divided into three age groups: Group 1 (≤30 years), Group 2 (>30 and ≤35 years), and Group 3 (>35 years). The logistic regression results indicated that differences in IVF outcomes across female age groups were not statistically significant (Group 1 vs 3: β = -0.09, p = 0.81, OR = 0.92; Group 2 vs 3: β = -0.09, p = 0.76, OR = 0.91). Conversely, male age was significantly associated with pregnancy outcomes. Compared with males in Group 3, those in Group 1 and Group 2 were less likely to experience unsuccessful IVF outcomes (Group 1 vs 3: β = -0.71, p = 0.04, OR = 0.49; Group 2 vs 3: β = -0.66, p = 0.03, OR = 0.52).
Reproductive characteristics between pregnant and not pregnant women
Analysis of reproductive characteristics indicated no significant differences in infertility duration (p = 0.353), cause of infertility (p = 0.669), or number of previous IVF/ICSI treatment cycles (p = 0.850) between the groups. A statistically significant variance was noted in infertility type (p = 0.030) between pregnant and non-pregnant women (Table 2).
IVF treatment characteristics between pregnant and not pregnant women
IVF treatment characteristic analysis showed no significant differences in cycle type (p = 0.553; Table 3), FSH (p = 0.971), prolactin (PRL) (p = 0.381), estradiol (E2) (p = 0.372), testosterone (T) (p = 0.766), progesterone (P) (p = 0.663), total dose of gonadotropin (Gn) (p = 0.493), and duration of Gn used (p = 0.186) between the groups. Statistically significant differences were observed in basal serum LH level (p = 0.039), endometrial thickness on the day of embryo transfer(p = 0.005), number of embryos transferred (p < 0.001), and top-quality embryos rate (p < 0.001).
Psychological characteristics and Sleep status between pregnant and not pregnant women
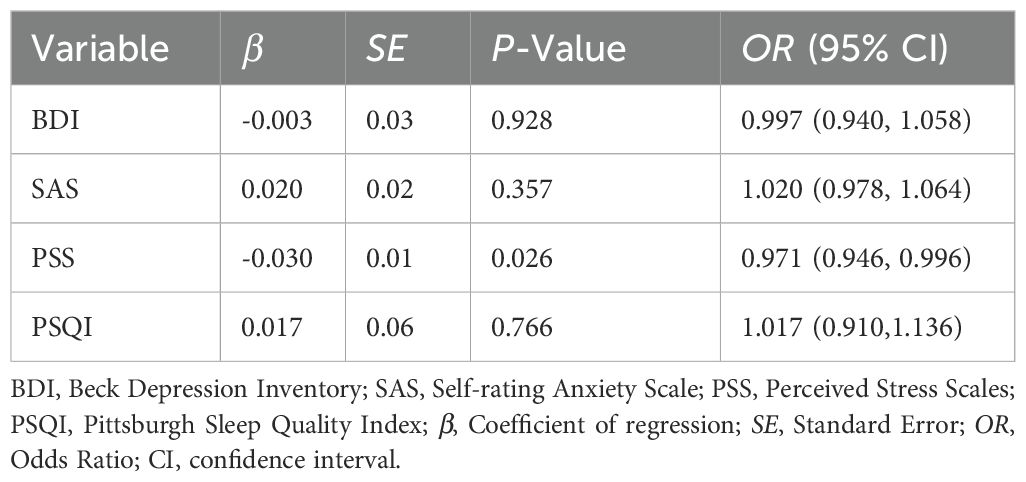
The result of logistic regression indicated that only PSS had a significant β coefficient with p-value of 0.026 (see Table 4). A lower p-value indicates stronger evidence against the null hypothesis that PSS’ β coefficient is zero. This suggests that the observed result in the current sample is a rare event under the null hypothesis, implying that the null hypothesis may be rejected. Therefore, PSS is a significant predictor of pregnancy in this study. Specifically, participants with higher levels of perceived stress were less likely to become pregnant. In contrast, the predictive effects of depression (BDI score, p = 0.928), anxiety (SAS score, p = 0.357), and sleep quality (PSQI score, p = 0.766) were not significant.
Discussion
Since the landmark birth of the first IVF baby in the United Kingdom in 1978, millions upon millions of children have been born since that time who simply could not have been without the development of IVF (23). IVF technology has seen widespread adoption for treating infertility, experiencing rapid advancements. Nonetheless, the current clinical success rates for IVF/ICSI are relatively modest, prompting considerable research interest in strategies to enhance clinical pregnancy rates in the realm of reproductive medicine. Our prospective study identified female age, male partner age, infertility type, basal LH, endometrial thickness on the day of embryo transfer, number of embryos transferred, embryo quality, and perceived stress level as significant factors influencing IVF pregnancy success in this cohort. Psychological factors like depression, anxiety, and poor sleep quality did not show a significant link. Perceived stress was the only significant psychological predictor, negatively impacting pregnancy chances. This study's strength lies in its comprehensive approach, examining social, physiological, and psychological variables that might affect the outcome of IVF-assisted pregnancies, thereby providing a theoretical foundation for strategies aimed at enhancing clinical pregnancy rates.
In our cohort of 493 participants, the clinical pregnancy rate reached 55.2%, modestly exceeding the 49.1% live birth rate per embryo transfer under 35 years old reported in the CDC's 2022 US national ART data. Given that our study exclusively assessed clinical pregnancy outcomes—without tracking subsequent live births—this discrepancy is methodologically expected. It further underscores a key limitation of our research: the absence of longitudinal follow-up for birth outcomes.
Consistent with existing literature, our study corroborates that the ages of both partners are significantly associated with differing clinical pregnancy outcomes. The fecundity of women decreases gradually but significantly beginning approximately at age 32 years and decreases more rapidly after age 37 years. The age-related decline in fertility is accompanied by significant increases in the rates of disorders that impair fertility, such as leiomyomas, tubal disease, and endometriosis, and an increased risk of aneuploidy and spontaneous abortion (24). However, the predictive effect of male age appears to be more pronounced in this study, whereas few studies have specifically examined the impact of paternal age on ART outcomes. Some studies have shown that advancing paternal age is associated with a lower probability of IVF/ICSI pregnancy (25), other studies have not observed a similar association (26–28). Recent investigations have elucidated the detrimental effects of paternal age on the timing of blastocyst formation and HCG levels, For every increase of one year, men had a 6% increased probability that the competent blastocyst was transferred on day 6 compared to day 5,and further they showed that the mean difference in hCG values when comparing paternal age group 30-34, 35–39 and 40–45 with the age group 25–29 in those receiving COS treatment, all showed significantly lower adjusted values for older men (29). These investigators demonstrate that after age 40 years sperm exhibit increased DNA fragmentation, chromatin decondensation and aneuploidy rates, and in IVF there are more canceled embryo transfers, lower clinical pregnancy rates and greater miscarriage rates (30).
The core findings of our investigation underscore the basal serum luteinizing hormone (LH) levels, endometrial thickness on the day of embryo transfer, the number of embryos transferred, and the proportion of top-quality embryos as pivotal factors associated with varied pregnancy outcomes. Intriguingly, an elevation in the basal serum LH levels has been identified as a prognosticator for the non-achievement of clinical pregnancy in women. Many studies have delved into the intricate role of hormone levels in determining the outcomes of IVF and intracytoplasmic sperm injection (ICSI) treatments. One area of focus has been that higher levels of basal LH, AMH, estradiol (E2) on gonadotropin-releasing hormone antagonist (GnRH-ant) start day and lower levels of LH on GnRH-ant start day had negative association with outcomes, including number of oocytes retrieved, 2PN, available embryos, incidence of OHSS, chemical pregnancy, clinical pregnancy, abortion and live birth; and the specific thresholds ratio of serum LH on the day of GnRH-ant administration to basal LH levels (hLH/bLH) may predict the success of IVF/ICSI outcomes more reliably (31). Another research found that the LH levels were correlated with clinical pregnancy and live births. The highest clinical pregnancy rate (25%) was achieved in women with low LH (< 2IU/l); whereas the miscarriage rate was almost similar in all the groups, indicating other factors that could influence the miscarriage rate exist. The pregnancy rate was the lowest (16%) in women with high LH levels (> 8IU/L); however, none of the results had statistical significance (32). Another study on serum LH measurements during the follicular phase in IVF cycles using GnRH agonist protocols indicates that suppressed levels of LH do not predict ovarian response or pregnancy outcomes (33). Consequently, the predictive effect of LH on pregnancy outcomes is still indeterminate in various studies. Furthermore, in our reach, the remaining basal serum hormonal level had no predictive value for pregnancy outcomes. This is consistent with previous studies. Basal levels of progesterone and testosterone have been scrutinized for their predictive value regarding ovarian response and IVF success. While elevated basal progesterone levels correlate with the incidence of preovulatory progesterone rise, they do not necessarily impact clinical pregnancy rates (34). Similarly, basal testosterone levels show a positive association with ovarian reserve markers and response, yet they fall short of predicting pregnancy outcomes (35, 36).
This investigation elucidated that augmented endometrial thickness at the time of transfer, the transfer of multiple embryos, and a heightened proportion of top-quality embryos show potential protective associations for the attainment of clinical pregnancy in females. The mean endometrial thickness observed on the day of embryo transfer was 11mm in the pregnancy cohort compared to 10.5mm in the non-pregnancy cohort. The relationship between endometrial thickness (EMT) and IVF outcomes, including live birth rates (LBR) and pregnancy losses, has been a focal point of numerous studies. The exact influence of EMT, as measured by ultrasonography, on IVF success rates remains a subject of debate due to inconsistencies across retrospective and prospective studies, confounded by factors such as age, estradiol levels, and oocyte count (37–39). Data from multiple sources, including the Canadian Assisted Reproductive Technology Registry and various fertility centers in China, indicate that increasing EMT is generally associated with improved clinical pregnancy rates and LBR, with a notable plateau effect beyond certain thickness thresholds (37, 40). What is he optimal EMT threshold that optimizes the IVF outcome? The results are controversial. Fresh embryo transfer cycles show an increase in LBR until an EMT of 10–12 mm, whereas in frozen embryo transfer (FET) cycles, the benefits plateau after 7–10 mm. Conversely, an EMT less than 6 mm is clearly linked with diminished LBR in both fresh and FET cycles (37). Another research reveals that clinical pregnancy rate, live birth rate and miscarriage rate may achieve their optimal level when EMT ≥ 12 mm, but some adverse pregnancy outcomes would be observed when EMT ≥15 mm especially for clinical pregnancy in fresh cycles (41). Despite these findings, the independent predictive value of EMT for IVF success is minimal, suggesting that while EMT is a factor, it should not be the sole criterion for clinical decision-making in IVF treatments (42).
Moreover, our investigation revealed strong associations between both embryo quality and the number of embryos transferred with clinical pregnancy outcomes, aligning with previous research findings. Similar studies have identified a non-linear relationship between the number of top-quality blastocysts (TQB) and successful pregnancy outcomes. Specifically, the odds of achieving an ongoing pregnancy or live birth increased by approximately 11% or more for each additional TQB, up to five TQB. Beyond this point, the outcomes plateaued, suggesting a diminishing return on pregnancy success after the transfer of five blastocysts (43). The contention over whether the augmentation in the number of embryos transferred is associated with improved pregnancy rates continues to be a subject of discussion. Another similar research supports that in women ≤42 years, transferring a single euploid blastocyst resulted in pregnancy rates similar to those of transferring 2 untested blastocysts while dramatically reducing the risk of twins (44). More and more studies emphasize the importance of single blastocyst transfer to minimize the risk of multiple pregnancies and enhance live birth rates, regardless of patient age or blastocyst quality. The American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology have updated guidelines to reduce multiple pregnancies in IVF cycles, advocating for elective single-embryo transfers, particularly in women under 38, and suggesting preimplantation genetic testing to lower the risk of multiples (45).
Our analysis revealed that fertility-related characteristics, including the duration of infertility, its causes, and the number of preceding IVF/ICSI treatment cycles exhibited no significant association with clinical pregnancy rates. However, the type of infertility (based on the history of previous pregnancy) displayed a statistically significant correlation. This observation is in harmony with the results of some previous investigations, where these factors were associated with clinical pregnancy in several large-scale prospective studies. There was a significant decrease in age-adjusted livebirth rates with increasing duration of infertility between 1 and 12 years. Women who had had any previous pregnancy had a significantly higher livebirth rate than women with no previous pregnancies. The livebirth rate per treatment cycle, per egg collection, or embryo transfer was highest in the first cycle and decreased significantly with increasing number of previous treatment cycles (5). Notwithstanding the substantial participant count in our study, the sample size may still fall short of the requisite magnitude for a meaningful prediction of clinical pregnancy outcomes.
Lifestyle attributes such as BMI, educational attainment, income levels, smoking habits, tea, cola consumption, and alcohol intake were found to bear no significant relation to the outcomes of clinical pregnancy. Findings from multiple studies indicate that elevated BMI, particularly obesity, in women has been associated with poorer ART outcomes. A new approach, MitoScore, BMI, and body fat percentage were significantly lower in pregnant women as compared to non-pregnant women, they suggest that MitoScore, BMI, and body fat percentage could act as critical parameters in determining the success of ART (46). Not only spontaneous fertility and pregnancy outcomes after IVF, live birth rates both in IVF and ICSI cycles could be influenced by male and/or female BMI (47). However, conventional assessments of embryo morphology and quality did not significantly differ across BMI categories (48). Interestingly, the source of the oocytes (donor or non-donor) didn't sway the overall interpretation, but obesity in conjunction with polycystic ovary syndrome had a detrimental (49). The overarching consensus is that while elevated BMI may impact ART outcomes, its role as a sole determinant remains nuanced and multifactorial, pointing to the necessity of further research, especially on the benefits of weight reduction before undergoing ART.
The relationship between lifestyle choices and fertility outcomes, particularly in the context of IVF treatments, is a topic of growing research interest. A significant focus across several studies is on the consumption of caffeine and its potential impact on fertility. One New Zealand-based cross-sectional study examined the lifestyle and dietary habits of 250 women preparing for fertility treatment, revealing that a substantial proportion of these women engaged in poor lifestyle choices, notably high rates of alcohol (50.8%) and caffeine consumption (86.8%) (50). Another study delved into the specific effects of caffeine on IVF outcomes, using serum and follicular fluid samples from 619 women. While it found no direct link between coffee or tea consumption and pregnancy rates, it did observe potential adverse effects of caffeine on reproductive processes. Specifically, as serum caffeine levels increased, the number of eggs retrieved decreased, with higher coffee intake being associated with more aborted pregnancies (51). In contrast, a study involving 2474 couples and 4716 IVF cycles concluded that neither female nor male caffeine consumption impacted IVF outcomes significantly, such as live birth rates, fertilization rates, or implantation rates. A separate investigation into 2817 fertile women showed no discernible adverse effect of caffeine on time to conceive, further emphasizing the complexity of the caffeine-fertility relationship (52). Beyond caffeine, other lifestyle factors like smoking have also been scrutinized for their potential effects on fertility. One study pointedly demonstrated the negative impact of active smoking in women on both ovarian reserve and IVF outcomes. Active smokers had a decreased ovarian response to hyperstimulation, reflected in fewer mature oocytes retrieved, and a considerably lower clinical pregnancy rate when compared to non-smokers (53). However, neither caffeine nor smoking showed a significant association with pregnancy rates in the current study. One potential explanation is that unbalanced sample among subgroups related to smoking and drinking tea or cola. In conclusion, the connection between these lifestyles and successful fertility outcomes requires further research for definitive conclusions. The overarching sentiment, however, is the critical need for awareness and advice about healthy lifestyle choices for individuals planning to undergo fertility treatments.
IVF and ART are accompanied by significant emotional and psychological challenges. Our findings indicate that psychological states, especially perceived stress, might impact the rates of clinical pregnancy, being consistent with a meta-research’s result (54). It's noteworthy that perceived stress is not limited to the individual undergoing treatment. In IVF couples, research has shown that both partners may suffer more stress and emotional problems. This turbulence may even have biochemical indicators, as elevated cytokine levels in both partners have been associated with unsuccessful IVF attempts (55). There is also some indirect evidence to support this finding that the resilience of pregnant women in the IVF circle was significantly higher than that of non-pregnant women (56). Interestingly, this buffering effect seems to wane after unsuccessful IVF attempts (57). It could be inferred that a higher level of resilience may improve the success rate of IVF by buffering the increasing perceived stress, though with an unsustainable effect. Moreover, previous studies found the controversial influence of sleep quality and emotional problems on the outcome of IVF (58–60). The current study suggested that sleep quality and emotional problems may not be determining factors of IVF’s success. Specifically, the predictive effects of depression, anxiety, as well as sleep quality, which are regarded as stress-related health problems (61), were not significant. Compared with given emotional disorders or sleep disorders, subjectively perceived stress has a stronger connection to IVF’s outcome. Further research could explore whether resilience plays a buffering role in this connection.
Limitations
This study has several limitations. Most notably, its single-center design may restrict the generalizability of the findings, while the relatively restricted sample size could further compromise the universality of our conclusions. This necessitates subsequent multi-center studies with expanded cohorts for validation. Additionally, although this study investigated the relationship between psychological factors, sleep quality, and pregnancy outcomes, the assessment was confined to the pre-IVF/ICSI treatment phase. Future research will incorporate dynamic evaluations at critical junctures throughout the assisted reproductive continuum—specifically during pre-oocyte retrieval, pre-embryo transfer, and peri-pregnancy confirmation periods. Through expanded sample sizes and enhanced data collection, we seek to comprehensively characterize the evolving trajectories of psychological states and sleep patterns during assisted reproduction. This integrated approach endeavors to establish an evidence base for clinical practice, providing targeted psychological interventions when indicated, with the ultimate objective of optimizing clinical pregnancy outcomes.
Conclusion
There are several significant factors associated with the likelihood of achieving clinical pregnancy following IVF/ICSI treatment. Clinical pregnancy success was significantly associated with younger age in both partners, secondary infertility, lower basal LH levels, increased endometrial thickness at embryo transfer, transfer of more embryos, higher proportion of top-quality embryos, and reduced perceived stress. No significant associations were observed with lifestyle factors (BMI, smoking, alcohol), other hormonal profiles, infertility history, or psychological states except perceived stress.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Medical Science Ethics Board of Affiliated Hospital of Jining Medical University (number: 2020C081). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CW: Conceptualization, Data curation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. Q-LL: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Y-SX: Investigation, Writing – original draft, Writing – review & editing. K-XC: Data curation, Writing – original draft, Writing – review & editing. Y-QZ: Writing – review & editing, Data curation, Investigation, Validation. LC: Data curation, Writing – original draft. YT: Writing – review & editing. A-JY: Data curation, Writing – original draft. ZL: Data curation, Writing – original draft. LZ: Data curation, Formal Analysis, Writing – original draft. LL: Conceptualization, Data curation, Project administration, Writing – original draft. TL: Conceptualization, Project administration, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the General Project of Shandong Natural Science Foundation (ZR2022MH094), the Key Research and Development of Jining City (2022YXNS092).
Acknowledgments
The authors would like to thank the study subjects for their participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. (2020) 113:533–5. doi: 10.1016/j.fertnstert.2019.11.025
2. Carson SA and Kallen AN. Diagnosis and management of infertility: A review. Jama. (2021) 326:65–76. doi: 10.1001/jama.2021.4788
3. Zhou Z, Zheng D, Wu H, Li R, Xu S, Kang Y, et al. Epidemiology of infertility in China: a population-based study. Bjog. (2018) 125:432–41. doi: 10.1111/1471-0528.14966
4. Sunderam S, Kissin DM, Crawford SB, Folger SG, Boulet SL, Warner L, et al. Assisted reproductive technology surveillance - United States, 2015. MMWR Surveill Summ. (2018) 67:1–28. doi: 10.15585/mmwr.ss6703a1
5. Templeton A, Morris JK, and Parslow W. Factors that affect outcome of in-vitro fertilisation treatment. Lancet. (1996) 348:1402–6. doi: 10.1016/s0140-6736(96)05291-9
6. Banker M, Dyer S, Chambers GM, de Mouzon J, Zegers-Hochschild F, David Adamson G, et al. International Committee for Monitoring Assisted Reproductive Technologies (ICMART): world report on assisted reproductive technologies, 2013. Fertil Steril. (2021) 116:741–56. doi: 10.1016/j.fertnstert.2021.03.039
7. Dabbagh Rezaeiyeh R, Mehrara A, Mohammad Ali Pour A, Fallahi J, and Forouhari S. Impact of various parameters as predictors of the success rate of in vitro fertilization. Int J Fertil Steril. (2022) 16:76–84. doi: 10.22074/ijfs.2021.531672.1134
8. Rooney KL and Domar AD. The relationship between stress and infertility. Dialogues Clin Neurosci. (2018) 20:41–7. doi: 10.31887/DCNS.2018.20.1/klrooney
9. Eryilmaz OG, Devran A, Sarikaya E, Aksakal FN, Mollamahmutoğlu L, and Cicek N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J Assist Reprod Genet. (2011) 28:815–20. doi: 10.1007/s10815-011-9604-y
10. Pavone M, Hirshfeld-Cytron J, Lawson A, Smith K, and Klock S. Sleep distubances high in patients seeking fertility preservation. Fertility sterility. (2013) 100:S168. doi: 10.1016/j.fertnstert.2013.07.1463
11. Goldstein CA, Lanham MS, Smith YR, and O'Brien LM. Sleep in women undergoing in vitro fertilization: a pilot study. Sleep Med. (2017) 32:105–13. doi: 10.1016/j.sleep.2016.12.007
12. Philipsen MT, Knudsen UB, Zachariae R, Ingerslev HJ, Hvidt JEM, and Frederiksen Y. Sleep, psychological distress, and clinical pregnancy outcome in women and their partners undergoing in vitro or intracytoplasmic sperm injection fertility treatment. Sleep Health. (2022) 8:242–8. doi: 10.1016/j.sleh.2021.10.011
13. Li QL, Wang C, Cao KX, Zhang L, Xu YS, Chang L, et al. Sleep characteristics before assisted reproductive technology treatment predict reproductive outcomes: a prospective cohort study of Chinese infertile women. Front Endocrinol (Lausanne). (2023) 14:1178396. doi: 10.3389/fendo.2023.1178396
14. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, and Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
15. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, and Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. (2016) 25:52–73. doi: 10.1016/j.smrv.2015.01.009
16. Ng KC, Wu LH, Lam HY, Lam LK, Nip PY, Ng CM, et al. The relationships between mobile phone use and depressive symptoms, bodily pain, and daytime sleepiness in Hong Kong secondary school students. Addict Behav. (2020) 101:105975. doi: 10.1016/j.addbeh.2019.04.033
17. Beck AT and Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. (1974) 7:151–69.
18. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. (1971) 12(6):371–9. doi: 10.1016/S0033-3182(71)71479-0
19. Zung WW, Magruder-Habib K, Velez R, and Alling W. The comorbidity of anxiety and depression in general medical patients: a longitudinal study. J Clin Psychiatry. (1990) 51:77–80.
20. Leung DY, Lam TH, and Chan SS. Three versions of Perceived Stress Scale: validation in a sample of Chinese cardiac patients who smoke. BMC Public Health. (2010) 10:513. doi: 10.1186/1471-2458-10-513
21. Cohen S, Kamarck T, and Mermelstein R. A global measure of perceived stress. J Health Soc Behav. (1983) 24:385–96. doi: 10.2307/2136404
22. Cohen S and Williamson GM. Stress and infectious disease in humans. Psychol Bull. (1991) 109:5–24. doi: 10.1037/0033-2909.109.1.5
23. Niederberger C, Pellicer A, Cohen J, Gardner DK, Palermo GD, O'Neill CL, et al. Forty years of IVF. Fertil Steril. (2018) 110:185–324.e5. doi: 10.1016/j.fertnstert.2018.06.005
24. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. (2014) 101:633–4. doi: 10.1016/j.fertnstert.2013.12.032
25. Klonoff-Cohen HS and Natarajan L. The effect of advancing paternal age on pregnancy and live birth rates in couples undergoing in vitro fertilization or gamete intrafallopian transfer. Am J Obstet Gynecol. (2004) 191:507–14. doi: 10.1016/j.ajog.2004.01.035
26. Paulson RJ, Milligan RC, and Sokol RZ. The lack of influence of age on male fertility. Am J Obstet Gynecol. (2001) 184:818–22. doi: 10.1067/mob.2001.113852
27. Bellver J, Garrido N, Remohí J, Pellicer A, and Meseguer M. Influence of paternal age on assisted reproduction outcome. Reprod BioMed Online. (2008) 17:595–604. doi: 10.1016/s1472-6483(10)60305-7
28. Spandorfer SD, Avrech OM, Colombero LT, Palermo GD, and Rosenwaks Z. Effect of parental age on fertilization and pregnancy characteristics in couples treated by intracytoplasmic sperm injection. Hum Reprod. (1998) 13:334–8. doi: 10.1093/humrep/13.2.334
29. Borgstrøm MB, Grøndahl MLT, Klausen TW, Danielsen AK, Thomsen T, Bentin-Ley UB, et al. Is paternal age associated with transfer day, developmental stage, morphology, and initial hCG-rise of the competent blastocyst leading to live birth? A multicenter cohort study. PloS One. (2022) 17:e0270664. doi: 10.1371/journal.pone.0270664
30. Kaarouch I, Bouamoud N, Madkour A, Louanjli N, Saadani B, Assou S, et al. Paternal age: Negative impact on sperm genome decays and IVF outcomes after 40 years. Mol Reprod Dev. (2018) 85:271–80. doi: 10.1002/mrd.22963
31. Wei Y, Luan T, Shen J, Zhang JJ, Zhang J, Su Y, et al. LH on GnRH-ant day to basal LH affects the IVF/ICSI outcome of PCOS women undergoing GnRH-antagonist protocol. Int J Gynaecol Obstet. (2024) 164:624–32. doi: 10.1002/ijgo.15131
32. Bansal S, Singh N, Gupta P, Malhotra N, and Mahendru R. Does basal luteinizing hormone help predict the fate of in vitro fertilization? JBRA Assist Reprod. (2016) 20:66–71. doi: 10.5935/1518-0557.20160016
33. Cabrera RA, Stadtmauer L, Mayer JF, Gibbons WE, and Oehninger S. Follicular phase serum levels of luteinizing hormone do not influence delivery rates in in vitro fertilization cycles down-regulated with a gonadotropin-releasing hormone agonist and stimulated with recombinant follicle-stimulating hormone. Fertil Steril. (2005) 83:42–8. doi: 10.1016/j.fertnstert.2004.06.050
34. Mutlu MF, Erdem M, Mutlu I, Bulut B, and Erdem A. Elevated basal progesterone levels are associated with increased preovulatory progesterone rise but not with higher pregnancy rates in ICSI cycles with GnRH antagonists. Eur J Obstet Gynecol Reprod Biol. (2017) 216:46–50. doi: 10.1016/j.ejogrb.2017.06.044
35. Xiao S, Li Y, Long L, Luo C, and Mai Q. Basal serum testosterone levels correlate with ovarian reserve and ovarian response in cycling women undergoing in vitro fertilization. Gynecol Endocrinol. (2016) 32:51–4. doi: 10.3109/09513590.2015.1076784
36. Sun B, Wang F, Sun J, Yu W, and Sun Y. Basal serum testosterone levels correlate with ovarian response but do not predict pregnancy outcome in non-PCOS women undergoing IVF. J assisted Reprod Genet. (2014) 31:829–35. doi: 10.1007/s10815-014-0246-8
37. Mahutte N, Hartman M, Meng L, Lanes A, Luo ZC, and Liu KE. Optimal endometrial thickness in fresh and frozen-thaw in vitro fertilization cycles: an analysis of live birth rates from 96,000 autologous embryo transfers. Fertil Steril. (2022) 117:792–800. doi: 10.1016/j.fertnstert.2021.12.025
38. Mathyk B, Schwartz A, DeCherney A, and Ata B. A critical appraisal of studies on endometrial thickness and embryo transfer outcome. Reprod BioMed Online. (2023) 47:103259. doi: 10.1016/j.rbmo.2023.103259
39. Liao SJ, Wang R, Hu C, Pan WJ, Pan W, Yu D, et al. Analysis of endometrial thickness patterns and pregnancy outcomes considering 12,991 fresh IVF cycles. BMC Med Inform Decis Mak. (2021) 21:176. doi: 10.1186/s12911-021-01538-2
40. Moshkalova G, Karibayeva I, Kurmanova A, et al. Endometrial thickness and live birth rates after IVF: a systematic review. Acta Biomed. (2023) 94:e2023152. doi: 10.23750/abm.v94i3.14437
41. Xu J, Zhang S, Jin L, Mao Y, Shi J, Huang R, et al. The effects of endometrial thickness on pregnancy outcomes of fresh IVF/ICSI embryo transfer cycles: an analysis of over 40,000 cycles among five reproductive centers in China. Front Endocrinol (Lausanne). (2021) 12:788706. doi: 10.3389/fendo.2021.788706
42. Griesinger G, Trevisan S, and Cometti B. Endometrial thickness on the day of embryo transfer is a poor predictor of IVF treatment outcome. Hum Reprod Open. (2018) 2018:hox031. doi: 10.1093/hropen/hox031
43. Xiong F, Sun Q, Li G, Yao Z, Chen P, Wan C, et al. Association between the number of top-quality blastocysts and live births after single blastocyst transfer in the first fresh or vitrified-warmed IVF/ICSI cycle. Reprod BioMed Online. (2020) 40:530–7. doi: 10.1016/j.rbmo.2020.01.005
44. Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. (2013) 100:100–7.e1. doi: 10.1016/j.fertnstert.2013.02.056
45. Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril. (2021) 116:651–4. doi: 10.1016/j.fertnstert.2021.06.050
46. Arora H, Collazo I, Eisermann J, Hendon N, Kuchakulla M, Khodamoradi K, et al. Association between mitoScore, BMI, and body fat percentage as a predictive marker for the outcome of in-vitro fertilization (IVF). Cureus. Jul. (2022) 14:e27367. doi: 10.7759/cureus.27367
47. Qi L, Liu YP, Wang SM, Shi H, Chen XL, Wang NN, et al. Abnormal BMI in male and/or female partners are deleterious for embryonic development and pregnancy outcome during ART process: A retrospective study. Front Endocrinol (Lausanne). (2022) 13:856667. doi: 10.3389/fendo.2022.856667
48. Bellver J. BMI. and miscarriage after IVF. Curr Opin Obstet Gynecol. (2022) 34:114–21. doi: 10.1097/gco.0000000000000778
49. Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M, et al. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update. (2019) 25:439–51. doi: 10.1093/humupd/dmz011
50. Gormack AA, Peek JC, Derraik JG, Gluckman PD, Young NL, and Cutfield WS. Many women undergoing fertility treatment make poor lifestyle choices that may affect treatment outcome. Hum Reprod. (2015) 30:1617–24. doi: 10.1093/humrep/dev094
51. Al-Saleh I, El-Doush I, Grisellhi B, and Coskun S. The effect of caffeine consumption on the success rate of pregnancy as well various performance parameters of in-vitro fertilization treatment. Med Sci Monit. (2010) 16:Cr598–605.
52. Choi JH, Ryan LM, Cramer DW, Hornstein MD, and Missmer SA. Effects of caffeine consumption by women and men on the outcome of in vitro fertilization. J Caffeine Res. (2011) 1:29–34. doi: 10.1089/jcr.2011.0001
53. Freour T, Masson D, Mirallie S, Jean M, Bach K, Dejoie T, et al. Active smoking compromises IVF outcome and affects ovarian reserve. Reprod BioMed Online. (2008) 16:96–102. doi: 10.1016/s1472-6483(10)60561-5
54. Matthiesen SM, Frederiksen Y, Ingerslev HJ, and Zachariae R. Stress, distress and outcome of assisted reproductive technology (ART): a meta-analysis. Hum Reprod. (2011) 26:2763–76. doi: 10.1093/humrep/der246
55. Haimovici F, Anderson JL, Bates GW, Racowsky C, Ginsburg ES, Simovici D, et al. Stress, anxiety, and depression of both partners in infertile couples are associated with cytokine levels and adverse IVF outcome. Am J Reprod Immunol. (2018) 79:e12832. doi: 10.1111/aji.12832
56. Santa-Cruz DC, Caparros-Gonzalez RA, Romero-Gonzalez B, Peralta-Ramirez MI, Gonzalez-Perez R, and García-Velasco JA. Hair cortisol concentrations as a biomarker to predict a clinical pregnancy outcome after an IVF cycle: A pilot feasibility study. Int J Environ Res Public Health. (2020) 17(9):3020. doi: 10.3390/ijerph17093020
57. Fernandez-Ferrera C, Llaneza-Suarez D, Fernandez-Garcia D, Castañon V, Llaneza-Suarez C, and Llaneza P. Resilience, perceived stress, and depressed mood in women under in vitro fertilization treatment. Reprod Sci Mar. (2022) 29:816–22. doi: 10.1007/s43032-021-00685-1
58. Ražić Pavičić A, Jakšić N, Jakovina T, Skočić Hanžek M, Miškulin I, and Gregurek R. The role of psychological factors in the outcome of in vitro fertilization in women with primary infertility. Psychiatr Danub. (2022) 34:104–14.
59. Liu Z, Zheng Y, Wang B, Li J, Qin L, Li X, et al. The impact of sleep on in vitro fertilization embryo transfer outcomes: a prospective study. Fertil Steril. (2023) 119:47–55. doi: 10.1016/j.fertnstert.2022.10.015
60. Cesta CE, Viktorin A, Olsson H, Johansson V, Sjölander A, Bergh C, et al. Depression, anxiety, and antidepressant treatment in women: association with in vitro fertilization outcome. Fertil Steril. (2016) 105:1594–1602.e3. doi: 10.1016/j.fertnstert.2016.01.036
Keywords: infertile women, psychological factor, stress, assisted reproductive technology (ART), reproductive outcomes
Citation: Wang C, Li Q-L, Xu Y-S, Cao K-X, Zhang Y-Q, Chang L, Tong Y, Yang A-J, Liu Z, Zhang L, Lin L and Liu T (2025) Interplay of endocrine and psychological factors in IVF/ICSI outcomes: a prospective cohort analysis. Front. Endocrinol. 16:1596664. doi: 10.3389/fendo.2025.1596664
Received: 20 March 2025; Accepted: 30 September 2025;
Published: 24 October 2025.
Edited by:
Gedis Grudzinskas, Independent Researcher, London, United KingdomReviewed by:
Saba Amin, Shri Venkateshwara University, IndiaMeruyert Suleimenova, PERSONA International Clinical Center for Reproductology, Kazakhstan
Copyright © 2025 Wang, Li, Xu, Cao, Zhang, Chang, Tong, Yang, Liu, Zhang, Lin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tour Liu, bWlrZWJvbml0YUBob3RtYWlsLmNvbQ==; Li Lin, bF9kb2N0b3IyMDIxQDE2My5jb20=; Lin Zhang, anlmeXpsQDE2My5jb20=
 Chao Wang
Chao Wang Qian-Ling Li
Qian-Ling Li Yun-Shuai Xu3
Yun-Shuai Xu3 Liang Chang
Liang Chang Ai-Jun Yang
Ai-Jun Yang Lin Zhang
Lin Zhang Tour Liu
Tour Liu