- 1Department of Pediatrics, Vittore Buzzi Children’s Hospital, Department of Biomedical and Clinical Science, Università di Milano, Milan, Italy
- 2Department of Biomedical and Clinical Sciences, “L. Sacco”, Biological Chemistry and Nutritional Biochemistry Lab, Università di Milano, , Milan, Italy
The usefulness, effectiveness, and safety of advanced hybrid closed-loop systems (AHCLs) in early-stage 3 type 1 diabetes (T1D) are unknown. We report 9 months of continuous glucose monitoring (CGM) outcomes in a patient with early-stage 3 T1D treated with the Tandem t:slim Control-IQ® system. A 13-year-old girl, affected by celiac disease and Hashimoto’s thyroiditis, was diagnosed with T1D without symptoms (fasting c-peptide: 1.77 ng/ml; HbA1c: 6.4%) following an outpatient T1D screening program. She wore a CGM at diagnosis to closely monitor her glucose profile. After 6 months, when the time in range (TIR) fell below 80%, the Tandem t:slim Control-IQ® was initiated. Standardized CGM metrics, as well as instances of severe hypoglycemia and diabetic ketoacidosis (DKA), were recorded. CGM data guided the initiation of early insulin therapy. Tandem t:slim Control-IQ® system proved effective from the onset of T1D, with a low insulin requirement (0.1 U/kg/day, < 10 units/day) and maintained good metabolic control (TIR > 80%) without severe hypoglycemia or DKA. Based on our experience, we suggest a two-step approach for monitoring and treating patients with early stage 3 type 1 diabetes: first, positioning CGM, and second, when TIR falls below 80%, considering the addition of an AHCLs, even if the patient has a low insulin requirement.
Introduction
The diagnosis of type 1 diabetes (T1D) in presymptomatic stages remains rare (1). Without screening, most diagnoses are made at stage 3, when symptoms appear due to hyperglycemia, and up to 40% of cases are diagnosed during diabetic ketoacidosis (DKA) (1, 2). Currently, no data are available about the fluctuation of blood glucose levels over 24 h, as measured by continuous glucose monitoring (CGM) in the earliest stages of the disease, particularly when diabetes-related symptoms are absent. These data could be instrumental in guiding timely detection and the initiation of prompt therapy of T1D, leading to substantial improvements in diabetes management and future care.
The role of advanced hybrid closed-loop systems (AHCLs) in diabetes is expanding. AHCLs have been shown to be superior to insulin pump therapy, sensor-augmented pumps, and multiple daily insulin in most studies involving the pediatric population (3). However, data on the usefulness and effectiveness of AHCLs in the early stages of diabetes, when insulin requirements are low and residual insulin secretion is present, are still lacking.
In 2023, the Italian Parliament approved a law (Italian Republic Law 130/2023) introducing nationwide screening for T1D and coeliac disease in the general population aged 1–17 years, as part of a public health program aimed at reducing the impact of these chronic diseases (4). Despite this important initial step, several key questions remain, particularly concerning how to manage early diagnoses.
We report the case of a pubertal Caucasian 13-year-old girl, diagnosed with early stage 3 T1D during a screening program, who presented without diabetes-related symptoms. She used CGM during the first 6 months of the disease, followed by an AHCL (Tandem t:slim Control-IQ ® and Dexcom G6®). We reported 9 months of CGM data (time in range (TIR), time above range (TAR), and time below range (TBL)), as well as safety outcomes (severe hypoglycemia, DKA).
Results
A pubertal 13-year-old girl (weight: 54 kg; BMI: + 0.47 standard deviation score (SDS)), affected by celiac disease since age 9 and Hashimoto’s thyroiditis since age of 11 and treated with levotyroxine (1.2 mg/kg/day) orally, presented a fasting glucose level of 114 mg/dl detected during her annual routine screening, in the absence of diabetes-related symptoms. No family history of autoimmune disorders, including T1D, was reported.
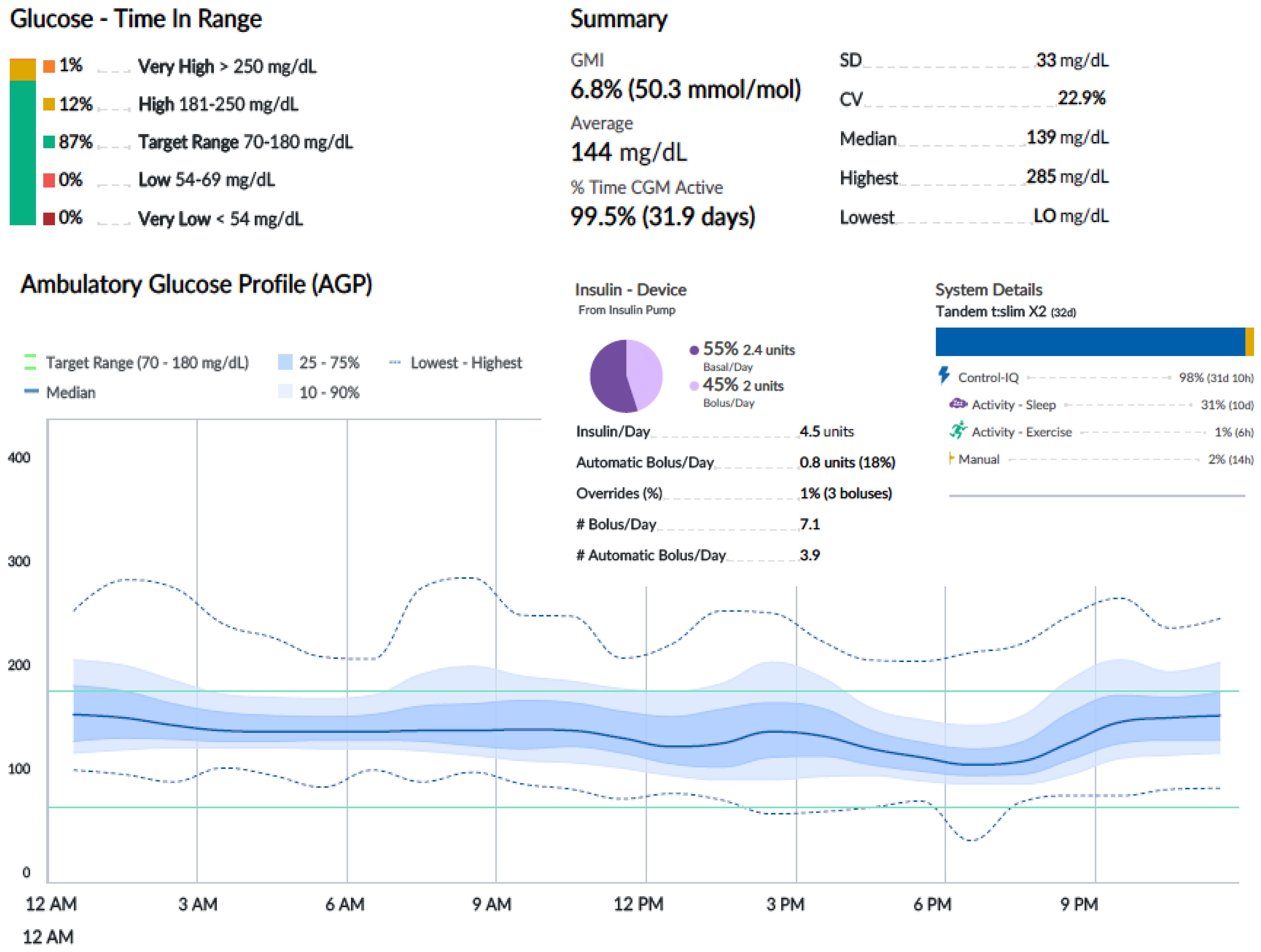
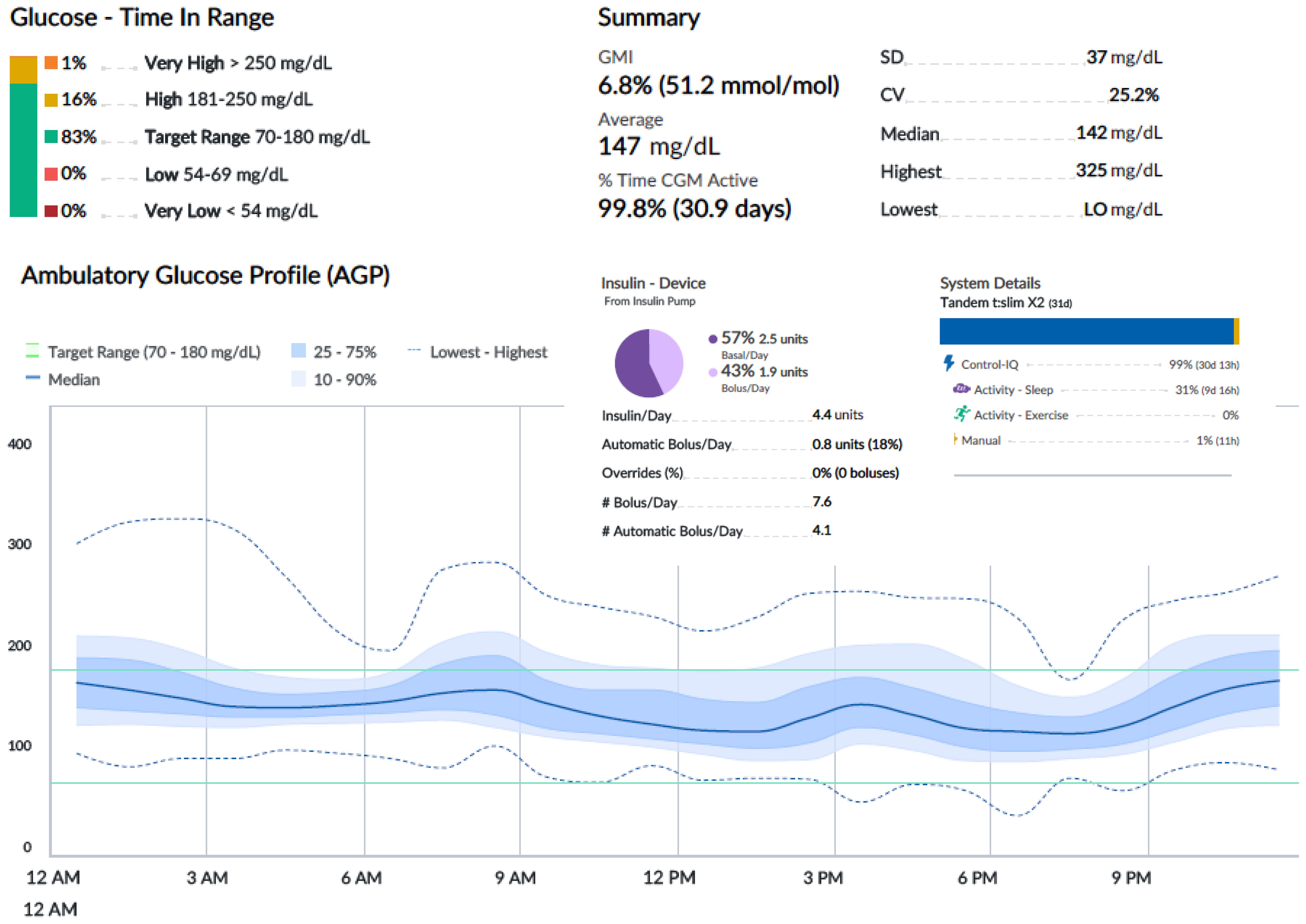
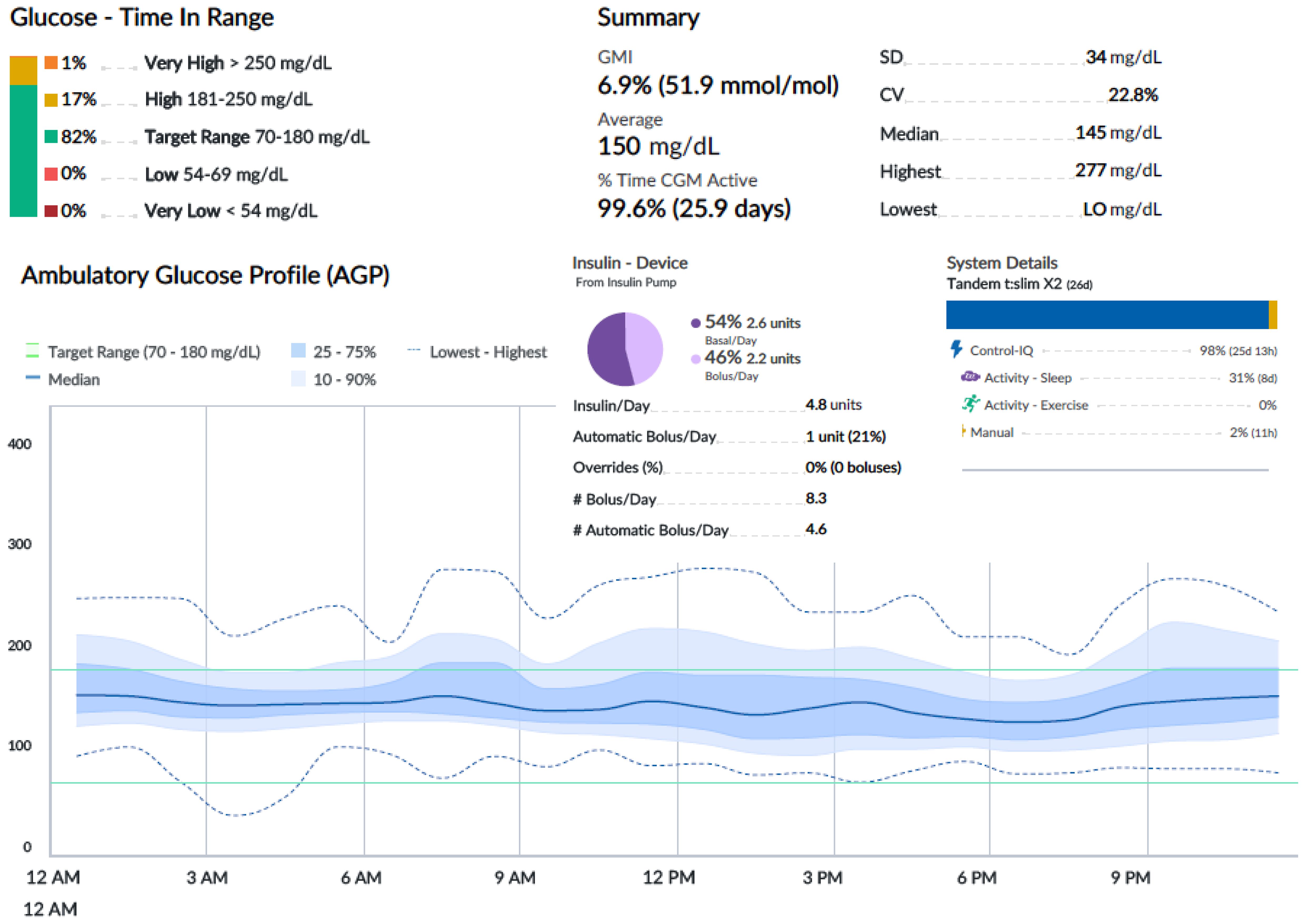
An oral glucose tolerance test showed a fasting blood glucose level of 113 mg/dl and a 2-h plasma glucose level of 278 mg/dl. HbA1c was 6.4% (46 mmol/mol). Two out of three diabetes-related autoantibodies were positive: GAD-ab: 19.23 U/ml (> 10 positive), ZNT8-ab: 1,760 U/ml (> 15 positive), ab anti-insulin: 2.23 U/ml (> 10 positive). Fasting c-peptide was 1.77 ng/ml. The girl was provided a Guardian™ Sensor 3 Medtronic® and was educated by a diabetes specialist nurse to monitor her glucose profile at home. Two weeks later, CGM data showed a time in range of 92%, TAR 180–250 of 8%, TAR > 250 mg/dl of 0%. HbA1c was 6.3% (45 mmol/mol) (Figure 1A). Time in tight range (70–140 mg/dl) was 72%, and TAR > 140 mg/dl was 28%. Considering the 24-h profile and CGM metric, we decided to proceed with follow-up visits without initiating insulin therapy, while continuing patient-centered education on the disease and use of technology. During follow-up, CGM showed a decrease in TIR and an increase in TAR (Figure 1B). Six months later, postprandial and overnight hyperglycemia (glycemia > 200 mg/dl) were frequently detected by CGM and confirmed by capillary blood glucose measurements, with a reduction in TIR to < 80% (74%) and a TAR > 26% (26% in the 180–250-mg/dl range) (Figure 1B). Upon observing the worsening of the 24-h glucose profile, she was promptly admitted to the Pediatric Emergency Room—without DKA—and subsequently to the Pediatric Clinic to receive personalized education in disease management, carbohydrate counting, and use of technology from a diabetes specialist nurse and dietitian. HbA1c was 6.4% (46 mmol/mol). Tandem t:slim Control-IQ® and Dexcom G6® were initiated at 0.1 U/kg/day (insulin requirement: 4.5 insulin units/day; insulin correction factor: 1:500; carbohydrate ratio: 1:130; sleep activity enabled; weight: 54.1 kg; BMI: + 0.47 SDS). During the 3-month follow-up, TAR gradually increased to 94%. In parallel, TAR decreased to 6% (with > 250 mg/dl accounting for 2%), and no hypoglycemic events occurred (TBL: 0%) throughout the period. After 3 months of AHCL use, HbA1c was 6.7% (50 mmol/mol). The insulin requirement decreased to 0.07 units/kg/day, corresponding to 3.6 U/day. The CGM active time was 99.5%, with 99.8% in automode.
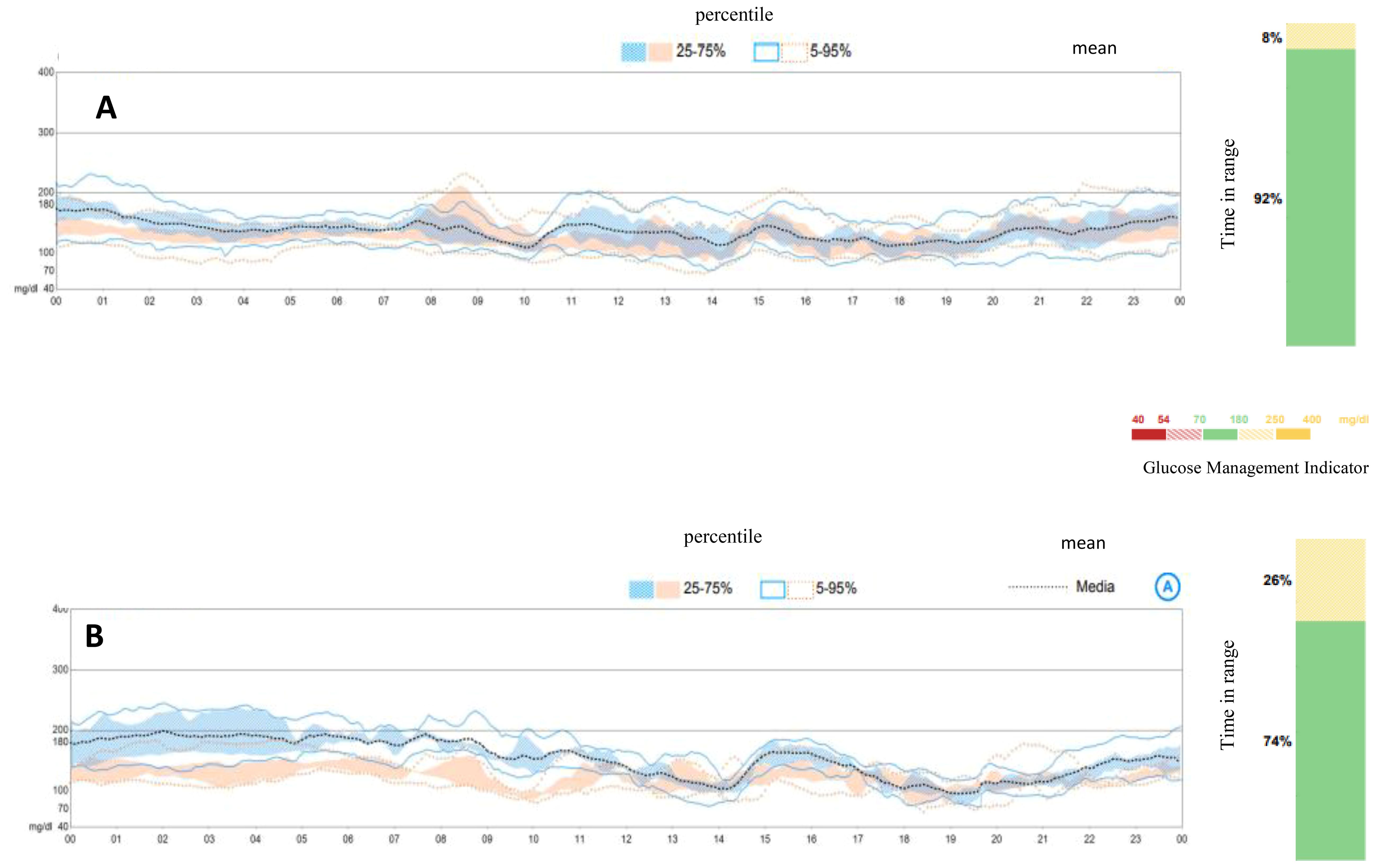
Figure 1. Ambulatory glucose profile and standardized CGM metrics before initiating AIDs. (A) Ambulatory glucose profile at diagnosis of early stage 3 type 1 diabetes. (B) Ambulatory glucose profile at 6 months from diagnosis, at the initiation of Tandem t:slim Control-IQ.
Figures 2-4 show 30-day ambulatory glucose profiles during the first months of follow-up, indicating optimal metabolic control (TIR > 80%) throughout the period. No episodes of severe hypoglycemia or diabetic ketoacidosis were observed.
Discussion
In this report, we describe the use of CGM and the Tandem t:slim Control-IQ® system in early stage 3 T1D. Recent advancements have highlighted the utility of CGM systems for monitoring the early stages of T1D (5). Based on our experience, CGM was effective in monitoring the 24-h glucose profile and provided valuable information for initiating insulin therapy. A time-in-range of 80% was chosen as the cutoff for starting insulin therapy based on our clinical experience. The use of the Tandem t:slim Control-IQ ® system from the onset of the disease aligns with the most recent consensus guidelines, which recommend that youth be offered the most advanced insulin delivery technology that is available, affordable, and appropriate (6). In our patient, the Tandem t:slim Control-IQ® system effectively maintained good metabolic control, despite a low daily insulin requirement, generally between 4 and 5 units/day. Notably, based on our experience, the Tandem t:slim Control-IQ® system functions effectively even when the total daily insulin dose is less than 10 units, without any safety concerns. No episodes of severe hypoglycemia, DKA, or other symptoms of diabetes were observed during the 3-month follow-up. A limitation of this approach is the direct comparison of CGM metrics before (Guardian 3) and during AHCL (Dexcom G6), although both sensors offer high accuracy and are approved for use in patients with T1D. Finally, the approach of first positioning CGM and then introducing AHCL in the early stages of the disease facilitates gradual patient education on the use of technology, which is essential in reducing future dropout.
The advent of screening for T1D presents the opportunity to diagnose the condition at earlier stages (stages 1 and 2) before the clinical disease onset (stage 3) (7). Recently, a consensus guideline was published, defining the staging criteria of T1D, the associated risk of progression, and the purpose of monitoring for any child or adolescent who has tested positive for islet autoantibodies (7). Monitoring for individuals in prestage 1 and stages 1–3 of T1D is determined by islet autoantibody status, glycemic status, diabetes-related symptoms, and insulin requirement. Individuals diagnosed before stage 3 T1D require ongoing monitoring for disease progression, including regular assessments of glucose levels (7). The earlier the diagnosis is made in the disease process, the sooner interventions can be initiated to prevent hyperglycemia. It is well known that maintaining blood glucose levels as close to normal as possible is essential for delaying or preventing complications (8). Of outstanding importance, individuals diagnosed with stage 2 T1D should be offered the opportunity to participate in clinical trials and receive access to disease-modifying agents or approved therapies aimed at delaying or halting disease progression (9).
Introducing technology earlier than usual in T1D management may increase the initial cost of diabetes care (10). However, considering the positive impact of AHCLs and CGMs on T1D health outcomes, these short-term costs may be offset by long-term savings. Comprehensive cost-effectiveness analyses are needed to support optimal T1D care in an era of increasing reliance on therapeutic technology and expanding T1D screening efforts.
Conclusion
Based on our experience, the described two-step approach to monitoring and treating presymptomatic type 1 diabetes proved useful and effective, with no safety issues observed. First, CGM is initiated, and the patient is educated on glucose monitoring. Second, if TIR drops below < 80%, the use of an advanced AHCL should be considered, even in patients with low insulin requirements. It is important to note, however, that the findings from this case report cannot be generalized to all individuals with early-stage 3 T1D, as they are based on the clinical experience of our research group. We recommend that all treatment options be thoroughly discussed and that patient-centered care be prioritized.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the patient for the publication of this case report.
Author contributions
CM: Supervision, Formal Analysis, Methodology, Writing – original draft, Conceptualization. MM: Investigation, Writing – original draft. FR: Writing – original draft, Investigation. AP: Writing – original draft, Data curation. AH: Writing – original draft, Investigation. AB: Investigation, Writing – original draft. GZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bonifiacio E, Winkler C, Achenbach P, and Ziegler A. Effect of population-wide screening for presymptomatic early-stage type 1 diabetes on paediatric clinical care. Lancet Diabtes Endocrinol. (2024) 12:376–8. doi: 10.1016/S2213-8587(24)00101-3
2. Mameli C, Scaramuzza A, Macedoni M, Marano G, Frontino G, Luconi E, et al. Type 1 diabetes onset in Lombardy region, Italy, during the COVID-19 pandemic: The double-wave occurrence. EClinicalMedicine. (2021) 39:101067. doi: 10.1016/j.eclinm.2021.101067
3. Zeng B, Gao L, Yang Q, Jia H, and Sun F. Automated insulin delivery systems in children and adolescents with type 1 diabetes: A systematic review and meta-analysis of outpatient randomized controlled trials. Diabetes Care. (2023) 46:2300–7. doi: 10.2337/dc23-0504
4. Gazzetta Ufficiale della Repubblica Italiana. Legge 15 settembre 2023, n.130. In: GU Serie Generale n. 226 (2023). Available at: https://www.gazzettaufficiale.it/eli/id/2023/09/27/23G00140/sg.
5. Chobot A, Piona C, Bombaci B, Kamińska-Jackowiak O, and Mancioppi V. Passanisi S exploring the continuous glucose monitoring in pediatric diabetes: current practices, innovative metrics, and future implications. Children (Basel). (2024) 11:907. doi: 10.3390/children11080907
6. Biester T I, Berget C, Boughton C, Cudizio L, Ekhlaspour L, Hilliard ME, et al. International society for pediatric and adolescent diabetes clinical practice consensus guidelines 2024: diabetes technologies – insulin delivery. Horm Res Paediatr. (2024) 97:636–62.
7. Phillip M, Achenbach P, Addala A, Albanese-O’Neill A, Battelino T, Bell KJ, et al. Consensus guidance for monitoring individuals with islet autoantibody-positive pre-stage 3 type 1 diabetes. Diabetes Care. (2024) 47:1276–98. doi: 102337/dci24-0042
8. American Diabetes Association Professional Practice Committee. Prevention or delay of diabetes and associated comorbidities: standards of care in diabetes-2025. Diabetes Care. (2025) 48:S50–8. doi: 10.2337/dc25-S003
9. Mameli C, Triolo TM, Chiarelli F, Rewers M, Zuccotti G, and Simmons KM. Lessons and gaps in the prediction and prevention of type 1 diabetes. Pharmacol Res. (2023) 193:106792. doi: 10.1016/j.phrs.2023.106792
Keywords: type 1 diabetes, screening, CGM, CSII, children
Citation: Mameli C, Macedoni M, Redaelli F, Petitti A, Hajro A, Bosetti A and Zuccotti G (2025) Case Report: Usefulness, effectiveness, and safety of an advanced hybrid closed-loop system in a child with early identification of type 1 diabetes. Front. Endocrinol. 16:1598264. doi: 10.3389/fendo.2025.1598264
Received: 22 March 2025; Accepted: 09 May 2025;
Published: 03 June 2025.
Edited by:
Maurizio Delvecchio, University of L’Aquila, ItalyReviewed by:
Stefano Passanisi, University of Messina, Messina, ItalySergio Di Molfetta, Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari, Italy
Copyright © 2025 Mameli, Macedoni, Redaelli, Petitti, Hajro, Bosetti and Zuccotti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Mameli, Y2hpYXJhLm1hbWVsaUB1bmltaS5pdA==
 Chiara Mameli
Chiara Mameli Maddalena Macedoni1
Maddalena Macedoni1 Gianvincenzo Zuccotti
Gianvincenzo Zuccotti