- 1Department of Physiology, Faculty of Basic Medical Sciences, Federal University of Health Sciences, Ila-Orangun, Nigeria
- 2Department of Medical Physiology, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Nigeria
- 3Environmental Health and Safety, Osun State Ministry of Health, Osogbo, Nigeria
- 4Department of Physiology, University of Ilorin, Ilorin, Nigeria
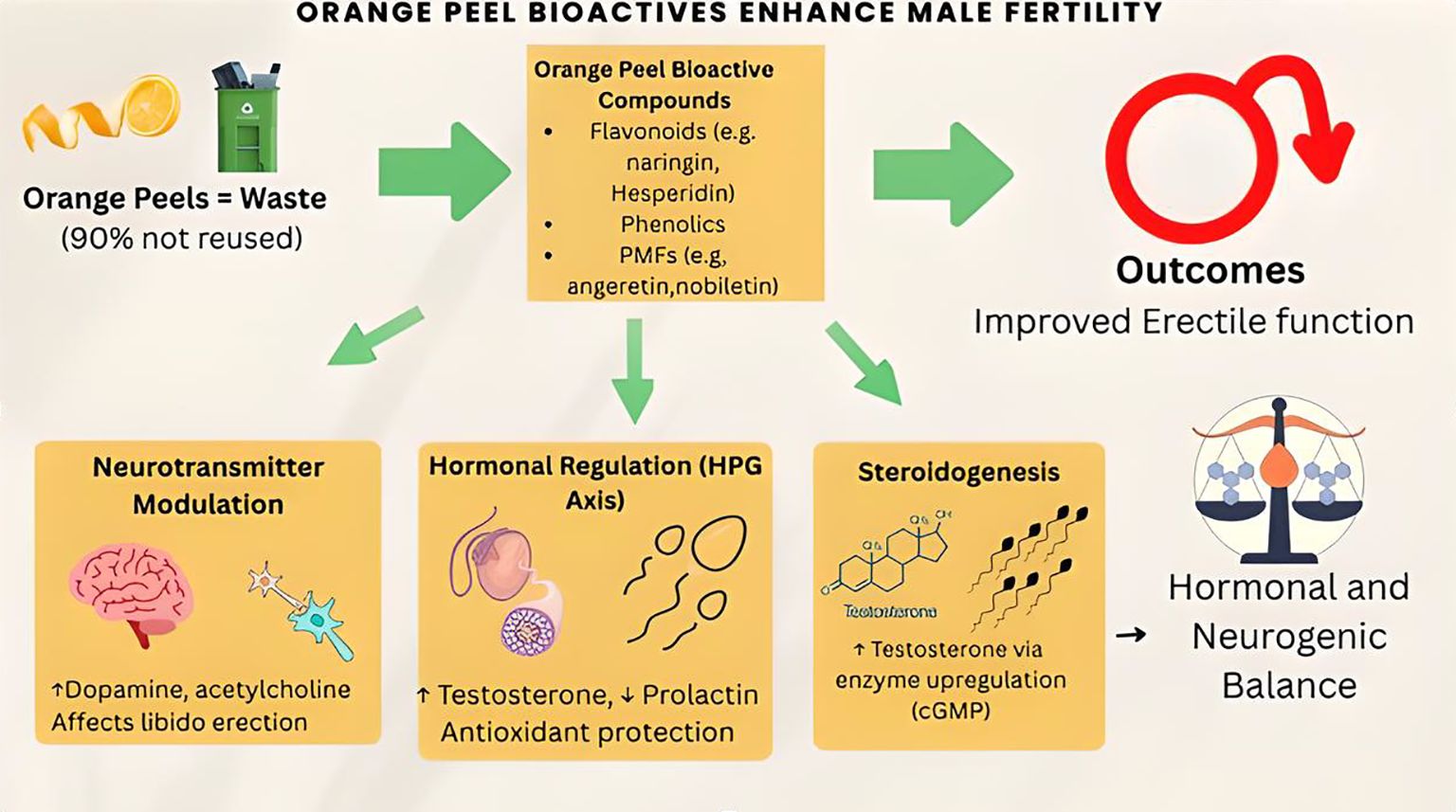
Large amounts of citrus wastes, especially orange peels, are produced globally, and they are an environmental menace in different parts of the world because of their perishability. However, various research findings have shown that orange peels are a rich source of vitamins, fibers, phenols, and other bioactive compounds. This makes orange peel a promising therapeutic option for managing various disease conditions. Despite these health benefits, only about 10% of orange peel produced as waste is recycled and reused. Hence, the review was designed to explore the beneficial roles of orange peel bioactive compounds on male sexual function. This review, therefore, explore the aphrodisiac effect of orange peel bioactive compounds by reviewing recent publications that detail the association of orange peel bioactive compounds with male sexual functions regarding its potential roles in erectile function regulation, testosterone levels, and semen characteristics. Bioactive compounds from orange peel contributed positively to sexual and erectile functions. This was mediated via a hormonal-dependent mechanism and upregulation of steroidogenic enzymatic activities. It also improves endothelial functions by stimulating the secretion and release of nitric oxide, which is important for erection. Furthermore, it acts by mediating the activities of monoamine neurotransmitters, which are responsible for the motor activities involved in sexual function. Orange peel bioactive compounds enhance male sexual functions via multiple pathways. Hence, orange peels offer great potential for developing functional food products with health and economic benefits.
1 Introduction
The current mounting rate of environmental pollution has significantly raised different researchers’ interest in the efficient use and recycling of agricultural residues and waste. Agricultural activities account for a considerable amount of waste annually, which, if not effectively managed, can result in socio-economic, environmental, and health problems (1). It was reported that about one-third of the food produced for human consumption is lost or wasted annually (2). The estimated 931 million tonnes of food waste generated yearly, of which 61% comes from households, 26% from food services (restaurants), and 13% from retail, make up 17% of the world’s total waste (3). As shown in Figure 1, the annual food waste in millions of tonnes for the top ten nations worldwide revealed that the China, India, Nigeria, and United States produce the most food waste annually, with 91,646,213 tonnes, 68,760,163 tonnes, 37,900,000 tonnes, and 19,359,951 tonnes, respectively (4). The residues (such as peels and seeds) arising during household consumption and industrial processing of different agricultural products, such as vegetables and fruits, result in huge waste (5).
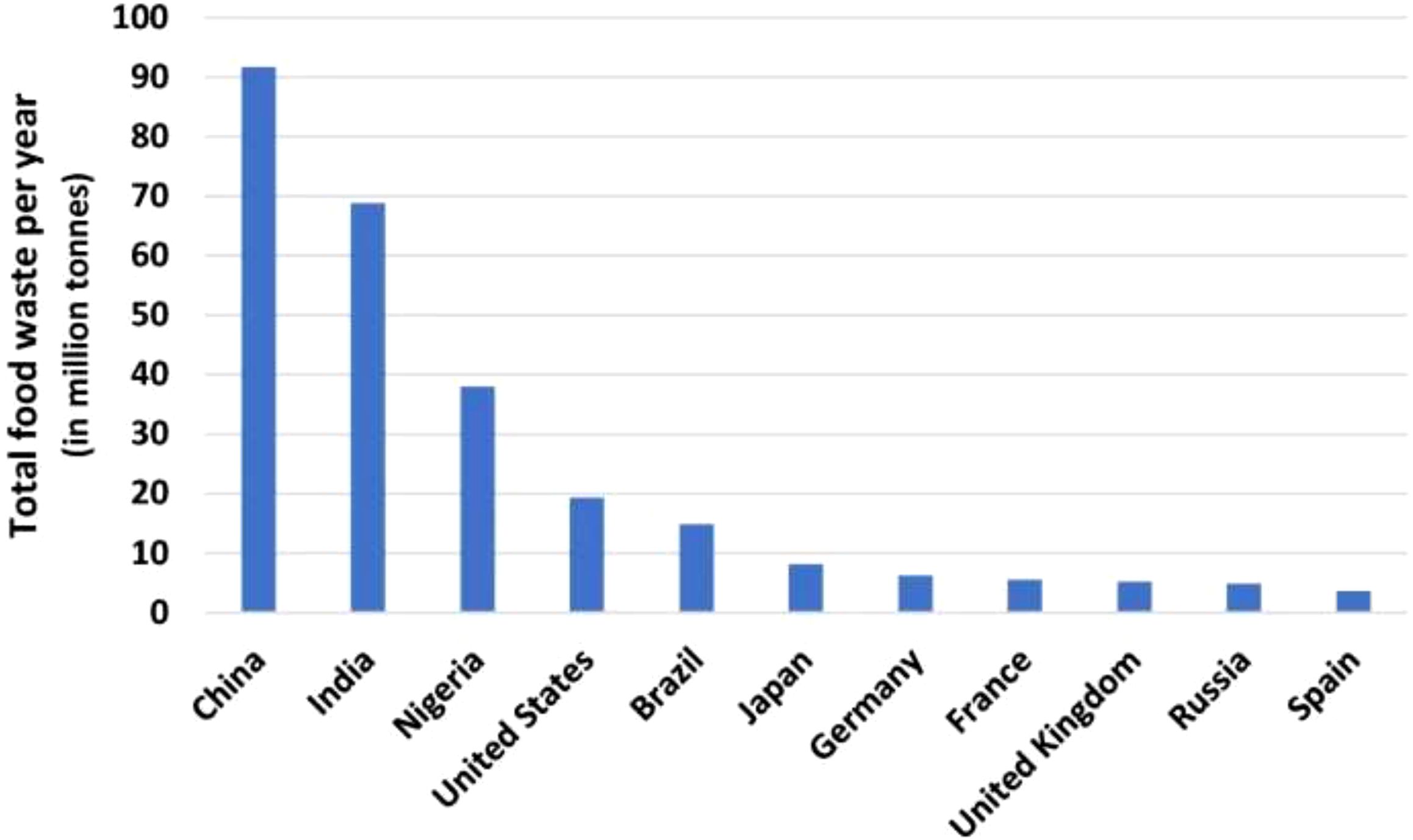
Figure 1. The top eleven countries with the highest annual food waste (4).
Recently, the food processing industry has rapidly grown and, at the same time, has significantly increased food waste globally (6), valued at around $1 trillion, with the US singlehandedly generating 60 million metric tonnes per year (3). Regrettably, waste generated from fruits such as peels, which are rich in beneficial compounds, contributes significantly to this waste. These agricultural wastes are mainly disposed of in rivers, landfills, and open dumps, leading to air, soil, and water pollution. Unfortunately, despite the nutritional value of this generated waste, only about 10% of the waste is recycled and reused. This contributes significantly to the increased rate of economic losses and environmental pollution.
Based on the above, it is important to establish effective strategies for the optimal utilization of the generated agricultural waste. Organic residues contain different bioactive molecules, such as phenolic compounds, which are important for various biological activities responsible for human health (7). The extraction, identification, and investigation of these bioactive molecules will contribute significantly to the use of agricultural waste and prevent economic losses and environmental pollution.
Among these agro-based waste products, orange peel is gradually becoming the most researched waste product owing to its rich phytochemical profile (8). The primary orange peel-producing countries are Brazil, China, India, the USA, and Mexico (9). Within Europe, Spain, Italy, Greece, and Turkey are major orange producers and waste generators, particularly in the Mediterranean region. Other countries that generate large amount of orange waste include Egypt and South Africa (10).These nations collectively account for a significant portion of global orange production, leading to large volumes of orange peel generated during processing and consumption. The generated orange peels are considered as waste and have been identified as the primary substance disrupting the cleanliness of urban areas. Orange peel is a by-product obtained after juice extraction or other orange processing activities. This by-product is perishable, and proper disposal is a significant challenge faced by pollution monitoring agencies and processing industries (11). Hence, there is a need to utilize orange peel to produce value-added food products. Using orange peels will provide health benefits and resolve environmental pollution caused by orange peel perishability. Interestingly, some countries consider orange peel a perfect sweet and chewy snack, especially when consumed with dark chocolate (6). Also, scientists are exploring the rich phytochemistry of this agricultural waste to develop functional foods and dietary supplements. The antibacterial property of orange peel has made it a natural preservative that could replace artificial ones in different food products (12). In fact, orange peel has been proven to be effective for managing complications arising from disorders such as cardiovascular dysfunction (13), diabetes (14), gonadotoxicity (15) and cancer (16). Importantly, the exploitation of orange peel in food will not only lead to the cost-effective, innovative therapeutic option, it also improves the value of functional and nutraceutical food products (17). Aside from the health benefits, the recovery of the orange peel will also reduce the global waste burden. Orange peel has been valorized for its phenolic content, e.g., phenolic acid and flavonoids. It has been established that phenolic compounds of natural origin are more desired due to their potent antioxidant properties in food systems or living organisms for various maladies (12). Hence, this review article aimed at establishing the aphrodisiac and fertility-enhancing properties of orange peel bioactive compounds.
2 Orange peel: an insight
Orange is the world’s most popular fruit and belongs to the Citrus genus. It is the largest citrus producer, accounting for more than half of all the citrus fruits produced globally. Orange can be classified into sweet orange (Citrus sinensis), sour or bitter orange (Citrus aurantium), mandarin orange and tangerine varieties, Citrus reticulate, and Hybrid oranges. Sweet oranges are the most important in production volumes and cultivation areas (18). In most markets, juice made from sweet oranges is regarded as orange juice.
An orange is a ball-like fruit protected by waxy skin called the peel. The orange peel comprises a thin outer layer known as the flavedo and a thicker inner layer referred to as the albedo (19). The flavedo consists of the carotenoids that account for the fruit’s characteristic color (20), and vesicles (small sacs/cavities) that contain the peel oil. This peel oil accounts for the fruit’s fresh aroma. On the other hand, the white spongy inner albedo is made up of different substances such as flavonoids, d-limolene, limon, and pectin (21).
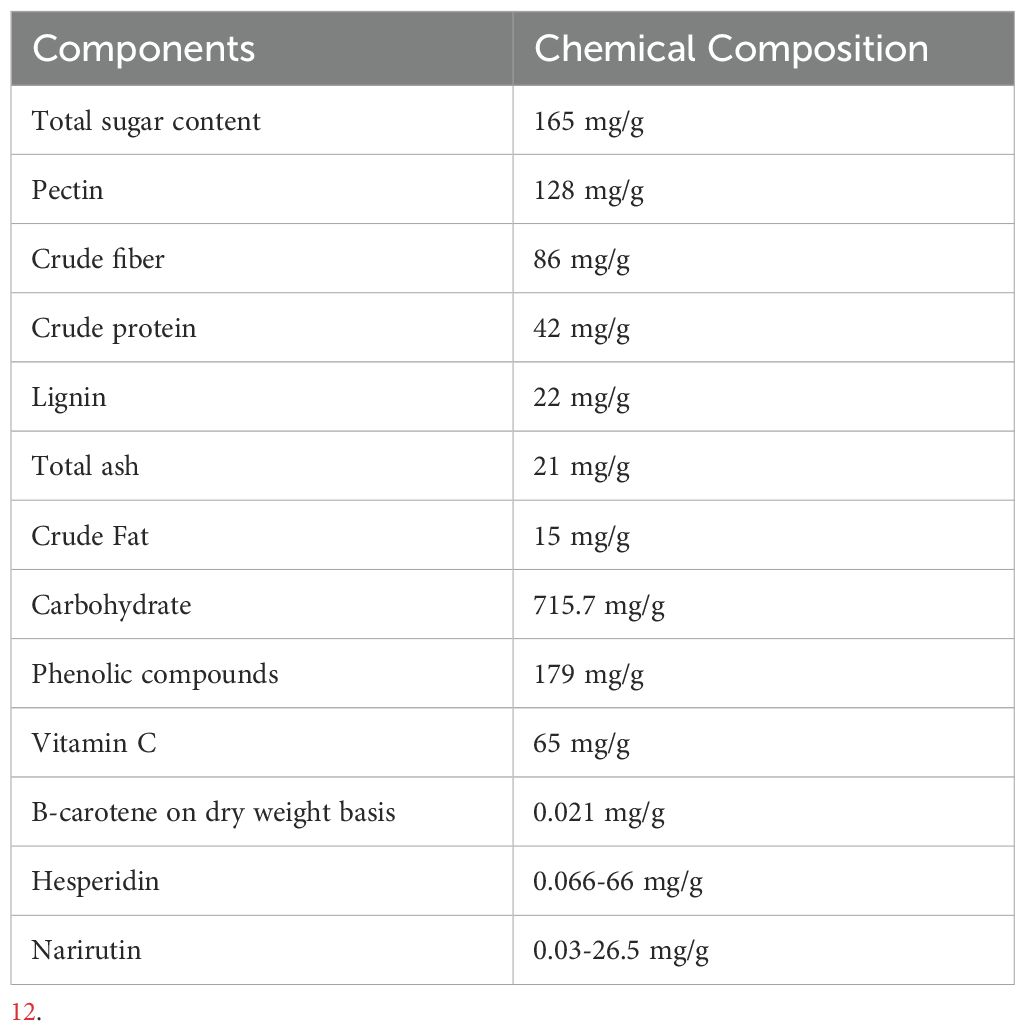
As shown in Table 1, orange peels are excellent sources of bioactive compounds such as essential oils, polyphenols, fibers, minerals, pectin, and monosaccharides (22). The essential oil contains different terpenes, with limonene as the primary compound. It also contains oxygenated compounds such as alcohols, aldehydes, and esters (23). Polyphenolic compounds are other bioactive substances in the form of phenolic acids, flavonoids, and their derivatives (23, 24). Additionally, orange peels are rich in sugars, which can be as free monosaccharides, disaccharides (sucrose), or polymerized to cellulose (glucose), hemicellulose, and pectin. Pectin is a heteropolysaccharide rich in galacturonic acid (22).
Of these bioactive compounds, the most important are the flavonoids, and the most prevalent are hesperidin, naringin, and rutinose (25). Hesperidin is abundantly found all over the peel, whereas naringin is predominant in the flavedo portion (26). Other important flavonoids include neohesperidin, didymin, narirutin, eriocitrin, and tangeretin. Another important bioactive substance is polymethoxylated flavone (PMF), a mixture of 5.44% hydroxylated PMFs and 75.1% non-hydroxylated (27). PMFs such as nobiletin, nestin, and tangerine are majorly present in the flavedo portion of the essential oil, but they are less frequent than flavanones. PMFs are commonly present in sweet and bitter oranges, such as nobiletin and tangeretin (28).
3 Orange peel bioactive components extraction methods
Orange peel extraction has been a focal point for developing a valuable strategy to exploit waste material from the orange juice industry (29). These methods are designed to optimize bioactive compound yields and quality, including essential oils, pectin, and d-limonene, and contribute to environmental sustainability. Several sophisticated techniques have been developed with unique advantages, drawbacks, and utility.
3.1 Supercritical fluid extraction
Supercritical fluid extraction (SFE) represents one of the greenest extraction methods. It employs supercritical carbon dioxide (ScCO2), which is frequently supplemented with ethanol, as an organic solvent of choice (30). This approach is based on controlled temperature and pressure conditions and thus delivers good quality products with a low environmental footprint. Ghadiri et al. (31) demonstrated that optimizing SFE parameters such as temperature, pressure, and extraction time yields essential oils with superior quality while reducing energy consumption and solvent waste. Similarly, Ortiz-Sanchez et al. (32) reported that SFE outperformed conventional methods in selectively isolating bioactive molecules like hesperidin, making it a preferred choice for high-value products.
This process requires a pump for CO2, a pressure cell that will contain the sample (orange peel), a restrictor to regulate control flow and pressure of the extraction fluid. The extracted fluid is pumped to a heating zone to ensure it is heated to supercritical conditions. This fluid will then move to the extraction vessel where it is immediately diffused into the solid matrix where the materials to be extracted will be dissolved. The dissolved material is then swept into a separator from the extraction cell where it will settle out. The CO2 can then be cooled, re-compressed, or discharged into the atmosphere.
The major advantage of this method is it selectivity and efficiency in extracting specific compounds from mixtures, while also minimizing the use of organic solvents and energy consumption. However, it requires high operational cost, which can be a barrier to widespread adoption, particularly in specialized fields.
3.2 Microwave-assisted extraction
Microwave-assisted extraction (MAE) has become a trend in terms of energy efficiency and shortened extraction time. One major advantage of this method is that it is rapid since the peel will quickly heats and the matrix of orange peel will quickly breaks down, liberating bioactive compounds more efficiently. The microwave extraction method requires heating the orange peel via the use of radiation energy that has short wavelength and high frequency. The water present in the peel absorb the microwave’s energy to increase the internal temperature. There is a rapid increase in temperature of the vascular bundle and glandular cell system and this temperature can be maintained until the internal pressure exceeds the capacity of the cell wall to expand, causing the cell to rupture and the spices located inside the cell to flow out of the cell wall, transfer to the surrounding extraction medium. Benassi et al. (33) showed that MAE yielded higher values of pectin yields at lower energy expenditure compared to other strategies, including hot-water extraction and Rapid Solid-Liquid Dynamic (RSLD) extraction. Furthermore, Murador et al. (34) investigated the potential of ionic liquids based on MAEs to improve the bioaccessibility of carotenoids and chlorophylls and thus showcased the potentially broad applicability for nutraceutical purposes.
The advantages of this method of extracting bioactive compounds from orange peel include its fast extraction times and higher yields, along with reduced solvent consumption. However, there is possibility of analyte degradation and the need for specialized equipment that are quite expensive.
3.3 Ultrasound-assisted extraction
Ultrasound-assisted extraction (UAE) is a non-thermal extraction method that uses sound waves to enhance the extraction of bioactive compounds from plant materials. It leverages acoustic cavitation, where the high-frequency sound waves create tiny bubbles that implode, generating localized heat and pressure. This process disrupts cell walls, facilitating the release of desired compounds into the solvent. This approach is particularly useful due to its small solvent uptake and ability to retain heat-sensitive molecules. Li et al. (35) have shown the role of UAE in obtaining phenolics and pectin from orange waste with the yield of high-quality extract for nutraceutical and pharmaceutical purposes. UAE’s low power demand and operational ease confer practicality for industrial-scale development.
3.4 Hot-water extraction
Although hot-water extraction is one of the oldest methods, it continues to be extensively employed, especially in pectin extraction. It is based on using water as a solvent at specific temperatures and pH conditions to perform the reactions in high yields with a low environmental footprint. Benassi et al. (33) also pointed out the ability of this solution, emphasizing its applicability for industrial-scale applications where simplicity and low cost are necessary.
3.5 Solvent-based extraction
Although less environmentally acceptable than green options, solvent-extractable-based extraction remains valuable in specific applications. Siddiqui et al. (36) highlighted bio-solvents’ advantages, including their potential to replace expendable heptane and, consequently, the environmental impact. This method is still feasible for compound mining, such as d-limonene, especially after the optimization of efficiency and safety.
4 Male sexual function
Sexual function is the ability to experience sexual pleasure or react sexually and is characterized by the ability to move through the stages of sexual desire, arousal, and orgasm without difficulty. Male sexual function is regulated by a complex interplay between the psychological and physiological mechanisms that significantly impact the overall life quality (37). This mechanism involves the activities of the neurological, cardiovascular, and endocrine systems. Hence, any alterations in these systems can impair male sexual function, leading to sexual dysfunction. Therefore, sexual dysfunction is not a singular disorder but a multifaceted issue that disrupts life quality (38). One of the most prevalent sexual dysfunction is erectile dysfunction, an inability of the penis to achieve and sustain erection for a satisfactory sexual experience (39, 40). Hence, penile erection plays a dominant role in maintaining sexual function.
Penile erection is a highly intricate process requiring the congenial relationship between the psychological, endocrine, neurological, and cardiovascular aspects of the body systems (40). Neuromuscular activities play a central role in penile erection mechanisms, and they involve an interplay between the hormones, neurotransmitters, autonomic nervous system, and nitric oxide (NO). Once there is sexual desire or arousal, the nervous system will stimulate the release of NO (a potent vasodilator) via dopamine-oxytocin-NO signaling (41, 42), leading to the relaxation of the smooth muscles present in the corpus cavernosal via the NO- cyclic guanosine monophosphate (cGMP) dependent pathway (43, 44). Consequently, an increase in blood flow to the corpus cavernosum and a decrease in local venous return occurs, leading to penile erection.
4.1 Neurobiology of male sexual function and orange peel bioactive compounds
4.1.1 Erectogenic enzymes and orange peel bioactive compounds
Sexual desire, also referred to as libido, is the first stage of the sexual cycle, and it can be defined as the drive to engage in sexual activity or the interest in sexual objects and activities. On the other hand, sexual arousal is a psychological and physiological response that occurs as a result of exposure to sexual stimuli or preparation for sexual intercourse (penile erection) (45). The mechanisms underlying sexual arousal are complex and involve different cerebral circuits involved in the release of neurochemicals and sex hormones. The ascending pathways consist of five neurochemical systems: dopamine, serotonin, acetylcholine, norepinephrine, and histamine. These neurochemicals are majorly involved in the arousal of the forebrain. (46).
Dopamine is a monoamine neurotransmitter that facilitates sexual motivation and penile erection via its action on the oxytocinergic neurons that are present in the hypothalamic paraventricular nucleus (PCN) and the pro-erectile sacral parasympathetic nucleus of the spinal cord (42, 47). Also, serotonin is another monoamine neurotransmitter that is responsible for maintaining sexual activities. Unlike dopamine, serotonin inhibits sexual function (48). In fact, the selective serotonin reuptake inhibitor class of antidepressants has been shown to impair penile erection and sexual desire (49, 50). Another important monoamine neurotransmitter involved in sexual function is acetylcholine. Acetylcholine has been shown to have an excitatory effect on penile erection by stimulating the relaxation of the cavernosal smooth muscles (51). The presence of cholinergic nerves and receptors in the corpus cavernosum (46, 52) further substantiates the important roles of acetylcholine in penile erection.
Additionally, acetylcholine stimulates the release of dopamine responsible for sexual motor activities (53). Norepinephrine or noradrenaline is also a monoamine neurotransmitter, and it can also be considered a stress hormone. The noradrenergic neurons in the brain are part of the neurochemical system with excitatory effects on mental alertness, sexual arousal, and the reward system (46). Histamine is also a neurotransmitter that stimulates the hypothalamic ventromedial nucleus, leading to the modulation of sexual behavior (54). In fact, histamine antagonists have been implicated in loss of libido and erectile dysfunction (55). In sum, monoamine neurotransmitters play a dominant role in sexual arousal.
Orange peel fertility-enhancing abilities can be associated with their modulatory effects on monoamine neurotransmitter activities. Orange peel flavonoids such as heptamethoxyflavone have been shown to increase the hippocampal brain-derived neurotrophic factor (BDNF) expression (56) via extracellular signal-regulated kinases 1/2 (ERK1/2) and cAMP response element-binding protein (CREB) signaling (57). In the same vein, Sawamoto et al. (58) revealed that heptamethoxyflavone stimulated BDNF synthesis and release in the C6 glioma cells. Another important orange peel flavonoid that plays a key role in sexual function via BDNF synthesis is hesperidin. Hesperidin is an antidepressant, and its antidepressive activities have been shown to increase the production of BDNF in different animal behavioral tests (59, 60). This BDNF synthesis has a role to play in penile erection, and together with its receptors, BDNF is a key regulatory protein in the male genitourinary system and can be a therapeutic target for the management of secondary penile dysfunction (56). BDNF exhibits this fertility-enhancing capacity by modulating the activities of monoamine neurotransmitters (61–63). This was also confirmed by the study of Juric et al. (64), which established a positive relationship between BDNF concentration and monoaminergic system activities.
Additionally, naringenin, a dietary flavonoid present in orange peel, has been shown to increase the activities of the monoamine neurotransmitters by acting as a monoamine oxidase inhibitor (65). Monoamine oxidase inhibitors are chemicals that break down monoamine oxidases, enzymes that breakdown monoamine neurotransmitters in the brain. Hence, via its monoamine oxidase inhibitor activities, naringenin increases the level of monoamine neurotransmitters in the brain, thereby increasing sexual function. Therefore, one of the mechanisms of action associated with the fertility-enhancing properties of orange peel bioactive compounds is via their modulatory effects on monoamine neurotransmitter activities.
4.1.2 Reproductive hormones and Orange peel bioactive compounds
Male reproductive hormone concentration is majorly regulated by the hypothalamus via the hypothalamic-pituitary-gonadal (HPG) axis. HPG axis is a closed-loop relationship between the hypothalamus, pituitary, and testis. The hypothalamus synthesizes gonadotropin-releasing hormone (GnRH), which acts on the pituitary gland to produce luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These two hormones act on the testis, but while LH stimulates the Leydig cells to produce testosterone (a major sex hormone in males), FSH stimulates spermatogenesis via its stimulatory effect on the Sertoli cells. The produced testosterone sends negative feedback to the pituitary and/or hypothalamus to inhibit their secretions. Also, the synthesized testosterone can be converted via aromatization in the brain to produce estrogen. Estrogen and testosterone are important for neural circuit development, sex-specific behaviors, and orientation (46). The activities of this closed-loop relationship can be disrupted by the hypothalamic-pituitary-adrenal (HPA) axis which becomes activated during stressful conditions. This HPA axis inhibit the HPG-axis by stimulating the synthesis and release of gonadotropin inhibitory hormone from the hypothalamus (37).
Testosterone binds with the androgen receptors (ARs) located in the brain; it can also be converted by 5‐alpha‐reductase to dihydrotestosterone (DHT) before binding to ARs. Also, it can be converted to estradiol via the aromatase pathway and bind to estrogen receptors. After binding, testosterone acts to masculinize (increase male‐typical behaviors) and defeminize (reduce female‐typical responses) behavioral development in males (46). The ARs are widely and extensively found in the medial pre-optic area (MPOA), suprachiasmatic nucleus, sexually dimorphic nucleus, and the third interstitial nucleus of the anterior hypothalamus, which are all involved in sexual behavior.
Sexual hormones regulate sexual arousal by maintaining cerebral integration between the somatic and autonomic sexual systems. Also, they assist with the ascent of sexual reflexes from the spinal cord to the cerebrum and the consequent activation of the autonomic centers leading to the organization of genital stimulation (66). Furthermore, testosterone or androgens play dominant roles in stimulating and maintaining male sexual function. Mainly, the development and growth of the penile tissue and erectile function depend on the optimal circulatory level of androgens. Supportively, androgen deficiency has been associated with penile tissue atrophy, dorsal nerve structure changes, endothelial morphology changes, reduced trabecular smooth muscle content, and alterations in the extracellular matrix (46).
Furthermore, testosterone has been shown to play key roles in sexual interest and activity. This is because loss of testosterone has been established to cause loss of libido (67, 68). The mechanism involved in libido is associated with androgens’ influence on the hypothalamic paraventricular nucleus, which is an integration center between the central and peripheral autonomic nervous systems (46).
On the other hand, prolactin is responsible for the sexual gratification that follows sexual acts (69). Prolactin is important for the refractoriness and loss of sexual drive that follows a complete sexual cycle (70). The inhibitory effect of prolactin on male function could be associated with its effect on dopamine. This is because the upregulation of prolactin receptor gene expression and prolactin-secreting lactotrophs in the anterior pituitary gland can downregulate pituitary dopamine receptor expression (71). This downregulation reduces the sensitivity of lactotrophs to dopamine’s inhibitory effects, thereby leading to hyperprolactinemia, where prolactin levels are chronically high, potentially causing various issues like infertility, and other hormonal imbalances.
On the other hand, orange peel bioactive compounds have been proven to enhance sexual functions via hormone-dependent mechanisms (15). Okesina et al. (72) established that naringin can stimulate androgen production via the HPG-axis-dependent pathway. Similarly, hesperidin has been established to enhance fertility indices by stimulating the HPG-axis to produce testosterone and suppressed prolactin secretion in a rat model of testicular dysfunction (73). Similarly, naringenin maintains hormonal balance by acting as an estrogen agonist when the level of circulatory estrogen is low and has an anti-estrogenic effect in estrogen-rich states (75). Finally, the flavonoids present in orange peel could regulate the concentration of androgens in the serum and mediate their biological effects, majorly by maintaining the HPG-axis, androgen synthesis and metabolism, androgen receptors expression, and antioxidant effects (74). Hence, orange peel bioactive compounds, especially flavonoids, regulate the HPG-axis to preserve male sexual functions and prevent psychosexual dysfunction.
4.1.3 Erectile function and orange peel bioactive components
Penile erection is a complex process modulated peripherally by neurovascular, nonadrenergic, and noncholinergic mechanisms and the central nervous system (76, 77). Psychological factors and androgens regulate the erectile response and may proceed through psychogenic or reflexogenic neuronal pathways (78).
Penile erection is triggered by external erotic inputs via the five senses (Sight, Sound, Smell, Taste, and Touch), which are processed in the MPOA of the hypothalamus (79). In response to these inputs, the hypothalamus stimulates the release of monoamines and reproductive hormones such as GnRH, oxytocin, and substance P to stimulate penile erection. These reproductive hormones project to the thoracolumbar sympathetic nerve fibers at the level of T11–L2 and S2‐S4 parasympathetic nerve fibers to maintain erectile function (78).
Once external inputs stimulate the sacral parasympathetic nerves, the information is carried via the pelvic plexus to the corpora cavernosa to stimulate the release of nitric oxide (NO), the main vasoactive neurotransmitter in the penile tissue (80).
Orange peel has been established as a penile erectile function enhancement agent (12). This activity can be traced to some of the phenolic compounds present in the citrus species. Generally, flavonoids contain a C2‐3 double bond, a keto group C4, and hydroxyls at C3′ and/or C4, making them a potent fertility-enhancing agent (81). Flavonoid intake has been shown to reduce the occurrence of erectile dysfunction (82). Tangeretin is a polymethoxylated flavone, 4′,5,6,7,8-pentamethoxyflavone that has been shown to improve erectile function by modulating NO concentration (83). Similarly, naringin has been shown to act as a phosphodiesterase type 5 (PDE5) inhibitor (84). By inhibiting PDE5 activities, naringin prevented the breakdown of cGMP, thereby enhancing the activities of NO/cGMP signaling to stimulate penile erection. Also, since arginase competes with the NO/cGMP pathway for L-arginine, the inhibitory effect of naringin on arginase suggests that naringin ensures the availability of L-arginine for NO synthesis, thereby improving penile erection. Furthermore, according to Laila et al. (82), hesperidin can prevent erectile dysfunction and also enhance penile erection. Hence, phenolic compounds from orange peel can be explored as aphrodisiac agent.
5 Testicular functions and orange peel extract bioactive compounds
5.1 Spermatogenesis and Orange peel extract bioactive compounds
Spermatogenesis is a process of cell differentiation to produce fertilizing sperm, which can fuse with an ovum during fertilization to form a zygote. Spermatogenesis occurs in the testis’s Sertoli cells that isolate male germ cells from the interstitium by their tight junctions and provide nutrients for the meiosis process. (85). Orange peel bioactive compounds have been established to enhance spermatogenesis and improve sperm quality. Findings from Okesina et al. (86) and Butchi Akondi et al. (87) revealed that naringin improved sperm quality by increasing sperm count, morphology, and motility Similarly, naringenin improved total and progressive motility, viability, and membrane functionality (88). These invivo findings can be supported by those from our insilico study that reported that didymin, hesperidin, neohesperidin, naringin, narirutin, tangeretin, sinensetin, vicenin 2, and eriocitrin upregulate the activities of Arp2/3 complex via their inhibitory effect on arpin. Arp2/3 complex is responsible for maintaining acrosomal reaction, capacitation, and numerous phases of spermatogenesis, such as acrosome biogenesis, flagellum formation, and nuclear processes, such as synaptonemal complex formation (15). Also, these bioactive compounds improved the blood-testis barrier (responsible for preventing the flow of toxins while ensuring the availability of nutrients to the spermatogonial stem cells) by preserving Arp2/3 complex activities.
Also, compounds from orange peel, especially the flavonoids, are potent antioxidants and could account for their sperm quality-enhancing properties. Cellular oxidative stress occurs when the antioxidant capacity is overwhelmed by the production of oxidants such as reactive oxygen species (ROS) (89). The belief that oxidative stress is an essential factor contributing to male infertility began in the 1920s. A group of scientists from the University of Cambridge demonstrated for the first time the toxic effect of lipid peroxidation on sperm function (90). Sperm cells were first identified as susceptible to oxidative damage (91). At this junction, it is important to mention that sperm cells also generate a small amount of ROS, which is vital for capacitation and fertilization. However, excess ROS is detrimental to sperm function, and it has been shown to rapidly and irreversibly impair sperm motility in humans (92).
Citrus flavonoids are potent free radical scavengers that neutralize ROS and restore redox homeostasis in testes. For example, Okesina et al. (86) and Dong et al. (93) revealed that naringin supplementation upregulated antioxidant activities (e.g., superoxide dismutase (SOD) and catalase) and downregulated pro-oxidant expression (malondialdehyde (MDA). These effects protect testicular cells from oxidative stress and maintain sperm quality and function. Furthermore, Choi et al. (94) established the antioxidant properties of hesperidin using an in vitro model, while Ortiz et al. (95) reported the antioxidant activities of neohesperidin. Hence, bioactive compounds from orange peel can preserve spermatogenesis and may improve sperm quality.
5.2 Steroidogenesis and orange peel bioactive compounds
The multi-step process of converting cholesterol into steroid hormones is referred to as steroidogenesis. Steroidogenesis is restricted to the Leydig cells in the testis, where cholesterol is converted to testosterone. Testosterone in embryonic life is responsible for the development of male sex organs by stimulating the growth of Wolffian ducts to form epididymis, vas deferens, and seminal vesicles. Additionally, testosterone contributes to the development of genital tubercle to form the penis, scrotum, and prostate (95). At puberty, testosterone ensures brain masculinization, male sexual behavior development, spermatogenesis, external genitalia maturation, and regulation of gonadotropins. Hence, testosterone is the major male reproductive hormone, and all male sexual functions depend on its availability.
Bioactive compounds from orange peel have been shown to maintain male sexual functions by improving testosterone synthesis. For example, naringenin increased serum testosterone concentration after 10 weeks of oral administration in rats (96). Similarly, it was shown to abolish the decrease in serum testosterone and inhibin B following chemotherapeutic drug treatment (97). Similarly, hesperidin glycoside ameliorated vanadium-induced decline in testosterone synthesis (98). The ability of these bioactive compounds to stimulate testosterone synthesis could be associated with their ability to upregulate steroidogenic enzymatic activities. Hesperidin, for example, has been shown to upregulate the activities of steroidogenic acute regulatory protein, CYP11A1, CYP17A1, and 17β-Hydroxysteroid dehydrogenases (73). Hence, bioactive compounds from orange peel maintain male sexual function by modulating steroidogenic enzymatic activities.
6 Conclusion and future perspective
In conclusion, orange peels, frequently thrown away as trash in the citrus industry, can be recycled into valuable components that promote health. Orange peels are an inexpensive bioactive chemical source that modulates enzymes that are therapeutic targets in managing male sexual dysfunction. Therefore, this review identified different orange peel bioactive compounds and explored different methods of extraction such as supercritical fluid extraction, microwave-assisted extraction, ultrasound-assisted extraction, hot-water extraction, and solvent-based extraction. Additionally, we established the effect of these bioactive compounds on male sexual functions. As stated in this review, orange peel bioactive chemicals can improve male sexual functions by increasing the activities of the erectogenic enzymes, penile erection via the NO/cGMP signaling, libido, steroidogenesis or testosterone production, and spermatogenesis. Hence, these bioactive compounds may replace the expensive existing drugs for managing male sexual disorders. However, one of the significant challenges of exploring natural flavonoids is bioavailability. Hence, nanoformulations should be created as efficient delivery systems due to the potential therapeutic strength of orange peel bioactive. Nanocarriers such as nanoparticles and liposomes will improve the solubility, stability, and bioavailability of these agents.
Author contributions
AO: Resources, Conceptualization, Visualization, Project administration, Funding acquisition, Formal Analysis, Validation, Data curation, Supervision, Writing – original draft, Investigation, Software, Methodology, Writing – review & editing. GB: Validation, Data curation, Visualization, Supervision, Project administration, Methodology, Formal Analysis, Investigation, Software, Resources, Writing – review & editing, Funding acquisition. AA: Software, Investigation, Resources, Visualization, Funding acquisition, Formal Analysis, Validation, Methodology, Writing – review & editing, Data curation, Supervision, Project administration. LO: Resources, Validation, Data curation, Conceptualization, Project administration, Visualization, Writing – review & editing, Methodology, Supervision, Investigation, Funding acquisition, Software, Formal Analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fraia SD, Sharmila VD, Banu JR, and Massarotti N. A comprehensive review on upcycling of food waste into value added products towards a circular economy: Holistic approaches and life cycle assessments. Trends Food Sci Technology. (2024) 143:104288. doi: 10.1016/j.tifs.2023.104288
2. Schiebel CS, Bueno LR, Pargas RB, de Mello Braga LLV, da Silva KS, Fernandes ACVU, et al. Exploring the biological activities and potential therapeutic applications of agro-industrial waste products through non-clinical studies: A systematic review. Sci total Environ. (2024) 950:175317. doi: 10.1016/j.scitotenv.2024.175317
3. Kolawole ID, Kolawole GO, Sanni-manuel BA, Kolawole SK, Ewansiha JV, Kolawole VA, et al. Economic impact of waste from food, water, and agriculture in Nigeria: challenges, implications, and applications—a review. Discov Environ. (2024) 2:51. doi: 10.1007/s44274-024-00086-6
4. Jones ER, Van Vliet MTH, Qadir M, and Bierkens MFP. Country-level and gridded estimates of wastewater production, collection, treatment and reuse. Earth Syst Sci. (2021) 13:237–54. doi: 10.5194/essd-13-237-2021
5. García-Mahecha M, Soto-Valdez H, Carvajal-Millan E, Madera-Santana TJ, Lomelí-Ramírez MG, and Colín-Chávez C. Bioactive compounds in extracts from the agro-industrial waste of mango. Molecules (Basel Switzerland). (2023) 28:458. doi: 10.3390/molecules28010458
6. Okesina KB, Odetayo AF, Adeyemi WJ, Okesina AA, Bassey GE, and Olayaki LA. Naringin prevents diabetic-induced dysmetabolism in male wistar rats by modulating GSK-3 activities and oxidative stress-dependent pathways. Cell Biochem biophysics. (2024). doi: 10.1007/s12013-024-01444-0
7. Bala S, Garg D, Sridhar K, Inbaraj BS, Singh R, Kamma S, et al. Transformation of agro-waste into value-added bioproducts and bioactive compounds: micro/nano formulations and application in the agri-food-pharma sector. Bioengineering (Basel Switzerland). (2023) 10:152. doi: 10.3390/bioengineering10020152
8. Parmar HS, Dixit Y, and Kar A. Fruit and vegetable peels: Paving the way towards the development of new generation therapeutics. Drug discoveries Ther. (2010) 4.
9. Negrea M, Cocan I, Jianu C, Alexa E, Berbecea A, Poiana MA, et al. Valorization of citrus peel byproducts: A sustainable approach to nutrient-rich jam production. Foods (Basel Switzerland). (2025) 14:1339. doi: 10.3390/foods14081339
10. Suri S, Singh A, and Nema PK. Current applications of citrus fruit processing waste: A scientific outlook. Appl Food Res. (2022) 2:100050. doi: 10.1016/j.afres.2022.100050
11. Varmie EB and Thakur M. Utilization of citrus processing waste: A review. Pharma Innov J. (2021) 10:682–97.
12. Munir H, Yaqoob S, Awan KA, Imtiaz A, Naveed H, Ahmad N, et al. Unveiling the chemistry of citrus peel: insights into nutraceutical potential and therapeutic applications. Foods (Basel Switzerland). (2024) 13:1681. doi: 10.3390/foods13111681
13. Maqbool Z, Khalid W, Atiq HT, Koraqi H, Javaid Z, Alhag SK, et al. Citrus waste as source of bioactive compounds: extraction and utilization in health and food industry. Molecules (Basel Switzerland). (2023) 28:1636. doi: 10.3390/molecules28041636
14. Olayaki LA, Okesina KB, Jesubowale JD, Ajibare AJ, and Odetayo AF. Orange peel extract and physical exercise synergistically ameliorate type 2 diabetes mellitus-induced dysmetabolism by upregulating GLUT4 concentration in male wistar rats. J medicinal Food. (2023) 26:470–9. doi: 10.1089/jmf.2023.0061
15. Odetayo AF, Ajibare AJ, Okesina KB, Akhigbe TM, Olugbogi EA, and Olayaki LA. Orange peel ethanolic extract and physical exercise prevent testicular toxicity in streptozocin and high fat diet-induced type 2 diabetes rats via Nrf2/NF-kB signaling: In silico and in vivo studies. Heliyon. (2024) 10:e39780. doi: 10.1016/j.heliyon.2024.e39780
16. Wang L, Wang J, Fang L, Zheng Z, Zhi D, Wang S, et al. Anticancer activities of citrus peel polymethoxyflavones related to angiogenesis and others. BioMed Res Int. (2014) 2014:453972. doi: 10.1155/2014/453972
17. Khalil RK, Sharaby MR, and Abdelrahim DS. Novel active edible food packaging films based entirely on citrus peel wastes. Food Hydrocolloids. (2023) 134:107961. doi: 10.1016/j.foodhyd.2022.107961
18. Seminara S, Bennici S, Di Guardo M, Caruso M, Gentile A, La Malfa S, et al. Sweet orange: evolution, characterization, varieties, and breeding perspectives. Agriculture. (2023) 13:264. doi: 10.3390/agriculture13020264
19. Afifi SM, Gök R, Eikenberg I, Krygier D, Rottmann E, Stübler AS, et al. Comparative flavonoid profile of orange (Citrus sinensis) flavedo and albedo extracted by conventional and emerging techniques using UPLC-IMS-MS, chemometrics and antioxidant effects. Front Nutr. (2023) 10:1158473. doi: 10.3389/fnut.2023.1158473
20. Kato M, Ikoma Y, Matsumoto H, Sugiura M, Hyodo H, and Yano M. Accumulation of carotenoids and expression of carotenoid biosynthetic genes during maturation in citrus fruit. Plant Physiol. (2004) 134:824–37. doi: 10.1104/pp.103.031104
21. Nieto G, Fernández-López J, Pérez-Álvarez JA, Peñalver R, Ros-Berruezo G, and Viuda-Martos M. Valorization of citrus co-products: recovery of bioactive compounds and application in meat and meat products. Plants. (2021) 10:1069. doi: 10.3390/plants10061069
22. Brezo-Borjan T, Švarc-Gajić J, Morais S, Delerue-Matos C, Rodrigues F, Lončarević I, et al. Chemical and biological characterisation of orange (Citrus sinensis) peel extracts obtained by subcritical water. Processes. (2023) 11:1766. doi: 10.3390/pr11061766
23. Senit JJ, Velasco D, Manrique AG, Sanchez-Barba M, Toledo JM, Santos VE, et al. Orange peel waste upstream integrated processing to terpenes, phenolics, pectin and monosaccharides: Optimization approaches. Ind Crops Products. (2019) 134:370–81. doi: 10.1016/j.indcrop.2019.03.060
24. Rathod NB, Elabed N, Punia S, Ozogul F, Kim S-K, and Rocha JM. Recent developments in polyphenol applications on human health: A review with current knowledge. Plants. (2023) 12:1217. doi: 10.3390/plants12061217
25. Deo S and Sakhale BK. A review on potential of bioactive compounds obtained from processing waste of various fruits and vegetables. Int J Pure Appl Biosci. (2018) 6:680–6. doi: 10.18782/2320-7051.6742
26. Wang X, Li S, Wei CC, Huang J, Pan MH, Shahidi F, et al. Anti-inflammatory effects of polymethoxyflavones from citrus peels: A review. J Food Bioactives. (2018) 3:76–86. doi: 10.31665/JFB.2018.3150
28. Donia T, Dabbour NM, and Loutfy SA. Hesperidin: Advances on Resources, Biosynthesis Pathway, Bioavailability, Bioactivity, and Pharmacology. In: Handbook of Dietary Flavonoids. Springer International Publishing, Cham (2023). p. 1–55.
29. Mohsin A, Hussain M, Zaman W, Mohsin M, Zhang J, Liu Z, et al. Advances in sustainable approaches utilizing orange peel waste to produce highly value-added bioproducts. Crit Rev Biotechnol. (2021) 42:1284–1303. doi: 10.1080/07388551.2021.2002805
30. Herzyk F, Piłakowska-Pietras D, and Korzeniowska M. Supercritical extraction techniques for obtaining biologically active substances from a variety of plant byproducts. Foods. (2024) 13:1713. doi: 10.3390/foods13111713
31. Ghadiri K, Raofie F, Qomi M, and Davoodi A. Response surface methodology for optimization of supercritical fluid extraction of orange peel essential oil. Pharmaceut Biomed Res. (2020) 64, 303–312.
32. Ortiz-Sanchez M, Agudelo-Patiño T, and Cardona Alzate CA. Maximizing the hesperidin extraction using supercritical carbon dioxide and ethanol: theoretical prediction and experimental results. Processes. (2024) 1211, 2457.
33. Benassi L, Alessandri I, and Vassalini I. Assessing green methods for pectin extraction from waste orange peels. Molecules. (2021) 26:1766. doi: 10.3390/molecules26061766
34. Murador D, De Souza Mesquita L, Neves B, Braga A, Martins P, Zepka L, et al. Bioaccessibility and cellular uptake by Caco-2 cells of carotenoids and chlorophylls from orange peels: A comparison between conventional and ionic liquid mediated extractions. Food Chem. (2020) 339:127818. doi: 10.1016/j.foodchem.2020.127818
35. Li Q, Putra NR, Rizkiyah DN, Abdul Aziz AH, Irianto I, and Qomariyah L. Orange pomace and peel extraction processes towards sustainable utilization: A short review. Molecules. (2023) 28:3550. doi: 10.3390/molecules28083550
36. Siddiqui SA, Pahmeyer MJ, Assadpour E, and Jafari SM. Extraction and purification of d-limonene from orange peel wastes: Recent advances. Ind Crops Products. (2022) 177:114484. doi: 10.1016/j.indcrop.2021.114484
37. Odetayo AF, Akhigbe RE, Bassey GE, Hamed MA, and Olayaki LA. Impact of stress on male fertility: role of gonadotropin inhibitory hormone. Front Endocrinol. (2024) 14:1329564. doi: 10.3389/fendo.2023.1329564
38. Kandeel FR, Koussa VK, and Swerdloff RS. Male sexual function and its disorders: physiology, pathophysiology, clinical investigation, and treatment. Endocrine Rev. (2001) 22:342–88. doi: 10.1210/edrv.22.3.0430
39. Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, et al. Male infertility. Lancet (London England). (2021) 397:319–33. doi: 10.1016/S0140-6736(20)32667-2
40. Saikia Q, Adhikari K, Begum T, Dutta S, Hazarika A, and Kalita JC. Erectile dysfunction: basics and its management using plant products. Egyptian J Basic Appl Sci. (2024) 11:25–41. doi: 10.1080/2314808X.2023.2300560
41. Melis MR and Argiolas A. Oxytocin, erectile function and sexual behavior: last discoveries and possible advances. Int J Mol Sci. (2021) 22:10376. doi: 10.3390/ijms221910376
42. Odetayo AF and Olayaki LA. Omega 3 fatty acid improves sexual and erectile function in BPF-treated rats by upregulating NO/cGMP signaling and steroidogenic enzymes activities. Sci Rep. (2023) 13:18060. doi: 10.1038/s41598-023-45344-4
43. Adeyemi DH, Odetayo AF, Hamed MA, and Akhigbe RE. Impact of COVID 19 on erectile function. Aging male: Off J Int Soc Study Aging Male. (2022) 25:202–16. doi: 10.1080/13685538.2022.2104833
44. de Souza ILL, Ferreira EDS, Vasconcelos LHC, Cavalcante FA, and da Silva BA. Erectile dysfunction: key role of cavernous smooth muscle cells. Front Pharmacol. (2022) 13:895044. doi: 10.3389/fphar.2022.895044
45. Motofei IG. A dual physiological character for sexual function: libido and sexual pheromones. BJU Int. (2009) 104:1702–8. doi: 10.1111/j.1464-410X.2009.08610.x
46. Calabrò RS, Cacciola A, Bruschetta D, Milardi D, Quattrini F, Sciarrone F, et al. Neuroanatomy and function of human sexual behavior: A neglected or unknown issue? Brain Behav. (2019) 9:e01389. doi: 10.1002/brb3.v9.12
47. Akhigbe RE, Hamed MA, Odetayo AF, Akhigbe TM, and Oyedokun PA. Zinc improves sexual and erectile function in HAART-treated rats via the upregulation of erectogenic enzymes and maintenance of redox balance. Aging male: Off J Int Soc Study Aging Male. (2023) 26:2205517. doi: 10.1080/13685538.2023.2205517
48. Croft HA. Understanding the role of serotonin in female hypoactive sexual desire disorder and treatment options. J sexual Med. (2017) 14:1575–84. doi: 10.1016/j.jsxm.2017.10.068
49. Atmaca M. Selective serotonin reuptake inhibitor-induced sexual dysfunction: current management perspectives. Neuropsychiatr Dis Treat. (2020) 16:1043–50. doi: 10.2147/NDT.S185757
50. Moses TEH and Javanbakht A. Resolution of selective serotonin reuptake inhibitor-associated sexual dysfunction after switching from fluvoxamine to fluoxetine. J Clin Psychopharmacol. (2023) 43:71–3. doi: 10.1097/JCP.0000000000001636
51. Jung J, Jo HW, Kwon H, and Jeong NY. Clinical neuroanatomy and neurotransmitter-mediated regulation of penile erection. Int neurourology J. (2014) 18:58–62. doi: 10.5213/inj.2014.18.2.58
52. Blanco R, Saenz de Tejada I, Goldstein I, Krane RJ, Wotiz HH, and Cohen RA. Cholinergic neurotransmission in human corpus cavernosum. II. Acetylcholine synthesis. Am J Physiology-Heart Circulatory Physiol. (1988) 254:H468–72. doi: 10.1152/ajpheart.1988.254.3.H468
53. Cookson J, Hodgson R, and Wildgust HJ. Prolactin, hyperprolactinaemia and antipsychotic treatment: a review and lessons for treatment of early psychosis. J Psychopharmacol (Oxford England). (2012) 26:42–51. doi: 10.1177/0269881112442016
54. Dupré C, Lovett-Barron M, Pfaff DW, and Kow LM. Histaminergic responses by hypothalamic neurons that regulate lordosis and their modulation by estradiol. Proc Natl Acad Sci United States America. (2010) 107:12311–6. doi: 10.1073/pnas.1006049107
55. Meston CM and Frohlich PF. The neurobiology of sexual function. Arch Gen Psychiatry. (2000) 57:1012–30. doi: 10.1001/archpsyc.57.11.1012
56. Tan X, Zhao L, and Tang Y. The function of BDNF and its receptor in the male genitourinary system and its potential clinical application. Curr Issues Mol Biol. (2023) 45:110–21. doi: 10.3390/cimb45010008
57. Wang H, Zhang Y, and Qiao M. Mechanisms of extracellular signal-regulated kinase/cAMP response element-binding protein/brain-derived neurotrophic factor signal transduction pathway in depressive disorder. Neural regeneration Res. (2013) 8:843–52.
58. Sawamoto A, Okuyama S, Nakajima M, and Furukawa Y. Citrus flavonoid 3, 5, 6, 7, 8, 3′, 4′-heptamethoxyflavone induces BDNF via cAMP/ERK/CREB signaling and reduces phosphodiesterase activity in C6 cells. Pharmacol Rep. (2019) 71:653–8. doi: 10.1016/j.pharep.2019.03.006
59. Donato F, de Gomes MG, Goes ATR, Borges Filho C, Del Fabbro L, Antunes MS, et al. Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: possible role of l-arginine-NO-cGMP pathway and BDNF levels. Brain Res Bull. (2014) 104:19–26. doi: 10.1016/j.brainresbull.2014.03.004
60. Kim J, Wie MB, Ahn M, Tanaka A, Matsuda H, and Shin T. Benefits of hesperidin in central nervous system disorders: a review. Anat Cell Biol. (2019) 52:369–77. doi: 10.5115/acb.19.119
61. Knipper M, da Penha Berzaghi M, Blöchl A, Breer H, Thoenen H, and Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci. (1994) 6:668–71. doi: 10.1111/j.1460-9568.1994.tb00312.x
62. Narita M, Aoki K, Takagi M, Yajima Y, and Suzuki T. Implication of brain-derived neurotrophic factor in the release of dopamine and dopamine-related behaviors induced by methamphetamine. Neuroscience. (2003) 119:767–75. doi: 10.1016/S0306-4522(03)00099-X
63. Angoa-Pérez M, Anneken JH, and Kuhn DM. The role of brain-derived neurotrophic factor in the pathophysiology of psychiatric and neurological disorders. J Psychiatry Psychiatr Disord. (2017) 1:252–69. doi: 10.26502/jppd.2572-519X0025
64. Juric DM, Miklic S, and Carman-Krzan M. Monoaminergic neuronal activity up-regulates BDNF synthesis in cultured neonatal rat astrocytes. Brain Res. (2006) 1108:54–62. doi: 10.1016/j.brainres.2006.06.008
65. Yi LT, Li CF, Zhan X, Cui CC, Xiao F, Zhou LP, et al. Involvement of monoaminergic system in the antidepressant-like effect of the flavonoid naringenin in mice. Progress Neuro-psychopharmacol Biolog Psych. (2010) 347, 1223–1228.
66. Krassioukov A and Elliott S. Neural control and physiology of sexual function: effect of spinal cord injury. Topics spinal cord injury Rehabil. (2017) 23:1–10. doi: 10.1310/sci2301-1
67. Rastrelli G, Corona G, and Maggi M. Testosterone and sexual function in men. Maturitas. 112. (2018), 46–52. doi: 10.1016/j.maturitas.2018.04.004
68. Corona G and Maggi M. The role of testosterone in male sexual function. Rev endocrine Metab Disord. (2022) 23:1159–72. doi: 10.1007/s11154-022-09748-3
69. Krysiak R, Drosdzol-Cop A, Skrzypulec-Plinta V, and Okopien B. Sexual function and depressive symptoms in young women with elevated macroprolactin content: a pilot study. Endocrine. (2016) 53:291–8. doi: 10.1007/s12020-016-0898-5
70. Krüger TH, Haake P, Haverkamp J, Krämer M, Exton MS, Saller B, et al. Effects of acute prolactin manipulation on sexual drive and function in males. J Endocrinol. (2003) 179:357–65. doi: 10.1677/joe.0.1790357
71. Fatai OA and Aribidesi OL. Effect of bisphenol F on sexual performance and quality of offspring in Male Wistar rats. Ecotoxicology Environ Saf. (2022) 244:114079. doi: 10.1016/j.ecoenv.2022.114079
72. Okesina KB, Odetayo AF, Adeyemi WJ, Okesina AA, Bassey GE, and Olayaki LA. Naringin prevents diabetic-induced dysmetabolism in male wistar rats by modulating GSK-3 activities and oxidative Stress-Dependent Pathways. Cell Biochem Biophys. (2024) 824, 3559–3571.
73. Khamis T, Hegazy AA, El-Fatah SSA, Abdelfattah ER, Abdelfattah MMM, Fericean LM, et al. Hesperidin mitigates cyclophosphamide-induced testicular dysfunction via altering the hypothalamic pituitary gonadal axis and testicular steroidogenesis, inflammation, and apoptosis in male rats. Pharm (Basel Switzerland). (2023) 16:301. doi: 10.3390/ph16020301
74. Hu X, Li X, Deng P, Zhang Y, Liu R, Cai D, et al. The consequence and mechanism of dietary flavonoids on androgen profiles and disorders amelioration. Critical Rev Food Sci Nutr. (2023) 6332, 11327–11350.
75. Kim S and Park TI. Naringenin: a partial agonist on estrogen receptor in T47D-KBluc breast cancer cells. Int J Clin Exp Med. (2013) 6:890–9.
76. Burnett AL. Role of nitric oxide in the physiology of erection. Biol Reprod. (1995) 52:485–9. doi: 10.1095/biolreprod52.3.485
77. Lue TF. Erectile dysfunction. New Engl J Med. (2000) 342:1802–13. doi: 10.1056/NEJM200006153422407
78. Burnett AL. The role of nitric oxide in erectile dysfunction: implications for medical therapy. J Clin hypertension (Greenwich Conn.). (2006) 8:53–62.
79. Fabbri A, Aversa A, and Isidori A. Erectile dysfunction: an overview. Hum Reprod Update. (1997) 3:455–66. doi: 10.1093/humupd/3.5.455
80. Maggi M, Filippi S, Ledda F, Magini A, and Forti G. Erectile dysfunction: from biochemical pharmacology to advances in medical therapy. Eur J Endocrinol. (2000) 143:143–54. doi: 10.1530/eje.0.1430143
81. Kuppusamy UR and Das NP. Effects of flavonoids on cyclic AMP phosphodiesterase and lipid mobilization in rat adipocytes. Biochem Pharmacol. (1992) 44:1307–15. doi: 10.1016/0006-2952(92)90531-M
82. Laila IMI, Kassem SH, and Diab MSEM. Ameliorative effect of hesperidin against high dose sildenafil-induced liver and testicular oxidative stress and altered gene expression in male rats. Lab Anim Res. (2023) 39:22. doi: 10.1186/s42826-023-00173-4
83. Chiangsaen P, Maneesai P, Kukongviriyapan U, Tong-un T, Ishida W, Prachaney P, et al. Tangeretin ameliorates erectile and testicular dysfunction in a rat model of hypertension. Reprod Toxicol (Elmsford N.Y.). (2020) 96:1–10.
84. Akintunde JK, Akintola TE, Aliu FH, Fajoye MO, and Adimchi SO. Naringin regulates erectile dysfunction by abolition of apoptosis and inflammation through NOS/cGMP/PKG signalling pathway on exposure to Bisphenol-A in hypertensive rat model. Reprod Toxicol. (2020). doi: 10.1016/j.reprotox.2020.05.007
85. Linn E, Ghanem L, Bhakta H, Greer C, and Avella M. Genes regulating spermatogenesis and sperm function associated with rare disorders. Front Cell Dev Biol. (2021) 9:634536. doi: 10.3389/fcell.2021.634536
86. Okesina KB, Odetayo AF, Adeyemi WJ, Ajibare AJ, Okesina AA, and Olayaki LA. Naringin from sweet orange peel improves testicular function in high fat diet-induced diabetic rats by modulating xanthine oxidase/uric acid signaling and maintaining redox balance. Lab Anim Res. (2024) 40:5. doi: 10.1186/s42826-024-00188-5
87. Butchi Akondi R, Kumar P, Annapurna A, and Pujari M. Protective effect of rutin and naringin on sperm quality in streptozotocin (STZ) induced type 1 diabetic rats. Iranian J Pharm research: IJPR. (2011) 10:585–96.
88. Mehdipour M, Daghigh Kia H, Najafi A, Mohammadi H, and Álvarez-Rodriguez M. Effect of crocin and naringenin supplementation in cryopreservation medium on post-thaw rooster sperm quality and expression of apoptosis associated genes. PloS One. (2020) 15:e0241105. doi: 10.1371/journal.pone.0241105
89. Odetayo AF and Olayaki LA. Omega 3 fatty acids preserve testicular function by ameliorating BPF-induced dysthyroidism: role of p53/Bcl-2 signaling and proton pump activities. JBRA assisted Reprod. (2024) 28:471–82. doi: 10.5935/1518-0557.20240033
90. Jones R, Mann T, and Sherins R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril. (1979) 31:531–7. doi: 10.1016/S0015-0282(16)43999-3
91. Gharagozloo P and Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod (Oxford England). (2011) 26:1628–40. doi: 10.1093/humrep/der132
92. Aitken RJ, Drevet JR, Moazamian A, and Gharagozloo P. Male infertility and oxidative stress: A focus on the underlying mechanisms. Antioxidants (Basel Switzerland). (2022) 11:306. doi: 10.3390/antiox11020306
93. Dong PY, Liang SL, Li L, Liu J, Zhang SE, Klinger FG, et al. Naringin regulates intestinal microorganisms and serum metabolites to promote spermatogenesis. Food Funct. (2023) 14:3630–40. doi: 10.1039/D3FO00123G
94. Choi S-S, Lee S-H, and Lee K-A. A comparative study of hesperetin, hesperidin and hesperidin glucoside: antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants. (2022) 11:1618. doi: 10.3390/antiox11081618
95. Ortiz AC, Fideles SOM, Reis CHB, Bellini MZ, Pereira ESBM, Pilon JPG, et al. Therapeutic effects of citrus flavonoids neohesperidin, hesperidin and its aglycone, hesperetin on bone health. Biomolecules. (2022) 12:626. doi: 10.3390/biom12050626
96. Adana MY, Akang EN, Naidu ECS, Aniekan PI, Kouame K, Offor U, et al. Testicular microanatomical and hormonal alterations following use of antiretroviral therapy in Sprague Dawley rats: Role of Naringenin. Andrologia. (2018) 50:e13137.
97. Fouad AA, Refaie MMM, and Abdelghany MI. Naringenin palliates cisplatin and doxorubicin gonadal toxicity in male rats. Toxicol Mech Methods. (2019) 29:67–73. doi: 10.1080/15376516.2018.1512180
98. Vijaya Bharathi B, Jaya Prakash G, Krishna KM, Ravi Krishna CH, Sivanarayana T, Madan K, et al. Protective effect of alpha glucosyl hesperidin (G-hesperidin) on chronic vanadium induced testicular toxicity and sperm nuclear DNA damage in male Sprague Dawley rats. Andrologia. (2015) 47:568–78. doi: 10.1111/and.2015.47.issue-5
Keywords: antioxidant, citrus peel, erectile dysfunction, flavonoids, phenolic compounds, traditional medicine
Citation: Odetayo AF, Bassey GE, Adeyemi AL and Olayaki LA (2025) From waste to health: exploring the male sexuality enhancing activities of the bioactive components of orange peel. Front. Endocrinol. 16:1598545. doi: 10.3389/fendo.2025.1598545
Received: 23 March 2025; Accepted: 24 June 2025;
Published: 11 July 2025.
Edited by:
Etienne Marbaix, Université Catholique de Louvain, BelgiumReviewed by:
Jessica Elizabeth Pineda-Lozano, University of Guadalajara, MexicoCarmen Alejandrina Virgen-Carrillo, Instituto de Investigaciones en Comportamiento Alimentario y Nutriciónn, Mexico
Copyright © 2025 Odetayo, Bassey, Adeyemi and Olayaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adeyemi Fatai Odetayo, YWRleWVtaW9kZXRheW9AZ21haWwuY29t; YWRleWVtaS5vZGV0YXlvQGZ1aHNpLmVkdS5uZw==
 Adeyemi Fatai Odetayo
Adeyemi Fatai Odetayo Grace Edet Bassey2
Grace Edet Bassey2