- 1Department of Pediatrics, University Hospital Centre Zagreb, Zagreb, Croatia
- 2School of Medicine, University of Zagreb, Zagreb, Croatia
- 3Department of Radiology, University Hospital Centre Zagreb, Zagreb, Croatia
- 4Department of Gynaecology and Obstetrics, University Hospital Centre Zagreb, Zagreb, Croatia
Objectives: Hypothalamic hamartoma (HH) is an important cause of central precocious puberty (CPP) in young children but is rarely described in infants. Interpretation of laboratory data could be difficult because gonadotropins and estradiol levels often overlap in healthy infants with mini-puberty and children with HH. Extremely elevated estradiol levels are mostly described in girls with peripheral precocious puberty.
Case presentation: We present a 5.5-month-old girl with vaginal bleeding, significantly elevated estradiol levels (up to 3,974 pmol/L), elevated gonadotropins, and right ovarian cyst. Laboratory and radiologic evaluation revealed the HH as a cause of CPP. Immediately after the start of treatment with depot gonadotropin-releasing hormone analogue, age-appropriate undetectable levels of estradiol were achieved, with ovarian cyst regression and cessation of pubertal changes.
Conclusion: If observed in the period of mini-puberty, high levels of estradiol accompanied by unsuppressed gonadotropins can complicate the discrimination between central and peripheral precocious puberty. This challenge emerges particularly due to the absence of the negative feedback mechanism in children with HH. This is the first report identifying extremely high estradiol levels as part of the phenotypic spectrum of HH in infants.
1 Introduction
Pubertal changes in female infants are most observed as part of benign premature telarche, which rarely requires further laboratory evaluation (1). However, central or peripheral precocious puberty can also occur, although central precocious puberty in infants is extremely rare (1, 2).
Hypothalamic hamartoma (HH) is a well-recognized cause of central precocious puberty (CPP), particularly in very young children, with an average age of initial symptoms of around 1.5 years (3, 4). Estradiol levels in healthy girls and those with CPP are usually only slightly elevated in this age group. In contrast, significant elevation has been described in premature infants or girls with ovarian tumors or functional cysts (5–8).
We present a 5.5-month-old female infant who presented with vaginal bleeding and was found to have extremely elevated estradiol levels and CPP caused by HH.
2 Case presentation
A 5.5-month female infant was referred for further evaluation due to prolonged vaginal bleeding. She is the third child of healthy, unrelated parents, born from an uneventful pregnancy at 38 + 2 weeks of gestation (birth weight 3,380 g, + 0.37 SDS; length 51 cm, +0.17 SDS). Her previous medical history was unremarkable, and developmental milestones were age appropriate. There were no data on previous infection, trauma, topical use of estrogen-containing cosmetic products, or sexual precocity in family members. Bloody vaginal discharge resolved spontaneously after 5 days. On physical examination, her height and weight were 1.19 SDS and 0.31 SDS, respectively. The breast development was Tanner stage II, with enlarged, normally pigmented nipples and no pubic or axillary hair. There were no caffe-au-lait spots, but large light beige discoloration with irregular margins, covering the left abdominal area, up to the left rib cage, was noted. Endocrine laboratory evaluation showed elevated basal gonadotropins with markedly elevated estradiol levels, measured during, and following the resolution of vaginal bleeding (Table 1). Serum tumor markers (α-fetoprotein, β-subunit human chorionic gonadotropin, carcinoembryonic antigen, cancer antigen 125, carbohydrate antigen 19–9) and thyroid function tests (free thyroxine, thyroid-stimulating hormone) were normal. Pelvic ultrasound revealed a slightly enlarged uterus measuring 1.96 × 1.45 × 3.09 cm with an endometrial thickness of 3.1 mm. No follicles, cysts, or tumors were detected in the ovaries (right ovary 1.58 × 0.82 cm; left ovary 1.54 × 1.14 cm). Because of extremely high estradiol levels, autonomous ovarian secretion was also considered, so both brain and pelvic MRIs were performed on the 24th day after bleeding. Estradiol levels, measured simultaneously in two different laboratories, were about four times higher than initial (Table 1). Pelvic MRI revealed a cyst in the right ovary measuring 17 mm, and repeated pelvic ultrasound also described an avascular right ovarian cyst 20 × 14 mm. However, a brain MRI confirmed the diagnosis of HH (isointense peduncular lesion originating from mammillary bodies, 6 mm in diameter) (Figure 1).
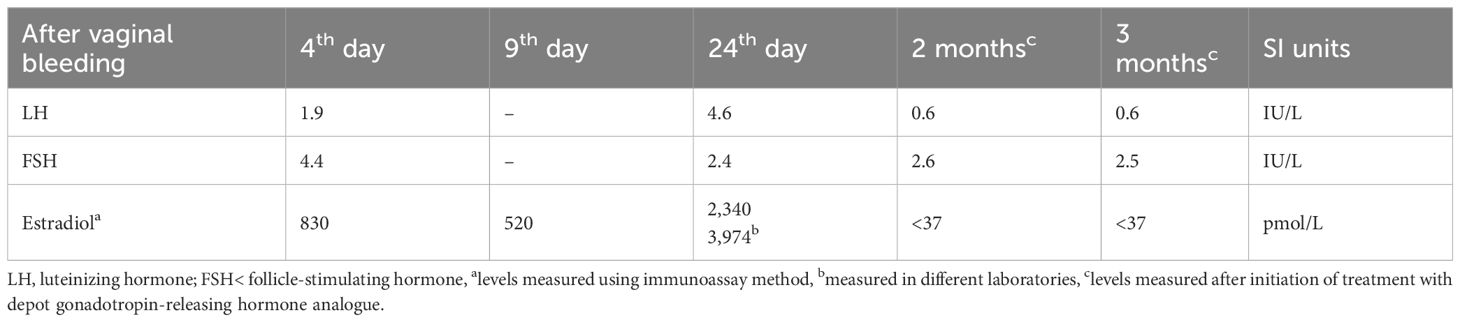
Table 1. Laboratory data of a female infant with central precocious puberty caused by hypothalamic hamartoma.
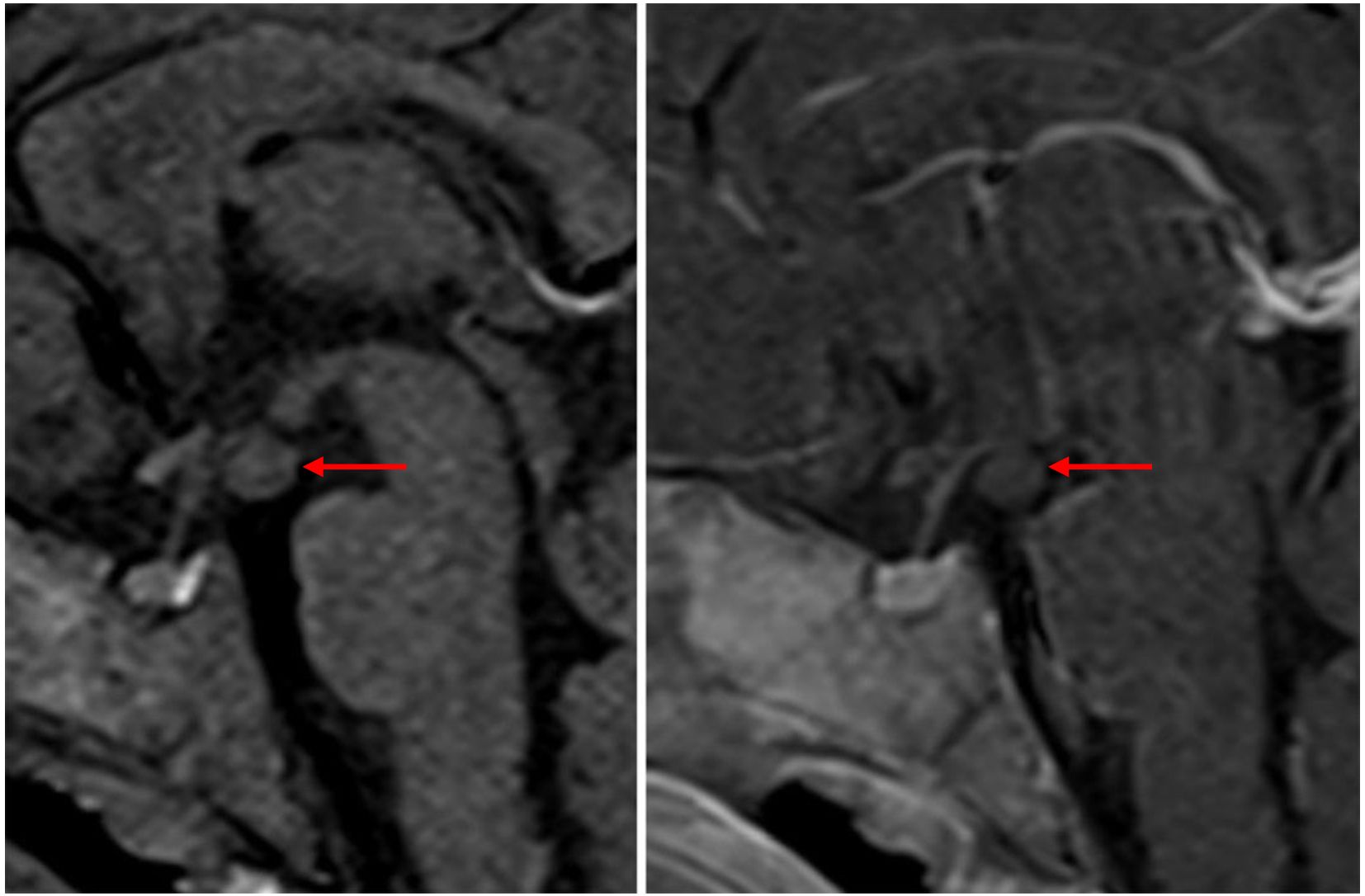
Figure 1. T1-weighted sagittal MR images in a 5.5-month-old infant with central precocious puberty and exaggerated estradiol secretion before and after gadolinium administration demonstrated a hypothalamic hamartoma. A rounded mass is located below the floor of the third ventricle, projecting into the suprasellar cistern behind the infundibulum of the pituitary gland. It is isointense to grey matter on T1 weighted images and does not enhance after gadolinium administration.
Diagnosis of central precocious puberty due to HH was made, and the treatment with monthly gonadotropin-releasing hormone analogue was commenced. One month after the treatment initiation, the estradiol levels were undetectable and the cyst in the right ovary subsided completely.
On follow-up, 4 months after treatment initiation, gradual breast tissue regression was noticed, and estradiol levels remained low. The developmental milestones are in the normal range, and no seizures are noticed.
Written informed consent was obtained from the proband’s parents, and Institutional Ethical Board approval for publication of data was acquired.
3 Discussion
The hypothalamic–pituitary–gonadal axis (HPGA) is active in infancy, leading to cyclic pituitary and gonadal secretion characterized as mini-puberty (1). During mini-puberty, the levels of luteinizing hormone (LH), follicle-stimulating hormone, and estradiol reach the pubertal range, but besides breast tissue enlargement, the development of other visible changes is rare (9). Vaginal bleeding is seldom described as a manifestation of exaggerated mini-puberty in premature infants (7). If there is a progression of clinical symptoms or more severe pubertal changes than age-appropriate are present, it is important to investigate potential underlying pathological causes.
Diagnosing precocious puberty in infants is often challenging due to difficulties interpreting laboratory data. Basal levels of LH in CPP and healthy infants with mini-puberty are indistinguishable. The response to gonadotropin-releasing analogue stimulation testing may be more pronounced during infancy, and the results have not been well established, leading to overlap between CPP and benign forms of precocious puberty in infants (1).
Estradiol levels during mini-puberty in healthy infants reach up to 100 pmol/L (5); however, much higher estradiol levels were reported in preterm infants as exaggerated mini-puberty manifestation. Extreme forms of mini-puberty have been reported exclusively in premature infants. It is speculated that this phenomenon could present an adaptive mechanism that promotes linear growth and maturation of reproductive organs and target tissues or indicate an immaturity of negative feedback control of HPGA (7, 10). All reported patients with exaggerated mini-puberty presented with vaginal bleeding, elevated levels of gonadotropins and estradiol. Most of them had ovarian cysts on pelvic ultrasound examinations and normal findings on brain MRI (7). High estradiol levels in preterm infants are also reported in ovarian hyperstimulation syndrome (11). Pituitary activity during infancy can rarely lead to ovarian hyperstimulation with the formation of functional ovarian cysts. Ovarian hyperstimulation was also described in gonadotroph adenomas (only two reported in children), with estradiol levels as high as 31,731 pmol/L and multicystic enlarged ovaries (12).
In a girl presenting with central precocious puberty at an early age, it is mandatory to exclude HH. According to the presentation on MRI scans, the HH can be classified as either pedunculated or sessile, with CPP being more common in the former (4). There are several plausible explanations of the HH effect on pubertal development. These include the activation of normal hypothalamic tissue due to compression, anatomical communication and secretion of paracrine factors, or autonomic pulsatile release of gonadotropin-release hormone from HH (4). The sequence of pubertal changes and levels of gonadotropins and sex hormones in children with HH are similar to normal puberty. The use of gonadotropin-releasing hormone agonists is the first-line treatment of CPP in children with HH (13).
Among infants with HH, basal levels of gonadotropins and estradiol are usually in the normal pubertal range (14). Higher than normal levels of estradiol are rarely reported in infants with HH (15). Our patient presented with extremely elevated estradiol levels, with cyclical increased levels mimicking the normal menstrual cycle. However, gonadotropin levels were not suppressed, which supported the diagnosis of CPP. Such high estradiol levels are usually described only in patients with peripheral precocious puberty, where excessive hormone secretion originates from either gonads or adrenal glands, while gonadotropin levels are suppressed (14). Excessively high estradiol levels were described in prepubertal girls with ovarian steroid cell tumors (estradiol levels ranging from 348.8 to 2,420.9 pmol/L) (6). Elevated levels of estradiol (680 pmol/L) and unsuppressed gonadotropin levels were also reported by Feilberg Jørgensen et al. in an 18-month girl with HH and juvenile granulosa cell tumor. The authors speculated that continuous hormonal stimulation from an early age by HH might have contributed to ovarian tumor development (8). However, to the best of our knowledge, there is no report of such excessive estradiol secretion caused exclusively by HH and suppressed immediately with gonadotropin-releasing hormone analogue treatment.
Our patient had supranormal estradiol levels (5–39 times above the upper range for healthy female infants), but despite this, gonadotropin levels were not suppressed. Our results align with the patient described by Feilberg Jørgensen et al. and illustrate the loss of negative feedback mechanism in HH (8).
We can speculate that our patient, in addition to HH, may also have endogenous ovarian dysfunction leading to a propensity for ovarian cyst formation and high estradiol level production, similar to ovarian hyperstimulation syndrome. However, due to rarity of HH finding in infancy, further studies should confirm if such high estradiol levels are incidental findings or are characteristic pattern of secretion in immature hyperstimulated ovaries of young infants.
With our case report presentation, we would like to emphasize that even extreme estradiol levels can be a phenotypic spectrum of HH in infants and that interpreting laboratory data of infant with CPP can pose difficulties in this age group.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
MV: Writing – review & editing, Writing – original draft. DB: Writing – review & editing. KD: Writing – review & editing. IJ: Writing – review & editing. MB: Writing – review & editing. NK: Writing – original draft, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We sincerely thank the patient’s parents for granting permission to publish this information.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kaplowitz PB and Lee PA. Females with breast development before three years of age. Endocrinol Metab Clin North Am. (2024) 53:195–201. doi: 10.1016/j.ecl.2024.01.002
2. Lanciotti L, Cofini M, Leonardi A, Penta L, and Esposito S. Up-to-date review about minipuberty and overview on hypothalamic-pituitary-gonadal axis activation in fetal and neonatal life. Front Endocrinol (Lausanne). (2018) 9:410. doi: 10.3389/fendo.2018.00410
3. Corbet Burcher G, Liang H, Lancaster R, Cross JH, Tisdall M, Varadkar S, et al. Neuropsychiatric profile of paediatric hypothalamic hamartoma: systematic review and case series. Dev Med Child Neurol. (2019) 61:1377–85. doi: 10.1111/dmcn.14241
4. Alomari SO, Houshiemy MNE, Bsat S, Moussalem CK, Allouh M, and Omeis IA. Hypothalamic hamartomas: A comprehensive review of the literature - Part 1: Neurobiological features, clinical presentations and advancements in diagnostic tools. Clin Neurol Neurosurg. (2020) 197:106076. doi: 10.1016/j.clineuro.2020.106076
5. Frederiksen H, Johannsen TH, Andersen SE, Albrethsen J, Landersoe SK, Petersen JH, et al. Sex-specific estrogen levels and reference intervals from infancy to late adulthood determined by LC-MS/MS. J Clin Endocrinol Metab. (2020) 105:754–68. doi: 10.1210/clinem/dgz196
6. Chu CH, Wang WD, Wang SY, Chao TK, Su RY, and Lin CM. Ovarian steroid cell tumor causing isosexual pseudoprecocious puberty in a young girl: an instructive case and literature review. BMC Endocr Disord. (2022) 22:41. doi: 10.1186/s12902-022-00956-1
7. Lattuada M, Molinari S, Nicolosi ML, Doni D, Lui C, Passoni P, et al. Exaggerated mini-puberty in a preterm girl: a case report and review of literature. J Pediatr Endocrinol Metab. (2022) 35:1309–15. doi: 10.1515/jpem-2022-0179
8. Feilberg Jørgensen N, Brock Jacobsen B, Ahrons S, and Starklint H. An association of hypothalamic hamartoma, central precocious puberty and juvenile granulosa cell tumour in early childhood. Horm Res. (1998) 49:292–4. doi: 10.1159/000023189
9. Rohayem J, Alexander EC, Heger S, Nordenström A, and Howard SR. Mini-puberty, physiological and disordered: consequences, and potential for therapeutic replacement. Endocr Rev. (2024) 45:460–92. doi: 10.1210/endrev/bnae003
10. Kiviranta P, Kuiri-Hänninen T, Saari A, Lamidi ML, Dunkel L, and Sankilampi U. Transient postnatal gonadal activation and growth velocity in infancy. Pediatrics. (2016) 138:e20153561. doi: 10.1542/peds.2015-3561
11. Altuntas N, Turkyilmaz C, Yuce O, Kulali F, Hirfanoglu IM, Onal E, et al. Preterm ovarian hyperstimulation syndrome presented with vaginal bleeding: a case report. J Pediatr Endocrinol Metab. (2014) 27:355–8. doi: 10.1515/jpem-2013-0166
12. Halupczok J, Kluba-Szyszka A, Bidzińska-Speichert B, and Knychalski B. Ovarian hyperstimulation caused by gonadotroph pituitary adenoma–review. Adv Clin Exp Med. (2015) 24:695–703. doi: 10.17219/acem/25212
13. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, ESPE-LWPES GnRH Analogs Consensus Conference Group, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. (2009) 123:e752–62. doi: 10.1542/peds.2008-1783
14. Bourayou R, Giabicani E, Pouillot M, Brailly-Tabard S, and Brauner R. Premature pubarche before one year of age: distinguishing between mini-puberty variants and precocious puberty. Med Sci Monit. (2015) 21:955–63. doi: 10.12659/MSM.893139
Keywords: hypothalamic hamartoma, central precocious puberty, estradiol, mini-puberty, case report
Citation: Vinkovic M, Braovac D, Dumic Kubat K, Jovanovic I, Banovic M and Krnic N (2025) Case Report: Exaggerated estradiol secretion in an infant with hypothalamic hamartoma. Front. Endocrinol. 16:1598734. doi: 10.3389/fendo.2025.1598734
Received: 23 March 2025; Accepted: 09 September 2025;
Published: 25 September 2025.
Edited by:
Brenda Kohn, New York University, United StatesReviewed by:
David William Cooke, Johns Hopkins University, United StatesHavva Nur Kendirci, Hittite University, Türkiye
Copyright © 2025 Vinkovic, Braovac, Dumic Kubat, Jovanovic, Banovic and Krnic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maja Vinkovic, bWFqYS52aW5rb3ZpYzIyQGdtYWlsLmNvbQ==
 Maja Vinkovic
Maja Vinkovic Duje Braovac
Duje Braovac Katja Dumic Kubat
Katja Dumic Kubat Ivan Jovanovic3
Ivan Jovanovic3 Maja Banovic
Maja Banovic Nevena Krnic
Nevena Krnic