- 1The First Clinical College, Wuhan University of Science and Technology, Wuhan, China
- 2Department of Endocrinology, Tianyou Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, China
Gene mutations in the Janus kinase/signal transducer and activator of transcription signaling (JAK/STAT) pathway can promote the occurrence of type 2 diabetes mellitus (T2DM) and autoimmune diseases. We report on two patients with T2DM (a mother and her adult son) with concomitant Hashimoto’s thyroiditis and autoimmune diseases. The son was diagnosed with systemic sclerosis and antiphospholipid syndrome, while the mother was diagnosed with primary biliary cholangitis. Both diagnoses occurred simultaneously. These cases highlight that, in clinical practice, careful symptom assessment, thorough history-taking, standardized physical examination, and obtaining a detailed family history are important. This reduces the misdiagnosis and missed diagnosis rates, enabling early diagnosis and treatment, thereby improving patient outcomes. While genetic testing was not performed in these two patients, this represents a potential direction for future research.
1 Introduction
Type 2 diabetes mellitus (T2DM) is a disease caused by a complex interplay of genetic, epigenetic, and environmental factors and is characterized by insulin resistance and insufficient insulin secretion. Research has shown that T2DM clusters in families, and specific causative genes and susceptibility genes associated with the disease have been identified (1). The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway is an important intracellular signaling pathway discovered in recent years. Numerous studies have shown that there is dysregulation of the JAK/STAT signaling pathway in T2DM and autoimmune diseases. The combination of T2DM with autoimmune diseases is rarely reported, particularly in the case of T2DM in an immediate family member with multiple autoimmune diseases. These cases suggest that genetic factors may regulate the co-occurrence of T2DM and autoimmune diseases. Detection of the JAK/STAT signaling pathway genes is a direction worthy of further research.
2 Case presentation
2.1 Case 1
Presenting complaint: A 55-year-old man presented on May 10, 2024, with bilateral hand numbness over 1 year, worsened by dry mouth, polydipsia, and polyuria over 4 months. Blurred vision occurred 1 week prior to admission. The outpatient point-of-care capillary blood glucose was 15.2 mmol/L. Urinalysis showed glucose 1+ and protein 1+. He was admitted for management of T2DM and suspected diabetic nephropathy. His mental status, appetite, sleep, bowel habits, physical strength, and body weight were normal.
Examination: Vitals: temperature, 36.4°C; pulse, 74/min (regular); blood pressure (BP), 140/89 mmHg; respiratory rate (RR), 20/min (regular). His BMI was 22 kg/m2. General: alert, ambulated independently, appeared fatigued, but no icterus. His skin showed multiple hypopigmented macules on the forehead, periorbital areas, mouth corners, neck, and bilateral dorsal hands. Lymphatics: no palpable superficial lymphadenopathy. Cardiopulmonary: auscultation clear. Abdomen: soft, non-tender, no rebound tenderness, and no masses. Sclerodactyly with fingertip swelling was noted in both hands, but no lower limb edema. Dorsalis pedis (DP) pulses were palpable and symmetric bilaterally. Neuromuscular examination revealed normal limb muscle strength and tone.
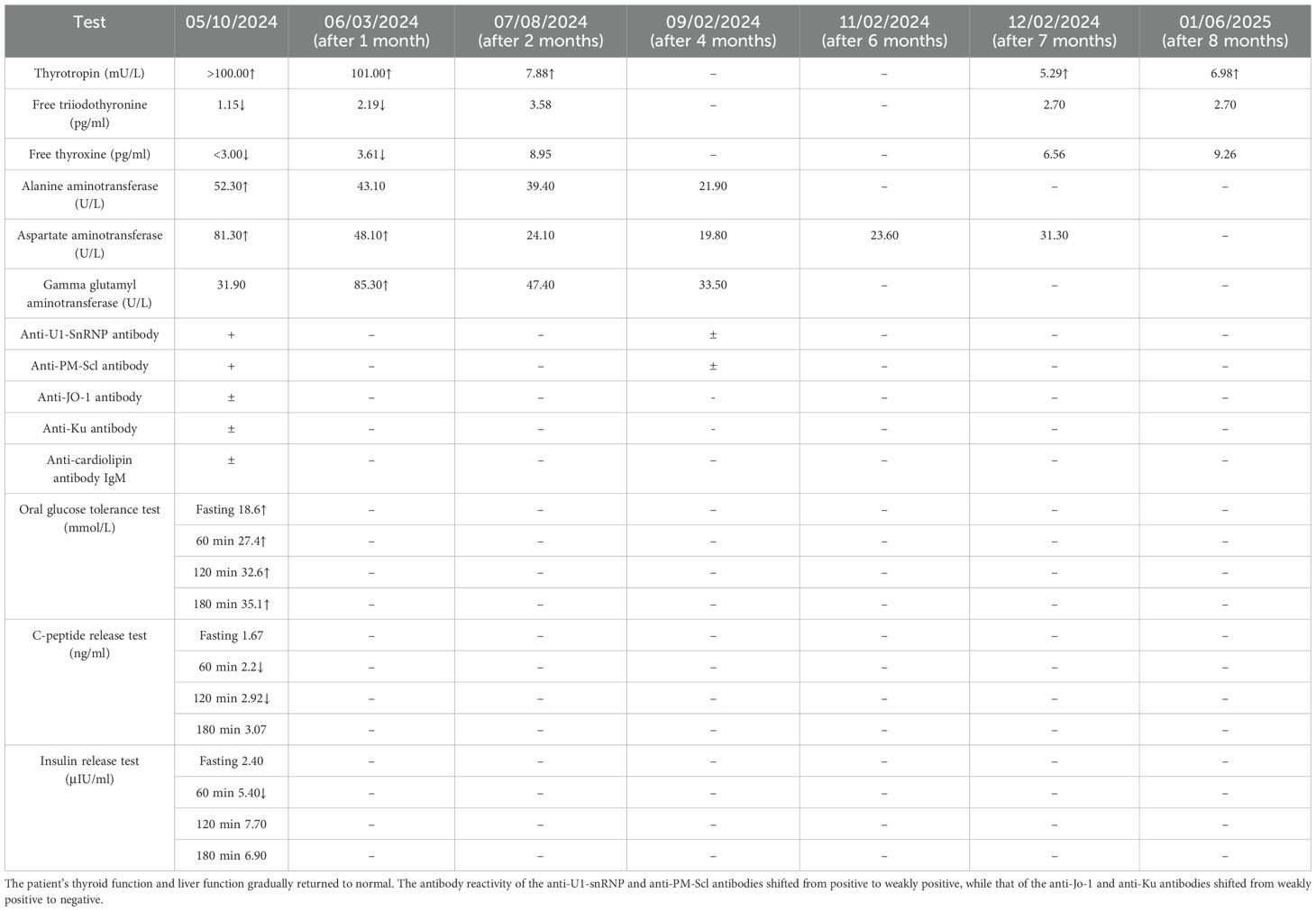
Investigations: glycosylated hemoglobin (HbA1c), 14.5% ↑; free triiodothyronine (FT3), 1.15 pg/ml ↓; free thyroxine (FT4), <3.00 pg/ml ↓; thyrotropin (thyroid-stimulating hormone, TSH), >100.000 mU/L ↑; anti-thyroglobulin antibody, 182.41 IU/ml (+); and anti-U1-snRNP antibody (+), anti-PM-Scl antibody (+), anti-Jo-1 antibody (±), anti-Ku antibody (±), and anti-cardiolipin anti-IgM (+). Other investigation results are shown in Table 1. Imaging: thyroid ultrasound (with cervical lymph nodes), solid hypoechoic nodule in the right lobe of the thyroid gland of TI-RADS (Thyroid Imaging Reporting and Data System) grade III, and bilateral cervical lymph nodes visible. Screening for diabetic complications: no abnormal changes found on fundus photography; the sensory examination suggests that vibration, touch and temperature sensation are normal. Doppler flowmetry of the extremities: right lower extremity posterior tibial artery (PT) ankle–branchial index (ABI) of 1.10 and DP ABI of 1.10; left lower extremity PT ABI of 1.19 and DP ABI of 1.19.
Diagnoses: T2DM, type 2 diabetic nephropathy (stage IIIB), type 2 diabetic peripheral neuropathy, hypothyroidism (Hashimoto’s thyroiditis, HT), overlap syndrome [connective tissue disease: systemic sclerosis (SSc) and antiphospholipid syndrome], vitiligo, thyroid nodule, and liver dysfunction.
Treatments: 1) Type 2 diabetes: mixed protamine zinc recombinant human insulin lispro injection (50 R), 26 IU, subcutaneous injection, twice a day; acarbose capsule, two capsules, oral before food. 2) Liver dysfunction: bicyclol tablets, one tablet, three times/day. 3) Type 2 diabetic nephropathy: BaiLingJiaoNang, four capsules, three times/day. 4) Hypothyroidism: levothyroxine sodium tablets, 50 μg, once a day. 5) Autoimmune diseases: total glucosides of White Peony capsules, two capsules, twice a day; hydroxychloroquine sulfate tablets, one tablet, twice a day; prednisone, 10 mg, twice a day. 6) Peripheral neuropathy: vitamin B1 tablets, one tablet, once a day; mecobalamin tablets, one tablet, once a day. 7) Prevention of osteoporosis: calcitriol capsules, one capsule, once a day; calcium carbonate and vitamin D3 tablets, one tablet, once a day.
Follow-up: Regular follow-up visits were conducted after discharge. The multiple hyperpigmented macules on the patient’s skin resolved completely (Figure 1). The patient’s thyroid function and liver function gradually returned to normal. The antibody reactivity of the anti-U1-snRNP and anti-PM-Scl antibodies shifted from positive to weakly positive, while that of the anti-Jo-1 and anti-Ku antibodies shifted from weakly positive to negative (Table 1).
2.2 Case 2
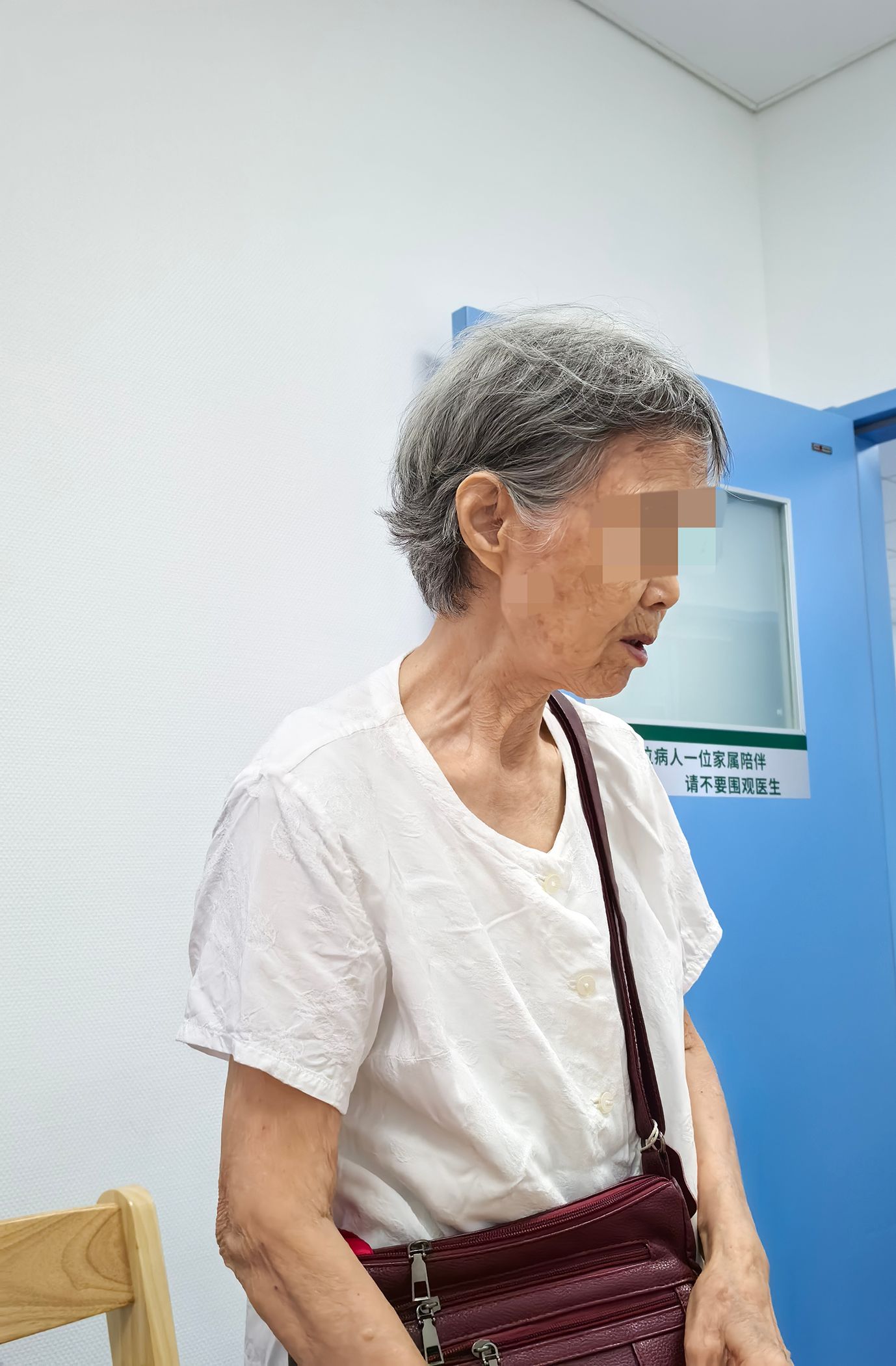
Presenting complaint: An 81-year-old woman (Figure 2) presented on May 20, 2024, with 6 months of hyperglycemia and 1 month of intermittent fatigue. During the course of the disease, the patient gradually developed bilateral blurred vision, foamy urine, heaviness in the bilateral lower limbs, dry mouth, polydipsia, and intermittent numbness of limbs. She was admitted for management of T2DM. Since the disease onset, the patient’s mental status, appetite, and sleep have been poor, but her bowel habits were normal. She developed a significantly reduced physical endurance and a 6-kg unintentional weight loss over the past year.
Examination: Vitals: temperature, 35.9°C; pulse, 74/min (regular); RR, 20/min (regular); BP, 132/74 mmHg; BMI, 16 kg/m2. General: alert, ambulated independently, appeared fatigued, and no icterus. Lymphatics: no palpable superficial lymphadenopathy. Cardiopulmonary: auscultation clear. Abdomen: soft, non-tender, no rebound tenderness, no masses, no right lower extremity edema, but mild edema over the left foot dorsum. Neuromuscular: normal limb muscle strength and tone.
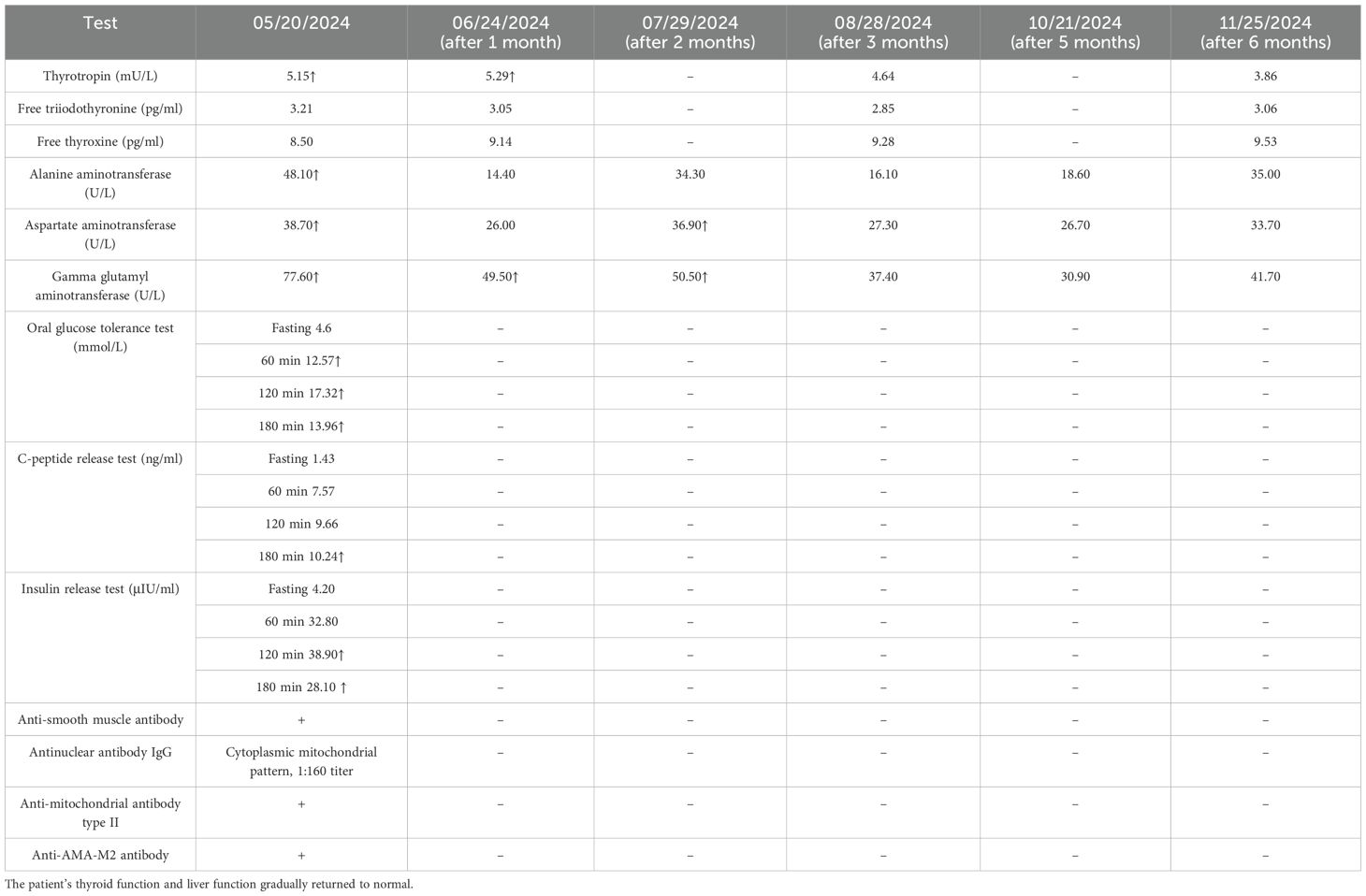
Investigations: HbA1c, 6.2% ↑; β-hydroxybutyric acid, 0.49 mmol/L ↑; TSH, 5.149 mU/L ↑; FT3, 3.21 pg/ml; FT4, 8.5 pg/ml; anti-thyroglobulin antibody, 342.50 IU/ml (+); and anti-AMA-M2 antibody (+), anti-mitochondrial antibody type II (+), anti-smooth muscle antibodies (+), and antinuclear antibody IgG (cytoplasmic mitochondrial pattern, 1:160 titer). Other investigation results are shown in Table 2. Imaging: dual-energy X-ray bone mineral density (BMD) T-value, less than −2.5. Thyroid ultrasound: increased thyroid blood flow signal and small solid nodule in the left lobe of the thyroid gland, TI-RADS grade II. Diabetes mellitus specialist examination: in both eyes, the fundus image did not show abnormal changes; the sensory examination suggests that vibration, touch and temperature sensation are normal, and cool temperature sensation is normal. Doppler flowmetry of the extremities suggests left ABIs of 0.93 and 0.93 (for PT and DP, respectively) and right ABIs of 0.93 and 1.08 (for PT and DP, respectively).
Diagnoses: T2DM, type 2 diabetic peripheral neuropathy, type 2 diabetic ketosis, type 2 diabetic nephropathy, autoimmune liver disease (primary biliary cholangitis, PBC), HT, thyroid nodule, senile osteoporosis, thrombocytopenia, and liver dysfunction.
Treatments: 1) Type 2 diabetes: alogliptin benzoate tablets, 12.5 mg, taken orally before breakfast. 2) Autoimmune liver disease (PBC): ursodeoxycholic acid capsules, two capsules, twice a day. 3) Hypothyroidism: levothyroxine sodium tablets, 1/4 tablet, every other day for treatment. 4) Osteoporosis: calcitriol capsules, one capsule, once a day; calcium carbonate and vitamin D3 tablets, one tablet, once a day; desucumab injection, 60 mg, semi-annual treatment. 5) Peripheral neuropathy: mecobalamin tablets, one tablet, once a day. 6) Thrombocytopenia: caffeic acid tablets, three times/day. 7) Liver dysfunction: HuGanKeLi, one sachet, three times/day; bicyclol tablets, one tablet, three times/day.
Follow-up: Regular follow-up visits were conducted after discharge. The patient’s thyroid function and liver function gradually returned to normal. The follow-up results are shown in Table 2.
3 Discussion
The final diagnoses of case 1 were T2DM, HT, and connective tissue disease (SSc and antiphospholipid syndrome). The final diagnoses of case 2 were T2DM, HT, and autoimmune liver disease (PBC). Although both patients had preserved β-cell function and negative diabetes-associated autoantibodies, they do not meet the diagnostic criteria for latent autoimmune diabetes in adults (LADA) (2). It is necessary to be vigilant of the possibility of LADA. Therefore, ruling out LADA requires longitudinal monitoring. During follow-up, long-term monitoring of C-peptide and diabetes-associated autoantibodies in these patients is required.
The conditions of the two patients in this report might be related to the JAK/STAT signaling pathway. This pathway has three main components: cytokine receptors, Janus kinases (JAKs), and signal transducers and activators of transcription (STATs) (3). JAKs are non-receptor tyrosine kinases composed of four members: JAK1, JAK2, JAK3, and TYK2. Dimerized kinases bind cytokine receptors, phosphorylating the intracellular tyrosine residues to propagate activation signals. Of these, JAK1, JAK2, and TYK2 are ubiquitously expressed, whereas the expression of JAK3 is restricted to the bone marrow and the lymphatic system (4). The human STAT family consists of seven members: STAT1, STAT2, STAT3, STAT4, STAT5a/b, and STAT6 (5).
Gene mutations in the JAK/STAT signaling pathway can drive the release of interferons (IFNs) and other cytokines. These IFNs and other cytokines can further enhance the activation of the JAK/STAT signaling pathway, establishing a pathogenic positive feedback loop that promotes multi-organ damage. IFNs are cytokines that can be classified into three groups: type I (IFN-α, IFN-β, IFN-δ, IFN-ϵ, IFN-κ, IFN-τ, IFN-ω, and IFN-ζ), type II (IFN-λ), and type III ( IFN-λ) (6). After transcriptional activation and mRNA translation, type I IFNs (IFN-I) are secreted by immune cells to adjacent cells and bind to two receptor subunits: IFN-α receptor 1 (IFNAR1) and IFNAR2. These two receptors are respectively associated with TYK2 and JAK1 (7). The dimerization of the receptor initiates the autophosphorylation of JAK1. JAK1 subsequently phosphorylates and activates the STAT1 and STAT2 proteins. These proteins form a complex with interferon regulatory factor 9 (IRF9), eventually forming a well-characterized complex, IFN-stimulated gene factor 3 (ISGF3). ISGF3 translocates into the nucleus and binds to the IFN-stimulated response elements (ISREs) in the promoters of genes, thereby promoting the transcription of IFN-stimulated genes (ISGs) (8). JAKs also mediate the signaling pathways of various other cytokines, including interleukin-2 (IL-2), IL-4, IL-6, IL-7, IL-9, IL-10, IL-12, IL-13, IL-15, and IL-21 (9).
Numerous studies have shown that mutations in the STAT-related genes can lead to the occurrence of autoimmune diseases. For instance, gain-of-function (GOF) mutations in STAT1 amplify IFN-α/β signaling, predisposing to disorders ranging from autoimmune thyroiditis to systemic lupus erythematosus (10, 11). In thyroiditis, infiltrating pro-inflammatory cytokines (e.g., TNF-α and IFN-γ) induce inflammasome activation and thyrocyte apoptosis (12). Similarly, the STAT3 P471R variant hyperactivates the T helper 17 (Th17) pathway, increasing IL-17 production and triggering autoimmunity (13). Conversely, STAT5B deficiency impairs regulatory T-cell function, facilitating multi-organ autoimmune manifestations such as eczema, juvenile idiopathic arthritis, and immune thrombocytopenia (14). A meta-analysis further implicates the STAT4 rs7574865 T-allele in the increased risk of SSc and antiphospholipid syndrome (15).
The onset of SSc is associated with the abnormal increase of cytokines. An elevated IL-4 is a key mediator. It synergizes with IL-13 and transforming growth factor beta (TGF-β) to amplify the inflammatory and pro-fibrotic responses, facilitating the pathogenic T cell-fibroblast crosstalk (16, 17). Phosphorylated STAT1/3 levels increase in peripheral blood T cells and monocytes, and cells with STAT3 phosphorylation are also found in skin sections of patients with SSc (18). Such IL-4-driven signaling promotes fibrosis via JAK/STAT transcriptional programs. The level of IFN-I are also associated with severe cutaneous, pulmonary, and musculoskeletal involvement in patients with SSc (19). Critically, the anti-IFNAR monoclonal antibody anifrolumab suppresses disease activity (20), confirming the central role of IFN-I in SSc.
IFN-γ plays a central role in the pathogenesis of vitiligo. IFN-γ acts on keratinocytes to induce the secretion of the serum chemokines CXC ligand 9 (CXCL9) and CXCL10. These chemokines recruit the CXC receptor 3 (CXCR3) CD8+ T cells. The activated CXCR3 CD8+ T cells can induce melanocyte apoptosis and IFN-γ secretion (21).
IFN-I promote liver injury in both metabolic and cholestatic contexts. In fatty liver diseases, they amplify Toll-like receptor 4 signaling in macrophages and recruit CD8+ T cells (22, 23). In PBC, portal tract immune cells overexpress IFN-I (24), indicating a common effector mechanism.
The involvement of the JAK/STAT signaling pathway in the pathogenesis of T2DM is increasingly recognized. Genetic variants in JAK2 exemplify this link: the allele/genotype frequencies of rs10974914 and rs10815157 differ significantly between diabetic and control cohorts, with the rs10974914-AA genotype increasing the T2DM risk while the rs10815157-C allele conferring protection (25). Mechanistically, JAK2 overexpression amplifies the insulin promoter activity (26), whereas phosphorylated STAT3 impairs the insulin signaling and glucose uptake in skeletal muscle, driving insulin resistance (27). Complementary evidence implicates STAT4 polymorphisms (e.g., rs7574865) in T2DM susceptibility among Chinese Han populations (28).
4 Conclusion
These cases underscore that, in clinical diagnosis and treatment, it is important to closely observe the symptoms and signs of patients with familial T2DM and to pay attention to the autoimmune manifestations for early diagnosis and treatment. The association of T2DM and autoimmune diseases with the JAK/STAT signaling pathway has been well studied, and patients with T2DM combined with autoimmune diseases may have specific JAK/STAT variants. The characteristics of these cases suggest a possible genetic predisposition, and genetic testing of these patients could help identify potential genetic risks. Neither patient underwent genetic testing. Future research could investigate the association of specific JAK/STAT variants with T2DM complicated with autoimmune diseases through targeted genetic screening.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Tianyou Hospital Affiliated to Wuhan University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XS: Investigation, Conceptualization, Writing – original draft. YY: Supervision, Writing – review & editing. HJ: Writing – original draft. XL: Writing – original draft. YQ: Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1599546/full#supplementary-material
References
1. Zhu Y, Wang W, and Li Q. Research progress on genetic predisposition and associated genes of type 2 diabetes. Chin J Prev Contr Chron Dis. (2015) 23(3):229–32.
2. Qiu J, Xiao Z, Zhang Z, Luo S, and Zhou Z. Latent autoimmune diabetes in adults in China. Front Immunol. (2022) 13:977413. doi: 10.3389/fimmu.2022.977413
3. Wang Y and Leng J. The relationship between JAK-STAT signaling pathway and painful diabetic peripheral neuropathy. Chin J Diffic and Compl Cas. (2018) 17:1176–8.
4. Gao Q, Liang X, Shaikh AS, Zang J, Xu W, and Zhang Y. JAK/STAT signal transduction: promising attractive targets for immune, inflammatory and hematopoietic diseases. Curr Drug Targets. (2018) 19:487–500. doi: 10.2174/1389450117666161207163054
5. Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, and Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell communication Signaling. (2017) 15:1–3. doi: 10.1186/s12964-017-0177-y
6. Li S, Gong M, Zhao F, Shao J, Xie Y, Zhang Y, et al. Type I interferons: Distinct biological activities and current applications for viral infection. Cell Physiol Biochem. (2018) 51:2377–96. doi: 10.1159/000495897
7. Platanias LC. Mechanisms of type-i- and type-II-interferon-mediated signalling. Nat Rev Immunol. (2005) 5:375–86. doi: 10.1038/nri1604
8. Lukhele S, Boukhaled GM, and Brooks DG. Type I interferon signaling, regulation and gene stimulation in chronic virus infection. Semin Immunol. (2019) 43:101277. doi: 10.1016/j.smim.2019.05.001
9. Stark GR, Cheon H, and Wang Y. Responses to cytokines and interferons that depend upon jaks and stats. Cold Spring Harbor Perspect Biol. (2017) 10. doi: 10.1101/cshperspect.a028555
10. Boisson-Dupuis S, Kong XF, Okada S, Cypowyj S, Puel A, Abel L, et al. Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr Opin Immunol. (2012) 24:364–78. doi: 10.1016/j.coi.2012.04.011
11. Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood J Am Soc Hematol. (2016) 127:3154–64. doi: 10.1182/blood-2015-11-679902
12. Guo Q, Wu Y, Hou Y, Liu Y, Liu T, Zhang H, et al. Cytokine secretion and pyroptosis of thyroid follicular cells mediated by enhanced NLRP3, NLRP1, NLRC4, and AIM2 inflammasomes are associated with autoimmune thyroiditis. Front Immunol. (2018) 9:1197. doi: 10.3389/fimmu.2018.01197
13. Wienke J, Janssen W, Scholman R, Spits H, van Gijn M, Boes M, et al. A novel human STAT3 mutation presents with autoimmunity involving Th17 hyperactivation. Oncotarget. (2015) 6:20037. doi: 10.18632/oncotarget.5042
14. Kanai T, Jenks J, and Nadeau KC. The STAT5b pathway defect and autoimmunity. Front Immunol. (2012) 3:234. doi: 10.3389/fimmu.2012.00234
15. Liang YL, Wu H, Shen X, Li PQ, Yang XQ, Liang L, et al. Association of STAT4 rs7574865 polymorphism with autoimmune diseases: a meta-analysis. Mol Biol Rep. (2012) 39:8873–82. doi: 10.1007/s11033-012-1754-1
16. Gasparini G, Cozzani E, and Parodi A. Interleukin-4 and interleukin-13 as possible therapeutic targets in systemic sclerosis. Cytokine. (2020) 125:154799. doi: 10.1016/j.cyto.2019.154799
17. Higashioka K, Kikushige Y, Ayano M, Kimoto Y, Mitoma H, Kikukawa M, et al. Generation of a novel CD30+ B cell subset producing GM-CSF and its possible link to the pathogenesis of systemic sclerosis. Clin Exp Immunol. (2020) 201:233–43. doi: 10.1111/cei.13477
18. Kitanaga Y, Imamura E, Nakahara Y, Fukahori H, Fujii Y, Kubo S, et al. In vitro pharmacological effects of peficitinib on lymphocyte activation: a potential treatment for systemic sclerosis with JAK inhibitors. Rheumatology. (2020) 59:1957–68. doi: 10.1093/rheumatology/kez526
19. Liu X, Mayes MD, Tan FK, Wu M, Reveille JD, Harper BE, et al. Correlation of interferon-inducible chemokine plasma levels with disease severity in systemic sclerosis. Arthritis Rheumatism. (2012) 65:226–35. doi: 10.1002/art.37742
20. Guo X, Higgs BW, Bay-Jensen AC, Karsdal MA, Yao Y, Roskos LK, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 MAB, anifrolumab, in adult patients with systemic sclerosis. J Invest Dermatol. (2015) 135:2402–9. doi: 10.1038/jid.2015.188
21. Zhao Y, Lu N, Wang X, Chen W, and Zhang L. Exploration of the immunological pathogenesis of vitiligo. Chin J Dermato Venerol Integ Trad W Med. (2022) 21:381–3.
22. Zhai Y, Qiao B, Gao F, Shen X, Vardanian A, Busuttil RW, et al. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. (2008) 47:199–206. doi: 10.1002/hep.21970
23. Ghazarian M, Revelo XS, Nøhr MK, Luck H, Zeng K, Lei H, et al. Type I interferon responses drive intrahepatic T cells to promote metabolic syndrome. Sci Immunol. (2017) 2. doi: 10.1126/sciimmunol.aai7616
24. Bae HR, Hodge DL, Yang G, Leung PSC, Chodisetti SB, Valencia JC, et al. The interplay of type I and type II interferons in murine autoimmune cholangitis as a basis for sex-biased autoimmunity. Hepatology. (2018) 67:1408–19. doi: 10.1002/hep.29524
25. Zhang Y, Lin C, Chen R, Luo L, Huang J, Liu H, et al. Association analysis of SOCS3, JAK2 and STAT3 gene polymorphisms and genetic susceptibility to type 2 diabetes mellitus in Chinese population. Diabetol Metab Syndrome. (2022) 14:4. doi: 10.1186/s13098-021-00774-w
26. Zhang Y, Zhou B, Deng B, Zhang F, Wu J, Wang Y, et al. Amyloid-β induces hepatic insulin resistance in vivo via JAK2. Diabetes. (2013) 62:1159–66. doi: 10.2337/db12-0670
27. Mashili F, Chibalin AV, Krook A, and Zierath JR. Constitutive STAT3 phosphorylation contributes to skeletal muscle insulin resistance in type 2 diabetes. Diabetes. (2013) 62:457–65. doi: 10.2337/db12-0337
Keywords: type 2 diabetes, hypothyroidism, Hashimoto’s thyroiditis, autoimmune disease, case report
Citation: Su X, Yang Y, Jiang H, Lai X and Qu Y (2025) A case report of familial type 2 diabetes mellitus combined with hypothyroidism and multiple autoimmune diseases. Front. Endocrinol. 16:1599546. doi: 10.3389/fendo.2025.1599546
Received: 25 March 2025; Accepted: 11 July 2025;
Published: 04 August 2025.
Edited by:
Chris Wincup, King’s College Hospital NHS Foundation Trust, United KingdomReviewed by:
Sanja Medenica, Clinical Center of Montenegro, MontenegroLu-Ting Wang, MacKay Children’s Hospital, Taiwan
Jiajia Ni, Shanghai University of Technology, China
Copyright © 2025 Su, Yang, Jiang, Lai and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Yang, eWFuZ3lhbmcxMDAzQHd1c3QuZWR1LmNu
 Xuan Su
Xuan Su Yang Yang1,2*
Yang Yang1,2*