- 1Faculty of Medicine, Mansoura University, Mansoura, Egypt
- 2Faculty of Medicine, Assiut University, Assiut, Egypt
- 3Faculty of Medicine, Hashemite University, Zarqa, Jordan
- 4College of Medicine, King Edward Medical University, Lahore, Pakistan
- 5Faculty of Medicine, Yarmouk University, Irbid, Jordan
- 6Faculty of Medicine, Zagazig University, Zagazig, Egypt
- 7Department of Medicine, Houston Methodist/Willowbrook Hospital, Houston, TX, United States
- 8Department of Medicine, Division of Endocrinology, Diabetes, and Metabolism, College of Medicine, University of Illinois Chicago, Chicago, IL, United States
- 9Department of Medicine, Jesse Brown Veterans Affairs Medical Center, Chicago, IL, United States
- 10Department of Kinesiology and Nutrition, College of Applied Health Sciences, University of Illinois Chicago, Chicago, IL, United States
Background: Ultra-rapid-acting insulin (URAI) improves glycemic control by reducing variability; however, optimal strategies for its use, especially within hybrid closed-loop (HCL) insulin delivery systems, remain unclear. This meta-analysis assesses the efficacy and safety of combining URAI with HCL systems in maintaining the euglycemic range and reducing glycemic excursions.
Methods: We systematically searched PubMed, Scopus, Cochrane Library, Web of Science, and related article citations for relevant studies. Outcomes assessed included time in range (TIR), time below range (TBR), and time above range (TAR) during overall 24-hour periods, daytime, nighttime, postprandial, and post-exercise periods, as well as adverse events. Dichotomous outcomes were summarized using risk ratios (RR), and continuous outcomes were pooled using mean differences (MD) presented with 95% confidence intervals (CI).
Results: URAI showed a modest, statistically non-significant improvement in TIR (70–180 mg/dL) compared to standard insulin (MD 0.87%, 95% CI [-0.21 to 1.85], P = 0.12). Importantly, glycemic variability significantly improved with URAI, as demonstrated by reductions in the coefficient of variation (CV) (MD -0.78%, 95% CI [-1.44 to -0.12], P = 0.02). The combination of URAI with HCL systems significantly reduced hypoglycemia (TBR <70 mg/dL: MD -0.32%, 95% CI [-0.56 to -0.13], P = 0.002). However, overall reductions in TAR >250 mg/dL and TAR >180 mg/dL were statistically non-significant.
Conclusion: The integration of URAI with HCL demonstrates encouraging improvements in glycemic outcomes, notably reduced glucose variability and nighttime hypoglycemia risk. However, further research with larger sample sizes is essential to confirm these benefits and establish broader clinical recommendations.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024594375, identifier CRD42024594375.
1 Introduction
The 21st century has seen an increase in the prevalence of diabetes, which has become a global public health concern. Once common in Western nations, diabetes has now struck globally, driven by widespread consumption of calorie-dense, nutrient-poor diets combined and increasingly sedentary lifestyles (1). According to the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019, approximately 460 million individuals across all age groups were affected by diabetes, according to estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019, ranking it as the seventh-leading cause of death and disability globally (2).
Recent technological advancements have significantly transformed diabetes management, notably through the development of automated insulin delivery (AID) systems designed to reduce disease burden and enhance glycemic control for individuals with type 1 diabetes (3). Among these innovations is the hybrid closed-loop (HCL) system, commonly referred to as an “artificial pancreas,” which integrates an insulin pump, a continuous glucose monitor (CGM), and a sophisticated algorithm running on a computer or smartphone (4). HCL systems, often integrated into AID devices, automatically determine and administer basal insulin doses, whereas mealtime insulin boluses require manual input regarding meal size and timing (5). Currently, these insulin administration systems represent the pinnacle of insulin delivery technology available for type 1 diabetes management, significantly improving glucose control and lowering hypoglycemia risk (6, 7). Commercially available HCL systems, such as the Medtronic 670G/780G, Tandem t:slim X2 Control IQ, and CamAPS FX systems, have obtained regulatory approval from the United States Food and Drug Administration (FDA) and European Conformity (CE) certification, supported by robust clinical trial data (8, 9).
Rapid-Acting Insulin Analogues (RAIAs) like aspart, lispro, and glulisine exhibit faster absorption kinetics compared to regular human insulin, yet managing optimal post-prandial glucose (PPG) remains challenging (10, 11). Consequently, newer ultra-rapid-acting insulins (URAIs) have been developed, including faster-acting insulin aspart (FIAsp; marketed as Fiasp, approved in 2017) and ultra-rapid lispro insulin (URLi; marketed as Lyumjev, approved in 2020) (12, 13).
Previous randomized controlled trials (RCTs) evaluating glycemic control with Fiasp and URLi in HCL systems have produced mixed results compared to conventional RAIAs (14–16). To address these inconsistencies, this systematic review and meta-analysis aim to comprehensively assess the effectiveness and safety of Fiasp and URLi within HCL systems among patients with type 1 diabetes.
2 Methods
2.1 Protocol registration
Our systematic review protocol was registered in PROSPERO (registration ID: CRD42024594375). This systematic review and meta-analysis were conducted following the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the Cochrane Handbook for Systematic Reviews and Meta-Analysis (17, 18).
2.2 Data sources & search strategy
We systematically searched the Web of Science, SCOPUS, PubMed (MEDLINE), and Cochrane Central Register of Controlled Trials (CENTRAL) databases from their inception through December 2024 without applying any search filters. The detailed approach and results are outlined in Supplementary Table 1.
2.3 Eligibility criteria
We included RCTs based on the following PICO criteria: Patients were individuals diagnosed with type 1 diabetes; interventions involved automated insulin delivery systems using URAIs (Lispro or Aspart); comparators were automated insulin delivery systems using standard insulin (Lispro or Aspart); outcomes focused on CGM data, specifically the time in range (TIR).
Studies were excluded if they met any of the following criteria (1): non-human or in vitro studies; (2) overlapping or duplicate datasets; (3) book chapters, reviews, commentaries, letters to the editor, or clinical guidelines; and (4) publications not available in English.
2.4 Study selection
Search results from all the databases were imported to Rayyan (19), and duplicates were manually removed. Four authors (A.N., M.M.A., A.S.G., and A.M.) independently screened the remaining articles, with disagreements resolved by a fifth reviewer (M.S.R.). The screening process consisted of two stages: initial assessment of titles and abstracts to identify relevant studies, followed by full-text screening to confirm eligibility according to predefined inclusion criteria for subsequent qualitative and quantitative analyses.
2.5 Data extraction
Data extraction was independently performed by four reviewers (A.N., M.M.A., A.S.G., and A.M.) using a standardized Excel template. Extracted information included study characteristics (study design, country, number of centers, study setting, total participants, population details, intervention type, comparators, and follow-up duration), baseline patient data (group sample sizes, age, BMI, sex, diabetes duration, HbA1c levels, and total daily insulin doses), and clinical outcomes (time in range [70–180 mg/dL and 70–140 mg/dL], time below range [<70 mg/dL and <54 mg/dL], time above range [>180 mg/dL and >250 mg/dL], total daily insulin dose, basal insulin dose, bolus insulin dose, coefficient of variation, standard deviation of sensor glucose, mean sensor glucose, severe hypoglycemia events, diabetic ketoacidosis incidents, and infusion site reactions).
The primary outcome of this meta-analysis was time in range (70–180 mg/dL), as assessed by continuous glucose monitoring (CGM) data. Secondary outcomes included time in range (70–140 mg/dL), time below range at thresholds <70 mg/dL and <54 mg/dL, and time above range at thresholds >180 mg/dL and >250 mg/dL. Additional glycemic control measures included mean glucose, glucose standard deviation (SD), and coefficient of variation (CV). We also assessed total daily insulin dose (TDD) and the frequency of adverse events (hypoglycemia events, diabetic ketoacidosis incidents, infusion site reactions, and device-related adverse events) to evaluate treatment safety. Any disagreements among reviewers were resolved through consensus discussions.
2.6 Risk of bias and certainty of evidence
Four reviewers (A.N., M.M.A., A.S.G., and A.M.) independently evaluated the methodological quality of included studies using the Cochrane Risk of Bias 2 (ROB2) tool (20). Assessments covered potential biases attributed to the randomization process, deviations from intended interventions, missing outcomes, measuring outcomes, and selective reporting of results. Each outcome was assessed individually, with all decisions clearly justified and documented. Any discrepancies between reviewers were resolved through discussion and consensus. To appraise the quality of evidence, we utilized the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines (21, 22). Any discrepancies were settled through discussion.
2.7 Statistical analysis
Statistical analyses were performed using RevMan version 4.5.1 software (23). Study results were pooled using risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, both presented with 95% confidence intervals (CI). A random-effects model was utilized when significant heterogeneity was identified (I2 > 50% detected using the Chi-square and I2 tests); otherwise, a fixed-effect model was used. Sensitivity analyses were conducted to investigate and resolve identified heterogeneity. We used the available data in the trials, and when both intention-to-treat (ITT) and per-protocol (PP) analyses were reported, we prioritized the ITT data. Median and interquartile range data were converted to means and standard deviations using the Meta-Analysis Accelerator calculator (24). Meta-regression analysis was performed when at least ten studies reported on a specific outcome and moderator (25) using OpenMeta (Analyst) software. An omnibus p-value of <0.05 indicated a statistically significant association (26). Subgroup analyses were carried out whenever feasible. Publication bias was evaluated for primary outcomes reported by ten or more studies using funnel plots, with symmetrical distribution indicating a lower risk of publication bias (27).
3 Results
3.1 Literature search
A systematic search was conducted across four databases (PubMed, Scopus, Web of Science, and Cochrane Library), yielding 1845 articles, of which 330 duplicates were excluded. After duplicate removal, 1045 articles underwent title and abstract screening. Of these, 21 studies qualified for full-text assessment, resulting in the inclusion of 12 clinical trials and one secondary analysis. The study selection process is detailed in the PRISMA flow diagram (Figure 1).
3.2 Characteristics of included studies
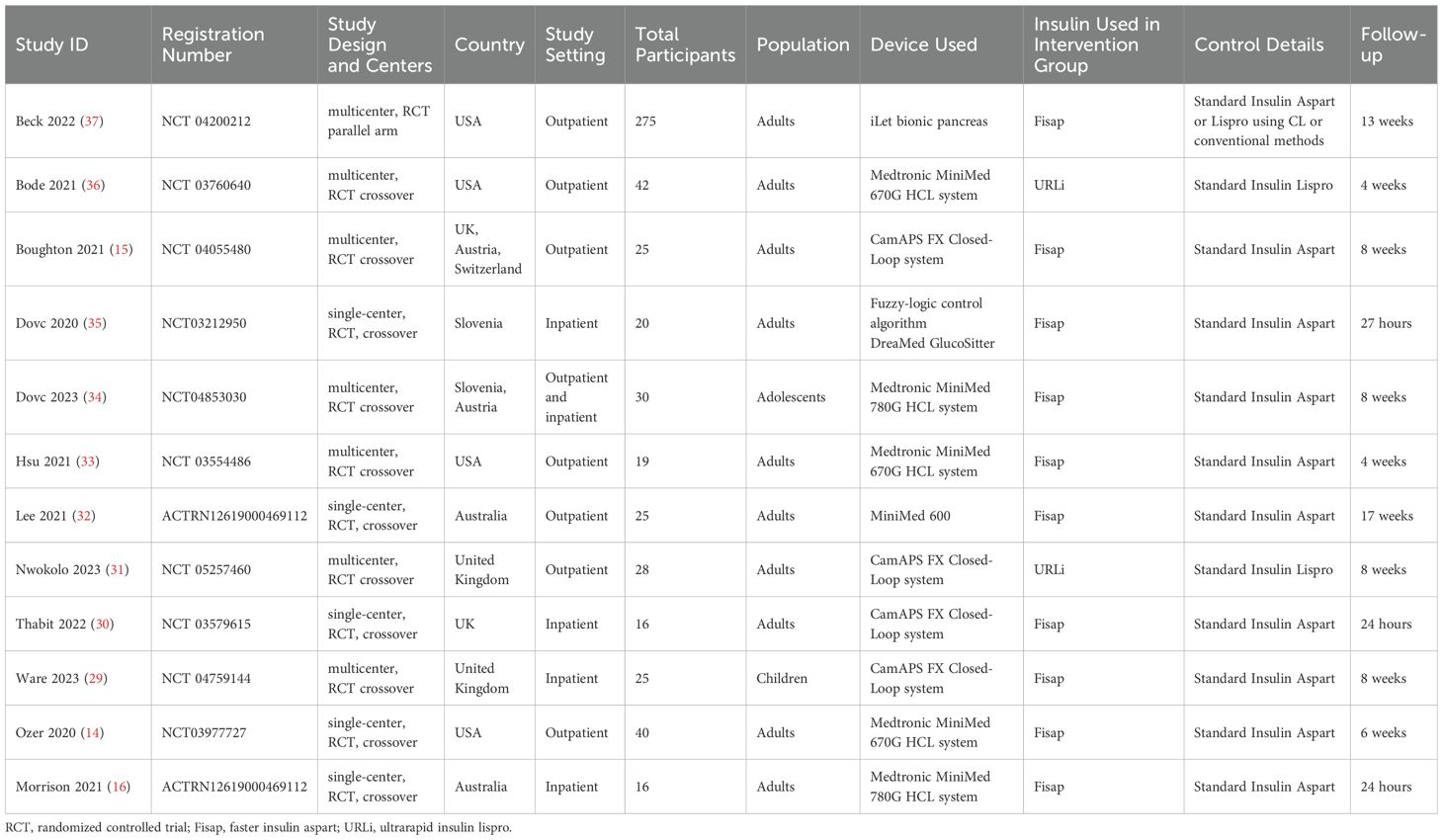
Of the 12 studies included in the review (14, 16, 28–37), only one was a parallel-arm randomized controlled trial (RCT) (Beck 2022) (37), while the remaining studies utilized a crossover design. Three trials were conducted in inpatient settings (Thabit 2022, Ware 2023, and Morrison 2021) (16, 29, 30). Two trials specifically involved children and adolescents (Dovc 2020, Ware 2023) (29, 35), whereas the others enrolled adult populations.
Regarding HCL systems, four trials employed the CamAPS FX system (Nwokolo 2023, Thabit 2022, Ware 2023, Boughton 2021) (15, 29–31), three utilized Medtronic 670G (Ozer 2020, Bode 2021, Hsu 2021) (14, 33, 36), and two evaluated Medtronic 780G (Morrison 2021, Dovc 2023) (16, 34). Two studies examined ultra-rapid insulin lispro (URLi) (Bode 2021, Nwokolo 2023) (31, 36), while the remaining studies assessed faster-acting insulin aspart (Fiasp). Follow-up durations across the studies ranged widely, from 24 hours to 17 weeks (Tables 1, 2).
3.3 Risk of bias assessment
For crossover trials, we considered potential carryover effects. All included crossover studies implemented appropriate washout periods or randomized sequence allocation to minimize such bias. Risk of bias specific to crossover designs was evaluated using the ROB2 model tailored for crossover trials. Among the 11 crossover studies evaluated, they all demonstrated a low risk of bias across all domains, including randomization processes, carryover effects, deviations from intended interventions, missing outcome data, measuring of the outcomes, and selective result reporting, except for Ozer 2020. The study by Ozer et al. (14) raised concerns regarding the randomization method, as details about randomization procedures and allocation concealment were not reported. Additionally, Beck 2020 presented some concerns related to deviations from intended interventions due to an increased number of unscheduled visits in the intervention group during the COVID-19 pandemic (Supplementary Figure 1). A GRADE evidence profile demonstrates the certainty of evidence in Table 3.
3.4 Primary outcomes
3.4.1 Time in range
Pooled analysis of TIR within the 70–180 mg/dL range showed a slight, non-significant improvement in response to ultra-rapid insulin [mean difference (MD) 0.87%, 95% CI (-0.21 to 1.85), P = 0.12, I² = 0%] compared to standard insulin (Figure 2a). Analysis of TIR within the tighter range of 70–140 mg/dL also indicated no significant improvement [MD 0.65%, 95% CI (-0.7 to 2.0), P = 0.35, I² = 0%] (Figure 2b). Similarly, subgroup analyses for daytime and nighttime TIR (70–180 mg/dL) revealed statistically non-significant changes in ultra-rapid insulin users during daytime [MD 0.97%, 95% CI (-0.8 to 2.74), P = 0.28, I² = 61%] but a significant difference during nighttime [MD -1.9%, 95% CI (-2.47 to -1.33), P = 0.0001, I² = 13%] (Figures 2c, d). However, removing the Bode 2022 study resolved heterogeneity and indicated a significant improvement for daytime TIR (70–180 mg/dL) with ultra-rapid insulin [MD 2.05%, 95% CI (0.55 to 3.56), P = 0.007, I² = 0%] (Supplementary Figure 2).

Figure 2. Forest plots of time in range (TIR): (a) All day TIR 70-180 mg/dl, (b) TIR 70-140 mg/dl, (c) Daytime TIR 70-180 mg/dl, (d) TIR 70-180 nighttime.
Meta-regression analysis assessing the influence of diabetes duration and HbA1c levels on TIR (70–180 mg/dL) revealed no statistically significant associations (p > 0.05) (Supplementary Table 2, Supplementary Figures 3, 4). Assessment of publication bias demonstrated a symmetrical distribution of the effect size, suggesting minimal risk of publication bias (Supplementary Figure 5).
3.5 Secondary outcomes:
3.5.1 Time below range
Analysis of TBR <54 mg/dL showed a slight but non-significant reduction with ultra-rapid insulin [MD -0.05%, 95% CI (-0.11 to 0.01), P = 0.11, I² = 0%] (Figure 3a). However, daytime and nighttime TBR <54 mg/dL percentages were significantly reduced in ultra-rapid insulin users [daytime: MD -0.1%, 95% CI (-0.13 to -0.06), P = 0.0001, I² = 0%; nighttime: MD 0.09%, 95% CI (0.02 to 0.16), P = 0.01, I² = 0%] (Supplementary Figures 6a, b). For TBR <70 mg/dL, the meta-analysis demonstrated a significant overall reduction with ultra-rapid insulin [MD -0.34%, 95% CI (-0.56 to -0.13), P = 0.002, I² = 0%] (Figure 3b). Daytime TBR <70 mg/dL showed a significant improvement with ultra-rapid insulin [MD -0.53%, 95% CI (-0.85 to -0.2), P = 0.002, I² = 50%] (Supplementary Figure 7a), whereas nighttime data revealed no significant difference [MD 0.13%, 95% CI (-0.43 to 0.69), P = 0.65, I² = 86%] (Supplementary Figure 7b).

Figure 3. Forest plots of Time below range (TBR): (a) All day TBR 54 mg/dl, (b) All day TBR 70 mg/dl.
Sensitivity analysis indicated that excluding the Bode 2022 study eliminated heterogeneity in daytime TBR <70 mg/dL, resulting in continued significance favoring ultra-rapid insulin [MD -0.31%, 95% CI (-0.61 to -0.01), P = 0.04, I² = 0%] (Supplementary Figure 8). Similarly, removing Boughton 2021 from nighttime TBR <70 mg/dL resolved heterogeneity and shifted results significantly toward ultra-rapid insulin [MD -0.3%, 95% CI (-0.45 to -0.16), P = 0.0001, I² = 0%] (Supplementary Figure 9).
Meta-regression analyses assessing the influence of diabetes duration and HbA1c levels on TBR <70 mg/dL revealed no significant associations (p > 0.05) (Supplementary Table 1, Supplementary Figures 10, 11). Assessment for publication bias showed an asymmetrical distribution, indicating possible publication bias (Supplementary Figure 12).
3.5.2 Time above range
Analysis of TAR >250 mg/dL and TAR >180 mg/dL demonstrated non-significant overall reductions with ultra-rapid insulin [TAR >250 mg/dL: MD -0.36%, 95% CI (-1.24 to 0.52), P = 0.42, I² = 48%; TAR >180 mg/dL: MD -0.34%, 95% CI (-1.48 to 0.79), P = 0.56, I² = 0%] (Figures 4a, b). Daytime TAR >250 mg/dL also showed no significant difference [MD -1.42%, 95% CI (-3.9 to 1.07), P = 0.26, I² = 96%] (Supplementary Figure 13a), while nighttime TAR >250 mg/dL was significantly higher with ultra-rapid insulin [MD 0.35%, 95% CI (0.11 to 0.5), P = 0.005, I² = 0%] (Supplementary Figure 13b). Analysis of daytime TAR >180 mg/dL revealed no significant difference [MD -0.29%, 95% CI (-2.06 to 1.48), P = 0.75, I² = 58%] (Supplementary Figure 14a), whereas nighttime TAR >180 mg/dL significantly increased in ultra-rapid insulin users compared to standard insulin [MD 2.19%, 95% CI (1.66 to 2.72), P = 0.0001, I² = 30%] (Supplementary Figure 14b).

Figure 4. Forest plots of Time above range (TAR): (a) All day TAR 250 mg/dl, (b) All day TAR 180 mg/dl.
Sensitivity analysis indicated that removing the Dovc 2023 study resolved heterogeneity for TAR >250 mg/dL [MD -0.22%, 95% CI (0.75 to 0.32), P = 0.43, I² = 0%] (Supplementary Figure 15). Exclusion of Bode 2022 resolved heterogeneity resulting in lower daytime TAR >180 mg/dL in ultra-rapid insulin users [MD -1.4%, 95% CI (-2.96 to 0.16), P = 0.08, I² = 0%] (Supplementary Figure 16).
Meta-regression analysis found no significant relationship between TAR >180 mg/dL and diabetes duration or HbA1c levels (Supplementary Table 2, Supplementary Figures 17, 18). Publication bias evaluation for TAR >180 mg/dL using funnel blot showed an asymmetrical distribution of effect size, suggesting the presence of publication bias (Supplementary Figure 19).
3.5.3 Glycemic variability
Ultra-rapid insulin showed non-significant reductions in mean glucose levels compared to standard insulin across all-day [MD -0.28 mg/dL, 95% CI (-0.95 to 0.39), P = 0.42, I² = 0%], daytime [MD -1.42 mg/dL, 95% CI (-3.97 to 1.13), P = 0.27, I² = 0%], and nighttime periods [MD 23.59 mg/dL, 95% CI (-4.19 to 51.36), P = 0.10, I² = 99%] (Figure 5a, Supplementary Figures 20a, b). Similarly, glucose standard deviation (SD) showed a non-significant downward trend [MD -1.48, 95% CI (-3.03 to 0.08), P = 0.06, I² = 0%] (Figure 5b). However, the coefficient of variation (CV) demonstrated a significant improvement with ultra-rapid insulin for both all-day [MD -0.78, 95% CI (-1.44 to -0.12), P = 0.02, I² = 0%] and daytime periods [MD -1.08, 95% CI (-2.07 to -0.08), P = 0.03, I² = 24%] (Figures 5c, Supplementary Figures 21a), though nighttime CV showed no significant differences [MD -0.71, 95% CI (-1.78 to 0.35), P = 0.19, I² = 0%] (Supplementary Figure 21b).

Figure 5. Forest plots of glycemic variability: (a) All day mean glucose, (b) Standard deviation of glucose, (c) All day glucose coefficient variation (CV).
Heterogeneity in nighttime mean glucose levels was resolved upon excluding the Ware 2023 study [MD 0.1 mg/dL, 95% CI (-1.03 to 1.24), P = 0.86, I² = 0%] (Supplementary Figure 22). Removing Beck 2022 from daytime CV resolved the heterogeneity and changed results to non-significance [MD -0.4 mg/dl, 95% CI [-1.57 to 0.77], P = 0.5, I2 = 0%] (Supplementary Figure 23). Meta-regression analyses showed no significant relationships between mean glucose levels and either diabetes duration or HbA1c levels (Supplementary Table 2, Supplementary Figures 24, 25). Funnel plot assessment for publication bias in mean glucose outcomes revealed an asymmetrical distribution of effect size, indicating possible publication bias (Supplementary Figure 26).
3.5.4 Insulin total daily insulin dose
Ultra-rapid insulin demonstrated a trend toward reducing total daily insulin doses, approaching statistical significance [MD 0.52 Units, 95% CI (-0.03 to 1.07), P = 0.07, I² = 0%] (Figure 6a). Bolus insulin doses were significantly lower in ultra-rapid insulin users [MD 0.87 Units, 95% CI (0.48 to 1.26), P = 0.0001, I² = 0%] (Figure 6b). Additionally, basal insulin delivery was significantly reduced among ultra-rapid insulin users [MD -0.4 Units, 95% CI (-0.68 to -0.11), P = 0.006, I² = 0%] (Figure 6c).

Figure 6. Forest plots of Insulin dose: (a) Total daily insulin dose (TDD), (b) Bolus insulin dose, (c) Basal insulin dose.
3.5.5 Adverse events
Regarding safety outcomes, the overall frequency of adverse events was significantly higher with ultra-rapid insulin [Risk Ratio (RR) 1.38, 95% CI (1.21 to 1.69), P = 0.002, I² = 21%] (Supplementary Figure 27a). Infusion site reactions also occurred more frequently in the ultra-rapid insulin group [RR 2.77, 95% CI (1.5 to 5.12), P = 0.001, I² = 29%] (Supplementary Figure 27b). Conversely, no significant differences were observed between ultra-rapid and standard insulin groups regarding hypoglycemic events [RR 0.35, 95% CI (0.10 to 1.29), P = 0.12], diabetic ketoacidosis (DKA) events [RR 4.70, 95% CI (0.23 to 96.7), P = 0.32], or device-related events [RR 1.24, 95% CI (0.87 to 1.77), P = 0.23, I² = 19%] (Supplementary Figures 27c–e).
3.5.6 Post-meal glycemic control
During the two-hour post-lunch period, ultra-rapid insulin did not significantly reduce blood glucose incremental area under the curve (IAUC) [MD -30.1, 95% CI (-68.45 to 8.24), P = 0.12, I² = 0%] (Supplementary Figure 28a). However, ultra-rapid insulin significantly lowered mean glucose levels within two hours following breakfast [MD -57.57 mg/dL, 95% CI (-108.74 to -6.39), P = 0.03, I² = 65%], but not after dinner [MD -33.06 mg/dL, 95% CI (-74.43 to 8.32), P = 0.12, I² = 4%] (Supplementary Figures 28b, c). Furthermore, the four-hour blood glucose area under the curve (AUC) following lunch did not significantly differ between ultra-rapid insulin and standard insulin [MD -42.97, 95% CI (-128.27 to 42.33), P = 0.32, I² = 9%] (Supplementary Figure 29a). Similar non-significant findings were observed for breakfast [MD -9.27, 95% CI (-84.05 to 65.5), P = 0.81, I² = 0%] and dinner [MD -42.97, 95% CI (-128.27 to 42.33), P = 0.32, I² = 9%] (Supplementary Figures 29b, c).
Regarding post-meal TIR (70–180 mg/dL), ultra-rapid acting insulin showed no significant improvement compared to standard insulin after lunch [MD 0.9%, 95% CI (-3.0 to 4.8), P = 0.65, I² = 0%], breakfast [MD 3.94%, 95% CI (-0.7 to 8.58), P = 0.10, I² = 34%], or dinner [MD 1.42%, 95% CI (-2.78 to 5.62), P = 0.51, I² = 71%] (Supplementary Figures 30a–c). Similarly, ultra-rapid insulin did not significantly reduce post-meal TBR <70 mg/dL at lunch [MD 0.55%, 95% CI (-0.47 to 1.56), P = 0.29, I² = 0%], breakfast [MD 0.35%, 95% CI (-0.51 to 1.2), P = 0.43, I² = 52%], or dinner [MD 0.34%, 95% CI (-0.71 to 1.0), P = 0.52, I² = 7%] (Supplementary Figures 31a–c). Additionally, ultra-rapid insulin showed non-significant reductions in TAR >180 mg/dL following lunch [MD -1.35%, 95% CI (-5.63 to 2.94), P = 0.54, I² = 0%], breakfast [MD -3.47%, 95% CI (-11.39 to 4.44), P = 0.39, I² = 62%], and dinner [MD -1.41%, 95% CI (-9.54 to 6.73), P = 0.73, I² = 67%] (Supplementary Figures 32a–c).
3.5.7 Post-exercise glycemic control
Ultra-rapid acting insulin showed no significant improvement in TIR (70–180 mg/dL) within two hours post-exercise [MD 3.77%, 95% CI [-9.37 to 1.84], P = 0.19, I2 = 99%] (Supplementary Figure 33a). Similarly, there were no significant differences between ultra-rapid and standard insulin regarding mean glucose levels [MD 0.34 mg/dl, 95% CI [-0.71 to 1.39], P = 0.52, I2 = 0%] (Supplementary Figure 33b), TBR (<70 mg/dl) [MD 0.4%, 95% CI [-0.64 to 1.44], P = 0.5, I2 = 0%] (Supplementary Figure 33c), TAR (>180 mg/dl) [MD 0%, 95% CI [-8.95 to 8.95], P = 1%] (Supplementary Figure 33d), or TAR (>250 mg/dl) [MD -2%, 95% CI [-4.33 to 0.33], P = 0.09] (Supplementary Figure 33e). Sensitivity analysis removing Morrison 2022 resolved heterogeneity for TIR (70–180 mg/dL) post-exercise, resulting in outcomes that no longer favored ultra-rapid insulin [MD -2.37%, 95% CI [-9.86 to 5.12], P = 0.54. I2 = 0] (Supplementary Figure 34).
3.5.8 Psychological outcomes
Ultra-rapid-acting insulin did not significantly influence psychological outcomes as measured by the Hypoglycemia Fear Scale [MD 0.11, 95% CI [-3.68 to 3.89], P = 0.96, I2 = 0%] (Supplementary Figure 35a). On the other hand, Insulin Delivery Systems: Perspectives, Ideas, Reflections, and Expectations [INSPIRE score] showed a near-significant increase among ultra-rapid insulin users [MD 3.98, 95% CI [-0.24 to 8.2], P = 0.06, I2 = 20] (Supplementary Figure 35b).
3.6 Subgroup analysis
We conducted subgroup analyses based on study location, population subgroups, device type, insulin type, and study duration. Subgroup analysis for closed-loop devices showed no significant changes in TIR (70-180 mg/dl) among patients using the CamAPX FX [MD 0.56%, 95% CI (-2.11, 3.22), P=0.68, I²=0%], Medtronic 670G [MD -0.23%, 95% CI (-2.52, 2.06), P=0.85, I²=28%], or Medtronic 870G [MD 1.31%, 95% CI (-1.83, 4.45), P=0.41, I²=0%] devices (Supplementary Figure 36). In insulin subgroup analyses, Fiasp showed a borderline significant increase in TIR (70-180 mg/dl) [MD 1.20%, 95% CI (-0.04, 2.43), P=0.06, I²=0%], while URLi demonstrated no significant effect [MD -0.19%, 95% CI (-2.43, 2.05), P=0.87, I²=20%]; the difference between these two insulins was also not significant (Supplementary Figure 37). Additional details can be found in Supplementary Table 3.
4 Discussion
Our systematic review and meta-analysis comprehensively assessed the impact of integrating ultra-rapid-acting insulin (URAI) with hybrid closed-loop (HCL) systems, providing a direct comparison to standard insulin across key glycemic metrics and safety outcomes, including TIR, TBR, and TAR metrics, mean glucose levels, glucose variability, insulin dosage, adverse events, hypoglycemia, and DKA. Although our findings indicated no significant differences between URAI and standard insulin in terms of TIR (both 70-180 mg/dL and 70-140 mg/dL) or TAR (both >180 mg/dL and >250 mg/dL), URAI demonstrated a clinically meaningful advantage by significantly reducing the time spent in mild hypoglycemia (<70 mg/dL), with a mean difference of 0.33%. Moreover, URAI significantly lowered glucose variability (coefficient of variation) by an average of 0.79%, suggesting more stable glycemic control. Conversely, the mean glucose level and total daily insulin dose did not differ significantly between the groups. Importantly, adverse events, particularly infusion site reactions, occurred significantly more frequently with URAI, underscoring the necessity for clinicians to balance the glycemic benefits of URAI against the increased risk of local adverse reactions. However, reassuringly, rates of severe hypoglycemia, DKA, and related serious events were not increased with URAI. These insights highlight the potential clinical utility of URAI in enhancing glycemic stability while emphasizing careful monitoring of infusion-site tolerability during clinical application.
A previous meta-analysis by Stamati et al. (11) evaluating URAI, specifically Fiasp, and URLi, in patients with T1DM utilizing continuous subcutaneous insulin infusion (CSII) systems reported the superiority of URAI analogs over rapid-acting insulin analogs (RAIAs) in increasing time spent within normoglycemia (70–180 mg/dL). This finding contrasts with the results of our current analysis. However, consistent with our findings, their analysis demonstrated a significant reduction in time spent in hypoglycemia (<70 mg/dL) alongside an increased incidence of infusion site reactions in the URAI group. Notably, Stamati’s meta-analysis did not clearly define statistical significance nor explicitly include hybrid closed-loop (HCL) systems, limiting direct comparability with our study.
Additionally, two simultaneous but separate meta-analyses individually assessing Fiasp and URLi against rapid insulin analogs or placebo reported no discernible differences in TIR or mean glucose levels. Interestingly, URLi was associated with fewer hypoglycemic events but a higher frequency of infusion site reactions, whereas Fiasp showed no notable differences in adverse events compared to rapid aspart insulin (38, 39). Our results differ from these later studies primarily due to methodological variations, including individual analyses of each URAI and the inclusion of placebo-controlled trials in these two meta-analyses. Furthermore, neither study specifically evaluated URAI performance within HCL systems, emphasizing the uniqueness and clinical relevance of our analysis to real-world applications and device-specific glycemic outcomes.
Given their unique pharmacokinetic profiles, the advent of faster insulin analogs (URAI) was anticipated to substantially enhance closed-loop system performance by accelerating insulin action onset and offset following delivery, thus enabling better glycemic control (40). Contrary to these expectations, our meta-analysis indicated that URAI’s primary advantages were limited to significant reductions in time spent below the glycemic target range and a notable improvement in glucose variability, as reflected by the reduced coefficient of variation. However, these benefits were offset by an increased frequency of adverse events, particularly infusion site reactions. Notably, our findings challenge prior predictions (11), demonstrating that URAI exacerbated, rather than reduced, infusion site complications in clinical practice. To address this limitation, ongoing research is exploring innovative strategies such as improving insulin delivery techniques through the implementation of infusion site modifications, including adjusting the insertion angle and the infusion protocols in AID systems and integrating novel technologies, including insulin infusion site warming devices, to enhance insulin absorption efficiency and minimize adverse reactions (41–43). These ongoing investigations underscore the critical need for continuous optimization of insulin delivery methods to fully harness the potential benefits of URAI within closed-loop systems.
To enhance clinical applicability and interpretation, our meta-analysis incorporated focused subgroup analyses of two prominent HCL systems extensively studied in individual trials: the Cam-APS FX Closed-Loop system and the Medtronic MiniMed 670G HCL system. These devices are among the earliest commercially approved and widely adopted technologies in diabetes management (44). Thus, their detailed performance evidence using URAI is required. Interestingly, despite the Cam-APS FX being the only licensed system to utilize ultra-rapid-acting insulin (URAI) analogs (45), our analysis uncovered a lack of substantial glycemic improvement coupled with a higher frequency of adverse events when using URAI analogs with this system. Conversely, the Medtronic MiniMed 670G demonstrated superior performance by achieving meaningful improvements in glycemic outcomes by reducing time spent in hypoglycemia (<70 mg/dl) and glucose variability without increasing adverse events such as hypoglycemia, DKA, or infusion site reactions. This finding is clinically significant, highlighting potential device-specific interactions with URAI that could influence both efficacy and safety profiles. These observations underscore the importance of device selection in clinical practice and call for further targeted research into optimizing URAI utilization within specific HCL systems to maximize patient outcomes and minimize potential risks.
We recognize several limitations in our current study that warrant consideration when interpreting the findings. Primarily, our conclusions rely heavily on the available primary evidence, which, despite an extensive and systematic literature review, is limited to a small number of RCTs with short duration and relatively small sample sizes, with only two RCTs having data for URLi. Most included studies used a crossover design, which may introduce carryover, period, or learning effects. While most trials implemented washout periods and proper randomization to minimize these biases, this design may limit generalizability and requires cautious interpretation. The included studies varied significantly in terms of quality and consistency, potentially affecting the robustness of our outcomes. Specifically, while we evaluated infusion site reactions as a safety outcome, the underlying mechanisms driving these adverse events remain unclear due to a lack of detailed reporting in the primary studies. Additionally, our analysis faced constraints in terms of generalizability, given the scarcity of data addressing the effects of URAI in patients with type 2 diabetes mellitus (T2DM) and pediatric populations; notably, we identified only two studies involving children and none involving patients with non-insulin-dependent diabetes. Thus, future research should prioritize the generation of robust real-world evidence from broader patient cohorts, including individuals with T2DM and pediatric groups. Detailed investigations into the long-term outcomes and patient-specific factors affecting efficacy and safety are crucial. Such studies will provide invaluable insights, enabling clinicians to make more informed decisions and ultimately optimizing the clinical application of URAI in hybrid closed-loop systems and enhancing patients’ treatment satisfaction and quality of life.
5 Conclusion
Our systematic review and meta-analysis demonstrated that the use of URAIs instead of standard insulin within HCL systems provides clinically meaningful improvements in glycemic control, particularly by significantly reducing nighttime hyperglycemia, both at moderate (TAR >180 mg/dL) and severe (TAR >250 mg/dL) levels. These findings have important clinical implications, suggesting that URAI may be particularly beneficial in optimizing overnight glycemic control, a period often challenging for patients with diabetes. Depending on our comparison between URAIs and standard insulin, individualized treatment selection is crucial; Despite offering improved glycemic stability and reduced hypoglycemia, the cost appears to be an increase in infusion site reactions, necessitating careful risk-benefit assessment. To further validate and expand upon these promising results, future research should prioritize conducting robust trials with larger patient cohorts, extended follow-up periods, and targeted inclusion of populations currently underrepresented in existing studies, notably individuals with type 2 diabetes mellitus and pediatric patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
MR: Investigation, Software, Conceptualization, Writing – review & editing, Writing – original draft, Formal analysis, Methodology, Data curation. RR: Methodology, Investigation, Writing – review & editing, Writing – original draft, Software, Formal analysis, Conceptualization. BE: Investigation, Writing – original draft, Formal analysis, Writing – review & editing, Data curation, Methodology, Conceptualization. BA: Writing – review & editing, Conceptualization, Writing – original draft, Formal analysis, Methodology, Data curation, Investigation. AN: Writing – review & editing, Formal analysis, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. MA: Data curation, Formal analysis, Conceptualization, Writing – review & editing, Methodology, Writing – original draft, Investigation. AG: Data curation, Conceptualization, Writing – original draft, Methodology, Investigation, Writing – review & editing, Formal analysis. AM: Conceptualization, Writing – review & editing, Methodology, Writing – original draft, Investigation, Data curation, Formal analysis. MM: Writing – original draft, Data curation, Methodology, Investigation, Writing – review & editing, Conceptualization, Formal analysis. BA: Data curation, Writing – original draft, Formal analysis, Methodology, Investigation, Conceptualization, Writing – review & editing. BL: Writing – original draft, Formal analysis, Writing – review & editing, Investigation, Methodology, Data curation, Conceptualization. AM: Supervision, Project administration, Validation, Writing – review & editing, Funding acquisition, Writing – original draft, Conceptualization, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the National Institute of Health grant number R01HL161386 (PI: AMM).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1600157/full#supplementary-material
References
1. Hruby A and Hu FB. The epidemiology of obesity: A big picture. Pharmacoeconomics. (2015) 33:673–89. doi: 10.1007/s40273-014-0243-x
2. Diseases GBD and Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
3. Jennings P and Hussain S. Do-it-yourself artificial pancreas systems: A review of the emerging evidence and insights for healthcare professionals. J Diabetes Sci Technol. (2020) 14:868–77. doi: 10.1177/1932296819894296
4. Kesavadev J, Saboo B, Krishna MB, and Krishnan G. Evolution of insulin delivery devices: from syringes, pens, and pumps to DIY artificial pancreas. Diabetes Ther. (2020) 11:1251–69. doi: 10.1007/s13300-020-00831-z
5. Daly A, Hartnell S, Boughton CK, and Evans M. Hybrid closed-loop to manage gastroparesis in people with type 1 diabetes: a case series. J Diabetes Sci Technol. (2021) 15:1216–23. doi: 10.1177/19322968211035447
6. Karageorgiou V, Papaioannou TG, Bellos I, Alexandraki K, Tentolouris N, Stefanadis C, et al. Effectiveness of artificial pancreas in the non-adult population: A systematic review and network meta-analysis. Metabolism. (2019) 90:20–30. doi: 10.1016/j.metabol.2018.10.002
7. Weisman A, Bai JW, Cardinez M, Kramer CK, and Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. (2017) 5:501–12. doi: 10.1016/S2213-8587(17)30167-5
8. Leelarathna L, Choudhary P, Wilmot EG, Lumb A, Street T, Kar P, et al. Hybrid closed-loop therapy: Where are we in 2021? Diabetes Obes Metab. (2021) 23:655–60. doi: 10.1111/dom.14273
9. Peacock S, Frizelle I, and Hussain S. A systematic review of commercial hybrid closed-loop automated insulin delivery systems. Diabetes Ther. (2023) 14:839–55. doi: 10.1007/s13300-023-01394-5
10. Home PD. The pharmacokinetics and pharmacodynamics of rapid-acting insulin analogues and their clinical consequences. Diabetes Obes Metab. (2012) 14:780–8. doi: 10.1111/j.1463-1326.2012.01580.x
11. Stamati A, Karagiannis T, Tsapas A, and Christoforidis A. Efficacy and safety of ultra-rapid insulin analogues in insulin pumps in patients with Type 1 Diabetes Mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract. (2022) 193:110144. doi: 10.1016/j.diabres.2022.110144
12. Administration USFaD. FIASP or insulin aspart (2019). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208751s010s011lbl.pdf. (Accessed March 3, 2025)
13. Administration USFaD. LYUMJEV (insulin lispro-aabc) injection, for subcutaneous or intravenous use (2020). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761109Orig1s000lbl.pdf. (Accessed March 3, 2025)
14. Ozer K, Cooper AM, Ahn LP, Waggonner CR, and Blevins TC. Fast acting insulin aspart compared with insulin aspart in the medtronic 670G hybrid closed loop system in type 1 diabetes: an open label crossover study. Diabetes Technol Ther. (2021) 23:286–92. doi: 10.1089/dia.2020.0500
15. Boughton CK, Hartnell S, Thabit H, Poettler T, Herzig D, Wilinska ME, et al. Hybrid closed-loop glucose control with faster insulin aspart compared with standard insulin aspart in adults with type 1 diabetes: A double-blind, multicentre, multinational, randomized, crossover study. Diabetes Obes Metab. (2021) 23:1389–96. doi: 10.1111/dom.14355
16. Morrison D, Zaharieva DP, Lee MH, Paldus B, Vogrin S, Grosman B, et al. Comparable glucose control with fast-acting insulin aspart versus insulin aspart using a second-generation hybrid closed-loop system during exercise. Diabetes Technol Ther. (2022) 24:93–101. doi: 10.1089/dia.2021.0221
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
18. Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, and Welch VA. Cochrane handbook for systematic reviews of interventions version 6.5 (updated august 2024). Cochrane. (2024) 2024. doi: 10.1002/9781119536604
19. Ouzzani M, Hammady H, Fedorowicz Z, and Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
20. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
21. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
22. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ, et al. What is “quality of evidence” and why is it important to clinicians? BMJ. (2008) 336:995–8. doi: 10.1136/bmj.39490.551019.BE
23. Dolinoy DC. The agouti mouse model: an epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr Rev. (2008) 66 Suppl 1:S7–11. doi: 10.1111/j.1753-4887.2008.00056.x
24. Abbas A, Hefnawy MT, and Negida A. Meta-analysis accelerator: a comprehensive tool for statistical data conversion in systematic reviews with meta-analysis. BMC Med Res Methodol. (2024) 24:243. doi: 10.1186/s12874-024-02356-6
25. Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI, Health Outcomes P, et al. Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract. (2009) 63:1426–34. doi: 10.1111/j.1742-1241.2009.02168.x
26. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, and Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Software. (2012) 49:1–15. doi: 10.18637/jss.v049.i05
27. Lin L and Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74:785–94. doi: 10.1111/biom.12817
28. Haliloglu B, Boughton CK, Lakshman R, Ware J, Nwokolo M, Thabit H, et al. Postprandial glucose excursions with ultra-rapid insulin analogs in hybrid closed-loop therapy for adults with type 1 diabetes. Diabetes Technol Ther. (2024) 26:449–56. doi: 10.1089/dia.2023.0509
29. Ware J, Allen JM, Boughton CK, Cezar A, Hartnell S, Wilinska ME, et al. Hybrid closed-loop with faster insulin aspart compared with standard insulin aspart in very young children with type 1 diabetes: A double-blind, multicenter, randomized, crossover study. Diabetes Technol Ther. (2023) 25:431–6. doi: 10.1089/dia.2023.0042
30. Thabit H, Mubita W, Rubio J, Karuppan M, Schofield J, Willinska ME, et al. Comparison of faster-acting aspart with insulin aspart under conditions mimicking underestimation or missed meal boluses in type 1 diabetes using closed-loop insulin delivery. Diabetes Obes Metab. (2023) 25:1121–4. doi: 10.1111/dom.14942
31. Nwokolo M, Lakshman R, Hartnell S, Alwan H, Ware J, Allen JM, et al. CamAPS FX hybrid closed-loop with ultra-rapid lispro compared with standard lispro in adults with type 1 diabetes: A double-blind, randomized, crossover study. Diabetes Technol Ther. (2023) 25:856–63. doi: 10.1089/dia.2023.0262
32. Lee MH, Paldus B, Vogrin S, Morrison D, Zaharieva DP, Lu J, et al. Fast-acting insulin aspart versus insulin aspart using a second-generation hybrid closed-loop system in adults with type 1 diabetes: A randomized, open-label, crossover trial. Diabetes Care. (2021). 44:2371–8. doi: 10.2337/figshare.14900547
33. Hsu L, Buckingham B, Basina M, Ekhlaspour L, von Eyben R, Wang J, et al. Fast-acting insulin aspart use with the miniMed(TM) 670G system. Diabetes Technol Ther. (2021) 23:1–7. doi: 10.1089/dia.2020.0083
34. Dovc K, Bergford S, Frohlich-Reiterer E, Zaharieva DP, Potocnik N, Muller A, et al. A comparison of faster insulin aspart with standard insulin aspart using hybrid automated insulin delivery system in active children and adolescents with type 1 diabetes: A randomized double-blind crossover trial. Diabetes Technol Ther. (2023) 25:612–21. doi: 10.1089/dia.2023.0178
35. Dovc K, Piona C, Yesiltepe Mutlu G, Bratina N, Jenko Bizjan B, Lepej D, et al. Faster compared with standard insulin aspart during day-and-night fully closed-loop insulin therapy in type 1 diabetes: A double-blind randomized crossover trial. Diabetes Care. (2020) 43:29–36. doi: 10.2337/dc19-0895
36. Bode B, Carlson A, Liu R, Hardy T, Bergenstal R, Boyd J, et al. Ultrarapid lispro demonstrates similar time in target range to lispro with a hybrid closed-loop system. Diabetes Technol Ther. (2021) 23:828–36. doi: 10.1089/dia.2021.0184
37. Beck RW, Russell SJ, Damiano ER, El-Khatib FH, Ruedy KJ, Balliro C, et al. A multicenter randomized trial evaluating fast-acting insulin aspart in the bionic pancreas in adults with type 1 diabetes. Diabetes Technol Ther. (2022) 24:681–96. doi: 10.1089/dia.2022.0167
38. Dutta D, Mohindra R, Mahajan K, and Sharma M. Performance of fast-acting aspart insulin as compared to aspart insulin in insulin pump for managing type 1 diabetes mellitus: A meta-analysis. Diabetes Metab J. (2023) 47:72–81. doi: 10.4093/dmj.2022.0035
39. Dutta D, Nagendra L, Bhattacharya S, and Sharma M. Efficacy and safety of ultra-rapid lispro insulin in managing type-1 and type-2 diabetes: A systematic review and meta-analysis. Indian J Endocrinol Metab. (2023) 27:467–75. doi: 10.4103/ijem.ijem_225_23
40. Boughton CK and Hovorka R. New closed-loop insulin systems. Diabetologia. (2021) 64:1007–15. doi: 10.1007/s00125-021-05391-w
41. Diaz-Balzac CA, Pillinger D, and Wittlin SD. Continuous subcutaneous insulin infusions: closing the loop. J Clin Endocrinol Metab. (2023) 108:1019–33. doi: 10.1210/clinem/dgac746
42. Gradel AKJ, Porsgaard T, Lykkesfeldt J, Seested T, Gram-Nielsen S, Kristensen NR, et al. Factors affecting the absorption of subcutaneously administered insulin: effect on variability. J Diabetes Res. (2018) 2018:1205121. doi: 10.1155/2018/1205121
43. Cengiz E, Weinzimer SA, Sherr JL, Tichy EM, Carria L, Cappiello D, et al. Faster in and faster out: accelerating insulin absorption and action by insulin infusion site warming. Diabetes Technol Ther. (2014) 16:20–5. doi: 10.1089/dia.2013.0187
44. Rodriguez-Sarmiento DL, Leon-Vargas F, and Garcia-Jaramillo M. Artificial pancreas systems: experiences from concept to commercialisation. Expert Rev Med Devices. (2022) 19:877–94. doi: 10.1080/17434440.2022.2150546
Keywords: closed-loop systems, ultra fast acting insulin analogs, type 1 diabetes, glucose variability, meta-analysis
Citation: Rakab MS, Rateb RM, Elsalakawi BH, Al Zoubi BM, Nazir A, Abu-Laila MM, Ghanem AS, Maamoun A, Mattar M, Ataallah B, Layden BT and Mahmoud AM (2025) Ultra-rapid lispro or fast-acting aspart compared to standard insulin lispro and aspart using closed-loop insulin therapy: a systematic review and meta-analysis of randomized control trials. Front. Endocrinol. 16:1600157. doi: 10.3389/fendo.2025.1600157
Received: 25 March 2025; Accepted: 22 May 2025;
Published: 06 June 2025.
Edited by:
Hamid Reza Baradaran, Iran University of Medical Sciences, IranReviewed by:
Benli Su, Second Hospital of Dalian Medical University, ChinaSeyyed Amir Yasin Ahmadi, Iran University of Medical Sciences, Iran
Copyright © 2025 Rakab, Rateb, Elsalakawi, Al Zoubi, Nazir, Abu-Laila, Ghanem, Maamoun, Mattar, Ataallah, Layden and Mahmoud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abeer M. Mahmoud, YW1haG1vNEB1aWMuZWR1
†These authors have contributed equally to this work
 Mohamed Saad Rakab
Mohamed Saad Rakab Rahma Mogahed Rateb2†
Rahma Mogahed Rateb2† Basel Hatem Elsalakawi
Basel Hatem Elsalakawi Abubakar Nazir
Abubakar Nazir Brian T. Layden
Brian T. Layden Abeer M. Mahmoud
Abeer M. Mahmoud