- 1Department of Nuclear Medicine, Nanjing BenQ Medical Center, The Affiliated BenQ Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Radiology, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
Background: The associations between high-density lipoprotein cholesterol (HDL-C) levels and the risk of sarcopenia are inconclusive. This study aimed to investigate the association between HDL-C levels and chest computed tomography (CT)-defined low muscle mass in older adults and its sex-related differences.
Methods: This prospective study involved 1995 participants aged ≥50 years. The muscle area of the bilateral erector spinae muscles was measured at the T12 level on a single CT image. Linear regression analysis was used to evaluate the effects of related factors on muscle area. Multivariate logistic regression and restricted cubic spline (RCS) analysis were used to analyze the relationships between HDL-C quartile and low muscle mass in all participants and in the male and female subgroups.
Results: An increased HDL-C level was associated with a greater risk of lower muscle area overall (β=-1.91, 95% CI: -2.95 to -0.87) and in male participants (β=-3.16, 95% CI: -4.70– -1.61), whereas no significant difference was found in the female subgroup (P > 0.05). A higher continuous HDL-C level was associated with a greater risk of low muscle mass in all participants (odds ratio (OR) =2.28, 95% confidence interval (CI): 1.51–3.45) and in the male subgroup (OR=3.28, 95% CI: 1.84–5.87) after adjustment for confounders, whereas no significant difference was found in the female subgroup (P>0.05). Furthermore, the RCS model showed similar results regarding the relationship between HDL-C levels and the risk of low muscle mass.
Conclusions: Higher HDL-C levels were associated with a significantly greater risk of low muscle mass, particularly in older male adults. HDL-C levels are useful in identifying older individuals who are at risk for low muscle mass.
Introduction
Sarcopenia is a progressive skeletal muscle disorder characterized by accelerated loss of muscle mass and function (1). Sarcopenia is an age-related process that commonly occurs in older adults and is affected by concurrent risk factors and genetic and lifestyle factors throughout the life course (2). Sarcopenia has been shown to predict negative outcomes, including increased risk of osteoporosis, falls and disability, cardiovascular disorders, diabetes, and even mortality (3). In the context of global aging, sarcopenia is becoming an important public health challenge.
Few studies have reported the association between high-density lipoprotein cholesterol (HDL-C) and the risk of sarcopenia (4, 5). Several studies have demonstrated that high levels of HDL-C increase the risk of sarcopenia (4, 5). However, a study reported that sarcopenic patients had significantly lower HDL-C levels than nonsarcopenic patients did (6). Furthermore, one study indicated that there was no significant difference in HDL-C levels between sarcopenia patients and nonsarcopenia patients (7). Those studies indicated that the association between HDL-C and sarcopenia was inconclusive.
In our country, CT lung cancer screening is recommended for subjects aged 50–80 years (8). Subjects at such ages are also susceptible to sarcopenia. It would be interesting to assess low muscle mass during CT chest scans in a timely manner. Computed tomography (CT) is an effective imaging technique for estimating muscle mass by measuring the skeletal muscle cross-sectional area (SMA) (9). However, few studies have reported that chest CT reveals sarcopenia or low muscle mass (10, 11). A recent study demonstrated that the SMA and SMA/height2 at the T12 level were strongly associated with whole-body skeletal muscle mass (BSM), revealing that they could be used as surrogates to diagnose sarcopenia (10). However, to our knowledge, studies assessing the associations between HDL-C and chest CT-defined low muscle mass and whether sex differences exist remain insufficient. Thus, this study aimed to explore the associations between HDL-C levels and chest computed tomography-defined low muscle mass in older adults and its sex-related differences.
Materials and methods
Study population
This was a cross-sectional study based on 1995 individuals who received routine chest CT scans for lung cancer screening at the Affiliated Hospital of Nanjing University of Chinese Medicine. All participants were aged ≥ 50 years. Individuals who had a history of malignant tumors, severe liver disease and renal dysfunction, rheumatic disease or thyroid disease, which may affect muscle or body metabolism, were excluded from this study. The study was approved by the Ethics Committee of the Affiliated Hospital of Nanjing University of Chinese Medicine. Written informed consent was waived because of the retrospective design.
Collection of general and blood test data
Information, including age, sex, alcohol consumption status, smoking status, and body mass index information, was collected from the electronic medical system. The following parameters were measured in blood samples after at least 8 h of fasting: high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), total cholesterol (TC), aspartate aminotransferase (AST), albumin, creatinine, and blood glucose. All of this information was collected from May 2023 to October 2023.
Measurement of muscle area on unenhanced chest CT
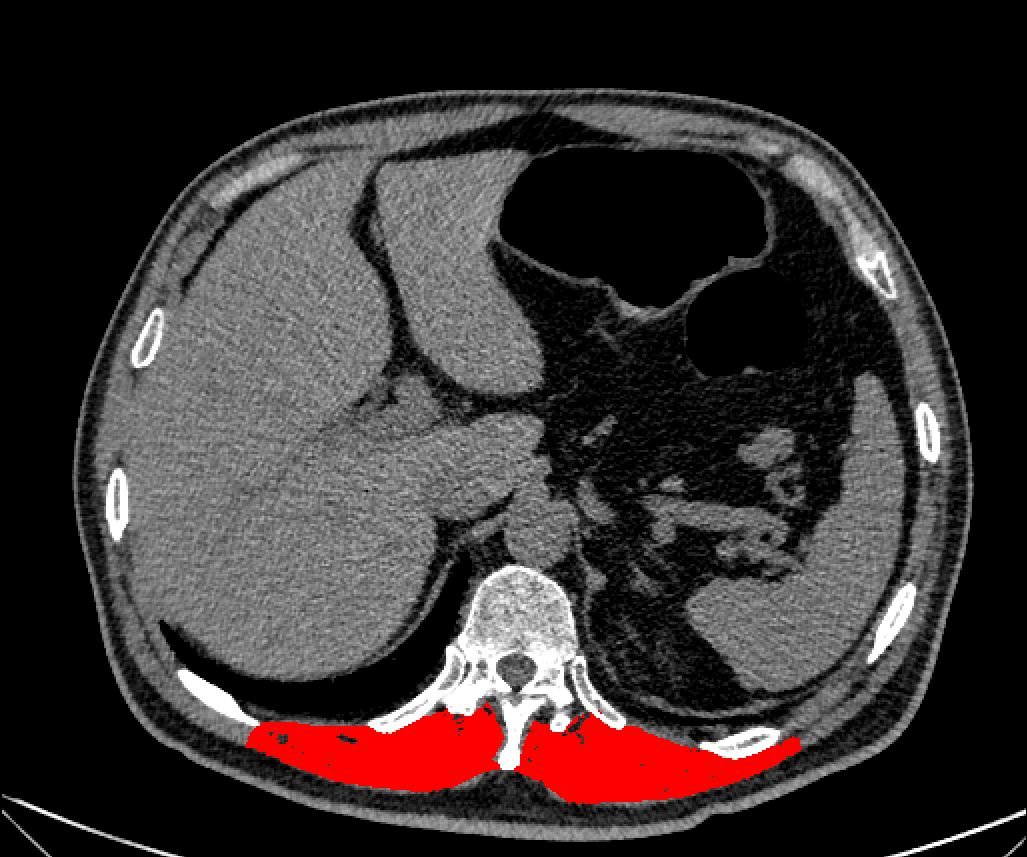
Unenhanced chest CT was performed for all participants. The CT acquisition parameters were as follows: 120 kV, tube current of 100–120 mAs, slice thickness of 0.625 mm and 512×512 pixels. On a single sagittal CT image at the T12 vertebra level, bilateral erector spinae muscles (iliocostalis, longissimus, and spinalis) were measured via ImageJ software, as shown in Figure 1. The muscle tissue thresholds were set at -29–150 (12, 13). Then, ImageJ software automatically segments the muscles within the defined area according to the muscle tissue thresholds, with manual correction of the tissue boundaries when necessary. The 25th percentile has been used as the cutoff point in some studies (14, 15) (Lera et al., 2018; Kidd et al., 2024). Therefore, low muscle mass was defined on the criteria of muscle mass < 25.0 cm² in men and < 20.0 cm2 in women (calculated from the 25th percentile of muscle area in men and women). Muscle mass was determined from May 2023 to May 2024.
Statistical methods
Statistical analyses were performed via SPSS 25.0 software (IBM Corp., Armonk, NY, USA). Continuous and categorical variables are expressed as the means and standard deviations and percentages, respectively. All the subjects were divided into four groups according to HDL-C quartile, and quartile 1 (Q1) served as a reference. One-way analysis of variance or nonparametric rank sum tests were used to compare the levels of related indicators between different groups. Linear regression analysis was used to evaluate the effects of HDL-C on muscle area. Multivariable logistic regression and restricted cubic spline (RCS) analysis were used to analyze the relationships between HDL-C quartile and low muscle mass in all participants and in the male and female subgroups. Three models were analyzed: Model 1 was adjusted for age, sex and body mass index; Model 2 was further adjusted for liver function (AST), renal function (creatinine), diabetes, and albumin. Model 3 was further adjusted for LDL-C, TC and TG. P < 0.05 was considered statistically significant.
Results
Characteristics of the subjects divided by HDL-C quartile
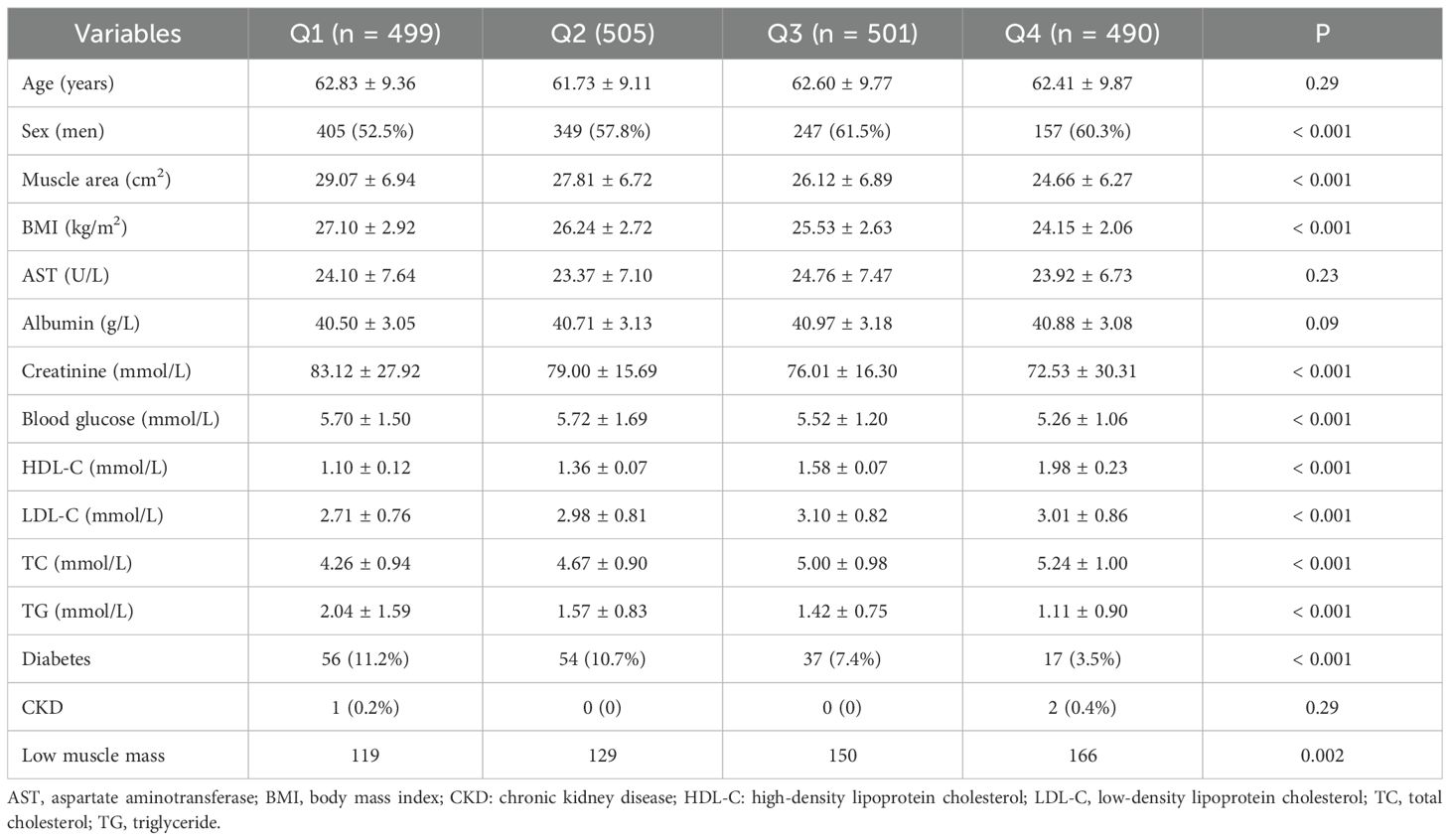
The clinical characteristics of the subjects are described in Table 1. A total of 1995 subjects were classified into four groups according to the quartile of HDL-C levels. Muscle area, number of individuals with low muscle mass, BMI, creatinine, LDL-C, total cholesterol (TC), total triglyceride (TG), blood glucose, and number of individuals with diabetes were significantly different among the four groups (P < 0.05). Compared with those in the Q1 group, the muscle area, BMI, creatinine, TG, blood glucose, and number of diabetic patients in the Q2, Q3 and Q4 groups were significantly lower, whereas the levels of LDL-c, TC, and low muscle mass were significantly greater. The prevalence of low muscle mass in men and women on the basis of the interquartile range of HDL-C is shown in Figure 2. The prevalence of low muscle mass increased with increasing HDL-C levels in the male and female subgroups. Compared with individuals in the Q1 group, female participants in the Q2 and Q3 groups with higher HDL-C levels had a greater prevalence of low muscle mass, whereas the prevalence of low muscle mass in the Q4 group was lower than that in the Q3 group.
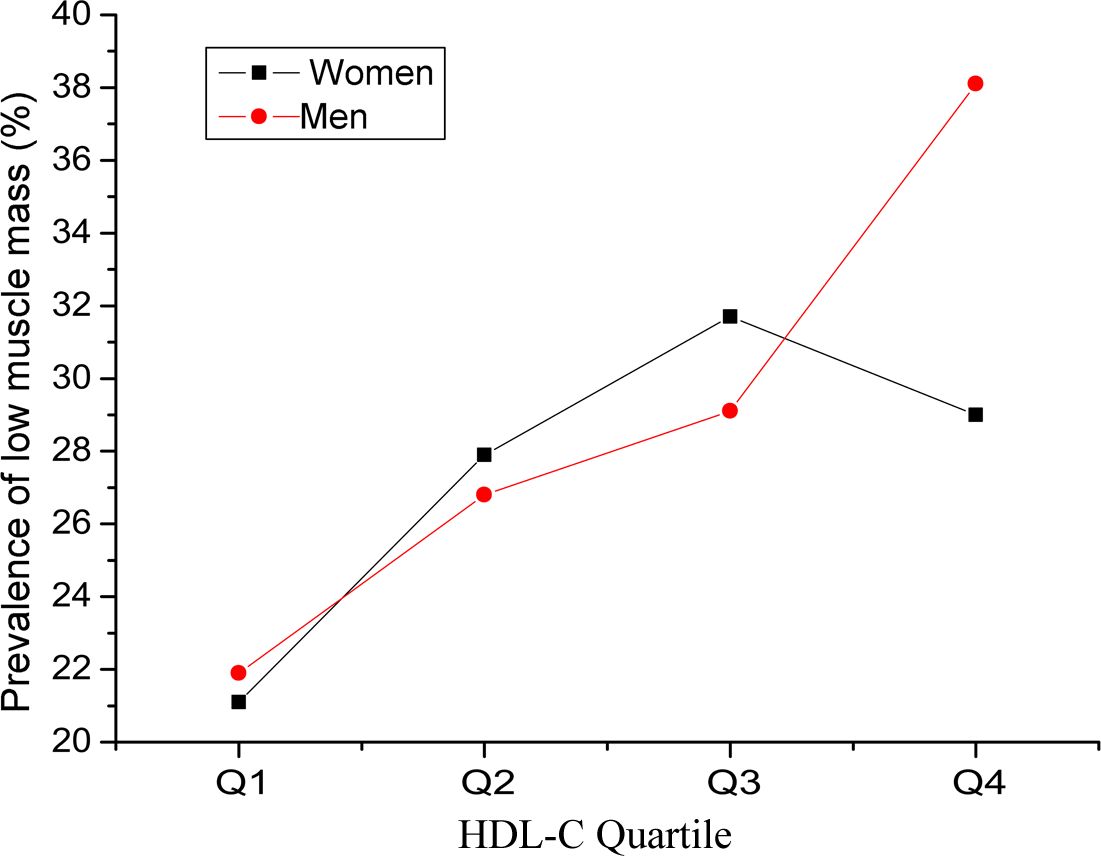
Figure 2. Prevalence of low muscle mass in men and women on the basis of the interquartile range of high-density lipoprotein cholesterol (HDL-C).
Associations between serum lipids, age, BMI and the risk of lower muscle area
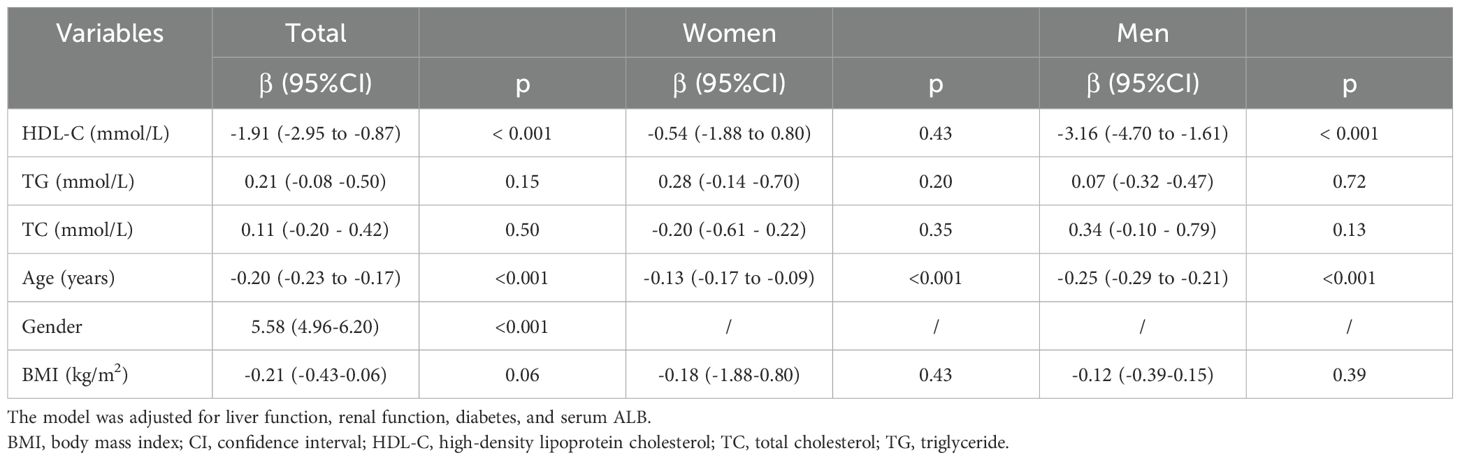
The associations between serum lipid levels, age, BMI and muscle area after adjusting for liver function, renal function, diabetes, and serum ALB are presented in The linear regression results show that an increased HDL-C level was associated with a greater risk of a decreased muscle area overall (β =-1.91, 95% CI: -2.95–0.87) and in male participants (β = -3.16, 95% CI: -4.70– -1.61), whereas no significant difference was found in the female subgroup (β = -0.54, 95% CI: -1.88–0.80, P > 0.05) (Table 2). Moreover, older age was associated with decreases in muscle area in all participants (β = -0.20, 95% CI: -0.23–0.17), males (β = -0.25, 95% CI: -0.29–0.21) and females (β = -0.13, 95% CI: -0.17–0.09) (P < 0.001). No significant correlations were detected between TG or TC levels or BMI and muscle area (P > 0.05).
Association between HDL-C level and risk of low muscle mass
The associations between HDL-C levels and the risk of low muscle mass are presented in Table 3. A greater continuous HDL-C level was associated with a greater risk of low muscle mass in overall participants (Model 1, OR = 2.01, 95% CI: 1.41–2.89; Model 2, OR = 2.18, 95% CI: 1.50–3.16; Model 3, OR = 2.28, 95% CI: 1.51–3.45) and the male subgroup (Model 1, OR = 2.58, 95% CI: 1.58–4.22; Model 2, OR = 3.02, 95% CI: 1.79–5.09; Model 3, OR = 3.28, 95% CI: 1.84–5.87) after adjustment for different possible confounders, while no significant difference was found in the female subgroup (P>0.05). For female subjects older than 60 years, higher continuous HDL-C levels were associated with a greater risk of low muscle mass in Model 1 (OR = 2.19, 95% CI: 1.07–4.48) and Model 2 (OR = 2.06, 95% CI: 1.00–4.27) but not in Model 3 (OR = 2.01, 95% CI: 1.08–4.70).
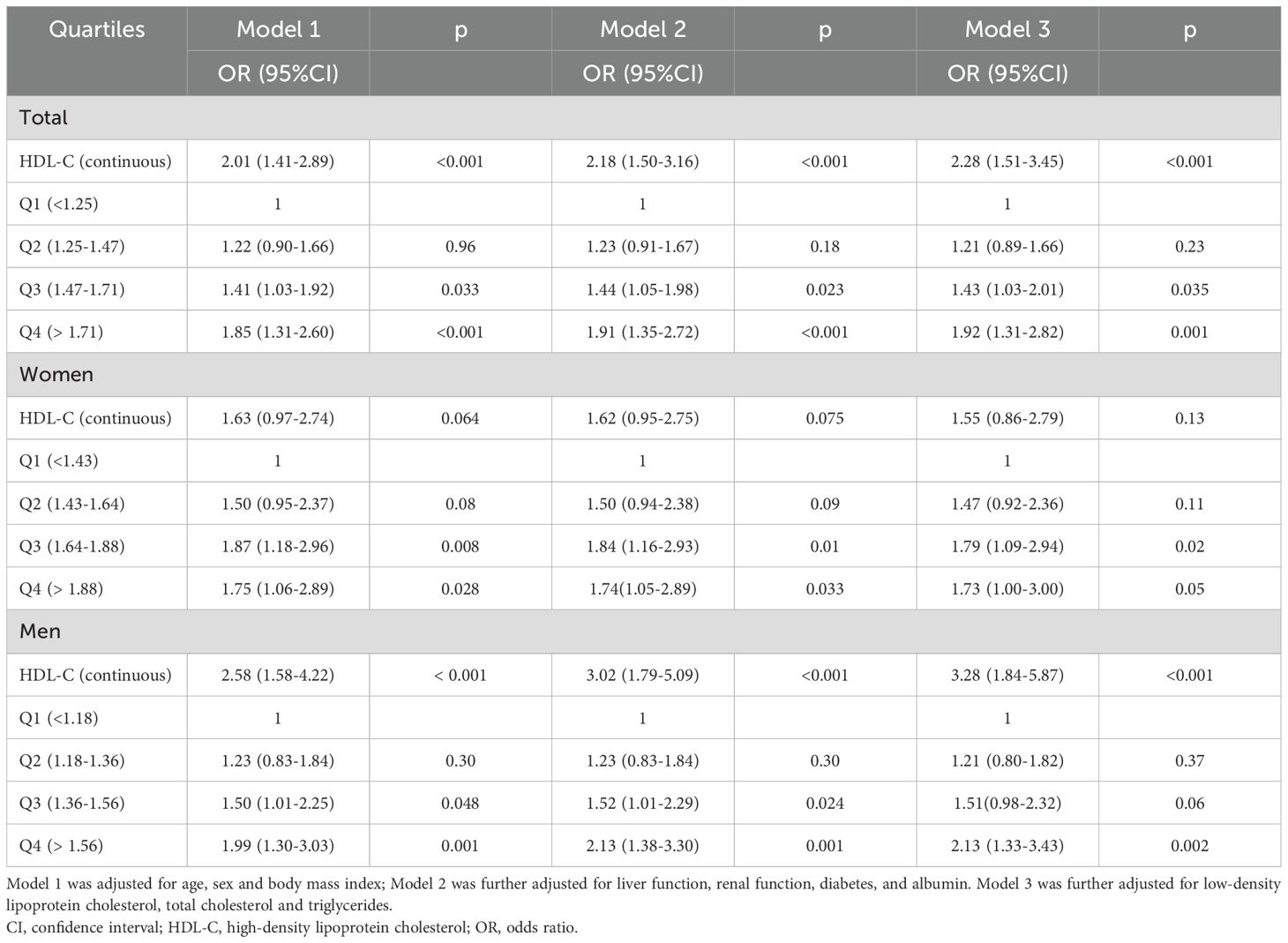
Table 3. Association between HDL-C and the risk of low muscle mass according to logistic regression analysis.
All the subjects were then divided into four groups according to the HDL-C quartiles, and quartile 1 (Q1) served as a reference. Compared with individuals in the reference Q1 group, overall, participants in the Q3 and Q4 groups with higher HDL-C levels had a greater risk of low muscle mass (OR = 1.43, 95% CI: 1.03–2.01; OR = 1.92, 95% CI: 1.31–2.82). No significant difference was found in the hazard of lower muscle mass between the Q2 group and the reference Q1 group. The subgroup analysis for male and female participants revealed similar results.
Furthermore, the RCS model revealed a linear relationship between HDL-C and the risk of low muscle mass in all subjects (Figure 3A) and in the male subgroup (Figure 3B) (P <0.001), and these results implied that the risk of low muscle mass increased with increasing HDL-C levels, while this linear relationship was not found in the female subgroup (Figure 3C) (P>0.05). The dose–response relationships between HDL-C and the risk of low muscle mass were consistent with the results of the logistic model.
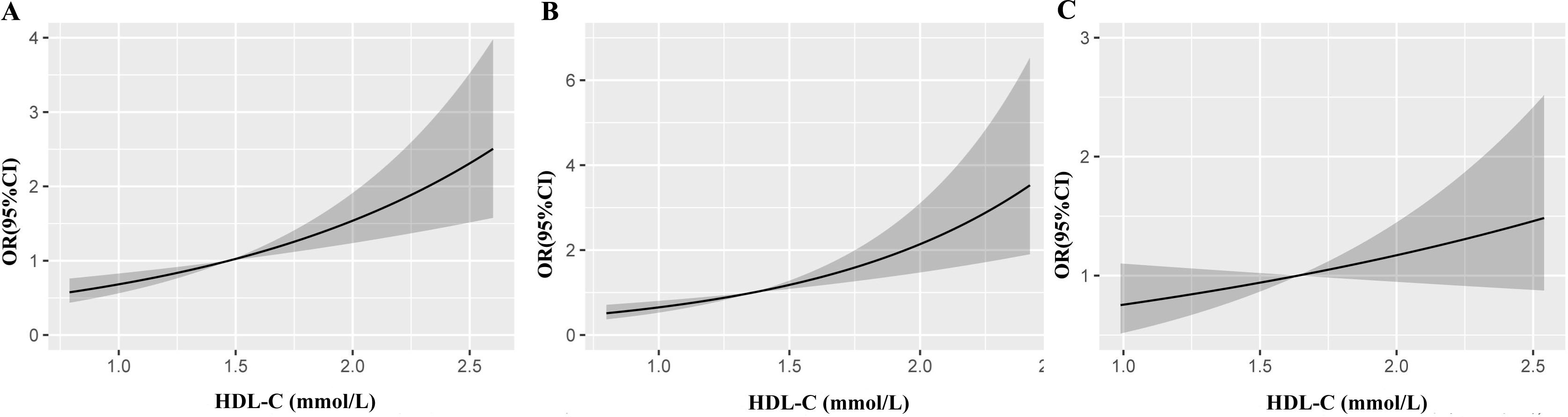
Figure 3. Restricted cubic splines show the multivariable adjusted odds ratio for the risk of low muscle mass according to high-density lipoprotein cholesterol (HDL-C) in the total population (A), men (B) and women (C). Age, sex and body mass index, liver function, renal function, diabetes, low-density lipoprotein cholesterol, triglyceride, albumin and total cholesterol were adjusted. CI, confidence interval; OR, odds ratio.
Discussion
This cross-sectional study of older Chinese adults demonstrated that higher HDL-C levels were associated with a significantly greater risk of low muscle mass, as measured by chest computed tomography. In addition, higher HDL-C levels had a more significant effect on low muscle mass risk in older male adults than in females. Furthermore, the RCS model revealed a linear relationship between HDL-C and the risk of low muscle mass in all subjects and in the male subgroup, whereas this linear relationship was not found in the female subgroup. Our study provides new evidence of the negative effect of very high HDL-C on muscle mass in older adults and suggests that the HDL-C level is an important monitoring index for identifying older individuals who are at risk for low muscle mass.
In this study, higher HDL-C levels were associated with a significantly greater risk of low muscle mass in older Chinese adults, which is consistent with the findings of a subset of extant studies (5, 16, 17). A longitudinal study encompassing 4031 elderly Chinese individuals indicated that older adults with higher HDL-C levels (>70 mg/dl) were at a significantly increased risk of developing sarcopenia and having low grip strength (5). Another 4-year longitudinal study conducted on 7,415 Chinese middle-aged and older adults indicated that, with each 1-unit increase (1SD = 15.4 mg/dL) in HDL-C, the risk of developing sarcopenia increased by 42% at the 4-year follow-up (16). A cross-sectional study including 4302 patients also revealed that sarcopenia patients had lower TG and LDL-C levels but higher HDL-C levels (17).
Nonetheless, findings from certain studies are inconsistent with our findings. For example, data from the National Health and Nutrition Examination Survey (NHANES) involving 4,636 subjects revealed that, in comparison with the control group, the sarcopenia group presented higher LDL-C, TG and total cholesterol (TC) levels and lower HDL-C levels (P <0.05) (18). A probable explanation for the different trends reported in the studies described above is that the impact of HDL-C on sarcopenia is a long-term process, as is the case with cardiovascular disease (19). Although HDL-C is generally acknowledged as the “good cholesterol” beneficial to cardiovascular health, pharmaceutical trials that aim to increase the level of circulating HDL-C have shown a limited capacity to reduce cardiovascular risk (20, 21). Owing to the brevity of cross-sectional studies’ duration, longitudinal associations were insufficiently captured, leading to biased estimates in opposite directions (17, 18). Additional factors contributing to the different outcomes include sample size, the characteristics of the research subjects, and inadequate adjustment for confounding factors. Our findings were consistent with recent longitudinal studies, which reinforced the evidence that high HDL-C levels increase the risk of sarcopenia (5, 16).
Both extremely low and high levels of HDL-C are related to a higher rate of mortality (in terms of total, coronary heart disease and stroke) and an elevated concentration of inflammatory factors (22). This provides an explanation for the differences in the results of the abovementioned observational studies (5, 16–18). A potential interpretation for the association between exceedingly high HDL-C and the risk of low mass is that individuals with exceedingly high HDL-C levels, genetic variants such as CETP (23), scavenger receptor class B member 1 (SCARB1) (24), the hepatic lipase gene (LIPC) (25), and ATP-binding cassette subfamily A member 1 (ABCA1) (26) might be carried. These genetic variants not only increase HDL cholesterol levels but also affect human physiology and thereby potentially increase the risk of disease or death. Mendelian randomization studies may be useful for revealing the causal relationship between high HDL-C and sarcopenia risk. Another possible explanation is that HDL is commonly known for its cardioprotective benefits, since it has anti-inflammatory, antioxidative, antithrombotic, and cytoprotective characteristics (27, 28). Modifications in the HDL lipidome and proteome, including oxidation and glycation, are capable of altering the composition of HDL. HDL subsequently becomes a proinflammatory and atherogenic molecule that is harmful in a variety of pathologies (29–31). Future studies are needed to investigate the effects of high levels of HDL-C on inflammation. Mounting evidence shows that the composition and function of HDL-C are more critical determinants than HDL-C levels for disease outcome, not only in coronary heart disease but also in other conditions (32). The conformation and function of HDL might also be modified in those who have extremely high HDL-C. A hypothesis could be that for those with extremely high HDL-C, the function of HDL-C is undermined so that HDL-C cannot operate properly and instead has detrimental effects (33). Whether there is a causal relationship between extremely high HDL-C levels and increased risk of low muscle mass remains an important unresolved question and demands further in-depth investigation.
We detected heterogeneity in the dose–response association between HDL-C levels and the risk of sarcopenia across different sexes. Our study revealed a linear relationship between HDL-C and the risk of low muscle mass in all subjects and in the male subgroup, whereas this relationship was weak in the female subgroup. The possible mechanisms are unknown. Such sex differences were also observed in some studies (16, 34). A possible explanation for this difference could be the modifying effect of sex hormones on the relationship between HDL-C and sarcopenia (35). With increasing age, the decrease in androgen levels curtails skeletal muscle protein synthesis (36). Similarly, a reduction in estrogen levels may be correlated with an increase in TNF-α, IL-6, and a range of other inflammatory factors, and it can also induce mitochondrial dysfunction, thereby leading to a decrease in muscle mass (37). The decrease in estrogen in women may be greater than the decrease in androgen in men. Estrogen may play a more important role in muscle than does HDL-C in women. Therefore, the linear relationship between HDL-C and sarcopenia in women was diminished. However, as categorical data, the Q2 of HDL-C indicated a high risk of low muscle mass. The relationship between HDL-C may be complicated. Future research is expected to explore the causal mechanisms of this sex-related relationship.
However, there were certain limitations. First, owing to the observational study design, we were unable to establish a causal association between HDL-C levels and muscle mass. We only discuss possible reasons for the association between exceedingly high HDL-C and low muscle mass. To elucidate the pathophysiological underpinnings of these findings, additional prospective or longitudinal studies are imperative. Second, several guidelines have shown that the diagnosis of sarcopenia should be established on the basis of the criteria of low appendicular skeletal muscle mass and compromised muscle function (3). However, owing to insufficient data availability, functional parameters such as handgrip strength were not evaluated in our study. Third, this study focused on the Chinese elderly population. Given the physiological and environmental variances across different ethnic groups, the generalizability of the findings may be limited, and additional research involving multiethnic populations is imperative. Fourth, our population included people who underwent CT lung cancer scans. There were no specific predispositions to lung cancer except for age. To some extent, our population can represent the general population. Although this scan is recommended for all subjects older than 50 years, selection bias cannot be avoided. In addition, we discuss possible mechanisms by which high HDL-C is related to low muscle mass, and further studies are needed to confirm these hypotheses. Furthermore, although the research endeavored to account for potential confounding variables, other variables that could influence the relationship between HDL-C and muscle mass, such as dietary habits and physical exercise, were not considered.
Our research revealed a linear correlation between HDL-C levels and the risk of sarcopenia among older individuals, and higher HDL-C levels were significantly associated with an increased risk of low muscle mass. Increasing HDL-C levels had a more significant effect on low muscle mass risk in older male individuals. HDL-C may be useful in identifying older individuals with sarcopenia or for managing sarcopenia in older adults.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Affiliated Hospital of Nanjing University of Chinese Medicine (No. 2017NL-137-05). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this study is a retrospective study.
Author contributions
WZ: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. YL: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. YZ: Data curation, Formal analysis, Writing – original draft. DZ: Data curation, Formal analysis, Writing – original draft. JW: Data curation, Formal analysis, Writing – review & editing. XC: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Aging. (2019) 48:601. doi: 10.1093/ageing/afz046
2. Dennison EM, Sayer AA, and Cooper C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat Rev Rheumatol. (2017) 13:340–7. doi: 10.1038/nrrheum.2017.60
3. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012
4. ella CA, Nelson MC, Unkart JT, Miljkovic I, and Allison MA. Skeletal muscle area and density are associated with lipid and lipoprotein cholesterol levels: The Multi-Ethnic Study of Atherosclerosis. J Clin Lipidol. (2020) 14:143–53. doi: 10.1016/j.jacl.2020.01.002
5. Hua N, Qin C, Wu F, Wang A, Chen J, and Zhang Q. High-density lipoprotein cholesterol level and risk of muscle strength decline and sarcopenia in older adults. Clin Nutr. (2024) 43:2289–95. doi: 10.1016/j.clnu.2024.08.017
6. Li JJ, Chen Y, Zhang H, Li M, and Wang L. Association between blood lipid profile and sarcopenia in elderly people in one community in Suzhou. Chin J Multiple Organ Dis Elder. (2023) 22:6–11. doi: 10.11915/j.issn.1671-5403.2023.01.002
7. Guo YS, Xue Q, Weu YN, Liu J, and Wang J. Prevalence and risk factors for Sarcopenia in obese Elderly Adults. Chin Gen Pract. (2021) 24:3048–53. doi: 10.12114/j.issn.1007-9572.2021.00.405
8. Chinese Expert Group on Early Diagnosis and Treatment of Lung Cancer. China lung oncology group. China national lung cancer screening guideline with low-dose computed tomography (2023 version). Zhongguo Fei Ai Za Zhi. (2023) 26:1–9. doi: 10.3779/j.issn.1009-3419.2023.102.10
9. Nemec U, Heidinger B, Sokas C, Chu L, and Eisenberg RL. Diagnosing sarcopenia on thoracic computed tomography: quantitative assessment of skeletal muscle mass in patients undergoing transcatheter aortic valve replacement. Acad Radiol. (2017) 24:1154–61. doi: 10.1016/j.acra.2017.02.008
10. Tan L, Ji G, Bao T, Fu H, Yang L, and Yang M. Diagnosing sarcopenia and myosteatosis based on chest computed tomography images in healthy Chinese adults. Insights Imaging. (2021) 12:163. doi: 10.1186/s13244-021-01106-2
11. Kaplan SJ, Zhao KL, Koren M, Bentov I, Reed MJ, and Pham TN. Thresholds and mortality associations of paraspinous muscle sarcopenia in older trauma patients. JAMA Surg. (2020) 155:662–4. doi: 10.1001/jamasurg.2020.0435
12. Park J, Gil JR, Shin Y, Won SE, Huh J, You MW, et al. Reliable and robust method for abdominal muscle mass quantification using CT/MRI: An explorative study in healthy subjects. PloS One. (2019) 14:e0222042. doi: 10.1371/journal.pone.0222042
13. Kim HK, kW K, Kim EH, Lee MJ, Bae SJ, Ko Y, et al. Age-related changes in muscle quality and development of diagnostic cutoff points for myosteatosis in lumbar skeletal muscles measured by CT scan. Clin Nutr. (2021) 40:4022–8. doi: 10.1016/j.clnu.2021.04.017
14. Kidd AC, Cowell GW, Martin GA, Ferguson J, Fennell DA, Evison M, et al. The prevalence and prognostic significance of Sarcopenia and Adipopenia in Pleural Mesothelioma. Cancer Treat Res Commun. (2024) 42:100856. doi: 10.1016/j.ctarc.2024.100856
15. Lera L, Albala C, Leyton B, Márquez C, Angel B, Saguez R, et al. Reference values of hand-grip dynamometry and the relationship between low strength and mortality in older Chileans. Clin Interv Aging. (2018) 13:317–24. doi: 10.2147/CIA.S152946
16. Wang M, Yang Z, and Zhai H. Association of high-density lipoprotein cholesterol with sarcopenia in chinese community-dwelling middle-aged and older adults: evidence from 4-year longitudinal study. Gerontology. (2024) 70:812–22. doi: 10.1159/000538980
17. Yin M, Zhang H, Liu Q, Ding F, Deng Y, Hou L, et al. Diagnostic performance of clinical laboratory indicators with sarcopenia: results from the west China health and aging trend study. Front Endocrinol (Lausanne). (2021) 12:785045. doi: 10.3389/fendo.2021.785045
18. Huang H, Yu X, Jiang S, Wang C, Chen Z, Chen D, et al. The relationship between serum lipid with sarcopenia: Results from the NHANES 2011–2018 and bidirectional Mendelian randomization study. Exp Gerontol. (2024) 196:112560. doi: 10.1016/j.exger.2024.112560
19. Duncan MS, Vasan RS, and Xanthakis V. Trajectories of blood lipid concentrations over the adult life course and risk of cardiovascular disease and all-cause mortality: observations from the framingham study over 35 years. J Am Heart Assoc. (2019) 8:e011433. doi: 10.1161/JAHA.118.011433
20. Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. (2017) 376:1933–42. doi: 10.1056/NEJMoa1609581
21. Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. (2012) 367:2089–99. doi: 10.1056/NEJMoa1206797
22. Mazidi M, Mikhailidis DP, and Banach M. Associations between risk of overall mortality, cause-specific mortality and level of inflammatory factors with extremely low and high high-density lipoprotein cholesterol levels among American adults. Int J Cardiol. (2019) 276:242–7. doi: 10.1016/j.ijcard.2018.11.095
23. Agerholm-Larsen B, Nordestgaard BG, Steffensen R, Jensen G, and Tybjaerg-Hansen A. Elevated HDL cholesterol is a risk factor for ischemic heart disease in white women when caused by a common mutation in the cholesteryl ester transfer protein-encoding gene. Circulation. (2000) 101:1907–12. doi: 10.1161/01.CIR.101.16.1907
24. Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. (2016) 351:1166–71. doi: 10.1126/science.aad3517
25. Andersen RV, Wittrup HH, Tybjaerg-Hansen A, Steffensen R, Schnohr P, and Nordestgaard BG. Hepatic lipase mutations, elevated high-density lipoprotein cholesterol, and increased risk of ischemic heart disease: the Copenhagen City Heart Study. J Am Coll Cardiol. (2003) 41:1972–82. doi: 10.1016/S0735-1097(03)00407-8
26. Frikke-Schmidt R, Nordestgaard BG, Jensen GB, Steffensen R, and Tybjaerg-Hansen A. Genetic variation in ABCA1 predicts ischemic heart disease in the general population. Arterioscler Thromb Vasc Biol. (2008) 28:180–6. doi: 10.1161/ATVBAHA.107.153858
27. Brites F, Martin M, Guillas I, and Kontush A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. (2017) 8:66–77. doi: 10.1016/j.bbacli.2017.07.002
28. Xepapadaki E, Zvintzou E, Kalogeropoulou C, Filou S, and Kypreos KE. The antioxidant function of HDL in atherosclerosis. Angiology. (2020) 71:112–21. doi: 10.1177/0003319719854609
29. Ansell BJ, Fonarow GC, and Fogelman AM. The paradox of dysfunctional high-density lipoprotein. Curr Opin Lipidol. (2007) 18:427–34. doi: 10.1097/MOL.0b013e3282364a17
30. Ertek S. High-density lipoprotein (HDL) dysfunction and the future of HDL. Curr Vasc Pharmacol. (2018) 16:490–8. doi: 10.2174/1570161115666171116164612
31. HB G, Rao VS, and Kakkar VV. Friend turns foe: transformation of anti-inflammatory HDL to proinflammatory HDL during acute-phase response. Cholesterol. (2011) 2011:274629. doi: 10.1155/2011/274629
32. Márquez AB, Nazir S, and van der Vorst EPC. High-density lipoprotein modifications: A pathological consequence or cause of disease progression. Biomedicines. (2020) 8:549. doi: 10.3390/biomedicines8120549
33. Madsen CM, Varbo A, and Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. (2017) 38:2478–86. doi: 10.1093/eurheartj/ehx163
34. Bi B, Dong X, Yan M, Zhao Z, Liu R, Li S, et al. Dyslipidemia is associated with sarcopenia of elderly individuals: a meta-analysis. BMC Geriatr. (2024) 24:181. doi: 10.1186/s12877-024-04761-4
35. Liao Y, Peng Z, Chen L, Zhang Y, Cheng Q, Nüssler AK, et al. Prospective views for whey protein and/or resistance training against age-related sarcopenia. Aging Dis. (2019) 10:157–73. doi: 10.14336/AD.2018.0325
36. Rolland Y, Dray C, Vellas B, and Barreto PS. Current and investigational medications for the treatment of sarcopenia. Metabolism. (2023) 149:155597. doi: 10.1016/j.metabol.2023.155597
Keywords: low muscle mass, sarcopenia, muscle area, high-density lipoprotein cholesterol, chest CT
Citation: Zhang W, Liu Y, Zhang Y, Zhang D, Wang J and Chen X (2025) Associations between high-density lipoprotein cholesterol levels and computed tomography-defined low muscle mass in older adults and sex-related differences. Front. Endocrinol. 16:1600431. doi: 10.3389/fendo.2025.1600431
Received: 03 April 2025; Accepted: 16 May 2025;
Published: 11 June 2025.
Edited by:
Lynda Bourebaba, Wroclaw University of Environmental and Life Sciences, PolandReviewed by:
Laura Pérez Campos Mayoral, Benito Juárez Autonomous University of Oaxaca, MexicoShibo Wei, Gwangju Institute of Science and Technology, Republic of Korea
Copyright © 2025 Zhang, Liu, Zhang, Zhang, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Chen, Y2h4d2luQDE2My5jb20=
†These authors have contributed equally to this work
 Weixiao Zhang1†
Weixiao Zhang1† Yongkang Liu
Yongkang Liu Xiao Chen
Xiao Chen