- 1Graduate School, Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang, China
- 2Heilongjiang University of Chinese Medicine, School of Basic Medical Sciences, Harbin, Heilongjiang, China
Type 2 diabetes mellitus (T2DM) is a complex metabolic disorder, and its management has evolved from mere glycemic control to multitarget metabolic regulation. Sodium–glucose cotransporter 2 inhibitors (SGLT2is) have demonstrated extensive pleiotropic effects in treating T2DM, and its complications through unique mechanisms. SGLT2is promote urinary glucose excretion, leading to a negative energy balance that triggers lipid metabolic reprogramming and fuel switching in the body. This process significantly reduces visceral fat deposition and improves insulin resistance and the inflammatory status. Additionally, SGLT2is provide a metabolic foundation for cardiovascular, hepatic, and renal protection through multiple pathways, including remodeling cardiac structure, enhancing myocardial metabolism, reducing uric acid levels, and alleviating renal hypoxia. With respect to combination therapy, the pairing of SGLT2is with other hypoglycemic agents and cardiovascular protective drugs has synergistic effects; however, potential adverse reactions should also be considered. Future research should investigate the precise application and long-term safety of SGLT2is as well as develop individualized treatment strategies on the basis of patients’ metabolic phenotypes, complications, and drug tolerability to maximize clinical benefits for patients. This review systematically explores the significant roles of SGLT2is in metabolic regulation, cardiovascular protection, and combination therapy, with the aim of providing a comprehensive foundation for optimizing individualized treatment strategies in T2DM management.
1 Introduction
Diabetes has emerged as a significant public health challenge globally in the 21st century, with its widespread prevalence not only imposing a heavy disease burden but also exerting substantial socioeconomic pressure (1). Epidemiological forecasts predict that the number of diabetes patients worldwide will rise to 578 million by 2030 (2). Among the types of diabetes, type 2 diabetes mellitus (T2DM) is the most prevalent, and its pathogenesis is complex, involving dysregulation of glucose metabolism in the body due to the interaction of various factors, such as the social environment and genetics (3). The complications of T2DM combined with cardiovascular or kidney disease increase the risk of hospitalization and mortality. The previous treatment plan for T2DM focused on restoring pancreatic beta-cell function and supplementing insulin injections, as one of the important mechanisms of T2DM is insulin resistance. Sodium–glucose cotransporter 2 inhibitors (SGLT2is) achieve glucose-lowering effects through a unique renal mechanism of action. They selectively inhibit the reabsorption of glucose in the renal tubules, promoting urinary glucose excretion independent of pancreatic β-cell function. Additionally, SGLT2is can improve the uptake and utilization of glucose in peripheral tissues and increase insulin sensitivity (4, 5). Several landmark large-scale clinical trials, including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI, have demonstrated that SGLT2is can significantly reduce the risk of cardiovascular death and improve cardiorenal outcomes in the T2DM population (6–8).
The specific molecular mechanisms by which SGLT2is exert these multiple metabolic benefits remain an important area of current research. A growing body of evidence suggests that SGLT2is have significant pleiotropic effects on weight management, adipose tissue remodeling, cardiovascular protection, and renal protection. The focus of this review is the metabolic benefits of long-term SGLT2i treatment, its correlation with reductions in fat mass, changes in adipokines and lipoprotein profiles, and its cardiovascular benefits and hepatic and renal protective effects in relation to visceral fat and inflammatory factors. Finally, we propose that personalized treatment strategies and future research directions should be emphasized.
2 Metabolic regulatory effects of SGLT2is
2.1 Weight management and adipose tissue remodeling
SGLT2is inhibit the SGLT2 receptor in the proximal tubule of the kidney, promoting urinary glucose excretion (approximately 60–90 grams of glucose per day), resulting in an energy loss of approximately 200–300 kcal daily, which mimics a low-calorie state in the body. This negative energy balance can sometimes trigger compensatory increases in food intake, thereby reducing the anticipated weight loss effect (9). However, related studies indicate that this compensatory response does not significantly affect the efficacy of SGLT2is (10–12). The weight loss effect of SGLT2is is typically assessed through changes in BMI. An elevated BMI is typically associated with obesity, and prolonged obesity is often closely linked to high-sugar and high-fat diets. Poor dietary habits serve as a potential driving force for the development and progression of metabolic diseases. A series of pathological changes induced by obesity in the body, ranging from microvascular and macrovascular lesions to organ pathological alterations, are potential factors that exacerbate disease progression. For example, pathophysiological changes such as chronic low-grade inflammation, abnormal lipid metabolism, and insulin resistance associated with obesity collectively form the important pathogenic basis for heart failure with preserved ejection fraction (HFpEF) (13). In clinical practice, BMI is frequently used as a significant indicator for disease risk assessment. For example, studies by Said et al. showed that higher BMI can serve as an independent predictor of new-onset heart failure (HF) in patients with T2DM (14). However, BMI cannot reflect changes in body fat distribution or variations in the weight of different parts of the body, such as skeletal muscle and muscle mass. Analyses of the DELIVER, EMPEROR-Reserved, and CANDLE trials indicated that although patients with higher BMIs experience more significant symptom improvement (15), the therapeutic effects of SGLT2is in reducing the risk of cardiovascular death and other aspects are independent of baseline BMI (15–17).
The weight loss induced by SGLT2is is associated primarily with a reduction in water content and a decrease in fat mass (18). Initially, due to increased urine output, the reduction in water content is more pronounced; however, the long-term weight loss effect is related to a reduction in fat mass. In a 12-week randomized controlled trial, dapagliflozin was associated with weight loss related to a decrease in lean body mass and body water content, with no significant change in fat mass observed (19). However, in longer-term treatments (such as 24 weeks and 102 weeks), dapagliflozin not only reduced body water content but also significantly decreased fat mass (with reductions of 1.48 kg and 2.80 kg, respectively), and these effects were correlated with the duration of treatment (20, 21). The reduction in fat mass is partly attributed to the decrease in visceral adipose tissue and subcutaneous adipose tissue (22–24). SGLT2is can reduce liver fat (25, 26), the thickness of epicardial adipose tissue (EAT) (27, 28), and perirenal fat (29), among other types of tissue.
SGLT2is can alter adipose tissue structure by converting white adipose tissue into brown adipose tissue (BAT) or beige adipose tissue through browning, thereby increasing energy expenditure to adapt to the negative energy balance induced by SGLT2is. Studies have shown that SGLT2is can reduce lipid content in perirenal WAT, inguinal WAT, and epididymal WAT in mice and upregulate the expression of uncoupling protein 1 (UCP1) (30–32), thermogenesis-related genes (such as Prdm16 and Irisin) (32), and the mRNA expression of beige adipose-selective genes (such as Cd137 and Tmem26) (31). Uncoupling protein 1 (UCP1) is a crucial thermogenic regulator that can uncouple substrate oxidation from ATP synthesis, thereby generating heat. The increased expression of UCP1 reflects an increase in BAT quantity and active mitochondrial function (33). Although beige adipose tissue does not contribute to overall energy expenditure as much as BAT does, it has additional metabolic benefits in terms of glucose and lipid clearance as well as anti-inflammatory effects (34).
2.2 Lipid metabolism reprogramming and liver protection
SGLT2is decrease insulin secretion, and increase glucagon secretion, thereby improving β-cell function and tissue sensitivity to insulin. Substrate utilization for body metabolism shifts from carbohydrates to lipids (35). The reduction in insulin and increase in glucagon reflect the decreased inhibition of lipolysis and increased activation of hormone-sensitive lipase (HSL), which promotes lipolysis. The activation of the sympathetic nervous system stimulates β-adrenergic receptors, thereby promoting lipolysis. Although the substantial loss of glucose increases hepatic gluconeogenesis, leading to an increase in endogenous glucose production, the ultimate reduction in blood glucose concentration indicates a general shift in the body toward lipid metabolism. Fat breakdown releases a large amount of free fatty acids (FFAs), which are converted into ketone bodies through hepatic fatty acid oxidation. This process reduces lipid accumulation in the liver (Figure 1).
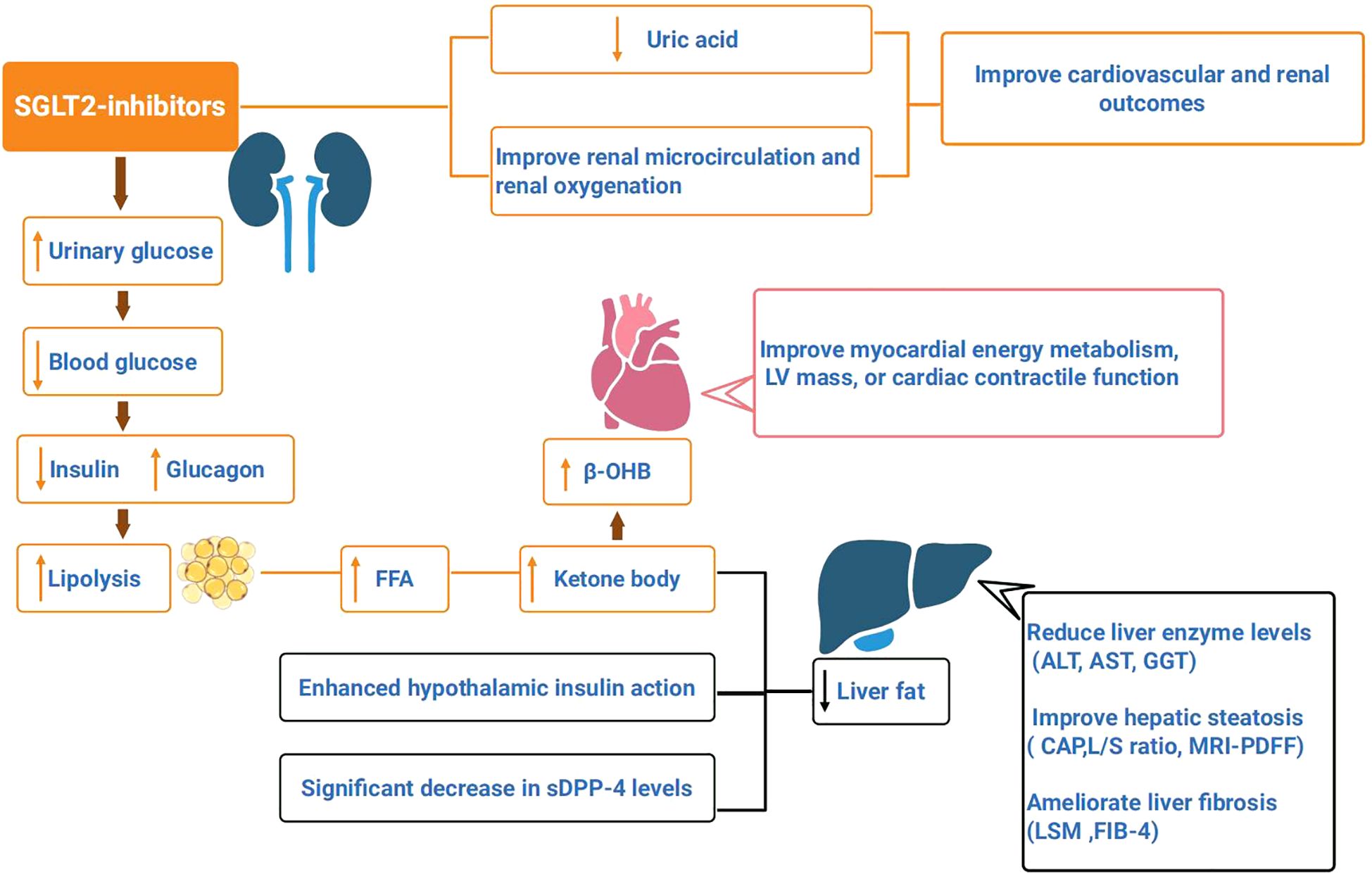
Figure 1. SGLT2is promote urinary glucose excretion, reduce blood glucose concentration. Reduced insulin secretion and increased glucagon can promote fat breakdown, which produces a large amount of free fatty acids (FFA), that are oxidized by liver fatty acids to form ketone bodies, and reduce liver fat. Enhanced insulin action in the hypothalamus, along with a significant decrease in serum soluble dipeptidyl peptidase-4 (sDPP-4) levels, can lead to a reduction in liver fat. SGLT2is have been shown to reduce liver enzyme levels (ALT, AST, GGT) in patients with nonalcoholic fatty liver disease(NAFLD), improve hepatic steatosis (controlled attenuation parameter (CAP), L/S ratio, MRI-PDFF), and ameliorate liver fibrosis (liver stiffness measurement (LSM), FIB-4 index). SGLT2is lead to an elevated circulating level of β-hydroxybutyrate (β-OHB), thereby improving myocardial energy metabolism, LV mass, or cardiac contractile function. SGLT2is can reduce uric acid levels, improve renal microcirculation and oxygenation, and improve cardiovascular and renal outcomes in T2DM.
The improvement of insulin resistance also requires long-term treatment with SGLT2is. Studies have shown that 4 weeks of empagliflozin treatment has no significant effect on skeletal muscle free fatty acid or glucose uptake (18). After 8 weeks of dapagliflozin treatment, no significant improvement in insulin sensitivity was observed in tissues such as the liver, skeletal muscle, or myocardium (25). However, 12 weeks of empagliflozin treatment increased hepatic insulin sensitivity (26). A recent meta-analysis by Li et al. reported that SGLT2is can improve insulin resistance in patients with T2DM complicated by nonalcoholic fatty liver disease (NAFLD), significantly reducing HOMA-IR levels (MD [95% CI]; -0.66 [-0.99, -0.32], p = 0.0001). The duration of treatment in the 11 included studies was more than 12 weeks, with the longest duration reaching 72 weeks (36). Additionally, studies have shown that combination therapy with dapagliflozin and exenatide (EXE) can reduce hepatocellular lipids (HCLs) (-4.4%, P < 0.05), and changes in HCLs are associated with a reduction in visceral adipose tissue, independent of glycemic control (37).
SGLT2is can improve the levels of adipokines and inflammatory factors, but the benefits of SGLT2is remain uncertain. A study by Dihoum et al. revealed that dapagliflozin significantly reduced CRP after 12 months of treatment (mean difference -1.96; 95% CI -3.68 to -0.24, p = 0.026), but other inflammatory factors (tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), IL-6, and interleukin 10 (IL-10)) were not significantly improved (38). A meta-analysis by Buttice et al. demonstrated that SGLT2is significantly increased adiponectin and reduced IL-6 and tumor necrosis factor receptor-1 (TNFR1), but there was no significant change in CRP levels (39). The anti-inflammatory mechanism of SGLT2is is controversial, as their anti-inflammatory effects are not prominent in some studies and involve complex physiological mechanisms. However, the reduction in inflammatory factors may be associated with a decrease in fat mass. For example, increased hepatic fatty acid oxidation increases circulating ketone body levels, and serum β-hydroxybutyrate can inhibit NLRP3 inflammasome activation, thereby reducing IL-1B levels (40). Additionally, fat and muscle are significant sources of IL-6 (41). Although studies have shown that a reduction in IL-6 is associated with higher baseline HbA1c levels (42), this may be because IL-6 can lead to increased hepatic glucose output, subsequently increasing blood glucose levels (43).
SGLT2is can improve the lipoprotein profile, with beneficial changes in the human body being an increase in high-density lipoprotein cholesterol (HDL-C) and a decrease in triglyceride (TG) levels (36, 44). These changes are often common, but research findings can sometimes be contradictory, particularly regarding changes in low-density lipoprotein cholesterol (LDL-C). Bechmann et al.’s meta-analysis of 60 RCTs, which were all the RCTs of SGLT2is published at that time, reported an increase in total cholesterol (TC) and LDL-C (44). Elevated LDL-C levels are harmful to the body and promote the development of atherosclerotic cardiovascular disease (ASCVD) (45). Rizos et al. reported that SGLT2is do not appear to improve arterial stiffness, with no favorable changes observed in either pulse wave velocity (PWV) or the augmentation index (AIx) (46). However, numerous studies have demonstrated that SGLT2is can reduce ASCVD events in patients with T2DM (47–49). Additionally, the meta-analysis by Li et al. did not reveal significant changes in TC or LDL-C, with the majority of patients included in this study also suffering from NAFLD and T2DM (36). Current evidence suggests that SGLT2is offer substantial cardiovascular benefits for patients with NAFLD.
Furthermore, SGLT2is reduce hepatic fat, thereby lowering the risk in patients with NAFLD. Enhanced insulin action in the hypothalamus (50), along with a significant decrease in serum soluble dipeptidyl peptidase-4 (sDPP-4) levels (51), can lead to a reduction in liver fat. sDPP-4 is secreted by hepatocytes and promotes hepatic fat synthesis by degrading glucagon-like peptide-1 (GLP-1) while also inducing adipose tissue inflammation and insulin resistance. In summary, SGLT2is promote lipolysis while simultaneously inhibiting hepatic fat synthesis. Moreover, SGLT2is have been shown to reduce liver enzyme levels (ALT, AST, GGT) in patients with NAFLD (36, 52, 53), improve hepatic steatosis (controlled attenuation parameter (CAP), L/S ratio, MRI-PDFF), and ameliorate liver fibrosis (liver stiffness measurement (LSM), FIB-4 index) (36, 53–56). It has been proposed that SGLT2is could serve as the first-line treatment for patients with T2DM complicated by NAFLD (26, 57).
2.3 Energy metabolism and fuel conversion
According to the “thrifty substrate” hypothesis, SGLT2is lead to an elevated circulating level of β-hydroxybutyrate (β-OHB), which then competes with acetyl-CoA from FFA oxidation and glucose-derived pyruvate for entry into the tricarboxylic acid (TCA) cycle. As an energy substrate, β-OHB consumes less oxygen, thereby increasing myocardial efficiency while reducing reactive oxygen species generated by excessive FFA oxidation rates, lowering oxidative stress, and improving mitochondrial function (58). However, some studies have raised doubts about the “thrifty substrate” hypothesis. Although elevated circulating levels of β-OHB increase the likelihood of myocardial substrate utilization, they do not significantly improve myocardial energy metabolism, LV mass, or cardiac contractile function. For example, a study by Gaborit et al. showed that after 12 weeks of treatment with empagliflozin, although the liver fat content (LFC) was significantly reduced by 27%, there was no significant effect on myocardial or epicardial fat, and myocardial energetics (PCr/ATP) also did not significantly change (59). A study by Pietschner et al. demonstrated that after 12 weeks of empagliflozin treatment in patients with stable chronic heart failure (CHF), although there was an overall reduction in blood pressure and improvement in vascular function, the increase in β-OHB levels partially offset these benefits (60). Another study showed that empagliflozin treatment for one month did not alter ketone body concentrations in patients hospitalized with acute HF, further confirming that SGLT2is do not increase the risk of diabetic ketoacidosis, as the initial phase of acute HF is already accompanied by a significant increase in circulating total ketone body (TKB) concentrations (61).
Overall, elevated ketone body levels may not significantly improve cardiac function in the early stages, but in most cases, they do not impair cardiac function or increase the risk of adverse cardiac events, demonstrating good safety. Cardiac energy metabolism is highly flexible and can switch between different energy sources on the basis of substrate availability. For example, one of the characteristics of heart failure with a reduced ejection fraction (HFrEF) is an increased reliance on ketone bodies in the context of reduced fatty acid and glucose oxidation (62). Oldgren et al. reported that in patients with type 2 diabetes without heart failure, although dapagliflozin treatment for 6 weeks did not increase cardiac fatty acid uptake or improve myocardial efficiency, it significantly reduced LV work (-0.095 [-0.145, -0.043] J/g/min) and LV oxygen consumption (-0.30 [-0.49, -0.12] J/g/min) (63). Although existing evidence suggests limitations to the “thrifty substrate” hypothesis, the potential role of ketone bodies in cardiovascular benefits cannot be denied.
2.4 Uric acid metabolism and oxidative stress
One of the mechanisms by which SGLT2is protect the kidneys is through reducing plasma uric acid (UA) levels, but this effect is not achieved by decreasing UA production but rather by increasing UA excretion (64). This process primarily involves urate transporter 1 (URAT1) and glucose transporter 9 (GLUT9). However, the effects of SGLT2is on URAT1 and GLUT9 remain controversial. For example, SGLT2is do not increase urate excretion in URAT1-deficient mice, and GLUT9 appears to be nonessential for the urate excretion effect of canagliflozin (65). When empagliflozin is combined with the URAT1 inhibitor benzbromarone, the effect is inferior to that of benzbromarone alone (66). Although the significant role of URAT1 in renal uric acid reabsorption cannot be overlooked, the uricosuric effect of SGLT2is may rely more on GLUT9 isoform 2. For example, studies have shown that luseogliflozin does not directly affect the activity of proteins such as URAT1, GLUT9 isoform 1, and OAT4, but an increase in the luminal glucose concentration stimulates GLUT9 isoform 2 and inhibits its uric acid reabsorption (67).
However, multiple studies have also indicated that the effects of SGLT2is on reducing UA levels are associated with baseline HbA1c levels: in patients with lower baseline HbA1c and higher baseline UA levels, the reduction in UA levels is more significant (68–71). This finding appears to contradict the role of GLUT9 in reducing uric acid reabsorption through a glucose-dependent mechanism. In summary, the mechanism by which SGLT2is promote uric acid excretion may not depend entirely on transporters expressed in renal tubular epithelial cells (such as URAT1 and GLUT9) but rather involves more complex physiological processes.
SGLT2is exert significant cardiovascular and renoprotective effects by reducing UA levels. Studies have shown that SGLT2is, when improving cardiovascular outcomes in patients with T2DM, are often accompanied by a significant decrease in UA levels, including heart failure (72–74), acute myocardial infarction (AMI) (75), and gout outcomes in atherosclerotic cardiovascular disease patients (76). Notably, the ability of SGLT2is to reduce UA levels and the risk of cardiovascular events is independent of the patient’s heart failure status (77). A recent meta-analysis revealed that SGLT2is significantly reduce serum uric acid levels, with empagliflozin showing the most pronounced effect (-46.75 μmol/L) (78). In patients with type 2 diabetes mellitus (T2DM), baseline uric acid (UA) levels are closely associated with cardiorenal outcomes and the risk of mortality (79). Elevated serum uric acid (SUA) levels can increase the risk of cardiovascular mortality in patients with chronic kidney disease (CKD) (80) and are associated with increased risks of all-cause mortality and cardiovascular disease (CVD) mortality in diabetic patients (81).
Uric acid can also serve as a key clinical indicator of oxidative stress (82). Oxidative stress increases tissue oxygen consumption and impairs mitochondrial function. Studies have shown that SGLT2is improve mitochondrial biogenesis by activating the AMPK/SIRT1/PGC-1α pathway, thereby reducing oxidative stress (83, 84). Oxidative stress increases tissue oxygen consumption and impairs mitochondrial function. Studies have shown that SGLT2is improve mitochondrial biogenesis by activating the AMPK/SIRT1/PGC-1α pathway, thereby reducing oxidative stress (85, 86). Furthermore, long-term SGLT2i treatment can stimulate renal erythropoietin secretion or hypoxia-inducible factor (HIF), promoting erythropoiesis (87–89). This improvement in renal oxygenation may confer significant renal benefits, as hypoxic injury is a common mechanism leading to adverse renal outcomes (90–92). Several mediation analyses from the EMPA-REG OUTCOME and CANVAS trials have indicated that changes in hematocrit and hemoglobin levels mediate a substantial portion of the benefits of SGLT2is (93, 94), with their effects surpassing those of changes in urate and the urinary albumin-to-creatinine ratio (UACR) in improving renal outcomes (94).
3 Clinical significance of metabolic regulation
3.1 Cardiovascular protection
SGLT2is have been incorporated into the HF treatment guidelines as a Class I recommended medication and are applicable to all HF patients across the spectrum of ejection fractions (95). Research indicates that SGLT2is can reduce cardiovascular events and mortality in elderly or frail, high-risk T2DM patients with HF (96), decrease HF events in CKD patients (97), and, through combination therapy, improve metabolism in symptomatic adult congenital heart disease (ACHD) patients (98). These studies not only expand the clinical application scope of SGLT2is but also provide in-depth insights into their potential mechanisms of action. SGLT2is can remodel the left ventricle, improving its structure and function. A meta-analysis by Savage et al. demonstrated that SGLT2is can improve left ventricular function in patients with HF, and a trend toward improvement in left atrial-related indices was also observed (99).
Empagliflozin also has similar cardioprotective effects in patients with prediabetes (100). The cardiovascular benefits of SGLT2is are independent of glycemic control, and a 6-month treatment failed to improve left atrial function in high-risk patients (101). Interestingly, one study found that for patients with acute decompensated heart failure (ADHF), empagliflozin treatment for 5 days improved left atrial volume (102). The inconsistency in therapeutic efficacy may be associated with baseline disease conditions and the patient’s own organic changes, which suggests the importance of personalized treatment in the future. Moreover, SGLT2is can reduce NT-proBNP levels in HF patients, with canagliflozin showing the most significant effect (103). In terms of quality of life (QoL), SGLT2is significantly improved quality of life, as assessed by the Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OSS), after 3 months of treatment (104). Compared with HFpEF, empagliflozin is superior in improving exercise capacity and QoL in patients with heart failure with a reduced ejection fraction (HFrEF) (105).
In patients with myocardial infarction (MI), SGLT2is significantly reduced the hospitalization rate for heart failure (HF) (106–109), regardless of whether the MI occurred recently or in the past (110). A meta-analysis by Mukhopadhyay et al. indicated that SGLT2is reduced the risk of major adverse cardiovascular events (MACEs) in patients with T2DM but did not affect MI or stroke (111). A meta-analysis of 13 randomized controlled trials by Liang et al. further confirmed that SGLT2is significantly reduced the risk of nonfatal myocardial infarction by 12% in patients with T2DM but had no significant effect on the risk of nonfatal stroke. Notably, the study by Liang et al. included four studies from the analysis by Mukhopadhyay et al., with a larger sample size, rendering the results more representative. Research by Asham et al. demonstrated that SGLT2is could reduce all-cause mortality (OR, 0.55; 95% CI, 0.38–0.81; P = 0.002; I² = 0%) and improve the left ventricular ejection fraction (SMD, 0.36; 95% CI, 0.02–0.70; P = 0.04; I² = 62%) (108). A recent study by Jia et al. reported for the first time that SGLT2is can reduce the risks of HF combined with cardiovascular death, all-cause mortality, severe arrhythmias, and renal injury while improving left ventricular function (112). This finding contradicts previous findings that SGLT2is do not significantly improve the risks of all-cause mortality, cardiovascular death, or all-cause hospitalization (106, 107, 110). Although there are some differences in the results of various meta-analyses, some studies have been included repeatedly. On the basis of the current evidence, SGLT2is can significantly reduce the HF hospitalization rate in MI patients and may improve all-cause mortality and MACEs, but further precise evaluation is still needed.
SGLT2is also exert cardiovascular protective effects in patients with atrial fibrillation (AF) and arrhythmias. Studies have shown that SGLT2is can reduce the risk of arrhythmias or atrial fibrillation in patients with T2DM (113). Additionally, SGLT2is can decrease all-cause mortality (RR, 0.37; 95% CI, 0.28–0.50), heart failure (RR, 0.66; 95% CI, 0.53–0.83), stroke (RR, 0.76; 95% CI, 0.66–0.88), and cardiovascular mortality (RR, 0.57; 95% CI, 0.44–0.74) in T2DM patients with AF (114). SGLT2i therapy can also prevent the recurrence of AF after catheter ablation in patients with T2DM (115). However, for high-risk patients (such as those with concurrent HF or CKD, although SGLT2is have potential metabolic benefits), SGLT2i therapy does not significantly reduce the risk of AF occurrence (116).
3.2 Potential for combination therapy
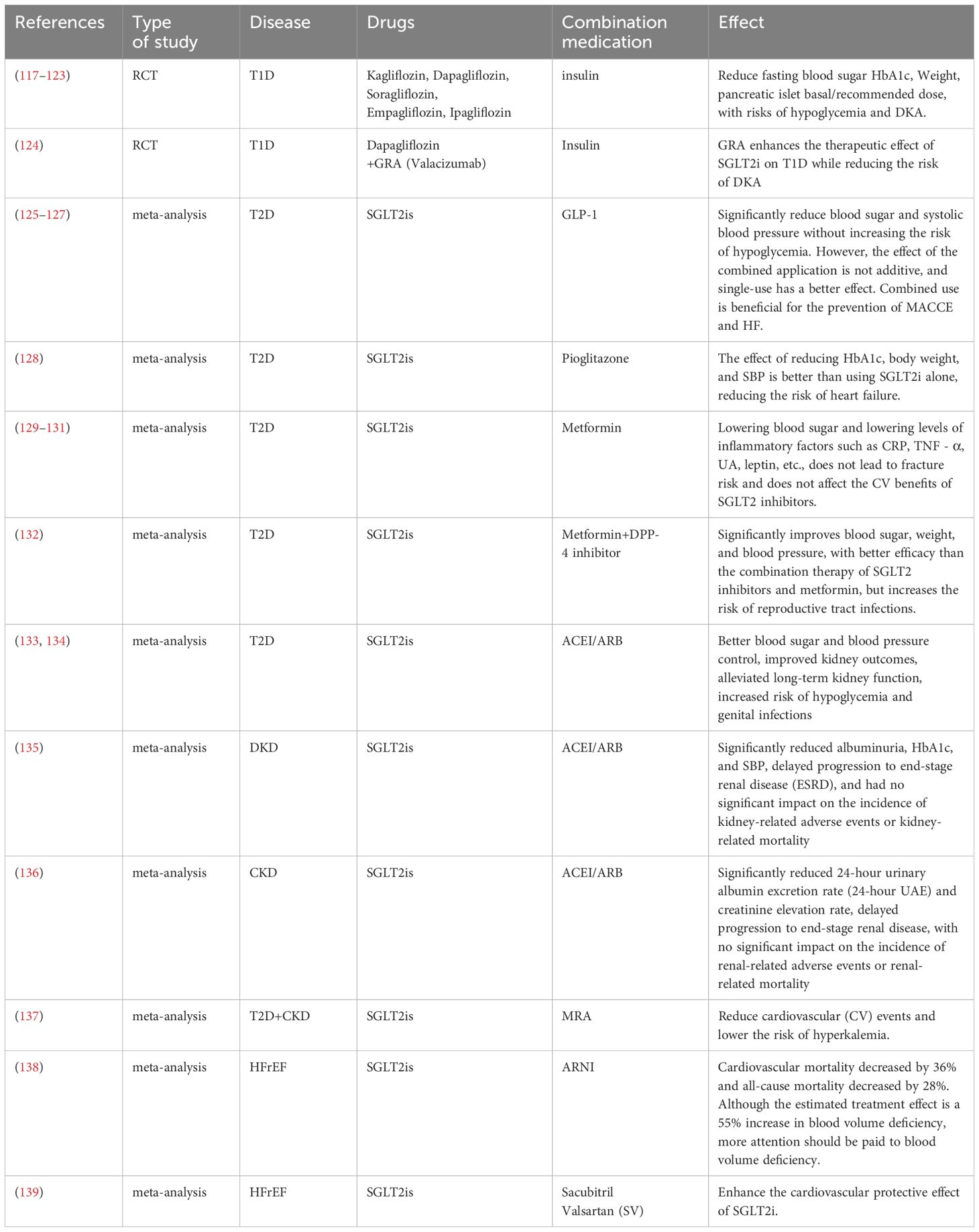
As an adjunct to insulin therapy, SGLT2i therapy can effectively reduce blood glucose levels, decrease body weight, and reduce insulin dosage requirements in patients with type 1 diabetes (T1D). However, its potential adverse effects, such as hypoglycemia and diabetic ketoacidosis (DKA), require careful evaluation. Multiple clinical studies have shown that there are differences in the safety profiles of various SGLT2is: canagliflozin (100/300 mg) increased the incidence of ketoacidosis-related adverse events during an 18-week treatment period (117); dapagliflozin (5/10 mg) was associated with some mild adverse reactions, such as nasopharyngitis or urinary tract infections, and had a lower incidence of severe hypoglycemia during a 24-week treatment period, although the risk of DKA still warrants caution (118, 119); and the 52-week treatment outcomes of sotagliflozin (400 mg) indicated risks of DKA and hypoglycemia (120, 121). In contrast, the risk of DKA with empagliflozin is dose-dependent (10 mg: 4.3%; 25 mg: 3.3%; 2.5 mg: 0.8%) and does not increase the risk of hypoglycemia (122). Additionally, ipragliflozin (50 mg) did not cause significant safety concerns during the 24-week treatment period (123). Recent studies have shown that the combination of dapagliflozin (10 mg) with a glucagon receptor antagonist (GRA) increases glucose-lowering efficacy and reduces the risk of DKA. However, the study had a small sample size (n=12), and larger-scale trials are needed for validation (124). On the basis of the existing evidence, ipragliflozin demonstrates a superior safety profile, whereas a low dose of empagliflozin (2.5 mg) may be a preferable option for patients at risk of hypoglycemia.
As SGLT2is are glucose-lowering, their combined effects with other hypoglycemic drugs are noteworthy. There may be crosstalk between the mechanisms of action of these hypoglycemic agents, and whether their combination results in additive or diminished effects warrants further exploration to optimize blood glucose management. The combination with GLP-1 receptor agonists does not lead to hypoglycemia, and the effects are independent. Both drugs individually have protective effects on the cardiovascular system and kidneys, and their combination does not interfere with each other (125, 126). However, in the prevention of major adverse cardiovascular and cerebrovascular events (MACCEs) and heart failure, their combination has synergistic effects (127). When combined with pioglitazone, SGLT2is have additive effects in terms of hypoglycemia and weight reduction and further reduce the risk of HF (128). When used in combination with metformin, SGLT2is significantly reduce inflammatory markers through anti-inflammatory mechanisms (129) and do not increase the risk of fractures (130). Moreover, the combination does not affect the cardiovascular benefits of SGLT2is (131). Additionally, triple therapy (SGLT2i + metformin + DPP-4 inhibitor) can provide better glycemic control but may increase the risk of genital infections (132). Therefore, when a combination regimen is selected clinically, it is necessary to balance efficacy and safety and to individualize treatment strategies.
The combination of SGLT2is with renin–angiotensin system blockers, including ACE inhibitors and ARBs, has synergistic effects on the treatment of type 2 diabetes mellitus (T2DM). This combination not only enhances glucose-lowering and blood pressure-lowering effects but also significantly improves renal outcomes, although it may increase the risk of hypoglycemia and genital infections (133, 134). In patients with diabetic kidney disease (DKD) and CKD, the combination of SGLT2is with ACEIs/ARBs effectively protects renal function and slows the progression of kidney disease (135, 136). Furthermore, the combination of SGLT2is with mineralocorticoid receptor antagonists (MRAs) provides additional cardiovascular benefits for patients with T2DM and CKD while reducing the risk of hyperkalemia (137). More notably, combination therapy with SGLT2i and angiotensin receptor–neprilysin inhibitor (ARNI) has demonstrated significant efficacy in patients with HFrEF: it reduces the risk of the composite endpoint of heart failure hospitalization or cardiovascular death by 32%, decreases cardiovascular death by 36%, and decreases all-cause mortality by 28%, although it may increase the risk of hypovolemia (138). Combination with sacubitril-valsartan (SV) can also enhance cardiovascular protection in HFrEF patients (139). These findings provide important evidence for optimizing cardiorenal protection strategies in patients with T2DM (Table 1).
4 Future directions and challenges
Although SGLT2is have demonstrated significant pleiotropic effects in the treatment of T2DM and related complications, their clinical application still faces numerous challenges. Future research needs to further optimize treatment strategies and explore the underlying mechanisms involved. Currently, the efficacy of SGLT2is is heterogeneous across different patient populations, and future studies should focus on screening biomarkers to predict patients’ responses to SGLT2is. Additionally, precision medicine models based on artificial intelligence and big data may help optimize dosing regimens and achieve individualized treatment. The pleiotropic mechanisms of SGLT2is have not been fully elucidated, particularly the specific pathways through which they affect adipose tissue remodeling, myocardial energy metabolism, and renal protection. Future research should integrate metabolomics, imaging, and molecular biology techniques to further elucidate the underlying mechanisms of SGLT2is in organ protection. Additionally, the impact of SGLT2is on emerging fields, such as the gut microbiota and immune regulation, also warrants exploration.
In conclusion, the potential of SGLT2is in the management of T2DM remains to be fully explored. Future research should integrate basic and clinical sciences to advance the development of personalized treatment strategies and address existing challenges, thereby maximizing long-term benefits for patients.
5 Summary
SGLT2is exhibit extensive pleiotropic effects in the treatment of T2DM and its complications through a unique mechanism. This article systematically reviews the significant roles of SGLT2is in metabolic regulation, cardiovascular protection, and combination therapy. It also proposes that, on the basis of the metabolic pleiotropy of SGLT2is, the development of individualized treatment strategies is the future trend in the application of SGLT2is. The effects of combination therapy can be synergistic, additive, or independent and carry risks of adverse events such as hypoglycemia, DKA, and local tissue inflammation. Therefore, evaluating the treatment duration, as well as the dosage form and dosage of the medication, are critical factors in targeted therapy. Future research should further explore the precise application of SGLT2is in different populations as well as their long-term safety and their protective mechanisms for specific organs.
In summary, SGLT2is are not only efficient hypoglycemic agents but also multifunctional metabolic modulators, offering a new therapeutic paradigm for the management of T2DM and its complications. With further research, the clinical application prospects of SGLT2is will become even broader, resulting in increased benefits for patients.
Author contributions
QW: Conceptualization, Project administration, Writing – review & editing, Methodology, Writing – original draft. JZ: Project administration, Methodology, Conceptualization, Writing – original draft, Writing – review & editing. DL: Writing – review & editing. FZ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the seventh batch of national senior traditional Chinese medicine experts' academic experience inheritance guidance teacher and inheritor project of the State Administration of Traditional Chinese Medicine [Guozhong Medicine Renjiaofa (2022) No. 76].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. (2018) 41:963–70. doi: 10.2337/dc17-1962
2. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
3. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. (2020) 21:6275. doi: 10.3390/ijms21176275
4. Kalra S, Shetty KK, Nagarajan VB, and Ved JK. Basic and clinical pharmaco-therapeutics of SGLT2 inhibitors: a contemporary update. Diabetes Ther. (2020) 11:813–33. doi: 10.1007/s13300-020-00789-y
5. Kaneto H, Obata A, Kimura T, Shimoda M, Kinoshita T, Matsuoka T, et al. Unexpected pleiotropic effects of SGLT2 inhibitors: pearls and pitfalls of this novel antidiabetic class. Int J Mol Sci. (2021) 22:3062. doi: 10.3390/ijms22063062
6. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
7. Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377:644–57. doi: 10.1056/NEJMoa1611925
8. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2019) 380:347–57. doi: 10.1056/NEJMoa1812389
9. Devenny JJ, Godonis HE, Harvey SJ, Rooney S, Cullen MJ, and Pelleymounter MA. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obes Silver Spring Md. (2012) 20:1645–52. doi: 10.1038/oby.2012.59
10. Yoshida A, Matsubayashi Y, Nojima T, Suganami H, Abe T, Ishizawa M, et al. Attenuation of weight loss through improved antilipolytic effect in adipose tissue via the SGLT2 inhibitor tofogliflozin. J Clin Endocrinol Metab. (2019) 104:3647–60. doi: 10.1210/jc.2018-02254
11. Tsukagoshi-Yamaguchi A, Koshizaka M, Ishibashi R, Ishikawa K, Ishikawa T, Shoji M, et al. Metabolomic analysis of serum samples from a clinical study on ipragliflozin and metformin treatment in Japanese patients with type 2 diabetes: exploring human metabolites associated with visceral fat reduction. Pharmacotherapy. (2023) 43:1317–26. doi: 10.1002/phar.2884
12. Rajeev SP, Roberts CA, Brown E, Sprung VS, Harrold JA, Halford JCG, et al. No evidence of compensatory changes in energy balance, despite reductions in body weight and liver fat, during dapagliflozin treatment in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, cross-over trial (ENERGIZE). Diabetes Obes Metab. (2023) 25:3621–31. doi: 10.1111/dom.15257
13. Hathorn B, Haykowsky MJ, Almandoz J, Pandey A, Sarma S, Hearon CM Jr., et al. Insights into the role of obesity in heart failure with preserved ejection fraction pathophysiology and management. Can J Cardiol. (2025) S0828–282X(25)00199–0. doi: 10.1016/j.cjca.2025.03.015
14. Said F, Arnott C, Voors AA, Heerspink HJL, and Ter Maaten JM. Prediction of new-onset heart failure in patients with type 2 diabetes derived from ALTITUDE and CANVAS. Diabetes Obes Metab. (2024) 26:2741–51. doi: 10.1111/dom.15592
15. Adamson C, Kondo T, Jhund PS, De Boer RA, Cabrera Honorio JW, Claggett B, et al. Dapagliflozin for heart failure according to body mass index: the DELIVER trial. Eur Heart J. (2022) 43:4406–17. doi: 10.1093/eurheartj/ehac481
16. Sezai A, Tanaka A, Imai T, Kida K, Sekino H, Murohara T, et al. Comparing the effects of canagliflozin vs. Glimepiride by body mass index in patients with type 2 diabetes and chronic heart failure: A subanalysis of the CANDLE trial. Biomedicines. (2022) 10:1656. doi: 10.3390/biomedicines10071656
17. Sattar N, Butler J, Lee MMY, Harrington J, Sharma A, Zannad F, et al. Body mass index and cardiorenal outcomes in the EMPEROR-preserved trial: principal findings and meta-analysis with the DELIVER trial. Eur J Heart Fail. (2024) 26:900–9. doi: 10.1002/ejhf.3221
18. Voigt JH, Lauritsen KM, Pedersen SB, Hansen TK, Møller N, Jessen N, et al. Four weeks SGLT2 inhibition improves beta cell function and glucose tolerance without affecting muscle free fatty acid or glucose uptake in subjects with type 2 diabetes. Basic Clin Pharmacol Toxicol. (2024) 134:643–56. doi: 10.1111/bcpt.13991
19. Fadini GP, Bonora BM, Zatti G, Vitturi N, Iori E, Marescotti MC, et al. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovasc Diabetol. (2017) 16:42. doi: 10.1186/s12933-017-0529-3
20. Hussain M, Atif M, Babar M, and Akhtar L. Comparison of efficacy and safety profile of empagliflozin versus dapagliflozin as add on therapy in type 2 diabetic patients. J Ayub Med Coll Abbottabad JAMC. (2021) 33:593–7.
21. Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. (2012) 97:1020–31. doi: 10.1210/jc.2011-2260
22. Yamamoto C, Miyoshi H, Ono K, Sugawara H, Kameda R, Ichiyama M, et al. Ipragliflozin effectively reduced visceral fat in Japanese patients with type 2 diabetes under adequate diet therapy. Endocr J. (2016) 63:589–96. doi: 10.1507/endocrj.EJ15-0749
23. Sezai A, Sekino H, Unosawa S, Taoka M, Osaka S, and Tanaka M. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc Diabetol. (2019) 18:76. doi: 10.1186/s12933-019-0877-2
24. Cefalu WT, Leiter LA, Yoon K-H, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. (2013) 382:941–50. doi: 10.1016/S0140-6736(13)60683-2
25. Latva-Rasku A, Honka M-J, Kullberg J, Mononen N, Lehtimäki T, Saltevo J, et al. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: a randomized, double-blind, placebo-controlled study with 8-week treatment in type 2 diabetes patients. Diabetes Care. (2019) 42:931–7. doi: 10.2337/dc18-1569
26. Hiruma S, Shigiyama F, and Kumashiro N. Empagliflozin versus sitagliptin for ameliorating intrahepatic lipid content and tissue-specific insulin sensitivity in patients with early-stage type 2 diabetes with non-alcoholic fatty liver disease: A prospective randomized study. Diabetes Obes Metab. (2023) 25:1576–88. doi: 10.1111/dom.15006
27. Cinti F, Leccisotti L, Sorice GP, Capece U, D’Amario D, Lorusso M, et al. Dapagliflozin treatment is associated with a reduction of epicardial adipose tissue thickness and epicardial glucose uptake in human type 2 diabetes. Cardiovasc Diabetol. (2023) 22:349. doi: 10.1186/s12933-023-02091-0
28. Bouchi R, Terashima M, Sasahara Y, Asakawa M, Fukuda T, Takeuchi T, et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: A pilot study. Cardiovasc Diabetol. (2017) 16:32. doi: 10.1186/s12933-017-0516-8
29. Cuatrecasas G, De Cabo F, Coves MJ, Patrascioiu I, Aguilar G, Cuatrecasas G, et al. Dapagliflozin added to metformin reduces perirenal fat layer in type 2 diabetic patients with obesity. Sci Rep. (2024) 14:10832. doi: 10.1038/s41598-024-61590-6
30. Wei D, Liao L, Wang H, Zhang W, Wang T, and Xu Z. Canagliflozin ameliorates obesity by improving mitochondrial function and fatty acid oxidation via PPARα in vivo and in vitro. Life Sci. (2020) 247:117414. doi: 10.1016/j.lfs.2020.117414
31. Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, Chen G, et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. (2017) 20:137–49. doi: 10.1016/j.ebiom.2017.05.028
32. Xu L, Xu C, Liu X, Li X, Li T, Yu X, et al. Empagliflozin induces white adipocyte browning and modulates mitochondrial dynamics in KK cg-ay/J mice and mouse adipocytes. Front Physiol. (2021) 12:745058. doi: 10.3389/fphys.2021.745058
33. Oelkrug R, Polymeropoulos ET, and Jastroch M. Brown adipose tissue: physiological function and evolutionary significance. J Comp Physiol [B]. (2015) 185:587–606. doi: 10.1007/s00360-015-0907-7
34. Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, et al. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab. (2015) 22:279–90. doi: 10.1016/j.cmet.2015.06.004
35. Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. (2014) 124:499–508. doi: 10.1172/JCI72227
36. Li H, Hou Y, Xin W, Ding L, Yang Y, Zhang Y, et al. The efficacy of sodium-glucose transporter 2 inhibitors in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Pharmacol Res. (2025) 213:107647. doi: 10.1016/j.phrs.2025.107647
37. Harreiter J, Just I, Leutner M, Bastian M, Brath H, Schelkshorn C, et al. Combined exenatide and dapagliflozin has no additive effects on reduction of hepatocellular lipids despite better glycaemic control in patients with type 2 diabetes mellitus treated with metformin: EXENDA, a 24-week, prospective, randomized, placebo-controlled pilot trial. Diabetes Obes Metab. (2021) 23:1129–39. doi: 10.1111/dom.14319
38. Dihoum A, Brown AJ, McCrimmon RJ, Lang CC, and Mordi IR. Dapagliflozin, inflammation and left ventricular remodelling in patients with type 2 diabetes and left ventricular hypertrophy. BMC Cardiovasc Disord. (2024) 24:356. doi: 10.1186/s12872-024-04022-7
39. Buttice L, Ghani M, Suthakar J, Gnanalingham S, Carande E, Kennedy BWC, et al. The effect of sodium-glucose cotransporter-2 inhibitors on inflammatory biomarkers: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. (2024) 26:2706–21. doi: 10.1111/dom.15586
40. Paramel Varghese G, Folkersen L, Strawbridge RJ, Halvorsen B, Yndestad A, Ranheim T, et al. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc. (2016) 5:e003031. doi: 10.1161/JAHA.115.003031
41. Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. (1997) 82:4196–200. doi: 10.1210/jcem.82.12.4450
42. Gohari S, Ismail-Beigi F, Mahjani M, Ghobadi S, Jafari A, Ahangar H, et al. The effect of sodium-glucose co-transporter-2 (SGLT2) inhibitors on blood interleukin-6 concentration: a systematic review and meta-analysis of randomized controlled trials. BMC Endocr Disord. (2023) 23:257. doi: 10.1186/s12902-023-01512-1
43. Tsigos C, Papanicolaou DA, Kyrou I, Defensor R, Mitsiadis CS, and Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J Clin Endocrinol Metab. (1997) 82:4167–70. doi: 10.1210/jcem.82.12.4422
44. Bechmann LE, Emanuelsson F, Nordestgaard BG, and Benn M. SGLT2-inhibition increases total, LDL, and HDL cholesterol and lowers triglycerides: meta-analyses of 60 randomized trials, overall and by dose, ethnicity, and drug type. Atherosclerosis. (2024) 394:117236. doi: 10.1016/j.atherosclerosis.2023.117236
45. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the european atherosclerosis society consensus panel. Eur Heart J. (2017) 38:2459–72. doi: 10.1093/eurheartj/ehx144
46. Rizos EC, Tagkas CF, Asimakopoulos A-GI, Tsimihodimos V, Anastasiou G, Rizzo M, et al. The effect of SGLT2 inhibitors and GLP1 receptor agonists on arterial stiffness: a meta-analysis of randomized controlled trials. J Diabetes Complications. (2024) 38:108781. doi: 10.1016/j.jdiacomp.2024.108781
47. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Lond Engl. (2019) 393:31–9. doi: 10.1016/S0140-6736(18)32590-X
48. Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CSP, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. J Am Heart Assoc. (2020) 9:e014908. doi: 10.1161/JAHA.119.014908
49. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: A meta-analysis. JAMA Cardiol. (2021) 6:148. doi: 10.1001/jamacardio.2020.4511
50. Kullmann S, Hummel J, Wagner R, Dannecker C, Vosseler A, Fritsche L, et al. Empagliflozin improves insulin sensitivity of the hypothalamus in humans with prediabetes: a randomized, double-blind, placebo-controlled, phase 2 trial. Diabetes Care. (2022) 45:398–406. doi: 10.2337/dc21-1136
51. Aso Y, Kato K, Sakurai S, Kishi H, Shimizu M, Jojima T, et al. Impact of dapagliflozin, an SGLT2 inhibitor, on serum levels of soluble dipeptidyl peptidase-4 in patients with type 2 diabetes and non-alcoholic fatty liver disease. Int J Clin Pract. (2019) 73:e13335. doi: 10.1111/ijcp.13335
52. Parveen R, Hussain S, Saini S, Khan P, Saha N, and Nidhi N. Effect of ipragliflozin on liver enzymes in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Expert Opin Pharmacother. (2024) 25:925–35. doi: 10.1080/14656566.2024.2360078
53. Zafar Y, Rashid AM, Siddiqi AK, Ellahi A, Ahmed A, Hussain HU, et al. Effect of novel glucose lowering agents on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. (2022) 46:101970. doi: 10.1016/j.clinre.2022.101970
54. Ong Lopez AMC and Pajimna JAT. Efficacy of sodium glucose cotransporter 2 inhibitors on hepatic fibrosis and steatosis in non-alcoholic fatty liver disease: an updated systematic review and meta-analysis. Sci Rep. (2024) 14:2122. doi: 10.1038/s41598-024-52603-5
55. Malandris K, Papandreou S, Avgerinos I, Karagiannis T, Paschos P, Michailidis T, et al. Comparative efficacy of glucose-lowering drugs on liver steatosis as assessed by means of magnetic resonance imaging in patients with type 2 diabetes mellitus: Systematic review and network meta-analysis. Horm Athens Greece. (2023) 22:655–64. doi: 10.1007/s42000-023-00493-z
56. Zhou P, Tan Y, Hao Z, Xu W, Zhou X, and Yu J. Effects of SGLT2 inhibitors on hepatic fibrosis and steatosis: A systematic review and meta-analysis. Front Endocrinol. (2023) 14:1144838. doi: 10.3389/fendo.2023.1144838
57. Jang H, Kim Y, Lee DH, Joo SK, Koo BK, Lim S, et al. Outcomes of various classes of oral antidiabetic drugs on nonalcoholic fatty liver disease. JAMA Intern Med. (2024) 184:375. doi: 10.1001/jamainternmed.2023.8029
58. Marton A, Kaneko T, Kovalik J-P, Yasui A, Nishiyama A, Kitada K, et al. Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nat Rev Nephrol. (2021) 17:65–77. doi: 10.1038/s41581-020-00350-x
59. Gaborit B, Ancel P, Abdullah AE, Maurice F, Abdesselam I, Calen A, et al. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: the EMPACEF study. Cardiovasc Diabetol. (2021) 20:57. doi: 10.1186/s12933-021-01237-2
60. Pietschner R, Kolwelter J, Bosch A, Striepe K, Jung S, Kannenkeril D, et al. Effect of empagliflozin on ketone bodies in patients with stable chronic heart failure. Cardiovasc Diabetol. (2021) 20:219. doi: 10.1186/s12933-021-01410-7
61. Voorrips SN, Boorsma EM, Beusekamp JC, DE-Boer RA, Connelly MA, Dullaart RPF, et al. Longitudinal changes in circulating ketone body levels in patients with acute heart failure: A post hoc analysis of the EMPA-response-AHF trial. J Card Fail. (2023) 29:33–41. doi: 10.1016/j.cardfail.2022.09.009
62. Selvaraj S, Fu Z, Jones P, Kwee LC, Windsor SL, Ilkayeva O, et al. Metabolomic profiling of the effects of dapagliflozin in heart failure with reduced ejection fraction: DEFINE-HF. Circulation. (2022) 146:808–18. doi: 10.1161/CIRCULATIONAHA.122.060402
63. Oldgren J, Laurila S, Åkerblom A, Latva-Rasku A, Rebelos E, Isackson H, et al. Effects of 6 weeks of treatment with dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on myocardial function and metabolism in patients with type 2 diabetes: A randomized, placebo-controlled, exploratory study. Diabetes Obes Metab. (2021) 23:1505–17. doi: 10.1111/dom.14363
64. Lytvyn Y, Škrtić M, Yang GK, Yip PM, Perkins BA, and Cherney DZI. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. (2015) 308:F77–83. doi: 10.1152/ajprenal.00555.2014
65. Novikov A, Fu Y, Huang W, Freeman B, Patel R, van Ginkel C, et al. SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am J Physiol Renal Physiol. (2019) 316:F173–85. doi: 10.1152/ajprenal.00462.2018
66. Suijk DLS, van Baar MJB, van Bommel EJM, Iqbal Z, Krebber MM, Vallon V, et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Clin J Am Soc Nephrol CJASN. (2022) 17:663–71. doi: 10.2215/CJN.11480821
67. Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, Nakanishi T, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. (2014) 35:391–404. doi: 10.1002/bdd.1909
68. Ouchi M, Oba K, Kaku K, Suganami H, Yoshida A, Fukunaka Y, et al. Uric acid lowering in relation to HbA1c reductions with the SGLT2 inhibitor tofogliflozin. Diabetes Obes Metab. (2018) 20:1061–5. doi: 10.1111/dom.13170
69. Chino Y, Kuwabara M, and Hisatome I. Factors influencing change in serum uric acid after administration of the sodium-glucose cotransporter 2 inhibitor luseogliflozin in patients with type 2 diabetes mellitus. J Clin Pharmacol. (2022) 62:366–75. doi: 10.1002/jcph.1970
70. Li J, Badve SV, Zhou Z, Rodgers A, Day R, Oh R, et al. The effects of canagliflozin on gout in type 2 diabetes: a post-hoc analysis of the CANVAS program. Lancet Rheumatol. (2019) 1:e220–8. doi: 10.1016/S2665-9913(19)30078-5
71. McDowell K, Welsh P, Docherty KF, Morrow DA, Jhund PS, de Boer RA, et al. Dapagliflozin reduces uric acid concentration, an independent predictor of adverse outcomes in DAPA-HF. Eur J Heart Fail. (2022) 24:1066–76. doi: 10.1002/ejhf.2433
72. Davies MJ, Trujillo A, Vijapurkar U, Damaraju CV, and Meininger G. Effect of canagliflozin on serum uric acid in patients with type 2 diabetes mellitus. Diabetes Obes Metab. (2015) 17:426–9. doi: 10.1111/dom.12439
73. Zhang C, Ren W, Lu X, Feng L, Li J, and Zhu B. Empagliflozin’s role in early tubular protection for type 2 diabetes patients. Mol Med Camb Mass. (2024) 30:112. doi: 10.1186/s10020-024-00881-0
74. Nagao M, Sasaki J, Tanimura-Inagaki K, Sakuma I, Sugihara H, Oikawa S, et al. Ipragliflozin and sitagliptin differentially affect lipid and apolipoprotein profiles in type 2 diabetes: the SUCRE study. Cardiovasc Diabetol. (2024) 23:56. doi: 10.1186/s12933-024-02149-7
75. Shimizu W, Kubota Y, Hoshika Y, Mozawa K, Tara S, Tokita Y, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: The EMBODY trial. Cardiovasc Diabetol. (2020) 19:148. doi: 10.1186/s12933-020-01127-z
76. Sridhar VS, Cosentino F, Dagogo-Jack S, McGuire DK, Pratley RE, Cater NB, et al. Effects of ertugliflozin on uric acid and gout-related outcomes in persons with type 2 diabetes and cardiovascular disease: post hoc analyses from VERTIS CV. Diabetes Obes Metab. (2024) 26:5336–46. doi: 10.1111/dom.15895
77. Diallo A, Diallo MF, Carlos-Bolumbu M, and Galtier F. Uric acid-lowering effects of sodium-glucose cotransporter 2 inhibitors for preventing cardiovascular events and mortality: a systematic review and meta-analysis. Diabetes Obes Metab. (2024) 26:1980–5. doi: 10.1111/dom.15483
78. Sridharan K and Alkhidir MMOH. Hypouricemic effect of sodium glucose transporter-2 inhibitors: a network meta-analysis and meta-regression of randomized clinical trials. Expert Rev Endocrinol Metab. (2025) 20:139–46. doi: 10.1080/17446651.2025.2456504
79. Verma S, Ji Q, Bhatt DL, Mazer CD, Al-Omran M, Inzucchi SE, et al. Association between uric acid levels and cardio-renal outcomes and death in patients with type 2 diabetes: a subanalysis of EMPA-REG OUTCOME. Diabetes Obes Metab. (2020) 22:1207–14. doi: 10.1111/dom.13991
80. Luo Q, Xia X, Li B, Lin Z, Yu X, and Huang F. Serum uric acid and cardiovascular mortality in chronic kidney disease: a meta-analysis. BMC Nephrol. (2019) 20:18. doi: 10.1186/s12882-018-1143-7
81. Li B, Chen L, Hu X, Tan T, Yang J, Bao W, et al. Association of serum uric acid with all-cause and cardiovascular mortality in diabetes. Diabetes Care. (2023) 46:425–33. doi: 10.2337/dc22-1339
82. Corso LML, Wing RR, Tate DF, Espeland MA, Blanchard BE, and McCaffery JM. Uric acid as a predictor of weight gain and cardiometabolic health in the study of novel approaches to weight gain prevention (SNAP) study. Int J Obes 2005. (2022) 46:1556–9. doi: 10.1038/s41366-022-01131-1
83. Yang X, Liu Q, Li Y, Tang Q, Wu T, Chen L, et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-pgc-1α signalling pathway. Adipocyte. (2020) 9:484–94. doi: 10.1080/21623945.2020.1807850
84. Tanaka S, Sugiura Y, Saito H, Sugahara M, Higashijima Y, Yamaguchi J, et al. Sodium–glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int. (2018) 94:912–25. doi: 10.1016/j.kint.2018.04.025
85. Faucon A-L, Flamant M, Metzger M, Boffa J-J, Haymann J-P, Houillier P, et al. Extracellular fluid volume is associated with incident end-stage kidney disease and mortality in patients with chronic kidney disease. Kidney Int. (2019) 96:1020–9. doi: 10.1016/j.kint.2019.06.017
86. Tsai Y-C, Tsai J-C, Chen S-C, Chiu Y-W, Hwang S-J, Hung C-C, et al. Association of fluid overload with kidney disease progression in advanced CKD: a prospective cohort study. Am J Kidney Dis Off J Natl Kidney Found. (2014) 63:68–75. doi: 10.1053/j.ajkd.2013.06.011
87. Mazer CD, Hare GMT, Connelly PW, Gilbert RE, Shehata N, Quan A, et al. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation. (2020) 141:704–7. doi: 10.1161/CIRCULATIONAHA.119.044235
88. Bessho R, Takiyama Y, Takiyama T, Kitsunai H, Takeda Y, Sakagami H, et al. Hypoxia-inducible factor-1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci Rep. (2019) 9:14754. doi: 10.1038/s41598-019-51343-1
89. Packer M. Mutual antagonism of hypoxia-inducible factor isoforms in cardiac, vascular, and renal disorders. JACC Basic Transl Sci. (2020) 5:961–8. doi: 10.1016/j.jacbts.2020.05.006
90. Nangaku M. Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol. (2006) 17:17–25. doi: 10.1681/ASN.2005070757
91. Singh DK, Winocour P, and Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol. (2008) 4:216–26. doi: 10.1038/ncpneph0757
92. Pruijm M, Milani B, Pivin E, Podhajska A, Vogt B, Stuber M, et al. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int. (2018) 93:932–40. doi: 10.1016/j.kint.2017.10.020
93. Wanner C, Nangaku M, Kraus BJ, Zinman B, Mattheus M, Hantel S, et al. How do SGLT2 inhibitors protect the kidney? A mediation analysis of the EMPA-REG OUTCOME trial. Nephrol Dial Transplant. (2024) 39:1504–13. doi: 10.1093/ndt/gfae032
94. Li J, Neal B, Perkovic V, De Zeeuw D, Neuen BL, Arnott C, et al. Mediators of the effects of canagliflozin on kidney protection in patients with type 2 diabetes. Kidney Int. (2020) 98:769–77. doi: 10.1016/j.kint.2020.04.051
95. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2023) 44:3627–39. doi: 10.1093/eurheartj/ehad195
96. Aldafas R, Crabtree T, Alkharaiji M, Vinogradova Y, and Idris I. Sodium-glucose cotransporter-2 inhibitors (SGLT2) in frail or older people with type 2 diabetes and heart failure: a systematic review and meta-analysis. Age Ageing. (2024) 53:afad254. doi: 10.1093/ageing/afad254
97. Theodorakopoulou MP, Alexandrou M-E, Tsitouridis A, Kamperidis V, Pella E, Xanthopoulos A, et al. Effects of sodium-glucose co-transporter 2 inhibitors on heart failure events in chronic kidney disease: a systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. (2024) 10:329–41. doi: 10.1093/ehjcvp/pvae003
98. Das BB and Niu J. A systematic review and meta-analysis of the safety and efficacy of SGLT2 inhibitors in chronic heart failure in ACHD patients. Am J Cardiovasc Drugs Drugs Dev Interv. (2025) 25:231–40. doi: 10.1007/s40256-024-00697-7
99. Savage P, Watson C, Coburn J, Cox B, Shahmohammadi M, Grieve D, et al. Impact of SGLT2 inhibition on markers of reverse cardiac remodelling in heart failure: Systematic review and meta-analysis. Esc Heart Fail. (2024) 11:3636–48. doi: 10.1002/ehf2.14993
100. Afshani MR, Torfi E, Akiash N, Jahanshahi A, Mohamadi A, and Sherafat O. Effect of empagliflozin on left ventricular volumes in type 2 diabetes or prediabetes heart failure patients with reduced ejection fraction. Acta Cardiol. (2024) 79:419–25. doi: 10.1080/00015385.2023.2240130
101. Pourafkari M, Connelly KA, Verma S, Mazer CD, Teoh H, Quan A, et al. Empagliflozin and left atrial function in patients with type 2 diabetes mellitus and coronary artery disease: insight from the EMPA-HEART CardioLink-6 randomized clinical trial. Cardiovasc Diabetol. (2024) 23:319. doi: 10.1186/s12933-024-02344-6
102. Bogoviku J, Nguyen TD, Westphal JG, Aftanski P, Moebius-Winkler S, Haertel F, et al. Acute effects of empagliflozin on left atrial and ventricular filling parameters using echocardiography-a subanalysis of the EMPAG-HF trial. Eur Heart J Cardiovasc Pharmacother. (2025) 11:190–7. doi: 10.1093/ehjcvp/pvaf003
103. Wang N, Wu Z, Ren J, Zheng X, and Han X. SGLT2 inhibitors in patients with heart failure: a model-based meta-analysis. Clin Pharmacokinet. (2024) 63:1667–78. doi: 10.1007/s40262-024-01443-7
104. Oriecuia C, Tomasoni D, Sala I, Bonfioli GB, Adamo M, Gussago C, et al. Sodium glucose co-transporter 2 inhibitors and quality of life in patients with heart failure: a comprehensive systematic review and meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. (2024) 10:147–57. doi: 10.1093/ehjcvp/pvad088
105. Yan Q, Chen X, Yu C, and Yin Y. Long-term surrogate cardiovascular outcomes of SGLT2 inhibitor empagliflozin in chronic heart failure: A systematic review and meta-analysis. BMC Cardiovasc Disord. (2024) 24:663. doi: 10.1186/s12872-024-04316-w
106. Xiong B, He L, Zhang A, and Ling Z. Effect of sodium glucose cotransporter 2 inhibitors on all cause death and rehospitalization for heart failure in patients with acute myocardial infarction. Sci Rep. (2024) 14:30148. doi: 10.1038/s41598-024-81954-2
107. Ahmed M, Jain H, Javaid H, Ahsan A, Szilagyi S, Ahmad A, et al. Efficacy of sodium-glucose cotransporter-2 inhibitors in patients with acute myocardial infarction: A meta-analysis of randomised controlled trials. Endocrinol Diabetes Metab. (2024) 7:e514. doi: 10.1002/edm2.514
108. Asham H, Ghaffari S, Taban-Sadeghi M, and Entezari-Maleki T. Efficacy and safety of SGLT-2 inhibitors in acute myocardial infarction: A systematic review and meta-analysis. J Clin Pharmacol. (2025) 65:303–17. doi: 10.1002/jcph.6149
109. Idowu A, Adebolu O, Wattanachayakul P, Obomanu E, Shah S, Lo KB, et al. Cardiovascular outcomes of sodium-glucose co-transporter 2 inhibitors use after myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Curr Probl Cardiol. (2024) 49:102648. doi: 10.1016/j.cpcardiol.2024.102648
110. Scardini PG, Shih Katsuyama E, Armani Prata A, Marques Fernandes J, Ken Fukunaga C, Falco Neto W, et al. Impact of sodium–glucose cotransporter-2 inhibitors in patients with recent versus previous myocardial infarction: a systematic review and meta-analysis. Cardiovasc Diabetol. (2025) 24:73. doi: 10.1186/s12933-024-02540-4
111. Mukhopadhyay P, Sanyal D, Chatterjee P, Pandit K, and Ghosh S. SGLT2 inhibitors: effect on myocardial infarction and stroke in type 2 diabetes. J Clin Endocrinol Metab. (2023) 108:2134–40. doi: 10.1210/clinem/dgad113
112. Jia Q, Zuo A, Song H, Zhang C, Fu X, Hu K, et al. Effects of sodium-glucose cotransporter-2 inhibitors in myocardial infarction patients: a systematic review and meta-analysis. Diabetes Obes Metab. (2025) 27:1276–86. doi: 10.1111/dom.16122
113. Xu B, Kang B, and Zhou J. Sodium glucose cotransporter 2 inhibitors with cardiac arrhythmias in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized placebo-controlled trials. Clin Res Cardiol Off J Ger Card Soc. (2024) 113:910–23. doi: 10.1007/s00392-024-02386-6
114. Ameer MZ, Rehman AU, Amjad Z, Khan S, Ameer F, Shirwany HAK, et al. Cardiovascular outcomes with SGLT-2 inhibitors in individuals with diabetes and co-existing atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. (2025) 426:133083. doi: 10.1016/j.ijcard.2025.133083
115. Abdelhadi NA, Ragab KM, Elkholy M, Koneru J, Ellenbogen KA, and Pillai A. Impact of sodium-glucose co-transporter 2 inhibitors on atrial fibrillation recurrence post-catheter ablation among patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. (2025) 36:673–82. doi: 10.1111/jce.16544
116. Zhang H-D, Ding L, Mi L-J, Zhang A-K, Zhang K, Jiang Z-H, et al. Sodium-glucose co-transporter-2 inhibitors for the prevention of atrial fibrillation: a systemic review and meta-analysis. Eur J Prev Cardiol. (2024) 31:770–9. doi: 10.1093/eurjpc/zwad356
117. Henry RR, Thakkar P, Tong C, Polidori D, and Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. (2015) 38:2258–65. doi: 10.2337/dc15-1730
118. Dandona P, Mathieu C, Phillip M, Hansen L, Griffen SC, Tschöpe D, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. (2017) 5:864–76. doi: 10.1016/S2213-8587(17)30308-X
119. Mathieu C, Dandona P, Gillard P, Senior P, Hasslacher C, Araki E, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care. (2018) 41:1938–46. doi: 10.2337/dc18-0623
120. Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the north american inTandem1 study. Diabetes Care. (2018) 41:1970–80. doi: 10.2337/dc18-0343
121. Danne T, Pettus J, Giaccari A, Cariou B, Rodbard H, Weinzimer SA, et al. Sotagliflozin added to optimized insulin therapy leads to lower rates of clinically relevant hypoglycemic events at any HbA1c at 52 weeks in adults with type 1 diabetes. Diabetes Technol Ther. (2019) 21:471–7. doi: 10.1089/dia.2019.0157
122. Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care. (2018) 41:2560–9. doi: 10.2337/dc18-1749
123. Kaku K, Isaka H, Sakatani T, and Toyoshima J. Efficacy and safety of ipragliflozin add-on therapy to insulin in Japanese patients with type 1 diabetes mellitus: a randomized, double-blind, phase 3 trial. Diabetes Obes Metab. (2019) 21:2284–93. doi: 10.1111/dom.13807
124. Boeder SC, Thomas RL, Le Roux MJ, Giovannetti ER, Gregory JM, and Pettus JH. Combination SGLT2 inhibitor and glucagon receptor antagonist therapy in type 1 diabetes: A randomized clinical trial. Diabetes Care. (2025) 48:52–60. doi: 10.2337/dc24-0212
125. Apperloo EM, Neuen BL, Fletcher RA, Jongs N, Anker SD, Bhatt DL, et al. Efficacy and safety of SGLT2 inhibitors with and without glucagon-like peptide 1 receptor agonists: A SMART-C collaborative meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. (2024) 12:545–57. doi: 10.1016/S2213-8587(24)00155-4
126. Neuen BL, Fletcher RA, Heath L, Perkovic A, Vaduganathan M, Badve SV, et al. Cardiovascular, kidney, and safety outcomes with GLP-1 receptor agonists alone and in combination with SGLT2 inhibitors in type 2 diabetes: a systematic review and meta-analysis. Circulation. (2024) 150:1781–90. doi: 10.1161/CIRCULATIONAHA.124.071689
127. Wright AK, Carr MJ, Kontopantelis E, Leelarathna L, Thabit H, Emsley R, et al. Primary prevention of cardiovascular and heart failure events with SGLT2 inhibitors, GLP-1 receptor agonists, and their combination in type 2 diabetes. Diabetes Care. (2022) 45:909–18. doi: 10.2337/dc21-1113
128. Anson M, Henney AE, Zhao SS, Ibarburu GH, Lip GYH, Cuthbertson DJ, et al. Effect of combination pioglitazone with sodium-glucose cotransporter-2 inhibitors or glucagon-like peptide-1 receptor agonists on outcomes in type 2 diabetes: a systematic review, meta-analysis, and real-world study from an international federated database. Diabetes Obes Metab. (2024) 26:2606–23. doi: 10.1111/dom.15576
129. Cao Y, Liang N, Liu T, Fang J, and Zhang X. Effect of SGLT2 inhibitors and metformin on inflammatory and prognostic biomarkers in type 2 diabetes patients. Endocr Metab Immune Disord Drug Targets. (2023) 23:530–47. doi: 10.2174/1871530322666220827150054
130. Qian B-B, Chen Q, Li L, and Yan C-F. Association between combined treatment with SGLT2 inhibitors and metformin for type 2 diabetes mellitus on fracture risk: a meta-analysis of randomized controlled trials. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. (2020) 31:2313–20. doi: 10.1007/s00198-020-05590-y
131. Neuen BL, Arnott C, Perkovic V, Figtree G, de Zeeuw D, Fulcher G, et al. Sodium-glucose co-transporter-2 inhibitors with and without metformin: a meta-analysis of cardiovascular, kidney and mortality outcomes. Diabetes Obes Metab. (2021) 23:382–90. doi: 10.1111/dom.14226
132. Li M, Wang S, and Wang X. Efficacy and safety of triple therapy with SGLT-2 inhibitor, DPP-4 inhibitor, and metformin in type 2 diabetes: a meta-analysis. Altern Ther Health Med. (2023) 29:320–6.
133. Cai Y, Shi W, and Xu G. The efficacy and safety of SGLT2 inhibitors combined with ACEI/ARBs in the treatment of type 2 diabetes mellitus: a meta-analysis of randomized controlled studies. Expert Opin Drug Saf. (2020) 19:1497–504. doi: 10.1080/14740338.2020.1817378
134. Tian B, Deng Y, Cai Y, Han M, and Xu G. Efficacy and safety of combination therapy with sodium-glucose cotransporter 2 inhibitors and renin-angiotensin system blockers in patients with type 2 diabetes: A systematic review and meta-analysis. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. (2022) 37:720–9. doi: 10.1093/ndt/gfab048
135. Woodhams LM, Chalmers L, Sim TF, Yeap BB, Schlaich MP, Schultz C, et al. Efficacy and safety of sodium glucose cotransporter 2 inhibitors plus standard care in diabetic kidney disease: a systematic review and meta-analysis. J Diabetes Complications. (2023) 37:108456. doi: 10.1016/j.jdiacomp.2023.108456
136. Liu T, Li R, Wang X, Gao X, and Zhang X. Benefits of SGLT2 inhibitors combining with renin-angiotensin-system blockers on cardiovascular outcomes in chronic kidney disease patients: A systemic review and meta-analysis. Med Clin (Barc). (2022) 159:65–72. doi: 10.1016/j.medcli.2021.09.031
137. Tsukamoto S, Morita R, Yamada T, Urate S, Azushima K, Uneda K, et al. Cardiovascular and kidney outcomes of combination therapy with sodium-glucose cotransporter-2 inhibitors and mineralocorticoid receptor antagonists in patients with type 2 diabetes and chronic kidney disease: a systematic review and network meta-analysis. Diabetes Res Clin Pract. (2022) 194:110161. doi: 10.1016/j.diabres.2022.110161
138. Huang Y, Fang C, Zhang Y, Ma L, Zhou H, and Ye H. Effectiveness and safety of angiotensin receptor-neprilysin inhibitor and sodium-glucose cotransporter-2 inhibitors for patients with heart failure with reduced ejection fraction: a meta-analysis. J Cardiovasc Med Hagerstown Md. (2023) 24:123–31. doi: 10.2459/JCM.0000000000001426
Keywords: SGLT2is, metabolic regulation, type 2 diabetes, cardiovascular protection, combination therapy
Citation: Wu Q, Zhang J, Zhang F and Li D (2025) SGLT2 inhibitors as metabolic modulators: beyond glycemic control in type 2 diabetes. Front. Endocrinol. 16:1601633. doi: 10.3389/fendo.2025.1601633
Received: 28 March 2025; Accepted: 02 June 2025;
Published: 24 June 2025.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Mona Mashayekhi, Vanderbilt University Medical Center, United StatesGaocan Ren, China Academy of Chinese Medical Sciences, China
Irtiza Hasan, University of Florida, United States
Copyright © 2025 Wu, Zhang, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuli Zhang, ZnVsaTc1MDVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Qian Wu
Qian Wu Jian Zhang
Jian Zhang Fuli Zhang2*
Fuli Zhang2*