- Department of Gynecology, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou, Henan, China
Background: Polycystic ovary syndrome (PCOS) is associated with increased risk of gestational diabetes mellitus (GDM), but reliable early predictive biomarkers remain lacking. This study investigated the predictive value of serum Sortilin, HMGB1, and galanin-like peptide (GALP) for GDM development in PCOS pregnancies.
Methods: This prospective cohort study enrolled 139 PCOS patients. Serum Sortilin, HMGB1 and GALP levels were measured by ELISA at 8-12 weeks. GDM was diagnosed at 24-28 weeks using 75g OGTT (IADPSG criteria). Predictive performance was assessed using multivariable logistic regression and receiver operating characteristic (ROC) curve analysis, with adjustment for maternal age, BMI, and lipid profiles.
Results: The PCOS-GDM group (n=60) showed significantly higher levels of all biomarkers versus controls (n=79) (all p<0.001). GALP (aOR=1.55, 95%CI:1.05-1.92) and HMGB1 (aOR=1.65, 95%CI:1.50-1.79) independently predicted GDM after adjustment. The combined model achieved superior prediction (AUC=0.84, 95%CI:0.74-0.94) versus individual markers.
Conclusion: Serum GALP and HMGB1 are promising early predictors of GDM in PCOS pregnancies, with combined assessment offering optimal risk stratification. These findings may facilitate timely intervention in high-risk populations.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine and metabolic disorders affecting women of reproductive age, with a prevalence ranging from 5% to 15% (1–4). Characterized by heterogeneous clinical manifestations, PCOS typically presents with menstrual irregularities, chronic anovulation, hyperandrogenism, and insulin resistance (5–7). Beyond its reproductive implications, PCOS is associated with a spectrum of metabolic comorbidities, including type 2 diabetes mellitus, dyslipidemia, and increased cardiovascular risk, underscoring its long-term health burden (8–10). In the context of pregnancy, women with PCOS are at increased risk for a range of adverse pregnancy outcomes, such as early miscarriage, gestational hypertension, preeclampsia, and preterm delivery (11–13).
Gestational diabetes mellitus (GDM) is defined as the first onset or diagnosis of glucose metabolism abnormalities during pregnancy (14, 15). With the updating of diagnostic criteria, increasing proportion of advanced maternal age, and improved nutritional status, its incidence continues to rise. GDM poses significant threats to both maternal and fetal health in both short- and long-term perspectives (16–18). However, due to its insidious early symptoms, diagnosis typically relies on oral glucose tolerance tests during the second trimester, by which time adverse effects have already been established (19, 20). Therefore, early prediction and intervention are crucial. The pathogenesis of pregnancy complications in PCOS patients is currently believed to be closely associated with the interplay between endocrine dysfunction, chronic inflammatory state, and metabolic abnormalities (21–23). Studies demonstrate that PCOS, as a significant risk factor for GDM, is characterized by pre-existing endocrine and metabolic disturbances prior to pregnancy (13, 24). When superimposed with physiological hormonal changes and exacerbated insulin resistance during gestation, these women face a higher susceptibility to GDM (25, 26).
Despite growing understanding of these risks, reliable serological biomarkers for early pregnancy screening of high-risk populations remain lacking in clinical practice (22, 27). Early identification of GDM risk in PCOS patients is critical for implementing timely clinical interventions and improving perinatal outcomes (28, 29). Consequently, exploring and validating serum biomarkers capable of predicting GDM in this population has emerged as an urgent and significant clinical need. Early identification of GDM risk in PCOS pregnancies is clinically important, as it allows for timely interventions to prevent adverse maternal and fetal outcomes. This study focuses on investigating potential predictive biomarkers for GDM in PCOS pregnancies, aiming to establish an early warning system that could facilitate personalized clinical management strategies.
Recent studies have revealed that Sortilin, a member of the Vps10p receptor family, participates in glucose homeostasis regulation by modulating lipid metabolism and insulin signaling pathways (30, 31). Meanwhile, high-mobility group box 1 (HMGB1), as a critical proinflammatory mediator, exacerbates insulin resistance through activation of the TLR4/NF-κB pathway (32). Additionally, the neuroendocrine peptide galanin-like peptide (GALP) is involved in glucose metabolism regulation by influencing feeding behavior, energy metabolism, and insulin sensitivity (33, 34). Notably, PCOS patients exhibit characteristic endocrine-metabolic disturbances, which may lead to distinctive alterations in the expression profiles of these biomarkers in serum (17, 35).
However, the predictive value of combined detection of Sortilin, HMGB1, and GALP for assessing GDM risk in PCOS patients remains unclear, and the synergistic mechanisms underlying their roles in the progression from PCOS to GDM have not been systematically investigated. A comprehensive exploration of the dynamic changes in these biomarkers and their predictive potential for GDM development would not only contribute to elucidating the pathogenesis of PCOS-associated GDM but, more importantly, could provide novel molecular targets and predictive strategies for the early identification of high-risk individuals in clinical practice. Therefore, this study aims to evaluate the predictive value of serum Sortilin, HMGB1, and GALP levels measured in early pregnancy for the subsequent development of GDM in women with PCOS. The primary outcome is to determine whether these biomarkers independently or jointly predict GDM onset in this high-risk population. The secondary outcome is to explore the potential pathophysiological relevance and dynamic changes of these biomarkers during pregnancy in PCOS patients, thereby providing insights into novel mechanisms and early intervention strategies.
Methods
Study population
This prospective cohort study enrolled 139 pregnant women with PCOS who attended Henan Provincial People’s Hospital between May 2019 and December 2023. The study protocol was approved by the Institutional Review Board of Henan Provincial People’s Hospital, and all participants provided written informed consent. The sample size was determined based on previously reported GDM incidence rates in PCOS populations. Using a two-sample proportion comparison formula with α=0.05 and β=0.2 (power=80%), the minimum required sample size was calculated to be 120 subjects. Ultimately, 139 participants were recruited to ensure adequate statistical power, accounting for potential attrition.
Inclusion and exclusion criteria
Inclusion criteria: (1) Age 18-40 years; (2) Diagnosis of PCOS according to the Guidelines for the Diagnosis and Treatment of Polycystic Ovary Syndrome (36); (3) Singleton pregnancy; (4) No pre-existing hypertension, diabetes mellitus, cardiovascular diseases, or other chronic medical conditions prior to pregnancy; (5) Regular antenatal care at our institution with complete follow-up data through at least 28 weeks of gestation.
Exclusion criteria: (1) Pre-pregnancy diagnosis of diabetes mellitus, hypertension, or major organ dysfunction; (2) Comorbid malignancies, neurological disorders, or severe psychiatric conditions; (3) Multiple gestation pregnancy; (4) Incomplete clinical data.
Diagnostic criteria and methodology for GDM
This study employed standardized diagnostic criteria for GDM in accordance with the Guidelines for the Diagnosis and Treatment of Diabetes in Pregnancy (37). All participants underwent a 75g oral glucose tolerance test (OGTT) between 24-28 weeks of gestation following an 8-10 hour overnight fast, with venous blood samples collected at fasting, 1-hour, and 2-hour post-glucose load intervals. Plasma glucose levels were analyzed using an automated biochemical analyzer, with GDM diagnosis confirmed if any single threshold was met: fasting ≥5.1 mmol/L, 1-hour ≥10.0 mmol/L, or 2-hour ≥8.5 mmol/L. To ensure methodological rigor and data reliability, all procedures were conducted by uniformly trained laboratory personnel under strict quality control protocols, thereby minimizing variability and enhancing the clinical validity of the diagnostic outcomes. This standardized approach not only aligns with international consensus but also provides a robust foundation for investigating potential biomarkers and their association with GDM development in high-risk populations.
Clinical data collection
Baseline clinical and biochemical information was collected at the time of enrollment. Demographic data included maternal age and pre-pregnancy body mass index (BMI), calculated as weight in kilograms divided by the square of height in meters (kg/m²). Fasting blood samples were also used to measure insulin levels and serum total testosterone using standardized laboratory methods at the institutional clinical laboratory. Gestational age was determined based on the last menstrual period and confirmed by first-trimester ultrasound.
Measurement of serum biomarkers
Fasting venous blood samples (5 mL) were obtained from each participant during the first trimester of pregnancy, specifically between 8 and 12 weeks of gestation. Blood samples were collected using EDTA-containing tubes and immediately placed on ice. Within 30 minutes of collection, samples were centrifuged at 3000 rpm for 15 minutes at 4°C to separate the serum. The serum aliquots were stored at −80°C until batch analysis. Serum concentrations of Sortilin, high mobility group box 1 (HMGB1), and Galanin-like Peptide (GALP) were quantified using ELISA kits: Sortilin (Cat. No. EK1234, R&D Systems), HMGB1 (Cat. No. ST51011, Sigma-Aldrich), and GALP (Cat. No. CSB-EQ027578HU, Cusabio), following the manufacturer’s protocols. The minimum detection limits for Sortilin, HMGB1, and GALP, respectively. All samples were analyzed in duplicate to ensure reliability, and the intra-assay and inter-assay coefficients of variation were maintained below 10%. In a subset of samples, quantitative validation was performed using liquid chromatography–tandem mass spectrometry (LC-MS/MS) to confirm peptide specificity and concentration accuracy.
Statistical analysis
All statistical analyses were performed using R software or SPSS Continuous variables were tested for normality using the Shapiro-Wilk test. Normally distributed data were expressed as mean ± standard deviation and compared using the Student’s t-test. Non-normally distributed data were presented as median and interquartile range (IQR), and compared using the Mann–Whitney U test. Correlations between serum biomarker levels and clinical parameters were assessed using Pearson or Spearman correlation coefficients, depending on data distribution. Univariate and multivariate logistic regression analyses were conducted to identify independent predictors of gestational diabetes mellitus (GDM). Multicollinearity among predictors was assessed using the variance inflation factor (VIF). The robustness of the multivariate models was tested by performing sensitivity analyses excluding outliers based on Cook’s distance. Internal validation of the model was performed using 10-fold cross-validation. Model calibration was examined using the Hosmer–Lemeshow goodness-of-fit test. The predictive performance of each biomarker, as well as their combined model, was evaluated using receiver operating characteristic (ROC) curve analysis. The area under the curve (AUC) and 95% confidence intervals (CIs) were calculated. Comparisons between AUCs were performed using DeLong’s test. A two-sided p-value < 0.05 was considered statistically significant.
Results
Comparative analysis of serum biomarkers in PCOS patients with and without GDM
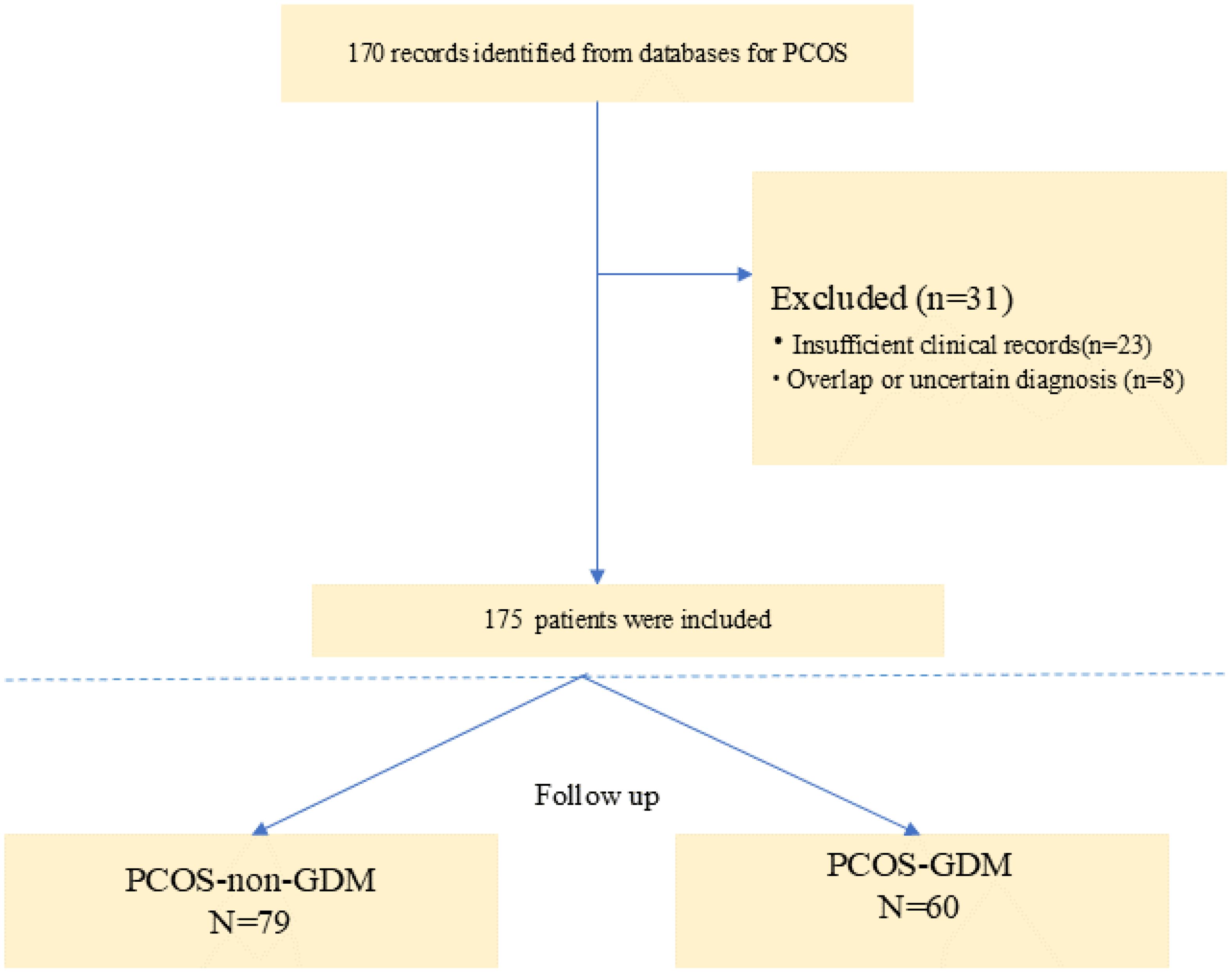
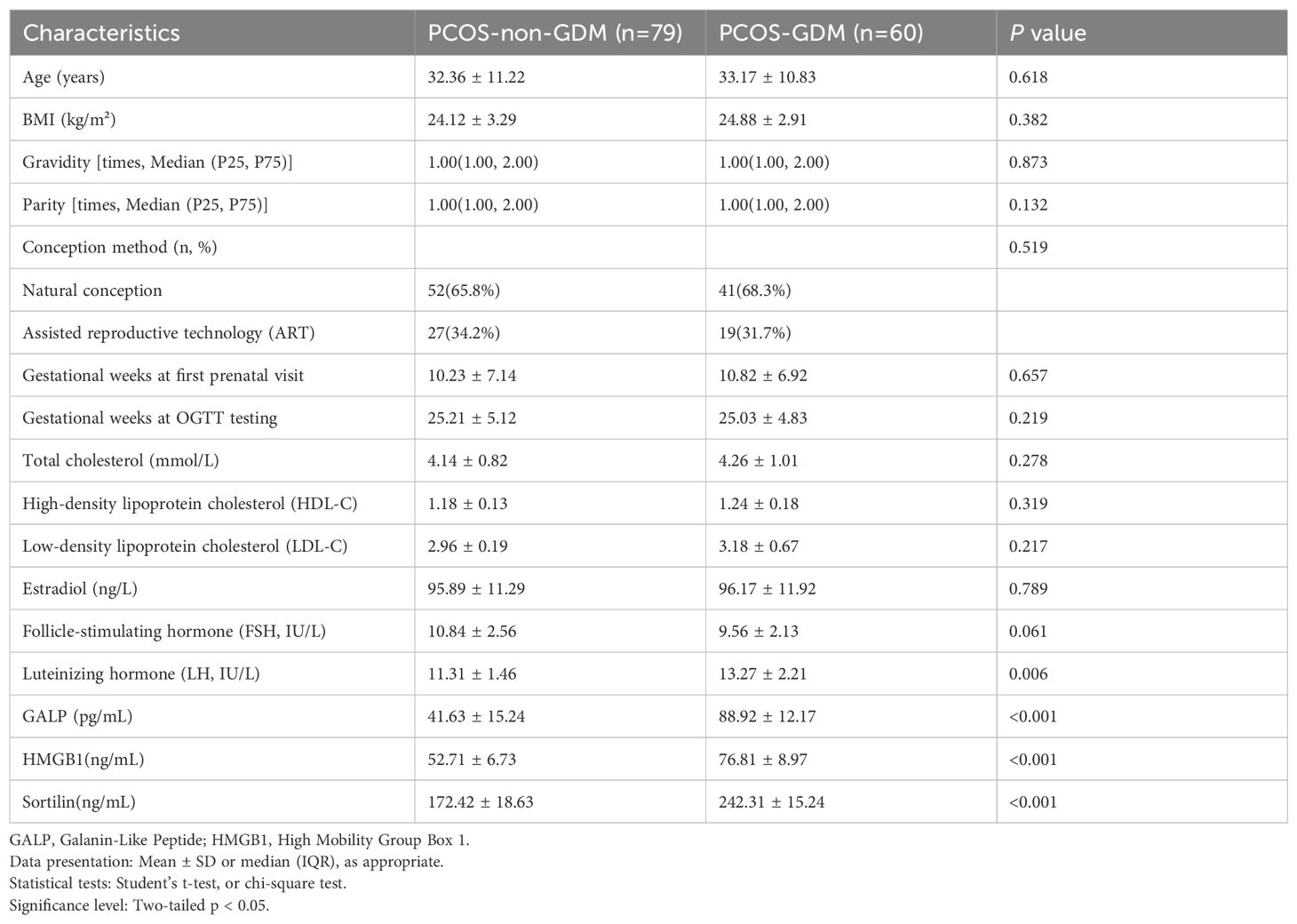
The study included a total of 139 baseline patients with PCOS who were prospectively followed during pregnancy (Figure 1). Based on oral glucose tolerance testing at 24-28 gestational weeks, participants were stratified into two groups: 79 women with PCOS-non-GDM (56.8%) and 60 women with PCOS-GDM (43.2%). As presented in Table 1, the demographic and biochemical characteristics were compared between groups. No significant differences were observed in age (32.36 ± 11.22 vs 33.17 ± 10.83 years, p=0.618), BMI (24.12 ± 3.29 vs 24.88 ± 2.91 kg/m², p=0.382), or obstetric history including gravidity (median=1.00 [IQR:1.00-2.00] for both groups, p=0.873) and parity (median=1.00 [IQR:1.00-2.00] for both groups, p=0.132). Conception methods showed similar distribution between groups (natural conception: 65.8% vs 68.3%; ART: 34.2% vs 31.7%, p=0.519). Notably, the PCOS-GDM group demonstrated significantly higher levels of all three investigated biomarkers compared to PCOS-non-GDM controls: GALP (88.92 ± 12.17 vs 41.63 ± 15.24 pg/mL, p<0.001), HMGB1 (76.81 ± 8.97 vs 52.71 ± 6.73 ng/mL, p<0.001), and Sortilin (242.31 ± 15.24 vs 172.42 ± 18.63 ng/mL, p<0.001). LH levels were also significantly elevated in the GDM group (13.27 ± 2.21 vs 11.31 ± 1.46 IU/L, p=0.006), while other hormonal and lipid parameters showed no statistically significant differences between groups.
Risk factors for GDM development in PCOS patients
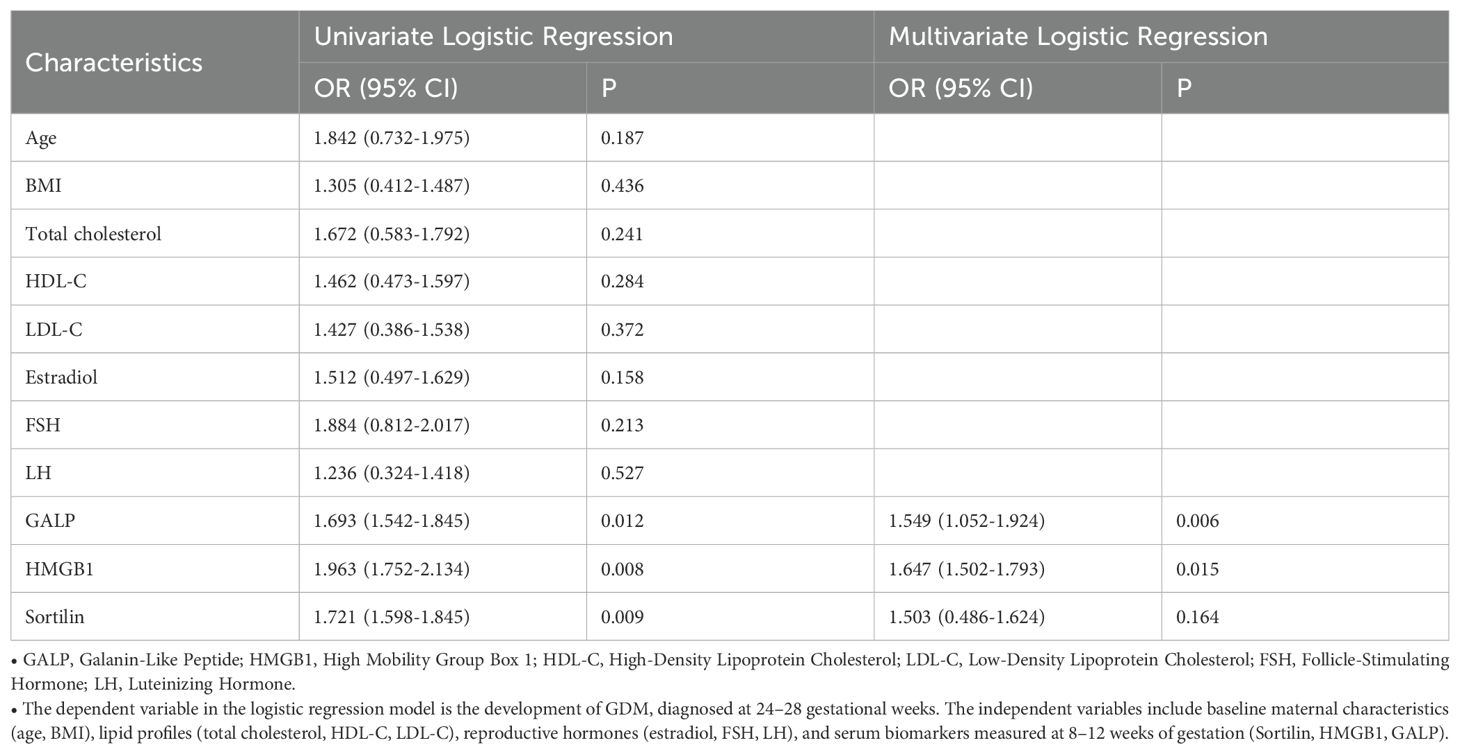
Logistic regression analysis revealed that elevated serum levels of GALP and HMGB1 were significantly associated with increased risk of GDM in women with PCOS (Table 2). In univariate analysis, GALP showed an OR of 1.693 (95%CI: 1.542-1.845, p=0.012), while HMGB1 demonstrated an even stronger association (OR 1.963, 95% CI 1.752-2.134, p=0.008). After adjusting for potential confounders including age, BMI and lipid profiles in multivariate analysis, both biomarkers remained statistically significant independent predictors: GALP maintained an adjusted OR (aOR) of 1.549 (95% CI 1.052-1.924, p=0.006) and HMGB1 showed an aOR of 1.647 (95% CI 1.502-1.793, p=0.015). Notably, Sortilin, while significant in univariate analysis (OR 1.721, 95% CI 1.598-1.845, p=0.009), lost statistical significance after adjustment (aOR 1.503, 95% CI 0.486-1.624, p=0.164). Traditional metabolic parameters including BMI (p=0.436), total cholesterol (p=0.241) and LDL-C (p=0.372) failed to show significant associations in either analysis.
ROC curve analysis
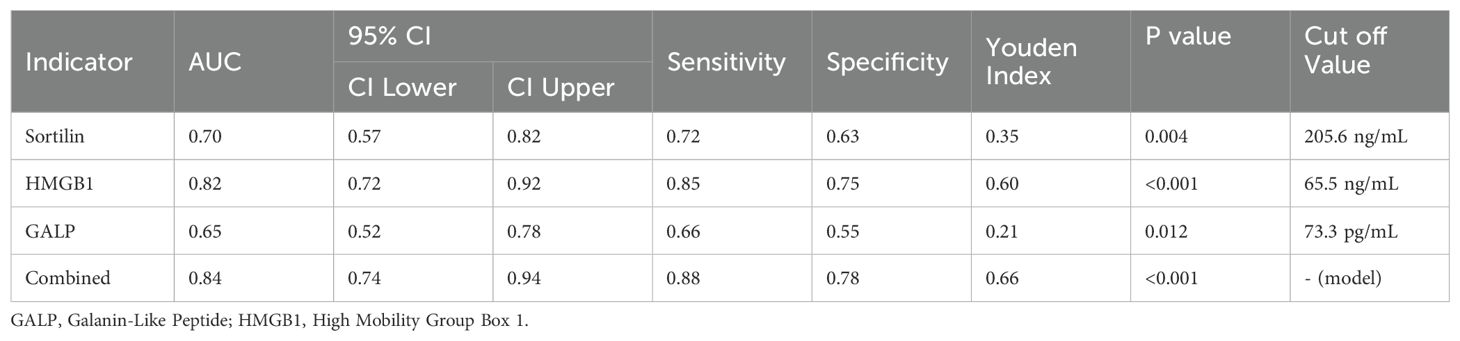
ROC curve analysis was conducted to evaluate the discriminative ability of each serum biomarker for GDM prediction (Figure 2). The AUC represents the overall ability of the test to discriminate between patients who developed GDM and those who did not. The area under the curve (AUC) for Sortilin was 0.70 (95% CI: 0.57–0.82, p = 0.004), with an optimal cut-off value of 205.6 ng/mL, yielding a sensitivity of 72% and specificity of 63%. HMGB1 exhibited superior diagnostic performance, with an AUC of 0.82 (95% CI: 0.72–0.92, p < 0.001) and a cut-off value of 65.5 ng/mL, achieving 85% sensitivity and 75% specificity. GALP showed a modest predictive capacity, with an AUC of 0.65 (95% CI: 0.52–0.78, p = 0.012) and an optimal threshold of 73.3 pg/mL, corresponding to a sensitivity of 66% and specificity of 55%. Importantly, the combined multi-marker model significantly outperformed individual markers, achieving an AUC of 0.84 (95% CI: 0.74–0.94, p < 0.001), with 88% sensitivity and 78% specificity at the optimal probability threshold determined by logistic regression. These results underscore the clinical utility of GALP and HMGB1 as early predictive biomarkers, particularly when used in combination for risk stratification in PCOS pregnancies For clarity and clinical interpretability, AUC values were retained within the ROC plots to facilitate intuitive visual comparison between biomarkers, while detailed statistical metrics are provided in Table 3.
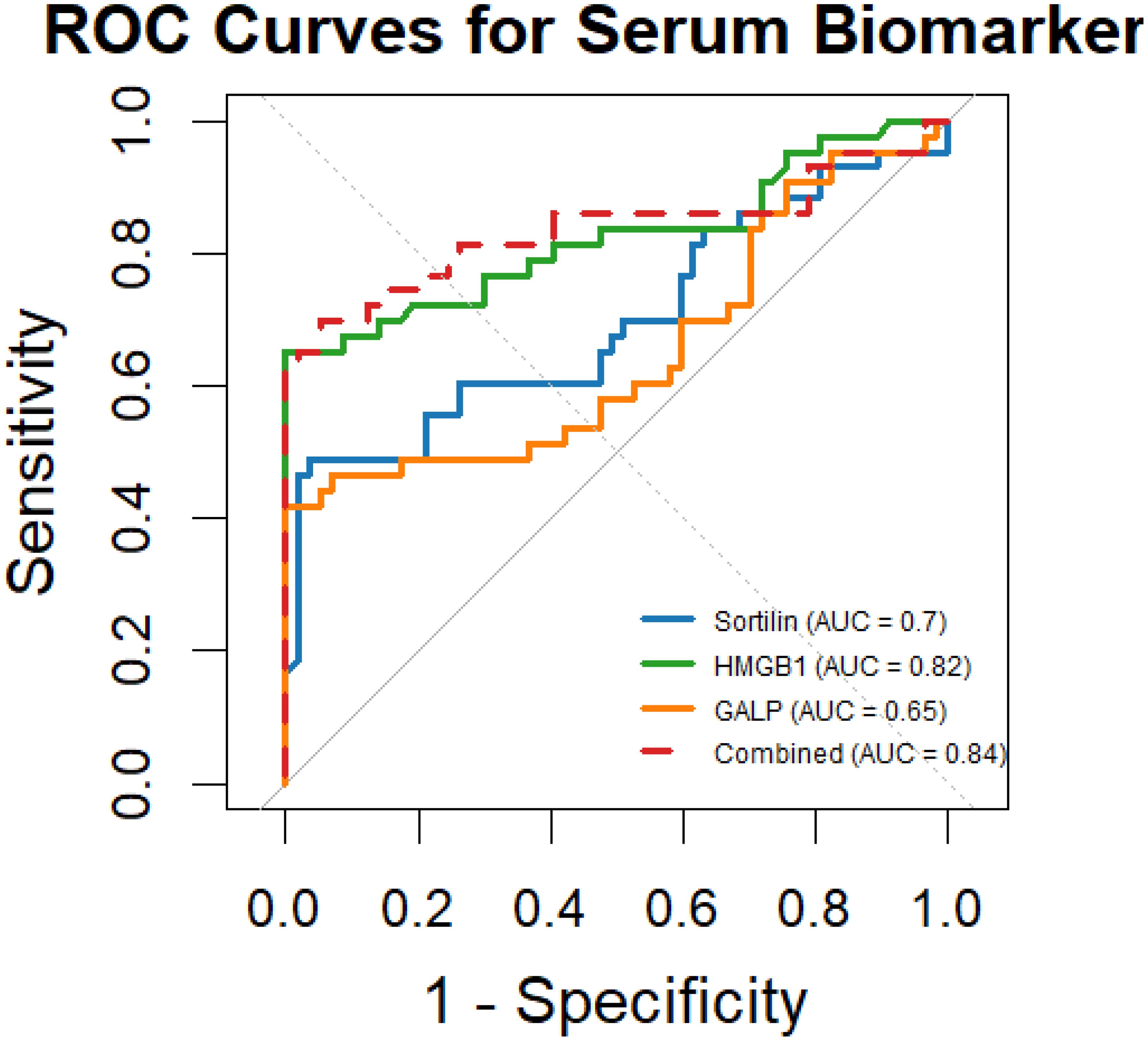
Figure 2. ROC curves showing the predictive performance of serum Sortilin, HMGB1, and GALP for GDM risk in PCOS pregnancies. AUC values are displayed in the figure for visual comparison of each biomarker’s discriminative ability. Corresponding p-values and Youden indices are detailed in Table 3. GALP, Galanin-Like Peptide; HMGB1, High Mobility Group Box 1.
Discussion
This study demonstrates that elevated serum GALP (aOR 1.55) and HMGB1 (aOR 1.65) independently predict GDM development in PCOS pregnancies, with combined biomarker assessment showing superior predictive accuracy (AUC 0.84). These novel markers outperformed conventional metabolic parameters, highlighting their potential for early risk stratification.
PCOS is a complex endocrine-metabolic disorder featuring ovulatory dysfunction, hyperandrogenemia, and insulin resistance (IR), with clinical manifestations including menstrual irregularities, infertility, and metabolic disturbances (38–40). Recognized as a major risk factor for GDM by leading guidelines, PCOS confers risk through synergistic mechanisms: pre-pregnancy IR compounded by pregnancy-induced hormonal changes (estrogen, progesterone, placental lactogen) that further impair insulin sensitivity (41–43). The magnitude of this risk has been quantified in multiple studies. A comprehensive meta-analysis demonstrated that PCOS patients face a 2.8-3.7 fold increased risk of developing GDM compared to healthy pregnant women (95% CI: 2.4-4.1) (44). The 2012 ASRM consensus statement further estimated that 40-50% of PCOS pregnancies are complicated by GDM (45). These findings underscore the critical need for enhanced screening and early intervention strategies in PCOS pregnancies to mitigate the substantial risk of GDM and its associated adverse outcomes. Future research should focus on elucidating precise molecular mechanisms and developing targeted preventive approaches for this high-risk population.
Sortilin is a multifunctional peptide hormone that plays a regulatory role in glucose and lipoprotein metabolism, insulin sensitivity, arterial wall inflammation, and calcification (30, 46). It is implicated in the pathogenesis of cardiovascular and metabolic diseases (47). Studies have demonstrated significantly elevated serum Sortilin levels in patients with diabetes mellitus, suggesting that elevated Sortilin levels may be associated with the onset of diabetes. In this study, serum Sortilin levels were markedly higher in patients with PCOS complicated by GDM compared to the control group. However, after adjusting for covariates, elevated Sortilin levels were not identified as an independent risk factor for GDM. Previous research has indicated that Sortilin plays a crucial role in regulating glucose homeostasis and insulin signaling (48). Sortilin and glucose transporter 4 (GLUT4) are co-expressed in adipocytes and muscle cells, where they are essential for glucose storage, transport, and homeostasis maintenance (49). Studies have shown that Sortilin knockout reduces diet-induced obesity and glycolysis in mice (50). Additionally, upregulated Sortilin expression has been observed in insulin resistance models, suggesting that Sortilin overexpression may be associated with glucose metabolism abnormalities and insulin resistance (51, 52). These findings imply that increased Sortilin expression may contribute to the pathogenesis of GDM by exacerbating glucose and lipid metabolism disorders and insulin resistance. However, the precise mechanisms underlying this relationship require further investigation.
Galanin-like peptide (GALP), a neuropeptide involved in energy metabolism and reproductive regulation, may influence the risk of GDM in PCOS patients through multiple mechanisms (34, 53). Research indicates that GALP modulates gonadotropin secretion via the hypothalamic-pituitary-ovarian (HPO) axis, while the characteristic LH/FSH ratio imbalance and hyperandrogenemia in PCOS patients may further exacerbate IR, a core pathogenic mechanism of GDM (34). Additionally, GALP enhances insulin sensitivity in adipose tissue and skeletal muscle, and its downregulation may contribute to glucose metabolism disorders, thereby increasing GDM susceptibility (53). Animal studies have demonstrated that GALP deficiency exacerbates high-fat diet-induced glucose intolerance, and the observed reduction in GALP levels in obese PCOS patients may promote chronic inflammation and lipid metabolism dysregulation, collectively driving GDM development (54). Although current evidence suggests a potential association between GALP and PCOS-related GDM, further clinical studies are required to elucidate its precise mechanistic role and evaluate its feasibility as either a predictive biomarker or therapeutic target.
HMGB-1, a potent proinflammatory protein, plays a pivotal role in inflammatory responses by promoting immune cell maturation, migration, and proinflammatory cytokine secretion (55). In pregnant women with PCOS, elevated HMGB-1 levels both reflect and exacerbate the chronic low-grade inflammatory state characteristic of this condition. Mechanistically, HMGB-1 contributes to GDM development by impairing insulin signaling cascades while simultaneously inducing pathological apoptosis in placental tissues. Furthermore, it amplifies inflammatory mediators through positive feedback mechanisms that directly compromise pancreatic β-cell function (56), thereby promoting insulin resistance (as quantified by HOMA-IR) and establishing a self-perpetuating cycle that progressively disrupts glucose homeostasis during pregnancy (57).
The integration of Sortilin, HMGB1, and GALP into a first-trimester predictive panel (AUC 0.84) enables a paradigm shift from reactive GDM diagnosis to proactive prevention in PCOS pregnancies. This multi-analyte approach identifies high-risk patients 12-16 weeks before conventional diagnosis, allowing timely initiation of pathophysiology-targeted interventions: metabolic management for Sortilin elevation, anti-inflammatory strategies for HMGB1, and endocrine modulation for GALP abnormalities. The panel’s clinical implementation could substantially reduce the 46% GDM incidence in this high-risk population by facilitating risk-stratified interventions ranging from intensified lifestyle counseling to pharmacologic prophylaxis.
Limitations and future directions
Our study has several important limitations that warrant consideration. First, the single-center nature of this research, while ensuring procedural consistency, may limit the generalizability of our findings to broader populations with diverse ethnic, socioeconomic, or clinical backgrounds. Second, although our sample size (n = 139) met the requirements for primary statistical analyses, the moderate number of participants may have limited the power to detect subtle associations or perform more granular subgroup analyses. Third, the observational design of this study introduces the potential for selection bias, and residual confounding cannot be excluded. Although we adjusted for key covariates such as maternal age, BMI, and HOMA-IR, we lacked data on important lifestyle-related confounders, including detailed dietary intake, physical activity levels, and sleep quality, all of which may influence both biomarker levels and the risk of GDM. Fourth, biomarkers were measured only once in early pregnancy, which precludes the evaluation of dynamic longitudinal changes across gestation that could offer additional insights into the pathophysiological trajectory of GDM in PCOS. Finally, postpartum follow-up data were not collected, which limits our ability to assess whether these biomarkers are predictive of long-term metabolic outcomes beyond pregnancy.
Future studies should focus on validating these biomarkers in larger, multi-ethnic cohorts while incorporating serial measurements across pregnancy to better understand their temporal relationships with GDM development. Mechanistic studies are needed to elucidate how GALP and HMGB1 contribute to GDM pathogenesis in PCOS, potentially informing targeted prevention strategies. Translationally, research should prioritize developing clinically feasible assays and determining optimal cutoff values for risk stratification in diverse clinical settings.
Conclusions
In conclusion, this study identified serum GALP and HMGB1 as independent early-pregnancy biomarkers associated with an increased risk of developing GDM in women with PCOS. Notably, the combined use of GALP and HMGB1 demonstrated superior predictive performance over traditional clinical indicators such as BMI and fasting glucose, suggesting their potential utility for early risk stratification. These findings underscore the promise of incorporating GALP and HMGB1 into clinical screening algorithms to enable earlier identification and intervention in high-risk PCOS pregnancies. However, given the single-center design and moderate sample size, further large-scale, multicenter prospective studies are needed to validate these biomarkers and establish standardized cutoff values for clinical application.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Henan Provincal People Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HL: Conceptualization, Writing – original draft. X-HZ: Conceptualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors extend their sincere appreciation to all participants for their involvement in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen Y, Chen J, Li Y, Wu Y, Wu X, Zhang H, et al. Insulin-like peptide 5 is associated with insulin resistance in women with polycystic ovary syndrome. J Diabetes Complications. (2023) 37:108493. doi: 10.1016/j.jdiacomp.2023.108493
2. Zhu L and Wang L. Serum asprosin levels are increased and associated with insulin resistance in patients with polycystic ovary syndrome: A retrospective case-control study. Med (Baltimore). (2023) 102:e33526. doi: 10.1097/MD.0000000000033526
3. Biernacka-Bartnik A, Kocełak P, Owczarek AJ, Choręza PS, Markuszewski L, Madej P, et al. The cut-off value for HOMA-IR discriminating the insulin resistance based on the SHBG level in women with polycystic ovary syndrome. Front Med (Lausanne). (2023) 10:1100547. doi: 10.3389/fmed.2023.1100547
4. Kim AE, Applebaum J, Lee IT, Kim EK, Jang M, Dokras A, et al. Variable adoption of polycystic ovary syndrome-related infertility guidelines in the United States: A retrospective cohort and survey study. Fertil Steril. (2025) 8:S0015-0282(25)00151-7. doi: 10.1016/j.fertnstert.2025.03.003
5. Rehman F, Muslim Khan M, Lnu A, Lnu N, Lnu R, and Zeb S. Comparative analysis of psychosocial outcomes and quality of life among nulligravida, primigravida, and multigravida women diagnosed with polycystic ovary syndrome (PCOS). Cureus. (2025) 17:e79895. doi: 10.7759/cureus.79895
6. Hofmann K, Decrinis C, Bitterlich N, Bachmann A, and Stute P. Health-related quality of life and mental state in women with polycystic ovary syndrome and migration or minority background - A cross-sectional study. J Migr Health. (2025) 11:100313. doi: 10.1016/j.jmh.2025.100313
7. Kanina A, Stener-Victorin E, Butwicka A, Oberg AS, Rosenqvist MA, and Cesta CE. Adverse cardiometabolic outcomes in men with sisters with polycystic ovary syndrome. J Clin Endocrinol Metab. (2025). doi: 10.1210/clinem/dgaf121
8. Gambineri A, Rosa S, Pandurevic S, Cecchetti C, Rotolo L, Dionese P, et al. Evolution of cardiovascular risk factors and the risk for cardiovascular events in a Caucasian population with polycystic ovary syndrome. Eur J Endocrinol. (2025) 192:210–9. doi: 10.1093/ejendo/lvaf027
9. Sabeti Akbar-Abad M, Majidpour M, Sargazi S, Ghasemi M, and Saravani R. Unraveling the role of cathepsin B variants in polycystic ovary syndrome: insights from a case-control study and computational analyses. Reprod Sci. (2025). doi: 10.1007/s43032-025-01806-w
10. Chen P, Liu Q, Shi H, Liu Z, and Yang X. Choline metabolism disorder induced by Prevotella is a risk factor for endometrial cancer in women with polycystic ovary syndrome. Mol Biol Rep. (2025) 52:285. doi: 10.1007/s11033-025-10392-8
11. Khamaiseh K, Bdeir R, Abukbeer MM, Khamaiseh R, Nassar A, Al-Sawadha DN, et al. Impact of polycystic ovary syndrome hyperandrogenic phenotypes A and non-hyperandrogenic D on pregnancy outcomes after in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI). Int J Womens Health. (2025) 17:561–9. doi: 10.2147/IJWH.S500692
12. Moreira T, Leal C, Barreiro M, Tome A, and Vale-Fernandes E. Predictors of pregnancy after artificial insemination in women with polycystic ovary syndrome. JBRA Assist Reprod. (2025). doi: 10.5935/1518-0557.20240095
13. Huang C, Yan Y, Mei J, Jiang Y, Sun H, and Xing J. The impact of long-acting Gonadotropin-releasing hormone agonist pretreatment on the clinical pregnancy outcomes of hormone replacement therapy-frozen embryo transfer in recurrent implantation failure patients with and without polycystic ovary syndrome: a retrospective clinical study. BMC Pregnancy Childbirth. (2025) 25:133. doi: 10.1186/s12884-025-07264-1
14. Syngelaki A, Wright A, Gomez Fernandez C, Mitsigiorgi R, and Nicolaides KH. First-trimester prediction of gestational diabetes mellitus based on maternal risk factors. BJOG. (2025). doi: 10.1111/1471-0528.18110
15. Wei L, Fang C, Jiang Y, Zhang H, Gao P, Zhou X, et al. The role of placental MFF-mediated mitochondrial fission in gestational diabetes mellitus. Diabetes Metab Syndr Obes. (2025) 18:541–54. doi: 10.2147/DMSO.S484002
16. Xia L, Yang Z, Mu Q, Ji Y, and Lyu J. Risk factors for gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. Diabetes Metab Syndr Obes. (2025) 18:565–81. doi: 10.2147/DMSO.S502043
17. Chen YS and Li DZ. Gestational diabetes mellitus and group B streptococcus maternal colonization. Eur J Obstet Gynecol Reprod Biol. (2025) 307:275. doi: 10.1016/j.ejogrb.2025.02.043
18. Naz S, Jamal S, Jaffar A, Azam I, Chandir S, Qureshi R, et al. Development and validation of a Non-INvaSive Pregnancy RIsk ScoRE (INSPIRE) for the screening of high-risk pregnant women for gestational diabetes mellitus in Pakistan. BMJ Public Health. (2024) 2:e000920. doi: 10.1136/bmjph-2024-000920
19. Sonaglioni A, Casieri F, Nicolosi GL, Bianchi S, and Lombardo M. Comprehensive assessment of biventricular and biatrial myocardial strain parameters at 4 years postpartum in a cohort of women with previous gestational diabetes mellitus. J Clin Med. (2025) 14. doi: 10.3390/jcm14041271
20. Ruiz-Martinez ML, Gomez-Diaz RA, Valdez Gonzalez AL, Ángeles Mejía S, Mondragón González R, Díaz Flores M, et al. Association of oxidative stress markers with incident hyperglycemia in gestational diabetes mellitus in an educational intervention. Nutrients. (2025) 17(4):680. doi: 10.3390/nu17040680
21. Rubin SC, Bibi M, Breborowicz A, Chau P, and Keltz MD. Transvaginal ovarian drilling for polycystic ovary syndrome prior to in vitro fertilization dramatically improves embryo yield, implantation, and ongoing pregnancy rates. J Assist Reprod Genet. (2024). doi: 10.1007/s10815-024-03362-9
22. Chang YT, Chen MJ, Lin WS, Lin CH, and Chang JC. Adverse pregnancy outcomes in patients with polycystic ovary syndrome with pre-conceptional hyperandrogenism: A multi-institutional registry-based retrospective cohort study. J Clin Med. (2024) 14. doi: 10.3390/jcm14010123
23. Wang Z, Fleisch A, Rifas-Shiman SL, Calafat AM, James-Todd T, Coull BA, et al. Associations of maternal per- and polyfluoroalkyl substance plasma concentrations during pregnancy with offspring polycystic ovary syndrome and related characteristics in project viva. Environ Res. (2025) 268:120786. doi: 10.1016/j.envres.2025.120786
24. Ge H, Huang D, Tan L, Luo D, Zhou L, Liu H, et al. Metabolic profiles of pregnancy with polycystic ovary syndrome: insights into maternal-fetal metabolic communication. J Clin Endocrinol Metab. (2025) 27:dgaf189. doi: 10.1210/clinem/dgaf057
25. Fu X, Cao W, Ye F, Bei J, Du Y, and Wang L. Astaxanthin compound nutrient improved insulin resistance, hormone levels, embryo quality and pregnancy outcomes in polycystic ovary syndrome patients undergoing in vitro fertilization/intracytoplasmic sperm injection. Drug Discov Ther. (2024) 18:296–302. doi: 10.5582/ddt.2024.01036
26. Valdimarsdottir R, Vanky E, Elenis E, Ahlsson F, Lindström L, Junus K, et al. Polycystic ovary syndrome and gestational diabetes mellitus association to pregnancy outcomes: A national register-based cohort study. Acta Obstet Gynecol Scand. (2025) 104:119–29. doi: 10.1111/aogs.v104.1
27. Shao S, Xu Q, Zi Y, Zheng X, Chen S, Qin C, et al. The genetic association between polycystic ovary syndrome and the risk of hypertensive disorders of pregnancy: A Mendelian randomization study. Eur J Obstet Gynecol Reprod Biol. (2025) 305:351–5. doi: 10.1016/j.ejogrb.2024.12.043
28. Wang Y, Li R, Sun B, Song W, Zhao X, and Hu Y. Relationship of serum nesfatin-1 and insulin-like growth factor-1 levels with adverse pregnancy outcomes in patients with polycystic ovary syndrome. J Coll Physicians Surg Pak. (2024) 34:1167–71. doi: 10.29271/jcpsp.2024.10.1167
29. Li YT, Li CL, Yang H, Huang L, Liu JJ, Zheng XY, et al. Correlation between acupuncture dose and pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization-embryo transfer: a systematic review. BMC Complement Med Ther. (2024) 24:407. doi: 10.1186/s12906-024-04695-9
30. Alarslan P and Doruk M. Serum sortilin levels as a biomarker for metabolic and hormonal dysregulation in polycystic ovary syndrome. J Pers Med. (2025) 15. doi: 10.3390/jpm15020070
31. Choi YJ, Nam YA, Hyun JY, Yu J, Mun Y, Yun SH, et al. Impaired chaperone-mediated autophagy leads to abnormal SORT1 (sortilin 1) turnover and CES1-dependent triglyceride hydrolysis. Autophagy. (2025) 21:827–39. doi: 10.1080/15548627.2024.2435234
32. Oza D, Ivich F, Deprey K, Bittner K, Bailey K, Goldman S, et al. Treatment of acute liver injury through selective tropism of high mobility group box 1 gene-silenced large peritoneal macrophages. ACS Nano. (2025) 19(12):12102–18. doi: 10.1021/acsnano.4c18345
33. Sanadgol E, Zendehdel M, Vazir B, Rassouli A, and Haghbinnazarpak H. Central administration of galanin-like peptide (GALP) causes short-term orexigenic effects in broilers: Mediatory role of NPY1 and D1 receptors. Neurosci Lett. (2025) 844:138042. doi: 10.1016/j.neulet.2024.138042
34. Demirpence M, Yilmaz Yasar H, Karakoyun I, and Girgin EM. Galanin-like peptide and its correlation with androgen levels in patients with polycystic ovary syndrome. Endokrynol Pol. (2023) 74:197–202. doi: 10.5603/EP.a2023.0026
35. Li Y, Yang S, Huang Z, Zhang Y, Guan H, and Fan J. Free triiodothyronine and risk of gestational diabetes mellitus: an observational study and Mendelian randomization analysis. Nutr Metab (Lond). (2025) 22:17. doi: 10.1186/s12986-025-00905-4
36. Teede HJ, Tay CT, Laven J, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J Clin Endocrinol Metab. (2023) 108:2447–69. doi: 10.1210/clinem/dgad463
37. Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res Clin Pract. (2022) 183:109050. doi: 10.1016/j.diabres.2021.109050
38. Yazdanpanah Z, Nasrabadi MH, Cheraghi E, and Salehipour M. The ameliorative effect of myo-inositol on apoptosis-related genes expression in cumulus cells of women with polycystic ovary syndrome undergoing ICSI and its relationship with the quality of oocyte and embryo. Naunyn Schmiedebergs Arch Pharmacol. (2025). doi: 10.1007/s00210-025-03952-z
39. Yuan J, Li Z, Yu Y, Wang X, and Zhao Y. Natural compounds in the management of polycystic ovary syndrome: a comprehensive review of hormonal regulation and therapeutic potential. Front Nutr. (2025) 12:1520695. doi: 10.3389/fnut.2025.1520695
40. Rahim S and Pergolizzi J Jr. The potential role of glucagon-like peptide-1 (GLP-1) agonists for polycystic ovary syndrome. Cureus. (2025) 17:e77998. doi: 10.7759/cureus.77998
41. Jiang Y, Li Y, and Huang Y. Circulating cytokines levels and the risk of polycystic ovary syndrome: A Mendelian randomization analysis. Med (Baltimore). (2025) 104:e41359. doi: 10.1097/MD.0000000000041359
42. Wang K, Bu Z, Ge X, Wang F, Zhang M, and Guo Y. Hyperandrogenism increases late spontaneous miscarriage in polycystic ovary syndrome women due to cervical insufficiency? A propensity-score matching study. BMC Pregnancy Childbirth. (2025) 25:222. doi: 10.1186/s12884-025-07342-4
43. Saleem Azam S, Vasudevan S, Saqib Bukhari W, Thadhani J, Tasneem H, Singh S, et al. Reproductive endocrine disorders: A comprehensive guide to the diagnosis and management of infertility, polycystic ovary syndrome, and endometriosis. Cureus. (2025) 17:e78222. doi: 10.7759/cureus.78222
44. Bahri Khomami M, Boyle JA, Tay CT, Vanky E, Teede HJ, Joham AE, et al. Polycystic ovary syndrome and adverse pregnancy outcomes: Current state of knowledge, challenges and potential implications for practice. Clin Endocrinol (Oxf). (2018) 88:761–9. doi: 10.1111/cen.2018.88.issue-6
45. Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. (2012) 97:28–38 e25. doi: 10.1016/j.fertnstert.2011.09.024
46. Cheng G, Liu J, Zhang H, Cui Y, Xu S, and Wang L. Sortilin as a culprit in the atherosclerosis plaque progression: evidence from clinical and experimental studies. Curr Mol Med. (2025). doi: 10.2174/0115665240342078250114165535
47. Almoyad MAA, Wahab S, Mohanto S, and Khan NJ. Repurposing drugs to modulate sortilin: structure-guided strategies against atherogenesis, coronary artery disease, and neurological disorders. ACS Omega. (2024) 9:18438–48. doi: 10.1021/acsomega.4c00470
48. Ek M, Nilvebrant J, Nygren PA, Stahl S, Lindberg H, and Lofblom J. An anti-sortilin affibody-peptide fusion inhibits sortilin-mediated progranulin degradation. Front Immunol. (2024) 15:1437886. doi: 10.3389/fimmu.2024.1437886
49. Xu J, Shen CJ, Ooi JD, Tang YS, Xiao Z, Yuan QJ, et al. Serum sortilin is associated with coronary artery calcification and cardiovascular and cerebrovascular events in maintenance hemodialysis patients. Kidney Dis (Basel). (2021) 7:503–13. doi: 10.1159/000517304
50. El-Khodary NM, Dabees H, and Werida RH. Folic acid effect on homocysteine, sortilin levels and glycemic control in type 2 diabetes mellitus patients. Nutr Diabetes. (2022) 12:33. doi: 10.1038/s41387-022-00210-6
51. Ye S, Wang B, Zhou Y, Sun Q, and Yang X. Sortilin 1 regulates hepatocellular carcinoma progression by activating the PI3K/AKT signaling. Hum Exp Toxicol. (2022) 41:9603271221140111. doi: 10.1177/09603271221140111
52. Jakobsen TS, Østergaard JA, Kjolby M, Birch EL, Bek T, Nykjaer A, et al. Sortilin inhibition protects neurons from degeneration in the diabetic retina. Invest Ophthalmol Vis Sci. (2023) 64:8. doi: 10.1167/iovs.64.7.8
53. Liu M, Zhang X, Sun Z, Wang H, Sun X, and Zhang W. Serum levels of galanin-like peptide and alarin are highly correlated with polycystic ovary syndrome. Sci Rep. (2025) 15:9881. doi: 10.1038/s41598-025-93354-1
54. Li Y, Zhi W, Haoxu D, Qing W, Ling C, Ping Y, et al. Effects of electroacupuncture on the expression of hypothalamic neuropeptide Y and ghrelin in pubertal rats with polycystic ovary syndrome. PloS One. (2022) 17:e0259609. doi: 10.1371/journal.pone.0259609
55. Yun BH, Kim S, Chon SJ, Kim GH, Choi YS, Cho S, et al. High mobility group box-1 promotes inflammation in endometriotic stromal cells through Toll-like receptor 4/nuclear factor-kappa B. Am J Transl Res. (2021) 13:1400–10.
56. Hu M, Zhang Y, Lu Y, Han J, Guo T, Cui P, et al. Regulatory mechanisms of HMGB1 and its receptors in polycystic ovary syndrome-driven gravid uterine inflammation. FEBS J. (2023) 290:1874–906. doi: 10.1111/febs.v290.7
Keywords: polycystic ovary syndrome, gestational diabetes mellitus, biomarkers, HMGB1, galanin-like peptide
Citation: Li H and Zhao X (2025) Predictive value of serum sortilin, HMGB1, and galanin-like peptide for gestational diabetes mellitus in women with polycystic ovary syndrome. Front. Endocrinol. 16:1602622. doi: 10.3389/fendo.2025.1602622
Received: 30 March 2025; Accepted: 25 April 2025;
Published: 19 May 2025.
Edited by:
Mayank Choubey, NYU Grossman Long Island School of Medicine, United StatesCopyright © 2025 Li and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Li, Z3JleWxlZTE5ODhAMTYzLmNvbQ==
 Hui Li*
Hui Li* Xinghao Zhao
Xinghao Zhao