- Department of Pediatrics, Endocrinology, and Diabetology with Cardiology Division, Medical University of Bialystok, Białystok, Poland
Introduction: Maintaining endogenous insulin secretion in type 1 diabetes (T1D) long after its onset, and thus the need for early diagnosis and searching for factors preserving the secretory function of β-cells, has become an important goal of current research. The aim of the study was to evaluate C-peptide secretion in T1D children with at least 1 year disease duration and to investigate the potential role of body mass index (BMI) and betatrophin on residual β-cell function. We also assessed factors that may affect betatrophin levels.
Methods: 121 children and adolescents suffering from T1D were divided into groups based on: clinical significance of C-peptide; BMI-SDS <1 and ≥1; and disease duration to compare C-peptide and betatrophin levels and determine the importance of these changes.
Results: Of the children recruited, 44 (36.36%) had clinically significant C-peptide (> 0.23 ng/ml), and compared with the group with clinically insignificant C-peptide they had later onset (P<.001), shorter duration of illness (P<.001), lower daily insulin requirement (P=.025), lower mean HbA1c over the past year (P=.002), higher betatrophin levels (P=.019), and BMI-SDS at diagnosis (P=.013). Betatrophin levels correlated positively with C-peptide (P=.043) while negatively with patient’s age (P<.001), BMI-SDS (P=.010), disease duration (P=.006), HbA1c level at sampling (P=.022), average HbA1c level over the past year (P=.006), and basal insulin (P=.001).
Conclusion: The positive significant relationship between betatrophin and C-peptide concentrations may indicate betatrophin as a potential biomarker of long-lasting residual β-cell function. Negative correlation with BMI identifies the ongoing need to maintain an appropriate body mass.
1 Introduction
Type 1 diabetes (T1D) is a chronic metabolic disease characterized by autoimmune destruction of pancreatic β-cells, leading to endogenous insulin deficiency. At present, the basic method of treating this disorder is exogenous supplementation of insulin with hormone analogues through multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII). Unfortunately, current treatment methods for children with diabetes, despite the continuous improvement of glucose control systems such as continuous glucose monitoring (CGM) or closed-loop insulin pumps, are not always sufficient to maintain normoglycemia. Preservation of residual β-cell function has been shown to be associated with better glycemic control and has a protective effect on both acute and long-term complications of diabetes. This prompts the search for factors that enhance β-cell function, or at least maintain their longer survival as well as the search for biomarkers of the remission phase. The discovery of methods and factors to expand the secretory function of pancreatic islets would provide new therapeutic options that would not only improve metabolic parameters, but also have a positive impact on patients’ quality of life (1).
The biomarker commonly used to assess pancreatic β-cell function is C-peptide. It is generated from the processing of preproinsulin molecules into insulin and C-peptide and abnormalities in this process, have clinical relevance to T1D (2). Shortly after initiating treatment, partial remission is usually observed (3). This period is characterized by preserved C-peptide secretion and better glycemic control with lower daily insulin requirements (DIR). According to International Society for Pediatric and Adolescent Diabetes (ISPAD), remission criteria include HBA1c <7% with DIR <0.5 U/kg/24h (4). However, after the relapse, C-peptide level begins to drop dramatically and is usually undetectable after the clinical onset (5). Nonetheless, it is already proved that C-peptide can remain at residual levels in some patients for years (6). The Diabetes Control and Complications Trial (DCCT) indicates that sustained C-peptide concentrations are associated with lower rates of hypoglycemia, lower HbA1c levels, reduced insulin dose requirements and lower incidents of diabetes complications, such as retinopathy and nephropathy (7). These findings suggest that even patients with long-standing and advanced disease may benefit from interventions to protect β-cell function or to prevent complications.
Numerous studies have been conducted to find factors influencing the presence and duration of partial remission phase (5, 8, 9). Unfortunately, little is known about the factors that predispose for a preservation of C-peptide, when partial remission phase ends. In recent years, several new candidate biomarker of residual β-cell function have emerged. One of the potentially significant factors could be betatrophin, a hormone alternatively referred to as angiopoietin-like protein 8 (ANGPTL8), hepatocellular carcinoma-associated protein (TD26), or lipazin, which is synthesized primarily in the liver and moderately in adipose tissue, adrenal glands, duodenum, and small intestine (10). Betatrophin has been studied mainly in patients with early T1D, but its elevated blood levels have also been observed in adults with long-term diabetes (11). However, little research has been conducted on this topic in children. Studies on mice have shown a significant effect of betatrophin in serum on the increase in triglyceride levels and a positive effect on the proliferation of pancreatic β-cells (12, 13). Betatrophin has been found to induce positive activators of Ki67 protein signaling and proliferation, such as cyclin A1, cyclin F, and E2F2, and inhibit suppressors cdkn1a and cdkn2a in pancreatic β-cells. Furthermore, overexpression of betatrophin levels improves glucose tolerance and lowers fasting blood glucose levels (14). In the presence of high glucose concentrations, insulin induces an increase in betatrophin levels, which activates β-cells, their proliferation, and stimulates them to release insulin, take up glucose, and enter the cells. Once glycemia is normalized, the positive feedback is stopped, and insulin-induced betatrophin production is inhibited (14).
Studies on human population explaining the role of betatrophin in glucose metabolism and in insulin resistance are few and have not yet fully clarify the issue. It has been found that betatrophin levels are increased in children with T1D compared to their healthy peers, particularly at the onset of the disease, when betatrophin levels may increase up to threefold, and high serum concentrations persist in the early stages of the disease. The downward trend in serum betatrophin concentrations starts after approximately one year (5), which may be related to the exploitation of pancreatic islets with the duration of the disease. Studies conducted on Asian Indians showed that patients with excess body weight (BMI > 25 kg/m2) had lower blood betatrophin levels than normal weight patients, regardless of whether they were type 2 diabetics (T2D) or patients with normal glucose tolerance. Furthermore, betatrophin showed a strong negative correlation with waist circumference, insulin resistance, fasting and 2-hour postprandial glucose, and HbA1c, while it showed a positive correlation with fasting C-peptide (15). It was hypothesized that the increased betatrophin concentration in patients suffering from diabetes was not due to insulin deficiency but to insulin resistance (11). When a patient with T1D has genetic susceptibility factors and contributing environmental factors such as obesity, chronic inflammation, hormonal changes, or a long duration of the disease, a condition called double diabetes may occur, in which the clinical features of both types of diabetes overlap (16).
Many publications have shown C-peptide secretion during remission in children with diabetes, but scarcely any studies have addressed the period after remission and several years of disease in this population, when C-peptide may continue to be secreted. In our previous work, we indicated that above 30% of children with long-standing diabetes have clinically significant C-peptide levels (>0.23 ng/ml according to DCCT recommendations) and we confirmed that it involves better metabolic control in these patients (17).
The aim of the current study was to assess residual β-cell function in children with type 1 diabetes, with at least 1 year of disease duration, who had exited remission phase and to investigate the significance of BMI and its effect on C-peptide secretion. Additionally, we decided to test the hypothesis, that there would be a difference in betatrophin concentration between individuals with and without clinically significant C-peptide level. Subsequently, we aimed to assess the correlation between circulating betatrophin and clinical factors that may affect its levels. We decided not to include patients with disease duration of less than 1 year in our analysis because the patients’ condition was not yet stabilized, treatment decisions (CSII or MDI) were still pending, and they had significantly higher betatrophin levels at that time, which may have confounded our analyses.
2 Materials and methods
Our retrospective study, involving data collected between January 2020 and December 2022, included 121 children and adolescents aged 3–18 years (12.98 ± 3.54 years), patients of the Diabetes Outpatient Clinic for Children of the University Children’s Clinical Hospital in Bialystok, Poland, who were diagnosed with type 1 diabetes according to ISPAD criteria (18), with the disease duration of at least 1 year and not meeting the criteria for partial remission according to ISPAD: HBA1c <7% with DIR < 0.5 U/kg/24h (4). Exclusion criteria were the diagnosis of diabetes other than type 1, age over 18 years and the duration of the disease less than 1 year and meeting the remission phase criteria.
The data of 121 patients (61 boys, 60 girls) were analyzed. The diabetes duration ranged from 1 to 15 years (5.47 ± 3.89 years). All children were treated with intensive insulin therapy: 26 (21.49%) - with MDI, 95 (78.51%) - treated with CSII using an insulin pump. Anthropometric data have been assessed. Height and weight were measured using a medical scale and an anthropometer in the morning in light clothing. Body weight was measured to the nearest 100 g, and height to the nearest 0.5 cm. BMI was calculated by dividing children’s weight in kilograms by their height in meters squared. To adjust for age and gender, the BMI-SDS was calculated using sex-specific and age-specific BMI growth charts based on Polish OLAF study (19). BMI-SDS < 1 indicates normal body weight, while BMI-SDS ≥ 1 signifies overweight and obesity according to WHO (20).
A blood sample (2.7 cm per clot) was taken to perform the test. C-peptide level was used as an indicator of the maintenance of endocrine pancreatic function due to its secretion in equimolar proportions with insulin. The concentration of fasting C-peptide was determined using the traditional method, as well as the modern ultrasensitive C-peptide ELISA method (Mercodia, Sylveniusgatanm, Sweden) using ELx 800 Automated Microplate Reader, Bio-Tek Instruments, Vermont, USA with a lower detection limit of < 2.5 pmol/L, which corresponds to 0.0076 ng/ml. Clinically significant fasting C-peptide levels according to DCCT were established at > 0.23 ng/ml (7). Betatrophin concentration was assessed using the ELISA method (BioVendor, Brno, Czech Republic) which was specific for human betatrophin and had a sensitivity of 0.244 ng/ml. HbA1c levels were determined using monoclonal antibodies in a biochemical analyzer (Co bas, Integra 800, Roche, Switzerland). Additionally, an analysis of clinical data was performed to assess the factors influencing the maintenance of residual C-peptide, hence endogenous insulin secretion: patient’s age, disease duration, age of onset, duration of clinical remission, DIR, HbA1c level at diagnosis, at collection and average value over the last year (average of three or four measurements taken every three months), BMI-SDS at disease onset, and actual BMI-SDS.
For the purpose of our analysis and comparison of various parameters, patients were categorized into groups based on the secretion of clinically significant (N=44) and insignificant (N=77) C-peptide levels; BMI-SDS < 1 (N=85) and ≥ 1 (N=36); and into 3 groups according to the duration of the disease: the first - up to 2 years (N=35), the second - 2–5 years (N=34) and the third - more than 5 years (N=52).
3 Statistical analysis
Statistical analysis was performed using Statistica version 13.3 (StatSoft Krakow, Poland). The Kolmogorov-Smirnov test of normality with Lillefors correction and Shapiro-Wilk test were used to determine whether all continuous variables have a normal distribution. All examined variables with a parametric distribution were evaluated using the unpaired Student’s T-test to compare the differences between the groups. As several of the study parameters were not normally distributed, the two-tailed Mann-Whitney U-test was used to correlate continuous variables. To assess the relationship between two qualitative variables, Pearson correlation was used for parametric data and Spearman rank coefficient for nonparametric data. Multiple linear regression analysis was performed to detect independent determinants of C-peptide levels. All data are expressed as mean ± SD or median with interquartile range (IQR), as appropriate. Relative risk was evaluated with the use of Fishers’exact test, and calculation of 95% confidence interval with Koopman asymptomatic score. For Student’s T-test, with α=.05, assumed medium effect size (0.5) and our population size, estimated power of the test was calculated as.803-.837 depending on the subgroup sizes. Subsequently, for correlation analysis, with our sample size, α=.05 and medium effect size (0.3), estimated power of the test reaches up to.95. For linear multiple regression with six predictors, our sample size, α=.05 and assumed medium effect size (0.15) the calculated power was estimated at.895.
Differences were considered statistically significant with P-value <.05.
4 Ethical approval
The study protocol was approved by the Ethical Committee of the Medical University of Bialystok (R-I-002/402/2019). Written informed consent was obtained from parents/legal guardians as well as from patients aged > 16 years.
5 Results
The study covered 121 Caucasian children, with almost equal participation of both sexes (50.41% boys). Clinical and biochemical characteristics of the study group were collected in Table 1.
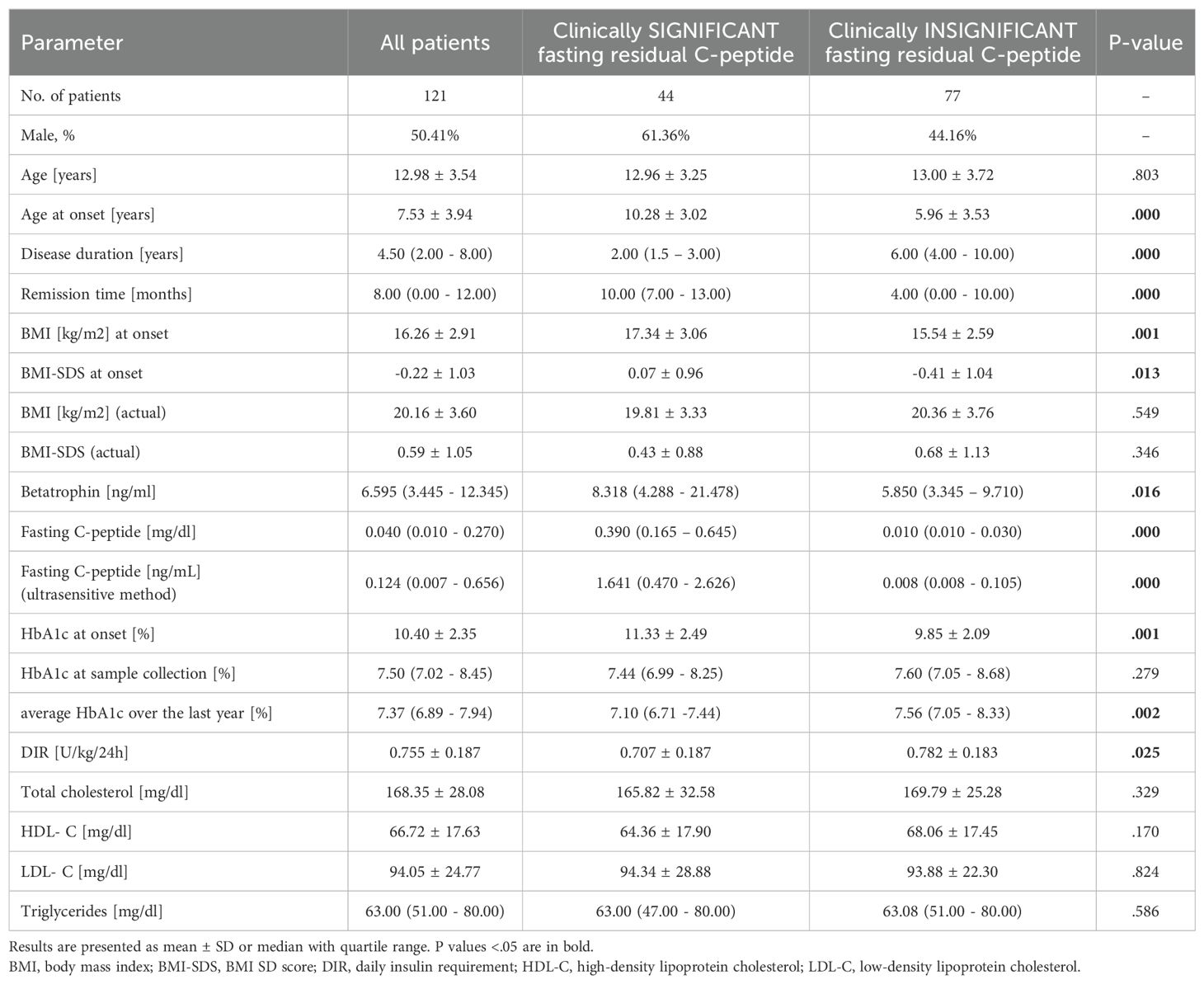
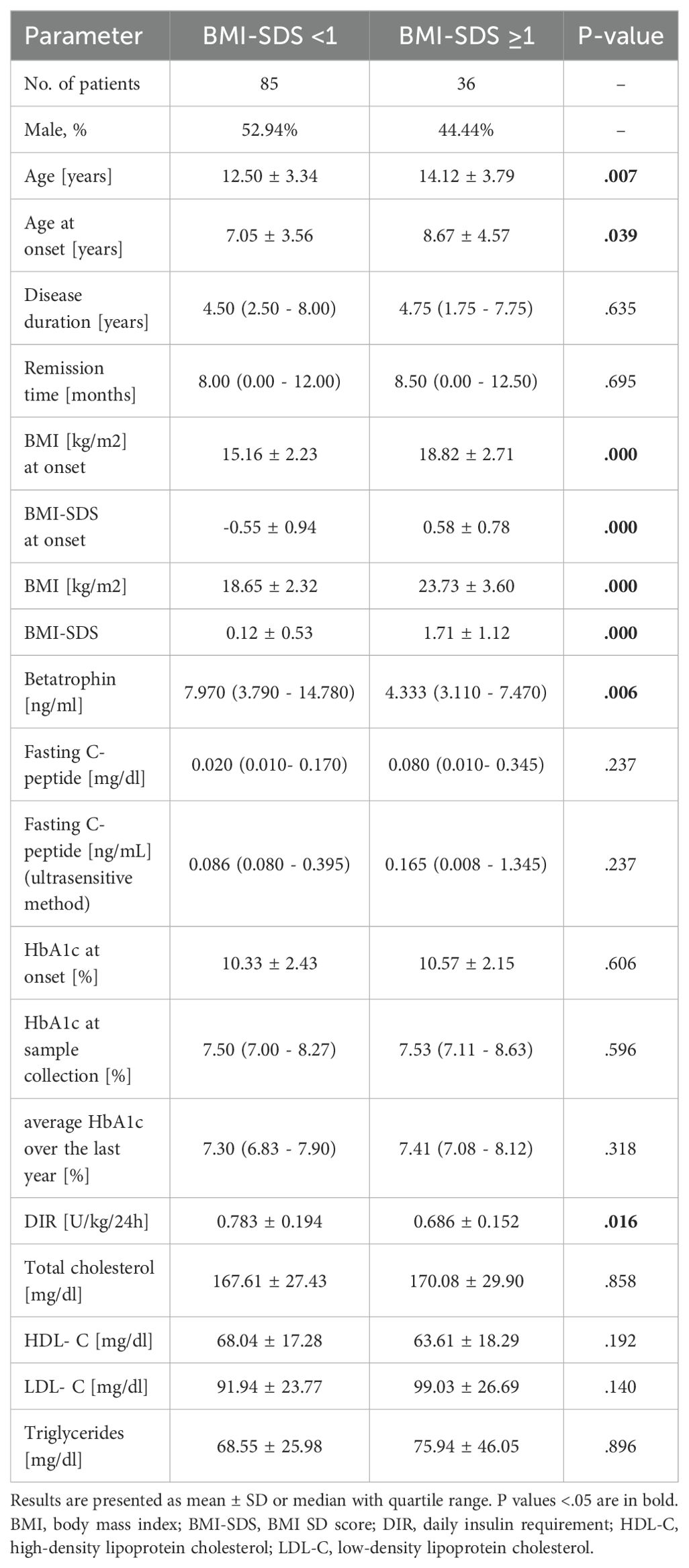
Table 1. Clinical and biochemical characteristics of all study patients and comparison of groups with clinically significant and insignificant fasting residual C-peptide.
The ultrasensitive method showed detectable levels of C-peptide in 54 (44.63%) individuals, with the lowest detection limit of 0.0076 ng/ml found. Clinically significant C-peptide level (> 0.23 ng/ml) was found in 44 (36.36%) patients. The mean age of children in both groups (clinically significant vs. insignificant C-peptide) was comparable, but patients with higher C-peptide levels were older at diagnosis (10.28 ± 3.02 vs. 5.96 ± 3.53 years, P <.001). As might be expected, children with preserved clinically significant C-peptide had a shorter disease duration (P <.001) and a longer mean remission time (P <.001). The BMI-SDS value at the beginning of the disease was higher in the group of patients with significant C-peptide level than in the remaining group of patients (0.07 ± 0.96 vs. -0.41 ± 1.04, P = .013), yet no such difference was found in the current BMI-SDS (P = .346). We also found statistically significant lower average HbA1c from the last year (P = .002) as well as lower DIR (P = .025) and higher betatrophin concentrations (P = .016) in patients with clinically significant compared to subjects with clinically insignificant residual fasting C-peptide levels (Table 1).
There were noticeable differences between the groups of patients with BMI-SDS <1 and ≥1 in betatrophin concentrations 7.970 (3.790 - 14.780) vs. 4.332 (3.110 - 7.470) ng/ml, P = .006 (Table 2, Figure 1A). Patients with BMI-SDS <1 were younger (12.50 ± 3.34 vs. 14.12 ± 3.79, P = .007) had earlier disease onset (7.05 ± 3.56 vs. 8.67 ± 4.57, P = .039) and surprisingly, had higher DIR (0.783 ± 0.194 vs. 0.686 ± 0.152, P = 0.016) than patients with greater body weight. The analysis did not reveal any statistically significant differences in C-peptide levels between these groups (P = .237) (Figure 1B). It should be added that already at the time of diagnosis there was a significant difference in BMI and BMI-SDS values between these groups (both P <.001), which could have affected both C-peptide and betatrophin levels from the very beginning of the disease.
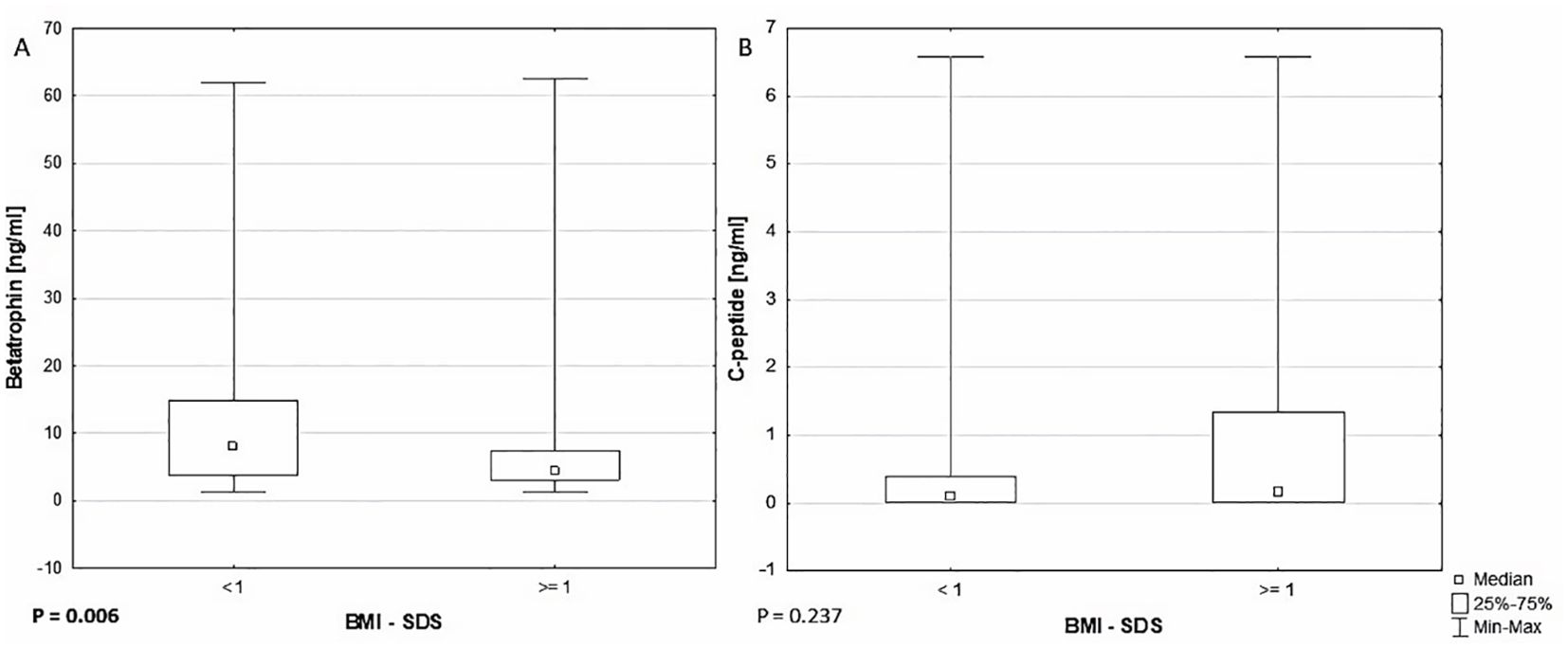
Figure 1. Comparison of (A) betatrophin (P = .006) and (B) C-peptide (P = .237) levels between BMI-SDS <1 and ≥1 groups.
In the subgroups according to the duration of diabetes, statistically significant difference in betatrophin levels was observed exclusively between the groups with disease duration up to 2 years and greater than 5 years (P = .035) (Figure 2A). It should be noted, however, that children with diabetes of a shorter duration were usually younger (11.71 ± 3.47 vs. 14.38 ± 3.04 years old, P <.001), diagnosed at a later age (often during puberty) (10.14 ± 3.50 vs. 5.36 ± 3.30 years old, P <.001), and had lower DIR (.672 ± .155 vs.796 ± .179 U/kg/24h, P = .003). C-peptide values showed a decreasing trend with disease duration: in the group with disease duration <2 years (0.966 (0.288 - 2.087) ng/ml), 2–5 years (0.118 (.008 - 0.288) ng/ml), and >5 years (0.008 (0.008 - 0.095) ng/ml) (Figure 2B).

Figure 2. Differences in betatrophin (A) and C-peptide (B) concentrations among groups with disease duration < 2 years, 2-5years and > 5 years. P values <.05 are in bold.
Interestingly, the results of the statistical analysis showed a positive correlation between the levels of betatrophin and C-peptide (R = 0.185, P = 0.043). It was found that the level of betatrophin is negatively correlated with the patient’s body weight (R = -0.383, P <.001) as well as with BMI (R = -0.385, P <.001) and BMI-SDS (R = -.0233, P = .010), the duration of the disease (R = -0.249, P = 0.006), patient’s age (R = -0.373, P <0.001), HbA1c at sample collection (R = -0.208, P = 0.022), average HbA1c over the last year (R = -0.248, P = 0.006), and basal insulin (R = -0.314, P = .001) (Table 3, Figures 3A–D). No correlation was found between the level of betatrophin and the level of triglycerides (TG), total cholesterol (TC), neither high density lipoprotein cholesterol (HDL-C) nor low density lipoprotein cholesterol (LDL-C) (respectively: P = 0.903; P = 0.468; P = 0.921; P = 0.667).
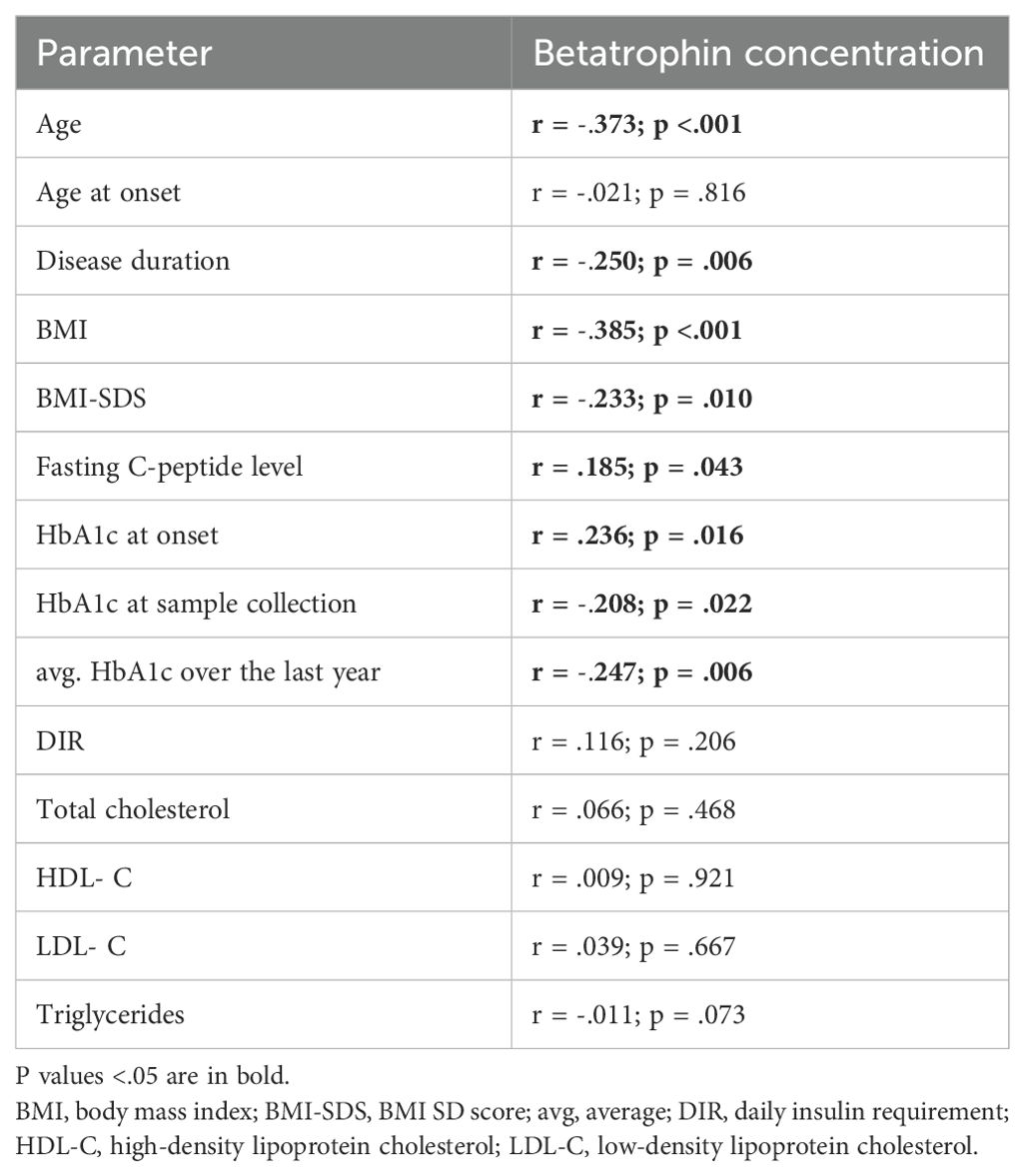
Table 3. Correlation analysis between betatrophin concentrations and studied variables within the entire study group.
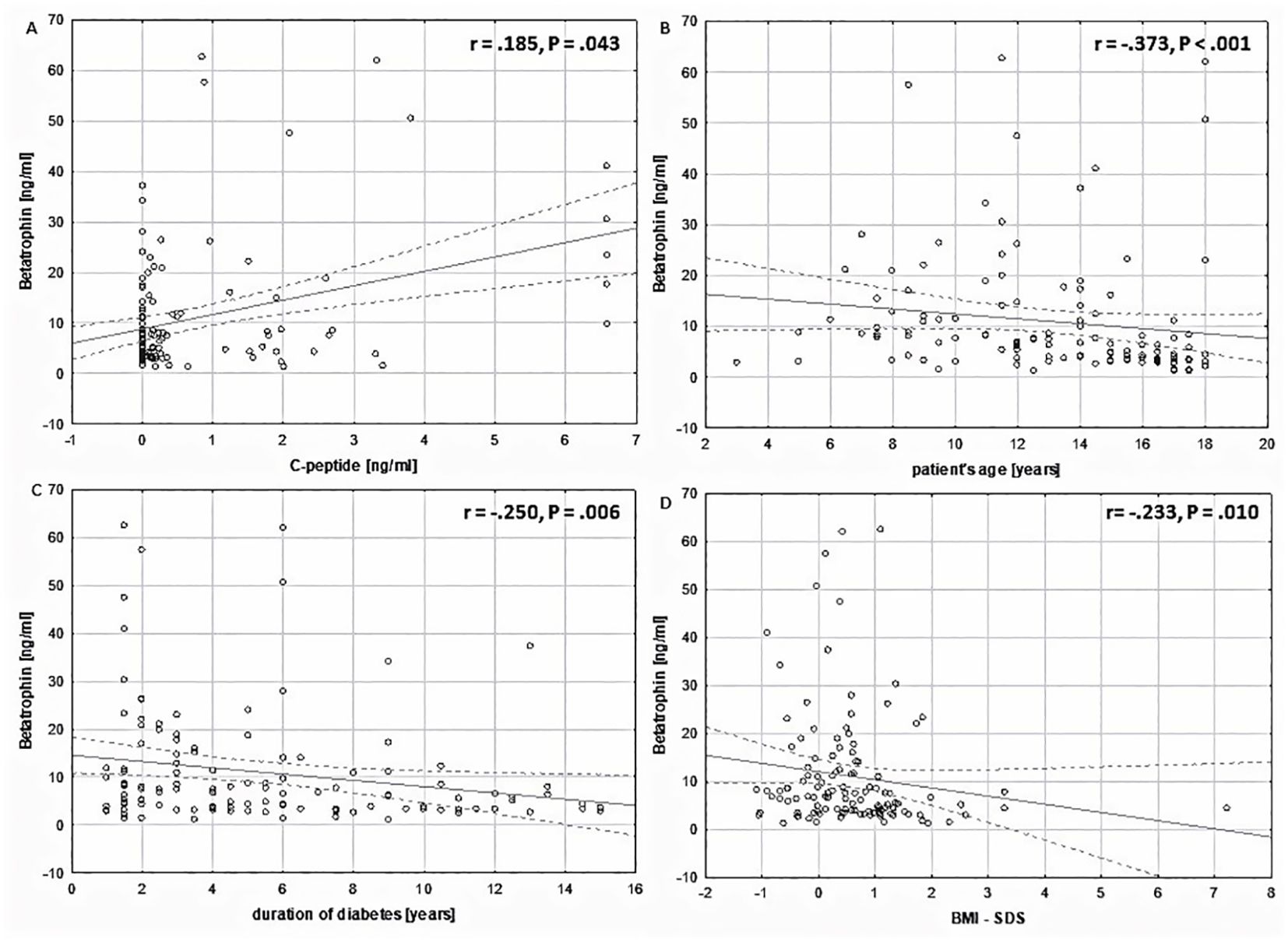
Figure 3. Correlation analysis of plasma betatrophin concentrations (ng/ml) in relation to the studied variables within the entire study group. (A) in relation to C-peptide levels (ng/ml) (Spearman r = .185, P = .043). (B) in relation to patient’s age (Spearman r = -.373, P <.001). (C) in relation to duration of diabetes (Spearman r = -.250, P = .006). (D) in relation to BMI-SDS (Spearman r= -.233, P = .010).
Regression analysis was performed and showed that greater betatrophin levels (β=.263, P=.003), older age at disease onset (β=.316, P=.004) and undeniably duration of remission (β=.194, P=.035) were factors determining higher concentrations of preserved C-peptide (Table 4).
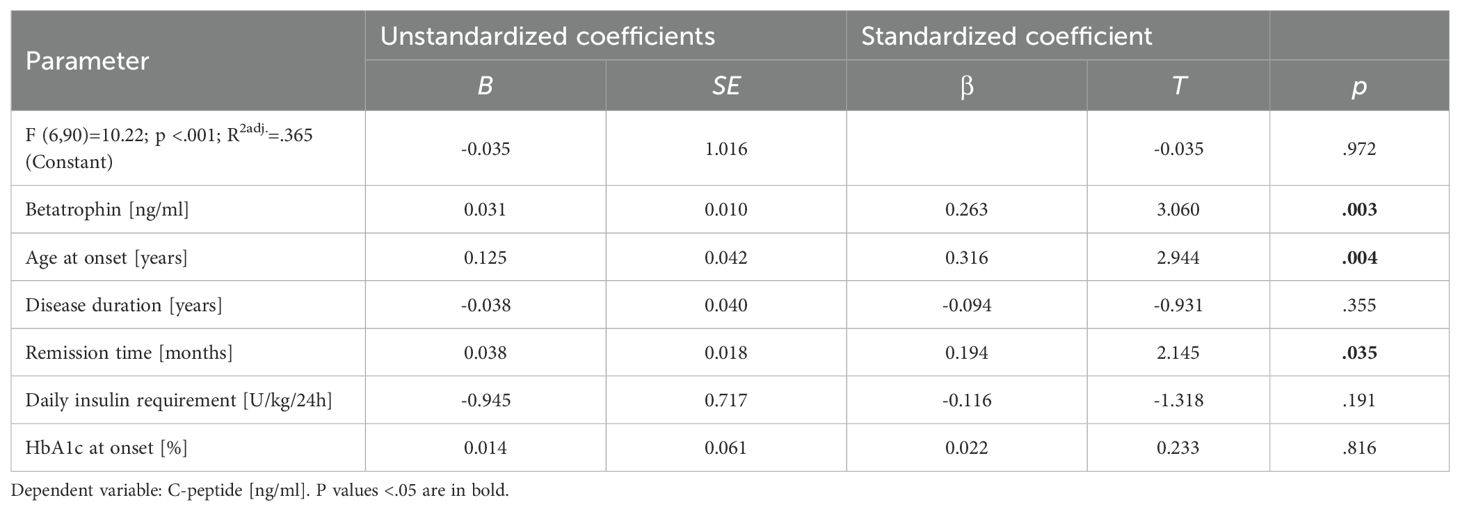
Table 4. Linear regression of variables independently affecting C-peptide levels in youths with type 1 diabetes at least one year after diagnosis.
6 Discussion
The aim of our study was to determine whether children suffering from type 1 diabetes for at least 1 year, beyond the remission phase can still secrete C-peptide in clinically significant amounts and factors that may affect its levels. Among the recruited children, 44 (36.36%) had detectable, clinically significant levels of C-peptide. We also aimed to assess the potential role of betatrophin as a biomarker of residual β-cell function. We reported that betatrophin levels may be influenced by body weight, age or duration of the disease. To our knowledge, for the first time we present a positive correlation between the concentration of betatrophin and C-peptide in the discussed population, with betatrophin being one of the strongest predictors among the factors correlating with C-peptide.
The study by Gokulakrishnan et al. did show a positive correlation between betatrophin and C-peptide, but included a population with T2D (15). Another study showed an increase in both betatrophin and C-peptide in patients with T2D, but no clear relationship between these two parameters was observed. However, the same study showed a positive correlation between betatrophin and C-peptide in non-diabetics (21). C-peptide levels are thought to reflect residual insulin reserves (22). Studies confirm the correlation between detectable C-peptide levels in long-term diabetes and good metabolic control, and additionally, maintained residual pancreatic function seems to play an important role in preventing serious complications such as hypoglycemia, nephropathy or retinopathy (23). The interdependence between betatrophin and C-peptide may indicate a positive effect of betatrophin on pancreatic secretion as well as on remaining in the partial remission phase. As with C-peptide levels, betatrophin levels decrease with longer disease duration, but not as rapidly. In addition, we found that betatrophin levels were negatively correlated with HbA1c at the time of sample collection and the average HbA1c level over the past year. This findings is consistent with the notion that higher betatrophin levels may contribute to better glycemic control in the pediatric T1D population. On the contrary, the study by Espes et al. suggests that although betatrophin levels are increased in patients with type 1 diabetes, its levels do not correlate with C-peptide levels, glycemic control, or insulin requirements (11).
Additionally, obesity appears to have an impact on betatrophin levels, but there are discrepancies in the results. The first studies did not show a significant changes in ANGPTL8 between obese and normal weight participants (24, 25). A little later, a study was published presenting higher levels of ANGPTL8 among obese people compared to a lean control group (26). Increased levels of betatrophin were observed in obese teenagers compared to subjects with normal body weight, regardless of gender (27). In another study, a statistically significant positive correlation between BMI and betatrophin levels in patients, in general, in partial remission, and 1.5 years after the diagnosis of T1D was observed, but there was no correlation at the onset of the disease (5). Conversely, circulating betatrophin levels were significantly reduced in people with obesity and reduced even more in participants with impaired glucose tolerance and T2D. They were also found to be positively correlated with a quantitative index of insulin sensitivity and HDL-C levels (28). Tuhan et al. have also reported reduced betatrophin levels in obese patients as well as those with insulin resistance in the pediatric population (29). Our findings regarding the correlation between the metabolic parameter both BMI and BMI-SDS and the level of circulating betatrophin are consistent with the results of the latter. Moreover, as indicated by numerous scientific reports, obesity may cause a state of chronic inflammation, which may affect the functioning of the organs producing betatrophin, among others the liver, and thus alters its concentrations (30). It can be assumed that obesity, coexisting with T1D, and its dysregulatory effect on the body may reduce the secretion of betatrophin and accelerate the loss of pancreatic β-cells, manifested by a faster decrease in C-peptide (31), which could imply that maintaining a healthy body weight may contribute to prolonging the residual function of pancreatic cells. Nevertheless, studies have also raised the issue of a negative correlation between betatrophin and insulin resistance, which may suggest that not the increased body weight itself, but the insulin resistance present in obesity may have an impact on the decrease in betatrophin concentration (29, 32).
Most previous studies reported increased levels of betatrophin at the onset of the disease. Similarly to the others we detected high values of betatrophin concentrations in children up to the second year of the disease, however, the longer the duration of diabetes, the concentrations decrease. Villalba et al. showed increased betatrophin concentrations in children and adolescents with T1D up to three times at the time of diagnosis when compared to control subjects. They also found a tendency for betatrophin levels to decrease from the twelfth month of the disease (P = .057) (5). Increased levels of betatrophin were also observed in adolescent Saudi females diagnosed with T1D, compared to their healthy peers (33). Another analysis also found elevated levels of circulating betatrophin, this time in a population of adults with T1D (11). However, the influence of disease duration on betatrophin concentration has not been studied. Studies have shown that betatrophin levels are also elevated in T2D patients and may be influenced by the duration of the disease (25, 34–36). This suggests that hyperglycemia may play a key role in increasing betatrophin levels in diabetes at least at the beginning of the disease. It is alluring to speculate that increased betatrophin concentrations may have had a temporary positive effect on β-cell stimulation at the onset of the disease or that the lack of betatrophin causes excessive reduction of residual C-peptide. However, in the long term, it does not protect against the progressive loss of residual C-peptide. Nevertheless, in-depth researches on the mechanism of action of betatrophin may enable treatment with this molecule in the future.
In contrast to other studies, we did not obtain any significant correlation between betatrophin and the lipid profile in the analyzed group. Ghasemi et al. found that in diabetic patients the level of betatrophin was positively correlated with the levels of TG and TC with statistical significance (37). Circulating betatrophin concentrations have been shown to be significantly lower in patients with dyslipidemia characterized by high TG levels or low HDL-C levels (38). A cross-sectional study conducted in a Chinese population presented that circulating concentrations of full-length betatrophin were significantly lower in patients without dyslipidemia compared with patients with documented dyslipidemia. The mentioned betatrophin concentrations were positively correlated with the levels of non-HDL-C, TG and TC, and negatively with the concentration of HDL-C (39). In another study, circulating ANGPTL8 concentrations were an important factor positively associated with fasting hypertriglyceridemia in diabetic patients, while negatively correlated with levels of HDL-C (40).
More recently, betatrophin has been extensively studied for its ability to promote β-cell proliferation, nevertheless the cause and significance of increased betatrophin levels in type 1 diabetes remain undetermined. Its correlation with metabolic and endocrine diseases such as diabetes or obesity is of great interest. Many studies have been conducted in the pediatric population in remission of T1D, as well as in long-lasting disease. We decided to focus on a relatively understudied group, i.e. differences between children fresh out of clinical remission compared to those with longer duration of disease in the search for still secreting C-peptide, in the context of the importance of betatrophin as a potential marker of preserved residual beta-cell function and its differences between overweight and normal-weight children. One of the possible implications of our findings for future research is that stimulating pancreatic β-cells proliferation through betatrophin to prolong insulin or residual C-peptide secretion to maintain blood glucose levels within desired limits in T1D patients would be a beneficial mechanism, but its presence remains to be determined.
6.1 Limitations
Limitations of this study include its observational design and the lack of measurement of some variables. Therefore, we cannot analyze changes between ANGPTL8 levels at diagnosis and at sampling in relation to, for example, C-peptide or changes in HbA1c, nor the rate of decline in individual patients due to the limited number of events. Due to the retrospective nature of the above study, the CGM results, i.e. time in range (TIR), time above range (TAR), time below range (TBR), glucose management indicator (GMI) or coefficient of variability (CV), which are increasingly appearing in newer publications on T1D, are not available and we cannot refer to betatrophin concentration in terms of short-term glycemia. However, we believe that the significance of transient glucose fluctuations is not as important for fluctuations in the concentration of the studied molecule as persistent metabolic control, which is reflected by HbA1c.
7 Conclusion
A large part of the pediatric type 1 diabetes population can still secrete clinically significant amounts of C-peptide even in the presence of long-term disease, which may promote better metabolic control. Therefore, it is important to search for methods and factors that influence the extension of pancreatic secretory function- betatrophin may be one of the possibilities. Recent studies indicate the presence of elevated betatrophin levels in people with type 1 diabetes. The most important finding of our study is that circulating betatrophin levels correlate positively with C-peptide, which, combined with the negative association of betatrophin with HbA1c, may suggest that betatrophin contributes to better glycemic control. The main clinical implication of our results is the potential use of betatrophin as a future biomarker of residual β-cell function. Additionally, excess body weight appears to have a negative impact on betatrophin levels in children with T1D, thus maintaining a healthy body weight may protect against betatrophin deprivation, which may lead to improved glycemic outcomes. Many variables, such as nutritional status, duration of the disease, lipid or metabolic profile, may influence changes in both C-peptide and betatrophin concentrations. At this time, betatrophin may not be an independent marker, but mainly in correlation with C-peptide it could provide information about endogenous insulin production. We believe that further studies involving a larger, more homogeneous population of children with type 1 diabetes are necessary to clarify the exact role of betatrophin as a biomarker of pancreatic secretion.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study protocol was approved by the Ethical Committee of the Medical University of Bialystok (R-I-002/402/2019). Written informed consent was obtained from parents/legal guardians as well as from patients aged > 16 years.
Author contributions
EO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Visualization. MK: Conceptualization, Writing – original draft, Investigation, Visualization. MJ-S: Conceptualization, Formal analysis, Investigation, Writing – review & editing, Data curation, Methodology. AB: Project administration, Resources, Supervision, Validation, Writing – review & editing. BG-O: Supervision, Validation, Writing – review & editing, Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Data curation, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research and the APC was funded by Medical University of Bialystok.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. DiMeglio LA, Evans-Molina C, and Oram RA. Type 1 diabetes. Lancet. (2018) 391:2449–62. doi: 10.1016/S0140-6736(18)31320-5
2. Sims EK, Bahnson HT, Nyalwidhe J, Haataja L, Davis AK, Speake C, et al. Proinsulin secretion is a persistent feature of type 1 diabetes. Diabetes Care. (2019) 42:258–64. doi: 10.2337/dc17-2625
3. Buckingham B, Cheng P, Beck RW, Kollman C, Ruedy KJ, Weinzimer SA, et al. CGM-measured glucose values have a strong correlation with C-peptide, HbA1c and IDAAC, but do poorly in predicting C-peptide levels in the two years following onset of diabetes. Diabetologia. (2015) 58:1167–74. doi: 10.1007/s00125-015-3559-y
4. Couper JJ, Haller MJ, Greenbaum CJ, Ziegler AG, Wherrett DK, Knip M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Stages of type 1 diabetes in children and adolescents. Pediatr Diabetes. (2018) 19:20–7. doi: 10.1111/pedi.2018.19.issue-S27
5. Villalba A, Fonolleda M, Murillo M, Rodriguez-Fernandez S, Ampudia RM, Perna-Barrull D, et al. Partial remission and early stages of pediatric type 1 diabetes display immunoregulatory changes. A pilot study. Transl Res. (2019) 210:8–25. doi: 10.1016/j.trsl.2019.03.002
6. Harsunen M, Haukka J, Harjutsalo V, Mars N, Syreeni A, Härkönen T, et al. Residual insulin secretion in individuals with type 1 diabetes in Finland: longitudinal and cross-sectional analyses. Lancet Diabetes Endocrinol. (2023) 11:465–73. doi: 10.1016/S2213-8587(23)00123-7
7. Steffes MW, Sibley S, Jackson M, and Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. (2003) 26:832–6. doi: 10.2337/diacare.26.3.832
8. Szypowska A, Groele L, Wysocka-Mincewicz M, Mazur A, Lisowicz L, Ben-Skowronek I, et al. Factors associated with preservation of C-peptide levels at the diagnosis of type 1 diabetes. J Diabetes Complications. (2018) 32:570–4. doi: 10.1016/j.jdiacomp.2018.03.009
9. Kara Ö, Esen İ, and Tepe D. Factors influencing frequency and duration of remission in children and adolescents newly diagnosed with type 1 diabetes. Med Sci Monit. (2018) 24:5996–6001. doi: 10.12659/MSM.908450
10. Leiherer A, Ebner J, Muendlein A, Brandtner EM, Zach C, Geiger K, et al. High betatrophin in coronary patients protects from cardiovascular events. Atherosclerosis. (2020) 293:62–8. doi: 10.1016/j.atherosclerosis.2019.11.011
11. Espes D, Lau J, and Carlsson PO. Increased circulating levels of betatrophin in individuals with long-standing type 1 diabetes. Diabetologia. (2014) 57:50–3. doi: 10.1007/s00125-013-3071-1
12. Yamada H, Saito T, Aoki A, Asano T, Yoshida M, Ikoma A, et al. Circulating betatrophin is elevated in patients with type 1 and type 2 diabetes. Endocr J. (2015) 62:417–21. doi: 10.1507/endocrj.EJ14-0525
13. Xu F, Chen Y, Wang N, and Sun K. Bacteria-derived recombinant human ANGPTL8/betatrophin significantly increases the level of triglyceride. Protein J. (2019) 38:472–8. doi: 10.1007/s10930-019-09825-8
14. Tseng YH, Yeh YH, Chen WJ, and Lin KH. Emerging regulation and function of betatrophin. Int J Mol Sci. (2014) 15:23640–57. doi: 10.3390/ijms151223640
15. Gokulakrishnan K, Manokaran K, Pandey GK, Amutha A, Ranjani H, Anjana RM, et al. Relationship of betatrophin with youth onset type 2 diabetes among Asian Indians. Diabetes Res Clin Pract. (2015) 109:71–6. doi: 10.1016/j.diabres.2015.04.028
16. Giuffrida FM, Bulcão C, Cobas RA, Negrato CA, Gomes MB, Dib SA, et al. Double-diabetes in a real-world sample of 2711 individuals: associated with insulin treatment or part of the heterogeneity of type 1 diabetes? Diabetol Metab Syndr. (2016) 8:28. doi: 10.1186/s13098-016-0143-7
17. Kalinowska A, Orlińska B, Panasiuk M, Jamiołkowska M, Zasim A, Florys B, et al. Assessment of preservation of beta-cell function in children with long-standing type 1 diabetes with “ultrasensitive c-peptide” method. Pediatr Endocrinol Diabetes Metab. (2017) 23:130–8. doi: 10.18544/PEDM-23.03.0084
18. Libman I, Haynes A, Lyons S, Pradeep P, Rwagasor E, Tung JY, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. (2022) 23:1160–74. doi: 10.1111/pedi.13454
19. Kułaga Z, Litwin M, Tkaczyk M, Palczewska I, Zajączkowska M, Zwolińska D, et al. Polish 2010 growth references for school-aged children and adolescents. Eur J Pediatr. (2011) 170:599–609. doi: 10.1007/s00431-010-1329-x
20. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, and Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. (2007) 85:660–7. doi: 10.2471/blt.07.043497
21. Abu-Farha M, Abubaker J, Noronha F, Al-Khairi I, Cherian P, Alarouj M, et al. Lack of associations between betatrophin/ANGPTL8 level and C-peptide in type 2 diabetic subjects. Cardiovasc Diabetol. (2015) 14:112. doi: 10.1186/s12933-015-0277-1
22. Jamiołkowska-Sztabkowska M, Głowińska-Olszewska B, and Bossowski A. C-peptide and residual β-cell function in pediatric diabetes - state of the art. Pediatr Endocrinol Diabetes Metab. (2021) 27:123–33. doi: 10.5114/pedm.2021.107165
23. Lam A, Dayan C, and Herold KC. A little help from residual β cells has long-lasting clinical benefits. J Clin Invest. (2021) 131. doi: 10.1172/JCI143683
24. Fenzl A, Itariu BK, Kosi L, Fritzer-Szekeres M, Kautzky-Willer A, Stulnig TM, et al. Circulating betatrophin correlates with atherogenic lipid profiles but not with glucose and insulin levels in insulin-resistant individuals. Diabetologia. (2014) 57:1204–8. doi: 10.1007/s00125-014-3208-x
25. Hu H, Sun W, Yu S, Hong X, Qian W, Tang B, et al. Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care. (2014) 37:2718–22. doi: 10.2337/dc14-0602
26. Abu-Farha M, Sriraman D, Cherian P, AlKhairi I, Elkum N, Behbehani K, et al. Circulating ANGPTL8/betatrophin is increased in obesity and reduced after exercise training. PloS One. (2016) 11:e0147367. doi: 10.1371/journal.pone.0147367
27. Hammad MM, Channanath AM, Abu-Farha M, Rahman A, Al Khairi I, Cherian P, et al. Adolescent obesity and ANGPTL8: correlations with high sensitivity C-reactive protein, leptin, and chemerin. Front Endocrinol (Lausanne). (2023) 14:1314211. doi: 10.3389/fendo.2023.1314211
28. Gómez-Ambrosi J, Pascual E, Catalán V, Rodríguez A, Ramírez B, Silva C, et al. Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J Clin Endocrinol Metab. (2014) 99:E2004–9. doi: 10.1210/jc.2014-1568
29. Tuhan H, Abacı A, Anık A, Çatlı G, Küme T, Çalan Ö, et al. Circulating betatrophin concentration is negatively correlated with insulin resistance in obese children and adolescents. Diabetes Res Clin Pract. (2016) 114:37–42. doi: 10.1016/j.diabres.2016.02.008
30. Onalan E, Bozkurt A, Gursu MF, Yakar B, and Donder E. Role of betatrophin and inflammation markers in type 2 diabetes mellitus, prediabetes and metabolic syndrome. J Coll Physicians Surg Pak. (2022) 32:303–7. doi: 10.29271/jcpsp.2022.03.303
31. Kurpiewska E, Ciężki S, Jamiołkowska-Sztabkowska M, Polkowska A, Starosz A, Grubczak K, et al. Excessive BMI is associated with higher C-peptide level at recognition but also with its greater loss in two years clinical observation in children with new onset type 1 diabetes. Front Immunol. (2023) 14:176403. doi: 10.3389/fimmu.2023.1176403
32. Wu S, Gao H, Ma Y, Fu L, Zhang C, and Luo X. Characterisation of betatrophin concentrations in childhood and adolescent obesity and insulin resistance. Pediatr Diabetes. (2016) 17:53–60. doi: 10.1111/pedi.12233
33. Mohammedsaeed W and Binjawhar D. Dyslipidemia and ANGPTL8 evaluation in young females with Type 1 diabetes mellitus. Endocrine. (2024) 86:564–73. doi: 10.1007/s12020-024-03909-x
34. Chen X, Lu P, He W, Zhang J, Liu L, Yang Y, et al. Circulating betatrophin levels are increased in patients with type 2 diabetes and associated with insulin resistance. J Clin Endocrinol Metab. (2015) 100:E96–100. doi: 10.1210/jc.2014-2300
35. Liu D, Qu H, Wang H, Duan Y, Liu F, Feng Z, et al. Relationship between serum betatrophin levels and the first-phase of glucose-stimulated insulin secretion. Obes Res Clin Pract. (2018) 12:9–15. doi: 10.1016/j.orcp.2016.08.004
36. Fu Z, Berhane F, Fite A, Seyoum B, Abou-Samra AB, and Zhang R. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci Rep. (2014) 4:5013. doi: 10.1038/srep05013
37. Ghasemi H, Tavilani H, Khodadadi I, Saidijam M, and Karimi J. Circulating betatrophin levels are associated with the lipid profile in type 2 diabetes. Chonnam Med J. (2015) 51:115–9. doi: 10.4068/cmj.2015.51.3.115
38. Gómez-Ambrosi J, Pascual-Corrales E, Catalán V, Rodríguez A, Ramírez B, Romero S, et al. Altered concentrations in dyslipidemia evidence a role for ANGPTL8/betatrophin in lipid metabolism in humans. J Clin Endocrinol Metab. (2016) 101:3803–11. doi: 10.1210/jc.2016-2084
39. Yang S, Jiao X, Huo X, Zhu M, Wang Y, Fang X, et al. Association between circulating full-length angiopoietin-like protein 8 and non-high-density lipoprotein cholesterol levels in Chinese non-diabetic individuals: a cross-sectional study. Lipids Health Dis. (2018) 17:161. doi: 10.1186/s12944-018-0802-9
Keywords: T1D (Type 1 diabetes), children, C-peptide, obesity, betatrophin, islet beta (β) cells
Citation: Odyjewska E, Kupińska M, Jamiołkowska-Sztabkowska M, Bossowski A and Głowińska-Olszewska B (2025) Residual beta-cell function in children with type 1 diabetes after a partial remission phase – a possible relation between C-peptide and betatrophin. Front. Endocrinol. 16:1602777. doi: 10.3389/fendo.2025.1602777
Received: 31 March 2025; Accepted: 28 May 2025;
Published: 18 June 2025.
Edited by:
Ping Wang, Michigan State University, United StatesReviewed by:
Viva Ma, Becton Dickinson, United StatesTamer A. Addissouky, University of Menoufia, Egypt
Copyright © 2025 Odyjewska, Kupińska, Jamiołkowska-Sztabkowska, Bossowski and Głowińska-Olszewska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara Głowińska-Olszewska, YmFyYmFyYS5nbG93aW5za2Etb2xzemV3c2thQHVtYi5lZHUucGw=
 Emilia Odyjewska
Emilia Odyjewska Monika Kupińska
Monika Kupińska Milena Jamiołkowska-Sztabkowska
Milena Jamiołkowska-Sztabkowska Artur Bossowski
Artur Bossowski Barbara Głowińska-Olszewska
Barbara Głowińska-Olszewska