- 1Rutgers University, New Brunswick, NJ, United States
- 2Neurocrine Biosciences, San Diego, CA, United States
- 3Trinity Life Sciences, Waltham, MA, United States
Background: Glucocorticoid (GC) therapies treat many chronic conditions such as congenital adrenal hyperplasia (CAH), but long-term use carries risks of side effects (e.g., skeletal, cardiometabolic, mental health issues) that can negatively impact clinical and economic outcomes. Consequently, patients and providers seek to balance the lowest efficacious dose and side effect risk. To our knowledge, no research has analyzed US payer coverage decisions on medications that reduce GC reliance.
Objective: To understand the significance and implications of US payer perceptions and coverage/access decisions for therapies reducing GC doses, which may be of relevance to new therapies for CAH.
Methods: A literature review was paired with primary market research to identify and characterize 5 GC-reducing therapies to evaluate payer coverage policies. No identified therapies were currently approved or studied in CAH. Qualitative interviews (n=13) were also conducted across managed care organizations, pharmacy benefit managers, and managed Medicaid payers to supplement publicly available information.
Results: GC-reducing therapies were desirable and therapeutically beneficial from payer perspectives based on market research of payer coverage policies; all therapies were covered in place of or in addition to GCs. Despite premium pricing vs. low-cost alternatives, all therapies evaluated were covered by some or all payers with prior authorization to label indication or trial criteria. Qualitative interviews revealed that payers clearly understood the clinical burden of long-term GC use; however, the economic burden was less understood. Payers stated that GC reduction is a secondary decision-making driver due to the focus on trial primary endpoints, contracting dynamics, lack of competitors, and small trial sample sizes. A subset of payers was interested in GC reduction data as a primary endpoint for rare diseases without treatment alternatives and in pediatric populations.
Conclusions: Despite the premium price over GCs, GC-reducing therapies were covered in place of or in addition to GCs. Payers acknowledged the clinical value of reducing long-term GC use. Understanding what payers perceive as important criteria for coverage of GC-reducing medication may aid clinicians in evaluating utilization management criteria, such as step therapy, and increase access to medications aiming to reduce the patient burden associated with long-term GC use. This is particularly important in CAH where there is a high unmet need due to lifelong exposure to supraphysiologic doses of GCs.
Highlights
Steroids are common treatments used in many diseases for different benefits such as to decrease inflammation or to replace hormones when a person’s body may not make enough. However, their use may cause side effects that can last a long time. The way insurance companies think about medications that reduce steroid dose, and how their decisions impact whether to cover a medication that reduces steroid dose, is not well understood. This study shows that insurance companies are concerned about side effects of steroids. Medications that reduced steroid dose were covered along with steroids or offered in place of steroids.
Introduction
Glucocorticoid (GC) therapies are synthetic analogs of natural steroid hormones produced by the adrenal cortex that can be given systemically as a treatment for many diseases (1). While GC therapy is critical in the treatment of numerous inflammatory and immunologic conditions, chronic GC use carries significant risks of long-term comorbidities, including osteoporosis, adrenal suppression, metabolic disorders, immunosuppression, neuropsychiatric effects, and cardiovascular disease (1, 2). The use of long-term GCs and GC-related comorbidities have the potential to cause a substantial economic burden for patients and payers.
Although the economic burden of GC use has not been well studied, recent evidence suggests the economic cost is considerable, particularly in patients who take higher GC doses (3–5). Indeed, in a systematic literature review of disease states with long-term GC exposure (e.g., autoimmune diseases, asthma, lung diseases) healthcare costs increased correspondingly with GC dose (4). Costs ranged from approximately $5,700 in low-dose GC users (<7.5 mg prednisone or equivalent [30 mg hydrocortisone equivalents (HCe)]/day) to $29,000 in high-dose GC users (>15 mg prednisone or equivalent [60 mg HCe]/day) in per-annum incremental costs relative to non-users (4). Furthermore, in systemic lupus erythematosus (SLE), low-dose GC users (<7.5 mg prednisone [30 mg HCe] or equivalent/day) had mean incremental healthcare costs of $21,869 vs. $45,360 in high-dose GC users (>15 mg prednisone or equivalent [60 mg HCe]/day) (3). GC-related adverse events can also be particularly costly; for example in a systematic literature review of 47 studies assessing adverse events with GCs, 1-year per-patient costs were highest for stroke ($36,390 [2024 US dollars]), non-fatal myocardial infarction ($39,470), and fractures ($27,372) (5). Additionally, the use of GCs has been associated with reductions in health-related quality of life, which increases in magnitude as patients fill more GC prescriptions (6).
Congenital adrenal hyperplasia is a group of autosomal recessive disorders associated with impaired cortisol synthesis and includes classic and non-classic forms. Classic congenital adrenal hyperplasia, herein referred to as CAH, relies on long-term use of GCs. The most common cause of CAH is mutations in the CYP21A2 gene that leads to 21-hydroxylase deficiency, which is associated with hyperandrogenism and variable degrees of cortisol and aldosterone deficiency (7–9). The classical form of CAH is typically diagnosed through prenatal or postnatal screening techniques, with an incidence of approximately 1 in 15,000 to 1 in 17,000 live births, which defines CAH as a rare disease under the Orphan Drug Act (10); the serious manifestations of the condition start at birth and require lifelong GC therapy with doses above physiologic levels, which can lead to long-term health challenges that can impact cardiometabolic risk, bone health, and quality of life, among other health domains (11, 12). CAH management involves a delicate balance between the risks associated with hyperandrogenism and those associated with chronic GC overexposure (11, 12). The variability in response to GCs in patients with CAH may lead to iatrogenic Cushing syndrome or adrenal crisis, which makes it challenging for clinicians and patients to manage the disease, ensure treatment adherence, and control adverse impacts of androgen excess (11, 12).
Since GCs have historically been the only medications used in CAH to manage an excess of adrenocorticotropic hormone (ACTH) and adrenal androgens, as well as replace cortisol, there is considerable unmet need in this patient population, particularly for patients who have burdensome GC dose-related adverse effects (13). However, novel treatments that reduce GC dose in other disease states where GCs are commonly prescribed, such as belimumab for SLE and sarilumab for polymyalgia rheumatics (PMR), have emerged (14–20). While most of these branded therapies have evidence to support that they can lower the required GC doses, they often also have utilization management criteria (e.g., prior authorization, step therapy) required for use (21).
To our knowledge, no research has been conducted to characterize United States (US) payer coverage decisions on GC-reducing medications and at the time of the analysis, no GC-reducing therapies were available for the treatment of CAH. To aid the optimization of treatment in patients with CAH, the objective of this study was to understand how payers perceive GC-reducing therapies in other disease states and make decisions related to their coverage and access as a proxy for future GC-reducing therapies in CAH.
Methods
Study design
A 2-phase approach was used in this study. First, a review of literature and market research were used to evaluate GC-reducing therapies and their payer coverage policies. Next, a cross-sectional study was conducted to understand health plans’ stated perspectives on the value of GC-reducing therapies, and to better characterize perspectives derived from their management actions (e.g., coverage criteria) of these therapies.
Identification of therapies
GC-reducing therapies were identified and analyzed (Figure 1). First, approximately 45 potential therapies that allow for a reduction in GC dose/utilization were identified from prior assessments, including a review of the literature and primary market research. Indications in which very high levels of GCs are typically used (>40 mg/m2/day HCe) were excluded to align with similar GC doses (< 40 mg/m2/day HCe) used in CAH. The therapies that had indications for which GCs were the primary treatment until specialized products became covered were then analyzed. As noted, at the time of analysis, no GC-reducing therapies were available for the treatment of CAH and, thus, none of the identified GC-reducing therapies were currently approved or studied in CAH. The therapies were selected based on the following criteria:
● GC reduction as a primary or secondary endpoint in one or more pivotal Phase 3 clinical trials in the US or as an endpoint in an observational study or other publication (non-clinical trial).
● Epidemiology of the therapy’s indication represented either a rare disease (defined as having a prevalence of less than 200,000 patients in the US) or a subset of a non-rare disease.
● Covered under US commercial 2023 health plans designated as pharmacy or medical.
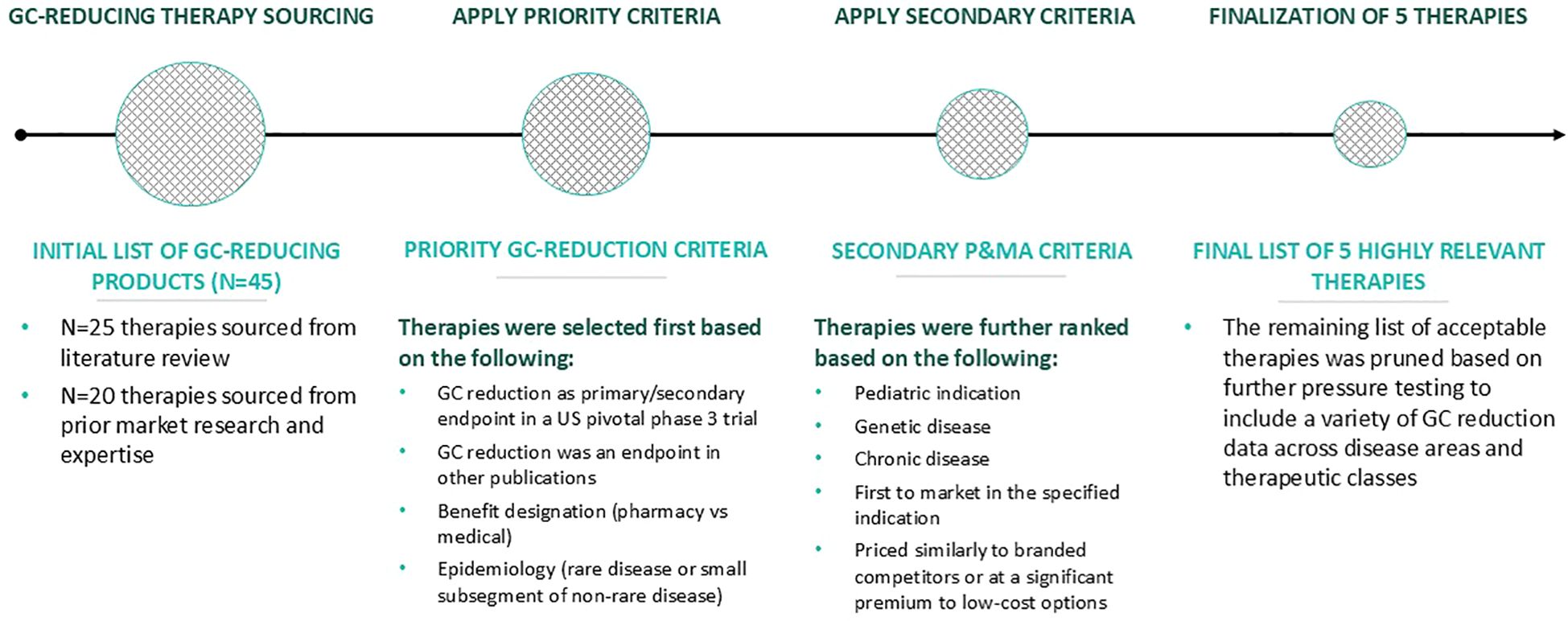
Figure 1. Approach to identifying GC-reducing therapies for evaluation. GC, glucocorticoid; P&MA, pricing and market access.
Therapies were subsequently ranked in order of those meeting the highest to lowest number of the following preferred criteria:
● First therapy to receive Food and Drug Administration (FDA) approval in its specified treatment indication.
● Indicated to treat pediatric patients.
● Indicated in a genetic disease.
● Indicated in a chronic disease.
● Priced similarly to branded competitors or priced at a substantial premium to lower-cost options.
Based on this assessment and ranking, 5 therapies that included a variety of GC reduction data across diverse disease areas/therapeutic classes were analyzed in greater detail. Additional secondary research for the 5 selected therapies included indication, clinical evidence package, and payer policies (formulary status and prior authorization criteria) across 5 large national commercial plans (Aetna, Humana, Anthem, United HealthCare, and Cigna).
Cross-sectional study (qualitative interviews)
Due to the limited information on the impact of GC reduction on multi-factorial formulary coverage decisions available through the literature review and market research, direct interviews were conducted with US decision-makers of national and regional managed care organizations (MCOs), pharmacy benefit managers (PBMs), and managed Medicaid health plans.
Payers were invited to participate through a healthcare research panel. Prior to participation, payers completed a self-administered, web-enabled screener to confirm eligibility to participate in the study. Payers who were eligible to participate must have met the following criteria:
● Voting member of a Pharmacy and Therapeutics Committee in their organization.
● At least 3 years and no more than 30 years of experience in their current position.
● Familiarity with at least 3 of the 5 selected therapy’s indication/therapeutic areas, with a self-assessed familiarity rating of at least 4 out of a possible 7 (scale 1 to 7, where 1 = least familiar and 7 = extremely familiar).
● Familiarity with their organization’s coverage policies for at least 3 of the 5 selected therapies in their specified indication/therapeutic areas, with a self-assessed familiarity rating of at least 4 out of a possible 7.
After the screening, eligible payers were invited to participate in a 45-minute in-depth telephone interview to capture their experiential background, familiarity with the selected GC-reducing therapies and relevant therapeutic areas, and insight regarding coverage and reimbursement decision-making. All interviews were conducted between December 2023 and January 2024.
Standard protocol approvals, registrations, and informed consent
This study was reviewed and determined to be exempt by ADVARRA, a central Institutional Review Board, and in compliance with ethical standards. All payers provided written consent via an online screener prior to participating in the qualitative interviews and received compensation for study participation.
Data analysis
Qualitative data derived from interviews were reviewed and assessed in aggregate and categorized according to key themes identified. Descriptive statistics were performed to describe the study population and quantitative responses captured during the interview, including responses captured on a 7-point Likert scale (1 = least familiar or knowledgeable and 7 = extremely familiar or knowledgeable). A higher score denoted more knowledge or familiarity with the topic of the survey question.
Results
Literature review and market research
Characteristics and coverage dynamics of selected GC-reducing therapies
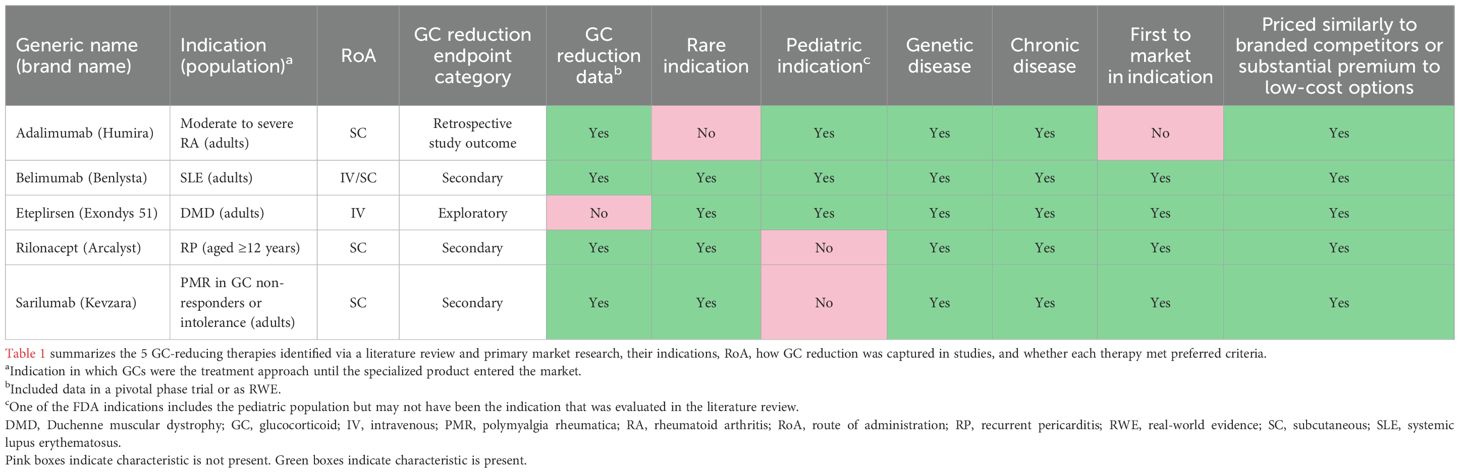
Based on the assessment and ranking of GC-reducing therapies, the 5 therapies selected were adalimumab (Humira®), belimumab (Benlysta™), eteplirsen (Exondys 51™), rilonacept (Arcalyst®), and sarilumab (Kevzara®) (Table 1).
All selected therapies had broad market access, were indicated in chronic diseases, and were in indications where the current treatment approach was GCs until a specialized product entered the market and had been covered throughout the years if it had successfully replaced or reduced GC use. The therapies included were purposefully diverse and had unique characteristics that made payer decision-making multi-factorial. Although adalimumab was not first to market in its specified indication or indicated in a rare disease, it was selected because it is the only approved rheumatoid arthritis (RA) therapy to demonstrate steroid reduction in a US-based trial and indicated in a subset of patients (those with moderate to severe RA) (22). Eteplirsen, indicated for Duchenne muscular dystrophy (DMD), was selected, partly due to it being the only selected therapy covered under the medical benefit due to its intravenous (IV) administration.
Three of the five therapies had evidence of GC reduction. Adalimumab, rilonacept, and sarilumab demonstrated significant reductions in GC use or GC elimination in their respective studies, while belimumab demonstrated non-significant GC dose reduction and eteplirsen had indirect GC reduction data available (Table 1).
Despite being priced at a substantial premium to low-cost GCs, all therapies evaluated were covered by some, if not all, of the 5 large national commercial plans with prior authorization using FDA indication or trial criteria as conditions for coverage (Table 2).
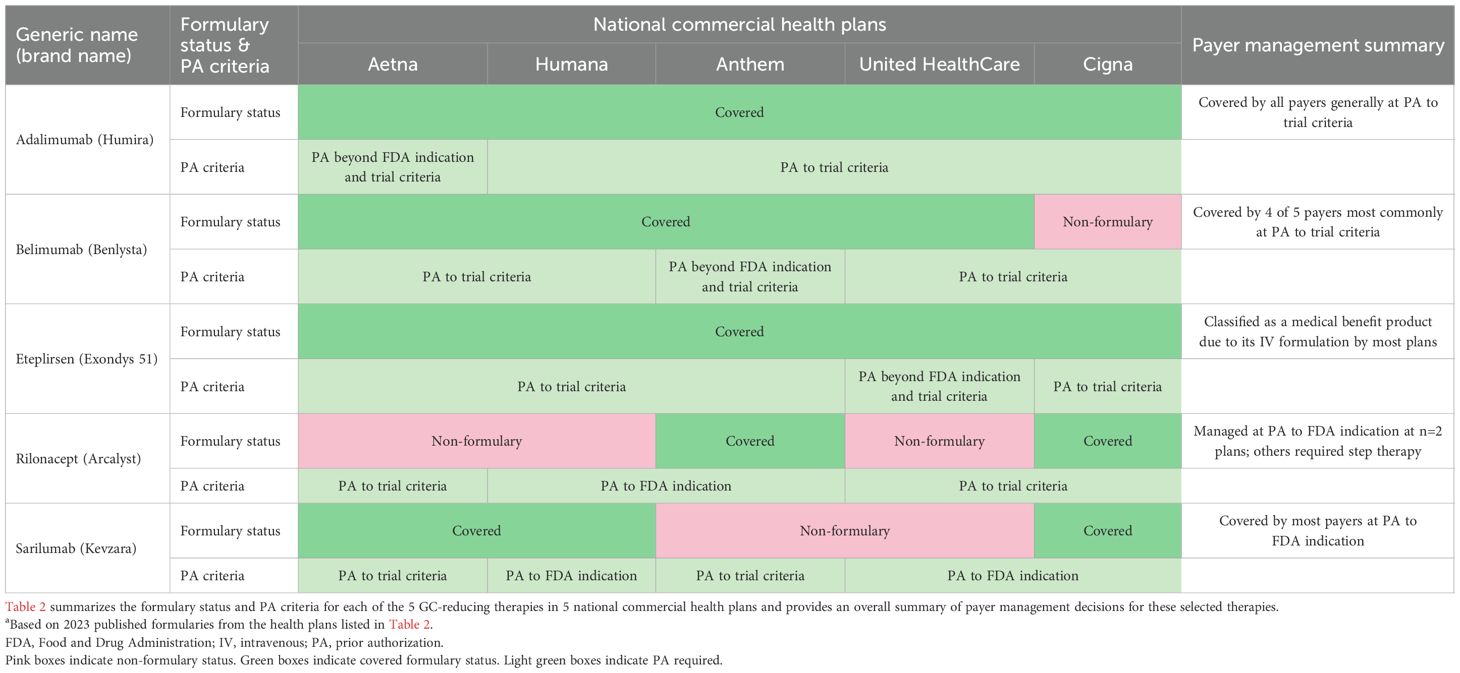
Table 2. Payer formulary and coverage policies of selected payer-covered GC-reducing therapies evaluateda.
Adalimumab
All plans currently cover adalimumab; similarly, all plans enforce trial criteria for adalimumab formulary coverage in RA, including failure, contraindication, and intolerance to a disease-modifying therapy.
Belimumab
Belimumab is covered on Aetna, Humana, Anthem, and United HealthCare and is non-formulary on Cigna. Plans enforce belimumab’s inclusion/exclusion criteria from its clinical trials.
Eteplirsen
All plans currently cover eteplirsen; all plans manage eteplirsen based on its trial inclusion/exclusion criteria, except United HealthCare, which is the only plan that adopted requirements beyond the trial criteria, as it also requires submission of a North Star Ambulatory Assessment or Gower’s test score.
Rilonacept
Rilonacept is non-formulary at Aetna, Humana, and United HealthCare. Aetna and United HealthCare manage rilonacept based on trial criteria, which specifies that patients either currently receive or demonstrate failure to one or more common therapies (including GCs). Humana manages rilonacept according to its labeled FDA indication, requiring a confirmed diagnosis of recurrent pericarditis (RP) demonstrated by symptoms and history of RP episodes. Rilonacept is covered by Anthem and Cigna; Anthem manages rilonacept by its FDA indication and Cigna manages rilonacept based on trial criteria.
Sarilumab
Sarilumab is covered on Aetna, Humana, and Cigna, while it is non-formulary at Anthem and United HealthCare. Most plans cover sarilumab following FDA indication criteria and require a demonstration of an inadequate patient response or intolerance to GCs.
Cross-sectional study (qualitative interviews with payers)
Payer characteristics
A total of 14 US payers were screened; 13 payers were invited to complete the qualitative interviews. Among them, 4 were national MCO payers, 3 were regional MCO payers, 3 were PBM representatives, and 3 were managed Medicaid payers. The characteristics of payers who participated in the qualitative interviews are provided in Supplementary Table 1.
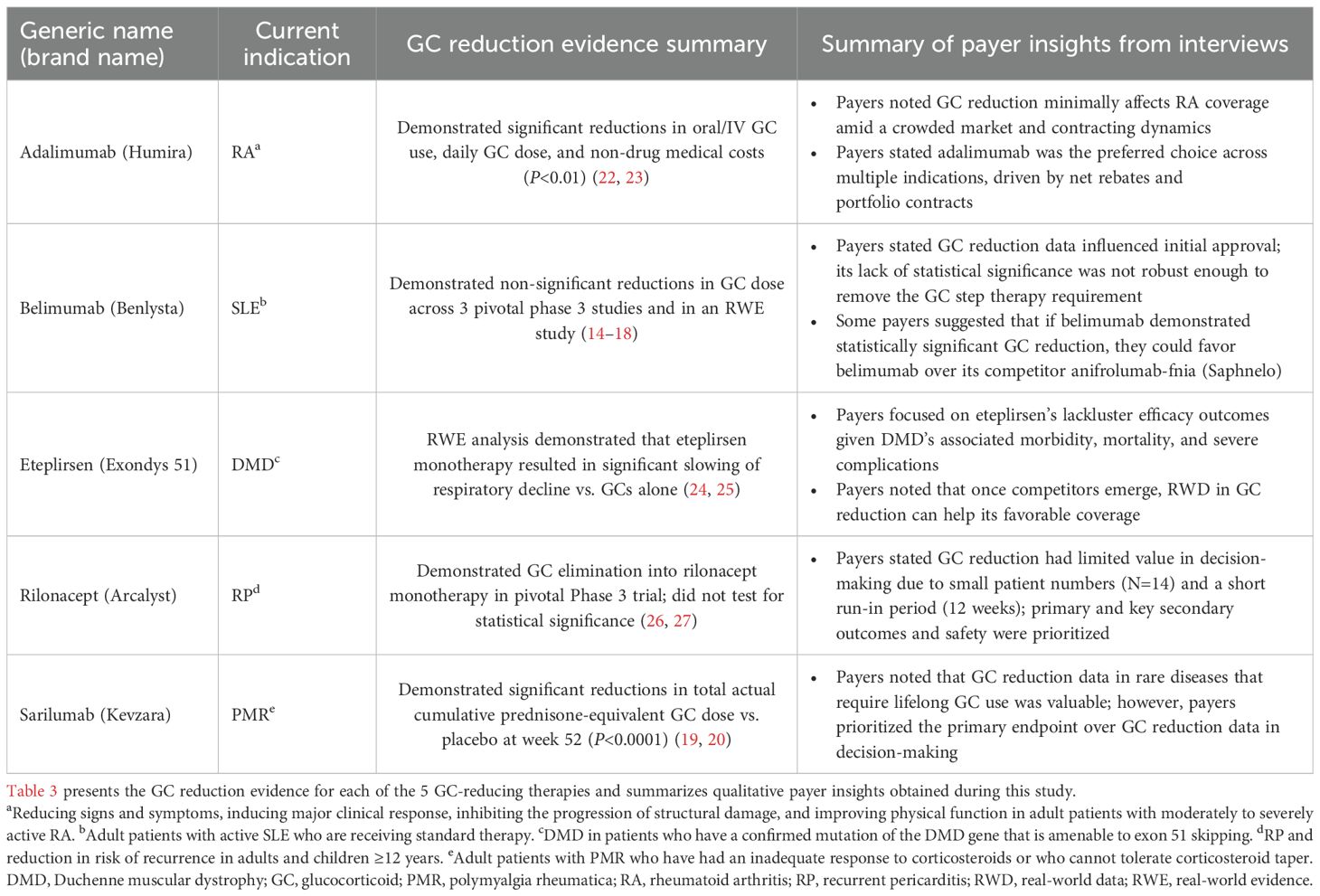
Summary of payer insights on the management of selected therapies
The summary of payer insights on coverage and formulary management of select GC-reducing therapies is presented in Table 3. When crafting coverage criteria and formulary structure, payers stated that they placed primary focus on each trial’s safety outcomes and primary endpoints. Although GC dose reduction data were meaningful to payers, it was not the primary driver in their decision-making due to the following reasons: none of the select GC-reducing therapies had GC reduction as a primary or secondary outcome, some GC reduction data were not statistically significant, trial design to assess GC reduction had too small of a sample for too short of a time period, and implications of contracting dynamics and rebates.
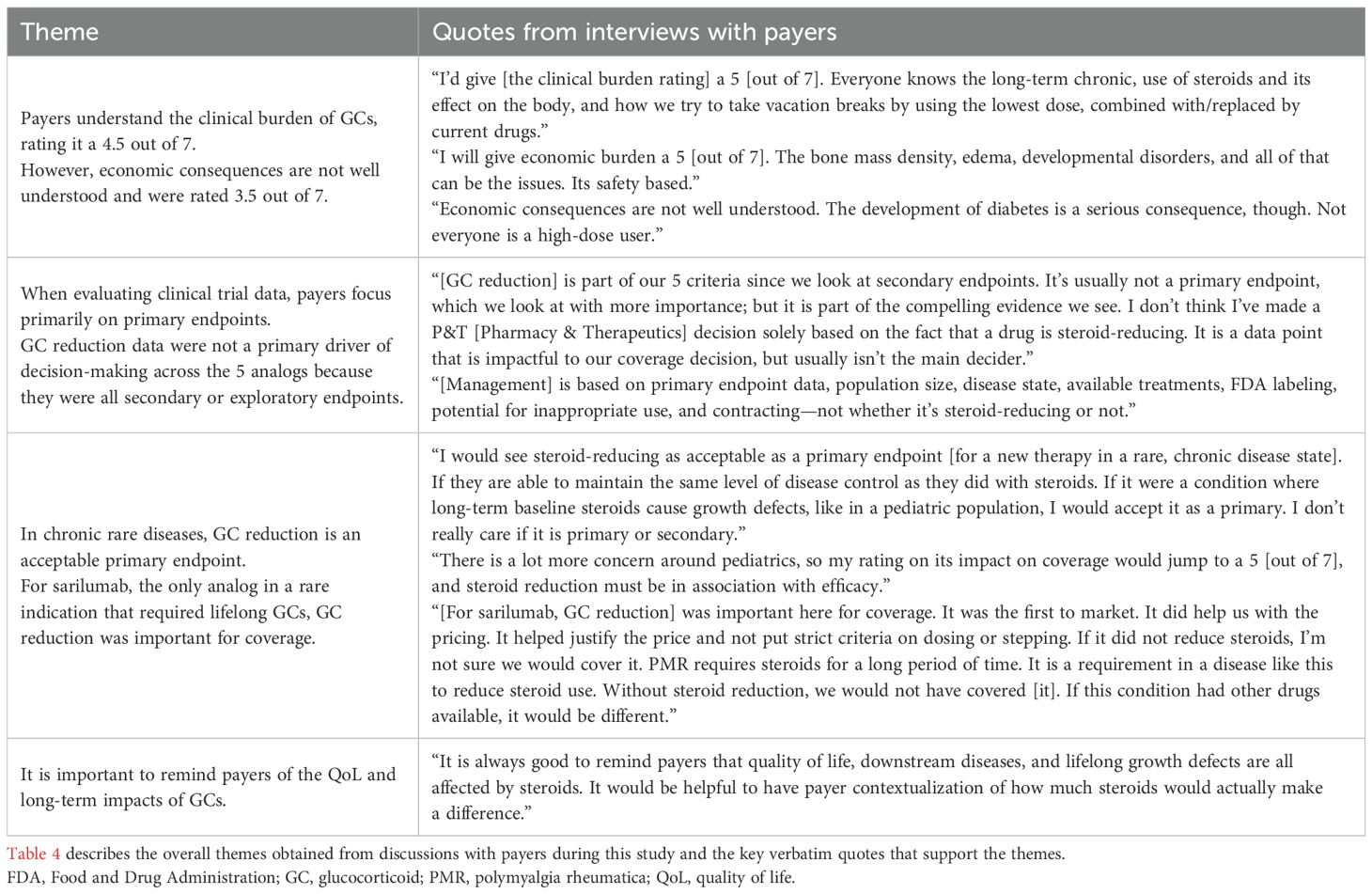
Qualitative insights from payers on the impact of long-term GC use and the importance of GC reduction
All payers recognized the moderate clinical and economic burden associated with long-term GC use in chronic conditions due to their downstream complications (Table 4). When payers were asked to rate the clinical burden and economic burden associated with long-term maintenance treatment of GCs for the management of chronic conditions on a scale of 1 to 7 (with 1 being very low burden and 7 being very high burden), the mean (standard deviation [SD]) payer perception rating of the clinical burden was a 4.9 (1.0; n=12) out of 7, while the perception of the economic burden was rated as a 3.5 (1.0; n=13) out of 7. All payers understood the clinical burden of GCs, noting that GC side effects were a significant contributor to the clinical burden in patients, requiring the need to use the lowest possible dose or replace GCs with other therapies. Although the economic consequences are not well understood by payers, they stated that the side effects of GCs can also carry an economic burden for payers.
Payers were asked about the extent to which GC reduction data impacted their decision-making about each of the 5 therapies and indications presented (Table 4). While GC reduction impacted payer decision-making to some degree for all 5 therapies, GC reduction data were noted as having the largest impact on sarilumab. Indeed, sarilumab’s indication in a rare disease, PMR, and the available GC reduction data that demonstrated the impact of long-term GC use were factors to this.
In contrast, payers noted that GC reduction had the lowest impact on their decision-making regarding eteplirsen, which was due to concerns about eteplirsen’s lack of efficacy in DMD and poor safety outcomes. Payers also noted that rilonacept and adalimumab demonstrating GC reduction in their respective trials was a secondary consideration in their coverage decisions, with the primary efficacy and safety endpoints being the primary consideration. Notably, regarding therapies with multiple competitors in their therapeutic area, such as adalimumab, payers were most concerned with pricing and contracting. Nonetheless, payers stated they had an interest in future studies that include GC reduction as a primary endpoint, especially if the therapy maintained the same level of disease control as GCs, and thought robust GC reduction data were particularly relevant in cases of rare, chronic diseases without specific alternative treatment options and in pediatric populations. Lastly, payers agreed education on the value of GC reduction would increase their understanding of the clinical and economic impact of GCs.
Other considerations of payers regarding therapy management
Throughout the survey, payers were asked to consider and discuss the expected impact on therapy management of label indication, efficacy, and safety; disease education; messaging; cost offsets; and guidelines/key opinion leader input. Payers thought that label indication, efficacy, and safety would have a very high impact on therapy management due to viewing FDA labeling as the most influential driver of coverage and management and the potential to include trial inclusion and exclusion criteria depending on competitor dynamics and budget impact. Payers also expected disease education to have a very high impact on therapy management, noting that it is crucial in payer coverage and management outcomes and understanding the clinical burden of long-term GC use. Next, they noted the importance of first-to-market therapies in diseases with high unmet need and cost offsets allowing for preferential tiering of therapies. Lastly, payers rated guidelines/key opinion leader input as having a neutral impact on management, citing that guidelines rarely trigger a Pharmacy & Therapeutic Committee re-review and subsequent policy changes unless there are major changes in the efficacy and/or safety of a therapy.
Discussion
To our knowledge, this is the first publication that characterizes US payer perspectives regarding the clinical burden of GCs when making formulary decisions on GC-reducing medications. Our qualitative interviews with payers revealed that reduction of negative clinical outcomes accompanying long-term GC use was clinically important when making their coverage decisions on the 5 selected GC-reducing therapies. Despite carrying high costs, all selected medications were covered in most national commercial health plans in place of, or in addition to, GCs.
The 5 selected GC-reducing therapies were purposefully diverse, and each had unique characteristics that made payer decision-making multi-factorial and complex, including factors such as pricing and contracting agreements/dynamics. Importantly, none of the selected therapies included GC reduction as a primary endpoint in their respective clinical trials. Given that payers reported they evaluate new therapies based on their primary endpoints and had never evaluated a therapy with GC reduction as a primary endpoint, they were interested in seeing future studies with GC reduction as a primary endpoint.
Other factors that may contribute to payer decision-making included safety and real-world evidence. Payers reported in this survey that safety has a high impact on therapy management. Although this study did not specifically review safety data or real-world evidence, these factors could influence payer attitudes. When GC reduction data serves as a primary or secondary endpoint in a trial, especially in a population with high unmet need (e.g., in a rare condition, a pediatric population, or in a disease area with limited therapeutic alternatives), GC reduction may serve as a key driver in payer decision-making.
Ultimately, payer decisions on medication coverage and access can impact a clinician’s ability to optimize patient care. The medications reviewed in this study were covered under most of the insurance policies but their use often required criteria that had to be met, or a trial of step therapy. While uncertainty in access or coverage may delay treatment or potentially allow further disease progression, clinicians can advocate for coverage of essential therapies that have clinical value to improve patient outcomes. Payers in this study reported trials with GC reduction as a primary endpoint would be particularly important in cases of rare, chronic diseases without specific alternative treatment options and in pediatric populations, highlighting their priorities and identifying a path for clinicians and payers to align on the clinical relevance and access to medications in these rare, persistent diseases where chronic use of GCs has shown numerous adverse effects on patients (28–33).
One such rare disease is CAH, which impacts pediatric and adult populations. Under a GC-only treatment paradigm for CAH, to restore the HPA axis negative feedback loop and reduce excess ACTH and adrenal androgen production, supraphysiologic doses of GCs are typically required and may be associated with additional risks and side effects including decreased growth rates, impaired final adult height, and reduced bone health (28, 29, 34–37). Risk of bone fractures, cardiovascular disease, and mental health problems due to exposure to GCs, as well as adrenal androgens, are also common in CAH (38–40). The negative clinical outcomes of chronic GC use highlight an urgent need for clinicians to address their adverse effects, minimize long-term usage and dose, and find safe and effective therapeutic alternatives. Striking the appropriate balance between clinical need and access to medications is crucial. While it can be challenging for clinicians when GC-reducing therapies are not adequately covered by health plans in the US, healthcare providers often have the ability to influence payer coverage by substantiating the clinical need for the medications they prescribe. In rare conditions where payers understand the value of GC reduction, new therapeutic options can offer value to clinicians, payers, and patients by mitigating long-term safety concerns while maintaining disease control.
Limitations
The limitations of this research should be considered alongside the results. First, due to the study’s cross-sectional research design, recall or social desirability bias may be present. However, the questions in the web-enabled screener and qualitative interviews were framed to help mitigate these biases and payers were asked to provide responses to the best of their ability. Additionally, this study recruited a relatively small sample, reducing its generalizability. However, the sample was well distributed between a variety of organizations (e.g., national and regional MCOs, PBM representatives, and Medicaid). Also, some additional factors may contribute to coverage decision-making including the drug’s entire safety profile, long-term real-world data, adherence, or perspectives from patients and clinicians. Providing an individual risk-benefit analysis of each identified GC-reducing therapy would have provided respondents with additional perspective to consider but was out of scope for the current study based on time restrictions. The 5 GC-reducing therapies were identified based on prespecified criteria and were used to proxy how payers may consider a GC-reducing therapy for CAH. However, applications of GC-reducing therapies vary by disease state and potential for disease modification, which may impact payer coverage decisions, and thus, cannot be completely proxied or captured and limit generalizability. Lastly, the opinions expressed by the payers in the qualitative interviews do not necessarily reflect the opinions or data findings of the sponsor or all payers.
Conclusions
Our research shows that each GC-reducing therapy evaluated was covered in place of, or in addition to, GCs despite carrying a premium price. While GC reduction was meaningful to payers, it was a secondary driver in coverage decision-making due to contracting dynamics, lack of competitors, small sample size in trials, and most importantly, payers’ focus on the trials’ primary endpoints. Payers acknowledged the clinical and economic value of reducing long-term GC use and may consider GC reduction data in their decision-making. Future studies with GC reduction as a primary endpoint in rare and chronic diseases that affect pediatric populations may inform access decision-making. Clinicians who understand how payers evaluate GC-reducing therapies and payer priorities can help shape more patient-centered coverage decisions and better align treatment decisions to ensure optimal patient management.
Author's note
Previously presented as poster at AMCP Nexus 2024, Las Vegas, NV, Oct 14-17.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by ADVARRA Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AK: Writing – original draft, Writing – review & editing. HK: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. BL: Writing – original draft, Writing – review & editing. NH: Writing – original draft, Writing – review & editing. SC: Writing – original draft, Writing – review & editing. HC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Acknowledgments
Trinity Life Sciences led the study and designed the survey, both collected the data and handled all administrative tasks involved in prospective data collection, analyzed the data, and developed the insights. Cencora provided medical writing and editorial support.
Conflict of interest
AK discloses consulting fees from Neurocrine Biosciences. HK is a shareholder and employee of Neurocrine Biosciences. MM is an employee of Trinity Life Sciences, which received consulting fees to design and conduct the research. BL is an employee of Trinity Life Sciences, which received consulting fees to design and conduct the research. NH is an employee of Trinity Life Sciences, which received consulting fees to design and conduct the research. SC is a shareholder and employee of Neurocrine Biosciences. HC is a shareholder and employee of Neurocrine Biosciences.
The authors declare that this study received funding from Neurocrine Biosciences, Inc. Neurocrine Biosciences collected the data and handled all administrative tasks involved in prospective data collection, analyzed the data, and developed the insights. Neurocrine Biosciences provided input when appropriate for drafts or insight interpretation.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1603701/full#supplementary-material
References
1. Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. (2013) 9:30. doi: 10.1186/1710-1492-9-30
2. Judd LL, Schettler PJ, Brown ES, Wolkowitz OM, Sternberg EM, Bender BG, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry. (2014) 171:1045–51. doi: 10.1176/appi.ajp.2014.13091264
3. Chen SY, Choi CB, Li Q, Yeh WS, Lee YC, Kao AH, et al. Glucocorticoid use in patients with systemic Lupus erythematosus: association between dose and health care utilization and costs. Arthritis Care Res (Hoboken). (2015) 67:1086–94. doi: 10.1002/acr.22574
4. Rice JB, White AG, Scarpati LM, Wan G, and Nelson WW. Long-term systemic corticosteroid exposure: A systematic literature review. Clin Ther. (2017) 39:2216–29. doi: 10.1016/j.clinthera.2017.09.011
5. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, and Bass D. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. (2011) 33:1413–32. doi: 10.1016/j.clinthera.2011.09.009
6. Sullivan PW, Ghushchyan VH, Globe G, and Sucher B. Health-related quality of life associated with systemic corticosteroids. Qual Life Res. (2017) 26:1037–58. doi: 10.1007/s11136-016-1435-y
7. Merke DP and Auchus RJ. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. (2020) 383:1248–61. doi: 10.1056/NEJMra1909786
8. Turcu AF and Auchus RJ. Adrenal steroidogenesis and congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. (2015) 44:275–96. doi: 10.1016/j.ecl.2015.02.002
9. Claahsen-van der Grinten HL, Speiser PW, Ahmed SF, Arlt W, Auchus RJ, Falhammar H, et al. Congenital adrenal hyperplasia-current insights in pathophysiology, diagnostics, and management. Endocr Rev. (2022) 43:91–159. doi: 10.1210/endrev/bnab016
10. Reisch N, Willige M, Kohn D, Schwarz HP, Allolio B, Reincke M, et al. Frequency and causes of adrenal crises over lifetime in patients with 21-hydroxylase deficiency. Eur J Endocrinol. (2012) 167:35–42. doi: 10.1530/EJE-12-0161
11. Auchus RJ and Arlt W. Approach to the patient: the adult with congenital adrenal hyperplasia. J Clin Endocrinol Metab. (2013) 98:2645–55. doi: 10.1210/jc.2013-1440
12. Kamoun M, Feki MM, Sfar MH, and Abid M. Congenital adrenal hyperplasia: Treatment and outcomes. Indian J Endocrinol Metab. (2013) 17:S14–7. doi: 10.4103/2230-8210.119491
13. Mallappa A and Merke DP. Management challenges and therapeutic advances in congenital adrenal hyperplasia. Nat Rev Endocrinol. (2022) 18:337–52. doi: 10.1038/s41574-022-00655-w
15. Collins CE, Dall’Era M, Kan H, Macahilig C, Molta C, Koscielny V, et al. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med. (2016) 3:e000118. doi: 10.1136/lupus-2015-000118
16. Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzóvá D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. (2011) 63:3918–30. doi: 10.1002/art.30613
17. Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. (2011) 377:721–31. doi: 10.1016/S0140-6736(10)61354-2
18. Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, et al. Efficacy and safety of subcutaneous belimumab in systemic Lupus erythematosus: A fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. (2017) 69:1016–27. doi: 10.1002/art.40049
20. Spiera RF, Unizony S, Warrington KJ, Sloane J, Giannelou A, Nivens MC, et al. Sarilumab for relapse of polymyalgia rheumatica during glucocorticoid taper. N Engl J Med. (2023) 389:1263–72. doi: 10.1056/NEJMoa2303452
21. Linnerooth S, Penley B, Sauvageau G, Ha J, Beal A, Craven J, et al. Methodology for conducting a comprehensive product review in managed care. J Managed Care Specialty Pharm. (2023) 29:237–43. doi: 10.18553/jmcp.2023.29.3.237
22. Spivey CA, Winthrop KL, Griffith J, Kaplan CM, Qiao Y, Postlethwaite AE, et al. Retrospective analysis of the impact of adalimumab initiation on corticosteroid utilization and medical costs among biologic-naive patients with rheumatoid arthritis. Rheumatol Ther. (2020) 7:133–47. doi: 10.1007/s40744-019-00184-5
25. Khan N, Eliopoulos H, Han L, Kinane TB, Lowes LP, Mendell JR, et al. Eteplirsen treatment attenuates respiratory decline in ambulatory and non-ambulatory patients with duchenne muscular dystrophy. J Neuromuscul Dis. (2019) 6:213–25. doi: 10.3233/JND-180351
27. Klein AL, Imazio M, Cremer P, Brucato A, Abbate A, Fang F, et al. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med. (2021) 384:31–41. doi: 10.1056/NEJMoa2027892
28. Cordeiro GV, Silva IN, Goulart EM, Goulart EMA, das Chagas AJ, Kater CE, et al. Final height in congenital adrenal hyperplasia: the dilemma of hypercortisolism versus hyperandrogenism. Arq Bras Endocrinol Metabol. (2013) 57:126–31. doi: 10.1590/S0004-27302013000200005
29. Sarafoglou K, Addo OY, Turcotte L, Otten N, Wickremasinghe A, Pittock S, et al. Impact of hydrocortisone on adult height in congenital adrenal hyperplasia-the Minnesota cohort. J Pediatr. (2014) 164:1141–1146.e1. doi: 10.1016/j.jpeds.2014.01.011
30. Andela CD, Staufenbiel SM, Joustra SD, Pereira AM, van Rossum EFC, and Biermasz NR. Quality of life in patients with adrenal insufficiency correlates stronger with hydrocortisone dosage, than with long-term systemic cortisol levels. Psychoneuroendocrinology. (2016) 72:80–6. doi: 10.1016/j.psyneuen.2016.06.015
31. Schulz J, Frey KR, Cooper MS, Zopf K, Ventz M, Diederich S, et al. Reduction in daily hydrocortisone dose improves bone health in primary adrenal insufficiency. Eur J Endocrinol. (2016) 174:531–8. doi: 10.1530/EJE-15-1096
32. Ragnarsson O, Mattsson AF, Monson JP, Nyström HF, Åkerblad AC, Kołtowska-Häggström M, et al. The relationship between glucocorticoid replacement and quality of life in 2737 hypopituitary patients. Eur J Endocrinol. (2014) 171:571–9. doi: 10.1530/EJE-14-0397
33. Lovas K, Gjesdal CG, Christensen M, Wolff AB, Almås B, Svartberg J, et al. Glucocorticoid replacement therapy and pharmacogenetics in Addison’s disease: effects on bone. Eur J Endocrinol. (2009) 160:993–1002.
34. Muirhead S, Sellers EA, Guyda H, and Canadian Pediatric Endocrine Group. Indicators of adult height outcome in classical 21-hydroxylase deficiency congenital adrenal hyperplasia. J Pediatr. (2002) 141:247–52. doi: 10.1067/mpd.2002.126601
35. Pijnenburg-Kleizen KJ, Thomas CMG, Otten BJ, et al. Long-term follow-up of children with classic congenital adrenal hyperplasia: suggestions for age dependent treatment in childhood and puberty. J Pediatr Endocrinol Metab. (2019) 32:1055–63. doi: 10.1515/jpem-2019-0006
36. Jaaskelainen J and Voutilainen R. Growth of patients with 21-hydroxylase deficiency: an analysis of the factors influencing adult height. Pediatr Res. (1997) 41:30–3. doi: 10.1203/00006450-199701000-00005
37. Espinosa Reyes TM, Leyva González G, Domínguez Alonso E, and Falhammar H. Bone mass in young patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr. (2021) 94:1–8. doi: 10.1159/000515833
38. Hummel SR, Sadler S, Whitaker MJ, Ara RM, Dixon S, Ross RJ, et al. A model for measuring the health burden of classic congenital adrenal hyperplasia in adults. Clin Endocrinol (Oxf). (2016) 85:361–98. doi: 10.1111/cen.13060
39. Falhammar H, Frisén L, Hirschberg AL, Norrby C, Almqvist C, Nordenskjöld A, et al. Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: A Swedish population-based national cohort study. J Clin Endocrinol Metab. (2015) 100:3520–8. doi: 10.1210/JC.2015-2093
Keywords: glucocorticoid-reducing therapies, payer, congenital adrenal hyperplasia, survey, corticosteroids, glucocorticoid dose reduction
Citation: Khattab A, Kim H, Mulrooney M, Leinwand B, Hadker N, Cicero S and Cheng H (2025) Optimizing glucocorticoid therapy in congenital adrenal hyperplasia and analog conditions: the intersection of dose reduction, patient care, and coverage in the US. Front. Endocrinol. 16:1603701. doi: 10.3389/fendo.2025.1603701
Received: 31 March 2025; Accepted: 18 August 2025;
Published: 09 September 2025.
Edited by:
Henrik Falhammar, Karolinska Institutet (KI), SwedenReviewed by:
Mimi Kim, University of Southern California, United StatesChamila Lakmini Balagamage, Birmingham Women’s and Children’s Hospital, United Kingdom
Copyright © 2025 Khattab, Kim, Mulrooney, Leinwand, Hadker, Cicero and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyunwoo Kim, aGtpbUBuZXVyb2NyaW5lLmNvbQ==
 Ahmed Khattab
Ahmed Khattab Hyunwoo Kim
Hyunwoo Kim Mary Mulrooney
Mary Mulrooney Brian Leinwand
Brian Leinwand Nandini Hadker
Nandini Hadker Samantha Cicero2
Samantha Cicero2