- Department of Children’s Heart Center, Fuwai Central China Cardiovascular Hospital, Zhengzhou University Central China Fuwai Hospital, Zhengzhou, China
Background: Residual cholesterol (RC), a key indicator of lipid metabolism disorders, has been increasingly implicated in atherosclerotic progression. However, its association with vulnerable thin-cap fibroatheromas (TCFA) in non-culprit coronary lesions (NCCLs) and the subsequent risk of major adverse cardiovascular events (MACE) remains insufficiently explored.
Methods: In this prospective observational study conducted between June 2022 and September 2023, patients diagnosed with TCFA within NCCLs were followed for at least 12 months. Participants were grouped according to MACE occurrence. Spearman correlation and multivariate logistic regression were used to examine associations between RC levels, plaque vulnerability features, and MACE.
Results: RC showed significant correlations with key vulnerability markers—negatively with fibrous cap thickness (rs = -0.61, P < 0.001) and positively with lipid arc (rs = 0.75, P < 0.001). In univariate analysis, elevated RC was associated with a 1.88-fold increased risk of MACE. RC remained an independent risk factor in multivariate analysis (OR = 1.127, 95% CI: 1.101–1.593, P = 0.031). ROC analysis yielded moderate predictive value (AUC = 0.720).
Conclusion: Elevated RC is associated with greater plaque vulnerability and increased MACE risk in patients with NCCL-TCFA. These findings suggest RC’s potential role in cardiovascular risk stratification, warranting further validation in larger studies.
1 Introduction
Cardiovascular disease (CVD) remains a leading cause of death and disability worldwide, posing significant challenges to public health and healthcare resources due to its high prevalence and mortality rates. Despite advancements in diagnostic and therapeutic strategies, early intervention targeting the progression of CVD is critical for preventing major adverse cardiovascular events (MACE). Among patients with coronary artery disease (CAD), non-culprit lesions (NCCL) have been identified as a major source of MACE (1–3), with their rupture often leading to acute coronary syndromes (ACS) or even cardiac death (4, 5).
In the progression of NCCL, thin-cap fibroatheromas (TCFA) represent the most common type of vulnerable plaque (6). TCFA is characterized by features such as a thin fibrous cap, a large lipid core, infiltration of inflammatory cells, and the presence of calcified nodules (7). TCFAs are among the most prevalent types of vulnerable plaques observed in NCCLs (8). Studies have estimated that TCFAs may be present in up to 40% of angiographically non-obstructive NCCLs, and they are independently associated with future adverse cardiovascular outcomes even when not flow-limiting (9–11). This underscores the clinical importance of identifying high-risk features in NCCLs, which are often overlooked compared to culprit lesions.
The vulnerability of TCFA not only determines the likelihood of plaque rupture but is also closely associated with the occurrence of cardiovascular events (12–15). Therefore, identifying key factors that influence TCFA vulnerability and its progression to MACE is of paramount importance for optimizing CVD prevention strategies. It is also important to consider the current clinical context in which NCCL management varies depending on the type of acute myocardial infarction (AMI) (16–18). In STEMI patients, revascularization of severe NCCLs is typically class IA recommended, whereas in NSTEMI, the evidence is less definitive and generally regarded as class IIa (19). Furthermore, the use of fractional flow reserve (FFR) has emerged as a key tool in guiding the need for intervention, yet it primarily assesses functional ischemia and may not fully reflect the structural vulnerability of plaques such as TCFAs (17). Therefore, understanding anatomical high-risk features remains crucial in complementing physiology-based decisions.
Residual cholesterol (RC), an independent marker of metabolic dysfunction, has gained increasing attention in recent years (20–22). RC is calculated by subtracting high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) from total cholesterol (TC) and reflects the accumulation of triglyceride-rich lipoproteins, such as very-low-density lipoproteins and chylomicron remnants (23–25). Previous studies have demonstrated that elevated RC is strongly associated with the development and progression of atherosclerotic diseases (26, 27). Its potential mechanisms include inducing endothelial dysfunction, activating inflammatory responses, and promoting lipid deposition. However, systematic research investigating the relationship between RC, TCFA vulnerability in NCCL, and MACE remains lacking (1, 28–30). On the other hand, RC may drive both systemic inflammation and direct atherogenic effects, supporting its evaluation in non-culprit lesion settings (24, 31). Our study specifically focuses on this underexplored association in the context of NCCLs, rather than culprit lesions traditionally targeted during revascularization.
Therefore, this study aims to explore whether elevated RC—as a marker of remnant lipoprotein burden—contributes to structural vulnerability in NCCL-TCFAs and increases the risk of MACE. Given the increasing recognition of non-obstructive yet high-risk plaques as contributors to future events, our work seeks to fill a critical gap in understanding the metabolic and anatomical mechanisms underlying NCCL instability. The primary outcome of this study is to assess the association between RC levels and the occurrence of MACE in patients with NCCL-TCFA. The secondary outcomes include evaluating the correlation between RC and TCFA features (fibrous cap thickness, lipid arc, and plaque composition), as well as assessing RC’s potential value as a predictive biomarker for MACE risk stratification.
2 Methods
2.1 Study population
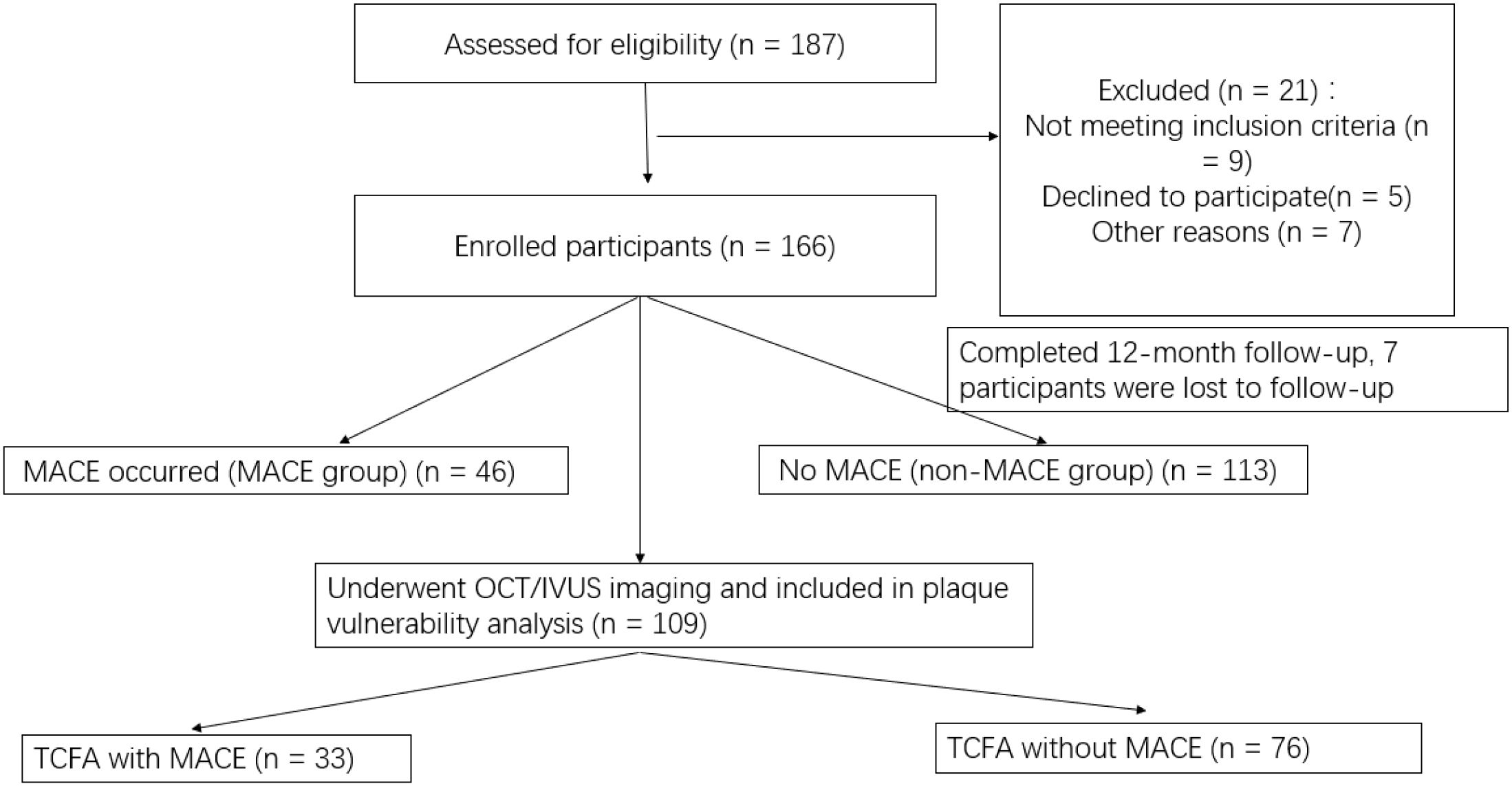
This was a single-center, prospective observational cohort study conducted at Fuwai Central China Cardiovascular Hospital between June 2022 and September 2023. The study protocol was approved by the Institutional Research Ethics Committee of Fuwai Central China Cardiovascular Hospital, and all procedures complied with the Declaration of Helsinki. All participants provided written informed consent prior to inclusion. Given that this was an observational study with no interventional component, no randomization was applied. Patients were enrolled consecutively based on predefined inclusion and exclusion criteria and were classified retrospectively into MACE and non-MACE groups according to whether they experienced major adverse cardiovascular events during the 12-month follow-up period. Of the 187 patients assessed for eligibility, 21 were excluded based on predefined criteria. An additional 7 participants were lost to follow-up due to incomplete imaging or laboratory data, resulting in 159 patients included in the final analysis. In total, 159 patients were included in the final analysis, of whom 46 patients experienced MACE (MACE group) and 113 patients did not (non-MACE group) (See Figure 1).
2.2 Inclusion criteria
All eligible patients were enrolled consecutively during the study period to reduce selection bias. The inclusion criteria were as follows: (1) confirmation of non-culprit lesions (NCCL) by coronary angiography, defined as coronary lesions not causing the patient’s current symptoms; (2) identification of TCFA within NCCL through imaging examinations, such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT); (3) completion of imaging evaluations and blood biochemical tests during follow-up; (4) a follow-up period of no less than 12 months; and (5) provision of written informed consent and voluntary participation in the study.
2.3 Exclusion criteria
The exclusion criteria included any of the following: (1) patients with acute coronary syndromes (ACS) who had not received stabilized treatment; (2) those with severe cardiac, pulmonary, hepatic, or renal dysfunction or other serious systemic diseases; (3) patients with malignant tumors or confirmed infectious diseases; (4) those unable to complete imaging examinations or blood testing; and (5) patients with poor adherence to the study or those lost to follow-up.
2.4 Patient grouping
Patients were divided into two groups based on whether they experienced major adverse cardiovascular events (MACE) during the follow-up period. The MACE group included patients with MACE events attributed to NCCL, such as cardiac death, nonfatal myocardial infarction, and unplanned coronary revascularization. The non-MACE group consisted of patients who did not experience these events during the follow-up period. The MACE group included patients who experienced MACE, defined as cardiac death, nonfatal myocardial infarction, or unplanned coronary revascularization, during follow-up.
2.5 Data collection
2.5.1 Baseline characteristics
Clinical baseline characteristics of all patients were collected, including sex, age, body mass index (BMI), smoking status, alcohol consumption, diabetes, hypertension, dyslipidemia, and family history of cardiovascular disease. These data were obtained from the patients’ electronic medical records and hospitalization records. To ensure accuracy, the baseline characteristics were independently verified and cross-checked by two researchers.
Medication use at baseline was recorded, including statins, dual antiplatelet therapy (DAPT), beta-blockers, and ACE inhibitors/ARBs. In our cohort, 82.4% of patients were on statins, and 91.2% received antiplatelet therapy during hospitalization.
2.5.2 Biochemical parameters
Venous blood samples were collected from patients upon admission to measure biochemical parameters, including: residual cholesterol (RC), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), blood glucose, and glycated hemoglobin (HbA1c). RC was calculated as TC minus HDL-C and LDL-C. TC and TG were measured using enzymatic methods, HDL-C and LDL-C were measured using direct assays, while blood glucose and HbA1c were determined using the glucose oxidase method and high-performance liquid chromatography, respectively. All blood tests were performed after an 8-hour overnight fast and analyzed at a hospital-certified central laboratory. Each sample was tested in duplicate to ensure accuracy, with the average value used for analysis.
2.5.3 Imaging parameters
All patients underwent coronary angiography and plaque imaging assessments using optical coherence tomography (OCT) or intravascular ultrasound (IVUS). Plaque characteristics analyzed included the thinnest fibrous cap thickness, measured by OCT to evaluate the risk of plaque rupture, and the maximum lipid arc, representing the largest angle of the lipid core in cross-sectional views. Lumen stenosis rate was calculated as the ratio of the minimal lumen area (MLA) at the lesion site to the normal reference lumen area. Additional parameters such as calcified nodules, macrophage infiltration, cholesterol crystals, and microvessel density were identified or qualitatively assessed based on imaging data. Imaging analyses were independently conducted by two experienced radiologists, and any discrepancies were resolved by a third radiologist. Among the enrolled patients, 89 underwent optical coherence tomography (OCT) and 70 underwent intravascular ultrasound (IVUS). In cases where both modalities were available, OCT data were prioritized for fibrous cap thickness and lipid arc evaluation due to its superior spatial resolution.
In our study, TCFA was defined based on established OCT and IVUS criteria. For OCT, TCFA was characterized by a lipid-rich plaque with a lipid arc >180° and a fibrous cap thickness <65 μm. For IVUS-based assessments, virtual histology-defined TCFA was identified by a necrotic core in contact with the lumen in the presence of thin fibrous cap, as per standard definitions.
2.5.4 Data quality control
To ensure the quality of collected data, several measures were implemented. First, all data were double-entered by independent research assistants and subjected to cross-checking for consistency. Regular multicenter meetings were held to standardize data collection and processing procedures, ensuring uniformity across study sites. Additionally, a dedicated data management system was established to enable real-time monitoring, review, and quality assessment of key data points.
2.6 Statistical analysis
All statistical analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY) and R version 4.2.1. Normality of continuous variables was assessed using the Shapiro–Wilk test. Data with a normal distribution were presented as mean ± standard deviation (SD) and compared using the independent samples t-test. Non-normally distributed variables were reported as median with interquartile ranges (IQR) and analyzed using the Mann–Whitney U test. Categorical variables were expressed as frequencies (percentages) and compared using the chi-square test or Fisher’s exact test, depending on the expected counts. Correlations between RC and vulnerable plaque features (e.g., fibrous cap thickness, lipid arc) were assessed using Spearman’s rank correlation coefficient (rs). To evaluate the independent association between RC and MACE, multivariate logistic regression was conducted, adjusting for potential confounders (e.g., age, hypertension, diabetes). Results were reported as odds ratios (OR) with corresponding 95% confidence intervals (CI). Predictive performance of RC was assessed using receiver operating characteristic (ROC) curve analysis, with the area under the curve (AUC) interpreted as follows: AUC > 0.7 indicates moderate prediction, and AUC > 0.8 indicates good prediction ability. All statistical tests were two-tailed, and P-values < 0.05 were considered statistically significant.
The primary hypothesis was that RC is independently associated with both greater TCFA plaque vulnerability and increased risk of MACE in patients with non-culprit coronary lesions. Based on previous literature and assuming an expected effect size (OR) of 1.9, a statistical power of 80% (β = 0.20), and a two-sided α level of 0.05, the minimum sample size required for logistic regression analysis was calculated using the formula for proportions in two independent groups. This yielded a minimum required sample size of 136 patients. Considering a 15% loss to follow-up rate, we aimed to recruit at least 156 participants, ultimately including 159.
To address potential confounding, variables known to influence MACE risk—including age, sex, diabetes, hypertension, lipid parameters, and smoking history—were included in the multivariate logistic regression model. Variable selection was based on clinical relevance and univariate screening (P < 0.10). To evaluate multicollinearity among independent variables, Variance Inflation Factor (VIF) was calculated. All variables included in the final model had VIF values less than 2, indicating an acceptable level of collinearity.
3 Results
3.1 Baseline characteristics comparison between MACE and non-MACE groups
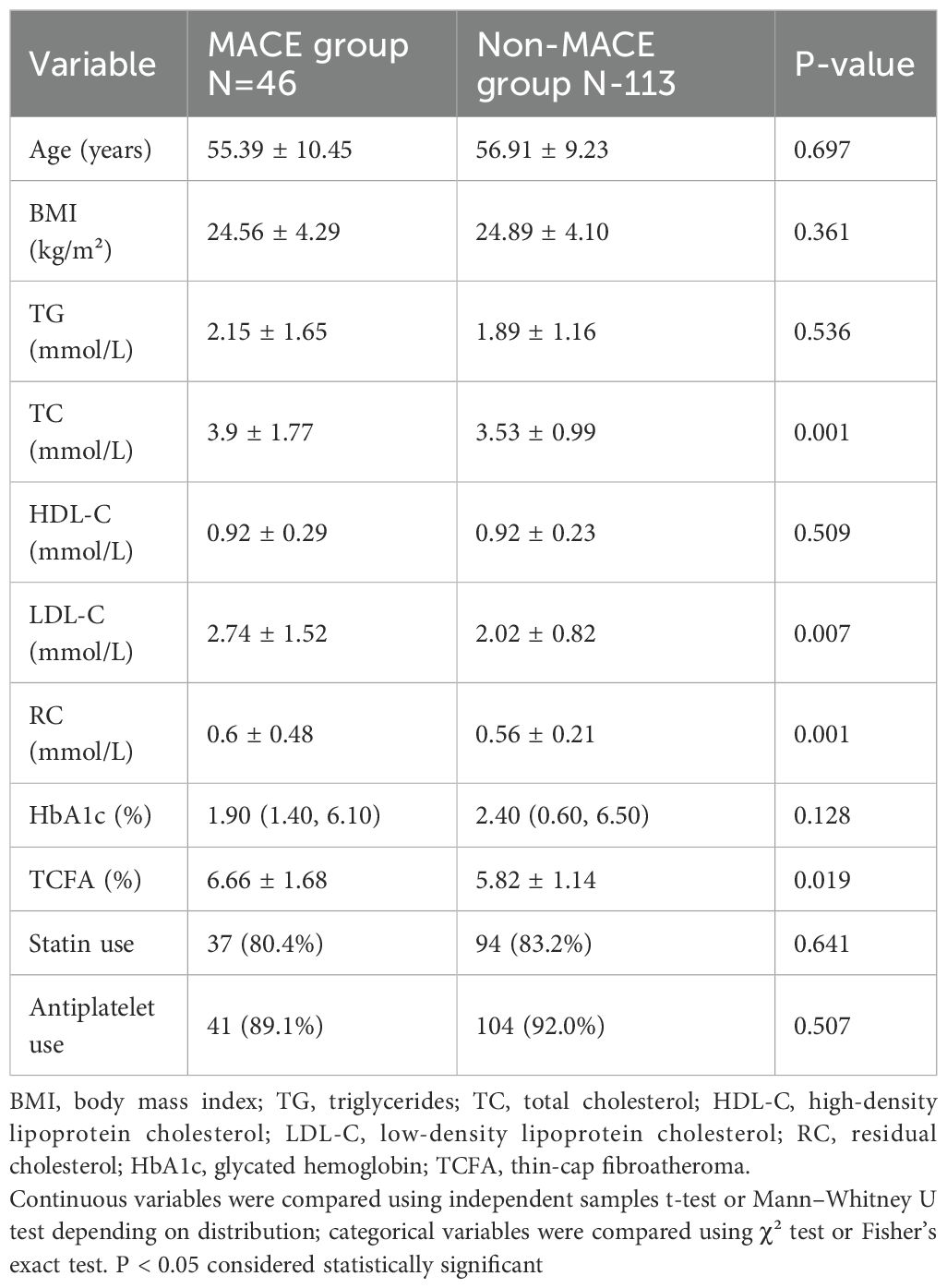
In the comparison of baseline data between patients with MACE (N=46) and those without MACE (N=113), no significant differences were observed in age (55.39 ± 10.45 years vs. 56.91 ± 9.23 years, p=0.697), BMI (24.56 ± 4.29 kg/m² vs. 24.89 ± 4.10 kg/m², p=0.361), TG (2.15 ± 1.65 mmol/L vs. 1.89 ± 1.16 mmol/L, p=0.536), HDL-C (0.92 ± 0.29 mmol/L vs. 0.92 ± 0.23 mmol/L, p=0.509), or HbA1c (1.90% vs. 2.40%, p=0.128). However, the MACE group exhibited significantly higher levels of TC (3.9 ± 1.77 mmol/L vs. 3.53 ± 0.99 mmol/L, p=0.001), LDL-C (2.74 ± 1.52 mmol/L vs. 2.02 ± 0.82 mmol/L, p=0.007), RC (0.6 ± 0.48 mmol/L vs. 0.56 ± 0.21 mmol/L, p=0.001), and TCFA (6.66 ± 1.68% vs. 5.82 ± 1.14%, p=0.019) compared to the non-MACE group. Additionally, there were no significant differences in baseline medication use between groups, with statin use observed in 37 (80.4%) of MACE patients versus 94 (83.2%) of non-MACE patients (P = 0.641), and antiplatelet use in 41 (89.1%) versus 104 (92.0%) respectively (P = 0.507) (Table 1).
3.2 The baseline data of the TCFA group with MACE and the TCFA group without MACE variable
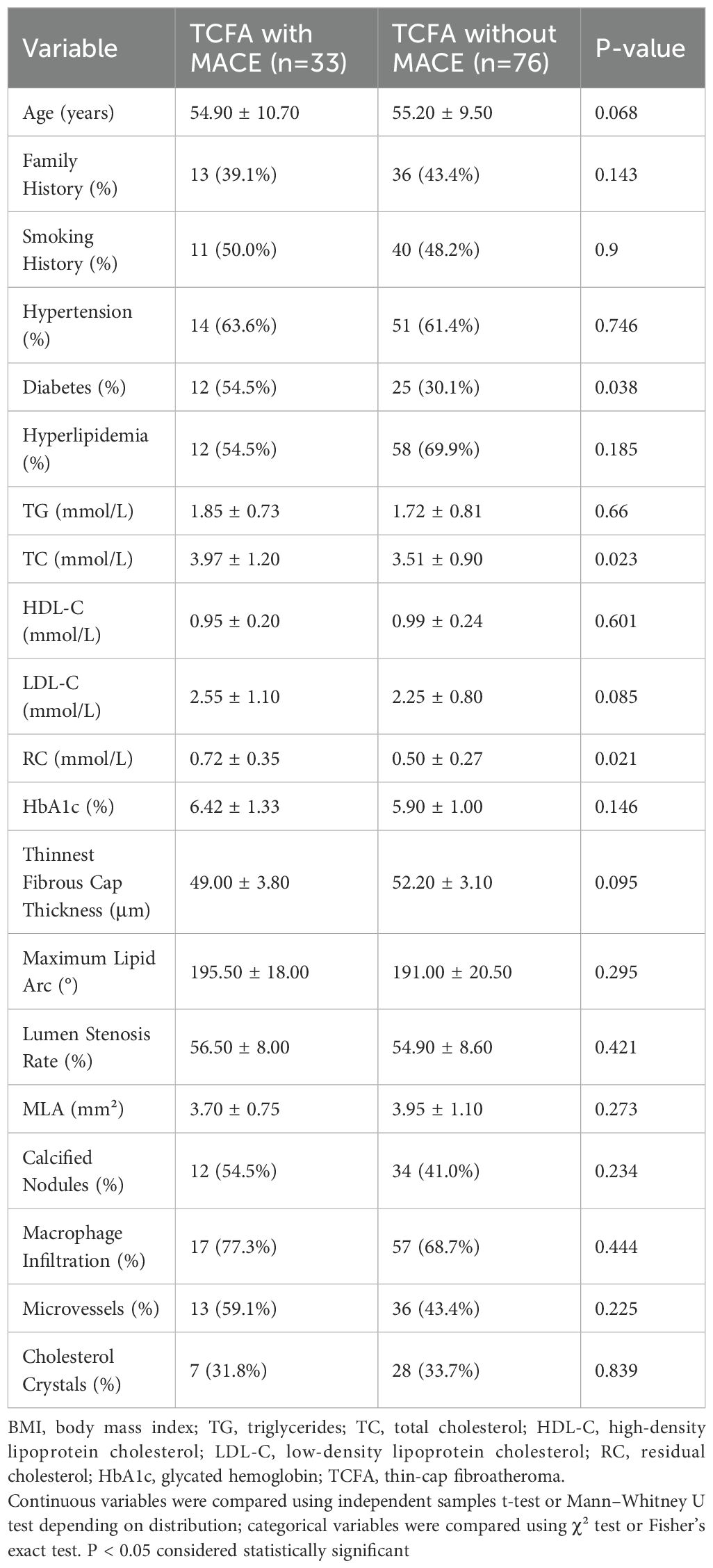
A total of 109 patients with confirmed TCFA and complete high-quality OCT/IVUS imaging were included in a subgroup analysis of plaque vulnerability characteristics (Table 2), derived from the overall study cohort of 159 participants. In the comparison of baseline data between the TCFA with MACE group (n=33) and the TCFA without MACE group (n=76), no significant differences were observed in age (54.90 ± 10.70 years vs. 55.20 ± 9.50 years, p=0.068), family history (39.1% vs. 43.4%, p=0.143), smoking history (50.0% vs. 48.2%, p=0.9), hypertension (63.6% vs. 61.4%, p=0.746), hyperlipidemia (54.5% vs. 69.9%, p=0.185), TG (1.85 ± 0.73 mmol/L vs. 1.72 ± 0.81 mmol/L, p=0.66), HDL-C (0.95 ± 0.20 mmol/L vs. 0.99 ± 0.24 mmol/L, p=0.601), LDL-C (2.55 ± 1.10 mmol/L vs. 2.25 ± 0.80 mmol/L, p=0.085), HbA1c (6.42 ± 1.33% vs. 5.90 ± 1.00%, p=0.146), thinnest fibrous cap thickness (49.00 ± 3.80 μm vs. 52.20 ± 3.10 μm, p=0.095), maximum lipid arc (195.50 ± 18.00° vs. 191.00 ± 20.50°, p=0.295), lumen stenosis rate (56.50 ± 8.00% vs. 54.90 ± 8.60%, p=0.421), MLA (3.70 ± 0.75 mm² vs. 3.95 ± 1.10 mm², p=0.273), calcified nodules (54.5% vs. 41.0%, p=0.234), macrophage infiltration (77.3% vs. 68.7%, p=0.444), microvessels (59.1% vs. 43.4%, p=0.225), or cholesterol crystals (31.8% vs. 33.7%, p=0.839). However, the TCFA with MACE group had a significantly higher prevalence of diabetes (54.5% vs. 30.1%, p=0.038), as well as higher levels of TC (3.97 ± 1.20 mmol/L vs. 3.51 ± 0.90 mmol/L, p=0.023) and RC (0.72 ± 0.35 mmol/L vs. 0.50 ± 0.27 mmol/L, p=0.021) compared to the TCFA without MACE group. (Table 2).
3.3 Association between residual cholesterol and TCFA characteristics
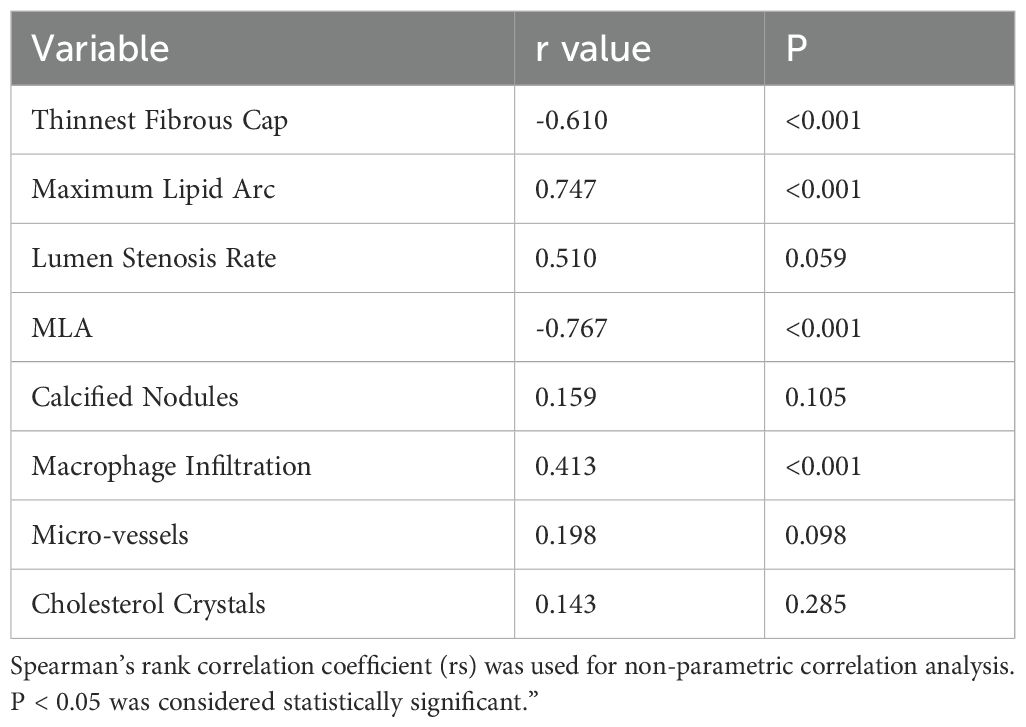
In the correlation analysis of TCFA plaque characteristics with RC and NCCLs, the results showed that the thinnest fibrous cap thickness was significantly negatively correlated with RC (r = -0.610, p < 0.001), while the maximum lipid arc was significantly positively correlated with RC (r = 0.747, p < 0.001). The lumen stenosis rate showed a positive trend but did not reach statistical significance (r = 0.510, p = 0.059), and MLA was significantly negatively correlated with RC (r = -0.767, p < 0.001). Additionally, macrophage infiltration was significantly positively correlated with RC (r = 0.413, p < 0.001), whereas calcified nodules (r = 0.159, p = 0.105), microvessels (r = 0.198, p = 0.098), and cholesterol crystals (r = 0.143, p = 0.285) did not show statistically significant correlations with RC (Table 3).
3.4 Multivariate logistic regression for MACE in non-culprit lesion TCFA
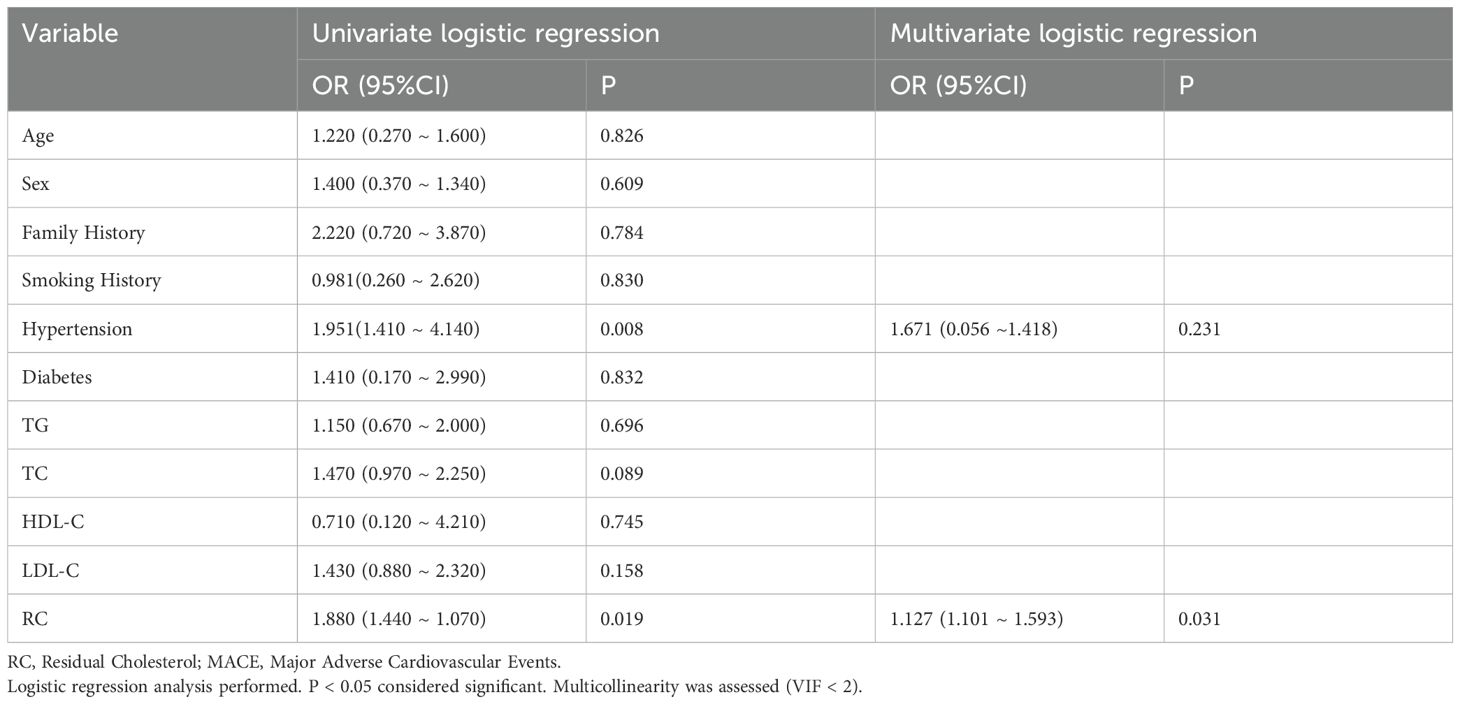
In the multivariate logistic regression analysis for the occurrence of MACE in TCFA within NCCLs, univariate analysis showed that hypertension (OR = 1.951, 95%CI: 1.410 ~ 4.140, p = 0.008) and RC (remnant cholesterol, OR = 1.880, 95%CI: 1.440 ~ 1.070, p = 0.019) were significantly associated with the occurrence of MACE. However, age, sex, family history, smoking history, diabetes, TG, TC, HDL-C, and LDL-C did not show significant correlations (p > 0.05). In the multivariate logistic regression analysis, RC remained an independent predictor of MACE (OR = 1.127, 95%CI: 1.101 ~ 1.593, p = 0.031), while the significance of hypertension was lost (OR = 1.671, 95%CI: 0.056 ~ 1.418, p = 0.231) (Table 4).
3.5 Predictive value of residual cholesterol for MACE in TCFA: ROC curve analysis
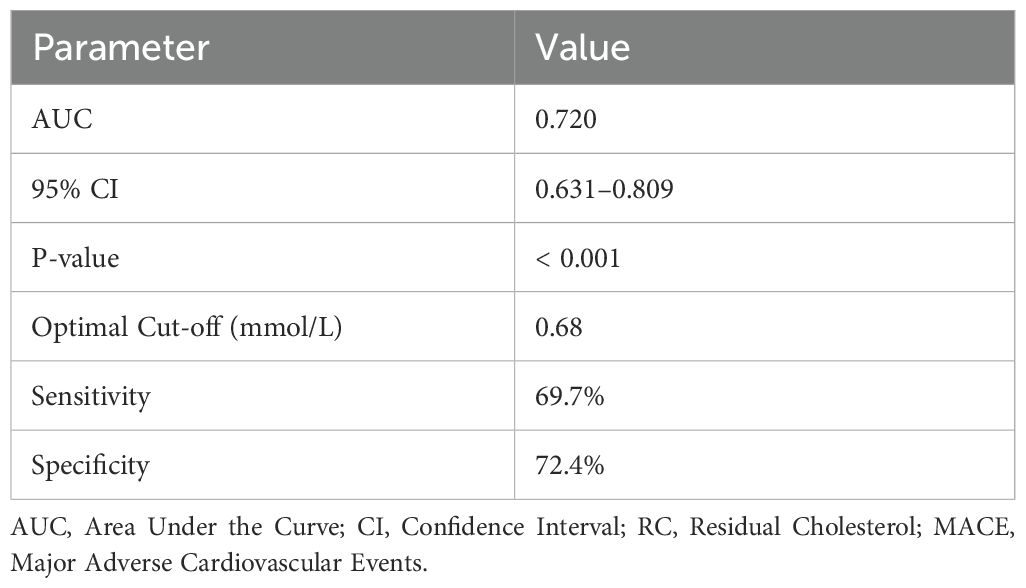
Figure 1 depicts the receiver operating characteristic (ROC) curve analysis evaluating the predictive value of RC for MACE occurring in TCFA of NCCLs. The ROC curve demonstrated moderate predictive performance, with an area under the curve (AUC) of 0.720, indicating RC as a potential biomarker for risk stratification and prognosis of MACE in patients with TCFA in NCCLs (Figure 2; Table 5).
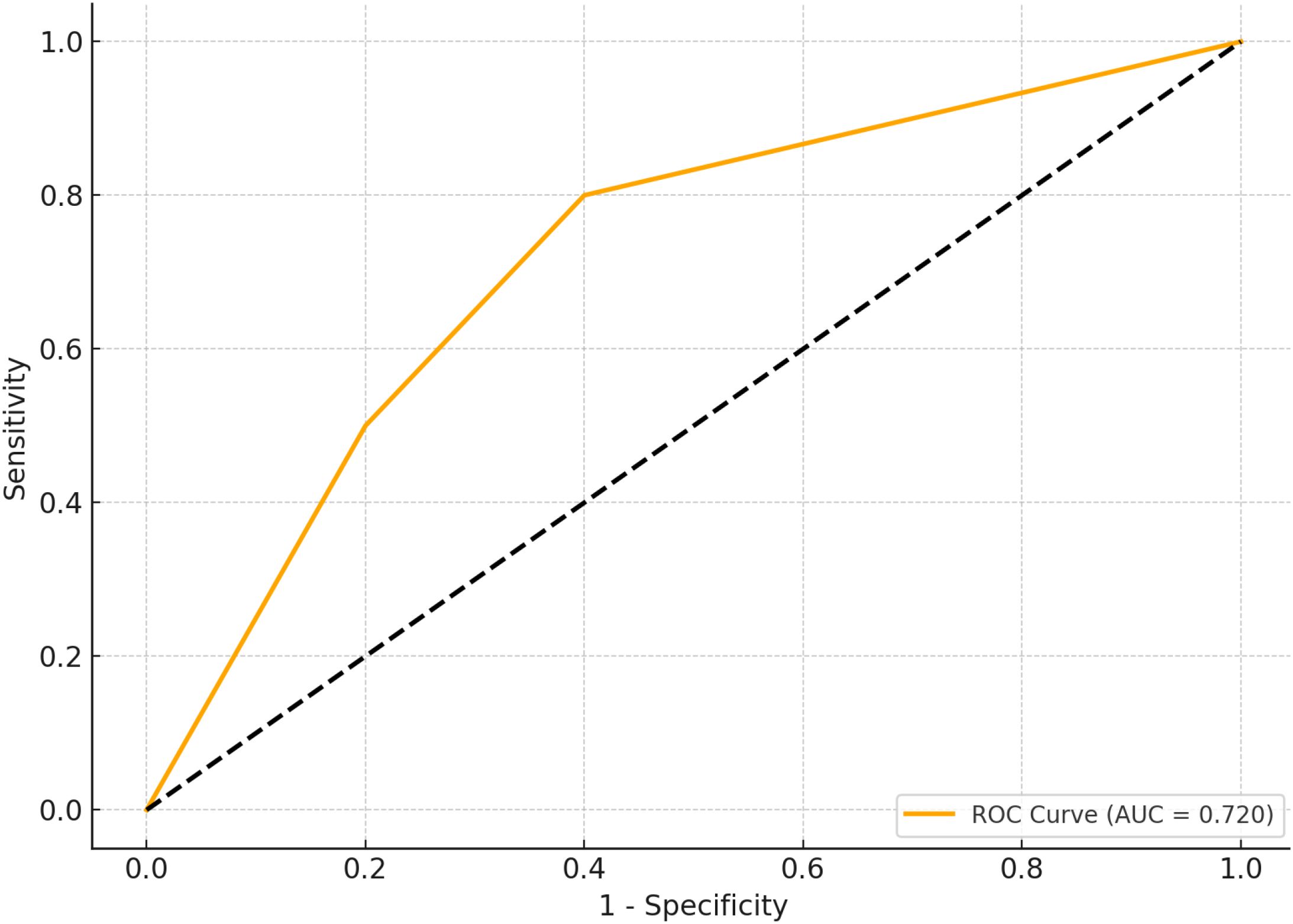
Figure 2. ROC curve simulation for RC predictive value in MACE. Figure 1 presents the simulated receiver operating characteristic (ROC) curve for residual cholesterol (RC) in predicting the occurrence of major adverse cardiovascular events (MACE) within thin-cap fibroatheromas (TCFA) of non-culprit lesions (NCCLs). ROC analysis conducted to assess predictive performance. Significance determined at P < 0.05.
4 Discussion
In this study, we observed that elevated RC was significantly associated with features of plaque vulnerability, including thinner fibrous caps and larger lipid arcs in TCFAs of NCCLs. Moreover, RC emerged as an independent risk factor for MACE, with a modest predictive ability demonstrated by ROC analysis (AUC = 0.720). These findings underscore the potential role of RC in identifying high-risk NCCLs and guiding secondary prevention strategies. This study explored the relationship between RC and the vulnerability of TCFAs in NCCLs, as well as its association with the risk of MACE, offering new perspectives for targeted intervention in vulnerable NCCL plaques. Our findings clarify, for the first time, the impact of RC on critical features of TCFA vulnerability—specifically, fibrous cap thickness and lipid arc—and identify RC as an independent risk factor for MACE. These results suggest that RC may serve not only as a predictive marker for NCCL vulnerability and cardiovascular event risk, but also as a strategic target for refining CVD prevention and intervention. Furthermore, by integrating imaging data with biochemical indices, this study reveals potential mechanisms of RC in the progression of vulnerable NCCLs, providing fresh evidence for investigating the role of RC in atherosclerotic diseases.
Although RC was treated as a continuous variable in this study, our results raise the question of whether a threshold effect may exist—i.e., a critical level of RC above which the risk of MACE increases disproportionately (32, 33). Prior studies suggest that RC > 0.8 mmol/L may confer elevated risk even in patients with well-controlled LDL-C, but further work is needed to define optimal cut-off values across diverse populations. Compared with other emerging biomarkers of residual cardiovascular risk such as lipoprotein(a) [Lp(a)] and apolipoprotein B (apoB), RC has the advantage of being routinely available from standard lipid panels and easy to calculate (34, 35). While Lp(a) and apoB have been associated with MACE in various cohorts, RC reflects the cholesterol content of remnant lipoproteins and may uniquely capture postprandial and triglyceride-rich atherogenic burden (36, 37). Future studies comparing the relative predictive value and combinatory models of these markers are warranted.
4.1 Influence of residual cholesterol on TCFA vulnerability
RC, an important indicator of lipid metabolism, is closely associated with the onset and progression of atherosclerosi (38)s. In this study, we found that RC levels significantly affect the key vulnerability features of TCFAs. Elevated RC may exacerbate TFCA instability through the following mechanisms:
First, RC is primarily composed of triglyceride-rich lipoprotein particles (e.g., very-low-density lipoproteins and chylomicron remnants). Metabolic abnormalities can lead to the accumulation of these remnants in the bloodstream (7, 8). Given their high arterial wall permeability, these particles can easily penetrate the endothelium and undergo oxidation, inducing local inflammatory responses and macrophage activation, thereby expanding the lipid core and thinning the fibrous cap (10, 11). Additionally, RC may promote further instability of the fibrous cap by increasing endothelial cell permeability and enhancing smooth muscle cell chemotaxis (9).
In this study, RC levels were significantly and negatively correlated with the thinnest fibrous cap thickness, suggesting that elevated RC may directly compromise plaque integrity by accelerating collagen degradation and inhibiting fibrous cap formation, ultimately increasing the risk of plaque rupture (39). Simultaneously, the significant positive correlation between RC levels and the maximum lipid arc implies that higher RC may drive lipid core enlargement, further raising the probability of plaque rupture and potentially exacerbating local inflammation in a self-reinforcing cycle (40, 41). Therefore, the pivotal role of RC in lipid metabolism and atherosclerotic progression indicates that its impact on TCFA vulnerability chiefly involves thinning of the fibrous cap and expansion of the lipid core. These findings provide new evidence for clarifying the mechanisms by which RC affects coronary artery lesions and suggest that RC may serve as a potential biomarker for evaluating plaque vulnerability.
4.2 Residual cholesterol and MACE risk
Our study indicates that RC significantly influences the incidence of MACE, with elevated RC serving as an independent risk factor for the progression of TCFAs in NCCLs to MACE (42, 43). RC holds notable potential value in NCCL risk management, especially regarding its synergistic effect with diabetes on the progression of vulnerable plaques.
RC and diabetes may jointly accelerate the formation and deterioration of vulnerable plaques. Patients with diabetes often exhibit insulin resistance and metabolic disorders, further elevating serum RC and the accumulation of triglyceride-rich particles (44, 45). Previous research suggests that the pro-inflammatory and pro-oxidative effects of RC in diabetic patients are more pronounced, as evidenced by increased lipid core expansion, persistent inflammatory cell infiltration, and a more rapid thinning of the fibrous cap. This synergistic process may explain why both diabetes and RC emerged as independent risk factors for MACE in our study (46–48). Elevated RC not only correlates closely with TCFA vulnerability, but may also increase MACE risk through direct effects on hemodynamics and the coronary microcirculation (49). The accumulation of RC can lead to endothelial dysfunction, endothelial cell apoptosis, and local inflammatory spread, further aggravating hemodynamic abnormalities in the affected coronary regions (50, 51). These mechanisms likely play a pivotal role in driving NCCL plaque progression toward MACE.
Given the clinical relevance of RC, this study suggests that RC could serve as a potential target in NCCL risk management. On the one hand, dynamic monitoring of RC levels may facilitate the early identification of high-risk patients (52, 53). On the other hand, interventions aimed at RC—such as optimizing lipid-lowering therapies or modulating lipid metabolic pathways—could offer effective strategies for the precise prevention of plaque vulnerability and MACE risk (54). Moreover, incorporating RC into cardiovascular risk assessment models could enhance the identification of high-risk individuals, thereby aiding in the refinement of preventative strategies against cardiovascular events. Therefore, the strong association between RC and MACE indicates that RC is not only a critical marker of NCCL risk but also a promising target for precision interventions in cardiovascular disease, offering a new avenue for optimizing vulnerability management in NCCLs (55–60). However, given the modest AUC and the relatively small sample size, these findings should be interpreted cautiously. The predictive performance of RC requires further validation in larger prospective cohorts with sufficient power.
4.3 Residual cholesterol, pro-inflammatory pathways, and immune modulation
Emerging evidence suggests that the impact of RC on atherosclerotic plaque vulnerability may extend beyond lipid accumulation and include inflammatory and immune-modulatory mechanisms (61). Notably, RC is known to induce endothelial dysfunction, partly by stimulating the expression of vascular endothelial growth factor (VEGF), a potent pro-inflammatory mediator that promotes vascular permeability and neovascularization within plaques (62). This process may enhance fibrous cap thinning and increase microvessel density, contributing to plaque instability (63, 64). Given that low-density lipoprotein (LDL) particles—particularly oxidized LDL—act as immunogenic triggers, the atherosclerotic plaque may in part represent a chronic immune response to lipid antigens (65, 66). These findings suggest that RC-mediated pathways intersect with vascular inflammation and immune modulation, underscoring the complex interplay between metabolic and immunological drivers of plaque vulnerability.
4.4 Study limitations
Despite providing novel insights, this study has several limitations that must be acknowledged: First, the single-center design and relatively small sample size may limit the generalizability and statistical power, especially for subgroup analyses and ROC evaluation. Larger multicenter cohorts are needed to validate our findings. Second, although our multivariate regression model adjusted for key clinical variables, the observational and non-randomized nature of the study means residual confounding cannot be fully excluded. Important unmeasured factors such as inflammatory markers, medication adherence, or dietary patterns may still influence outcomes. Third, no external validation cohort was used to confirm the predictive performance of RC for MACE, which should be addressed in future research. Fourth, although we assessed multicollinearity using VIF and found no significant collinearity, the relatively small event number may increase the risk of model overfitting. Finally, imaging assessments (OCT and IVUS) are subject to operator dependency and interpretation bias, even though image analysis was independently conducted by two experienced radiologists. Imaging assessments predominantly relied on OCT and IVUS, which, despite their high accuracy in quantifying plaque characteristics, may carry a degree of subjectivity. More advanced imaging modalities or artificial intelligence–aided analysis could be employed in future studies to minimize potential biases.
5 Conclusion
In conclusion, in this prospective observational study, elevated RC levels were associated with increased vulnerability of TCFAs and a higher incidence of MACE in non-culprit coronary lesions. These findings suggest that RC may serve as a potential biomarker for risk stratification, particularly in identifying patients with metabolically active, high-risk plaques. However, due to the non-randomized and observational nature of the study, these associations should be interpreted with caution, and no causal inferences can be drawn. Further prospective, multicenter studies with larger cohorts and mechanistic validation are warranted to confirm the predictive utility and clinical significance of RC in cardiovascular risk assessment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of Fuwai Central China Cardiovascular Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FW: Conceptualization, Writing – original draft. QL: Conceptualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The author expresses his profound gratitude to the participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Caller T, Fardman A, and Gerber Y. Atherosclerotic cardiovascular diseases are associated with incident metastatic and nonmetastatic cancer. JACC CardioOncol. (2024) 6:949–61. doi: 10.1016/j.jaccao.2024.07.020
2. Trillini M, Jenssen TG, and Martin WP. The future of glucagon-like peptide-1 receptor agonists in cardiovascular-kidney-metabolic diseases. Nephrol Dial Transplant. (2025). doi: 10.1093/ndt/gfaf008
3. Jing C, Wu Y, and Zhang Y. Epigenetic regulation and post-translational modifications of ferroptosis-related factors in cardiovascular diseases. Clin Epigenetics. (2025) 17:4. doi: 10.1186/s13148-024-01809-5
4. Liu G, Yu X, and Cui C. The pleiotropic effects of PCSK9 in cardiovascular diseases beyond cholesterol metabolism. Acta Physiol (Oxf). (2025) 241:e14272. doi: 10.1111/apha.v241.2
5. Gersh FL, Lavie CJ, and O'Keefe JH. Intervention is needed in PCOS for prevention of cardiovascular diseases. Eur J Prev Cardiol. (2025). doi: 10.1093/eurjpc/zwaf007
6. Marovic I, Udovicic M, and Rudan D. Prevalence and factors associated with potential clinically significant drug-drug interactions in patients with cardiovascular diseases at hospital admission. Acta Pharm. (2024) 74:693–708. doi: 10.2478/acph-2024-0038
7. Ahn SG, Raggi P, and Samady H. LDL cholesterol levels and thin cap fibroatheromas: A dynamic and complex puzzle. Atherosclerosis. (2015) 243:179–80. doi: 10.1016/j.atherosclerosis.2015.08.046
8. Loland KH, Bleie O, and Strand E. Effect of folic acid supplementation on levels of circulating Monocyte Chemoattractant Protein-1 and the presence of intravascular ultrasound derived virtual histology thin-cap fibroatheromas in patients with stable angina pectoris. PloS One. (2013) 8:e70101. doi: 10.1371/journal.pone.0070101
9. Matsuo Y, Kubo T, and Okumoto Y. Circulating malondialdehyde-modified low-density lipoprotein levels are associated with the presence of thin-cap fibroatheromas determined by optical coherence tomography in coronary artery disease. Eur Heart J Cardiovasc Imaging. (2013) 14:43–50. doi: 10.1093/ehjci/jes094
10. Wykrzykowska JJ, Diletti R, and Gutierrez-Chico JL. Plaque sealing and passivation with a mechanical self-expanding low outward force nitinol vShield device for the treatment of IVUS and OCT-derived thin cap fibroatheromas (TCFAs) in native coronary arteries: report of the pilot study vShield Evaluated at Cardiac hospital in Rotterdam for Investigation and Treatment of TCFA (SECRITT). EuroIntervention. (2012) 8:945–54. doi: 10.4244/EIJV8I8A144
11. Toutouzas K, Karanasos A, and Riga M. Optical coherence tomography assessment of the spatial distribution of culprit ruptured plaques and thin-cap fibroatheromas in acute coronary syndrome. EuroIntervention. (2012) 8:477–85. doi: 10.4244/EIJV8I4A75
12. Ma M, Lv D, and Wu X. Association between normal weight obesity and comorbidities and events of cardiovascular diseases among adults in South China. PloS One. (2025) 20:e0316346. doi: 10.1371/journal.pone.0316346
13. Moon KZ, Rahman MH, and Alam MJ. Unraveling the interplay between cardiovascular diseases and alcohol use disorder: A bioinformatics and network-based exploration of shared molecular pathways and key biomarkers validation via western blot analysis. Comput Biol Chem. (2024) 115:108338. doi: 10.1016/j.compbiolchem.2024.108338
14. Li X, Zhao C, and Liu M. Sociodemographic index-age differences in the global prevalence of cardiovascular diseases, 1990-2019: a population-based study. Arch Public Health. (2025) 83:2. doi: 10.1186/s13690-024-01454-7
15. Rashki Kemmak A, Etemad L, and Haghighizadeh A. Cost Effectiveness Analysis Rivaroxaban Plus Aspirin versus Aspirin alone in Treatment Cardiovascular Diseases: A Systematic Review. Med J Islam Repub Iran. (2024) 38:106.
16. Xu X, Fang C, and Jiang S. Functional or anatomical assessment of non-culprit lesions in acute myocardial infarction. EuroIntervention. (2025) 21:e217–28. doi: 10.4244/EIJ-D-24-00720
17. Milzi A, Dettori R, and Marx N. Quantitative flow ratio (QFR) identifies functional relevance of non-culprit lesions in coronary angiographies of patients with acute myocardial infarction. Clin Res Cardiol. (2021) 110:1659–67. doi: 10.1007/s00392-021-01897-w
18. Sulimov DS, Abdel-Wahab M, and Richardt G. Fractional flow reserve in acute myocardial infarction: A guide for non-Culprit lesions? Cardiol Ther. (2015) 4:39–46. doi: 10.1007/s40119-015-0040-4
19. Dai J, Zhao J, and Xu X. Long-term prognostic implications of non-culprit lesions in patients presenting with an acute myocardial infarction: is it the angiographic stenosis severity or the underlying high-risk morphology? Circulation. (2025) 151:1098–110. doi: 10.1161/CIRCULATIONAHA.124.071855
20. Bucher K, Belli S, and Wunderli-Allenspach H. P-glycoprotein in proteoliposomes with low residual detergent: the effects of cholesterol. Pharm Res. (2007) 24:1993–2004. doi: 10.1007/s11095-007-9326-0
21. Pedersen TR, Assmann G, and Bassand JP. Reducing residual cardiovascular risk: the relevance of raising high-density lipoprotein cholesterol in patients on cholesterol-lowering treatment. Diabetes Vasc Dis Res. (2006) 3:S1–S12. doi: 10.3132/dvdr.2006.011
22. Achedume AC, Nwoha PU, and Adepoju AY. Biochemical studies of the effect of gossypol consumption on cholesterol and residual glucose in fed and protein-energy deficient rats. Contraception. (1994) 50:391–5. doi: 10.1016/0010-7824(94)90026-4
23. Zhang H, Zhang C, and Zhang Y. The role of residual inflammatory risk and LDL cholesterol in patients with in-stent restenosis undergoing percutaneous coronary intervention. J Clin Lipidol. (2024) 18:e746–55. doi: 10.1016/j.jacl.2024.05.009
24. Liao J, Qiu M, and Su X. The residual risk of inflammation and remnant cholesterol in acute coronary syndrome patients on statin treatment undergoing percutaneous coronary intervention. Lipids Health Dis. (2024) 23:172. doi: 10.1186/s12944-024-02156-3
25. Sucato V, Comparato F, and Ortello A. Residual cardiovascular risk: role of remnants cholesterol, monocyte/HDL ratio and lipoprotein ratios on personalized cardiovascular prevention. J Pers Med. (2024) 14. doi: 10.3390/jpm14050460
26. Amrani S, Zemouri KC, and Bouguerra KA. Relationship between blood groups and cardiovascular diseases: insights from an Algerian inpatient study. Cureus. (2024) 16:e75624. doi: 10.7759/cureus.75624
27. Rashki M, Ghasemzadeh Rahbardar M, and Boskabady MH. Nutritional advantages of walnut (Juglans regia L.) for cardiovascular diseases: A comprehensive review. Food Sci Nutr. (2025) 13:e4526.
28. Yang X, Fan Q, and Shen C. Pregnancy loss was associated with the increased risk of cardiovascular diseases in middle-aged women: evidence from the China health and retirement longitudinal study. Glob Heart. (2025) 20:1. doi: 10.5334/gh.1386
29. Li J, Li Y, and Song G. Revolutionizing cardiovascular research: Human organoids as a Beacon of hope for understanding and treating cardiovascular diseases. Mater Today Bio. (2025) 30:101396. doi: 10.1016/j.mtbio.2024.101396
30. Kamarudin NA, Wan Puteh SE, and Abd Manaf MR. Trends in cardiovascular diseases and costs among type 2 diabetes mellitus (T2DM) patients in Malaysia: A cohort study of 240, 611 public hospital inpatients. Cureus. (2024) 16:e75531. doi: 10.7759/cureus.75531
31. Yang L, Yue Q, and Fang F. Effect of dual residual risk of cholesterol and inflammation on all-cause mortality in patients with cardiovascular disease. Cardiovasc Diabetol. (2023) 22:96. doi: 10.1186/s12933-023-01826-3
32. Shah PK. Residual risk and high-density lipoprotein cholesterol levels: is there a relationship? Rev Cardiovasc Med. (2011) 12:e55–9.
33. Carey VJ, Bishop L, and Laranjo N. Contribution of high plasma triglycerides and low high-density lipoprotein cholesterol to residual risk of coronary heart disease after establishment of low-density lipoprotein cholesterol control. Am J Cardiol. (2010) 106:757–63. doi: 10.1016/j.amjcard.2010.05.002
34. Wong ND, Chuang J, and Zhao Y. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. (2015) 9:525–32. doi: 10.1016/j.jacl.2015.05.003
35. Toth PP, Jones SR, and Slee A. Relationship between lipoprotein subfraction cholesterol and residual risk for cardiovascular outcomes: A post hoc analysis of the AIM-HIGH trial. J Clin Lipidol. (2018) 12:741–747 e11. doi: 10.1016/j.jacl.2018.03.077
36. Suzuki K, Oikawa T, and Nochioka K. Elevated serum non-HDL (High-density lipoprotein) cholesterol and triglyceride levels as residual risks for myocardial infarction recurrence under statin treatment. Arterioscler Thromb Vasc Biol. (2019) 39:934–44. doi: 10.1161/ATVBAHA.119.312336
37. Fujihara Y, Nakamura T, and Horikoshi T. Remnant lipoproteins are residual risk factor for future cardiovascular events in patients with stable coronary artery disease and on-statin low-density lipoprotein cholesterol levels <70 mg/dL. Circ J. (2019) 83:1302–8.
38. Saybolt MD, Lilly SM, and Patel D. The vulnerable artery: early and rapid deposition of lipid in coronary arteries is associated with subsequent development of thin-cap fibroatheromas. EuroIntervention. (2016) 11:e1612–8. doi: 10.4244/EIJV11I14A312
39. Dolla WJ, House JA, and Marso SP. Stratification of risk in thin cap fibroatheromas using peak plaque stress estimates from idealized finite element models. Med Eng Phys. (2012) 34:1330–8. doi: 10.1016/j.medengphy.2011.12.024
40. Schneiderman J, Wilensky RL, and Weiss A. Diagnosis of thin-cap fibroatheromas by a self-contained intravascular magnetic resonance imaging probe in ex vivo human aortas and in situ coronary arteries. J Am Coll Cardiol. (2005) 45:1961–9. doi: 10.1016/j.jacc.2004.09.080
41. Hong MK, Mintz GS, and Lee CW. A three-vessel virtual histology intravascular ultrasound analysis of frequency and distribution of thin-cap fibroatheromas in patients with acute coronary syndrome or stable angina pectoris. Am J Cardiol. (2008) 101:568–72. doi: 10.1016/j.amjcard.2007.09.113
42. Chen A, Chang X, and Bian X. Construction and comparison of machine learning-based risk prediction models for major adverse cardiovascular events in perimenopausal women. Int J Gen Med. (2025) 18:11–20. doi: 10.2147/IJGM.S497416
43. Li Q, Xu S, and Shen J. The nonlinear association between lipoprotein(a) and major adverse cardiovascular events in acute coronary syndrome patients with three-vessel disease. Sci Rep. (2025) 15:1720. doi: 10.1038/s41598-025-86154-0
44. Troum OM, Duong M, and Obermeyer K. Treatment-emergent major adverse cardiovascular and thromboembolic events were infrequent during clinical trials of pegloticase. Rheumatol (Oxford). (2025). doi: 10.1093/rheumatology/keaf017
45. Bitar W, Helve J, and Haapio M. Risk of major adverse cardiovascular events in home dialysis compared with in-center hemodialysis. Clin J Am Soc Nephrol. (2025) 20:81–7. doi: 10.2215/CJN.0000000579
46. Li B, Shaikh F, and Younes H. Prediction of major adverse cardiovascular events in patients with peripheral artery disease using circulating immunomodulatory proteins. Biomedicines. (2024) 12. doi: 10.3390/biomedicines12122842
47. Adusumalli S, McCarthy CP, and Magaret CA. Multiple biomarkers to predict major adverse cardiovascular events in patients with coronary chronic total occlusions. Am J Cardiol. (2025). doi: 10.1016/j.amjcard.2024.12.037
48. Toyama T, Kasama S, and Sato M. The most important prognostic factors for predicting major adverse cardiovascular, cerebrovascular, and renal events during 5-year follow-up of patients with chronic kidney disease with or without haemodialysis. Nucl Med Commun. (2025). doi: 10.1097/MNM.0000000000001943
49. Miller RJ, Bednarski B, and Cui Y. The relationship between quantitative ischemia, early revascularization, and major adverse cardiovascular events: A multicenter study. JACC Adv. (2025) 4:101440. doi: 10.1016/j.jacadv.2024.101440
50. Lee DY, Moon JS, and Jung I. Risk acceleration by gout on major adverse cardiovascular events and all-cause death in patients with diabetes and chronic kidney disease. Diabetes Obes Metab. (2025). doi: 10.1111/dom.16165
51. Smith C, Sim M, and Ilyas Z. Automated abdominal aortic calcification and major adverse cardiovascular events in people undergoing osteoporosis screening: the Manitoba Bone Mineral Density Registry. J Bone Miner Res. (2025). doi: 10.1093/jbmr/zjae208
52. Gitmez M, Guzel T, and Kis M. The performance of the NAPLES prognostic score in predicting one-year mortality and major adverse cardiovascular events (MACEs) after transcatheter aortic valve implantation in patients with severe aortic stenosis. Kardiol Pol. (2025). doi: 10.33963/v.phj.103230
53. Lin CH, Liu ZY, and Chu PH. A multitask deep learning model utilizing electrocardiograms for major cardiovascular adverse events prediction. NPJ Digit Med. (2025) 8:1. doi: 10.1038/s41746-024-01410-3
54. Zhou S, Qiu M, and Wang K. Triglyceride to high density lipoprotein cholesterol ratio and major adverse cardiovascular events in ACS patients undergoing PCI. Sci Rep. (2024) 14:31752. doi: 10.1038/s41598-024-82064-9
55. Ozbaltan OC, Cakmak S, and Sogut O. Predictive value of NT-proBNP for major adverse cardiovascular events within a 6-month period in patients with acute coronary syndrome. Ir J Med Sci. (2024). doi: 10.1007/s11845-024-03849-5
56. Rose SJ, Hartnett J, and Estep ZJ. Measurement of breast artery calcification using an artificial intelligence detection model and its association with major adverse cardiovascular events. PloS Digit Health. (2024) 3:e0000698. doi: 10.1371/journal.pdig.0000698
57. Li B, Shaikh F, and Younes H. Identification and evaluation of angiogenesis-related proteins that predict major adverse cardiovascular events in patients with peripheral artery disease. J Cardiovasc Dev Dis. (2024) 11. doi: 10.3390/jcdd11120402
58. Yu J, Peng X, and Zhou R. Development and validation of an interpretable machine learning model to predict major adverse cardiovascular events after noncardiac surgery in geriatric patients: a prospective study. Int J Surg. (2024). doi: 10.1097/JS9.0000000000002203
59. Khalil M, Sinnott SM, and Civieri G. Accelerated development of cardiovascular risk factors mediates risk for major adverse cardiovascular events in posttraumatic stress disorder. Brain Behav Immun. (2024). doi: 10.1016/S0735-1097(24)03845-2
60. Chen TL, Huang JY, and Lin HY. Risk of major adverse cardiovascular events and venous thromboembolic events between patients with psoriasis or psoriatic arthritis on tumor necrosis factor inhibitors, interleukin-17 inhibitors, interleukin-12/23 inhibitors and interleukin-23 inhibitors: an emulated target trial analysis. J Am Acad Dermatol. (2024).
61. Dulak J, Jozkowicz A, and Guevara I. Gene transfer of vascular endothelial growth factor and endothelial nitric oxide synthase–implications for gene therapy in cardiovascular diseases. Pol J Pharmacol. (1999) 51:233–41.
62. Liu F, Hidru TH, and Gao R. Cancer patients with potential eligibility for vascular endothelial growth factor antagonists use have an increased risk for cardiovascular diseases comorbidities. J Hypertens. (2020) 38:426–33. doi: 10.1097/HJH.0000000000002277
63. Boullier A, Hennuyer N, and Tailleux A. Increased levels of high-density lipoprotein cholesterol are ineffective in inhibiting the development of immune responses to oxidized low-density lipoprotein and atherosclerosis in transgenic rabbits expressing human apolipoprotein (apo) A-I with severe hypercholesterolaemia. Clin Sci (Lond). (2001) 100:343–55. doi: 10.1042/cs1000343
64. Islam S, Gutin B, and Treiber F. Association of apolipoprotein A phenotypes and oxidized low-density lipoprotein immune complexes in children. Arch Pediatr Adolesc Med. (1999) 153:57–62. doi: 10.1001/archpedi.153.1.57
65. Miller YI, Chang MK, and Binder CJ. Oxidized low density lipoprotein and innate immune receptors. Curr Opin Lipidol. (2003) 14:437–45. doi: 10.1097/00041433-200310000-00004
Keywords: residual cholesterol, non-culprit lesion, thin-cap fibroatheroma, major adverse cardiovascular events, CVD (cardio vascular disease)
Citation: Wang F and Li Q (2025) Association between residual cholesterol and vulnerable non-culprit lesions progressing to major adverse cardiovascular events. Front. Endocrinol. 16:1603907. doi: 10.3389/fendo.2025.1603907
Received: 01 April 2025; Accepted: 30 June 2025;
Published: 15 August 2025.
Edited by:
Yanmei Chen, Southern Medical University, ChinaReviewed by:
Giuseppe Murdaca, University of Genoa, ItalyRehab H. Werida, Damanhour University, Egypt
Copyright © 2025 Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengfeng Wang, d2FuZ2ZlbmdmZW5nc2pua0AxNjMuY29t
 Fengfeng Wang
Fengfeng Wang Qun Li
Qun Li