- 1Chengdu Integrated TCM and Western Medical Hospital, Department of Oncology, Chengdu, Sichuan, China
- 2Cancer Center, West China Hospital, Sichuan University, Chengdu, China
Adipose-derived mesenchymal stem cells (ADSCs), multipotent stromal cells abundant in adipose tissue, exhibit remarkable plasticity in modulating systemic metabolism, inflammatory responses, and immune homeostasis. Their bidirectional interactions with the tumor microenvironment (TME) position them as both accomplices and antagonists in cancer progression, offering unique therapeutic opportunities. ADSCs also hold significant potential for clinical application in the fields of regenerative medicine, tissue engineering, and gene engineering. This review synthesizes the impacts and challenges of ADSCs involving metabolic regulation, tumor modulation, immunomodulation, regenerative medicine and genetic engineering therapies—while elucidating underlying molecular mechanisms and signaling pathways, clinical studies, applications and challenges.
1 Introduction
Adipose-derived mesenchymal stem cells (ADSCs), a subset of mesenchymal stem cells (MSCs), are multipotent stromal cells isolated from the stromal vascular fraction (SVF) of adipose tissue (1). These cells share fundamental molecular and functional similarities with other mesenchymal stem cells (MSCs), such as bone marrow-derived mesenchymal stem cells (BMSCs), including expression of characteristic surface markers (CD105, CD44, CD73, CD90, CD146) and absence of hematopoietic lineage markers (CD13, CD19, CD45, HLA-DR) (2). Like other MSCs, ADSCs exhibit self-renewal capacity and multilineage differentiation potential (3). Beyond differentiation, ADSCs possess immunomodulatory properties mediated through paracrine secretion of anti-inflammatory cytokines (e.g., IL-10, TGF-β) and direct cell-cell interactions making them a promising candidate for regenerative medicine and reconstructive therapies (4, 5). Furthermore, adipose-derived mesenchymal stem cell exosomes (ADSC-Exos), enriched with diverse bioactive molecules including miRNAs, proteins, and lipids, significantly contribute to anti-inflammatory responses and immune regulation, while their cell-free transplantation circumvents risks of immune rejection and tumorigenesis associated with traditional stem cell therapies (6). ADSCs are easily accessible and minimally invasive procedures such as liposuction (7, 8). And their abundance in adipose tissue, coupled with low donor-site morbidity, positions ADSCs as a clinically viable resource for applications including anti-inflammatory, immunomodulatory, metabolic regulation and therapeutic potential (9, 10). Current research on ADSCs remains predominantly confined to elucidating basic molecular mechanisms, with insufficient preclinical validation and clinical translation to establish systematic frameworks or practical guidelines. To address these limitations, this study adopts a translational pipeline spanning “basic medical research (molecular pathways) → preclinical testing (in vitro/animal models) → clinical trials → therapeutic applications” to comprehensively evaluate ADSCs’ roles and challenges in metabolic regulation (diabetes, obesity), tumor modulation (pro-/anti-tumor effects), immunomodulation (autoimmune diseases, transplantation), regenerative medicine (tissue repair, osteoarthritis, cardiac/neural regeneration), and genetic engineering therapies.
2 Metabolic regulation
2.1 Diabetes mellitus
Diabetes mellitus (DM), encompassing both type I and type II forms with distinct pathogenic mechanisms, is characterized by persistent hyperglycemia; inadequate glycemic control frequently leads to severe complications such as retinopathy, neuropathy, and vasculopathy, all of which profoundly compromise patient health. Type 1 diabetes mellitus (T1DM) is primarily characterized by autoimmune-mediated destruction of pancreatic β-cells, while type 2 diabetes mellitus (T2DM) manifests as a triad of insulin resistance in target organs (skeletal muscle, liver, and adipose tissue), chronic low-grade inflammation driven by pro-inflammatory cytokines (e.g., TNF-α, IL-6) secreted from macrophages and other immune cells, and progressive β-cell dysfunction due to glucolipotoxicity and endoplasmic reticulum stress (11). ADSCs-mediated improvement of hyperglycemia may encompass islet β-cell regeneration via differentiation into insulin-producing cells or promotion of endogenous β-cell proliferation, modulation of hepatic metabolism toward enhanced glucose utilization, attenuation of chronic inflammation through anti-inflammatory cytokine secretion, and amelioration of insulin resistance (IR) in peripheral tissues via regulation of lipid homeostasis and insulin signaling pathways (12–15). ADSCs transplantation facilitates the restoration of islet function through the promotion of β-cell regeneration, attenuation of apoptosis and inflammation, and enhancement of islet vascularization (12, 16). Mechanistically , ADSCs mitigate insulin resistance in T2DM by suppressing chronic inflammation via polarization of pro-inflammatory M1 macrophages to anti-inflammatory M2 phenotypes, a process mediated by interleukin-10 (IL-10) and inhibition of NLRP3 inflammasome activation (17, 18) . In T1DM, ADSCs restore immune homeostasis by downregulating pathogenic Th1/Th17 responses and promoting regulatory T-cell (Treg) expansion, thereby attenuating autoimmune β-cell destruction (19, 20) . Molecularly , ADSC-Exos deliver microRNAs (e.g., miR-146a) that inhibit NF-κB and STAT3 signaling, reducing cytokine storms in pancreatic islets (21) . Additionally, ADSCs enhance β-cell survival and function via paracrine secretion of hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) to promote β-cell proliferation and reduce apoptosis (22) . Preclinically , in streptozotocin-induced T1DM rodent models, systemic ADSC administration improves glycemic control by restoring β-cell mass, reducing hyperglycemia (22, 23). In high-fat-diet-induced T2DM mice, ADSCs alleviate insulin resistance via AMPK/SIRT1-mediated enhancement of mitochondrial metabolism in adipose tissue and skeletal muscle (24, 25). Clinically , phase II trials in T2DM patients demonstrate that umbilical cord-derived MSCs (UC-MSCs) reduce HbA1c by 1.5–2.0% and improve insulin sensitivity, correlating with decreased serum TNF-α and increased adiponectin levels (26) . For T1DM, prior case reports have indicated that the infusion of in vitro-differentiated insulin-producing cells derived from ADSCs into patients led to sustained stabilization of blood glucose levels and glycosylated hemoglobin (HbA1c) (27), and a prospective dual-arm clinical trial demonstrated that autologous ADSCs combined with bone marrow-derived hematopoietic stem cells (BM-HSCs) enable sustained glycemic regulation (28). Other clinical trials report transient C-peptide preservation and reduced exogenous insulin requirements, though long-term efficacy remains inconsistent (29) . Challenges include donor-dependent variability in ADSC secretome potency, risks of iPSC-derived β-cell teratoma formation due to residual pluripotency , and fibrotic complications linked to TGF-β overactivation during immunomodulation (30) . Emerging strategies to address these limitations involve biomaterial encapsulation for targeted pancreatic delivery and IFN-γ preconditioning to enhance immunosuppressive capacity (31) .
2.2 Obesity and metabolic syndrome
Obesity induces a decline in ADSC activity, with studies demonstrating a 40% reduction in ADSC activity in obese populations compared to healthy individuals, directly impairing tissue regenerative capacity and potentially triggering metabolic disturbances such as arteriosclerosis and insulin resistance (32). Under obese conditions, ADSCs exhibit a shift toward a pro-inflammatory secretory profile characterized by increased secretion of IL-1β, IL-6, and IL-8 cytokines, accompanied by a concomitant reduction in immunomodulatory, insulin-sensitizing, and weight-regulatory adipokines (e.g., IL-10, TGF-β, and adiponectin), thereby exacerbating metabolic dysfunction (33–35). And Yu Meng et al.’s study demonstrates that obesity impairs the structural integrity and functional capacity of ADSCs’ mitochondria, potentially mediated in part through miRNA-induced regulation of mitochondrial genes, leading to an escalation in oxidative stress (36). Moreover, studies in diet-induced obesity models have established that PD-L1 upregulation contributes to T cell dysfunction, characterized by a marked impairment in cytolytic activity (37). Beyond investigations in animal models, a clinical study involving 47 reproductive-aged African women demonstrated that serum from overweight/obese individuals (with or without metabolic syndrome) significantly impaired ADSCs proliferation and migration, linked to elevated IL6 levels, and induced lipid accumulation during osteogenic differentiation, highlighting systemic inflammatory dysregulation as a key driver of ADSC functional decline in metabolic disorders (38). Although the reciprocal interactions between obesity and ADSCs remain incompletely elucidated, inspired by existing research, ADSCs may hold broad clinical application prospects in obesity and obesity-related diseases. ADSC-derived exosomes ameliorate obesity-associated metabolic dysregulation by promoting M2 macrophage polarization, enhancing white adipose tissue beiging, and improving insulin sensitivity through STAT3-mediated arginase-1 activation (39). Numerous in vivo studies have demonstrated the efficacy of ADSCs in promoting weight loss and ameliorating hyperlipidemia (40–42). Non-alcoholic fatty liver disease (NAFLD), a spectrum ranging from hepatic steatosis to non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and hepatocellular carcinoma, is closely associated with obesity and metabolic syndrome (43, 44). Studies indicate that ADSCs mitigate NAFLD progression by homing to damaged liver tissue, enhancing hepatocyte regeneration, and exerting anti-inflammatory/antioxidant effects through their self-renewal and multipotent differentiation capabilities (45, 46). Although current studies on the mechanisms and preclinical research of ADSCs in obesity and metabolic disorders suggest their potential therapeutic roles, translating these findings into clinical applications remains a protracted process. This transition necessitates rigorous optimization of multiple procedures, including standardized isolation, purification, expansion, and clinical validation of ADSCs, as well as comprehensive safety and efficacy evaluations in human trials.
3 Tumor-related application
3.1 ADSCs and tumor microenvironment
ADSCs exhibit a multifaceted and bidirectional relationship with the tumor microenvironment (TME), a heterogeneous ecosystem comprising immune cells, stromal fibroblasts, extracellular matrix (ECM), and metabolic mediators that collectively drive tumor progression and therapeutic resistance (47, 48) . A vitro study demonstrates that bidirectional crosstalk between human adipose-derived stem cells (ADSCs) and malignant melanoma cells (MMCs) in an indirect co-culture model significantly enhances tumor-promoting behaviors, including migration, invasion, and angiogenesis, via upregulation of pro-angiogenic factors (VEGF, IL-8, CCL2), matrix metalloproteinases (e.g., MMP-2), and oncogenic mediators (CXCL12, PTGS2, IL-6, HGF) (49, 50). ADSCs are recruited to the TME via tumor-secreted chemotactic signals, such as CCL2 and CXCL12, where they undergo functional reprogramming to adopt pro-tumorigenic roles (49, 51). Within the TME, ADSCs contribute to immunosuppression by secreting anti-inflammatory cytokines (e.g., IL-10, TGF-β) and exosomes that dampen cytotoxic T-cell activity, promote regulatory T-cell (Treg) expansion, polarize macrophages toward an immune-tolerant M2 phenotype, inhibit of dendritic cells differentiation, promoting immune escape of tumor cells (18, 19) . The TME reciprocally shapes ADSC behavior through metabolic crosstalk. Hypoxia and nutrient deprivation in the TME drive ADSCs to release fatty acids and lipid metabolites, which tumor cells exploit to fuel oxidative phosphorylation (OXPHOS) and mitigate metabolic stress (52) . This lipid transfer is further amplified by ADSC-derived exosomal miRNAs (e.g., miR-21, miR-155), which activate oncogenic pathways such as PI3K/Akt and Wnt/β-catenin in adjacent tumor cells (53, 54) . Notably, obesity exacerbates this metabolic symbiosis, as high-fat diets enhance lipid availability in the TME, impairing CD8+ T-cell function and accelerating tumor growth—a mechanism conserved across murine and human cancers (55–57) . Furthermore, a vitro study demonstrates MSCs promote reversible metastatic enhancement in breast carcinoma through TME-driven CCL5/RANTES secretion, which activates CCR5-mediated paracrine signaling to potentiate cancer cell motility, invasion, and distant dissemination (58). In contrast to their tumor-promoting effects, MSCs have also been shown to inhibit the progression of malignancies such as leukemia and hepatocellular carcinoma; therefore, as multipotent stromal cells, MSCs can secrete context-dependent factors within the tumor microenvironment that either suppress or exacerbate neoplastic growth through bidirectional modulation of oncogenic signaling pathways (59). Beyond their established roles in promoting tumorigenesis, progression, and metastasis, ADSCs are also closely associated with chemoresistance to antitumor agents.
3.2 ADSCs and chemoresistance
ADSCs drive chemoresistance in malignancies through multifaceted mechanisms within the TME. Primarily, ADSCs engage in bidirectional crosstalk with tumor cells via paracrine signaling, exemplified by the TSG-6/COX-2 axis: Tumor necrosis factor alpha-stimulated gene/protein 6 (TSG-6) upregulates cyclooxygenase-2 (COX-2) expression, fostering prostaglandin E2 (PGE2)-mediated angiogenesis, immunosuppression, and apoptosis evasion, a mechanism validated by the abrogation of chemoresistance upon TSG-6 silencing in ADSCs (60–63). Furthermore , ADSCs secrete interleukin-6 (IL-6), which activates STAT3 in tumor cells to enhance DNA repair and upregulate multidrug resistance (MDR) transporters such as P-glycoprotein (64, 65). Concurrently , metabolic reprogramming induced by ADSCs—such as the release of platinum-induced polyunsaturated fatty acids (PIFAs) to scavenge reactive oxygen species (ROS)—protects tumor cells from cytotoxic damage, a process reversible through COX-1/thromboxane synthase inhibition (66). Additionally , ADSCs remodel the extracellular matrix (ECM) via matrix metalloproteinase (MMP)-mediated stiffening, creating physical barriers that impede drug penetration while promoting hypoxia-driven angiogenesis through HIF-1α/VEGF activation (67). Critically , ADSCs enhance tumor cell stemness by inducing epithelial-mesenchymal transition (EMT) and cancer stem cell (CSC) phenotypes via Wnt/β-catenin and Notch signaling, thereby amplifying self-renewal capacity and therapeutic resistance (68, 69). Notably , targeting these pathways—such as COX-2/PGE2 inhibition, TSG-6 neutralization, or metabolic disruption of PIFA synthesis—holds therapeutic potential to restore chemosensitivity. In summary , ADSCs orchestrate a pro-survival TME through integrated paracrine, metabolic, and structural adaptations, highlighting the urgency of developing stroma-targeted adjuvants to overcome multidrug resistance in oncology.
3.3 Tumor related application
Although ADSCs may exhibit pro-tumorigenic effects, including tumor initiation, progression, and metastatic dissemination through crosstalk with the tumor microenvironment, emerging evidence paradoxically highlights their significant clinical potential as anti-neoplastic agents through immunomodulatory mechanisms and targeted therapeutic delivery. Preclinical studies demonstrate their capacity to suppress tumor growth in glioblastoma (70), hepatocellular carcinoma (71, 72), colon cancer (73, 74) and other tumor models (75). Meanwhile, the homing capacity of ADSCs to tumor niches positions them as ideal cellular vectors for targeted drug delivery. Engineered ADSCs overexpressing TNF-α or TRAIL via CRISPR/Cas9 have shown enhanced tumor-specific cytotoxicity in murine models, selectively localizing to metastatic sites while minimizing off-target effects (76, 77). Additionally, ADSC-derived exosomes loaded with chemotherapeutic agents (e.g., doxorubicin) or oncolytic viruses achieve precise intratumoral delivery, overcoming biological barriers and reducing systemic toxicity (78, 79). Recent advances highlight their utility in photodynamic therapy, where photoactivated ADSCs release reactive oxygen species (ROS) to induce localized tumor cell death, synergizing with checkpoint inhibitors to amplify anti-PD-1/PD-L1 efficacy (80). ADSCs, in addition to their direct/indirect antitumor effects, have also demonstrated positive clinical effects in managing toxic side effects associated with chemoradiotherapy, including cisplatin-induced fertility impairment (81), salivary gland dysfunction following radiotherapy for head and neck tumors (resulting in xerostomia) (80, 82), and chemotherapy-induced granulocytopenia through secreting Granulocyte Colony-Stimulating Factor (G-CSF (83, 84). Ongoing research focuses on bioengineering strategies to “lock” ADSCs in anti-tumor states through metabolic priming (e.g., mTOR inhibition) or epigenetic modulation to suppress pro-metastatic gene networks . These innovations underscore ADSCs’ versatility as modular therapeutic platforms, though rigorous characterization of their spatiotemporal dynamics within the TME remains critical to mitigate context-dependent risks.
4 Immunomodulatory effects
ADSCs exhibit multifaceted immunomodulatory properties in the treatment of autoimmune diseases and mitigation of post-transplant immune rejection, primarily through paracrine signaling and direct cell-cell interactions. Mechanistically , ADSCs secrete anti-inflammatory cytokines (e.g., IL-10, TGF-β) and induce regulatory T-cell (Treg) expansion while suppressing pro-inflammatory Th17 and effector T-cell activation (19). Additionally, ADSCs upregulate PD-1/PD-L1 interactions to induce T-cell anergy and apoptosis (85). In vitro studies support above results and also demonstrate that mesenchymal stem cells (MSCs) exert immunomodulatory effects by suppressing T-cell proliferation, cytotoxicity, and Th1/Th2 cytokine secretion, inhibiting dendritic cell maturation via Notch pathway activation, and attenuating B-cell proliferation and antibody production (86). We examine the latest research progress on the immunoregulatory effects of adipose-derived mesenchymal stem cells in autoimmune disorders and graft-versus-host disease (GVHD).
4.1 Autoimmune disorders
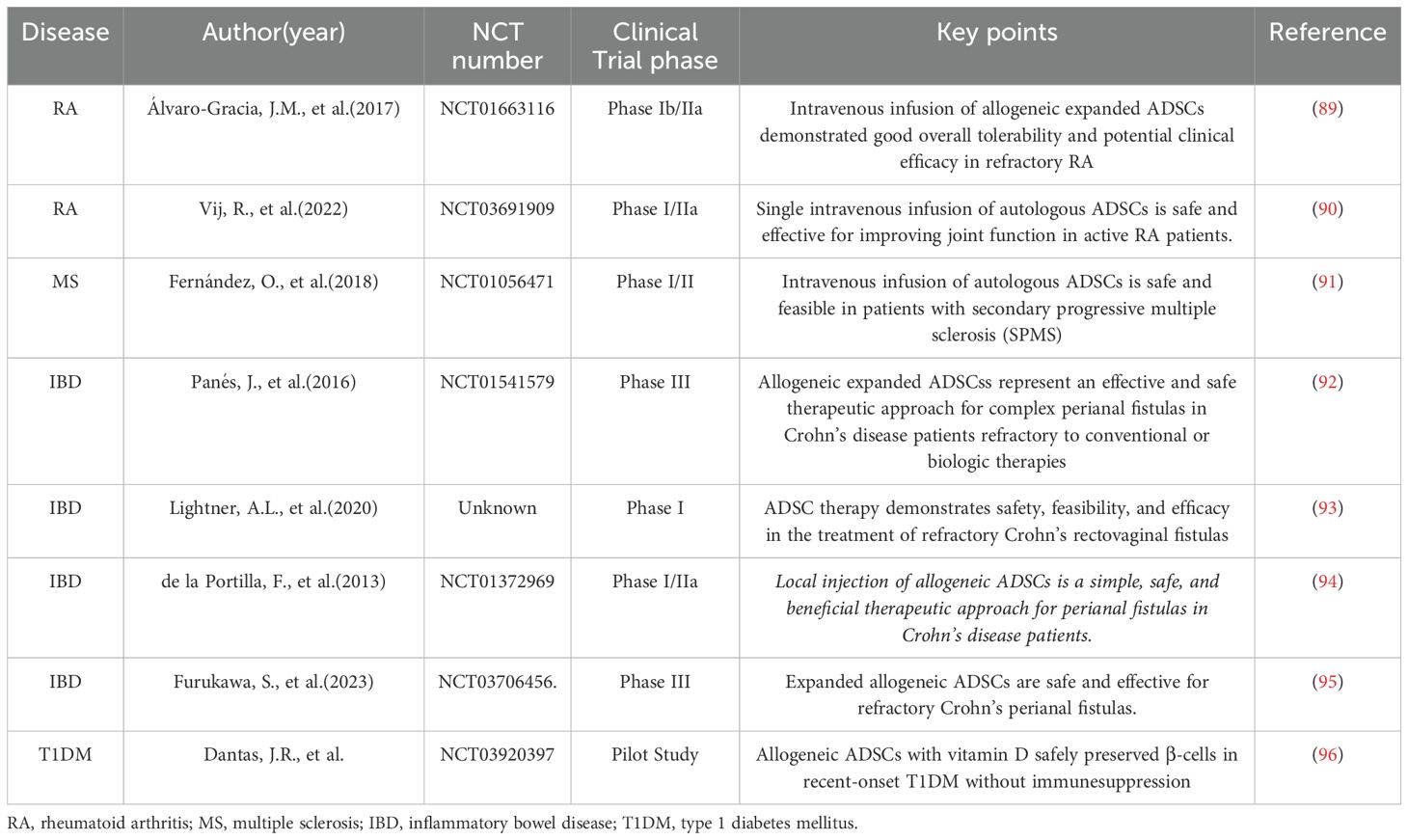
Autoimmune disorders, encompassing various chronic organ-specific and systemic diseases caused by immune system malfunction that mistakenly attacks the body’s own cells and tissues, affect approximately 8-10% of the population, resulting in significant health impairments, elevated mortality rates, and substantial medical burdens (87, 88). As summarized in Table 1, numerous preclinical and clinical studies have investigated the therapeutic potential of adipose-derived mesenchymal stem cells (ADSCs) in managing various autoimmune disorders. Rheumatoid arthritis (RA), multiple sclerosis (MS), inflammatory bowel disease (IBD), and type 1 diabetes mellitus (T1DM) represent the most prevalent systemic autoimmune conditions globally. These chronic disorders share a common pathogenesis characterized by dysregulated immune responses leading to persistent organ inflammation and progressive tissue damage. Current standard treatments, including non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, immunosuppressants, and chemotherapeutic agents such as methotrexate (MTX), often present significant limitations. Given their demonstrated anti-inflammatory properties and immunomodulatory capabilities, ADSCs have emerged as a promising therapeutic alternative for autoimmune diseases, offering the potential to restore immune homeostasis while avoiding the detrimental side effects of conventional pharmacotherapies. This unique biological profile positions ADSC-based therapy as a novel paradigm in the evolving landscape of autoimmune disease management.
4.2 Graft-versus-host disease
Acute and chronic graft-versus-host disease (GVHD) are common yet challenging clinical complications following allogeneic hematopoietic stem cell transplantation and solid organ transplantation (such as ocular grafts, kidneys, etc.). Given the immunomodulatory and anti-inflammatory properties of adipose-derived mesenchymal stem cells, they hold potential for combating GVHD (97–99). Animal model studies have clarified the effectiveness of ADSCs against bone marrow aplasia in GVHD (100), while another animal study demonstrated that subconjunctival injection of ADSCs significantly improved post-transplant corneal integrity and promoted wound healing, highlighting their clinical potential in this context (101). Beyond animal research, clinical data on ADSCs for GVHD management have also been reported. A single-arm clinical study involving pediatric patients with refractory bronchiolitis obliterans syndrome (BoS) following allogeneic hematopoietic stem cell transplantation (allo-HSCT) showed that ADSCs could be safely administered (102). Additionally, a phase I/II study indicated that combining ADSCs with immunosuppressive therapy is feasible and safe for chronic GVHD post allo-HSCT, with potential positive impacts on disease progression (103). More studies are warranted to understand the potential benefits of ADSCs in GVHD.
5 Regenerative medicine and tissue engineering
ADSCs have emerged as a promising therapeutic tool in regenerative medicine and tissue engineering due to their multifaceted mechanisms of action. Mechanistically, ADSCs possess the ability to self-renew and exhibit multipotent differentiation capacity, differentiating into various cell lineages such as fibroblasts, myocytes, chondrocytes, adipocytes, and immune cells (e.g., macrophages or lymphocytes) under specific microenvironmental cues. This versatility endows ADSCs with broad applications in various medical fields.
In plastic surgery and wound repair, ADSCs promote tissue regeneration through paracrine secretion of angiogenic factors (e.g., VEGF, PDGF, bFGF), which stimulate neovascularization (104–106). Additionally, they exhibit immunomodulatory properties by suppressing pro-inflammatory cytokines (TNF-α, IFN-γ) and upregulating anti-inflammatory mediators (IL-10, IL-4) (107–109). These mechanisms facilitate wound healing and tissue repair by enhancing keratinocyte migration and proliferation, modulating extracellular matrix (ECM) remodeling, and suppressing apoptosis (110–112). Animal models of full-thickness skin defects and ischemic flaps have demonstrated accelerated wound closure, increased capillary density, and reduced fibrosis in ADSC-treated groups, attributed to enhanced angiogenesis and attenuated oxidative stress (113–115). ADSCs have also shown promise in repairing radiation-induced/UV-induced tissue damage (116–119), chronic diabetic ulcers (120–123), and in aesthetic procedures such as facial volumization and breast reconstruction, where their adipogenic potential of improving graft retention and reducing scar formation (124–128).
In osteochondral repair, ADSCs exhibit robust osteogenic potential primarily through paracrine signaling, immunomodulation, and direct differentiation. Paracrine activity enables ADSCs to secrete osteoinductive factors, including BMPs, VEGF, and IGF-1, which synergistically enhance angiogenesis and osteoblast differentiation (129). ADSC-derived exosomes deliver osteogenic miRNAs that activate Wnt/β-catenin and BMP/Smad pathways, upregulating master regulators Runx2 and Osterix (130–133). Furthermore, ADSCs can modulate the immune system to inhibit osteoclastogenesis in conditions like osteoporosis and foster a pro-regenerative microenvironment in osteoarthritic joints (134). Under osteoinductive conditions, ADSCs directly differentiate into osteoblasts, marked by upregulated ALP, collagen type I, and OCN expression, culminating in mineralized matrix deposition (3, 135). These mechanisms have been corroborated across diverse preclinical models, with in vitro and in vivo studies demonstrating enhanced bone formation and reduced osteoclast activity.
ADSCs also exhibit therapeutic potential in myocardial repair and regeneration. Through paracrine secretion of bioactive molecules and exosomes enriched with cardioprotective miRNAs, ADSCs attenuate oxidative stress, activate PI3K/Akt and HIF-1α pathways, enhance cardiomyocyte survival, and stimulate angiogenesis (136–139). Furthermore, ADSCs can modulate the phenotype of monocytes/macrophages towards a more anti-inflammatory state, potentially beneficially influencing the duration and intensity of the inflammatory response post-myocardial infarction (140). Preclinical studies in rodent models have revealed improvements in left ventricular ejection fraction, reduction in infarct size, and increased amount of highly vascularized granulation tissue in the border zone (141).
In neural repair and regeneration, ADSCs possess inherent multipotency, characterized by their trilineage differentiation potential and robust secretion of neurotrophic mediators. These mediators orchestrate neuroprotection, axonal sprouting, and synaptogenesis in models of neural trauma (142–145). The immunomodulatory axis of ADSCs attenuates neuroinflammation and microglial activation by downregulating pro-inflammatory cytokines and upregulating anti-inflammatory interleukin-10 (146, 147). In vitro, ADSCs exhibit neurogenic differentiation potential under inductive conditions, evidenced by upregulation of neuronal markers and electrophysiological properties indicative of functional neuronal networks (148–150). Preclinical in vivo studies have demonstrated enhanced functional recovery through various signaling pathways and modulation of glial scar formation (151–154).
Moreover, ADSCs and their bioactive derivatives exhibit multifaceted anti-aging and regenerative potential. At the molecular level, ADSCs modulate senescence-associated pathways via paracrine signaling, releasing exosomes enriched with miRNAs, growth factors, and anti-inflammatory cytokines (105, 108, 155, 156). These components collectively suppress oxidative stress, inhibit the senescence-associated secretory phenotype, and enhance mitochondrial biogenesis. Studies have explored the role of ADSCs in aging-related conditions such as skin aging, alopecia, and cognitive dysfunction, with promising results suggesting potential for mitigating age-related inflammation, improving skin elasticity, and wound healing (157, 158).
6 In genetic engineering: applications and prospects
ADSCs have garnered significant interest in genetic engineering due to their inherent plasticity, ease of isolation, and robust expansion potential. Gene-editing technologies, such as CRISPR/Cas9, TALENs, and viral/non-viral vector systems, enable precise manipulation of ADSCs to enhance their therapeutic efficacy or confer novel functionalities. Genetic modification of ADSCs enhances their therapeutic paracrine signaling by co-upregulating regenerative/immunoregulatory factors (e.g., VEGF, HGF, IL-10) while suppressing pro-inflammatory cytokine expression, thereby amplifying paracrine-mediated tissue regeneration through balanced immunomodulation and matrix remodeling (159). For instance, CRISPR-mediated upregulation of VEGF in ADSCs has demonstrated enhanced angiogenesis in preclinical models of ischemic cardiomyopathy and diabetic wounds. Similarly, ADSCs engineered to express neurotrophic factors (e.g., brain-derived neurotrophic factor, BDNF) show promise in neural regeneration by promoting axonal growth and synaptic plasticity in spinal cord injury models (160). Another emerging application involves modifying ADSCs to improve their homing efficiency and survival in hostile microenvironments. Knockdown of pro-apoptotic genes (e.g., BAX) or overexpression of chemokine receptors (e.g., CXCR4) via lentiviral transduction enhances their engraftment at injury sites (161, 162). Furthermore, ADSCs can be reprogrammed to act as targeted delivery vehicles for therapeutic genes or RNA-based therapies (163, 164). For example, ADSCs transfected with oncolytic viruses or tumor-suppressor genes (e.g., p53, PTEN) exhibit synergistic anti-tumor effects by selectively localizing to tumor stroma and inducing apoptosis in malignant cells while sparing healthy tissues (165). The integration of synthetic biology platforms with ADSCs opens avenues for dynamic, condition-responsive therapies. Engineered gene circuits, such as hypoxia-inducible promoters or inflammation-sensitive switches, allow ADSCs to autonomously release therapeutic payloads (e.g., anti-inflammatory cytokines, matrix metalloproteinase inhibitors) in response to disease-specific cues. This approach minimizes off-target effects and maximizes spatiotemporal precision, as demonstrated in rheumatoid arthritis models where IL-1β-responsive ADSCs suppressed joint inflammation only in active disease states (166). Prospectively, ADSCs hold transformative potential in addressing genetic disorders through ex vivo gene correction. Autologous ADSCs edited to rectify monogenic mutations (e.g., in COL1A1 for osteogenesis imperfecta or CFTR for cystic fibrosis) could be reimplanted to restore functional tissue homeostasis (167). Additionally, ADSCs engineered to express chimeric antigen receptors (CARs) or T-cell engagers are being explored in adoptive cell therapies for cancers, leveraging their tumor-tropic properties to enhance localized immune activation. Despite these advancements, challenges persist in ensuring the safety and scalability of genetically modified ADSCs. Risks of off-target edits, immune rejection of engineered cells, and long-term genomic instability necessitate rigorous preclinical validation. Moreover, standardized protocols for GMP-compliant manufacturing, quality control of edited clones, and regulatory frameworks for clinical translation remain underdeveloped. Collaborative efforts among geneticists, bioengineers, and clinicians are critical to overcoming these barriers and unlocking the full potential of ADSCs in next-generation gene therapies. In summary, ADSCs serve as a versatile platform for genetic engineering, bridging regenerative medicine and precision therapeutics. Their applications span targeted gene delivery, dynamic microenvironment modulation, and autologous cell-based gene correction, with translational prospects in oncology, degenerative diseases, and genetic disorders. Continued innovation in gene-editing tools and biofabrication technologies will likely propel ADSCs to the forefront of personalized and programmable medicine.
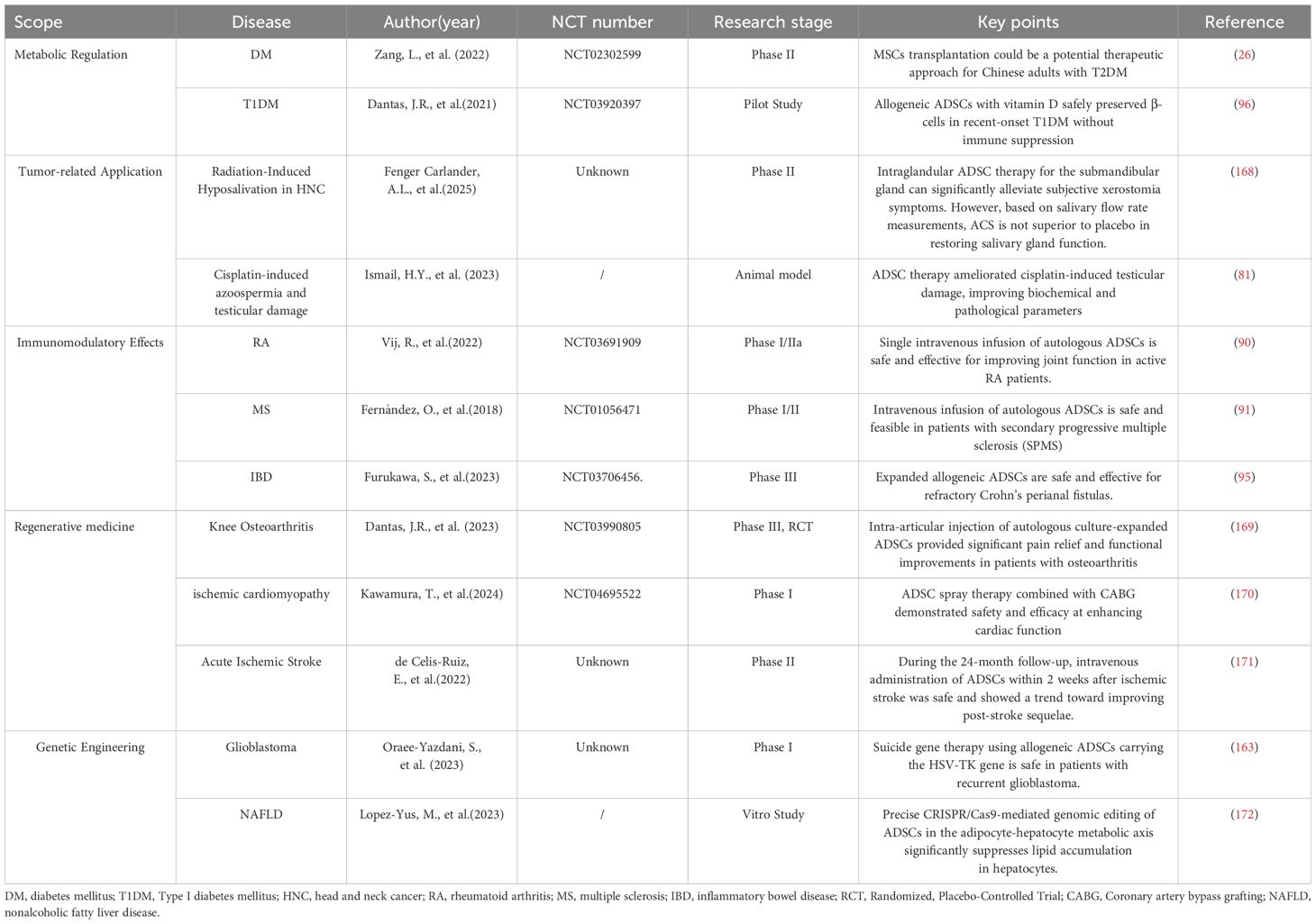
Despite their therapeutic promise in metabolic regulation, immunomodulation, and tissue regeneration (as exemplified by key studies summarized in Table 2), the clinical application of ADSCs faces several critical challenges. First, heterogeneity in cell populations derived from the stromal vascular fraction (SVF) and variability in isolation/expansion protocols compromise batch-to-batch consistency, potentially affecting therapeutic reproducibility. Standardized Good Manufacturing Practice (GMP)-compliant protocols, coupled with advanced single-cell sequencing to identify subpopulation-specific biomarkers, may enhance quality control. Second , long-term safety concerns persist, particularly regarding the potential pro-tumorigenic effects of ADSCs in pre-existing malignancies or their unintended differentiation post-transplantation. Rigorous preclinical studies using lineage-tracing models and tumor-prone animal cohorts, alongside real-time molecular monitoring in clinical trials, are essential to elucidate these risks. Third , the pleiotropic mechanisms underpinning ADSCs’ efficacy—such as paracrine signaling, exosome-mediated communication, and dynamic crosstalk with immune cells—remain incompletely mapped. Integrated multi-omics approaches (e.g., proteomic profiling of secretomes and CRISPR-based functional screens) could clarify dominant pathways like PI3K/AKT and Wnt/β-catenin, enabling targeted therapeutic optimization. Fourth , clinical translation is hindered by a paucity of robust Phase III randomized controlled trials (RCTs) and suboptimal delivery routes (e.g., systemic infusion vs. localized injection). Adaptive trial designs, route-specific pharmacokinetic studies, and international consortium-led registries may accelerate evidence generation. By addressing these challenges through interdisciplinary innovation and rigorous regulatory oversight, ADSCs could transition from experimental therapies to standardized clinical tools.
Author contributions
LW: Conceptualization, Writing – original draft, Writing – review & editing. XJ: Data curation, Formal analysis, Writing – original draft. FZ: Funding acquisition, Investigation, Writing – original draft. PD: Investigation, Methodology, Project administration, Writing – review & editing. ZL: Resources, Software, Supervision, Writing – review & editing. YL: Supervision, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. (2018) 36:1111–26. doi: 10.1016/j.biotechadv.2018.03.011
2. Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. (2013) 15:641–8. doi: 10.1016/j.jcyt.2013.02.006
3. Bunnell BA. Adipose tissue-derived mesenchymal stem cells. Cells. (2021) 10. doi: 10.3390/cells10123433
4. Gentile P. Adipose-derived mesenchymal stem cells, cell-based therapies, and biomaterials as new regenerative strategies in plastic surgery. Biomedicines. (2022) 10. doi: 10.3390/biomedicines10081875
5. Naderi N, Combellack EJ, Griffin M, Sedaghati T, Javed M, Findlay MW, et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int Wound J. (2017) 14:112–24. doi: 10.1111/iwj.2017.14.issue-1
6. Dong Z, Fu Y, Cai Z, Dai H, and He Y. Recent advances in adipose-derived mesenchymal stem cell-derived exosomes for regulating macrophage polarization. Front Immunol. (2025) 16:1525466. doi: 10.3389/fimmu.2025.1525466
7. MacGregor KA, Rodriguez-Sanchez N, Barwell ND, Gallagher IJ, Moran CN, and Di Virgilio TG. Human subcutaneous adipose tissue sampling using a mini-liposuction technique. J Vis Exp. (2021) 175). doi: 10.3791/62635-v
8. Simonacci F, Bertozzi N, Grieco MP, and Raposio E. From liposuction to adipose-derived stem cells: indications and technique. Acta BioMed. (2019) 90:197–208.
9. Sabol RA, Bowles AC, Côté A, Wise R, Pashos N, Bunnell BA, et al. Therapeutic potential of adipose stem cells. Adv Exp Med Biol. (2021) 1341:15–25.
10. Heo JS, Choi Y, and Kim HO. Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cells Int. (2019) 2019:7921760. doi: 10.1155/2019/7921760
11. Hasnain SZ, Prins JB, and McGuckin MA. Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes. J Mol Endocrinol. (2016) 56:R33–54. doi: 10.1530/JME-15-0232
12. Hu J, Fu Z, Chen Y, Tang N, Wang L, Wang F, et al. Effects of autologous adipose-derived stem cell infusion on type 2 diabetic rats. Endocr J. (2015) 62:339–52. doi: 10.1507/endocrj.EJ14-0584
13. Yu S, Cheng Y, Zhang L, Yin Y, Xue J, Li B, et al. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res Ther. (2019) 10:333. doi: 10.1186/s13287-019-1474-8
14. Xie M, Hao HJ, Cheng Y, Xie ZY, Yin YQ, Zhang Q, et al. Adipose-derived mesenchymal stem cells ameliorate hyperglycemia through regulating hepatic glucose metabolism in type 2 diabetic rats. Biochem Biophys Res Commun. (2017) 483:435–41. doi: 10.1016/j.bbrc.2016.12.125
15. Deng Z, Xu H, Zhang J, Yang C, Jin L, Liu J, et al. Infusion of adipose−derived mesenchymal stem cells inhibits skeletal muscle mitsugumin 53 elevation and thereby alleviates insulin resistance in type 2 diabetic rats. Mol Med Rep. (2018) 17:8466–74. doi: 10.3892/mmr.2018.8901
16. Wang X, Liu C, Li S, Xu Y, Chen P, Liu Y, et al. Hypoxia precondition promotes adipose-derived mesenchymal stem cells based repair of diabetic erectile dysfunction via augmenting angiogenesis and neuroprotection. PloS One. (2015) 10:e0118951. doi: 10.1371/journal.pone.0118951
17. Shree N and Bhonde RR. Conditioned media from adipose tissue derived mesenchymal stem cells reverse insulin resistance in cellular models. J Cell Biochem. (2017) 118:2037–43. doi: 10.1002/jcb.25777
18. Zhang X, Jiang Y, Huang Q, Wu Z, Pu H, Xu Z, et al. Exosomes derived from adipose-derived stem cells overexpressing glyoxalase-1 protect endothelial cells and enhance angiogenesis in type 2 diabetic mice with limb ischemia. Stem Cell Res Ther. (2021) 12:403. doi: 10.1186/s13287-021-02475-7
19. Li X, Lu X, Sun D, Wang X, Yang L, Zhao S, et al. Adipose-derived mesenchymal stem cells reduce lymphocytic infiltration in a rabbit model of induced autoimmune dacryoadenitis. Invest Ophthalmol Vis Sci. (2016) 57:5161–70. doi: 10.1167/iovs.15-17824
20. Yaochite JN, de Lima KW, Caliari-Oliveira C, Palma PV, Couri CE, Simões BP, et al. Multipotent mesenchymal stromal cells from patients with newly diagnosed type 1 diabetes mellitus exhibit preserved in vitro and in vivo immunomodulatory properties. Stem Cell Res Ther. (2016) 7:14. doi: 10.1186/s13287-015-0261-4
21. Shang Q, Bai Y, Wang G, Song Q, Guo C, Zhang L, et al. Delivery of adipose-derived stem cells attenuates adipose tissue inflammation and insulin resistance in obese mice through remodeling macrophage phenotypes. Stem Cells Dev. (2015) 24:2052–64. doi: 10.1089/scd.2014.0557
22. Tan Y, Nie W, Chen C, He X, Xu Y, Ma X, et al. Mesenchymal stem cells alleviate hypoxia-induced oxidative stress and enhance the pro-survival pathways in porcine islets. Exp Biol Med (Maywood). (2019) 244:781–8. doi: 10.1177/1535370219844472
23. Rahavi H, Hashemi SM, Soleimani M, Mohammadi J, and Tajik N. Adipose tissue-derived mesenchymal stem cells exert in vitro immunomodulatory and beta cell protective functions in streptozotocin-induced diabetic mice model. J Diabetes Res 2015. (2015) p:878535. doi: 10.1155/2015/878535
24. Sanap A, Bhonde R, and Joshi K. Conditioned medium of adipose derived Mesenchymal Stem Cells reverse insulin resistance through downregulation of stress induced serine kinases. Eur J Pharmacol. (2020) 881:173215. doi: 10.1016/j.ejphar.2020.173215
25. Piao C, Wang Y, Lu X, Liu T, Ma Y, Li Y, et al. Met-Exo attenuates mitochondrial dysfunction after hepatic ischemia-reperfusion injury in rats by modulating AMPK/SIRT1 signaling pathway. Free Radic Biol Med. (2024) 213:430–42. doi: 10.1016/j.freeradbiomed.2024.01.049
26. Zang L, Li Y, Hao H, Liu J, Cheng Y, Li B, et al. Efficacy and safety of umbilical cord-derived mesenchymal stem cells in Chinese adults with type 2 diabetes: a single-center, double-blinded, randomized, placebo-controlled phase II trial. Stem Cell Res Ther. (2022) 13:180. doi: 10.1186/s13287-022-02848-6
27. Dave SD, Trivedi HL, Chooramani SG, and Chandra T. Management of type 1 diabetes mellitus using in vitro autologous adipose tissue trans-differentiated insulin-making cells. BMJ Case Rep. (2013) 2013. doi: 10.1136/bcr-2013-200226
28. Thakkar UG, Trivedi HL, Vanikar AV, and Dave SD. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy. (2015) 17:940–7. doi: 10.1016/j.jcyt.2015.03.608
29. Taylor PN, Collins KS, Lam A, Karpen SR, Greeno B, Walker F, et al. C-peptide and metabolic outcomes in trials of disease modifying therapy in new-onset type 1 diabetes: an individual participant meta-analysis. Lancet Diabetes Endocrinol. (2023) 11:915–25. doi: 10.1016/S2213-8587(23)00267-X
30. Griffin M, Ryan CM, Pathan O, Abraham D, Denton CP, and Butler PE. Characteristics of human adipose derived stem cells in scleroderma in comparison to sex and age matched normal controls: implications for regenerative medicine. Stem Cell Res Ther. (2017) 8:23. doi: 10.1186/s13287-016-0444-7
31. Khorsandi L, Khodadadi A, Nejad-Dehbashi F, and Saremy S. Three-dimensional differentiation of adipose-derived mesenchymal stem cells into insulin-producing cells. Cell Tissue Res. (2015) 361:745–53. doi: 10.1007/s00441-015-2140-9
32. Louwen F, Ritter A, Kreis NN, and Yuan J. Insight into the development of obesity: functional alterations of adipose-derived mesenchymal stem cells. Obes Rev. (2018) 19:888–904. doi: 10.1111/obr.12679
33. Serena C, Keiran N, Ceperuelo-Mallafre V, Ejarque M, Fradera R, Roche K, et al. Obesity and type 2 diabetes alters the immune properties of human adipose derived stem cells. Stem Cells. (2016) 34:2559–73. doi: 10.1002/stem.2429
34. Silva KR and Baptista LS. Adipose-derived stromal/stem cells from different adipose depots in obesity development. World J Stem Cells. (2019) 11:147–66. doi: 10.4252/wjsc.v11.i3.147
35. Silva KR, Liechocki S, Carneiro JR, Claudio-da-Silva C, Maya-Monteiro CM, Borojevic R, et al. Stromal-vascular fraction content and adipose stem cell behavior are altered in morbid obese and post bariatric surgery ex-obese women. Stem Cell Res Ther. (2015) 6:72. doi: 10.1186/s13287-015-0029-x
36. Meng Y, Eirin A, Zhu XY, Tang H, Chanana P, Lerman A, et al. Obesity-induced mitochondrial dysfunction in porcine adipose tissue-derived mesenchymal stem cells. J Cell Physiol. (2018) 233:5926–36. doi: 10.1002/jcp.v233.8
37. Eljaafari A, Pestel J, Le Magueresse-Battistoni B, Chanon S, Watson J, Robert M, et al. Adipose-tissue-derived mesenchymal stem cells mediate PD-L1 overexpression in the white adipose tissue of obese individuals, resulting in T cell dysfunction. Cells. (2021) 10. doi: 10.3390/cells10102645
38. Seboko AM, Conradie MM, Kruger MJ, Ferris WF, Conradie M, and van de Vyver M. Systemic factors during metabolic disease progression contribute to the functional decline of adipose tissue-derived mesenchymal stem cells in reproductive aged females. Front Physiol. (2018) 9:1812. doi: 10.3389/fphys.2018.01812
39. Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. (2018) 67:235–47. doi: 10.2337/db17-0356
40. Lee CW, Hsiao WT, and Lee OK. Mesenchymal stromal cell-based therapies reduce obesity and metabolic syndromes induced by a high-fat diet. Transl Res. (2017) 182:61–74.e8. doi: 10.1016/j.trsl.2016.11.003
41. Liu GY, Liu J, Wang YL, Liu Y, Shao Y, Han Y, et al. Adipose-derived mesenchymal stem cells ameliorate lipid metabolic disturbance in mice. Stem Cells Transl Med. (2016) 5:1162–70. doi: 10.5966/sctm.2015-0239
42. Saleh F, Itani L, Calugi S, Dalle Grave R, and El Ghoch M. Adipose-derived mesenchymal stem cells in the treatment of obesity: A systematic review of longitudinal studies on preclinical evidence. Curr Stem Cell Res Ther. (2018) 13:466–75. doi: 10.2174/1574888X13666180515160008
43. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
44. Quek J, Chan KE, Wong ZY, Tan C, Tan B, Lim WH, et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2023) 8:20–30. doi: 10.1016/S2468-1253(22)00317-X
45. Pan F, Liao N, Zheng Y, Wang Y, Gao Y, Wang S, et al. Intrahepatic transplantation of adipose-derived stem cells attenuates the progression of non-alcoholic fatty liver disease in rats. Mol Med Rep. (2015) 12:3725–33. doi: 10.3892/mmr.2015.3847
46. Niu Q, Wang T, Wang Z, Wang F, Huang D, Sun H, et al. Adipose-derived mesenchymal stem cell-secreted extracellular vesicles alleviate non-alcoholic fatty liver disease via delivering miR-223-3p. Adipocyte. (2022) 11:572–87. doi: 10.1080/21623945.2022.2098583
48. Paganelli A, Rossi E, and Magnoni C. The dark side of adipose-derived mesenchymal stromal cells in cutaneous oncology: roles, expectations, and potential pitfalls. Stem Cells Dev. (2022) 31:593–603. doi: 10.1089/scd.2022.0189
49. Preisner F, Leimer U, Sandmann S, Zoernig I, Germann G, and Koellensperger E. Impact of human adipose tissue-derived stem cells on Malignant melanoma cells in an in vitro co-culture model. Stem Cell Rev Rep. (2018) 14:125–40. doi: 10.1007/s12015-017-9772-y
50. Kamat P, Schweizer R, Kaenel P, Salemi S, Calcagni M, Giovanoli P, et al. Human adipose-derived mesenchymal stromal cells may promote breast cancer progression and metastatic spread. Plast Reconstr Surg. (2015) 136:76–84. doi: 10.1097/PRS.0000000000001321
51. Midavaine É., Côté J, and Sarret P. The multifaceted roles of the chemokines CCL2 and CXCL12 in osteophilic metastatic cancers. Cancer Metastasis Rev. (2021) 40:427–45. doi: 10.1007/s10555-021-09974-2
52. Siltari A, Syvälä H, Lou YR, Gao Y, and Murtola TJ. Role of lipids and lipid metabolism in prostate cancer progression and the tumor’s immune environment. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14174293
53. Varela-López A, Vera-Ramírez L, Giampieri F, Navarro-Hortal MD, Forbes-Hernández TY, Battino M, et al. The central role of mitochondria in the relationship between dietary lipids and cancer progression. Semin Cancer Biol. (2021) 73:86–100. doi: 10.1016/j.semcancer.2021.01.001
54. Noorolyai S, Shajari N, Baghbani E, Sadreddini S, and Baradaran B. The relation between PI3K/AKT signaling pathway and cancer. Gene. (2019) 698:120–8. doi: 10.1016/j.gene.2019.02.076
55. Jiralerspong S and Goodwin PJ. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. (2016) 34:4203–16. doi: 10.1200/JCO.2016.68.4480
56. Piening A, Ebert E, Gottlieb C, Khojandi N, Kuehm LM, Hoft SG, et al. Obesity-related T cell dysfunction impairs immunosurveillance and increases cancer risk. Nat Commun. (2024) 15:2835. doi: 10.1038/s41467-024-47359-5
57. Dyck L, Prendeville H, Raverdeau M, Wilk MM, Loftus RM, Douglas A, et al. Suppressive effects of the obese tumor microenvironment on CD8 T cell infiltration and effector function. J Exp Med. (2022) 219. doi: 10.1084/jem.20210042
58. Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumor stroma promote breast cancer metastasis. Nature. (2007) 449:557–63. doi: 10.1038/nature06188
59. Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, and Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. (2007) 21:304–10. doi: 10.1038/sj.leu.2404489
60. Kang CM, Kim HK, Kim H, and Lee WJ. Cyclooxygenase-2 (COX-2) expression in solid pseudopapillary tumor of the pancreas: a pilot study. Pancreas. (2011) 40:159–61. doi: 10.1097/MPA.0b013e3181f74ca5
61. Sui H, Zhou S, Wang Y, Liu X, Zhou L, Yin P, et al. COX-2 contributes to P-glycoprotein-mediated multidrug resistance via phosphorylation of c-Jun at Ser63/73 in colorectal cancer. Carcinogenesis. (2011) 32:667–75. doi: 10.1093/carcin/bgr016
62. Day AJ and Milner CM. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. (2019) 78-79:60–83. doi: 10.1016/j.matbio.2018.01.011
63. Yang SJ, An JH, Park SM, Lee JH, Chae HK, Lee KM, et al. Enhanced expression of cyclooxygenase-2 related multi-drug resistance gene in melanoma and osteosarcoma cell lines by TSG-6 secreted from canine adipose-derived mesenchymal stem/stromal cells. Vet Med Sci. (2021) 7:968–78. doi: 10.1002/vms3.v7.3
64. Chu Y, Wang Y, Peng W, Xu L, Liu M, Li J, et al. STAT3 activation by IL-6 from adipose-derived stem cells promotes endometrial carcinoma proliferation and metastasis. Biochem Biophys Res Commun. (2018) 500:626–31. doi: 10.1016/j.bbrc.2018.04.121
65. Wei HJ, Zeng R, Lu JH, Lai WF, Chen WH, Liu HY, et al. Adipose-derived stem cells promote tumor initiation and accelerate tumor growth by interleukin-6 production. Oncotarget. (2015) 6:7713–26. doi: 10.18632/oncotarget.v6i10
66. Roodhart JM, Daenen LG, Stigter EC, Prins HJ, Gerrits J, Houthuijzen JM, et al. Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell. (2011) 20:370–83. doi: 10.1016/j.ccr.2011.08.010
67. Parvaneh S, Miklós V, Páhi ZG, Szűcs D, Monostori T, Póliska S, et al. Chemoresistance in pancreatic cancer: the role of adipose-derived mesenchymal stem cells and key resistance genes. Int J Mol Sci. (2025) 26. doi: 10.3390/ijms26010390
68. Iser IC, Ceschini SM, Onzi GR, Bertoni AP, Lenz G, and Wink MR. Conditioned medium from adipose-derived stem cells (ADSCs) promotes epithelial-to-mesenchymal-like transition (EMT-like) in glioma cells in vitro. Mol Neurobiol. (2016) 53:7184–99. doi: 10.1007/s12035-015-9585-4
69. Lazennec G and Lam PY. Recent discoveries concerning the tumor - mesenchymal stem cell interactions. Biochim Biophys Acta. (2016) 1866:290–9. doi: 10.1016/j.bbcan.2016.10.004
70. Babaei A, Yazdi AT, Ranji R, Bahadoran E, Taheri S, Nikkhahi F, et al. Therapeutic effects of exosomal miRNA-4731-5p from adipose tissue-derived stem cells on human glioblastoma cells. Arch Med Res. (2024) 55:103061. doi: 10.1016/j.arcmed.2024.103061
71. Serhal R, Saliba N, Hilal G, Moussa M, Hassan GS, El Atat O, et al. Effect of adipose-derived mesenchymal stem cells on hepatocellular carcinoma: In vitro inhibition of carcinogenesis. World J Gastroenterol. (2019) 25:567–83. doi: 10.3748/wjg.v25.i5.567
72. Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, et al. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. (2008) 18:500–7. doi: 10.1038/cr.2008.40
73. Iplik ES, Ertugrul B, Kozanoglu I, Baran Y, and Cakmakoglu B. An answer to colon cancer treatment by mesenchymal stem cell originated from adipose tissue. Iran J Basic Med Sci. (2018) 21:465–8.
74. Kao TC, Lee HH, Higuchi A, Ling QD, Yu WC, Chou YH, et al. Suppression of cancer-initiating cells and selection of adipose-derived stem cells cultured on biomaterials having. specific nanosegments. J BioMed Mater Res B Appl Biomater. (2014) 102:463–76. doi: 10.1002/jbm.b.v102.3
75. Kucerova L, Altanerova V, Matuskova M, Tyciakova S, and Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. (2007) 67:6304–13. doi: 10.1158/0008-5472.CAN-06-4024
76. Choi SA, Hwang SK, Wang KC, Cho BK, Phi JH, Lee JY, et al. Therapeutic efficacy and safety of TRAIL-producing human adipose tissue-derived mesenchymal stem cells against experimental brainstem glioma. Neuro Oncol. (2011) 13:61–9. doi: 10.1093/neuonc/noq147
77. Jung PY, Ryu H, Rhee KJ, Hwang S, Lee CG, Gwon SY, et al. Adipose tissue-derived mesenchymal stem cells cultured at high density express IFN-β and TRAIL and suppress the growth of H460 human lung cancer cells. Cancer Lett. (2019) 440-441:202–10. doi: 10.1016/j.canlet.2018.10.017
78. Babaei A, Soleimanjahi H, Soleimani M, and Arefian E. Mesenchymal stem cells loaded with oncolytic reovirus enhances antitumor activity in mice models of colorectal cancer. Biochem Pharmacol. (2021) 190:114644. doi: 10.1016/j.bcp.2021.114644
79. Xiao J, Zeng L, Ding S, Chen Y, Zhang X, Bian XW, et al. Tumor-tropic adipose-derived mesenchymal stromal cell mediated bi(2) se(3) nano-radiosensitizers delivery for targeted radiotherapy of non-small cell lung cancer. Adv Healthc Mater. (2022) 11:e2200143.
80. Wu Y, Li S, Chen Y, He W, and Guo Z. Recent advances in noble metal complex based photodynamic therapy. Chem Sci. (2022) 13:5085–106. doi: 10.1039/D1SC05478C
81. Ismail HY, Shaker NA, Hussein S, Tohamy A, Fathi M, Rizk H, et al. Cisplatin-induced azoospermia and testicular damage ameliorated by adipose-derived mesenchymal stem cells. Biol Res. (2023) 56:2. doi: 10.1186/s40659-022-00410-5
82. Jakobsen KK, Carlander AF, Grønhøj C, Todsen T, Melchiors J, Paaske N, et al. Effectiveness and safety of mesenchymal stem/stromal cell for radiation-induced hyposalivation and xerostomia in previous head and neck cancer patients (MESRIX-III): a study protocol for a single-center, double-blinded, randomized, placebo-controlled, phase II study. Trials. (2023) 24:567. doi: 10.1186/s13063-023-07594-5
83. Ueda T, Fujita A, Ogawa R, Itoh Y, Fukunaga Y, Shimada T, et al. Adipose-derived stromal cells grown on a hydroxyapatite scaffold can support hematopoiesis in regenerated bone marrow in vivo. Cell Biol Int. (2014) 38:790–8. doi: 10.1002/cbin.10254
84. Avila-Portillo LM, Aristizabal F, Riveros A, Abba MC, and Correa D. Modulation of adipose-derived mesenchymal stem/stromal cell transcriptome by G-CSF stimulation. Stem Cells Int 2020. (2020) p:5045124. doi: 10.1155/2020/5045124
85. Zhou K, Guo S, Tong S, Sun Q, Li F, Zhang X, et al. Immunosuppression of human adipose-derived stem cells on T cell subsets via the reduction of NF-kappaB activation mediated by PD-L1/PD-1 and gal-9/TIM-3 pathways. Stem Cells Dev. (2018) 27:1191–202. doi: 10.1089/scd.2018.0033
86. Zargarani S, Tavaf MJ, Soltanmohammadi A, Yazdanpanah E, Baharlou R, Yousefi B, et al. Adipose-derived mesenchymal stem cells ameliorates experimental autoimmune encephalomyelitis via modulation of Th1/Th17 and expansion of Th2/Treg responses. Cell Biol Int. (2024) 48:1124–37. doi: 10.1002/cbin.12171
87. Ghorbani F, Abbaszadeh H, Mehdizadeh A, Ebrahimi-Warkiani M, Rashidi MR, and Yousefi M. Biosensors and nanobiosensors for rapid detection of autoimmune diseases: a review. Mikrochim Acta. (2019) 186:838. doi: 10.1007/s00604-019-3844-4
88. Zharkova O, Celhar T, Cravens PD, Satterthwaite AB, Fairhurst AM, and Davis LS. Pathways leading to an immunological disease: systemic lupus erythematosus. Rheumatol (Oxford). (2017) 56:i55–66. doi: 10.1093/rheumatology/kew427
89. Álvaro-Gracia JM, Jover JA, García-Vicuña R, Carreño L, Alonso A, Marsal S, et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicenter, dose escalation, randomized, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. (2017) 76:196–202.
90. Vij R, Stebbings KA, Kim H, Park H, and Chang D. Safety and efficacy of autologous, adipose-derived mesenchymal stem cells in patients with rheumatoid arthritis: a phase I/IIa, open-label, non-randomized pilot trial. Stem Cell Res Ther. (2022) 13:88. doi: 10.1186/s13287-022-02763-w
91. Fernández O, Izquierdo G, Fernández V, Leyva L, Reyes V, Guerrero M, et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PloS One. (2018) 13:e0195891. doi: 10.1371/journal.pone.0195891
92. Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomized, double-blind controlled trial. Lancet. (2016) 388:1281–90.
93. Lightner AL, Dozois EJ, Dietz AB, Fletcher JG, Friton J, Butler G, et al. Matrix-delivered autologous mesenchymal stem cell therapy for refractory rectovaginal crohn’s fistulas. Inflammation Bowel Dis. (2020) 26:670–7. doi: 10.1093/ibd/izz215
94. de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, and Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. (2013) 28:313–23. doi: 10.1007/s00384-012-1581-9
95. Furukawa S, Mizushima T, Nakaya R, Shibata M, Yamaguchi T, Watanabe K, et al. Darvadstrocel for complex perianal fistulas in Japanese adults with crohn’s disease: A phase 3 study. J Crohns Colitis. (2023) 17:369–78. doi: 10.1093/ecco-jcc/jjac144
96. Dantas JR, Araújo DB, Silva KR, Souto DL, de Fátima Carvalho Pereira M, Luiz RR, et al. Adipose tissue-derived stromal/stem cells + cholecalciferol: a pilot study in recent-onset type 1 diabetes patients. Arch Endocrinol Metab. (2021) 65:342–51. doi: 10.20945/2359-3997000000368
97. Kim KW, Moon SJ, Park MJ, Kim BM, Kim EK, Lee SH, et al. Optimization of adipose tissue-derived mesenchymal stem cells by rapamycin in a murine model of acute graft-versus-host disease. Stem Cell Res Ther. (2015) 6:202. doi: 10.1186/s13287-015-0197-8
98. Engela AU, Hoogduijn MJ, Boer K, Litjens NH, Betjes MG, Weimar W, et al. Human adipose-tissue derived mesenchymal stem cells induce functional de-novo regulatory T cells with methylated FOXP3 gene DNA. Clin Exp Immunol. (2013) 173:343–54. doi: 10.1111/cei.12120
99. Blanco B, Herrero-Sánchez MD, Rodríguez-Serrano C, García-Martínez ML, Blanco JF, Muntión S, et al. Immunomodulatory effects of bone marrow versus adipose tissue-derived mesenchymal stromal cells on NK cells: implications in the transplantation setting. Eur J Haematol. (2016) 97:528–37. doi: 10.1111/ejh.2016.97.issue-6
100. Nishi Y, Murakami A, Murayama Y, Tsukahara N, Okamoto S, Nakachi S, et al. Adipose tissue-derived mesenchymal stem cells ameliorate bone marrow aplasia related with graft-versus-host disease in experimental murine models. Transpl Immunol. (2019) 55:101205. doi: 10.1016/j.trim.2019.03.004
101. Rusch RM, Inagaki E, Taniguchi H, Sakakura S, Tamai R, Nonaka H, et al. Adipose-derived mesenchymal stromal cells: A study on safety and efficacy in ocular inflammation. Ocul Surf. (2024) 34:523–34. doi: 10.1016/j.jtos.2024.11.001
102. Mohseni R, Mahdavi Sharif P, Behfar M, Modaresi MR, Shirzadi R, Mardani M, et al. Evaluation of safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells in pediatric bronchiolitis obliterans syndrome (BoS) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Stem Cell Res Ther. (2023) 14:256. doi: 10.1186/s13287-023-03498-y
103. Jurado M, De La Mata C, Ruiz-García A, López-Fernández E, Espinosa O, Remigia MJ, et al. Adipose tissue-derived mesenchymal stromal cells as part of therapy for chronic graft-versus-host disease: A phase I/II study. Cytotherapy. (2017) 19:927–36. doi: 10.1016/j.jcyt.2017.05.002
104. Han Y, Ren J, Bai Y, Pei X, and Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. (2019) 109:59–68. doi: 10.1016/j.biocel.2019.01.017
105. Liu L, Gao J, Yuan Y, Chang Q, Liao Y, and Lu F. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol Int. (2013) 37:551–60. doi: 10.1002/cbin.10097
106. Ding Y, Song M, Huang R, and Chen W. Adipose-mesenchymal stem cell-derived extracellular vesicles enhance angiogenesis and skin wound healing via bFGF-mediated VEGF expression. Cell Tissue Bank. (2024) 26:2. doi: 10.1007/s10561-024-10150-3
107. Li P, Cao L, Liu T, Lu X, Ma Y, and Wang H. The effect of adipose-derived stem cell (ADSC)-exos on the healing of autologous skin grafts in miniature pigs. Int J Mol Sci. (2025) 26. doi: 10.3390/ijms26020479
108. Yin X, Pang C, Bai L, Zhang Y, and Geng L. Adipose-derived stem cells promote the polarization from M1 macrophages to M2 macrophages. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. (2016) 32:332–8.
109. Mizuno H, Tobita M, and Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. (2012) 30:804–10. doi: 10.1002/stem.v30.5
110. Song Y, You Y, Xu X, Lu J, Huang X, Zhang J, et al. Adipose-derived mesenchymal stem cell-derived exosomes biopotentiated extracellular matrix hydrogels accelerate diabetic wound healing and skin regeneration. Adv Sci (Weinh). (2023) 10:e2304023. doi: 10.1002/advs.202304023
111. Zhang N, Yu X, Li W, Zhang K, Yu J, and Liu T. Extracellular vesicles derived from adipose-derived stem cells facilitate frostbite wound healing by regulating SOCS3 expression. Curr Stem Cell Res Ther. (2023) 18:528–39. doi: 10.2174/1574888X17666220715094504
112. An Y, Lin S, Tan X, Zhu S, Nie F, Zhen Y, et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. (2021) 54:e12993. doi: 10.1111/cpr.12993
113. Silveira BM, Ribeiro TO, Freitas RS, Carreira ACO, Gonçalves MS, Sogayar M, et al. Secretome from human adipose-derived mesenchymal stem cells promotes blood vessel formation and pericyte coverage in experimental skin repair. PloS One. (2022) 17:e0277863. doi: 10.1371/journal.pone.0277863
114. Abbasi-Kangevari M, Ghamari SH, Safaeinejad F, Bahrami S, and Niknejad H. Potential therapeutic features of human amniotic mesenchymal stem cells in multiple sclerosis: immunomodulation, inflammation suppression, angiogenesis promotion, oxidative stress inhibition, neurogenesis induction, MMPs regulation, and remyelination stimulation. Front Immunol. (2019) 10:238. doi: 10.3389/fimmu.2019.00238
115. Lei XX, Xu PC, Zhang L, Pang MR, Tian J, and Cheng B. Effects of human adipose-derived mesenchymal stem cells and platelet-rich plasma on healing of wounds with full-thickness skin defects in mice. Zhonghua Shao Shang Za Zhi. (2018) 34:887–94.
116. Huayllani MT, Ruiz-Garcia H, Boczar D, Avila FR, Lu X, Rinker BD, et al. Adipose-derived stem cells therapy for radiation-induced skin injury. Ann Plast Surg. (2021) 87:639–49. doi: 10.1097/SAP.0000000000003039
117. Yao C, Zhou Y, Wang H, Deng F, Chen Y, Zhu X, et al. Adipose-derived stem cells alleviate radiation-induced dermatitis by suppressing apoptosis and downregulating cathepsin F expression. Stem Cell Res Ther. (2021) 12:447. doi: 10.1186/s13287-021-02516-1
118. Zha J, Pan Y, Liu X, Zhu H, Liu Y, and Zeng W. Exosomes from hypoxia-pretreated adipose-derived stem cells attenuate ultraviolet light-induced skin injury via delivery of circ-Ash1l. Photodermatol Photoimmunol Photomed. (2023) 39:107–15. doi: 10.1111/phpp.12857
119. Liu Y, Wang Y, Yang M, Luo J, Zha J, Geng S, et al. Exosomes from hypoxic pretreated ADSCs attenuate ultraviolet light-induced skin injury via GLRX5 delivery and ferroptosis inhibition. Photochem Photobiol Sci. (2024) 23:55–63. doi: 10.1007/s43630-023-00498-y
120. Gu Y, Mu Z, Chen Y, Wu C, Shi J, and Bai N. Therapeutic potential of ADSCs in diabetic wounds: a proteomics-based approach. Front Cell Dev Biol. (2024) 12:1468220. doi: 10.3389/fcell.2024.1468220
121. Wang Z, Feng C, Liu H, Xia Y, Shan M, and Hao Y. Hypoxia-induced adipose derived stem cells-derived exosomes promote diabetic wound healing through circ-0001747/miR-199a-5p/HIF-1α axis. Arch Dermatol Res. (2025) 317:456. doi: 10.1007/s00403-025-03921-9
122. Uzun E, Güney A, Gönen ZB, Özkul Y, Kafadar İH, Günay M, et al. Intralesional allogeneic adipose-derived stem cells application in chronic diabetic foot ulcer: Phase I/2 safety study. Foot Ankle Surg. (2021) 27:636–42. doi: 10.1016/j.fas.2020.08.002
123. Carstens MH, Quintana FJ, Calderwood ST, Sevilla JP, Ríos AB, Rivera CM, et al. Treatment of chronic diabetic foot ulcers with adipose-derived stromal vascular fraction cell injections: Safety and evidence of efficacy at 1 year. Stem Cells Transl Med. (2021) 10:1138–47. doi: 10.1002/sctm.20-0497
124. Bashir MM, Sohail M, Bashir A, Khan FA, Jan SN, Imran M, et al. Outcome of conventional adipose tissue grafting for contour deformities of face and role of ex vivo expanded adipose tissue-derived stem cells in treatment of such deformities. J Craniofac Surg. (2018) 29:1143–7. doi: 10.1097/SCS.0000000000004367
125. Gentile P, Kothari A, Casella D, and Calabrese C. Fat graft enhanced with adipose-derived stem cells in aesthetic breast augmentation: clinical, histological, and instrumental evaluation. Aesthet Surg J. (2020) 40:962–77. doi: 10.1093/asj/sjz292
126. Gentile P. Breast silicone gel implants versus autologous fat grafting: biomaterials and bioactive materials in comparison. J Clin Med. (2021) 10. doi: 10.3390/jcm10153310
127. Gentile P. New strategies in plastic surgery: autologous adipose-derived mesenchymal stem cells contained in fat grafting improves symptomatic scars. Front Biosci (Landmark Ed). (2021) 26:255–7. doi: 10.52586/4940
128. Choi JW, Kim SC, Park EJ, Lee JA, and Jeong WS. Positive effect of incubated adipose-derived mesenchymal stem cells on microfat graft survival. J Craniofac Surg. (2018) 29:243–7. doi: 10.1097/SCS.0000000000004071
129. An C, Cheng Y, Yuan Q, and Li J. IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann BioMed Eng. (2010) 38:1647–54. doi: 10.1007/s10439-009-9892-x
130. Zhang X, Ma Y, Fu X, Liu Q, Shao Z, Dai L, et al. Runx2-modified adipose-derived stem cells promote tendon graft integration in anterior cruciate ligament reconstruction. Sci Rep. (2016) 6:19073. doi: 10.1038/srep19073
131. Xie Q, Xie Q, Wang Z, Zhou H, Yu Z, Huang Y, Sun H, et al. The role of miR-135-modified adipose-derived mesenchymal stem cells in bone regeneration. Biomaterials. (2016) 75:279–94. doi: 10.1016/j.biomaterials.2015.10.042
132. Tkach M and Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. (2016) 164:1226–32. doi: 10.1016/j.cell.2016.01.043
133. Zhang X, Yang M, Lin L, Chen P, Ma KT, Zhou CY, et al. Runx2 overexpression enhances osteoblastic differentiation and mineralization in adipose–derived stem cells in vitro and in vivo. Calcif Tissue Int. (2006) 79:169–78. doi: 10.1007/s00223-006-0083-6
134. González-Cubero E, González-Fernández ML, Esteban-Blanco M, Pérez-Castrillo S, Pérez-Fernández E, Navasa N, et al. The therapeutic potential of adipose-derived mesenchymal stem cell secretome in osteoarthritis: A comprehensive study. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms252011287
135. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. (2002) 13:4279–95. doi: 10.1091/mbc.e02-02-0105
136. Danieli P, Malpasso G, Ciuffreda MC, Cervio E, Calvillo L, Copes F, et al. Conditioned medium from human amniotic mesenchymal stromal cells limits infarct size and enhances angiogenesis. Stem Cells Transl Med. (2015) 4:448–58. doi: 10.5966/sctm.2014-0253
137. Liu XH, Bai CG, Xu ZY, Huang SD, Yuan Y, Gong DJ, et al. Therapeutic potential of angiogenin modified mesenchymal stem cells: angiogenin improves mesenchymal stem cells survival under hypoxia and enhances vasculogenesis in myocardial infarction. Microvasc Res. (2008) 76:23–30. doi: 10.1016/j.mvr.2008.02.005
138. Mazo M, Gavira JJ, Pelacho B, and Prosper F. Adipose-derived stem cells for myocardial infarction. J Cardiovasc Transl Res. (2011) 4:145–53. doi: 10.1007/s12265-010-9246-y
139. Wang L, Deng J, Tian W, Xiang B, Yang T, Li G, et al. Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: an MR imaging study of rat hearts. Am J Physiol Heart Circ Physiol. (2009) 297:H1020–31. doi: 10.1152/ajpheart.01082.2008
140. Ter Horst EN, Naaijkens BA, Krijnen PA, Van Der Laan AM, Piek JJ, and Niessen HW. Induction of a monocyte/macrophage phenotype switch by mesenchymal stem cells might contribute to improved infarct healing postacute myocardial infarction. Minerva Cardioangiol. (2013) 61:617–25.
141. Otto Beitnes J, Øie E, Shahdadfar A, Karlsen T, Müller RM, Aakhus S, et al. Intramyocardial injections of human mesenchymal stem cells following acute myocardial infarction modulate scar formation and improve left ventricular function. Cell Transplant. (2012) 21:1697–709. doi: 10.3727/096368911X627462
142. Mawrie D, Bhattacharjee K, Sharma A, Sharma R, Bhattacharyya J, Bhattacharjee H, et al. Human orbital adipose tissue-derived mesenchymal stem cells possess neuroectodermal differentiation and repair ability. Cell Tissue Res. (2019) 378:531–42. doi: 10.1007/s00441-019-03072-0
143. Chen B, Wang L, Pan X, Jiang S, Hu Y, et al. Adipose-derived stem cells modified by TWIST1 silencing accelerates rat sciatic nerve repair and functional recovery. Hum Cell. (2024) 37:1394–404. doi: 10.1007/s13577-024-01087-6
144. Aras Y, Sabanci PA, Kabatas S, Duruksu G, Subasi C, Erguven M, et al. The effects of adipose tissue-derived mesenchymal stem cell transplantation during the acute and subacute phases following spinal cord injury. Turk Neurosurg. (2016) 26:127–39.
145. Han C, Zhang L, Song L, Liu Y, Zou W, Piao H, et al. Human adipose-derived mesenchymal stem cells: a better cell source for nervous system regeneration. Chin Med J (Engl). (2014) 127:329–37. doi: 10.3760/cma.j.issn.0366-6999.20120064
146. Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. (2010) 20:510–8. doi: 10.1038/cr.2010.44
147. Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G, et al. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. (2012) 33:136–43. doi: 10.1016/j.it.2011.11.004
148. Avola R, Graziano ACÉ, Pannuzzo G, and Cardile V. Human mesenchymal stem cells from adipose tissue differentiated into neuronal or glial phenotype express different aquaporins. Mol Neurobiol. (2017) 54:8308–20. doi: 10.1007/s12035-016-0312-6
149. Bertani N, Malatesta P, Volpi G, Sonego P, and Perris R. Neurogenic potential of human mesenchymal stem cells revisited: analysis by immunostaining, time-lapse video and microarray. J Cell Sci. (2005) 118:3925–36. doi: 10.1242/jcs.02511
150. Gao S, Guo X, Zhao S, Jin Y, Zhou F, Yuan P, et al. Differentiation of human adipose-derived stem cells into neuron/motoneuron-like cells for cell replacement therapy of spinal cord injury. Cell Death Dis. (2019) 10:597. doi: 10.1038/s41419-019-1772-1
151. Hayat R, Manzoor M, and Hussain A. Wnt signaling pathway: A comprehensive review. Cell Biol Int. (2022) 46:863–77. doi: 10.1002/cbin.11797
152. Cerpa W, Toledo EM, Varela-Nallar L, and Inestrosa NC. The role of Wnt signaling in neuroprotection. Drug News Perspect. (2009) 22:579–91. doi: 10.1358/dnp.2009.22.10.1443391
153. Madani Neishaboori A, Eshraghi A, Tasouji Asl A, Shariatpanahi M, Yousefifard M, and Gorji A. Adipose tissue-derived stem cells as a potential candidate in treatment of Alzheimer’s disease: A systematic review on preclinical studies. Pharmacol Res Perspect. (2022) 10:e00977. doi: 10.1002/prp2.v10.4
154. Escalhão CCM, Ramos IP, Hochman-Mendez C, Brunswick THK, Souza SAL, Gutfilen B, et al. Safety of allogeneic canine adipose tissue-derived mesenchymal stem cell intraspinal transplantation in dogs with chronic spinal cord injury. Stem Cells Int 2017. (2017) p:3053759. doi: 10.1155/2017/3053759
155. An Y, Zhao J, Nie F, Qin Z, Xue H, Wang G, et al. Exosomes from adipose-derived stem cells (ADSCs) overexpressing miR-21 promote vascularization of endothelial cells. Sci Rep. (2019) 9:12861. doi: 10.1038/s41598-019-49339-y
156. Niada S, Giannasi C, Magagnotti C, Andolfo A, and Brini AT. Proteomic analysis of extracellular vesicles and conditioned medium from human adipose-derived stem/stromal cells and dermal fibroblasts. J Proteomics. (2021) 232:104069. doi: 10.1016/j.jprot.2020.104069
157. Guo S, Wang T, Zhang S, Chen P, Cao Z, Lian W, et al. Adipose-derived stem cell-conditioned medium protects fibroblasts at different senescent degrees from UVB irradiation damages. Mol Cell Biochem. (2020) 463:67–78. doi: 10.1007/s11010-019-03630-8
158. Park BS, Kim WS, Choi JS, Kim HK, Won JH, Ohkubo F, et al. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. BioMed Res. (2010) 31:27–34. doi: 10.2220/biomedres.31.27
159. Rostami M, Haidari K, and Shahbazi M. Genetically engineered adipose mesenchymal stem cells using HIV-based lentiviral vectors as gene therapy for autoimmune diseases. Cell Reprogram. (2018) 20:337–46. doi: 10.1089/cell.2018.0006
160. Yang M, Sun JY, Ying CC, Wang Y, and Guo YL. Adipose-derived stem cells modified by BDNF gene rescue erectile dysfunction after cavernous nerve injury. Neural Regener Res. (2020) 15:120–7. doi: 10.4103/1673-5374.264464
161. Li J, Deng T, Zhu S, Xie P, Wang W, Zhou H, et al. The SDF-1/CXCR4 axis is involved in adipose-derived stem cell migration. Neurourol Urodyn. (2024) 43:2279–89. doi: 10.1002/nau.25571
162. Kim M, Kim DI, Kim EK, and Kim CW. CXCR4 overexpression in human adipose tissue-derived stem cells improves homing and engraftment in an animal limb ischemia model. Cell Transplant. (2017) 26:191–204. doi: 10.3727/096368916X692708
163. Oraee-Yazdani S, Tavanaei R, Rostami F, Hajarizadeh A, Mehrabadi M, Akhlaghpasand M, et al. Suicide gene therapy using allogeneic adipose tissue-derived mesenchymal stem cell gene delivery vehicles in recurrent glioblastoma multiforme: a first-in-human, dose-escalation, phase I clinical trial. J Transl Med. (2023) 21:350. doi: 10.1186/s12967-023-04213-4
164. Zubkova ES, Beloglazova IB, Ratner EI, Dyikanov DT, Dergilev KV, Menshikov MY, et al. Transduction of rat and human adipose-tissue derived mesenchymal stromal cells by adeno-associated viral vector serotype DJ. Biol Open. (2021) 10. doi: 10.1242/bio.058461
165. Vilalta M, Dégano IR, Bagó J, Aguilar E, Gambhir SS, Rubio N, et al. Human adipose tissue-derived mesenchymal stromal cells as vehicles for tumor bystander effect: a model based on bioluminescence imaging. Gene Ther. (2009) 16:547–57. doi: 10.1038/gt.2008.176
166. Li B, Zhang Y, Li M, Zhao X, Xie H, Guo X, et al. Genetic correction of adipose tissue-derived mesenchymal stem cells mediated by TALEN targeting the GDF5 gene. Int J Mol Med. (2018) 41:2397–405. doi: 10.3892/ijmm.2018.3442
167. Liu Y, Wang Z, Ju M, Zhao Y, Jing Y, Li J, et al. Modification of COL1A1 in autologous adipose tissue-derived progenitor cells rescues the bone phenotype in a mouse model of osteogenesis imperfecta. J Bone Miner Res. (2021) 36:1521–34. doi: 10.1002/jbmr.4326
168. Fenger Carlander AL, Jakobsen KK, Todsen T, Paaske N, Østergaard Madsen AK, Bendtsen SK, et al. Long-term effectiveness and safety of mesenchymal stromal cell therapy for radiation-induced hyposalivation in head and neck cancer survivors: A randomized phase II trial. Clin Cancer Res. (2025) 31:824–31. doi: 10.1158/1078-0432.CCR-24-2663
169. Kim KI, Lee MC, Lee JH, Moon YW, Lee WS, Lee HJ, et al. Clinical efficacy and safety of the intra-articular injection of autologous adipose-derived mesenchymal stem cells for knee osteoarthritis: A phase III, randomized, double-blind, placebo-controlled trial. Am J Sports Med. (2023) 51:2243–53. doi: 10.1177/03635465231179223
170. Kawamura T, Yoshioka D, Kawamura A, Misumi Y, Taguchi T, Mori D, et al. Safety and therapeutic potential of allogeneic adipose-derived stem cell spray transplantation in ischemic cardiomyopathy: a phase I clinical trial. J Transl Med. (2024) 22:1091. doi: 10.1186/s12967-024-05816-1
171. de Celis-Ruiz E, Fuentes B, Alonso de Leciñana M, Gutiérrez-Fernández M, Borobia AM, Gutiérrez-Zúñiga R, et al. Final results of allogeneic adipose tissue-derived mesenchymal stem cells in acute ischemic stroke (AMASCIS): A phase II, randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. Cell Transplant. (2022) 31:9636897221083863. doi: 10.1177/09636897221083863
172. Lopez-Yus M, Frendo-Cumbo S, Del Moral-Bergos R, Garcia-Sobreviela MP, Bernal-Monterde V, Rydén M, et al. CRISPR/Cas9-mediated deletion of adipocyte genes associated with NAFLD alters adipocyte lipid handling and reduces steatosis in hepatocytes in vitro. Am J Physiol Cell Physiol. (2023) 325:C1178–c1189. doi: 10.1152/ajpcell.00291.2023
Keywords: adipose-derived mesenchymal stem cells (ADSCs), metabolic regulation, tumor microenvironment (TME), metabolic syndrome, immunomodulation, drug delivery systems
Citation: Wang L, Jiang X, Zhao F, Duan P, Li Z and Luo Y (2025) A review of adipose-derived mesenchymal stem cells‘ impacts and challenges: metabolic regulation, tumor modulation, immunomodulation, regenerative medicine and genetic engineering therapies. Front. Endocrinol. 16:1606847. doi: 10.3389/fendo.2025.1606847
Received: 06 April 2025; Accepted: 12 May 2025;
Published: 29 May 2025.
Edited by:
Antonino Belfiore, University of Catania, ItalyReviewed by:
Ayşegül Doğan, Yeditepe University, TürkiyeLi Li, LMU Munich University Hospital, Germany
Chunmin Li, Shanghai General Hospital, China
Copyright © 2025 Wang, Jiang, Zhao, Duan, Li and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiqiao Luo, eWlxaWFvbHVvQHdjaHNjdS5jbg==
 Lihong Wang
Lihong Wang Xiaorui Jiang1
Xiaorui Jiang1 Zhengguang Li
Zhengguang Li Yiqiao Luo
Yiqiao Luo