- 1Department of General Surgery, Affiliated Hospital of Hebei University, Baoding, Hebei, China
- 2Basic Research Key Laboratory of General Surgery for Digital Medicine, Affiliated Hospital of Hebei University, Baoding, Hebei, China
- 3Department of Ophthalmology, Baoding No.1 Central Hospital, Baoding, Hebei, China
Objective: To synthesize evidence on Horner syndrome (HS) as a complication of ultrasound-guided ablation therapy for thyroid nodules, including its incidence, mechanisms, risk factors, and prevention strategies, to enhance ablation safety and guide future research.
Data sources: Web of Science, PubMed, Cochrane Library, and Embase.
Review methods: Based on the framework of the PRISMA-ScR, a search was conducted in databases up to December 31, 2024.
Results: Twelve articles were included, covering Microwave Ablation (MWA), Radiofrequency Ablation (RFA), High-Intensity Focused Ultrasound (HIFU), and Percutaneous Ethanol Injection (PEI). HS incidence rates varied: MWA 0.4%-4.2%, RFA 0.1%-1.5%, HIFU 1.5%-6.7%, with PEI incidence unspecified due to insufficient data. HS mechanisms included thermal injury to the cervical sympathetic chain, nerve damage from ethanol extravasation, and mechanical compression. Risk factors included ablation zones adjacent to the middle cervical ganglion (MCG), improper ablation parameter settings (such as excessively high power or prolonged duration), and nodule locations near the inferior thyroid artery. Prevention strategies emphasized precise preoperative ultrasound localization of the CSC and MCG, optimization of the isolation belt technique, timely adjustment of ablation parameters, real-time monitoring of symptoms, and avoiding the ablation probe tip from extending beyond the nodule edge.
Conclusion: HS is a rare but serious complication with varying incidence rates by technique. Risk can be reduced through precise assessment, meticulous techniques, and technological innovations. Future prospective studies are needed to clarify incidence rates, long-term prognosis, and refine clinical practice guidelines.
1 Introduction
Ultrasound-guided thyroid ablation therapy is a non-surgical treatment modality that achieves in situ elimination or volume reduction of thyroid nodules and metastatic lymph nodes by inducing coagulative necrosis through physical or chemical methods, followed by subsequent absorption by the body (1). The primary techniques include thermal ablation modalities such as Microwave Ablation (MWA), Radiofrequency Ablation (RFA), High-intensity Focused Ultrasound (HIFU), and Laser Ablation (LA), as well as chemical ablation via Percutaneous Ethanol Injection/Percutaneous Lauromacrogol Injection (PEI/PLI) (2). This approach offers multiple clinical advantages, including scar-free intervention, high precision, short procedural duration, confirmed therapeutic efficacy, minimal complications, and optimal preservation of thyroid function (2).
However, the complex cervical anatomy poses inherent risks, particularly potential injury to the cervical sympathetic chain (CSC) during ablation procedures, which may lead to Horner Syndrome (HS) (3). Current major clinical guidelines and consensus statements on ablation therapy make no mention of this complication (2, 4, 5), though the Society of Interventional Radiology categorizes HS as a severe complication in their clinical practice guidelines (6). While the reported incidence of post-ablation HS remains low (fewer than 20 documented cases to date), its occurrence can significantly impact patients’ quality of life and psychological well-being. This review, for the first time, comprehensively integrates existing evidence to elucidate the incidence rate, pathomechanisms, risk factors, and preventive strategies for HS, thereby enhancing the safety profile of ultrasound-guided ablation therapy. Furthermore, it explores future research directions to optimize clinical implementation of this technology.
2 Materials and methods
Given limitations in the quantity and quality of available literature, methodological heterogeneity, and challenges in data extraction, a systematic review or meta-analysis was deemed unfeasible. Consequently, this study adopted the PRISMA extension for Scoping Reviews (PRISMA-ScR) as the methodological framework (7). Through comprehensive literature review and structured group discussions, the following key research questions were formulated: (1) Incidence rates of Horner Syndrome (HS) associated with different ablation techniques. (2) Pathomechanisms underlying HS development post-ablation. (3) Identifiable risk factors contributing to HS occurrence. (4) Evidence-based strategies for HS prevention.
2.1 Literature search strategy
A comprehensive computerized search was conducted across multiple databases, including Web of Science, PubMed, Cochrane Library, and Embase, covering records from their inception to December 31, 2024. The search strategy incorporated a combination of controlled vocabulary (e.g., MeSH terms) and free-text terms to maximize sensitivity. Key search terms included: (thyroid) AND (Horner Syndrome) AND (ablation therapy OR microwave ablation OR radiofrequency ablation OR high-intensity focused ultrasound OR laser ablation OR percutaneous ethanol injection OR percutaneous lauromacrogol injection). To ensure exhaustive coverage, a snowballing method was implemented by manually reviewing reference lists of included studies to identify additional relevant publications.
2.2 Inclusion and exclusion criteria
Inclusion Criteria: (1) Studies reporting ultrasound-guided thyroid ablation therapy using any of the following techniques: MWA, RFA, HIFU, LA, PEI or PLI. (2) Documented occurrence of HS as a post-procedural complication.
Exclusion Criteria: (1) Non-primary research literature, including dissertations/theses, conference abstracts, review articles, or meta-analyses. (2) Cases with pre-existing HS prior to ablation therapy. (3) Studies failing to report HS-related outcomes. (4) Animal studies or in vitro experiments. (5) Duplicate publications or studies with inaccessible full texts.
2.3 Article selection process
The study selection process was independently conducted by two researchers. First, retrieved citations were organized and duplicates were removed using EndNote software (Clarivate Analytics). Initial screening was performed by reviewing titles and abstracts against predefined inclusion and exclusion criteria. Subsequently, full-text articles of potentially eligible studies were assessed for final eligibility. Following independent evaluations, the researchers cross-checked their selection results. Any discrepancies were resolved through consultation with a senior investigator to achieve consensus. A detailed flowchart of the selection process is provided in Figure 1.
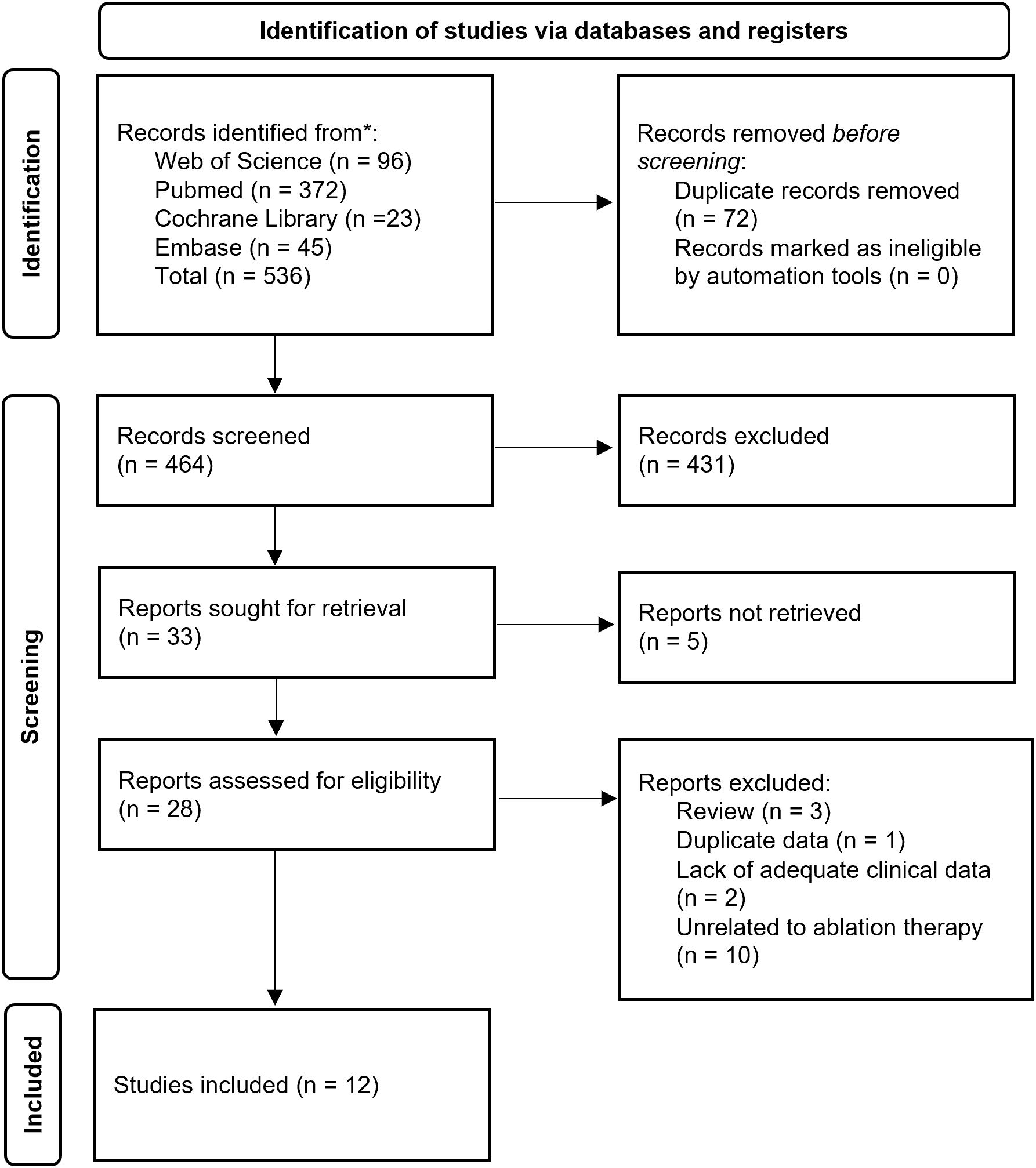
Figure 1. Preferred Reporting Items for Scoping Reviews diagram resembling electronic database search and inclusion/exclusion process of the review. *Date of last search December 31, 2024.
3 Results
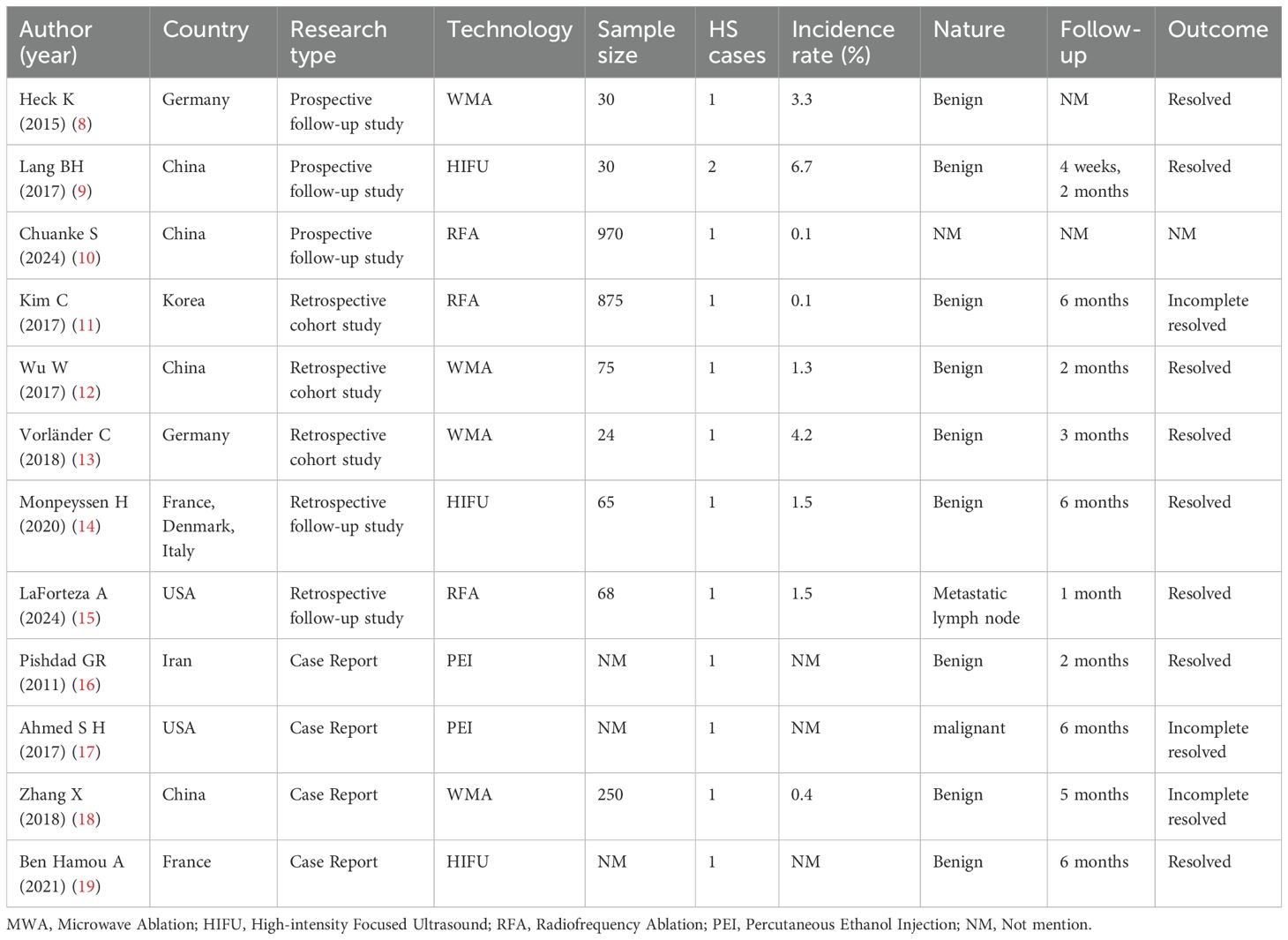
A total of 12 studies (8–19) were included, comprising 3 prospective studies (8–10), 5 retrospective studies (11–15), and 4 case reports (16–19), collectively documenting 13 cases of HS following ultrasound-guided thyroid ablation. All studies were published in English. The distribution of ablation techniques was as follows: MWA accounted for 4 studies (8, 12, 13, 18), RFA for 3 studies (10, 11, 15), HIFU for 3 studies (9, 14, 19), and PEI for 2 studies (16, 17). Notably, no cases associated with LA or PLI were identified. The baseline characteristics of the included studies, including patient demographics, ablation technology, and clinical outcomes, are comprehensively summarized in Table 1. The methodological quality of the included studies was evaluated using the JBI Scoping Review Appraisal Tool (20). The results indicated that five retrospective studies carried a risk of selection bias (e.g., non-random grouping), three prospective studies might have compromised statistical power due to small sample sizes, and case reports were constrained by single-center designs.
3.1 MWA
The four studies investigating MWA-related HS reported the following outcomes: (1) A prospective follow-up study (8) conducted at a single center evaluated MWA for benign thyroid nodules. Among 30 treated patients, 1 case of HS (incidence rate: 3.3%) was observed, with symptoms resolving completely during follow-up. (2) A retrospective case-control study (12) involving 75 patients undergoing MWA for benign thyroid nodules identified 1 HS case (incidence: 1.3%), with full symptom resolution occurring within 2 months. (3) A retrospective cohort study (13) comparing MWA and radiofrequency ablation (RFA) for benign thyroid nodules reported 1 HS case among 24 MWA-treated patients (incidence: 4.2%), demonstrating complete symptom resolution by 3 months post-procedure. (4) A case report (18) documented 1 HS occurrence (incidence: 0.4%) in a cohort of 75 MWA-treated patients with benign nodules, noting partial symptom alleviation at 5-month follow-up.
3.2 RFA
The three studies examining RFA-related HS reported the following findings: (1) A prospective follow-up study (10) involving 970 patients undergoing RFA for thyroid nodules documented 1 HS case (incidence: 0.1%), though recovery status was not specified. (2) A retrospective cohort study (11) comparing RFA outcomes in benign thyroid nodules versus recurrent thyroid carcinoma (total cohort: 875 patients) identified 1 HS case (incidence: 0.1%), with partial symptom resolution observed at 6-month follow-up. (3) A retrospective follow-up study (15) evaluating 68 patients receiving RFA for metastatic lymph nodes in thyroid cancer reported 1 HS occurrence (incidence: 1.5%), demonstrating complete symptom resolution within 1 month post-procedure.
3.3 HIFU
The three studies investigating HIFU-related HS reported the following outcomes: (1) A prospective follow-up study (9) conducted at a single center evaluated HIFU for refractory Graves’ disease. Among 30 treated patients, 2 cases of HS (incidence: 6.7%) were observed, with complete symptom resolution occurring at 4 weeks and 2 months post-procedure, respectively. (2) A multicenter retrospective follow-up study (14) assessing HIFU for benign thyroid nodules identified 1 HS case (incidence: 1.5%) in a cohort of 65 patients, demonstrating full symptom resolution by 6-month follow-up. (3) A case report (19) documented HS development in a patient undergoing HIFU for a benign thyroid nodule, with complete symptom resolution achieved 6 months post-treatment.
3.4 PEI
The two case reports detailing PEI-associated HS are summarized as follows: (1) A case report (16) documented HS in a patient following PEI treatment for metastatic papillary thyroid carcinoma, with partial symptom resolution observed during follow-up. (2) Another case report (17) described HS development after PEI administration targeting level III metastatic lymph nodes, demonstrating partial symptom resolution at 6-month follow-up.
4 Discussion
HS, first described in 1869 by Swiss ophthalmologist Johann Friedrich Horner (21), is a clinical triad caused by disruption of the oculosympathetic pathway (OSP). The classic triad comprises ipsilateral ptosis, miosis, and facial anhidrosis. The OSP originates in the hypothalamus and traverses a three-neuron chain to innervate ocular structures, regulating pupillary dilation, eyelid elevation, and ocular motility (3). Of particular relevance to iatrogenic injury is the second-order neuron of this pathway, which constitutes the CSC (3).
Anatomically, the CSC lies superficial to the longus colli muscles, lateral to the vertebral bodies, and deep to the prevertebral fascia. It comprises the superior, middle, and inferior cervical sympathetic ganglia interconnected by nerve fibers. Direct injury to the CSC during thyroid surgery has been documented as a cause of HS (22). While ultrasound-guided ablation therapy demonstrates significant promise for thyroid nodule management, its invasive nature necessitates careful consideration of complications. Although rare, HS has emerged as a clinically significant complication warranting heightened vigilance in interventional practice.
4.1 Incidence of HS
As of the search cutoff date, only 13 cases of HS following ablation therapy have been reported. The incidence rates ranged from 0.4% to 4.2% for MWA, 0.1% to 1.5% for RFA, and 1.5% to 6.67% for HIFU. The two case reports on PEI did not specify cohort sizes, precluding calculation of HS incidence for this modality. However, a retrospective cohort study (full text unavailable) (23) mentioned 1 HS case among 250 PEI-treated patients (incidence: 0.4%). Due to the limited number of reported cases and the absence of high-quality randomized controlled trial, current estimates of HS incidence may be subject to inaccuracies or selection bias. Future prospective, large-scale studies are required to establish more precise epidemiological profiles of this complication.
4.2 Pathogenetic mechanisms
Thermal ablation techniques share a common mechanism of inducing tumor cell death through protein denaturation and coagulative necrosis via hyperthermia (24). Elevating tissue temperature to 41°C may increase blood flow and enhance ion diffusion across cell membranes (25). Within the hyperthermic range (41°C–48°C), cellular sensitivity to thermal injury intensifies (26), leading to protein unfolding/aggregation and impaired DNA damage repair (27). Irreversible cellular damage at these temperatures typically requires 30 minutes to 1 hour of exposure (28). At higher temperatures (48–60°C), severe protein denaturation occurs (25), with irreversible thermal injury achievable within seconds. Temperatures exceeding 60°C cause near-instantaneous protein denaturation and coagulative necrosis (28). Thermal energy propagation to the CSC during ablation may induce such neurothermal damage (8–12, 14, 18).
Ethanol exerts its ablative effects through tissue dehydration, protein degradation, and thromboembolic effects, which may inadvertently damage adjacent neural structures (29).
Secondary mechanisms include: (1) Mechanical injury: Post-procedural edema or inflammatory responses may compress or traction the CSC, impairing neural conduction (8, 18). (2) Vascular compromise: Ablation-induced vascular injury may disrupt CSC blood supply, resulting in ischemic neuropathy and functional loss (18, 19).
4.3 Risk factors
Ablation of nodules or lesions adjacent to the CSC, particularly the MCG, warrants heightened caution (9, 12, 14, 19). The MCG, the smallest ganglion within the CSC, exhibits an ultrasound visualization rate of approximately 50.4%. Anatomically, it is typically located posterior to the carotid sheath and anterior to the longus colli muscles at the C3–C7 vertebral levels, with mean dimensions of 3.8–6.3 mm in width, 1.7–2.1 mm in height, and 6.3 ± 10.5 mm in length (30). HS risk escalates when ablation probe tip approaches the MCG (8, 14) or when the MCG is erroneously targeted during ablation (11). Additionally, large tumor volumes, particularly benign nodules encasing the carotid sheath, increase susceptibility to MCG injury (11). Notably, the MCG resides in proximity to the inferior thyroid artery, whose branches may supply blood to the ganglion (31). Consequently, ablation near the inferior thyroid artery risks compromising the MCG’s vascular supply, potentially inducing neural ischemia.
Carlander et al. (32) demonstrated in a rat model that localized energy delivery from ultrasound devices can induce neural dysfunction, with the extent of nerve injury dependent on thermal exposure duration. Consequently, prolonged ablation duration, higher power settings, or excessive ablation at a single site may amplify thermal accumulation within the ablation zone, thereby increasing the risk of collateral thermal damage to adjacent tissues (13, 14, 19). Tumor tissues, characterized by lower thermal conductivity and impaired heat dissipation, are prone to localized hyperthermia during ablation, leading to necrotic cell death due to their heightened thermosensitivity. In contrast, normal tissues exhibit superior heat dispersion capabilities owing to higher thermal conductivity (24). When the ablation probe tip extends beyond the thyroid nodule margin into surrounding soft tissues, rapid thermal conduction to adjacent structures may occur, potentially damaging the CSC or MCG (8, 13).
The heat sink effect—the phenomenon whereby heat is absorbed and dissipated by highly conductive structures (e.g., blood vessels, airways)—varies across ablation modalities. MWA exhibits less susceptibility to this effect compared to RFA (33), resulting in a more confined thermal field and consequently higher risks to periprocedural tissues in RFA (13). The differences in the risk of inducing HS among different ablation techniques may be attributed to their thermal field distribution characteristics, and further verification is needed regarding the impact of heat conduction patterns on neural injury.
During PEI, ethanol may diffuse into cervical tissues via the needle tract, exerting cytotoxic effects that can induce HS, recurrent laryngeal nerve palsy, and cervical pain (34). In one case report (16), post-PEI cervical ecchymosis—a direct indicator of ethanol extravasation—provided clinical evidence supporting the hypothesis that ethanol leakage contributes to neural injury. The incidence of HS following PEI cannot be accurately estimated due to the lack of large-sample studies. Existing cases suggest that ethanol extravasation may trigger HS through neurotoxic effects, and further research is needed to investigate its dose-effect relationship.
Furthermore, while swallowing facilitates intraprocedural nodule visualization, involuntary swallowing or neck movement during ablation may displace the ablation probe tip, compromising procedural accuracy and safety (19). Anatomical variations also pose risks: communicating branches between the recurrent laryngeal nerve and sympathetic trunk exist in some individuals, and injury to these anastomotic fibers or the recurrent laryngeal nerve itself may disrupt CSC conduction (12, 35).
4.4 Preventive strategies
For thermal ablation procedures, meticulous selection of device parameters and treatment zones is critical to avoid complications such as HS (14). Preprocedural evaluation should include detailed assessment of the spatial relationship between thyroid nodules and the MCG (8, 9, 13, 19). Real-time ultrasound-guided localization of the MCG and preoperative mapping of the CSC trajectory are essential to minimize intraprocedural neural injury (8, 11, 13).
Technical parameters and ablation strategies should be optimized based on nodule size and location to mitigate risks to adjacent critical structures (11, 13). The “moving shot technique”—a dynamic ablation approach involving sequential probe repositioning—can prevent excessive thermal accumulation at a single site (12, 13). For nodules in proximity to neural structures, incomplete ablation may be considered, prioritizing neural preservation over maximal volume reduction (8, 12).
The creation of a hydrodissection-induced fluid barrier between the tumor and critical neural structures (8, 12, 13, 19) can mitigate thermal injury risks by maintaining perineural temperatures below 45°C (distinct from the ablation zone temperature) (36). Rational application of this isolation technique not only prevents unintended thermal diffusion and minimizes postprocedural adhesion risks but also expands interfascial spaces to enhance procedural maneuverability (37).
During ablation, precise probe positioning must ensure the ablation probe tip remains entirely within the nodule, avoiding protrusion beyond the lesion margins (11, 13). Advanced probe designs incorporating distal choke collars or integrated cooling systems may further reduce thermal dispersion and heat sink effects, thereby lowering complication rates (38).
Continuous intraprocedural monitoring for early signs of HS, particularly ocular or conjunctival discomfort/pain, is critical. Such symptoms may serve as early predictive indicators, facilitating real-time identification of “danger zones,” especially those adjacent to the CSC (19). Employing general anesthesia (rather than local anesthesia) enables intraoperative assessment of eyelid ptosis—a key diagnostic marker of HS—allowing immediate procedural adjustments (18).
Preprocedural patient education must emphasize detailed disclosure of potential complications, including HS, with explicit documentation of informed consent obtained prior to intervention (11, 13).
4.5 Future directions
Based on the existing evidence, future research should focus on the following directions: (1) Establishing a risk prediction model for HS, integrating ultrasonic anatomical parameters with ablation energy settings; (2) Evaluating the neuroprotective effects of novel cooled probes; (3) Conduct long-term follow-up to clarify the impact of HS on the ablation population.
Emerging technologies hold transformative potential for optimizing ablation safety and efficacy. Augmented reality systems, which convert 2D imaging into 3D holographic reconstructions, may enable precise preoperative mapping of thyroid anatomy (including tumors, vasculature, and neural structures) through the integration of artificial intelligence-driven segmentation and 3D-printed anatomical models, thereby enhancing preprocedural planning and real-time intraoperative navigation (39–41).
Further innovation lies in combining fluid barrier techniques with contrast-enhanced lymphography to dynamically track lymphatic drainage patterns. This hybrid approach could improve metastatic lymph node detection accuracy and facilitate the development of standardized protocols for pre-ablation assessment and management of cervical lymph node metastases (42).
Blood perfusion rate significantly influences ablation efficacy. Systematic investigation of perfusion heterogeneity across thyroid nodule subtypes could enable personalized parameter optimization (e.g., power output, duration) to achieve optimal therapeutic outcomes (43). Development of predictive models for estimating post-ablation volume reduction ratios would support clinicians in formulating tailored ablation strategies and providing evidence-based prognostic counseling to patients (44, 45).
Advanced multi-layer thermal monitoring systems capable of real-time temperature mapping across tissue layers, anatomical regions, and adjacent organs are urgently needed. Concurrent innovation in thermoprotective materials and precision insulation technologies may further enhance procedural safety by containing thermal spread within predefined ablation zones (43).
5 Conclusions
Although HS following thyroid ablation exhibits a relatively low incidence rate, its potential impact on patient quality of life necessitates heightened clinical vigilance. The risk of HS can be effectively mitigated through optimized ablation techniques, enhanced preoperative anatomical mapping, and real-time intraoperative monitoring of critical neural structures. These refinements further improve the safety and efficacy profiles of ultrasound-guided percutaneous ablation therapy for thyroid nodules. Future high-quality prospective studies are imperative to establish precise epidemiological data on HS incidence, characterize long-term neurological sequelae, and validate risk stratification protocols. Such evidence will strengthen evidence-based clinical guidelines and advance personalized therapeutic strategies in interventional thyroidology.
Author contributions
TX: Writing – original draft, Writing – review & editing, Methodology, Project administration, Conceptualization. YF: Writing – review & editing, Writing – original draft, Conceptualization, Data curation. XJ: Formal Analysis, Writing – original draft, Data curation. XR: Data curation, Writing – original draft. JZ: Writing – original draft, Data curation. QS: Supervision, Writing – review & editing, Conceptualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Baldwin CK, Natter MB, Patel KN, and Hodak SP. Minimally invasive techniques for the management of thyroid nodules. Endocrinol Metab Clin North Am. (2022) 51:323–49. doi: 10.1016/j.ecl.2022.01.001
2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
3. Martin TJ. Horner syndrome: A clinical review. ACS Chem Neurosci. (2018) 9:177–86. doi: 10.1021/acschemneuro.7b00405
4. Papini E, Monpeyssen H, Frasoldati A, and Hegedüs L. European thyroid association clinical practice guideline for the use of image-Guided ablation in benign thyroid nodules. Eur Thyroid J. (2020) 9:172–85. doi: 10.1159/000508484
5. Society of Tumor Ablation Therapy of the Chinese Anti-Cancer Association, Ablation Expert Committee of the Chinese Society of Clinical Oncology (CSCO), Chinese Medical Doctor Association College of Interventionalists Tumor Ablation Committee, and Chinese Bethune Spirit Research Association Endocrinology and Diabetes Branch Interventional Endocrine Committee. Expert consensus on thermal ablation of papillary thyroid cancer (2024 edition). Zhonghua Nei Ke Za Zhi. (2024) 63:355–64. doi: 10.3760/cma.j.cn112138-20231104-00296
6. Cardella JF, Kundu S, Miller DL, Millward SF, and Sacks D. Society of Interventional Radiology. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. (2009) 20:S189–191. doi: 10.1016/j.jvir.2009.04.035
7. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-scR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
8. Heck K, Happel C, Grünwald F, and Korkusuz H. Percutaneous microwave ablation of thyroid nodules: effects on thyroid function and antibodies. Int J Hyperthermia. (2015) 31:560–7. doi: 10.3109/02656736.2015.1032371
9. Lang BH, Woo YC, Wong IY, and Chiu KW. Single-session high-intensity focused ultrasound treatment for persistent or relapsed graves disease: preliminary experience in a prospective study. Radiology. (2017) 285:1011–22. doi: 10.1148/radiol.2017162776
10. Chuanke S, Ming L, Zhideng Y, and Huan L. A 6-year single-center prospective follow-up study of the efficacy of radiofrequency ablation for thyroid nodules. Front Endocrinol (Lausanne). (2024) 15:1402380. doi: 10.3389/fendo.2024.1402380
11. Kim C, Lee JH, Choi YJ, Kim WB, Sung TY, and Baek JH. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. (2017) 27:3128–37. doi: 10.1007/s00330-016-4690-y
12. Wu W, Gong X, Zhou Q, Chen X, and Chen X. Ultrasound-guided percutaneous microwave ablation for solid benign thyroid nodules: comparison of MWA versus control group. Int J Endocrinol. (2017) 2017:9724090. doi: 10.1155/2017/9724090
13. Vorländer C, David Kohlhase K, Korkusuz Y, Erbelding C, Luboldt W, Baser I, et al. Comparison between microwave ablation and bipolar radiofrequency ablation in benign thyroid nodules: differences in energy transmission, duration of application and applied shots. Int J Hyperthermia. (2018) 35:216–25. doi: 10.1080/02656736.2018.1489984
14. Monpeyssen H, Ben Hamou A, Hegedüs LG, Ghanassia É, Juttet P, Persichetti A, et al. High-intensity focused ultrasound (HIFU) therapy for benign thyroid nodules: a 3-year retrospective multicenter follow-up study. Int J Hyperthermia. (2020) 37:1301–9. doi: 10.1080/02656736.2020.1846795
15. LaForteza A, Persons E, Hussein M, Luo X, Issa PP, Jishu J, et al. Treatment outcomes in patients with papillary thyroid cancer undergoing radiofrequency ablation of metastatic lymph nodes. Gland Surg. (2024) 13:1752–8. doi: 10.21037/gs-24-285
16. Pishdad GR, Pishdad P, and Pishdad R. Horner’s syndrome as a complication of percutaneous ethanol treatment of thyroid nodule. Thyroid. (2011) 21:327–8. doi: 10.1089/thy.2010.0386
17. Ahmed SH, Sanfield JA, and Freitas JE. Horner syndrome after percutaneous ethanol injection for treatment of metastatic papillary thyroid carcinoma: case report and review of the literature. AACE Clin Case Rep. (2017) 3:e140–3. doi: 10.4158/EP161336.CR
18. Zhang X, Ge Y, Ren P, Liu J, and Chen G. Horner syndrome as a complication after thyroid microwave ablation: Case report and brief literature review. Med (Baltimore). (2018) 97:e11884. doi: 10.1097/MD.0000000000011884
19. Ben Hamou A and Monpeyssen H. Horner’s syndrome during high-intensity focused ultrasound ablation for a benign thyroid nodule. AACE Clin Case Rep. (2021) 7:164–8. doi: 10.1016/j.aace.2020.11.035
20. Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Implement. (2021) 19:3–10. doi: 10.1097/XEB.0000000000000277
21. Fustes OJH, Kay CSK, Lorenzoni PJ, Ducci RD, Werneck LC, and Scola RH. Horner syndrome: tribute to Professor Horner on his 190th birthday. Arq Neuropsiquiatr. (2021) 79:647–9. doi: 10.1590/0004-282X-ANP-2020-0483
22. Smith I and Murley RS. Damage to the cervical sympathetic system during operations on the thyroid gland. Br J Surg. (1965) 52:673–5. doi: 10.1002/bjs.1800520909
23. Bartos M, Pomorski L, and Narebski J. The treatment of solitary thyroid nodules in non-toxic goiter with 96% ethanol injections. Wiad Lek. (1999) 52:432–40.
24. Bianchi L, Cavarzan F, Ciampitti L, Cremonesi M, Grilli F, and Saccomandi P. Thermophysical and mechanical properties of biological tissues as a function of temperature: a systematic literature review. Int J Hyperthermia. (2022) 39:297–340. doi: 10.1080/02656736.2022.2028908
25. Jaque D, Martínez Maestro L, del Rosal B, Haro-Gonzalez P, Benayas A, Plaza JL, et al. Nanoparticles for photothermal therapies. Nanoscale. (2014) 6:9494–530.
26. Behrouzkia Z, Joveini Z, Keshavarzi B, Eyvazzadeh N, and Aghdam RZ. Hyperthermia: how can it be used? Oman Med J. (2016) 31:89–97. doi: 10.5001/omj.2016.19
27. Kok HP, Cressman ENK, Ceelen W, Brace CL, Ivkov R, Grüll H, et al. Heating technology for Malignant tumors: a review. Int J Hyperthermia. (2020) 37:711–41. doi: 10.1080/02656736.2020.1779357
28. Chu KF and Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. (2014) 14:199–208. doi: 10.1038/nrc3672
29. Shiina S, Tagawa K, Niwa Y, Unuma T, Komatsu Y, Yoshiura K, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients[J. AJR Am J Roentgenol. (1993) 160:1023–8. doi: 10.2214/ajr.160.5.7682378
30. Park C, Suh CH, Shin JE, and Baek JH. Characteristics of the middle cervical sympathetic ganglion: A systematic review and meta-analysis. Pain Physician. (2018) 21:9–18.
31. Solomon P, Irish J, and Gullane P. Horner’s syndrome following a thyroidectomy. J Otolaryngol. (1993) 22:454–6.
32. Carlander J, Johansson K, Lindström S, Velin AK, Jiang CH, and Nordborg C. Comparison of experimental nerve injury caused by ultrasonically activated scalpel and electrosurgery. Br J Surg. (2005) 92:772–7. doi: 10.1002/bjs.4948
33. Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev BioMed Eng. (2010) 38:65–78. doi: 10.1615/critrevbiomedeng.v38.i1.60
34. Gharib H, Hegedüs L, Pacella CM, Baek JH, and Papini E. Clinical review: Nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab. (2013) 98:3949–57. doi: 10.1210/jc.2013-1806
35. Reeve TS, Coupland GA, Johnson DC, and Buddee FW. The recurrent and external laryngeal nerves in thyroidectomy. Med J Aust. (1969) 1:380–2. doi: 10.5694/j.1326-5377.1969.tb92166.x
36. Sag AA and Husain AM. Thermal protection strategies and neuromonitoring during ablation. Semin Intervent Radiol. (2022) 39:157–61. doi: 10.1055/s-0042-1745795
37. Wei Y, Zhao ZL, Niu Y, Peng LL, Li Y, and Yu MA. Ultrasound imaging of the perithyroid fascial space: a comparative analysis with anatomical correlations. Sci Rep. (2025) 15:4503. doi: 10.1038/s41598-025-88306-8
38. Cavagnaro M, Amabile C, Bernardi P, Pisa S, and Tosoratti N. A minimally invasive antenna for microwave ablation therapies: design, performances, and experimental assessment. IEEE Trans BioMed Eng. (2011) 58:949–59. doi: 10.1109/TBME.2010.2099657
39. Evans M, Kang S, Bajaber A, Gordon K, and Martin C 3rd. Augmented reality for surgical navigation: A review of advanced needle guidance systems for percutaneous tumor ablation. Radiol Imaging Cancer. (2025) 7:e230154. doi: 10.1148/rycan.230154
40. Bojunga J and Trimboli P. Thyroid ultrasound and its ancillary techniques. Rev Endocr Metab Disord. (2024) 25:161–73. doi: 10.1007/s11154-023-09841-1
41. Zhang Y, Wang MY, Wang LK, Zhang S, Sun H, and Liu J. Preliminary study of 3D printing technology for extracorporeal positioning guide assisted ultrasound-guided microwave ablation of the liver. Expert Rev Med Devices. (2023) 20:1227–33. doi: 10.1080/17434440.2023.2277233
42. Back K, Kim TH, Lee J, Kim JS, Choe JH, Oh YL, et al. Optimal value of lymph node ratio and metastatic lymph node size to predict risk of recurrence in pediatric thyroid cancer with lateral neck metastasis. J Pediatr Surg. (2023) 58:568–73. doi: 10.1016/j.jpedsurg.2022.07.010
43. Bini F, Pica A, Marinozzi F, Giusti A, Leoncini A, and Trimboli P. Model-optimizing radiofrequency parameters of 3D finite element analysis for ablation of benign thyroid nodules. Bioengineering (Basel). (2023) 10:1210. doi: 10.3390/bioengineering10101210
44. Li Z, Nie W, Liu Q, Lin M, Li X, Zhang J, et al. A prognostic model for thermal ablation of benign thyroid nodules based on interpretable machine learning. Front Endocrinol (Lausanne). (2024) 15:1433192. doi: 10.3389/fendo.2024.1433192
Keywords: thyroid, Horner syndrome (HS), ablation therapy, complication, scoping review
Citation: Xie T, Fu Y, Jin X, Ren X, Zhang J and Sun Q (2025) Horner syndrome as a complication of ultrasound-guided ablation therapy for thyroid nodules: a scoping review. Front. Endocrinol. 16:1607214. doi: 10.3389/fendo.2025.1607214
Received: 07 April 2025; Accepted: 09 May 2025;
Published: 04 June 2025.
Edited by:
Pia Pace-Asciak, University of Toronto, CanadaReviewed by:
Erivelto Martinho Volpi, Hospital Alemão Oswaldo Cruz, BrazilCopyright © 2025 Xie, Fu, Jin, Ren, Zhang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Sun, aGRmeXNxQDE2My5jb20=
 Tianhao Xie
Tianhao Xie Yan Fu
Yan Fu Xiaoshi Jin1,2
Xiaoshi Jin1,2 Xiangxiang Ren
Xiangxiang Ren