- 1Yunnan Arrhythmia Research Center, Division of Cardiology, the First People’s Hospital of Yunnan Province, the Affiliated Hospital of Kunming University of Science and Technology, Kunming, China
- 2Department of Biopharmaceuticals and Tianjin Key Laboratory on Technologies Enabling Development of Clinical Therapeutics and Diagnostics, School of Pharmacy, Tianjin Medical University, Tianjin, China
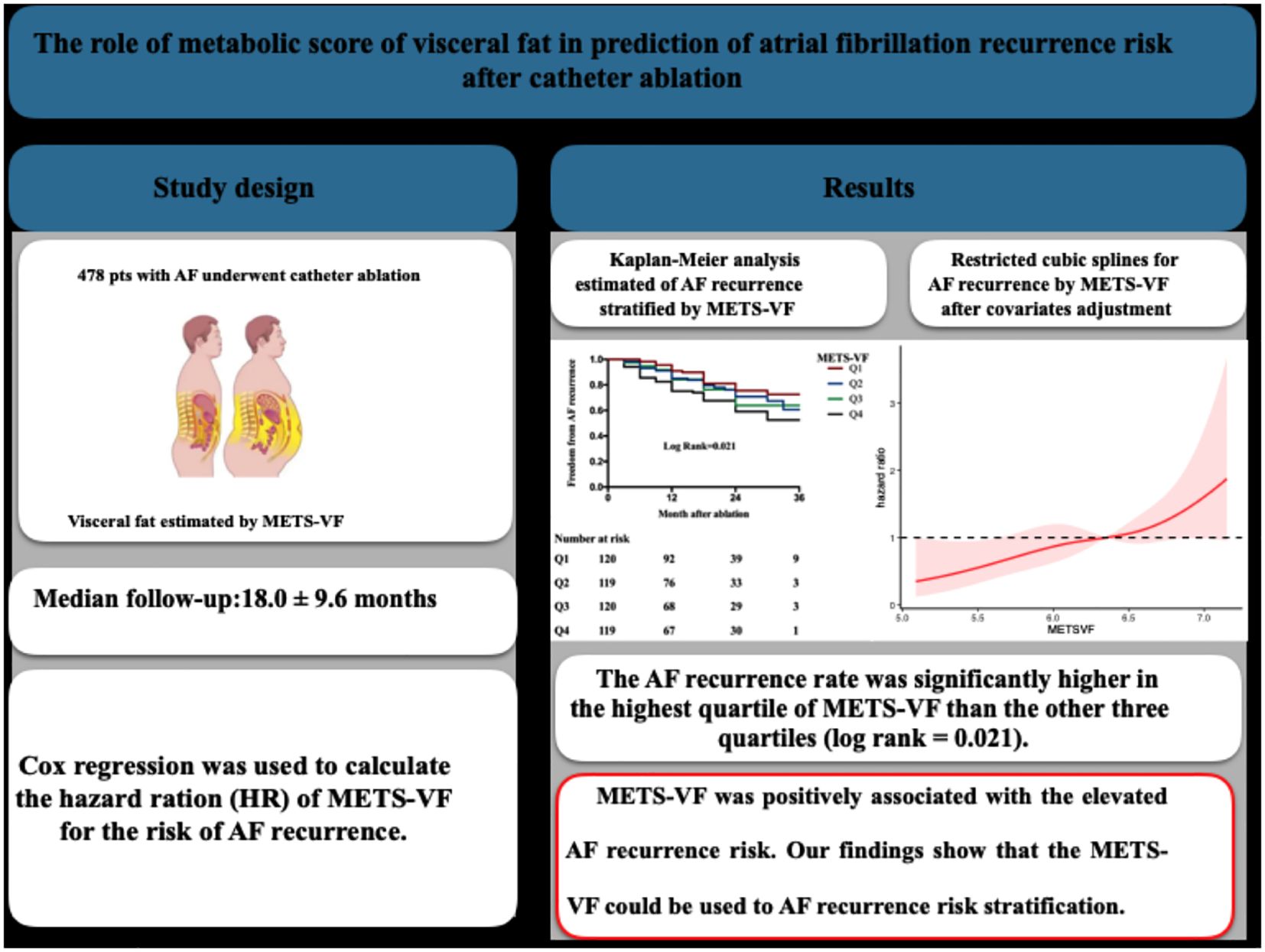
Background: Previous studies show that visceral fat tissue (VAT) play an important role in atrial fibrillation (AF). The metabolic score of visceral fat (METS-VF), a new surrogate to estimate VAT, is associated with cardiovascular mortality risk. In this study, we try to investigate the association between METS-VF and the risk of AF recurrence after catheter ablation.
Methods: 478 consecutive patients underwent catheter ablation were obtained and used to assess the relationship between METS-VF and the risk of AF recurrence. Cox regression was used to calculate the hazard ration (HR) of METS-VF for the risk of AF recurrence. Restricted cubic splines (RCS) was used to assessed the linear relationship between METS-VF and the AF recurrence risk.
Results: A total of 112(23.4%) patients experienced AF recurrence during 18.0 ± 9.6 months follow-up. The AF recurrence rate was significantly higher in the highest quartile of METS-VF than the other three quartiles (log rank = 0.021). In the univariate cox regression, LAD, and MET-VF were associated with AF recurrence (p<0.0001). In the multiple Cox regression results, compared with the participants with lowest METS-VF (Q1), the hazard ratio (HR) (95% CI) for the AF recurrence risk was 1.29 (0.73, 2.29) for Q2 (p=0.39), 1.59 (0.88 – 2.87) for Q3 (p=0.12), and 2.22 (1.20, 4.12) for Q4 (p<0.01) respectively.
Conclusions: METS-VF was positively associated with the elevated AF recurrence risk. Our findings show that the METS-VF could be used to AF recurrence risk stratification.
Graphical Abstract. This is the first study investigating the relationship between visceral adipose tissue (VAT) surrogates, such as lipid accumulation product (LAP), cardiometabolic index (CMI), and metabolic score for visceral fat (METS-VF), and atrial fibrillation (AF) recurrence following ablation. METS-VF, a reliable surrogate reflecting the VAT, was positively associated with elevated AF recurrence after ablation. METS-VF could be used to screen individuals with an elevated risk of AF recurrence in clinical practice.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, affecting over 33 million people worldwide (1, 2). It significantly increases the risk of stroke, heart failure, cardiovascular hospitalization, and all-cause mortality (3). Although catheter ablation is an effective rhythm control strategy, AF recurrence remains common, with rates ranging from 24% to 39% (4, 5). Identifying robust predictors of recurrence is crucial for improving long-term outcomes after ablation. Among the known risk factors, obesity—in particular the accumulation of visceral adipose tissue (VAT)—has been increasingly recognized as a key contributor to the development and progression of AF (6). However, direct imaging of VAT via CT, MRI, or dual-energy X-ray absorptiometry (DXA) is often impractical in routine clinical settings due to cost and limited accessibility (7, 8). To overcome this limitation, several surrogate indices have been proposed, including the lipid accumulation product (LAP), the cardiometabolic index (CMI), and the metabolic score for visceral fat (METS-VF). Unlike traditional markers, METS-VF integrates multiple dimensions of metabolic health—such as insulin resistance, BMI, and lipid metabolism—to provide a more comprehensive estimation of visceral fat burden (9). However, the association between METS-VF and AF recurrence after ablation remains largely unexplored. In this study, we aimed to investigate the predictive value of METS-VF for AF recurrence following catheter ablation. In addition, we compared its performance with those of other VAT-related indicators [i.e., LAP, CMI, and the metabolic score for insulin resistance (METS-IR)] using time-dependent receiver operating characteristic (ROC) curves to determine whether METS-VF offers superior prognostic utility.
Methods
Study population
We enrolled 478 patients who underwent the first radiofrequency catheter ablation for AF between January 2021 and March 2024 at the First People’s Hospital of Yunnan Province (Kunming, China). The exclusion criteria were as follows: 1) moderate-to-severe valvular disease; 2) uncontrolled thyroid dysfunction; 3) left atrial thrombosis; 4) acute coronary syndrome, myocardial infarction, and cardiac surgery in the previous 3 months; 5) contraindication of anticoagulation; 6) pregnancy; 7) hepatic or renal failure; and 8) patients who died or who were lost to follow-up. The study is in compliance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Yunnan First People’s Hospital.
Data collection and definitions
Clinical data including age, sex, BMI, waist circumference, hypertension (HT), diabetes mellitus (DM), history of stroke, heart failure (HF), and type of AF (paroxysmal AF or persistent AF) were collected. The CHA2DS2-VASc score was calculated for each patient. Serum blood biomarkers such as fasting plasma glucose (fGLU), creatinine, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were determined. Transthoracic echocardiography was employed to record the left atrial diameter (LAD) and the left ventricular ejection fraction (EF). The VAT surrogates were calculated as follows: BMI = weight (kg)/height2 (m2); WHtR = waist (cm)/height (cm); LAP(women) = TG (mmol/L) * [WC (cm) − 58]; LAP(men) = TG (mmol/L) * [WC (cm) − 65]; CMI = TG (mmol/L)/HDL-C (mmol/L) * WHtR; METS-IR = Ln[2 × FPG (mg/dl) + TG (mg/dl)] × BMI (kg/m2)/Ln [HDL-C (mg/dl); and METS-VF = 4.466 + 0.011 × [Ln(METS-VF)]3 + 3.239 × [Ln(WHtR)]3 + 0.319 × Sex (men = 1, women= 0) + 0.594 × [Ln(Age) (years)] (10).
Electrophysiology study and catheter ablation
The details regarding electrophysiology and catheter ablation are available in our previous study (11). In brief, an open irrigated catheter (ST Catheter, Biosense-Webster, Diamond Bar, CA, USA) was used to perform circumferential pulmonary vein (PV) isolation. Additional ablations in the superior vena cava or other non-PV triggers were performed when mappable AF triggers were available.
Follow-up
Patients were followed up at the outpatient department at 3, 6, 9, and 12 months after ablation and every 6 months thereafter, for a medium follow-up duration of 18 ± 9.6 months. At each visit, a 12-lead electrocardiogram and 24-h Holter monitoring were performed. When a patient showed symptoms of palpitations, the Holter data were obtained to evaluate arrhythmia. AF recurrence was defined as any episode of AF or atrial tachycardia lasting more than 30 s.
Statistical analysis
Descriptive statistics are reported as frequencies and percentages for categorical variables and as medians with interquartile ranges (Q1–Q3) for continuous variables. Continuous variables were compared using the Wilcoxon rank-sum test or the Kruskal–Wallis test, while categorical variables were compared using the chi-square test. Kaplan–Meier survival curves were generated to visualize the time to AF recurrence stratified by the METS-VF quartiles, and differences between groups were assessed using the log-rank test. The association between the clinical/metabolic variables and AF recurrence was assessed using Cox proportional hazards regression. Univariable Cox models were performed first, followed by multivariable models adjusting for potential confounders. The final multivariate model included age, sex, BMI, stroke, HT, HF, cardiovascular disease (CVD), DM, TC, LDL-C, HDL-C, triglycerides (Tg), fGLU, and LAD. Restricted cubic spline (RCS) modeling was used to explore the dose–response relationship and the potential non-linearity between METS-VF and AF recurrence risk. Time-dependent ROC curve analysis was conducted at 12, 24, and 36 months to evaluate and compare the predictive performance of METS-VF, CMI, LAP, and METS-IR. The area under the curve (AUC) values were compared using DeLong’s test. All data analyses were performed using R or SPSS. A p < 0.05 was considered statistically significant.
Results
Baseline characteristics
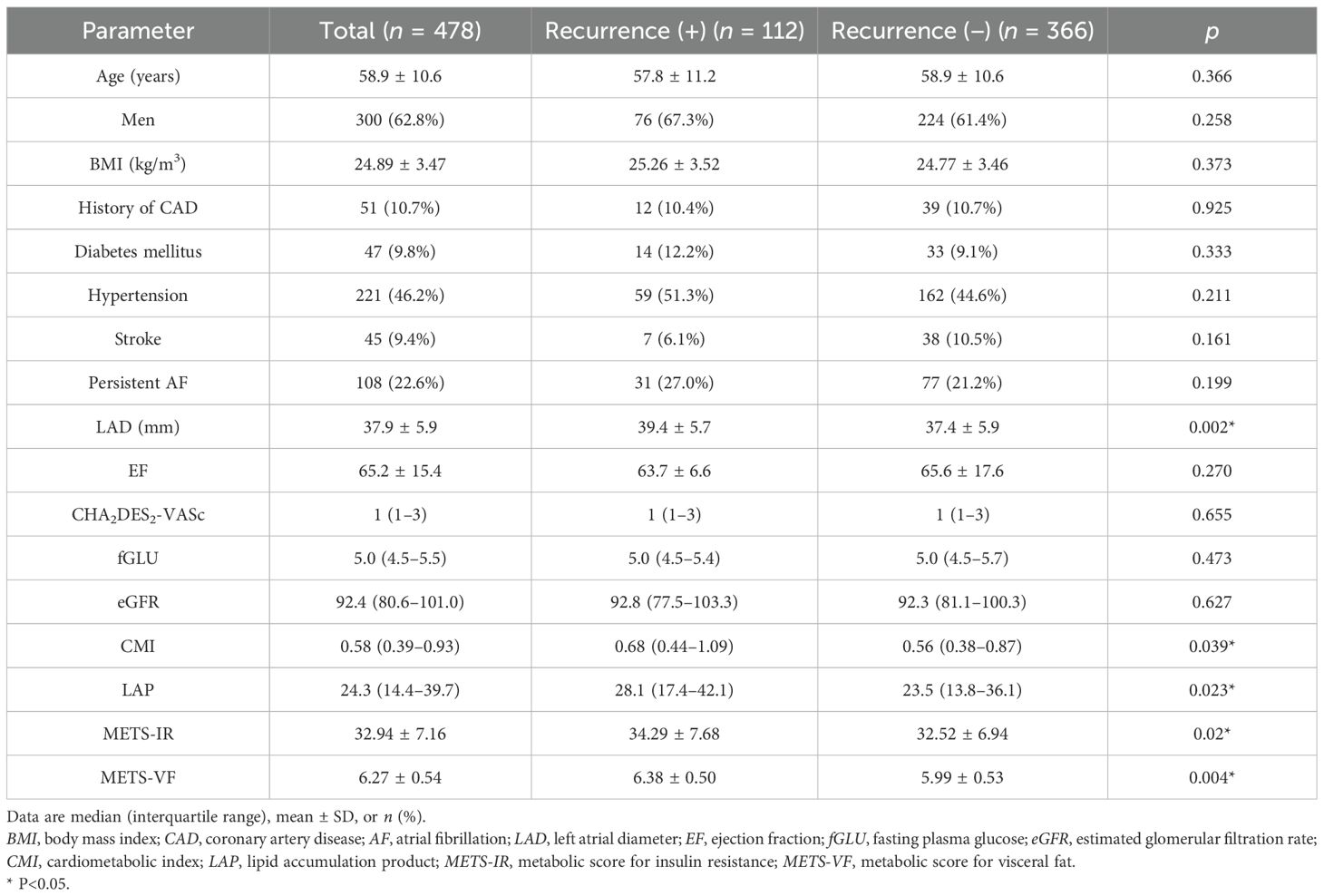
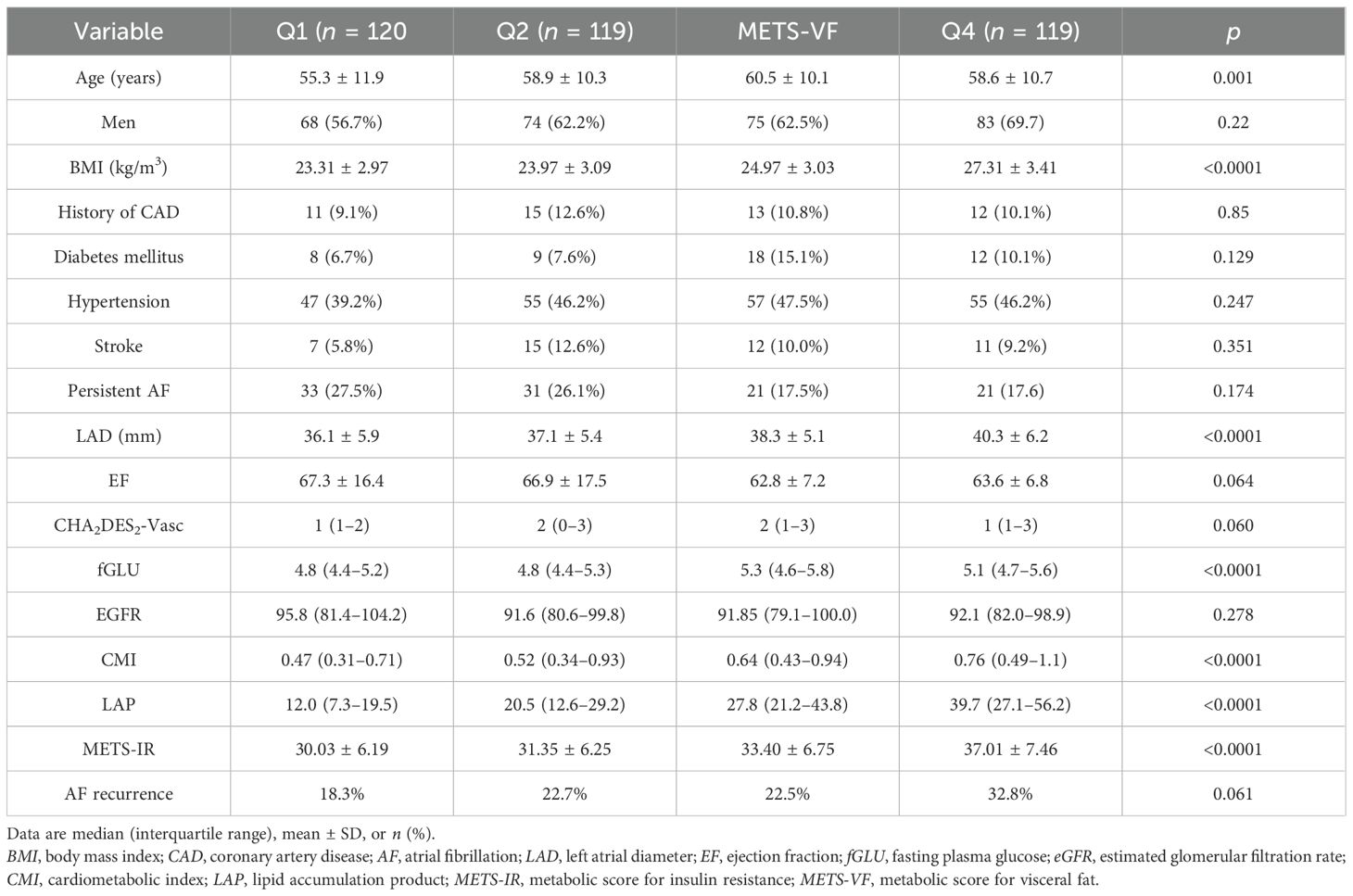
This study included 478 patients (age, 58.9 ± 10.6 years; 62.8% men) with either paroxysmal (n = 370) or persistent AF (n = 108). The demographic characteristics, the clinical and laboratory data, and the VAT surrogates are summarized in Table 1. After a mean follow-up period of 18.0 ± 9.6 months, AF recurrence was observed in 112 (23.4%) patients. As shown in Table 1, patients with AF recurrence exhibited larger LAD (39.4 ± 5.7 vs. 37.4 ± 5.9, p = 0.002), higher CMI (0.68 vs. 0.56, p = 0.039), higher LAP (28.1 vs. 23.5, p = 0.023), higher MET-IR (34.29 ± 7.68 vs. 32.52 ± 6.94, p = 0.02), and higher METS-VF (6.38 ± 0.50 vs. 5.99 ± 0.53, p = 0.004) compared to patients without AF recurrence. Furthermore, patients were stratified into four groups according to the METS-VF quartiles (Table 2). The quartile thresholds for METS-VF were determined as 5.90, 6.35, and 6.68. Patients in the highest quartile of METS-VF had higher BMI, larger LAD, and higher fGLU, CMI, LAP, and METS-IR than those in the other three groups (all p < 0.0001).
Cox regression and Kaplan–Meier analyses
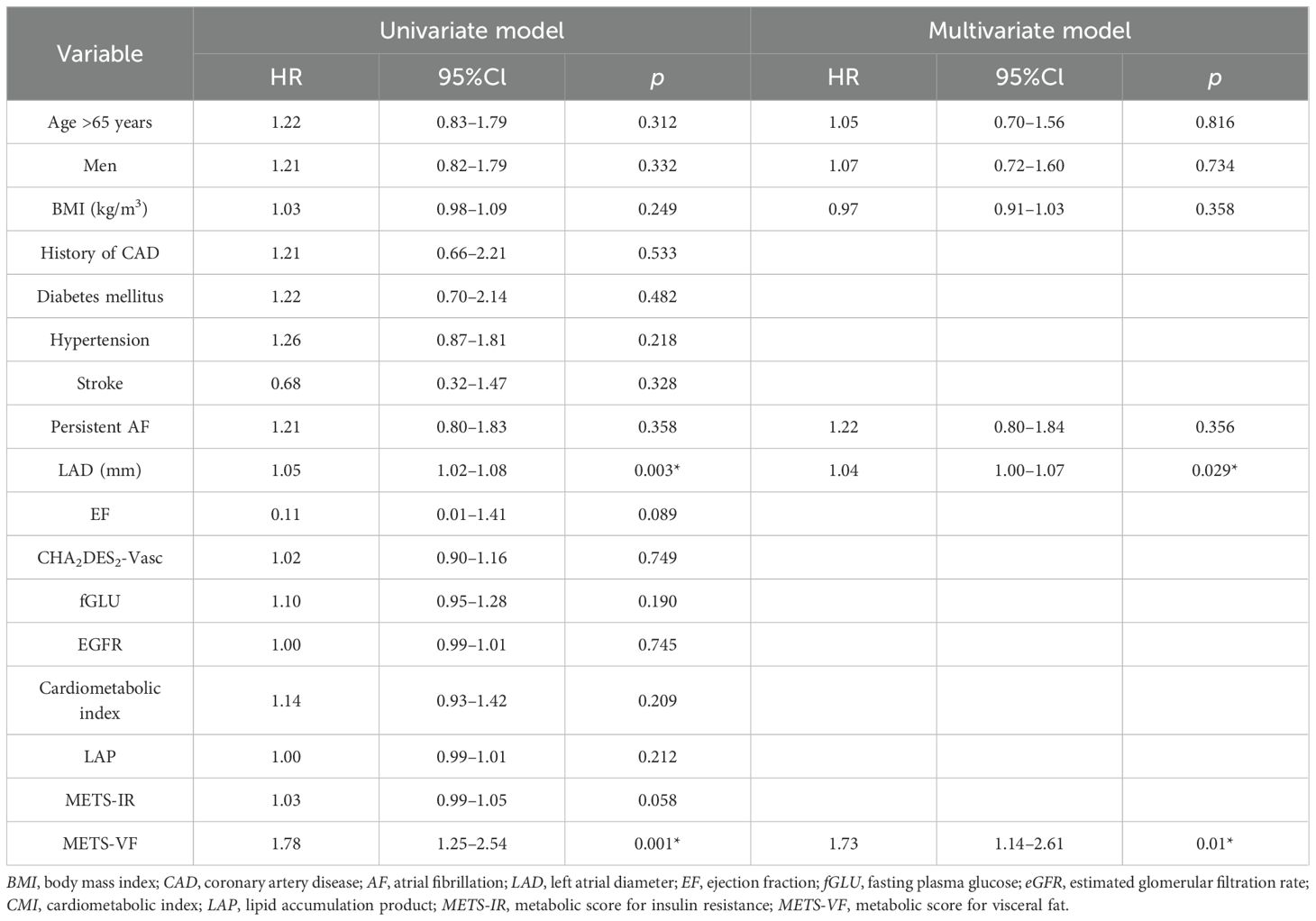
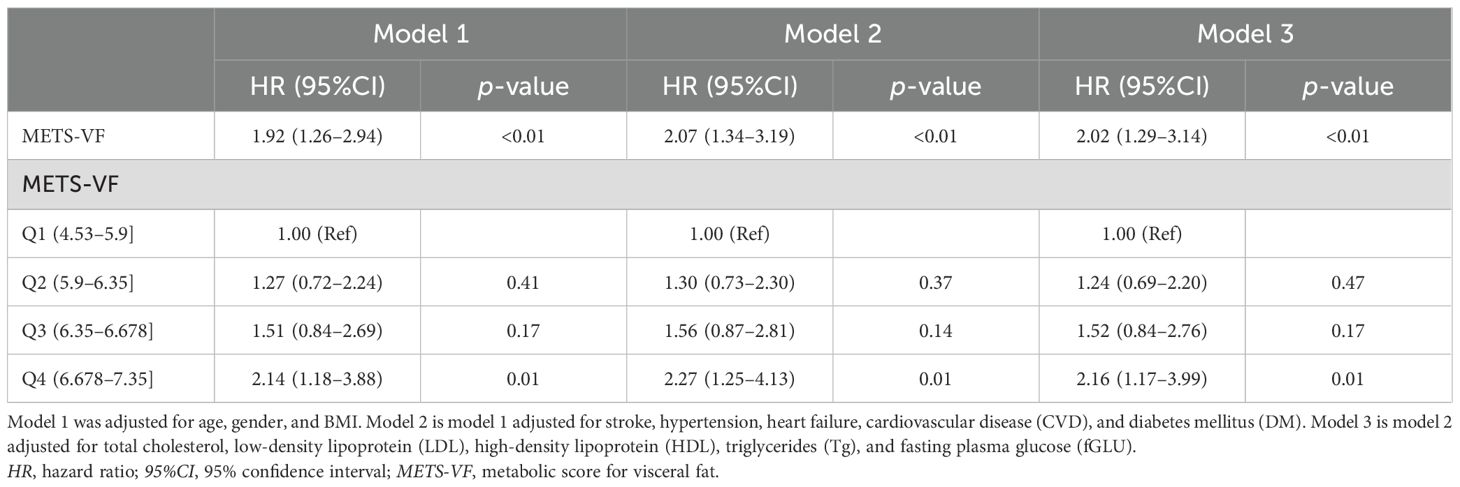
Kaplan–Meier analysis was performed to estimate the time to AF recurrence after catheter ablation across the different METS_VF risk strata (Figure 1). Kaplan–Meier analysis revealed a significant difference in AF recurrence across the METS-VF quartiles (log-rank p = 0.021), with higher METS-VF scores associated with an increased risk of recurrence following catheter ablation. Cox proportional hazards regression models were then utilized to identify whether METS-VF and other metabolic indices were independently associated with AF recurrence over time. In the univariable Cox regression analysis (Table 3), several clinical and metabolic variables were assessed for their association with AF recurrence following catheter ablation. Among them, LAD and a higher METS-VF were independently associated with the recurrence of AF after ablation. Multivariable Cox regression (Table 3) confirmed METS-VF as an independent predictor of AF recurrence after adjusting for confounders. To further evaluate risk stratification, METS-VF was analyzed as a categorical variable using quartiles (Table 4). In all three adjustment models, patients in the highest quartile (Q4) showed a significantly elevated risk of AF recurrence compared with those in Q1.
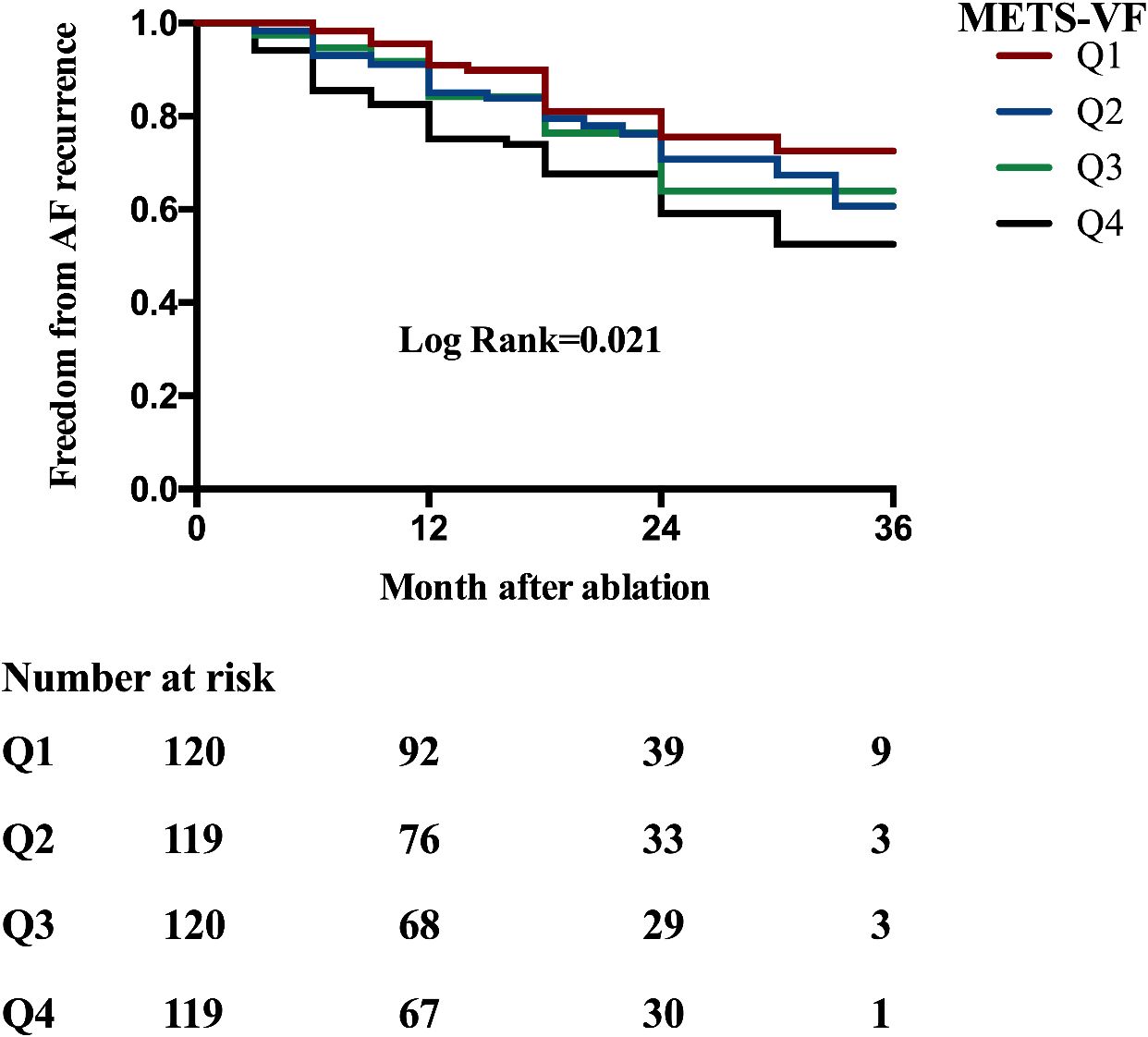
Figure 1. Kaplan–Meier analysis estimating AF recurrence in patients with AF undergoing catheter ablation stratified by the METS-VF quartiles (overall log-rank = 0.021). AF, atrial fibrillation; METS-VF, metabolic score for visceral fat.
Predictive performance of METS-VF vs. comparator models
To evaluate the discriminative ability of METS-VF in predicting AF recurrence after catheter ablation, time-dependent ROC curve analyses were conducted and the results compared against three established metabolic indices: CMI, LAP, and METS-IR. Figure 2 shows the time-dependent ROC curves generated at 12, 24, and 36 months of follow-up. The corresponding AUC values are presented in Table 5. Comparisons were performed using DeLong’s test to assess statistical significance. At the 12-month mark, METS-VF demonstrated significantly better predictive performance than LAP (p = 0.027), CMI (p = 0.003), and METS-IR (p = 0.013). However, the differences in the AUCs between METS-VF and the comparator models at 24 and 36 months were not statistically significant (all p > 0.3).
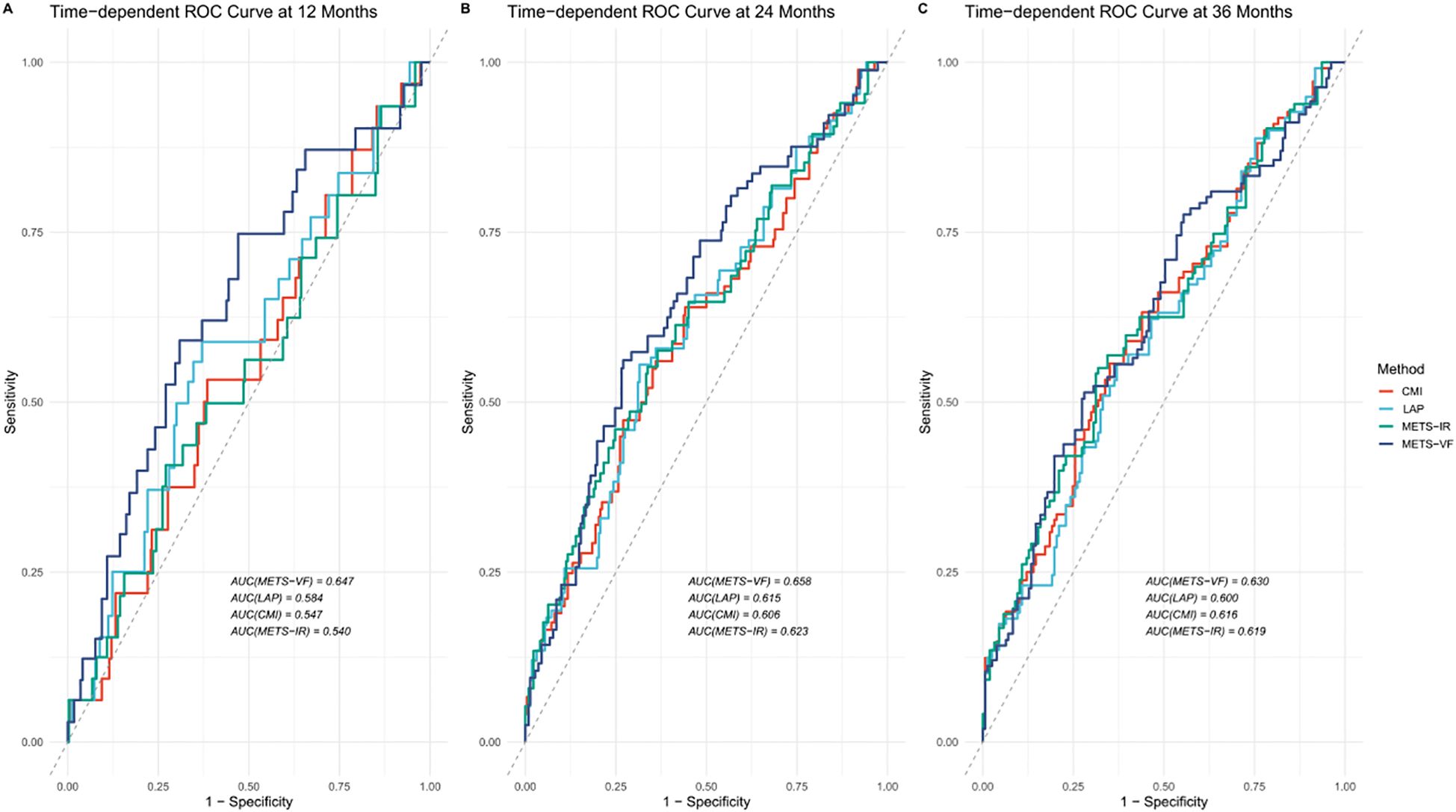
Figure 2. Time-dependent receiver operating characteristic (ROC) curves for predicting atrial fibrillation (AF) recurrence after catheter ablation. METS-VF, metabolic score for visceral fat; LAP, lipid accumulation product; CMI, cardiometabolic index; METS-IR, metabolic score for insulin resistance. (A) ROC curves for predicting AF one- year recurrence after catheter ablation; (B) ROC curves for predicting AF two- year recurrence after catheter ablation; (C) ROC curves for predicting AF three year recurrence after catheter ablation.
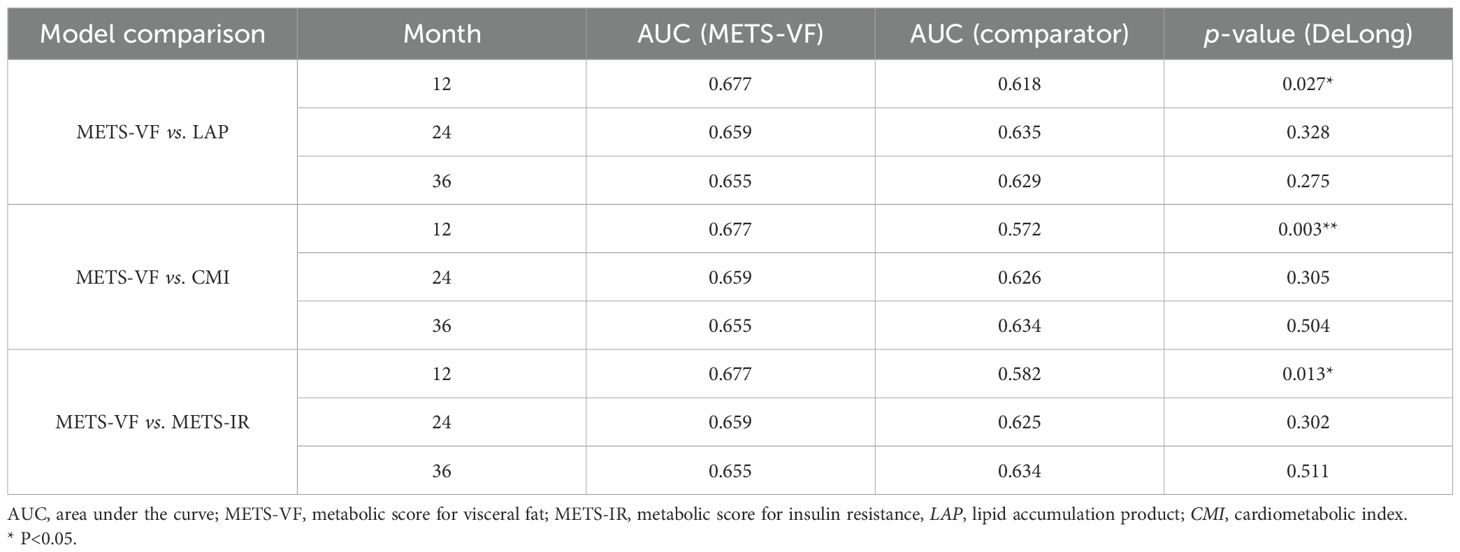
Table 5. Comparison of the predictive performance of METS-VF and the comparator models at different time points.
Dose–response relationship between METS-VF and AF recurrence
The association between METS-VF and AF recurrence was further examined using RCS analysis. As shown in Figure 3, a linear and positive dose–response relationship was observed between METS-VF and the risk of AF recurrence. The overall association was statistically significant (p = 0.02); however, no evidence of non-linearity was detected (p = 0.62). This suggests that higher METS-VF scores are linearly associated with an increased risk of AF recurrence following catheter ablation.
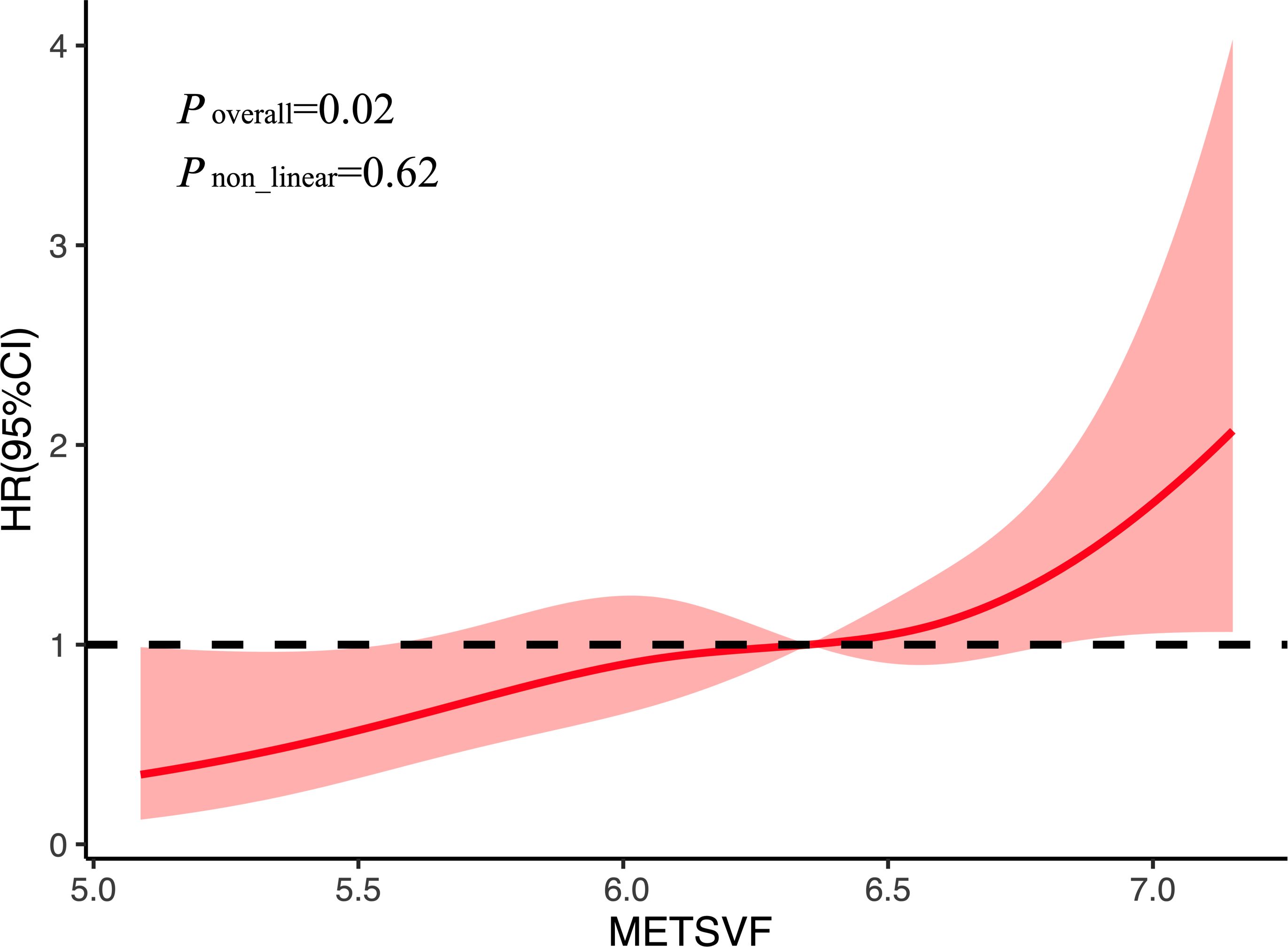
Figure 3. Restricted spline curves for AF recurrence by METS-VF after adjustment for covariates. AF, atrial fibrillation; METS-VF, metabolic score for visceral fat; HR, hazard ratio; 95%CI, 95% confidence interval.
Discussion
To our knowledge, this is the first study that evaluated the association between METS-VF and AF recurrence following catheter ablation. It was found that METS-VF is a strong independent predictor of AF recurrence, with a clear dose-dependent increase in recurrence risk observed across the METS-VF quartiles. This association remained significant after adjusting for conventional clinical and metabolic confounders. Although obesity is a well-established risk factor for AF (12), previous studies have reported the so-called obesity paradox, where individuals with obesity (as defined by their BMI) sometimes exhibit better prognoses compared with their lean counterparts (13). This paradox may have stemmed from the limitations of BMI, which does not distinguish between fat distribution types. Unlike general obesity measured using BMI, visceral adiposity (VAT-driven obesity) is ectopically deposited around critical organs such as the heart, liver, and skeletal muscle (14, 15), contributing to metabolic dysregulation, insulin resistance, and systemic inflammation—all of which are pathophysiological pathways that promote AF substrate formation. Direct VAT quantification through CT or MRI is often impractical in clinical practice due to cost, radiation exposure, and operator dependency (6, 7). Therefore, surrogate indices such as LAP, CMI, and METS-VF have been proposed for the estimation of VAT in a noninvasive and accessible manner (16). Of these, METS-VF offers a distinct advantage as it integrates anthropometric measurements (BMI and waist circumference), lipid profiles, and fasting glucose, capturing the composite metabolic burden more comprehensively than LAP or CMI alone. METS-VF, as a new surrogate used to estimate VAT, is useful for the evaluation of cardiometabolic health and has shown better performance in estimating VAT compared with other surrogates (17, 18). Kapoor et al. validated METS-VF as a reliable, easily available, and inexpensive surrogate for the measurement of VAT; thus, METS-VF exhibited correlations with different diseases such as metabolic disease, cardiovascular disease, and chronic kidney disease (19–22).
In this study, METS-VF demonstrated superior short-term predictive performance for AF recurrence compared with LAP, CMI, and METS-IR, as evidenced by the higher AUC values in the time-dependent ROC analysis and the statistically significant differences confirmed by DeLong’s test. This finding highlights the clinical utility of METS-VF in early post-ablation risk stratification. Furthermore, the RCS analysis revealed a linear dose–response relationship between METS-VF and the risk of AF recurrence, without evidence of a nonlinear threshold effect. This suggests that even moderate increases in METS-VF may incrementally elevate the risk of recurrence. Such a continuous risk gradient supports the use of METS-VF not only as a categorical stratification tool but also as a quantitative biomarker for individualized recurrence risk prediction.
Mechanistically, the components of METS-VF—specifically the markers of insulin resistance, adiposity, and dyslipidemia—may collectively contribute to atrial structural and electrical remodeling, increased atrial fibrosis, and enhanced arrhythmogenic substrate formation. Insulin resistance, a key metabolic abnormality reflected by an elevated METS-VF, has been linked to impaired atrial energy metabolism, increased oxidative stress, and enhanced fibrotic signaling through TGF-β1 pathways (23). These changes can lead to mitochondrial dysfunction and abnormal calcium handling in atrial myocytes, ultimately shortening the action potential duration and facilitating arrhythmogenesis (24). Visceral adiposity, particularly epicardial adipose tissue (EAT), has been shown to exert paracrine effects on the adjacent atrial myocardium, releasing pro-inflammatory cytokines (e.g., TNF-α), adipokines (e.g., leptin and resistin), and fibrotic mediators that promote atrial fibrosis, conduction slowing, and electrical dispersion (25). Recent imaging studies have demonstrated that EAT infiltration is associated with increased low-voltage areas and high recurrence after catheter ablation (26, 27). Recent studies have also suggested that adipose tissue-derived extracellular vesicles (EVs) can carry microRNAs and pro-fibrotic proteins that directly modulate atrial gene expression, indicating a potential epigenetic link between metabolic status and AF substrate progression (28). These interlinked metabolic pathways provide a plausible biological explanation for the observed association. Given its ease of calculation, cost-effectiveness, and predictive capability, METS-VF may serve as a practical tool for clinicians to identify patients at higher risk of AF recurrence after catheter ablation, facilitating personalized follow-up strategies and potential early interventions.
Study limitation
This study has several limitations. Firstly, this is a retrospective study based on patients who were referred for AF ablation in a single center, which needs validation in other populations. Secondly, the mechanisms between VAT and AF recurrence were not fully understood, and more basic research is needed to investigate these.
Conclusion
In this study, METS-VF, a reliable surrogate reflecting VAT, was positively associated with increased AF recurrence after ablation. METS-VF could be used to screen those individuals with an increased risk of AF recurrence in clinical practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Yunnan First People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YM: Data curation, Funding acquisition, Writing – original draft, Conceptualization. XG: Validation, Methodology, Writing – review & editing. JS: Writing – review & editing, Formal analysis, Data curation. XK: Writing – review & editing, Validation, Methodology. XZ: Validation, Writing – review & editing, Methodology. FW: Validation, Writing – review & editing, Methodology. TM: Investigation, Writing – review & editing. YC: Writing – review & editing, Validation. JG: Writing – review & editing, Data curation. PW: Writing – review & editing, Methodology. JL: Writing – review & editing, Formal analysis, Data curation. JF: Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Health Commission of Yunnan Province (2023-KHRCBZ-B18 to Yazhe Ma), Yunnan Fundamental Research Projects (grant no. 202401CF070012 to Yazhe Ma).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
2. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. (2014) 129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119
3. Cotté FE, Chaize G, Gaudin AF, Samson A, Vainchtock A, Fauchier L, et al. Burden of stroke and other cardiovascular complications in patients with atrial fibrillation hospitalized in France. Europace. (2016) 18:501–7. doi: 10.1093/europace/euv248
4. Chen H, Li C, Han B, Xiao F, Yi F, Wei Y, et al. Circumferential pulmonary vein isolation with vs without additional low-voltage-area ablation in older patients with paroxysmal atrial fibrillation: A randomized clinical trial. JAMA Cardiol. (2023) 8:765–72. doi: 10.1001/jamacardio.2023.1749
5. Wu G, Huang H, Cai L, Yang Y, Liu X, Yu B, et al. Long-term observation of catheter ablation vs. pharmacotherapy in the management of persistent and long-standing persistent atrial fibrillation (CAPA study). Europace. (2021) 23:731–9. doi: 10.1093/europace/euaa356
6. Gawałko M, Saljic A, Li N, Abu-Taha I, Jespersen T, Linz D, et al. Adiposity-associated atrial fibrillation: molecular determinants, mechanisms, and clinical significance. Cardiovasc Res. (2023) 119:614–30. doi: 10.1093/cvr/cvac093
7. Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, et al. Dual-energy x-ray absorptiometry for quantification of visceral fat. Obes (Silver Spring). (2012) 20:1313–8. doi: 10.1038/oby.2011.393
8. Neeland IJ, Grundy SM, Li X, Adams-Huet B, and Vega GL. Comparison of visceral fat mass measurement by dual-x-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: the Dallas heart study. Nutr Diabetes. (2016) 6:e221. doi: 10.1038/nutd.2016.28
9. Wang H, Chen Y, Sun G, Jia P, Qian H, and Sun Y. Validity of cardiometabolic index, lipid accumulation product, and body adiposity index in predicting the risk of hypertension in Chinese population. Postgrad Med. (2018) 130:325–33. doi: 10.1080/00325481.2018.1444901
10. Bello-Chavolla OY, Antonio-Villa NE, Vargas-Vázquez A, Viveros-Ruiz TL, Almeda-Valdes P, Gomez-Velasco D, et al. Metabolic Score for Visceral Fat (METS-VF), a novel estimator of intra-abdominal fat content and cardio-metabolic health. Clin Nutr. (2020) 39:1613–21. doi: 10.1016/j.clnu.2019.07.012
11. Zhang X, Kuang X, Gao X, Xiang H, Wei F, Liu T, et al. RESCUE-AF in patients undergoing atrial fibrillation ablation: the RESCUE-AF trial. Circ Arrhythm Electrophysiol. (2019) 12:e007044. doi: 10.1161/CIRCEP.118.007044
12. Sha R, Baines O, Hayes A, Tompkins K, Kalla M, Holmes AP, et al. Impact of obesity on atrial fibrillation pathogenesis and treatment options. J Am Heart Assoc. (2024) 13:e032277. doi: 10.1161/JAHA.123.032277
13. Badheka AO, Rathod A, Kizilbash MA, Garg N, Mohamad T, Afonso L, et al. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med. (2010) 123:646–51. doi: 10.1016/j.amjmed.2009.11.026
14. Tutor AW, Lavie CJ, Kachur S, Milani RV, and Ventura HO. Updates on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. (2023) 78:2–10. doi: 10.1016/j.pcad.2022.11.013
15. Cesaro A, De Michele G, Fimiani F, Samson A, Vainchtock A, Fauchier L, et al. Visceral adipose tissue and residual cardiovascular risk: a pathological link and new therapeutic options. Front Cardiovasc Med. (2023) 10:1187735. doi: 10.3389/fcvm.2023.1187735
16. Sun Q, Ren Q, Du L, Chen S, Wu S, Zhang B, et al. Cardiometabolic Index (CMI), Lipid Accumulation Products (LAP), Waist Triglyceride Index (WTI) and the risk of acute pancreatitis: a prospective study in adults of North China. Lipids Health Dis. (2023) 22:190. doi: 10.1186/s12944-023-01948-3
17. Torun C, Ankaralı H, Caştur L, Uzunlulu M, Erbakan AN, Akbaş MM, et al. Is metabolic score for visceral fat (METS-VF a better index than other adiposity indices for the prediction of visceral adiposity. Diabetes Metab Syndr Obes. (2023) 16:2605–15. doi: 10.2147/DMSO.S421623
18. Kapoor N, Jiwanmall SA, Nandyal MB, Kattula D, Paravathareddy S, Paul TV, et al. Metabolic score for visceral fat (METS-VF) estimation - A novel cost-effective obesity indicator for visceral adipose tissue estimation. Diabetes Metab Syndr Obes. (2020) 13:3261–7. doi: 10.2147/DMSO.S266277
19. Antonio-Villa NE, Juárez-Rojas JG, Posadas-Sánchez R, Reyes-Barrera J, and Medina-Urrutia A. Visceral adipose tissue is an independent predictor and mediator of the progression of coronary calcification: a prospective sub-analysis of the GEA study. Cardiovasc Diabetol. (2023) 22:81. doi: 10.1186/s12933-023-01807-6
20. Zhu Y, Zou H, Guo Y, Luo P, Meng X, Li D, et al. Associations between metabolic score for visceral fat and the risk of cardiovascular disease and all-cause mortality among populations with different glucose tolerance statuses. Diabetes Res Clin Pract. (2023) 203:110842. doi: 10.1016/j.diabres.2023.110842
21. Feng L, Chen T, Wang X, Xiong C, Chen J, Wu S, et al. Metabolism score for visceral fat (METS-VF): A new predictive surrogate for CKD risk. Diabetes Metab Syndr Obes. (2022) 15:2249–58. doi: 10.2147/DMSO.S370222
22. Yang R, Kuang M, Qiu J, Yu C, Sheng G, and Zou Y. Assessing the usefulness of a newly proposed metabolic score for visceral fat in predicting future diabetes: results from the NAGALA cohort study. Front Endocrinol (Lausanne). (2023) 14:1172323. doi: 10.3389/fendo.2023.1172323
23. Giraldo-Gonzalez GC, Roman-Gonzalez A, Cañas F, and Garcia A. Molecular Mechanisms of Type 2 Diabetes-Related Heart Disease and Therapeutic Insights. Int J Mol Sci. (2025) 26:4548. doi: 10.3390/ijms26104548
24. Chan YH, Chang GJ, Lai YJ, Chen WJ, Chang SH, Hung LM, et al. Atrial fibrillation and its arrhythmogenesis associated with insulin resistance. Cardiovasc Diabetol. (2019) 18:125. doi: 10.1186/s12933-019-0928-8
25. Krishnan A, Chilton E, Raman J, Saxena P, McFarlane C, Trollope AF, et al. Are Interactions between Epicardial Adipose Tissue, Cardiac Fibroblasts and Cardiac Myocytes Instrumental in Atrial Fibrosis and Atrial Fibrillation? Cells. (2021) 10:2501.
26. Huber AT, Fankhauser S, Wittmer S, Chollet L, Lam A, Maurhofer J, et al. Epicardial adipose tissue dispersion at CT and recurrent atrial fibrillation after pulmonary vein isolation. Eur Radiol. (2024) 34:4928–38. doi: 10.1007/s00330-023-10498-2
27. Umar A, Hocking J, Massin SZ, Suszko A, Wintersperger BJ, and Chauhan VS. Association of cardiac CT-derived epicardial adipose tissue with atrial fibrillation in patients without left atrial fibrosis as defined by endocardial voltage mapping. J Cardiovasc Electrophysiol. (2025) 36:489–500. doi: 10.1111/jce.16566
Keywords: metabolic score of visceral fat, atrial fibrillation, visceral obesity, catheter ablation, independent risk factor
Citation: Ma Y, Gao X, Sun J, Kuang X, Zhang X, Wei F, Ma T, Cui Y, Guo J, Wu P, Liu J and Fan J (2025) The role of metabolic score for visceral fat in the prediction of atrial fibrillation recurrence risk after catheter ablation. Front. Endocrinol. 16:1607497. doi: 10.3389/fendo.2025.1607497
Received: 07 April 2025; Accepted: 07 August 2025;
Published: 02 September 2025.
Edited by:
Yang Zou, Jiangxi Provincial People’s Hospital, ChinaReviewed by:
Ma Jianhua, Nanjing Medical University, ChinaShiming He, Jiangxi Provincial People’s Hospital, China
Copyright © 2025 Ma, Gao, Sun, Kuang, Zhang, Wei, Ma, Cui, Guo, Wu, Liu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Fan, ZmFuajkxM0BzaW5hLmNvbQ==; Jiangwen Liu, MjAyMDIwMzAyMDAyOUB3aHUuZWR1LmNu
†These authors share first authorship
 Yazhe Ma
Yazhe Ma Xiaolong Gao1†
Xiaolong Gao1†