- Department of Endocrinology, The Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, Enshi, Hubei, China
Background: Diabetic foot ulcers (DFUs), a common complication of diabetes, are often accompanied by delayed wound healing, pain, psychological distress, and sleep disturbances.
Objective: To evaluate the effectiveness of personalized continuous care (PCC) compared to routine care in improving wound healing, symptom severity, and psychological/sleep outcomes in DFU patients.
Methods: A retrospective cohort study of 60 DFU patients (2021–2024) compared PCC (n=30) with routine care (n=30). Outcomes assessed included wound area reduction, granulation tissue coverage, symptom scores (ulceration, necrosis, pain), and validated psychological (SDS, SAS) and sleep (AIS) scales.
Results: The PCC group showed superior wound healing (40.51% vs. 27.43% area reduction; 61.66% vs. 46.32% granulation coverage, p<0.05), lower symptom scores (ulceration: 3.18 ± 0.45 vs. 4.46 ± 0.6; pain: 2.01 ± 0.29 vs. 3.45 ± 0.58, p<0.01), and improved psychological (SDS: 32.1 ± 3.88 vs. 44.87 ± 4.05; SAS: 30.36 ± 3.77 vs. 43.25 ± 4.56, p<0.001) and sleep outcomes (AIS: 8.23 ± 0.6 vs. 11.33 ± 0.94, p<0.001).
Conclusion: PCC enhances DFU wound healing, alleviates symptoms, and improves psychological well-being and sleep quality, supporting its integration routine clinical practice.
1 Introduction
Diabetes mellitus (DM) affects 537 million adults worldwide, with projections indicating an increase to 783 million by 2045 (1). In China, the prevalence of diabetes among adults has reached 12.4%, representing the largest diabetic population globally, with over 140 million people affected as of 2021 (2). Among DM patients, 15% to 25% develop diabetic foot ulcers (DFUs), which represent a primary cause of lower-limb amputation and diminished quality of life (3, 4). Chronic hyperglycemia, neuropathy, and poor blood circulation impair wound healing, while psychological stress and sleep disorders further exacerbate these outcomes (5–7). Diabetic foot disease, a complication closely associated with diabetes, is one of the leading causes of amputation (7). As such, diabetic foot ulcers represent a major public health burden that demands urgent and effective management strategies.
As a common yet severe complication, diabetic foot disease—including DFUs—imposes long-term physical and psychological burdens on patients (8–10). DFUs typically manifests as local skin rupture, ulceration, and infection in the lower extremities, potentially leading to tissue necrosis and osteomyelitis (11). In severe cases, they may necessitate amputation or even threaten life. Persistent skin damage, ulceration, and infection around the ulcer render wound healing extremely difficult. According to the latest guidelines from the International Working Group on Diabetic Foot (IWGDF 2023), effective DFU management requires a multidisciplinary and individualized approach that addresses not only wound care but also systemic and psychosocial factors (12). Management of chronic wounds represents a critical challenge in clinical care, as the healing process is influenced not only by physiological factors but also by patients’ psychological states and sleep quality. Numerous studies have demonstrated that psychological stress and depression activate the hypothalamic-pituitary-adrenal (HPA) axis, increasing cortisol secretion, which suppresses immune function and delays tissue repair (13, 14). Similarly, sleep disturbances are associated with reduced growth hormone production and elevated levels of inflammatory cytokines, such as IL-6 and TNF-α, which contribute to poor wound healing outcomes (15). Sleep disorders further exacerbate healing delays by disrupting growth hormone secretion and increasing the release of inflammatory cytokines (e.g., IL-6, TNF-α). This highlights the need for care strategies that go beyond physical treatment and address the full complexity of DFU-related complications.
Although conventional nursing models maintain wound stability through basic measures such as debridement and anti-infection therapy, they often overlook systematic interventions for patients’ psychological and sleep needs, potentially becoming a latent blind spot affecting treatment efficacy. Therefore, effective treatment and nursing care for DFUs are of paramount importance (16–18). Among various therapeutic approaches, individualized continuous nursing care is recognized as a vital component of DFU management (19). The core of individualized continuous nursing care lies in providing personalized, comprehensive, and sustained medical services to meet the nursing needs of patients at different stages and with diverse requirements. This care model emphasizes comprehensive patient assessment, formulation of targeted treatment plans based on disease characteristics and lifestyle, and continuous improvement of health status through regular follow-up and plan adjustment (20–22). Therefore, addressing emotional well-being and sleep disturbances is crucial for promoting optimal wound healing outcomes in DFU patients.
Consequently, the study focuses on adult patients with Wagner grade 3–4 diabetic foot ulcers of more than four weeks’ duration, with stable comorbidities and independent mobility. This specific population was selected to ensure homogeneity in disease severity and functional status, thereby enhancing the comparability of outcomes (23), thereby providing a more scientific basis for clinical practice (24). In this context, personalized continuous care may offer a promising solution to bridge the current gaps in DFU nursing practices.
2 Materials and methods
2.1 General information
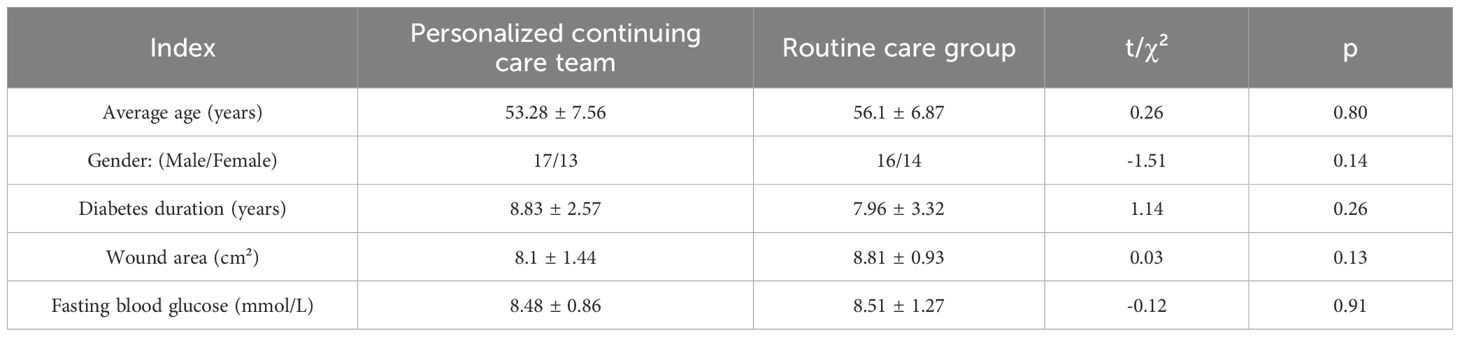
This retrospective cohort study was conducted from January 2021 to December 2024 at the Department of Endocrinology, The Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, a tertiary care facility located in a central urban area of Hubei Province, China. A total of 60 patients with diabetic foot ulcers who met the study criteria were included. The sample size (n=60) was based on available eligible cases and is consistent with similar retrospective studies, with post hoc analysis indicating sufficient statistical power to detect group differences and routine care were formed based on clinical care pathways in use during the study period. The group assignment was non-randomized and depended on the care model followed by the treating team. The PCC model was introduced in mid-2022 as part of a hospital-wide nursing improvement initiative; patients treated before this or who opted out received routine care. Patient data were collected through systematic review of the hospital’s electronic medical record (EMR) system. Key variables such as demographic information, wound characteristics, psychological scale scores, and treatment history were extracted using a standardized data collection form (Table 1). All data were anonymized before analysis. Patient identifiers were removed, and access to data was restricted to the research team to ensure confidentiality. Ethical approval was obtained from the hospital ethics committee (Approval No.: 20220618), with waived informed consent due to the retrospective nature.
2.2 Inclusion and exclusion criteria
Inclusion criteria (1): age ≥18 years (2); confirmed diagnosis of DFU with Wagner grade 3–4 ulcer lasting >4 weeks (3); stable comorbid conditions (e.g., controlled hypertension or heart disease without hospitalization in the past 3 months) (4); ability to ambulate independently without mobility aids. Exclusion criteria (1): end-stage organ failure (2); active malignancy (3); cognitive impairment or psychiatric illness interfering with participation.
2.3 Interventions
Patients in the personalized continuous care group receive individualized care plans tailored to their specific conditions. Specifically, these include: 1) regular wound cleaning and dressing changes, whereby personalized protocols are developed based on wound characteristics to maintain cleanliness and facilitate healing; 2) routine foot examinations, aimed at detecting and addressing lesions or abnormalities promptly to prevent complications; 3) nutritional support, involving customized dietary plans to ensure adequate nutrient intake and promote wound repair; 4) rehabilitation training, which designs exercise and physical therapy regimens to restore foot function and mobility; 5) psychological support, providing counseling to help patients manage emotional distress such as anxiety and depression during treatment; and 6) sleep guidance, offering strategies to improve sleep quality and mitigate the negative impacts of insomnia on healing.
In contrast, routine care followed the hospital’s standard DFU treatment protocol based on national clinical guidelines.: wound cleansing and dressing changes performed according to conventional protocols to maintain wound dryness and cleanliness, along with routine medications such as antibiotics and analgesics to control infection and manage pain.
2.4 Observation Indicators
Wound healing was assessed using two indicators: wound area reduction and granulation tissue coverage. Wound area was calculated with the formula area = πr², where r represents the radius, based on standardized clinical measurements. Granulation tissue coverage was visually estimated by trained wound care nurses during dressing changes, using a standardized assessment protocol and wound photographs. Two experienced assessors independently verified the results for consistency. Symptom severity, including ulceration, tissue necrosis (rot), and wound pain, was rated on a 5-point Likert scale, with higher scores indicating greater severity. Assessments were performed by clinical nursing staff during routine care. Psychological status was evaluated using the validated Chinese versions of the Self-Rating Anxiety Scale (SAS) and the Self-Rating Depression Scale (SDS), each containing 20 items scored on a 4-point scale. Scores ≥53 on SAS and ≥50 on SDS indicated significant anxiety and depression, respectively. These scales were administered in Chinese at baseline and after 4 weeks by trained nurses. Sleep quality was assessed with the Chinese version of the Athens Insomnia Scale (AIS), an 8-item tool covering sleep induction, awakenings, duration, quality, and daytime functioning. Each item is rated from 0 (no issue) to 3 (severe issue); total scores <4 indicate no sleep disturbance, 4–6 suggest suspected insomnia, and >6 indicate clinical insomnia. AIS was also administered at baseline and post-intervention by trained staff.
2.5 Statistical methods
SPSS statistical software was used for data analysis. Descriptive statistics were expressed as mean ± standard deviation, and t-test was utilized for comparing continuous variables between two groups, and the chi-squared test was used for count data comparison. In addition to p-values, effect sizes (Cohen’s d) were calculated for key continuous outcomes to assess the clinical significance of group differences. The P value for statistically significant differences was set at <0.05.
3 Result
3.1 Wound healing status
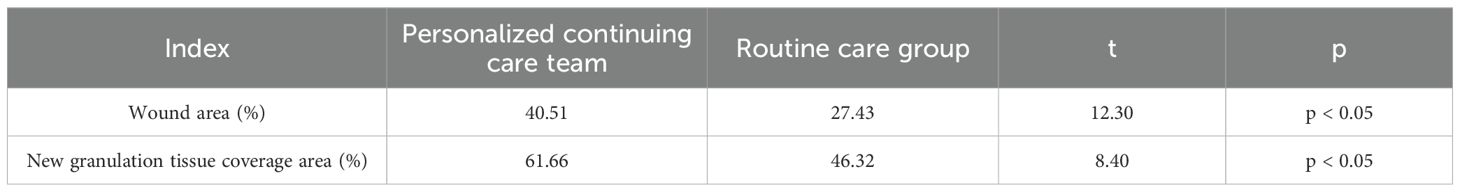
Following nursing intervention, the personalized continuous care group demonstrated significant advantages in wound healing. At the end of treatment, the wound area in the personalized continuous care group was significantly reduced, with an average reduction of 40.51% compared to a 27.43% decrease in the routine care group (p < 0.05). In terms of new granulation tissue coverage, the personalized continuous care group achieved 61.66%, while the routine care group achieved 46.32% (p < 0.05) (Table 2).
3.2 Symptom score comparison
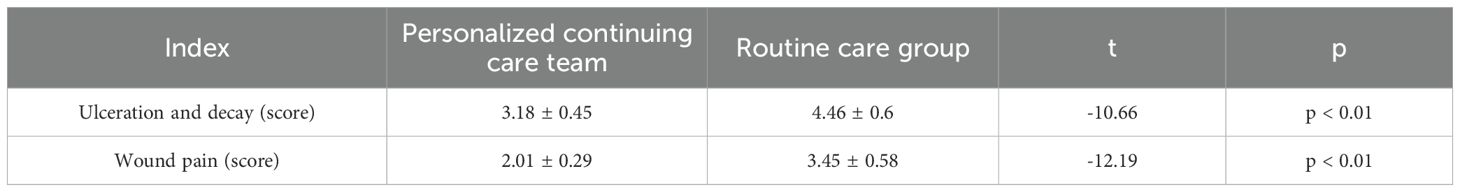
Following nursing intervention, the symptom scores (ulceration, gangrene, wound pain) in the personalized continuous care group were significantly lower than those in the routine care group. The ulceration and gangrene score were 3.18 ± 0.45 in the personalized continuous care group vs. 4.46 ± 0.6 in the routine care group (p < 0.01); the wound pain score was 2.01 ± 0.29 vs. 3.45 ± 0.58 (p < 0.01). Symptom improvement (ulceration, gangrene, wound pain) in the personalized continuous care group was significantly greater than in the routine care group (Table 3).
3.3 Patient psychological score
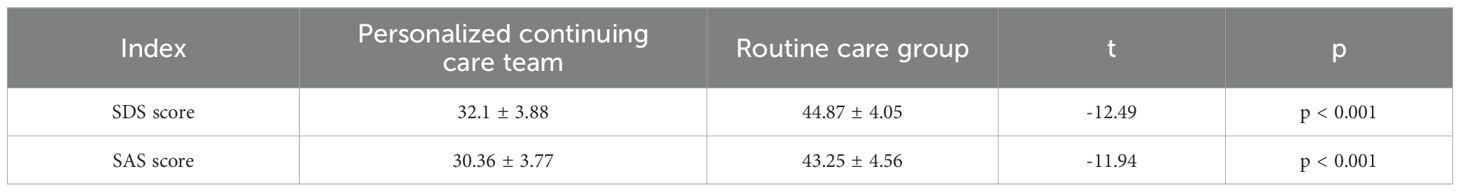
Following nursing intervention, the Self-Rating Depression Scale (SDS) and Self-Rating Anxiety Scale (SAS) scores in the personalized continuous care (PCC) group were significantly lower than those in the routine care group. The SDS score was 32.1 ± 3.88 in the PCC group and 44.87 ± 4.05 in the routine care group (p < 0.001); the SAS score was 30.36 ± 3.77 in the PCC group and 43.25 ± 4.56 in the routine care group (p < 0.001) (Table 4).
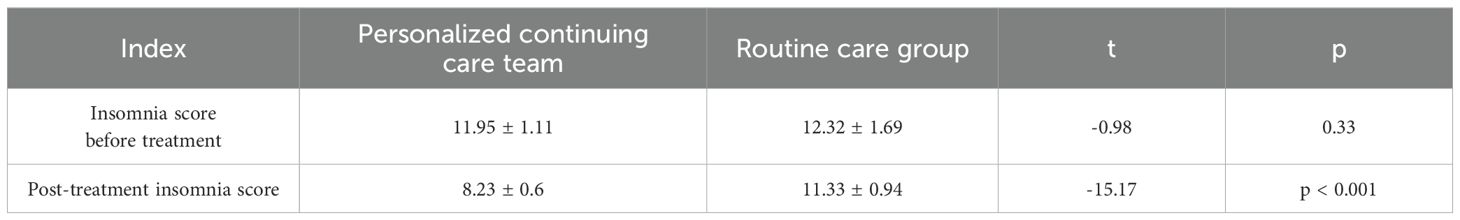
3.4 Sleep score
Following nursing intervention, the Athens Insomnia Scale (AIS) score in the personalized continuous care (PCC) group decreased significantly, with a statistically significant difference compared to baseline (pre-treatment: 11.95 ± 1.11 vs. post-treatment: 8.23 ± 0.6, p < 0.001). By contrast, the routine care group showed no significant difference (pre-treatment: 12.32 ± 1.69 vs. post-treatment: 11.33 ± 0.94, *p> 0.05) (Table 5).
4 Discussion
Diabetic foot ulcer (DFU), one of the common and severe complications in patients with diabetes, is often accompanied by challenges such as delayed wound healing, pain, psychological distress, and sleep disorders during treatment. Low-frequency ultrasound, as an adjunctive therapy for chronic wounds, has demonstrated remarkable efficacy, particularly in improving the healing rates of DFUs (25). Additionally, local insulin injections have been shown to positively influence DFU healing by promoting granulation tissue formation (26). Through a retrospective study involving 60 patients, we found that the personalized continuous care (PCC) group exhibited significant advantages in wound healing, symptom scores, psychological well-being, and sleep quality compared with the routine care group. Specifically, wound healing in the PCC group outperformed that in the routine care group. Reductions in wound area and increases in new granulation tissue coverage—key indicators of wound healing—were notably more pronounced in the PCC group. This superiority may be attributed to the comprehensive assessment of patients’ wound conditions in PCC, which enables the formulation of targeted treatment plans. Such plans include measures like regular wound debridement, dressing changes, foot examinations, nutritional support, rehabilitation training, and other interventions, all of which collectively promote the progression of the wound healing process.
In addition, patients in the personalized continuous care (PCC) group had significantly lower symptom scores than those in the routine care group, indicating that personalized continuous care can effectively alleviate symptoms such as ulceration, gangrene, and wound pain. This may be attributed to the timely diagnosis and management of symptoms in PCC, as well as its focus on patients’ psychological and sleep status, thereby improving their overall symptom burden. In terms of psychological and sleep outcomes, the PCC group exhibited significantly lower scores on the Self-Rating Depression Scale (SDS), Self-Rating Anxiety Scale (SAS), and Athens Insomnia Scale (AIS) compared with the routine care group, suggesting that personalized continuous care can notably enhance patients’ mental health and sleep quality. This improvement might stem from the psychological support and sleep guidance provided in PCC, which help patients manage emotional distress and sleep disorders during treatment, thus improvi Overall, personalized continuous care demonstrates significant advantages in the management of diabetic foot ulcer (DFU) patients, as it promotes wound healing, alleviates symptoms, and improves mental health and sleep quality. ng their mental state and sleep quality. A separate study has also demonstrated that a combined treatment regimen outperforms standard care in promoting diabetic foot ulcer healing (27), further confirming that systematic personalized care can significantly improve wound healing and quality of life in these patients. Overall, personalized continuous care demonstrates significant advantages in the management of diabetic foot ulcer (DFU) patients, as it promotes wound healing, alleviates symptoms, and improves mental health and sleep quality. These findings align with the 2023 IWGDF guidelines, which emphasize patient-centered, integrated care strategies to improve wound healing outcomes and reduce the risk of recurrence (12). However, this study has limitations, including a small sample size and a retrospective design. Additionally, the study did not evaluate indicators such as cholesterol levels, statin treatment rates for cholesterol management, glucose-lowering therapies with GLP-1 receptor agonists or SGLT2 inhibitors, and smoking status—factors that may substantially influence patient care strategies and treatment outcomes. Therefore, future research should prioritize these key indicators to conduct a more comprehensive assessment of the efficacy and safety of personalized continuous care in DFU management. Specifically, large-sample, multi-center randomized controlled trials are necessary to further validate the roles of these factors in diabetic foot ulcer care and their impact on clinical outcomes.
This study has several limitations. Notably, key confounding factors such as HbA1c levels, comorbidities, smoking status, and medication adherence were not included in the analysis due to incomplete retrospective data. These variables may influence wound healing and psychological outcomes, and their absence limits the ability to fully control for potential bias.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Ethics Committee of The Central Hospital of Enshi Tujia and Miao Autonomous Prefecture (Approval No.: 20220618). And the study was conducted in strict accordance with the Declaration of Helsinki and relevant ethical guidelines. The requirement for informed consent was waived due to the retrospective nature of the study, which involved anonymized clinical data and posed no more than minimal risk to the participants. The research content involved in this research meets the requirements of medical ethics and academic morality of the hospital, and the research content is reasonable, the risks are controllable, and there are no violations. The relevant research carried out is in line with the safe, standardized and true scientific research guiding principles, and in line with the requirements of the clinical research ethics code.
Author contributions
YM: Methodology, Formal analysis, Data curation, Writing – original draft, Software, Conceptualization. JW: Supervision, Writing – review & editing, Investigation, Project administration, Validation, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DFU, Diabetic foot ulcers; DM, Diabetes mellitus; HPA, Hypothalamic-pituitary-adrenal; PCC, Personalized Continuous Care.
References
1. Cloete L. Diabetes mellitus: an overview of the types, symptoms, complications and management. Nurs Stand. (2022) 37:61–6. doi: 10.7748/ns.2021.e11709
2. Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and treatment of diabetes in China, 2013-2018. Jama. (2021) 326:2498–506. doi: 10.1001/jama.2021.22208
3. Mponponsuo K, Sibbald RG, and Somayaji R. A comprehensive review of the pathogenesis, diagnosis, and management of diabetic foot infections. Adv Skin Wound Care. (2021) 34:574–81. doi: 10.1097/01.ASW.0000791876.10485.d4
4. Mathew JK and Sathyalakshmi S. ExpACVO-Hybrid Deep learning: Exponential Anti Corona Virus Optimization enabled Hybrid Deep learning for tongue image segmentation towards diabetes mellitus detection. BioMed Signal Process Control. (2023) 83:104635. doi: 10.1016/j.bspc.2023.104635
5. Akça Doğan D and Pehlivan S. Diabetic foot care training and the presence of nurses in Turkish YouTube videos. Prim Care Diabetes. (2022) 16:430–4. doi: 10.1016/j.pcd.2022.03.010
6. Scain SF, Franzen E, and Hirakata VN. Effects of nursing care on patients in an educational program for prevention of diabetic foot. Rev Gaucha Enferm. (2018) 39:e20170230. doi: 10.1590/1983-1447.2018.20170230
7. Aalaa M, Sanjari M, Shahbazi S, Shayeganmehr Z, Abooeirad M, Amini MR, et al. Diabetic foot workshop: Improving technical and educational skills for nurses. Med J Islam Repub Iran. (2017) 31:8. doi: 10.18869/mjiri.31.8
8. Al-Kaabi JM, Al Maskari F, Cragg P, Afandi B, and Souid AK. Illiteracy and diabetic foot complications. Prim Care Diabetes. (2015) 9:465–72. doi: 10.1016/j.pcd.2015.04.008
9. Hughes DR, Filar C, and Mitchell DT. Nurse practitioner scope of practice and the prevention of foot complications in rural diabetes patients. J Rural Health. (2022) 38:994–8. doi: 10.1111/jrh.12599
10. Edmonds ME. The diabetic foot: pathophysiology and treatment. Clin Endocrinol Metab. (1986) 15:889–916. doi: 10.1016/S0300-595X(86)80079-2
11. Ju HH, Momin R, Cron S, Jularbal J, Alford J, and Johnson C. A nurse-led telehealth program for diabetes foot care: feasibility and usability study. JMIR Nurs. (2023) 6:e40000. doi: 10.2196/40000
12. Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, and Lipsky BA. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. (2020) 36 Suppl 1:e3266. doi: 10.1002/dmrr.v36.S1
13. Armario A, Belda X, Gagliano H, Fuentes S, Molina P, Serrano S, et al. Differential hypothalamic-pituitary-adrenal response to stress among rat strains: methodological considerations and relevance for neuropsychiatric research. Curr Neuropharmacol. (2023) 21:1906–23. doi: 10.2174/1570159X21666221129102852
14. Malek H, Ebadzadeh MM, Safabakhsh R, and Razavi A. Mathematical analysis of the role of pituitary-adrenal interactions in ultradian rhythms of the HPA axis. Comput Biol Med. (2021) 135:104580. doi: 10.1016/j.compbiomed.2021.104580
15. da Silva JGM, de Melo IMF, Alves ÉR, de Oliveira GM, da Silva AA, Cavalcanti IMF, et al. Melatonin and bacterial cellulose regulate the expression of inflammatory cytokines, VEGF, PCNA, and collagen in cutaneous wound healing in diabetic rats. Polymers (Basel). (2024) 16(18):2611. doi: 10.3390/polym16182611
16. Wendling S and Beadle V. The relationship between self-efficacy and diabetic foot self-care. J Clin Transl Endocrinol. (2015) 2:37–41. doi: 10.1016/j.jcte.2015.01.001
17. Rastogi A, Hiteshi P, Bhansali AA, and Jude EB. Virtual triage and outcomes of diabetic foot complications during Covid-19 pandemic: A retro-prospective, observational cohort study. PloS One. (2021) 16:e0251143. doi: 10.1371/journal.pone.0251143
18. Peterson JM and Virden MD. Improving diabetic foot care in a nurse-managed safety-net clinic. J Am Assoc Nurse Pract. (2013) 25:263–71. doi: 10.1111/j.1745-7599.2012.00786.x
19. Zhao N, Xu J, Zhou Q, Hu J, Luo W, Li X, et al. Screening behaviors for diabetic foot risk and their influencing factors among general practitioners: a cross-sectional study in Changsha, China. BMC Prim Care. (2023) 24:68. doi: 10.1186/s12875-023-02027-3
20. Aalaa M, Malazy OT, Sanjari M, Peimani M, and Mohajeri-Tehrani M. Nurses’ role in diabetic foot prevention and care; a review. J Diabetes Metab Disord. (2012) 11:24. doi: 10.1186/2251-6581-11-24
21. Dardari D, Franc S, Charpentier G, Orlando L, Bobony E, Bouly M, et al. Hospital stays and costs of telemedical monitoring versus standard follow-up for diabetic foot ulcer: an open-label randomised controlled study. Lancet Reg Health Eur. (2023) 32:100686. doi: 10.1016/j.lanepe.2023.100686
22. Nayeri ND, Samadi N, Mehrnoush N, Allahyari I, Bezaatpour F, and NaseriAsl M. Experiences of nurses within a nurse-led multidisciplinary approach in providing care for patients with diabetic foot ulcer. J Family Med Prim Care. (2020) 9:3136–41. doi: 10.4103/jfmpc.jfmpc_1008_19
23. Walsh SM and Sage RA. Depression and chronic diabetic foot disability. A case report of suicide. Clin Podiatr Med Surg. (2002) 19:493–508. doi: 10.1016/S0891-8422(02)00019-8
24. Shin L, Bowling FL, Armstrong DG, and Boulton AJM. Saving the diabetic foot during the COVID-19 pandemic: A tale of two cities. Diabetes Care. (2020) 43:1704–9. doi: 10.2337/dc20-1176
25. Kruse D, Morgan K, Christensen J, Derner BS, and Sachs B. Treatment of nonhealing diabetic foot wounds with vaporous hyperoxia therapy in conjunction with standard wound care. J Am Podiatr Med Assoc. (2023) 113(2):20–259. doi: 10.7547/20-259
26. Zhang Z and Lv L. Effect of local insulin injection on wound vascularization in patients with diabetic foot ulcer. Exp Ther Med. (2016) 11:397–402. doi: 10.3892/etm.2015.2917
Keywords: diabetic foot ulcer, personalized continuous care, psychological distress, sleep quality, wound healing
Citation: Mi Y and Wang J (2025) Effectiveness of personalized continuous care in wound care of patients with diabetic foot ulcers. Front. Endocrinol. 16:1612047. doi: 10.3389/fendo.2025.1612047
Received: 15 April 2025; Accepted: 12 June 2025;
Published: 04 July 2025.
Edited by:
Calvin Omolo, United States International University - Africa, KenyaReviewed by:
Agus Santosa, Muhammadiyah University Purwokerto, IndonesiaKabange Kasumbwe, Durban University of Technology, South Africa
Copyright © 2025 Mi and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Wang, d2FuZ2o1MjY3OUBvdXRsb29rLmNvbQ==
 Yan Mi
Yan Mi Jing Wang
Jing Wang