- Department of Obstetrics and Gynecology, Faculty of Medicine, Bursa Uludag University, Bursa, Türkiye
Background: Infertility rates have been rising globally, necessitating accurate assessment tools for ovarian reserve. Anti-Müllerian hormone (AMH) is a key biomarker for evaluating ovarian reserve, yet age-stratified reference data remain limited. Establishing an AMH nomogram could enhance fertility counseling and treatment planning.
Objective: To develop an age-stratified AMH nomogram to improve the understanding of ovarian reserve across reproductive ages and assist in comparing individual AMH values with age-specific thresholds, aiding in the baseline infertility work-up
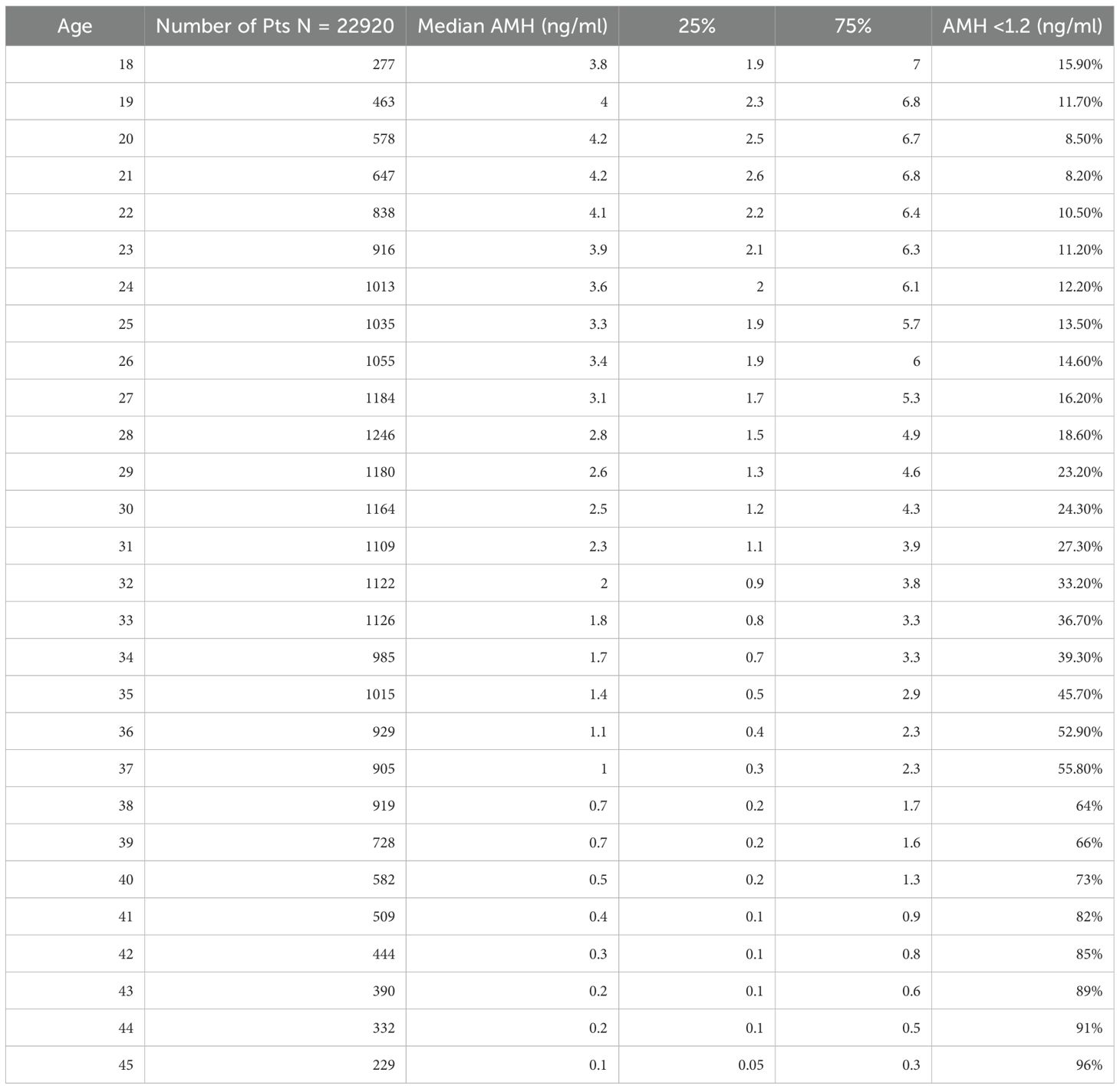
Methods: This retrospective cohort study analyzed AMH test results from a tertiary university hospital’s electronic database between April 2015 and June 2024. Data were collected from various departments, excluding women younger than 18 or older than 45 years. Median AMH levels and interquartile ranges were calculated for each age group. The prevalence of diminished ovarian reserve (DOR), defined as AMH <1.2 ng/mL, was determined. Statistical analyses, including correlation testing and subgroup comparisons across different clinical settings, were performed using SPSS version 22 (IBM Corp., Armonk, NY, USA).
Results: A total of 22,920 AMH results were analyzed after excluding patients outside the 18–45 age range and those with incomplete data. More than half of the AMH tests were from women aged 24–33 years. The results demonstrated a significant negative correlation between age and AMH levels, with a median AMH value dropping below 1.2 ng/mL by age 36. The prevalence of DOR increased from 15.9% at age 18 to 96% at age 45. Additionally, women from the Endometriosis Unit had significantly lower AMH levels (median 1.6 ng/mL) compared to other departments (median 2.03 ng/mL). The age-stratified AMH distribution remained consistent even when patients from ART (Assisted Reproductive Technology) centers, REI (Reproductive Endocrinology and Infertility), and the Endometriosis Unit were excluded.
Conclusion: This study provides an age-stratified AMH nomogram that can serve as a valuable tool for clinicians to assess ovarian reserve more accurately. The sharp decline in AMH levels, particularly after age 36, emphasizes the need for timely fertility evaluations and interventions, particularly in populations at risk for diminished ovarian reserve, such as those with endometriosis.
Background
The global infertility rate has been rising dramatically over the past few decades (1). Various factors contribute to infertility, including male factor, tubal factor, unexplained infertility, endometriosis, advanced age, and diminished ovarian reserve (2). Regardless of the underlying cause, a comprehensive infertility work-up for all couples remains essential. This evaluation includes a detailed history from both partners, physical examination, semen analysis, tubal assessment, ultrasonographic evaluation of the female pelvic anatomy, and ovarian reserve testing (3). Assessing ovarian reserve is a critical step in determining the appropriate treatment plan.
urrent practice primarily evaluates ovarian reserve using two key parameters: anti-Müllerian hormone (AMH) levels and antral follicle count (AFC) (4, 5). AMH is a glycoprotein hormone secreted by the granulosa cells of preantral and small antral follicles in the ovaries. It serves as a reliable endocrine marker of the remaining follicular pool, reflecting a woman’s reproductive potential. The antral follicle count is a useful, easy-to-perform, and cost-effective method, though it is ultrasound- and operator-dependent (6, 7). Additionally, conditions such as endometriomas may interfere with accurate AFC measurement. In such cases, AMH levels become particularly important for assessing ovarian reserve (8).
The existing literature provides substantial data on AFC and AMH concerning infertility evaluation, predicting controlled ovarian hyperstimulation (COH) outcomes, and forecasting cumulative live birth rates (9, 10). However, an equally important factor that influences all assisted reproductive technology (ART) outcomes is the woman’s age (11). Unfortunately, the literature lacks clarity regarding age-stratified ovarian reserve parameters.
A recent study presents age-stratified AFC outcomes to guide patients, offering AFC results by age, which can help in comparing ovarian reserve against age-matched thresholds (12). Similarly, another study from China provides quartiles and median AMH levels by age for a healthy population (13). To contribute to this growing body of knowledge, we aim to establish an AMH nomogram. This will make it easier to inform patients about their ovarian reserve, comparing their results with those of women in the same age group during their baseline infertility work-up.
Methods
Study design and ethical approval
This retrospective cohort study was conducted at a tertiary university hospital, utilizing an electronic database. Ethics approval for the study was obtained from the Bursa Uludag University Clinical Trials Ethical Committee with the number 2024-19/5.
Data collection and patient enrollment
The hospital’s electronic database was screened for AMH results collected between April 2015 and June 2024. AMH values from all departments, including Obstetrics and Gynecology (Ob&Gyn), were included. Women younger than 18 years or older than 45 years at the time of testing were excluded. Data collected included patient age at the time of testing and the department where the test was ordered. Records with incomplete or insufficient data were excluded from the analysis.
AMH analysis
The hospital’s standard protocol for AMH testing involved morning blood sample collection, typically while patients were fasting, by trained laboratory staff. The samples were processed under the appropriate conditions for AMH assays. Over the 10-year study period, the AMH levels were measured using the “Beckman Coulter Access II” enzymatic immunoassay, and results were recorded in ng/mL.
Statistical analysis
AMH levels were stratified by the patient’s age, ranging from 18 to 45 years. Median AMH levels were calculated for each age group, along with the 25th and 75th percentiles. According to the Poseidon Classification (14), diminished ovarian reserve (DOR) was defined as an AMH level below 1.2 ng/mL, and the percentage of women with DOR was calculated for each age group. To minimize bias, a separate analysis was performed, excluding patients who had been seen in infertility clinics. All statistical analyses were performed using SPSS software version 22.0 (IBM Corp., Armonk, NY, USA).
Results
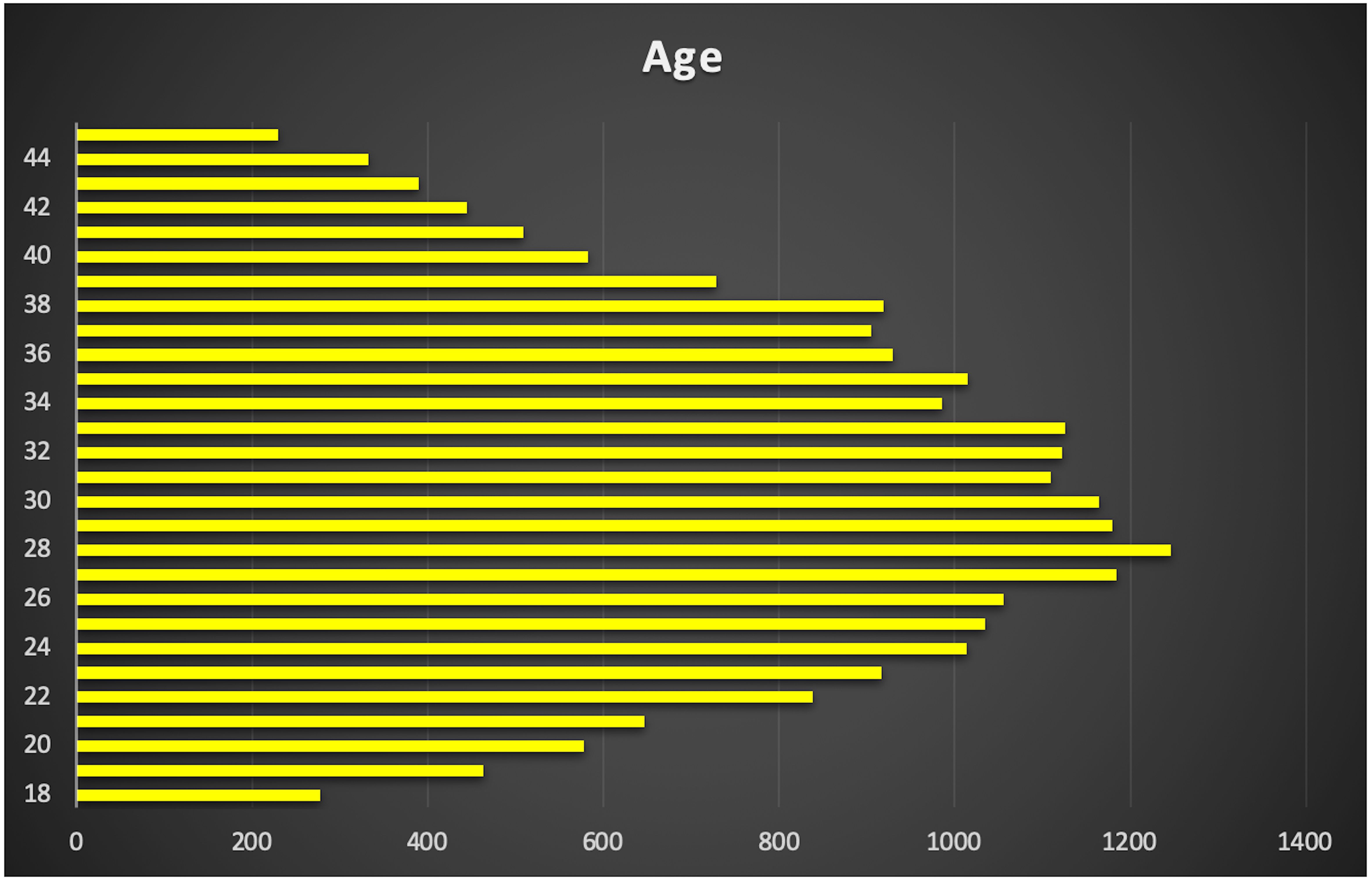
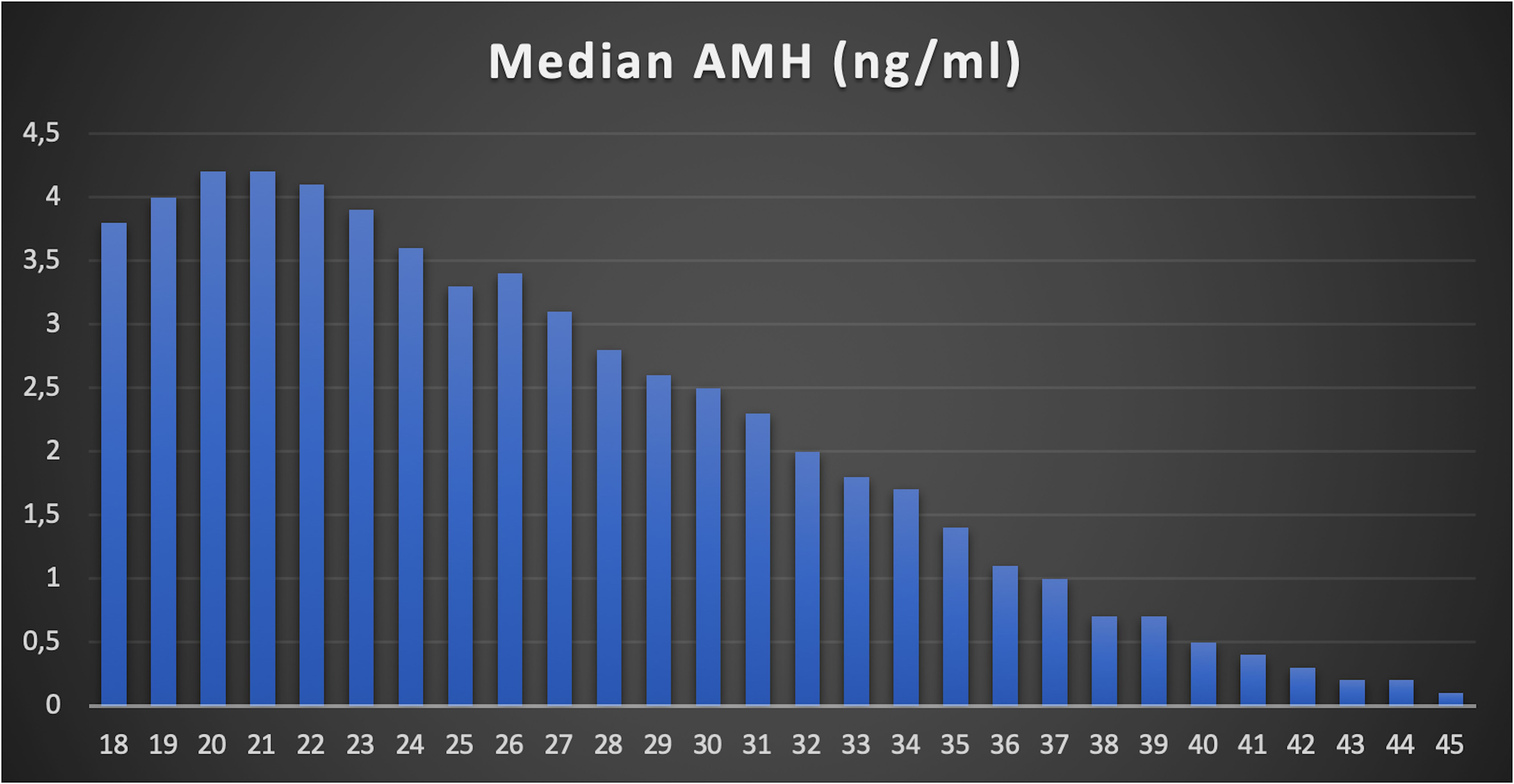
A total of 24,587 AMH results were retrieved from the hospital’s electronic database spanning from April 2015 to June 2024. After excluding patients below 18 and above 45 years old, along with those with incomplete data, 22,920 AMH results were included in the final analysis (Table 1). More than half of these results were from women aged between 24 and 33 years (Figure 1). Correlation analysis revealed a significant inverse relationship between AMH levels and age; as age increased, AMH levels progressively declined (Figure 2). By the age of 36, the median AMH values fell below 1.2 ng/ml. The prevalence of diminished ovarian reserve (DOR) was 15.9% at age 18, escalating to almost 96% by the age of 45 (Table 1, Figure 3).
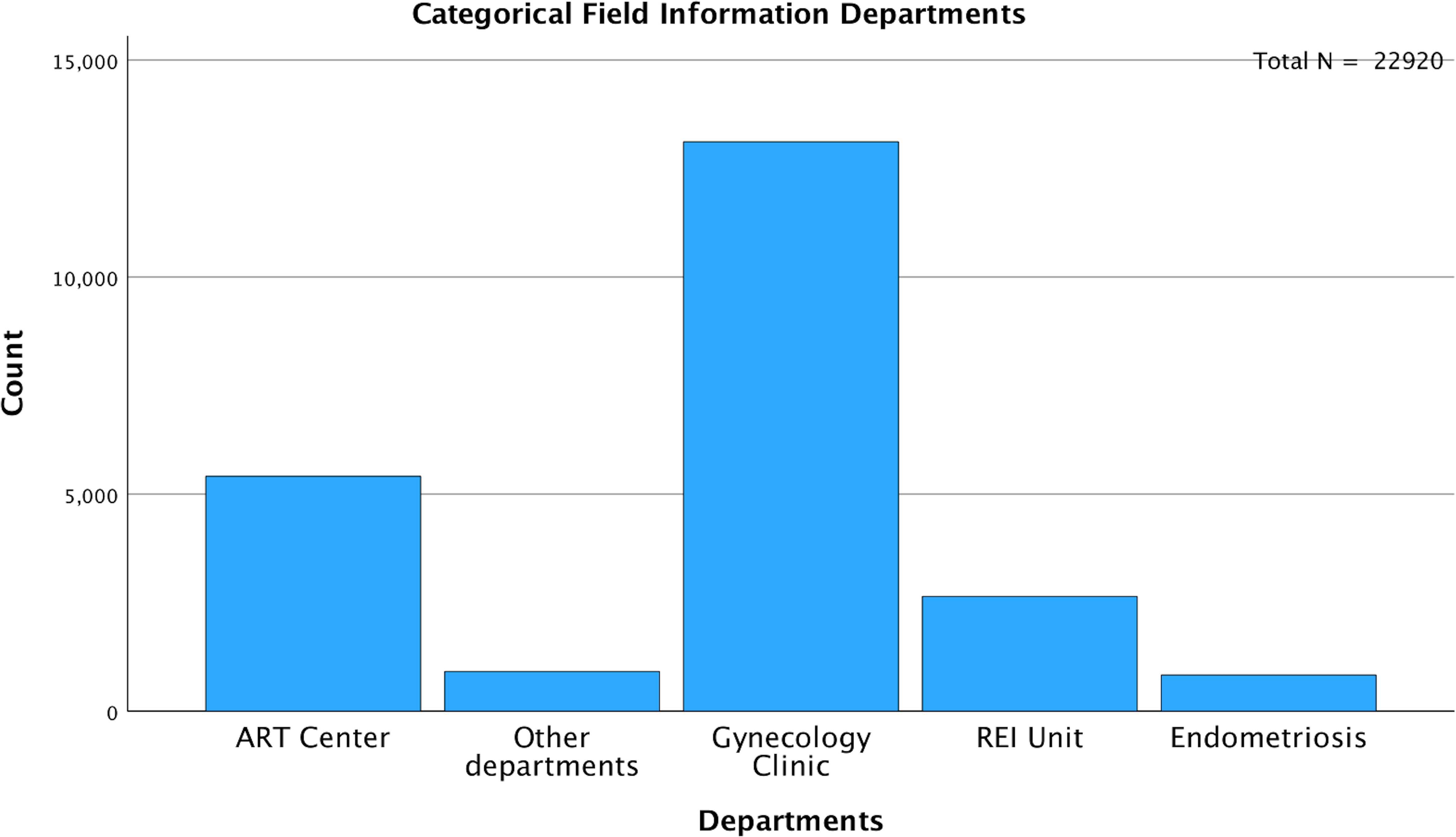
Among the entire cohort, 5,413 (23.6%) samples were gathered from the ART Center, 2,644 (11.5%) from the REI unit, and 834 (3.6%) from the Endometriosis Center. The remaining 14,029 (61.2%) samples were from departments including gynecology, endocrinology, dermatology, and internal medicine (Figure 4). To minimize bias, an additional analysis was conducted after excluding patients from the ART Center, REI unit, and Endometriosis Center (N=14,029). The age-stratified median AMH values in the excluded population (Gynecology Clinic: N=13,114, Other Departments: N=915) were comparable to those from the ART Center (N=5,413), REI unit (N=2,644), and Endometriosis Center (N=834) (Table 2).
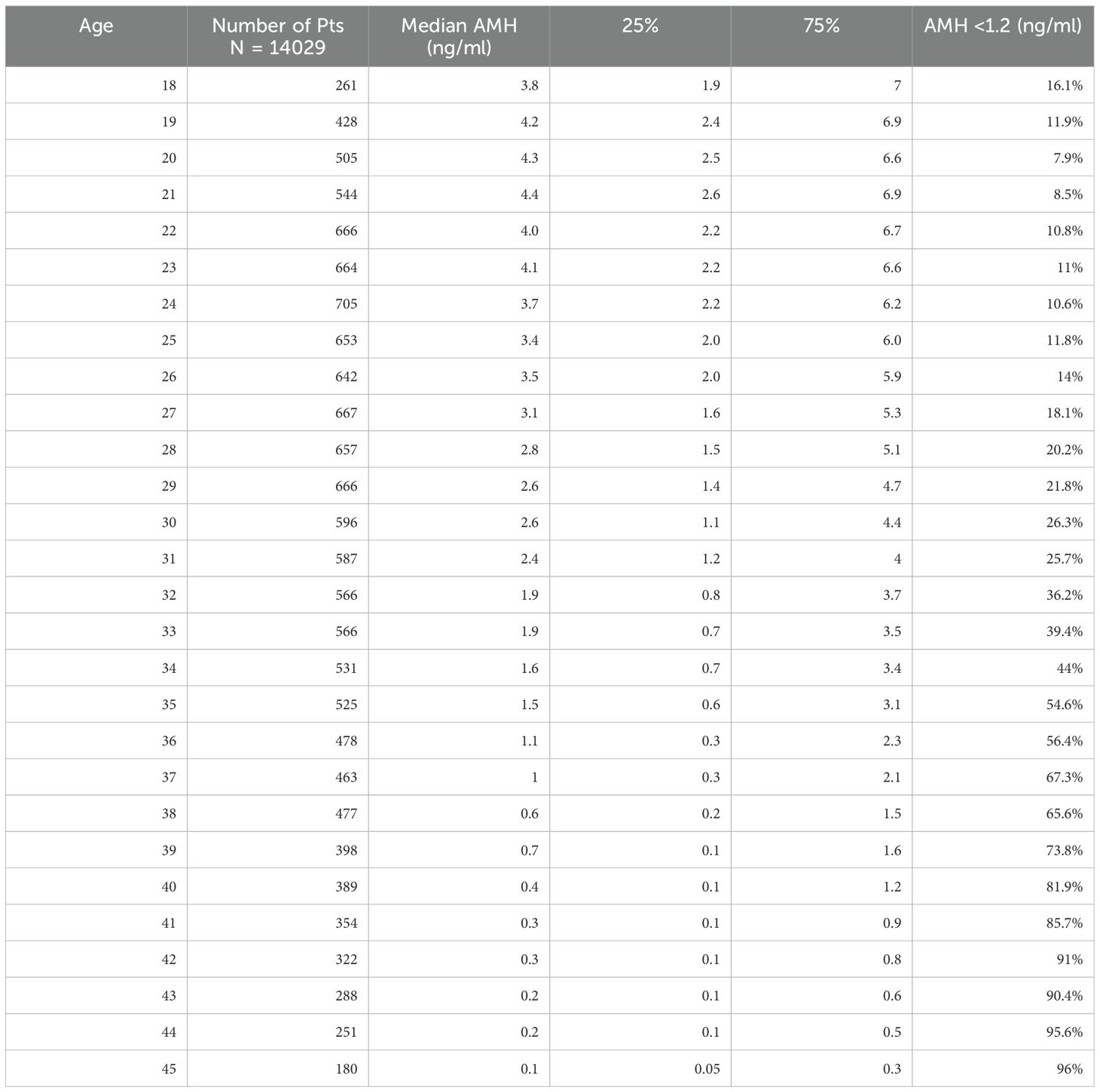
Table 2. Age stratified AMH values and presence of DOR (ART Center, REI and Endometriosis Unit Excluded).
9.
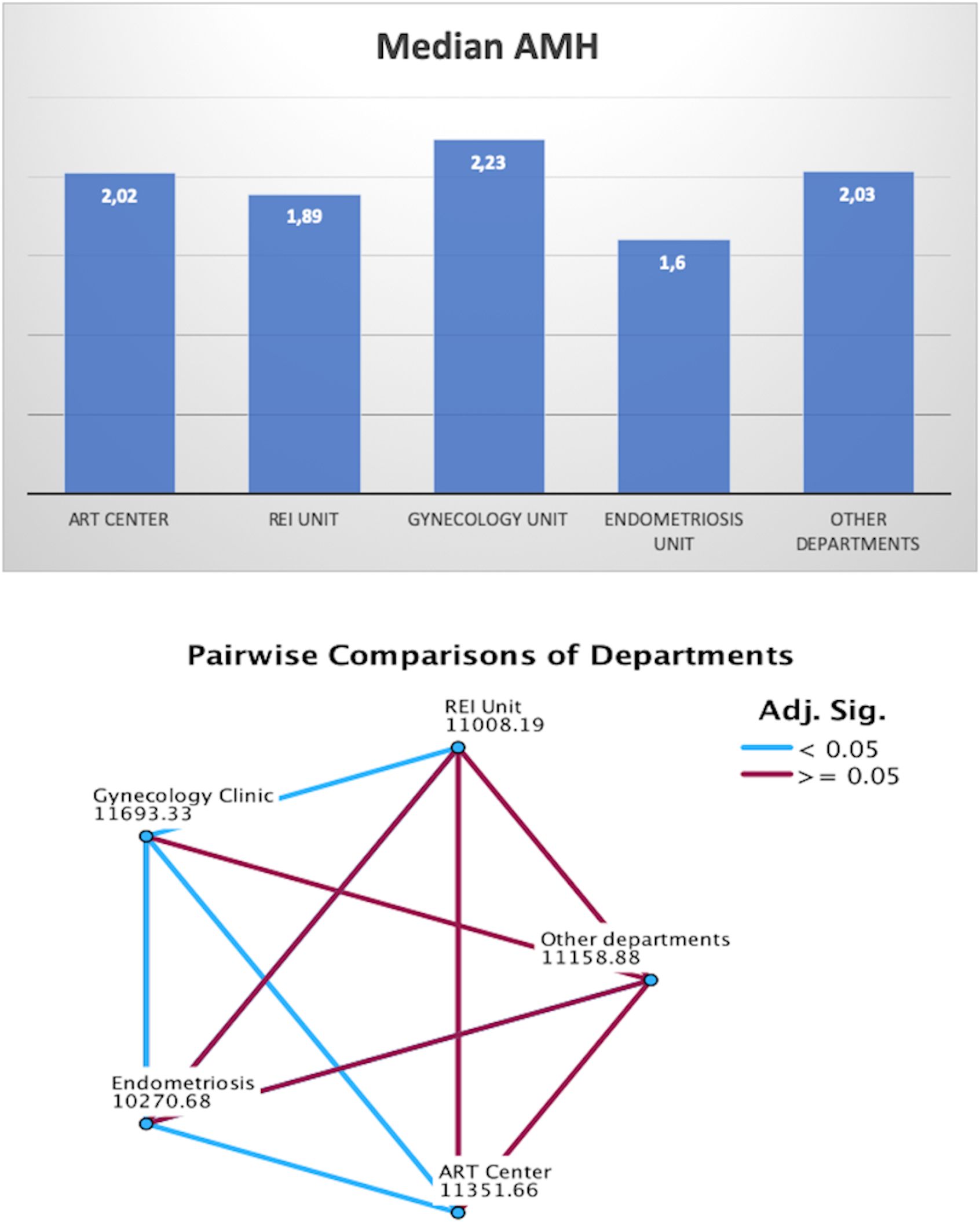
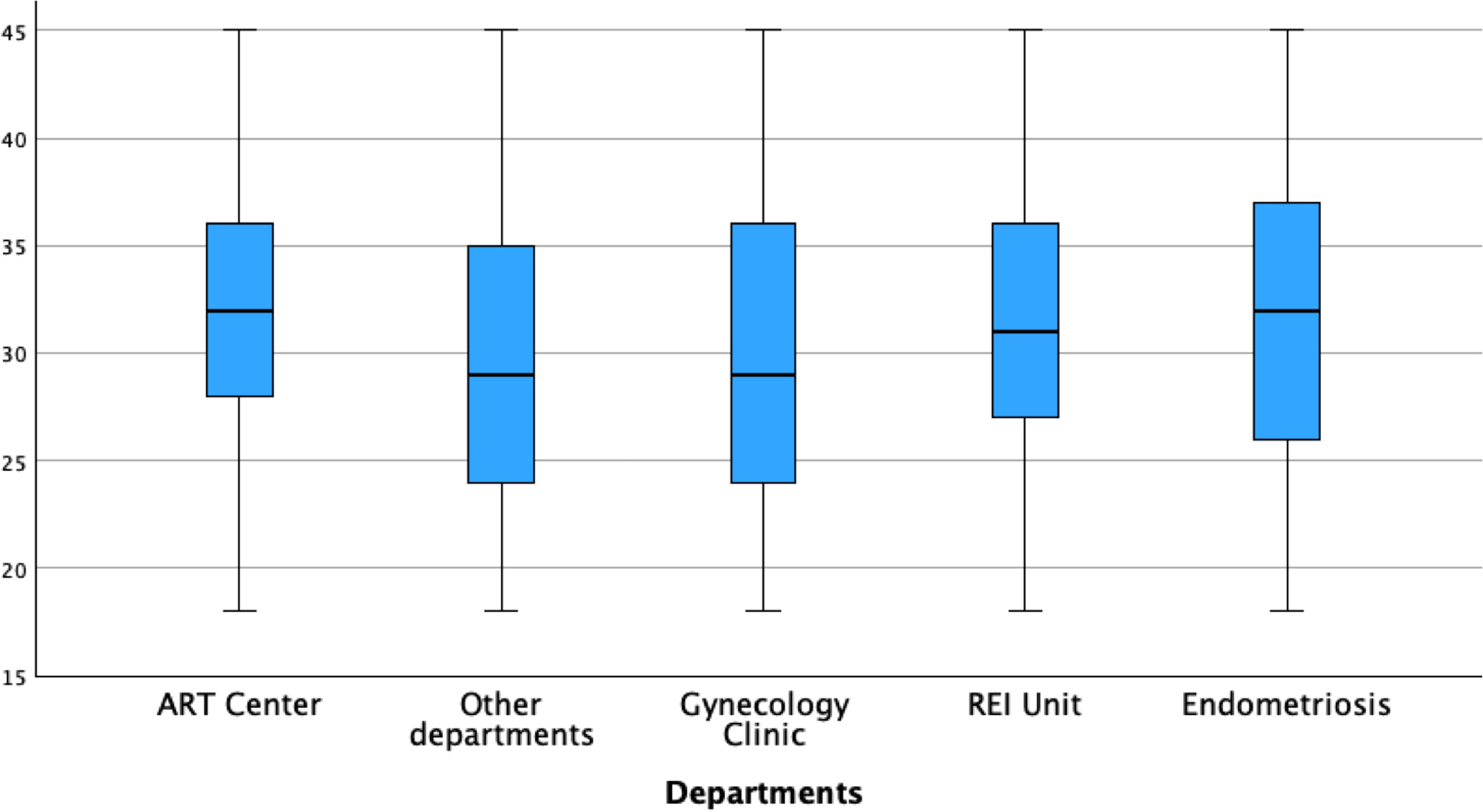
When stratifying AMH values by department, the Endometriosis Center exhibited the lowest median AMH levels (1.6 ng/ml), followed by the REI Unit (1.89 ng/ml), ART Center (2.02 ng/ml), Other Departments (2.03 ng/ml), and Gynecology Clinic (2.23 ng/ml) (Figure 5). Pairwise comparisons of AMH values between departments were illustrated in Figure 5. The average age of women was slightly higher in the Endometriosis Center (31.9 ± 6.8 years) compared to other departments (REI Unit: 31.3 ± 5.8 years, Gynecology Clinic: 30.1 ± 7.1 years, ART Center: 31.6 ± 5.2 years, Other Departments: 29.8 ± 7.3 years) (Figure 6).
Discussion
The results of this study provide further insights into the relationship between anti-Müllerian hormone (AMH) levels and age in a diverse population. AMH is a well-established marker of ovarian reserve, and its use in clinical practice continues to expand as we gain a deeper understanding of its role in assessing fertility potential (15). In this study, we have stratified AMH values by age and demonstrated significant age-related decline in AMH levels, supporting existing evidence in the literature (16–18). Specifically, by the age of 36, the median AMH value drops below 1.2 ng/mL, a commonly used threshold for diminished ovarian reserve (DOR) (19, 20). The prevalence of DOR rises steeply with age, from 15.9% at age 18 to nearly 96% by age 45, illustrating the rapid decline in ovarian reserve, particularly after the mid-30s.
The stratified data further reveal that the median AMH levels remain relatively stable during the early reproductive years, peaking around age 20 and then gradually declining thereafter. By age 30, a noticeable shift occurs, with AMH levels dropping from a median of 2.5 ng/mL at age 30 to 1.4 ng/mL by age 35. These findings are in line with prior studies that demonstrate a sharp decrease in AMH during the late 30s, reflecting the accelerated loss of ovarian follicles during this period (21).
Furthermore, the study compared AMH values across different clinical departments, shedding light on the impact of specific conditions like endometriosis on ovarian reserve. Women treated in the Endometriosis Unit had the lowest median AMH levels, which were significantly lower than those in the Gynecology Clinic, ART Center, or REI Unit. This observation is consistent with the hypothesis that endometriosis, particularly when associated with ovarian involvement (endometriomas), may accelerate ovarian reserve depletion through inflammatory or mechanical damage to the ovarian tissue (22–24). These findings underline the importance of early fertility assessment in women with endometriosis to offer timely interventions, such as fertility preservation.
The exclusion of patients from infertility-specific clinics (ART Center, REI Unit, and Endometriosis Center) revealed similar age-stratified AMH patterns in the general population, reinforcing the general applicability of the AMH nomogram. The similarity in results across these subpopulations suggests that AMH testing can reliably assess ovarian reserve regardless of the clinical setting, though certain conditions, like endometriosis, warrant special consideration.
These age-stratified AMH data provide clinicians with a valuable tool to counsel patients about their reproductive potential. The ability to compare a patient’s AMH level with age-specific reference ranges allows for more personalized fertility assessments. For example, a 30-year-old woman with an AMH of 2.5 ng/mL may be reassured that her ovarian reserve is within the expected range for her age, while a woman of the same age with an AMH 1.4 ng/mL could be counseled about the potential for diminished ovarian reserve and options for fertility preservation or more immediate intervention.
The clinical implications of these findings are profound. AMH testing can help guide decisions about fertility treatment, timing of family planning, and the need for interventions such as oocyte cryopreservation. Moreover, for patients undergoing assisted reproductive technologies (ART), age-stratified AMH data can assist in predicting ovarian response to stimulation and tailoring treatment protocols.
One notable strength of our study is the large sample size, which includes 22,920 AMH results collected over a nearly decade-long period. This has allowed us to provide robust age-stratified AMH data, contributing valuable information to existing nomograms. Our study population is unique in its inclusion of patients from a wide range of clinical settings, including infertility clinics, gynecology, endocrinology, and even departments not primarily focused on reproductive health. This diversity enhances the generalizability of our findings.
Despite the strengths of this study, including the large sample size and inclusion of diverse clinical populations, there are some limitations. First, the study is retrospective and relies on electronic medical records of a tertiary hospital, which may introduce selection bias. Additionally, while AMH is a reliable marker of ovarian reserve, it does not provide a complete picture of fertility potential. Other factors, such as antral follicle count (AFC), FSH levels, and the woman’s overall health, should be considered in conjunction with AMH levels when assessing fertility.
In conclusion, the age-stratified AMH nomogram presented in this study offers a valuable resource for both clinicians and patients. By providing detailed reference ranges across reproductive ages, this nomogram facilitates more accurate assessment of ovarian reserve and empowers women to make informed decisions about their reproductive health. Future research should focus on the longitudinal implications of AMH levels and explore the integration of these nomograms into broader fertility assessment protocols, particularly in populations with specific conditions like endometriosis or polycystic ovary syndrome.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethics approval for the study was obtained from the Bursa Uludag University Clinical Trials Ethical Committee with the number 2024-19/5. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
KA: Data curation, Conceptualization, Writing – original draft, Methodology, Formal analysis. IK: Writing – review & editing, Methodology. BK: Writing – original draft, Data curation. AT: Resources, Writing – original draft, Data curation. IT: Writing – original draft, Data curation, Methodology. GU: Writing – review & editing, Conceptualization, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, and Wu QJ. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging (Albany NY). (2019) 11:10952–91. doi: 10.18632/aging.102497
2. Recent advances in medically assisted conception. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. (1992) 820:1–111.
3. Practice Committee of the American Society for Reproductive Medicine. Electronic address:YXNybUBhc3JtLm9yZw==; Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: a committee opinion. Fertil Steril. (2021) 116:1255–65. doi: 10.1016/j.fertnstert.2021.08.038
4. Tal R and Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. (2017) 217:129–40. doi: 10.1016/j.ajog.2017.02.027
5. The Unexplained Infertility guideline group, Romualdi D, Ata B, Bhattacharya S, Bosch E, Costello M, et al. Evidence-based guideline: Unexplained Infertility. Hum Reprod. (2023) 38(10):1881–90. Available online at: https://www.eshre.eu/guideline/UI.
6. Broekmans FJ, de Ziegler D, Howles CM, Gougeon A, Trew G, and Olivennes F. The antral follicle count: practical recommendations for better standardization. Fertil Steril. (2010) 94:1044–51. doi: 10.1016/j.fertnstert.2009.04.040
7. Fleming R, Seifer DB, Frattarelli JL, and Ruman J. Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Reprod BioMed Online. (2015) 31:486–96. doi: 10.1016/j.rbmo.2015.06.015
8. Lima ML, Martins WP, Coelho Neto MA, Nastri CO, Ferriani RA, and Navarro PA. Assessment of ovarian reserve by antral follicle count in ovaries with endometrioma. Ultrasound Obstet Gynecol. (2015) 46:239–42. doi: 10.1002/uog.2015.46.issue-2
9. Tal R, Seifer DB, Tal R, Granger E, Wantman E, and Tal O. AMH highly correlates with cumulative live birth rate in women with diminished ovarian reserve independent of age. J Clin Endocrinol Metab. (2021) 106:2754–66. doi: 10.1210/clinem/dgab168
10. Li HW, Lee VC, Lau EY, Yeung WS, Ho PC, and Ng EH. Role of baseline antral follicle count and anti-Mullerian hormone in prediction of cumulative live birth in the first in vitro fertilisation cycle: a retrospective cohort analysis. PloS One. (2013) 8:e61095. doi: 10.1371/journal.pone.0061095
11. Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, and Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400–135 treatment cycles. Hum Reprod. (2011) 26:1768–74. doi: 10.1093/humrep/der106
12. Balachandren N, Kastora SL, Yasmin E, Saridogan E, Jurkovic D, Learner HE, et al. An age-related nomogram for antral follicle count: an observational study of 3821 women. Reprod BioMed Online. (2024) 49:104295. doi: 10.1016/j.rbmo.2024.104295
13. Du X, Ding T, Zhang H, Zhang C, Ma W, Zhong Y, et al. Age-specific normal reference range for serum anti-müllerian hormone in healthy chinese han women: A nationwide population-based study. Reprod Sci. (2016) 23:1019–27. doi: 10.1177/1933719115625843
14. Humaidan P, Alviggi C, Fischer R, and Esteves SC. The novel POSEIDON stratification of ‘Low prognosis patients in Assisted Reproductive Technology’ and its proposed marker of successful outcome. F1000Res. (2016) 5:2911. doi: 10.12688/f1000research
15. Cedars MI. Evaluation of female fertility-AMH and ovarian reserve testing. J Clin Endocrinol Metab. (2022) 107:1510–9. doi: 10.1210/clinem/dgac039
16. Kotlyar AM and Seifer DB. Ethnicity/race and age-specific variations of serum AMH in women-A review. Front Endocrinol (Lausanne). (2021) 11:593216. doi: 10.3389/fendo.2020.593216
17. Hawkins Bressler L and Steiner A. Anti-Müllerian hormone as a predictor of reproductive potential. Curr Opin Endocrinol Diabetes Obes. (2018) 25:385–90. doi: 10.1097/MED.0000000000000440
18. Broer SL, Broekmans FJ, Laven JS, and Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. (2014) 20:688–701. doi: 10.1093/humupd/dmu020
19. Drakopoulos P, Bardhi E, Boudry L, Vaiarelli A, Makrigiannakis A, Esteves SC, et al. Update on the management of poor ovarian response in IVF: the shift from Bologna criteria to the Poseidon concept. Ther Adv Reprod Health. (2020) 14:2633494120941480. doi: 10.1177/2633494120941480
20. Esteves SC, Yarali H, Vuong LN, Carvalho JF, Özbek İY, Polat M, et al. Antral follicle count and anti-Müllerian hormone to classify low-prognosis women under the POSEIDON criteria: a classification agreement study of over 9000 patients. Hum Reprod. (2021) 36:1530–41. doi: 10.1093/humrep/deab056
21. Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. (2012) 97:4650–5. doi: 10.1210/jc.2012-1440
22. Sanchez AM, Viganò P, Somigliana E, Panina-Bordignon P, Vercellini P, and Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update. (2014) 20:217–30. doi: 10.1093/humupd/dmt053
23. Kasapoglu I, Ata B, Uyaniklar O, Seyhan A, Orhan A, Yildiz Oguz S, et al. Endometrioma-related reduction in ovarian reserve (ERROR): a prospective longitudinal study. Fertil Steril. (2018) 110:122–7. doi: 10.1016/j.fertnstert.2018.03.015
Keywords: AMH, ovarian reserve, age stratification, diminished ovarian reserve, nomogram, infertility, endometriosis
Citation: Aslan K, Kasapoglu I, Kosan B, Tunali A, Tellioglu I and Uncu G (2025) Age-stratified anti-Müllerian hormone (AMH) nomogram: a comprehensive cohort study including 22.920 women. Front. Endocrinol. 16:1612194. doi: 10.3389/fendo.2025.1612194
Received: 15 April 2025; Accepted: 06 June 2025;
Published: 20 June 2025.
Edited by:
Michal Zikan, Charles University, CzechiaCopyright © 2025 Aslan, Kasapoglu, Kosan, Tunali, Tellioglu and Uncu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gurkan Uncu, Z3VuY3VAdWx1ZGFnLmVkdS50cg==
†ORCID: Kiper Aslan, orcid.org/0000-0002-9277-7735
Isil Kasapoglu, orcid.org/0000-0002-1953-2475
Bahadir Kosan, orcid.org/0009-0003-9154-1880
Gurkan Uncu, orcid.org/0000-0001-7660-8344
 Kiper Aslan
Kiper Aslan Isil Kasapoglu
Isil Kasapoglu Bahadir Kosan†
Bahadir Kosan† Aylin Tunali
Aylin Tunali Ilayda Tellioglu
Ilayda Tellioglu