- 1Department of Andrology, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
- 2Department of Surgery, Hai’an Hospital of Traditional Chinese Medicine, Nantong, China
- 3Department of Andrology, Nanjing Hospital of Traditional Chinese Medicine, Nanjing Hospital of Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
Objective: This study aimed to explore the differences of the psychological states and total testosterone (TT) in patients with erectile dysfunction (ED) across various age groups.
Methods: A total of 1411 ED patients were enrolled from the Department of Andrology, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine from September 2018 to September 2021. The SCL-90 was used to evaluate the psychological condition of patients while the 5-item international index of erectile function (IIEF-5) questionnaire was applied to estimate the severity of ED. The serum TT level of patients was also measured. ED patients were divided into three groups (group A: 20–30 years old; group B: 31–40 years old; group C: 41–50 years old). In addition, patients in each group were divided into three groups including mild group (12<IIEF-5<21), moderate group (8<IIEF-5<11), and severe group (IIEF-5<7). The level of TT and SCL-90 scores were compared between groups. Finally, relationships between SCL-90, STAI scores and TT were explored.
Results: (1) Differences of TT between groups with different ages: The TT level of group A was higher than that of group B and C (P<0.05). (2) Differences of TT between patients with different ED severity in each age group: The TT level of severe ED patients was lower than that of mild ED patients in group B (P<0.05) while the TT level of severe ED patients was lower than that of mild and moderate ED patients in group C (P<0.05). (3) Differences of SCL-90 and STAI scores between patients with different severity in each age group: The factor scores of anxiety, psychoticism, obsession, interpersonal relationship, depression and total scores of SCL-90 of group A and B were higher than those of group C (P<0.05). (4) Differences of SCL-90 and STAI scores between patients with different severity in each age group: 1) The factor scores of hostility, anxiety, phobia, paranoid, psychoticism, obsession, depression and total scores of SCL-90 of mild ED patients were lower than those of moderate and severe ED patients in group B (P<0.05); 2) The factor scores of anxiety and other of SCL-90, as well as the state anxiety scores of STAI of mild ED patients were lower than those of severe ED patients in group C (P<0.05). (5) The level of TT was positively related to IIEF-5 scores of ED patients (r = 0.06; P = 0.02). Both SCL-90 (r = -0.08; P < 0.01) and STAI (r = -0.06; P = 0.04) scores were negatively associated with IIEF-5 scores of ED patients. STAI scores were positively related to SCL-90 scores of ED patients (r = 0.64; P < 0.01).
Conclusion: Age-stratified results demonstrate a pronounced differential impact of TT and psychological factors on ED. Compared with younger patients, serum TT has more significant effects on elderly patients with ED. For young ED patients, the influences of psychological factors are significantly higher than that of elderly patients, and young patients show more severe anxiety and depression.
1 Introduction
Erectile dysfunction (ED), a common male disease, is considered as a complex and multifactorial disease, which seriously affects the quality of life of patients (1, 2). At present, ED is divided into organic, psychological and mixed according to its causes (2, 3). ED is affected by various factors, which have different effects on patients of different ages, such as vascular, neurological, psychological, and endocrine hormones (4). Epidemiological evidences from multinational cohort studies consistently demonstrate a significantly higher prevalence of ED in elderly males (>40 years) compared to younger populations. The prevalence of ED in elderly males reaches 37% (5–7). The influencing factors for ED were focused on elderly patients over 40 years old, while the research on young ED patients was ignored (8).
For young patients with ED, the prevalence of common ED related complications is much lower than that of older patients (9). Young ED patients (≤40 years) exhibit significantly lower prevalence rates of comorbidities: hypogonadism (10.8% vs 38.7%), hypertension (15.6% vs 29%), cardiovascular disease (3.3% vs 16%) compared to older their older patients (≥40 years) (9–11). However, the number of young patients under the age of 40 seeking treatment for ED is gradually increasing (12). The ED consultation rates among men under 40 years, rise from 5% of total cases in 2010 to 15% in 2015 (8). Testosterone mediates penile vasodilation via nitric oxide synthase activation (13), however, the correlation between total testosterone (TT) levels and ED remains inconclusive. This discrepancy may reflect the multifactorial nature of ED pathogenesis across different age groups. The characteristics of the overlap of testosterone deficiency symptoms with other diseases make the diagnosis of testosterone deficiency and its related comorbidities prone to missed diagnosis or overtreatment (14–16). TT plays a key role in male sexual response, and symptoms associated with low testosterone such as hypogonadism, are also important risk factors for ED (17–20). However, the age-related TT decline (21) contradicts increased ED incidence in younger adults (22), suggesting multifactorial pathophysiology.
In addition, the psychological factors are key risk factors for the occurrence and development of ED (23–25). A retrospective study involving 3500 patients aged 18–48 showed that depression and anxiety were important predictors of young ED patients (26). The incidence of anxiety and depression symptoms in ED patients are significantly higher than that in healthy people (17.1% vs 12.9%) (27). These evidences indicate that there may be a bidirectional effect between psychological factors and ED (28–31). Given the age-dependent roles of testosterone deficiency and psychological distress in ED pathogenesis, in this cross-sectional study, TT levels and psychological assessments were collected from 1,411 ED patients stratified by age. The objective was to elucidate the differential contributions of TT and psychogenic factors to ED across distinct age groups.
2 Materials and methods
2.1 Participants
This study was approved by the ethical committee of Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine. Moreover, written informed consents were obtained from all patients. A total of 1411 ED patients were enrolled from the Department of Andrology, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine from September 2018 to September 2021.
The patients enrolled in this study were aged 20–50 years, who had a regular sexual partner and regular sexual life for more than 6 months. Those with organic abnormalities of penis and testis, combined with other types of sexual dysfunction, sexual partners with sexual dysfunction, previous psychiatric history, organic mental disorders or mental disorders were excluded.
2.2 Instruments
2.2.1 Serum TT
Fasting venous blood was drawn from the subjects at 8:00 in the morning, and the TT level was detected by chemiluminescence assay (normal value range: 248.00~835.00ng/dL).
2.2.2 Symptom checklist 90
SCL-90 is a self-reported symptom scale, which is often used to evaluate psychopathological symptoms. The scale includes 10 main symptom dimensions: somatization, obsession, interpersonal relationship, depression, anxiety, hostility, phobia, paranoid, psychoticism and other. In this study, each question has a score of 0-4. The lower the score, the better the psychological health condition.
2.2.3 State-trait anxiety inventory
STAI is a self-report questionnaire consisting of 2 scales with 20 questions. Questions 1–20 are state anxiety scale, which is used to assess immediate or recent experiences and feelings. Questions 21–40 are trait anxiety scale, which is used to assess people’s regular emotional experience. Each question has a score of 1-4.
2.3 Statistical analysis
ED patients were divided into three groups (group A: 20–30 years old, n=704; group B: 31–40 years old, n=457; group C: 41–50 years old, n=250). The TT level and psychological evaluation data were analyzed by ANOVA and Tukey’s post hoc-test for pairwise comparisons (LSD test) in each group. ED patients in each age group were divided into three groups including mild group (12<IIEF-5<21), moderate group (8<IIEF-5<11), and severe group (IIEF-5<7). The data of TT level and psychological evaluation of ED patients with different levels of severity were analyzed by ANOVA and Tukey’s post hoc-test for pairwise comparisons (LSD test) in different age groups. Finally, relationships between SCL-90, STAI scores and TT were explored. All analyses were carried out with SPSS 25 statistical package (IBM Corporation, Armonk, NY, USA), and P<0.05 was considered statistically significant.
3 Result
3.1 Comparison of TT in each age group
Compared with 704 patients in group A, 457 patients in group B and 250 patients in group C had significantly lower TT levels. [A (447.35 ± 127.46) ng/dL VS B (430.62 ± 116.14) ng/dL, C (425.72 ± 93.47) ng/dL)] (P<0.05).
3.2 Comparison of TT in patients with different ED severity in each age group
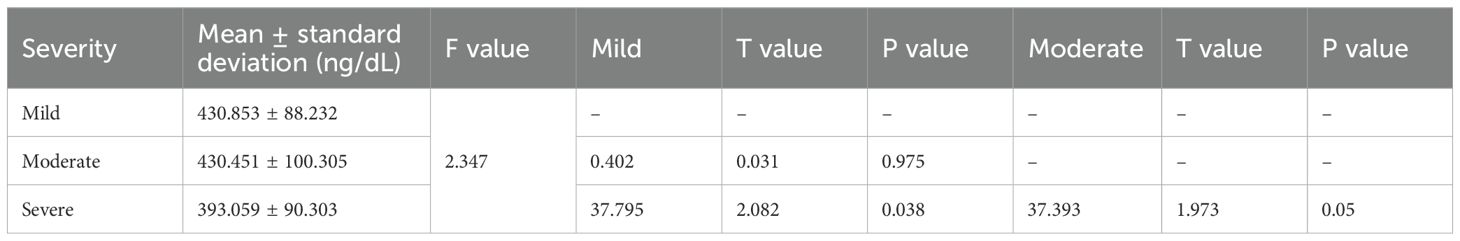
3.2.1 Group A
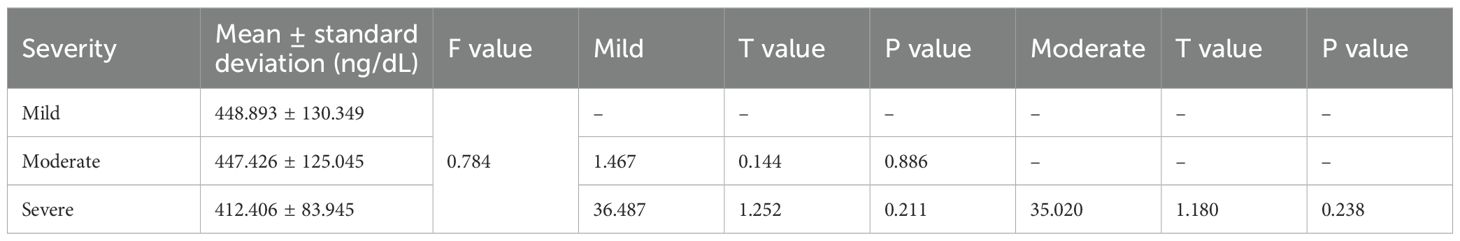
There was no statistically significant difference in the TT level between 443 patients with mild ED and 241 patients with moderate ED and 20 patients with severe ED (P>0.05) (Table 1).
3.2.2 Group B
Compared with that in 224 patients with mild ED, the level of TT was significantly declined in 40 patients with severe ED (P<0.05). There was no statistically significant difference in the TT level between 193 patients with moderate ED and patients with mild or severe ED (P>0.05) (Table 2).
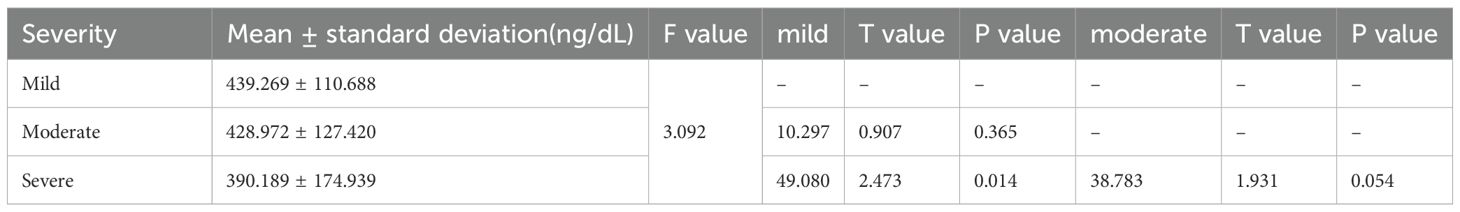
3.2.3 Group C
Compared with that in 128 patients with mild ED and 89 patients with moderate ED, the level of TT was significantly declined in 33 patients with severe ED (P<0.05). No statistically significant difference was observed in the TT level among mild and moderate patients (P>0.05) (Table 3).
3.3 Comparison of SCL-90 and STAI scores in each age group
The factor scores of anxiety, psychoticism, obsession, interpersonal relationship, depression and total scores of SCL-90 of group A and B were higher than those of group C (P<0.05). The factor scores of trait anxiety, state anxiety and total scores of STAI of group A and B were higher than those of group C (P<0.05) (Table 4).
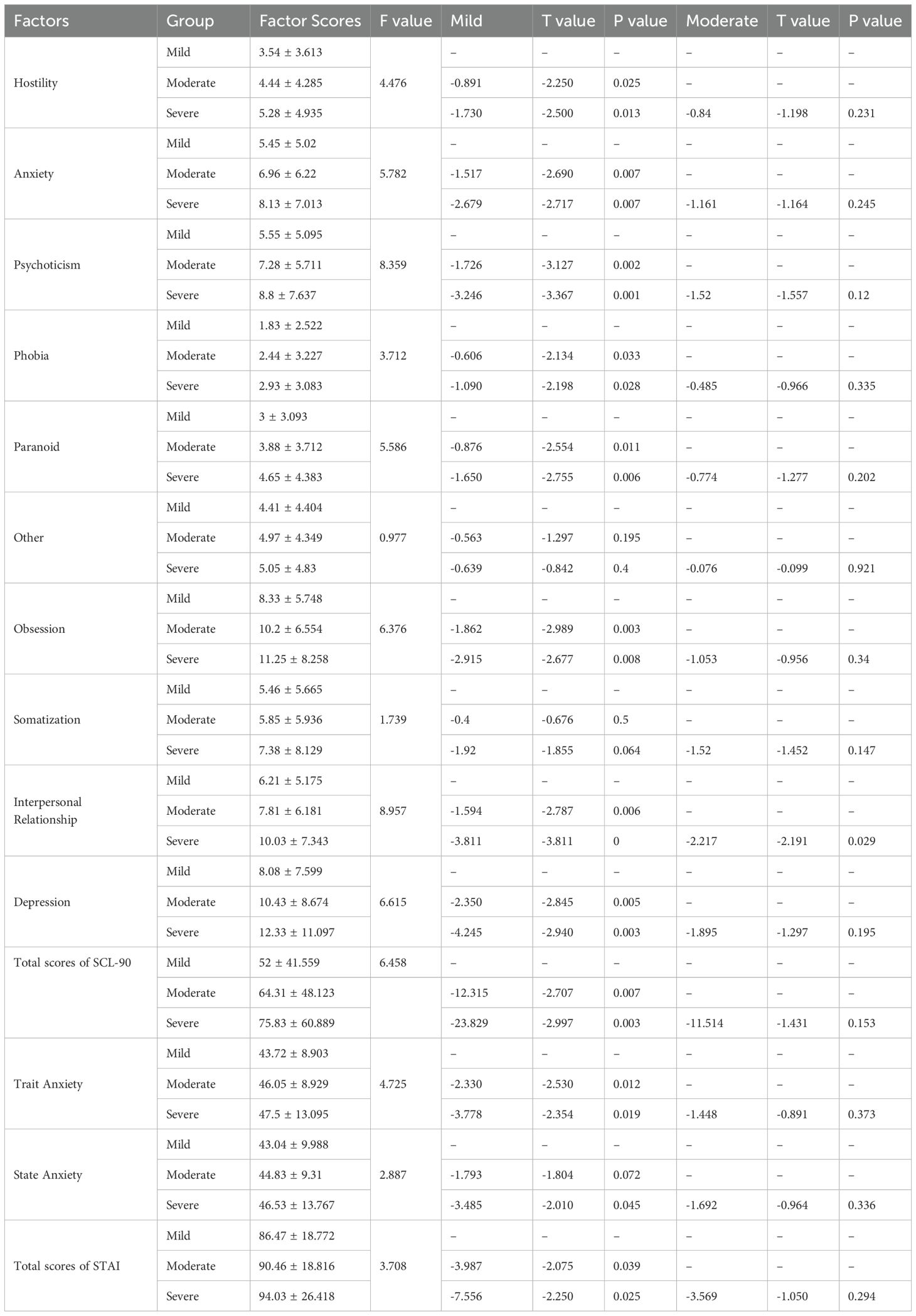
3.4 Differences of SCL-90 and STAI scores between patients with different ED severity in each age group
3.4.1 Group A
There were no significant differences in SCL-90 and STAI scores of mild, moderate and severe ED patients (P>0.05).
3.4.2 Group B
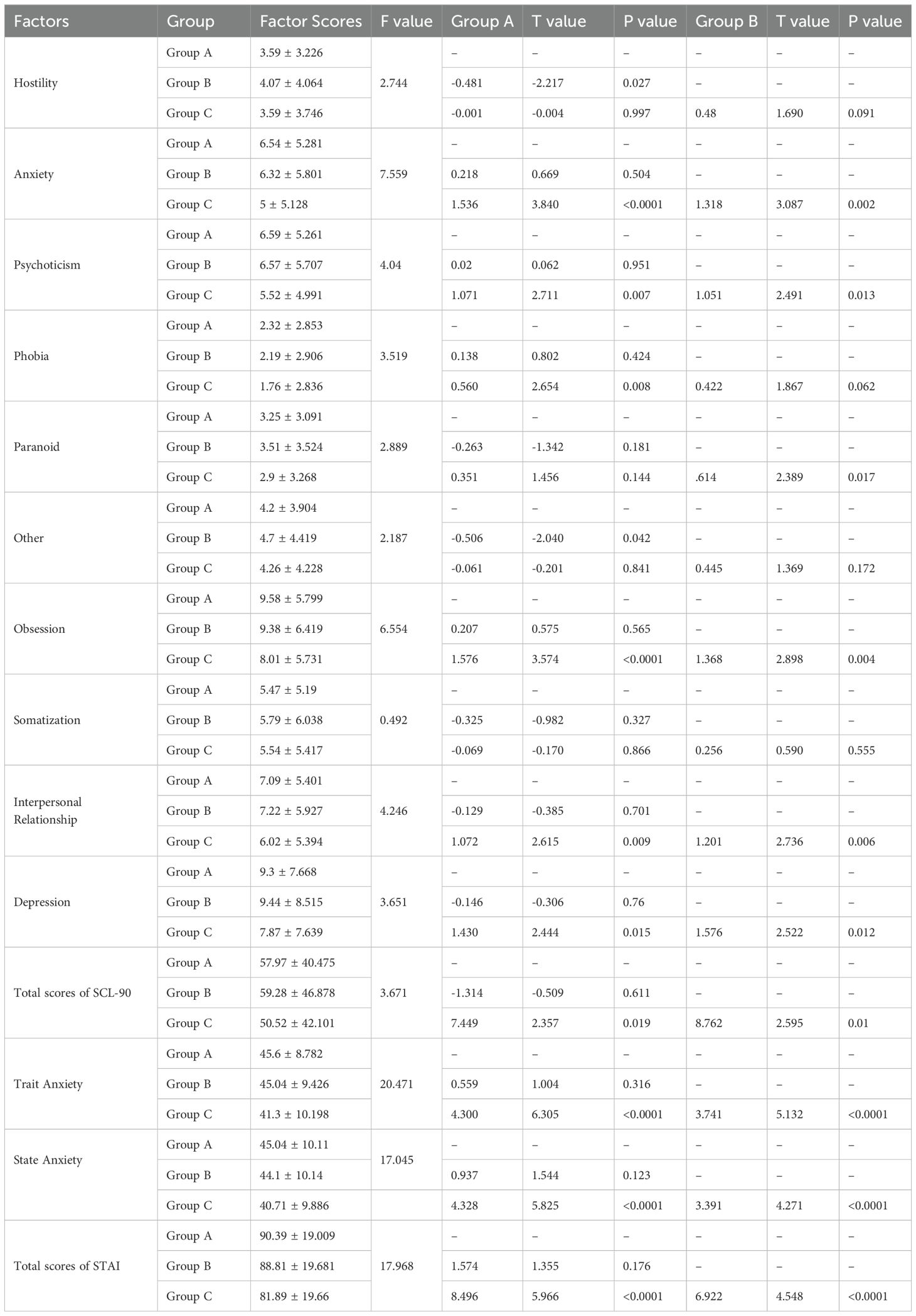
There were no significant differences in SCL-90 and STAI scores of moderate and severe ED patients (P>0.05). The factor scores of hostility, anxiety, phobia, paranoid, psychoticism, obsession, depression and total scores of SCL-90 of mild ED patients were lower than those of moderate and severe ED patients (P<0.05). The factor scores of trait anxiety, state anxiety and total scores of STAI of mild ED patients were lower than those of moderate and severe ED patients (P<0.05) (Table 5).
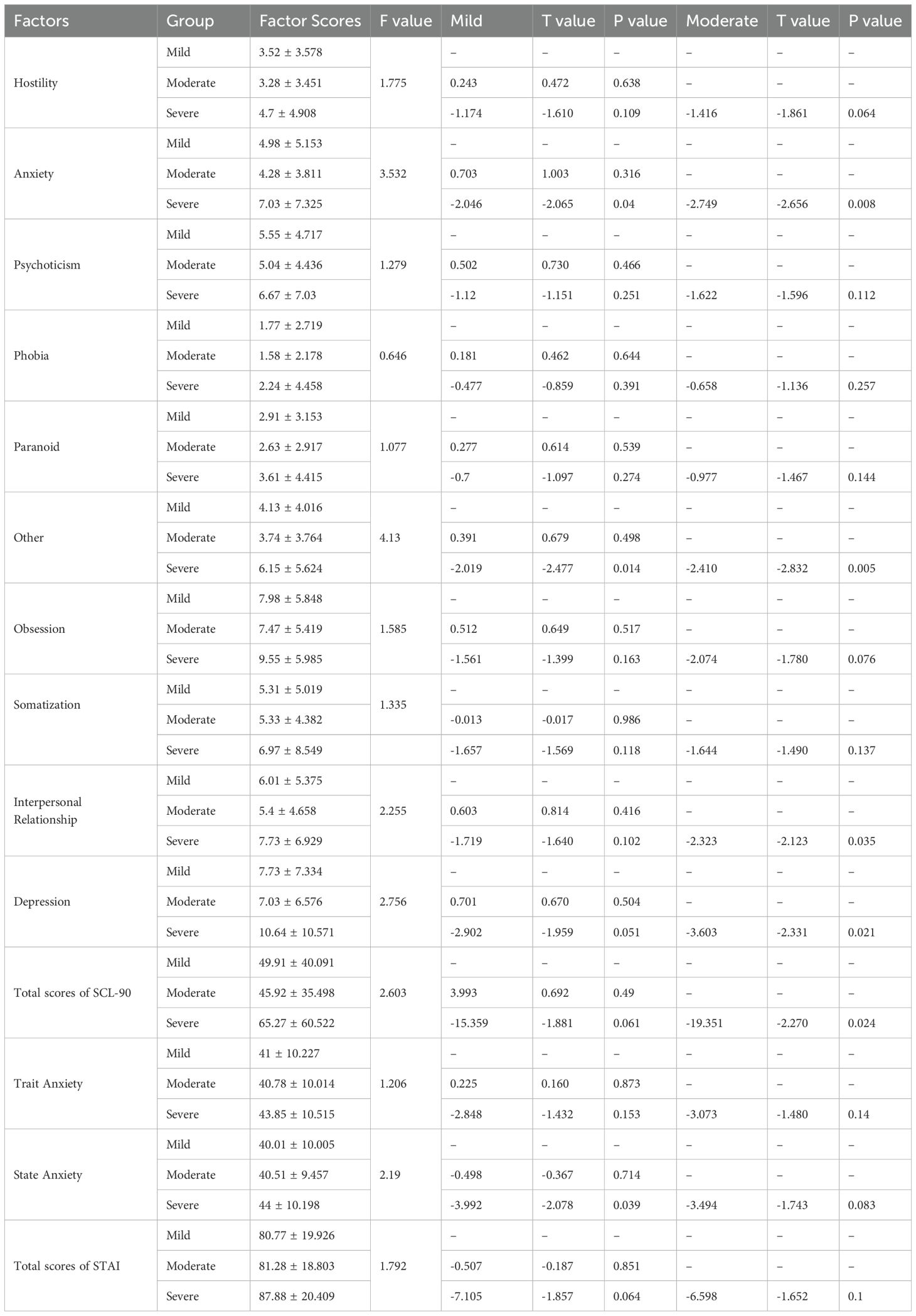
3.4.3 Group C
There were no significant differences in SCL-90 and STAI scores of mild and moderate ED patients (P>0.05). The factor scores of anxiety and other of SCL-90, as well as the state anxiety scores of STAI of mild ED patients were lower than those of severe ED patients (P<0.05). Furthermore, the factor scores of interpersonal relationship, depression and total scores of SCL-90 of moderate ED patients were higher than those of severe ED patients (P<0.05) (Table 6).
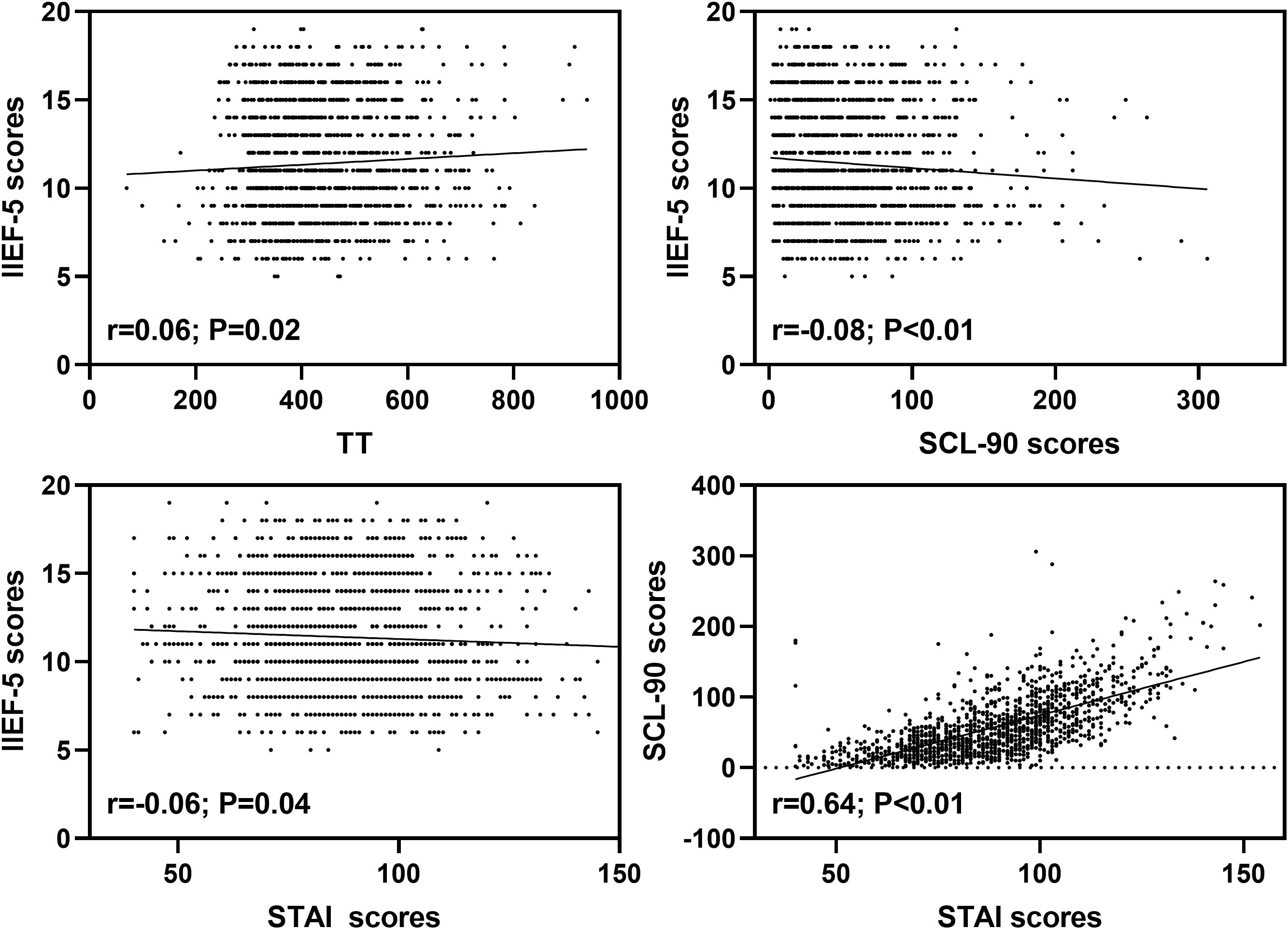
3.5 Relationships between SCL-90, STAI scores and TT
The level of TT was positively related to IIEF-5 scores of ED patients (r = 0.06; P = 0.02). In addition, both SCL-90 (r = -0.08; P < 0.01) and STAI (r = -0.06; P = 0.04) scores were negatively associated with IIEF-5 scores of ED patients. Moreover, STAI scores were positively related to SCL-90 scores of ED patients (r = 0.64; P < 0.01) (Figure 1).
4 Discussion
ED, as a common male disease, seriously affects the sexual quality of life of patients and their sexual partners (32). It has been found that the incidence rate of ED is gradually increasing with age, and main patients are over 40 years old (33). However, a recent population-based study has demonstrated that with the change of people’s living habits, the ED population is becoming younger (34). Consequently, elucidating the incidence trends in younger populations and conducting age-stratified comparative analyses of disease manifestations could enable early intervention and precision medicine approaches.
TT is a key factor in regulating male sexual response, and low TT level is related to low libido in male (35, 36). Meanwhile, testosterone-deficient metabolic syndrome is significantly associated with severe ED (12). This study revealed lower TT levels in patients with severe ED compared to those with mild ED. Low TT level is considered as a sentinel manifestation of metabolic syndrome, and is also closely related to penile vascular injury, diabetes and other causes of ED (37). In this study, there was a significant correlation between TT and age: TT level showed a decline with age in ED patients. However, in patients aged 20–30 years with ED, there was no significant difference in TT levels among patients with ED of different levels of ED severity. Only older patients with higher ED severity showed significantly lower TT levels. This paradoxical finding, which contradicts previous studies reporting increasing ED prevalence among younger populations, suggests that non-hormonal etiological factors may play a more predominant role in ED pathogenesis in young patients compared to older individuals. TT level, which gradually decreases with age, may cause the patient’s sexual desire to decline, thus reducing their attention to sexual life. In addition, the decrease of TT level and the progress of TT-related comorbidities make the ED severity of elderly patients higher. While the precise pathogenesis remains to be elucidated, one plausible explanation is that as low TT level does not exist for a long time in young patients, it may not cause low testosterone related comorbidities (38). Although isolated low TT factor may not manifest as overt ED symptoms, routine testosterone monitoring remains clinically warranted in elderly patients, given the well-established association between hypogonadism and multiple age-related comorbidities.
It is undeniable that psychological factors play a key role in the occurrence and development of ED (39–41). Accumulated studies have established that a bidirectional relationship between psychological distress and ED: chronic anxiety and depression serves as a risk factor for ED pathogenesis, and ED caused by diverse etiology is also easy to cause psychological disorders of various severity (42–44). Our results demonstrate that the psychological burden of ED manifests acutely in younger populations, exhibiting consistent psychological problems regardless of ED severity. An age-stratified study (N = 948) revealed that 85.2% of ED cases among young males (<40 years) were primarily attributable to psychological etiology (45). Failed sexual behavior may trigger anxiety, decreased self-confidence, and subsequent avoidance behavior, thereby significantly elevating the likelihood of sexual behavior failure. Especially for adolescents with limited sexual experience demonstrate greater vulnerability to the negative consequences of sexual performance difficulties compared to middle-aged and elderly individuals (46). These psychological disturbances primarily manifest as persistent self-doubt, which significantly compromise subsequent sexual performance. This bidirectional interaction forms a self-perpetuating pathogenic cycle.
Our findings demonstrate an age-dependent attenuation in the correlation between psychological comorbidities and ED severity. Specifically, while patients aged 31–40 years with mild ED exhibited significant anxiety and depression symptoms, this psychological-disturbance gradient diminished progressively with advancing age. Only severe ED cases demonstrated pronounced psychological comorbidities in older age groups (≥41 years). There was a certain correlation between the decline of sexual life frequency and low life satisfaction, especially in young people and people over 60 years old (47, 48). However, for the elderly, sexual intercourse is not the key to maintaining sexual activity (49). Advanced-age patients typically demonstrate lower sexual activity frequency compared to younger populations, which may be attributed to age-related testosterone decline. Consequently, mild ED symptoms often fail to elicit significant anxiety in elderly patients.
Collectively, our findings delineate that psychological factor emerge as the primary etiological contributors in younger cohorts, whereas age-related hypogonadism and metabolic syndrome progressively assume dominant roles in older populations. While this study provides novel insights into age-related etiological differences in ED, several limitations warrant acknowledgment: (1) owing to the clinical recruitment process, the distribution of participants across age strata deviated from the anticipated equal allocation; (2) the reliance on self-reported psychological assessments could introduce recall bias. Future studies should address these gaps through broader inclusion criteria, and clinician-administered psychometric tools.
5 Conclusion
Endocrine and psychological factors exert distinct influences on ED across different ages. Young patients exhibit a heightened vulnerability to psychological distress, including anxiety and depression, despite generally presenting with milder ED severity. This phenomenon stems from the heightened sociosexual demands associated with maintaining intimate partnerships. Consequently, targeted psychological interventions should be prioritized in clinical management to mitigate both the symptoms of ED and their associated psychosocial sequelae in this population. The prevalence and severity of ED escalate with advancing age, due to age-related declines in TT levels and the progressively increasing incidence of related comorbidities. Comprehensive management of ED in elderly patients necessitates a multifactorial etiological analysis and the implementation of individualized, multidisciplinary treatment strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethical committee of Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZY: Investigation, Visualization, Resources, Funding acquisition, Validation, Data curation, Writing – review & editing, Formal Analysis, Project administration, Supervision, Software, Conceptualization, Writing – original draft, Methodology. CL: Validation, Funding acquisition, Conceptualization, Resources, Investigation, Visualization, Writing – review & editing, Project administration, Writing – original draft, Supervision, Methodology, Formal Analysis, Data curation, Software. XH: Resources, Supervision, Methodology, Conceptualization, Validation, Investigation, Data curation, Writing – review & editing, Funding acquisition, Writing – original draft, Formal Analysis, Software, Project administration, Visualization. TL: Formal Analysis, Supervision, Project administration, Data curation, Methodology, Writing – review & editing, Writing – original draft, Resources, Conceptualization, Investigation, Visualization, Funding acquisition, Software, Validation. YX: Software, Data curation, Resources, Conceptualization, Writing – review & editing, Funding acquisition, Investigation, Writing – original draft, Visualization, Methodology, Validation, Formal Analysis, Project administration, Supervision. QW: Formal Analysis, Visualization, Funding acquisition, Project administration, Resources, Data curation, Validation, Conceptualization, Supervision, Writing – review & editing, Methodology, Software, Writing – original draft, Investigation. JC: Writing – original draft, Data curation, Supervision, Methodology, Conceptualization, Software, Investigation, Resources, Formal Analysis, Validation, Writing – review & editing, Funding acquisition, Visualization, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The work was supported by the grants of: Jiangsu Traditional Chinese Medicine Science and Technology Development Plan Project (No. MS2022023; MS2023021); Key Project of Natural Science Foundation of Nanjing University of Chinese Medicine (No. XZR2024015); Research Project of Jiangsu Society of Traditional Chinese Medicine (No. ZXFZ2024026); Excellent Young Doctor Training Program of Jiangsu Province Hospital of Chinese Medicine (No. 2023QB0126).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shamloul R and Ghanem H. Erectile dysfunction. Lancet (London England). (2013) 381:153–65. doi: 10.1016/S0140-6736(12)60520-0
3. Domes T, Najafabadi BT, Roberts M, Campbell J, Flannigan R, Bach P, et al. Canadian Urological Association guideline: Erectile dysfunction. Can Urol Assoc J. (2021) 15:310–22. doi: 10.5489/cuaj.7572
4. Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, Hakim LS, et al. Erectile dysfunction: AUA guideline. J Urol. (2018) 200:633–41. doi: 10.1016/j.juro.2018.05.004
5. Zhang X, Yang B, Li N, and Li H. Prevalence and risk factors for erectile dysfunction in chinese adult males. J Sexual Med. (2017) 14:1201–8. doi: 10.1016/j.jsxm.2017.08.009
6. Rosen RC, Fisher WA, Eardley I, Niederberger C, Nadel A, and Sand M. The multinational Men’s Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. (2004) 20:607–17. doi: 10.1185/030079904125003467
7. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, and McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. (1994) 151:54–61. doi: 10.1016/S0022-5347(17)34871-1
8. Rastrelli G and Maggi M. Erectile dysfunction in fit and healthy young men: psychological or pathological? Trans Androl Urol. (2017) 6:79–90. doi: 10.21037/tau.2016.09.06
9. Huang IS, Mazur DJ, Kahn BE, Kate Keeter M, Desai AS, Lewis K, et al. Risk factors for hypogonadism in young men with erectile dysfunction. J Chin Med Assoc. (2019) 82:477–81. doi: 10.1097/JCMA.0000000000000099
10. Buvat J, Maggi M, Gooren L, Guay AT, Kaufman J, Morgentaler A, et al. Endocrine aspects of male sexual dysfunctions. J Sex Med. (2010) 7:1627–56. doi: 10.1111/j.1743-6109.2010.01780.x
11. Corona G, Lee DM, Forti G, O’Connor DB, Maggi M, O’Neill TW, et al. Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS). J Sex Med. (2010) 7:1362–80. doi: 10.1111/j.1743-6109.2009.01601.x
12. Capogrosso P, Ventimiglia E, Boeri L, Cazzaniga W, Chierigo F, Pederzoli F, et al. Age at First Presentation for Erectile Dysfunction: Analysis of Changes over a 12-yr Period. Eur Urol Focus. (2019) 5:899–905. doi: 10.1016/j.euf.2018.02.006
13. Huh JS, Chung BH, Hong CH, Ryu JK, Kim JH, Han WK, et al. The effects of testosterone replacement on penile structure and erectile function after long-term castration in adult male rats. Int J Impotence Res. (2018) 30:122–8. doi: 10.1038/s41443-017-0010-6
14. Barkin J. Erectile dysfunction and hypogonadism (low testosterone). Can J Urol. (2011) 18 Suppl:2–7. doi: 10.1038/sj.ijir.3901030
15. Barbonetti A, D’Andrea S, and Francavilla S. Testosterone replacement therapy. Andrology. (2020) 8:1551–66. doi: 10.1111/andr.12774
16. Lee H, Hwang EC, Oh CK, Lee S, Yu HS, Lim JS, et al. Testosterone replacement in men with sexual dysfunction. Cochrane Database Syst Rev. (2024) 1:Cd013071. doi: 10.1002/14651858
17. Onyeji IC and Clavijo RI. Testosterone replacement therapy and erectile dysfunction. Int J Impotence Res. (2022) 34:698–703. doi: 10.1038/s41443-021-00512-w
18. Kuchakulla M, Narasimman M, Soni Y, Leong JY, Patel P, and Ramasamy R. A systematic review and evidence-based analysis of ingredients in popular male testosterone and erectile dysfunction supplements. Int J Impotence Res. (2021) 33:311–7. doi: 10.1038/s41443-020-0285-x
19. Blute M, Hakimian P, Kashanian J, Shteynshluyger A, Lee M, and Shabsigh R. Erectile dysfunction and testosterone deficiency. Front Hormone Res. (2009) 37:108–22. doi: 10.1159/000176048
20. Elkhoury FF, Rambhatla A, Mills JN, and Rajfer J. Cardiovascular health, erectile dysfunction, and testosterone replacement: controversies and correlations. Urology. (2017) 110:1–8. doi: 10.1016/j.urology.2017.07.030
21. Travison TG, Araujo AB, Kupelian V, O’Donnell AB, and McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. (2007) 92:549–55. doi: 10.1210/jc.2006-1859
22. Pantazis A, Franco I, and Gitlin J. Erectile dysfunction in adolescents and young adults. Curr Urol Rep. (2024) 25:225–32. doi: 10.1007/s11934-024-01213-9
23. Sevim M, Alkis O, Kartal İG, and Aras B. A factor not to be ignored in post-COVID-19 erectile dysfunction; psychological effect, a prospective study. Andrologia. (2022) 54:e14443. doi: 10.1111/and.14443
24. Ma J, Zhang Y, Bao B, Chen W, Li H, and Wang B. Prevalence and associated factors of erectile dysfunction, psychological disorders, and sexual performance in primary vs. secondary infertility men. Reprod Biol Endocrinol: RB&E. (2021) 19:43. doi: 10.1186/s12958-021-00720-5
25. Bentsen IL, Giraldi AG, Kristensen E, and Andersen HS. Systematic review of sexual dysfunction among veterans with post-traumatic stress disorder. Sexual Med Rev. (2015) 3:78–87. doi: 10.1002/smrj.47
26. Jern P, Gunst A, Sandnabba K, and Santtila P. Are early and current erectile problems associated with anxiety and depression in young men? A retrospective self-report study. J Sex Marital Ther. (2012) 38:349–64. doi: 10.1080/0092623X.2012.665818
27. Manalo TA, Biermann HD, Patil DH, and Mehta A. The temporal association of depression and anxiety in young men with erectile dysfunction. J Sexual Med. (2022) 19:201–6. doi: 10.1016/j.jsxm.2021.11.011
28. Liu Q, Zhang Y, Wang J, Li S, Cheng Y, Guo J, et al. Erectile dysfunction and depression: A systematic review and meta-analysis. J Sexual Med. (2018) 15:1073–82. doi: 10.1016/j.jsxm.2018.05.016
29. Yuan P, Chen Y, Sun T, Cui L, Wei Y, Li T, et al. Exploring potential genes and mechanisms linking erectile dysfunction and depression. Front Endocrinol. (2023) 14:1221043. doi: 10.3389/fendo.2023.1221043
30. Xiao Y, Xie T, Peng J, Zhou X, Long J, Yang M, et al. Factors associated with anxiety and depression in patients with erectile dysfunction: a cross-sectional study. BMC Psychol. (2023) 11:36. doi: 10.1186/s40359-023-01074-w
31. Özkent MS, Hamarat MB, Taşkapu HH, Kılınç MT, Göger YE, and Sönmez MG. Is erectile dysfunction related to self-esteem and depression? A prospective case-control study. Andrologia. (2021) 53:e13910. doi: 10.1111/and.13910
32. Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European association of urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. Eur Urol. (2021) 80:333–57. doi: 10.1016/j.eururo.2021.06.007
33. Mobley DF, Khera M, and Baum N. Recent advances in the treatment of erectile dysfunction. Postgrad Med J. (2017) 93:679–85. doi: 10.1136/postgradmedj-2016-134073
34. Pozzi E, Capogrosso P, Chierigo F, Pederzoli F, Ventimiglia E, Boeri L, et al. Clinical profile of young patients with erectile dysfunction: preliminary findings of a real-life cross-sectional study. Eur Urol Focus. (2020) 6:184–9. doi: 10.1016/j.euf.2018.10.003
35. Corona G, Isidori AM, Aversa A, Burnett AL, and Maggi M. Endocrinologic control of men’s sexual desire and arousal/erection. J Sexual Med. (2016) 13:317–37. doi: 10.1016/j.jsxm.2016.01.007
36. Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology. (2020) 8:970–87. doi: 10.1111/andr.12770
37. Rastrelli G, Corona G, and Maggi M. Testosterone and sexual function in men. Maturitas. (2018) 112:46–52. doi: 10.1016/j.maturitas.2018.04.004
38. Calzo JP, Austin SB, Charlton BM, Missmer SA, Kathrins M, Gaskins AJ, et al. Erectile dysfunction in a sample of sexually active young adult men from a U.S. Cohort: demographic, metabolic and mental health correlates. J Urol. (2021) 205:539–44. doi: 10.1097/JU.0000000000001367
39. Lu Y, Fan S, Cui J, Yang Y, Song Y, Kang J, et al. The decline in sexual function, psychological disorders (anxiety and depression) and life satisfaction in older men: A cross-sectional study in a hospital-based population. Andrologia. (2020) 52:e13559. doi: 10.1111/and.13559
40. Ciaccio V and Di Giacomo D. Psychological factors related to impotence as a sexual dysfunction in young men: A literature scan for noteworthy research frameworks. Clinics Pract. (2022) 12:501–12. doi: 10.3390/clinpract12040054
41. Althof SE and Needle RB. Psychological factors associated with male sexual dysfunction: screening and treatment for the urologist. Urol Clinics North America. (2011) 38:141–6. doi: 10.1016/j.ucl.2011.02.003
42. Dewitte M, Bettocchi C, Carvalho J, Corona G, Flink I, Limoncin E, et al. A psychosocial approach to erectile dysfunction: position statements from the european society of sexual medicine (ESSM). Sexual Med. (2021) 9:100434. doi: 10.1016/j.esxm.2021.100434
43. Yang Y, Song Y, Lu Y, Xu Y, Liu L, and Liu X. Associations between erectile dysfunction and psychological disorders (depression and anxiety): A cross-sectional study in a Chinese population. Andrologia. (2019) 51:e13395. doi: 10.1111/and.13395
44. Molina-Leyva A, Molina-Leyva I, Almodovar-Real A, Ruiz-Carrascosa JC, Naranjo-Sintes R, and Jimenez-Moleon JJ. Prevalence and associated factors of erectile dysfunction in patients with moderate to severe psoriasis and healthy population: A comparative study considering physical and psychological factors. Arch Sexual Behav. (2016) 45:2047–55. doi: 10.1007/s10508-016-0757-8
45. Caskurlu T, Tasci AI, Resim S, Sahinkanat T, and Ergenekon E. The etiology of erectile dysfunction and contributing factors in different age groups in Turkey. Int J Urol. (2004) 11:525–9. doi: 10.1111/j.1442-2042.2004.00837.x
46. Hedon F. Anxiety and erectile dysfunction: a global approach to ED enhances results and quality of life. Int J Impot Res. (2003) 15 Suppl 2:S16–19. doi: 10.1038/sj.ijir.3900994
47. Vasconcelos P, Paúl C, Serruya SJ, Ponce de León RG, and Nobre P. A systematic review of sexual health and subjective well-being in older age groups. Pan Am J Public Health. (2022) 46:e179. doi: 10.26633/RPSP.2022.179
48. Jackson SE, Firth J, Veronese N, Stubbs B, Koyanagi A, Yang L, et al. Decline in sexuality and wellbeing in older adults: A population-based study. J Affect Disord. (2019) 245:912–7. doi: 10.1016/j.jad.2018.11.091
Keywords: erectile dysfunction, age, psychological factors, anxiety, depression, testosterone
Citation: Yang Z, Lu C, Wang Q, Liu T, Xu Y, Huang X and Chen J (2025) Age-related differences of serum total testosterone and psychological factors in patients with erectile dysfunction. Front. Endocrinol. 16:1615402. doi: 10.3389/fendo.2025.1615402
Received: 21 April 2025; Accepted: 16 October 2025;
Published: 30 October 2025.
Edited by:
Yemin Wang, University of British Columbia, CanadaReviewed by:
Jing Chen, Tongde Hospital of Zhejiang Province, ChinaJia Zhang, Nanjing Medical University, China
Copyright © 2025 Yang, Lu, Wang, Liu, Xu, Huang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinfei Huang, aHhmdXBAMTYzLmNvbQ==; Jianhuai Chen, amlhbmh1YWljaGVuQDEyNi5jb20=
†These authors share first authorship
 Zhaoxu Yang1†
Zhaoxu Yang1† Jianhuai Chen
Jianhuai Chen