- 1Department of Gynecology Obstetric and Reproductive Medicine, AP-HM La Conception University Hospital, Marseille, France
- 2Public Health Department, AP-HM Aix Marseille University, Marseille, France
- 3Biochemistry Department, Hôpital de la Conception, AP-HM, Marseille, France
- 4IMBE, Aix Marseille Univ, Avignon Univ, CNRS, IRD, Marseille, France
Objective: To investigate the impact of LH levels in the late follicular phase during the GnRH antagonist protocol on embryo implantation and IVF live birth rate (LBR) after fresh embryo transfer (ET).
Design: Retrospective cohort study.
Subjects: Women who underwent controlled ovarian stimulation (COS) with a GnRH antagonist protocol at a Reproductive Medicine Center in a University Teaching Hospital between January 2020 and December 2022.
Exposure: Monocentric study involving 544 IVF cycles with the GnRH antagonist protocol. Four groups were stratified based on preovulatory LH levels: Q1: LH < 25th percentile, Q2: LH 25-50th percentile, Q3: LH 50-75th percentile, and Q4: LH > 75th percentile.
Main outcome measures: The primary outcome was the live birth rate after fresh embryo transfer. Secondary outcomes included embryo implantation, clinical pregnancy, and early pregnancy loss rates.
Results: During the late follicular phase, estradiol levels were significantly correlated with preovulatory LH levels (P = 0.03). The number of retrieved oocytes, 2PNs (two pronuclei), and usable embryos were similar across the groups. No significant differences were observed between the groups regarding implantation rate, clinical pregnancy rate, early pregnancy loss, and LBR (P > 0.05).
Conclusion: In the antagonist protocol, pre-ovulatory LH levels had no impact on embryo implantation or IVF outcomes. A low LH level in the late follicular phase is not an indication for embryo freeze-all.
Introduction
The GnRH antagonist protocol is recommended for IVF ovulation stimulation due to its comparable efficacy and higher safety compared to GnRH agonist protocols (1). GnRH antagonists bind to specific receptors on the pituitary gland, inhibiting endogenous luteinizing hormone (LH). During ovarian stimulation, GnRH antagonists prevent an early rise in LH, thereby reducing the cycle cancellation rate.
LH, secreted by the pituitary gland, is essential for normal follicular development and oocyte maturation in the menstrual cycle (2). Exogenous LH supplementation is necessary for folliculogenesis in cases of hypogonadotropic hypogonadism (3–5). However, the role of LH in embryo implantation remains controversial, particularly in women undergoing GnRH antagonist protocols (6–8). Indeed, GnRH antagonists induce pronounced suppression of LH during ovarian stimulation (9). Some studies suggest that profound suppression of LH during the follicular phase may negatively affect IVF outcomes with fresh embryo transfer (10–12). However, there is no consensus on the LH threshold that would be deleterious for embryo implantation. Zhou et al. suggested canceling fresh embryo transfers in the < 25th percentile group of LH levels (<1.62 mIU/mL in normal responders, <2.25 mIU/mL in PCOS women, and 2.14 mIU/mL in poor responders) (12). Conversely, other studies have not found such negative impacts of LH levels during the follicular phase (6, 13, 14). The impact of LH levels on endometrial receptivity could be either direct, through LH’s effect on the endometrium, or indirect, through estradiol or progesterone levels at the end of the follicular phase. Another hypothesis would be the direct impact of Antagonists on IVF results through action on the endometrium by immunomodulation.
This study aimed to evaluate the impact of LH levels at the end of the follicular phase on embryo implantation and live birth rate.
Materials and methods
Study design and patients
This retrospective study was conducted in a single IVF Unit at a University Teaching Hospital. We included all women aged 18–43 years undergoing a fresh embryo transfer using the GnRH antagonist protocol between January 2020 and December 2022. Exclusion criteria included hypothalamic or pituitary amenorrhea, indication for freeze-all (OHSS, increased progesterone, etc.), and missing core data. IVF cycles were stratified into four groups based on preovulatory LH levels.
Study protocol
All women underwent a COS cycle using a fixed GnRH antagonist protocol. Women were pre-treated with estradiol (oral or transdermal patch) starting on the 25th day of the cycle preceding the stimulation cycle, lasting 5–10 days. Recombinant follicle-stimulating hormone (r-FSH) (without and with LH activity like Menopur) was administered on day 2 or 3 of the menstrual cycle, with the starting dose adapted to age, BMI, AMH concentration, and antral follicle count. The GnRH antagonist was started on day 5 of ovarian stimulation. From day 7 of stimulation, cycles were monitored by transvaginal ultrasonography and measurement of LH, progesterone, and estradiol. Recombinant-LH was not used in the cycles analyzed. When at least three follicles measured more than 17 mm, recombinant human chorionic gonadotropin (hCG) was administered at a dose of 250 micrograms to induce oocyte maturation. For poor responders, dual triggering was used. Oocyte retrieval was performed 36 hours after hCG administration. Fertilization was performed with conventional IVF or ICSI using fresh or thawed sperm.
Serum LH was measured at the last monitoring visit before hCG triggering, (on the day of triggering (J0), the day before (J–1), or two days before (J–2), depending on the individual monitoring schedule.
Hormone assay
Serum LH levels were measured using the Atellica IM LH test (SIEMENS Healthineers). The intra- and inter-assay coefficients of variation were 2.7% and 3.7%, respectively.
Fresh embryo transfer and luteal phase support
Fresh embryo transfers were primarily performed on day 2 or 3, with some on day 5. The embryos transferred were among the usable embryos (defined as diploid embryos used for fresh ET or freezing). The number of embryos transferred (one or two) was determined by embryo grading (15), the woman’s medical history, and the couple’s choice. According to Griesinger et al., oral dydrogesterone 30 mg/day was prescribed for LPS after fresh ET starting on the day of oocyte pick-up (16).
Outcome measures
The primary outcome was the live birth rate after fresh ET. Secondary outcomes included clinical pregnancy, defined as the detection of fetal heart activity by transvaginal ultrasonography 5 weeks after embryo transfer; implantation rate, calculated as the number of intrauterine gestational sacs observed by transvaginal ultrasonography divided by the total number of transferred embryos; and early pregnancy loss, defined as the spontaneous loss of clinical pregnancy before 12 weeks of gestation.
Statistical analysis
We determined that including 236 cycles would provide 80% power at a two-tailed alpha level of 0.05 to detect a significant difference in LBR (absolute difference was 12.1%) between the group with the lowest preovulatory LH levels and the group with the highest preovulatory LH levels, with an OR of 2.56. First, a descriptive analysis of the entire sample was performed. Categorical variables were presented as proportions and numbers, while quantitative variables were presented as mean ± standard deviation or median and quartiles. For each variable, the proportion of missing data was specified. A comparative analysis was conducted according to quartiles of LH levels at the last blood test. Qualitative variables were compared using Chi-square or Fisher’s exact tests according to application conditions, and quantitative variables were compared using a one-way ANOVA test followed by Bonferroni post-hoc tests for multiple comparisons. Pregnancy outcomes were also compared using the same procedure. The results of the analysis are presented as bar charts with their 95% confidence intervals (95% CI). The significance threshold was set at 5%. All analyses were carried out using IBM SPSS® Statistics version 20 software.
Ethical statement
The study protocol was approved by the local ethics committee (PADS23-71).
Results
A total of 552 IVF cycles with fresh ET were included in the study. Preovulatory LH level data were missing for 8 cycles, which were excluded from the analysis. Consequently, 544 IVF cycles with fresh ET were analyzed. The women included had a mean age of 35.3 ± 4.6 years, a mean BMI of 25.8 ± 5.2 kg/m², and 17% were smokers (n = 94).
The IVF cycles were categorized into four groups according to the interquartile range of preovulatory LH levels: Q1 = LH < 1.7 UI/L; Q2 = LH 1.7-2.9 UI/L; Q3 = LH 2.9-4.8 UI/L; Q4 = LH > 4.8 UI/L (Figure 1). The characteristics of the women in each of the four groups are detailed in Table 1. No significant differences were found among the groups regarding age, BMI, tobacco use, duration of infertility, type of infertility (primary or secondary), and cause of infertility. The causes of male and female infertility were comparable across all groups. The percentage of women with diminished ovarian reserve was 31.3% (n = 31) in Q1, 43% (n = 40) in Q2, 40% (n = 40) in Q3, and 37.4% (n = 34) in Q4 (p= 0,5). The proportion of poor responders was comparable across the four LH quartiles (31–43%), with no statistically significant difference between groups. The percentages of tubal anomalies were 16.2% (n = 16) in Q1, 5.4% (n = 5) in Q2, 12% (n = 12) in Q3, and 6.6% (n = 6) in Q4, with no statistical significance.
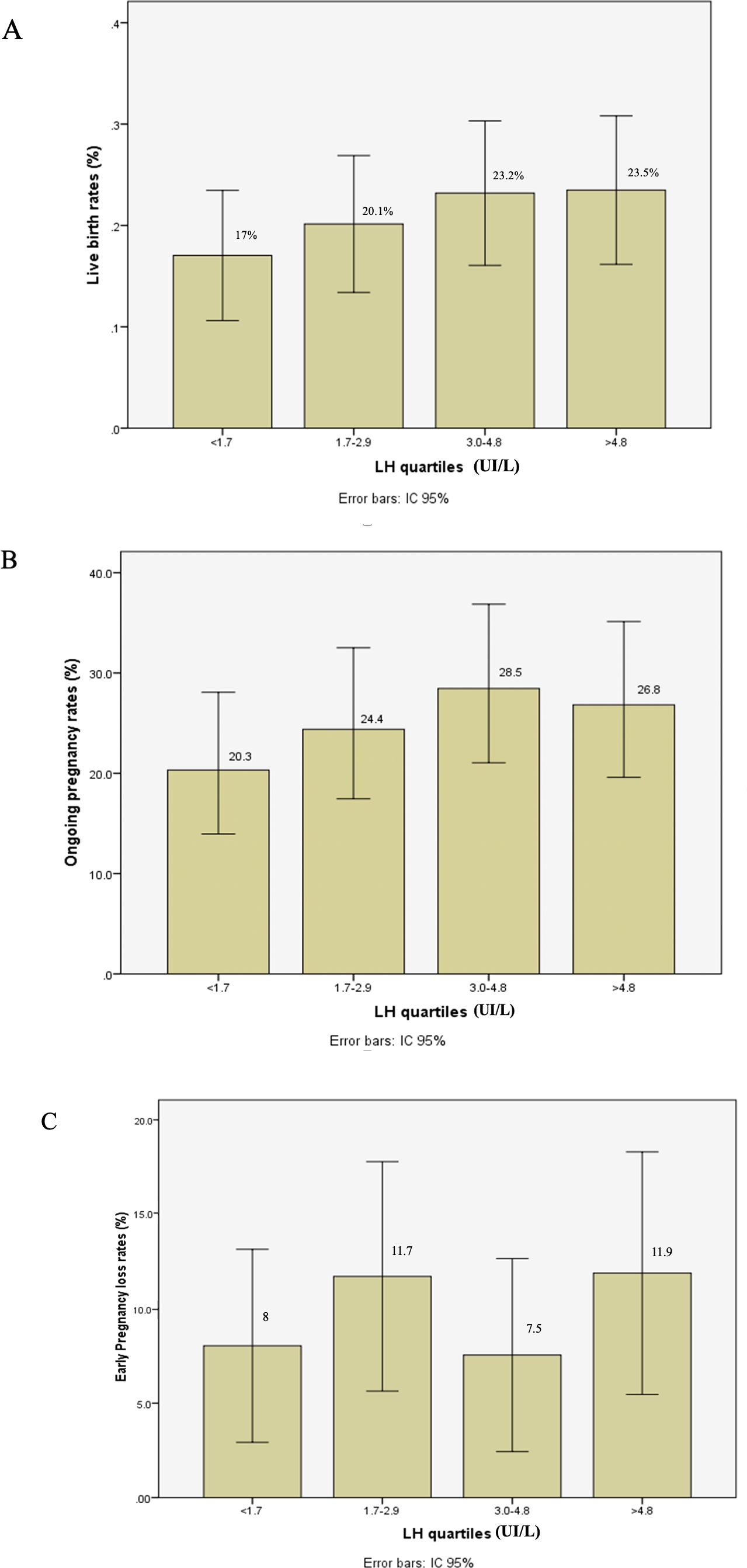
Figure 1. Live birth rate (A), ongoing pregnancy rate (B), and early pregnancy loss rate (C) after fresh embryo transfer according to LH levels in late follicular phase during GnRH antagonist protocol.
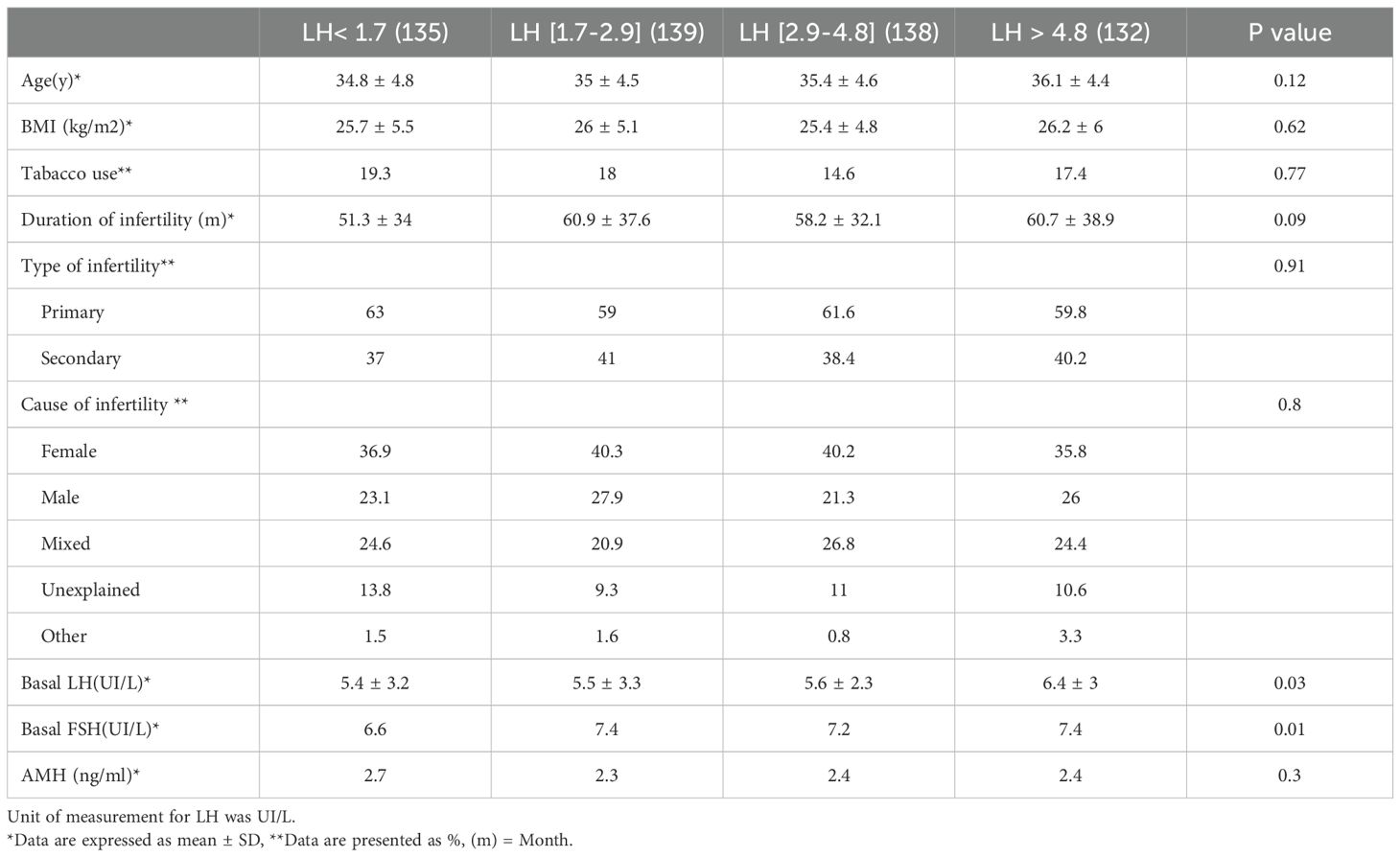
Table 1. Characteristics of women underwent GnRH antagonist protocol based on late follicular phase LH levels.
The mean level of basal LH before stimulation was significantly higher in Q4 compared to Q. Mean AMH levels were 2.7 ± 2.3 ng/mL in Q1, 2.3 ± 2 ng/mL in Q2, 2.4 ± 1.8 ng/mL in Q3, and 2.4 ± 1.7 ng/mL in Q4 (P = 0.3). Significant differences were observed between Q1 and Q4 regarding preovulatory estradiol levels (1837.5 ± 1025.6 pg/mL vs. 2159.9 ± 827.2 pg/mL; P = 0.025) and between Q2 and Q4 in terms of the duration of stimulation (11.1 ± 1.7 days vs. 10.5 ± 1.7 days; P = 0.026) and the total dose of gonadotropin (3303.8 ± 1310.3 UI vs. 2875.4 ± 1291.9 UI; P = 0.04). Other COS parameters, such as preovulatory progesterone levels, endometrial thickness, type of gonadotropin used (with or without LH activity), and the method of ART, were comparable across all groups (Table 2).
The number of oocytes retrieved, and the number of usable embryos were similar across all groups. IVF outcomes are presented in Table 3 and Figure 1. After fresh embryo transfer, there were no significant differences among the four groups regarding implantation rate, clinical pregnancy rate, early pregnancy loss, and live birth rate.
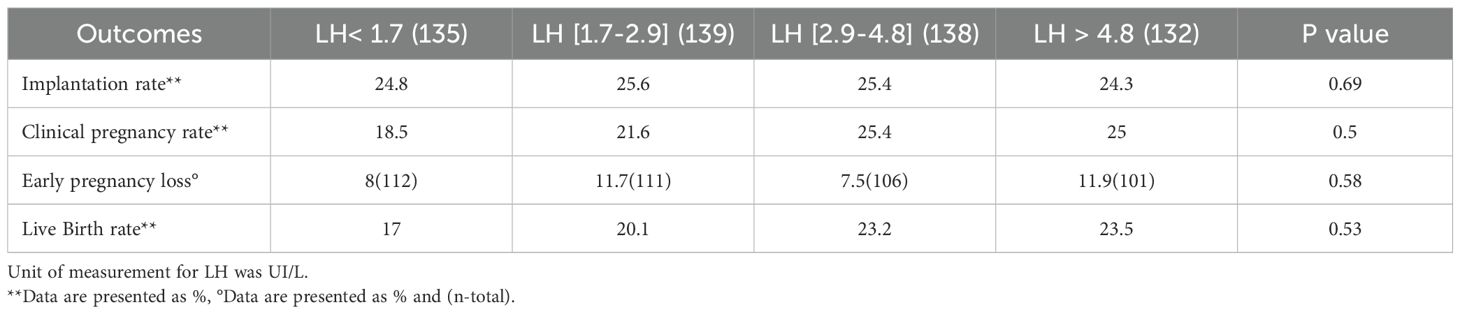
Table 3. Outcomes of GnRH antagonist cycles after fresh embryo transfer based on late follicular phase LH levels.
Discussion
In our study of 544 GnRH antagonist cycles, the late follicular LH level did not influence the implantation rate or LBR after fresh ET. The increase in preovulatory estradiol levels was associated with the preovulatory LH level. Our outcome supports the conclusion from other literature that shows pre-ovulatory estradiol levels do not make a difference on IVF outcomes (17–19). Despite the correlation between LH and estradiol levels, Huang et al. found no impact of peri-implantation estradiol levels on IVF pregnancy outcomes (18).
Taken together, these findings suggest that although LH and estradiol levels are associated, their fluctuations do not appear to significantly affect clinical outcomes in IVF.
Some studies have suggested that low LH levels at the end of the follicular phase might negatively affect the endometrium and IVF outcomes with GnRH antagonists and fresh ET (10–12, 20). If confirmed, this hypothesis could make low LH levels in the late follicular phase an indication for embryo freeze-all.
Shoham et al. proposed an “LH window” necessary for follicular development, emphasizing the need for a minimum circulating endogenous LH level for adequate ovarian steroidogenesis (21). Adding “LH activity” during COS is recommended for women with a poor response to stimulation despite normal ovarian reserve. Conforti et al. found that women with a hypo-response to ovarian stimulation supplemented with recombinant LH (r-LH) had significantly higher clinical pregnancy rates, implantation rates, and number of oocytes retrieved than those who underwent r-FSH alone (22).
However, our data, together with most recent findings, suggest that low LH levels in antagonist cycles do not necessarily compromise oocyte quality or endometrial receptivity, and therefore should not automatically trigger a freeze-all approach.
This body of evidence highlights that while low LH levels may raise concerns, the clinical need for intervention such as embryo freeze-all remains debatable and context-dependent.
Regarding studies that observed an impact of LH levels on embryo implantation, the debate focuses on the LH threshold with conflicting results (10–12, 20). Few studies have investigated the effect of LH levels on LBR after fresh embryo transfer with the GnRH antagonist protocol. Our findings are consistent with those of Merviel et al., who found no significant difference in clinical outcomes between different LH levels on the day of hCG administration, with an arbitrary threshold of 0.5 IU/L (6). Similarly, Griesinger et al. reported no association between LH concentrations on day 8 of stimulation and ongoing pregnancy rates (13). Bosch et al. as well as Doody et al., also observed no differences in clinical pregnancy rates (23, 24).
In a study of 426 cycles, Eftekhar et al. reported no relationship between LH levels during GnRH antagonist cycles and pregnancy outcomes, though they did observe a higher implantation rate in the LH 2.60–4.60 IU/L group compared to the LH <1.49 IU/L group, but no significant difference between the LH <1.49 IU/L group and the LH >4.6 IU/L group (14).
These consistent findings reinforce our conclusion that there is no clear or universally applicable LH threshold predictive of live birth in antagonist cycles.
Conversely, Luo et al. showed that LH levels <4 IU/L significantly reduced LBR (38% vs 51.5%; P<0.05) and increased early pregnancy loss rate after fresh embryo transfer, without affecting the implantation rate. They included 1480 women undergoing COS with GnRH antagonist, who were arbitrarily divided into “low” and “high” LH groups with a cutoff of 4 IU/L (20). In the study of Benmachiche et al., 322 infertile women were included and underwent IVF with GnRH antagonist protocol and fresh ET. Above an LH threshold of 1.6 IU/L, early pregnancy loss rate decreased and the ongoing pregnancy rate and LBR increased. The absolute difference between the highest LH group (LH >1.6 IU/L) and the lowest LH group (LH<0.6 IU/L) was 13.4%, 12.1%, and 12% in ongoing pregnancy rate, LBR, and early pregnancy loss rate, respectively (P<0.05) (11). These discrepancies may be explained by differences in study design and patient populations. Luo et al. included a larger cohort with a higher proportion of PCOS women and used an arbitrary LH cutoff of 4 IU/L, whereas Benmachiche et al. applied a lower threshold of 1.6 IU/L. In addition, the LH assays and the timing and dosing of antagonist administration varied between studies, which may also account for heterogeneity in the reported outcomes.
These discrepancies highlight that the thresholds proposed in the literature can be considered specific to the study population rather than universal biological thresholds.
In a retrospective study, Chen et al. found that LH ≤0.8 mIU/ml during COS with GnRH antagonist was associated with a significantly higher early pregnancy loss rate (31.1%, n=19 vs 16.6%, n=36; P = 0.012) but no significant differences in LBR or implantation rate (10). There is variability in LH levels during COS with the antagonist protocol, with differences described between the first day of the cycle and after introduction of GnRH antagonists (25). For long-agonist protocol, Lahoud et al. observed that a drop in LH levels of >50% from early to mid-follicular phase resulted in a significantly lower live birth rate per cycle (22.2% vs 15.8%; P<0.05) (26). Despite the use of the same dose of GnRH antagonists, women do not have the same pituitary response. Kol et al. defined “normal” responders as those with LH levels greater than 50% of the pre-injection level 24 hours after the first GnRH antagonist injection and “over-suppressed” if less than 50%. About a quarter of women were “over-suppressed” in their study, though they did not investigate the impact on LBR (25). This variability in LH levels may be due to interindividual differences in pituitary response to GnRH antagonists, potentially explained by polymorphisms in the GnRH receptor (GnRHR). In a study of 269 women, Weng et al. found that genetic variants in the GnRHR gene could modulate LH release and affect ovarian stimulation outcomes (27).
LH receptor polymorphisms appear to have no impact on clinical outcomes or IVF LBR. In a study of 1183 women in whom genotyping of the FSH receptor and LH receptor polymorphisms was performed, Pirtea et al. found no association between gonadotropin receptor polymorphisms and LBR or implantation rate (28).
Altogether, these findings suggest that variability in LH response may be influenced by genetic and pharmacological factors, but these do not consistently translate into differences in live birth outcomes.
To understand the mechanism of action of GnRH antagonists, Murase et al. reported that prolonged GnRH antagonist treatment inhibits LH beta subunit mRNA and GnRH-R mRNA expression, like GnRH agonists (29). In a review, Maggi et al. reported that the GnRH/GnRH-R system is also expressed in female reproductive tissues, in addition to the pituitary gland. The expression of this system in the human endometrium supports its physiological role in the processes of embryo implantation (30). A comparative transcriptomic analysis study of the endometrium in 9 women with adenomyosis before and after GnRH agonist treatment suggested that GnRH agonists significantly alter immune system-associated signal transduction in the endometrium, a hypothesis that remains unexplored for GnRH antagonists (31).
These mechanistic data highlight the need for future research investigating how GnRH antagonists might influence endometrial gene expression and immune modulation, potentially mediating subtle effects on implantation.
Despite the retrospective and monocentric nature of our study, as well as the heterogeneity of the population, our sample was sufficiently large, even though we had few women with LH levels below 0.6 IU/L.
We acknowledge as a major limitation the retrospective cohort design, which may introduce selection bias and limits causal inference.
Overall, our study adds value by providing an analysis of a large sample of late follicular phase LH levels, with a focus on the results of fresh embryo transfers, which helps to guide clinical decision-making. Our findings suggest that low late follicular LH levels alone should not be used as a criterion to systematically opt for a freeze-all strategy. Instead, clinicians can continue to perform fresh transfers even in cases of low LH, provided that no other clinical or biological factors indicate otherwise. This can help avoid unnecessary delays in treatment and reduce patient burden.
However, as this is a descriptive study based on statistical associations, it cannot establish a causal relationship between late follicular LH levels and IVF outcomes. Therefore, our results should be interpreted with caution. Further prospective multicenter and mechanistic studies are needed to confirm these findings and to better understand the pathways underlying LH variability and its potential impact on endometrial receptivity.
In conclusion, our study indicates that preovulatory LH levels during cycles with GnRH antagonists are not correlated with IVF outcomes. Therefore, monitoring LH levels during antagonist protocols may be of limited use in deciding between fresh transfer or freeze all, and a low LH level at the end of the follicular phase does not necessarily require freezing all.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by local ethics committee Of Aix Marseille Université (PADS23-71). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NJ: Writing – review & editing, Writing – original draft. AZ: Supervision, Writing – original draft, Validation. CF: Validation, Writing – review & editing, Formal Analysis. LM: Validation, Writing – review & editing. CB: Validation, Writing – review & editing. JP: Writing – review & editing, Validation, Supervision. BC: Methodology, Conceptualization, Validation, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. The ESHRE Guideline Group on Ovarian Stimulation, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI†. Hum Reprod Open. (2020) 2020:hoaa009. doi: 10.1093/hropen/hoaa009
2. Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. (2001) 179:39−46. doi: 10.1016/S0303-7207(01)00469-5
3. Shoham Z, Balen A, Patel A, and Jacobs HS. Results of ovulation induction using human menopausal gonadotropin or purified follicle-stimulating hormone in hypogonadotropic hypogonadism patients. Fertil Steril. (1991) 56:1048−53. doi: 10.1016/S0015-0282(16)54715-3
4. Shoham Z, Mannaerts B, Insler V, and Coelingh-Bennink H. Induction of follicular growth using recombinant human follicle-stimulating hormone in two volunteer women with hypogonadotropic hypogonadism. Fertil Steril. (1993) 59:738−42. doi: 10.1016/S0015-0282(16)55852-X
5. Balasch J, Miro F, Burzaco I, Casamitjana R, Ballesca JL, Puerto B, et al. The role of luteinizing hormone in human follicle development and oocyte fertility: evidence from in-vitro fertilization in a woman with long-standing hypogonadotrophic hypogonadism and using recombinant human follicle stimulating hormone. Hum Reprod. (1995) 10:1678–83. doi: 10.1093/oxfordjournals.humrep.a136154
6. Merviel P, Antoine JM, Mathieu E, Millot F, Mandelbaum J, and Uzan S. Luteinizing hormone concentrations after gonadotropin-releasing hormone antagonist administration do not influence pregnancy rates in in vitro fertilization–embryo transfer. Fertil Steril. (2004) 82:119−25. doi: 10.1016/j.fertnstert.2003.11.040
7. Cedrin-Durnerin I. Recombinant human LH supplementation during GnRH antagonist administration in IVF/ICSI cycles: a prospective randomized study. Hum Reprod. (2004) 19:1979−84. doi: 10.1093/humrep/deh369
8. Frydman R, Cornel C, De Ziegler D, Taieb J, Spitz IM, and Bouchard P. Prevention of premature luteinizing hormone and progesterone rise with a gonadotropin-releasing hormone antagonist, Nal-Glu, in controlled ovarian hyperstimulation. Fertil Steril. (1991) 56:923−7. doi: 10.1016/S0015-0282(16)54666-4
9. Diedrich K, Diedrich C, Santos E, Zoll C, Al-Hasani S, Reissmann T, et al. Suppression of the endogenous luteinizing hormone surge by the gonadotrophin-releasing hormone antagonist Cetrorelix during ovarian stimulation. Hum Reprod. (1994) 9:788−91. doi: 10.1093/oxfordjournals.humrep.a138597
10. Chen CD, Chiang YT, Yang PK, Chen MJ, Chang CH, Yang YS, et al. Frequency of low serum LH is associated with increased early pregnancy loss in IVF/ICSI cycles. Reprod BioMed Online. (2016) 33:449−57. doi: 10.1016/j.rbmo.2016.07.001
11. Benmachiche A, Benbouhedja S, Zoghmar A, and Humaidan P. Low LH level on the day of gnRH agonist trigger is associated with reduced ongoing pregnancy and live birth rates and increased early miscarriage rates following IVF/ICSI treatment and fresh embryo transfer. Front Endocrinol. (2019) 10:639. doi: 10.3389/fendo.2019.00639
12. Zhou R, Dong M, Huang L, Zhu X, Wei J, Zhang Q, et al. Association between serum LH levels on hCG trigger day and live birth rate after fresh embryo transfer with GnRH antagonist regimen in different populations. Front Endocrinol 5 juill. (2023) 14:1191827. doi: 10.3389/fendo.2023.1191827
13. Griesinger G, Shapiro DB, Kolibianakis EM, Witjes H, and Mannaerts BM. No association between endogenous LH and pregnancy in a GnRH antagonist protocol: part II, recombinant FSH. Reprod BioMed Online. (2011) 23:457−65. doi: 10.1016/j.rbmo.2011.06.016
14. Eftekhar M, Hoseini M, and Tabibnejad N. Is there a relationship between luteinizing hormone levels and ART outcome in GnRH antagonist protocols? A retrospective cross-sectional study. Indian J Endocrinol Metab. (2021) 25:563. doi: 10.4103/ijem.ijem_331_21
15. Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology, Balaban B, Brison D, Calderon G, Catt J, Conaghan J, et al. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. (2011) 26:1270−83. doi: 10.1016/j.rbmo.2011.02.001
16. Griesinger G, Blockeel C, Kahler E, Pexman-Fieth C, Olofsson JI, Driessen S, et al. Dydrogesterone as an oral alternative to vaginal progesterone for IVF luteal phase support: A systematic review and individual participant data meta-analysis. PloS One. (2020) 15:e0241044. doi: 10.1371/journal.pone.0241044
17. Li R, Gong F, and Qiao j. Correlation of LH level and steroid concentrations in GnRH antagonist protocol: A sub-analysis of Ganirelix phase III study of China. J Gynecol Obstet Hum Reprod. (2022) 515102363:2022. doi: 10.1016/j.jogoh.2022.102363
18. Liu Y, Li J, Zhang W, and Guo Y. Association between serum oestradiol level on the hCG administration day and neonatal birthweight after IVF-ET among 3659 singleton live births. Sci Rep 16 mars. (2021) 11:6084. doi: 10.1038/s41598-021-85692-7
19. Huang P, Ou Y, Tang N, Chen J, Wen Q, Li J, et al. Peri-implantation estradiol level has no effect on pregnancy outcome in vitro fertilization- embryo transfer. Front Endocrinol 12 févr. (2024) 15:1326098. doi: 10.3389/fendo.2024.1326098
20. Luo Y, Liu S, Su H, Hua L, Ren H, Liu M, et al. Low serum LH levels during ovarian stimulation with gnRH antagonist protocol decrease the live birth rate after fresh embryo transfers but have no impact in freeze-all cycles. Front Endocrinol. (2021) 12:640047. doi: 10.3389/fendo.2021.640047
21. Shoham Z. The clinical therapeutic window for luteinizing hormone in controlled ovarian stimulation. Fertil Steril. (2002) 77:1170−7. doi: 10.1016/S0015-0282(02)03157-6
22. Conforti A, Esteves SC, Di Rella F, Strina I, De Rosa P, Fiorenza A, et al. The role of recombinant LH in women with hypo-response to controlled ovarian stimulation: a systematic review and meta-analysis. Reprod Biol Endocrinol déc. (2019) 17:18. doi: 10.1186/s12958-019-0460-4
23. Bosch E, Escudero E, Crespo J, Simón C, Remohí J, and Pellicer A. Serum luteinizing hormone in patients undergoing ovarian stimulation with gonadotropin-releasing hormone antagonists and recombinant follicle-stimulating hormone and its relationship with cycle outcome. Fertil Steril. (2005) 84:1529−32. doi: 10.1016/j.fertnstert.2005.05.040
24. Doody KJ, Devroey P, Leader A, Witjes H, and Mannaerts BM. No association between endogenous LH and pregnancy in a GnRH antagonist protocol: part I, corifollitropin alfa. Reprod BioMed Online. (2011) 23:449−56. doi: 10.1016/j.rbmo.2011.06.015
25. Kol S. Individualized treatment from theory to practice: the private case of adding LH during gnRH antagonist-based stimulation protocol. Clin Med Insights Reprod Health. (2014) 8:59−64. doi: 10.4137/CMRH.S17788
26. Lahoud R, Al-Jefout M, Tyler J, Ryan J, and Driscoll G. A relative reduction in mid-follicular LH concentrations during GnRH agonist IVF/ICSI cycles leads to lower live birth rates. Hum Reprod Oxf Engl. (2006) 21:2645−9. doi: 10.1093/humrep/del219
27. Weng SL, Tzeng SL, Lee CI, Liu CH, Huang CC, Yang SF, et al. Association between gnRH receptor polymorphisms and luteinizing hormone levels for low ovarian reserve infertile women. Int J Env Res Public Health. (2021) 18:7006. doi: 10.3390/ijerph18137006
28. Pirtea P, De Ziegler D, Marin D, Sun L, Tao X, Ayoubi JM, et al. Gonadotropin receptor polymorphisms (FSHR N680S and LHCGR N312S) are not predictive of clinical outcome and live birth in assisted reproductive technology. Fertil Steril. (2022) 118:494−503. doi: 10.1016/j.fertnstert.2022.06.011
29. Murase M, Uemura T, Gao M, Inada M, Funabashi T, and Hirahara F. GnRH antagonist-induced down-regulation of the mRNA expression of pituitary receptors: comparisons with gnRH agonist effects. Endocr J. (2005) 52:131−7. doi: 10.1507/endocrj.52.131
30. Maggi R, Cariboni AM, Marelli MM, Moretti RM, Andrè V, Marzagalli M, et al. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum Reprod Update. (2016) 22:358−81. doi: 10.1093/humupd/dmv059
Keywords: IVF, GnRH antagonist, embryo implantation, live birth rate, luteinizing hormone (LH), fresh embryo transfer
Citation: Jelassi N, Zimmermann A, Faust C, Miquel L, Buffat C, Perrin J and Courbiere B (2025) Impact of late follicular luteinizing hormone levels on live birth rate after fresh embryo transfer: a retrospective cohort study in GNRH antagonist cycles. Front. Endocrinol. 16:1615525. doi: 10.3389/fendo.2025.1615525
Received: 21 April 2025; Accepted: 27 October 2025;
Published: 07 November 2025.
Edited by:
María Laura Ribeiro, CONICET Centro de Estudios Farmacológicos y Botánicos (CEFYBO), ArgentinaReviewed by:
Kequan Lin, Zhejiang University, ChinaAijun Yang, Affiliated Hospital of Jining Medical University, China
Copyright © 2025 Jelassi, Zimmermann, Faust, Miquel, Buffat, Perrin and Courbiere. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nada Jelassi, amVsYXNzaW5hZGFAaG90bWFpbC5jb20=
 Nada Jelassi
Nada Jelassi Appoline Zimmermann1
Appoline Zimmermann1 Laura Miquel
Laura Miquel