- 1Department of Nursing, People’s Hospital of Chengyang District, Qingdao, Shandong, China
- 2Department of Endocrinology, People’s Hospital of Chengyang District, Qingdao, Shandong, China
Background: To investigate the correlation between thyroid immune-related adverse events (irAEs) and thyroid autoantibodies in cancer patients treated with immune checkpoint inhibitors (ICIs).
Methods: A retrospective analysis was conducted on 316 cancer patients (139 females, 177 males; median age 64.0 [56.0–71.0] years) treated at Qingdao Chengyang District People’s Hospital from January 2018 to December 2023. Patients were divided into a euthyroid group (n = 158) and a thyroid irAEs group (n = 158) based on the occurrence of thyroid dysfunction post-ICI therapy. The researchers received at least one treatment with ICIs, and after the initial treatment, they underwent at least one or more tests for thyroid hormone levels, TPOAb, TRAb, and TgAb, with an interval of 4 weeks or more for each test. Thyroid hormone levels and autoantibodies (TPOAb, TRAb, TgAb) were measured. Clinical characteristics and baseline thyroid autoantibodies were evaluated for their association with thyroid irAEs.
Results: Thyroid irAEs included subclinical thyrotoxicosis (19.94%, n = 63), clinical thyrotoxicosis (2.53%, n = 8), subclinical hypothyroidism (6.01%, n = 19), and clinical hypothyroidism (21.52%, n = 68). Baseline thyroid autoantibodies were positive in 28.48% (n = 45) of the irAEs group versus 5.70% (n = 9) in the euthyroid group (P < 0.001). Post-ICI treatment, the thyrotoxicosis group exhibited higher TRAb titers but lower TPOAb titers and TSH levels compared to the hypothyroidism group (P < 0.05). Logistic regression identified pre-treatment TRAb positivity (OR=6.927, 95% CI: 1.817–32.724, P=0.002) and TPOAb positivity (OR = 7.128, 95% CI: 1.877–37.225, P = 0.001) as risk factors for thyroid irAEs.
Conclusion: Patients with malignant tumors who had high levels of TPOAb and/or TRAb before treatment were more likely to develop thyroid immune-related adverse events (irAEs) after treatment. The importance of screening for baseline thyroid autoantibodies in predicting thyroid irAEs needs to be clearly understood, and close monitoring and notification to patients should be carried out, along with prior intervention.
1 Introduction
In recent years, cancer immunotherapy, particularly immune checkpoint inhibitors (ICIs), has emerged as a breakthrough treatment for malignancies. ICIs target cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) or programmed cell death-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) pathways to disrupt tumor immune evasion (1). While ICIs are generally well-tolerated, they can induce immune-related adverse events (irAEs) affecting the gastrointestinal tract, endocrine system, skin, and other organs (2). Endocrine irAEs frequently involve thyroid dysfunction (including hypothyroidism with or without transient thyrotoxicosis), occurring in 40% of patients (3, 4). Combination PD-1/CTLA-4 inhibitors pose the highest risk, followed by PD-1 inhibitors alone (4, 5).
Thyroid autoantibodies—anti-thyroid peroxidase antibody (TPOAb), thyrotropin receptor antibody (TRAb), and anti-thyroglobulin antibody (TgAb)—have shown inconsistent associations with thyroid irAEs. Some studies suggest TPOAb/TgAb positivity predicts ICI-related thyroid dysfunction (6–9), while others report no correlation (10). Notably, TRAb’s role remains unexplored (11). To understand whether the occurrence of thyroid irAEs in tumor patients after ICI treatment is related to the patients’ baseline thyroid antibodies, this study investigates the relationship between thyroid autoantibodies and irAEs post-ICI therapy, providing a theoretical basis for clinical intervention. In order to understand whether the occurrence of thyroid immune-related adverse events (irAEs) in tumor patients after using immune checkpoint inhibitors (ICIs) is related to their baseline thyroid antibodies, this study explored the correlation between the occurrence of thyroid irAEs in tumor patients after using ICIs and the levels of thyroid autoantibodies, in order to provide a theoretical basis for intervention treatment.
2 Materials and methods
2.1 Study population
Patients (n = 316) treated with ICIs (Tislelizumab, Pembroliumab, and Xindiliumab) at Qingdao Chengyang District People’s Hospital (2018–2023) were retrospectively analyzed. Based on thyroid irAEs post-ICI, they were stratified into euthyroid and irAEs groups, with the latter further subclassified into thyrotoxicosis (subclinical/clinical) and hypothyroidism (subclinical/clinical). The required sample size was estimated using Kendall’s rule (10 times the variable count). With 20 variables, the baseline sample size was 200; after a 10% buffer for invalid responses, the final target was ≥ 220 cases. Therefore, the inclusion of 316 participants was sufficient for the sample size. Inclusion criteria: The investigator received at least 1 ICIs treatment, and at least 2 or more thyroid hormone levels and TPOAb, TRAb and TgAb tests after initial treatment, with an interval of more than 2 weeks between each test. Exclusion criteria: Prior ICI use, pre-existing thyroid dysfunction, central hypothyroidism, incomplete data, or confounding factors (e.g., tyrosine kinase inhibitors, non-thyroidal illness syndrome, radiation thyroiditis). Ethical approval was obtained (No. 2023100812); informed consent was waived for retrospective data.
2.2 Methods
2.2.1 Data collection
Demographics, ICI type, tumor type, and thyroid function tests, including free thyroxine (FT4), reference range 12.0-22.0pmol/l, free triiodothyronine (FT3), reference range 3.5-6.8pmol/l, thyroid-stimulating hormone (TSH), reference range 0.4-4.2uIU/ml, Thyroid stimulating hormone receptor antibody (TRAb), reference range0-1.75IU/L, anti-thyroid peroxidase antibody (TPOAb), reference range 0–34 IU/ml, anti-thyroglobulin antibody (TgAb), reference range 0–115 IU/ml, were recorded. Thyroid function was monitored every 4 weeks (every 2 weeks if abnormal).
2.2.2 Diagnostic criteria
To minimize selection bias, case allocation followed these three principles (12) (1): At least 50 patient cases were collected annually (2); Cases were evenly distributed across each quarter of the year (3); Monthly case contributions accounted for no less than 10% of the total. Patient selection flowchart: Eligible cancer patients were those who received immune checkpoint inhibitor (ICI) therapy. Methods for Assessing Thyroid Function and Severity of Thyroid Injury: According to relevant guidelines for the diagnosis and treatment of thyroid diseases, thyroid injury is classified into the following five categories (1): Thyrotoxicosis: Elevated FT3 (normal reference range: 3.5–6.8 pmol/L) and/or elevated FT4 (normal reference range: 12.0–22.0 pmol/L), with decreased TSH (normal reference range: 0.4–4.2 μIU/mL) (2). Hypothyroidism: FT3 and/or FT4 below the normal reference range, with TSH above the normal reference range (3). Subclinical thyrotoxicosis or subclinical hypothyroidism**: FT3 and FT4 within the normal reference range, with TSH below or above the normal reference range (4). Antibody-positive euthyroidism: Normal TSH, FT3, and FT4, with positive TPOAb (normal range: 0–34 IU/mL) and/or positive TgAb (normal range: 0–115 IU/mL) and/or positive TRAb (normal range: 0–1.75 IU/L) (5). Normal thyroid function: All parameters (TSH, FT3, FT4, and thyroid antibodies) within their respective normal reference ranges (13). The inclusion criteria required: Baseline thyroid function tests, including antibody assessment; At least one post-treatment thyroid function evaluation; Complete collection of essential demographic and clinical data. Thyrotoxicosis: Subclinical: TSH < lower limit of normal (LLN), normal FT4/FT3. Clinical: TSH < LLN, elevated FT4/FT3. Hypothyroidism: Subclinical: TSH > upper limit of normal (ULN), normal FT4. Clinical: TSH > ULN, FT4 < LLN.
2.3 Statistical analysis
Statistical analyses were performed using SPSS 27.0 (IBM Corp.). Continuous variables were assessed for normality using Shapiro-Wilk tests. Normally distributed data were presented as mean ± standard deviation (SD) and compared using Student’s t-test, while non-normally distributed data were expressed as median (interquartile range, Q1-Q3) and analyzed with Mann-Whitney U test. Categorical variables were compared using χ2 test or Fisher’s exact test, as appropriate. To address potential type I error inflation in multiple comparisons, we applied Bonferroni correction when appropriate, with adjusted P-values reported for primary outcomes. Binary logistic regression analysis was conducted to identify independent risk factors for immune-related adverse events (irAEs), incorporating clinically relevant covariates and variables showing P < 0.10 in univariate analyses. Model assumptions were verified including absence of multicollinearity (variance inflation factors < 5) and adequate goodness-of-fit (Hosmer-Lemeshow test P > 0.05). A two-tailed P-value < 0.05 was considered statistically significant, except where multiple comparison adjustments were applied.
3 Results
3.1 Clinical characteristics
Table 1 presented a comparative analysis of baseline characteristics between the euthyroid group (n = 158) and the thyroid irAEs group (n = 158) in 316 cancer patients treated with immune checkpoint inhibitors (ICIs). No significant differences were observed in sex distribution (47.48% vs. 49.37% male, P = 0.282) or median age (66.0 vs. 62.5 years, P = 0.095) between groups. The majority received PD-1/PD-L1 inhibitors (100% in euthyroid vs. 92.41% in irAEs group), with combination CTLA-4/PD-1/PD-L1 therapy exclusively administered to the irAEs group (7.59%, P = 0.513 by Fisher’s exact test). Tumor types differed significantly (P = 0.032), with lung cancer predominating in the euthyroid group (78.48% vs. 58.23%) and gynecologic cancers more frequent in the irAEs group (12.02% vs. 1.90%).
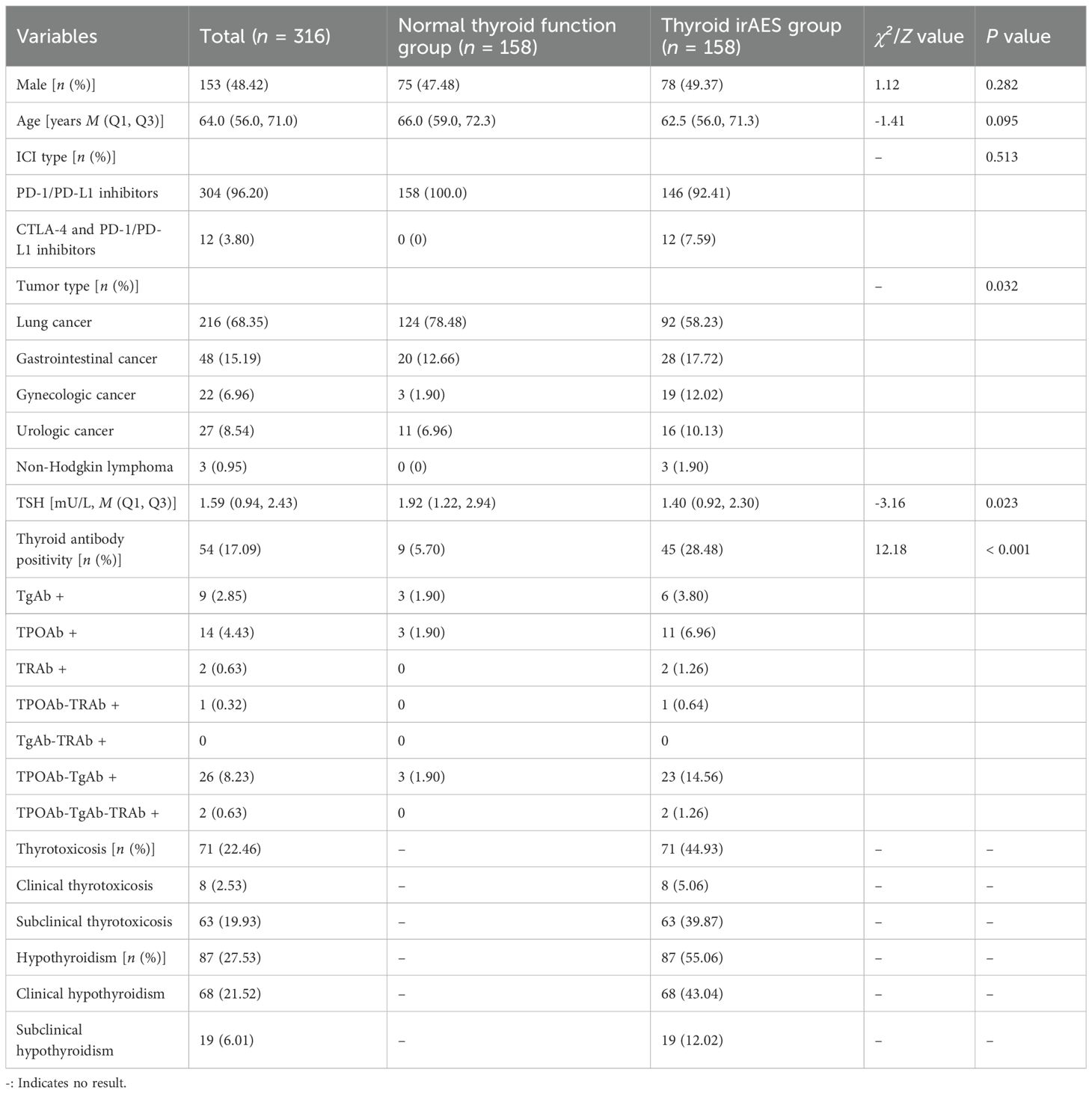
Table 1. Comparison of general information between the normal thyroid function group and the thyroid irAEs group.
Key thyroid-related parameters revealed lower baseline TSH levels in the irAEs group (median 1.40 vs. 1.92 mU/L, P = 0.023) and markedly higher thyroid autoantibody positivity (28.48% vs. 5.70%, P<0.001). Notably, combined TPOAb-TgAb positivity was prevalent in the irAEs group (14.56% vs. 1.90%). Among irAEs subtypes, hypothyroidism (55.06%) was more common than thyrotoxicosis (44.93%), with clinical hypothyroidism constituting 43.04% of cases.
3.2 Autoantibody profiles
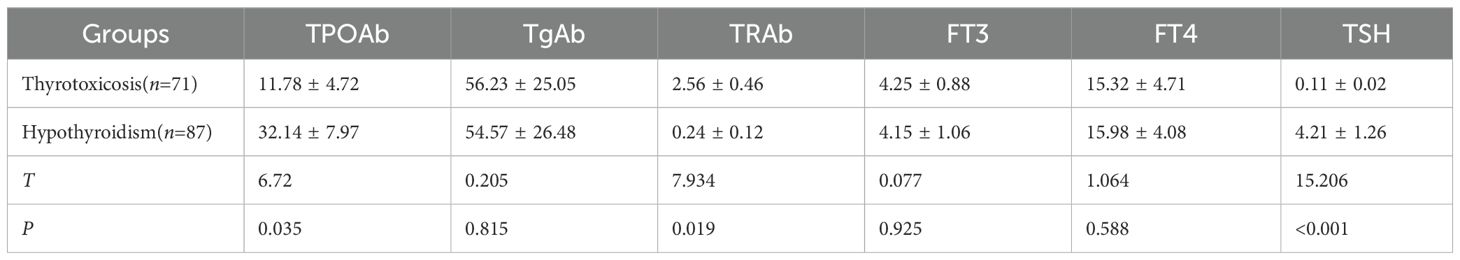
Table 2 compared thyroid hormone profiles and autoantibody levels between the thyrotoxicosis (n = 71) and hypothyroidism (n = 87) subgroups of patients who developed thyroid immune-related adverse events (irAEs) following immune checkpoint inhibitor (ICI) therapy. The data reveal distinct immunological patterns between these two manifestations of thyroid dysfunction.
Patients with thyrotoxicosis demonstrated significantly lower thyroid peroxidase antibody (TPOAb) levels (11.78 ± 4.72 IU/mL vs 32.14 ± 7.97 IU/mL, P = 0.035) but markedly higher thyrotropin receptor antibody (TRAb) titers (2.56 ± 0.46 IU/L vs 0.24 ± 0.12 IU/L, P = 0.019) compared to the hypothyroidism group. These findings suggest different underlying pathophysiological mechanisms, with TRAb potentially driving the thyrotoxicosis phenotype while TPOAb predominates in hypothyroidism. Notably, thyroglobulin antibody (TgAb) levels showed no significant intergroup difference (56.23 ± 25.05 IU/mL vs 54.57 ± 26.48 IU/mL, P = 0.815).
As expected, thyroid-stimulating hormone (TSH) levels were profoundly suppressed in thyrotoxicosis (0.11 ± 0.02 mIU/L) versus elevated in hypothyroidism (4.21 ± 1.26 mIU/L, P < 0.001). Free thyroid hormones (FT3 and FT4) showed no statistically significant differences between groups, though the numerical values align with the clinical definitions of these conditions.
These results highlighted the importance of differentiating between thyrotoxicosis and hypothyroidism in ICI-related thyroid irAEs, as they exhibit distinct autoantibody profiles that may inform both pathogenesis and clinical management.
3.3 Risk factors for thyroid irAEs
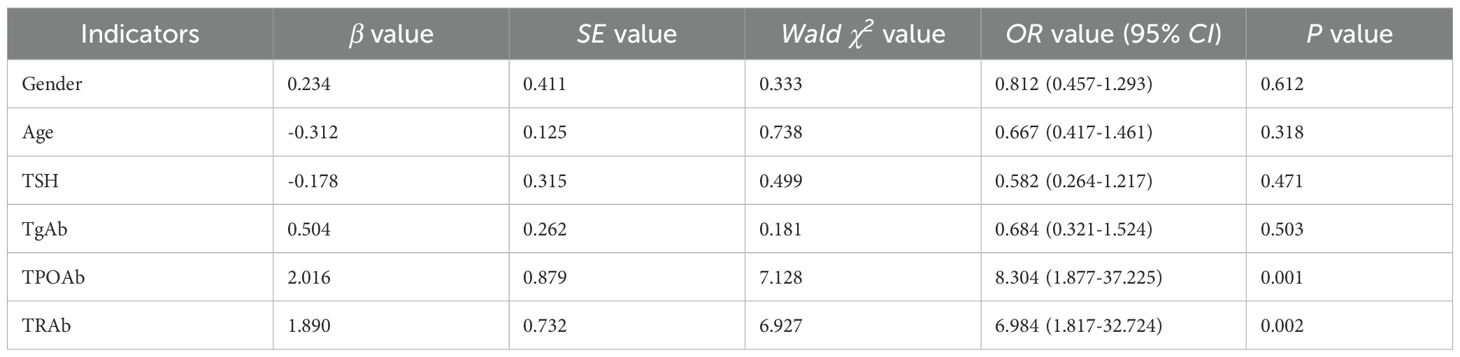
Table 3 presented the logistic regression analysis of risk factors for developing thyroid immune-related adverse events (irAEs) following immune checkpoint inhibitor therapy. The analysis identified pre-treatment thyroid autoantibodies as the most significant predictors of thyroid dysfunction, while demographic and other clinical factors showed no significant association.
Notably, thyroid peroxidase antibody (TPOAb) positivity demonstrated the strongest association with thyroid irAEs (OR=8.30, 95% CI: 1.88-37.23, P = 0.001), followed by thyrotropin receptor antibody (TRAb) positivity (OR=6.98, 95% CI: 1.82-32.72, P = 0.002). These findings suggest that pre-existing thyroid autoimmunity substantially increases susceptibility to ICI-induced thyroid dysfunction. In contrast, thyroglobulin antibody (TgAb) status (OR = 0.68, 95% CI: 0.32-1.52, P = 0.503), age (OR = 0.67, 95% CI: 0.42-1.46, P = 0.318), gender (OR = 0.81, 95% CI: 0.46-1.29, P = 0.612), and baseline TSH levels (OR = 0.58, 95% CI: 0.26-1.22, P = 0.471) were not statistically significant risk factors in this cohort.
These results emphasize the critical importance of pre-treatment thyroid autoantibody screening, particularly for TPOAb and TRAb, as these markers may help identify patients at highest risk for developing thyroid irAEs during ICI therapy. The differential predictive values of specific autoantibodies suggest distinct pathophysiological mechanisms underlying different forms of ICI-related thyroid dysfunction.
4 Discussion
In recent years, immune checkpoint inhibitors (ICIs) have been widely adopted in the treatment of various malignancies, significantly improving patient prognosis. However, while enhancing antitumor immunity, these agents can induce excessive immune responses in normal tissues, leading to immune-related adverse events (irAEs) (14). Endocrine organs—particularly the pituitary, thyroid, and pancreas—are frequent targets of such irAEs. The role of baseline immune status in predisposing cancer patients to thyroid irAEs remains controversial.
Several studies have demonstrated that patients with pre-existing thyroid autoantibodies (e.g., TPOAb, TRAb, TgAb) are more susceptible to thyroid dysfunction following PD-1 inhibitor monotherapy or combination PD-1/CTLA-4 blockade (15–17). In contrast, other reports suggest that thyroid irAE development correlates poorly with autoantibody titers (10, 18), implying a more complex interplay between multiple immune pathways (16, 19). Our findings align with the former perspective, as baseline thyroid autoantibody positivity was significantly higher in the irAEs group than in euthyroid controls (28.48% vs. 5.70%, P<0.001). This supports the hypothesis that pre-treatment autoimmunity primes the thyroid for ICI-mediated injury. TgAb was produced after thyroglobulin enters the bloodstream and can bind with thyroglobulin to form complexes, which have a destructive effect on thyroid follicular epithelial cells. Unlike TPOAb, thyroglobulin is not expressed on the surface of thyroid cells, limiting the antibody-dependent cell-mediated cytotoxicity of TgAb. A positive result is only observed in 60% of patients with autoimmune thyroid diseases (20).
The prognostic implications of specific autoantibodies remain unclear. Prior research indicates that while both TPOAb and TgAb predict thyroid irAEs, their clinical trajectories differ: TgAb positivity often precedes thyrotoxicosis, whereas TPOAb-positive patients tend to progress from transient thyrotoxicosis to persistent hypothyroidism (21). Our data further refine this model by revealing significantly higher TRAb titers in thyrotoxicosis cases versus hypothyroidism (P < 0.05), alongside lower TPOAb/TSH levels—a pattern suggesting TRAb’s potential role in Graves’-like thyrotoxicosis. Notably, TgAb levels showed no difference for the two groups, contradicting earlier reports that identified TgAb as a risk factor for PD-1 inhibitor-induced thyroiditis (8). Sex disparities in irAE susceptibility remain unclear; while Muir et al. (22) reported higher predisposition in females, our analysis—consistent with reference (8)—found no sex-based association.
The pathophysiology of ICI-induced thyroiditis likely involves both cellular and humoral immunity. Murine models demonstrate CD4+ T-cell infiltration in thyroid tissue during irAEs, implicating Th1/Th17-driven inflammation in destructive thyroiditis (23). Human studies similarly link thyroid peroxidase (TPO)- and thyroglobulin (Tg)-specific CD8+ T cells to Hashimoto’s-like damage (24). Intriguingly, Tg-directed immunity may dominate in PD-1 inhibitor-associated cases (8), as evidenced by marked TgAb/TPOAb elevation post-treatment in a hypothyroidism patient (25). These observations collectively underscore thyroid irAEs as a multifaceted immune phenomenon warranting mechanistic exploration.
Several limitations should be acknowledged in this study. Firstly, the single-center, retrospective design may limit generalizability and introduce selection bias. Second, as a cross-sectional analysis, we could not establish causal relationships between thyroid autoantibodies (TPOAb/TRAb) and thyroid dysfunction outcomes following ICI therapy - the observed associations require validation through prospective intervention studies. Third, potential confounding factors including concurrent medications and pre-existing autoimmune conditions may influence results. This study identifies pre-treatment TPOAb/TRAb as practical biomarkers for thyroid irAE risk stratification, advocating their routine screening before ICI initiation. However, as a single-center retrospective analysis, this work cannot establish causality or delineate autoantibody-specific risks for thyrotoxicosis versus hypothyroidism. Prospective multicenter studies with serial immune profiling are needed to validate these findings and guide personalized monitoring protocols.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The People’s Hospital of Chengyang Distric Ethics Review Board (ERB). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YJ: Writing – original draft, Conceptualization, Data curation. MX: Writing – original draft, Methodology, Project administration. XR: Writing – original draft, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
A special thanks to all of participants freely giving their time to make this and other possible studies. We also grateful to all those who helped us in our research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
2. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Prim. (2020) 6:38. doi: 10.1038/s41572-020-0160-6
3. Muir CA, Tsang VHM, Menzies AM, Clifton-Bligh RJ, and Tsang VHM. Immune related adverse events of the thyroid - A narrative review. Thyroid. (2020) 30:1458–69. doi: 10.1089/thy.2020.0032
4. Muir CA, Tsang VHM, Menzies AM, and Clifton-Bligh RJ. Immune related adverse events of the thyroid - A narrative review. Front Endocrinol (Lausanne). (2022) 13:886930. doi: 10.3389/fendo.2022.886930
5. Wu L, Xu Y, Wang X, Cheng X, Zhang Y, Wang Y, et al. Thyroid dysfunction after immune checkpoint inhibitor treatment in a single-center Chinese cohort: a retrospective study. Endocrine. (2023) 81:123–33. doi: 10.1007/s12020-023-03323-9
6. Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. (2019) 5:376–83. doi: 10.1001/jamaoncol.2018.5860
7. Sakakida T, Ishikawa T, Uchino J, Chihara Y, Komori S, Asai J, et al. Clinical features of immune-related thyroid dysfunction and its association with outcomes in patients with advanced Malignancies treated by PD-1 blockade. Oncol Lett. (2019) 18:2140–7. doi: 10.3892/ol.2019.10466
8. Kimbara S, Fujiwara Y, Iwama S, Ohashi K, Kuchiba A, Arima H, et al. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci. (2018) 109:3583–90. doi: 10.1111/cas.13800
9. Izawa N, Shiokawa H, Onuki R, Hamaji K, Morikawa K, Saji H, et al. The clinical utility of comprehensive measurement of autoimmune disease-related antibodies in patients with advanced solid tumors receiving immune checkpoint inhibitors: a retrospective study. ESMO Open. (2022) 7:100415. doi: 10.1016/j.esmoop.2022.100415
10. Yano S, Ashida K, Nagata H, Ohe K, Wada N, Takeichi Y, et al. Nivolumab-induced thyroid dysfunction lacking antithyroid antibody is frequently evoked in Japanese patients with Malignant melanoma. BMC Endocr Disord. (2018) 18:36. doi: 10.1186/s12902-018-0267-x
11. Gao Y, Ye T, Wu LG, Xu Y, Wang X, Cheng XQ, et al. The association between baseline TPOAb and/or TgAb positivity and thyroid immune-related adverse events in patients with Malignancies following treatment with immune checkpoint inhibitors. Zhonghua Yi Xue Za Zhi. (2024) 104:963–9. doi: 10.3760/cma.j.cn112137-20231011-00706
12. Bahn RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. (2011) 17:456–520. doi: 10.4158/ep.17.3.456
13. Gong WW, Zhou FY, and Guo QH. Clinical characteristics and risk factors of programmed death-1 inhibitors associated with thyroid gland injury. Zhonghua nei ke za zhi. (2023) 62:176–81. doi: 10.3760/cma.j.cn112138-20220329-00220
14. Muir CA, Menzies AM, Clifton-Bligh R, and Tsang VHM. Thyroid toxicity following immune checkpoint inhibitor treatment in advanced cancer. Thyroid. (2020) 30:1458–69. doi: 10.1089/thy.2020.0032
15. Iwama S, Kobayashi T, Yasuda Y, Okuji T, Ito M, Ando M, et al. Increased risk of thyroid dysfunction by PD-1 and CTLA-4 blockade in patients without thyroid autoantibodies at baseline. J Clin Endocrinol Metab. (2022) 107:e1620–30. doi: 10.1210/clinem/dgab829
16. Okada N, Iwama S, Okuji T, Kobayashi T, Yasuda Y, Wada E, et al. Anti-thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. Br J Cancer. (2020) 122:771–7. doi: 10.1038/s41416-020-0736-7
17. Kobayashi T, Iwama S, Yasuda Y, Okada N, Tsunekawa T, Onoue T, et al. Patients with antithyroid antibodies are prone to develop destructive thyroiditis by nivolumab: A prospective study. J Endocr Soc. (2018) 2:241–51. doi: 10.1210/js.2017-00432
18. Mazarico I, Capel I, Giménez-Palop O, Albert L, Berges I, Luchtenberg F, et al. Low frequency of positive antithyroid antibodies is observed in patients with thyroid dysfunction related to immune check point inhibitors. J Endocrinol Invest. (2019) 42:1443–50. doi: 10.1007/s40618-019-01058-x
19. Chang L-S, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, and Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. (2019) 40:17–65. doi: 10.1210/er.2018-00006
20. Hu YL, Li X, Fang HS, Ye XH, Shen MP, and Wu XH. Comparison of diagnostic performance of thyroid autoantibodies and high-resolution ultrasound in euthyroid Hashimoto’s thyroiditis. Zhonghua Yi Xue Za Zhi. (2021) 101:2537–43. doi: 10.3760/cma.j.cn112137-20201120-03154
21. Zhou X, Iwama S, Kobayashi T, Ando M, and Arima H. Risk of thyroid dysfunction in PD-1 blockade is stratified by the pattern of tgAb and TPOAb positivity at baseline. J Clin Endocrinol Metab. (2023) 108:e1056–62. doi: 10.1210/clinem/dgad231
22. Muir CA, Clifton-Bligh RJ, Long GV, Scolyer RA, Lo SN, Carlino MS, et al. Thyroid immune-related adverse events following immune checkpoint inhibitor treatment. J Clin Endocrinol Metab. (2021) 106:e3704–13. doi: 10.1210/clinem/dgab263
23. Yasuda Y, Iwama S, Sugiyama D, Okuji T, Kobayashi T, Ito M, et al. CD4(+) T cells are essential for the development of destructive thyroiditis induced by anti-PD-1 antibody in thyroglobulin-immunized mice. Sci Transl Med. (2021) 13. doi: 10.1126/scitranslmed.abb7495
24. Ehlers M, Thiel A, Bernecker C, Porwol D, Papewalis C, Willenberg HS, et al. Evidence of a combined cytotoxic thyroglobulin and thyroperoxidase epitope-specific cellular immunity in Hashimoto’s thyroiditis. J Clin Endocrinol Metab. (2012) 97:1347–54. doi: 10.1210/jc.2011-2178
Keywords: neoplasms, thyroid gland, immune checkpoint inhibitors, immune-related adverse events, anti-thyroid peroxidase antibody, thyrotropin receptor antibody, anti-thyroglobulin antibody
Citation: Jiao Y, Xu M and Rao X (2025) Correlation between different levels of thyroid autoantibodies and immune checkpoint inhibitor-associated thyroid dysfunction. Front. Endocrinol. 16:1620718. doi: 10.3389/fendo.2025.1620718
Received: 30 April 2025; Accepted: 28 July 2025;
Published: 13 August 2025.
Edited by:
Poupak Fallahi, University of Pisa, ItalyReviewed by:
Dmitry Aleksandrovich Zinovkin, Gomel State Medical University, BelarusJavier Ismael Altamirano García, National Institute of Medical Sciences and Nutrition Salvador Zubirán, Mexico
Victor Valsecchi, Federal University of São Paulo, Brazil
Copyright © 2025 Jiao, Xu and Rao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaopang Rao, ZG9jcnhwQDEyNi5jb20=
 Yanyan Jiao1
Yanyan Jiao1 Xiaopang Rao
Xiaopang Rao