- 1Bioorganic Research Institute, Suntory Foundation for Life Sciences, Kyoto, Japan
- 2Department of Chemistry, Biology, and Environmental Science, Faculty of Science, Nara Women’s University, Nara, Japan
Background: Cathepsin D is an aspartic protease responsible for the proteolytic processing of vitellogenin at the early stages of folliculogenesis in oviparous vertebrates. Previously, we identified a multifunctional neuropeptide, tachykinin (TK), as an inducer of cathepsin D that promotes vitellogenic follicle growth in the ascidian Ciona intestinalis type A (Ciona robusta), a sister group of vertebrates. However, no regulatory factor for cathepsin D in the ovary has been identified in vertebrates. Moreover, the involvement of cathepsin D in mammalian folliculogenesis has yet to be investigated.
Methods: Ovaries of 2-week-old ICR mice were used. Cathepsin D gene expression in the ovaries was examined by real-time PCR. Localization of cathepsin D was shown by immunostaining of ovarian sections. Cathepsin D activity was measured using supernatants from the homogenized ovaries. Mouse follicle growth was evaluated using three-dimensional follicle culture system.
Results: Immunohistochemical analysis revealed that cathepsin D is co-localized with TK receptors in granulosa cells of secondary follicles in the ovaries of two-week-old mice, which are sexually immature and in which the hypothalamus–pituitary–gonadal (HPG) axis is not yet functional. TK treatment of the ovaries significantly increased cathepsin D gene expression and its proteolytic activity. Moreover, inhibition of cathepsin D markedly suppressed the secondary follicle growth.
Conclusion: Collectively, these results indicate that cathepsin D plays essential roles in mouse secondary follicle growth. Recently, we also demonstrated that ovarian TKs promote secondary follicle growth in mice, primarily in a paracrine/autocrine manner. Combined with these findings, the present study leads us to propose an evolutionary scenario in which TK–cathepsin D signaling functions as a conserved mechanism for HPG axis-independent follicle growth across chordates, which may be more broadly conserved than the vertebrate-specific, HPG axis-dependent systems.
1 Introduction
In mammals, follicular development progresses through a well-defined sequence of stages: primordial, primary, secondary, pre-antral, antral, and Graafian follicles (1–5). This process is largely classified into gonadotropin-independent and gonadotropin-dependent phases. In postpubertal females, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), secreted from the anterior pituitary in response to hypothalamic gonadotropin-releasing hormone (GnRH), play essential roles in promoting the growth and maturation of pre-antral to antral follicles through the hypothalamus–pituitary–gonadal (HPG) axis. In contrast, the growth of early-stage follicles—including primordial, primary, and secondary follicles—is independent of gonadotropins and occurs prior to the onset of HPG axis functions (6, 7).
Tachykinins (TKs) are a conserved family of neuropeptides found across chordates, including ascidians. In mammals, TKs function in diverse physiological contexts, acting as neuromodulators and peripheral hormones that regulate pain, inflammation, gastrointestinal motility, immune response, hematopoiesis, and reproductive processes (8–10). Mammalian TKs include substance P (SP), neurokinin A (NKA), neurokinin B (NKB), and hemokinin-1/endokinins (HK-1/EKs), which exert their effects through specific G-protein-coupled receptors: TACR1, TACR2, and TACR3 (8, 11–13). Our previous study demonstrated that ovarian TKs promote secondary follicle growth in mice, primarily through autocrine/paracrine signaling (14). TKs stimulate the expression of cyclooxygenase-2 (COX-2) via the JAK1–STAT3 pathway in granulosa cells, resulting in elevated production of prostaglandins E2 (PGE2) and F2α (PGF2α). Moreover, we previously demonstrated that TK specifically triggered the growth of vitellogenic follicles via induction of the expression of several proteases, including cathepsin D, in the ascidian Ciona intestinalis type A (Ciona robusta), a sister group of vertebrates (15, 16). These findings verified that cathepsin D acts as a direct downstream effector of TK signaling for early-stage follicles in Ciona.
Cathepsin D is a lysosomal aspartic protease widely conserved across metazoans (17–19), and plays vital roles in protein turnover, activation of hormones and growth factors, enzymatic processing, and apoptosis. In oviparous vertebrates, cathepsin D is known to facilitate the breakdown of yolk precursors such as vitellogenin, thereby providing energy for oocyte growth (20–22). Despite these known functions, the physiological role and regulatory mechanisms of cathepsin D in mammalian folliculogenesis remain largely unexplored.
In this study, we revealed the involvement of TK-induced cathepsin D in secondary follicle development in mice, and provides new insight into a conserved HPG axis-independent mechanism of early-stage folliculogenesis.
2 Materials and methods
2.1 Animals
This study was approved by the Suntory animal ethics committee and the Animal Care Committee of Nara Women’s University, and all animals were maintained in accordance with committee guidelines for the care and use of laboratory animals. This study was approved by the Suntory animal ethics committee (APRV000340). ICR mice were purchased from Japan SLC Inc (Kyoto, Japan). All of the mice were euthanized with CO2 asphyxiation.
2.2 Immunohistochemistry
2-week-old mouse ovaries were fixed at 25°C for 15 min in Bouin fluid. The fixed ovaries were embedded in paraffin, and the ovaries were cut into 7-µm sections. Immunostaining using primary antibodies (CathepsinD(R-20) and NK-1R(H-83); Santa Cruz Biotechnology, CA, USA) was performed as previously described (14). The immunoreactivity was visualized with an indirect immunofluorescence technique using secondary antibodies (Alexa 488 donkey anti-rabbit IgG and alexa 568 donkey anti-goat IgG; Life Technology, Carlsbad, CA, USA) diluted with blocking buffer (1:500; v/v). Coverslips were mounted in Fluorosafe mounting medium (Merck, Darmstadt, Germany), and the sections of ovaries were viewed using an Olympus BX51 photomicroscope (Olympus, Tokyo, Japan) equipped with epifluorescence. Because auto-fluorescence was detected in the ovarian sections, a WIB long-pass filter cube (Olympus) was used for observation of cathepsin D-derived fluorescent signals.
2.3 Real-time PCR
The real-time PCR was performed using CFX96 Real-time System and SsoAdvancedTM Universal SYBR Green Supermix (Bio-Rad laboratories, Hercules, CA, USA). Total volume of the real-time PCR reaction mixture was 20 µl consisting of 100 ng template cDNA, each 500 nM primer, and 10 µl SsoAdvancedTM Universal SYBR Green Supermix. The real-time PCR program was at 95°C for 30 sec, and 44 cycles of at 95°C for 15 sec and at 60°C for 30 sec. The melting curve analyses for amplified PCR products were performed to confirm the absence of primer dimers. To evaluate the gene expressional level, we analyzed using ΔΔCt method that represents the induction level of target genes in the ovaries treated with TACR1, 2, and 3 agonists or antagonists (Supplementary Table 1). Ct value represents the PCR cycle number when the PCR product is arrived at determined level, and ΔCt shows the difference between Ct values using PCR products prepared from the agonists-treated and the antagonists-treated ovaries. Subsequently, ΔΔCt was calculated using ΔCt of β-actin gene between the agonists-treated and antagonists-treated ovaries to normalize the real-time PCR result. The primers used for the real-time PCR were designed using Primer-blast web tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/), and their sequences are shown in Supplementary Table 2.
2.4 Measurement of cathepsin D activity in the ovary
The half-piece ovaries were incubated in the presence of TACR1, 2, and 3 agonists or antagonists (Supplementary Table 1). The agonist-treated or antagonist-treated ovaries were homogenized in Cathepsin D assay buffer included in the Cathepsin D Assay Kit (Sigma-Aldrich, St. Louis, MO, USA, for the Cathepsin D assay), and centrifuged at 15000 x g at 4°C for 5 min. The supernatants were frozen with liquid nitrogen, and stored at -80°C until use. The cathepsin D activity was evaluated using the Cathepsin D Assay Kit (Sigma-Aldrich) according to the manufacturer’s instruction. The difference of cathepsin D activities between agonist-treated and antagonist-treated ovaries was calculated.
2.5 Three-dimensional follicle culture system
Co-cultivation of mouse secondary follicles with theca/interstitial cells using collagen gel (Cellmatrix Type I-A; Nitta Gelatin, Inc., Osaka, Japan) was performed as described in previous reports (23, 24). In brief, 0.2% collagen gel containing 10% FBS (Thermo Fisher Scientific Inc.), 100 U/ml penicillin, 0.1 mg/ml streptomycin (Nacalai Tesque Inc., Kyoto, Japan), and Dulbecco’s Modified Eagle Medium components (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) were used to culture the secondary follicles. Approximately 20–30 secondary follicles with 100 μm-diameter and 6 × 104/well-theca/interstitial cells were co-cultured at 37°C in 5% CO2 in air and 100% humidity for 5 days in a 96-well plate with no ligand or pepstatin A as a cathepsin D inhibitor. Morphological change of each follicle was observed using an inverted microscopy Olympus CK2 (Olympus). Short and long diameters of oocytes and follicles were measured using an image analysis tool, ImageJ (https://imagej.nih.gov/ij/). Oocyte and follicle growth were calculated differential length of diameters between oocytes and follicles culturing for 0 day and 5 days.
2.6 Statistical analysis
Results are shown as mean ± S.E.M. Data were analyzed by t test with Welch’s correction or one-way ANOVA and turkey’s multiple comparison test. Differences were accepted as significant for P < 0.05.
3 Results
3.1 Localization of cathepsin D in the mouse ovary
All TK receptors (TACR1, -2, and -3), are found to be colocalized in the granulosa cells of the inner layer within secondary follicles, and their activation triggers the growth of these follicles (14). Immunostaining of sections from 2-week-old mouse ovaries revealed that cathepsin D is localized in the granulosa cells of the inner layer of secondary follicles, showing strong spatial correspondence with the immunoreactivity of TACR1 (Figure 1). Additionally, TACR1 was shown to be co-localized with both TACR2 and 3 in the granulosa cells of the inner layer of secondary follicles in our previous study (14), indicating that cathepsin D is co-localized with all three TACRs.
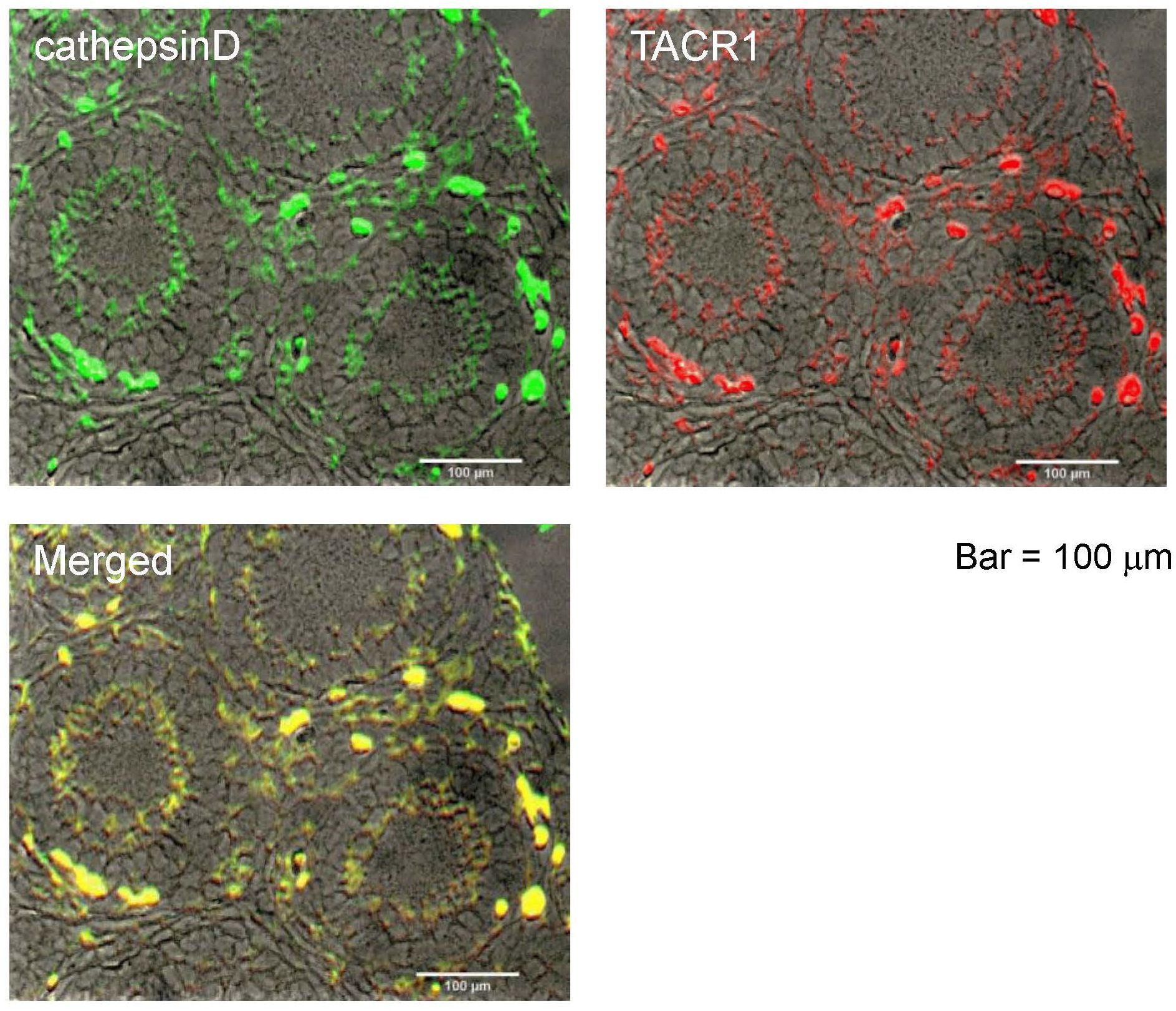
Figure 1. Localization of cathepsin D in the ovaries of 2-weeks old mouse. Immunostaining of cathepsin D on a section of the ovary is shown by green signal, while immunostaining of TACR1 is shown by red signal. Yellow signal in the merged image shows the co-localization of cathepsin D and TACR1. Scale bars indicate 100 μm.
3.2 Downregulation of cathepsin D gene expression and activity by TK receptor antagonists
We conducted a microarray analysis on 2-week-old mouse ovaries treated with either TK receptor agonists or antagonists to identify factors induced by TK (14; accession no. GSE213246). The microarray data suggested that the cathepsin D gene was also downregulated in ovaries treated with TK receptor antagonists. To verify the changes in cathepsin D gene expression, we performed real-time PCR on cDNAs prepared from the 2-week-old mouse ovaries treated with either TK receptor agonists or antagonists. Real-time PCR revealed a 2.1-fold increase in cathepsin D gene expression in ovaries treated with TK receptor agonists for one day, compared to those treated with antagonists (Figure 2). However, after more than two days, the expression levels of the cathepsin D gene were comparable between the ovaries treated with agonists and those treated with antagonists (Figure 2, Supplementary Table 3). These data indicated that the activation of TK receptors transiently upregulated cathepsin D gene expression, which was rapidly downregulated thereafter. Subsequently, we assessed the protease activity of cathepsin D in the 2-week-old mouse ovaries treated with TK receptor agonists or antagonists. These assays indicated that ovaries treated with TK receptor agonists exhibited higher protease activity compared to those treated with antagonists (Figure 3, Supplementary Table 4), proving that TKs stimulate the production of cathepsin D in the ovaries. Notably, cathepsin D activity remained consistently higher in ovaries treated with TK receptor agonists than in those treated with antagonists, despite comparable levels of cathepsin D gene expression in both groups after more than two days. Together with the immunostaining results, these findings suggest that the increase in cathepsin D activity is directly triggered by TK receptor activation in granulosa cells of the inner layer of secondary follicles.
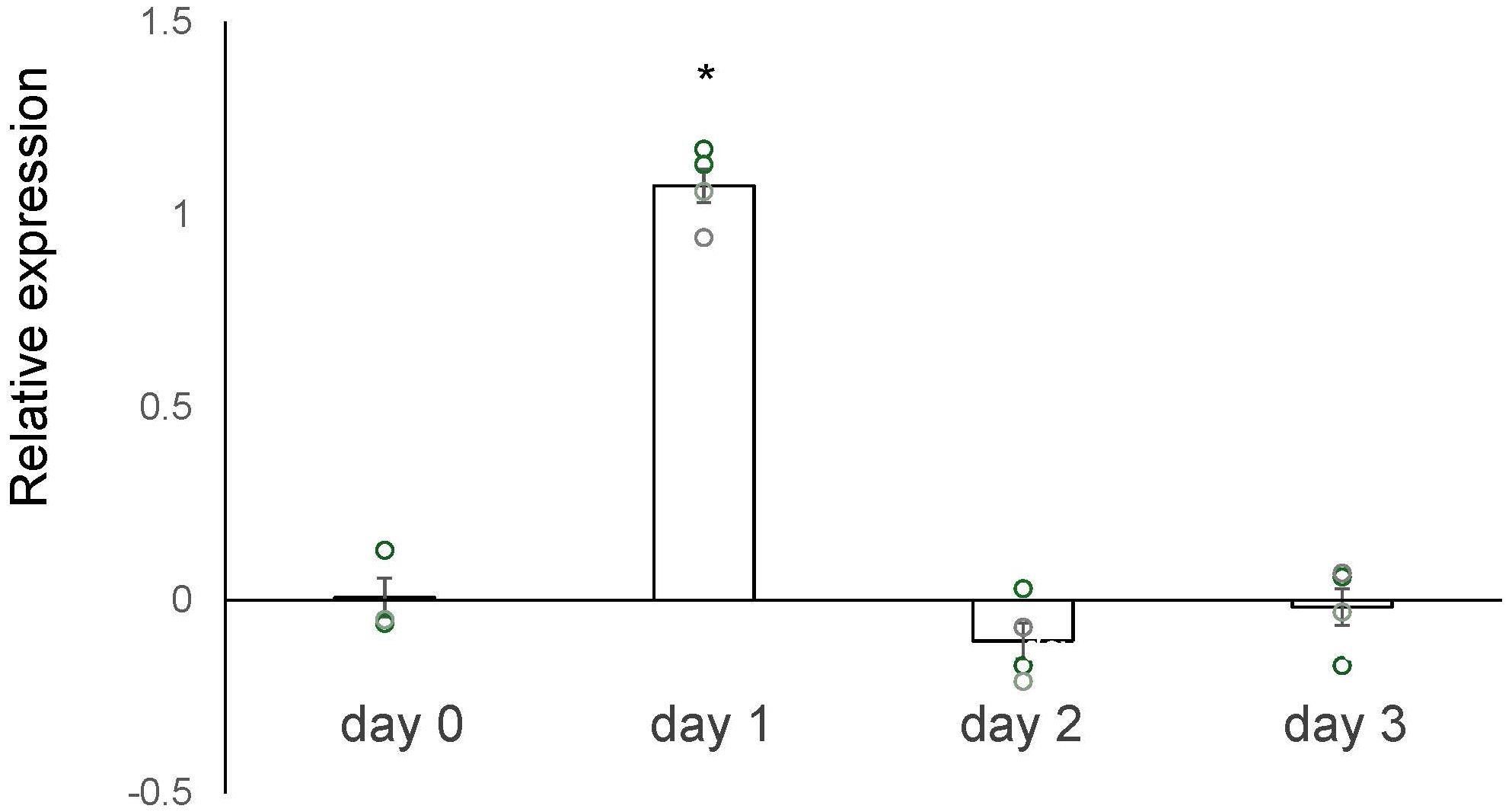
Figure 2. Real-time PCR-based quantification of the cathepsin D gene expression. The vertical axis represents the relative level of the cathepsin D gene expression in the ovary treated with TK receptor agonists (each agonist at 1 μM), compared to that treated with TK receptor antagonists (each antagonist at 1 μM). The induction level of gene expression was calculated from the ΔΔCt values using β actin gene in the condition of presence of TK receptor agonists or TK receptor antagonists. Each point represents the mean ± S.E.M for three or four independent experiments. Significant differences (P< 0.05 vs. TK antagonist-treated group with t-test) are indicated by asterisks.
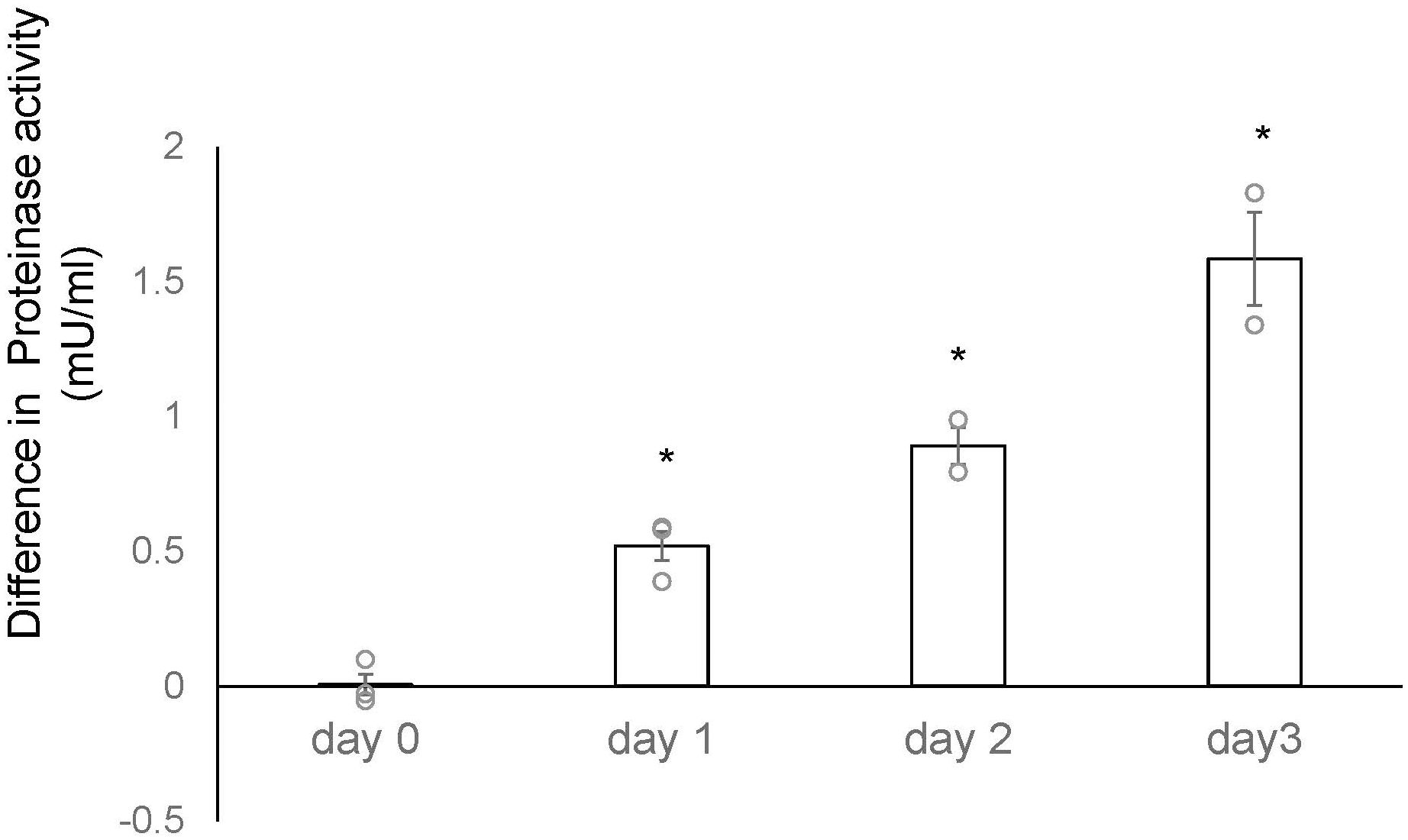
Figure 3. Time-course analysis of differences in cathepsin D activity between 2-week-old mouse ovaries treated with TK receptor agonists and those with antagonists. The vertical axis represents the difference in proteinase activity between ovaries cultured with TK receptor agonists and those cultured with TK receptor antagonists. Cathepsin D activity was gradually upregulated in the ovary treated with TK receptor agonists (each agonist at 1 μM) compared to those treated with TK receptor antagonists (each antagonist at 1 μM). Each point represents the mean ± S.E.M for two independent experiments. Significant differences (P< 0.05 vs. TK receptor antagonist-treated group with t-test) are indicated by asterisks.
3.3 Suppression of follicle growth by cathepsin D inhibitor
Subsequently, we evaluated the impact of cathepsin D on secondary follicle growth by in vitro morphological assays using three-dimensional follicle culture (14, 23, 24). We utilized an aspartic protease, pepstatin A, as a cathepsin D inhibitor. Of particular interest is that the follicle growth was suppressed by approximately 80% in the presence of pepstatin A, while normal growth occurred in its absence (Figure 4). Similarly, the growth of oocytes within the secondary follicle was also obstructed by pepstatin A (Figure 4). The inhibitory effect of pepstatin A on cathepsin D activity indicates that cathepsin D is critical for secondary follicle growth, such as the role of TKs. Overall, these results verified that mouse TKs induce the secondary follicle growth via the upregulation of the gene expression and enzymatic activity of cathepsin D in the mouse ovary.
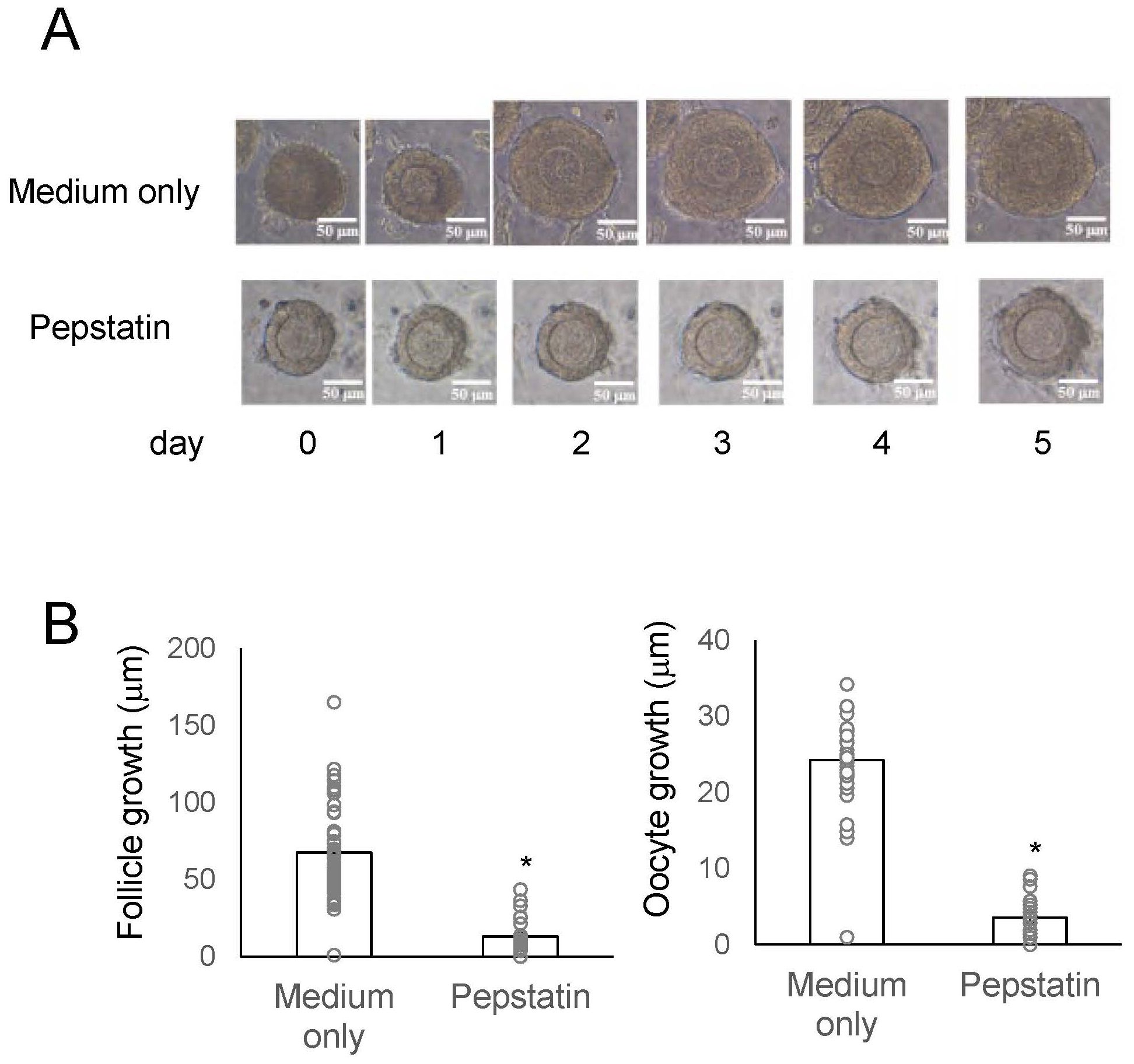
Figure 4. Effects of a cathepsin D inhibitor on secondary follicle growth in three-dimensional follicle culture. (A) Secondary follicles isolated from the mouse ovary were cultured within a collagen gel with theca cells for 5 days. The follicles were untreated (medium only) or treated with 8 μg/ml-pepstatin A (cathepsin D inhibitor). Bars indicate 50 μm. (B) The size of the follicles grown in three-dimensional culture in the presence or absence of 8 μg/ml-pepstatin for 5 days was measured. Oocyte growth is represented as the increase in oocyte diameter from Day 0 to Day 5, calculated by subtracting the diameter at Day 0 from that at Day 5. Likewise, follicle growth is represented as the increase in follicle diameter over the same period. Each point represents the mean ± S.E.M. Significant differences (P< 0.05 vs. medium only group with t-test) are indicated by asterisks.
4 Discussion
Our current study reveals a novel role for cathepsin D in the growth of mouse secondary follicles. Cathepsin D promotes follicle growth through the proteolysis of vitellogenin, a process previously observed in various oviparous vertebrates and invertebrates (20, 21, 25, 26). Vitellogenin serves as a precursor protein of egg yolk, which is stored in oocytes and utilized as a nutrient source during egg laying and early embryonic development. In contrast, the vitellogenin gene has been lost in viviparous species during the mammalian evolution (27), indicating that in viviparous organisms, cathepsin D may contribute to reproductive functions beyond yolk protein processing. Cathepsin D is also known to play a vital role in maintaining cellular homeostasis. In humans, it regulates protein turnover by degrading misfolded and aggregated proteins and facilitates apoptosis under conditions of proteostatic stress (19). In mouse follicles, granulosa cells undergo substantial proliferation as follicles develop from the secondary to pre-antral stage, necessitating quality control and remodeling for normal growth. It is therefore plausible that cathepsin D contributes to these processes during follicular development in mice.
Recent advancements of follicle culture systems in vitro have been significant, with mature follicles successfully generated from pluripotent stem cells in mice (28, 29). The culture medium used for follicle maturation includes factors such as GDF9, BMP15, FSH, EGF, and hCG, all of which are essential contributors to the development of mature follicles. Nevertheless, the molecular mechanisms in each cell that comprising the follicle during its development and maturation are not yet well characterized. As follicles develop, granulosa cells undergo processes of formation, proliferation, and multilayering, with specialized subtypes such as cumulus cells emerging. These granulosa cells are believed to shift their functions across both temporal and spatial dimensions. It has been proposed that TK-cathepsin D signaling specifically contributes to the proliferation and stratification of granulosa cells within secondary follicles. In the future, the increasing use of single-cell transcriptomic technologies is expected to shed light on the distinct temporal and spatial functions of individual granulosa cell populations.
Interestingly, the timing of cathepsin D gene upregulation in mouse secondary follicles does not align with the peak of its protease activity (Figures 2, 3). Cathepsin D is initially synthesized as pre-pro-cathepsin D from its mRNA (20, 21, 25, 26). Following the removal of the signal peptide, pro-cathepsin D is transported into intracellular vesicles, where it undergoes proteolytic maturation into the active form through the action of cysteine proteases and other cathepsins (e.g., cathepsins B and L) within endosomes and lysosomes (17–19). This post-translational processing is in a good agreement with the observed gap between cathepsin D gene expression and its enzymatic activity. Consistently, in Drosophila ovaries, cathepsin D gene expression is elevated during vitellogenesis, whereas mature cathepsin D protein accumulates to higher levels during follicular atresia (21). Eventually, the protease activity is retained in the ovaries during atresia, despite lower gene expression levels (21). These findings support the notion that in mouse secondary follicles, cathepsin D activity in granulosa cells is regulated not only at the transcriptional level but also through precursor processing at the post-translational level.
Ascidians are aquatic organisms found globally, classified within the phylum Urochordata and the superphylum Chordata, and are among the closest relatives of vertebrates (30–32). In Ciona, cathepsin D is localized in the test cells that surround oocytes within vitellogenic follicles (15, 16, 33, 34). The geometric arrangement of these test cells closely resembles that of granulosa cells surrounding oocytes in vertebrate follicles (35), suggesting that several functional characteristics are at least in part conserved between ascidian test cells and vertebrate granulosa cells (Figure 5). Notably, Ciona TK (CiTK) promotes the growth of vitellogenic follicles in Ciona through an increase in cathepsin D levels (16). Combined with these findings, the present study suggests that the essential mechanism underlying TK-cathepsin D-mediated early-stage follicle growth is essentially conserved throughout chordates and have originated at least in a common ancestor of vertebrates and urochordates (Figure 5). Conversely, the regulatory mechanisms governing TK–cathepsin-mediated follicular development differ between the mouse and ascidian. In the ascidian, CiTK is secreted from the central nervous system, reflecting a “centralized” neuroendocrine regulation. In contrast, in the mouse ovary, TKs are synthesized within the follicle and act through a “locally autonomous” paracrine or autocrine signaling system. Consequently, it is postulated that this locally autonomous TKergic system emerged alongside the evolutionary advancement and functional specialization of vertebrate tissues.
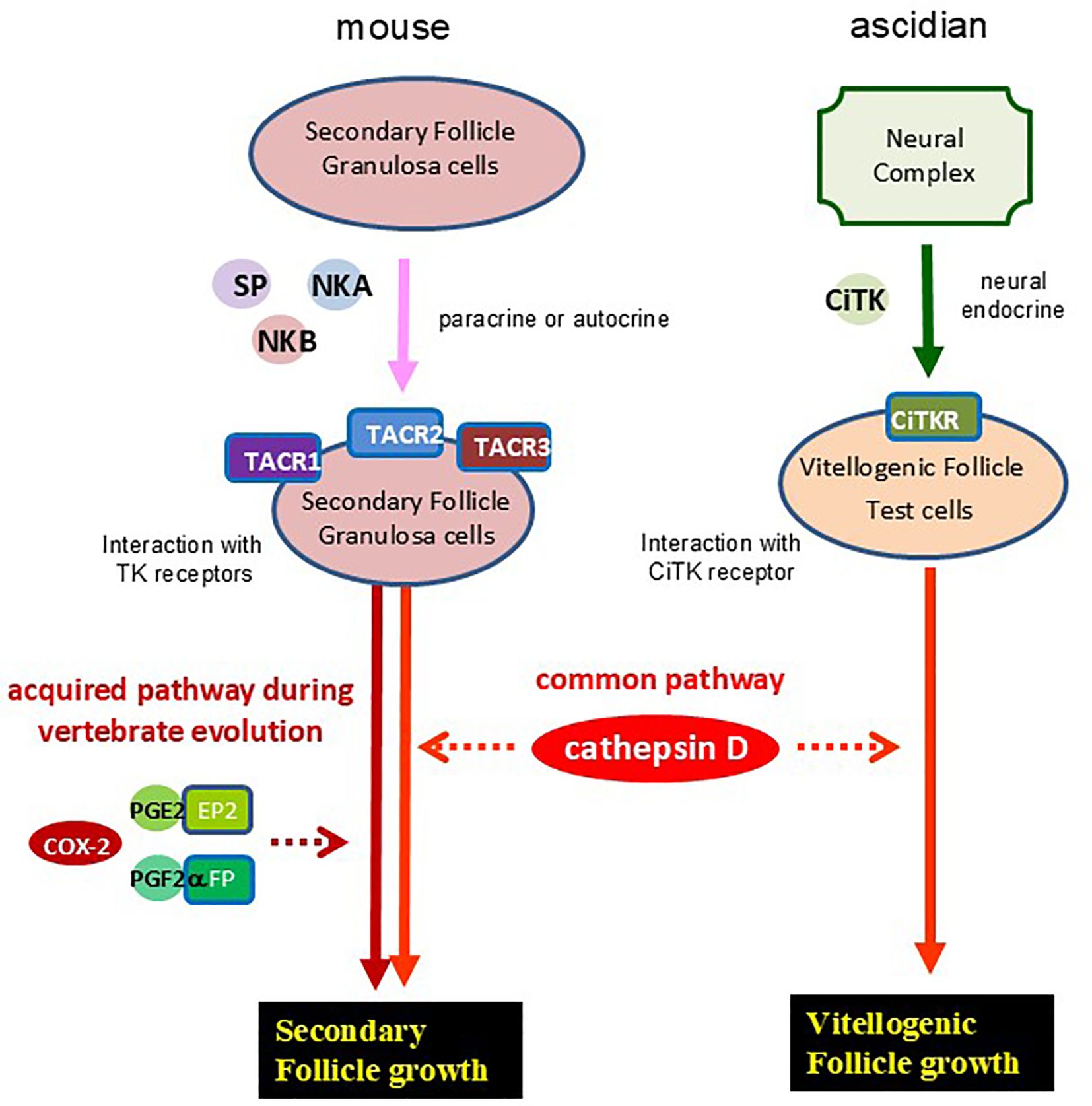
Figure 5. An evolutionary hypothesis of TKergic follicle growth in chordates. Mouse TKs (SP, NKA, and NKB) are secreted from granulosa cells of secondary follicles, and selectively interact with TK receptors (TACR1, -2, and -3) at the granulosa cells with autocrine or paracrine. Activation of TK receptors induces secondary follicle growth, which is mediated by cathepsin D activation and interaction between prostaglandins (PGE2 and PGF2α) and their receptors (EP2 and FP, respectively) (14). On the other hand, Ciona TK (CiTK) is secreted from the ascidian neural complex, and is secreted to the ovary via a neuroendocrine pathway. Activation of CiTK receptor (CiTKR) induces the cathepsin D gene expression, leading to the vitellogenic follicle growth in Ciona (15, 16).
Recently, we demonstrated that PGE2 and PGF2α are crucial for the growth of TK-mediated secondary follicles in mice (14). TKs induce the upregulation of the COX-2 gene, which encodes prostaglandin H2 (PGH2) synthase, in mouse ovaries, and increase of PGH2 synthesis leads to the production of PGE2 and PGF2α (14). Combined with these findings, the present study indicates that TKs facilitate the growth of secondary follicles in mice through both prostaglandin and cathepsin D signaling pathways. In contrast, the previous gene expression profile revealed that the COX-2 gene, unlike the cathepsin D gene, was not upregulated by treatment of Ciona ovaries with CiTK. These results indicate that the TK-cathepsin D signaling pathway involved in early follicular development is evolutionarily conserved across chordates, whereas the TK-COX-2 signaling pathway has been acquired by limited chordates. Likewise, the HPG axis, a key regulatory system of follicle growth, is restricted to vertebrates. Thus, the TK-cathepsin D system is likely to be more broadly conserved, compared with both the TK-COX-2 system and the HPG axis. In contrast, the substrate of cathepsin D in the TKergic follicle growth is not conserved among chordates. In viviparous species, developing embryos receive nutrients directly from the maternal body, which diminishes the necessity for substantial yolk reserves within ovarian follicles. This shift in reproductive strategy might have reduced the functional relevance of yolk-associated proteins, such as vitellogenin, during follicle development. Consequently, it is hypothesized that, during the evolutionary transition from oviparous to viviparous species, the cathepsin D substrate in TKergic follicle growth shifted from vitellogenin to other proteins.
Our previous study demonstrated that the JAK1–STAT3 signaling promotes COX-2 gene expression in granulosa cells of mouse secondary follicles (14). In contrast, the signaling pathway responsible for inducing cathepsin D gene expression in these cells remains unidentified. Indeed, the functional relationship between the TK-COX-2 signaling and TK-cathepsin D signaling has not been found in any animals. In MCF−7 human breast cancer cells, the cathepsin D gene expression is regulated by multiple pathways. Estrogen directly induces cathepsin D expression through estrogen-responsive elements in the gene promoter region (36, 37), while growth factors like IGF-I, TGFα, and EGF also elevate cathepsin D level via alternative mechanisms, including MAPK-mediated activation of estrogen receptor and Sp1 binding to GC-rich promoter regions in MCF-7 cells (37). Nevertheless, the signaling pathways for regulation of cathepsin D gene expression remain poorly characterized in most other cell types and tissues. Future studies will aim to elucidate the TK–cathepsin D signaling pathway in granulosa cells of mouse secondary follicles in greater detail.
5 Conclusion
We have provided original evidence for a secondary follicle growth process mediated by the TKergic cathepsin D activation. Our findings will enhance the understanding of not only gonadotropin-independent follicle growth mechanisms in mammals but also the evolutionary pathways of follicle growth systems throughout chordates.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The animal study was approved by Suntory animal ethics committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
TK: Data curation, Investigation, Methodology, Validation, Writing – original draft. MA: Data curation, Investigation, Methodology, Resources, Validation, Writing – original draft. KY: Data curation, Investigation, Resources, Writing – original draft. HS: Conceptualization, Data curation, Funding acquisition, Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science (http://www.jsps.go.jp/english/index.html), Japan (grant no.: JP22H02658; to HS).
Acknowledgments
We thank Prof Makoto Suematsu and Prof Shigetada Nakanishi for providing insightful comments on the research. We are also grateful to Dr Saori Itami for the three-dimensional follicle culture system support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1621348/full#supplementary-material
References
1. McGee EA and Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. (2000) 21:200–14. doi: 10.1210/edrv.21.2.0394
2. Orisaka M, Tajima K, Tsang BK, and Kotsuji F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res. (2009) 2:9. doi: 10.1186/1757-2215-2-9
3. Robker RL, Hennebold JD, and Russell DL. Coordination of ovulation and oocyte maturation: A good egg at the right time. Endocrinology. (2018) 159:3209–18. doi: 10.1210/en.2018-00485
4. Duffy DM, Ko C, Jo M, Brannstrom M, and Curry TE. Ovulation: parallels with inflammatory processes. Endocr Rev. (2019) 40:369–416. doi: 10.1210/er.2018-00075
5. Orisaka M, Miyazaki Y, Shirafuji A, Tamamura C, Tsuyoshi H, Tsang BK, et al. The role of pituitary gonadotropins and intraovarian regulators in follicle development: A mini-review. Reprod Med Biol. (2021) 20:169–75. doi: 10.1002/rmb2.12371
6. Maggi R, Cariboni AM, Marelli MM, Moretti RM, Andrè V, Marzagalli M, et al. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum Reprod Update. (2016) 22:358–81. doi: 10.1093/humupd/dmv059
7. Xie Q, Kang Y, Zhang C, Xie Y, Wang C, Liu J, et al. The role of kisspeptin in the control of the hypothalamic-pituitary-gonadal axis and reproduction. Front Endocrinol (Lausanne). (2022) 13:925206. doi: 10.3389/fendo.2022.925206
8. Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martín JD, et al. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. (2004) 11:2045–81. doi: 10.2174/0929867043364748
9. Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, and Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. (2014) 94:265–301. doi: 10.1152/physrev.00031.2013
10. Campo A, Dufour S, and Rousseau K. Tachykinins, new players in the control of reproduction and food intake: A comparative review in mammals and teleosts. Front Endocrinol (Lausanne). (2022) 13:1056939. doi: 10.3389/fendo.2022.1056939
11. Satake H and Kawada T. Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors. Curr Drug Targets. (2006) 7:963–74. doi: 10.2174/138945006778019273
12. Satake H, Aoyama M, Sekiguchi T, and Kawada T. Insight into molecular and functional diversity of tachykinins and their receptors. Protein Pept Lett. (2013) 20:615–27. doi: 10.2174/0929866511320060002
13. Nässel DR, Zandawala M, Kawada T, and Satake H. Tachykinins: neuropeptides that are ancient, diverse, widespread and functionally pleiotropic. Front Neurosci. (2019) 13:1262. doi: 10.3389/fnins.2019.01262
14. Kawada T, Aoyama M, Matsubara S, Osugi T, Sakai T, Kirimoto S, et al. Ovarian tachykinin signaling system induces the growth of secondary follicles during the gonadotropin-independent process. J Biol Chem. (2025) 301:108375. doi: 10.1016/j.jbc.2025.108375
15. Aoyama M, Kawada T, Fujie M, Hotta K, Sakai T, Sekiguchi T, et al. A novel biological role of tachykinins as an up-regulator of oocyte growth: identification of an evolutionary origin of tachykininergic functions in the ovary of the ascidian, Ciona intestinalis. Endocrinology. (2008) 149:4346–56. doi: 10.1210/en.2008-0323
16. Aoyama M, Kawada T, and Satake H. Localization and enzymatic activity profiles of the proteases responsible for tachykinin-directed oocyte growth in the protochordate, Ciona intestinalis. Peptides. (2012) 34:186–92. doi: 10.1016/j.peptides.2011.07.019
17. Fusek M and Vetvicka V. Dual role of cathepsin D: ligand and protease. BioMed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2005) 149:43–50. doi: 10.5507/bp.2005.003
18. Benes P, Vetvicka V, and Fusek M. Cathepsin D–many functions of one aspartic protease. Crit Rev Oncol Hematol. (2008) 68:12–28. doi: 10.1016/j.critrevonc.2008.02.008
19. Mijanovic O, Petushkova AI, Brankovic A, Turk B, Solovieva AB, Nikitkina AI, et al. Cathepsin D-managing the delicate balance. Pharmaceutics. (2021) 13:837. doi: 10.3390/pharmaceutics13060837
20. Carnevali O, Cionna C, Tosti L, Lubzens E, and Maradonna F. Role of cathepsins in ovarian follicle growth and maturation. Gen Comp Endocrinol. (2006) 146:195–203. doi: 10.1016/j.ygcen.2005.12.007
21. Leyria J, Fruttero LL, Nazar M, and Canavoso LE. The role of dmCatD, a cathepsin D-like peptidase, and acid phosphatase in the process of follicular atresia in dipetalogaster maxima (Hemiptera: reduviidae), a vector of Chagas’ Disease. PloS One. (2015) 10:e0130144. doi: 10.1371/journal.pone.0130144
22. Sharma L, Pipil S, Rawat VS, and Sehgal N. Role of cathepsins B and D in proteolysis of yolk in the catfish Clarias gariepinus. Fish Physiol Biochem. (2022) 48:749–65. doi: 10.1007/s10695-022-01062-8
23. Itami S, Yasuda K, Yoshida Y, Matsui C, Hashiura S, Sakai A, et al. Co-culturing of follicles with interstitial cells in collagen gel reproduce follicular development accompanied with theca cell layer formation. Reprod Biol Endocrinol. (2011) 9:159. doi: 10.1186/1477-7827-9-159
24. Aoyama M, Shiraishi A, Matsubara S, Horie K, Osugi T, Kawada T, et al. Identification of a new theca/interstitial cell-specific gene and its biological role in growth of mouse ovarian follicles at the gonadotropin-independent stage. Front Endocrinol (Lausanne). (2019) 10:553. doi: 10.3389/fendo.2019.00553
25. Yilmaz O, Prat F, Ibañez AJ, Amano H, Koksoy S, and Sullivan CV. Estrogen-induced yolk precursors in European sea bass, Dicentrarchus labrax: Status and perspectives on multiplicity and functioning of vitellogenins. Gen Comp Endocrinol. (2015) 221:16–22. doi: 10.1016/j.ygcen.2015.01.018
26. Li H and Zhang S. Functions of vitellogenin in eggs. Results Probl Cell Differ. (2017) 63:389–401. doi: 10.1007/978-3-319-60855-6_17
27. Ohishi Y, Arimura S, Shimoyama K, Yamada K, Yamauchi S, Horie T, et al. Egg yolk protein homologs identified in live-bearing sharks: co-opted in the lecithotrophy-to-matrotrophy shift? Genome Biol Evol. (2023) 15:evod028. doi: 10.1093/gbe/evad028
28. Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. (2016) 539:299–303. doi: 10.1038/nature20104
29. Yoshino T, Suzuki T, Nagamatsu G, Yabukami H, Ikegaya M, Kishima M, et al. Generation of ovarian follicles from mouse pluripotent stem cells. Science. (2021) 373:6552. doi: 10.1126/science.abe0237
30. Delsuc F, Brinkmann H, Chourrout D, and Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. (2006) 439:965–8. doi: 10.1038/nature04336
31. Denoeud F, Henriet S, Mungpakdee S, Aury JM, Da Silva C, Brinkmann H, et al. Plasticity of animal genome architecture unmasked by rapid evolution of a pelagic tunicate. Science. (2010) 330:1381–5. doi: 10.1126/science.1194167
32. Satoh N, Rokhsar D, and Nishikawa T. Chordate evolution and the three-phylum system. Proc Biol Sci. (2014) 281:20141729. doi: 10.1098/rspb.2014.1729
33. Prodon F, Chenevert J, and Sardet C. Establishment of animal-vegetal polarity during maturation in ascidian oocytes. Dev Biol. (2006) 290:297–311. doi: 10.1016/j.ydbio.2005.11.025
34. Matsubara S, Shiraishi A, Osugi T, Kawada T, and Satake H. Fractionation of Ovarian Follicles and in vitro Oocyte Maturation and Ovulation Assay of Ciona intestinalis Type A. Bio Protoc. (2020) 10:e3577. doi: 10.21769/BioProtoc.3577
35. Sakai T, Yamamoto T, Watanabe T, Hozumi A, Shiraishi A, Osugi T, et al. Characterization of a novel species-specific 51-amino acid peptide, PEP51, as a caspase-3/7 activator in ovarian follicles of the ascidian. Front Endocrinol (Lausanne). (2023) 14:1260600. doi: 10.3389/fendo.2023.1260600
36. Rochefort H. Mechanism of the overexpression of the cathepsin D gene in breast cancer and consequences in the metastatic process. C R Seances Soc Biol Fil. (1991) 185:415–21.
Keywords: tachykinin, cathepsin D, follicle growth, immature follicle, granulosa cell
Citation: Kawada T, Aoyama M, Yasuda K and Satake H (2025) Cathepsin D mediates tachykinin-induced secondary follicle growth independent of the hypothalamic–pituitary–gonadal axis in mice. Front. Endocrinol. 16:1621348. doi: 10.3389/fendo.2025.1621348
Received: 30 April 2025; Accepted: 20 May 2025;
Published: 05 June 2025.
Edited by:
Shogo Haraguchi, Showa University, JapanCopyright © 2025 Kawada, Aoyama, Yasuda and Satake. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Honoo Satake, c2F0YWtlQHN1bmJvci5vci5qcA==
†Present address: Masato Aoyama, NIPRO CORPORATION, Shiga, Japan
‡These authors have contributed equally to this work
 Tsuyoshi Kawada
Tsuyoshi Kawada Masato Aoyama2†‡
Masato Aoyama2†‡ Honoo Satake
Honoo Satake