- 1Institute of Biomedical Engineering and Nanomedicine, National Health Research Institutes, Miaoli,, Taiwan
- 2Department of Biological Science and Technology, National Yang Ming Chiao Tung University, Hsinchu, Taiwan
- 3Metal Industries Research & Development Centre, Kaohsiung, Taiwan
Diabetic peripheral neuropathy (DPN) is a debilitating complication of diabetes that affects nearly half of diabetic patients and manifests as chronic pain, sensory loss, and motor dysfunction. The limited efficacy of traditional pharmacological treatments, coupled with their side effects, has intensified the search for alternative therapies that not only mitigate symptoms but also delay the progression of diabetes-related neural complications. In this review, the potential of transcranial magnetic stimulation (TMS) as a nonpharmacological intervention for DPN is explored, with a focus on the ability of TMS to delay neural inflammation—a key factor in the progression of DPN—rather than directly treating diabetes. TMS has shown promising results in alleviating neuropathic pain, promoting nerve regeneration, and regulating autonomic nervous function, making it a strong candidate for delaying adverse neural outcomes. Other neuromodulation techniques, such as spinal cord stimulation (SCS), decompression nerve surgery (DNS), and transcranial direct current stimulation (tDCS), have also been examined for their efficacy in treating DPN. While TMS has significant therapeutic potential for protecting neural function and delaying inflammation, further research is needed to optimize treatment protocols and understand their long-term benefits. This review emphasizes the translational potential of neuromodulation technologies in delaying the progression of diabetes-induced neural damage, underscoring the need for further studies to translate these therapies into clinical practice.
1 Introduction
1.1 Diabetes and diabetic neuropathy
Diabetes mellitus (DM) is a common condition that has been prevalent for many years. The International Diabetes Federation (IDF) reported that in 2021, the global prevalence of diabetes in adults was 10.5% (1). The IDF suggested that this percentage will continue to rise. They reported that by the year 2045, 12.2% of people worldwide will be confirmed to have diabetes (1). Diabetes is a chronic metabolic disease caused by elevated blood glucose levels (2, 3). There are two possible etiologies of diabetes: a lack of insulin or insulin insufficiency (4, 5). Insulin is a hormone that facilitates the transfer of glucose from the blood into cells to further provide energy for metabolism (6–8). A lack of insulin or insulin insufficiency destroys this important process and may lead to diabetes.
The etiologies mentioned above can result in two main types of diabetes (9–11). Type 1 diabetes mellitus (T1DM) occurs when a patient’s own immune system antibodies attack and destroy insulin-producing β-cells in the pancreas, resulting in less insulin production (12, 13); however, it is unclear what exactly causes T1DM. Some findings have suggested that genetic or environmental factors are responsible for T1DM, whereas other findings have suggested that viruses could also play a role, as supported by case studies and blood test results (14–17). On the other hand, type 2 diabetes mellitus (T2DM) can be caused by either insufficient insulin production or insulin resistance (18–23). Research shows that a family history of T2DM increases an individual’s risk of developing the disease for genetic reasons (24–26). Furthermore, consuming high-fat foods, insufficient exercise, being overweight, being stressed, and having high blood lipid and cholesterol levels are factors that can cause T2DM (27). Studies have even shown that changes in the gut microbiota can be a cause of T2DM (28, 29). The gut microbiome is responsible for controlling fat accumulation and the progression of obesity-related diseases, making the gut microbiome a significant factor in T2DM development. When the microbiota of the gut is not regulated properly, a large amount of short-chain fatty acids are produced by bacteria, which can lead to impaired glucose metabolism and the occurrence of insulin resistance, ultimately resulting in T2DM (30, 31).
Both types of diabetes increase the glucose concentration in the blood. These changes can lead to several problems related to the circulatory system. Issues such as eye disease, kidney disease, and nerve damage can occur (32, 33). There is also a risk of severe circulatory system problems, such as heart disease, strokes, and diseases affecting blood flow in the limbs (34–36). Diabetic peripheral neuropathy (DPN) affects almost half of DM patients globally, making it a difficult, persistent challenge in DM treatment. DPN leads to problems such as lower limb pain and amputations, irregular heartbeats, and heart tissue breakdown, directly affecting everyday tasks and quality of life (37–39). Among patients with DPN, 20 to 30% also suffer from neuropathic pain (40, 41). This type of pain is hard to address because it usually gradually worsens and becomes more severe. To relieve pain, people often need medicines or other treatments (42, 43). The current understanding of DPN is based on abnormalities in glucose metabolism (44). A relatively high level of glucose triggers the activity of alternative metabolic pathways (45). These pathways and processes include the polyol and protein kinase C pathway, hexosamine and glucosamine formation, the accumulation of advanced glycation end products, and the anaerobic glycolytic process. One of these processes, alone or in combination, can lead to the beginning and progression of DPN (41, 45).
1.2 Drug treatment of diabetes and DPN
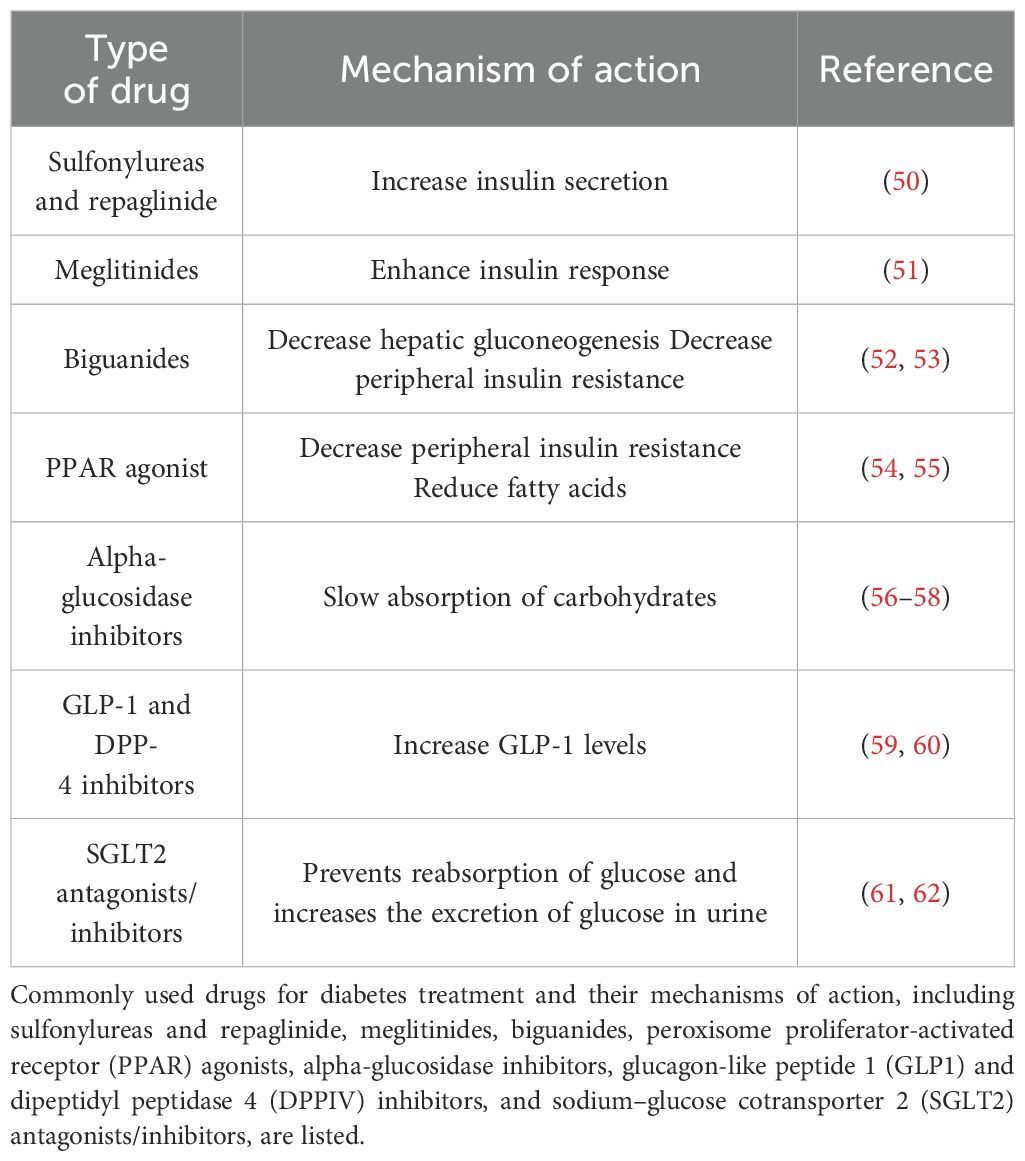
Presently, common therapeutic classes of drugs for diabetes include sulfonylureas, meglitinides, biguanides, peroxisome proliferator-activated receptor (PPAR) agonists, α-glucosidase inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium–glucose cotransporter 2 (SGLT2) inhibitors (46–49), as shown in Table 1. People with T2DM usually take sulfonylureas and meglitinides, which are insulin secretagogues. Insulin secretions facilitate the release of insulin by interacting with sulfonylurea receptors on pancreatic β-cells (50, 63, 64). Biguanides do not impact insulin release directly. The mechanism of action of biguanides involves decreasing the number of insulin-resistant cells. Biguanides also reduce the uptake of glucose in the intestine, which helps maintain glucose levels in the blood, prevents glucose delivery into the blood and facilitates the absorption of more glucose by cells (65, 66).
PPAR agonists act as insulin sensitizers, activating transcription factors within the superfamily of hormone receptors, including PPARα, PPARγ, and PPARβ/δ. PPARs regulate metabolic functions and maintain energy stability, with PPARγ increasing cellular sensitivity to insulin and increasing glucose metabolism (54, 67, 68). Alpha-glucosidase inhibitors act by temporarily delaying the intestinal absorption of carbohydrates, resulting in a slower entry of glucose from food into the bloodstream, thereby inhibiting postprandial blood glucose levels (56, 69–71). Commonly used α-glucosidase inhibitors in clinical trials include acarbose, miglitol, and voglibose.
GLP-1 is a peptide consisting of 36 amino acids that plays crucial roles in various diabetes-related metabolic processes (72, 73). These roles include stimulating insulin secretion, lowering blood glucose levels, reducing gastric emptying, inhibiting food intake, and regulating rodent β-cell proliferation (59, 74, 75). DPP-4, a serine protease, degrades numerous peptides containing GLP-1 within biological organisms (60, 76). Therefore, the use of GLP-1 receptor agonists and DPP-4 inhibitors to increase GLP-1 levels and the half-life of GLP-1 in the body effectively lowers blood glucose levels (77, 78). SGLT2, which is found in the proximal convoluted tubule, is responsible for glucose reabsorption along with passive transport proteins, facilitated glucose transporters, and active cotransporter proteins (79). SGLT2 inhibitors prevent glucose reabsorption in the proximal convoluted tubule and promote its excretion in the urine, thereby reducing blood glucose concentrations (61, 80, 81). Various drugs are used to prevent diabetes. The different strategies are illustrated in Figure 1.
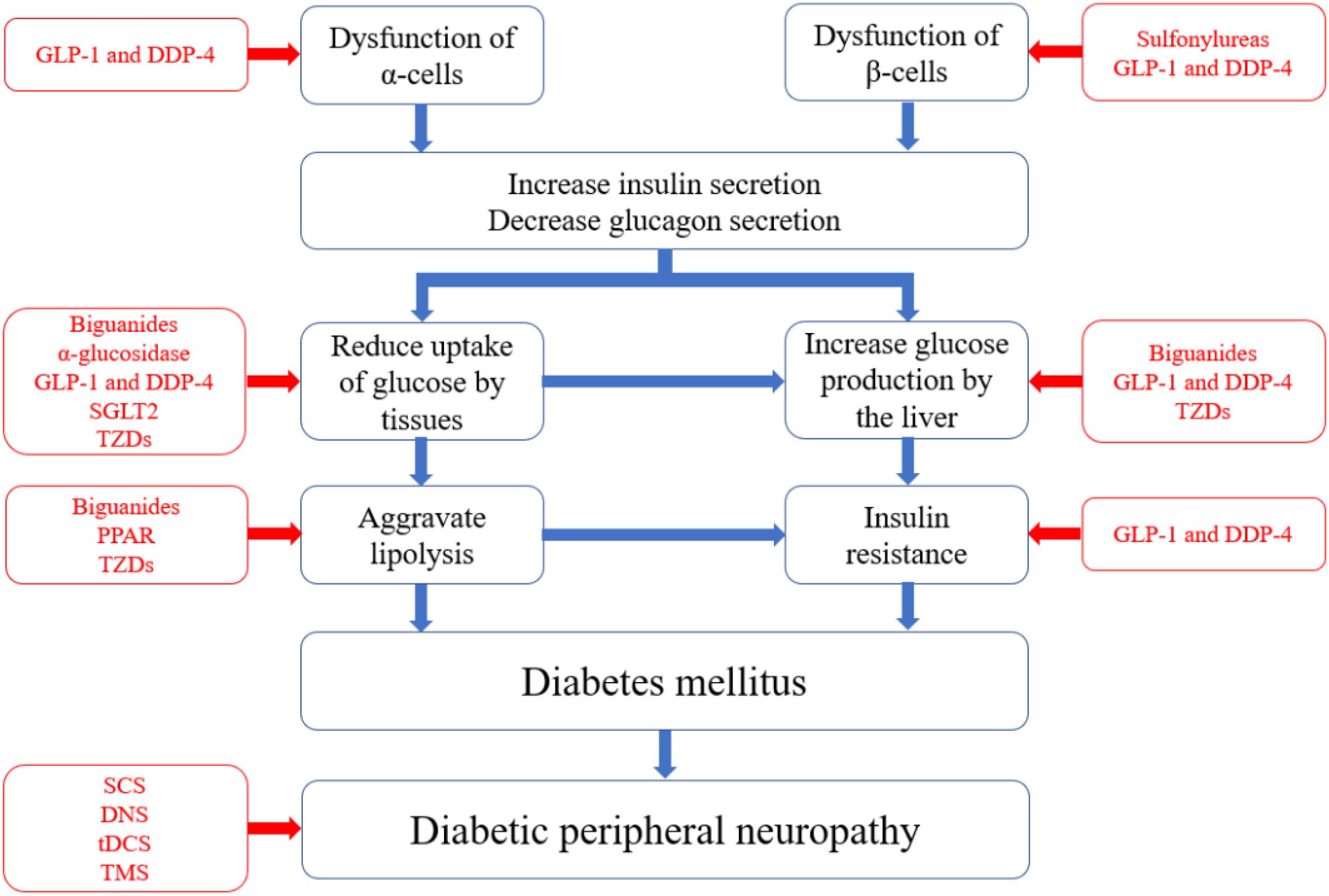
Figure 1. Pathophysiology of diabetes and corresponding pharmacological treatment. The blue boxes in the figure represent the pathological pathways of diabetes, starting from the dysfunction of α and β cells and leading to elevated blood glucose levels, insulin resistance, and ultimately diabetes. Each component can independently or synergistically contribute to the development and worsening of diabetes. The red boxes indicate the types of medications that target specific pathological pathways.
There are three classes of Food and Drug Administration (FDA)-approved drugs for treating DPN: pregabalin, duloxetine, and tapentadol. However, some experts challenge the approval of tapentadol because it is categorized as an opioid (82–84). Pregabalin binds to the α2δ subunit of calcium channels, reducing calcium influx into nerve endings and altering neurotransmitter release (85–87). Pregabalin is excreted primarily unchanged in the urine. Therefore, extra care is needed when administering pregabalin to people with kidney problems (88, 89). Duloxetine is a selective serotonin and norepinephrine reuptake inhibitor. It can cause side effects, including nausea, dizziness, and somnolence, when taken. Combining duloxetine with pregabalin, which has a different mechanism of action, has shown good clinical efficacy (90–92).
1.3 Nondrug treatment for diabetes and diabetic neuropathy
Medicines may help alleviate diabetes and DPN, but they also have notable side effects. We found that different nonpharmacological methods can be used to manage diabetes and DPN more safely. For example, diet control and exercise prevent people from developing diabetes (93–95). To alleviate and suppress DPN, methods such as spinal cord stimulation (SCS), decompression nerve surgery (DNS), transcranial direct current stimulation (tDCS), and transcranial magnetic stimulation (TMS) have also been used (96–99), shown as Figure 2.
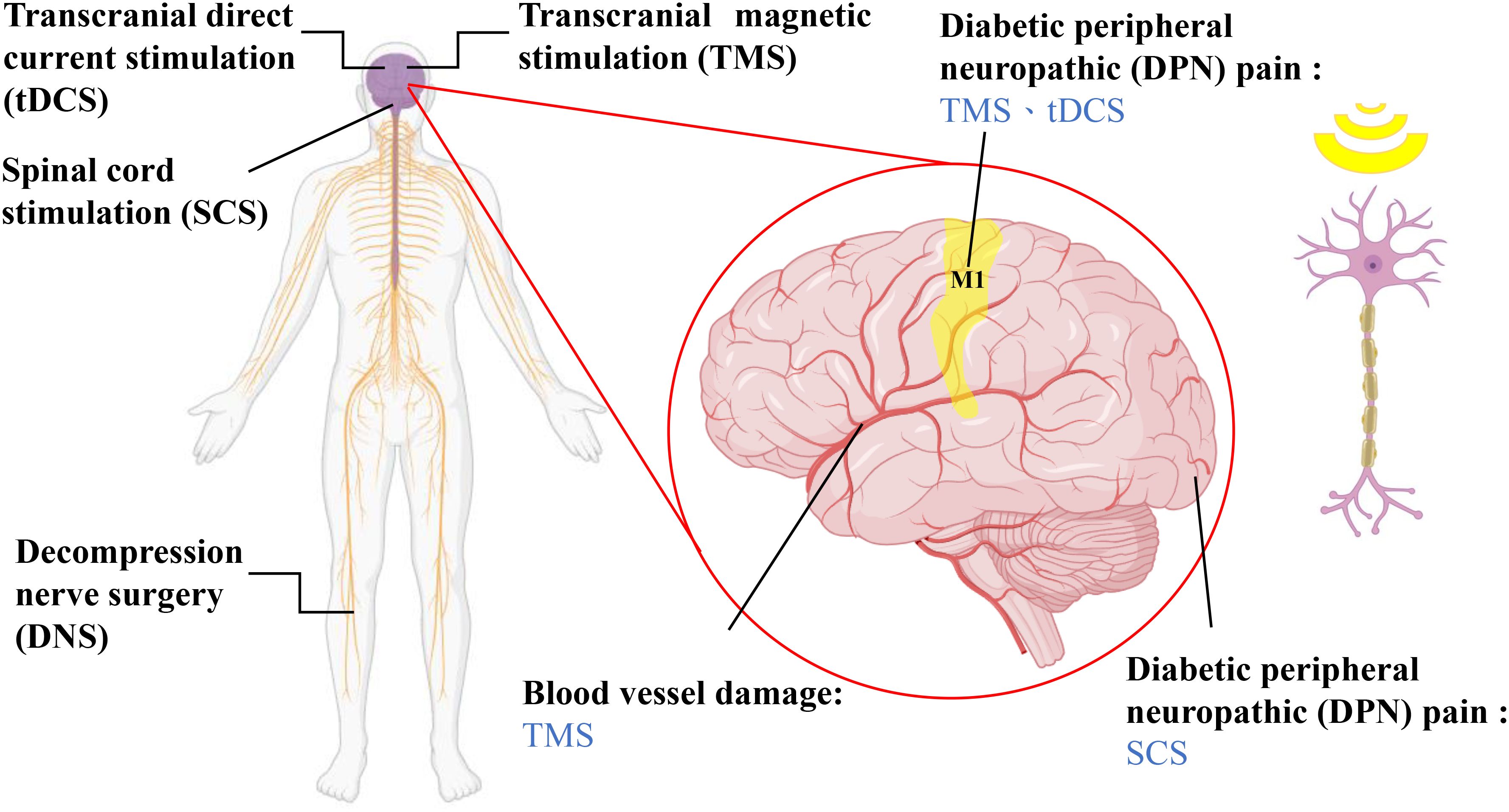
Figure 2. Nondrug treatments for diabetic neuropathy and the location of treatment. SCS involves placing electrodes on the dorsal side of the dura mater of the brain, utilizing fixed-frequency electrical stimulation to mask pain sensations (64). DNS is a surgical method that can alleviate chronic neuropathic pain caused by prolonged nerve compression in the lower limbs according to several observational studies, although its effectiveness has yet to be confirmed (56). tDCS and TMS are both noninvasive neuromodulatory techniques that can modulate neural functions in the brain, including through pain inhibition, depression control, and the relief of autonomic nerve dysfunction (74, 96, 100, 101). The magnified image on the right illustrates the brain regions that each therapy interferes with and the types of neuropathic conditions the therapies treat.
TMS is a new treatment for DPN and has gained much attention because of research on the use of TMS for neuromodulation, nerve repair, and stimulation of neural pathways (102–104). TMS is a noninvasive method of localized cortical stimulation originally applied for central nervous system disorders (105, 106). However, by altering stimulation patterns and parameters, TMS can be used to assess the integrity of the CST and the degree of damage to motor conduction pathways (107, 108). Additionally, TMS can promote functional recovery after nerve injury by influencing brain blood flow and oxidative stress levels (109, 110). In TMS, a magnetic field is produced by altering the stimulation coil current to create an induced electrical field in the brain. When the current meets a certain limit, the axon hillock or interneurons are triggered. This shift in neuron responsiveness aids in adjusting brain activity (100). TMS is effective in treating different complications of diabetes, including painful conditions, depression, autonomic neuropathy, and vascular damage (102). However, as a relatively new therapy, limited research is available. Therefore, the aims of this review are to summarize the current research on the use of TMS in the treatment of DPN and compare DPM with other nonpharmacological treatment modalities.
1.4 Research motivation and objectives
Despite the availability of numerous pharmacological and nonpharmacological strategies for the management of diabetes and its complications, DPN remains a prevalent and debilitating condition that significantly impairs patients’ quality of life. Current pharmacotherapies for DPN are often associated with limited efficacy, adverse effects, and poor long-term outcomes, whereas conventional nonpharmacological approaches yield inconsistent therapeutic benefits. These limitations underscore the urgent need for alternative modalities that are both effective and well tolerated. In recent years, TMS, a noninvasive neuromodulation technique, has emerged as a promising treatment option for various neurological and pain-related disorders. Preliminary investigations suggest that TMS may exert beneficial effects on DPN through mechanisms involving cortical reorganization, pain modulation, and neurovascular regulation. However, evidence supporting its application in DPN remains fragmented, and its efficacy relative to that of other nonpharmacological interventions has not been systematically examined. Therefore, this review aims to synthesize the current literature on the use of TMS in treating DPN, evaluate its therapeutic potential in comparison with other nonpharmacological modalities, and elucidate the underlying mechanisms that may contribute to its clinical effects. This review aims to address an important gap in existing studies and inform future research directions and clinical approaches for managing diabetic neuropathy.
2 Exercise and diet in the treatment and management of diabetes
2.1 Mechanisms of action of alleviation and treatment methods and approaches for controlling diabetes in the diet
2.1.1 Impact of diet on diabetes
A high-fat diet serves as a standard method to induce obesity in animal models. Excessive intake of saturated fats leads to the overexpansion of adipocytes in adipose tissue, resulting in hypoxia. Hypoxia triggers a cascade of responses, including the activation of hypoxia-inducible factor 1 (HIF-1) gene expression. HIF-1, a transcription factor, becomes activated in low-oxygen environments, leading to the overexpression of other proteins, such as c-Jun N-terminal kinase and IκB kinase. In turn, inflammatory responses are induced in cells (111). Inflammation typically manifests as the production of proinflammatory cytokines, which further exacerbate the inflammatory process (111, 112). Inflammation causes a chain reaction in cells, which leads to difficulty in insulin regulation. Insulin usually controls fat breakdown by reducing the influence of hormone-sensitive lipases within fat-storing cells. However, when these cells resist insulin, stored fats are transferred into free fatty acids (113), move into the bloodstream and are taken up by various organs. This process further causes these organs to resist insulin and leads to T2DM (101, 111, 113).
2.1.2 Dietary approaches for the prevention and alleviation of diabetes
It is clear that what we eat greatly impacts early diabetes development. Eating properly, such as choosing low-fat foods, limiting carbohydrates, or following a ketogenic diet, can effectively prevent and manage diabetes (111, 113, 114). A healthy diet involves the consumption of daily essential nutrients, such as water, protein, fat, carbohydrates, and other micronutrients, to maintain overall health. According to the World Health Organization recommendations for a healthy diet, individuals should consume 400 grams of fruits and vegetables per day, as well as legumes, grains, and nuts. The fat content should be kept below 30% of the total caloric intake, and the intake of simple sugars should be limited to less than 10% of the total caloric intake. Daily caloric intake should be set on the basis of individual energy expenditure (115).
In contrast to a high-fat diet, a low-fat diet restricts the consumption of fats, including cholesterol (116). By reducing the risk of obesity and insulin resistance, a low-fat diet helps prevent diabetes (117–119). For diabetes patients, reducing fat intake can effectively alleviate the development of insulin resistance in organs. A carbohydrate-restricted diet (CRD) involves the replacement of high-carbohydrate foods with higher protein- or fat-containing foods and fiber-rich vegetables (120–122). CRD can effectively control blood sugar levels, and studies suggest that reducing carbohydrate intake can lower the risk of developing T2DM (120–123). Therefore, for patients who are unresponsive to medications that lower glucose levels, a diet limited in carbohydrates is a practical choice. For example, a ketogenic diet causes the body to produce ketones, similar to when the body is fasting (124). This type of diet makes the body favor ketones over glucose for energy (124, 125). Ketogenic diets have garnered significant popularity in contemporary times and are increasingly employed for the management of diabetes. An intriguing aspect of a ketogenic diet is its ability to effectively reduce blood glucose levels (126, 127). Research findings indicate that individuals following a ketogenic diet may require only half the amount of insulin needed before initiating this dietary regimen (124, 128, 129). For example, a four-month research study revealed a decrease in antidiabetic medicine use with a ketogenic diet. Some patients could even stop using antidiabetic medications entirely (130). As a result, experts view a ketogenic diet as a tool that can help regulate blood glucose, assisting those with diabetes.
2.2 Mechanisms of action of exercise in the treatment of diabetes and exercise modalities
2.2.1 Impact of exercise on diabetes
Exercise and diet control are ways to lower weight. Losing weight with activity and less caloric intake can help approximately 80% of T2DM patients (93–95). Furthermore, regular exercise can alleviate inflammatory symptoms in muscle cells, reduce cellular insulin resistance, and stimulate the activity of adenosine monophosphate-activated protein kinase (AMPK). In turn, AMPK activity facilitates the translocation of glucose transporter 4 (GLUT4) to the cell membrane, contributing to the control of blood glucose levels (131–133).
2.2.2 Exercise modalities for the prevention and alleviation of diabetes
Among all exercise modalities, aerobic exercise, resistance training, and high-intensity interval training (HIIT) are the most common. Aerobic exercise involves rhythmic activities, such as walking and running, which involve large muscle groups (134–136). A minimum of 150 minutes per week of moderate to vigorous aerobic exercise can increase VO2max cardiac output (134, 137). The risks of heart issues and death are greatly lower in patients with T2DM who exercise (138). Additionally, aerobic exercise helps balance fat and other body-building substances. Furthermore, exercise lowers hemoglobin A1c (HbA1c) levels (139, 140). In contrast to the fat-reducing effects of aerobic exercise, resistance training focuses more on altering the structure of the body’s muscle tissue through the use of weight machines, free weights, and resistance bands (141). Resistance training improves health indicators such as bone density, blood pressure, blood lipids, insulin sensitivity, and muscle strength by 10–15% (142–144). This effect not only slows the progression of diabetes in elderly patients but also contributes to a reduction in the risk associated with other diseases.
As a popular workout style, in HIIT, individuals push their limits in short, intense workouts of approximately 10 minutes (145, 146). HIIT is a useful method for controlling blood fat and glucose levels (147, 148). HIIT even increases muscle power and the response to insulin (147–149). One HIIT workout is short and might be a good fit for T2DM patients willing to take on a challenge. However, HIIT is not suitable for everyone. Some experts believe that combined aerobic and resistance workouts could work better (150). This combined workout regimen could lead to more positive results in terms of insulin response and HbA1c reduction than either type alone (151–153). The efficacy of a combined exercise strategy in terms of reducing the risk of cardiovascular diseases has been demonstrated (153, 154). In conclusion, a mixed aerobic and resistance regimen could be suitable for T2DM patients for whom HIIT is too difficult.
2.3 Limitations of exercise and diet in the treatment and management of diabetes
While exercise and dietary interventions are widely acknowledged as foundational nonpharmacological approaches for managing type 2 diabetes, their effectiveness is subject to several important limitations. One key constraint is the influence of concurrent antidiabetic medications, such as metformin, SGLT2 inhibitors, or insulin, which can alter baseline glycemic levels and potentially obscure or exaggerate the true effects of lifestyle changes (155, 156). Additionally, individual behavioral and lifestyle factors, including smoking, alcohol use, chronic stress, poor sleep quality, and prolonged sedentary behavior, can negatively impact insulin sensitivity and systemic inflammation, thereby confounding the outcomes of exercise- and diet-based interventions.
3 Diabetic neuropathy alleviation and treatment
3.1 Spinal cord stimulation
In the past, it was usually difficult to efficiently ease pain with the standard medication for DPN (43, 157). This standard treatment is often associated with substantial side effects and low tolerance (158, 159). To reduce our dependence on medicines, the search for different treatment methods or adjunct therapies is now an important part of research. Compared with traditional medicine, SCS provides pain relief that lasts eight times longer (160). SCS is a method in which the sense of pain is replaced with sensory paresthesia. The electrodes are placed on the dorsal surface of the spinal cord. Then, a certain strength of electrical current is sent through the spinal cord and masks the sensation of pain (161, 162). With technological advancements, SCS is no longer limited to the original 40~60 Hz low-frequency stimulation; a unique waveform at 10 kHz can be utilized to alleviate the pain associated with DPN without inducing sensory disturbances. The evidence suggests that, following high-frequency SCS stimulation, 87.5% of patients experienced at least a 50% reduction in pain during a six-month follow-up (163). Furthermore, more than half of patients experienced a reduction in pain intensity of over 50%, even five years after treatment (164). The supporting evidence confirms the effectiveness of the SCS.
3.2 Decompression nerve surgery
DPN is characterized by symptoms of regular, long-term nerve pressure, including discomfort, a burning sensation, a pin-and-needle sensation, and a loss of sensation (165, 166). Because of these parallels, some studies have suggested that nerve compression causes DPN (167). The affected areas in the human body include three nerves located in the legs, including the distal fibula on the lateral side of the lower leg, the intersection of the leg with the posterior heel on the medial side, and the junction of the short extensor muscle of the thumb with the branch of the deep peroneal nerve (168). These nerves are often compressed because of the narrow tunnel they pass through at these locations. Thus, DNS is widely used to prevent pain due to DPN (99, 169), but the effectiveness of DNS is still a matter of debate. Indeed, most researchers have proposed the effectiveness of DNS simply on the basis of observations (99). Future research is needed to further clarify the feasibility and efficacy of DNS in the treatment of DPN.
3.3 Transcranial direct current stimulation
3.3.1 Mechanism of action of tDCS
tDCS is a noninvasive brain stimulation technique, and its efficacy was confirmed in rodents several decades ago (170). Anodal tDCS can increase cortical excitability, whereas cathodal stimulation can reduce cortical excitability (171, 172). The effects of tDCS are rapid, with cortical excitability typically being influenced within seconds of stimulation, and prolonged stimulation can significantly extend the duration of cortical modulation (173–175). Rather than inducing changes in only single brain cells, tDCS has a greater impact on whole-brain networks (176). Several cells in our bodies, such as endothelial cells, immune cells, and brain cells, are sensitive to changes in electrical fields. The activities of these cells are manipulated when tDCS is applied (177). These findings indicate that tDCS can influence the development of certain diseases by affecting these cells.
3.3.2 Application of tDCS in DPN
tDCS successfully lowers pain in people suffering from DPN-related discomfort. tDCS adjusts brain activity, specifically in the left dorsolateral prefrontal cortex (DLPFC) and the primary motor cortex (M1) (178). Anodal stimulation of M1 can inhibit thalamic activity, thereby regulating the pain threshold and intensity (179). The DLPFC is one of the most commonly activated brain regions during pain episodes (180). Therefore, disrupting the involvement of the DLPFC in pain perception through electrical stimulation can significantly alleviate patients’ pain. Additionally, stimulation of regions such as the medial prefrontal cortex, anterior insula, anterior cingulate cortex, and bilateral amygdala can reduce negative emotions such as anxiety and depression (181). The use of tDCS to alleviate pain associated with DPN has been considered feasible in numerous studies (182). However, the impact of tDCS remains inconsistent. The effectiveness of tDCS can be affected by age, genetic factors, medicines, lifestyle factors, and even physical activities. Moreover, even the same patient can experience different results at different time points (183). Determining the right level of stimulation for each patient can be challenging when tDCS is applied in clinical practice.
3.4 Transcranial magnetic stimulation
3.4.1 Principles and mechanism of action of TMS
TMS, a noninvasive cortical modulation technique, was initially employed for functional localization in the central nervous system (109). Over time, with changes in coil shape, stimulation patterns, and other parameters, TMS is now being utilized for treating central and peripheral neuropathologies, evaluating the activity of the corticospinal tract, and determining the extent of damage to motor functions and conduction pathways (184, 185). Additionally, TMS can influence local cerebral blood flow to alter oxidative stress levels, promoting functional recovery in the lower limbs following nerve injury (109).
In a TMS, magnetic field pulses are created via electrical currents following Faraday’s law. These shifting magnetic fields pass through the skull to affect the intended area, producing currents in the process (186). When the intensity of electrical current flow increases to a certain level, nerves in the focus area are stimulated. TMS adjusts neuron signals and modulates brain activity (100, 187). The intensity of the magnetic field follows the inverse square law, so the intensity decreases rapidly with depth. To stimulate deeper areas of the brain, a stronger intensity is required at the surface (188). To address this, various coil shapes have been developed, including circular, figure-eight, quadruple butterfly, and Hesed coils (H-coils) (188, 189), as shown in Figure 3. The determination of coil shape depends on the specific brain region area and depth. Circular coils are characterized by their simple design and ease of manufacturing (190, 191). However, the circular electric field it generates cannot achieve focused stimulation. Although techniques have been developed to focus the electric field by altering the winding angle and density of the coil (192, 193), its focusing capability remains inferior to that of other coil designs. In comparison, figure-8 coils, which consist of two adjacent circular coils, can generate a focused electric field at the intersection point beneath the coils (194, 195), allowing for deeper and more precise stimulation than circular coils. Compared with figure-eight coils, quadruple butterfly coils, which are derived from figure-eight coils, are composed of four circular coils and are more commonly used for stimulating long fiber structures (197, 198). Finally, the more novel H-coils, compared with the aforementioned designs, are larger in size and have a more complex winding pattern. This structure allows the electric field to penetrate deeper into the brain with a slower rate of field attenuation (199, 200), although this comes at the cost of reduced focusing ability. In addition, the TMS pattern includes single-pulse TMS, paired-pulse TMS, and repetitive pulse TMS (rTMS) (202). This parameter affects the results of the TMS as well. Single-pulse TMS is commonly used for specific cortical site stimulation because of its effectiveness in measuring the cortical response to each pulse (187, 203). Paired-pulse TMS employs two paired pulses to stimulate the same or different cortical areas, serving to assess functional connectivity between two regions (204). rTMS induces cortical effects through a continuous sequence of pulses, allowing control over the produced effects and duration through the adjustment of stimulation intensity, frequency, and time (205, 206). TMS can be used for long-lasting, high or low activation or blockage of central and peripheral nerves. This allows focused stimulation of connections between brain areas. To date, encouraging results of TMS have been shown for diagnosing and treating central and peripheral nerve injuries. Even though the use of TMS in DPN patients is not widespread, TMS still offers significant promise as a treatment option.
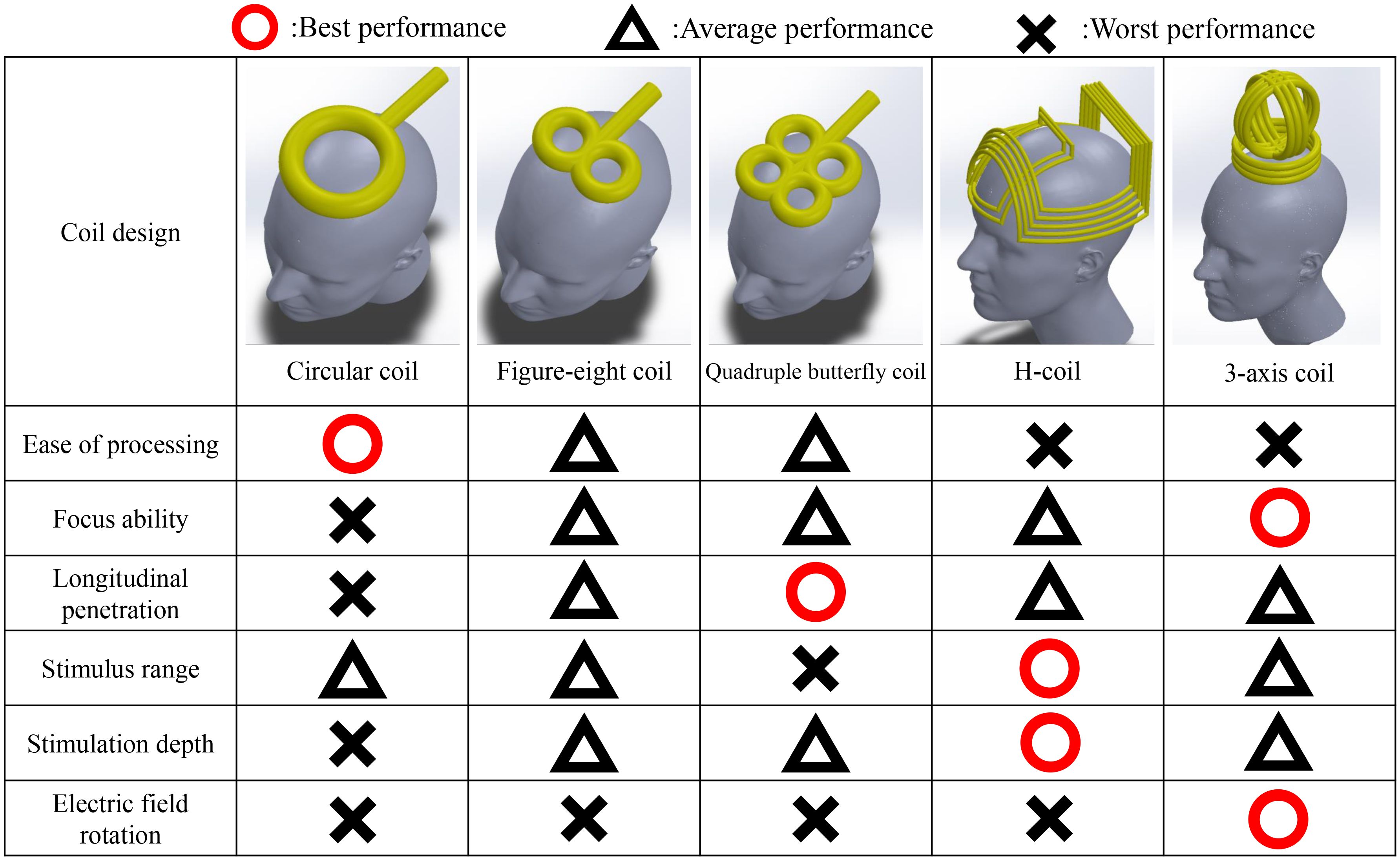
Figure 3. Performance comparison of coil designs. Since the development of the TMS, the design of the coil has been improved many times. Currently, four coil designs are most commonly used (188, 189), each with distinct advantages and disadvantages, as illustrated in the figure. The circular coil was the earliest design and is relatively easy to manufacture, although it lacks precision and depth in stimulation (190–193). The figure-8 coil was developed as an improvement over the circular coil, enhancing both the focus and penetration depth (194–196). The quadruple butterfly coil, optimized for increased stimulation depth along a vertical axis, provides the highest focus and penetration depth among the four designs but covers a relatively narrow area (197, 198). Finally, the H-coil, in contrast to earlier designs, is considered capable of achieving greater stimulation depth and coverage owing to its skull-encompassing structure, allowing for stimulation across a wider range of brain regions (199, 200). The 3-axis coil provides a stimulation range similar to that of traditional circular coils but overcomes the difficulty in achieving focal stimulation. By adjusting the current output of the three coil sets, this coil enables rotation of the electric field to finely tune the stimulation location (201).
3.4.2 Value of TMS in diagnosing DPN
DPN originates in the early stages of diabetes, as elevated blood glucose levels following dietary intake lead to the onset and subsequent progression of DPN (207). Therefore, American Diabetes Association (ADA) scholars emphasize DPN screening in patients exhibiting early symptoms of diabetes (208). In the early phase of DPN, smaller nerve groups are usually targeted first, which could affect pain sensation. However, because small and large nerve fibers work collaboratively, clear signs of this nerve damage might not be observable. Thus, DPN examinations should cover both nerve types to better identify small nerve damage (207). According to the ADA 2022 Clinical Practice Guidelines, the recommended protective sensory testing methods can detect only severe sensory loss (209). Hence, more accurate early screening methods are needed for a comprehensive assessment. The TMS is likely to assist in addressing these related issues.
TMS alone is not sensitive enough to serve as a standalone technique for evaluating DPN. However, TMS can be utilized to detect motor-evoked potentials when combined with other assessment methods, such as functional magnetic resonance imaging (fMRI), electroencephalography (EEG), and Doppler ultrasound monitoring (210, 211). This integrated approach can help establish potential connections between various functional parameters, aiding in a more comprehensive evaluation and diagnosis of DPN patients (212, 213). Combined with the above methods, TMS can be used to detect motor-evoked potentials, which are crucial for assessing a patient’s neural and muscular function. Through TMS, key parameters such as nerve conduction velocity, excitability thresholds, and response times can be observed, facilitating the identification of abnormalities in the nervous system (109, 210). In addition, fMRI provides details about brain activity and connections. These findings reveal how the nervous system processes pain and sensory information in diabetes patients (186, 210). EEG detects changes in brain electrical activity related to pain perception, which is crucial for evaluating central nervous system abnormalities in DPN patients. Doppler ultrasound, which is used to monitor vascular health and focuses on blood flow velocity and microcirculation, is essential for diagnosing vascular or nerve lesions in DPN patients. TMS can stimulate specific brain regions, and when combined with fMRI, neural circuit responses can be observed in real time; when combined with EEG, fMRI can synchronously record changes in brain electrical activity; and when combined with Doppler ultrasound, fMRI can assess blood flow changes in response to neural stimulation. This multimodal approach helps to comprehensively assess and diagnose nerve and vascular abnormalities in early-stage DPN patients. Therefore, the TMS plays a significant role in this multifaceted assessment process.
3.4.3 Application of TMS in neural protection and regeneration
In patients with DPN, the lower extremities typically manifest as the initial site of neuropathic changes, progressing from sensory impairments to the emergence of motor symptoms as the condition worsens (214). The main pathological pathways of DPN in the presence of elevated blood glucose levels include the polyol, hexosamine, and protein kinase C pathways, as well as oxidative stress and inflammatory responses that act on neural origins, all of which contribute to impaired nerve function (215). Studies have shown that, after 8–12 weeks of a high-fructose diet, C57BL/6 N mice exhibit significant hippocampal neuroinflammatory responses; these responses activate glial cells and astrocytes, leading to neuroglial proliferation and a substantial decrease in the number of hippocampal neurons and newborn neurons (216). Matrix metalloproteinases (MMPs), which are downstream effectors of hyperglycemia and oxidative stress, play dual roles in extracellular matrix remodeling and neuroregeneration. Under controlled activation, MMPs facilitate axonal sprouting and the structural reorganization necessary for nerve regeneration by promoting the degradation of inhibitory matrix components and enabling the migration of neural progenitor cells (217).
On the basis of the aforementioned mechanisms, TMS coils have been used in several studies to generate magnetic field pulses and stimulate the heads of rats with gray matter injuries. This approach can restore the regenerative capacity of neurons in the hypothalamic region (218). Research has demonstrated that rTMS promotes remyelination in demyelinated white matter regions by increasing the proliferation, migration, and differentiation of oligodendrocyte precursor cells (219). Moreover, evidence suggests that rTMS can increase the expression of brain-derived neurotrophic factor, which in turn activates the TrkB signaling cascade—a critical pathway involved in synaptic plasticity, axonal growth, and the survival of injured neurons (220–222). These findings collectively indicate that TMS, as a neuromodulatory technique, can promote neural regeneration.
3.4.4 TMS for alleviating neuropathic pain in DPN patients
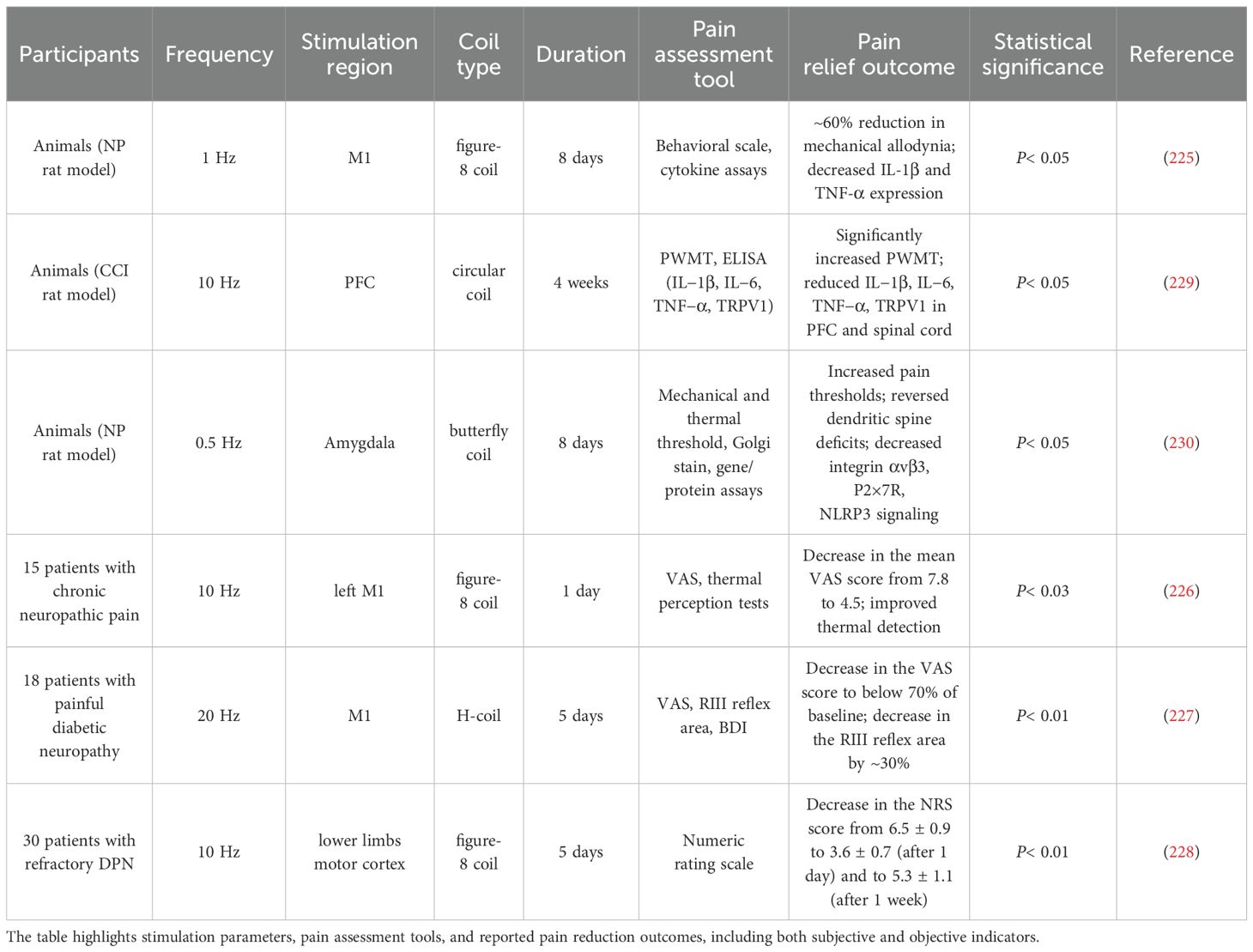
Various approaches have previously been used to treat neuropathic pain caused by DPN, including SCS, DNS, tDCS, or conventional pharmaceutical interventions; however, several clinical challenges are still faced with these treatments. Issues such as discomfort during treatment, a pain relief rate less than 50%, and significant side effects associated with drug therapy have been reported (223). TMS is able to directly stimulate the thalamus, which serves as the pain integration center, through cortical brain activity. When stimulated, the thalamus blocks the sensory transmission pathway from the spinal cord to the thalamus, reducing the patient’s sensitivity to pain (224). Research findings indicate that for chronic pain resulting from spinal cord injury, low-frequency (1-Hz) stimulation of the M1 region of the brain can inhibit cortical excitability, thereby increasing the pain threshold and alleviating pain perception (225). Stimulation of the M1 region has also been shown to mitigate abnormal temperature perception caused by chronic neuropathic pain (226). Research has shown that the use of an H-coil for high-frequency (20 Hz) stimulation of deeper and broader areas is effective in relieving lower limb pain in patients (227). Additionally, focused stimulation of the lower limb motor cortex region via a figure-8 coil can reduce pain sensitivity in patients over a period of five weeks (228). The analgesic efficacy of rTMS in DPN has been supported by multiple studies involving varying designs and stimulation protocols. As summarized in Table 2, both animal and human studies have reported significant reductions in pain scores and the levels of inflammatory markers, with some trials reporting reductions of up to 60% in allodynia or subjective pain intensity. These outcomes highlight the therapeutic potential of rTMS; however, given the diversity in coil types, stimulation frequencies, and cortical targets employed across studies, further investigations are warranted to optimize stimulation parameters and identify patient-specific predictors of responses.
3.4.5 TMS for treating depression induced by DPN
Depression is an emotional disorder characterized by features such as loss of pleasure, despair, intensified guilt, and physical distress (231). The pathological mechanisms underlying this psychological illness are complex and involve responses to stress, neural structure and function, and immune–neurological imbalances, all of which are associated with dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis (232). The hippocampus, a crucial regulator of stress responses, undergoes neuronal damage or apoptosis due to HPA axis dysregulation induced by hyperthyroidism (233). TMS has been demonstrated to significantly improve mood, cognition, motor function, and sensation (221, 234). TMS also influences neurotransmitters and the endocrine response of the HPA axis in patients with depression (235). Therefore, the use of TMS to restore HPA axis homeostasis and prevent hippocampal neuronal apoptosis has become a therapeutic strategy for treating depression. The feasibility of this approach has been acknowledged in studies using high-frequency (10 Hz) TMS in Sprague–Dawley rats (236). Additionally, research findings suggest that peripheral neuropathic pain caused by diabetes is a factor contributing to depression. Consequently, the use of TMS to inhibit M1 and the descending pain system to treat muscle-related pain may have an adjunctive effect on diabetes-induced depression (237). Although the mechanisms underlying diabetes-induced depression remain unclear, TMS is practical for modulating depressive symptoms under high-glucose conditions. TMS can also be employed to assess the efficacy of antidepressant medications in patients, serving as an auxiliary therapeutic tool.
3.4.6 Application of TMS in the treatment of diabetic autonomic neuropathy
Diabetic autonomic neuropathy (DAN) affects the cardiovascular, gastrointestinal, and urogenital systems, with approximately 20% of diabetic patients experiencing DAN, often accompanied by other peripheral neuropathies (238). Autonomic neuropathy occurring in the cardiovascular system may lead to tachycardia, hypotension, dizziness, and motor impairment, even resulting in a loss of control over the sympathetic and parasympathetic nerves of the heart (239). When the gastrointestinal tract is affected, symptoms such as esophageal dysmotility and gastroparesis may result. DAN can also disrupt the urogenital system, causing recurrent urinary tract infections, pyelonephritis, and bladder and sexual dysfunction (240).
Currently, the understanding of how the cerebral cortex controls autonomic nervous system function remains uncertain. However, studies have shown that the use of TMS to stimulate the sympathetic nervous system can induce transient changes in the cardiovascular system (241). In another study, TMS was utilized to stimulate M1, and the neural connection between the brain and kidneys was demonstrated. The findings indicated a significant increase in urinary protein levels in both diabetic and nondiabetic individuals after stimulation, suggesting a potential link between the brain and renal autonomic functions (242). While the ability of TMS to influence the autonomic nervous system has been demonstrated, further research is needed to determine whether such effects can have therapeutic benefits for patients.
3.4.7 TMS for addressing vascular damage in DPN patients
Cardiovascular diseases and microvascular complications contribute to the increased incidence and mortality of diabetes patients (243). DPN induces endothelial cell proliferation in blood vessels, leading to thickening of the capillary basement membrane. These structural changes result in narrowed blood vessels, reducing the blood supply to neuronal fibers (244). Among these, the nerve–vascular barrier and oxygen tension of dorsal root nerves and other peripheral nerve trunks are lower, increasing their susceptibility to microvascular changes (245). The dorsal root nerves, which act as a system to regulate blood flow, are adversely affected when damaged, disrupting blood flow regulation and exacerbating the process of vascular damage. Endothelial dysfunction can also trigger neuropathies; in diabetes, hyperglycemia-induced adhesion of endothelial cells or thrombus formation can lead to blood vessel obstruction, causing ischemic damage to neuronal fibers (246).
Studies have shown that TMS can induce angiogenesis and significantly increase cerebral blood flow (247). Additionally, stimulation of brain regions with low-frequency (1-Hz) TMS increases oxygen consumption and metabolic rates by approximately 28%, which is correlated with increased blood flow (248). Although there is currently no research on the use of TMS to modulate vascular blood flow and endothelial cell proliferation in DPN patients, future studies in this area are highly anticipated.
4 Conclusion and future challenges
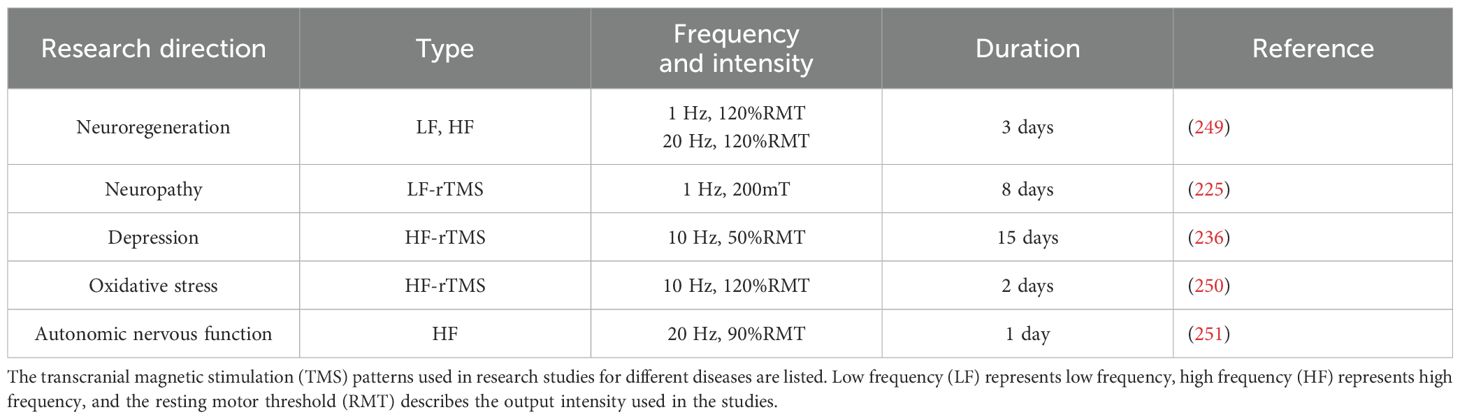
DPN affects approximately 50% of diabetic patients and results in chronic pain, sensory loss, and motor dysfunction. Although approved by the FDA, traditional pharmacological treatments, such as pregabalin and duloxetine, provide limited relief for approximately 20–30% of patients and are often accompanied by significant side effects. This necessitates the exploration of alternative therapeutic approaches, particularly nonpharmacological interventions. In this review, the potential of TMS as a noninvasive treatment for delaying neural inflammation and mitigating the symptoms of DPN is highlighted. TMS has shown promise in reducing pain sensitivity by up to 50% over several weeks of treatment and has demonstrated potential in promoting nerve regeneration and regulating autonomic nervous function. As outlined in Table 3, the efficacy of TMS is influenced by various stimulation parameters, such as frequency and intensity, with low-frequency (1 Hz) stimulation being effective in reducing cortical excitability and alleviating pain, whereas high-frequency (10 Hz) stimulation shows benefits in mood regulation. The flexibility of coil designs, including figure-eight and H-coils, further expands the application of TMS across different neuropathic conditions.
Other neuromodulation techniques, such as SCS, have demonstrated an 87.5% success rate in reducing pain intensity by at least 50% after six months of treatment, with pain relief being maintained in 55% of patients five years posttreatment. Similarly, DNS and tDCS present alternative therapeutic options, although their clinical applications remain limited owing to inconsistent outcomes and a lack of large-scale clinical trials. In contrast, TMS is a noninvasive intervention with a relatively low incidence of adverse effects. Reported side effects include transient headaches, scalp discomfort, and, in rare cases, seizures, particularly in individuals with a history of epilepsy or those undergoing prolonged high-frequency stimulation. To integrate these therapies into routine clinical practice, large-scale, randomized controlled trials are needed to establish the long-term efficacy, safety, and cost-effectiveness of TMS and other neuromodulation approaches. Moreover, advanced diagnostic tools such as fMRI and EEG could assist in the personalization of these treatments, enabling more precise and effective interventions for DPN patients. Table 3 outlines the various frequencies and intensities used in TMS for different neurological conditions, emphasizing the need for the standardization of these parameters to optimize patient outcomes. As the global prevalence of diabetes is projected to rise to 12.2% by 2045, addressing the challenges of DPN through innovative therapies such as TMS will become increasingly important. While neuromodulation techniques, especially TMS, show significant therapeutic potential, the application of TMS in the treatment of DPN remains relatively limited, and comprehensive treatment protocols and long-term data are still lacking. In light of the multifactorial nature of DPN, and considering that the effectiveness of rTMS may vary significantly with factors such as age, gender, and cortical thickness, integrative approaches that combine rTMS with existing pharmacological, nutritional, or exercise-based interventions should be explored in future research. For example, combining rTMS with agents that enhance neuroplasticity or reduce inflammation may yield synergistic effects. Similarly, coupling rTMS with aerobic or resistance training, both of which are known to improve glucose metabolism and nerve function, may help optimize therapeutic outcomes. These multimodal strategies represent promising directions for developing personalized and noninvasive treatments that address both the neurological and metabolic aspects of diabetic neuropathy. The broader implications of this work suggest that TMS could serve as a complementary tool in clinical neurology and diabetes care, particularly for patients who are unresponsive to pharmacological treatments. The integration of TMS into multidisciplinary care plans could enhance treatment personalization, reduce long-term healthcare costs associated with diabetes complications, and potentially prevent the progression of neuropathic symptoms in high-risk populations. In conclusion, while TMS and other neuromodulation techniques represent promising frontiers in the nonpharmacological treatment of DPN, optimizing treatment protocols and expanding clinical trials will be key to the broader adoption of these techniques. With the increasing burden of diabetes worldwide, advancing these therapies could significantly improve the quality of life of millions of diabetic patients suffering from neuropathy.
Author contributions
CL: Formal Analysis, Investigation, Methodology, Writing – original draft. C-WL: Validation, Writing – original draft, Writing – review & editing. G-LW: Validation, Writing - review & editing, Visualization. SY: Methodology, Writing – original draft. C-MH: Methodology, Writing - original draft. Z-HL: Writing – original draft. P-CH: Writing – original draft. L-DL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported in part by the National Science and Technology Council of Taiwan under grant numbers NSTC 114-2221-E- 400-001-MY3, NSTC 113–2221-E-400–003, NSTC 113-2218-E-007-026, 112-2221-E-005–042 and 111-2221-E-005-018; by the National Health Research Institutes of Taiwan under grant numbers BN-114-PP-15, BN- 114-GP-02, BN-114-GP-03 and BN-114-GP-05; by the Ministry of Economic Affairs of Taiwan under grant numbers 114-EC-17-A-22–1906 and 114-EC-17-A22-1905; and by the Metal Industries Research & Development Centre of Taiwan under grant number K00069651K and the Southern Taiwan Science Park Bureau, Ministry of Science and Technology under grant number 113CH-1-03.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Amelia R. The correlation between Body Mass Index and self-efficacy with blood glucose level in Type 2 Diabetes Mellitus. Advanced Sci Lett. (2017) 23:3606–9. doi: 10.1166/asl.2017.9192
3. Deshmukh CD, Jain A, and Nahata B. Diabetes mellitus: a review. Int J Pure Appl Biosci. (2015) 3:224–30.
4. Khursheed R, Singh SK, Wadhwa S, Kapoor B, Gulati M, Kumar R, et al. Treatment strategies against diabetes: Success so far and challenges ahead. Eur J Pharmacol. (2019) 862:172625. doi: 10.1016/j.ejphar.2019.172625
6. Petersen MC and Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. (2018) 98(4):2133–223. doi: 10.1152/physrev.00063.2017
7. Cheatham B and Kahn CR. Insulin action and the insulin signaling network. Endocrine Rev. (1995) 16:117–42. doi: 10.1210/edrv-16-2-117
8. Pirola L, Johnston A, and Van Obberghen E. Modulation of insulin action. Diabetologia. (2004) 47:170–84. doi: 10.1007/s00125-003-1313-3
9. Kumar R, Saha P, Kumar Y, Sahana S, Dubey A, and Prakash O. A review on diabetes mellitus: type1 & Type2. World J Pharm Pharm Sci. (2020) 9:838–50. doi: 10.20959/wjpps202010-17336
10. Lebovitz HE. Diagnosis, classification, and pathogenesis of diabetes mellitus. J Clin Psychiatry. (2001) 62:5–9.
11. Blair M. Diabetes mellitus review. Urologic Nurs. (2016) 6(1):27–36. doi: 10.7257/1053-816X.2016.36.1.27
12. Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. (2017) 3:1–17. doi: 10.1038/nrdp.2017.16
14. Rewers M and Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. (2016) 387:2340–8. doi: 10.1016/S0140-6736(16)30507-4
15. Haller MJ, Atkinson MA, and Schatz D. Type 1 diabetes mellitus: etiology, presentation, and management. Pediatr Clinics. (2005) 52:1553–78. doi: 10.1016/j.pcl.2005.07.006
16. Redondo MJ, Fain PR, and Eisenbarth GS. Genetics of type 1A diabetes. Recent Prog hormone Res. (2001) 56:69–89. doi: 10.1210/rp.56.1.69
17. Hämäläinen A-M and Knip M. Autoimmunity and familial risk of type 1 diabetes. Curr Diabetes Rep. (2002) 2:347–53. doi: 10.1007/s11892-002-0025-2
18. Acharjee S, Ghosh B, Al-Dhubiab BE, and Nair AB. Understanding type 1 diabetes: etiology and models. Can J Diabetes. (2013) 37:269–76. doi: 10.1016/j.jcjd.2013.05.001
19. DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. (2015) 1:1–22. doi: 10.1038/nrdp.2015.19
21. Jiménez-Osorio AS, González-Reyes S, and Pedraza-Chaverri J. Natural Nrf2 activators in diabetes. Clinica Chimica Acta. (2015) 448:182–92. doi: 10.1016/j.cca.2015.07.009
22. Chang-Chen K, Mullur R, and Bernal-Mizrachi E. β-cell failure as a complication of diabetes. Rev Endocrine Metab Disord. (2008) 9:329–43. doi: 10.1007/s11154-008-9101-5
23. Leibowitz G, Kaiser N, and Cerasi E. β-Cell failure in type 2 diabetes. J Diabetes Invest. (2011) 2:82–91. doi: 10.1111/j.2040-1124.2010.00094.x
24. Willemsen G, Ward KJ, Bell CG, Christensen K, Bowden J, Dalgård C, et al. The concordance and heritability of type 2 diabetes in 34,166 twin pairs from international twin registers: the discordant twin (DISCOTWIN) consortium. Twin Res Hum Genet. (2015) 18:762–71. doi: 10.1017/thg.2015.83
25. Malecki MT. Genetics of type 2 diabetes mellitus. Diabetes Res Clin Pract. (2005) 68:S10–21. doi: 10.1016/j.diabres.2005.03.003
26. Barroso I. Genetics of type 2 diabetes. Diabetic Med. (2005) 22:517–35. doi: 10.1111/j.1464-5491.2005.01550.x
27. Pillon NJ, Loos RJ, Marshall SM, and Zierath JR. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell. (2021) 184:1530–44. doi: 10.1016/j.cell.2021.02.012
28. Cunningham A, Stephens J, and Harris D. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. (2021) 13:1–13. doi: 10.1186/s13099-021-00446-0
29. Larsen N, Vogensen FK, van den Berg FWJ, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. (2010) 5:e9085. doi: 10.1371/journal.pone.0009085
30. Campo L, Eiseler S, Apfel T, and Pyrsopoulos N. Fatty liver disease and gut microbiota: a comprehensive update. J Clin Trans Hepatol. (2019) 7:56. doi: 10.14218/JCTH.2018.00008
31. Tai N, Wong FS, and Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocrine Metab Disord. (2015) 16:55–65. doi: 10.1007/s11154-015-9309-0
32. Danaei G, Lawes CM, Vander Hoorn S, Murray CJ, and Ezzati M. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet. (2006) 368:1651–9. doi: 10.1016/S0140-6736(06)69700-6
33. Dou L and Jourde-Chiche N. Endothelial toxicity of high glucose and its by-products in diabetic kidney disease. Toxins. (2019) 11:578. doi: 10.3390/toxins11100578
34. Forouhi NG and Wareham NJ. Epidemiology of diabetes. Medicine. (2019) 47:22–7. doi: 10.1016/j.mpmed.2018.10.004
35. Gerstein H. Glucose: a continuous risk factor for cardiovascular disease. Diabetic Med. (1997) 14:S25–31. doi: 10.1002/(SICI)1096-9136(199708)14:3+<S25::AID-DIA441>3.0.CO;2-1
36. Ruderman NB, Williamson JR, and Brownlee M. Glucose and diabetic vascular disease 1. FASEB J. (1992) 6:2905–14. doi: 10.1096/fasebj.6.11.1644256
37. Braffett BH, Gubitosi-Klug RA, Albers JW, Feldman EL, Martin CL, White NH, et al. Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes. (2020) 69:1000–10. doi: 10.2337/db19-1046
38. Mezal RJ and El Rasyid H. Arrhythmia mechanism on diabetes mellitus: a narrative review. Bioscientia Medicina: J Biomedicine Trans Res. (2022) 6:1764–72. doi: 10.37275/bsm.v6i5.511
39. Lopaschuk GD. Metabolic abnormalities in the diabetic heart. Heart failure Rev. (2002) 7:149–59. doi: 10.1023/A:1015328625394
40. Albers JW and Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. (2014) 14:1–11. doi: 10.1007/s11910-014-0473-5
41. Singh R, Kishore L, and Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res. (2014) 80:21–35. doi: 10.1016/j.phrs.2013.12.005
42. Tesfaye S, Vileikyte L, Rayman G, Sindrup SH, Perkins BA, Baconja M, et al. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes/metabolism Res Rev. (2011) 27:629–38. doi: 10.1002/dmrr.v27.7
43. Chong MS and Hester J. Diabetic painful neuropathy: current and future treatment options. Drugs. (2007) 67:569–85. doi: 10.2165/00003495-200767040-00006
44. Stino AM and Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Invest. (2017) 8:646–55. doi: 10.1111/jdi.2017.8.issue-5
45. Mizukami H and Osonoi S. Collateral glucose-utlizing pathwaya in diabetic polyneuropathy. Int J Mol Sci. (2020) 22:94. doi: 10.3390/ijms22010094
46. Padhi S, Nayak AK, and Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomedicine Pharmacotherapy. (2020) 131:110708. doi: 10.1016/j.biopha.2020.110708
47. Sivakumar PM, Prabhawathi V, Zarrabi A, Akthar S, and Prabhakar PK. Current trends in the therapeutic strategies for diabetes management. Curr Medicinal Chem. (2021) 28:4616–37. doi: 10.2174/0929867328666210218183914
48. Blahova J, Martiniakova M, Babikova M, Kovacova V, Mondockova V, and Omelka R. Pharmaceutical drugs and natural therapeutic products for the treatment of type 2 diabetes mellitus. Pharmaceuticals. (2021) 14:806. doi: 10.3390/ph14080806
49. Ahuja V and Chou C-H. Novel therapeutics for diabetes: uptake, usage trends, and comparative effectiveness. Curr Diabetes Rep. (2016) 16:1–10. doi: 10.1007/s11892-016-0744-4
50. Seino S, Sugawara K, Yokoi N, and Takahashi H. β-Cell signalling and insulin secretagogues: A path for improved diabetes therapy. Diabetes Obes Metab. (2017) 19:22–9. doi: 10.1111/dom.2017.19.issue-S1
51. Sola D, Rossi L, Carnevale Schianca GP, Maffioli P, Bigliocca M, Mella R, et al. State of the art paper Sulfonylureas and their use in clinical practice. Arch Med Sci. (2015) 11:840–8. doi: 10.5114/aoms.2015.53304
52. Quillen DM, Samraj G, and Kuritzky L. Improving management of type 2 diabetes mellitus: 2. Biguanides. Hosp Pract. (1999) 34:41–4. doi: 10.1080/21548331.1999.11443925
53. Bourron O, Daval M, Hainault I, Hajduch E, Servant JM, Gautier JF, et al. Biguanides and thiazolidinediones inhibit stimulated lipolysis in human adipocytes through activation of AMP-activated protein kinase. Diabetologia. (2010) 53:768–78. doi: 10.1007/s00125-009-1639-6
54. Tyagi S, Gupta P, Saini AS, Kaushal C, and Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. (2011) 2:236–40. doi: 10.4103/2231-4040.90879
55. Thangavel N, Al Bratty M, Javed SA, Ahsan W, and Alhazmi HA. Targeting peroxisome proliferator-activated receptors using thiazolidinediones: strategy for design of novel antidiabetic drugs. Int J medicinal Chem. (2017) 2017:2017. doi: 10.1155/2017/1069718
56. Dirir AM, Daou M, Yousef AF, and Yousef LF. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem Rev. (2022) 21:1049–79. doi: 10.1007/s11101-021-09773-1
57. Wehmeier U and Piepersberg W. Biotechnology and molecular biology of the α-glucosidase inhibitor acarbose. Appl Microbiol Biotechnol. (2004) 63:613–25. doi: 10.1007/s00253-003-1477-2
58. Derosa G and Maffioli P. Mini-Special Issue paper Management of diabetic patients with hypoglycemic agents α-Glucosidase inhibitors and their use in clinical practice. Arch Med Sci. (2012) 8:899–906. doi: 10.5114/aoms.2012.31621
59. Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. (2019) 30:72–130. doi: 10.1016/j.molmet.2019.09.010
60. Lambeir A-M, Durinx C, Scharpé S, and De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. (2003) 40:209–94. doi: 10.1080/713609354
61. Scheen AJ. Pharmacodynamics, efficacy and safety of sodium–glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. (2015) 75:33–59. doi: 10.1007/s40265-014-0337-y
62. Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther. (2014) 5:355–66. doi: 10.1007/s13300-014-0089-4
63. Zimmerman BR. Sulfonylureas. Endocrinol Metab Clinics North America. (1997) 26:511–22. doi: 10.1016/S0889-8529(05)70264-4
64. Del Prato S and Pulizzi N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metabolism. (2006) 55:S20–7. doi: 10.1016/j.metabol.2006.02.003
65. García Rubiño ME, Carrillo E, Ruiz Alcalá G, Domínguez-Martín A, Marchal JA, and Boulaiz H. Phenformin as an anticancer agent: challenges and prospects. Int J Mol Sci. (2019) 20:3316. doi: 10.3390/ijms20133316
66. Tomlinson B, Patil NG, Fok M, Chan P, and Lam CWK. The role of sulfonylureas in the treatment of type 2 diabetes. Expert Opin Pharmacotherapy. (2022) 23:387–403. doi: 10.1080/14656566.2021.1999413
67. Bermúdez V, Finol F, Parra N, Parra M, Pérez A, Peñaranda L, et al. PPAR-γ agonists and their role in type 2 diabetes mellitus management. Am J Ther. (2010) 17:274–83. doi: 10.1097/MJT.0b013e3181c08081
68. Chandra A, Kaur P, Sahu SK, and Mittal A. A new insight into the treatment of diabetes by means of pan PPAR agonists. Chem Biol Drug Design. (2022) 100:947–67. doi: 10.1111/cbdd.v100.6
69. Joshi SR, Standl E, Tong N, Shah P, Kalra S, and Rathod R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin pharmacotherapy. (2015) 16:1959–81. doi: 10.1517/14656566.2015.1070827
70. Derosa G and Maffioli P. α-Glucosidase inhibitors and their use in clinical practice. Arch Med science: AMS. (2012) 8:899. doi: 10.5114/aoms.2012.31621
71. Hossain U, Das AK, Ghosh S, and Sil PC. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem Toxicol. (2020) 145:111738. doi: 10.1016/j.fct.2020.111738
72. Aaboe K, Krarup T, Madsbad S, and Holst JJ. GLP-1: physiological effects and potential therapeutic applications. Diabetes Obes Metab. (2008) 10:994–1003. doi: 10.1111/j.1463-1326.2008.00853.x
73. Nauck MA, Quast DR, Wefers J, and Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Mol Metab. (2021) 46:101102. doi: 10.1016/j.molmet.2020.101102
74. Ahrén B. GLP-1 for type 2 diabetes. Exp Cell Res. (2011) 317:1239–45. doi: 10.1016/j.yexcr.2011.01.010
75. Nauck M. Glucagon-like peptide 1 (GLP-1) in the treatment of diabetes. Hormone Metab Res. (2004) 36:852–8. doi: 10.1055/s-2004-826175
76. Gupta R, Walunj SS, Tokala RK, Parsa KV, Singh SK, and Pal M. Emerging drug candidates of dipeptidyl peptidase IV (DPP IV) inhibitor class for the treatment of type 2 diabetes. Curr Drug Targets. (2009) 10:71–87. doi: 10.2174/138945009787122860
77. Holst JJ. Treatment of type 2 diabetes mellitus with agonists of the GLP-1 receptor or DPP-IV inhibitors. Expert Opin emerging Drugs. (2004) 9:155–66. doi: 10.1517/14728214.9.1.155
78. Gilbert MP and Pratley RE. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front Endocrinol. (2020) 11:520041. doi: 10.3389/fendo.2020.00178
79. Gallo LA, Wright EM, and Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diabetes Vasc Dis Res. (2015) 12:78–89. doi: 10.1177/1479164114561992
80. Ferrannini E and Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. (2012) 8:495–502. doi: 10.1038/nrendo.2011.243
81. Hasan FM, Alsahli M, and Gerich JE. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res Clin Pract. (2014) 104:297–322. doi: 10.1016/j.diabres.2014.02.014
82. Pop-Busui R, Ang L, Boulton AJM, Feldman EL, Marcus RL, Mizokami-Stout K, et al. Diagnosis and treatment of painful diabetic peripheral neuropathy. Compendia. (2022) 2022:1–32. doi: 10.2337/db2022-01
83. Rafiullah M and Siddiqui K. Pharmacological treatment of diabetic peripheral neuropathy: an update. CNS Neurological Disorders-Drug Targets (Formerly Curr Drug Targets-CNS Neurological Disorders). (2022) 21:884–900. doi: 10.2174/1871527320666210303111939
84. Javed S, Alam U, and Malik RA. Treating diabetic neuropathy: present strategies and emerging solutions. Rev Diabetic studies: RDS. (2015) 12:63. doi: 10.1900/RDS.2015.12.63
85. Zhang S-S, Wu Z, Zhang L-C, Zhang Z, Chen R-P, Huang Y-H, et al. Efficacy and safety of pregabalin for treating painful diabetic peripheral neuropathy: a meta-analysis. Acta Anaesthesiologica Scandinavica. (2015) 59:147–59. doi: 10.1111/aas.2015.59.issue-2
86. Azmi S, ElHadd KT, Nelson A, Chapman A, Bowling FL, Perumbalath A, et al. Pregabalin in the management of painful diabetic neuropathy: a narrative review. Diabetes Ther. (2019) 10:35–56. doi: 10.1007/s13300-018-0550-x
87. Huffman C, Stacey BR, Tuchman M, Burbridge C, Li C, Parsons B, et al. Efficacy and safety of pregabalin in the treatment of patients with painful diabetic peripheral neuropathy and pain on walking. Clin J Pain. (2015) 31:946–58. doi: 10.1097/AJP.0000000000000198
88. Freynhagen R, Baron R, Kawaguchi Y, Malik RA, Martire DL, Parsons B, et al. Pregabalin for neuropathic pain in primary care settings: recommendations for dosing and titration. Postgraduate Med. (2021) 133:1–9. doi: 10.1080/00325481.2020.1857992
89. Toth C. Pregabalin: latest safety evidence and clinical implications for the management of neuropathic pain. Ther Adv Drug Saf. (2014) 5:38–56. doi: 10.1177/2042098613505614
90. Alam U, Sloan G, and Tesfaye S. Treating pain in diabetic neuropathy: current and developmental drugs. Drugs. (2020) 80:363–84. doi: 10.1007/s40265-020-01259-2
91. Wu C-S, Huang Y-J, Ko Y-C, and Lee C-H. Efficacy and safety of duloxetine in painful diabetic peripheral neuropathy: a systematic review and meta-analysis of randomized controlled trials. Systematic Rev. (2023) 12:53. doi: 10.1186/s13643-023-02185-6
92. Skljarevski V, Desaiah D, Zhang Q, Chappell AS, Detke MJ, Gross JL, et al. Evaluating the maintenance of effect of duloxetine in patients with diabetic peripheral neuropathic pain. Diabetes/metabolism Res Rev. (2009) 25:623–31. doi: 10.1002/dmrr.v25:7
93. Hamdy O, Goodyear LJ, and Horton ES. Diet and exercise in type 2 diabetes mellitus. Endocrinol Metab Clinics. (2001) 30:883–907. doi: 10.1016/S0889-8529(05)70220-6
94. Magkos F, Hjorth MF, and Astrup A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. (2020) 16:545–55. doi: 10.1038/s41574-020-0381-5
95. Broadbent E, Donkin L, and Stroh JC. Illness and treatment perceptions are associated with adherence to medications, diet, and exercise in diabetic patients. Diabetes Care. (2011) 34:338–40. doi: 10.2337/dc10-1779
96. Zeng H, Pacheco-Barrios K, Cao Y, Li Y, Zhang J, Yang C, et al. Non-invasive neuromodulation effects on painful diabetic peripheral neuropathy: a systematic review and meta-analysis. Sci Rep. (2020) 10:19184. doi: 10.1038/s41598-020-75922-9
97. Amato Nesbit S, Sharma R, Waldfogel JM, Zhang A, Bennett WL, Yeh H-C, et al. Non-pharmacologic treatments for symptoms of diabetic peripheral neuropathy: a systematic review. Curr Med Res Opin. (2019) 35:15–25. doi: 10.1080/03007995.2018.1497958
98. Slangen R, Schaper NC, Faber CG, Joosten EA, Dirksen CD, van Dongen RT, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two-center randomized controlled trial. Diabetes Care. (2014) 37:3016–24. doi: 10.2337/dc14-0684
99. Albers JW and Jacobson R. Decompression nerve surgery for diabetic neuropathy: a structured review of published clinical trials. Diabetes Metab syndrome obesity: Targets Ther. (2018) 11:493–514. doi: 10.2147/DMSO.S146121
100. Takahashi S, Ukai S, Tsuji T, Ueyama T, Kono M, Yamanaka N, et al. Reduction of cortical excitability and increase of thalamic activity in a low-frequency rTMS treatment for chronic tinnitus. Neurocase. (2015) 21:339–44. doi: 10.1080/13554794.2014.893000
101. Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, and Sul HS. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am J Physiology-Gastrointestinal Liver Physiol. (2007) 293:G1–4. doi: 10.1152/ajpgi.00554.2006
102. Xu X and Xu DS. Prospects for the application of transcranial magnetic stimulation in diabetic neuropathy. Neural Regener Res. (2021) 16:955–62. doi: 10.4103/1673-5374.297062
103. Yang S, Kwak SG, Choi G-S, and Chang MC. Short-term effect of repetitive transcranial magnetic stimulation on diabetic peripheral neuropathic pain. Pain Physician. (2022) 25:E203.
104. Charlton CS, Ridding MC, Thompson PD, and Miles TS. Prolonged peripheral nerve stimulation induces persistent changes in excitability of human motor cortex. J neurological Sci. (2003) 208:79–85. doi: 10.1016/S0022-510X(02)00443-4
105. Weber M and Eisen AA. and AAEM, Magnetic stimulation of the central and peripheral nervous systems. Muscle Nerve. (2002) 25:160–75. doi: 10.1002/mus.10038
106. Chaves AR, Snow NJ, Alcock LR, and Ploughman M. Probing the brain–body connection using transcranial magnetic stimulation (tms): Validating a promising tool to provide biomarkers of neuroplasticity and central nervous system function. Brain Sci. (2021) 11:384. doi: 10.3390/brainsci11030384
107. Tshala-Katumbay D, Eeg-Olofsson KE, Kazadi-Kayembe T, Tylleskär T, and Fällmar P. Analysis of motor pathway involvement in konzo using transcranial electrical and magnetic stimulation. Muscle Nerve. (2002) 25:230–5. doi: 10.1002/mus.10029
108. Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, et al. Descending motor pathways and cortical physiology after spinal cord injury assessed by transcranial magnetic stimulation: a systematic review. Brain Res. (2015) 1619:139–54. doi: 10.1016/j.brainres.2014.09.036
109. Beaulieu LD and Schneider C. Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control. A review. Neurophysiologie Clinique/Clinical Neurophysiol. (2013) 43:251–60. doi: 10.1016/j.neucli.2013.05.003
110. Medina-Fernández FJ, Escribano BM, Padilla-del-Campo C, Drucker-Colín R, Pascual-Leone Á., and Túnez I. Transcranial magnetic stimulation as an antioxidant. Free Radical Res. (2018) 52:381–9. doi: 10.1080/10715762.2018.1434313
111. Deed G, Barlow J, Kawol D, Kilov G, Sharma A, and Yu Hwa L. Diet and diabetes. Aust Family physician. (2015) 44:288–92.
112. He Q, Gao Z, Yin J, Zhang J, Yun Z, and Ye J. Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiology-Endocrinology Metab. (2011) 300:E877–85. doi: 10.1152/ajpendo.00626.2010
113. Jafaripour S, Sedighi S, Jokar MH, Aghaei M, and Moradzadeh M. Inflammation, diet, and type 2 diabetes: a mini-review. J Immunoassay Immunochemistry. (2020) 41:768–77. doi: 10.1080/15321819.2020.1750423
114. Awuchi CG, Echeta CK, and Igwe VS. Diabetes and the nutrition and diets for its prevention and treatment: A systematic review and dietetic perspective. Health Sci Res. (2020) 6:5–19.
115. W. H. Organization. Global status report on alcohol and health 2018. Geneva, Switzerland: World Health Organization (2019).
116. Mayer-Davis EJ. Low-fat diets for diabetes prevention. Diabetes Care. (2001) 24:613–3. doi: 10.2337/diacare.24.4.613
117. Sears B and Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. (2015) 14:121. doi: 10.1186/s12944-015-0123-1
118. Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, et al. A very low-carbohydrate, low–saturated fat diet for type 2 diabetes management: a randomized trial. Diabetes Care. (2014) 37:2909–18. doi: 10.2337/dc14-0845
119. Due A, Larsen TM, Hermansen K, Stender S, Holst JJ, Toubro S, et al. Comparison of the effects on insulin resistance and glucose tolerance of 6-mo high-monounsaturated-fat, low-fat, and control diets. Am J Clin Nutr. (2008) 87:855–62. doi: 10.1093/ajcn/87.4.855
120. Kirk JK, Graves DE, Craven TE, Lipkin EW, Austin M, and Margolis KL. Restricted-carbohydrate diets in patients with type 2 diabetes: a meta-analysis. J Am Dietetic Assoc. (2008) 108:91–100. doi: 10.1016/j.jada.2007.10.003
121. Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. (2015) 31:1–13. doi: 10.1016/j.nut.2014.06.011
122. Sainsbury E, Kizirian NV, Partridge SR, Gill T, Colagiuri S, and Gibson AA. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. (2018) 139:239–52. doi: 10.1016/j.diabres.2018.02.026
123. Meng Y, Bai H, Wang S, Li Z, Wang Q, and Chen L. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. (2017) 131:124–31. doi: 10.1016/j.diabres.2017.07.006
124. Kumar S, Behl T, Sachdeva M, Sehgal A, Kumari S, Kumar A, et al. Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus. Life Sci. (2021) 264:118661. doi: 10.1016/j.lfs.2020.118661
125. Alharbi A and Al-Sowayan NS. The effect of ketogenic-diet on health. Food Nutr Sci. (2020) 11:301–13. doi: 10.4236/fns.2020.114022
126. Mobbs CV, Mastaitis J, Isoda F, and Poplawski M. Treatment of diabetes and diabetic complications with a ketogenic diet. J Child Neurol. (2013) 28:1009–14. doi: 10.1177/0883073813487596
127. Tinguely D, Gross J, and Kosinski C. Efficacy of ketogenic diets on type 2 diabetes: a systematic review. Curr Diabetes Rep. (2021) 21:1–10. doi: 10.1007/s11892-021-01399-z
128. Ranjan A, Schmidt S, Damm-Frydenberg C, Holst JJ, Madsbad S, and Nørgaard K. Short-term effects of a low carbohydrate diet on glycaemic variables and cardiovascular risk markers in patients with type 1 diabetes: a randomized open-label crossover trial. Diabetes Obes Metab. (2017) 19:1479–84. doi: 10.1111/dom.2017.19.issue-10
129. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, and McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab. (2008) 5:1–9. doi: 10.1186/1743-7075-5-36
130. Dashti HM, Mathew TC, and Al-Zaid NS. Efficacy of low-carbohydrate ketogenic diet in the treatment of type 2 diabetes. Med Principles Pract. (2021) 30:223–35. doi: 10.1159/000512142
131. Bird SR and Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open sport Exercise Med. (2017) 2:e000143. doi: 10.1136/bmjsem-2016-000143
132. Chipkin SR, Klugh SA, and Chasan-Taber L. Exercise and diabetes. Cardiol Clinics. (2001) 19:489–505. doi: 10.1016/S0733-8651(05)70231-9
133. Jenkins DW and Jenks A. Exercise and diabetes: a narrative review. J Foot Ankle Surg. (2017) 56:968–74. doi: 10.1053/j.jfas.2017.06.019
134. Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. (2016) 39:2065. doi: 10.2337/dc16-1728
135. da Silva DE, Grande AJ, Roever L, Tse G, Liu T, Biondi-Zoccai G, et al. High-intensity interval training in patients with type 2 diabetes mellitus: a systematic review. Curr Atheroscl Rep. (2019) 21:1–10. doi: 10.1007/s11883-019-0767-9
136. Yang Z, Scott CA, Mao C, Tang J, and Farmer AJ. Resistance exercise versus aerobic exercise for type 2 diabetes: a systematic review and meta-analysis. Sports Med. (2014) 44:487–99. doi: 10.1007/s40279-013-0128-8
137. Aggarwala J, Sharma S, Saroochi JA, and Sarkar A. Effects of aerobic exercise on blood glucose levels and lipid profile in Diabetes Mellitus type 2 subjects. Al Ameen J Me d Sci. (2016) 9:65–9.
138. Sluik D, Buijsse B, Muckelbauer R, Kaaks R, Teucher B, Johnsen NF, et al. Physical activity and mortality in individuals with diabetes mellitus: a prospective study and meta-analysis. Arch Internal Med. (2012) 172:1285–95. doi: 10.1001/archinternmed.2012.3130
139. Zanuso S, Jimenez A, Pugliese G, Corigliano G, and Balducci S. Exercise for the management of type 2 diabetes: a review of the evidence. Acta diabetologica. (2010) 47:15–22. doi: 10.1007/s00592-009-0126-3
140. Afriani AB, Ginting SC, and Anggita GM. (2021). The effect of aerobic exercise on body weight and body fat percentage, in: ISMINA 2021: Proceedings of the 5th International Conference on Sports, Health, and Physical Education. Semarang, Central Java, Indonesia: EAI Publishing. p. 194.
141. Vikberg S, Sörlén N, Brandén L, Johansson J, Nordström A, Hult A, et al. Effects of resistance training on functional strength and muscle mass in 70-year-old individuals with pre-sarcopenia: a randomized controlled trial. J Am Med Directors Assoc. (2019) 20:28–34. doi: 10.1016/j.jamda.2018.09.011
142. Gordon B, Benson A, Bird S, and Fraser S. Resistance training improves metabolic health in type 2 diabetes: a systematic review. Diabetes Res Clin Pract. (2009) 83:157–75. doi: 10.1016/j.diabres.2008.11.024
143. Kraemer WJ, Ratamess NA, and French DN. Resistance training for health and performance. Curr sports Med Rep. (2002) 1:165–71. doi: 10.1249/00149619-200206000-00007
144. Morrissey MC, Harman EA, and Johnson MJ. Resistance training modes: specificity and effectiveness. Med Sci Sports Exercise. (1995) 27:648–60. doi: 10.1249/00005768-199505000-00006
145. Karlsen T, Aamot I-L, Haykowsky M, and Rognmo Ø. High intensity interval training for maximizing health outcomes. Prog Cardiovasc Dis. (2017) 60:67–77. doi: 10.1016/j.pcad.2017.03.006
146. Hough P. High-intensity interval training. In: Advanced personal training. Abingdon, UK: Routledge (2021). p. 171–203.
147. Jelleyman C, Yates T, O’Donovan G, Gray LJ, King JA, Khunti K, et al. The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev. (2015) 16:942–61. doi: 10.1111/obr.2015.16.issue-11
148. Khalafi M, Ravasi AA, Malandish A, and Rosenkranz SK. The impact of high-intensity interval training on postprandial glucose and insulin: A systematic review and meta-analysis. Diabetes Res Clin Pract. (2022) 186:109815. doi: 10.1016/j.diabres.2022.109815
149. Winding KM, Munch GW, Iepsen UW, Van Hall G, Pedersen BK, and Mortensen SP. The effect on glycaemic control of low-volume high-intensity interval training versus endurance training in individuals with type 2 diabetes. Diabetes Obes Metab. (2018) 20:1131–9. doi: 10.1111/dom.2018.20.issue-5
150. Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, and Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. (2003) 26:2977–82. doi: 10.2337/diacare.26.11.2977
151. Oliveira C, Simões M, Carvalho J, and Ribeiro J. Combined exercise for people with type 2 diabetes mellitus: a systematic review. Diabetes Res Clin Pract. (2012) 98:187–98. doi: 10.1016/j.diabres.2012.08.004
152. Figueira FR, Umpierre D, Casali KR, Tetelbom PS, Henn NT, Ribeiro JP, et al. Aerobic and combined exercise sessions reduce glucose variability in type 2 diabetes: crossover randomized trial. PloS One. (2013) 8:e57733. doi: 10.1371/journal.pone.0057733
153. Yavari A, Najafipoor F, Aliasgarzadeh A, Niafar M, and Mobasseri M. Effect of aerobic exercise, resistance training or combined training on glycaemic control and cardio-vascular risk factors in patients with type 2 diabetes. Biol Sport. (2012) 29:135–43. doi: 10.5604/20831862.990466
154. Schwingshackl L, Missbach B, Dias S, König J, and Hoffmann G. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetologia. (2014) 57:1789–97. doi: 10.1007/s00125-014-3303-z
155. D. P. P. R. Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New Engl J Med. (2002) 346:393–403. doi: 10.1056/NEJMoa012512
156. Amer BE, Abdelgalil MS, Hamad AA, Abdelsayed K, Elaraby A, Abozaid AM, et al. Metformin plus lifestyle interventions versus lifestyle interventions alone for the delay or prevention of type 2 diabetes in individuals with prediabetes: a meta-analysis of randomized controlled trials. Diabetol Metab Syndrome. (2024) 16:273. doi: 10.1186/s13098-024-01504-8
157. Rosenberg CJ and Watson JC. Treatment of painful diabetic peripheral neuropathy. Prosthetics orthotics Int. (2015) 39:17–28. doi: 10.1177/0309364614542266
158. Ziegler D. Treatment of diabetic polyneuropathy: update 2006. Ann New York Acad Sci. (2006) 1084:250–66. doi: 10.1196/annals.1372.008
159. Chang MC and Yang S. Diabetic peripheral neuropathy essentials: a narrative review. Ann palliative Med. (2023) 12:39098–398. doi: 10.21037/apm-22-693
160. van Beek M, Slangen R, Schaper NC, Faber CG, Joosten EA, Dirksen CD, et al. Sustained treatment effect of spinal cord stimulation in painful diabetic peripheral neuropathy: 24-month follow-up of a prospective two-center randomized controlled trial. Diabetes Care. (2015) 38:e132–4. doi: 10.2337/dc15-0740
161. Deer TR, Krames E, Mekhail N, Pope J, Leong M, Stanton-Hicks M, et al. The appropriate use of neurostimulation: new and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation: Technol at Neural Interface. (2014) 17:599–615. doi: 10.1111/ner.12204
162. Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation: Technol at Neural Interface. (2014) 17:515–50. doi: 10.1111/ner.12208
163. Galan V, Scowcroft J, Chang P, Li S, Staats P, Rotte A, et al. 10-kHz spinal cord stimulation treatment for painful diabetic neuropathy: results from post-hoc analysis of the SENZA-PPN study. Pain Manage. (2020) 10:291–300. doi: 10.2217/pmt-2020-0033
164. van Beek M, Geurts JW, Slangen R, Schaper NC, Faber CG, Joosten EA, et al. Severity of neuropathy is associated with long-term spinal cord stimulation outcome in painful diabetic peripheral neuropathy: five-year follow-up of a prospective two-center clinical trial. Diabetes Care. (2018) 41:32–8. doi: 10.2337/dc17-0983
165. Dong D and Liu H. Prevalence of carpal tunnel syndrome in patients with long-term type 2 diabetes mellitus. Heliyon. (2022) 8:e12615. doi: 10.1016/j.heliyon.2022.e12615
166. Papanas N, Stamatiou I, and Papachristou S. Carpal tunnel syndrome in diabetes mellitus. Curr Diabetes Rev. (2022) 18:16–9. doi: 10.2174/1573399817666210901114610
167. Chaudhry V, Stevens JC, Kincaid J, and So YT. Practice Advisory: utility of surgical decompression for treatment of diabetic neuropathy: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. (2006) 66:1805–8. doi: 10.1212/01.wnl.0000219631.89207.a9
168. Wood WA and Wood MA. Decompression of peripheral nerves for diabetic neuropathy in the lower extremity 3 3The authors have not received financial benefit or other compensation for any product or device mentioned in this article, nor do they own stock or any portion of any company mentioned herein. J Foot Ankle Surg. (2003) 42:268–75. doi: 10.1016/S1067-2516(03)00313-2
169. Ma F, Wang G, Wu Y, Xie B, and Zhang W. Improving effects of peripheral nerve decompression microsurgery of lower limbs in patients with diabetic peripheral neuropathy. Brain Sci. (2023) 13:558. doi: 10.3390/brainsci13040558
170. Bindman LJ, Lippold O, and Redfearn J. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. (1964) 172:369. doi: 10.1113/jphysiol.1964.sp007425
171. t. Gorman A. Differential patterns of activation of the pyramidal system elicited by surface anodal and cathodal cortical stimulation. J Neurophysiol. (1966) 29:547–64. doi: 10.1152/jn.1966.29.4.547
172. Paulus W. Transcranial direct current stimulation (tDCS). In: Supplements to Clinical neurophysiology, vol. 56. Amsterdam, Netherlands: Elsevier (2003). p. 249–54.
173. Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol. (2003) 114:589–95. doi: 10.1016/S1388-2457(02)00437-6
174. Flöel A. tDCS-enhanced motor and cognitive function in neurological diseases. Neuroimage. (2014) 85:934–47. doi: 10.1016/j.neuroimage.2013.05.098
175. Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. (2017) 128:56–92. doi: 10.1016/j.clinph.2016.10.087
176. Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, et al. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J Neurosci. (2011) 31:15284–93. doi: 10.1523/JNEUROSCI.0542-11.2011
177. Ruohonen J and Karhu J. tDCS possibly stimulates glial cells. Clin Neurophysiol. (2012) 123:2006–9. doi: 10.1016/j.clinph.2012.02.082
178. Zortea M, Ramalho L, Alves RL, Alves CFS, Braulio G, Torres ILdS, et al. Transcranial direct current stimulation to improve the dysfunction of descending pain modulatory system related to opioids in chronic non-cancer pain: an integrative review of neurobiology and meta-analysis. Front Neurosci. (2019) 13:1218. doi: 10.3389/fnins.2019.01218
179. García-Larrea L, Peyron R, Mertens P, Grégoire MC, Lavenne F, Le Bars D, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. (1999) 83:259–73. doi: 10.1016/S0304-3959(99)00114-1
180. Apkarian AV, Bushnell MC, Treede R-D, and Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. (2005) 9:463–84. doi: 10.1016/j.ejpain.2004.11.001
181. Nitsche MA, Boggio PS, Fregni F, and Pascual-Leone A. Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp Neurol. (2009) 219:14–9. doi: 10.1016/j.expneurol.2009.03.038
182. Ngernyam N, Jensen MP, Auvichayapat N, Punjaruk W, and Auvichayapat P. Transcranial direct current stimulation in neuropathic pain. J Pain Relief. (2013) Suppl 3:001. doi: 10.4172/2167-0846.S3-001
183. Monte-Silva K, Kuo M-F, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain stimulation. (2013) 6:424–32. doi: 10.1016/j.brs.2012.04.011
184. Hsu W-Y, Cheng C-H, Liao K-K, Lee I-H, and Lin Y-Y. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. (2012) 43:1849–57. doi: 10.1161/STROKEAHA.111.649756
185. Conti A, Raffa G, Granata F, Rizzo V, Germanò A, and Tomasello F. Navigated transcranial magnetic stimulation for somatotopic tractography of the corticospinal tract. Operative Neurosurg. (2014) 10:542–54. doi: 10.1227/NEU.0000000000000502
186. Hallett M. Transcranial magnetic stimulation and the human brain. Nature. (2000) 406:147–50. doi: 10.1038/35018000
187. Spampinato DA, Ibanez J, Rocchi L, and Rothwell J. Motor potentials evoked by transcranial magnetic stimulation: interpreting a simple measure of a complex system. J Physiol. (2023) 601:2827–51. doi: 10.1113/tjp.v601.14
188. Deng Z-D, Lisanby SH, and Peterchev AV. Electric field depth–focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain stimulation. (2013) 6:1–13. doi: 10.1016/j.brs.2012.02.005
189. Somaa FA, de Graaf TA, and Sack AT. Transcranial magnetic stimulation in the treatment of neurological diseases. Front Neurol. (2022) 13:793253. doi: 10.3389/fneur.2022.793253
190. Barker AT, Jalinous R, and Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. (1985) 325:1106–7. doi: 10.1016/S0140-6736(85)92413-4
191. Barker AT. An introduction to the basic principles of magnetic nerve stimulation. J Clin Neurophysiol. (1991) 8:26–37. doi: 10.1097/00004691-199101000-00005
192. Hsiao IN and Lin VW-H. Improved coil design for functional magnetic stimulation of expiratory muscles. IEEE Trans Biomed Eng. (2001) 48:684–94. doi: 10.1109/10.923786
193. Ruohonen J, Virtanen J, and Ilmoniemi RJ. Coil optimization for magnetic brain stimulation. Ann Biomed Eng. (1997) 25:840–9. doi: 10.1007/BF02684168
194. Ueno S, Tashiro T, and Harada K. Localized stimulation of neural tissues in the brain by means of a paired configuration of time-varying magnetic fields. J Appl Phys. (1988) 64:5862–4. doi: 10.1063/1.342181
195. Zhang N, Wang Z, Shi J, Ning S, Zhang Y, Wang S, et al. Theoretical analysis and design of an innovative coil structure for transcranial magnetic stimulation. Appl Sci. (2021) 11:1960. doi: 10.3390/app11041960
196. Rotenberg A, Horvath JC, and Pascual-Leone A. The Transcranial Magnetic Stimulation (TMS) Device and Foundational Techniques. In: Rotenberg A, Horvath JC, and Pascual-Leone A, editors. Transcranial Magnetic Stimulation. Springer New York, New York, NY(2014). p. 3–13.
197. Hsu K-H, Nagarajan SS, and Durand DM. Analysis of efficiency of magnetic stimulation. IEEE Trans Biomed Eng. (2003) 50:1276–85. doi: 10.1109/TBME.2003.818473
198. Afuwape OF, Oya H, Boes AD, and Jiles DC. Measurement and modeling of the effects of transcranial magnetic stimulation on the brain. IEEE Trans Magnetics. (2020) 57:1–5. doi: 10.1109/TMAG.2020.3008554
199. Roth Y, Zangen A, and Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol. (2002) 19:361–70. doi: 10.1097/00004691-200208000-00008
200. Zangen A, Roth Y, Voller B, and Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol. (2005) 116:775–9. doi: 10.1016/j.clinph.2004.11.008
201. Navarro de Lara LI, Daneshzand M, Mascarenas A, Paulson D, Pratt K, Okada Y, et al. A 3-axis coil design for multichannel TMS arrays. Neuroimage. (2021) 224:117355. doi: 10.1016/j.neuroimage.2020.117355
202. Rotenberg A, Horvath JC, and Pascual-Leone A. The transcranial magnetic stimulation (TMS) device and foundational techniques. New York, NY, USA: Springer (2014).
203. Thut G, Northoff G, Ives JR, Kamitani Y, Pfennig A, Kampmann F, et al. Effects of single-pulse transcranial magnetic stimulation (TMS) on functional brain activity: a combined event-related TMS and evoked potential study. Clin Neurophysiol. (2003) 114:2071–80. doi: 10.1016/S1388-2457(03)00205-0
204. Rothwell J. Paired-pulse investigations of short-latency intracortical facilitation using TMS in humans. Electroencephalogr Clin Neurophysiol Suppl. (1999) 51:113–9.
205. Fitzgerald PB, Fountain S, and Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. (2006) 117:2584–96. doi: 10.1016/j.clinph.2006.06.712
206. Peterchev AV. Direction and directionality: the neural effects of transcranial magnetic stimulation coil orientation and pulse waveform. Biol Psychiatry: Cogn Neurosci Neuroimaging. (2024) 9:739–41. doi: 10.1016/j.bpsc.2024.06.010
207. Pafili K, Papanas N, and Ziegler D. Neuropathy in diabetes:one cannot begin it too soonVol. pp. . Los Angeles, CA: SAGE Publications Sage CA(2018) p. 752–4.
208. Pop-Busui R, Boulton AJM, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. (2017) 40:136. doi: 10.2337/dc16-2042
209. A. D. A. P. P. Committee. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. (2022) 45:S17–38. doi: 10.2337/dc22-S002
210. Vucic S, Chen K-HS, Kiernan MC, Hallett M, Benninger D, Di Lazzaro V, et al. Clinical diagnostic utility of transcranial magnetic stimulation in neurological disorders. Updated report of an IFCN committee. Clin Neurophysiol. (2023) 150:131–75. doi: 10.1016/j.clinph.2023.03.010
211. Mancuso M, Cruciani A, Sveva V, Casula EP, Brown K, Rothwell JC, et al. Somatosensory input in the context of transcranial magnetic stimulation coupled with electroencephalography: An evidence-based overview. Neurosci Biobehav Rev. (2023) 155:105434. doi: 10.1016/j.neubiorev.2023.105434
212. Derosiere G, Vassiliadis P, and Duque J. Advanced TMS approaches to probe corticospinal excitability during action preparation. Neuroimage. (2020) 213:116746. doi: 10.1016/j.neuroimage.2020.116746
213. Selvarajah D, Wilkinson ID, Fang F, Sankar A, Davies J, Boland E, et al. Structural and functional abnormalities of the primary somatosensory cortex in diabetic peripheral neuropathy: a multimodal MRI study. Diabetes. (2019) 68:796–806. doi: 10.2337/db18-0509
214. Tesfaye S, Boulton AJM, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. (2010) 33:2285–93. doi: 10.2337/dc10-1303
215. Gonçalves NP, Vægter CB, Andersen H, Østergaard L, Calcutt NA, and Jensen TS. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol. (2017) 13:135–47. doi: 10.1038/nrneurol.2016.201
216. Li J-M, Yu R, Zhang L-P, Wen S-Y, Wang S-J, Zhang X-Y, et al. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: a benefit of short-chain fatty acids. Microbiome. (2019) 7:1–14. doi: 10.1186/s40168-019-0713-7
217. Sango K, Mizukami H, Horie H, and Yagihashi S. Impaired axonal regeneration in diabetes. perspective on the underlying mechanism from in vivoand in vitroexperimental studies. Front Endocrinol. (2017) 8:12. doi: 10.3389/fendo.2017.00012
218. Arias-Carrión O, Verdugo-Díaz L, Feria-Velasco A, Millán-Aldaco D, Gutiérrez AA, Hernández-Cruz A, et al. Neurogenesis in the subventricular zone following transcranial magnetic field stimulation and nigrostriatal lesions. J Neurosci Res. (2004) 78:16–28. doi: 10.1002/jnr.20235
219. Sherafat MA, Heibatollahi M, Mongabadi S, Moradi F, Javan M, and Ahmadiani A. Electromagnetic field stimulation potentiates endogenous myelin repair by recruiting subventricular neural stem cells in an experimental model of white matter demyelination. J Mol Neurosci. (2012) 48:144–53. doi: 10.1007/s12031-012-9791-8
220. Deveci S, Matur Z, Kesim Y, Senturk G, Sargın-Kurt G, Ugur SA, et al. Effect of the brain-derived neurotrophic factor gene Val66Met polymorphism on sensory-motor integration during a complex motor learning exercise. Brain Res. (2020) 1732:146652. doi: 10.1016/j.brainres.2020.146652
221. Fitzsimmons SM, Oostra E, Postma TS, van der Werf YD, and van den Heuvel OA. Repetitive transcranial magnetic stimulation-induced neuroplasticity and the treatment of psychiatric disorders: state of the evidence and future opportunities. Biol Psychiatry. (2023) 94(1):1–11. doi: 10.1016/j.biopsych.2023.11.016
222. Wang H-Y, Crupi D, Liu JJ, Stucky A, Cruciata G, Di Rocco A, et al. Repetitive transcranial magnetic stimulation enhances BDNF–TrkB signaling in both brain and lymphocyte. J Neurosci. (2011) 31:11044–54. doi: 10.1523/JNEUROSCI.2125-11.2011
223. Yekkirala AS, Roberson DP, Bean BP, and Woolf CJ. Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov. (2017) 16:545–64. doi: 10.1038/nrd.2017.87
224. Gustin SM, Wrigley PJ, Youssef AM, McIndoe L, Wilcox SL, Rae CD, et al. Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. PAIN®. (2014) 155:1027–36. doi: 10.1016/j.pain.2014.02.008
225. Toledo RS, Stein DJ, Sanches PRS, da Silva LS, Medeiros HR, Fregni F, et al. rTMS induces analgesia and modulates neuroinflammation and neuroplasticity in neuropathic pain model rats. Brain Res. (2021) 1762:147427. doi: 10.1016/j.brainres.2021.147427
226. Lefaucheur J, Drouot X, Menard-Lefaucheur I, Keravel Y, and Nguyen J. Motor cortex rTMS in chronic neuropathic pain: pain relief is associated with thermal sensory perception improvement. J Neurology Neurosurg Psychiatry. (2008) 79:1044–9. doi: 10.1136/jnnp.2007.135327
227. Onesti E, Gabriele M, Cambieri C, Ceccanti M, Raccah R, Di Stefano G, et al. H-coil repetitive transcranial magnetic stimulation for pain relief in patients with diabetic neuropathy. Eur J Pain. (2013) 17:1347–56. doi: 10.1002/j.1532-2149.2013.00320.x
228. Abdelkader AA, El Gohary AM, Mourad HS, and El Salmawy DA. Repetitive TMS in treatment of resistant diabetic neuropathic pain. Egyptian J Neurology Psychiatry Neurosurg. (2019) 55:1–9. doi: 10.1186/s41983-019-0075-x
229. Xu Z, Zhong Q, Xing F, Zhu Y, Hu Y, Huang M, et al. rTMS and TENS relieve neuropathic pain in CCI model rats by modulating central nervous system TRPV1 and neuroinflammation. Mediators Inflammation. (2024) 2024:8500317. doi: 10.1155/mi/8500317
230. Zhang Z, Hou Z, Han M, Guo P, Chen K, Qin J, et al. Amygdala-targeted relief of Neuropathic Pain: efficacy of Repetitive Transcranial Magnetic Stimulation in NLRP3 pathway suppression. Mol Neurobiol. (2024) 61:8904–20. doi: 10.1007/s12035-024-04087-7
231. Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, and Lönnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. (2008) 106:1–27. doi: 10.1016/j.jad.2007.06.006
232. Lee AL, Ogle WO, and Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord. (2002) 4:117–28. doi: 10.1034/j.1399-5618.2002.01144.x
233. Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol. (2010) 20:1–17. doi: 10.1016/j.euroneuro.2009.08.003
234. Rossi S, Hallett M, Rossini PM, Pascual-Leone A, and S. o. T. C. Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. (2009) 120:2008–39. doi: 10.1016/j.clinph.2009.08.016
235. Baeken C, De Raedt R, Bossuyt A, Van Hove C, Mertens J, Dobbeleir A, et al. The impact of HF-rTMS treatment on serotonin2A receptors in unipolar melancholic depression. Brain stimulation. (2011) 4:104–11. doi: 10.1016/j.brs.2010.09.002
236. Zhao L, Ren H, Gu S, Li X, Jiang C, Li J, et al. rTMS ameliorated depressive-like behaviors by restoring HPA axis balance and prohibiting hippocampal neuron apoptosis in a rat model of depression. Psychiatry Res. (2018) 269:126–33. doi: 10.1016/j.psychres.2018.08.017
237. Cardinal TM, Antunes LC, Brietzke AP, Parizotti CS, Carvalho F, De Souza A, et al. Differential neuroplastic changes in fibromyalgia and depression indexed by up-regulation of motor cortex inhibition and disinhibition of the descending pain system: an exploratory study. Front Hum Neurosci. (2019) 13:138. doi: 10.3389/fnhum.2019.00138
238. Vinik AI, Maser RE, Mitchell BD, and Freeman R. Diabetic autonomic neuropathy. Diabetes Care. (2003) 26:1553–79. doi: 10.2337/diacare.26.5.1553
239. Duque A, Mediano MFF, De Lorenzo A, and Rodrigues LF Jr. Cardiovascular autonomic neuropathy in diabetes: Pathophysiology, clinical assessment and implications. World J Diabetes. (2021) 12:855–67. doi: 10.4239/wjd.v12.i6.855
240. Vinik AI and Erbas T. Chapter 22 - Diabetic autonomic neuropathy. In: Buijs RM and Swaab DF, editors. Handbook of Clinical Neurology, vol. 117. Amsterdam, Netherlands: Elsevier (2013). p. 279–94.
241. Kaur M, Michael JA, Hoy KE, Fitzgibbon BM, Ross MS, Iseger TA, et al. Investigating high-and low-frequency neuro-cardiac-guided TMS for probing the frontal vagal pathway. Brain stimulation. (2020) 13:931–8. doi: 10.1016/j.brs.2020.03.002
242. Lupica R, Donato V, Lacquaniti A, Cernaro V, Lucisano S, Grasso G, et al. Proteinuric effect of transcranial magnetic stimulation in healthy subjects and diabetic patients with Stage 3–4 CKD. Nephrol Dialysis Transplant. (2014) 29:573–9. doi: 10.1093/ndt/gft454
243. Vas PR and Edmonds ME. Early recognition of diabetic peripheral neuropathy and the need for one-stop microvascular assessment. Lancet Diabetes Endocrinol. (2016) 4:723–5. doi: 10.1016/S2213-8587(16)30063-8
244. Nukada H. Ischemia and diabetic neuropathy. Handb Clin Neurol. (2014) 126:469–87. doi: 10.1016/B978-0-444-53480-4.00023-0
245. Kobayashi M and Zochodne DW. Diabetic neuropathy and the sensory neuron: new aspects of pathogenesis and their treatment implications. J Diabetes Invest. (2018) 9:1239–54. doi: 10.1111/jdi.2018.9.issue-6
246. Chapouly C, Yao Q, Vandierdonck S, Larrieu-Lahargue F, Mariani JN, Gadeau A-P, et al. Impaired Hedgehog signalling-induced endothelial dysfunction is sufficient to induce neuropathy: implication in diabetes. Cardiovasc Res. (2016) 109:217–27. doi: 10.1093/cvr/cvv263
247. Pahk K and Lee S-H. Effects of repetitive transcranial magnetic stimulation on improving cerebral blood flow in patients with middle cerebral artery steno-occlusion. Acta Neurologica Belgica. (2023) 124:1–8.
248. Mesquita RC, Faseyitan OK, Turkeltaub PE, Buckley EM, Thomas A, Kim MN, et al. Blood flow and oxygenation changes due to low-frequency repetitive transcranial magnetic stimulation of the cerebral cortex. J Biomed Optics. (2013) 18:067006–6. doi: 10.1117/1.JBO.18.6.067006
249. Gersner R, Kravetz E, Feil J, Pell G, and Zangen A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals. J Neurosci. (2011) 31:7521–6. doi: 10.1523/JNEUROSCI.6751-10.2011
250. Kaur M, Michael JA, Hoy KE, Fitzgibbon BM, Ross MS, Iseger TA, et al. High-frequency repetitive transcranial magnetic stimulation improves functional recovery by inhibiting neurotoxic polarization of astrocytes in ischemic rats. J Neuroinflamm. (2020) 17:150. doi: 10.1186/s12974-020-01747-y
Keywords: transcranial magnetic stimulation (TMS), diabetic peripheral neuropathy (DPN), neural inflammation, nonpharmacological interventions, neuromodulation
Citation: Lee C, Lai C-W, Wu G-L, Yen S, Huang C-M, Liu Z-H, Hu P-C and Liao L-D (2025) Review of nonpharmacological interventions for delaying the effects of cerebral neuropathy caused by diabetes. Front. Endocrinol. 16:1621448. doi: 10.3389/fendo.2025.1621448
Received: 08 May 2025; Accepted: 04 August 2025;
Published: 27 August 2025.
Edited by:
Mithun Rudrapal, Vignan’s Foundation for Science, Technology and Research, IndiaReviewed by:
Pei Shang, Mayo Clinic, United StatesKoyel Kar, BCDA College of Pharmacy and Technology, India
Tanishk Saini, Birla Institute of Technology, Mesra, India
Copyright © 2025 Lee, Lai, Wu, Yen, Huang, Liu, Hu and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lun-De Liao, bGRsaWFvQG5ocmkuZWR1LnR3; Z3MzMzYudHdAZ21haWwuY29t
 Chi Lee1,2
Chi Lee1,2 Chih-Mao Huang
Chih-Mao Huang Lun-De Liao
Lun-De Liao