- 1Department of Nephrology, The First People’s Hospital of Hangzhou Lin’an District, Hangzhou, China
- 2Department of Traditional Chinese Medicine, Jiande First People’s Hospital, Jiande, Hangzhou, China
- 3Department of Nephrology, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Zhejiang Key Laboratory of Research and Translation for Kidney Deficiency-Stasis-Turbidity Disease, Zhejiang-Macau International Joint Laboratory of Integrated Traditional Chinese and Western Medicine for Nephrology and Immunology, Hangzhou, China
Diabetic nephropathy (DN) stands as a prominent microvascular complication of diabetes mellitus and presents a significant global health challenge. Despite advancements in glycemic control and renin-angiotensin system inhibition, current treatments merely delay disease progression without targeting fundamental pathological processes. This review explores gut microbiota modulation as a promising treatment strategy for DN through probiotic supplementation, dietary interventions, and fecal microbiota transplantation(FMT) protocols. The gut microbiota, integral to the “gut-kidney axis,” is critically implicated in DN pathogenesis. DN is associated with gut dysbiosis—characterized by reduced microbial diversity, depletion of beneficial short-chain fatty acid (SCFA)-producing bacteria, and proliferation of opportunistic pathogens. This dysbiosis impairs gut barrier integrity, fostering systemic inflammation and the accumulation of uremic toxins like indoxyl sulfate. Furthermore, translocated bacterial lipopolysaccharides activate Toll-like receptors and the NLRP3 inflammasome, exacerbating kidney damage and fibrosis. Interventions targeting the microbiota, including dietary strategies (e.g., enhancing fermentable fibers, low-protein diets) and FMT, show promise in preclinical and early clinical studies, though FMT requires stringent safety and donor screening protocols. Significant challenges persist, such as managing inter-individual microbiota variability for personalized therapies, fully elucidating molecular mechanisms like SCFA-GPR43 signaling, and leveraging multiomics for biomarker discovery. Advancing microbiota-focused interventions for DN towards microbiome-centered precision medicine necessitates addressing standardization, deepening mechanistic understanding, and validating combination therapies, heralding a potential shift from traditional nephroprotective approaches.
1 Introduction
Diabetic nephropathy (DN) is a severe microvascular complication of diabetes mellitus and is the leading cause of end-stage renal disease (ESRD) worldwide, accounting for approximately 40% of ESRD cases (1, 2). The increasing global prevalence of diabetes has led to a corresponding increase in the incidence of DN, which presents a pressing clinical challenge.
Histologically, DN is characterized by progressive glomerulosclerosis, tubulointerstitial fibrosis, and abnormal extracellular matrix deposition, resulting in irreversible nephron loss and functional decline (3, 4). Although intensive glycemic control and renin-angiotensin system RAS inhibition remain therapeutic cornerstones, they predominantly decelerate clinical deterioration rather than halting disease progression. Notably, these interventions show limited capacity to repair existing renal structural damage, especially in late-stage nephropathy (3, 5). This significant gap underscores the urgent need to both investigation of new pathogenic mechanisms and development of targeted therapies. The gut-kidney axis now constitutes a critical framework for understanding DN pathophysiology. This bidirectional communication network is characterized by complex interactions involving microbial metabolites, immune system regulation, and inflammatory pathways (6, 7). Supporting this concept, clinical investigations consistently demonstrate characteristic microbial imbalances in DN patients. Key features include reduced microbial ecological diversity, decreased beneficial butyrate-producing bacteria (e.g., Roseburia and Faecalibacterium), increased lipopolysaccharide (LPS)-producing gram-negative organisms (notably Enterobacteriaceae), and elevated urease-producing microbes (7–10). These alterations compromise intestinal barrier integrity, facilitating bacterial endotoxin translocation and toll-like receptor 4-mediated pro-inflammatory cascades, thereby amplifying oxidative stress and fibrogenic responses within the renal parenchyma (3, 11, 12). Additionally, aberrant gut microbial metabolism contributes to peripheral insulin resistance and accelerates advanced glycation end product formation, exacerbating glomerular basement membrane thickening and podocyte dysfunction (8, 13, 14).
Microbiota-targeted therapeutic strategies hold considerable promise for the management of DN. Preclinical investigations have demonstrated that selective probiotic supplementation, particularly with Lactobacillus and Bifidobacterium species, or prebiotic administration, effectively restores microbial homeostasis, enhances colonic short-chain fatty acid (SCFA) production, and subsequently inhibits the TGF-β1/Smad3 signaling pathway in the kidneys (9, 11, 15). These interventions attenuate epithelial–mesenchymal transition in tubular epithelial cells and reduce pathological collagen deposition, which are key pathological features of DN (11, 15).
Translational studies and early phase clinical trials have indicated that targeted microbiota transplantation protocols and structured dietary fiber supplementation significantly reduce albuminuria, improve insulin sensitivity, and ameliorate dyslipidemia in patients (3, 16). Notably, specific gut microbiota-derived metabolites, such as indolepropionic acid and trimethylamine-N-oxide, correlate strongly with the severity of renal fibrosis, suggesting their potential as clinically relevant biomarkers for the early detection and therapeutic monitoring of DN management (3, 8, 17). Furthermore, targeted microbiome-based interventions may provide a promising translational approach to overcome current limitations in the management of DN. These novel therapeutic strategies have the potential to offer effective treatment options for clinically challenging complication (3, 18). This review examines therapeutic targeting of the gut microbiota in DN management through probiotics, dietary modifications, and FMT.
2 Molecular mechanisms by which the gut microbiota influences DN
2.1 Pathological interactions mediated by the gut–kidney axis via microbial metabolite imbalance
2.1.1 SCFA depletion
Metabolic dysregulation and barrier disruption SCFAs, notably butyrate and propionate, modulate immune responses by activating G protein-coupled receptors (GPR43/41) and inhibiting histone deacetylase (HDAC) activity, leading to pro-inflammatory cytokine transcription suppression (19–21). These metabolites are crucial to maintain intestinal barrier integrity by enhancing the expression of tight junction proteins such as zona occludens-1, occludin, and claudin-1, and stimulating mucin production from goblet cells (4, 13, 22). In patients with DN, quantitative assessments reveal a 30–50% reduction in intestinal SCFA levels, which negatively correlates with gut permeability markers (22–24). Metagenomic analyses indicate significant reductions (2.5–3.8-fold) in key butyrate-producing bacteria, including Faecalibacterium prausnitzii and Roseburia species, in patients with DN patients compared to healthy controls (23–25). The functional relevance of these changes has been highlighted by interventional studies in which sodium butyrate supplementation (200 mg/kg/day) in diabetic C57BL mice with 14-day quercetin pretreatment restored colonic tight junction expression and decreased circulating lipopolysaccharide levels by 42% (26).
2.1.2 Uremic toxin accumulation and nephrotoxicity mechanisms
Protein-bound uremic toxins significantly contribute to the progression of DN. Indoxyl sulfate (IS), a tryptophan-derived bacterial metabolite, induces pyroptotic cell death in renal tubular cells through aryl hydrocarbon receptor activation, triggering oxidative stress, NLRP3 inflammasome assembly, and caspase-1 activation (21, 26–28). P-Cresyl sulfate (pCS) induces renal tubular cell injury through oxidative stress and direct cytotoxic effects. The anti-proliferative impact of pCS on renal cells surpasses that of other uremic toxins (e.g., indoxyl sulfate, IS), potentially accelerating kidney disease progression via pro-fibrotic signaling pathway activation (29). pCS upregulates renal fibrosis markers and disrupts repair mechanisms of tubular epithelial cells, characterized by reduced autophagic flux and mitochondrial dysfunction. Systemic accumulation of pCS, correlated with elevated gut microbiota-derived precursor p-cresol levels, further exacerbates renal function decline in chronic kidney disease (CKD) patients. The nephrotoxicity of pCS is potentially compounded by inhibition of r renal membrane transporters, such as organic anion transporters 1/3 (OAT1/OAT3). Such inhibition curtails the efficient excretion of uremic toxins (including pCS), thus perpetuating a vicious cycle of toxin accumulation, heightened systemic inflammation, and progressive renal injury (30).Additionally, IS and p-cresyl sulfate activate the pro-fibrotic TGF-β1/Smad3 pathway, increasing renal cortical TGF-β1 expression approximately 3.2-fold and promoting interstitial fibrosis, effects that are abrogated by selective AhR antagonism (18, 31, 32). The clinical relevance of these mechanisms is supported by significantly higher circulating IS concentrations in patients with stage 3–4 DN compared to non-nephropathic individuals with diabetes, and these elevated concentrations strongly correlate with the rate of eGFR decline (18, 28, 32). A randomized controlled trial (NCT04111775) demonstrated that oral charcoal adsorbent AST-120 reduces serum IS concentrations by 58% and decreases the urinary protein-to-creatinine ratio by 29% after 12 weeks, providing evidence that targeting gut-derived uremic toxins offers potential therapeutic benefits for established DN (28, 29). Analysis of the seven key metabolites secreted by the gut microbiota in patients with DN is presented in Figure 1.
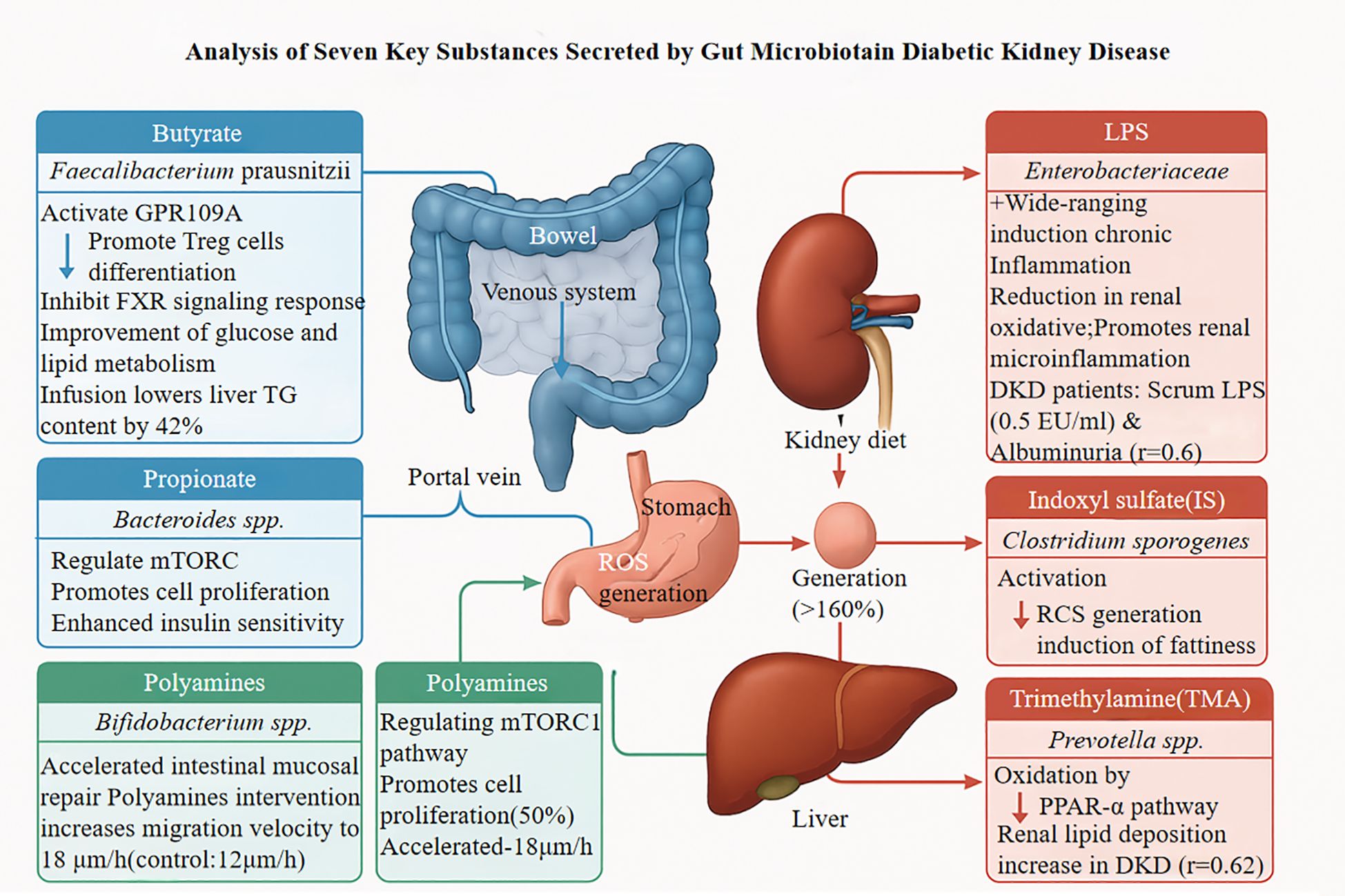
Figure 1. GRP109A, G Protein-Coupled Receptor 109A; FXR, Farnesoid X Receptor; TG, Triglycerides; LPS, Lipopolysaccharide; DKD, Diabetic Kidney Disease; TORC, Target of Rapamycin Complex; RCS, Reactive Carbonyl Species; TMA, Trimethylamine; PPAR-α, Peroxisome Proliferator-Activated Receptor α.
2.2 Molecular mechanisms of toll-like receptor activation and therapeutic strategies
2.2.1 TLR4 signaling in DN
Lipopolysaccharide (LPS), a key component of the outer membrane of gram negative bacteria, acts as a potent pathogen-associated molecule that triggers inflammatory responses. LPS recognition occurs through binding to membrane-bound or soluble CD14, forming a complex that interacts with the TLR4/MD-2 heterodimer in renal and immune cells (33). This interaction initiates a MyD88-dependent signaling cascade. MyD88, recruited to the activated receptor complex, functions as a scaffold for IL-1 receptor-associated kinases (IRAKs) and tumor necrosis factor receptor-associated factor 6 (TRAF6). The resulting proximal signaling complex activates inhibition of κB kinase (IKK), leading to phosphorylation and proteasomal degradation of inhibitory IκB proteins. This releases NF-κB p65 subunits, enabling their nuclear translocation and DNA binding (34, 35).
NF-κB activation upregulates pro-inflammatory mediators, including cytokines (IL-1β, IL-6, TNF-α) and chemokines (MCP-1), fostering a microenvironment that exacerbates kidney injury. The inflammatory response is self-amplifying, as NF-κB activation further increases TLR4 expression, creating a positive feedback loop (36). Efficient TLR4 signaling also depends on membrane microarchitecture modifications, such as lipid raft reorganization and the coordinated recruitment of adaptor proteins TIRAP/MAL and MyD88 to form multimeric signalosome (34, 37, 38).
2.2.2 Experimental evidence supporting TLR4 involvement
In vitro studies using LPS-stimulated macrophages have demonstrated substantial temporal increases in TLR4 protein expression (3.2-fold within 6 hours; p < 0.01), accompanied by enhanced expression of downstream intermediates, particularly MyD88 (2.8-fold) and phosphorylated NF-κB p65 (4.1-fold). These changes correlate with 2–3-fold increases in pro-inflammatory cytokine secretion compared to that in controls (20). Genetic studies employing CRISPR/Cas9-mediated TLR4 deletion attenuate LPS-induced NF-κB activity by 85% (p < 0.001) and suppress downstream inflammatory mediator expression by 70–90% (35, 39). Pharmacological interventions with the selective TLR4 inhibitor TAK-242 (1 μM) block IKKβ phosphorylation, reducing IL-6 and TNF-α production by 62% and 58%, respectively (40). Complementary investigations show that baicalein (10 μM) disrupts MyD88 homodimerization, reducing NF-κB nuclear translocation by 73% (p < 0.01) (41, 42). In vivo studies with TLR4-knockout mice demonstrated an approximately 50% reduction in renal macrophage infiltration and an 80% decrease in MCP-1 mRNA levels (p < 0.001) compared with wild-type controls under inflammatory conditions (43, 44). The TLR4/MyD88/NF-κB cascade mediates LPS-triggered inflammatory signaling, as depicted in Figure 2.
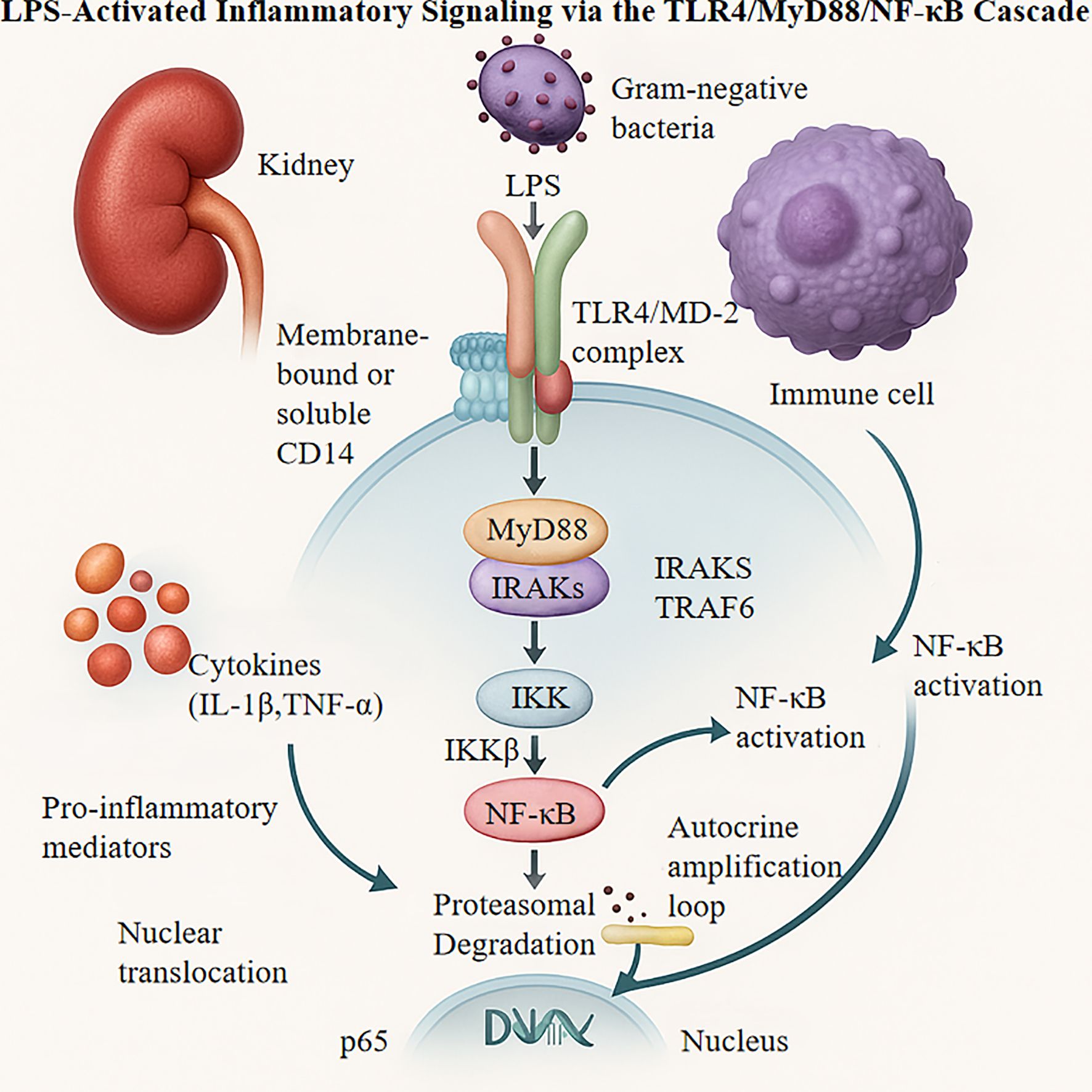
Figure 2. LPS, Lipopolysaccharide; TLR4, Toll-like Receptor 4; MD-2, Myeloid Differentiation Protein 2; IRAKS, IL-1 receptor-associated kinases; TRAF6, TNF Receptor Associated Factor 6; IKK, Inhibitor of NF-κB Kinase; MyD88, Myeloid Differentiation Primary Response Protein 88; CD14, Cluster of Differentiation 14.
2.2.3 Clinical implications and therapeutic considerations
The clinical relevance of toll-like receptor 4 (TLR4) signaling is evident in human disease states, particularly in alcohol-related liver pathology. Patients with this condition exhibit a 3–5-fold increase in serum lipopolysaccharide (LPS) concentration (p < 0.001). Hepatic TLR4 mRNA expression is strongly correlated with Child–Pugh scores, suggesting a mechanistic link between bacterial translocation, TLR4 activation, and progressive organ dysfunction (45, 46). Therapeutic interventions targeting this pathway show promise in preclinical studies. For instance, administration of andrographolide (5 mg/kg/day) over 4 weeks attenuates hepatic inflammation, reducing interleukin-6 (IL-6) concentrations by approximately 58% (p < 0.01) through inhibition of MyD88/IKKβ phosphorylation, with accompanying improvements in histological parameters (41, 42). However, TLR4-targeted therapeutics present significant challenges related to homeostatic functions. Prolonged TLR4 suppression increases susceptibility to opportunistic infections 2.3-fold, particularly Klebsiella pneumoniae, highlighting the need for selective modulation rather than complete inhibition (47). Recent advances in structural biology have facilitated the development of more sophisticated agents with improved specificity, as exemplified by eritoran (MD-2 binding Kd = 12 nM), which has advanced to Phase II clinical trials for conditions characterized by excessive inflammatory responses (34, 48).
2.3 Activation mechanisms of the NLRP3 inflammasome in a multidimensional regulatory network
2.3.1 Molecular activation network
The NLRP3 inflammasome functions as an integrated molecular complex that responds to diverse pathophysiological signals, including exogenous danger signals and endogenous metabolic perturbations. Activation primarily occurs through two convergent pathways initiated by stimuli such as monosodium urate crystals and extracellular ATP (49). The first pathway involves P2X7 receptor engagement, which triggers substantial potassium efflux and a three-fold increase in extracellular potassium concentration. This ionic dysregulation coincides with mitochondrial dysfunction, characterized by elevated production of reactive oxygen species (approximately 250% increase in MitoSOX fluorescence intensity) and permeability transition pore opening, further amplifying cellular oxidative stress (50). Concurrently, a second pathway functions through TLR4/MyD88-dependent signaling, culminating in NF-κB nuclear translocation. This transcriptional activation dramatically enhances NLRP3 expression, with a 34-fold increase in NLRP3 mRNA levels (51, 52). Notably, the gut microbiota potently regulates the sensitivity of the TLR4/MyD88 signaling pathway (observed approximately 2.8-fold differential expression) through the release of metabolites(e.g., hort-chain fatty acids, SCFAs) and structural components (e.g., lipopolysaccharide, LPS). This modulation consequently influences NLRP3 inflammasome transcriptional activation. Clinical studies indicate Bacteroidetes abundance exhibited a negative correlation with TLR4 signaling intensity (r = -0.76, p < 0.01). Conversely, Firmicutes, via the production of butyrate, suppressed NF-κB nuclear translocation efficiency by as up to 42% (53). These mechanisms converge during caspase-1 autocatalytic processing, generating functional p20/p10 subunits that increase three-fold relative to the baseline. Activated caspase-1 catalyzes pro-IL-1β cleavage, resulting in a 40-fold elevation in biologically active IL-1β secretion (54, 55).
2.3.2 Oxidative stress-driven regulatory mechanisms
The interplay between redox homeostasis and inflammasome regulation has been elucidated in experimental models using hexavalent chromium (Cr(VI)). At a concentration of 10 μM, Cr(VI) causes significant mitochondrial dysfunction in intestinal epithelial cells, demonstrated by a 40% decrease in mitochondrial membrane potential (JC-1 fluorescence ratio decreasing from 1.0 to 0.6). This dysfunction is associated with weakened antioxidant defenses, highlighted by a 50% reduction in superoxide dismutase activity and a 200% increase in malondialdehyde levels (p < 0.01) (55, 56). This oxidative milieu markedly enhances inflammasome component expression, as shown by immunoblot analyses indicating a 3.2-fold rise in NLRP3 protein and a 2.8-fold increase in ASC expression, leading to a 400% surge in IL-1β secretion. The NF-κB antagonist BAY11-7082 (5 μM) partially mitigates these effects, decreasing ASC expression by approximately 45% (p < 0.05), underscoring an oxidative stress-inflammatory signaling feedback loop (57, 58).
2.3.3 Emerging paradigms for precision-targeted intervention
Recent studies have introduced new methods to regulate inflammasome activity for potential therapeutic purposes. One such approach involves metabolic modulation using sodium propionate at a concentration of 10 mM, which has shown significant anti-inflammatory effects by reducing renal IL-1β levels by approximately 60%. This SCFA boosts the activity of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, leading to an 80% increase in nuclear translocation efficiency and an improvement in the GSH/GSSG ratio from 0.5 to 1.2, indicating restoration of redox homeostasis (58, 59). Another strategy involves disrupting the recognition of selective danger signals using dihydroartemisinin at a dose of 50 mg/kg, which effectively hinders the interactions between high-mobility group box 1 (HMGB1) and the TLR4 receptor with a dissociation constant (Kd) of 3.2 nM. In models of spinal cord injury, this intervention has been shown to decrease tissue IL-1β levels by 45% (p < 0.01) and enhance neurological function, as evidenced by a 38% increase in Basso, Beattie, and Bresnahan locomotor scores compared to control groups (60–62). These findings lay the groundwork for the development of targeted therapeutic approaches against inflammasome-mediated inflammatory conditions, thereby presenting promising avenues for future clinical application.
2.4 Pathological networks and targeted reversal of metabolic endotoxemia
2.4.1 Mechanisms of multi-organ damage
Metabolic endotoxemia triggers a complex pathophysiological cascade that results in multi-organ dysfunction through specific cellular and molecular pathways. A key aspect of this process is the metabolic alteration of macrophages via the CD14-TLR4 signaling pathway. Binding of lipopolysaccharide (LPS) to the CD14/TLR4 receptor complex induces significant changes in cellular bioenergetics and inflammatory reactions (63). LPS-induced TLR4 activation boosts glycolytic metabolism by doubling the expression of hexokinase 2 (HK2) (p < 0.01), which is associated with the polarization of pro-inflammatory M1 macrophages. Flow cytometry revealed an increase in the population of inducible nitric oxide synthase-positive (iNOS+) cells from approximately 15% to 65% after endotoxin exposure (p < 0.001) (64, 65). Concurrently, the CD14-TLR4 signaling pathway disrupts mitochondrial function by inhibiting peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α), leading to a 60% decrease in mitochondrial DNA copy number (p < 0.01) and a 50% reduction in cellular ATP production (p < 0.05). This dysfunction results in the accumulation of reactive oxygen species, with mitochondrial superoxide levels increasing to relative light units per milligram of protein, compared to 623 RLU/mg in control samples (p < 0.001) (66, 67). The interplay between heightened glycolysis and impaired mitochondrial oxidative phosphorylation establishes a “mitochondrial-glycolysis metabolic gap” that sustains oxidative stress and pro-inflammatory signaling, contributing to energy metabolism disturbances in various organ systems (68). Molecular targeting and organ regulation in DN are shown in Figure 3.
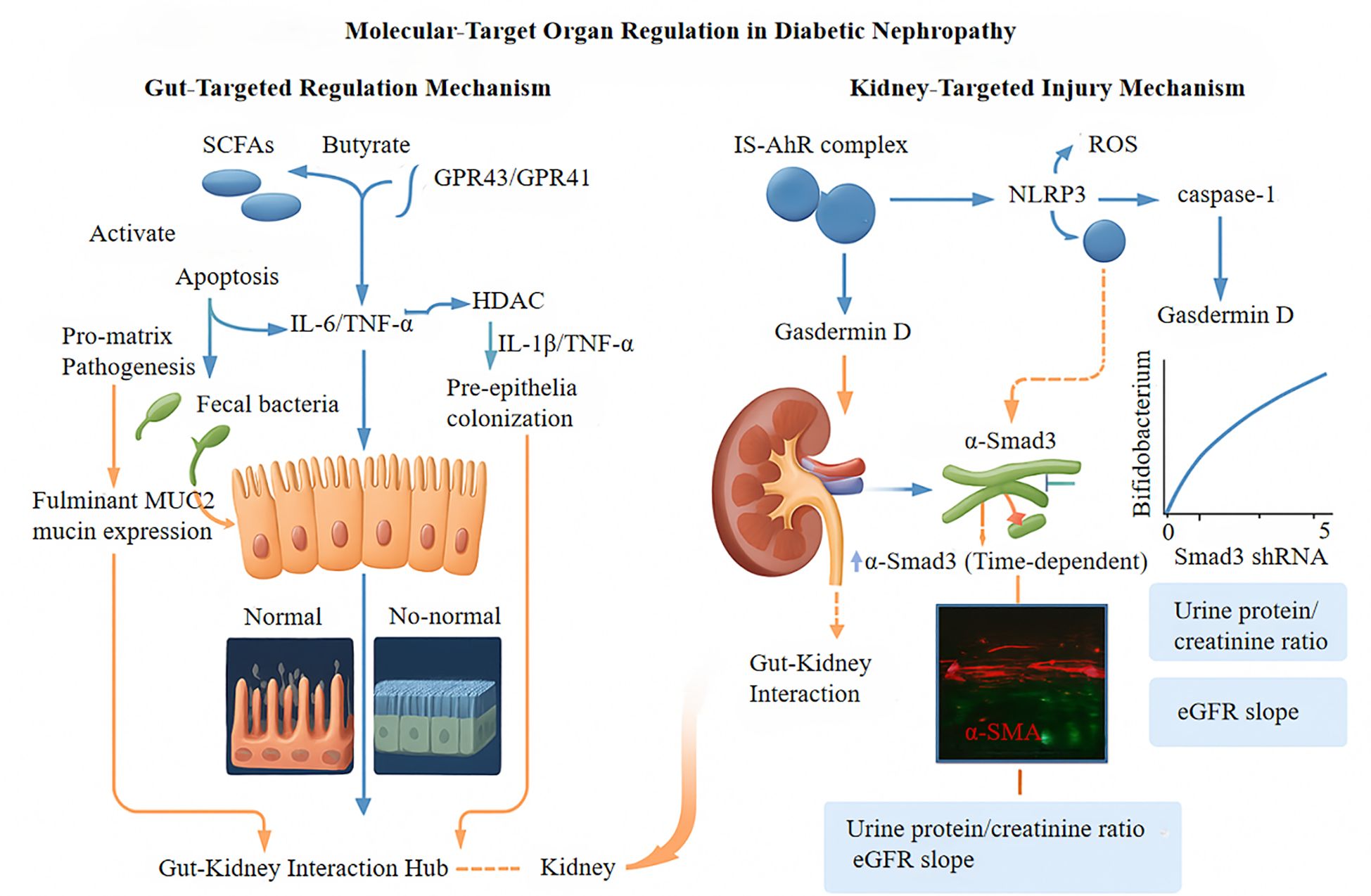
Figure 3. GPR43/GPR41, G Protein-Coupled Receptor 43/41; HDAC, Histone deacetylase; IL-6, Interleukin-6; TNF-α, Tumor Necrosis Factor-alpha; NLRP3, NLR Family Pyrin Domain Containing 3; IS, Indoxyl sulfate; AhR, Aryl hydrocarbon receptor; MUC2, Recombinant Mucin 2; Smad3 shRNA, Gene silencing tool; α-SMA, α-Smooth Muscle Actin; eGFR, Estimated Glomerular Filtration Rate.
2.4.2 Translational medicine applications
Clinical studies have confirmed the significance of metabolic endotoxemia in human pathophysiology. Research on patients with DN has shown a strong association between serum LPS levels and the urinary protein-to-creatinine ratio. Furthermore, examinations of renal tissue samples from patients compared to healthy individuals have revealed a three-fold increase in the density of TLR4-positive macrophage infiltration compared to healthy individuals (p < 0.001) (69–71). These clinical findings have led to the development of novel, targeted therapeutic strategies.
Luteolin, a flavonoid at a concentration of 20 μM, has demonstrated efficacy in rebalancing macrophage polarization, shifting the M1:M2 ratio from 4:1 to 1:2 (p < 0.01), primarily through activation of the peroxisome proliferator-activated receptor-gamma (PPARγ) pathway (72, 73). Luteolin also significantly improves mitochondrial function, as indicated by the restoration of mitochondrial membrane potential and the recovery of the JC-1 red/green fluorescence ratio from 0.3 to 1.8 (p < 0.001). This protective effect is accompanied by a 72% decrease in NF-κB p65 subunit phosphorylation (p < 0.01), suggesting inhibition of the TLR4/NF-κB inflammatory signaling pathway (74). Furthermore, luteolin enhances the stability of the mitochondrial network by upregulating optic atrophy protein 1 (OPA1) by 1.7-fold (p < 0.05), thereby improving mitochondrial dynamics and resilience against stress-induced fragmentation (75).
These results establish a mechanistic connection between metabolic reprogramming and organ dysfunction in metabolic endotoxemia, highlighting the PPARγ-HK2-PGC-1α signaling network as a crucial regulatory hub with significant therapeutic implications. This molecular pathway is a promising target for the development of comprehensive interventions that address both the inflammatory and metabolic abnormalities associated with endotoxemia (76, 77).
2.5 Bidirectional interaction and pathological mechanisms of the gut–kidney axis
The intricate interplay between renal function and intestinal microbiota involves metabolic, immune, and neuroendocrine signaling pathways, which play crucial roles in renal pathophysiology, especially in CKD.
2.5.1 Pathophysiological framework
A decline in renal function leads to changes in the gut microenvironment due to the buildup of nitrogenous waste products. This altered environment exerts selective pressure, reshaping the microbial community by reducing beneficial commensal bacteria such as Bifidobacterium and Lactobacillus and promoting opportunistic pathogens, especially within the phylum Proteobacteria (78, 79). Consequently, dysbiosis occurs, characterized by an overabundance of gram negative bacteria with increased endotoxin production capacity (80). This disruption is particularly notable in patients with DN. Metagenomic studies have indicated a 30% decrease in alpha diversity indices in patients with DN compared to healthy individuals, with a significant positive association between microbial diversity measures and estimated glomerular filtration rate (eGFR) (7, 81).
2.5.2 Molecular mechanisms of key pathological loops
IS, a protein-bound uremic toxin produced from microbial tryptophan metabolism, hinders enterocyte proliferation by activating the AhR and inhibiting the Wnt/β-catenin signaling pathway. Histological examination indicated a 52% decrease in Ki-67-positive cells in colonic crypts upon exposure to IS (82). Additionally, IS exposure reduces the levels of tight junction proteins (zonula occludens-1 and occludin), leading to a 3.8-fold increase in paracellular permeability (83). This compromised epithelial barrier facilitates the translocation of bacterial endotoxins into systemic circulation, establishing a cycle of “gut dysbiosis, barrier dysfunction, uremic toxin accumulation, and kidney deterioration.” Clinical investigations have revealed a significant association between serum IS levels and urinary albumin-to-creatinine ratios in patients with DN (81, 83).
A complex crosstalk involving Toll-like receptor 4 (TLR4), the NLRP3 inflammasome, and SCFAs underpins the molecular pathogenesis of DN. Ligand-dependent TLR4 activation (e.g., by advanced glycation end-products or LPS), initiates NF-κB-driven pro-inflammatory and fibrotic cascades and NLRP3 inflammasome priming. Oxidative stress-induced NLRP3 inflammasome activation (via TXNIP axis) and mitochondrial dysfunction critically mediates Caspase-1-dependent IL-1β maturation, exacerbating renal damage including mesangial injury, podocyte apoptosis, and tubulointerstitial fibrosis (84).Conversely, gut microbiota-derived SCFAs exert protective effects through multiple mechanisms: direct inhibition of TLR4 signaling (e.g., by attenuating MyD88/NF-κB phosphorylation); modulation of NLRP3 inflammasome activity, partly via GPR43 activation or HDAC inhibition, thereby disrupting essential pathways (e.g., mitochondrial ROS-TXNIP axis) (85). SCFAs also enhance intestinal barrier integrity to reduce systemic endotoxin exposure and thus mitigate TLR4/NLRP3 activation. The observed synergy between SCFAs and NLRP3 inhibitors (e.g., MCC950), potentially involving epigenetic modulation, highlights a novel therapeutic angle. This intricate regulatory network offers a compelling rationale for microbiota-targeted strategies (e.g., probiotic-induced SCFA enhancement) to concurrently dampen TLR4-initiated inflammation and NLRP3-amplified responses, ultimately ameliorating fibrotic remodeling in DN.
2.5.3 Novel interventional strategies and metabolic regulation
Fecal microbiota transplantation (FMT) holds promise for the disruption of pathological circuits. In rodent models of DN, FMT increases the Shannon diversity index of the intestinal microbiota by approximately 40%, leading to significant enrichment of SCFA-producing bacterial genera such as Roseburia and Faecalibacterium. These alterations are associated with notable enhancements in renal function parameters, including reductions in serum creatinine (25%) and blood urea nitrogen (18%) (86, 87). Gut dysbiosis notably hinders the conversion of primary bile acids to secondary bile acids, resulting in inadequate activation of the farnesoid X receptor (FXR). This disruption affects the FXR-FGF15/19 signaling axis, which governs hepatic glucose and lipid metabolism. Insufficient FXR levels are linked to a 37% decrease in hepatic glycogen content and a 2.1-fold increase in triglyceride accumulation, exacerbating systemic metabolic dysfunction (88, 89). Furthermore, altered microbial metabolites influence renal redox homeostasis by modulating the Keap1/Nrf2/ARE signaling pathway. This leads to reduced expression of crucial antioxidant enzymes, with a 45% decline in renal superoxide dismutase (SOD) activity and a 2.3-fold increase in malondialdehyde concentration (90). This oxidative environment promotes NLRP3 inflammasome activation (a 1.8-fold increase in caspase-1 activity), establishing a self-perpetuating cycle of oxidative stress and inflammation (91).
Future therapeutic strategies should encompass multifaceted approaches targeting the AhR-Wnt signaling axis, refining the FMT donor selection criteria, and exploring combination therapies. Preclinical studies indicate that the concurrent administration of Clostridium butyricum and sulforaphane enhances Nrf2 nuclear translocation while decreasing serum IS concentrations (90, 91), offering a more comprehensive therapeutic efficacy for renal disease.
3 Targeted interventions for DN via gut microbiota
3.1 Mechanisms and applications of probiotic therapy
3.1.1 Immunological homeostasis remodeling
Administration of probiotics has significant immunomodulatory effects and plays a crucial role in renoprotection against DN. A key aspect of these immunological effects is the restoration of T-cell homeostasis, particularly by modulating the regulatory T-cell (Treg) cell/T helper 17 cell (Th17) axis. Specific probiotic strains, such as Lactobacillus LA, reduce Th17-mediated renal inflammatory responses through the intricate regulation of cytokine networks (92). In-depth immunological analyses indicate that probiotic interventions notably decrease the secretion of pro-inflammatory cytokines like interleukin-6 (IL-6), IL-17, and tumor necrosis factor-alpha (TNF-α), while simultaneously increasing anti-inflammatory mediators such as IL-10 and interferon-gamma (IFN-γ). This immunomodulatory impact primarily occurs by inhibiting the Toll-like receptor 4/Nuclear factor-kappa B (TLR4/NF-κB) signaling pathway (93, 94).
Additionally, probiotic supplementation reduces circulating levels of IL-1β, IL-6, and TNF-α while enhancing the IL-10/IL-6 ratio. This collective effect helps correct the Th1/Th2 immune imbalance that is characteristic of DN (95, 96). The activation of peroxisome proliferator-activated receptor-gamma (PPAR-γ) is mechanistically implicated in mediating immunomodulatory effects. PPAR-γ agonists enhance Treg differentiation and function, leading to improved insulin sensitivity by suppressing the pro-inflammatory TNF-α/IL-1β/IL-6 signaling pathway (97). A pivotal study in the Journal of Clinical Investigation revealed that probiotics activate the PPAR-γ/CD36 pathway while inhibiting the TLR4/MyD88 pathway, resulting in decreased NF-κB phosphorylation in renal tissues (98, 99). This dual mechanism underlies the immunoregulatory effects of probiotic therapies in DN.
3.1.2 Metabolic reprogramming
SCFAs, particularly butyrate, play a crucial role as metabolic mediators of the beneficial effects of probiotics in DN. These metabolites, originating from microbes, activate G protein-coupled receptors 43 (GPR43) and 41 (GPR41) on intestinal epithelial cells, leading to enhanced PPAR-γ signaling (100). This signaling pathway contributes to enhanced insulin sensitivity and mitochondrial function, which are commonly impaired in DN.
Butyrate effectively reverses the disruption of the intestinal barrier induced by TNF-α/IFN-γ. Functional evaluations have demonstrated the restoration of transepithelial electrical resistance and the upregulation of tight junction proteins such as occludin and zonula occludens-1 (ZO-1) following butyrate treatment (101, 102).
Furthermore, butyrate inhibits HDAC activity, promoting the expression of genes crucial for mitochondrial biogenesis, including peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α) and nuclear respiratory factor 1 (NRF1). This epigenetic modulation enhances energy metabolism in renal tubular epithelial cells (103, 104). Moreover, probiotic interventions lead to a significant increase in circulating SCFA concentrations, particularly those of propionate and butyrate, which enhance insulin signaling by promoting the phosphorylation of insulin receptor substrate-1/phosphoinositide 3-kinase/protein kinase B (IRS-1/PI3K/Akt) through a GPR43-dependent mechanism (96, 105). Metabolic reprogramming is a key mechanism through which probiotic therapy mitigates the progression of DN.
3.1.3 Preservation of the gut–kidney axis
Probiotics maintain intestinal barrier integrity through multiple complementary mechanisms, collectively contributing to the preservation of the gut–kidney axis in DN. Probiotic administration upregulates gene expression and protein abundance of critical tight junction components, including occludin, ZO-1, and claudin-1, directly enhancing intestinal barrier function (92, 103, 106). Furthermore, probiotics repair the intestinal epithelial barrier by modulating the microRNA-29a/Claudin-1 regulatory axis, significantly reducing the translocation of gut-derived uremic toxins, such as IS, into the systemic circulation (104, 107). Probiotic interventions attenuate the release of metabolic endotoxins, including lipopolysaccharide-binding protein (LBP), by downregulating the TLR4/TIR-domain-containing adapter-inducing interferon-β (TRIF) signaling pathway (107, 108). This immunomodulation effectively suppresses the renal expression of IL-17A and monocyte chemoattractant protein-1 (MCP-1), decelerating glomerulosclerosis progression in experimental DN models, as reported by Kidney International (109, 110). Probiotics also counteract the inflammation-induced increases in intestinal epithelial permeability by modulating cytoskeletal regulatory elements, specifically the myosin light chain kinase-myosin light chain (MLCK-MLC) pathway (107, 111). Recent animal studies have demonstrated that probiotic administration inhibits MLCK/phosphorylated-MLC (p-MLC) signaling, thereby reducing intestinal permeability and circulating LBP concentrations. These effects translate to a significant mitigation of renal oxidative stress markers, including reduced malondialdehyde (MDA) levels and improved superoxide dismutase (SOD) activity (103, 112). Furthermore, combined administration of probiotics and dietary fiber enhances the abundance of butyrate-producing bacterial species, particularly Faecalibacterium prausnitzii. Through the activation of the butyrate-GPR109A signaling axis, this combination therapy effectively inhibits NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activation and reduces renal IL-18 secretion, providing additional renoprotection in DN (107, 113).
3.2 Precision nutritional interventions
Recent studies have revealed the multifaceted mechanisms by which dietary interventions modulate the gut–kidney axis in the management of DN. Resistant starch (RS3), particularly the lotus-derived variant, significantly enriches butyrate-producing bacteria (Faecalibacterium +318%, Roseburia +205%), activating the AMPK/Sirt1/PGC-1α signaling cascade. This activation ameliorates the imbalance in mitochondrial dynamics by modulating the expression of MFN2 and DRP1, ultimately improving renal tubular ATP production (114). Butyrate exerts metabolic benefits through dual epigenetic mechanisms: enhancing renal NAD+/NADH ratios with subsequent class II HDAC suppression and activating GPR43 to promote mitochondrial autophagy (115–117). Complementary fiber components have distinct renoprotective pathways. β-Glucan activates GLP-1 receptors, inducing renal vasodilation with reduced intraglomerular pressure, an effect amplified in FXR rs56163822 T allele carriers. Inulin inhibits the generation of secondary bile acids, particularly taurocholic acid, thereby attenuating TLR4/MyD88/NF-κB inflammatory signaling (114, 116, 118).
Protein metabolic remodeling also shows significant promise. Plant-based protein blends (soy:pea 3:1) reduce uremic toxin buildup by suppressing AhR receptors and enhancing UGT1A9 detoxification (119, 120). Very low-protein diets affect the tryptophan metabolic pathway and restore podocyte autophagy via mTORC1 inhibition. Restricting branched-chain amino acids boosts BCKDH complex activity and mitochondrial efficiency, reducing branched-chain α-keto acid accumulation and oxidative stress (121–124). Therefore, population-specific factors should be considered in clinical applications. Metabolomic models using urinary Neu5Ac and 2-HB effectively predicted low glycemic index intervention responsiveness (125, 126). Serum deoxycholic acid serves as a biomarker for dietary fiber resistance, and co-intervention with colesevelam improves the eGFR in affected patients (127, 128). Asian populations with Prevotella-rich microbiota show better plant protein utilization, but increased urinary oxalate excretion, requiring kidney stone risk monitoring. European groups with Bacteroide-dominant microbiota respond better to whey protein, which is influenced by LCT-13910 C/T polymorphism (120, 129–131).
Future research should focus on 1) integrating spatiotemporal multiomics to understand microbiome-metabolome-epigenome interactions, 2) analyzing gene-environment interactions using GWAS and CRISPR screening, and 3) validating the findings from organoid models to real-world evidence (119, 132). This framework combines mitochondrial dynamics, epigenetic regulation, and microbial metabolic networks into a comprehensive approach to manage DN, advancing precision nutritional medicine beyond current paradigms (115).
3.3 Advances in immune regulation through microbiota reconstruction therapy
3.3.1 FMT
Recent research has established critical parameters for optimizing donor selection during FMT. Comprehensive metagenomic analyses indicate that successful “super donors” must exhibit specific microbiological characteristics: a gut microbiota Shannon diversity index exceeding 3.5 (based on consensus gut microbiota diversity criteria where healthy adults demonstrate Shannon indices of 4.0-6.0, and certain studies report values above 3.5 as clinically normal in particular demographics) (32), reflecting robust ecological stability; butyrate synthesis-associated gene clusters comprising more than 0.1% of the total genetic material, supporting metabolic resilience; and a balanced Bacteroidetes-to-Firmicutes phylum ratio between 0.8–1.2, maintaining optimal energy metabolism (133, 134). Notably, FMT from metabolically healthy donors (homeostatic model assessment of insulin resistance, HOMA-IR <2.5) yields significantly greater improvements in recipient insulin sensitivity compared to conventional donors, as evidenced by differential Δ M-values (1.2 vs. 0.4 mg/kg/min). These findings suggest an intricate co-evolutionary relationship between host metabolic phenotypes and microbiota functionality (134, 135).
3.3.2 Molecular pathways of microbiota-organ interaction
In the context of nephroprotection, specific donor microbial signatures, particularly Akkermansia muciniphila abundance exceeding 4%, selectively attenuate the excessive activation of the renal TLR4/MyD88/NF-κB inflammatory cascade (P < 0.01). This protective effect is mediated through competitive inhibition of TLR4 receptors by the bacteria-derived Amuc_1100 protein (136, 137). Furthermore, microbiota reconstruction effectively restores indole-3-propionic acid (IPA) metabolic pathways, substantially enhancing podocyte autophagic activity, as demonstrated by a 2.4-fold increase in the LC3-II/LC3-I ratio. This process is fundamentally dependent on the activation of the AhR and subsequent upregulation of the autophagy-associated gene ATG5 (138, 139). Experimental studies in db/db mice show the remarkable efficacy of an 8-week FMT intervention, reducing the glomerulosclerosis index by approximately 57%. Mechanistic investigations have revealed that this therapeutic effect is closely correlated with altered tryptophan metabolism within the gut microbiota, characterized by a metabolic shift from the kynurenine pathway toward serotonin (5-HT) synthesis, resulting in a substantial 63% reduction in the kynurenine/5-HT ratio (139, 140).
3.3.3 Challenges in clinical translation and immune regulatory balance
An initial clinical trial targeting FMT-DN yielded promising results. Single colonoscopic FMT reduced 24-hour urinary protein excretion by 31%, with persistent effects for up to 12 weeks. The increased abundance of the donor-derived bacterium Dialister invisus was negatively correlated with recipient serum levels of soluble urokinase-type plasminogen activator receptor (suPAR) (137, 140). However, transient elevation of IL-17A levels in 12% of the participants highlighted the need for a comprehensive assessment of the Th17/Treg balance to monitor immune status (141, 142). Probiotics and FMT entail inherent risks including potential pathogen transmission via donor microbiota transfer and unintended modulation of host immune response, necessitating donor screening and post-treatment immune surveillance (143). Microbiota modulation may raise ethical controversies surrounding the concept of ‘microbial identity.’ The enduring stability of transplanted microbiome and lasting impacts on host-microbe symbiotic relationship remain to be fully elucidated. Within the United States, FMT is regulated as a drug by the Food and Drug Administration (FDA). Although two FMT products, Vowst and Rebyota, have received FDA approval, their indications are currently confined to recurrent Clostridioides difficile infection (rCDI), such as cases of antibiotic-unresponsive colitis and diarrhea, with no provision for use in DN. These limitations partly stem from ethical concerns about long-term effects of microbiota-directed therapies. For example, DN patients often present with comorbid metabolic derangements and immune imbalances. Although FMT holds the potential to indirectly alter disease progression via modifications to the gut-kidney axis, the enduring metabolic and immune sequelae are not yet fully elucidated. To date, no evidence in the literature suggests the FDA has formulated a distinct regulatory pathway for FMT as a treatment for DN. However, significant technical challenges remain in optimizing the viability of therapeutic bacterial communities. The recovery rate of viable bacteria in lyophilized microbiota formulations is currently limited to 45–60%. Recent advances in microencapsulation coating technologies have demonstrated the potential to substantially improve bacterial survival rates by approximately 82% (144, 145). These technological developments provide a robust foundation for the precise application of FMT in the management of immunometabolic disorders.
4 Other intervention strategies
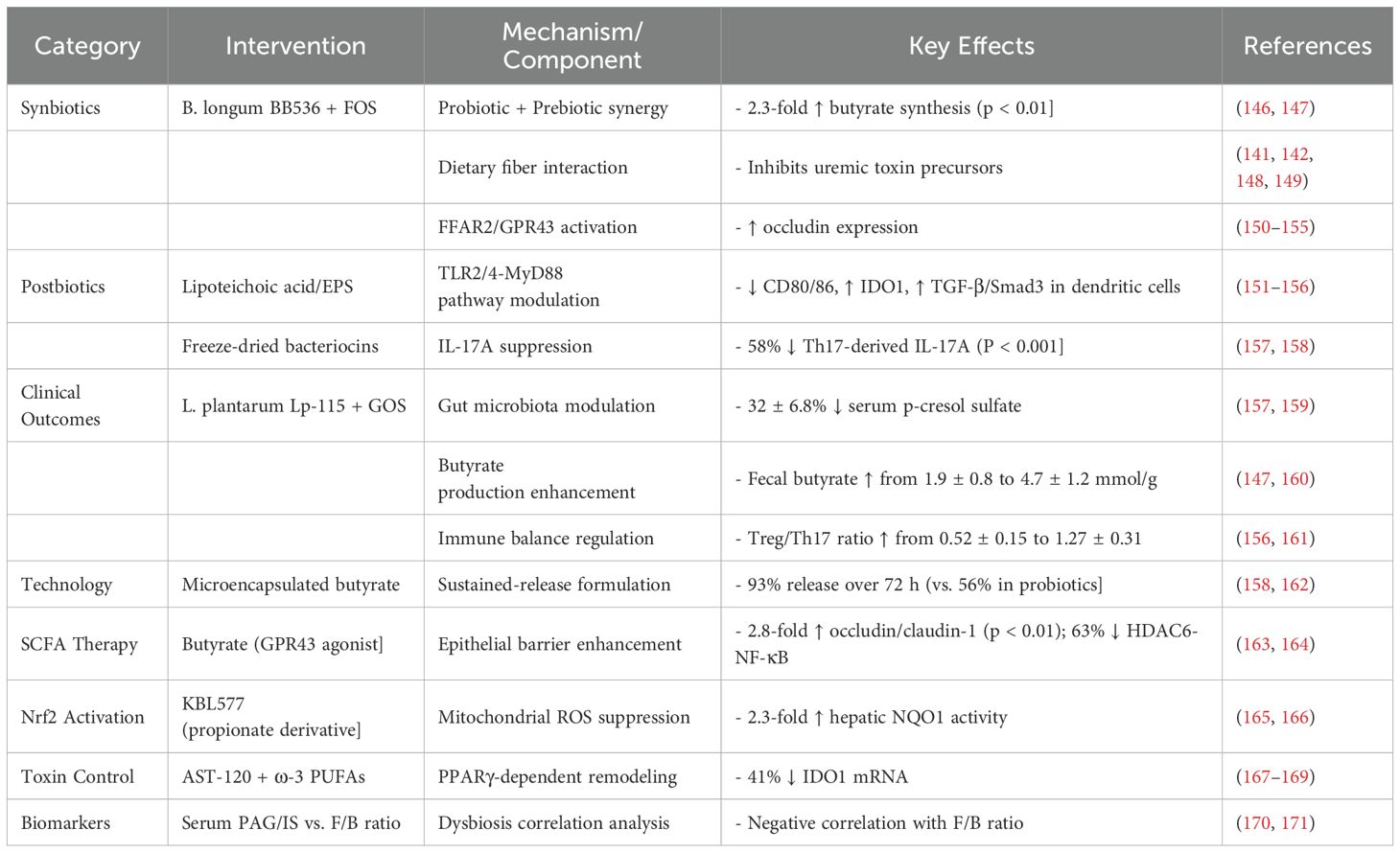
In CKD, synbiotics and postbiotics modulate the gut-kidney axis, evidenced by: 2.3-fold increase in butyrate synthesis; improved Treg/Th17 ratio (from 0.52 to 1.27); and activated of PPARγ/Nrf2 pathway. Microencapsulation significantly enhances sustained-release efficacy (e.g., 93% vs. 56% for unencapsulated forms). These interventions regulate gut microbiota, suppress uremic toxins, and reinforce intestinal barrier function, partly through microencapsulated therapies targeting SCFA receptors, which further contributes to improved butyrate synthesis and Treg/Th17 balance (Table 1).
Targeted bacteriophage therapy has emerged as a precise approach for modulating the microbiome through the selective lysis of urease-positive pathogens, such as Klebsiella pneumoniae. This strategy disrupts aromatic amino acid metabolic pathways while preserving the ecological stability of beneficial SCFA-producing microbiota such as Roseburia and Faecalibacterium, as evidenced by a 57% reduction in fecal p-cresyl sulfate without significant depletion of beneficial bacteria (172, 173). CRISPR-Cas9-engineered bacteriophages represent a significant technological advancement in microbiome manipulation. By delivering guide RNA targeting tnaA, these phages selectively disable tryptophanase-coding genes in urease-producing Escherichia coli, effectively blocking indole biosynthesis and downstream IS production at its metabolic source.
Animal studies confirm a 72% decrease in serum IS levels without any detectable off-target effects (174, 175). In contrast, conventional broad-spectrum antibiotics, which temporarily reduce uremic toxin levels, often cause the irreversible depletion of butyrate-producing bacteria, including Anaerostipes and Eubacterium hallii. This leads to intestinal barrier impairment and toxin level rebound effects, with post-intervention IS levels reaching 1.8-fold above baseline after 6 weeks (176, 177).
Sequential bacteriophage-FMT therapy was recently introduced for clinical translation (178). This dual-phase approach, which first eliminates pathogenic bacteria and then reintroduces functional microbiota, successfully reduces the estimated glomerular filtration rate (eGFR) decline by 34% in patients with stage 3 CKD (179, 180). The renoprotective effects significantly correlated with increased gut butyrate concentrations and reduced serum IL-6 levels. These innovations represent a novel paradigm for microbiome-immune axis interventions in the management of CKD progression (Figure 4).
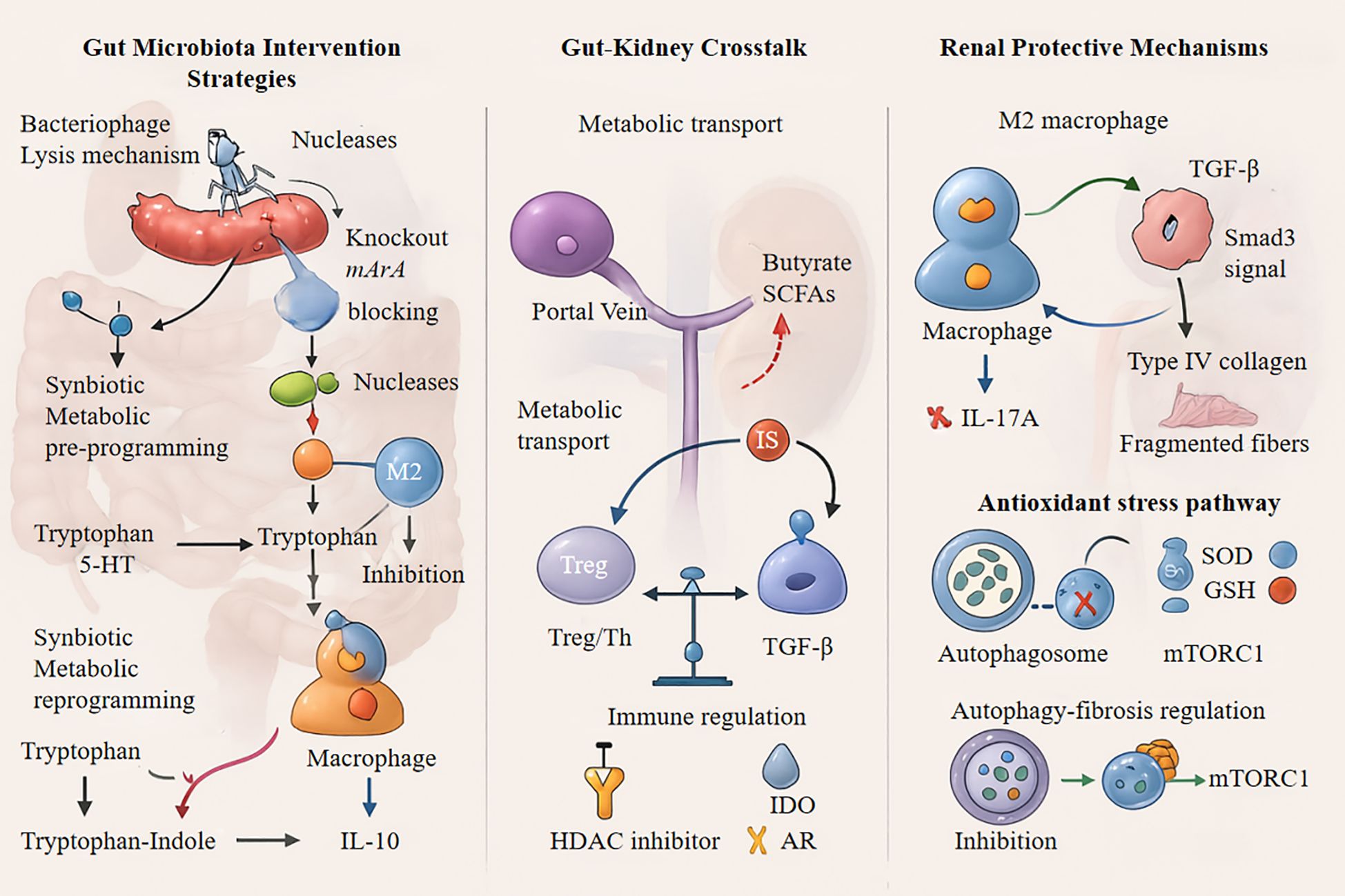
Figure 4. IL-10, Interleukin-10; SCFAs, Short-Chain Fatty Acids; TGF-β, Transforming Growth Factor-beta; HDAC, Histone Deacetylase; IDO, Indoleamine 2,3-Dioxygenase; IL-17A, Interleukin-17A; SOD, Superoxide Dismutase; GSH, Glutathione; MTORC1, Mechanistic Target of Rapamycin Complex 1, Treg, Regulatory T cells.
5 Challenges and future directions
5.1 Microbiota therapy challenges: from mechanisms to solutions
Despite promising progress, personalized gut microbiota therapies encounter significant challenges. A major hurdle is inter-individual variability in microbiota composition, which complicates the development of standardized treatment protocols. Approximately 40% of the variability in intervention efficacy is linked to host genotype, dietary habits, and baseline microbiota characteristics (Figure 3) (181, 182). Integrating host genome-metabolome-microbiome interaction networks using systems biology approaches significantly enhances the accuracy of efficacy prediction models. A deeper mechanistic understanding is required, especially concerning the molecular pathways through which microbiota-derived metabolites regulate immune cell differentiation.
SCFAs influence these processes via GPR43/GPR41, whereas microbiota-derived tryptophan metabolites modulate the Th17/Treg balance, necessitating further detailed studies (183, 184). Clinical translations face three critical challenges. First, evidence of its long-term safety is limited, with approximately 30% of FMT recipients experiencing immune-related adverse events (181, 185). Second, multiomics techniques require standardization, as combining metagenomics with single-cell metabolomics has uncovered previously undetected functional redundancy at the strain level (186, 187). Regulatory frameworks have not kept pace with technological advancements, which highlights the need for adaptable approval processes based on dynamic microbiota risk assessment (188).
Combined intervention strategies have a significant potential to address these challenges. For instance, a sequential treatment involving probiotics, a Mediterranean diet, and FMT activates the Akkermansia-mediated IL-22/REG3γ pathway, leading to substantial synergistic effects in promoting mucosal healing in models of inflammatory bowel disease (185, 189). These combined approaches offer promising prospects for clinical implementation, and there is a pressing need to develop a comprehensive three-dimensional intervention system that integrates strain tracking, dynamic immune monitoring, and predictive analytics driven by artificial intelligence (190).
5.2 Critical barriers to clinical application of FMT
Although FMT shows therapeutic potential for immune-related diseases, its clinical application faces several interconnected challenges. Technical delivery challenges represent the primary barrier. Administration routes significantly impact efficacy, as disease responsiveness varies between lower and upper gastrointestinal delivery approaches. Preparation variables (e.g., fresh vs. frozen samples) affect outcomes though microbial viability variations. Non-standardized manufacturing processes introduce safety concerns, exemplified by FDA warnings on multidrug-resistant organism transmission. The biological interaction between donor and recipient add complexity. Transplant success depends on microbiome compatibility, yet extreme inter-individual variability in immune status, genetics, and baseline microbiota hinders universal donor identification. Clinical evidence showed 30% post-FMT microbiota reversion to baseline suggests host ecosystem resilience may fundamentally limit treatment effectiveness (191). Long-term efficacy presents another significant challenge. Follow-up studies in metabolic disorders revealed that 60% of patients experience diminished metabolic benefits by 12 months post-treatment, with retreatment response rates below 50% of initial therapy (192). This decline may result from competitive exclusion or adaptation of transplanted microbiota in host environments. Standardization deficiencies compound these issues throughout therapeutic pipeline. Inconsistent donor screening protocols, operational variability in preservation media and administration protocols, and the absence of objective biomarker-driven efficacy criteria for complex conditions create significant heterogeneity in clinical practice and outcomes (193). Safety considerations demand careful attention. Although severe adverse events are rare, pathogen transmission risks (e.g., antimicrobial resistance genes, viruses, and hitherto unidentified pathogens) require long-term monitoring, particularly in immunosuppressed patients. Recent research further suggests that FMT may transiently perturb host metabolic or immune homeostasis, as evidenced by reports of extra-intestinal manifestations or autoimmune reactions. To advance FMT from experimental therapy to mainstream treatment requires developing both a comprehensive quality control framework and personalized prediction models based on microbial functional profiles rather than simply compositional data.
6 Conclusion
This review examined multiple intervention strategies targeting gut microbiota for the management of DN. Strain-specific probiotic modulation shows substantial potential, particularly when strain selection is guided by metabolic profiles and immune response patterns. Dietary interventions, including strategic fiber supplementation and protein restriction, effectively reshape the microbial composition and function, with evidence supporting their therapeutic efficacy by modulating SCFA and nitrogen metabolism pathways. FMT is the most comprehensive intervention that directly reprograms the gut ecosystem and has shown promising preliminary results in both experimental models and early clinical investigations.
These approaches achieve therapeutic effects through intersecting mechanisms such as restoration of the microbial ecological balance, normalization of microbiota-derived metabolite profiles, and amelioration of gut barrier dysfunction, which contributes to systemic inflammation. The clinical translation of gut microbiota-based therapies faces several fundamental challenges that warrant systematic investigation. First, the relationship between individualized microbiota profiles and treatment responses remains incompletely characterized, with considerable heterogeneity in baseline microbiota composition, confounding standardized approaches. Second, the molecular pathways underlying host-microbiota interactions with specific bacterial strains require further elucidation, particularly regarding the strain-specific effects on immune cell programming and metabolite production. Third, rigorous standardization of methodological approaches is necessary, including donor selection criteria for FMT, therapeutic endpoints, and long-term safety monitoring protocols to detect potential adverse immunological consequences.
Future research should focus on advancing the methodologies across multiple domains. Integrating multiomics technologies such as metagenomics, metabolomics, and single-cell sequencing with advanced organoid models presents unique opportunities to move from correlative associations to causal mechanistic insights. Rigorous multicenter randomized controlled trials with extended follow-up are crucial to validate the renoprotective effects of microbiota-targeted interventions and establish reliable biomarkers to predict treatment responses. These advancements collectively support the shift in diabetic kidney disease management from conventional empirical approaches to evidence-based, microbiome-informed precision medicine, which addresses the complex pathophysiology of the disease through personalized interventions. We urgently call for coordinated international efforts to: 1) Establish open-access biorepositories of multiomics data from diverse patient cohorts; 2) Prioritize organoid-microbiome co-culture systems for mechanistic discovery; 3) Implement adaptive trial designs incorporating real-time biomarker monitoring. These advancements collectively support the shift in diabetic kidney disease management from conventional empirical approaches to evidence-based, microbiome-informed precision medicine.
Author contributions
XW: Writing – original draft. XL: Writing – original draft. FG: Writing – review & editing. YJ: Writing – review & editing. CZ: Writing – review & editing. WeiZ: Writing – review & editing. WenZ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was sponsored by research grants from the Hangzhou Science and Technology Bureau (20241029Y164) and the Science and Technology Bureau of Lin’an District, Hangzhou (2022Y13).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. For the organ sections in the images generated for the article, we used both ChatGPT and Claude 3.7 Sonnet, developed by Anthropic.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nagase N, Ikeda Y, Tsuji A, Kitagishi Y, and Matsuda S. Efficacy of probiotics on the modulation of gut microbiota in the treatment of diabetic nephropathy. World J Diabetes. (2022) 13:150–60. doi: 10.4239/wjd.v13.i3.150
2. Fu S, Li F, Yu J, Ma S, Zhang L, and Cheng Y. Investigating the role of gut microbiota in diabetic nephropathy through plasma proteome mediated analysis. Sci Rep. (2025) 15:5457. doi: 10.1038/s41598-025-90306-7
3. Cheng G, Liu Y, Guo R, Wang H, Zhang W, and Wang Y. Molecular mechanisms of gut microbiota in diabetic nephropathy. Diabetes Res Clin Pract. (2024) 213:111726. doi: 10.1016/j.diabres
4. Cao Y, Lin JH, Hammes HP, and Zhang C. Cellular phenotypic transitions in diabetic nephropathy: An update. Front Pharmacol. (2022) 13:1038073. doi: 10.3389/fphar.2022.1038073
5. Hsieh CC, Lin CL, He JT, Chiang M, Wang Y, Tsai YC, et al. Administration of cytokine-induced myeloid-derived suppressor cells ameliorates renal fibrosis in diabetic mice. Stem Cell Res Ther. (2018) 9:183. doi: 10.1186/s13287-018-0915-0
6. Lu X, Ma J, and Li R. Alterations of gut microbiota in biopsy-proven diabetic nephropathy and a long history of diabetes without kidney damage. Sci Rep. (2023) 13:12150. doi: 10.1038/s41598-023-39444-4
7. Ni Y, Zheng L, Nan S, Ke L, Fu Z, and Jin J. Enterorenal crosstalks in diabetic nephropathy and novel therapeutics targeting the gut microbiota. Acta Biochim Biophys Sin (Shanghai). (2022) 54:1406–20. doi: 10.3724/abbs.2022140
8. Zhang Q, Zhang Y, Zeng L, Chen G, Zhang L, Liu M, et al. The role of gut microbiota and microbiota-related serum metabolites in the progression of diabetic kidney disease. Front Pharmacol. (2021) 12:757508. doi: 10.3389/fphar.2021.757508
9. Yan H, Zhang Y, Lin X, Huang J, Zhang F, Chen C, et al. Resveratrol improves diabetic kidney disease by modulating the gut microbiota-short chain fatty acids axis in db/db mice. Int J Food Sci Nutr. (2024) 75:264–76. doi: 10.1080/09637486.2024.2303041
10. Kim JE, Nam H, Park JI, Cho H, Lee J, Kim HE, et al. Gut microbial genes and metabolism for methionine and branched-chain amino acids in diabetic nephropathy. Microbiol Spectr. (2023) 11:e0234422. doi: 10.1128/spectrum.02344-22
11. Lu CC, Hu ZB, Wang R, Hong ZH, Lu J, Chen PP, et al. Gut microbiota dysbiosis-induced activation of the intrarenal renin-angiotensin system is involved in kidney injuries in rat diabetic nephropathy. Acta Pharmacol Sin. (2020) 41:1111–8. doi: 10.1038/s41401-019-0326-5
12. Wang S, Zhou Y, Zhang Y, He X, Zhao X, Zhao H, et al. Roscovitine attenuates renal interstitial fibrosis in diabetic mice through the TGF-β1/p38 MAPK pathway. BioMed Pharmacother. (2019) 115:108895. doi: 10.1016/j.biopha.2019.108895
13. Zhao H, Yang CE, Liu T, Zhang MX, Niu Y, Wang M, et al. The roles of gut microbiota and its metabolites in diabetic nephropathy. Front Microbiol. (2023) 14:1207132. doi: 10.3389/fmicb.2023.1207132
14. Fernandes R, Viana SD, Nunes S, and Reis F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim Biophys Acta Mol Basis Dis. (2019) 1865:1876–97. doi: 10.1016/j.bbadis.2018.09.032
15. Ghosh A, Muley A, Ainapure AS, Deshmane AR, and Mahajan A. Exploring the impact of optimized probiotic supplementation techniques on diabetic nephropathy: mechanisms and therapeutic potential. Cureus. (2024) 16:e55149. doi: 10.7759/cureus.55149
16. Pourheydar B, Samadi M, Habibi P, Nikibakhsh AA, and Naderi R. Renoprotective effects of tropisetron through regulation of the TGF-β1, p53 and matrix metalloproteinases in streptozotocin-induced diabetic rats. Chem Biol Interact. (2021) 335:109332. doi: 10.1016/j.cbi.2020.109332
17. Song X, Cui J, Li S, and Huang B. Causal relationships between gut microbiota, metabolites, and diabetic nephropathy: insights from a two-sample mendelian randomization analysis. Int J Nephrol Renovasc Dis. (2024) 17:319–32. doi: 10.2147/IJNRD.S489074
18. Tian E, Wang F, Zhao L, Sun Y, and Yang J. The pathogenic role of intestinal flora metabolites in diabetic nephropathy. Front Physiol. (2023) 14:1231621. doi: 10.3389/fphys.2023.1231621
19. Tian B, Huang P, Pan Y, Gu H, Yang K, Wei Z, et al. Tea polyphenols reduced obesity by modulating gut microbiota-SCFAs-barrier and inflammation in high-fat diet-induced mice. Mol Nutr Food Res. (2024) 68:e2400685. doi: 10.1002/mnfr.202400685
20. Dicks LMT. How important are fatty acids in human health and can they be used in treating diseases? Gut Microbes. (2024) 16:2420765. doi: 10.1080/19490976.2024.2420765
21. Du L, Chen C, Yang YH, Zheng Y, Li H, Wu ZJ, et al. Fucoxanthin alleviates lipopolysaccharide-induced intestinal barrier injury in mice. Food Funct. (2024) 15:6359–73. doi: 10.1039/d4fo00611a
22. Yang C, Chen J, Zhou H, Zeng D, Wan H, and Yang J. Therapeutic effect of Yinhuapinggan granules mediated through the intestinal flora in mice infected with the H1N1 influenza virus. Front Microbiol. (2024) 15:1394304. doi: 10.3389/fmicb.2024.1394304
23. Wu Y, Zhang X, Han D, Pi Y, Tao S, Zhang S, et al. Early life administration of milk fat globule membrane promoted SCFA-producing bacteria colonization, intestinal barriers and growth performance of neonatal piglets. Anim Nutr. (2021) 7:346–55. doi: 10.1016/j.aninu.2020.07.012
24. Lee TH, Park D, Kim YJ, Lee I, Kim S, Oh CT, et al. Lactobacillus salivarius BP121 prevents cisplatin-induced acute kidney injury by inhibition of uremic toxins such as indoxyl sulfate and p-cresol sulfate via alleviating dysbiosis. Int J Mol Med. (2020) 45:1130–40. doi: 10.3892/ijmm.2020.4495
25. Guo C, Li Q, Chen R, Fan W, Zhang X, Zhang Y, et al. Baicalein alleviates non-alcoholic fatty liver disease in mice by ameliorating intestinal barrier dysfunction. Food Funct. (2023) 14:2138–48. doi: 10.1039/d2fo03015b
26. Mi W, Hu Z, Xu L, Bian X, Lian W, Yin S, et al. Quercetin positively affects gene expression profiles and metabolic pathway of antibiotic-treated mouse gut microbiota. Front Microbiol. (2022) 13:983358. doi: 10.3389/fmicb.2022.983358
27. Rehman AU, Siddiqui NZ, Farooqui NA, Alam G, Gul A, Ahmad B, et al. Morchella esculenta mushroom polysaccharide attenuates diabetes and modulates intestinal permeability and gut microbiota in a type 2 diabetic mice model. Front Nutr. (2022) 9:984695. doi: 10.3389/fnut.2022.984695
28. Wang P, Wang T, Zheng X, Cui W, Shang J, and Zhao Z. Gut microbiota, key to unlocking the door of diabetic kidney disease. Nephrol (Carlton). (2021) 26:641–9. doi: 10.1111/nep.13874
29. Fan Z, Gao Y, Jiang N, Zhang F, Liu S, and Li Q. Immune-related SERPINA3 as a biomarker involved in diabetic nephropathy renal tubular injury. Front Immunol. (2022) 13:979995. doi: 10.3389/fimmu.2022.979995
30. Yang L, Xu L, Hao X, Song Z, Zhang X, Liu P, et al. An aldose reductase inhibitor, WJ-39, ameliorates renal tubular injury in diabetic nephropathy by activating PINK1/Parkin signaling. Eur J Pharmacol. (2024) 967:176376. doi: 10.1016/j.ejphar.2024.176376
31. Mao ZH, Gao ZX, Liu DW, Liu ZS, and Wu P. Gut microbiota and its metabolites - molecular mechanisms and management strategies in diabetic kidney disease. Front Immunol. (2023) 14:1124704. doi: 10.3389/fimmu.2023.1124704
32. Cai TT, Ye XL, Li RR, Chen H, Wang YY, Yong HJ, et al. Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice. Front Pharmacol. (2020) 11:1249. doi: 10.3389/fphar.2020.01249
33. Ni Y, Du H, Ke L, Zheng L, Nan S, Ni L, et al. Gut-kidney interaction reinforces dapagliflozin-mediated alleviation in diabetic nephropathy. Am J Physiol Cell Physiol. (2025) 328:C452–66. doi: 10.1152/ajpcell.00651.2024
34. Ciesielska A, Matyjek M, and Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. (2021) 78:1233–61. doi: 10.1007/s00018-020-03656-y
35. Shin S, Park J, Lee YE, Ko H, and Youn HS. Isobavachalcone suppresses the TRIF-dependent signaling pathway of Toll-like receptors. Arch Pharm (Weinheim). (2022) 355:e2100404. doi: 10.1002/ardp.202100404
36. Chen Z, Chen X, Zou Y, Zhou Y, Du J, Qin Y, et al. The immune function of TLR4–1 gene in Octopus sinensis revealed by RNAi and RNA-seq. Fish Shellfish Immunol. (2024) 154:109899. doi: 10.1016/j.fsi.2024.109899
37. Liu X, Wang Y, Wen X, Hao C, Ma J, and Yan L. Platelet rich plasma alleviates endometritis induced by lipopolysaccharide in mice via inhibiting TLR4/NF-κB signaling pathway. Am J Reprod Immunol. (2024) 91:e13833. doi: 10.1111/aji.13833
38. Zsengellér ZK and Gerard NP. The oxidation state of cysteine thiols on the ectodomain of TLR2 and TLR4 influences intracellular signaling. Immunobiology. (2020) 225:151895. doi: 10.1016/j
39. Li T, Zeng W, and Liu R. Effect of erdosteine on middle ear effusion in rats by mediating TLR4 signaling pathway. BioMed Res Int. (2021) 2021:9968907. doi: 10.1155/2021/9968907
40. Li S, Xie F, Shi K, Wang J, Cao Y, and Li Y. Gossypol ameliorates the IL-1β-induced apoptosis and inflammation in chondrocytes by suppressing the activation of TLR4/MyD88/NF-κB pathway via downregulating CX43. Tissue Cell. (2021) 73:101621. doi: 10.1016/j.tice.2021.101621
41. Ignacio BJ, Albin TJ, Esser-Kahn AP, and Verdoes M. Toll-like receptor agonist conjugation: A chemical perspective. Bioconjug Chem. (2018) 29:587–603. doi: 10.1021/acs.bioconjchem
42. Chen G, Wang X, Liu C, Zhang M, Han X, and Xu Y. The interaction of MD-2 with small molecules in huanglian jiedu decoction play a critical role in the treatment of sepsis. Front Pharmacol. (2022) 13:947095. doi: 10.3389/fphar.2022.947095
43. Wang K, Lei Q, Ma H, Jiang M, Yang T, Ma Q, et al. Phloretin protects bovine rumen epithelial cells from LPS-induced injury. Toxins (Basel). (2022) 14:337. doi: 10.3390/toxins14050337
44. Zhang FX and Xu RS. Juglanin ameliorates LPS-induced neuroinflammation in animal models of Parkinson's disease and cell culture via inactivating TLR4/NF-κB pathway. BioMed Pharmacother. (2018) 97:1011–9. doi: 10.1016/j.biopha.2017.08.132
45. Wu G, Yang Q, Yu Y, Lin S, Feng Y, Lv Q, et al. Taurine inhibits kupffer cells activation induced by lipopolysaccharide in alcoholic liver damaged rats. Adv Exp Med Biol. (2017) 975 Pt 2:789–800. doi: 10.1007/978-94-024-1079-2_61
46. Vila-Casahonda RG, Lozano-Aponte J, and Guerrero-Beltrán CE. HSP60-derived peptide as an LPS/TLR4 modulator: an in silico approach. Front Cardiovasc Med. (2022) 9:731376. doi: 10.3389/fcvm.2022.731376
47. Ota Y, Inagaki R, Takanashi Y, Uemachi H, Matsuda K, Matsuoka M, et al. Targeting tumor-associated macrophages with the immune-activating nanomedicine for achieving strong antitumor activity with rapid clearance from the body. ACS Nano. (2024) 18:23757–72. doi: 10.1021/acsnano.4c08811
48. Xu Y, Huang Y, and Cai S. Characterization and function analysis of interleukin-1 receptor-associated kinase-1 (IRAK-1) from Fenneropenaeus penicillatus. Fish Shellfish Immunol. (2017) 61:111–9. doi: 10.1016/j.fsi.2016.12.030
49. Tsai CM, Riestra AM, Ali SR, Fong JJ, Liu JZ, Hughes G, et al. Siglec-14 enhances NLRP3-inflammasome activation in macrophages. J Innate Immun. (2020) 12:333–43. doi: 10.1159/000504323
50. Dominic A, Le NT, and Takahashi M. Loop between NLRP3 inflammasome and reactive oxygen species. Antioxid Redox Signal. (2022) 36:784–96. doi: 10.1089/ars.2020.8257
51. Huang Z, Zhuang X, Xie C, Hu X, Dong X, Guo Y, et al. Exogenous hydrogen sulfide attenuates high glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome activation by suppressing TLR4/NF-κB pathway in H9c2 cells. Cell Physiol Biochem. (2016) 40:1578–90. doi: 10.1159/000453208
52. Luo ZL, Ren JD, Huang Z, Wang T, Xiang K, Cheng L, et al. The role of exogenous hydrogen sulfide in free fatty acids induced inflammation in macrophages. Cell Physiol Biochem. (2017) 42:1635–44. doi: 10.1159/000479405
53. Zhang L, Liu ZX, Liu YH, Chen Y, Chen J, and Lu CH. Auricularia auriculaPolysaccharides exert anti-inflammatory effects in hepatic fibrosis by the gut-liver axis and enhancing SCFA metabolism. J Agric Food Chem. (2025) 73:4617–29. doi: 10.1021/acs.jafc.4c07952
54. Krantz M, Eklund D, Särndahl E, Hedbrant A, and detailed molecular network map A. and model of the NLRP3 inflammasome. Front Immunol. (2023) 14:1233680. doi: 10.3389/fimmu.2023.1233680
55. Wang J, Wang L, Zhou J, Qin A, and Chen Z. The protective effect of formononetin on cognitive impairment in streptozotocin (STZ)-induced diabetic mice. BioMed Pharmacother. (2018) 106:1250–7. doi: 10.1016/j.biopha.2018.07.063
56. Xing C, Yang F, Lin Y, Shan J, Yi X, Ali F, et al. Hexavalent chromium exposure induces intestinal barrier damage via activation of the NF-κB signaling pathway and NLRP3 inflammasome in ducks. Front Immunol. (2022) 13:952639. doi: 10.3389/fimmu.2022.952639
57. Han C, Guan L, and Xu L. Protective effect of luteoloside against Toxoplasma gondii-induced liver injury through inhibiting TLR4/NF-κB and P2X7R/NLRP3 and enhancing Nrf2/HO-1 signaling pathway. Parasitol Res. (2023) 122:1333–42. doi: 10.1007/s00436-023-07833-3
58. Piippo N, Korhonen E, Hytti M, Kinnunen K, Kaarniranta K, and Kauppinen A. Oxidative stress is the principal contributor to inflammasome activation in retinal pigment epithelium cells with defunct proteasomes and autophagy. Cell Physiol Biochem. (2018) 49:359–67. doi: 10.1159/000492886
59. Liu J, Li X, Ding L, Li W, Niu X, and Gao D. GRK2 participation in cardiac hypertrophy induced by isoproterenol through the regulation of Nrf2 signaling and the promotion of NLRP3 inflammasome and oxidative stress. Int Immunopharmacol. (2023) 117:109957. doi: 10.1016/j.intimp.2023
60. Jiao B, Guo S, Yang X, Sun L, Sai L, Yu G, et al. The role of HMGB1 on TDI-induced NLPR3 inflammasome activation via ROS/NF-κB pathway in HBE cells. Int Immunopharmacol. (2021) 98:107859. doi: 10.1016/j.intimp.2021.107859
61. Li J, He J, He H, Wang X, Zhang S, He Y, et al. Sweet triterpenoid glycoside from Cyclocarya paliurus ameliorates obesity-induced insulin resistance through inhibiting the TLR4/NF-κB/NLRP3 inflammatory pathway. Curr Res Food Sci. (2024) 8:100677. doi: 10.1016/j.crfs.2024.100677
62. Balaha MF, Ahmed NJ, Almalki ZS, Alahmari AK, Alshehri AM, Soliman GA, et al. Epimedin A ameliorates DNFB-induced allergic contact dermatitis in mice: Role of NF-κB/NLRP3-driven pyroptosis, Nrf2/HO-1 pathway, and inflammation modulation. Life Sci. (2022) 302:120653. doi: 10.1016/j.lfs.2022.120653
63. Biedroń R, Peruń A, and Józefowski S. CD36 differently regulates macrophage responses to smooth and rough lipopolysaccharide. PloS One. (2016) 11:e0153558. doi: 10.1371/journal.pone
64. Chen S, Xia J, Zhang Y, and Zhan Q. IL35 attenuated LPS-induced acute lung injury by regulating macrophage polarization. Mol Biol Rep. (2022) 49:5811–20. doi: 10.1007/s11033-022-07293-5
65. Balic JJ, Albargy H, Luu K, Kirby FJ, Jayasekara WSN, Mansell F, et al. STAT3 serine phosphorylation is required for TLR4 metabolic reprogramming and IL-1β expression. Nat Commun. (2020) 11:3816. doi: 10.1038/s41467-020-17669-5
66. Yi Z, Wu Y, Zhang W, Wang T, Gong J, Cheng Y, et al. Activator-mediated pyruvate kinase M2 activation contributes to endotoxin tolerance by promoting mitochondrial biogenesis. Front Immunol. (2021) 11:595316. doi: 10.3389/fimmu.2020.595316
67. Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. (2016) 17:684–96. doi: 10.1016/j.celrep.2016.09.008
68. Kuang J, Fang J, Hu S, Yang X, and Fan X. Mechanism Of microRNA-218-5p in mitochondrial biogenesis of sepsis-induced acute kidney injury by the regulation Of PGC-1α. Shock. (2024) 62:426–36. doi: 10.1097/SHK.0000000000002410
69. Fuke N, Nagata N, Suganuma H, and Ota T. Regulation of gut microbiota and metabolic endotoxemia with dietary factors. Nutrients. (2019) 11:2277. doi: 10.3390/nu11102277
70. Chmielarz M, Bromke MA, Olbromski M, Środa-Pomianek K, Frej-Mądrzak M, Dzięgiel P, et al. Lipidomics analysis of human HMC3 microglial cells in an in vitro model of metabolic syndrome. Biomolecules. (2024) 14:1238. doi: 10.3390/biom14101238
71. Sharma M, Mohapatra J, Malik U, Nagar J, Chatterjee A, Ramachandran B, et al. Effect of pioglitazone on metabolic features in endotoxemia model in obese diabetic db/db mice. J Diabetes. (2017) 9:613–21. doi: 10.1111/1753-0407.12450
72. Huang J, Nong X, Chen Y, Zhang A, and Chen L. 3-O-trans-caffeoyloleanolic acid improves acute lung injury via anti-inflammation and antioxidative stress-involved PI3K/AKT pathway. Chem Biol Drug Des. (2021) 98:114–26. doi: 10.1111/cbdd.13856
73. Gu M and Pang Z. Luteolin inhibits inflammation and M1 macrophage polarization in the treatment of Pseudomonas aeruginosa-induced acute pneumonia through suppressing EGFR/PI3K/AKT/NF-κB and EGFR/ERK/AP-1 signaling pathways. Phytomedicine. (2025) 141:156663. doi: 10.1016/j.phymed.2025.156663
74. Yan S, Zhou M, Zheng X, Xing Y, Dong J, Yan M, et al. Anti-inflammatory effect of curcumin on the mouse model of myocardial infarction through regulating macrophage polarization. Mediators Inflamm. (2021) 2021:9976912. doi: 10.1155/2021/9976912
75. Cheng S, Zhou L, Wang WY, Zhang MJ, Yang QC, Da Wang W, et al. Mitochondria-loading erythrocytes transfer mitochondria to ameliorate inflammatory bone loss. Acta Biomater. (2025) 195:225–39. doi: 10.1016/j.actbio.2025.02.024
76. Wang Y, Zhang R, Chen Q, Lei Z, Shi C, Pang Y, et al. PPARγ Agonist pioglitazone prevents hypoxia-induced cardiac dysfunction by reprogramming glucose metabolism. Int J Biol Sci. (2024) 20:4297–313. doi: 10.7150/ijbs.98387
77. Zhang Q, Sun W, Li T, and Liu F. Polarization behavior of bone macrophage as well as associated osteoimmunity in glucocorticoid-induced osteonecrosis of the femoral head. J Inflammation Res. (2023) 16:879–94. doi: 10.2147/JIR.S401968
78. Cosola C, Rocchetti MT, di Bari I, Acquaviva PM, Maranzano V, Corciulo S, et al. An innovative synbiotic formulation decreases free serum indoxyl sulfate, small intestine permeability and ameliorates gastrointestinal symptoms in a randomized pilot trial in stage IIIb-IV CKD patients. Toxins (Basel). (2021) 13:334. doi: 10.3390/toxins13050334
79. Meijers B, Jouret F, and Evenepoel P. Linking gut microbiota to cardiovascular disease and hypertension: Lessons from chronic kidney disease. Pharmacol Res. (2018) 133:101–7. doi: 10.1016/j.phrs.2018.04.023
80. Rysz J, Franczyk B, Ławiński J, Olszewski R, Ciałkowska-Rysz A, and Gluba-Brzózka A. The impact of CKD on uremic toxins and gut microbiota. Toxins (Basel). (2021) 13:252. doi: 10.3390/toxins13040252
81. Holle J, Kirchner M, Okun J, Bayazit AK, Obrycki L, Canpolat N, et al. Serum indoxyl sulfate concentrations associate with progression of chronic kidney disease in children. PloS One. (2020) 15:e0240446. doi: 10.1371/journal.pone
82. Liu JR, Miao H, Deng DQ, Vaziri ND, Li P, and Zhao YY. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol Life Sci. (2021) 78:909–22. doi: 10.1007/s00018-020-03645-1
83. Brito JS, Borges NA, Anjos JSD, Nakao LS, Stockler-Pinto MB, Paiva BR, et al. Aryl hydrocarbon receptor and uremic toxins from the gut microbiota in chronic kidney disease patients: is there a relationship between them? Biochemistry. (2019) 58:2054–60. doi: 10.1021/acs.biochem.8b01305
84. Li YJ, Ma J, Loh YW, Chadban SJ, and Wu H. Short-chain fatty acids directly exert anti-inflammatory responses in podocytes and tubular epithelial cells exposed to high glucose. Front Cell Dev Biol. (2023) 11:1182570. doi: 10.3389/fcell.2023.1182570
85. Xiao N, He W, Chen S, Yao Y, Wu N, Xu M, et al. Protective effect of egg yolk lipids against dextran sulfate sodium-induced colitis: the key role of gut microbiota and short-chain fatty acids. Mol Nutr Food Res. (2024) 68:e2400048. doi: 10.1002/mnfr.202400048
86. Fang J, Yu CH, Li XJ, Yao JM, Fang ZY, Yoon SH, et al. Gut dysbiosis in nonalcoholic fatty liver disease: pathogenesis, diagnosis, and therapeutic implications. Front Cell Infect Microbiol. (2022) 12:997018. doi: 10.3389/fcimb.2022.997018
87. Tsuji K, Uchida N, Nakanoh H, Fukushima K, Haraguchi S, Kitamura S, et al. The gut-kidney axis in chronic kidney diseases. Diagnostics (Basel). (2024) 15:21. doi: 10.3390/diagnostics15010021
88. Li S, Zhuge A, Chen H, Han S, Shen J, Wang K, et al. Sedanolide alleviates DSS-induced colitis by modulating the intestinal FXR-SMPD3 pathway in mice. J Adv Res. (2025) 69:413–26. doi: 10.1016/j.jare.2024.03.026
89. Liu X, Li J, Shi M, Fu J, Wang Y, Kang W, et al. Melatonin improves cholestatic liver disease via the gut-liver axis. J Pineal Res. (2024) 76:e12929. doi: 10.1111/jpi.12929
90. Yang M, Gu Y, Li L, Liu T, Song X, Sun Y, et al. Bile acid-gut microbiota axis in inflammatory bowel disease: from bench to bedside. Nutrients. (2021) 13:3143. doi: 10.3390/nu13093143
91. Liu W, Zhang Y, Zheng M, Ye Y, Shi M, Wang X, et al. Polysaccharides in Medicinal and Food Homologous Plants regulate intestinal flora to improve type 2 diabetes: Systematic review. Phytomedicine. (2024) 134:156027. doi: 10.1016/j.phymed.2024.156027
92. Chen J, Wu S, Wu R, Ai H, Lu X, Wang J, et al. Essential oil from Artemisia argyi alleviated liver disease in zebrafish (Danio rerio) via the gut-liver axis. Fish Shellfish Immunol. (2023) 140:108962. doi: 10.1016/j.fsi.2023.108962
93. Gu Z, Zhu Y, Mei F, Dong X, Xia G, and Shen X. Tilapia head glycolipids protect mice against dextran sulfate sodium-induced colitis by ameliorating the gut barrier and suppressing NF-kappa B signaling pathway. Int Immunopharmacol. (2021) 96:107802. doi: 10.1016/j.intimp.2021.107802
94. Meng F, Hao P, and Du H. Regulatory T cells differentiation in visceral adipose tissues contributes to insulin resistance by regulating JAZF-1/PPAR-γ pathway. J Cell Mol Med. (2023) 27:553–62. doi: 10.1111/jcmm.17680
95. Wang Q, Zhang J, and Wang F. A study of the predictive value of Treg and Th1/Th2 cytokines on pregnancy outcome in patients with recurrent pregnancy loss. Altern Ther Health Med. (2023) 29:400–3.
96. Ribeiro FPB, de Luna Freire MO, de Oliveira Coutinho D, de Santana Cirilo MA, and de Brito Alves JL. Gut dysbiosis and probiotic therapy in chronic kidney disease: A comprehensive review. Probiotics Antimicrob Proteins. (2024). doi: 10.1007/s12602-024-10427-9
97. Rao Z, Zeng J, Li X, Peng L, Wang B, Luan F, et al. JFNE-A isolated from Jing-Fang n-butanol extract attenuates lipopolysaccharide-induced acute lung injury by inhibiting oxidative stress and the NF-κB signaling pathway via promotion of autophagy. Phytomedicine. (2022) 96:153891. doi: 10.1016/j.phymed.2021.153891
98. Noori M, Azimirad M, Ghorbaninejad M, Meyfour A, Zali MR, and Yadegar A. PPAR-γ agonist mitigates intestinal barrier dysfunction and inflammation induced by Clostridioides difficile SlpA in vitro. Sci Rep. (2024) 14:32087. doi: 10.1038/s41598-024-83815-4
99. Wu X, Lv Y, Li Z, and Yang Z. Serelaxin inhibits lipopolysaccharide-induced inflammatory response in cardiac fibroblasts by activating peroxisome proliferator-activated receptor-γ and suppressing the nuclear factor-kappa B signaling pathway. J Cardiovasc Pharmacol. (2023) 82:201–11. doi: 10.1097/FJC.0000000000001447
100. Li Y, Li ZX, Xie CY, Fan J, Lv J, Xu XJ, et al. Gegen Qinlian decoction enhances immunity and protects intestinal barrier function in colorectal cancer patients via gut microbiota. World J Gastroenterol. (2020) 26:7633–51. doi: 10.3748/wjg.v26.i48.7633
101. Huang X, Oshima T, Tomita T, Fukui H, and Miwa H. Butyrate alleviates cytokine-induced barrier dysfunction by modifying claudin-2 levels. Biol (Basel). (2021) 10:205. doi: 10.3390/biology10030205
102. Wang X, Wang J, Okyere SK, Huang R, Shao C, Yousif M, et al. Ageratina adenophora damages the rumen epithelium via inducing the expression of inflammatory factors in goats. J Anim Sci. (2024) 102:skad418. doi: 10.1093/jas/skad418
103. Hou Q, Zhu S, Zhang C, Huang Y, Guo Y, Li P, et al. Berberine improves intestinal epithelial tight junctions by upregulating A20 expression in IBS-D mice. BioMed Pharmacother. (2019) 118:109206. doi: 10.1016/j.biopha.2019.109206
104. Pan H, Hu T, He Y, Zhong G, Wu S, Jiang X, et al. Curcumin attenuates aflatoxin B1-induced ileum injury in ducks by inhibiting NLRP3 inflammasome and regulating TLR4/NF-κB signaling pathway. Mycotoxin Res. (2024) 40:255–68. doi: 10.1007/s12550-024-00524-7
105. Kuo YW, Huang YY, Tsai SY, Wang JY, Lin JH, Syu ZJ, et al. Probiotic formula ameliorates renal dysfunction indicators, glycemic levels, and blood pressure in a diabetic nephropathy mouse model. Nutrients. (2023) 15:2803. doi: 10.3390/nu15122803
106. Wu Z, Zeng H, Zhang L, Pu Y, Li S, Yuan Y, et al. Patchouli alcohol: a natural sesquiterpene against both inflammation and intestinal barrier damage of ulcerative colitis. Inflammation. (2020) 43:1423–35. doi: 10.1007/s10753-020-01219-8
107. Sun X, Cui Y, Su Y, Gao Z, Diao X, Li J, et al. Dietary fiber ameliorates lipopolysaccharide-induced intestinal barrier function damage in piglets by modulation of intestinal microbiome. mSystems. (2021) 6:e01374–20. doi: 10.1128/mSystems.01374-20
108. Lai Z, Lin L, Zhang J, and Mao S. Effects of high-grain diet feeding on mucosa-associated bacterial community and gene expression of tight junction proteins and inflammatory cytokines in the small intestine of dairy cattle. J Dairy Sci. (2022) 105:6601–15. doi: 10.3168/jds.2021-21355
109. Zhang D, Jing B, Chen ZN, Li X, Shi HM, Zheng YC, et al. Ferulic acid alleviates sciatica by inhibiting neuroinflammation and promoting nerve repair via the TLR4/NF-κB pathway. CNS Neurosci Ther. (2023) 29:1000–11. doi: 10.1111/cns.14060
110. Paul P, Kaul R, and Chaari A. Renal health improvement in diabetes through microbiome modulation of the gut-kidney axis with biotics: A systematic and narrative review of randomized controlled trials. Int J Mol Sci. (2022) 23:14838. doi: 10.3390/ijms232314838
111. Cheng G, Liu Y, Guo R, Wang H, Zhang W, and Wang Y. Molecular mechanisms of gut microbiota in diabetic nephropathy. Diabetes Res Clin Pract. (2024) 213:111726. doi: 10.1016/j.diabres.2024.111726
112. Fu R, Niu R, Zhao F, Wang J, Cao Q, Yu Y, et al. Exercise alleviated intestinal damage and microbial disturbances in mice exposed to fluoride. Chemosphere. (2022) 288:132658. doi: 10.1016/j.chemosphere.2021.132658
113. Ye L, Wang Y, Xiao F, Wang X, Li X, Cao R, et al. prausnitzii-derived extracellular vesicles attenuate experimental colitis by regulating intestinal homeostasis in mice. Microb Cell Fact. (2023) 22:235. doi: 10.1186/s12934-023-02243-7
114. Ruocco C, Segala A, Valerio A, and Nisoli E. Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress. Curr Opin Clin Nutr Metab Care. (2021) 24:88–95. doi: 10.1097/MCO.0000000000000704
115. Wang C, Peng Y, Zhang Y, Xu J, Jiang S, Wang L, et al. The biological functions and metabolic pathways of valine in swine. J Anim Sci Biotechnol. (2023) 14:135. doi: 10.1186/s40104-023-00927-z
116. Chen C, Naveed H, and Chen K. Research progress on branched-chain amino acid aminotransferases. Front Genet. (2023) 14:1233669. doi: 10.3389/fgene.2023.1233669
117. Zhao X, Gang X, Liu Y, Sun C, Han Q, and Wang G. Using metabolomic profiles as biomarkers for insulin resistance in childhood obesity: A systematic review. J Diabetes Res. (2016) 2016:8160545. doi: 10.1155/2016/8160545
118. Mehta NH, Huey SL, Kuriyan R, Peña-Rosas JP, Finkelstein JL, Kashyap S, et al. Potential mechanisms of precision nutrition-based interventions for managing obesity. Adv Nutr. (2024) 15:100186. doi: 10.1016/j.advnut.2024.100186
119. Wang DD and Hu FB. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol. (2018) 6:416–26. doi: 10.1016/S2213-8587(18)30037-8
120. Voruganti VS. Precision nutrition: recent advances in obesity. Physiol (Bethesda). (2023) 38:42–50. doi: 10.1152/physiol.00014.2022
121. Hong BV, Agus JK, Tang X, Zheng JJ, Romo EZ, Lei S, et al. Precision nutrition and cardiovascular disease risk reduction: the promise of high-density lipoproteins. Curr Atheroscler Rep. (2023) 25:663–77. doi: 10.1007/s11883-023-01148-5
122. Qi L. Nutrition for precision health: The time is now. Obes (Silver Spring). (2022) 30:1335–44. doi: 10.1002/oby.23448
123. Ye Z, Wang S, Zhang C, and Zhao Y. Coordinated modulation of energy metabolism and inflammation by branched-chain amino acids and fatty acids. Front Endocrinol (Lausanne). (2020) 11:617. doi: 10.3389/fendo.2020.00617
124. VanDerStad LR, Wyatt EC, and Vaughan RA. Excess branched-chain amino acids suppress mitochondrial function and biogenic signaling but not mitochondrial dynamics in a myotube model of skeletal muscle insulin resistance. Metabolites. (2024) 14:389. doi: 10.3390/metabo14070389
125. Fernicola J, Vyavahare S, Gupta SK, Kalwaghe A, Kosmac K, Davis A, et al. The role of branched chain ketoacid dehydrogenase kinase (BCKDK) in skeletal muscle biology and pathogenesis. Int J Mol Sci. (2024) 25:7601. doi: 10.3390/ijms25147601
126. Tang L, Yuan L, Yang G, Wang F, Fu M, Chen M, et al. Changes in whole metabolites after exenatide treatment in overweight/obese polycystic ovary syndrome patients. Clin Endocrinol (Oxf). (2019) 91:508–16. doi: 10.1111/cen.14056
127. Li X and Zhang HS. Amino acid metabolism, redox balance and epigenetic regulation in cancer. FEBS J. (2024) 291:412–29. doi: 10.1111/febs.16803
128. Robertson S, Clarke ED, Gómez-Martín M, Cross V, Collins CE, and Stanford J. Do precision and personalised nutrition interventions improve risk factors in adults with prediabetes or metabolic syndrome? A systematic review of randomised controlled trials. Nutrients. (2024) 16:1479. doi: 10.3390/nu16101479
129. Chen M, Miao G, Huo Z, Peng H, Wen X, Anton S, et al. Longitudinal profiling of fasting plasma metabolome in response to weight-loss interventions in patients with morbid obesity. Metabolites. (2024) 14:116. doi: 10.3390/metabo14020116
130. Kim JK, Hong S, Park J, and Kim S. Metabolic and transcriptomic changes in the mouse brain in response to short-term high-fat metabolic stress. Metabolites. (2023) 13:407. doi: 10.3390/metabo13030407
131. Leshem A, Segal E, and Elinav E. The gut microbiome and individual-specific responses to diet. mSystems. (2020) 5:e00665–20. doi: 10.1128/mSystems.00665-20
132. Pontoizeau C, Gaborit C, Tual N, Simon-Sola M, Rotaru I, Benoist M, et al. Successful treatment of severe MSUD in Bckdhb-/- mice with neonatal AAV gene therapy. J Inherit Metab Dis. (2024) 47:41–9. doi: 10.1002/jimd.12604
133. Yadegar A, Bar-Yoseph H, Monaghan TM, Pakpour S, Severino A, Kuijper EJ, et al. Fecal microbiota transplantation: current challenges and future landscapes. Clin Microbiol Rev. (2024) 37:e0006022. doi: 10.1128/cmr.00060-22
134. Ianiro G, Punčochář M, Karcher N, Porcari S, Armanini F, Asnicar F, et al. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat Med. (2022) 28:1913–23. doi: 10.1038/s41591-022-01964-3
135. Wortelboer K, Nieuwdorp M, and Herrema H. Fecal microbiota transplantation beyond Clostridioides difficile infections. EBioMedicine. (2019) 44:716–29. doi: 10.1016/j.ebiom.2019.05.066
136. Deng Q, Du L, Zhang Y, and Liu G. NEFAs influence the inflammatory and insulin signaling pathways through TLR4 in primary calf hepatocytes in vitro. Front Vet Sci. (2021) 8:755505. doi: 10.3389/fvets.2021.755505
137. Hou S, Yu J, Li Y, Zhao D, and Zhang Z. Advances in fecal microbiota transplantation for gut dysbiosis-related diseases. Adv Sci (Weinh). (2025) 12:e2413197. doi: 10.1002/advs.202413197
138. Li S, Zhao X, Lin F, Ni X, Liu X, Kong C, et al. Gut flora mediates the rapid tolerance of electroacupuncture on ischemic stroke by activating melatonin receptor through regulating indole-3-propionic acid. Am J Chin Med. (2022) 50:979–1006. doi: 10.1142/S0192415X22500409
139. Zhong Y, Cao J, Ma Y, Zhang Y, Liu J, and Wang H. Fecal microbiota transplantation donor and dietary fiber intervention collectively contribute to gut health in a mouse model. Front Immunol. (2022) 13:842669. doi: 10.3389/fimmu.2022.842669
140. Antonopoulos DA and Chang EB. Transplanting a microbial organ: the good, the bad, and the unknown. mBio. (2016) 7:e00572–16. doi: 10.1128/mBio.00572-16
141. de Andrade LS, Sardá FAH, Pereira NBF, Teixeira RR, Rodrigues SD, de Lima JD, et al. Effect of unripe banana flour on gut-derived uremic toxins in individuals undergoing peritoneal dialysis: A randomized, double-blind, placebo-controlled, crossover trial. Nutrients. (2021) 13:646. doi: 10.3390/nu13020646
142. Hayashi K, Uchida R, Horiba T, Kawaguchi T, Gomi K, and Goto Y. Soy sauce-like seasoning enhances the growth of Agathobacter rectalis and the production of butyrate, propionate, and lactate. Biosci Microbiota Food Health. (2024) 43:275–81. doi: 10.12938/bmfh.2023-103
143. Pezeshki B, Abdulabbas HT, Alturki AD, Mansouri P, Zarenezhad E, Nasiri-Ghiri M, et al. Synergistic interactions between probiotics and anticancer drugs: mechanisms, benefits, and challenges. Probiotics Antimicrob Proteins. (2025) 503:90–98. doi: 10.1007/s12602-025-10462-0
144. Chen Y, Zhiliang L, Jiaqu C, Xiaoqiong L, Shaoyi Z, Chunlian M, et al. Fecal microbiota and human intestinal fluid transplantation: methodologies and outlook. Front Med (Lausanne). (2022) 9:830004. doi: 10.3389/fmed.2022.830004
145. Antushevich H. Fecal microbiota transplantation in disease therapy. Clin Chim Acta. (2020) 503:90–8. doi: 10.1016/j.cca.2019.12.010
146. Iwashita Y, Ohya M, Yashiro M, Sonou T, Kawakami K, Nakashima Y, et al. Dietary changes involving bifidobacterium longum and other nutrients delays chronic kidney disease progression. Am J Nephrol. (2018) 47:325–32. doi: 10.1159/000488947
147. Aoe S, Nakamura F, and Fujiwara S. Effect of wheat bran on fecal butyrate-producing bacteria and wheat bran combined with barley on bacteroides abundance in Japanese healthy adults. Nutrients. (2018) 10:1980. doi: 10.3390/nu10121980
148. Nicese MN, Bijkerk R, Van Zonneveld AJ, Van den Berg BM, and Rotmans JI. Sodium butyrate as key regulator of mitochondrial function and barrier integrity of human glomerular endothelial cells. Int J Mol Sci. (2023) 24:13090. doi: 10.3390/ijms241713090
149. Beker BM, Colombo I, Gonzalez-Torres H, and Musso CG. Decreasing microbiota-derived uremic toxins to improve CKD outcomes. Clin Kidney J. (2022) 15:2214–9. doi: 10.1093/ckj/sfac154
150. Młynarska E, Budny E, Saar M, Wojtanowska E, Jankowska J, Marciszuk S, et al. Does the composition of gut microbiota affect chronic kidney disease? Molecular mechanisms contributed to decreasing glomerular filtration rate. Int J Mol Sci. (2024) 25:10429. doi: 10.3390/ijms251910429
151. Schwarz A, Philippsen R, and Schwarz T. Induction of regulatory T cells and correction of cytokine disbalance by short-chain fatty acids: implications for psoriasis therapy. J Invest Dermatol. (2021) 141:95–104.e2. doi: 10.1016/j.jid.2020.04.031
152. Schwarz A, Bruhs A, and Schwarz T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J Invest Dermatol. (2017) 137:855–64. doi: 10.1016/j.jid.2016.11.014
153. Haase S, Haghikia A, Wilck N, Müller DN, and Linker RA. Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology. (2018) 154:230–8. doi: 10.1111/imm.12933
154. Favero C, Giordano L, Mihaila SM, Masereeuw R, Ortiz A, and Sanchez-Niño MD. Postbiotics and kidney disease. Toxins (Basel). (2022) 14:623. doi: 10.3390/toxins14090623
155. Cui H, Wang N, Li H, Bian Y, Wen W, Kong X, et al. The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: a crosstalk between ancient "Yin-Yang" theory and modern immunology. Cell Commun Signal. (2024) 22:99. doi: 10.1186/s12964-024-01505-0
156. Yamaguchi T, Tsuji S, Akagawa S, Akagawa Y, Kino J, Yamanouchi S, et al. Clinical significance of probiotics for children with idiopathic nephrotic syndrome. Nutrients. (2021) 13:365. doi: 10.3390/nu13020365
157. Gholami A, Montazeri-Najafabady N, Ashoori Y, Kazemi K, Heidari R, Omidifar N, et al. The ameliorating effect of limosilactobacillus fermentum and its supernatant postbiotic on cisplatin-induced chronic kidney disease in an animal model. BMC Complement Med Ther. (2023) 23:243. doi: 10.1186/s12906-023-04068-8
158. Yan JB, Luo MM, Chen ZY, and He BH. The function and role of the Th17/Treg cell balance in inflammatory bowel disease. J Immunol Res. (2020) 2020:8813558. doi: 10.1155/2020/8813558
159. Azamian Y, Abdollahzad H, Rezaeian S, Rouhani MH, and Fatehi MH. The effect of synbiotic supplementation on plasma levels of advanced glycation end products and cardiovascular risk factors in hemodialysis patients: A double-blind clinical trial. Food Sci Nutr. (2024) 12:6864–72. doi: 10.1002/fsn3.4338
160. Zhao Y, Li S, Lessing DJ, and Chu W. The attenuating effects of synbiotic containing Cetobacterium somerae and Astragalus polysaccharide against trichlorfon-induced hepatotoxicity in crucian carp (Carassius carassius). J Hazard Mater. (2024) 461:132621. doi: 10.1016/j.jhazmat.2023.132621
161. Li X, Xiao S, Li F, Fang K, Wen J, and Gong H. Max interacting protein 1 induces IL-17-producing T helper/regulatory T imbalance in osteoarthritis by upregulating tectonic family member 2. Tissue Cell. (2022) 78:101906. doi: 10.1016/j.tice.2022.101906
162. Tsai CW, Huang HW, Lee YJ, and Chen MJ. Investigating the efficacy of kidney- protective lactobacillus mixture-containing pet treats in feline chronic kidney disease and its possible mechanism. Anim (Basel). (2024) 14:630. doi: 10.3390/ani14040630
163. Yang L, Lin H, Lin W, and Xu X. Exercise ameliorates insulin resistance of type 2 diabetes through motivating short-chain fatty acid-mediated skeletal muscle cell autophagy. Biol (Basel). (2020) 9:203. doi: 10.3390/biology9080203
164. Kobayashi M, Mikami D, Kimura H, Kamiyama K, Morikawa Y, Yokoi S, et al. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun. (2017) 486:499–505. doi: 10.1016/j.bbrc.2017.03.071
165. Stockler-Pinto MB, Soulage CO, Borges NA, Cardozo LFMF, Dolenga CJ, Nakao LS, et al. From bench to the hemodialysis clinic: protein-bound uremic toxins modulate NF-κB/Nrf2 expression. Int Urol Nephrol. (2018) 50:347–54. doi: 10.1007/s11255-017-1748-y
166. Shu C, Yan-Yan Z, Hai Z, Long-Kun D, Yue X, Man Y, et al. Anti-inflammatory effects of NaB and NaPc in Acinetobacter baumannii-stimulated THP-1 cells via TLR-2/NF-κB/ROS/NLRP3 pathway. Acta Pharm. (2022) 72:615–28. doi: 10.2478/acph-2022-0036
167. Yamaguchi J, Tanaka T, and Inagi R. Effect of AST-120 in chronic kidney disease treatment: still a controversy? Nephron. (2017) 135:201–6. doi: 10.1159/000453673
168. Wei J, Li R, Zhang P, Jin H, Zhang Z, Li Y, et al. Efficient selective removal of uremic toxin precursor by olefin-linked covalent organic frameworks for nephropathy treatment. Nat Commun. (2023) 14:2805. doi: 10.1038/s41467-023-38427-3
169. Thomas SS, Cha YS, and Kim KA. Protective effect of diet-supplemented and endogenously produced omega-3 fatty acids against HFD-induced colon inflammation in mice. Foods. (2022) 11:2124. doi: 10.3390/foods11142124
170. Choi SY, Kim S, Jeon JY, Kim MG, Lee SY, and Shin KH. Metabolomic profiles in patients with cervical cancer undergoing cisplatin and radiation therapy. Biomol Ther (Seoul). (2024) 32:379–89. doi: 10.4062/biomolther.2023.159
171. Hiraga Y, Kubota T, Katoh M, Horai Y, Suzuki H, Yamashita Y, et al. AST-120 treatment alters the gut microbiota composition and suppresses hepatic triglyceride levels in obese mice. Endocr Res. (2021) 46:178–85. doi: 10.1080/07435800.2021.1927074
172. Gryp T, De Paepe K, Vanholder R, Kerckhof FM, Van Biesen W, Van de Wiele T, et al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int. (2020) 97:1230–42. doi: 10.1016/j.kint.2020.01.028
173. Roson-Calero N, Ballesté-Delpierre C, Fernández J, and Vila J. Insights on current strategies to decolonize the gut from multidrug-resistant bacteria: pros and cons. Antibiotics (Basel). (2023) 12:1074. doi: 10.3390/antibiotics12061074
174. Yang CY, Chen TW, Lu WL, Liang SS, Huang HD, Tseng CP, et al. Synbiotics alleviate the gut indole load and dysbiosis in chronic kidney disease. Cells. (2021) 10:114. doi: 10.3390/cells10010114
175. El Haddad L, Mendoza JF, and Jobin C. Bacteriophage-mediated manipulations of microbiota in gastrointestinal diseases. Front Microbiol. (2022) 13:1055427. doi: 10.3389/fmicb.2022.1055427
176. Alvarenga L, Cardozo LFMF, Leal VO, Kemp JA, Saldanha JF, Ribeiro-Alves M, et al. Can resveratrol supplementation reduce uremic toxin plasma levels from the gut microbiota in nondialyzed patients with chronic kidney disease? J Ren Nutr. (2022) 32:685–91. doi: 10.1053/j.jrn.2022.01.010
177. Chen L, Shi J, Ma X, Shi D, and Qu H. Effects of microbiota-driven therapy on circulating indoxyl sulfate and P-cresyl sulfate in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Adv Nutr. (2022) 13:1267–78. doi: 10.1093/advances/nmab149
178. Kousgaard SJ, Nielsen HL, Kirk KF, and Thorlacius-Ussing O. Consumption of yoghurt favours remission after faecal microbiota transplantation for chronic pouchitis. Int J Colorectal Dis. (2020) 35:1955–8. doi: 10.1007/s00384-020-03648-1
179. Arteaga-Muller GY, Flores-Treviño S, Bocanegra-Ibarias P, Robles-Espino D, Garza-González E, Fabela-Valdez GC, et al. Changes in the progression of chronic kidney disease in patients undergoing fecal microbiota transplantation. Nutrients. (2024) 16:1109. doi: 10.3390/nu16081109
180. Zhong HJ, Xie X, Chen WJ, Zhuang YP, Hu X, Cai YL, et al. Washed microbiota transplantation improves renal function in patients with renal dysfunction: a retrospective cohort study. J Transl Med. (2023) 21:740. doi: 10.1186/s12967-023-04570-0
181. Speckmann B, Ehring E, Hu J, and Rodriguez Mateos A. Exploring substrate-microbe interactions: a metabiotic approach toward developing targeted synbiotic compositions. Gut Microbes. (2024) 16:2305716. doi: 10.1080/19490976.2024.2305716
182. Wang M, Song Z, Lai S, Tang F, Dou L, and Yang F. Depression-associated gut microbes, metabolites and clinical trials. Front Microbiol. (2024) 15:1292004. doi: 10.3389/fmicb.2024.1292004
183. Conti G, D'Amico F, Fabbrini M, Brigidi P, Barone M, and Turroni S. Pharmacomicrobiomics in anticancer therapies: why the gut microbiota should be pointed out. Genes (Basel). (2022) 14:55. doi: 10.3390/genes14010055
184. Polcz VE, Barrios EL, Larson SD, Efron PA, and Rincon JC. Charting the course for improved outcomes in chronic critical illness: therapeutic strategies for persistent inflammation, immunosuppression, and catabolism syndrome (PICS). Br J Anaesth. (2024) 133:260–3. doi: 10.1016/j.bja.2024.05.005
185. Strati F, Lattanzi G, Amoroso C, and Facciotti F. Microbiota-targeted therapies in inflammation resolution. Semin Immunol. (2022) 59:101599. doi: 10.1016/j.smim.2022.101599
186. Qusty N, Sarhan A, Taha M, Alshanqiti A, Almuteb AM, Alfaraidi AT, et al. The role of gut microbiota in the efficacy and side effect profile of biologic therapies for autoimmune diseases. Cureus. (2024) 16:e71111. doi: 10.7759/cureus.71111
187. Xu Z, Li W, Dong X, Chen Y, Zhang D, Wang J, et al. Precision medicine in colorectal cancer: Leveraging multi-omics, spatial omics, and artificial intelligence. Clin Chim Acta. (2024) 559:119686. doi: 10.1016/j.cca.2024.119686
188. Molefi T, Mabonga L, Hull R, Sebitloane M, and Dlamini Z. From genes to clinical practice: exploring the genomic underpinnings of endometrial cancer. Cancers (Basel). (2025) 17:320. doi: 10.3390/cancers17020320
189. Olatunji G, Kokori E, Yusuf I, Ayanleke E, Damilare O, Afolabi S, et al. Stem cell-based therapies for heart failure management: a narrative review of current evidence and future perspectives. Heart Fail Rev. (2024) 29:573–98. doi: 10.1007/s10741-023-10351-0
190. Seo H, Kim S, Beck S, and Song HY. Perspectives on microbiome therapeutics in infectious diseases: A comprehensive approach beyond immunology and microbiology. Cells. (2024) 13:2003. doi: 10.3390/cells13232003
191. Sahle Z, Engidaye G, Shenkute Gebreyes D, Adenew B, and Abebe TA. Fecal microbiota transplantation and next-generation therapies: A review on targeting dysbiosis in metabolic disorders and beyond. SAGE Open Med. (2024) 12:20503121241257486. doi: 10.1177/20503121241257486
192. Porcari S, Benech N, Valles-Colomer M, Segata N, Gasbarrini A, Cammarota G, et al. Key determinants of success in fecal microbiota transplantation: From microbiome to clinic. Cell Host Microbe. (2023) 31:712–33. doi: 10.1016/j.chom.2023.03.020
Keywords: diabetic kidney disease, gut microbiota, short-chain fatty acids (SCFAs), probiotics, fecal microbiota transplantation (FMT)
Citation: Wang X, Liu X, Gong F, Jiang Y, Zhang C, Zhou W and Zhang W (2025) Targeting gut microbiota for diabetic nephropathy treatment: probiotics, dietary interventions, and fecal microbiota transplantation. Front. Endocrinol. 16:1621968. doi: 10.3389/fendo.2025.1621968
Received: 02 May 2025; Accepted: 03 June 2025;
Published: 30 June 2025.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Soumik Das, Vellore Institute of Technology, IndiaXiangyu Zou, Shanghai Children’s Hospital, China
Copyright © 2025 Wang, Liu, Gong, Jiang, Zhang, Zhou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Zhang, MzA2NzYyODI3QHFxLmNvbQ==; Wei Zhou, MjM2MjAxMDRAcXEuY29t
†These authors have contributed equally to this work and share first authorship
 Xiaoran Wang
Xiaoran Wang Xinyin Liu
Xinyin Liu Fanghong Gong1
Fanghong Gong1 Wen Zhang
Wen Zhang